Introduction
Bronchiectasis is considered a chronic respiratory
disease that is characterized by anatomical changes (abnormal and
permanent dilatation of bronchi) associated with specific clinical
features, such as chronic cough, expectoration of large amounts of
purulent sputum and/or recurrent haemoptysis and represents the
final common pathway of different disease processes (1,2). In
recent years, the prevalence of bronchiectasis has increased
worldwide. There are significant differences in the prevalence of
bronchiectasis between countries and regions, which are related to
factors such as ethnicity and sociohygienic conditions. For
example, in the United States, the prevalence of bronchiectasis in
individuals aged ≥70 years and over is 272 per 100,000, whereas the
prevalence in individuals aged 18-34 years is 4.2 per 100,000.
Bronchiectasis is a common disease in Asian populations and its
incidence and prevalence have increased in recent years. In China,
for example, survey data from 2013 revealed that 1.2% (135/10,811)
of residents >40 years of age in the urban areas of seven
provinces and cities had been diagnosed with bronchiectasis and the
prevalence increased with age. Data on the specific current status
of bronchiectasis in South Asia (e.g., India, Pakistan, Bangladesh)
may be relatively scarce, but the prevalence and disease burden of
bronchiectasis may not be negligible considering the socioeconomic
conditions, healthcare level and demographic structure of the
region.
A high frequency of acute exacerbations of
bronchiectasis can lead to airway and systemic inflammation and is
associated with progressive lung injury, decreased quality of life,
accelerated decline in lung function and increased mortality
(3-5).
Therefore, reducing the frequency of exacerbations and/or
shortening the time to the first exacerbation is highly important
for reducing medical costs and improving patient prognosis.
Little is known about the pathobiology of acute
exacerbations of bronchiectasis and even the clinical
manifestations of this disease vary widely. Acute exacerbation of
bronchiectasis is usually considered caused by colonization and
infection by bacterial pathogens. Current treatment guidelines
recommend antibiotics to control acute exacerbations (6). In 2017, the European Respiratory
Society defined three or more acute exacerbations as the threshold
for starting long-term antibiotic treatment to prevent future
exacerbations (7). Infection and
colonization by Pseudomonas aeruginosa are the main causes
of bronchiectasis. There are also reports on the role of
nontuberculous mycobacteria, Staphylococcus aureus and fungi
in the prognosis of bronchiectasis. Studies on Haemophilus
influenzae and Klebsiella pneumoniae are limited
(8,9). With respect to the detection of
pathogens in patients with bronchiectasis, a number of patients
test negative for pathogens and in a few patients with a positive
pathogen test, the distribution of pathogens is different; thus, in
addition to P. aeruginosa, few pathogens have been well
studied (10,11). One study revealed that P.
aeruginosa infection led to poorer outcomes in patients with
bronchiectasis with long-term chronic infection, as indicated by an
increased risk of hospitalization and an increased frequency of
acute exacerbations (12). It is
still not clear whether there is a definite correlation between
infection by different pathogens and the number of acute
exacerbations, hospitalization rates, or mortality due to
bronchiectasis.
In the present study, the characteristics of
patients infected with pathogenic bacteria, the risk of acute
exacerbation and the risk of hospitalization within 1 year
following infection with different pathogenic microorganisms were
retrospectively observed to improve understanding and treat
bronchiectasis and to improve its prognosis.
Materials and methods
Research objects
The present study was a retrospective analysis of
522 patients with bronchiectasis admitted to the Department of
Respiratory and Critical Care Medicine at The Second Affiliated
Hospital of Jiaxing University (Zhejiang, China) between January
2019 and December 2022. The inclusion criteria were sputum or
alveolar lavage fluid samples positive for pathogenic bacteria and
imaging changes indicative of bronchiectasis on chest computed
tomography (CT). The diagnostic criteria for bronchiectasis were
based on the 2021 Chinese Expert Consensus on the Diagnosis and
Treatment of Bronchiectasis in Adults (13). The exclusion criteria were: i)
<18 years of age, ii) bronchiectasis caused by cystic fibrosis,
iii) chronic bacterial colonization, iv) loss to follow-up and v)
bronchiectasis caused by Mycobacterium tuberculosis infection.
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Jiaxing University
(Zhejiang, China; approval no. 2024SW110-01). The clinical data
from the patients were collected anonymously. The personally
identifiable information and privacy of the patients were
protected. The patients were verbally informed and agreed to
participate during the telephone follow-up; therefore, signing
informed consent was exempt.
Data acquisition
The present study analysed the clinical
characteristics of 522 patients with bronchiectasis, including age,
sex, clinical symptoms and signs, smoking history, alcohol
consumption history, comorbidities, medical history and pathogen
detection results in sputum or alveolar lavage fluid. Some
haematology indicators and biochemical parameters of the laboratory
examination for acute exacerbation of patients with bronchiectasis
at the time of treatment included C-reactive protein (CRP),
procalcitonin (PCT), white blood cell count, neutrophil count,
haemoglobin, platelet count, erythrocyte sedimentation rate,
duration of antibiotic use and number of hospitalization days
during acute exacerbation of bronchiectasis.
The start point of the studies was the date of
detection of positive pathogenic bacteria in the sputum or alveolar
lavage fluid of the patient. The patient was followed up by
telephone or in the clinic after 1 year. The number of
hospitalizations, the number of outpatient visits, the presence of
acute exacerbations of symptoms within 1 year and the need for
antibiotic treatment were recorded.
Acute exacerbation of bronchiectasis was defined as
a change in three or more of the following symptoms in patients
with bronchiectasis: Cough, a change in sputum volume, purulent
sputum, difficulty breathing, force or discomfort, or coughing up
blood; this change had to last at least 48 h, after which clinical
physicians needed to change the treatment plan for bronchiectasis
(usually referring to the prescription of antibiotics) (14).
Pathogenic bacteria detection methods.
Sputum culture
The patient coughs out respiratory secretions after
waking up in the morning on the first day after admission to the
hospital, puts the sample in a sterilized container and sends it to
the hospital for immediate examination. In accordance with the
traditional microbial standard culture method
(inoculation-culture-identification-drug sensitivity), for the
specific process, the samples were inoculated into a blood plate
(AnTuBio), chocolate plate (Komatsu Biologicals), or Shabao weak
plate (Komatsu Biologicals), incubated at 37˚C for 24 h and
colonies of bacteria were suspected to be subjected to Gram-stained
microscopic examination and then analysed by a fully automatic
microbiological analyser (MALDI-TOF MS; Biomerieux, France). The
suspected colonies were microscopically examined via Gram staining
and the pathogenic species were identified via a fully automated
microbiological analyser (matrix-assisted laser
desorption/ionization time-of-flight mass spectrometry; bioMérieux
SA).
