Occult breast cancer (OBC) is a rare presentation of
breast cancer in which axillary lymph node metastasis is the
primary or presenting symptom, and both clinical and imaging
examinations cannot identify the primary breast lesion. OBC is
estimated to comprise 0.3-1.0% of all breast cancer cases (1-3).
The most effective method of treating OBC is still controversial
because of these low incidence rates and the absence of randomized
controlled research that specifically addresses this cancer
subtype. Adenocarcinoma of the lymph nodes that has metastatic
spread is the primary clinical symptom of patients with OBC and is
supported by pathological investigation of the axillary lymph nodes
(4-6).
The exact diagnostic methodology for OBC is being continuously
improved (7). The initial symptoms
in individuals finally diagnosed with OBC are typically metastatic
tumors in the axillary lymph nodes or other locations (8,9).
Standard imaging diagnostic methods may have difficulty detecting
initial breast lesions, which lowers the rate of OBC diagnosis and
affects the clinical course of treatment and prognosis for patients
(7). These difficulties can
frequently result in misdiagnosis such that treatment is delayed
and patients face a worse prognosis. Thus, the early diagnosis of
OBC must be improved to improve patient survival and other
prognostic outcomes.
Preoperative primary breast cancer identification
can significantly impact the treatment and prognosis assessment of
patients with OBC, allowing doctors to choose the most appropriate
biopsy and chemotherapy treatment regimens (10). Various clinical methods are
currently used to detect and diagnose breast diseases (11). Mammography is among the most common
and effective diagnostic technologies, providing high detection
rates for early-stage tumors (11,12).
By digitizing images and employing different post-processing
technologies, mammography can improve diagnostic sensitivity and
specificity for OBC instances (13-15).
However, using a single imaging modality is often insufficient
owing to the influence of a range of factors on imaging findings
(16,17). Accordingly, mammography and
positron emission tomography/computed tomography (PET/CT) are often
combined to diagnose breast diseases reliably (18). Mammography is beneficial for
identifying benign and malignant breast lesions and detecting small
breast cancer lesions in the deep breast tissue (19,20).
PET/CT can help clarify the clinical staging of patients with
breast cancer but is insufficient to diagnose breast cancer when
used in isolation (21-23).
When primary breast lesions cannot be detected through mammography
and ultrasonography, magnetic resonance imaging (MRI) can be
considered an alternative imaging strategy (24). Enhanced MRI and mammography are
routinely used to identify breast cancer, but each has advantages
and disadvantages. Thus, increasing evidence indicates that they
should be combined for the best diagnostic results (25). Although it might be challenging to
