1. Introduction
Pelvic organ prolapse (POP) is a condition
characterized by the downward displacement of the uterus and
adjacent organs, such as the bladder and rectum, due to the
weakening of pelvic floor support and reduced structural tension.
This condition manifests clinically as uterine, urethral, bladder
and rectal prolapse and bulging of the anterior and posterior
vaginal walls. It is often associated with stress urinary
incontinence (1). Although POP can
affect adult women across all age groups, the exact prevalence
remains uncertain.
Epidemiological data reveal a significant
discrepancy between prevalence rates determined by prolapse
symptoms and those based on clinical examination, largely because
numerous women with POP have no obvious clinical symptoms. A
prospective cohort study involving 259 perimenopausal women found
an incidence rate of 26% at 1 year after menopause, rising to 40%
after 3 years (2). Furthermore,
30-76% of adult women are first diagnosed with vaginal or uterine
prolapse during routine gynecological exams, with 3-6% of these
cases showing prolapse that reaches or exceeds the hymen, although
only ~3% report subjective symptoms (3). Research indicates that 13% of women
in the US will require surgical intervention for POP, with most
surgeries occurring between the ages of 70 and 79 years. By 2050,
the number of women affected by POP will further increase (4). While several women with POP are
asymptomatic, others may experience swelling, discomfort, sexual
dysfunction and psychological distress in severe cases (5-7).
Therefore, POP has emerged as a significant health and social
problem, posing substantial risks to the well-being and quality of
life of adult women (8,9).
The present study reviews the risk factors,
molecular pathogenesis pathways [including matrix
metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs),
transforming growth factor β (TGF-β), advanced glycation end
products (AGEs)/receptor for AGE (AGE/RAGE), phosphoinositide
3-kinase/protein kinase B (PI3K/AKT), the fibulin family, lysyl
oxidase-like 1 (LOXL1), homeobox A11 (HOXA11), collagen α-1 (XVIII)
chain (COL18A1), Wnt signaling pathways and estrogen receptor α
(ERα)] and the therapeutic approaches of POP, aiming to enhance
clinicians' understanding of the progression of POP and to support
effective interventions and treatments.
2. Physiological structure and disease
stratification
Physiological structure
The pelvic floor is anatomically composed of
muscles, ligaments and fascia that provide crucial support to the
reproductive organs, the rectum and the bladder. The bony pelvis,
comprising the sacrum and two innominate bones, forms the skeletal
framework that stabilizes and encircles the pelvic floor (10). The pelvic fascia, levator muscles
and ligaments work in unison to support the pelvic organs.
Dysfunction in these structures has been shown to disrupt their
support points, leading to the descent of pelvic organs and
subsequent symptoms and pathologies (11,12).
A considerable body of research has emphasized that the molecular
alterations in connective tissue play a significant role in the
development of POP. The connective tissue of the pelvic floor,
including ligaments and fascia, is primarily made up of
extracellular matrix (ECM) components such as collagen, elastin and
other fibrous elements (13-15).
The network of cross-linked collagen and elastin fibers enables the
pelvic organs to maintain their shape and position, providing a
buffering capacity to withstand external pressure, thereby
preserving pelvic organ function (13).
Staging of POP
The International Urogynecological
Association/International Continence Society categorizes the stages
of POP into five levels, ranging from 0 to 4: Stage 0 signifies no
prolapse; stage 1 indicates that the most distal prolapse is 1 cm
above the hymen; stage 2 is characterized by the most distal
prolapse being 1 cm above to 1 cm below the hymen; stage 3 occurs
when the most distal portion of the vagina extends beyond the
hymenal plane by >1 cm, but the prolapse remains at least 2 cm
shorter than the total vaginal length; and stage 4 is defined by
complete prolapse or prolapse extending to within 2 cm of the total
length of the lower genital tract (16).
3. Risk factors for POP
The etiology of POP is multifaceted, influenced by a
combination of factors rather than a singular cause. Current
research identifies key risk factors as lifestyle, age, pregnancy
and parity, mode of delivery, estrogen levels, obesity,
intra-abdominal pressure, history of pelvic surgery and genetic
predispositions (Fig. 1).
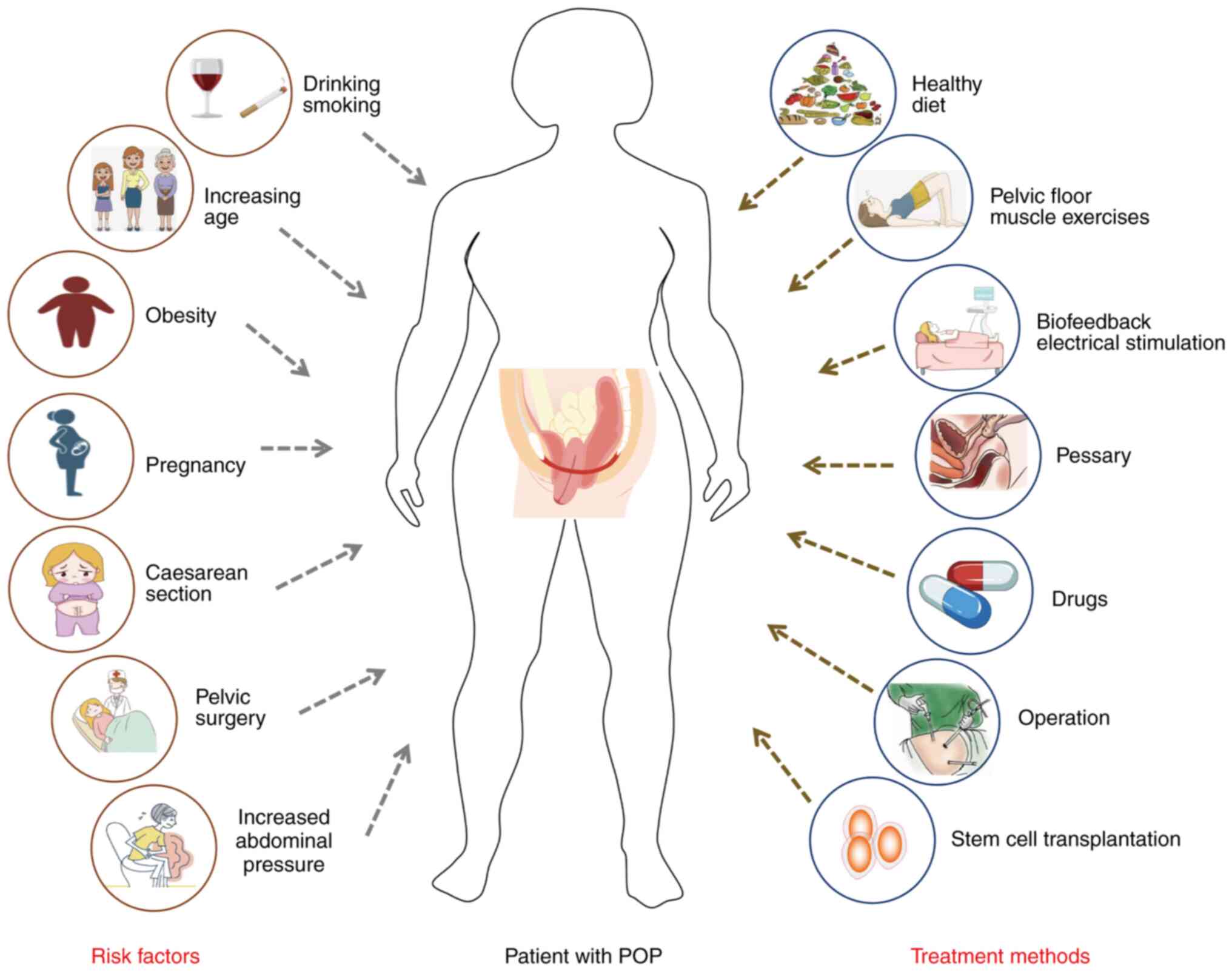 | Figure 1Risk factors and treatment of female
POP. There are a number of risk factors for female POP, including
lifestyle habits (drinking and smoking), age, obesity, pregnancy
and parity, caesarean section, history of gynecological surgery,
and increased abdominal pressure. Current treatments include diet
control, exercise, pelvic floor muscle exercises, biofeedback
electrical stimulation of the uterus, drugs, surgery and stem cell
transplantation. POP, pelvic organ prolapse. |
Lifestyle
Epidemiological and observational studies have
identified an association between POP and various lifestyle
factors, including alcohol consumption (17), coffee intake (18), smoking (19), physical activity (20), labor (21) and hypertension (22,23).
However, the association between these risk factors and POP remain
unclear, with scientific studies often yielding inconsistent or
contradictory conclusions (24).
To investigate the relationship between lifestyle factors,
metabolic factors and socioeconomic status with POP, Liu et
al (25) conducted a
two-sample Mendelian randomization study utilizing pooled data from
the largest existing genome-wide association study (GWAS). Their
findings suggest a positive association between genetically
predicted coffee consumption, strenuous physical activity,
high-density lipoprotein cholesterol and POP (25). Additionally, a cross-sectional
study involving 76 women with significant levator ani deficiency
found that 51 had symptomatic POP, while 25 did not. Severe levator
ani deficiency is associated with a higher incidence of symptomatic
POP, particularly among older women and smokers (26).
Age
Age is a significant risk factor for POP, with its
incidence rising as individuals age. A study by Patel et al
(27) observed that in menopausal
women, the incidence of POP increases by ~40% with each decade of
age. In the United States of America, an epidemiological study
revealed that the prevalence of POP is 26.5% among women aged
40-59, 36.8% in those aged 60-79 and 49.7% in women aged ≥80 years
(28). Beyond the influence of
smoking, Rostaminia et al (26) noted that women with noticeable
levator ani muscle defects but without POP are, on average, 18
years younger than those with POP and apparent muscle defects.
These results underscore age as a key factor in the development of
POP.
Pregnancy and parity
Pregnancy and childbirth also constitute major risk
factors for POP. Approximately two-thirds of first-time mothers
experience pelvic floor dysfunction (PFD) symptoms within a year
postpartum (29). Patel et
al (27) reported that the
risk of POP is 4 times higher in women who have given birth once
compared with nulliparous women, and this risk escalates to 8.4
times after 2 deliveries. As pregnancy progresses, the increasing
size and weight of the uterus places greater strain on the pelvic
floor support structures. Although the body initially compensates
for this strain, the continued growth of the fetus during the mid
to late stages of pregnancy can overwhelm these compensatory
mechanisms, leading to damage to the pelvic floor muscles (30,31).
Mode of delivery
Vaginal delivery is recognized as the risk factor
most closely associated with POP, likely due to the damage
inflicted on the connective tissues of the pelvic floor, muscles
and nerves during childbirth (32-34).
Compared with controls, vaginal delivery increases the likelihood
of developing POP by 4 to 11 times (35). This association may, in part, be
attributed to injury to the levator ani muscle (36). A longitudinal study of postpartum
PFD, which followed participants 5-10 years after their first
childbirth and conducted annual evaluations for up to 9 years,
found that the incidence of POP increases following vaginal
delivery but not cesarean section (37). A prospective cohort study further
demonstrated that women aged <60 years are at the highest risk
of POP due to vaginal delivery (38), while cesarean section and
nulliparity may act as protective factors (39). In the absence of subsequent vaginal
deliveries, cesarean section provides protection against POP,
whereas instrumental deliveries heighten the risk (40,41).
Estrogen levels
ERs are extensively distributed throughout the
pelvic floor tissues, including the cardinal ligaments, uterosacral
ligaments, levator ani muscles, posterior vaginal fornix and
vaginal wall. After menopause, systemic estrogen levels decline
significantly, leading to a marked reduction in serum estrogen and
ER content within the pelvic floor ligaments (42-44).
