Introduction
The prevalence of diabetes mellitus (DM) is on an
upward trajectory, with projections indicating an increase to over
700 million individuals by the year 2045(1). As of 2019, it is estimated that
>463 million individuals globally are afflicted with DM,
positioning the management and prevention of this condition as a
primary global health objective (2). Type 2 DM (T2DM) is characterized by
hyperglycemia, insulin resistance and compromised insulin
secretion, representing the predominant form of diabetes and
accounting for >90% of DM cases worldwide. This condition
impacts hundreds of millions globally (3). Despite the significant lifetime risk
associated with T2DM, accurately predicting and preventing the
disease in the general populace remains a significant
challenge.
Vitamin D, encompassing cholecalciferol (vitamin D3)
and ergocalciferol (vitamin D2), serves as a precursor to hormones
and plays a pivotal role in the regulation of calcium and phosphate
metabolism (4). The biosynthesis
of vitamin D initiates with the irradiation of 7-dehydrocholesterol
in the skin by ultraviolet B radiation under the influence of
strong sunlight, constituting the principal mechanism of vitamin D
production (5). The intake of
vitamin D and its protective effects against T2DM have been the
focus of extensive research.
Several strands of evidence suggest a potential role
for vitamin D in the prevention of T2DM. First, vitamin D may
regulate numerous processes implicated in the initiation of T2DM,
including the modulation of calcium ion concentration and the
generation of reactive oxygen species (ROS) (6,7).
Second, vitamin D is recognized for its role in maintaining normal
mitochondrial function, crucial for cellular bioenergetics
(8). Lastly, vitamin D has been
shown to mitigate inflammation, thereby aiding in the control of
insulin resistance (9).
Consequently, the proposition that vitamin D mitigates the onset of
T2DM can be elucidated by its multifaceted mechanisms of action, a
subject that has been rigorously examined within both clinical and
basic research spheres.
Nevertheless, the hypothesis that vitamin D status
could affect the risk of T2DM, despite its biological plausibility,
is met with consistently inconclusive results. The real-world
efficacy of vitamin D supplementation in diminishing the incidence
of new T2DM cases remains ambiguous, notwithstanding theoretical
rationale. This ambiguity underscores the critical necessity to
integrate the findings from extant clinical trials, highlighting
the imperative to rigorously evaluate the influence of vitamin D
consumption on the development of T2DM. Accordingly, the present
meta-analysis was designed to amalgamate the outcomes of clinical
investigations concerning the impact of vitamin D supplementation
on the progression of T2DM.
Materials and methods
Search strategy
A comprehensive search of English-language
literature was conducted through MEDLINE (https://pubmed.ncbi.nlm.nih.gov), EMBASE (https://www.embase.com) and the Cochrane Library
(https://www.cochranelibrary.com) for
studies published from January 2010 to December 2023. The search
strategy employed was ‘(diabetes* or
hyperglycemia*) and (vitamin D or
cholecalciferol* or ergocalciferol*)’, with a
restriction to randomized controlled trials.
Study selection criteria
Eligible studies were required to meet the following
inclusion criteria: i) Inclusion of an adult population; ii)
diagnosis of impaired glucose tolerance, prediabetes, or impaired
fasting glucose; iii) investigation of the effect of vitamin D
supplementation on the onset of T2DM, conversion to normoglycemia,
oral glucose tolerance test, fasting serum glucose (FSG) and
hemoglobin A1c (HbA1c) levels; iv) restriction to
randomized controlled trials (RCTs). Additionally, studies
involving participants diagnosed with T2DM, which also considered
combination therapy with other medications, such as calcium
supplements, omega-3 fatty acids, or statins, were included. Such
studies were specifically selected when they were designed to serve
as appropriate control groups, thereby facilitating the examination
of the effects of vitamin D. The screening and selection process of
the studies for inclusion in the analysis were conducted by two
independent reviewers.
To enhance the reliability, and validity of
meta-analysis research, the study protocol is registered on the
Open Science Framework. The study protocol is available in the Open
Science Framework (https://doi.org/10.17605/OSF.IO/XJ3EN).
Quality assessment methodology
To assess the quality of the RCTs, the Cochrane Risk
of Bias tool for randomized trials (ROB2) was utilized (10). This tool evaluates five domains of
potential bias: The randomization process, the blinding of
participants and personnel, the handling of missing outcome data,
the completeness of outcome data measurement, and the reporting of
selected outcomes. Each study was independently assessed by two
reviewers for its risk of bias, categorizing the risk level as
‘high’, ‘some concern’ or ‘low’.
Statistical analysis
Meta-analyses were performed utilizing Review
Manager software (RevMan, version 5.4.1; The Cochrane
Collaboration, 2020) for statistical analysis. The magnitude of the
effect was determined based on the mean difference (MD) along with
its 95% confidence interval (CI) for continuous outcomes. For
dichotomous outcomes, the risk ratio (RR) with its 95% CI was
calculated. An inverse-variance method and Mantel-Haenszel method
were applied to combine data for continuous outcomes and
dichotomous outcomes, respectively. Heterogeneity among studies was
quantified using the I2 statistic, with ≤25% indicating
low heterogeneity, 26-50% indicating moderate heterogeneity, and
>50% indicating high heterogeneity. Random effects meta-analysis
was used due to differences in patient baseline characteristics in
each study affecting treatment effectiveness.
Subgroup analyses were undertaken to explore the
influence of specific covariates on the outcomes, including body
mass index (BMI), ethnicity, baseline vitamin D deficiency,
concurrent calcium intake, dosage of vitamin D supplementation,
baseline vitamin D levels, and duration of vitamin D intake.
Publication bias was evaluated using a funnel plot and Egger's
test. Sensitivity analyses by sample size were also conducted to
verify the stability of the findings.
