Introduction
Diabetes mellitus (DM) is a disease characterized by
elevated blood glucose levels and is a prominent challenge for the
healthcare system due to its high prevalence (1,2). DM
can also result in vascular complications including corneal
neuropathy and cardiovascular disease (CVD) (3). Endothelial dysfunction caused by DM
is also characterized by increased vasoconstriction and a doubled
risk of developing CVD (4,5). CVD pathophysiology encompasses a
broad spectrum of disorders affecting the heart and blood vessels,
including coronary artery disease (CAD), heart failure, arterial
hypertension, atherosclerosis and stroke (6). An important risk factor for CV
mortality is chronic kidney disease. A number of studies have
previously reported that a lower estimated glomerular filtration
rate (eGFR) is strongly associated with CVD and all-cause mortality
in patients with DM (7,8). Increased cardiac-specific troponin
content and insulin resistance (IR) levels [Homeostasis Model
Assessment (HOMA)-IR] are also positively correlated with an
increased risk of CVD (9,10). Disease development in patients with
kidney neuropathy (DN) is induced by inflammation (11). Activation of nuclear factor NF-κB,
which serves crucial role in the inflammatory process in patients,
induces the production of inflammatory chemokines, adhesion
molecules and cytokines, such as TNF-α (12). DM is reported to increase the
expression levels of TNF-α and nuclear factor erythroid 2-related
factor 2 (Nrf2) (13). Induced
TNF-α contributes to the progression of certain diseases, including
psoriasis and rheumatoid arthritis (14). Nrf2 signaling is also involved in
attenuating inflammation-associated pathogenesis in certain
diseases, such as rheumatoid arthritis, asthma and atherosclerosis
(15).
Presently, echocardiography is the most common
diagnostic test used for identifying diabetic cardiomyopathy (DCM)
(16). This method enables the
concurrent identification of both structural and functional
alterations in the myocardium (17). However, the use of echocardiography
is not a cost-effective approach (18). Therefore, the development of new
tools for blood-based diagnosis is needed to allow the
identification of patients at risk of developing DCM. Although a
number of biomarkers to identify certain inflammatory processes are
currently well-known, more precise and novel molecular markers are
needed to facilitate the early diagnosis of DM (19). Non-coding RNAs (ncRNAs) can be
classified into small size ncRNAs, such as microRNAs (miRNAs) and
long ncRNAs (lncRNAs). These ncRNAs are functional RNA molecules
transcribed from the genome that are not translated into proteins
(20). miRNAs consist of 18-24
nucleotides and regulate gene expression by silencing genes at the
post-transcriptional level (21).
Altering ncRNA expression in diabetic patients is linked to the
pathophysiology of certain diseases and disorders including lung,
heart and kidney diseases (22,23).
Thus, ncRNAs could potentially act as therapeutic targets based on
their regulatory roles. Among miRNAs, miR-132 has been reported to
be a negative gene regulator that contributes to the complications
of DM (24). miR-132 targets Nrf2,
which induces renal injury (25)
and mediates the expression of genes involved in TGF-β signaling
and cell proliferation, such as Foxo3/p300, which regulates the
progression of fibrosis (26).
In response to myocardial infarction, locked nucleic
acid (LNA)-based anti-miR-132 treatment improves ejection fraction
and ameliorates cardiac dysfunction (27). Additionally, in patients with heart
failure, a synthetic LNA-based antisense oligonucleotide against
miR-132 was reported to reverse heart failure in vivo
(27). miR-133a also participates
in mediating glucose-induced cardiomyocyte hypertrophy in DM
(28). It is the most
downregulated miRNA in the failing heart (29), which protects the heart against
adverse remodeling (30). It has
been previously reported that miR-133a expression is significantly
reduced in the hearts of patients with DM (31). Reduced serum miR-133a levels are
significantly associated with increased autophagy markers, which
results in the exacerbation of DM-induced cardiac hypertrophy
(31). Increased miR-133a
expression could prevent hypertrophy in the hearts of patients with
DM by decreasing the expression of the early cardiac hypertrophy
marker β-myosin heavy chain (β-MHC) (32). The lnc-RNA megacluster (lnc-MGC),
which is typically >200 nucleotides in length and does not
encode proteins, plays a role in controlling DM-induced renal
fibrosis, which suggests its involvement in the pathogenesis of
kidney diseases (33). Human
lnc-MGC shares exons with other lncRNAs, such as maternally
expressed gene 8 and maternally expressed gene 9(34). lnc-MGC is upregulated under
conditions associated with DM, such as high glucose and TGF-β
levels (34). Inhibition of
lnc-MGC decreases the expression of key cluster kidney miRNAs (such
as miR-379, miR-494, miR-495 and miR-377) which triggers early DN
(35). lnc-MGC upregulation in
human cardiac fibroblasts under mechanical stress conditions was
also associated with the downregulation of miR-133a expression
(36). ncRNAs are useful as
biomarkers as they are easily accessible and can be extracted
through liquid biopsies from bodily fluids. They can detect changes
that occur as a disease advance and have high specificity for
tissue and cell type (37).
Compared with the production of novel antibodies to target protein
biomarkers, developing new assays for the detection of nucleic
acids requires less time and lower costs (38). Overall, this highlights the future
potential for the utility of ncRNAs in both clinical and
personalized medicine.
To date, the relationship between circulating
miR-133a, miR-132 and lnc-MGC expression levels in patients with DM
and CVDs, glycemic biomarkers in DN, and the inflammatory
biomarkers Nrf2 and TNF-α has not been reported. Thus, the present
study aimed to identify the potential of serum miR-133a, miR-132
and lnc-MGC to act as molecular biomarkers for DN and CVDs and
examine their interactions with inflammatory biomarkers. This has
the potential to contribute to predicting the progression of
diabetic cardiomyopathy and nephropathy in the future.
Materials and methods
Experimental design
A total of 200 type 2 diabetic patients who attended
the specialized DM and nephrology clinics at the Internal Medicine
Department of Beni-Suef University Hospital, (Beni-Suef, Egypt)
from November 2021-May 2022 provided written informed consent for
participation in the present study and agreed to the use of their
samples in scientific research. Eligible patients were classified
according to the value of their eGFR into six groups. Furthermore,
40 healthy subjects were included in the study as controls. The
study protocol followed the Declaration of Helsinki and good
clinical practice guidelines and was approved by the Ethics
Committee of Beni-Suef University Hospital (approval no.
BSU:7-2021; Beni-Suef, Egypt).
Healthy individuals, diabetic patients without
nephropathy [glycated hemoglobin (HbA1c) >6.5%; eGFR ≥60
ml/min/1.73 m2)], and diabetic patients with nephropathy
[HbA1c >6.5%; eGFR <60 ml/min/1.73 m2)] were
enrolled in the present study. The present study excluded patients
with any history of chronic and acute infections, diabetic
retinopathy, hepatic diseases, malignancy and other endocrine
dysfunctions. According to the clinical data and eGFR values,
enrolled participants were classified into seven groups as follows:
i) Healthy controls (n=40); ii) G1, eGFR ≥90 ml/min/1.73
m2 (n=35); iii) G2, eGFR 60-89 ml/min/1.73 m2
(n=30); iv) G3a, eGFR 45-59 ml/min/1.73 m2 (n=30); v)
G3b, eGFR 30-44 ml/min/1.73 m2 (n=35); vi) G4, eGFR
15-29 ml/min/1.73 m2 (n=30); and vii) G5, eGFR <15
ml/min/1.73 m2 (n=40). The G5 group of patients was
classed as being in kidney failure. The sample size for the present
study was determined based on several factors, including the
desired level of confidence, expected effect sizes of the
biomarkers, variability within the population and statistical power
considerations. Specifically, the aim was to achieve a power of 80%
to detect statistically significant differences in biomarker
expression levels between diabetic patients with and without
complications, as well as among different severity groups of
diabetic nephropathies. With each group comprising 30-40
participants and considering an effect size of 0.5, the sample size
was deemed sufficient for detecting medium to large effect
sizes.
Blood sample collection
A total of two blood samples (4 ml/sample) were
collected from healthy controls and diabetic participants after
overnight fasting. EDTA was used in the collection of one of the
blood samples and the other was collected using a plain collection
tube. Samples were incubated for 30 min at room temperature and
blood in the plain tubes was centrifuged at 4,000 x g at 4˚C for 20
min to isolate serum. Blood samples in EDTA were used for complete
blood count, DNA extraction and HbA1c level measurements. Samples
were stored at -80˚C until used.
Biochemical analyses
HbA1c was measured using a Stanbio™ Glycohemoglobin
(HBA1 and HBA1C) Pre-Fil™ Test (cat. no. SB-P350-50;
Stanbio), while fasting blood sugar (FBS), Na+,
Ca2+ and K+ levels were determined in serum
samples using a commercial SPINREACT diagnostic kit (cat. nos.