This estrogen-deficient environment causes alterations in collagen
composition and strength, as well as atrophy and degeneration of
pelvic floor tissues, making it increasingly difficult to maintain
the pelvic organs in their proper anatomical positions, thus
contributing to prolapse (44,45).
Postmenopausal women with POP exhibit significantly decreased serum
estrogen concentrations and pelvic floor ligament ER levels
compared with their non-POP counterparts (46). Additionally, a notable 1.5-2.5-fold
reduction in ERα levels has been observed in postmenopausal women
with POP compared with those in the group without POP, while
premenopausal women with POP show increased ERβ levels compared
with those without POP (47).
Obesity
A previous study indicated that a higher body mass
index significantly increases the likelihood of developing POP
(25). Obesity exacerbates the
strain on pelvic floor tissues, resulting in continuous damage to
pelvic floor muscles, nerves and other structures due to prolonged
mechanical stress and traction (48,49).
A study comparing 358 obese women with non-obese women of the same
age found a higher prevalence of POP in the obese group (91 vs.
22%) (50).
Increased abdominal pressure
Increased abdominal pressure is another
well-established risk factor for POP, often arising from conditions
such as obesity, chronic cough or persistent constipation.
Sustained elevation in abdominal pressure directly strains the
pelvic floor muscles, ligaments and other tissues, while chronic
mechanical stress disrupts the cellular microenvironment, leading
to an imbalance in cellular homeostasis and accelerating the
progression of POP (51-53).
Chronic constipation, in particular, maintains elevated tension in
the rectus abdominis and anal sphincter, perpetuating high
abdominal pressure. This ongoing pressure is a primary driver in
promoting or exacerbating reproductive tract prolapse. A study
investigating the prevalence and risk factors of symptomatic POP in
rural China included 25,864 rural women from February 2014 to March
2016. The results showed that 20.84% of women with POP had a
history of constipation for >1 year (19). Therefore, chronic constipation is
also one of the risk factors for POP in women (54,55).
History of pelvic surgery
A history of pelvic surgery heightens the risk of
developing POP. Gynecological surgeries, such as total
hysterectomy, pelvic mass removal and radical pelvic organ
resection, can damage the supporting structures of the pelvic
floor, including muscles, connective tissue and ligaments, thereby
inducing POP (37). A number of
studies show that vaginal vault prolapse often manifests 2-13 years
post-hysterectomy. The procedure not only removes the uterus but
also severs the ligaments, blood vessels and nerves essential for
maintaining pelvic floor function, thus compromising the pelvic
floor support system (56). A
cohort study involving 160,000 women post-hysterectomy found a 3.2%
risk of POP, compared with only 2% in the control group (32). Another retrospective study
indicated that POP could develop within 20 years following a
hysterectomy (57).
Genetic factors
Clinically, uterine prolapse can occasionally be
observed in young nulliparous women, or even in those without any
sexual history. A study by Buchsbaum et al (58) found that the degree of POP after
menopause is similar between women who have given birth and their
biological sisters who have not, highlighting genetics as an
important risk factor for POP. Jack et al (59) investigated 10 female patients under
the age of 55 with a positive family history of POP and discovered
that POP in these families exhibited an incomplete penetrance
inheritance pattern, with both parents potentially passing on the
trait. The risk of POP in such families is substantially higher
than in the general population. Another study utilizing the Swedish
Twin Registry, which included all known same-sex female twins born
between 1926 and 1958, indicated that genetic factors contribute to
the development of stress urinary incontinence and POP, although
environmental factors are also influential (60). Evidence-based research has shown
that women with a family history of POP have a 2.3-2.7 times higher
risk of developing the condition and a 1.4 times greater risk of
POP recurrence compared with those without such a history (30). A GWAS conducted by Allen-Brady
et al (61) identified six
single nucleotide polymorphisms (SNPs) significantly associated
with POP in participants from high-risk families, located at 4q21
(rs1455311), 8q24 (rs1036819), 9q22 (rs430794), 15q11 (rs8027714),
20p13 (rs1810636) and 21q22 (rs2236479). Additionally, numerous
studies have demonstrated that gene loci related to pelvic floor
tissue components, such as collagen-, elastin- and ECM-related
genes, are implicated in the pathogenesis of POP (62-64).
Other factors
Furthermore, studies involving subjectively reported
and objectively measured POP in ethnically diverse cohorts have
shown that Latino and white women are 4-5 times more likely to
develop symptomatic prolapse, as measured according to the POP
classification system, compared with African American women
(65). These results suggest that
race is also a factor influencing the occurrence of POP.
4. Molecular biological mechanisms of
POP
The supporting structures of female pelvic organs
primarily consist of connective tissues, including pelvic floor
nerves, muscles, ligaments and fascia (1). Fibroblasts within these connective
tissues secrete significant amounts of ECM, composed of structural
proteins (such as collagen and elastin), matrix adhesion molecules
(fibronectin and laminin) and proteoglycans (13,14).
Recent research into the pathogenesis of POP has largely focused on
the signaling molecules involved in collagen and elastin
biosynthesis, as well as ECM metabolic processes (Fig. 2). The relevant molecular mechanisms
underlying the occurrence and progression of POP are detailed in
Table I.
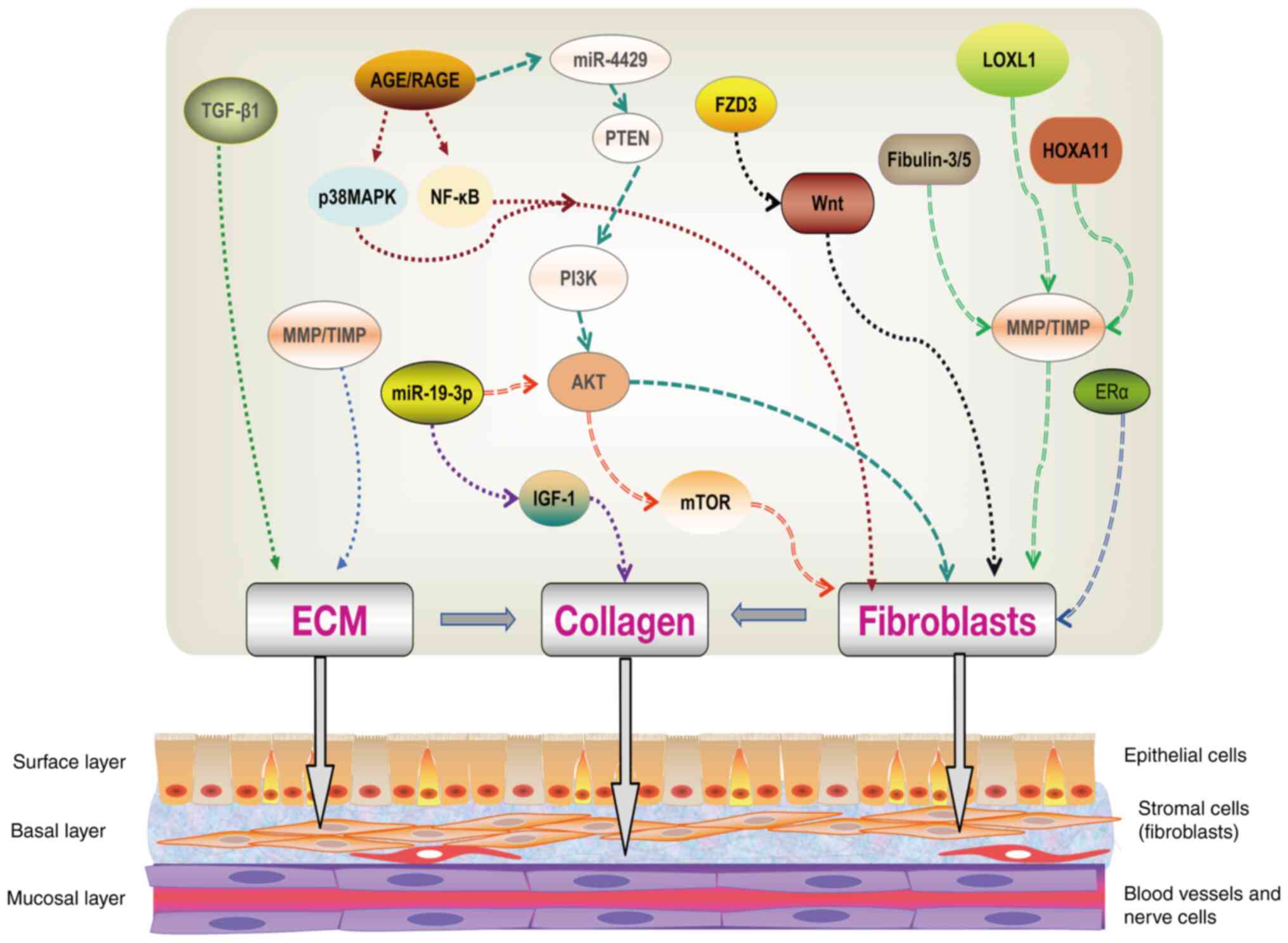 | Figure 2Molecular mechanism of POP. TGF-β1
and MMPs/TIMPs can regulate the occurrence of ECM, thereby
affecting the collagen synthesis of POP. AGEs/RAGE can regulate the
proliferation of vaginal fibroblasts by regulating the p38 MAPK and
NF-κB pathways. AGEs can also affect fibroblast apoptosis by
regulating the PTEN/PI3K/AKT pathway by targeting miR-4429.
miR-19-3 can affect the apoptosis of fibroblasts by regulating the
AKT/mTOR pathway, and can also target IGF-1 to regulate collagen
production. FZD3 can affect the viability and apoptosis of
fibroblasts by regulating the Wnt pathway. ERα can also affect
fibroblast viability and apoptosis, but the downstream mechanisms
are unclear. Fibulin-3/5, LOXL1 and HOXA11 can all regulate
fibroblasts by regulating MMPs/TIMPs, thereby affecting the
synthesis of collagen fibers. POP, pelvic organ prolapse; MMP,
matrix metalloproteinase; TIMP, tissue inhibitors of MMP; ECM,
extracellular matrix; TGF-β, transforming growth factor β; AGE,
advanced glycation end product; RAGE, receptor for AGE; PI3K,
phosphoinositide 3-kinase; AKT, kinase B; LOXL1, lysyl oxidase-like
1; IGF-1, insulin-like growth factor-1; FZD3, frizzled 3; ERα,
estrogen receptor α; miR, microRNA. |
 | Table IMolecular mechanism of pelvic organ
prolapse. |
Table I
Molecular mechanism of pelvic organ
prolapse.
| Molecules | Mechanism | Impact | (Refs.) |
|---|
| MMPs/TIMPs | In the tissues of
the uterosacral ligament and vaginal mucosa, increased expression
of MMPs and decreased expression of TIMPs led to an increase in
ECM, thereby inhibiting the synthesis of collagen fibers and
promoting their degradation. | Aggravating the
progression of POP. | (67-73) |
| TGF-β | In sacral ligament
tissue, the reduction of TGF-β1 expression led to an increase in
ECM, thereby inhibiting the synthesis of collagen fibers. | Aggravating disease
progression in POP patients with hysterectomy. | (76,77) |
| AGE/RAGE | In the vaginal
tissue of patients with POP, increased AGE levels regulated the p38
MAPK and NF-κB pathways, inhibited vaginal fibroblast proliferation
and reduced the expression of collagen I. Increased AGEs also
promoted fibroblast apoptosis by inhibiting the
miR-4429/PTEN/PI3K/AKT pathway. | Aggravating the
progression of POP. | (81-85) |
| PI3K/AKT | Mechanical strain
activated the PI3K/AKT pathway in human uterosacral ligament
fibroblasts, thereby promoting cell apoptosis and senescence and
reducing the production of collagen I. | Aggravating the
progression of POP. | (86) |
| Fibulin | Decreased
expression of Fibulin-3/5 promoted the upregulation of MMP-9
expression and increased elastic fiber dissolution. | Promoting the
occurrence or aggravation of POP. | (88-93) |
| LOXL1 | In the vagina,
LOXL1 deficiency affected MMPs, TIMPs and ECM components, thereby
promoting disordered arrangement of collagen fiber bundles. | Promoting the
occurrence or aggravation of POP. | (96,97) |
| HOXA11 | In the uterosacral
ligament, reduced expression of HOXA11 led to decreased expression
of collagen I and III and increased activation of MMP2 and MMP9,
and weakened the connective tissue. | Promoting the
occurrence or aggravation of POP. | (102,103) |
| miRNAs | In vaginal
fibroblasts from patients with POP, elevated levels of miRNA-19-3p
promoted the activation of the AKT/mTOR/ p70S6K pathway, which in
turn promoted autophagy and apoptosis in vaginal fibroblasts.