identify the primary breast lesions, most patients prefer to have a
total mastectomy along with axillary lymph node dissection
(26). Several retrospective
studies have detected no significant differences in predictive
outcomes associated with radiotherapy following breast-preserving
surgery or total mastectomy (2,13-15).
However, due to a scarcity of large-scale clinical research on OBC,
these patients have no clear diagnostic or therapeutic standards.
Because initial lesions are not apparent in the breast tissue of
individuals with OBC, the most appropriate local treatment
techniques are similarly uncertain, and it is unclear whether
radiotherapy can provide significant survival benefits to these
patients (27-29).
The objective of the current investigation was to examine the
clinicopathological characteristics, imaging manifestations and
therapy alternatives of OBC to establish a basis for developing
enhanced personalized treatment approaches for this rare form of
breast cancer.
A 51-year-old female patient initially presented
with a recently detected left axillary mass (3.1x1.5 cm) when the
patient first attended the China-Japan Union Hospital of Jilin
University (Changchun, China). No masses were palpable in either
breast, and there was no evidence of nipple discharge. No right
axillary lymph node enlargement was detected. Ultrasonography
revealed three homogenous hypoechoic left axillary masses with
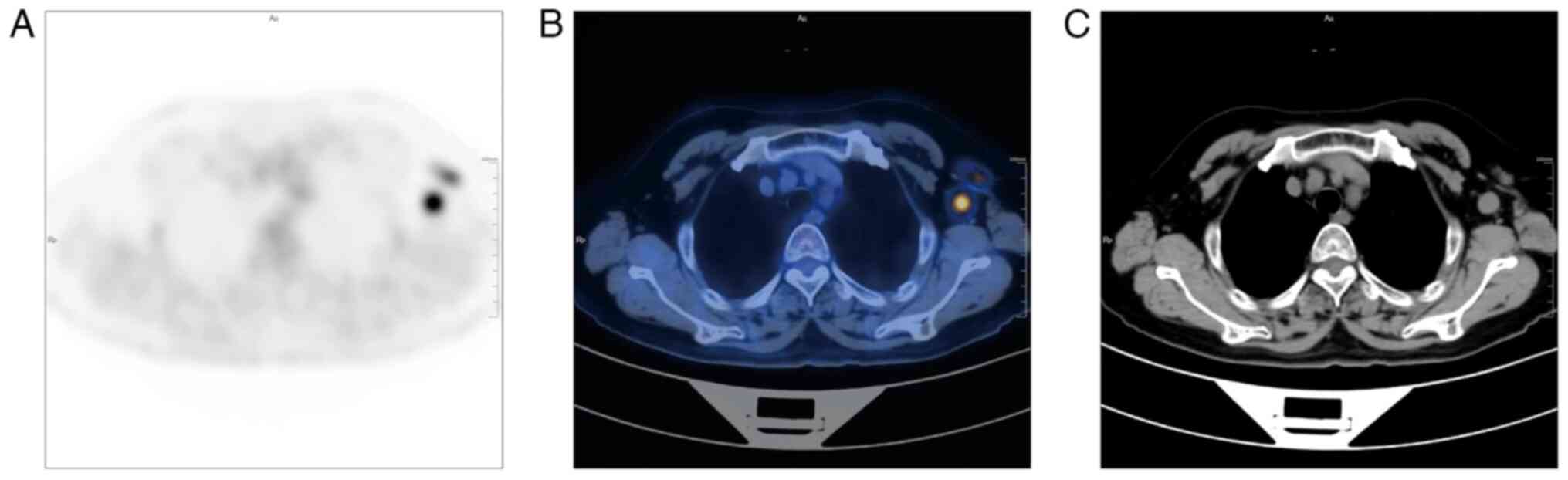
irregular margins, the largest measuring ~3.11x1.61 cm. PET/CT
scanning indicated an area of increased glucose metabolism
co-registered with the left axillary lymph nodes (SUVmax=9.56;
Fig. 1). There was no evidence of
aberrant glucose metabolism in the basal/myoepithelial layer of the
mammary gland. Mammography and ultrasonography were unable to
detect any anomalies in either breast. The patient underwent
fine-needle aspiration cytology (FNAC) for imaging-detected
indeterminate or suspicious lesions. Pathology results for the
analyzed left axillary lymph node were consistent with a diagnosis
of invasive ductal carcinoma. Immunohistochemistry results were as
follows: Ki-67+ (70%), estrogen receptor
(ER+) (90%), progesterone receptor (PR+)
(80%), HER-2 (score 2+), E-cadherin+, androgen Receptor
(AR+) (90%), CK5/6-, p63-,
calponin-, SOX10-, GATA-3+ and
gross cystic disease fluid protein 15 (GCDFP-15+). All
analyzed tumor markers were within the normal ranges, including
serum AFP, CEA, prostate-specific antigen, carbohydrate antigen
(CA) 19-9 and CA 15-3 levels. The individual did not disclose any
previous personal or familial instances of malignancies. Based on
the findings, the patient was diagnosed with OBC and subsequently
underwent a left breast nipple-sparing mastectomy with axillary
lymph node dissection.
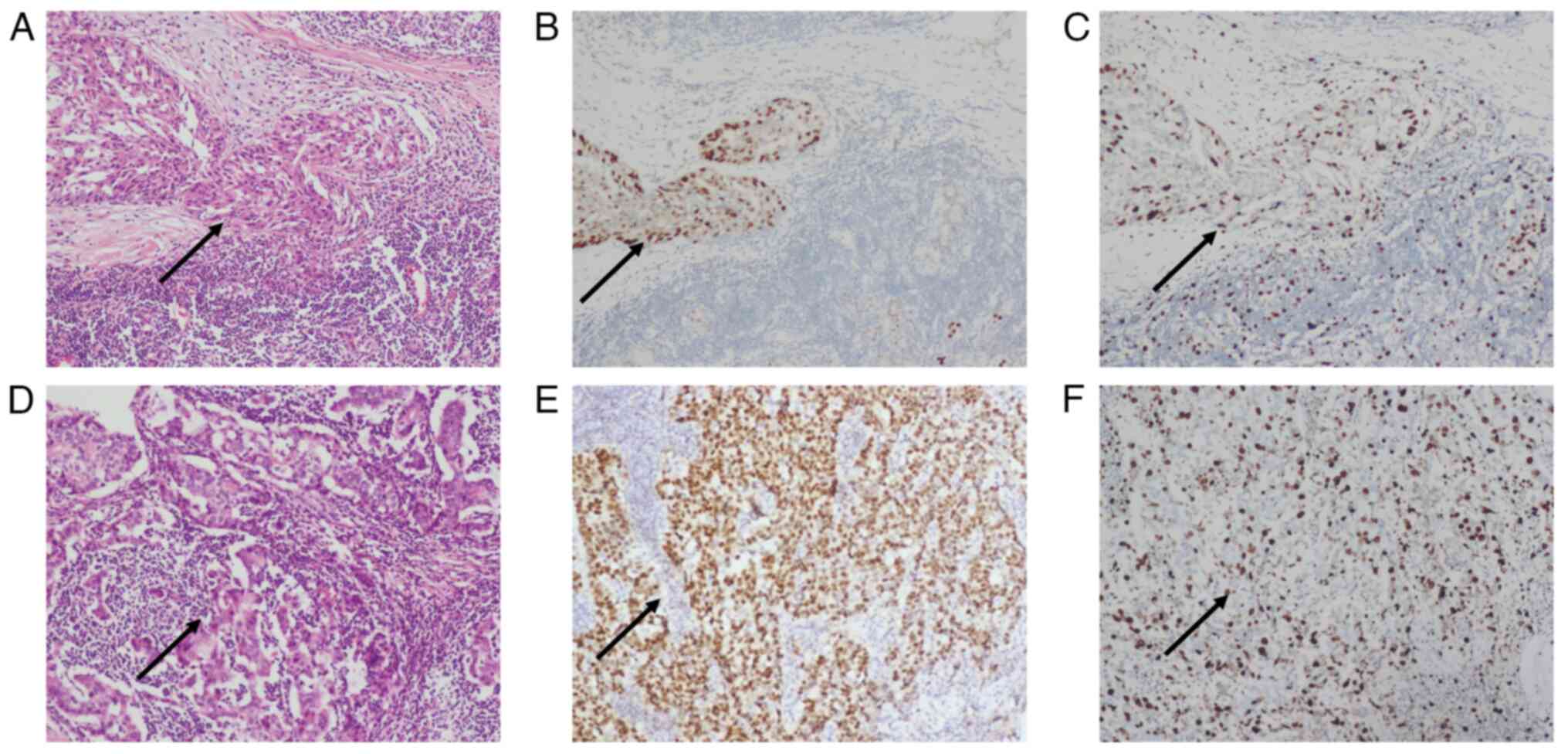
Post-surgical pathology revealed that the dissected
axillary lymph nodes exhibited invasive carcinoma and that the left
breast tumor was a predominantly intermediate-grade ductal
carcinoma in situ (DCIS) (Fig.