Metagenomic next-generation sequencing
(mNGS) technology
The bronchoalveolar lavage fluid (BALF) of each
patient was retained via bronchial lavage and sent to Fangliu
Biological Company (http://www.idtbio.com/) for the detection of
pathogenic bacteria via mNGS, which was performed in accordance
with the standard specifications of Fangliu Biological Company. The
mNGS samples were removed, preserved and transported according to
the standard specifications of the detection institution.
Sample pretreatment
i) The samples of sputum and BALF were removed from
the refrigerator at -80˚C and placed at room temperature (25˚C)
until they were dissolved. ii) The sample was placed in a 50˚C
water bath for 30 min after dissolution and then equilibrated at
room temperature (25˚C). iii) The sample (0.5 ml) was pipetted into
a clean 1.5 ml centrifuge tube.
Nucleic acid extraction and
determination of nucleic acid concentration
A bacterial genomic DNA extraction kit (Tiangen
Biotech Co., Ltd.; cat. no. DP302) was used for nucleic acid
extraction according to the manufacturer's protocols. The nucleic
acid concentration was determined via a micro UV spectrophotometer
(NanoDrop 2000; NanoDrop Technologies; Thermo Fisher Scientific,
Inc.), which was used to assess the purity of the samples.
Sequencing of all the nucleic acids of the microorganisms extracted
from the samples was performed via high-throughput sequencing. The
quality control for sequencing reads was conducted by removing
low-quality reads, adapter sequences, and duplicated or short
(<36 bp) reads. The remaining qualified reads were first mapped
to the human reference genome (hs37d5) using bowtie2 software and
then the non-human reads were aligned to the microorganism genome
database for pathogens identification.
Core respiratory pathogen nucleic acid
test
When the patient was admitted to The Second
Affiliated Hospital of Jiaxing University (Zhejiang, China), a
professional nurse instructed the patient to obtain sputum from the
deep respiratory tract and this was sent to the microbiology room
of the Laboratory Department and tested with the Crystalline Core
Respiratory Pathogenic Bacteria Nucleic Acid Detection Kit (Beijing
CapitalBio MedLab), which conducts real-time fluorescence detection
via the fluorescent dye doping method and amplifies the target
nucleic acid with an S-shaped curve under the action of the
polymerase with the function of chain replacement (which can react
at a constant temperature of 65˚C). Under the action of polymerase
with a chain-switching function (65˚C constant temperature reaction
is available), the amplified target nucleic acid will show an
S-shaped curve and then the target gene will be detected and
amplified.
Study group
A total of 522 patients with bronchiectasis were
included in the present study. They were scored on the
bronchiectasis severity index (BSI) (14), which gives 1 point for each of the
following: i) age ≥70 years; ii) forced expiratory volume in the
first second to be ≤50% of the predicted value; iii) previous
hospitalization for aggravation; iv) each acute exacerbation in the
past year; v) colonization by P. aeruginosa; vi)
dyspnoea-modified Medical Research Council score; and vii) chest CT
showing that more than three lung lobes were infiltrated. In the
present study, a score <4 indicated mild to moderate
bronchiectasis and a score ≥4 was considered severe. There were 282
patients in the mild to moderate group and 240 patients in the
severe group.
Statistical analysis
SPSS 27.0 software (IBM Corp.) was used for
processing and analysis. Continuous variables are described through
means, standard deviations and medians, whereas categorical
variables are presented as frequencies and percentages. The
χ2 test or Fisher's exact test (when the number of
expected cases was <5) was used to analyse categorical
variables. Student's t-test was performed to analyse standard
deviations and medians. Wilcoxon rank sum test was used to compare
nonnormally distributed continuous variables, which are presented
as medians (IQRs) and ranges. Univariate logistic regression
analysis was performed to identify risk factors for hospitalization
in the next year in adult patients diagnosed with bronchiectasis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Basic clinical characteristics of the
patients
Among 522 patients with bronchiectasis whose sputum
or alveolar lavage fluid test was positive for pathogens, 325
patients had positive sputum cultures and 197 patients had positive
alveolar lavage fluid cultures. The patients were mainly
middle-aged and elderly, with 91.8% being ≥50 years. The patients
in the severe group were significantly older than the patients in
the mild to moderate group (P<0.05). There was no significant
difference in sex between the groups (P>0.05). Among the common
clinical symptoms, chest tightness and bilateral pulmonary rales
were significantly more common in patients in the severe group than
in those in the mild to moderate group and the incidence of cough
was lower than that in the mild to moderate group (P<0.05).
There were no significant differences in cough, sputum, fever,
chest pain, or unilateral pulmonary rales between the two groups
(P>0.05). The proportion of smokers and drinkers in the severe
group was significantly lower than that in the mild to moderate
group (P<0.05). The severe group was more likely to have
comorbidities; less likely to have chronic obstructive pulmonary
disease, respiratory failure, ischaemic heart disease, diabetes and
rheumatoid arthritis; and more likely to have malignant tumours
(P<0.05). There was no significant difference in bronchial
asthma, interstitial lung disease, or hypertension between the two
groups (P>0.05). Table I lists
the specific data.
 | Table IBaseline characteristics and clinical
manifestations of patients with bronchiectasis. |
Table I
Baseline characteristics and clinical
manifestations of patients with bronchiectasis.