1001380, MD1001065 and #1001390 for Na+, Ca2+
and K+, respectively; Spinreact, S.A.U.). Blood uric
acid was measured using a commercial SPINREACT diagnostic kit (cat.
no. MD41001; Spinreact, S.A.U.). Serum activity of glutamate
pyruvate transaminase (sGPT) and glutamate oxaloacetate
transaminase (sGOT) enzymes were measured using a commercial
SPINREACT GOT/AST diagnostic kit (cat. no. MD41264; Spinreact,
S.A.U.) according to the manufacturer's instructions. Blood urea
(BUN reagent; cat. no. BK-443350D) and creatinine uric acid levels
(creatine reagent kit; cat. no. BK-472525D) were determined in the
serum samples (Diamond Diagnostics). Fasting insulin levels were
assayed using Diagnostic Products Corporation radioimmunoassay kits
(Coat-A-Count; cat. no. TKIN-4; Diagnostic Products Corporation).
Insulin resistance was investigated by calculating the HOMA-IR.
HOMA-IR=[(fasting insulin, µU/ml) x (fasting glucose,
mmol/l)]/22.5, where 22.5 is the normalizing factor.
The glomerular filtration rate (eGFR) was measured
based on serum creatinine levels and calculated according to the
Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)
equation. Moreover, the CKD-EPI value was calculated taking into
account sex and serum creatinine (39). Females: Creatinine ≤0.7 mg/dl:
eGFR=144x (creatinine/0.7)-0.329 x (0.993) age x 1.159
(for Black or African American individuals). Creatinine >0.7
mg/dl: eGFR=144 x (creatinine/0.7)-1.209 x (0.993) age x
1.159 (for Black or African American individuals). Males:
Creatinine ≤0.9 mg/dl: eGFR=141 x (creatinine/0.9)-0.411 x (0.993)
age x 1.159 (for Black or African American individuals). Creatinine
>0.9 mg/dl: eGFR=141 x (creatinine/0.9)-1.209 x (0.993) age x
1.159 (for Black or African American individuals). Adjustments for
Black or African American individuals were made in eGFR
calculations to account for higher average serum creatinine levels
in Black individuals for more accurate kidney function estimates
(40). The calculation used for
the rest of the individuals studied in this study who were not of
Black/African American ethnicity was based on the following CKD-EPI
creatinine-cystatin equation (41)
eGFR=135 x min (SCr/κ,1)α x max
(SCr/κ,1)-0.544 x min (Scys/0.8,1)-0.323 x
max (Scys/0.8,1)-0.778 x 0.9961Age x 0.963
[if female], where eGFR=estimated GFR in ml/min/1.73 m2,
SCr=standardized serum creatinine in mg/dl, Scys=standardized serum
cystatin C in mg/l, κ=0.7 (females) or 0.9 (males), α=-0.219
(females) or -0.144 (males), min=indicates the minimum of SCr/κ or
1, max=indicates the maximum of SCr/κ or 1, age=years. ELISA kits
were used to measure serum TNF-α (cat. no. SEA133Mu; Cloud-Clone
Corp.), cardiac troponin I (Human Cardiac Troponin IELISA Kit; cat.
no. ab200016; Abcam) and Nrf2 levels (cat no. #80593-1-PBS; Wuhan
Fine Biological Technology Co., Ltd.).
To investigate hyperglycemia-induced cardiac injury,
circulating creatine kinase (CK) activity, CK-myocardial band (MB)
activity, lactate dehydrogenase (LDH) activity and troponin I
levels were measured. Manufacturers' instructions were followed to
determine the ELISA CK-MB (Rat CK-MB ELISA kit; cat. no.
DEIA-FN285; Creative Diagnostics), troponin I (cat no.
ELH-Troponin1-1; RayBiotech, Inc.) and LDH (Rat LDH kit; cat. no.
LS-F5026; LifeSpan Biosciences, Inc.).
miR-133a, miR-132 and lnc-MGC
expression assays
miRNA was extracted from serum samples using the
Plus kit (cat. no. R2072; Zymo Research Corp.). Isolated RNA
samples were reverse-transcribed, then miR-133a, miR-132 and
lnc-MGC expression levels were determined via reverse-transcription
quantitative PCR (RT-qPCR) using the One-Step RT-PCR kit (cat. no.
12594100; Thermo Fisher Scientific, Inc.). The cycling conditions
for RT-qPCR started with initial HotStar Taq DNA Polymerase
activation step at 95˚C for 15 min, then 40 cycles each of three
steps (94˚C for 15 sec, 55˚C for 30 sec, and 70˚C for 30 sec), and
then the dissociation curve stage was added to verify specificity
of the PCR products. The primer sequences for GAPDH were obtained
from OriGene Technologies, Inc. (cat. no. HP205798) and were used
as internal reference controls. The primer sequences for miR-132,
miR-133a and lnc-MGC were designed using the National Center for
Biotechnology Information Primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The
primer sequences used were as follows: miR-123 (accession no.
NC_000017.11) forward (F), 5'-CGACCATGGCTGTAGACTGT-3' and reverse
(R), 5'-GTCTCCAGGGCAACCGTG-3'; miR-133a (accession no.
NC_000018.10) F, 5'-TTTGGTCCCCTTCAACC-3' and R,
5'-GAACATGTCTGCGTATCTCA-3'; lnc-MGC (accession no. MW802745) F,
5'-GCTACAGCTGGTTGAAGGG-3' and R, 5'-TGCTTTGCTAGAGCTGGTAAAATG-3';
small nucleolar RNA C/D box 68 (SNORD68; accession no.
NC_000016.10) F, 5'-CGTGATGACATTCTCCGGAATC-3' and R,
5'-AATCAGATGGAAAAGGGTTCAAATG-3; and GAPDH (accession no. NM_002046)
F, 5'-GTCTCCTCTGACTTCAACAGCGC-3' and R,
5'-ACCACCCTGTTGCTGTAGCCAA-3'. SNORD68 was used as an endogenous
housekeeping gene for miR-132 and miR-133a as its expression
remains stable and does not vary under different experimental
conditions or in different states of the same sample (for example,
‘disease’ vs. ‘normal’ samples) (42-44).
The relative quantities of each target gene were measured and
standardized against the specified internal control according to
the 2-∆∆Cq method (45).
Statistical analysis
Statistical analysis was conducted using SPSS
(version 16; SPSS, Inc.). Data were presented as mean ± SEM
(46) and statistical comparisons
were carried out using a one-way analysis of variance (ANOVA) with
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. Multiple testing corrections
were carried out using the false discovery rate (Table SI). Receiver operating
characteristic (ROC) curves were generated by plotting sensitivity
against 1-specificity at different cut-off values. The diagnostic
accuracy for each cut-off point was assessed using the area under
the ROC curve (AUC). A performance level of ≥50% was deemed
acceptable.
Results
Demographic indices and clinical
characteristics of the study patients
Based on the clinical characteristics, the study
participants were grouped into a healthy control group and six
patient groups (Table I). The age
range of the study participants was 41-65 years. The mean age of
the healthy group (43 years) was significantly lower compared with
that of the patient groups (mean range, 50-63 years; P=0.001). The
sex distribution of the individuals in the healthy and patient
groups was not significantly different. Patient groups demonstrated
significantly higher obesity indices as indicated by BMI, compared
with the body weights recorded in the healthy group (P=0.001).
Furthermore, diabetes-related parameters such as systolic blood
pressure (SBP), diastolic blood pressure (DBP), HbA1c, FBS and the
activity levels of sGPT and sGOT enzymes were significantly higher
in the patient groups compared with the healthy controls
(P<0.001), particularly in patient groups G3-G5. According to
the present results, both sGPT and sGOT enzyme level in healthy and
all diabetic groups were in the range from 21-27 U/l, which is
within the normal range of sGPT (7-56 U/l) sGOT (5-40 U/l)
(https://www.medicinenet.com/liver_blood_tests/article.htm).
 | Table IClinical characteristics of healthy
controls and groups of patients with diabetes and diabetic
nephropathy. |
Table I
Clinical characteristics of healthy
controls and groups of patients with diabetes and diabetic
nephropathy.
