G protein-coupled receptor 124 (GPR124) belongs to
the B2 class of adhesive trans-membrane signal transduction
proteins and is involved in a number of physiological and
biochemical reactions in the human body, such as regulating
endothelial cell (EC) function, participating in inflammation and
angiogenesis (1-4).
Cardiovascular and cerebrovascular diseases are general terms of
cardiovascular and cerebrovascular diseases, which generally refer
to ischemic or hemorrhagic diseases of the heart, brain and whole
body tissues caused by hyperlipidemia, blood viscosity,
atherosclerosis (AS) and hypertension (HTN) (5). There is evidence that GPR124 is
closely related to ischemic stroke, AS, HTN, cancer and other
diseases. In this paper, the biological characteristics of GPR124
were summarized, the main research progress in its related diseases
was introduced and its potential signal transduction and associated
pathways were highlighted.
The guanosine-binding protein coupled receptors
(GPCRs) are the largest family of transmembrane receptors in
eukaryotes and the most abundant protein family in the genome
(6). All GPCRs have a similar
structure, i.e., consisting of an extracellular N terminus, an
intracellular C terminus and a secondary trans-membrane helix,
TM-7, formed by connecting the extracellular and intracellular
loops (7,8). They transmit signals in response to a
variety of physical and chemical stimuli, including
neurotransmitters, hormones, local mediators, metabolism or
olfaction. GPCR has regulatory functions in almost all organ
systems and the dysregulation of GPCR signaling is related to the
pathogenesis of numerous diseases (9).
GPCRs can be divided into five categories: A, B1,
B2, C and F according to their genetic sequence and structural
similarity (10). GPR124 belongs
to the B2 family of adhesive GPCR. This receptor-related gene is
located in the 16q12.4 region of the rat chromosome and 8p11.22 in
the human chromosome, with 84% homology, encoding a protein of
1,331 amino acids (11). The
extracellular N-terminus of GPR124 has a leucine-rich repeat (LRR),
immunoglobulin domain, hormone binding domain (12). The C-terminal tail in the cell
membrane interacts with the Gβ1γ2 subunit and connects to the
molecular scaffold of the engulfment and cell motility-dedicator of
cytokinesis family and guanine nucleotide exchange factor, which
has a potentially extensive role in the signal transduction of
GPR124(13). GPR124 is a
transmembrane signal transduction protein, also known as tumor
endothelial marker 5 (TEM5). As it was first found in the
neovascular ECs of human colorectal cancer, it is closely related
to tumor and vascular biology research (14,15).
At present, the research on the orphan receptor GPR124 at home and
abroad is still in the initial stage and most studies have focused
on the central nervous system (CNS), cardiovascular and
cerebrovascular diseases and tumors.
GPR124 is an endothelial-specific receptor necessary
for normal pre-cerebrovascular formation and blood-brain barrier
(BBB) function in mouse embryos, mediating CNS angiogenesis
(16,17). Studies have shown that GPR124 has a
cellular autonomic function in the brain ECs, regulating the
embryonic forebrain and neural tube angiogenesis (14,18-20).
A clear lethal CNS-specific phenotype associated with GPR124
deletion is observed during embryonic development. This phenotype
is marked by impaired angiogenesis, hemorrhagic glomeruloid
malformations, developmental BBB defects and loss of expression of
the BBB marker glucose transporter 1 (Glut1, also referred to as
Slc2a1) (20). The result was also
confirmed in experiments on zebrafish (21). Similar developmental abnormalities
were seen in ECs after deleting β-catenin or dual-deleting
Wnt7a/Wnt7b, indicating a potential connection between GPR124 and
canonical WNT signaling (22,23).
Wnt ligands participate in a variety of signaling pathways that are
active throughout different stages of disease, during development
and during tissue homeostasis maintenance. Wnt family member 1
(WNT1)- and WNT7B-mediated synergistic Wnt signaling requires
frizzled class receptor 5 (FZD5), FZD8 and LDL receptor-related
protein 6 (LRP6), as well as the WNT7B co-receptors GPR124 and
reversion inducing cysteine-rich protein with Kazal motifs (RECK)
(24). Further studies, such as
genetic analyses, have shown that GPR124 typically stimulates
β-catenin signaling to cooperate with WNT7 to regulate angiogenesis
and is of great significance in the specific angiogenesis of the
CNS and the formation of the blood-brain barrier (25). GPR124 and Reck are part of the cell
surface protein complex and enable brain ECs to selectively respond
to Wnt7 (26,27). Dishevelled increases the local
concentrations of Wnt7 that are available for Frizzled signaling by
recruiting GPR124 and the related Reck-bound Wnt7 into dynamic
Wnt/Frizzled/Lrp5/6 signalosomes by polymerization (26). Studies have demonstrated that
GPR124 coactivates canonical Wnt signaling induced by Wnt7a and
Wnt7b via a Lrp coreceptor and Frizzled receptor, and that
GPR124-stimulated signaling coordinates with Norrin/Frizzled4
signaling to regulate CNS vascular development (28-30).
Therefore, GPR124 is an important regulatory factor for the
development of the neurovascular system.
The expression of GPR124 is induced by small GTPase
Rac and mediates contact inhibition of EC proliferation during
angiogenesis (31). A layer of
cells in the arterial lumen known as vascular ECs (VECs) is crucial
in the development of vascular disorders. They can release a
variety of proteins that govern thrombosis, smooth muscle cell
(SMC) migration and proliferation, vascular wall inflammation and
cell adhesion and migration-all of which are critical for
maintaining vascular homeostasis. VECs exhibit an active phenotype
or endothelial dysfunction, in response to adverse stimuli
(32). Abnormal EC proliferation
and angiogenesis may lead to the formation of cerebral
arteriovenous malformation, increasing the risk of ischemic stroke.
GPR124 conditional knockout in the endothelium of adult mice did
not affect steady-state BBB integrity, but resulted in BBB
disruption and microvascular bleeding in a mouse model of ischemic
stroke, accompanied by decreased cerebrovascular classical
WNT/β-catenin signaling (20).