| Characteristic | Total (n=522) | Mild to moderate
group (n=282) | Severe group
(n=240) | χ2 |
P-valuea |
|---|
| Age, years | | | | 62.93 | <0.001 |
|
<50 | 48 | 42 | 6 | | |
|
50-69 | 288 | 174 | 114 | | |
|
70-79 | 120 | 54 | 66 | | |
|
≥80 | 66 | 12 | 54 | | |
| Sex | | | | 0.30 | 0.59 |
|
Male | 222 | 123 | 99 | | |
|
Female | 300 | 159 | 141 | | |
| Clinical
characteristics | | | | | |
|
Cough and
expectoration | 444 | 220 | 224 | 0.07 | 0.79 |
|
Fever | 78 | 36 | 42 | 0.92 | 0.34 |
|
Chest
distress | 105 | 27 | 78 | 49.54 | <0.001 |
|
Pectoralgia | 18 | 9 | 9 | 0 | 1 |
|
Hemoptysis | 174 | 102 | 72 | 10.34 | 0.01 |
|
Pulmonary
rale (unilateral) | 78 | 45 | 33 | 3.69 | 0.08 |
|
Pulmonary
rale (bilateral) | 183 | 72 | 111 | 16.62 | <0.001 |
| Personal
history | | | | | |
|
Smoking | 108 | 63 | 45 | 6 | 0.02 |
|
Alcohol
drinking | 78 | 48 | 30 | 8.31 | 0.01 |
| Complications | | | | | |
|
None | 129 | 105 | 24 | 101.70 | <0.001 |
|
Chronic
obstructive pulmonary disease | 84 | 12 | 72 | 85.71 | <0.001 |
|
Respiratory
failure | 45 | 9 | 36 | 32.40 | <0.001 |
|
Bronchial
asthma | 6 | 3 | 3 | 0 | 1 |
|
Interstitial
pulmonary disease | 6 | 1 | 5 | 5.33 | 0.08 |
|
Ischemic
heart disease | 15 | 1 | 14 | 22.53 | <0.001 |
|
Hypertension | 153 | 81 | 72 | 1.06 | 0.36 |
|
Diabetes | 60 | 21 | 39 | 10.8 | 0.01 |
|
Rheumatoid
arthritis | 21 | 6 | 15 | 7.71 | 0.01 |
|
Malignant
tumour | 27 | 9 | 18 | 6.00 | 0.03 |
|
Miscellaneousb | 198 | 102 | 96 | 0.36 | 0.62 |
Laboratory values of patients with
different degrees of bronchiectasis
By comparing the complete blood count results and
serum albumin levels between the two groups, it was found that the
number of white blood cells, the number of neutrophils, the CRP
level, the PCT level and the erythrocyte sedimentation rate were
significantly greater in the severe group than in the mild to
moderate group (P<0.05). There was no significant difference in
haemoglobin (P>0.05). These specific data are shown in Table II.
 | Table IILaboratory indicators in patients
with bronchiectasis. |
Table II
Laboratory indicators in patients
with bronchiectasis.
| Index | Mild to moderate
group (n=282) | Severe group
(n=240) |
P-valuea |
|---|
| White blood cell
count, 109/l | 6.03±1.22 | 7.32±2.88 | <0.001 |
| Neutrophil count,
109/l | 4.35±1.86 | 5.24±2.74 | <0.001 |
| Hemoglobin,
g/l | 4.04±0.32 | 3.98±0.48 | 0.09 |
| reactive protein,
mg/l) | 23.0
(9.0-36.0)b | 29.0
[10.0-58.0]a | <0.001 |
| Procalcitonin,
ng/ml | 0.05±0.01 | 0.09±0.02 | <0.001 |
| Erythrocyte
sedimentation rate, mm/h | 21.35±4.46 | 28.02±5.22 | <0.001 |
Distribution characteristics of
pathogens in patients with bronchiectasis with positive pathogen
infections
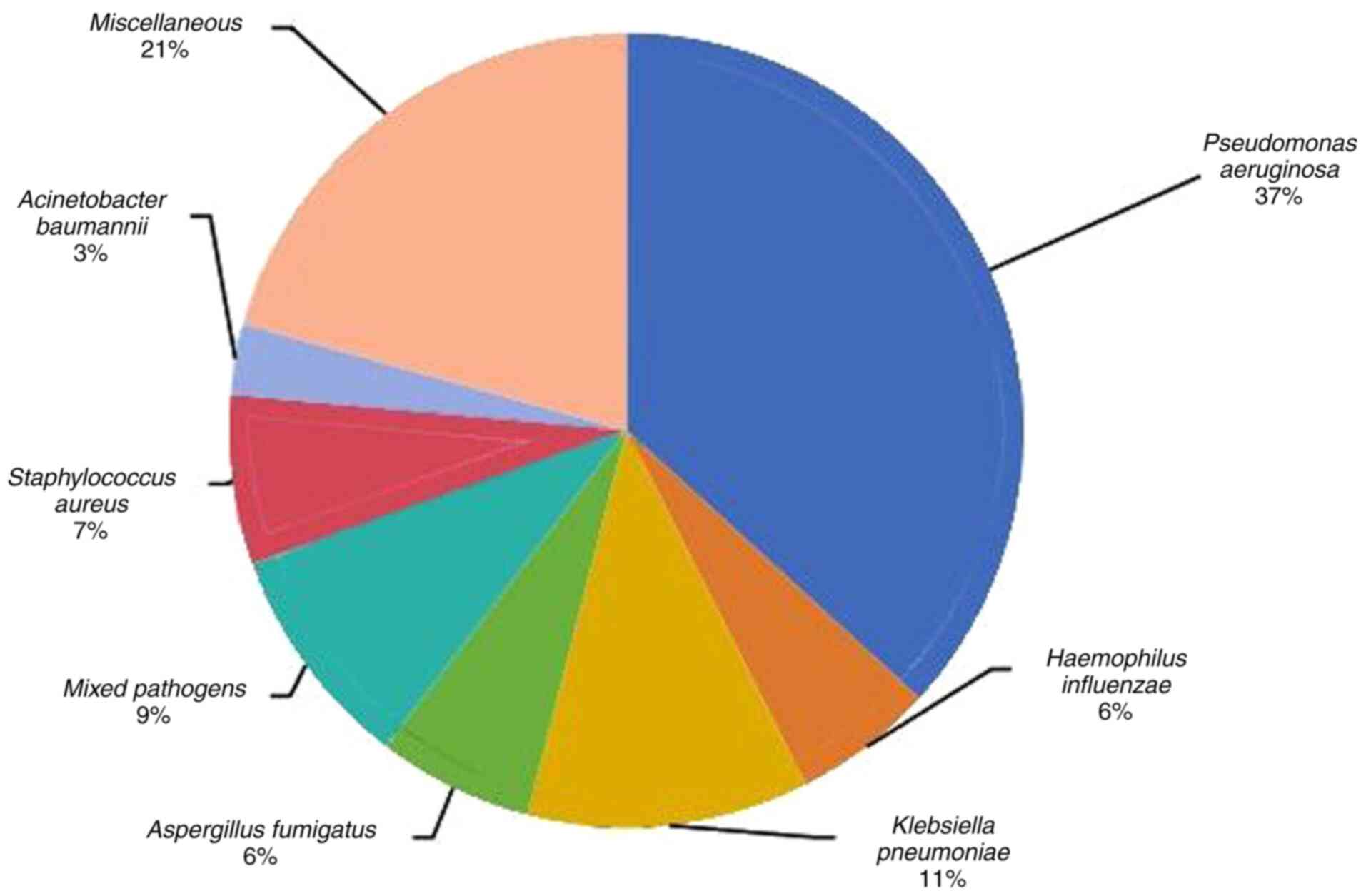
Through the analysis of 522 patients with
bronchiectasis whose sputum or alveolar lavage fluid was positive
for pathogenic bacteria, P. aeruginosa was detected in 192
patients (36.8%) and K. pneumoniae was detected in 60
patients (11.5%). There were 48 patients infected with a mixture of
pathogens (≥2 pathogens) (9.2%), 36 patients infected with
Staphylococcus aureus (6.9%), 33 patients infected with
Aspergillus fumigatus (6.3%), 30 patients infected with
Haemophilus influenzae (5.7%), 15 patients infected with
Acinetobacter baumannii (2.9%) and 108 patients infected
with other pathogens (20.7%). Other pathogens include Enterobacter
cloacae, Proteus mirabilis, Acinetobacter junii,
Pseudomonas putida, Corynebacterium striatum,
Streptococcus mitis, Schizophyllum, Nocardia
otitidiscaviarum and Stenotrophomonas maltophilia
(Fig. 1).