2A and D). Immunostaining
results confirmed tumor positivity for ER+ (60%)
(Fig. 2B), Ki-67+ (25%)
(Fig. 2C), GATA3+,
ER+ (90%) (Fig. 2E),
Ki-67+ (40%) (Fig. 2F),
PR+ (80%), HER-2 (score 2+) and E-Cadherin. By contrast,
tumor tissue was negative for mammaglobin, WT-1 and PAX-8 (Fig. 2). At 1 year after surgery, the
patient was discharged and was recurrence-free. The patient was
treated with adjuvant chemotherapy (Table I).
The present investigation was approved by the Ethics
Committee of China-Japan Union Hospital (grant no. 2023033009), and
the patient provided written informed consent for its
publication.
Occult breast cancer is a medical condition
infrequently encountered in clinical practice. The diagnostic
process for occult breast cancer is complicated by the challenge of
identifying the primary tumor site in affected patients. The
diagnosis and treatment of OBC have gained significant research
attention since Halsted's initial description of its symptoms,
therapy and natural progression (30). Confirming OBC diagnoses requires
patients to undergo an axillary mass puncture or mass excision
biopsy (31,36). When axillary tumors are
pathologically diagnosed as metastatic adenocarcinomas of the lymph
nodes, a clinical diagnosis of OBC should be considered (46). Bhatia et al revealed that a
high proportion of female OBC cases are positive for ER and/or PR
(47). Immunohistochemical
analyses of PR, ER and HER-2 status following the surgical excision
of axillary tumors should be considered as a means of aiding
patient diagnosis. The patient in the present case study was ER,
PR, and HER-2 positive. However, when lesions are negative for ER
and PR, it is impossible to rule out the diagnosis of OBC because
certain breast cancer cases are hormone receptor-independent
(33,48). Future advancements in diagnostic
methods and imaging modalities are anticipated to make it easier to
detect intramammary lesions, which have historically been
challenging to detect. The diagnosis and management of OBC remains
debatable due to these factors.
The percentage of cancer cases with cancer of
unknown primary (CUP) ranges from 5-10% (49,50),
and 10-40% of these patients have metastatic lesions limited to
lymph nodes (51). Only 1% of all
cancers have axillary lymph node metastases from an unidentified
origin (52). CUP has a higher
relative contribution to cancer mortalities because of its high
mortality rate (53). The greater
success in detecting primary tumors is probably the cause of the
declining incidence of CUP (54).
Histologically confirmed metastatic malignant tumors
whose primary site cannot be detected after complete clinical and
radiological examinations are called CUP (55,56).
After completing clinical and pathological diagnostic procedures,
the diagnosis of CUP relies on a multidisciplinary consultation to
ascertain whether the tumor symptoms align with metastases
indicative of CUP or original cancer (57). A subset of CUP, known as OBC, is a
metastatic breast cancer confirmed by biopsy that has no
recognizable primary breast tumor (56). Metastasis to the axillary and
cervical lymph nodes is frequently observed as an initial sign of
ovarian breast cancer (56).
Imaging and pathological evaluations to rule out a primary breast
tumor can be used to diagnose OBC. The incidence of OBC has been
declining due to the development of sophisticated diagnostic
techniques (58). Due to the
scarcity of data, there is currently insufficient information to
formulate comprehensive management standards for OBC. The diagnosis
and treatment of OBC pose a significant clinical challenge because
of the requirement of conducting a thorough physical examination,
utilizing specific radio diagnostic testing, and analyzing
pathologic and immunohistochemical findings (32,34).
The lack of an intelligent and personalized system to rapidly
detect patients with OBC in the early stages is among the existing
clinical practice issues.
Based on available findings, MRI has demonstrated
the capability to generate three-dimensional images, hence aiding
oncologists in detecting a primary breast lesion when conventional
breast imaging modalities have proven ineffective in identifying
the origin of the lesion. This has been observed in ~75% of the
cases (59). For the treatment of
OBC, mastectomy and breast preservation have been suggested
(35), with or without
lymphadenectomy, for diagnosis and locoregional control (26,60).
Most patients diagnosed with OBC initially seek medical input
regarding a mass in the armpit without any palpable breast mass
(61,62). Several factors can contribute to
the unusual presentation of OBC (62,63).