Elevated levels of miRNA-19-3p also targeted IGF-1, thereby
inhibiting the secretion of collagen I by vaginal fibroblasts. | Aggravating the
progression of POP. | (104,105) |
| Wnt | In vaginal wall
fibroblasts, FZD3 promoted fibroblast viability and ECM
degradation, and inhibited apoptosis by activating the Wnt
pathway. | Mitigating the
progression of POP. | (107,108) |
| ERα | In uterosacral
ligament fibroblasts, β-estradiol treatment promoted the expression
of ERα, PARP1 and Bcl-2, thereby reducing apoptosis. | Mitigating the
progression of POP. | (110) |
| Others | The expression
levels of heparinases and COL18A1 gene were increased in the
uterosacral ligament connective tissue of patients with POP, but
the specific mechanism was unclear. | Aggravating the
progression of POP. | (111,112) |
MMPs/TIMPs
MMPs are enzymes responsible for degrading ECM
proteins, while TIMPs act as their inhibitors. An imbalance between
MMPs and TIMPs can lead to collagen metabolism disorders, playing a
pivotal role in the development of POP (66). Studies have demonstrated a
significant upregulation of MMP1 and MMP9 expression in the
uterosacral ligament (USL) and vaginal mucosa tissues of patients
with POP (67-69).
Evidence indicates that an imbalance between MMPs and TIMPs can
impair pelvic connective tissue quality, contributing to POP
pathogenesis (70,71). It has been reported that collagen
content in the pelvic floor support structures of patients with POP
is not significantly decreased compared with that in individuals
without POP. Instead, accelerated collagen degradation appears to
be a key factor in the pathophysiology of POP (64). In a study by Hu et al
(72), anterior vaginal wall
tissue from 97 patients with POP and 35 controls was analyzed,
revealing significantly increased mRNA and protein expression of
MMP-1 and MMP-8 in patients with POP compared with controls.
Additionally, a marked reduction in the mRNA and protein levels of
type I and type III collagen, as well as TIMP-1, was observed in
the POP group (72). Zhu et
al (73) further investigated
USL tissues from 35 patients with POP and 20 controls, finding that
MMP2 and MMP9 protein and mRNA levels are significantly increased
in the POP group, while TIMP1 and TIMP2 levels are markedly lower.
Moreover, the study revealed that in patients with POP, the mRNA
and protein levels of collagen I and ERα are considerably reduced,
whereas the mRNA levels of Bax and Bad, as well as caspase-3
protein expression, are significantly higher compared with controls
(73). These results suggested
that POP is associated with ECM remodeling in USL tissue,
characterized by decreased collagen production, increased collagen
degradation and increased cellular apoptosis (73).
TGF-β
TGF-β is a multifunctional cytokine that plays a
critical role as both a proliferation inhibitor and mitogen for
mesenchymal-derived cells. It is a key molecule in fibrotic
diseases and is involved in the pathogenesis of pelvic organ
disorders (74,75). Qi et al (76) found that the expression levels of
connective tissue growth factor and TGF-β1, both closely linked to
collagen synthesis, were significantly reduced in the pubocervical
fascia tissue of patients with POP. Liu et al (77) extended this research by
investigating the role of TGF-β1 in the pathological processes of
POP, particularly focusing on the metabolic change in ECM within
the USL in patients with POP. This study involved 60 individuals
(30 with POP and 30 without POP) who had undergone hysterectomy for
benign reasons. Compared with the control group, patients with POP
exhibited significantly decreased expression levels of collagen,
elastin, TIMP-2 and TGF-β1 in their sacral ligament tissue, while
MMP-2/9 activity was markedly increased. Statistical analysis
revealed that TGF-β1 mRNA expression was inversely correlated with
the severity of POP, suggesting that the pathogenic characteristics
of POP include reduced ECM protein synthesis and increased
degradation in the USL (77). Due
to its potential to predict the severity of POP, TGF-β1 should be
considered a possible therapeutic target for treating persistent
POP.
AGEs/RAGE
AGEs are formed through the non-enzymatic glycation
and oxidation of proteins and lipids. AGEs influence collagen
metabolism and are implicated in ECM-related diseases, primarily
characterized by collagen metabolism disorders (78,79).
Chen et al (80) studied 44
patients with POP and 46 non-POP women, detecting AGEs and RAGE
levels in vaginal tissues. They found that patients with POP
exhibited higher protein expression of AGEs and lower levels of
type I collagen compared with the controls, although no significant
difference in RAGE expression was noted between the two groups.
Similar results were observed in vaginal tissue tests conducted on
POP rats, where AGEs were inversely correlated with type I collagen
content (80). Additionally,
sequencing of the complete RAGE gene in 24 patients with POP and 25
controls identified two SNPs (rs184003 and rs55640627) as potential
factors contributing to POP (80).
Vetuschi et al (81)
examined the anterior vaginal wall myometrium in 20 patients with
POP and 10 control patients undergoing treatment for uterine
fibromatosis. In POP samples, the myometrium displayed upregulation
of AGE, ERK1/2, Smad-2/3, MMP-3 and collagen III, while the control
group showed increased levels of Smad-7 and collagen I. RAGE
expression was minimal or absent in both groups. Their findings
suggested that AGEs and RAGE may play a significant role in the
pathophysiology of POP, although further research is needed to
clarify the mechanisms by which the AGEs-RAGE pathway contributes
to tissue degeneration and the fragility in POP (81). It has been indicated that AGEs
exert cytotoxic effects on human vaginal wall fibroblasts (VWFs),
inhibiting their viability and proliferation while inducing
apoptosis (82). Treatment of
primary cultured human VWFs derived from POP and non-POP tissues
with AGEs revealed that AGEs regulate the RAGE and/or p38 MAPK and
NF-κB pathways, thereby inhibiting VWF proliferation and reducing
type I collagen levels in patients with POP (83). Sima et al (84) further explored AGE-induced
apoptosis in human USL fibroblasts, identifying the microRNA
(miRNA/miR)-4429/PTEN/PI3K/AKT pathway as a key regulatory
mechanism in POP. Their findings suggested that AGEs induce
fibroblast apoptosis via this pathway (84). Moreover, their latest research
demonstrates that quercetin inhibits the AGE-induced downregulation
of the miR-4429/PTEN/PI3K/AKT pathway, thereby counteracting
AGE-induced fibroblast apoptosis (85).
PI3K/AKT pathway
In the development of POP, factors such as
mechanical strain, in addition to the regulatory effects of AGEs on
the PI3K/AKT pathway, play a significant role. Li et al
(86) exposed human USL
fibroblasts to mechanical tensile strain at an intensity of 5,333
µε and a frequency of 0.3 Hz, finding that this strain accelerates
cell death and senescence while reducing type I collagen formation
by activating the PI3K/AKT-mediated oxidative stress signaling
pathway. This process leads to the dysfunction of pelvic support,
resulting in the relaxation of the pelvic region and promoting the
onset of POP.
Fibulin
The fibulin family, a key component of the basement
membrane and elastic fibers, consists of extracellular
glycoproteins that facilitate tissue bridging and ECM assembly. The
seven members of this family are divided into two subgroups based
on their structural regions and size: Fibulin-1, 2 and 6 in the
first subgroup and fibulin-3, 4, 5 and 7 in the second subgroup
(87). Previous studies have shown
that fibulin-3 knockout mice develop elastic fiber abnormalities as
they age, such as abdominal wall hernias and POP, with increased
MMP activity detected (88).
Similarly, fibulin-5 gene knockout mice exhibit reduced elastic
fibers, upregulated MMP-9 expression and POP symptoms, which result
from the loss of negative feedback control of MMP-9 by fibulin-5,
leading to abnormal elastic fiber formation (89,90).
Genetic polymorphism analysis by Khadzhieva et al (91), involving 210 patients with severe
POP and 292 controls, revealed a strong association between POP and
high-frequency SNPs in fibulin-5 (SNP rs2018736 and rs12589592).
According to Zhao and Zhou (92),
it was further observed that although elastin expression levels
remain unchanged in patients with POP, defects in elastic fiber
remodeling due to reduced LOXL1 and fibulin-5 expression can
contribute to POP development. Moreover, fibulin-5 may protect
against or aid in the recovery from POP caused by childbirth or
elastase deficiency (93).
LOXL1
LOXL1, a key protein for postnatal elastic fiber
deposition, is highly expressed in the mouse reproductive tract,
with expression levels diminishing with age. LOXL1 deficiency
impairs elastic fiber recruitment to reproductive tissues after
delivery, leading to lower urinary tract dysfunction, paraurethral
pathology, vaginal wall thinning and POP (94). Liu et al (94) compared LOXL1 knockout mice with
wild-type mice and found that the former developed varying degrees
of POP by the second postpartum day, with some improvement by the
14th postpartum day, but a permanent pelvic floor prolapse
persisted. Li et al (95)
identified disordered collagen fibers in the vaginal tissue of
LOXL1 knockout mice, further indicating the presence of aberrant
ECM in this tissue. LOXL1 deficiency underlies numerous
pathological processes associated with ECM metabolic imbalances. In
a different study by Li et al (96), LOXL1 deficiency in the vagina was
shown to affect key MMPs (MMP2, -9 and -12), TIMPs (TIMP1-4) and
mRNA expression levels of ECM components (COL1a1, COL3a1, fibulin-5
and α-SMA). Additionally, a study by Kufaishi et al
(97) found a significant
reduction in the LOXL l and LOXL3 expression in the vaginal wall
cells of patients with severe POP, underscoring the crucial role of
the LOXL1 gene in maintaining elastic fiber structure and function,
with defects in this gene potentially leading to the onset or
worsening of POP.
HOXA11
HOXA11 plays a pivotal role in regulating the
expression of genes involved in ECM metabolism within reproductive
organs, maintaining tissue developmental plasticity in adult mice
and humans through its expression in the reproductive tract. The
USL, which provides essential support to the upper vagina and
uterus, benefits from the ability of HOXA11 to promote fibroblast
proliferation in the uterine fundal ligament (98). Disruptions in HOXA11 signaling may
hinder the functional development or regeneration of the USL after
trauma, leading to compromised biomechanical strength in the USL
and, ultimately, uterovaginal prolapse in susceptible women
(99,100). A study by Connell et al
(101) comparing MMP2, MMP9, type
III collagen and HOXA11 expression in the USL of patients with POP
vs. controls revealed a significant reduction in the HOXA11 and
type III collagen expression, alongside a notable increase in the
MMP2 levels in patients with POP. Similarly, Ma et al
(102) found that knocking down
HOXA11 in the USL and uterus of mice decreased type I and type III
collagen content while increasing MMP2 and MMP9 activation. Zhang
et al (103) further
demonstrated that reduced expression of the human homologous gene
HOXA11 and TGF-β1 in the USL of patients with POP contributes to
the disorder of ECM, highlighting their role in regulating the
downregulation of collagens and MMPs, which leads to POP. These
findings suggested that disruptions in the signaling pathways
involving HOXA11, collagen and MMPs are key contributors to the
onset and progression of POP by compromising connective tissue
integrity.
miRNAs
Emerging research also highlights the involvement of
certain miRNAs in the development of POP. Yin et al
(104) found that miR-19-3p is
upregulated in the tissues of women with POP, where it induces
autophagy and apoptosis via the AKT/mTOR/p70S6K pathway.