| Patient group | Age, years (mean ±
SEM) | Male, n | Female n | BMI,
kg/m2 (mean ± SEM) | Systolic blood
pressure, mmHg (mean ± SEM) | Diastolic blood
pressure, mmHg (mean ± SEM) | Fasting blood,
mg/dl (mean ± SEM) | Glycated
hemoglobin, % (mean ± SEM) | Insulin, µl U/l
(mean ± SEM) | Serum glutamic
pyruvic aminotransferase, u/l (mean ± SEM) | Serum glutamate
oxaloacetate transaminase, u/1 (mean ± SEM) |
|---|
| Healthy
controls | 43.0±1.20 | 22 | 18 | 28.3±0.36 | 124.5±1.13 | 82.8±0.62 | 82.1±0.98 | 4.7±0.06 | 11.1±0.10 | 21.8±0.7 | 24.1±0.6 |
| G1 |
50.8±0.73a | 15 | 17 |
33.3±0.61a | 126.2±0.99 | 83.0±0.74 |
170.2±4.59a |
8.8±0.12a |
10.4±0.10a | 22.9±0.8 | 24.4±0.82 |
| G2 |
55.6±1.16a | 18 | 17 |
31.8±0.79a | 131.0±1.65 | 87.1±0.92 |
196.0±9.22a |
9.6±0.18a |
8.4±0.06a | 23.9±1.16 | 26±0.83 |
| G3a |
60.1±0.98b | 14 | 16 |
31.1±0.48a |
141.7±2.01a | 87.7±1.08 |
184.5±9.36a |
9.0±0.19a |
8.05±0.05a | 22.6±1.17 | 26.6±1.11 |
| G3b |
62.7±0.99b | 19 | 14 |
33.8±0.73a |
143.2±1.73a |
89.5±1.10a |
187.5±7.06a |
9.7±0.13a |
7.9±0.04a | 22.7±0.85 | 27±0.85 |
| G4 |
63.7±1.04b | 19 | 13 |
30.5±0.57a |
147.9±2.57b |
91.6±1.73a |
168.6±3.78a |
9.4±0.12a |
7.8±0.06b |
25.1±0.85a | 26.7±0.82 |
| G5 |
58.3±1.15a | 24 | 14 |
32.1±0.80a |
146.4±4.04b |
90.1±2.27a |
178.8±7.59a |
9.1±0.27a |
7.9±0.06a |
22.5±1.1a | 23.8±1.01 |
High blood pressure was observed in the patients of
G1-G5 with values of 124-146 mmHg for SBP and 82-90 mmHg for DBP.
Moreover, there were significant increases in both FBS and HbA1c
levels in the diabetic groups compared with the healthy group.
Hyperglycemia-induced kidney
failure
Kidney function-related parameters including urine,
creatinine, urea, uric acid and mineral (Na+,
K+ and Ca2+) levels in serum, as well as GFR,
were measured (Table II). There
was a significant increase in the concentrations of urea, uric
acid, and creatinine in the patient urine in diabetic groups
(G1-G5) compared with the healthy group (P=0.001). These increases
were more pronounced in the diabetic groups G3b-5, where the
concentration of creatinine increased by 78, 233 and 711%
(P=0.001), respectively, compared with the healthy group. In G2
diabetic group, there was also a significant increase in serum
Na+, also known as hypernatremia, of 5.8% (P=0.001),
compared with the healthy group. This indicated the presence of a
common type of electrolyte abnormality due to osmotic
diuresis-induced hypotonic losses increasing serum Na+
levels (47). Similar to the
observed increase in Na+ levels, the level of serum
K+ was increased, and these increases were positively
correlated with the increase in diabetic indices. There was a
steady increase in the concentration across different diabetic
groups, recording high increased values for the G5 group (29.5%)
for K+ levels compared with the healthy group. On the
other hand, Ca2+ levels and GFR, a sensitive indicator
of kidney function (a low GFR value indicates that the kidneys are
not functioning properly), were significantly decreased (P=0.001)
compared with healthy controls. The G5 group demonstrated a lower
level of Ca2+ (7.9±0.14) compared with the normal serum
Ca2+ level of the healthy control group (9.3±0.07) and
the other diabetic groups (G1-G4) (8.9-9.1 mg/dl). In contrast to
the G1 group, there was a significant decrease in GFR across the
other diabetic groups and the lowest value was recorded in the G5
group (7.9 ml/min/1.73 m2).
 | Table IIKidney function tests of healthy
controls and groups of patients with diabetes and diabetic
nephropathy. |
Table II
Kidney function tests of healthy
controls and groups of patients with diabetes and diabetic
nephropathy.
| Patient group | Creatinine,
mg/dl | Urea, mg/dl | Uric acid,
mg/dl | Na+,
mEq/l | K+,
mEq/l | Ca2+,
mg/dl | Estimated
glomerular filtration rate, ml/min/1.73 m2 |
|---|
| Healthy
controls | 0.9± 0.01 | 22.9±0.55 | 4.31±0.08 | 140.4±0.76 | 4.4±0.07 | 9.3±0.07 | 93.0±0.97 |
| G1 | 0.94±0.02 | 24.7±0.70 |
4.95±0.13a | 144.2±0.93 | 4.4±0.08 | 9.1±0.09 | 93.1±0.44 |
| G2 | 1.0±0.03 | 28.0±0.76 |
5.39±0.13a | 144.3±0.91 | 4.4±0.09 | 8.9±0.11 |
69.5±0.96a |
| G3a | 1.2±0.03 |
36.3±1.29a |
5.88±0.14b |
147.7±1.06a | 4.5±0.09 |
8.6±0.12a |
52.1±0.83a |
| G3b |
1.6±0.05a |
51.3±2.17b |
6.30±0.16b |
148.6±1.46a | 4.6±0.08 | 8.9±0.11 |
39.7±0.72b |
| G4 |
3.0±0.09b |
90.5±2.53c |
6.31±0.17b |
148.1±1.83a |
6.11±0.06a | 8.9±0.12 |
20.2±0.47b |
| G5 |
7.3±0.38c |
114.2±4.43c |
6.51±0.18b |
134.3±0.85b |
5.7±0.13a |
7.9±0.14a |
7.9±0.45c |
Hyperglycemia increased the risk of
heart failure in the diabetic group
To investigate hyperglycemia-induced cardiac injury,
the circulating LDH and troponin levels and CK and CK-MB enzyme
activity were measured (Fig. 1).
Compared with the healthy control group, the diabetic patients,
mainly G5, demonstrated a marked increase in the majority of
measured cardiac-related parameters. The mean serum LDH cholesterol
(P<0.0001) and troponin (P<0.01) levels of diabetic patients
in the G5 group, were higher than those compared with the healthy
control group, respectively (Fig.
1A and B). Overall, a positive
correlation was demonstrated between LDH and HbA1C levels (r=0.48;
Table III). The mean serum CK
and CK-MB levels were also significantly higher in all diabetic
groups (G5) compared with the healthy control group (P<0.0001
and P<0.001, respectively) (Fig.
1C and D). For all the
aforementioned parameters, there was an increase demonstrated
across the diabetic groups, with the highest levels of CK and CK-MB
in the serum of diabetic patients in the G4 and G5 groups. Overall,
patients with increased levels of LDH, troponin, CK and CK-MB were
potentially associated with the risk of heart failure in the
diabetic groups.
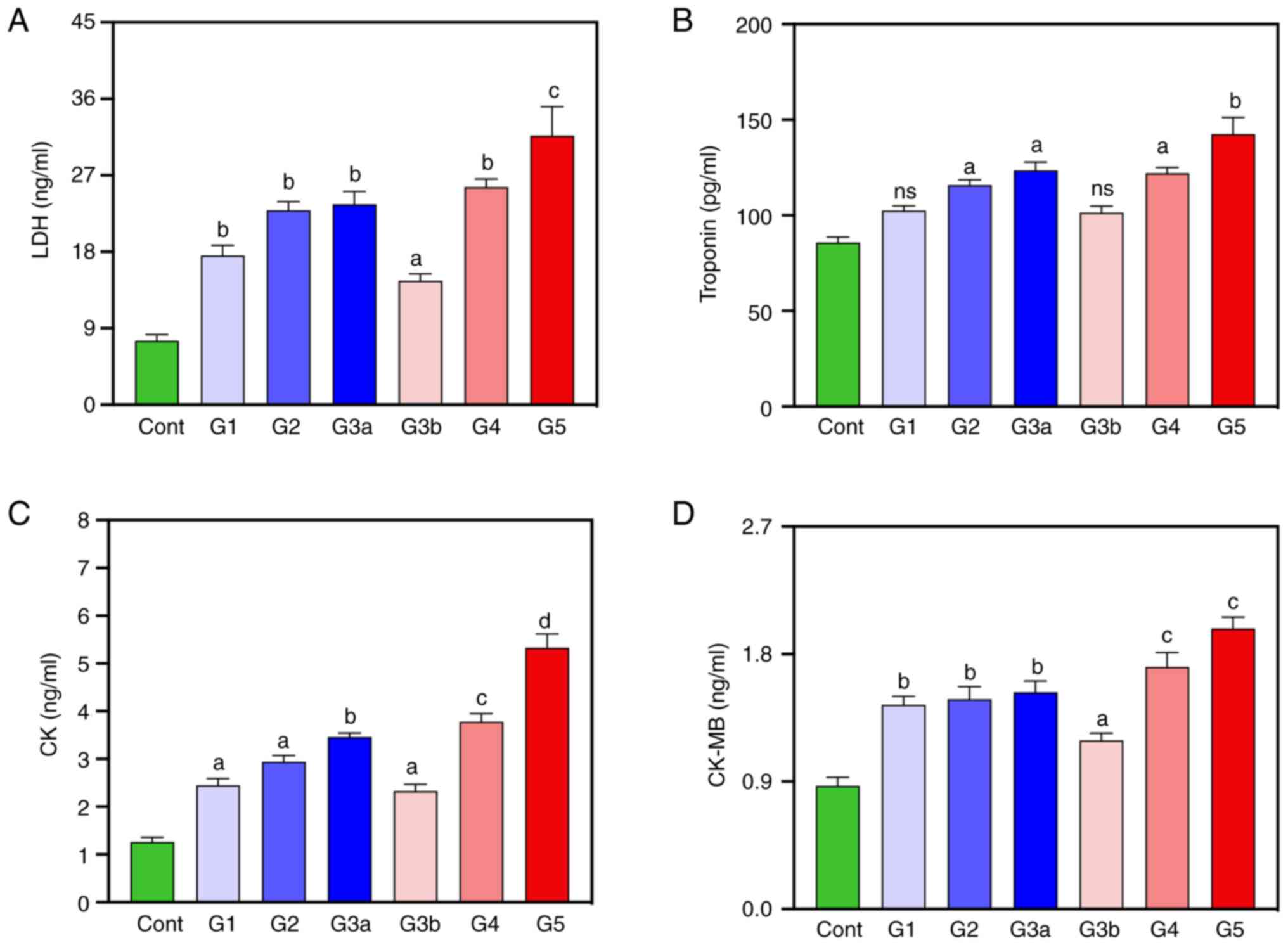 | Figure 1Cardiac profile of healthy controls
and diabetic patients. (A) LDH, (B) troponin, (C) CK and (D) CK-MB
levels. Data are expressed as mean ± SEM. (not significant),
aP<0.05, bP<0.01,
cP<0.001, dP<0.0001 vs. healthy
controls. LDH, lactate dehydrogenase; CK, creatine kinase, CK-MB,
CK-myocardial band; Cont, healthy controls; G1, diabetes without
kidney neuropathy; G2, diabetes with mild renal impairment;
G3a-diabetes with severe renal impairment, G3b, diabetes with
severe renal impairment and mild cardiovascular disease; G4
diabetes with severe renal impairment and moderate CVD; G4 diabetes
with severe renal impairment and severe cardiovascular disease. |
 | Table IIICorrelation analysis between
biochemical and molecular diabetics and molecular indicators of
diabetes and CVD of healthy individuals and groups of patients with
diabetes. |
Table III
Correlation analysis between
biochemical and molecular diabetics and molecular indicators of
diabetes and CVD of healthy individuals and groups of patients with
diabetes.