During ischemia, morphological analysis showed that GPR124 was
localized in focal adhesion, directly bound to actin and promoted
the formation and directed migration of vascular pericytes
(33). Studies have shown that the
Wnt7/Gpr124/Reck signaling pathway regulates EC function by
regulating VEGF expression (34,35).
Statistical analysis suggested that periodontitis was a risk factor
for ischemic stroke (36,37). The inflammatory markers caspase-1
and IL-6 have not only been identified as biomarkers of
periodontitis, but also as indicators of increased stroke risk.
Both can be detected in the bloodstream, which suggests a possible
worsening of the stroke process (36). This further confirmed the role of
GPR124 in ischemic stroke.
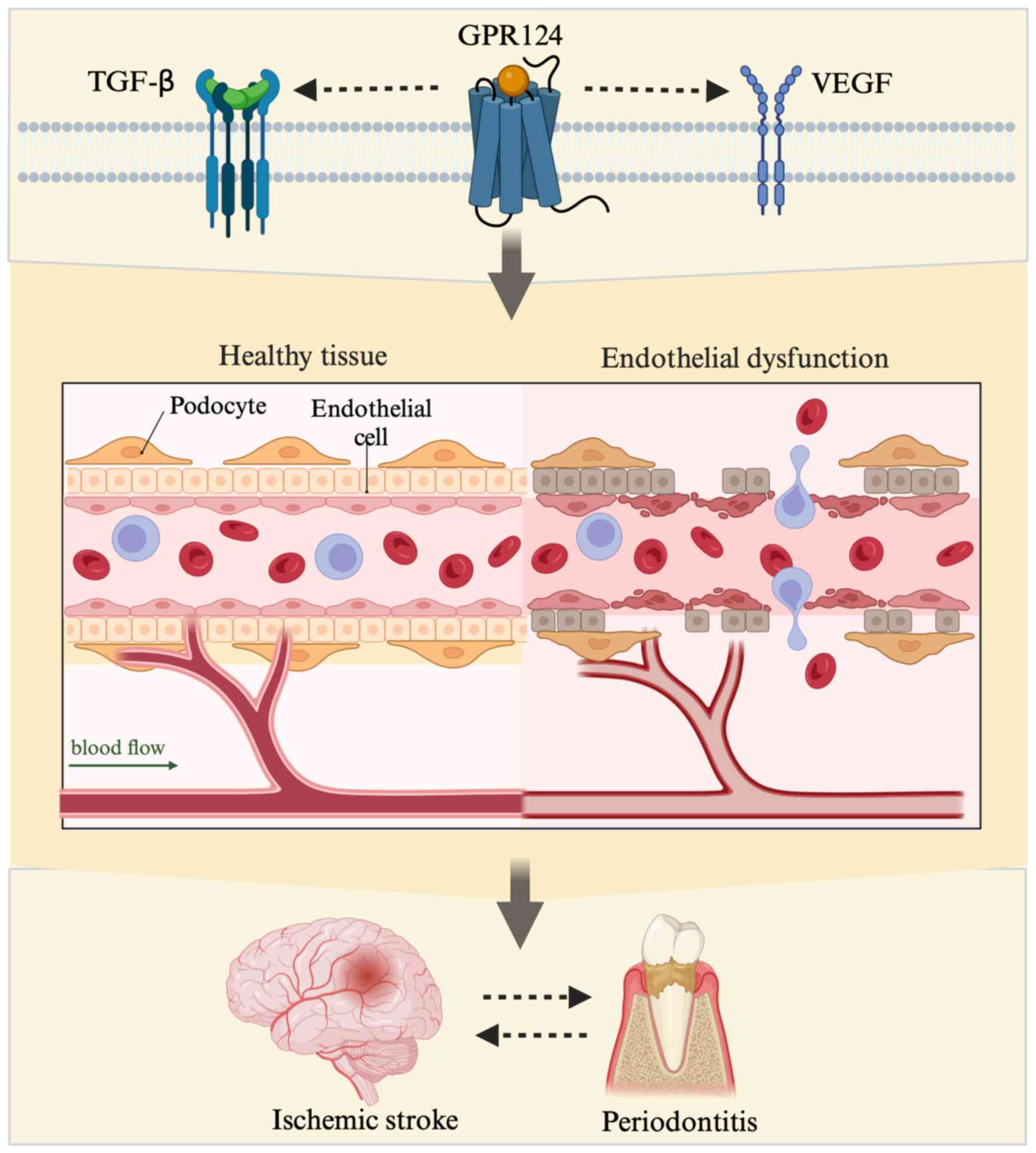
Studies revealed that TGF-β increases the expression
of GPR124 and that GPR124 ablation causes abnormal TGF-β pathway
activation, indicating that GPR124 has a function in regulating
TGF-β signaling (38). During CNS
angiogenesis, pericytes and astrocytes have been identified as
cellular sources of TGF-β (26).
TGF-β signaling in ECs has previously been shown to be associated
with CNS angiogenesis, as its absence leads to abnormal blood
vessel germination and bleeding (39). This suggests a potential
association of GPR124 with TGF-β in ischemic stroke (Fig. 1).
AS is considered to be a chronic inflammatory
disease that results in arterial plaque formation and vessel bed
stenosis, and is the major cause of cardiovascular disease
(40). Every stage of
atherosclerosis is influenced by vascular SMCs (VSMCs), and lineage
tracing research has shown that at least 30% of the cells in
atherosclerotic lesions are produced from VSMCs (41). It is thought that the proliferation
and migration of VSMCs are of great significance for the formation
of AS, among which inhibiting VSMC proliferation and migration has
a protective effect on arteries (42). GPR124 showed neuronal and
non-neuronal expression profiles (19). Overexpression of GPR124 in ECs
activated by inflammation or other microenvironmental stimuli can
induce increased expression of α-SM actin, a marker of SMCs,
suggesting that SMCs at the root of the aorta proliferate and
migrate to the intima after EC dysfunction, thereby aggravating
atherosclerotic plaques (43).