Moreover, miR-19-3p targets insulin-like growth factor-1 to inhibit
the secretion of collagen I in vaginal fibroblasts (104). Additionally, miR-30d and miR-181a
are overexpressed in women with POP, with their expression
inversely correlated with HOXA11 mRNA levels (105). These results suggest that
miR-19-3p, miR-30d and miR-181a may be significant players in the
pathology of POP, although further research is needed.
Wnt pathway
The reduction in collagen content in pelvic floor
tissues is considered to result from increased collagen degradation
by collagenase. Changes in the biochemical and ultrastructural
properties of collagen fibers are linked to the onset of POP
(106). A decrease in fiber
quantity leads to the relaxation of supporting structures (such as
ligaments and fascia), which eventually contributes to the
development of POP (71). The Wnt
signaling pathway is known to activate the proliferation and
differentiation of myofibroblasts in pelvic floor support tissues,
encouraging them to release collagen and synthesize connective
tissue. Xie et al (107)
performed RNA-sequencing on USL samples from women with POP and
controls, conducting pathway enrichment analysis on differentially
expressed genes. Their findings revealed that the canonical Wnt
receptor signaling pathway showed the most significant enrichment
(107). Li et al (108) isolated VWFs from patients with
POP and non-POP individuals and found that the frizzled class
receptor 3 promotes VWF activity, enhances ECM degradation and
inhibits apoptosis through the Wnt pathway in POP.
ERα
Nakad et al (109) analyzed blood samples from 33
women with advanced POP and 33 women without POP to examine the
rs2228480 G/A mutation in the ERα gene using PCR technology. Their
findings revealed that homozygous ERα rs2228480 G/A mutation is
present in 19.2% of women with POP and 0.0% of those without,
indicating a potential link between ERα gene mutations and an
increased risk of late-stage POP. In a different study, Xie et
al (110) investigated the
effects of β-estradiol on human USL fibroblasts derived from
patients with POP and non-POP individuals, finding that estrogen
treatment reverses mechanical stress-induced apoptosis and cell
death in both groups. This reversal is accompanied by increased
protein and mRNA levels of the anti-apoptotic factors poly
(ADP-ribose) polymerase 1 (PARP1) and Bcl-2, as well as elevated
expression of ERα, the target of PARP1(110). These studies suggest that ERα may
play a significant role in the onset and progression of POP.
Other molecules
One potential mechanism underlying uterine prolapse
involves ECM and connective tissue damage in the USL, potentially
caused by the presence of heparinase. A study indicated that liver
enzymes are more prevalent in the USL connective tissue of women
with uterine prolapse compared with those without (111), suggesting that increased
heparinase expression may weaken tissue strength and contribute to
prolapse.
Li et al (112) conducted a candidate gene
association study in China involving 48 patients with POP and 8
women without POP, identifying a significant association between
three COL18A1 SNPs (rs55690336, rs56335679 and rs1050351) and POP.
However, the precise mechanism linking COL18A1 to POP remains
unclear and requires further investigation.
Cox et al (113), utilizing the Michigan Genomics
Initiative (University of Michigan Institutional Review Board
project approval no. HUM00161910; approval date, 05.03.2019),
performed a GWAS involving 1,329 patients with POP and 16,383
controls, identifying four SNPs (rs12325192, rs9306894, rs1920568
and rs1247943) as potentially increasing susceptibility to
prolapse. These SNPs are located near the genes SALL1, GDF7, TBX5
and TBX5 respectively. Despite these findings, the specific
mechanisms through which these SNPs contribute to POP remain to be
elucidated.
5. Treatment methods for POP
Treatment for POP is generally categorized into
non-surgical and surgical approaches. Non-surgical treatments aim
to alleviate symptoms, enhance pelvic floor muscle strength and
support and prevent severe prolapse, thus avoiding or delaying the
need for surgical intervention. Current non-surgical options
include conservative lifestyle modifications, pelvic floor muscle
training, physical therapy and pharmacotherapy (114-116).
Surgical treatment, often considered the final recourse, remains
the most critical intervention for POP (Fig. 1). While surgery can effectively
address prolapse, potential complications, such as mesh erosion,
infection, exposure and postoperative pain, may arise (117-119).
Stem cell transplantation holds considerable promise as a treatment
for POP, but further research is necessary (120).
General treatment
Comprehensive health education and behavioral
guidance should be provided to patients with POP, emphasizing
weight management, the reduction of high-intensity physical
activities (such as lifting and carrying heavy objects), smoking
cessation and the timely management of chronic conditions such as
cough and constipation (121,122). Postpartum women should initiate
rehabilitation promptly and actively manage risk factors to prevent
the onset of POP. Lifestyle intervention can help women prevent the
occurrence and development of POP to a certain extent and help
patients with POP improve symptoms; however, lifestyle intervention
cannot fundamentally solve the problem. Individuals with POP are
encouraged to see a physical therapist or combine other methods to
treat POP.
Physiotherapy. Pelvic floor muscle
training (PFMT)
PFMT, commonly known as Kegel training, involves
instructing patients to consciously perform voluntary contractions
of the pelvic floor muscles. This practice strengthens muscle
tension and contraction, improving pelvic blood circulation
(123). First introduced by
American physician Arnold Kegel in 1948, PFMT is a traditional
non-surgical treatment and the primary method for pelvic floor
rehabilitation. Kegel exercises focus on tightening the anus and
vagina (124), and numerous
clinical studies have demonstrated that effective Kegel training
significantly alleviates POP symptoms (123,125). In a randomized controlled trial
involving patients with POP, PFMT was found to be more effective
than stress-reducing exercises in improving POP symptoms (5). However, in clinical applications, it
has been found that almost half of the patients cannot contract the
pelvic floor muscles correctly but contract the abdominal muscles
and gluteus maximus incorrectly. This not only fails to improve
symptoms but will aggravate the condition as well (112,126,127). Therefore, Kegel training is
rarely used independently in clinical practice, and is often used
in conjunction with biofeedback and other methods to improve the
symptoms of patients with POP.
Biofeedback electrical stimulation.
Electrical stimulation, often combined with biofeedback, uses
varying energies, frequencies and pulse widths to induce
contractions in the pelvic floor muscles (128). During this process, biofeedback
mechanisms, such as electromyography and pressure curves, provide
real-time information on pelvic floor muscle activity, allowing
patients to observe and correct their muscle function for optimal
training results (128-130).
Pelvic floor biofeedback therapy, which does not require external
stimulation, is safe, non-invasive and effectively reactivates
pelvic floor muscles and nerves damaged during childbirth, helping
to restore vaginal tightness (131). Extensive literature supports the
effectiveness of electrical stimulation in improving POP symptoms,
enhancing the strength of type I and II pelvic floor fibers, and
treating urinary incontinence (129,132). In current clinical practice,
biofeedback therapy is often combined with electrical stimulation
to treat patients with POP (129). Studies have shown that this
combined approach, when paired with lifestyle interventions, is
more effective at improving POP symptoms and muscle strength than
lifestyle changes or Kegel exercises alone (125,133). Despite the long-standing use of
electrical stimulation in pelvic floor rehabilitation, standardized
parameters and treatment protocols are still lacking, necessitating
further clinical research and exploration. Biofeedback electrical
stimulation has the advantages of being non-invasive, highly
efficient and safe. However, clinically, there are no unified
parameters and standard treatment courses for treatment. Pelvic
floor muscle electrical stimulation may cause a small amount of
vaginal bleeding (128,129). Therefore, for different patients
with POP, more individualized approaches should be considered, and
plans should be formulated according to the degree of each disease.
Clinical research and exploration still need to be carried out in
the future.
Pessary
Vaginal pessaries are a highly effective
non-surgical treatment option for POP (134), particularly suitable for
individuals who decline surgery, cannot undergo surgical treatment
due to medical reasons, wish to preserve fertility or have
experienced POP recurrence post-surgery. The choice of pessary type
depends on the severity of the prolapse (134,135). Ramsay et al (136) demonstrated a high success rate
with pessary use in treating POP, reporting an 87.5% success rate
for women aged 65-74 years after 1 year of use and an 80.8% success
rate for those >75 years old. Proper pessary fitting can
significantly alleviate most clinical symptoms of prolapse. In
fact, 85% of French gynecologists view the clinical application of
pessaries for POP as satisfactory, with 50% considering it the
first-line treatment for POP (137). While pessaries effectively
improve symptoms and quality of life for patients with POP,
long-term use may lead to side effects such as increased vaginal
secretions, mucosal ulcers and bleeding (119,138). Sarma et al (119) found that 56% of patients
experienced complications after prolonged pessary use, with painful
bleeding, leucorrhea, odor and erosion being the most common
issues. A prospective cohort study following 130 female patients
over 1 year revealed that while 70 patients continued to use
pessaries, some developed vaginal erosion complications (138). Ongoing research is evaluating new
pessary designs, with results from a study involving 15 adults with
stage 2-4 POP still pending (139). To mitigate the side effects
associated with long-term pessary use, it is recommended that
postmenopausal women with vaginal mucosal atrophy receive local
estrogen therapy, which can reduce complications (140,141).
Pessary is the first-line treatment for POP. It is a
simple, economical and effective treatment method with a high
success rate (136,142). Previous hysterectomy, pelvic
reconstruction surgery and posterior vaginal wall prolapse are the
main factors leading to the failure of pessary treatment (18). However, pessary treatment also has
its risks in the form of complications. Although most complications
are relatively minor and can be improved by temporary
discontinuation of the device, topical estrogen therapy or
attention to hygiene. However, when minor complications are not
controlled or the patient leaves the pessary in the vagina, more
serious complications may occur, such as vesicovaginal fistula
(143). Therefore, strengthening
guidance and regular follow-up are the main measures to avoid
related complications.
Medication
Topical estrogen preparations are effective in
improving vaginal mucosal atrophy and may play a role in the
treatment of postmenopausal POP (140). Some studies have shown that
preoperative use of local estrogen combined with pelvic floor
muscle training can reduce the incidence of cystitis within 1 month
postoperatively, and oral raloxifene may decrease the need for POP
surgery in women aged ≥60 years old (141). According to Vaccaro et al
(140), administering vaginal
estrogen preoperatively for 2-12 weeks restores vaginal cytology to
premenopausal levels without increasing the thickness of the
vaginal epithelium. However, randomized controlled trials provide
limited evidence regarding the effectiveness of estrogen in
preventing and treating POP, indicating a need for further
research. Additionally, Xie et al (144) revealed that Buzhong Yiqi
decoction combined with surgery demonstrates significant clinical
efficacy in treating rectal prolapse, suggesting that traditional
Chinese medicine may offer benefits in the management of POP.
Researchers found that Shenqi Wenyang vaginal dilation suppository
(145) and Guyuan Shengti
decoction (146) combined with
biofeedback electrical stimulation treatment can improve the
clinical symptoms of patients with POP, and also improve muscle
coordination and strengthen the pelvic floor muscles. Traditional
Chinese medicine compounds combined with biofeedback electrical
stimulation treatment brought a significantly improvement compared
with simple traditional Chinese medicine or physical therapy.