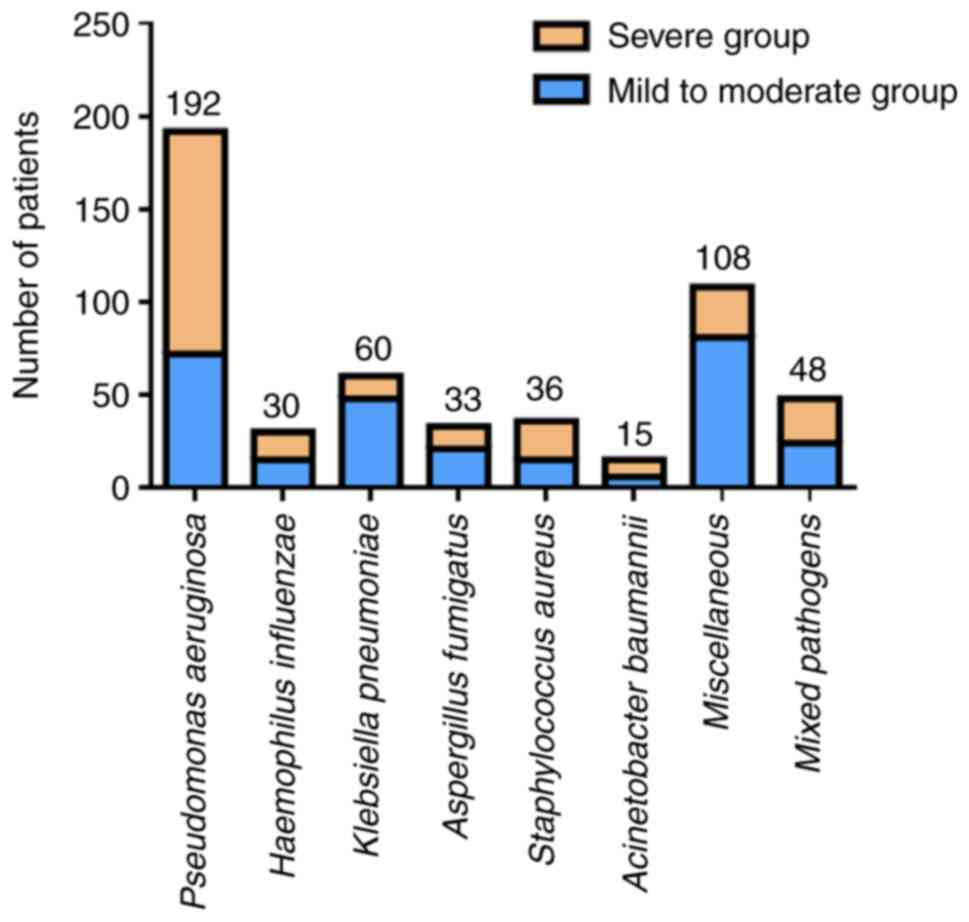
Compared with patients with mild to moderate
bronchiectasis, patients with severe bronchiectasis were more
likely to have P. aeruginosa infection but less likely to
have K. pneumoniae and other pathogens (P<0.05). There
was no significant difference between the two groups of patients in
terms of the detection of H. influenzae, A.
fumigatus, S. aureus, A. baumannii, or mixed
bacteria (P>0.05; Fig. 2).
Effects of different pathogenic
bacterial infections on the length of hospitalization and duration
of antibiotic use in patients with bronchiectasis
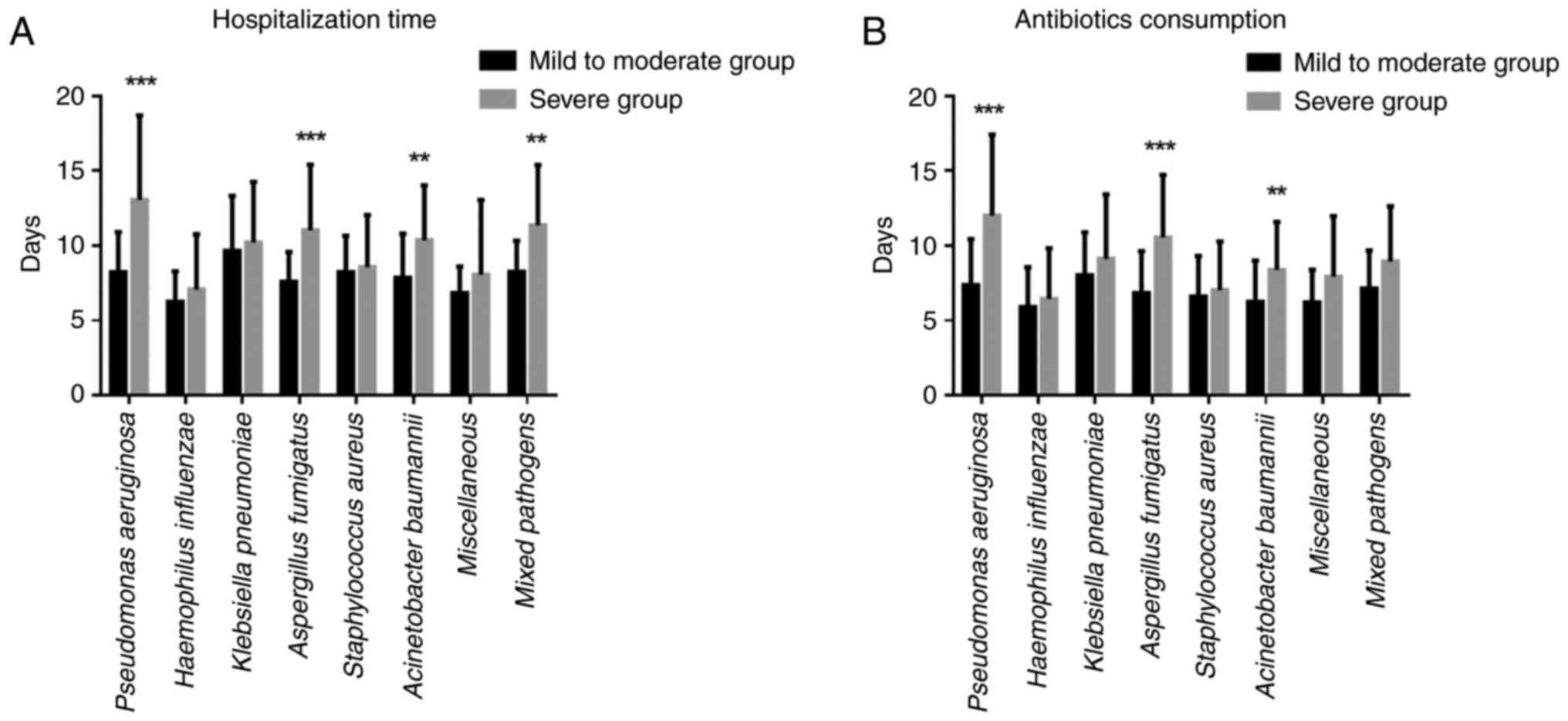
To study whether infection with different pathogens
affects the length of hospitalization, the number of
hospitalization days for patients infected with different pathogens
in the two groups was analysed. Compared with those in the mild to
moderate group, the length of hospitalization of patients in the
severe group infected with P. aeruginosa, A.