Results
Study selection and quality
assessment
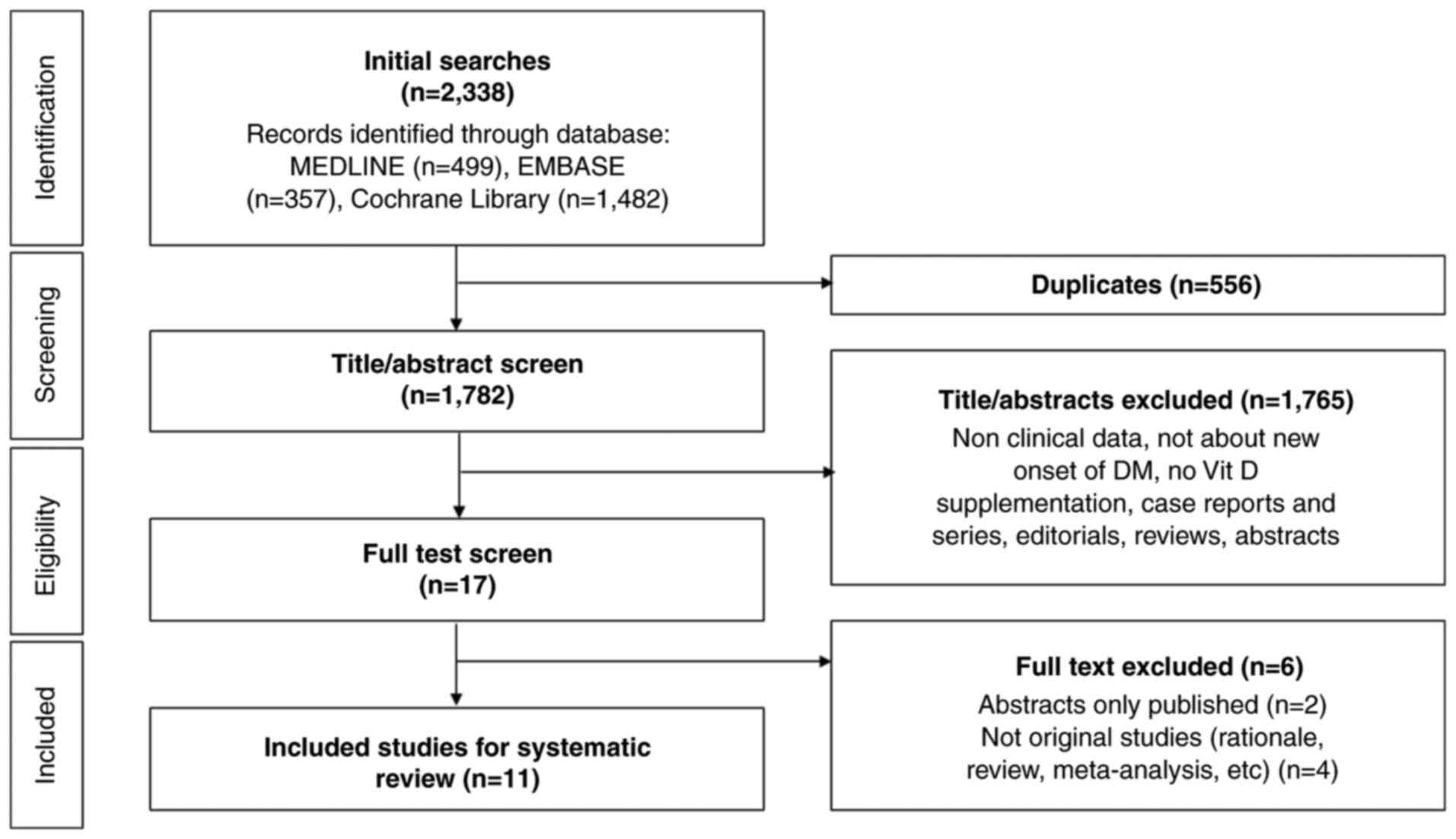
In the initial phase of the literature search, a
total of 2,338 potential studies were identified. The process of
study selection, adhering to the Preferred Reporting Items for
Systematic Reviews and Meta-Analyses guidelines, is delineated in
Fig. 1 (11). Subsequent to the preliminary
screening, a total of 556 studies were removed due to duplication.
An additional 1,765 studies were deemed ineligible and thus
excluded based on an assessment of their titles and abstracts,
which indicated a lack of direct relevance to the research query.
Upon a more detailed examination, involving the full-text review of
the 17 studies preliminarily selected, 6 were further excluded for
failing to satisfy the established inclusion criteria (12-17).
Consequently, the present systematic review ultimately incorporated
11 studies that conformed to the rigorous selection criteria.
In total, 11 RCTs were included in the final
analyses (18-28).
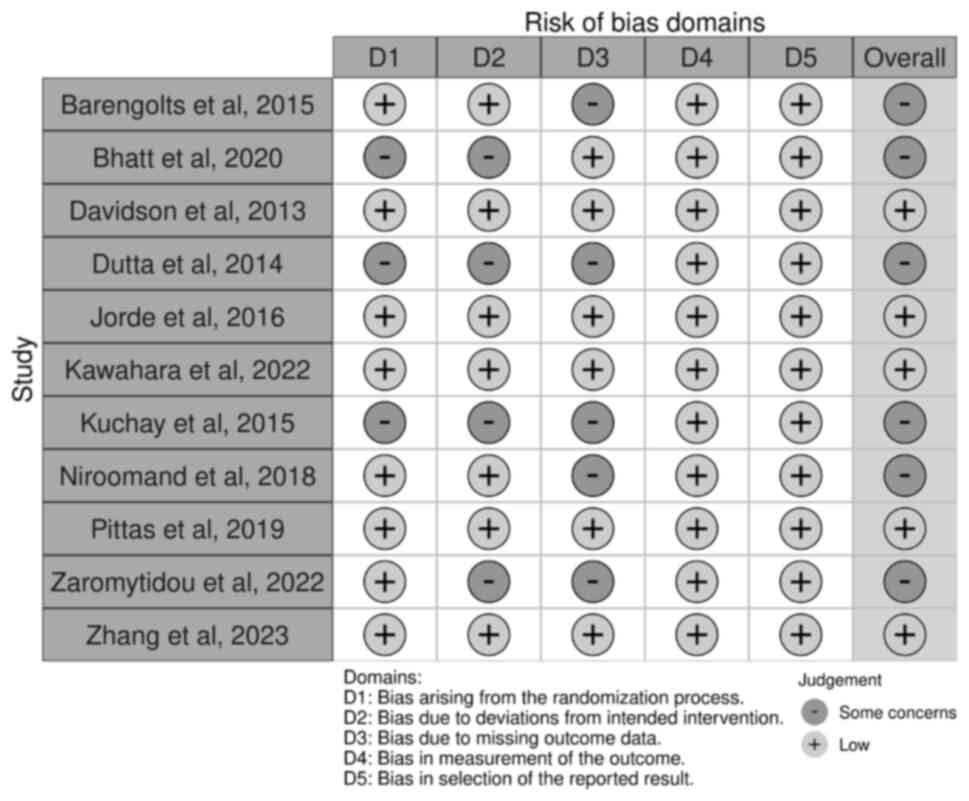
Upon evaluating the methodological quality of the included studies,
each demonstrated a low risk of bias when appraised utilizing the
ROB2 tool for randomized trials. The follow-up duration of all
trials ranged from 6 months to 5 years. The risk of bias is shown
in Fig. 2. All these trials were
conducted on prediabetic patients. The characteristics of the
included trials are shown in Tables
I and II. A total of 5,221
patients were included in the analyses, 2,619 patients were
supplemented with vitamin D and 2,602 patients were assigned to the
control group. In total, 9 trials used vitamin D3
(cholecalciferol) (19-22,24-28),
1 trial used vitamin D2 (ergocalciferol), and 1 trial
used eldecalcitol, a vitamin analog (23). A total of 10 trials reported the
progression of prediabetes to T2DM (18-27)
and 8 trials measured regression to normoglycemia (18-23,25,27).
Of those 10 trials, 5 trials progressed over 12 months (19,21-23,26),
3 trials included only Indians (19,21,24),
7 trials had mean BMI >30 (18-20,22,25-27),
and 3 trials used calcium carbonate or supplements along with
vitamin D (19,21,26).