Currently, there are no drugs with confirmed
efficacy for the treatment of POP. Short-term external use of
estrogen drugs can increase the thickness of the vaginal wall and
improve inflammation. It is often used clinically as an auxiliary
drug for other conservative treatments and before and after
surgery, and can improve POP to a certain extent (140,141). However, long-term treatment
effects and adverse reactions need to be confirmed by further
research. Traditional Chinese medicine has been used in combination
with pelvic floor rehabilitation therapy to treat mild POP
(145,146). This combination treatment has
important efficacy and few side effects, but more clinical research
is still needed to establish its therapeutic effects.
Surgical treatment
Surgical treatment for POP aims to repair defective
tissues and restore the anatomy and function of the pelvic floor
(117,135). Women with late-stage POP face a
higher risk of recurrence following native tissue repair compared
with those with early-stage POP; on the other hand, they may
experience improved sexual function, particularly in more advanced
cases. Due to the elevated risk of recurrence, it is advisable for
women with early-stage POP to postpone surgery until the condition
progresses (118). In current
clinical practice, the selection of a surgical method requires
careful consideration of various factors, including the location
and severity of the prolapse, patient age, overall health and
accompanying symptoms. The advent of new materials, such as
biological meshes, has expanded the surgical options available for
POP (147). In 2023, Dong et
al (148) reported a case of
transvaginal extraperitoneal uterine mesh fixation for treating
POP, with no interlaminar or mesh exposure issues observed during
postoperative follow-up. However, previous studies have documented
vaginal erosion rates ranging from 11.4-15.6% after transvaginal
POP mesh repair (149,150). The incidence of such
complications tends to increase significantly over time. For
instance, Hokenstad et al (57) reported erosion rates of 17% at 1
year, rising to 42% after 7 years. Moreover, the incidence of pain
following mesh surgery has been reported to exceed 9.1%.
Traditional surgeries (vaginal hysterectomy,
anterior and posterior vaginal wall repair and Manchester
operation) mostly involve repeated reinforcement of weak tissues,
which may not only distort or damage the anatomical structure and
fail to improve the upper vaginal defects, but also cause
postoperative vaginal discomfort and pain (151). In previous years, mesh repair has
been widely used in the treatment of POP and has achieved good
clinical results (120,152). The aim of this treatment is not
only to repair defects but also to strengthen the support of the
pelvic floor muscles and uterine ligaments. The addition of
synthetic mesh to the vagina can effectively improve the cure rate
of POP, but its complications cannot be ignored. The most common
complications are mesh erosion and infection (153,154). Given these concerns, the
development of more advanced and biocompatible graft materials is
essential, and further research is needed to evaluate the efficacy
and safety of these alternatives to traditional synthetic materials
for POP repair.
Stem cell transplantation
Stem cell therapy is emerging as a promising avenue
for the treatment of PFDs, although most studies remain in the
experimental stage, with limited in vivo research. For
example, scientists have successfully injected stem cells into the
urethral striated muscle of rats with stress urinary incontinence
to promote sphincter regeneration (155). Li et al (82) demonstrated that umbilical
cord-derived mesenchymal stem cells (MSCs) can inhibit the
cytotoxic effects of AGEs on the cells of patients with POP by
triggering anti-inflammatory responses and activating the
PI3K/AKT/PTEN signaling pathway. MSCs can promote new collagen
synthesis and have the ability to transform into smooth muscle
cells (156), which provides a
theoretical basis for repairing the weak vaginal wall of POP.
Currently, to the best of our knowledge, there are few studies
using stem cells to treat POP. At the same time, the mechanisms
underlying stem cell repair of pelvic floor tissue damage remain
incompletely understood, and challenges such as stem cell survival,
differentiation, ethical considerations, adverse reactions, complex
culture requirements and long treatment cycles persist. Thus,
further research is needed to support the clinical application of
stem cell therapy in POP treatment.
In addition, MSCs combined with tissue engineering
technology are regarded as a new approach to POP treatment
(157,158). MSCs use their powerful
immunomodulatory and paracrine abilities to promote successful
implantation, recruitment and integration of mesh. Mesh materials
provide a suitable living environment and mechanical support for
MSCs. The advantages of the two complement each other, which
promotes the biological patch developed by tissue engineering
technology. Good biocompatibility, mechanical stability, elasticity
and flexibility make it an ideal new therapeutic material (159). However, the current research on
MSCs is still in its infancy, the development of new materials also
needs to be improved, and further research is needed in the future.
MSCs in combination with mesh materials may bring new opportunities
for the treatment of POP.
6. Conclusions
PFD have emerged as a prominent concern in
obstetrics and gynecology both domestically and internationally.
Among these, POP is a particularly common condition that
significantly impacts the quality of life, mental health and social
interactions of adult women. The development of POP is complex,
involving multiple contributing factors rather than a single cause.
These risk factors include lifestyle, age, pregnancy parity, mode
of delivery, estrogen levels, abdominal pressure, pelvic surgery
history and genetic predispositions. Some of these factors act
independently, while others interact and collectively influence the
progression of POP, underscoring the importance of women being
mindful of these elements in daily life.
Research into the pathogenesis of POP remains
relatively sparse, with most studies focusing on various molecules
or signaling pathways related to fibroblasts, collagen and the ECM,
such as MMPs/TIMPs, TGF-β, AGEs/RAGE, PI3K/AKT, the fibulin family,
LOXL1, HOXA11, COL18A1, Wnt signaling pathways and ERα. Due to the
significant burden this disease places on women, particularly
postpartum women, it is crucial to conduct more comprehensive and
detailed research. With the advancement of research methodologies
and deepening scientific inquiries, it is anticipated that
understanding of the molecular biological mechanism driving the
development and progression of POP will expand. However, current
studies face several limitations. Primarily, much of the research
on the molecular mechanisms of POP is confined to isolated proteins
and pathways, with insufficient exploration of the upstream and
downstream interactions within these pathways, as well as a lack of
comprehensive cross-sectional studies of multiple pathways.
Additionally, much of the research on how specific molecules and
pathways influence POP remains at the level of cell and animal
models, lacking verification in clinical settings. Furthermore,
while changes in the expression levels of certain molecules (such
as TGF-β, ERα and MMPs) have been observed in patients with POP,
the research often fails to delve into the underlying mechanisms.
Future research should prioritize in-depth exploration of the
interactions among multiple proteins and pathways, while also
integrating animal studies with clinical research to uncover the
molecular mechanisms of POP more comprehensively in clinical
contexts.
For the treatment of POP, clinicians should
prioritize non-surgical approaches as the first-line treatment,
emphasizing early prevention, timely intervention and prompt
recovery. While PFMT has certain limitations, it offers significant
and effective benefits when practiced consistently under the
guidance of a clinician or rehabilitation therapist. Electrical
stimulation biofeedback, a conventional treatment for pelvic floor
muscle dysfunction, is particularly valuable for its emphasis on
individualized treatment plans. Pessary use remains an essential
option for elderly patients who cannot undergo surgery, often
serving as the only viable treatment for this population.
Pharmacological treatment presents a promising avenue, but further
research is needed to identify new therapeutic targets and develop
effective drugs. Stem cell transplantation holds considerable
therapeutic potential, yet more clinical trials are necessary to
establish its efficacy. Surgical treatment, while effective, is
associated with complications. The development and use of advanced
bionic and biological graft materials as alternatives to
traditional synthetic options for POP repair are ongoing, but
additional studies are required to assess their safety and
effectiveness. The pelvic floor plays a critical role in overall
health, and future pelvic floor rehabilitation efforts will likely
focus on simple, effective home-based treatments. There is also a
growing commitment to leveraging technological advancements to
provide sequential, precise and targeted training and treatment,
aiming to achieve optimal recovery of pelvic floor function.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JG and YL contributed to the acquisition, analysis
and interpretation of the data and drafted the manuscript. JH
contributed to the acquisition and analysis of the data. YW
contributed to the conception and design of the study and
critically revised the manuscript. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iglesia CB and Smithling KR: Pelvic Organ
Prolapse. Am Fam Physician. 96:179–185. 2017.PubMed/NCBI
|
|
2
|
Weintraub AY, Glinter H and Marcus-Braun
N: Narrative review of the epidemiology, diagnosis and
pathophysiology of pelvic organ prolapse. Int Braz J Urol. 46:5–14.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Collins S and Lewicky-Gaupp C: Pelvic
Organ Prolapse. Gastroenterol Clin North Am. 51:177–193.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Selihova MS, Ershov GV and Ershov AG:
Pelvic organ prolapse, a hidden epidemic of the 21st century. Adv
Gerontol. 34:431–437. 2021.PubMed/NCBI(In Russian).
|
|
5
|
Resende APM, Bernardes BT, Stüpp L,
Oliveira E, Castro RA, Girão MJBC and Sartori MGF: Pelvic floor
muscle training is better than hypopressive exercises in pelvic
organ prolapse treatment: An assessor-blinded randomized controlled
trial. Neurourol Urodyn. 38:171–179. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Roos AM, Thakar R, Sultan AH, de Leeuw JW
and Paulus AT: The impact of pelvic floor surgery on female sexual
function: A mixed quantitative and qualitative study. BJOG.
121:92–100; discussion 101. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kalata U, Jarkiewicz MM and Barcz EM:
Depression and anxiety in patients with pelvic floor disorders.
Ginekol Pol. 94:748–751. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Walker GJ and Gunasekera P: Pelvic organ
prolapse and incontinence in developing countries: review of
prevalence and risk factors. Int Urogynecol J. 22:127–135.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Al-Badr A, Saleem Z, Kaddour O, Almosaieed
B, Dawood A, Al-Tannir M, AlTurki F, Alharbi R and Alsanea N:
Prevalence of pelvic floor dysfunction: A Saudi national survey.
BMC Womens Health. 22(27)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eickmeyer SM: Anatomy and Physiology of
the Pelvic Floor. Phys Med Rehabil Clin N Am. 28:455–460.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McLeod LJ and Lee PE: Pelvic organ
prolapse. CMAJ. 195(E1013)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pelvic Organ Prolapse. Female Pelvic Med
Reconstr Surg. 23:353–364. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cecati M, Corradetti A, Sartini D, Pozzi
V, Giannubilo SR, Saccucci F, Ciavattini A and Emanuelli M:
Expression of extracellular matrix and adhesion proteins in pelvic
organ prolapse. Cell Mol Biol (Noisy-le-grand). 64:142–148.
2018.PubMed/NCBI
|
|
14
|
Tian Z, Li Q, Wang X and Sun Z: The
difference in extracellular matrix metabolism in women with and
without pelvic organ prolapse: A systematic review and
meta-analysis. BJOG. 131:1029–1041. 2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ying W, Hu Y and Zhu H: Expression of
CD44, Transforming Growth Factor-β, and matrix metalloproteinases
in women with pelvic organ prolapse. Front Surg.
9(902871)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Haylen BT, Maher CF, Barber MD, Camargo S,
Dandolu V, Digesu A, Goldman HB, Huser M, Milani AL, Moran PA, et
al: An International Urogynecological Association
(IUGA)/International Continence Society (ICS) Joint Report on the
Terminology for Female Pelvic Organ Prolapse (POP). Neurourol
Urodyn. 35:137–168. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhu L, Li L, Lang J, Xu T and Wong F:
Epidemiology of mixed urinary incontinence in China. Int J Gynaecol
Obstet. 109:55–58. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kato J, Nagata C, Miwa K, Ito N and
Morishige KI: Pelvic organ prolapse and Japanese lifestyle:
Prevalence and risk factors in Japan. Int Urogynecol J. 33:47–51.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Z, Xu T, Li Z, Gong J, Liu Q and Zhu L:
An epidemiologic study of pelvic organ prolapse in rural Chinese
women: A population-based sample in China. Int Urogynecol J.