The growth of primary intramammary lesions may have been hindered
due to immune-mediated inhibition, leading to relatively modest
lesions. Furthermore, fibrous mastitis is responsible for the
thickness of the breast tissue, which in turn hampers the
identification of minor breast lesions (64). In the current case, the lymph nodes
of the patient displayed indications of invasive cancer. A number
of metastatic cancer cells exhibited diffuse infiltration at the
microscopic scale, destroying the typical lymph node architecture.
Consequently, only limited quantities of lymphocytes were
observable amidst clusters of cancer cells. Tumor tissue morphology
within lymph nodes is typically similar to that in primary lesions,
with strip-like, papillary, acinar and clumpy presentations
(65-68).
In OBC cases where an axillary mass is the first symptom, lymph
nodes are generally nodular and diffusely infiltrated by tumor
cells, as in the present case, destroying the integrity of normal
lymphoid structures. The appearance of tumor nuclei is determined
mainly by the degree of differentiation. High-grade nuclei are
commonly found in poorly differentiated areas, including large
nuclei, vesicular nuclei, uneven chromatin and even tumor giant
cells. On the other hand, well-differentiated epithelial papillary
areas are predominantly composed of low-grade nuclei equal in size
to the nuclei of normal breast cells, with mitotic figures being
infrequent (45-49).
There is currently a lack of specific examination
procedures for OBC. Nevertheless, it is anticipated that future
developments in the field of medical imaging will contribute to the
identification of small breast lesions, thereby significantly
enhancing the probability of early detection (70,71).
Among various techniques, imaging techniques have emerged as
practical tools for detecting and monitoring responses to therapy
in patients with breast cancer (Table
II). Mammography and ultrasonography are the traditional breast
cancer screening approaches, but these fail to detect occult
lesions in some patients (72,73).
Compared to more conventional methods, full-field digital
mammography has shown significant gains in image quality and
contrast while exposing individuals to less radiation (74). Breast tumors and other high-density
lesions can be seen anatomically in the breast tissue using
mammography, which measures their size, density, borders and
development (19,75,76).
Combining digital breast tomosynthesis with grid
positioning technology can accurately diagnose microcalcifications
in patients with OBC with negative palpation, further improving
tumor detection rates (77-82).
MRI can detect undetectable lesions via color Doppler
ultrasonography or mammography while allowing for the observation
of internal blood flow, thereby providing a high level of detection
sensitivity (54-56).
As a result, it offers an efficient method of evaluating soft
tissue that is especially useful for identifying deep breast
lesions. It also enables the evaluation of the quantity, size and
extent of these lesions (57-59).
Prior MRI results have revealed OBC lesions that present as
lump-like regions of irregular circular enhancement or
non-lump-like areas of uneven enhancement (84,85).
Clinicians can successfully identify breast lesions in numerous
cases by using MRI, PET/CT and other systemic diagnostic techniques
while ruling out the possibility of other malignant tumor sources
in the body and spotting any metastases existing within other
tissues or organ compartments (86,87).
The sensitivity and specificity of PET-CT for axillary lymph node
metastasis are 95 and 65%, respectively, and it is commonly used to
evaluate patients with OBC with negative mammography results.
PET/CT is of particular value for the differential diagnosis of
OBC, given its ability to recognize axillary lymph node metastasis
and primary lesions of non-breast origin (88). In the present case, the patient
received PET/CT imaging, which revealed several enlarged
hypermetabolic left axillary lymph nodes. Pathology revealed that
these lymph nodes exhibited metastases, and the left breast tumor
was primarily intermediate-grade DCIS. When routine examination
results are inconclusive or difficult to interpret, PET/CT can
provide practical diagnostic assistance, particularly in
individuals with locally advanced or metastatic disease. Although a
number of investigations have suggested the potential efficacy of
PET/CT in detecting OBC, its utility in this specific patient
cohort is constrained due to the small size of lesions (89).
Breast-specific γ imaging (BSGI) is a
high-resolution imaging approach that can detect occult breast
lesions at sub-centimeter resolution with improved sensitivity and
specificity compared with MRI. BSGI enables the detection of small
tumor foci even in dense breast tissue (90,91).
The technique known as radioisotope occult lesion localization
involves administering a solution of water-soluble non-ionic iodine
contrast agent and radionuclide-labeled albumin gel close to the
suspected lesion location, followed by a localization biopsy under
the supervision of a γ-ray detector. Implementing this radionuclide
localization method reduces the possibility of requiring additional
surgical procedures while also considering the post-surgical
aesthetic outcomes and their impact on patient appearance (92,93).
However, radionuclides are limited by their short duration of
activity, such that they can only be injected within one day of the
operation (94-96).
If primary lesions in the breast tissue are successfully detected
in patients with OBC, they can receive a definitive diagnosis.
Nevertheless, there are cases where identifying
these initial abnormalities remains elusive, even after undergoing
numerous supplementary investigations (13,97).
In the case of these individuals, it is imperative to distinguish
between OBC and alternative types of malignancies, including
auxiliary breast cancer, thyroid carcinoma, lung cancer and
melanoma (33,98). The detection of accessory breast
cancer relies primarily on pertinent exams and a thorough clinical
history. The absence of accessory breast tissue identified during
ALND can be considered sufficient evidence to dismiss it as a
potential etiological factor for the disease (99). Tumor tissue in these cases with
exhibit morphological characteristics similar to those of the
primary tumor, and a primary focus may also be found via chest CT,
abdominal CT or other examinations (100). The tumor may be poorly
differentiated and difficult to detect based on its histological
morphology when it is organoid, acinar or lamellar, although
further immunohistochemistry analyses can aid in diagnosis
(3,69-71).