The median age of 10 trials was 59.5 years.
 | Table IDemographic features of randomized
controlled trials that evaluated the association between vitamin D
supplements and type 2 diabetes included in the meta-analysis. |
Table I
Demographic features of randomized
controlled trials that evaluated the association between vitamin D
supplements and type 2 diabetes included in the meta-analysis.
| | Patients | Age (years) | BMI
(kg/m2) | Female (%) | Baseline vitamin D
level (ng/ml) | |
|---|
| First author,
year | Ethnicity
(nation) | Vitamin D | Control | Vitamin D | Control | Vitamin D | Control | Vitamin D | Control | Vitamin D | Control | (Refs.) |
|---|
| Barengolts et
al, 2015 | African American
(United States) | 87 | 86 | 58.2±6 | 59.8±6.0 | 32.4±2.9 | 31.5±2.4 | 0 | 0 | 14.7±4.7 | 14.0±4.8 | (18) |
| Bhatt et al,
2020 | Indian (India) | 61 | 60 | 20-60 years | | 31.1±6.2 | 28.8±3.9 | 100% | 100% | 12±5.4 | 12.9±2.1 | (19) |
| Davidson et
al, 2013 | Latino and African
American (United States) | 56 | 53 | 52.3±8 | 52.5±7 | 32.1±4.7 | 32.9±4.3 | 64% | 71% | 22.0±4.5 | 22.0±4.8 | (20) |
| Dutta et al,
2014 | Indian (India) | 68 | 57 | 48.37±10.47 | 47.4±11.51 | 26.32±4.52 | 26.83±4.63 | 63.2% | 54.4% | 17.04±7.66 | 18±7.16 | (21) |
| Jorde et al,
2016 | Norwegian
(Norway) | 256 | 255 | 62.3±8.1 | 61.9±9.2 | 30.1±4.1 | 29.8±4.4 | 37.1% | 40% | 24.0±8.8 | 24.4±8.5 | (22) |
| Kawahara et
al, 2022 | Japanese
(Japan) | 630 | 626 | 61.1±8.8 | 61.4±9.1 | 24.1±2.7 | 24.5±1.8 | 45.7% | 45.2 % | 21±6.2 | 20.7±6.1 | (23) |
| Kuchay et
al, 2015 | Indian (India) | 64 | 65 | 47.6±9.5 | 48.5±11.8 | 25.9±2.6 | 25.2±3.1 | NA | NA | 19.8±15.5 | 18.9±13.4 | (24) |
| Niroomand et
al, 2019 | Iranian (Iran) | 81 | 81 | 45±14 | 48±11 | 31±6 | 32±6 | 77.8% | 75.3% | 12.3±6.6 | 12.7±6.3 | (25) |
| Pittas et
al, 2019 | Hispanic or Latino
(United States) | 1,211 | 1,212 | 59.6±9.9 | 60.4±10.0 | 32.0±4.5 | 32.1±4.4 | 44.7% | 45 % | 27.7±10.2 | 28.2±10.1 | (26) |
| Zaromytidou et
al, 2022 | Greek (Greece) | 45 | 45 | 73.1±7.16 | 74.03±7.63 | 29.90±4.16 | 30.29±4.14 | NA | NA | 19.98±6.73 | 19.85± 5.72 | (27) |
| Zhang et al,
2023 | Chinese
(China) | 60 | 62 | 56.5 (median, IQR:
48-62) | 56 (median, IQR:
50-65) | 26.07±3.26 | 25.55±3.32 | 66.67% | 70.97% | 26.23±8.30 | | (28) |
 | Table IICharacteristics of therapeutic
interventions of randomized controlled trials included in
meta-analysis. |
Table II
Characteristics of therapeutic
interventions of randomized controlled trials included in
meta-analysis.
| | Hb1Ac outcome
level | |
|---|
| First author,
year | Treatment
duration | Treatment | Control | Follow-up
duration | Vitamin D | Control | (Refs.) |
|---|
| Barengolts et
al, 2015 | 12 months | Vitamin
D2 50,000 IU/week and Vitamin D3 400
IU/day | Placebo/week and
Vitamin D3 400 IU/day | 12 months | 6.14±0.30 | 6.09±0.26 | (18) |
| Bhatt et al,
2020 | 78 weeks | Cholecalciferol
60,000 IU/week for eight weeks. After every 24 weeks blood 25(OH)D
levels were assessed. If subjects were still vitamin D deficient,
cholecalci-ferol 60,000 IU/week for eight weeks was repeated. If
25(OH)D level was normal, cholecalciferol 200 IU/day was given as
maintenance dose. Also, calcium carbonate 1 g/day was given. | Placebo and Calcium