GPR124 not only increases serum cholesterol and lipid deposition in
the aortic sinus, leading to EC dysfunction, but also exacerbates
the proliferation of VSMCs. After 16 weeks of high-fat diet in
apolipoprotein E knockout mice, mouse aortic SMCs showed obvious
signs of inflammatory activation and dedifferentiation (44). At the same time, the expression of
GPR124 in aortic SMCs increased significantly. Lipopolysaccharide
(LPS) is one of the inflammatory substances that aggravate the
progression of AS and LPS can lead to the dedifferentiation of
VSMCs. Inflammatory activation in a septicemia model constructed by
intraperitoneal injection of LPS led to characteristic changes in
the GPCR library, in which dedifferentiated SMC upregulated
GPR124(45). Cell experiments have
shown that in vitro culture of primary aortic SMCs not only
leads to the upregulation of genes of a dedifferentiated SMC
phenotype, such as intercellular adhesion molecule 1, vascular cell
adhesion molecule 1, proliferation marker Ki-67 or IL-6, but also
leads to upregulation of genes of the dedifferentiated SMC
phenotype. Furthermore, the expression frequency of GPR124
increased significantly. This confirms that GPR124 is involved in
the mechanism of VSMC dedifferentiation during atherosclerosis.
However, studies have also shown that GPR124 is lower in
atherosclerotic aortic dedifferentiated SMCs than in healthy aorta.
Based on the theory that oxidized low-density lipoprotein (mox-LDL)
can induce the transformation of VSMC from a ‘contractile’
phenotype to a ‘migration, proliferation and synthesis’ phenotype,
which is the core of intimal hyperplasia and atherosclerosis
formation, researchers found that VSMCs are transformed after
mox-LDL action and the expression of GPR124 is downregulated
(46).
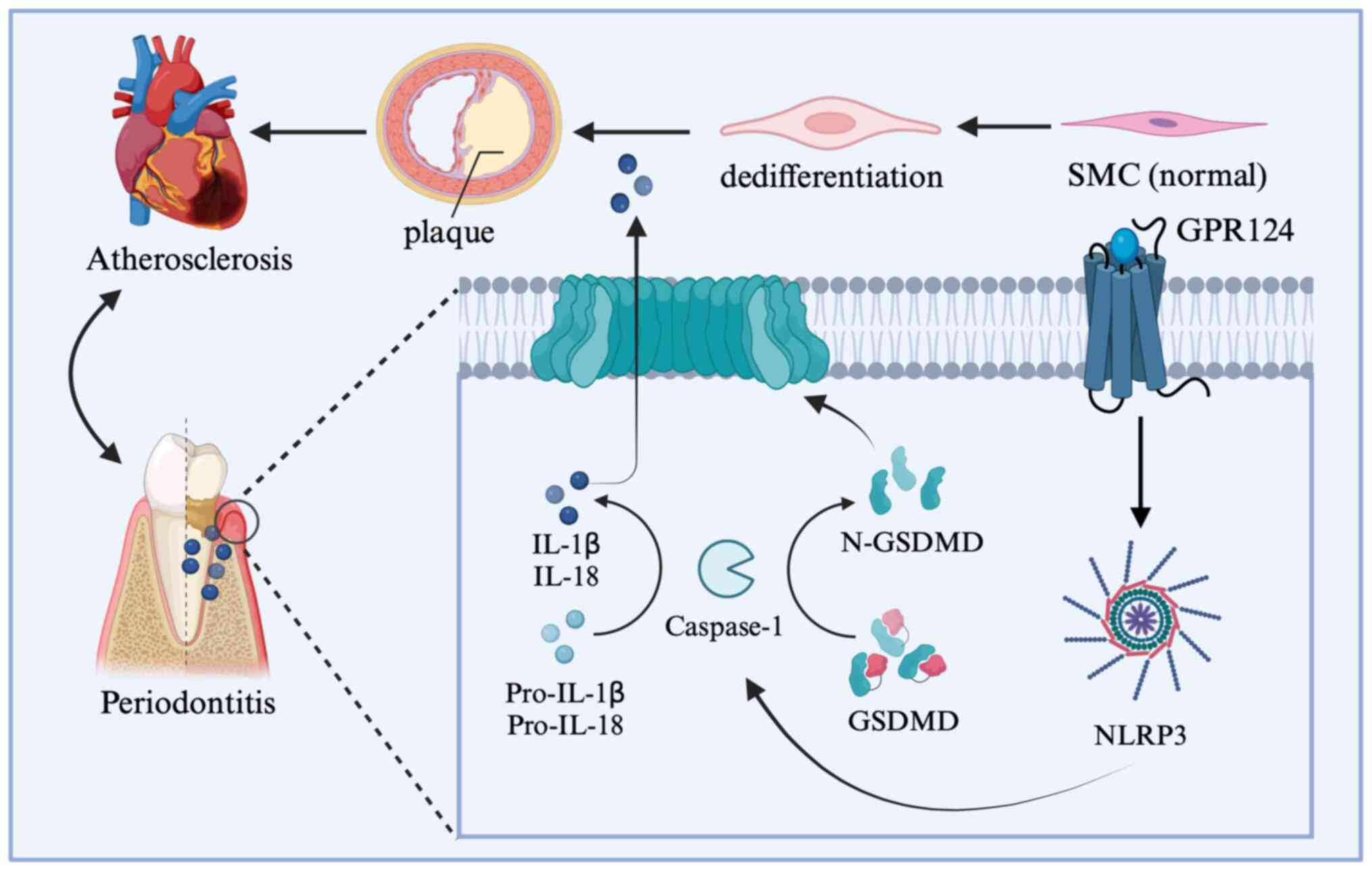
Studies have demonstrated the potential importance
of molecular and cellular mechanisms associated with NOD-, LRR- and
pyrin domain-containing protein 3 (NLRP3) inflammasome signaling in
the disease process (47).