When encountering inexplicable growth of axillary lymph nodes, it
is essential to evaluate the possibility of OBC as a potential
diagnosis. When breast and accessory breast examinations yield
negative results, and the clinical primary tumor site remains
uncertain, it is advisable to pursue pathological evaluation. Small
amounts of invasive ductal carcinoma tissue and tumor thrombus are
observed within the intravascular lymphatic vessels, suggesting a
potential case of OBC.
Studies of local treatment options suggest that
patients with OBC that do not undergo mastectomy can benefit from
radiotherapy (105,112). According to some reports,
systemic treatment is considered the most suitable approach for
patients with OBC who do not have a detectable primary breast
lesion (107,113,114). This approach involves treating
the problem as a systemic disease and enabling breast preservation.
This can have the benefit of alleviating the psychological distress
often associated with breast surgery. The prognosis results of
patients are not improved by local treatment, even in cases where
breast abnormalities are discovered (115). In the subset of patients with OBC
in whom digestive tract metastases are the first symptom, the
disease should be treated as a form of advanced breast cancer and
treated through appropriate combinations of chemotherapy, endocrine
therapy, targeted therapy and other systemic treatments (116-118).
If patients exhibit gastrointestinal bleeding, perforation,
obstruction or other difficult-to-treat complications, palliative
surgery can be performed to prolong the median survival duration of
this patient population (119).
Due to the systemic nature of breast cancer,
surgical intervention represents but a single component of the
comprehensive therapy approach. Adjuvant therapy options for
patients with OBC encompass a range of interventions, such as
endocrine therapy, chemotherapy, targeted therapy, targeted
regional radiation, and immunotherapy regimens. The selection of
these treatment modalities is guided by established treatment
guidelines for non-OBC patients and the specific clinical
characteristics and needs of the particular patient under
consideration. Neoadjuvant chemotherapy (NAC) for OBC can help
target axillary lymph nodes and facilitate their surgical removal
in these patients while preventing the development of
drug-resistant tumor cells, eliminating small metastatic foci,
reducing tumor cell activity and restricting metastatic spread
(120-123).
Some reports have suggested that NAC treatment for patients with
OBC can be administered based on the immunohistochemical staining
of axillary lymph node biopsy samples, patient age, ultrasound and
18F-FDG PET/CT imaging findings (123-127).
In general, managing individuals with OBC should encompass a
comprehensive treatment approach comprising surgical intervention,
chemotherapy, radiotherapy, endocrine therapy, targeted therapy and
other suitable interventions. When determining the appropriate
course of action, it is crucial to thoroughly consider
patient-specific factors, pathological classification, and staging
outcomes.
The identification of pertinent prognostic variables
in patients with OBC is ongoing. The prognosis of patients becomes
poorer as the number of metastatic axillary lymph nodes increases
in breast cancer (83-85).
There is also evidence that hormone receptor status, tumor marker
expression, primary tumor pathological type, number of axillary
lymph nodes, the timing of axillary lymph node diagnosis and the
presence or absence of distant supraclavicular metastasis are all
related to patient outcomes (86-88).
In individuals diagnosed with OBC, nodal status may offer value as
an independent predictor of poor outcomes (2,10,26),
and those patients harboring distant metastases exhibit a very poor
prognosis and a short survival interval. Vlastos et al
(35) also discovered a connection
between outcomes of patients with OBC and the quantity of positive
axillary lymph nodes. However, deciding between primary surgery and
simple lymph node dissection does not significantly affect patient
survival rates.
The 10 year survival rate of patients with atypical
axillary metastases is 50-71%, with this rate being slightly
improved compared with that associated with stage II disease
(35,69,128). Significant prognostic factors
include hormone receptor status and the number of involved axillary
lymph nodes (35,128). Generally, the 5 year overall
survival of patients with 1-3 involved lymph nodes tends to be
improved compared with that of patients with 4+ involved nodes
(35).
The presenting symptom in patients with OBC is
typically painless axillary lymph node enlargement, which may
coincide with distant tumor metastasis and paraneoplastic
neurological syndromes in some cases (131). Patients should undergo prompt
breast examination, ultrasonography and mammography when isolated
enlarged axillary lymph nodes are detected (132,133). Breast-conserving surgery,
mastectomy and ALND should be considered if primary breast lesions
are detected through these assessments (134-136).
If no primary breast lesion is detected, it is recommended that
patients undergo FNAC, core needle biopsy or other forms of
puncture biopsy (137-139).