30:1925–1932. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nygaard IE and Shaw JM: Physical activity
and the pelvic floor. Am J Obstet Gynecol. 214:164–171.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cattani L, Decoene J, Page AS, Weeg N,
Deprest J and Dietz HP: Pregnancy, labour and delivery as risk
factors for pelvic organ prolapse: A systematic review. Int
Urogynecol J. 32:1623–1631. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rogowski A, Bienkowski P, Tarwacki D,
Dziech E, Samochowiec J, Jerzak M and Baranowski W: Association
between metabolic syndrome and pelvic organ prolapse severity. Int
Urogynecol J. 26:563–568. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gava G, Alvisi S, Mancini I, Seracchioli R
and Meriggiola MC: Prevalence of metabolic syndrome and its
components in women with and without pelvic organ prolapse and its
association with prolapse severity according to the Pelvic Organ
Prolapse Quantification system. Int Urogynecol J. 30:1911–1917.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
American College of Obstetricians and
Gynecologists and the American Urogynecologic Society; INTERIM
UPDATE. This Practice Bulletin is updated as highlighted to reflect
the US Food and Drug Administration order to stop the sale of
transvaginal synthetic mesh products for the repair of pelvic organ
prolapse: Pelvic Organ Prolapse. Female Pelvic Med Reconstr Surg.
25:397–408. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu H, Wu W, Xiang W and Yuan J: Lifestyle
factors, metabolic factors and socioeconomic status for pelvic
organ prolapse: A Mendelian randomization study. Eur J Med Res.
28(183)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rostaminia G, Peck JD, Quiroz LH and
Shobeiri SA: Characteristics associated with pelvic organ prolapse
in women with significant levator ani muscle deficiency. Int
Urogynecol J. 27:261–267. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Patel DA, Xu X, Thomason AD, Ransom SB,
Ivy JS and DeLancey JO: Childbirth and pelvic floor dysfunction: An
epidemiologic approach to the assessment of prevention
opportunities at delivery. Am J Obstet Gynecol. 195:23–28.
2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu JM, Vaughan CP, Goode PS, Redden DT,
Burgio KL, Richter HE and Markland AD: Prevalence and trends of
symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol.
123:141–148. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lipschuetz M, Cohen SM,
Liebergall-Wischnitzer M, Zbedat K, Hochner-Celnikier D, Lavy Y and
Yagel S: Degree of bother from pelvic floor dysfunction in women
one year after first delivery. Eur J Obstet Gynecol Reprod Biol.
191:90–94. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Samimi P, Jones SH and Giri A: Family
history and pelvic organ prolapse: a systematic review and
meta-analysis. Int Urogynecol J. 32:759–774. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cheng C, Guo B, Li R, Wu W, Mi C and Li X:
Correlation between postpartum pelvic floor dysfunction and vaginal
microecological imbalance in late pregnancy. Zhong Nan Da Xue Xue
Bao Yi Xue Ban. 47:1608–1614. 2022.PubMed/NCBI View Article : Google Scholar : (In English,
Chinese).
|
|
32
|
Altman D, Falconer C, Cnattingius S and
Granath F: Pelvic organ prolapse surgery following hysterectomy on
benign indications. Am J Obstet Gynecol. 198:572.e1–e6.
2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Farrell SA, Baskett TF and Farrell KD: The
choice of elective cesarean delivery in obstetrics: A voluntary
survey of Canadian health care professionals. Int Urogynecol J
Pelvic Floor Dysfunct. 16:378–383. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao Y, Zou L, Xiao M, Tang W, Niu HY and
Qiao FY: Effect of different delivery modes on the short-term
strength of the pelvic floor muscle in Chinese primipara. BMC
Pregnancy Childbirth. 18(275)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mant J, Painter R and Vessey M:
Epidemiology of genital prolapse: observations from the Oxford
Family Planning Association Study. Br J Obstet Gynaecol.
104:579–585. 1997.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kearney R, Miller JM, Ashton-Miller JA and
DeLancey JO: Obstetric factors associated with levator ani muscle
injury after vaginal birth. Obstet Gynecol. 107:144–149.
2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Blomquist JL, Carroll M, Muñoz A and Handa
VL: Pelvic floor muscle strength and the incidence of pelvic floor
disorders after vaginal and cesarean delivery. Am J Obstet Gynecol.
222:62.e1–62.e8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kayembe AT, Kayembe CDKK, Bebele JK and
Tozin RR: Factors associated with genital prolapse to Saint Joseph
Hospital of Kinshasa. Pan Afr Med J. 40(234)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Reimers C, Siafarikas F, Stær-Jensen J,
Småstuen MC, Bø K and Ellström Engh M: Risk factors for anatomic
pelvic organ prolapse at 6 weeks postpartum: a prospective
observational study. Int Urogynecol J. 30:477–482. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Leijonhufvud A, Lundholm C, Cnattingius S,
Granath F, Andolf E and Altman D: Risks of stress urinary
incontinence and pelvic organ prolapse surgery in relation to mode
of childbirth. Am J Obstet Gynecol. 204:70.e1–e7. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Handa VL, Blomquist JL, McDermott KC,
Friedman S and Muñoz A: Pelvic floor disorders after vaginal birth:
effect of episiotomy, perineal laceration, and operative birth.
Obstet Gynecol. 119 (2 Pt 1):233–239. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fuermetz A, Schoenfeld M, Ennemoser S,
Muetzel E, Jeschke U and Jundt K: Change of steroid receptor
expression in the posterior vaginal wall after local estrogen
therapy. Eur J Obstet Gynecol Reprod Biol. 187:45–50.
2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rahn DD, Richter HE, Sung VW, Hynan LS and
Pruszynski JE: Effects of preoperative intravaginal estrogen on
pelvic floor disorder symptoms in postmenopausal women with pelvic
organ prolapse. Am J Obstet Gynecol. 229:309.e1–309.e10.
2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lara LA, Ribeiro da Silva A, Rosa-e-Silva
JC, Silva-de-Sá MF and Rosa-e-Silva AC: Estrogen receptor
expression and vessel density in the vagina wall in postmenopausal
women with prolapse. Tissue Cell. 46:159–164. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Vergeldt TF, Weemhoff M, IntHout J and
Kluivers KB: Risk factors for pelvic organ prolapse and its
recurrence: A systematic review. Int Urogynecol J. 26:1559–1573.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ewies AA, Thompson J and Al-Azzawi F:
Changes in gonadal steroid receptors in the cardinal ligaments of
prolapsed uteri: immunohistomorphometric data. Hum Reprod.
19:1622–1628. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zbucka-Kretowska M, Marcus-Braun N, Eboue
C, Abeguile G, Wolczynski S, Kottler ML and Von Theobald P:
Expression of estrogen receptors in the pelvic floor of pre- and
post-menopausal women presenting pelvic organ prolapse. Folia
Histochem Cytobiol. 49:521–527. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ramalingam K and Monga A: Obesity and
pelvic floor dysfunction. Best Pract Res Clin Obstet Gynaecol.
29:541–547. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
de Sam Lazaro S, Nardos R and Caughey AB:
Obesity and pelvic floor dysfunction: Battling the Bulge. Obstet
Gynecol Surv. 71:114–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Swift S, Woodman P, O'Boyle A, Kahn M,
Valley M, Bland D, Wang W and Schaffer J: Pelvic Organ Support
Study (POSST): The distribution, clinical definition, and
epidemiologic condition of pelvic organ support defects. Am J
Obstet Gynecol. 192:795–806. 2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Liu X, Rong Q, Liu Y, Wang J, Xie B and
Ren S: Relationship between high intra-abdominal pressure and
compliance of the pelvic floor support system in women without
pelvic organ prolapse: A finite element analysis. Front Med
(Lausanne). 9(820016)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Reddy J, Barber MD, Walters MD, Paraiso MF
and Jelovsek JE: Lower abdominal and pelvic pain with advanced
pelvic organ prolapse: A case-control study. Am J Obstet Gynecol.
204:537.e1–e5. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tan YH, Gillor M and Dietz HP: Abdominal
pressure and pelvic organ prolapse: Is there an association? Int
Urogynecol J. 33:337–342. 2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Matsuyama A, Kato K, Suzuki S, Nishiko Y,
Sai H, Ishiyama A, Kato T, Inoue S, Hirabayashi H and Hattori R:
Pelvic organ prolapse and inguinal hernia aggravated by ovarian
fibrothecoma with ascites. Nihon Hinyokika Gakkai Zasshi.
112:137–140. 2021.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
55
|
Spahlinger DM, Newcomb L, Ashton-Miller
JA, DeLancey JO and Chen L: Relationship between intra-abdominal
pressure and vaginal wall movements during Valsalva in women with
and without pelvic organ prolapse: Technique development and early
observations. Int Urogynecol J. 25:873–881. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chen CCG, Avondstondt AM, Khatry SK, Singh
M, Klasen EM, LeClerq SC, Katz J, Tielsch JM and Mullany LC:
Prevalence of symptomatic urinary incontinence and pelvic organ
prolapse among women in rural Nepal. Int Urogynecol J.
31:1851–1858. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Hokenstad ED, Glasgow AE, Habermann EB and
Occhino JA: Readmission and Reoperation After Surgery for Pelvic
Organ Prolapse. Female Pelvic Med Reconstr Surg. 23:131–135.
2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Buchsbaum GM, Duecy EE, Kerr LA, Huang LS,
Perevich M and Guzick DS: Pelvic organ prolapse in nulliparous
women and their parous sisters. Obstet Gynecol. 108:1388–1393.
2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Jack GS, Nikolova G, Vilain E, Raz S and
Rodríguez LV: Familial transmission of genitovaginal prolapse. Int
Urogynecol J Pelvic Floor Dysfunct. 17:498–501. 2006.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Altman D, Forsman M, Falconer C and
Lichtenstein P: Genetic influence on stress urinary incontinence
and pelvic organ prolapse. Eur Urol. 54:918–922. 2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Allen-Brady K, Cannon-Albright L, Farnham
JM, Teerlink C, Vierhout ME, van Kempen LCL, Kluivers KB and Norton
PA: Identification of six loci associated with pelvic organ
prolapse using genome-wide association analysis. Obstet Gynecol.
118:1345–1353. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gong R and Xia Z: Collagen changes in
pelvic support tissues in women with pelvic organ prolapse. Eur J
Obstet Gynecol Reprod Biol. 234:185–189. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jameson SA, Swaminathan G, Dahal S, Couri
B, Kuang M, Rietsch A, Butler RS, Ramamurthi A and Damaser MS:
Elastin homeostasis is altered with pelvic organ prolapse in
cultures of vaginal cells from a lysyl oxidase-like 1 knockout
mouse model. Physiol Rep. 8(e14436)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Campeau L, Gorbachinsky I, Badlani GH and
Andersson KE: Pelvic floor disorders: Linking genetic risk factors
to biochemical changes. BJU Int. 108:1240–1247. 2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Whitcomb EL, Rortveit G, Brown JS,
Creasman JM, Thom DH, Van Den Eeden SK and Subak LL: Racial
differences in pelvic organ prolapse. Obstet Gynecol.
114:1271–1277. 2009.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ra HJ and Parks WC: Control of matrix
metalloproteinase catalytic activity. Matrix Biol. 26:587–596.
2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Vulić M, Strinić T, Buković D, Tomić S,
Zupić T, Pavić M, Turcić P and Mihaljević S: Expression of matrix
metalloproteinase-1 in uterosacral ligaments tissue of women with
genital prolapse. Coll Antropol. 34:1411–1414. 2010.PubMed/NCBI
|
|
68
|
Strinic T, Vulic M, Tomic S, Capkun V,
Stipic I and Alujevic I: Matrix metalloproteinases-1, -2 expression
in uterosacral ligaments from women with pelvic organ prolapse.