| A, HbA1C |
|---|
| Correlation
analysis | HbA1C | LDH | miR-132 | miR-133a | lnc-MGC |
|---|
| r | 1.00 | 480.00b | 0.31b | -0.42b |
-459.00a |
| Significant
(2-tailed) | N/A | 0 | 0 | 0 | 0 |
| N | 238 | 70 | 179 | 171 | 177 |
| B, estimated
glomerular filtration rate |
| Correlation
analysis | HbA1C | LDH | miR-132 | miR-133a | lnc-MGC |
| r | -0.51a | -0.61a | -0.76a | 0.81b | 0.79a |
| Sig.
(2-tailed) | 0 | 0 | 0 | 0 | 0 |
| N | 238 | 70 | 179 | 171 | 177 |
| C, LDH |
| Correlation
analysis | HbA1C | LDH | miR-132 | miR-133a | lnc-MGC |
| r |
0.480* | 1 | 0.618 | -0.531a | -0.564a |
| Sig.
(2-tailed) | 0 | N/A | 0 | 0 | 0 |
| N | 70 | 70 | 70 | 69 | 70 |
| D, miR-132 |
| Correlation
analysis | HbA1C | LDH | miR-132 | miR-133a | lnc-MGC |
| r | 0.316a | 618 | 1 |
-0.576* | -0.624a |
| Sig.
(2-tailed) | 0 | 0 | N/A | 0 | 0 |
| N | 179 | 70 | 179 | 169 | 175 |
| E, miR-133a |
| Correlation
analysis | HbA1C | LDH | miR-132 | miR-133a | lnc-MGC |
| r | -571b | -0.551a | -620b | 1 | 0.643a |
| Sig.
(2-tailed) | 0 | 0 | 0 | N/A | 0 |
| N | 171 | 69 | 169 | 171 | 171 |
| F, lnc-MGC |
| Correlation
analysis | HbA1C | LDH | miR-132 | miR-133a | lnc-MGC |
| r | -0.459 | -0.529 | -0.631 | 743b | 1 |
| Sig.
(2-tailed) | 0 | 0 | 0 | 0 | N/A |
| N | 177 | 70 | 175 | 171 | 177 |
Inflammatory cytokine biomarkers
TNF-α and Nrf2 levels were measured as they are
cytokine biomarkers of inflammation (Fig. 2). These results demonstrated that
the levels of TNF-α were significantly increased in G2-G5 groups
compared with the healthy controls, with the G3a, G4 and G5 groups
demonstrating the highest levels of TNF-α. Nrf2 levels were
significantly reduced in all diabetic groups compared with the
control group, with the lowest values demonstrated in the G4 and G5
groups (-66.6 and -73.33%, respectively).
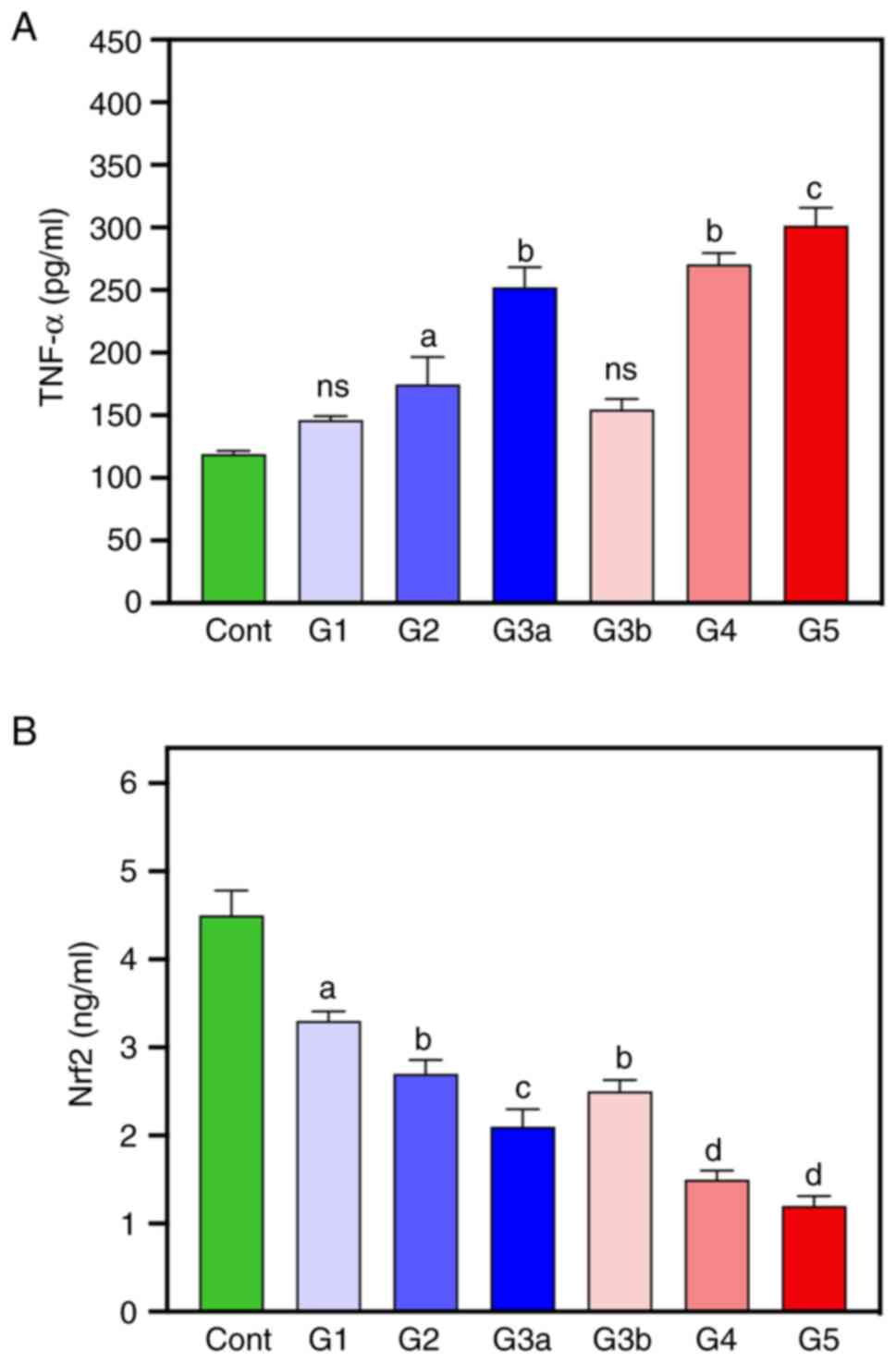 | Figure 2Inflammatory cytokine biomarkers were
measured in healthy controls and diabetic patients. (A) ‘TNF-α’ and
(B) Nrf2 levels were measured in healthy controls and diabetic
groups. Data are expressed as mean ± SEM. ns (not significant),
aP<0.05, bP<0.01,
cP<0.001, dP<0.0001 vs. healthy
controls. Nrf2, nuclear factor erythroid 2-related factor 2; Cont,
healthy controls; G1, diabetes without kidney neuropathy; G2,
diabetes with mild renal impairment; G3a-diabetes with severe renal
impairment, G3b, diabetes with severe renal impairment and mild
cardiovascular disease; G4 diabetes with severe renal impairment
and moderate cardiovascular disease; G4 diabetes with severe renal
impairment and severe cardiovascular disease. |
Expression patterns of ncRNAs as
pre-diagnostic molecular biomarkers for CVD
To investigate the molecular biomarkers for CVD,
expression levels of the ncRNAs miR-132, miR-133a and lnc-MGC were
measured using quantitative RT-qPCR (Fig. 3A-C). Lower expression levels of
miR-132 in blood were demonstrated in diabetic patients compared
with healthy controls (Fig. 3A).