carbonate 1 g/day | 78 weeks | 5.8±1.05 | 6.21±1.45 | (19) |
| Davidson et
al, 2013 | 12 months | Vitamin
D3 88,865 IU/week and allowed to continue (and
encouraged not to change) any current vitamin/mineral supplements
that they were taking. | Placebo | 12 months | 6.0 | 6.2 | (20) |
| Dutta et al,
2014 | 28.2±8.83
months | Vitamin
D3 60,000 IU/week for 8 weeks and then 60,000 IU/month
along with calcium carbonate 1250 mg/day (equivalent to elemental
calcium 500 mg) | Calcium
carbonate | 40 months | 6.34±0.84 | 6.43±1 | (21) |
| Jorde et al,
2016 | 5 years | Cholecalciferol
20,000 IU/week | Placebo | 5 years | 6.09±0.36 | 6.1±0.54 | (22) |
| Kawahara et
al, 2022 | 2.9 years | Eldecalcitol 0.75
µg/day | Placebo | Median 2.9 years,
IQR: 2.8-3.0 | 5.9±0.2 | 6.0±0.2 | (23) |
| Kuchay et
al, 2015 | 12 months | Cholecalciferol
60,000 IU weekly for 4 weeks and then 60,000 IU monthly followed,
12 months long totally | Nothing | 12 months | 5.7±0.4 | 6.0±0.3 | (24) |
| Niroomand et
al, 2019 | 6 months | Vitamin
D3 50,000 IU/week for 3 months, followed by 50,000
IU/month pearl per month for an additional period of 3 months | Placebo | 6 months | NA | NA | (25) |
| Pittas et
al, 2019 | 4 years | Vitamin
D3 4,000 IU/day | Placebo | median 2.5 years
IQR: 1.9-3.5 (treatment), 1.7-3.5 (control) | NA | NA | (26) |
| Zaromytidou et
al, 2022 | 12 months | Vitamin
D3 25,000 IU/week | Nothing | 12 months | 5.80±0.2 | 5.83±0.24 | (27) |
| Zhang et al,
2023 | 24 weeks | Vitamin
D3 1,600 IU/day | Placebo | 24 weeks | 5.68±0.57 | 5.64±0.38 | (28) |
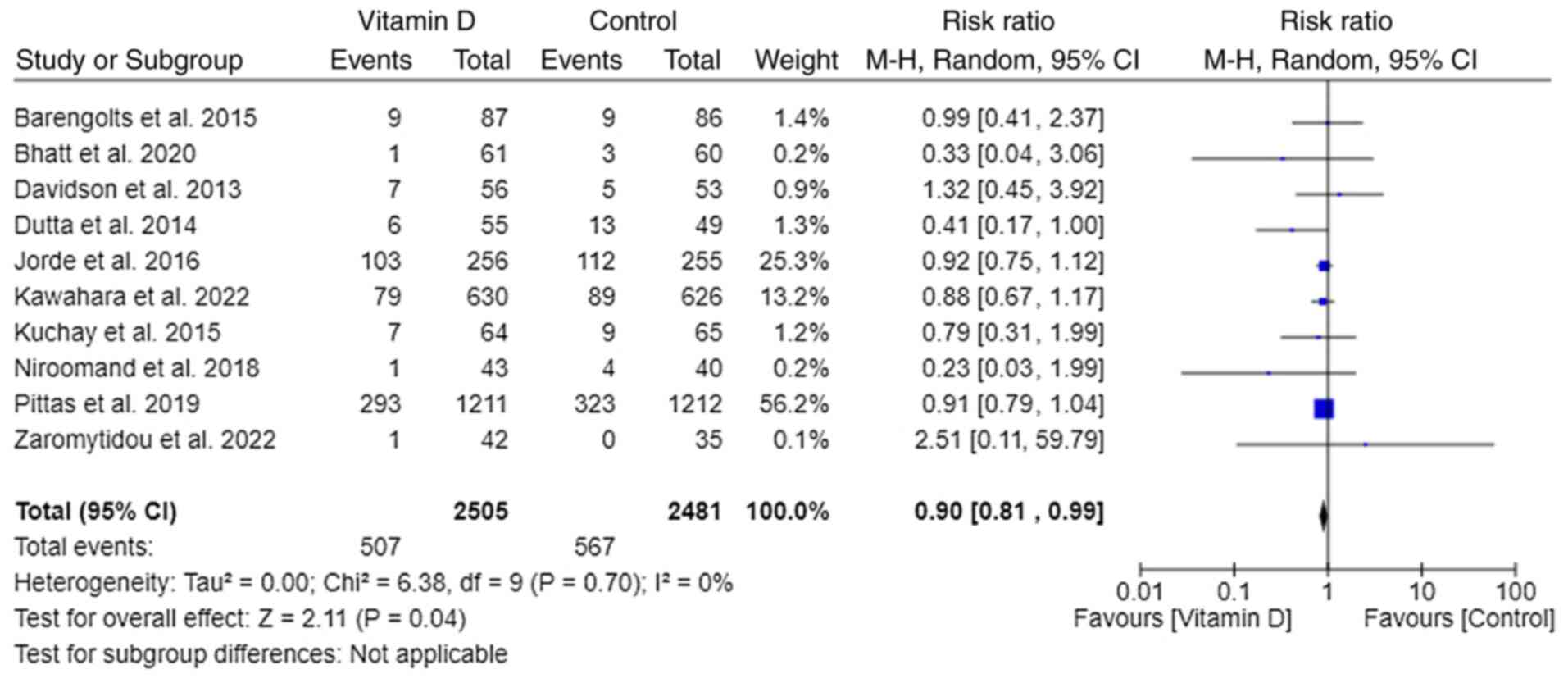
New onset of T2DM
A total of 10 out of 11 trials reported the
progression of prediabetes to T2DM (18-27).
Pooled data from all 10 trials reporting in RR revealed that
vitamin D supplementation in prediabetes patients decreased the
new-onset T2DM by 10% [RR, 0.90; 95% CI, (0.81, 0.99); P=0.04 and
I²=0%)] (Fig. 3).
Subgroup analyses were conducted to identify any
covariates for between-study heterogeneity. The pooled effect sizes
found in each subgroup (based on treatment duration, ethnicity,
mean BMI, baseline vitamin D level, inclusion of vitamin D
deficiency, median age and vitamin dose) were not significantly
different. Among the subgroups, the group that received only
vitamin D showed a statistically insignificant difference in the
incidence of T2DM compared with the control group [RR, 0.89; 95%
CI, (0.71, 1.11); P=0.30]. However, when the analysis included all
clinical trials that used a combination of calcium carbonate and
vitamin D supplements, a statistically significant difference was
observed [RR, 0.89; 95% CI, (0.79, 1.00); P=0.04] (data not
shown).
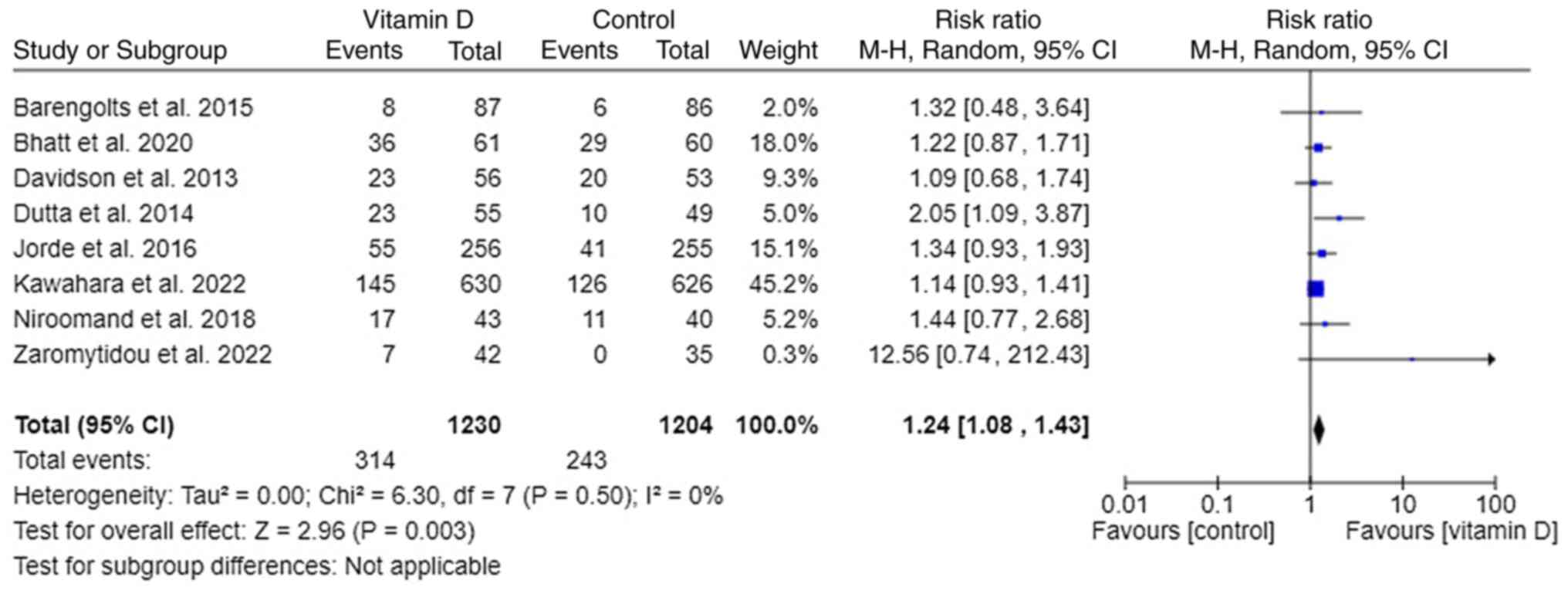
Regression to normoglycemia
A total of 8 out of 11 trials reported the
regression of prediabetes to normoglycemia (18-23,25,27). It
appeared that supplementing vitamin D to prediabetes patients leads
to normoglycemia significantly [RR, 1.24; 95% CI, (1.08, 1.43);
P=0.003; I²=0%] (Fig. 4). Subgroup
analyses were conducted to identify any covariates for
between-study heterogeneity. Pooled effect sizes found in these
subgroups did not differ significantly from each other.