fumigatus, A. baumannii, or mixed bacteria significantly
increased (P<0.05). There was no significant difference in the
hospitalization time of patients infected with H.
influenzae, K. pneumoniae, S. aureus, or other pathogens
between the two groups (P>0.05; Fig. 3A).
To study whether infection with different pathogens
affects the time at which patients take antibiotics, the difference
in antibiotic use between the two groups of patients infected with
different pathogens were analysed. Compared with those in the
mild-to-moderate group, the duration of antibiotic use in patients
with severe infections, including P. aeruginosa, A.
fumigatus and A. baumannii infections, was significantly
longer (P<0.05). There was no significant difference in the
duration of antibiotic use between the two groups of patients
infected with H. influenzae, K. pneumoniae, S.
aureus, mixed bacteria, or other pathogens (P>0.05; Fig. 3B).
Effects of different bacterial
infections on the number of acute exacerbations and
hospitalizations in patients with severe bronchiectasis
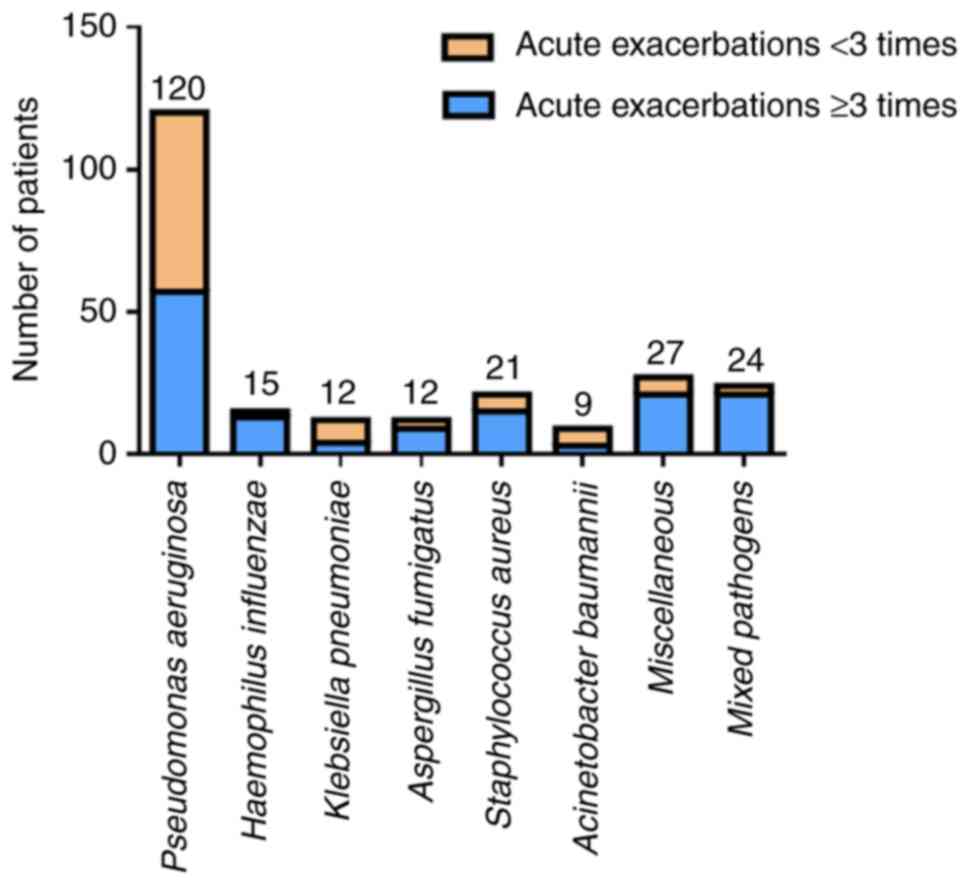
All the patients with severe bronchiectasis were
followed for 1 year to observe the effects of different pathogen
infections on the number of acute exacerbations and the number of
hospitalizations. The pathogens that caused ≥3 acute exacerbations
during the follow-up period were A. baumannii (66.7%), P.
aeruginosa (50%), K. pneumoniae (50%), S. aureus
(28.6%) and A. fumigatus (25%). By contrast, patients with
severe bronchiectasis caused by H. influenzae (13.33%),
other bacteria (22.22%), or mixed pathogens (22.50%) had fewer
acute exacerbations during the 1-year follow-up. The data are shown
in Fig. 4.