Serum miR-132 expression was steadily decreased across diabetic
groups and the lowest expression levels were demonstrated in the G4
and G5 groups of patients (-83.33 and -91.67%, respectively).
Compared with the healthy controls, the expression levels of
circulating miR-133a were significantly higher in diabetic patients
of G2-G5 who showed impaired cardiovascular function (increased LDH
and troponin I levels and CK and CK-MB enzyme activity) (Fig. 3B). The expression levels of serum
miR-133a in diabetic patients steadily increased across diabetic
groups and reached the highest expression level in patients in the
G5 group (Fig. 3B). There was an
increase in the expression levels of lnc-MGC in diabetic patients
compared with healthy controls, with the maximum lnc-MGC expression
level recorded in the G5 group (729.29%) (Fig. 3C). The expression level of miR-133a
(r=0.326; P<0.01) was negatively correlated with the levels of
LDH and HbA1C (Table III).
However, serum miR-132 was positively correlated with HbA1C
(r=0.382; P<0.01).
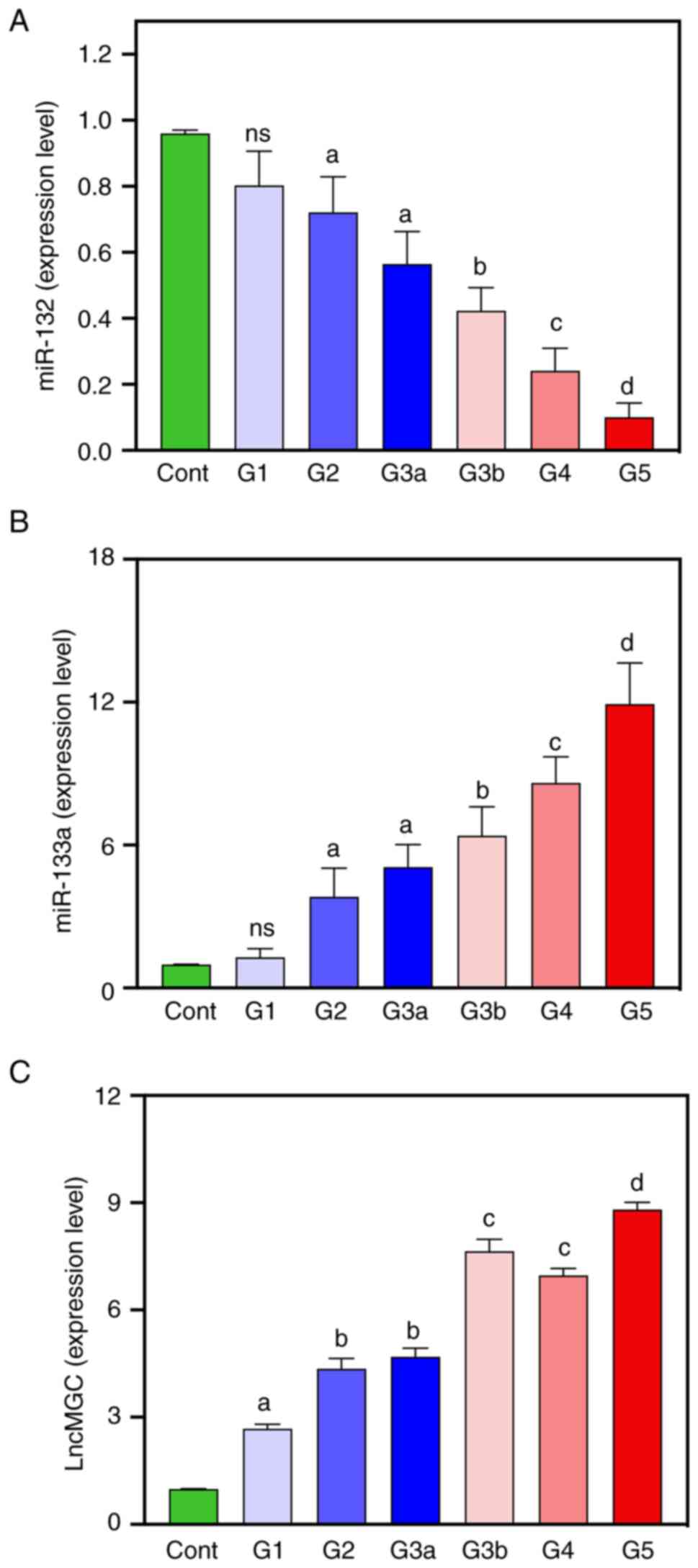 | Figure 3Measurement of non-coding RNA
expression levels in healthy controls and diabetic patients as
prediagnostic molecular biomarkers for human cardiovascular
disease. Expression levels of (A) miR-132, (B) miR-133a and (C)
Inc-MGC. Statistical analysis was performed using one-way ANOVA
followed by Tukey's post-hoc test to compare the significance among
all diabetic groups (G1-G5) vs. healthy controls. Data are
expressed as mean ± SEM. aP<0.05,
bP<0.01, cP<0.001,
dP<0.0001 vs. healthy controls. miR, microRNA;
lnc-MGC, long non-coding RNA megacluster; cont, healthy controls;
G1, diabetes without kidney neuropathy; G2, diabetes with mild
renal impairment; G3a-diabetes with severe renal impairment, G3b,
diabetes with severe renal impairment and mild cardiovascular
disease; G4 diabetes with severe renal impairment and moderate
cardiovascular disease; G4 diabetes with severe renal impairment
and severe cardiovascular disease. |
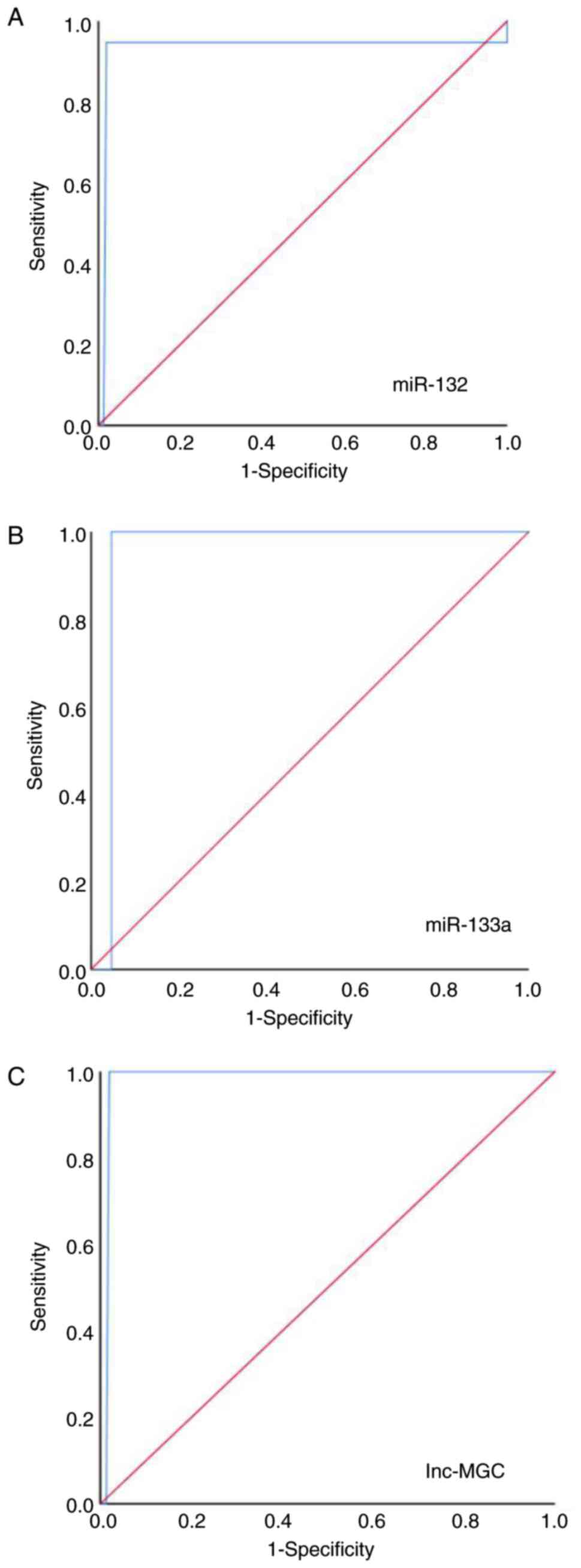
Given the upregulated expression levels of miR-132,
miR-133a and lnc-MGC in diabetic patients, particularly those of
G3-G5, their role as potential prognostic markers was investigated
using ROC curves. Serum miR-132 was differentially expressed in
participants with DN compared with healthy controls, with a 93.20%
sensitivity and a 100.00% specificity (AUC=0.93; 95% CI=0.886-0.95;
P<0.001; Fig. 4A). Similarly,
serum miR-133a expression levels also differentiated the patients
of G2-G5 with DN from healthy controls, with 100.00% sensitivity
and 100.00% specificity (AUC=0.998; 95% CI=0.993-1.01; P<0.001;
Fig. 4B), thus suggesting its
potential utility as a biomarker of CVD. Lnc-MGC was differentially
expressed in participants with DN compared with healthy controls,
with a 97.20% sensitivity and a 100.00% specificity (AUC=0.951; 95%
CI=0.891-0.954; P<0.001; Fig.