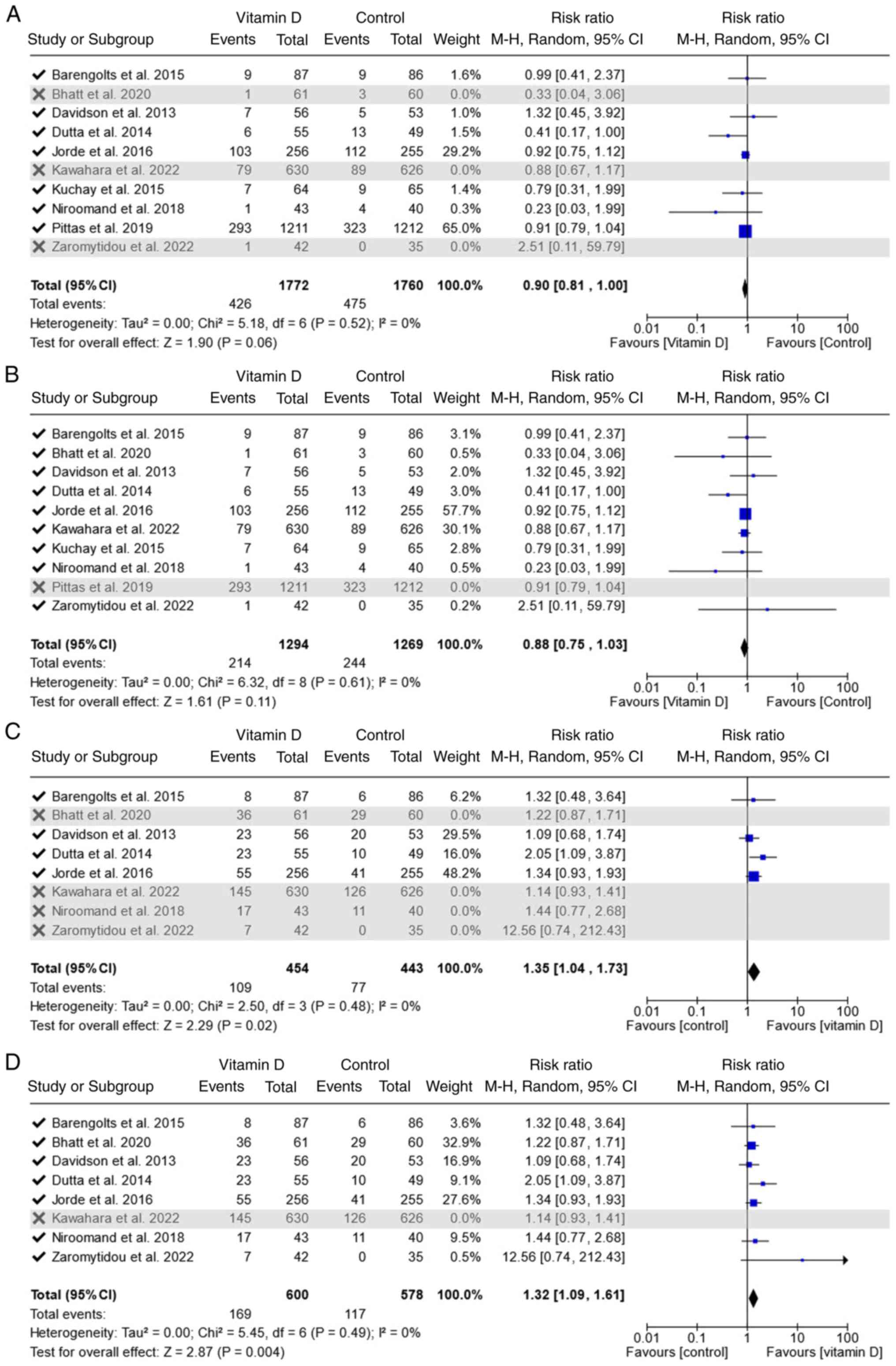
Sensitivity analysis
Sensitivity analysis was performed, and the results
are illustrated in Fig. 5. The
trials were added following the publication year, and since the
addition of Pittas et al (26), published in 2019, T2DM RR has
changed to significantly lower in Vitamin D group than in the
control group [RR, 0.90; 95% CI, (0.81, 1.00); P=0.06] (Fig. 5A). Exclusion of the largest trial
(26) didn't affect the
significance; it was still marginally significant [RR, 0.88; 95%
CI, (0.75, 1.03); P=0.11] (Fig.
5B). In regression to normoglycemia outcomes, RR changed
significantly when Jorde et al (22) was added [RR, 1.35; 95% CI, (1.04,
1.73); P=0.02] (Fig. 5C).
Exclusion of the largest trial (23) didn't affect the significance; it
was still significant [RR, 1.32; 95% CI, (1.09, 1.61); P=0.004]
(Fig. 5D).