If the patient is ultimately diagnosed with metastatic cancer,
immunohistochemical staining for ER, PR, HER-2, Ki-67 and GCDFP-15
should be performed together with other tests, including thyroid
ultrasound scans and CT scans of the chest, abdomen and pelvis to
detect or distinguish between OBC and other forms of metastatic
cancer (100,140,141). Each OBC patient's specific
conditions should be considered, and neoadjuvant chemotherapy,
adjuvant chemotherapy, radiotherapy and targeted therapy should be
explored as potential approaches to improving patient survival and
prognosis (142). OBC cases are
sporadic, and large-scale studies of populations of patients with
OBC are thus lacking, with most research instead consisting of
analyses of small patient cohorts (143). Accordingly, the basis for
diagnosing and treating this cancer type is relatively limited, and
additional evidence is needed to establish the most appropriate
clinical management of affected patients (142). The further investigation centered
on the pathogenesis and attributes of OBC to discern the
immunological elements that contribute to the expansion of primary
lesions in affected individuals holds promise for enhancing
targeted therapeutic interventions, finally improving prognostic
outcomes (29,143,144). The present comprehensive
discussion of current issues facing the diagnosis of OBC, the
importance of sentinel lymph nodes and internal mammary lymph nodes
in treating OBC and the standardized treatment of OBC will benefit
from rapid advances in artificial intelligence, sequencing and big
data technologies in clinical practice (145,146). Formulating robust diagnostic and
treatment guidelines will help patients with OBC receive a timely
and accurate diagnosis to begin treatment as quickly as possible.
An improved prognosis for patients with OBC will also result from
the continued use of targeted medications, immunotherapies and
other cutting-edge pharmacological drugs.
Not applicable.
Funding: No funding was received.
All data generated and/or analyzed during this study
are included in this published article.
CL and HX conceived the review and acquired data. CL
and HX participated in the process of writing and reviewing the
manuscript. CL and HX confirm the authenticity of all the raw data.
All authors contributed to the conception and revision of the
manuscript and approved its submission. All authors read and
approved the final manuscript.
The China-Japan Union Hospital Medical Ethics
Committee approved the present study (approval no. 2023033009).
Written informed consent was obtained during the initial data
collection for participation.
The patient and control subject consented to the
publication of this case report and accompanying images.
The authors declare that they have no competing
interests.
|
1
|
Baron PL, Moore MP, Kinne DW, Candela FC,
Osborne MP and Petrek JA: Occult breast cancer presenting with
axillary metastases: Updated Management. Arch Surg. 125:210–214.
1990.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sohn G, Son BH, Lee SJ, Kang EY, Jung SH,
Cho SH, Baek S, Lee YR, Kim HJ, Ko BS, et al: Treatment and
survival of patients with occult breast cancer with axillary lymph
node metastasis: A nationwide retrospective study. J Surg Oncol.
110:270–274. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Patel J, Nemoto T, Rosner D, Dao TL and
Pickren JW: Axillary lymph node metastasis from an occult breast
cancer. Cancer. 47:2923–2927. 1981.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang Y, Wu H and Luo Z: A retrospective
study of optimal surgical management for occult breast carcinoma:
Mastectomy or quadrantectomy? Medicine (Baltimore).
96(e9490)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yamaguchi H, Ishikawa M, Hatanaka K,
Uekusa T, Ishimaru M and Nagawa H: Occult breast cancer presenting
as axillary metastases. Breast. 15:259–262. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Abe S, Abe N, Noda M, Okano M, Tachibana
K, Yoshida S, Kiko Y, Hashimoto Y, Hatakeyama Y, Rokkaku Y and
Ohtake T: A Case of Occult Breast Cancer. Gan To Kagaku Ryoho.
44:1095–1097. 2017.PubMed/NCBI(In Japanese).
|
|
7
|
Walker GV, Smith GL, Perkins GH, Oh JL,
Woodward W, Yu TK, Hunt KK, Hoffman K, Strom EA and Buchholz TA:
Population-based analysis of occult primary breast cancer with
axillary lymph node metastasis. Cancer. 116:4000–4006.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Frattaroli FM, Carrara A, Conte AM and
Pappalardo G: Axillary metastasis as first symptom of occult breast
cancer: A case report. Tumori. 88:532–534. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu L, Zhang J, Chen M, Ren S, Liu H and
Zhang H: Anemia and thrombocytopenia as initial symptoms of occult
breast cancer with bone marrow metastasis: A case report. Medicine
(Baltimore). 96(e8529)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Woo SM, Son BH, Lee JW, Kim HJ, Yu JH, Ko
BS, Sohn G, Lee YR, Kim H, Ahn SH and Baek SH: Survival outcomes of
different treatment methods for the ipsilateral breast of occult
breast cancer patients with axillary lymph node metastasis: A
single center experience. J Breast Cancer. 16:410–416.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Coleman C: Early detection and screening
for breast cancer. Semin Oncol Nurs. 33:141–155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Anderson WF, Jatoi I and Devesa SS:
Assessing the impact of screening mammography: Breast cancer
incidence and mortality rates in Connecticut (1943-2002). Breast
Cancer Res Treat. 99:333–340. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nguyen QD, Randall JW, Harmon TS, Robinson
AS, Cotes C, Lee AE, Mahon BH and Sadruddin S: Detection of a
mammographically occult breast cancer with a challenging clinical
history. Cureus. 10(e3594)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee J and Nishikawa RM: Identifying women
with mammographically-occult breast cancer leveraging GAN-Simulated
Mammograms. IEEE Trans Med Imaging. 41:225–236. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tartter PI, Weiss S, Ahmed S, Kamath S,
Hermann G and Drossman S: Mammographically occult breast cancers.