4C). In addition, serum miR-132 expression levels were shown to
differentiate the of G3b, G4 and G5 stages of DN from the G2 and
G3a stages of DN with 43.90% sensitivity and a 94.80% specificity
(AUC=0.74; P<0.001; Fig. 5A).
Similarly, serum miR-133a and lnc-MGC expression level also showed
similar results (Fig. 5B-C).
Discussion
Patients with type 2 diabetes are prone to
developing heart conditions. Chronic hyperglycemia, as observed in
diabetics, can induce DN and potentially lead to chronic kidney and
heart failure (48). While several
biomarkers are currently available for monitoring DN and CVD, their
efficiency is limited by issues of specificity, sensitivity and
validation (49). Thus, the
effective diagnosis and early treatment of DN and CVDs requires the
identification of more sensitive molecular markers. In the present
study, it was demonstrated that serum expression levels of
miR-133a, miR-132 and lnc-MGC could potentially be used to detect
DN and CVD progression in DM patients with a high sensitivity.
In the present study, HbA1c, creatinine, urea, LDH,
troponin I, CK and CK-MB levels increased with GFR used as a
measure of CVD severity. In line with the findings of the present
study, the aforementioned cardiac biomarker levels were previously
reported to be significantly higher in diabetic individuals
compared with healthy individuals (50,51),
which can lead to impaired cardiac function (52-54).
Conversely, fasting insulin, Ca2+ and eGFR levels
decreased with DN severity. Fasting insulin and eGFR levels were
lower in all diabetic groups compared with the healthy group. A
decrease in GFR has been linked to increased renal damage and
reduced kidney function (55). In
diabetic patients, glucose loss through the kidneys induces
hyperosmotic urination that results in water and electrolyte loss
(56). Similar to the results
reported in the present study, the clinical presentation of DN has
been reported to include albuminuria, hyperkalemia, declining GFR
and high creatinine levels (2).
When acute myocardial infarction occurs, the inflammatory reaction
leads to the increase of serum inflammatory cytokine levels, such
as TNF-α and IL-1β (57).
Increased TNF-α levels can activate inflammatory signaling
pathways, contributing to complications of DM (58). The present study demonstrated that
diabetic patient groups G3-G5 exhibited significantly higher TNF-α
levels compared with the healthy group, with a marked variation in
TNF-α levels between the late and early stages of DN. A previous
study reported that patients with DM-induced chronic kidney disease
showed higher serum TNF-α levels (59). However, miR-133a may protect
myocardial cells by downregulating expression of the inflammatory
factors TNF-α and IL-6, which reduces the inflammatory response
(60). miR-132 can also target
Nrf2(25) and mediate genes
involved in TGF-β signaling and cell proliferation (26).
miRNAs serve a critical role in cardiovascular
diseases such as arrhythmias, hypertrophy, heart failure and
atherosclerosis (61). The present
study demonstrated a negative correlation between the development
of CVD and the expression levels of miR-132 and lnc-MGC, in
addition to the decreased expression of miR-133a. Both miR-132 and
miR-133 expression levels are increased in type 2 DM and CAD
(62). In addition, the potential
role of miR-132 and miR-133a in vascular pathologies has been
previously reported, as they were shown to modulate endothelial
cell function and angiogenesis (63,64).
Anti-miR-132 treatment improves cardiac function post-myocardial
infarction and reverses heart failure (27). miR-132 induces inhibition of PTEN
expression, activating the PI3K/Akt signal transduction pathway.
This mechanism facilitates cardiocyte proliferation and reduces
apoptosis and cardiac fibrosis (65). Treatment with miR-132 agomiR
suppresses the increase in collagen levels, TGF-β and α-smooth
muscle actin expression, which are elevated in the hearts of rats
with myocardial infarction-induced heart failure (66). Conversely, miR-133a exhibits
cardioprotective properties and can maintain lower LDH levels
(67) compared with those in
patients with diabetes (G2-G5). Moreover, miR-133a is positively
correlated with CK, CK-MB and cardiac troponin T (68,69).
miR-133a reduces cardiac hypertrophy in streptozotocin-induced
diabetic mice by inhibiting glucose-induced upregulation of
insulin-like growth factor-1 receptor (IGF1R) and serum- and
glucocorticoid-regulated kinase 1 (SGK1) (70). A previous study by Kambis et
al (71) reported that
overexpression of miR-133a in genetically modified mice prevented
DM-induced cardiac fibrosis and hypertrophy, which conferred
cardioprotective effects. miR-133a also mediates signaling of
myocyte enhancer factor 2C in diabetic cardiomyopathy, which is an
essential transcription factor underlying myocardial hypertrophy
and cardiac fibrosis (72).
lnc-MGC upregulation in human cardiac fibroblasts under mechanical
stress is associated with miR-133a downregulation (36). Lnc-MGC is upregulated in mouse
models of DN or mesangial cells treated with TGF-β1 or high glucose
(34).
In conclusion, ROC analysis results demonstrated
that serum miR-133a, miR-132 and lnc-MGC levels could potentially
be used to differentiate between DN and CVD cases from healthy
controls. Serum miR-133a exhibited high sensitivity and specificity
values of 99.32 and 100%, respectively, which could suggest that
miR-133a serum expression in DN patients may serve as a promising
circulating biomarker for detecting and monitoring the progression
of diabetes at the early stages of disease.
Overall, the aforementioned biomarkers could
potentially assist in predicting the progression of associated
complications. Although a correlation between the development of
CVD and the expression levels of miR-132, miR-133a and lnc-MGC was
demonstrated, the present study has limitations, such as the lack
of functional experiments. To address this limitation, in
vivo studies using genetically modified mouse models, such as
miR-132 or miR-133a knockouts or overexpression systems, could be
used to elucidate the roles of these miRNAs in cardiovascular
health. In vitro experiments with cardiomyocyte and
endothelial cell cultures could be used to assess the impact of
miR-132, miR-133a and lnc-MGC on proliferation, apoptosis and
fibrosis. Knockdown or overexpression experiments should be used to
investigate how lnc-MGC affects miR-133a levels and contributes to
cardiac fibrosis and hypertrophy. Luciferase assays and
CRISPR/Cas9-mediated gene editing should be used to identify target
genes, such as PTEN (miR-132), IGF1R and SGK1 (miR-133a), and
determine their molecular mechanisms of action. The development of
miRNA-based therapies, such as miR-132 inhibitors or miR-133a
mimics, and the evaluation of these therapies in preclinical and
clinical trials could potentially be used for the development of
treatments for cardiac fibrosis and other cardiovascular
complications in diabetes and related health conditions. Finally,
further large-scale studies and clinical trials are required, and
the functional impacts of the aforementioned markers on putative
target genes and pathways should be thoroughly evaluated.
Supplementary Material
P-values and false discover rates of
the comparisons between healthy individuals and groups of patients
with diabetes.
Acknowledgements
The authors extend their appreciation to the
Researchers Supporting Project number (grant no. RSP2024R376) King
Saud University, Riyadh, Saud Arabia.
Funding
Funding: The present study was funded by King Saud University
(grant no. RSP2024R376; Riyadh, Saudi Arabia).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GA, AA-M and NAH designed the study. GA, NAH, AA-M,
MYZ and AM performed the experiments. AM and NAH provided reagents
and analytic tools. GA, AM, AH and MYZ analyzed the data. GA and
NAH wrote the manuscript. All authors read and approved the final
version of the manuscript and agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. GA and NAH confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
This study was conducted in compliance with the
Declaration of Helsinki and ethical approval was provided by the
Ethics Committee of Beni-Suef University Hospital (approval no.