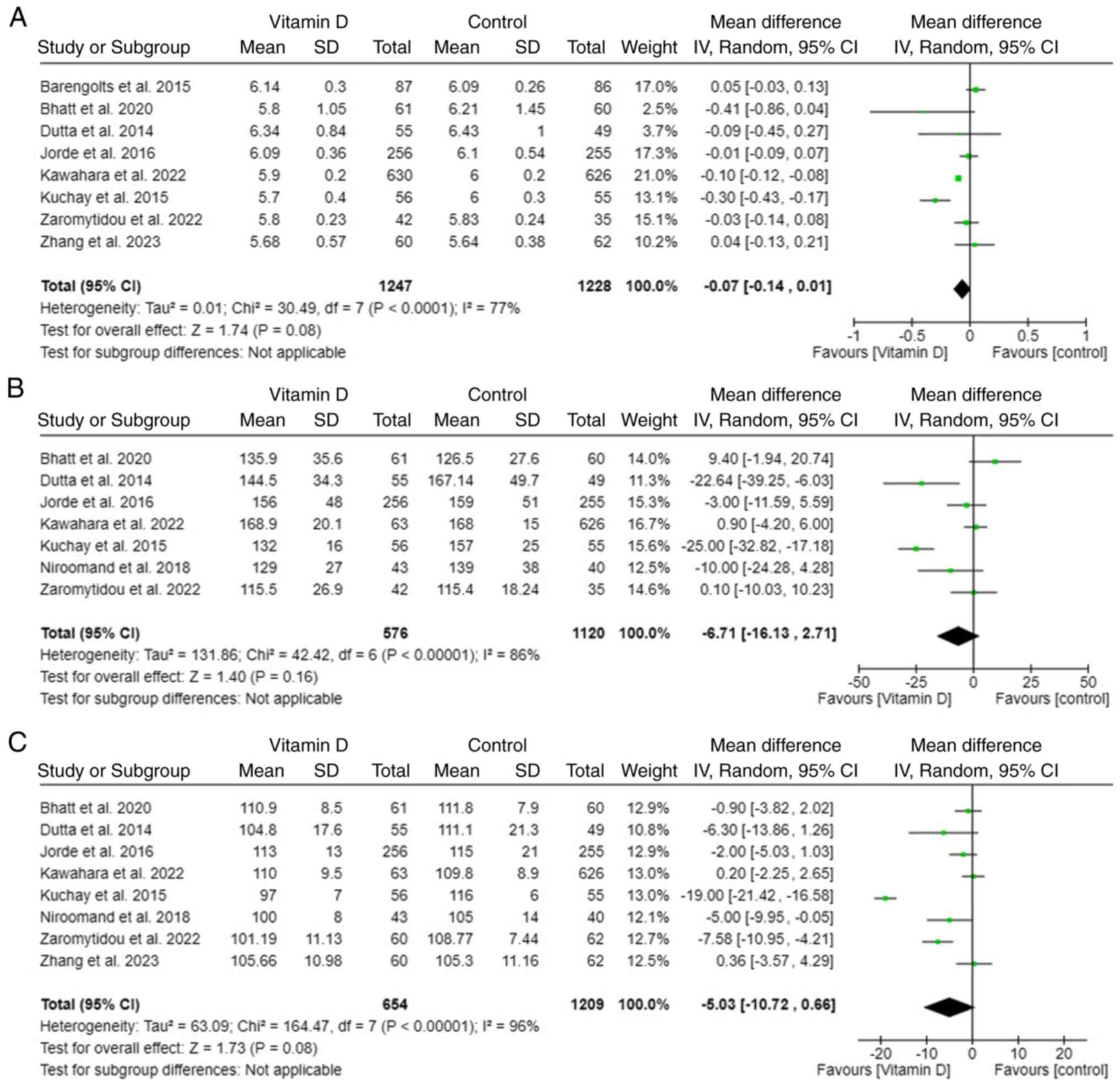
Secondary outcome
The definition of prediabetes is variant according
to the glycemic indices; raised glycosylated HbA1c, 2-h
plasma glucose (2OGT) and FSG. All three indices decreased after
supplementing vitamin D. All HbA1c, 2OGT, and FSG levels showed no
statistically significant reduction in the vitamin D supplement
group compared with the control group (Fig. 6).
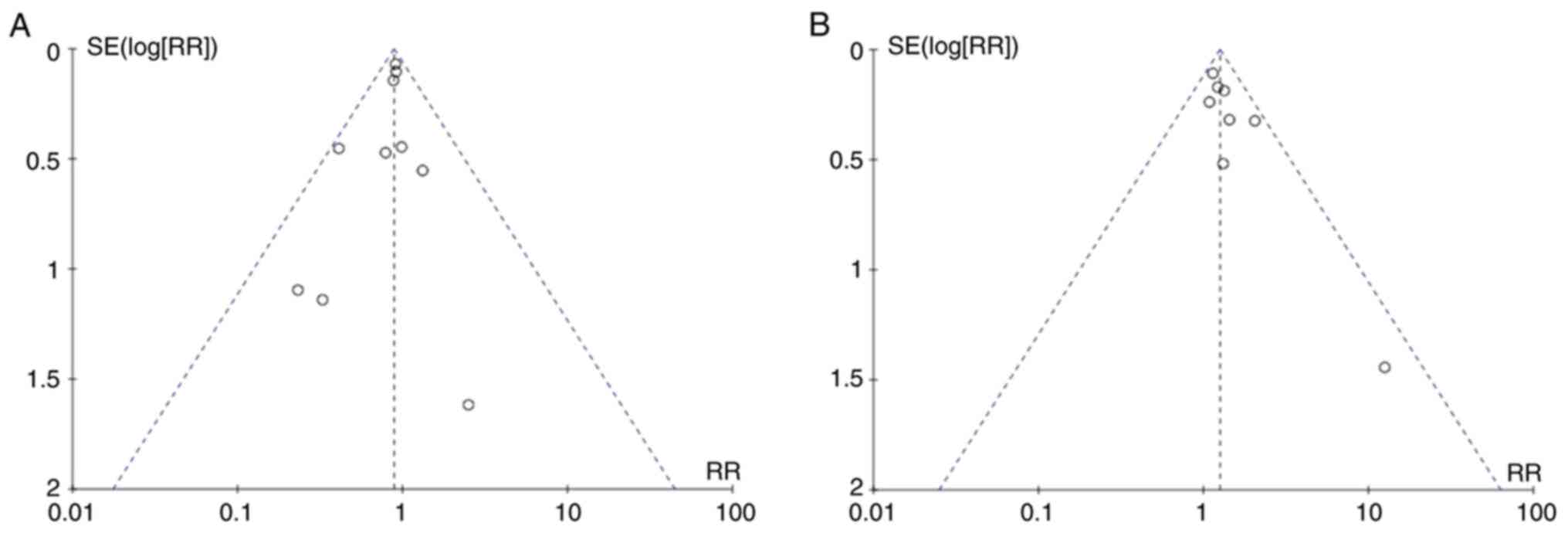
Publication bias
Visual inspection of the funnel plot revealed
relatively symmetrical distribution, indicating the absence of
publication bias. The funnel plots for onset of T2DM and regression
to normoglycemia are illustrated in Fig. 7A and B, respectively.
Discussion
Vitamin D treatment in prediabetes patients lowers
the risk of T2DM and regresses to normal glucose blood levels, as
revealed in the present meta-analysis of 11 randomized controlled
studies. Treating prediabetes patients with vitamin D had
significant effects compared with the control group in the subgroup
analyses without significant subgroup differences. It was expected
that long-term use of vitamin D would be effective, but the
subgroup analysis found that it had no interaction.
The trial, which lasted for 5 years, performed
subgroup analysis to examine the effect of vitamin D in subjects
with low baseline vitamin D levels of <20 ng/ml (22). However, there was no statistically
significant difference according to baseline vitamin D levels.