The pathogens most associated with hospitalization
within 1 year were A. baumannii (100%), K. pneumoniae
(83.33%), P. aeruginosa (58.33%) and A. fumigatus
(50%). Patients with severe bronchiectasis caused by H.
influenzae (13.33%), S. aureus (28.57%), mixed pathogens
(33.33%), or other bacteria (33.33%) had a low probability of being
hospitalized within 1 year of follow-up. The data are shown in
Table III.
 | Table IIIThe frequency of hospitalization in
patients with severe bronchiectasis infected by different pathogens
within 1 year. |
Table III
The frequency of hospitalization in
patients with severe bronchiectasis infected by different pathogens
within 1 year.
| Pathogen | Total | Number hospitalized
(%) | Hospitalized
(%) |
|---|
| Pseudomonas
aeruginosa | 120 | 50 (41.67) | 70 (58.33) |
| Haemophilus
influenzae | 15 | 13 (86.67) | 2 (13.33) |
| Klebsiella
pneumoniae | 12 | 2 (16.67) | 10 (83.33) |
| Aspergillus
fumigatus | 12 | 6 (50.00) | 6 (50.00) |
| Staphylococcus
aureus | 21 | 15 (71.43) | 6 (28.57) |
| Acinetobacter
baumannii | 9 | 0 (0.00) | 9 (100.00) |
| Miscellaneous | 27 | 18 (66.67) | 9 (33.33) |
| Mixed
pathogens | 24 | 16 (66.67) | 8 (33.33) |
Analysis of independent risk factors
for the development of acute exacerbations in patients with severe
bronchiectasis in the following year
In the severe group of patients with bronchiectasis,
the patients were followed up for 1 year to analyse the univariate
analysis of the associations with different pathogens in patients
with bronchiectasis with acute exacerbation. Compared with other
pathogens, A. baumannii and P. aeruginosa had significant adverse
effects on acute exacerbations in patients with bronchiectasis in
the severe group, as these two pathogens were positively associated
with the risk of acute exacerbations. Compared with infections
caused by other pathogens, infections caused by mixed pathogens,
S. aureus, or H. influenzae had fewer adverse effects
on patients in the severe group. The data are shown in Table IV.
 | Table IVUnivariate Cox risk regression
analysis of acute exacerbation in patients with severe
bronchiectasis. |
Table IV
Univariate Cox risk regression
analysis of acute exacerbation in patients with severe
bronchiectasis.
| Pathogen | Regression
coefficient | Standard error | Wald
χ2 | Odds ratio | 95% Confidence
interval | P-value |
|---|
| Acinetobacter
baumannii | 2.335 | 1.072 | 4.744 | 10.333 | 1.263-84.510 | 0.029 |
| Pseudomonas
aeruginosa | 1.022 | 0.292 | 12.262 | 2.779 | 1.568-4.925 | <0.001 |
| Klebsiella
pneumoniae | 0.021 | 0.595 | 0.001 | 1.021 | 0.318-3.278 | 0.972 |
| Aspergillus
fumigatus | -0.316 | 0.603 | 0.274 | 0.729 | 0.224-2.378 | 0.601 |
| Mixed
pathogens | -1.284 | 0.533 | 5.792 | 0.277 | 0.097-0.788 | 0.016 |
| Staphylococcus
aureus | -1.015 | 0.505 | 4.034 | 0.363 | 0.135-0.976 | 0.045 |
| Haemophilus
influenzae | -2.777 | 1.045 | 7.057 | 0.062 | 0.008-0.483 | 0.008 |
Discussion
P. aeruginosa is a longstanding cause of
adult bronchiectasis infections. However, infections caused by
pathogens other than P. aeruginosa account for more than
half of all bronchiectasis cases and there is a lack of in-depth
studies on the prognostic impact of these pathogens. The present
study described the distribution of pathogens in adults with
bronchiectasis. The most common pathogen was P. aeruginosa,
followed by K. pneumoniae. In the exacerbation stage of
adult bronchiectasis, among patients with severe bronchiectasis,
the pathogens A. baumannii, P. aeruginosa and A.
fumigatus are independent risk factors for future acute
exacerbation and hospitalization.
Among the patients with bronchiectasis who tested
positive for pathogens, those with severe bronchiectasis were
generally older than those with mild to moderate bronchiectasis,
indicating that the older the patients were, the more severe the
symptoms of pathogenic bacterial infection. Sex had no effect on
the severity of infection with pathogens in patients with
bronchiectasis, which is consistent with the literature (15,16).
In terms of common clinical symptoms, chest tightness and bilateral
pulmonary rales were more obvious in patients with severe
bronchiectasis caused by infection, indicating that chest tightness
and bilateral pulmonary rales have greater effects on the severity
of patients with bronchiectasis than other clinical symptoms. More
patients with mild to moderate bronchiectasis smoked and drank more
alcohol than patients with severe bronchiectasis. It is possible
that too little attention has been given to mild to moderate
bronchiectasis. Patients with mild to moderate bronchiectasis had
relatively few comorbidities and an increase in comorbidities
aggravated the disease progression of patients with bronchiectasis
caused by the pathogen. According to the laboratory data, the
changes in blood parameters were more obvious in patients with
severe bronchiectasis compared with patients with mild to moderate
bronchiectasis. This was also expected, as the clinical symptoms
and laboratory test indices were consistent with each other
(17). Therefore, it was
hypothesized that more publicity and education should be provided
to patients with bronchiectasis and that measures to prevent
pathogenic infection should be implemented for older patients with
more underlying diseases.
With respect to the distribution of pathogens in
adult patients with bronchiectasis, most researchers consider that
the most common pathogens are gram-negative bacteria, with P.
aeruginosa being the most common (18). In a cross-sectional study of 184
hospitalized patients with bronchiectasis in Hainan, China, Shi
et al (19) reported that
57.07% of patients had positive sputum microorganism results
according to culture; gram-negative bacteria were the most common
among the culture-positive pathogenic bacteria; and P.
aeruginosa (38.10%), K. pneumoniae (14.29%) and A.
baumannii (11.56%) were the most common gram-negative bacteria
in the culture. There are other similar reports on the aetiology of
patients with bronchiectasis. Wang et al (20) reported that gram-negative bacteria
were the most common bacteria and accounted for 76.8%, followed by
gram-positive bacteria (19.4%), whereas fungi were the least common
(accounting for 3.8%). Among the gram-negative bacteria, the top
three were P. aeruginosa (25.6%), A. baumannii
(17.5%) and K. pneumoniae (11.8%). However, several
researchers have put forwards a different point of view, concluding
that H. influenzae is the most common pathogen, followed by
P. aeruginosa (21). The
results of the present study revealed that among the 522 patients
with bronchiectasis who had positive pathogenic bacteria tests,
gram-negative bacteria were more common, with P. aeruginosa
being the most common, followed by K. pneumoniae, H.
influenzae and A. baumannii, accounting for 73% of all
pathogens, which was consistent with previous reports (22). In addition, concerning the
distribution of other pathogens, gram-positive bacteria accounted
for 9.8% of all the strains, mixed pathogens accounted for 9.2% of
all the strains and fungi accounted for 8% of all the strains;
these findings are also consistent with the most common pathogens
reported at home and abroad, but the distribution proportions were
slightly different. The possible reasons are that the prevalence of
pathogens in the population differs by region and climatic
environment. This may also be due to the different severities of
the conditions of the treated patients with bronchiectasis,
especially because the proportions of patients with severe
bronchiectasis were different (23).