Patients with atherosclerosis had higher expression of the NLRP3
inflammasome in their plaque and peripheral blood mononuclear
cells, suggesting a connection between the two conditions (48). At the molecular level, abnormal
activation of NLRP3 inflammasome and its resulting recruitment of
high circulating levels of IL-1β and IL-18 and macrophages to
aortic wall lesions promote the phenotypic transformation of VSMCs,
inducing foam-cell formation and increasing plaque instability
(49,50). Numerous studies have suggested that
the inflammasome NLRP3/Caspase-1/IL-1β pathway triggers an
inflammatory response in the blood vessel wall, leading to the
progression of atherosclerosis (51). However, the induced
endothelium-specific overexpression of GPR124 can promote
atherosclerosis by activating nitrosation, a process of converting
organic compounds into nitroso derivatives and NLRP3 inflammasome
signaling (43,52). The expression of GPR124 in AS mice
was increased at the same time as the level of oxidized low-density
lipoprotein (53). The progression
of AS is closely associated with several inflammatory diseases, one
of which is periodontitis (54).
In the pathological process of periodontitis, NLRP3 inflammasome
recruitment and trigger activation of Caspase-1, pro-inflammatory
factor IL-1β, IL-18 through chemotaxis to increase local
neutrophils, thus further destroying the periodontal membrane and
forming an environment suitable for the proliferation of
periodontal pathogens (55-58).
It will also activate osteoclasts and eventually lead to alveolar
bone resorption (59) (Fig. 2). Therefore, the relationship
between GPR124 and NLRP3/Caspase-1/IL-1β pathways may provide a new
treatment strategy for AS and improve the prognosis.
HTN is defined as systolic and diastolic blood
pressure greater than or equal to 140/90 mmHg (60). Consistent with the above-mentioned,
GPR124 is expressed in the heart, aorta, cerebrovascular system,
kidney and other organs (61,62).
GPR124 expression was significantly increased in the aorta of HTN
mice aged 6-8 weeks, and significantly increased in the kidneys and
left atrium at 10-12 weeks. Overall, GPR124 expression was
upregulated in the left ventricle and left atrium (63). This suggests that GPR124 may be
overexpressed after HTN progression to reduce heart damage caused
by HTN. VSMCs participate in the tissue structure of the vascular
wall and serve as one of the cells that mainly maintain vascular
tension (15). The proliferation
and hypertrophy of SMCs and the contractile changes of their
functions after stimulation will lead to the occurrence of
hypertension. GPR124 is expressed in aortic SMCs and skeletal SMCs,
which not only increases the expression during the differentiation
of skeletal SMCs, but also inhibits the inflammatory factors in
them. This suggests that GPR124 has a regulatory effect on blood
pressure. GPR124 also has a promoting effect on angiogenesis, which
is also related to the development of HTN. Poor periodontal health
was associated with an increased prevalence of hypertension, and
after periodontal treatment, the mean systolic blood pressure of
hypertensive patients with periodontitis was significantly reduced,
suggesting that periodontitis has an impact on blood pressure
control (64,65). At the same time, it has also been
suggested that HTN is related to peridental microecological
imbalance, suggesting that the influence of HTN leads to
microcirculation changes and subsequent periodontal tissue
ischemia, which leads to the progression and deterioration of
periodontitis (66). Further
research is needed on the potential association of GPR124 in
hypertension with periodontitis.
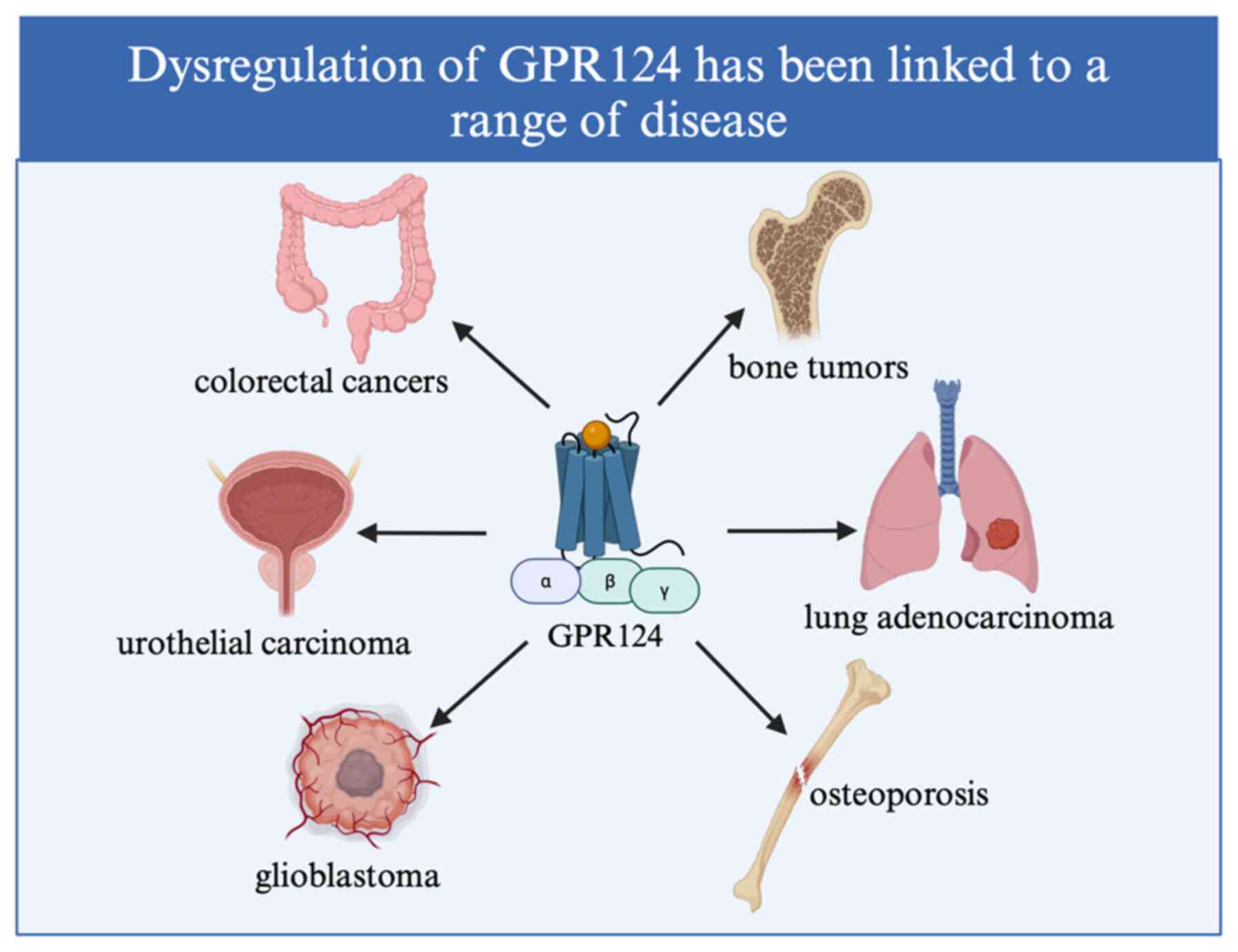
GPR124 is expressed in ECs and pericytes during
angiogenesis. Also, this receptor plays a role in EC migration and
differentiation without impacting cell proliferation. ECs create
the vasculature of the developing brain and express this receptor
at high levels (24). The
importance of GPR124 during development suggests that its abnormal
expression may be involved in tumor growth (67). Research has shown that GPR124
silencing in human ECs inhibits angiogenesis and tumor growth in
mouse xenograft tumors (33).