BSU:7-2021). The patients were provided with information about the
nature and goals of the study, signed an informed consent form and
agreed to the use of their samples in scientific research. Staff
members of Beni-Suef University are permitted to obtain ethical
approval through the University, which encompasses the University
Hospital as part of its broader institution.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnson RJ, Bakris GL, Borghi C, Chonchol
MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez
Lozada LG, et al: Hyperuricemia, acute and chronic kidney disease,
hypertension, and cardiovascular disease: Report of a scientific
workshop organized by the National Kidney Foundation. Am J Kidney
Dis. 71:851–865. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes: Standards of medical care
in diabetes-2020. Diabetes Care. 43 (Suppl 1):S14–S31.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jia G, Hill MA and Sowers JR: Diabetic
cardiomyopathy: An update of mechanisms contributing to this
clinical entity. Circ Res. 122:624–638. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kiss A, Arnold Z, Aykac I, Fee AJ,
Hallström S, Balogh F, Szekeres M, Szabo PL, Nagel F, Hamdani N, et
al: Tenascin C deficiency attenuates cardiac dysfunction,
endothelial dysfunction and fibrosis in diabetic cardiomyopathy
mice. J Mol Cell Cardiol. 173 (Suppl)(S99)2022.
|
|
5
|
Cheng YJ, Kanaya AM, Araneta MRG, Saydah
SH, Kahn HS, Gregg EW, Fujimoto WY and Imperatore G: Prevalence of
diabetes by race and ethnicity in the United States, 2011-2016.
JAMA. 322:2389–2398. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Frąk W, Wojtasińska A, Lisińska W,
Młynarska E, Franczyk B and Rysz J: Pathophysiology of
cardiovascular diseases: New insights into molecular mechanisms of
atherosclerosis, arterial hypertension, and coronary artery
disease. Biomedicines. 10(1938)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Weiner DE, Tighiouart H, Amin MG, Stark
PC, MacLeod B, Griffith JL, Salem DN, Levey AS and Sarnak MJ:
Chronic kidney disease as a risk factor for cardiovascular disease
and all-cause mortality: A pooled analysis of community-based
studies. J Am Soc Nephrol. 15:1307–1315. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ford I, Bezlyak V, Stott DJ, Sattar N,
Packard CJ, Perry I, Buckley BM, Jukema JW, de Craen AJ, Westendorp
RG and Shepherd J: Reduced glomerular filtration rate and its
association with clinical outcome in older patients at risk of
vascular events: Secondary analysis. PLoS Med.
6(e16)2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Somani YB, Uthman L, Aengevaeren VL,
Rodwell L, Lip GYH, Hopman MTE, Van Royen N, Eijsvogels TMH and
Thijssen DHJ: Exercise-induced release of cardiac troponin is
attenuated with repeated bouts of exercise: Impact of
cardiovascular disease and risk factors. Am J Physiol Heart Circ
Physiol. 324:H519–H524. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guo H, Wang C, Jiang B, Ge S, Cai J, Zhou
Y, Ying R, Zha K, Zhou J, Wang N, et al: Association of insulin
resistance and β-cell function with bone turnover biomarkers in
dysglycemia patients. Front Endocrinol (Lausanne).
12(554604)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Negeem Z, Moneim AA, Mahmoud B, Ahmed AE,
Abd El-Hameed AM, Eskandrani AA and Hasona NA: The implication of
miR-200a and miR-132 expression and their correlations with
NF-κB/TNF-alpha signaling in adults with diabetic nephropathy.
Saudi J Biol Sci. 31(103975)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mikuda N, Kolesnichenko M, Beaudette P,
Popp O, Uyar B, Sun W, Tufan AB, Perder B, Akalin A, Chen W, et al:
The IκB kinase complex is a regulator of mRNA stability. EMBO J.
37(e98658)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Battineni G, Sagaro GG, Chintalapudi N,
Amenta F, Tomassoni D and Tayebati SK: Impact of obesity-induced
inflammation on cardiovascular diseases (CVD). Int J Mol Sci.
22(4798)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pina T, Corrales A, Lopez-Mejias R,
Armesto S, Gonzalez-Lopez MA, Gómez-Acebo I, Ubilla B,
Remuzgo-Martínez S, Gonzalez-Vela MC, Blanco R, et al: Anti-tumor
necrosis factor-alpha therapy improves endothelial function and
arterial stiffness in patients with moderate to severe psoriasis: A
6-month prospective study. J Dermatol. 43:1267–1272.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim J, Cha YN and Surh YJ: A protective
role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in
inflammatory disorders. Mutat Res. 690:12–23. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hutyra M, Paleček T and Hromádka M: The
use of echocardiography in acute cardiovascular care. Summary of
the document prepared by the Czech society of cardiology. Cor et
Vasa. 60:e70–e88. 2018.
|
|
17
|
Paolillo S, Marsico F, Prastaro M, Renga
F, Esposito L, De Martino F, Di Napoli P, Esposito I, Ambrosio A,
Ianniruberto M, et al: Diabetic cardiomyopathy: Definition,
diagnosis, and therapeutic implications. Heart Fail Clin.
15:341–347. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cook CH, Praba AC, Beery PR and Martin LC:
Transthoracic echocardiography is not cost-effective in critically
ill surgical patients. J Trauma. 52:280–284. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tousoulis D, Papageorgiou N, Androulakis
E, Siasos G, Latsios G, Tentolouris K and Stefanadis C: Diabetes
mellitus-associated vascular impairment: Novel circulating
biomarkers and therapeutic approaches. J Am Coll Cardiol.
62:667–676. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Park EG, Ha H, Lee DH, Kim WR, Lee YJ, Bae
WH and Kim AH: Genomic analyses of non-coding RNAs overlapping
transposable elements and its implication to human diseases. Int J
Mol Sci. 23(8950)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dave VP, Ngo TA, Pernestig A-K, Tilevik D,
Kant K, Nguyen T, Wolff A and Bang DD: MicroRNA amplification and
detection technologies: Opportunities and challenges for point of
care diagnostics. Lab Invest. 99:452–469. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lekka E and Hall J: Noncoding RNA s in
disease. FEBS Lett. 592:2884–2900. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Soni DK and Biswas R: Role of non-coding
RNAs in post-transcriptional regulation of lung diseases. Front
Genet. 12(767348)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shi L, Zhang R, Li T, Han X, Yuan N, Jiang
L, Zhou H and Xu S: Decreased miR-132 plays a crucial role in
diabetic encephalopathy by regulating the GSK-3β/Tau pathway. Aging
(Albany NY). 13:4590–4604. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Civantos E, Bosch E, Ramirez E, Zhenyukh
O, Egido J, Lorenzo O and Mas S: Sitagliptin ameliorates oxidative
stress in experimental diabetic nephropathy by diminishing the
miR-200a/Keap-1/Nrf2 antioxidant pathway. Diabetes Metab Syndr
Obes. 10:207–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bijkerk R, de Bruin RG, van Solingen C,
van Gils JM, Duijs JMGJ, van der Veer EP, Rabelink TJ, Humphreys BD
and van Zonneveld AJ: Silencing of microRNA-132 reduces renal
fibrosis by selectively inhibiting myofibroblast proliferation.
Kidney Int. 89:1268–1280. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Foinquinos A, Batkai S, Genschel C,
Viereck J, Rump S, Gyöngyösi M, Traxler D, Riesenhuber M,
Spannbauer A, Lukovic D, et al: Preclinical development of a
miR-132 inhibitor for heart failure treatment. Nat Commun.
11(633)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ning F, Qiao Q, Tuomilehto J, Hammar N, Ho
S, Söderberg S, Zimmet PZ, Shaw JE, Nakagami T, Mohan V, et al:
Does abnormal insulin action or insulin secretion explain the
increase in prevalence of impaired glucose metabolism with age in
populations of different ethnicities? Diabetes Metab Res Rev.
26:245–253. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kumarswamy R and Thum T: Non-coding RNAs
in cardiac remodeling and heart failure. Circ Res. 113:676–689.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Matkovich SJ, Wang W, Tu Y, Eschenbacher
WH, Dorn LE, Condorelli G, Diwan A, Nerbonne JM and Dorn GW II:
MicroRNA-133a protects against myocardial fibrosis and modulates
electrical repolarization without affecting hypertrophy in
pressure-overloaded adult hearts. Circ Res. 106:166–175.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nandi SS, Duryee MJ, Shahshahan HR, Thiele
GM, Anderson DR and Mishra PK: Induction of autophagy markers is
associated with attenuation of miR-133a in diabetic heart failure
patients undergoing mechanical unloading. Am J Transl Res.
7:683–696. 2015.PubMed/NCBI
|
|
32
|
Krenz M and Robbins J: Impact of
beta-myosin heavy chain expression on cardiac function during
stress. J Am Coll Cardiol. 44:2390–2397. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Barrera-Chimal J and Jaisser F:
Pathophysiologic mechanisms in diabetic kidney disease: A focus on
current and future therapeutic targets. Diabetes Obes Metab. 22
(Suppl 1):S16–S31. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kato M, Wang M, Chen Z, Bhatt K, Oh HJ,
Lanting L, Deshpande S, Jia Y, Lai JY, O'Connor CL, et al: An
endoplasmic reticulum stress-regulated lncRNA hosting a microRNA
megacluster induces early features of diabetic nephropathy. Nat
Commun. 7(12864)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Allison SJ: Diabetic nephropathy: A lncRNA
and miRNA megacluster in diabetic nephropathy. Nat Rev Nephrol.