P. aeruginosa was the main infection in
patients with severe bronchiectasis, whereas K. pneumoniae
was the main infection in patients with mild to moderate
bronchiectasis. It was hypothesized that P. aeruginosa
exacerbates the acute symptoms of bronchiectasis. In addition, the
present study revealed that patients with bronchiectasis caused by
the same pathogen had different durations of hospitalization and
durations of antibiotic use. Patients with the same degree of
bronchiectasis had different lengths of hospitalization and
antibiotic use, depending on the pathogen. The duration of
hospitalization and antibiotic use were greatest for patients
infected with P. aeruginosa, which may be related to the
mechanism of action of P. aeruginosa on bronchiectasis
(24).
The present study involved a prognostic analysis of
the pathogens found in clinical work and revealed that P.
aeruginosa infection increased the risk of acute exacerbation
and hospitalization in future patients with bronchiectasis.
Additionally, A. baumannii, K. pneumoniae and A.
fumigatus adversely affect the prognosis of patients with
bronchiectasis (acute exacerbation, hospitalization). Long-term
macrolide antibiotics currently have the highest level of evidence
for reducing exacerbations (25).
Long-term oral antibiotics can also be considered for patients with
recurrent exacerbations who are otherwise optimally managed but
should not be prescribed routinely. The results of the present
study showed that patients with bronchiectasis caused by infection
with P. aeruginosa, H. influenzae, K.
pneumoniae, A. fumigatus, mixed pathogens, S.
aureus, or A. baumannii could develop acute
exacerbations in the future. Compared with patients infected with
other pathogens, patients infected with P. aeruginosa, K.
pneumoniae, A. fumigatus, or A. baumannii were
more likely to experience acute exacerbations, hospitalization, or
worsening of their conditions over 1 year. Depending on the
clinical situation, antibiotics are usually prescribed for 7-14
days. Intravenous antibiotics for severe exacerbations are usually
continued for at least 5 days and are often followed by oral
antibiotics for a total duration of 10-14 days (26). If the culture is positive for P.
aeruginosa, the common practice is to prescribe two weeks of
oral ciprofloxacin and then repeat the sputum culture (27). Furthermore, Foumani et al
(28) showed that the probiotics
were not effective in the improvement of clinically bronchiectasis,
consumption of antibiotics, the rate of pulmonary exacerbations
with or without the need for hospitalization, forced expiratory
volume in the first second (FEV1) and FEV1/forced vital capacity
(FVC) and microbiological pattern. Up to now, there has been no
sufficient evidence about the effects of probiotics on other
respiratory infections such as bronchiectasis (29).
A. baumannii is found mainly in critically
ill patients and infection most commonly manifests as
ventilator-associated pneumonia, followed by blood, skin and soft
tissue infections (30). The
incidence of multidrug-resistant A. baumannii is high. A
global drug resistance assessment revealed that ~45% of A.
baumannii isolates were multidrug-resistant strains, which is a
much greater percentage than that reported for other pathogens
(31). However, in the present
study, compared with patients infected with P. aeruginosa,
A. baumannii, K. pneumoniae, or A. fumigatus,
patients infected with H. influenzae, S. aureus, or
mixed pathogens had fewer adverse effects on the prognosis of
bronchiectasis (acute exacerbation, hospitalization), fewer acute
exacerbations and a lower risk of hospitalization.
The present study analysed the effects of different
pathogens on the prognosis of patients with bronchiectasis (acute
exacerbation, hospitalization). Compared with patients infected
with other pathogens, patients infected with P. aeruginosa,
A. baumannii, K. pneumoniae, or A. fumigatus
had a greater risk of acute exacerbation and/or hospitalization
within 1 year. More attention should be paid to their
anti-infective treatment, follow-up after discharge and health
education.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Health Bureau of
Zhejiang Province (grant no. 2024KY443) and Science and Technology
Bureau of Jiaxing (grant no 2022AD30028).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HQW and YYN conceived and designed the study. YYN
and XL designed the methodology and contributed to data
acquisition. YYN and XG confirm the authenticity of all the raw
data. XSL and XG performed the statistical analyses. YYN and HQW
drafted the manuscript. XG and HQW confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki (revised in 2013) and was approved by
The Second Affiliated Hospital of Jiaxing University (Zhejiang,
China; approval no. 2024SW110-01). Samples were obtained from all
patients with written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shteinberg M, Flume PA and Chalmers JD: Is
bronchiectasis really a disease? Eur Respir Rev.
29(190051)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
King P, Holdsworth S, Freezer N and Holmes
P: Bronchiectasis. Intern Med J. 36:729–737. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
King PT, Holdsworth SR, Freezer NJ,
Villanueva E and Holmes PW: Characterisation of the onset and
presenting clinical features of adult bronchiectasis. Respir Med.
100:2183–2189. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Polverino E, Dimakou K, Hurst J,
Martinez-Garcia MA, Miravitlles M, Paggiaro P, Shteinberg M,
Aliberti S and Chalmers JD: The overlap between bronchiectasis and
chronic airway diseases: State of the art and future directions.
Eur Respir J. 52(1800328)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mac Aogáin M, Xaverius Ivan F, Jaggi TK,
Richardson H, Shoemark A, Narayana JK, Dicker AJ, Koh MS, Lee KCH,
Thun How O, et al: Airway ‘resistotypes’ and clinical outcomes in
bronchiectasis. Am J Respir Crit Care Med. 210:47–62.
2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Berra G, Chappuis-Gisin É, Soccal PM and
Plojoux J: Inhaled antibiotics for the management of non cystic
fibrosis bronchiectasis. Rev Med Suisse. 13:2001–2004.
2017.PubMed/NCBI(In French).
|
|
7
|
Chalmers JD, Chang AB, Chotirmall SH, Dhar
R and McShane PJ: Bronchiectasis. Nat Rev Dis Primers.