Thus, it is suggested that GPR124 plays a crucial role in the
neovascularization of cancer. The first indication of GPR124's
overexpression came from tumor endothelial capillaries in human
colorectal malignancies (68).
Brain metastasis of cancer causes high mortality,
but the exact mechanisms underlying the metastasis remain unclear.
Brain metastasis of lung adenocarcinoma is caused by GPR124
activation of Wnt7-β-catenin signal transduction to enhance the
ability of pericytes to migrate across the endothelium for
trans-endothelial migration (69).
Similarly, patients with lung adenocarcinoma brain metastasis with
GPR124 mutations have an unfavorable prognosis (70). In addition, by downregulating the
protein levels of GPR124, VEGF, MMP-3 and MMP-9 in U-2OS cells with
drugs, blood metastasis and lymph node metastasis of bone tumors
can be inhibited (71). A study
used transcriptome datasets for a weighted gene co-expression
network analysis to identify networks and hub genes related to the
prognosis of gastric cancer. The results showed that in gastric
cancer, high expression of GPR124 was associated with poor
prognosis (72).
After menopause and in old age, osteoporosis is
common in women. Osteoporotic fractures are easy to produce and can
end in death or severe disability. Biomarker expression during
osteogenic differentiation in bone marrow mesenchymal stem cells
(BMSCs) was measured using western blotting and reverse
transcription-quantitative PCR. The findings demonstrated that
during the osteogenic differentiation of BMSCs, there was a
considerable increase in the expression of GPR124(73). The researchers further proposed
that GPR124 could promote the osteogenic differentiation of BMSCs
through the Wnt/β-catenin pathway, given the importance of the
typical Wnt/βcatenin signaling pathway in promoting osteoblast
differentiation and the association of GPR124 with the typical
Wnt/βcatenin signaling pathway (73,74).
BMSCs are important for the recovery of bone defects after
periodontitis (75). Future
studies on GPR124 regulation of BMSCs through the Wnt/βcatenin
signaling pathway to promote bone tissue regeneration have great
prospects. GPR124, also known as TEM5, is expressed in the
forebrain and spinal cord (76).
It is the first basic endothelial receptor as a regulator of
cerebral angiogenesis. GPR124 is one of the key molecules in the
differentiation and maturation of the BBB. BBB dysfunction is
associated with a variety of neurological diseases, including
stroke, multiple sclerosis and brain tumors. Genetic deletion of
GPR124 has been implicated in brain-specific regulation of
transforming growth factor-β (TGF-β) signaling. TGF-β signaling
pathway in ECs has been identified in the angiogenesis of the CNS.
A lack of the TGF-β pathway leads to vascular germination and
bleeding (19) (Fig. 3).
In conclusion, GPR124 is involved in the process of
cardiovascular and cerebrovascular diseases and cancers by
affecting EC function, regulating angiogenesis and mediating
inflammation, and the possible mechanism of GPR124 in systemic
diseases accompanied by periodontitis has been proposed. This makes
GPR124 a potential therapeutic target, particularly in cancer and
other vascular-related diseases. At the same time, GPR124 may also
affect neural development by regulating the TGF-β signaling
pathway, which is of great significance for the treatment and
research of nervous system diseases. However, the current research
on GPR124 and its mediated signaling pathway is still in the
preliminary stage, so it is of great significance to further
analyze and explore GPR124 and its related signaling pathways for
the study of related diseases.
Not applicable.
Funding: This research was funded by the National Natural
Science Foundation of China (grant no. 82201080) and High-level
Talents Project of Hainan Natural Science Foundation (grant no.
821RC687).
Not applicable.
WYL, YLD and DS: Investigation, Writing. YL:
Investigation and editing. ZLG: Funding acquisition, project
administration, supervision, writing-review & editing. Data
authentication is not applicable. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
All authors declare that they have no competing
interests.
|
1
|
America M, Bostaille N, Eubelen M, Martin
M, Stainier DYR and Vanhollebeke B: An integrated model for Gpr124
function in Wnt7a/b signaling among vertebrates. Cell Rep.
39(110902)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shu J, Wang C, Tao Y, Wang S, Cheng F,
Zhang Y, Shi K, Xia K, Wang R, Wang J, et al: Thermosensitive
hydrogel-based GPR124 delivery strategy for rebuilding blood-spinal
cord barrier. Bioeng Transl Med. 8(e10561)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lin WY, Zhou J and Guo ZL: Potential
mechanism and research progress of G protein couple TCGA d receptor
124 in periodontitis. J Hainan Med Coll. 1–12. 2024.
|
|
4
|
Ran QC, Long SR, Ye Y, Xie C, XuXiao ZL,
Liu YS, Pang HX, Sunchuri D, Teng NC and Guo ZL: Mining TCGA
database for prognostic genes in head and neck squamous cell
carcinoma microenvironment. J Dent Sci. 16:661–667. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
DISCHARGE Trial Group. Kofoed KF, Bosserdt
M, Maurovich-Horvat P, Rieckmann N, Benedek T, Donnelly P,
Rodriguez-Palomares J, Erglis A, Štěchovský C, et al: Comparative
effectiveness of initial computed tomography and invasive coronary
angiography in women and men with stable chest pain and suspected
coronary artery disease: Multicentre randomised trial. BMJ.