Maturitas. 64:132–135. 2009.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Dviri M, Leron E, Dreiher J, Mazor M and
Shaco-Levy R: Increased matrix metalloproteinases-1,-9 in the
uterosacral ligaments and vaginal tissue from women with pelvic
organ prolapse. Eur J Obstet Gynecol Reprod Biol. 156:113–117.
2011.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Wu JM, Visco AG, Grass EA, Craig DM,
Fulton RG, Haynes C, Weidner AC and Shah SH: Matrix
metalloproteinase-9 genetic polymorphisms and the risk for advanced
pelvic organ prolapse. Obstet Gynecol. 120:587–593. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wang X, Li Y, Chen J, Guo X, Guan H and Li
C: Differential expression profiling of matrix metalloproteinases
and tissue inhibitors of metalloproteinases in females with or
without pelvic organ prolapse. Mol Med Rep. 10:2004–2008.
2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Hu Y, Wu R, Li H, Gu Y and Wei W:
Expression and significance of metalloproteinase and collagen in
vaginal wall tissues of patients with pelvic organ prolapse. Ann
Clin Lab Sci. 47:698–705. 2017.PubMed/NCBI
|
|
73
|
Zhu YP, Xie T, Guo T, Sun ZJ, Zhu L and
Lang JH: Evaluation of extracellular matrix protein expression and
apoptosis in the uterosacral ligaments of patients with or without
pelvic organ prolapse. Int Urogynecol J. 32:2273–2281.
2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Rosenbloom J, Castro SV and Jimenez SA:
Narrative review: fibrotic diseases: Cellular and molecular
mechanisms and novel therapies. Ann Intern Med. 152:159–166.
2010.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Sun B, Zhou L, Wen Y, Wang C, Baer TM,
Pera RR and Chen B: Proliferative behavior of vaginal fibroblasts
from women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod
Biol. 183:1–4. 2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Qi XY, Hong L, Guo FQ, Fu Q, Chen L and Li
BS: Expression of transforming growth factor-beta 1 and connective
tissue growth factor in women with pelvic organ prolapse. Saudi Med
J. 32:474–478. 2011.PubMed/NCBI
|
|
77
|
Liu C, Wang Y, Li BS, Yang Q, Tang JM, Min
J, Hong SS, Guo WJ and Hong L: Role of transforming growth factor
β-1 in the pathogenesis of pelvic organ prolapse: A potential
therapeutic target. Int J Mol Med. 40:347–356. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hu H, Jiang H, Ren H, Hu X, Wang X and Han
C: AGEs and chronic subclinical inflammation in diabetes: Disorders
of immune system. Diabetes Metab Res Rev. 31:127–137.
2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ramasamy R, Yan SF and Schmidt AM:
Advanced glycation endproducts: From precursors to RAGE: Round and
round we go. Amino Acids. 42:1151–1161. 2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Chen Y, Huang J, Hu C and Hua K:
Relationship of advanced glycation end products and their receptor
to pelvic organ prolapse. Int J Clin Exp Pathol. 8:2288–2299.
2015.PubMed/NCBI
|
|
81
|
Vetuschi A, Pompili S, Gallone A,
D'Alfonso A, Carbone MG, Carta G, Festuccia C, Gaudio E, Colapietro
A and Sferra R: Immunolocalization of advanced glycation end
products, mitogen activated protein kinases, and transforming
growth Factor-β/Smads in pelvic organ prolapse. J Histochem
Cytochem. 66:673–686. 2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Li L, Sima Y, Wang Y, Zhou J, Wang L and
Chen Y: The cytotoxicity of advanced glycation end products was
attenuated by UCMSCs in human vaginal wall fibroblasts by
inhibition of an inflammatory response and activation of
PI3K/AKT/PTEN. Biosci Trends. 14:263–270. 2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Chen YS, Wang XJ, Feng W and Hua KQ:
Advanced glycation end products decrease collagen I levels in
fibroblasts from the vaginal wall of patients with POP via the
RAGE, MAPK and NF-κB pathways. Int J Mol Med. 40:987–998.
2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Sima Y, Li L, Xiao C, Xu L, Wang L and
Chen Y: Advanced glycation end products (AGEs) downregulate the
miR-4429/PTEN axis to promote apoptosis of fibroblasts in pelvic
organ prolapse. Ann Transl Med. 10(821)2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Sima Y, Li J, Xu L, Xiao C, Li L, Wang L
and Chen Y: Quercetin antagonized advanced glycated end products
induced apoptosis and functional inhibition of fibroblasts from the
prolapsed uterosacral ligament. Drug Discov Ther. 17:415–427.
2024.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Li BS, Guo WJ, Hong L, Liu YD, Liu C, Hong
SS, Wu DB and Min J: Role of mechanical strain-activated PI3K/Akt
signaling pathway in pelvic organ prolapse. Mol Med Rep.
14:243–253. 2016.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Argraves WS, Greene LM, Cooley MA and
Gallagher WM: Fibulins: Physiological and disease perspectives.
EMBO Rep. 4:1127–1131. 2003.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Rahn DD, Acevedo JF, Roshanravan S, Keller
PW, Davis EC, Marmorstein LY and Word RA: Failure of pelvic organ
support in mice deficient in fibulin-3. Am J Pathol. 174:206–215.
2009.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Budatha M, Roshanravan S, Zheng Q,
Weislander C, Chapman SL, Davis EC, Starcher B, Word RA and
Yanagisawa H: Extracellular matrix proteases contribute to
progression of pelvic organ prolapse in mice and humans. J Clin
Invest. 121:2048–2059. 2011.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Budatha M, Silva S, Montoya TI, Suzuki A,
Shah-Simpson S, Wieslander CK, Yanagisawa M, Word RA and Yanagisawa
H: Dysregulation of protease and protease inhibitors in a mouse
model of human pelvic organ prolapse. PLoS One.
8(e56376)2013.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Khadzhieva MB, Kamoeva SV, Chumachenko AG,
Ivanova AV, Volodin IV, Vladimirov IS, Abilev SK and Salnikova LE:
Fibulin-5 (FBLN5) gene polymorphism is associated with pelvic organ
prolapse. Maturitas. 78:287–292. 2014.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zhao BH and Zhou JH: Decreased expression
of elastin, fibulin-5 and lysyl oxidase-like 1 in the uterosacral
ligaments of postmenopausal women with pelvic organ prolapse. J
Obstet Gynaecol Res. 38:925–931. 2012.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Chin K, Wieslander C, Shi H, Balgobin S,
Montoya TI, Yanagisawa H and Word RA: Pelvic organ support in
animals with partial loss of fibulin-5 in the vaginal wall. PLoS
One. 11(e0152793)2016.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Liu X, Zhao Y, Gao J, Pawlyk B, Starcher
B, Spencer JA, Yanagisawa H, Zuo J and Li T: Elastic fiber
homeostasis requires lysyl oxidase-like 1 protein. Nat Genet.
36:178–182. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
95
|
Li Y, Nie N, Gong L, Bao F, An C, Cai H,
Yao X, Liu Y, Yang C, Wu B and Zou X: Structural, functional and
molecular pathogenesis of pelvic organ prolapse in patient and
Loxl1 deficient mice. Aging (Albany NY). 13:25886–25902.
2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Li Y, Wu B, An C, Jiang D, Gong L, Liu Y,
Liu Y, Li J, Ouyang H and Zou X: Mass cytometry and transcriptomic
profiling reveal body-wide pathology induced by Loxl1 deficiency.
Cell Prolif. 54(e13077)2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Kufaishi H, Alarab M, Drutz H, Lye S and
Shynlova O: Comparative characterization of vaginal cells derived
from premenopausal women with and without severe pelvic organ
prolapse. Reprod Sci. 23:931–943. 2016.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Connell KA, Guess MK, Chen HW, Lynch T,
Bercik R and Taylor HS: HOXA11 promotes fibroblast proliferation
and regulates p53 in uterosacral ligaments. Reprod Sci. 16:694–700.
2009.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Leppert PC: Tissue remodeling in the
female reproductive tract-a complex process becomes more complex:
The role of Hox genes. Biol Reprod. 86(98)2012.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Yılmaz N, Ozaksit G, Terzi YK, Yılmaz S,
Budak B, Aksakal O and Sahin FI: HOXA11 and MMP2 gene expression in
uterosacral ligaments of women with pelvic organ prolapse. J Turk
Ger Gynecol Assoc. 15:104–108. 2014.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Connell KA, Guess MK, Chen H, Andikyan V,
Bercik R and Taylor HS: HOXA11 is critical for development and
maintenance of uterosacral ligaments and deficient in pelvic
prolapse. J Clin Invest. 118:1050–1055. 2008.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Ma Y, Guess M, Datar A, Hennessey A,
Cardenas I, Johnson J and Connell KA: Knockdown of Hoxa11 in vivo
in the uterosacral ligament and uterus of mice results in altered
collagen and matrix metalloproteinase activity. Biol Reprod.
86(100)2012.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Zhang L, Dai F, Chen G, Wang Y, Liu S,
Zhang L, Xian S, Yuan M, Yang D, Zheng Y, et al: Molecular
mechanism of extracellular matrix disorder in pelvic organ
prolapses. Mol Med Rep. 22:4611–4618. 2020.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Yin Y, Qin M, Luan M and Xia Z: miR-19-3p
Promotes Autophagy and Apoptosis in Pelvic Organ Prolapse Through
the AKT/mTOR/p70S6K Pathway: Function of miR-19-3p on Vaginal
Fibroblasts by Targeting IGF-1. Female Pelvic Med Reconstr Surg.
27:e630–e638. 2021.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Jeon MJ, Kim EJ, Lee M, Kim H, Choi JR,
Chae HD, Moon YJ, Kim SK and Bai SW: MicroRNA-30d and microRNA-181a
regulate HOXA11 expression in the uterosacral ligaments and are
overexpressed in pelvic organ prolapse. J Cell Mol Med. 19:501–509.
2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Johanes I, Mihelc E, Sivasankar M and
Ivanisevic A: Morphological properties of collagen fibers in
porcine lamina propria. J Voice. 25:254–257. 2011.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Xie R, Xu Y, Fan S and Song Y:
Identification of differentially expressed genes in pelvic organ
prolapse by RNA-Seq. Med Sci Monit. 22:4218–4225. 2016.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Li J, Zhang J, Chu Z, Han H and Zhang Y:
FZD3 regulates the viability, apoptosis, and extracellular matrix
degradation of vaginal wall fibroblasts in pelvic organ prolapse
via the Wnt signaling pathway. J Biochem Mol Toxicol.
38(e23654)2024.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Nakad B, Fares F, Azzam N, Feiner B,
Zilberlicht A and Abramov Y: Estrogen receptor and laminin genetic
polymorphism among women with pelvic organ prolapse. Taiwan J
Obstet Gynecol. 56:750–754. 2017.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Xie T, Guo D, Guo T, Zhu Y, Li F, Zhang S,
Lang J and Sun Z: The protective effect of 17 β-estradiol on human
uterosacral ligament fibroblasts from postmenopausal women with
pelvic organ prolapse. Front Physiol. 13(980843)2022.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Ben-Zvi M, Herman HG, Schreiber L, Sagiv
R, Bar J, Condrea A and Ginath S: Expression of Heparanase in
uterosacral ligaments of women with or without uterine prolapse.
Eur J Obstet Gynecol Reprod Biol. 244:110–113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Li C, Gong Y and Wang B: The efficacy of
pelvic floor muscle training for pelvic organ prolapse: A
systematic review and meta-analysis. Int Urogynecol J. 27:981–992.