4(45)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang XX, He JH, Pan CX, He ZF, Li HM, Lin
ZH, Zhang XF, Cen LJ, Zhang RL, Shi MX and Guan WJ: Bacteria and
viruses and clinical outcomes of asthma-bronchiectasis overlap
syndrome: A cohort study. Clin Transl Allergy.
14(e12331)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang SH, Song MJ, Kim YW, Kwon BS, Lim SY,
Lee YJ, Park JS, Cho YJ, Lee JH, Lee CT and Kim HJ: Understanding
the effects of Haemophilus influenzae colonization on
bronchiectasis: A retrospective cohort study. BMC Pulm Med.
24(7)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Loong SK, Liam CK, Karunakaran R, Tan KK,
Mahfodz NH and AbuBakar S: Non-classical Bordetella sp. (closely
related to Bordetella hinzii and Bordetella pseudohinzii) lower
respiratory tract infection in a patient with extensive
bronchiectasis: A case report. J Int Med Res.
52(3000605231214464)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McShane PJ: Investigation and management
of bronchiectasis in nontuberculous mycobacterial pulmonary
disease. Clin Chest Med. 44:731–742. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chagas MDS, Trindade Dos Santos M, Argollo
de Menezes M and da Silva FAB: Boolean model of the gene regulatory
network of Pseudomonas aeruginosa CCBH4851. Front Microbiol.
14(1274740)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bronchiectasis Expert Consensus Writing
Group; Pulmonary Infection Assembly, Chinese Thoracic Society.
Expert consensus on the diagnosis and treatment of adult
bronchiectasis in China. Zhonghua Jie He He Hu Xi Za Zhi.
44:311–321. 2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
14
|
Costa JC, Machado JN, Ferreira C, Gama J
and Rodrigues C: The bronchiectasis severity index and FACED score
for assessment of the severity of bronchiectasis. Pulmonology.
(S2173-5115(17)30154-9)(2018): (Epub ahead of print).
|
|
15
|
Yang B, Kim BG, Han K, Jung JH, Kim JH,
Park DW, Kim SH, Kim EG, Sohn JW, Yoon HJ, et al: Systemic
sclerosis and risk of bronchiectasis: A nationwide longitudinal
cohort study. Arthritis Res Ther. 25(209)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jayaram L, King PT, Hunt J, Lim M, Park C,
Hu E, Dousha L, Ha P, Bartlett JB, Southcott AM, et al: Evaluation
of high dose N-acetylcysteine on airway inflammation and quality of
life outcomes in adults with bronchiectasis: A randomised
placebo-controlled pilot study. Pulm Pharmacol Ther.
84(102283)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen W, Ran S, Li C, Li Z, Wei N, Li J and
Li N: Elevated eosinophil counts in acute exacerbations of
bronchiectasis: Unveiling a distinct clinical phenotype. Lung.
202:53–61. 2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yong VFL, Soh MM, Jaggi TK, Mac Aogáin M
and Chotirmall SH: The microbial endocrinology of Pseudomonas
aeruginosa: Inflammatory and immune perspectives. Arch Immunol
Ther Exp (Warsz). 66:329–339. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shi LH, Cheng F, Gao BR, et al: Analysis
of clinical characteristics of 207 inpatients with bronchiectasis
in Hainan Province. Int J Respir. 43:595–603. 2023.
|
|
20
|
Wang R, Ding S, Lei C, Yang D and Luo H:
The contribution of Pseudomonas aeruginosa infection to
clinical outcomes in bronchiectasis: A prospective cohort study.
Ann Med. 53:459–469. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Finney LJ, Ritchie A, Pollard E, Johnston
SL and Mallia P: Lower airway colonization and inflammatory
response in COPD: A focus on Haemophilus influenzae. Int J
Chron Obstruct Pulmon Dis. 9:1119–1132. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Duff RM, Simmonds NJ, Davies JC, Wilson R,
Alton EW, Pantelidis P, Cox MJ, Cookson WO, Bilton D and Moffatt
MF: A molecular comparison of microbial communities in
bronchiectasis and cystic fibrosis. Eur Respir J. 41:991–993.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Miao XY, Ji XB, Lu HW, Yang JW and Xu JF:
Distribution of major pathogens from sputum and bronchoalveolar
lavage fluid in patients with noncystic fibrosis bronchiectasis: A
systematic review. Chin Med J (Engl). 128:2792–2797.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sabat AJ, Pantano D, Akkerboom V, Bathoorn
E and Friedrich AW: Pseudomonas aeruginosa and
Staphylococcus aureus virulence factors as biomarkers of
infection. Biol Chem. 402:1565–1573. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Polverino E, Goeminne PC, McDonnell MJ,
Aliberti S, Marshall SE, Loebinger MR, Murris M, Cantón R, Torres
A, Dimakou K, et al: European Respiratory Society guidelines for
the management of adult bronchiectasis. Eur Respir J.
50(1700629)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chang AB, Bell SC, Torzillo PJ, King PT,
Maguire GP, Byrnes CA, Holland AE, O'Mara P and Grimwood K:
extended voting group. Chronic suppurative lung disease and
bronchiectasis in children and adults in Australia and New Zealand
Thoracic Society of Australia and New Zealand guidelines. Med J
Aust. 202:21–23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pasteur MC, Bilton D and Hill AT: British
Thoracic Society Bronchiectasis non-CF Guideline Group. British
Thoracic Society guideline for non-CF bronchiectasis. Thorax. 65
(Suppl 1):i1–i58. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Alavi Foumani A, Jafari A, Tangestani
Nejad A, Jafarinejhad A, Ziyapour S, Keivanlou MH and Afzalipour M:
Effects of probiotics on clinical manifestations of bronchiectasis:
A randomized, triple blinded, placebo-controlled clinical trial.
Tanaffos. 22:221–229. 2023.PubMed/NCBI
|
|
29
|
Morrow LE, Kollef MH and Casale TB:
Probiotic prophylaxis of ventilator-associated pneumonia: A
blinded, randomized, controlled trial. Am J Respir Crit Care Med.
182:1058–1064. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sarshar M, Behzadi P, Scribano D, Palamara
AT and Ambrosi C: Acinetobacter baumannii: An ancient
commensal with weapons of a pathogen. Pathogens.
10(387)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yao Y, Chen Q and Zhou H: Virulence
factors and pathogenicity mechanisms of Acinetobacter
baumannii in respiratory infectious diseases. Antibiotics
(Basel). 12(1749)2023.PubMed/NCBI View Article : Google Scholar
|