379(e071133)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xu YM: Structural study of thermostable
mutation-assisted G protein-coupled receptors GLP-1R and GPR124
(unpublished PhD thesis). East China Normal University, 2021.
|
|
7
|
Lagerström MC and Schiöth HB: Structural
diversity of G protein-coupled receptors and significance for drug
discovery. Nat Rev Drug Discov. 7:339–357. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Carson-Walter E, Watkins D, Nanda A,
Vogelstein B, Kinzler KW and St Croix B: Cell surface tumor
endothelial markers are conserved in mice and humans. Cancer Res.
61:6649–6655. 2001.PubMed/NCBI
|
|
9
|
O'Hayre M, Degese MS and Gutkind JS: Novel
insights into G protein and G protein-coupled receptor signaling in
cancer. Curr Opin Cell Biol. 27:126–135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Janetzko J, Kise R, Barsi-Rhyne B, Siepe
DH, Heydenreich FM, Kawakami K, Masureel M, Maeda S, Garcia KC, von
Zastrow M, et al: Membrane phosphoinositides regulate
GPCR-β-arrestin complex assembly and dynamics. Cell.
185:4560–4573.e19. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dejana E and Nyqvist D: News from the
brain: The GPR124 orphan receptor directs brain-specific
angiogenesis. Sci Transl Med. 2(58ps53)2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fredriksson R, Gloriam DEI, Höglund PJ,
Lagerström MC and Schiöth HB: There exist at least 30 human
G-protein-coupled receptors with long Ser/Thr-rich N-termini.
Biochem Biophys Res Commun. 301:725–734. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hernández-Vásquez MN, Adame-García SR,
Hamoud N, Chidiac R, Reyes-Cruz G, Gratton JP, Côté JF and
Vázquez-Prado J: Cell adhesion controlled by adhesion G
protein-coupled receptor GPR124/ADGRA2 is mediated by a protein
complex comprising intersectins and Elmo-Dock. J Biol Chem.
292:12178–12191. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cullen M, Elzarrad MK, Seaman S, Zudaire
E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, et al:
GPR124, an orphan G protein-coupled receptor, is required for
CNS-specific vascularization and establishment of the blood-brain
barrier. Proc Natl Acad Sci USA. 108:5759–5764. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou R, Shen L, Yang C, Wang L, Guo H,
Yang P and Song A: Periodontitis may restrain the mandibular bone
healing via disturbing osteogenic and osteoclastic balance.
Inflammation. 41:972–983. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Weinsheimer S, Brettman AD, Pawlikowska L,
Wu DC, Mancuso MR, Kuhnert F, Lawton MT, Sidney S, Zaroff JG,
McCulloch CE, et al: G protein-coupled receptor 124 (GPR124) gene
polymorphisms and risk of brain arteriovenous malformation. Transl
Stroke Res. 3:418–427. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yuki K, Vallon M, Ding J, Rada CC, Tang
AT, Vilches-Moure JG, McCormick AK, Henao Echeverri MF, Alwahabi S,
Braunger BM, et al: GPR124 regulates murine brain embryonic
angiogenesis and BBB formation by an intracellular
domain-independent mechanism. Development.
151(dev202794)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Siqueira M, Francis D, Gisbert D, Gomes
FCA and Stipursky J: Radial glia cells control angiogenesis in the
developing cerebral cortex through TGF-β1 signaling. Mol Neurobiol.
55:3660–3675. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kuhnert F, Mancuso MR, Shamloo A, Wang HT,
Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC and
Kuo CJ: Essential regulation of CNS angiogenesis by the orphan G
protein-coupled receptor GPR124. Science. 330:985–989.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chang J, Mancuso M, Maier C, Liang X, Yuki
K, Yang L, Kwong JW, Wang J, Rao V, Vallon M, et al: Gpr124 is
essential for blood-brain barrier integrity in central nervous
system disease. Nat Med. 23:450–460. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Umans RA, Henson HE, Mu F, Parupalli C, Ju
B, Peters JL, Lanham KA, Plavicki JS and Taylor MR: CNS
angiogenesis and barriergenesis occur simultaneously. Dev Biol.
425:101–108. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Posokhova E, Shukla A, Seaman S, Volate S,
Hilton MB, Wu B, Morris H, Swing DA, Zhou M, Zudaire E, et al:
GPR124 functions as a WNT7-specific coactivator of canonical
β-catenin signaling. Cell Rep. 10:123–130. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Martin M, Vermeiren S, Bostaille N,
Eubelen M, Spitzer D, Vermeersch M, Profaci CP, Pozuelo E, Toussay
X, Raman-Nair J, et al: Engineered Wnt ligands enable blood-brain
barrier repair in neurological disorders. Science.
375(eabm4459)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alok A, Lei Z, Jagannathan NS, Kaur S,
Harmston N, Rozen SG, Tucker-Kellogg L and Virshup DM: Wnt proteins
synergize to activate β-catenin signaling. J Cell Sci.
130:1532–1544. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bostaille N, Gauquier A, Twyffels L and
Vanhollebeke B: Molecular insights into Adgra2/Gpr124 and reck
intracellular trafficking. Biol Open. 5:1874–1881. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Eubelen M, Bostaille N, Cabochette P,
Gauquier A, Tebabi P, Dumitru AC, Koehler M, Gut P, Alsteens D,
Stainier DYR, et al: A molecular mechanism for Wnt ligand-specific
signaling. Science. 361(eaat1178)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cho C, Smallwood PM and Nathans J: Reck
and Gpr124 are essential receptor cofactors for
Wnt7a/Wnt7b-specific signaling in mammalian CNS angiogenesis and
blood-brain barrier regulation. Neuron. 95:1056–1073.e5.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou Y and Nathans J: Gpr124 controls CNS
angiogenesis and blood-brain barrier integrity by promoting
ligand-specific canonical wnt signaling. Dev Cell. 31:248–256.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vallon M, Yuki K, Nguyen TD, Chang J, Yuan
J, Siepe D, Miao Y, Essler M, Noda M, Garcia KC and Kuo CJ: A
RECK-WNT7 receptor-ligand interaction enables isoform-specific
regulation of Wnt bioavailability. Cell Rep. 25:339–349.e9.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cho C, Wang Y, Smallwood PM, Williams J
and Nathans J: Molecular determinants in frizzled, reck, and Wnt7a
for ligand-specific signaling in neurovascular development. Elife.