Breast J. 5:22–25. 1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Prasad SN and Houserkova D: The role of
various modalities in breast imaging. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 151:209–218. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Planche K and Vinnicombe S: Breast imaging
in the new era. Cancer Imaging. 4:39–50. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sánchez LR, Treviño LMT and Sánchez GE:
Fusion of Digital Mammography with High-Resolution Breast PET: An
Application to Breast Imaging. In: 2nd EAI International Conference
on Smart Technology. Torres-Guerrero F, Neira-Tovar L and
Bacca-Acosta J (eds). Springer International Publishing, Cham,
pp111-125, 2023.
|
|
19
|
Fiorica JV: Breast cancer screening,
mammography, and other modalities. Clin Obstet Gynecol. 59:688–709.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rodriguez-Ruiz A, Lång K, Gubern-Merida A,
Broeders M, Gennaro G, Clauser P, Helbich TH, Chevalier M, Tan T,
Mertelmeier T, et al: Stand-Alone artificial intelligence for
breast cancer detection in mammography: Comparison with 101
radiologists. J Natl Cancer Inst. 111:916–922. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Groheux D, Espié M, Giacchetti S and
Hindié E: Performance of FDG PET/CT in the clinical management of
breast cancer. Radiology. 266:388–405. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Radan L, Ben-Haim S, Bar-Shalom R,
Guralnik L and Israel O: The role of FDG-PET/CT in suspected
recurrence of breast cancer. Cancer. 107:2545–2551. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zangheri B, Messa C, Picchio M, Gianolli
L, Landoni C and Fazio F: PET/CT and breast cancer. Eur J Nucl Med
Mol Imaging. 31 (Suppl 1):S135–S142. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gradishar WJ, Moran MS, Abraham J, Aft R,
Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, et
al: Breast Cancer, Version 3.2022, NCCN Clinical Practice
Guidelines in Oncology. J Natl Compr Canc Netw. 20:691–722.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao YF, Chen Z, Zhang Y, Zhou J, Chen JH,
Lee KE, Combs FJ, Parajuli R, Mehta RS, Wang M and Su MY: Diagnosis
of breast cancer using radiomics models built based on dynamic
contrast enhanced MRI combined with mammography. Front Oncol.
11(774248)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Merson M, Andreola S, Galimberti V,
Bufalino R, Marchini S and Veronesi U: Breast carcinoma presenting
as axillary metastases without evidence of a primary tumor. Cancer.
70:504–508. 1992.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Varadarajan R, Edge SB, Yu J, Watroba N
and Janarthanan BR: Prognosis of occult breast carcinoma presenting
as isolated axillary nodal metastasis. Oncology. 71:456–459.
2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
He M, Tang LC, Yu KD, Cao AY, Shen ZZ,
Shao ZM and Di GH: Treatment outcomes and unfavorable prognostic
factors in patients with occult breast cancer. Eur J Surg Oncol.
38:1022–1028. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Khandelwal AK and Garguilo GA: Therapeutic
options for occult breast cancer: A survey of the American Society
of Breast Surgeons and review of the literature. Am J Surg.
190:609–613. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Halsted WS: I. The results of radical
operations for the cure of carcinoma of the breast. Ann Surg.
46:1–19. 1907.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lloyd MS and Nash AG: ‘Occult’ breast
cancer. Ann R Coll Surg Engl. 83:420–424. 2001.PubMed/NCBI
|
|
32
|
Ofri A and Moore K: Occult breast cancer:
Where are we at? Breast. 54:211–215. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wong YP, Tan GC, Muhammad R and Rajadurai
P: Occult primary breast carcinoma presented as an axillary mass: A
diagnostic challenge. Malays J Pathol. 42:151–155. 2020.PubMed/NCBI
|
|
34
|
Xu R, Li J, Zhang Y, Jing H and Zhu Y:
Male occult breast cancer with axillary lymph node metastasis as
the first manifestation: A case report and literature review.
Medicine (Baltimore). 96(e9312)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vlastos G, Jean ME, Mirza AN, Mirza NQ,
Kuerer HM, Ames FC, Hunt KK, Ross MI, Buchholz TA, Buzdar AU and
Singletary SE: Feasibility of breast preservation in the treatment
of occult primary carcinoma presenting with axillary metastases.
Ann Surg Oncol. 8:425–431. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fayanju OM, Jeffe DB and Margenthaler JA:
Occult primary breast cancer at a comprehensive cancer center. J
Surg Res. 185:684–689. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Terada M, Adachi Y, Sawaki M, Hattori M,
Yoshimura A, Naomi G, Kotani H, Iwase M, Kataoka A, Onishi S, et
al: Occult breast cancer may originate from ectopic breast tissue
present in axillary lymph nodes. Breast Cancer Res Treat. 172:1–7.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kadowaki M, Nagashima T, Sakata H,
Sakakibara M, Sangai T, Nakamura R, Fujimoto H, Arai M, Onai Y,
Nagai Y, et al: Ectopic breast tissue in axillary lymph node.
Breast Cancer. 14:425–428. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Edlow DW and Carter D: Heterotopic
epithelium in axillary lymph nodes: Report of a case and review of
the literature. Am J Clin Pathol. 59:666–673. 1973.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Turner DR and Millis RR: Breast tissue
inclusions in axillary lymph nodes. Histopathology. 4:631–636.
1980.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Maiorano E, Mazzarol GM, Pruneri G,
Mastropasqua MG, Zurrida S, Orvieto E and Viale G: Ectopic breast
tissue as a possible cause of false-positive axillary sentinel
lymph node biopsies. Am J Surg Pathol. 27:513–518. 2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Locopo N, Fanelli M and Gasparini G:
Clinical significance of angiogenic factors in breast cancer.