2016.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Cox CK, Pandit A, Zawistowski M, Dutta D,
Narla G and Swenson CW: Genome-Wide association study of pelvic
organ prolapse using the michigan genomics initiative. Female
Pelvic Med Reconstr Surg. 27:502–506. 2021.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Halberthal-Cohen A, Burke YZ, Matanes E,
Amiel G and Lowenstein L: Modified colpocleisis for repair of
pelvic organ prolapse post-radical cystectomy. Int Urogynecol J.
31:409–410. 2020.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Lawson S and Sacks A: Pelvic floor
physical therapy and women's health promotion. J Midwifery Womens
Health. 63:410–417. 2018.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Lamvu G, Carrillo J, Witzeman K and
Alappattu M: Musculoskeletal considerations in female patients with
chronic pelvic pain. Semin Reprod Med. 36:107–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Barcz EM: Pelvic organ prolapse surgery.
What techniques should be used? Ginekol Pol. 94:771–772.
2023.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Dubinskaya ED, Gasparov AS, Babichevа IA
and Kolesnikova SN: Questions surrounding the optimal time for
surgical treatment of pelvic organ prolapse. Eur J Obstet Gynecol
Reprod Biol. 234:120–125. 2019.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Sarma S, Ying T and Moore KH: Long-term
vaginal ring pessary use: Discontinuation rates and adverse events.
BJOG. 116:1715–1721. 2009.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Mangir N, Aldemir Dikici B, Chapple CR and
MacNeil S: Landmarks in vaginal mesh development: polypropylene
mesh for treatment of SUI and POP. Nat Rev Urol. 16:675–689.
2019.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Blanchard V, Nyangoh-Timoh K, Fritel X,
Fauconnier A and Pizzoferrato AC: Importance of a pelvic floor
lifestyle program in women with pelvic floor dysfunctions: A pilot
study. J Gynecol Obstet Hum Reprod. 50(102032)2021.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Ryhtä I, Axelin A, Parisod H, Holopainen A
and Hamari L: Effectiveness of exercise interventions on urinary
incontinence and pelvic organ prolapse in pregnant and postpartum
women: Umbrella review and clinical guideline development. JBI Evid
Implement. 21:394–408. 2023.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Ho L, Macnab A, Matsubara Y, Peterson K,
Tsang B and Stothers L: Rating of pelvic floor muscle training
mobile applications for treatment of urinary incontinence in women.
Urology. 150:92–98. 2021.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Espiño-Albela A, Castaño-García C,
Díaz-Mohedo E and Ibáñez-Vera AJ: Effects of Pelvic-Floor Muscle
Training in Patients with Pelvic Organ Prolapse Approached with
Surgery vs. Conservative Treatment: A Systematic Review. J Pers
Med. 12(806)2022.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Due U, Brostrøm S and Lose G: Lifestyle
advice with or without pelvic floor muscle training for pelvic
organ prolapse: a randomized controlled trial. Int Urogynecol J.
27:555–563. 2016.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Basnet R: Impact of pelvic floor muscle
training in pelvic organ prolapse. Int Urogynecol J. 32:1351–1360.
2021.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Wang T, Wen Z and Li M: The effect of
pelvic floor muscle training for women with pelvic organ prolapse:
A meta-analysis. Int Urogynecol J. 33:1789–1801. 2022.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Lv A, Gai T, Zhang S, Feng Q and Li Y:
Electrical stimulation plus biofeedback improves urination
function, pelvic floor function, and distress after reconstructive
surgery: a randomized controlled trial. Int J Colorectal Dis.
38(226)2023.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Min L, Chunxue Y, Qiubo L, Xudong D, Yan
Z, Guifang Z, Kejia H, Tianzi G and Qing F: Effectiveness of
intravaginal electrical stimulation combined with electromyography
biofeedback-mediated pelvic floor muscle training for postpartum
symptomatic pelvic organ prolapse: Protocol for the PROSPECT
randomized trial. Trials. 23(131)2022.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Zhong F, Miao W, Yu Z, Hong L and Deng N:
Clinical effect of electrical stimulation biofeedback therapy
combined with pelvic floor functional exercise on postpartum pelvic
organ prolapse. Am J Transl Res. 13:6629–6637. 2021.PubMed/NCBI
|
|
131
|
Liu J, Yan W, Tang Y, Zhou Y, Yang S,
Xiang J, Zeng X, Xie F and Li X: Therapeutic effect of
proprioception training combined with pelvic floor electrical
stimulation biofeedback on postpartum pelvic floor dysfunction.
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 47:1253–1259. 2022.PubMed/NCBI View Article : Google Scholar : (In Chinese,
English).
|
|
132
|
Vonthein R, Heimerl T, Schwandner T and
Ziegler A: Electrical stimulation and biofeedback for the treatment
of fecal incontinence: A systematic review. Int J Colorectal Dis.
28:1567–1577. 2013.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Hagen S, Stark D, Glazener C, Dickson S,
Barry S, Elders A, Frawley H, Galea MP, Logan J, McDonald A, et al:
Individualised pelvic floor muscle training in women with pelvic
organ prolapse (POPPY): A multicentre randomised controlled trial.
Lancet. 383:796–806. 2014.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Shah SM, Sultan AH and Thakar R: The
history and evolution of pessaries for pelvic organ prolapse. Int
Urogynecol J Pelvic Floor Dysfunct. 17:170–175. 2006.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Qiu J and Jiang D: Pessaries for managing
pelvic organ prolapse in women. Am Fam Physician. 103:660–661.
2021.PubMed/NCBI
|
|
136
|
Ramsay S, Tu le M and Tannenbaum C:
Natural history of pessary use in women aged 65-74 versus 75 years
and older with pelvic organ prolapse: A 12-year study. Int
Urogynecol J. 27:1201–1207. 2016.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Vardon D, Martin-Lasnel M, Agostini A,
Fauvet R and Pizzoferrato AC: Vaginal pessary for pelvic organ
prolapse: A survey among french gynecological surgeons. J Gynecol
Obstet Hum Reprod. 50(101833)2021.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Robert M, Govan AJ, Lohani U and Uprety A:
Feasibility of using pessaries for treatment of pelvic organ
prolapse in rural Nepal. Int J Gynaecol Obstet. 136:325–330.
2017.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Strohbehn K, Wadensweiler P and Hanissian
P: A novel, collapsible, space-occupying pessary for the treatment
of pelvic organ prolapse. Int Urogynecol J. 34:317–319.
2023.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Vaccaro CM, Mutema GK, Fellner AN, Crisp
CC, Estanol MV, Kleeman SD and Pauls RN: Histologic and cytologic
effects of vaginal estrogen in women with pelvic organ prolapse: A
randomized controlled trial. Female Pelvic Med Reconstr Surg.
19:34–39. 2013.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Ismail SI, Bain C and Hagen S: Oestrogens
for treatment or prevention of pelvic organ prolapse in
postmenopausal women. Cochrane Database Syst Rev.
2010(Cd007063)2010.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Cundiff GW, Weidner AC, Visco AG, Bump RC
and Addison WA: A survey of pessary use by members of the American
urogynecologic society. Obstet Gynecol. 95 (6 Pt 1):931–935.
2000.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Rapp DE, Congleton JY, Boatman K, Karp N
and Krupski T: Bilateral ureterovaginal fistulas and vesicovaginal
fistula in setting of retained pessary. Urology. 141:e11–e13.
2020.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Xie Y, Fan Y, Yang C, Wan R, Cheng X, Yang
X, Hu Y and Deng C: Efficacy and safety of Buzhong Yiqi decoction
combined with surgery for rectal prolapse: A protocol for
systematic review and meta analysis. Medicine (Baltimore).
99(e22732)2020.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Li J, Lin M and Zhu S: Efficacy and safety
of Shenqi Wenyang vaginal dilation suppository and biofeedback and
pelvic floor electrical stimulation in the treatment of stage
andⅡpelvic organ prolapse. Chin Prac Med. 18:20–24. 2023.(In
Chinese).
|
|
146
|
Wang M: Clinical study of Guyuan Shengti
Decoction combined with biofeedback electric stimulation in the
treatment of female mild and moderate pelvic organ prolapse
(unpublished PhD thesis). Nanjing University of Traditional Chinese
Medicine, 2022.
|
|
147
|
Van der Aa F and De Ridder D: Vaginal
pelvic organ prolapse repair using mesh: Let's welcome science into
the mesh debate. Eur Urol. 74:177–178. 2018.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Dong Y, Wang Y, Wang L and Sun L:
Laparoscopically monitored transvaginal extraperitoneal uterine
fixation with mesh for pelvic organ prolapse. Asian J Surg.
46:3109–3110. 2023.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Ellington DR and Richter HE: Indications,
contraindications, and complications of mesh in surgical treatment
of pelvic organ prolapse. Clin Obstet Gynecol. 56:276–288.
2013.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Höfner K, Hampel C, Kirschner-Hermanns R,
Alloussi SH, Bauer RM, Bross S, Bschleipfer T, Goepel M, Haferkamp
A, Hüsch T, et al: Use of synthetic slings and mesh implants in the
treatment of female stress urinary incontinence and prolapse:
Statement of the Working Group on Urological Functional Diagnostics
and Female Urology of the Academy of the German Society of Urology.
Urologe A. 59:65–71. 2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
151
|
Debodinance P, Cosson M, Collinet P,
Boukerrou M, Lucot JP and Madi N: Synthetic meshes for transvaginal
surgical cure of genital prolapse: evaluation in 2005. J Gynecol
Obstet Biol Reprod (Paris). 35 (5 Pt 1):429–454. 2006.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
152
|
Barski D and Deng DY: Management of mesh
complications after SUI and POP Repair: Review and analysis of the
current literature. Biomed Res Int. 2015(831285)2015.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Mateu Arrom L, Errando Smet C, Gutierrez
Ruiz C, Araño P and Palou Redorta J: Pelvic organ prolapse repair
with mesh: Mid-Term efficacy and complications. Urol Int.
101:201–205. 2018.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Weintraub AY: Mesh Complications Following
POP Repair with or without Vaginal Hysterectomy. Isr Med Assoc J.
21:419–421. 2019.PubMed/NCBI
|
|
155
|
Ni J, Li H, Zhou Y, Gu B, Xu Y, Fu Q, Peng
X, Cao N, Fu Q, Jin M, et al: Therapeutic potential of human
adipose-derived stem cell exosomes in stress urinary
incontinence-an in vitro and in vivo study. Cell Physiol Biochem.
48:1710–1722. 2018.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Xu L, Sima Y, Xiao C and Chen Y: Exosomes
derived from mesenchymal stromal cells: A promising treatment for
pelvic floor dysfunction. Hum Cell. 36:937–949. 2023.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Hennes DMZB, Rosamilia A, Werkmeister JA,
Gargett CE and Mukherjee S: Endometrial SUSD2(+) mesenchymal
stem/stromal cells in tissue engineering: Advances in novel
cellular constructs for pelvic organ prolapse. J Pers Med.
11(840)2021.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Gargett CE, Gurung S, Darzi S, Werkmeister
JA and Mukherjee S: Tissue engineering approaches for treating
pelvic organ prolapse using a novel source of stem/stromal cells
and new materials. Curr Opin Urol. 29:450–457. 2019.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Wen J, Zhao Z, Fang F, Xiao J, Wang L,
Cheng J, Wu J and Miao Y: Prussian blue nanoparticle-entrapped
GelMA Gels Laden with mesenchymal stem cells as prospective
biomaterials for pelvic floor tissue repair. Int J Mol Sci.
24(2704)2023.PubMed/NCBI View Article : Google Scholar
|
