12(713)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhu Y, Liu C and Hallajzadeh J:
Understanding the roles of non-coding RNAs and exosomal non-coding
RNAs in diabetic nephropathy. Curr Mol Med: Apr 5, 2024 (Epub ahead
of print).
|
|
37
|
Lan H, Lu H, Wang X and Jin H: MicroRNAs
as potential biomarkers in cancer: Opportunities and challenges.
Biomed Res Int. 2015(125094)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Solier C and Langen H: Antibody-based
proteomics and biomarker research-current status and limitations.
Proteomics. 14:774–783. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Levey AS, Bosch JP, Lewis JB, Greene T,
Rogers N and Roth D: A more accurate method to estimate glomerular
filtration rate from serum creatinine: A new prediction equation.
Modification of diet in renal disease study group. Ann Intern Med.
130:461–470. 1999.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Skiba JH, Bansal AD, Peck Palmer OM and
Johnstone DB: Case report: Clinical consequences of adjusting
estimated GFR for black race. Gen Intern Med. 37:958–961.
2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Inker LA, Eneanya ND, Coresh J, Tighiouart
H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et
al: New creatinine- and cystatin C-based equations to estimate GFR
without race. N Engl J Med. 385:1737–1749. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ayeldeen G, Nassar Y, Ahmed H, Shaker O
and Gheita T: Possible use of miRNAs-146a and -499 expression and
their polymorphisms as diagnostic markers for rheumatoid arthritis.
Mol Cell Biochem. 449:145–156. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shaker OG, Abdelaleem OO, Mahmoud RH,
Abdelghaffar NK, Ahmed TI, Said OM and Zaki OM: Diagnostic and
prognostic role of serum miR-20b, miR-17-3p, HOTAIR, and MALAT1 in
diabetic retinopathy. IUBMB Life. 71:310–320. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pratama MY, Cavalletto L, Tiribelli C,
Chemello L and Pascut D: Selection and validation of miR-1280 as a
suitable endogenous normalizer for qRT-PCR analysis of serum
microRNA expression in hepatocellular carcinoma. Sci Rep.
10(3128)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kovesdy CP: Management of hyperkalaemia in
chronic kidney disease. Nat Rev Nephrol. 10:653–662.
2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liamis G, Liberopoulos E, Barkas F and
Elisaf M: Diabetes mellitus and electrolyte disorders. World J Clin
Cases. 2:488–496. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shu A, Du Q, Chen J, Gao Y, Zhu Y, Lv G,
Lu J, Chen Y and Xu H: Catalpol ameliorates endothelial dysfunction
and inflammation in diabetic nephropathy via suppression of
RAGE/RhoA/ROCK signaling pathway. Chem Biol Interact.
348(109625)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Davis KD, Aghaeepour N, Ahn AH, Angst MS,
Borsook D, Brenton A, Burczynski ME, Crean C, Edwards R,
Gaudilliere B, et al: Discovery and validation of biomarkers to aid
the development of safe and effective pain therapeutics: Challenges
and opportunities. Nat Rev Neurol. 16:381–400. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chen C, Lin X, Lin R, Huang H and Lu F: A
high serum creatine kinase (CK)-MB-to-total-CK ratio in patients
with pancreatic cancer: A novel application of a traditional marker
in predicting malignancy of pancreatic masses? World J Surg Oncol.
21(13)2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hsieh YS, Yeh MC, Lin YY, Weng SF, Hsu CH,
Huang CL, Lin YP and Han AY: Is the level of serum lactate
dehydrogenase a potential biomarker for glucose monitoring with
type 2 diabetes mellitus? Front Endocrinol (Lausanne).
13(1099805)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Aydogdu U, Yildiz R, Guzelbektes H, Coskun
A and Sen I: Cardiac biomarkers in premature calves with
respiratory distress syndrome. Acta Vet Hung. 64:38–46.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Apak I, Iltumur K, Taman Y and Kaya N:
Serum cardiac troponin T levels as an indicator of myocardial
injury in ischemic and hemorrhagic stroke patients. Tohoku J Exp
Med. 205:93–101. 2005.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Thygesen K, Alpert JS, Jaffe AS, Chaitman
BR, Bax JJ, Morrow DA and White HD: Executive Group on behalf of
the Joint European Society of Cardiology (ESC)/American College of
Cardiology (ACC)/American Heart Association (AHA)/World Heart
Federation (WHF) Task Force for the Universal Definition of
Myocardial Infarction. Fourth universal definition of myocardial
infarction (2018). Circulation. 138:e618–e651. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tervaert TWC, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer
E, et al: Pathologic classification of diabetic nephropathy. J Am
Soc Nephrol. 21:556–563. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ozougwu J, Obimba K, Belonwu C and
Unakalamba C: The pathogenesis and pathophysiology of type 1 and
type 2 diabetes mellitus. J Physiol Pathophysiol. 4:46–57.
2013.
|
|
57
|
Yaraee R, Ghazanfari T, Ebtekar M,
Ardestani SK, Rezaei A, Kariminia A, Faghihzadeh S, Mostafaie A,
Vaez-Mahdavi MR, Mahmoudi M, et al: Alterations in serum levels of
inflammatory cytokines (TNF, IL-1alpha, IL-1beta and IL-1Ra) 20
years after sulfur mustard exposure: Sardasht-Iran cohort study.
Int Immunopharmacol. 9:1466–1470. 2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Pickup JC and Crook MA: Is type II
diabetes mellitus a disease of the innate immune system?
Diabetologia. 41:1241–1248. 1998.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Abou-Elela DH, Emara MM, Abo El-Khair NT,
El-Edel RH and Fathy WM: Role of tumor necrosis factor alpha in
type 2 diabetic nephropathy. Menoufia Med J. 33:920–925. 2020.
|
|
60
|
Wang Y, Li L, Moore BT, Peng XH, Fang X,
Lappe JM, Recker RR and Xiao P: MiR-133a in human circulating
monocytes: A potential biomarker associated with postmenopausal
osteoporosis. PLoS One. 7(e34641)2012.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Small EM and Olson EN: Pervasive roles of
microRNAs in cardiovascular biology. Nature. 469:336–342.
2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
De Rosa S, Arcidiacono B, Chiefari E,
Brunetti A, Indolfi C and Foti DP: Type 2 diabetes mellitus and
cardiovascular disease: Genetic and epigenetic links. Front
Endocrinol (Lausanne). 9(2)2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wang CY, Tsai PY, Chen TY, Tsai HL, Kuo PL
and Su MT: Elevated miR-200a and miR-141 inhibit endocrine
gland-derived vascular endothelial growth factor expression and
ciliogenesis in preeclampsia. J Physiol. 597:3069–3083.
2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Chen J, Cao W, Asare PF, Lv M, Zhu Y, Li
L, Wei J, Gao H, Zhang H, Mao H, et al: Amelioration of cardiac
dysfunction and ventricular remodeling after myocardial infarction
by danhong injection are critically contributed by
anti-TGF-β-mediated fibrosis and angiogenesis mechanisms. J
Ethnopharmacol. 194:559–570. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhang CJ, Huang Y, Lu JD, Lin J, Ge ZR and
Huang H: Retracted: Upregulated microRNA-132 rescues cardiac
fibrosis and restores cardiocyte proliferation in dilated
cardiomyopathy through the phosphatase and tensin homolog-mediated
PI3K/Akt signal transduction pathway. J Cell Biochem.
120:1232–1244. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wang G, Wang R, Ruan Z, Liu L, Li Y and
Zhu L: MicroRNA-132 attenuated cardiac fibrosis in myocardial
infarction-induced heart failure rats. Biosci Rep.
40(BSR20201696)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Li N, Zhou H and Tang Q: miR-133: A
suppressor of cardiac remodeling? Front Pharmacol.
9(903)2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Clauss S, Wakili R, Hildebrand B, Kääb S,
Hoster E, Klier I, Martens E, Hanley A, Hanssen H, Halle M and
Nickel T: MicroRNAs as biomarkers for acute atrial remodeling in
marathon runners (the miRathon study-a sub-study of the munich
marathon study). PLoS One. 11(e0148599)2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Mooren FC, Viereck J, Krüger K and Thum T:
Circulating microRNAs as potential biomarkers of aerobic exercise
capacity. Am J Physiol Heart Circ Physiol. 306:H557–H563.
2014.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Feng B, Chen S, George B, Feng Q and
Chakrabarti S: miR133a regulates cardiomyocyte hypertrophy in
diabetes. Diabetes Metab Res Rev. 26:40–49. 2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kambis TN, Shahshahan HR, Kar S, Yadav SK
and Mishra PK: Transgenic expression of miR-133a in the diabetic
akita heart prevents cardiac remodeling and cardiomyopathy. Front
Cardiovasc Med. 6(45)2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Muñoz JP, Collao A, Chiong M, Maldonado C,
Adasme T, Carrasco L, Ocaranza P, Bravo R, Gonzalez L, Díaz-Araya
G, et al: The transcription factor MEF2C mediates cardiomyocyte
hypertrophy induced by IGF-1 signaling. Biochem Biophys Res Commun.
388:155–160. 2009.PubMed/NCBI View Article : Google Scholar
|