8(e47300)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gong DM, Zhang YL, Chen DY, Hong LJ, Han
F, Liu QB, Jiang JJ and Lu YM: Endothelial GPR124 exaggerates the
pathogenesis of atherosclerosis by activating inflammation. Cell
Physiol Biochem. 45:547–557. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li Q, Ouyang X and Lin J: The impact of
periodontitis on vascular endothelial dysfunction. Front Cell
Infect Microbiol. 12(998313)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen DY, Sun NH, Lu YP, Hong LJ, Cui TT,
Wang CK, Chen XH, Wang SS, Feng LL, Shi WX, et al: GPR124
facilitates pericyte polarization and migration by regulating the
formation of filopodia during ischemic injury. Theranostics.
9:5937–5955. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Y, Cho SG, Wu X, Siwko S and Liu M:
G-protein coupled receptor 124 (GPR124) in endothelial cells
regulates vascular endothelial growth factor (VEGF)-induced tumor
angiogenesis. Curr Mol Med. 14:543–554. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Parab S, Card OA, Chen Q, America M, Buck
LD, Quick RE, Horrigan WF, Levkowitz G, Vanhollebeke B and Matsuoka
RL: Local angiogenic interplay of Vegfc/d and Vegfa controls brain
region-specific emergence of fenestrated capillaries. Elife.
12(e86066)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fagundes NCF, Almeida APCPSC, Vilhena KFB,
Magno MB, Maia LC and Lima RR: Periodontitis as a risk factor for
stroke: A systematic review and meta-analysis. Vasc Health Risk
Manag. 15:519–532. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Orlandi M and Graziani Fand D'Aiuto F:
Periodontal therapy and cardiovascular risk. Periodontol 2000.
83:107–124. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Anderson KD, Pan L, Yang XM, Hughes VC,
Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue Y, Wei Y, et
al: Angiogenic sprouting into neural tissue requires Gpr124, an
orphan G protein-coupled receptor. Proc Natl Acad Sci USA.
108:2807–2812. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Engelhardt B and Liebner S: Novel insights
into the development and maintenance of the blood-brain barrier.
Cell Tissue Res. 355:687–699. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fan J and Watanabe T: Atherosclerosis:
Known and unknown. Pathol Int. 72:151–160. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sorokin V, Vickneson K, Kofidis T, Woo CC,
Lin XY, Foo R and Shanahan CM: Role of vascular smooth muscle cell
plasticity and interactions in vessel wall inflammation. Front
Immunol. 11(599415)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Doran AC, Meller N and McNamara CA: Role
of smooth muscle cells in the initiation and early progression of
atherosclerosis. Arterioscler Thromb Vasc Biol. 28:812–819.
2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang YL: Study on the molecular mechanism
of endothelial G protein-coupled receptor 124 in mediating the
pathological process of atherosclerosis (unpublished PhD thesis).
Zhejiang University, 2017.
|
|
44
|
Orr AW, Hastings NE, Blackman BR and
Wamhoff BR: Complex regulation and function of the inflammatory
smooth muscle cell phenotype in atherosclerosis. J Vasc Res.
47:168–180. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kaur H, Carvalho J, Looso M, Singh P,
Chennupati R, Preussner J, Günther S, Albarrán-Juárez J, Tischner
D, Classen S, et al: Author correction: Single-cell profiling
reveals heterogeneity and functional patterning of GPCR expression
in the vascular system. Nat Commun. 10(1448)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Karagiannis GS, Weile J, Bader GD and
Minta J: Integrative pathway dissection of molecular mechanisms of
moxLDL-induced vascular smooth muscle phenotype transformation. BMC
Cardiovasc Disord. 13(4)2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Huang Y, Xu W and Zhou R: NLRP3
inflammasome activation and cell death. Cell Mol Immunol.
18:2114–2127. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhu BW, Chen LM and Guo ZL: Review of
NLRP3 inflammasomes in periodontal diseases. Int J Stomatol.
46:450–455. 2019.(In Chinese).
|
|
49
|
Li B and Meng WY: Research progress of
NLRP3 inflammasome in peri-implantitis. J Oral Sci Res.
38:1119–1123. 2022.(In Chinese).
|
|
50
|
Burger F, Baptista D, Roth A, da Silva RF,
Montecucco F, Mach F, Brandt KJ and Miteva K: NLRP3 inflammasome
activation controls vascular smooth muscle cells phenotypic switch
in atherosclerosis. Int J Mol Sci. 23(340)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zeng W, Wu D, Sun Y, Suo Y, Yu Q, Zeng M,
Gao Q, Yu B, Jiang X and Wang Y: The selective NLRP3 inhibitor
MCC950 hinders atherosclerosis development by attenuating
inflammation and pyroptosis in macrophages. Sci Rep.