Pittas et al (26), the
largest trial, conducted a post-hoc subgroup analysis based on
baseline vitamin D level of 12 ng/ml, and only in participants with
a baseline vitamin D level <12 ng/ml, the effect of vitamin D
lowering T2DM HR was significantly greater compared with the
control group. These two trials suggested the possibility of an
interaction between baseline vitamin D levels and the effect of
vitamin D supplementation on the incidence of T2DM. However, the
present subgroup analysis showed no association with this and
baseline vitamin D levels.
Patterns of vitamin D deficiency among Indians have
been proposed (29). Also, Asian
Indians have one of the highest numbers of individuals with
pre-diabetes and diabetes among all major ethnic groups (30). The 3 trials were designed to find
the effect of vitamin D on prediabetes patients with a population
of Indians (19,21,24).
The result of the subgroup analysis in this meta-analysis was that
there was no interaction with ethnicity. There are not many trials
tested on Indians, and Bhatt et al (19) only included females. To find a
relation with ethnicity, it needs to be interpreted with caution,
and more extensive researches are needed.
Calcium is a common supplement that people take with
vitamin D. Depending on the symptoms of the patient, there are
several alternative suggestions to take calcium with vitamin D. A
recent study showed that whether used alone or in combination,
vitamin D and calcium supplementation do not exert meaningful
effects on all-cause mortality, cardiovascular mortality, major
adverse cardiovascular events or myocardial infarction (31). Moreover, vitamin D administration
may worsen the risk of stone formation in patients with
hypercalciuria (32). In
accordance with the present meta-analysis, vitamin D supplements
with calcium may help people with prediabetes avoid developing type
2 diabetes.
An interesting result has been drawn about the BMI.
Non-obese patients (BMI <30 kg/m2) had a significant
reduction in T2DM while obese patients did not. However, it is
controversial whether vitamin D has a relationship with obesity. A
different meta-analysis concluded that 25(OH)D level is inversely
associated with percentage body fat mass (PFM) but cholecalciferol
supplementation had no effect on PFM (33). Another meta-analysis proved that
cholecalciferol supplementation decreases the BMI and the waist
circumference, but does not statistically affect weight loss
(34).
Previous meta-analyses constantly revealed an
association between vitamin D supplementation and BMI. A
meta-analysis that was published in 2020, included 8 trials and
found the benefit of vitamin D supplementation on the prevention of
diabetes in non-obese patients (35) [mean BMI <30 kg/m2;
RR, 0.73; 95% CI, (0.57-0.92); I2=4%;
Pinteraction=0.048]. In a different meta-analysis that
was also released in 2020, subgroup analysis was carried out,
including the trials that provided vitamin D supplements 1,000
IU/day or less. As a result, patients with a mean baseline BMI
<30 kg/m2 reduced the risk of T2DM significantly
(36) [RR, 0.68; 95% CI,
(0.53-0.89); P=0.005; I2=0%,
Pinteraction=0.03]. Only non-obese patients gained
benefits from either of the two meta-analyses and proved a
significant subgroup difference. However, the present meta-analysis
found no interaction between BMI and vitamin D supplementation. The
difference of the present meta-analysis from the two previous ones
was that in the present meta-analysis, the large trial was
considered (23). The Kawahara
et al (23) study recruited
1,256 participants and was published in 2022. There should be
additional studies needed to identify the possibility that vitamin
D supplementation and BMI interact. However, the quality of
Kawahara et al (23) study
was shown to be favorable through the Cochrane Risk of Bias tool
for randomized trials (ROB2) test, thus the results of the present
meta-analysis are considered to be reliable.
There are several limitations to be considered.
First, the subgroup analyses used the mean data of the trials. For
example, the present study followed the median age of the trials,
59.5 years. It was expected that senior patients would benefit from
supplementing vitamin D, but there was no advantage for them. The
broad age range of the trials could be the reason for it. To
determine the relationship depending on the specific age range,
more research needs to be conducted. Second, some results of the
subgroup analyses should be interpreted cautiously because of the
lack of data. There were only three studies using calcium and
vitamin D, as well as those that targeted Indians. Third, the study
that resulted in no patients who regressed to normoglycemia was
included in the present meta-analysis (27). The RR and 95% CI of Zaromytidou
et al (27) were extremely
high, and the study was conducted with small numbers. Furthermore,
it would be the reason for the only asymmetry in the funnel
plot.
In conclusion, vitamin D supplement is associated
with a decreased risk of T2DM onset and an increase of reversion to
normoglycemia in prediabetic patients. Further studies are needed
to ensure the detailed effects of vitamin D long-term use, baseline
vitamin D levels, BMI and obesity on vitamin D benefits for
T2DM.
Acknowledgements
This work was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MSIT) (grant no. RS-2023-00253995).
Funding
Funding: The present study was supported by the research funds
for newly appointed professors of Jeonbuk National University in
2022.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SML, JH and JK designed the study. GS and YJK have
done systematic search and selected studies independently. JK and
SML confirmed the authenticity of all the raw data. JH and YJK
analyzed the data. GS, YJK and SML drafted the manuscript. JK and
JH supervised the study process and revised the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu Hanquan A and Teo Li Wen MR: Prevalence
of diabetic peripheral neuropathy in patients with type 2 diabetes
mellitus at a tertiary referral centre in Singapore. Proc Singap
Healthc. 30:265–70. 2020.
|
|
2
|
American Diabetes Association. Standards
of medical care in diabetes-2019 abridged for primary care
providers. Clin Diabetes. 37:11–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zheng Y, Ley SH and Hu FB: Global
aetiology and epidemiology of type 2 diabetes mellitus and its
complications. Nat Rev Endocrinol. 14:88–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kulda V: Vitamin D metabolism. Vnitr Lek.
58:400–404. 2012.PubMed/NCBI(In Czech).
|
|
5
|
Bouillon R: Optimal vitamin D
supplementation strategies. Endocrine. 56:225–226. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Berridge MJ: Vitamin D: A custodian of
cell signalling stability in health and disease. Biochem Soc Trans.
43:349–358. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Berridge MJ: Vitamin D cell signalling in
health and disease. Biochem Biophys Res Commun. 460:53–71.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Calton EK, Keane KN and Soares MJ: The
potential regulatory role of vitamin D in the bioenergetics of
inflammation. Curr Opin Clin Nutr Metab Care. 18:367–373.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wimalawansa SJ: Vitamin D deficiency is a
surrogate marker for visceral fat content, metabolic syndrome, type
2 diabetes and future metabolic complications. J Diabetes Metab
Disord Control. 3:6–13. 2016.
|
|
10
|
Higgins JPT, Savović J, Page MJ, Elbers RG
and Sterne JAC: Assessing risk of bias in a randomized trial. In:
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ and
Welch VA (eds). Cochrane Handbook for Systematic Reviews of
Interventions. 2nd edition. Wiley-Blackwell, pp205-228, 2019.
|
|
11
|
Moher D, Shamseer L, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P and Stewart LA: PRISMA-P Group.
Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015 statement. Syst Rev. 4(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Davidson MB, Duran P, Lee ML and Friedman
TC: High-dose vitamin D supplementation in people with prediabetes
and hypovitaminosis D. Diabetes Care. 36:260–266. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dutta D, Mondal SA, Choudhuri S, Maisnam
I, Hasanoor Reza AH, Bhattacharya B, Chowdhury S and Mukhopadhyay
S: Vitamin-D supplementation in prediabetes reduced progression to
type 2 diabetes and was associated with decreased insulin
resistance and systemic inflammation: an open label randomized
prospective study from Eastern India. Diabetes Res Clin Pract.
103:e18–23. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gonzalez S, Duran P, Friedman T and
Davidson M (eds): The effect of six months of vitamin D
supplemention in minorities with pre-diabetes and hypovitaminosis
D. Journal of investigative medicine: Lippincott Williams &
Wilkins 530 Walnut St, Philadelphia, PA 19106-3621 USA, 2012.
|
|
15
|
Kawahara T: Eldecalcitol, a vitamin D
analog, for diabetes prevention in impaired glucose tolerance (DPVD
study). Diabetes. 67 (Suppl 1):S120–LB. 2018.
|
|
16
|
Kawahara T, Suzuki G, Inazu T, Mizuno S,
Kasagi F, Okada Y and Tanaka Y: Rationale and design of diabetes
prevention with active vitamin D (DPVD): A randomised,
double-blind, placebo-controlled study. BMJ Open.
6(e011183)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kawahara T, Suzuki G, Inazu T, Mizuno S,
Kasagi F, Okada Y and Tanaka Y: DPVD clinical study group.
Eldecalcitol, a vitamin D analogue, for diabetes prevention in
impaired glucose tolerance: DPVD study. Diabetologia. 61 (Suppl
1)(S78)2018.
|
|
18
|
Barengolts E, Manickam B, Eisenberg Y,
Akbar A, Kukreja S and Ciubotaru I: Effect of high-dose vitamin D
repletion on glycemic control in African-American males with
prediabetes and hypovitaminosis D. Endocr Pract. 21:604–612.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bhatt SP, Misra A, Pandey RM, Upadhyay AD,
Gulati S and Singh N: Vitamin D supplementation in overweight/obese
Asian Indian women with prediabetes reduces glycemic measures and
truncal subcutaneous fat: A 78 weeks randomized placebo-controlled
trial (PREVENT-WIN trial). Sci Rep. 10(220)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Davidson MB, Duran P, Lee ML and Friedman
TC: High-dose vitamin D supplementation in people with prediabetes
and hypovitaminosis D. Diabetes Care. 36:260–266. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dutta D, Mondal SA, Choudhuri S, Maisnam
I, Reza AHH, Bhattacharya B, Chowdhury S and Mukhopadhyay S:
Vitamin-D supplementation in prediabetes reduced progression to
type 2 diabetes and was associated with decreased insulin
resistance and systemic inflammation: An open label randomized
prospective study from Eastern India. Diabetes Res Clin Pract.
103:e18–e23. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jorde R, Sollid ST, Svartberg J, Schirmer
H, Joakimsen RM, Njølstad I, Fuskevåg OM, Figenschau Y and
Hutchinson MY: Vitamin D 20,000 IU per week for five years does not
prevent progression from prediabetes to diabetes. J Clin Endocrinol
Metab. 101:1647–1655. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kawahara T, Suzuki G, Mizuno S, Inazu T,
Kasagi F, Kawahara C, Okada Y and Tanaka Y: Effect of active
vitamin D treatment on development of type 2 diabetes: DPVD
randomised controlled trial in Japanese population. BMJ.
377(e066222)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kuchay MS, Laway BA, Bashir MI, Wani AI,
Misgar RA and Shah ZA: Effect of vitamin D supplementation on
glycemic parameters and progression of prediabetes to diabetes: A
1-year, open-label randomized study. Indian J Endocrinol Metab.
19:387–392. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Niroomand M, Fotouhi A, Irannejad N and
Hosseinpanah F: Does high-dose vitamin D supplementation impact
insulin resistance and risk of development of diabetes in patients
with pre-diabetes? A double-blind randomized clinical trial.
Diabetes Res Clin Pract. 148:1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pittas AG, Dawson-Hughes B, Sheehan P,
Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C,
Chatterjee R, et al: Vitamin D supplementation and prevention of
type 2 diabetes. N Engl J Med. 381:520–530. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zaromytidou E, Koufakis T, Dimakopoulos G,
Drivakou D, Konstantinidou S, Antonopoulou V, Grammatiki M, Manthou
E, Iakovou I, Gotzamani-Psarrakou A and Kotsa K: The effect of
vitamin D supplementation on glycemic status of elderly people with
prediabetes: A 12-month open-label, randomized-controlled study.
Expert Rev Clin Pharmacol. 15:89–97. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang D, Zhong X, Cheng C, Su Z, Xue Y,
Liu Y, Zhang Y, Feng M, Xu Z, Zhao T, et al: Effect of vitamin D
and/or calcium supplementation on pancreatic β-cell function in
subjects with prediabetes: A randomized, controlled trial. J Agric
Food Chem. 71:347–357. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aparna P, Muthathal S, Nongkynrih B and
Gupta SK: Vitamin D deficiency in India. J Family Med Prim Care.
7:324–330. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shrivastava U, Misra A, Mohan V,
Unnikrishnan R and Bachani D: Obesity, diabetes and cardiovascular
diseases in India: Public health challenges. Curr Diabetes Rev.
13:65–80. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y, Li Y, Liu J, Wei X, Tan N, Zhang
J, Zhang J, Wang W and Wang Y: Association of vitamin D or calcium
supplementation with cardiovascular outcomes and mortality: A
meta-analysis with trial sequential analysis. J Nutr Health Aging.
25:263–270. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bargagli M, Ferraro PM, Vittori M,
Lombardi G, Gambaro G and Somani B: Calcium and vitamin D
supplementation and their association with kidney stone disease: A
narrative review. Nutrients. 13(4363)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Golzarand M, Hollis BW, Mirmiran P, Wagner
CL and Shab-Bidar S: Vitamin D supplementation and body fat mass: A
systematic review and meta-analysis. Eur J Clin Nutr. 72:1345–1357.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Perna S: Is vitamin D supplementation
useful for weight loss programs? A systematic review and
meta-analysis of randomized controlled trials. Medicina (Kaunas).
55(368)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang Y, Tan H, Tang J, Li J, Chong W, Hai
Y, Feng Y, Lunsford LD, Xu P, Jia D and Fang F: Effects of vitamin
D supplementation on prevention of type 2 diabetes in patients with
prediabetes: A systematic review and meta-analysis. Diabetes Care.
43:1650–1658. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Barbarawi M, Zayed Y, Barbarawi O, Bala A,
Alabdouh A, Gakhal I, Rizk F, Alkasasbeh M, Bachuwa G and Manson
JE: Effect of vitamin D supplementation on the incidence of
diabetes mellitus. J Clin Endocrinol Metab. 105:2857–2868.
2020.PubMed/NCBI View Article : Google Scholar
|