11(19305)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Xu Y, Fang X, Zhao Z, Wu H, Fan H, Zhang
Y, Meng Q, Rong Q, Fukunaga K, Guo Q and Liu Q: GPR124 induces
NLRP3 inflammasome-mediated pyroptosis in endothelial cells during
ischemic injury. Eur J Pharmacol. 962(176228)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wu H, Gao T, Cao Y, Diao J, Chang F, Qi J
and Wang C: Protective and therapeutic effects of Trianthema
portulacastrum against atherosclerosis in male albino rats via
G-protein-coupled receptor 124. AMB Express. 9(178)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Larvin H, Kang J, Aggarwal VR, Pavitt S
and Wu J: Risk of incident cardiovascular disease in people with
periodontal disease: A systematic review and meta-analysis. Clin
Exp Dent Res. 7:109–122. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Feng W, Liu B, Liu D, Hasegawa T, Wang W,
Han X, Cui J, Yimin Oda K, Amizuka N and Li M: Long-term
administration of high-fat diet corrects abnormal bone remodeling
in the tibiae of interleukin-6-deficient mice. J Histochem
Cytochem. 64:42–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
García-Hernández AL, Muñoz-Saavedra ÁE,
González-Alva P, Moreno-Fierros L, Llamosas-Hernández FE,
Cifuentes-Mendiola SE and Rubio-Infante N: Upregulation of proteins
of the NLRP3 inflammasome in patients with periodontitis and
uncontrolled type 2 diabetes. Oral Dis. 25:596–608. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yang L, Tao W, Xie C, Chen Q, Zhao Y,
Zhang L, Xiao X, Wang S and Zheng X: Interleukin-37 ameliorates
periodontitis development by inhibiting NLRP3 inflammasome
activation and modulating M1/M2 macrophage polarization. J
Periodontal Res. 59:128–139. 2024.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang Z, Feng X, Zhang G, Li H, Zhou F, Xie
Y, Li T, Zhao C, Luo W, Xiong Y and Wu Y: Artesunate ameliorates
ligature-induced periodontitis by attenuating NLRP3
inflammasome-mediated osteoclastogenesis and enhancing osteogenic
differentiation. Int Immunopharmacol. 123(110749)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Bai B, Yang Y, Wang Q, Li M, Tian C, Liu
Y, Aung LHH, Li PF, Yu T and Chu XM: NLRP3 inflammasome in
endothelial dysfunction. Cell Death Dis. 11(776)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gyselaers W, Dreesen P, Staelens AS,
Tomsin K, Bruckers L and Vonck S: First-trimester normotension is a
weak indicator of normal maternal cardiovascular function.
Hypertension. 80:343–351. 2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Calderón-Zamora L, Canizalez-Román A,
León-Sicairos N, Aguilera-Mendez A, Huang F, Hong E and Villafaña
S: Changes in expression of orphan receptors GPR99 and GPR107
during the development and establishment of hypertension in
spontaneously hypertensive rats. J Recept Signal Transduct Res.
41:558–565. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ngo T, Kufareva I, Coleman JLJ, Graham RM,
Abagyan R and Smith NJ: Identifying ligands at orphan GPCRs:
Current status using structure-based approaches. Br J Pharmacol.
173:2934–2951. 2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Calderón-Zamora L, Ruiz-Hernandez A,
Romero-Nava R, León-Sicairos N, Canizalez-Román A, Hong E, Huang F
and Villafaña S: Possible involvement of orphan receptors GPR88 and
GPR124 in the development of hypertension in spontaneously
hypertensive rat. Clin Exp Hypertens. 39:513–519. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Del PR, Pietropaoli D, Munoz-Aguilera E,
D'Aiuto F, Czesnikiewicz-Guzik M, Monaco A, Guzik TJ and Ferri C:
Periodontitis and hypertension: Is the association causal? High
Blood Press Cardiovasc Prev. 27:281–289. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Czesnikiewicz-Guzik M, Osmenda G,
Siedlinski M, Nosalski R, Pelka P, Nowakowski D, Wilk G,
Mikolajczyk TP, Schramm-Luc A, Furtak A, et al: Causal association
between periodontitis and hypertension: Evidence from Mendelian
randomization and a randomized controlled trial of non-surgical
periodontal therapy. Eur Heart J. 40:3459–3470. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Duan SZ: Research progress in oral
microbiota and cardio-cerebrovascular diseases. Stomatology.
41:577–582. 2021.(In Chinese).
|
|
67
|
Cherry AE, Vicente JJ, Xu C, Morrison RS,
Ong SE, Wordeman L and Stella N: GPR124 regulates microtubule
assembly, mitotic progression, and glioblastoma cell proliferation.
Glia. 67:1558–1570. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
St Croix B, Rago C, Velculescu V, Traverso
G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C,
Vogelstein B and Kinzler KW: Genes expressed in human tumor
endothelium. Science. 289:1197–1202. 2000.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Huang Q, Liu L, Xiao D, Huang Z, Wang W,
Zhai K, Fang X, Kim J, Liu J, Liang W, et al: CD44+ lung
cancer stem cell-derived pericyte-like cells cause brain metastases
through GPR124-enhanced trans-endothelial migration. Cancer Cell.
41:1621–1636.e8. 2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Dono A, Takayasu T, Yan Y, Bundrant BE,
Arevalo O, Lopez-Garcia CA, Esquenazi Y and Ballester LY:
Differences in genomic alterations between brain metastases and
primary tumors. Neurosurgery. 88:592–602. 2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wang Z, Li Y, Zhou F, Piao Z and Hao J:
β-elemene enhances anticancer bone neoplasms efficacy of paclitaxel
through regulation of GPR124 in bone neoplasms cells. Oncol Lett.
16:2143–2150. 2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Liu D, Zhou B and Liu R: A transcriptional
co-expression network-based approach to identify prognostic
biomarkers in gastric carcinoma. PeerJ. 8(e8504)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Ma J, Chen P and Wang R: G-protein-coupled
receptor 124 promotes osteogenic differentiation of BMSCs through
the Wnt/β-catenin pathway. In Vitro Cell Dev Biol Anim. 58:529–538.
2022.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Houschyar KS, Tapking C, Borrelli MR, Popp
D, Duscher D, Maan ZN, Chelliah MP, Li J, Harati K, Wallner C, et
al: Wnt pathway in bone repair and regeneration-what do we know so
far. Front Cell Dev Biol. 6(170)2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Luo J, Chen H, Wang G, Lyu J, Liu Y, Lin
S, Zhou M and Jiang X: CGRP-loaded porous microspheres protect
BMSCs for alveolar bone regeneration in the periodontitis
microenvironment. Adv Healthc Mater. 12(e2301366)2023.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Vallon M, Aubele P, Janssen KP and Essler
M: Thrombin-induced shedding of tumour endothelial marker 5 and
exposure of its RGD motif are regulated by cell-surface protein
disulfide-isomerase. Biochem J. 441:937–944. 2012.PubMed/NCBI View Article : Google Scholar
|