Breast Cancer Res Treat. 52:159–173. 1998.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Blanchard DK, Shetty PB, Hilsenbeck SG and
Elledge RM: Association of surgery with improved survival in stage
IV breast cancer patients. Ann Surg. 247:732–738. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Olson JA Jr, Morris EA, Van Zee KJ,
Linehan DC and Borgen PI: Magnetic resonance imaging facilitates
breast conservation for occult breast cancer. Ann Surg Oncol.
7:411–415. 2000.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sanuki JI, Uchida Y, Uematsu T, Yamada Y
and Kasami M: Axillary mass suspected to be occult breast
carcinoma: A case study of skipped axillary lymph node metastasis
from endometrial carcinoma in which core-needle biopsy was useful
for diagnosis. Breast Cancer. 16:72–76. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hulka CA, Smith BL, Sgroi DC, Tan L,
Edmister WB, Semple JP, Campbell T, Kopans DB, Brady TJ and
Weisskoff RM: Benign and malignant breast lesions: Differentiation
with echo-planar MR imaging. Radiology. 197:33–38. 1995.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bhatia SK, Saclarides TJ, Witt TR, Bonomi
PD, Anderson KM and Economou SG: Hormone receptor studies in
axillary metastases from occult breast cancers. Cancer.
59:1170–1172. 1987.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Poulakaki F: Occult Breast Cancer. In:
Breast Cancer Essentials. Rezai M, Kocdor MA and Canturk NZ (eds).
Springer International Publishing, Cham, pp667-674, 2021.
|
|
49
|
Van de Wouw AJ, Janssen-Heijnen ML,
Coebergh JW and Hillen HF: Epidemiology of unknown primary tumours;
incidence and population-based survival of 1285 patients in
Southeast Netherlands, 1984-1992. Eur J Cancer. 38:409–413.
2002.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Randén M, Rutqvist LE and Johansson H:
Cancer patients without a known primary: Incidence and survival
trends in Sweden 1960-2007. Acta Oncol. 48:915–920. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hemminki K, Bevier M, Hemminki A and
Sundquist J: Survival in cancer of unknown primary site:
Population-based analysis by site and histology. Ann Oncol.
23:1854–1863. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Pentheroudakis G, Lazaridis G and Pavlidis
N: Axillary nodal metastases from carcinoma of unknown primary
(CUPAx): A systematic review of published evidence. Breast Cancer
Res Treat. 119:1–11. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Brewster DH, Lang J, Bhatti LA, Thomson CS
and Oien KA: Descriptive epidemiology of cancer of unknown primary
site in Scotland, 1961-2010. Cancer Epidemiol. 38:227–234.
2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Binder C, Matthes KL, Korol D, Rohrmann S
and Moch H: Cancer of unknown primary-Epidemiological trends and
relevance of comprehensive genomic profiling. Cancer Med.
7:4814–4824. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Krämer A, Hübner G, Schneeweiss A,
Folprecht G and Neben K: Carcinoma of unknown primary-an orphan
disease? Breast Care (Basel). 3:164–170. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Barbieri E, Anghelone CAP, Gentile D, La
Raja C, Bottini A and Tinterri C: Metastases from occult breast
cancer: A case report of carcinoma of unknown primary Syndrome.
Case Rep Oncol. 13:1158–1163. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Krämer A, Bochtler T, Pauli C, Baciarello
G, Delorme S, Hemminki K, Mileshkin L, Moch H, Oien K, Olivier T,
et al: Cancer of unknown primary: ESMO Clinical Practice Guideline
for diagnosis, treatment and follow-up. Ann Oncol. 34:228–246.
2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Urban D, Rao A, Bressel M, Lawrence YR and
Mileshkin L: Cancer of unknown primary: A population-based analysis
of temporal change and socioeconomic disparities. Br J Cancer.
109:1318–1324. 2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
de Bresser J, de Vos B, van der Ent F and
Hulsewé K: Breast MRI in clinically and mammographically occult
breast cancer presenting with an axillary metastasis: A systematic
review. Eur J Surg Oncol. 36:114–119. 2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Van Ooijen B, Bontenbal M, Henzen-Logmans
SC and Koper PC: Axillary nodal metastases from an occult primary
consistent with breast carcinoma. Br J Surg. 80:1299–1300.
1993.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hawes D, Neville AM and Cote RJ: Detection
of occult metastasis in patients with breast cancer. Semin Surg
Oncol. 20:312–318. 2001.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Medina-Franco H and Urist MM: Occult
breast carcinoma presenting with axillary lymph node metastases.
Rev Invest Clin. 54:204–208. 2002.PubMed/NCBI
|
|
63
|
Owen HW, Dockerty MB and Gray HK: Occult
carcinoma of the breast. Surg Gynecol Obstet. 98:302–308.
1954.PubMed/NCBI
|
|
64
|
Chen YC, Chan CH, Lim YB, Yang SF, Yeh LT,
Wang YH, Chou MC and Yeh CB: Risk of breast cancer in women with
mastitis: A retrospective population-based cohort study. Medicina
(Kaunas). 56(372)2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ping J, Liu W, Chen Z and Li C: Lymph node
metastases in breast cancer: Mechanisms and molecular imaging. Clin
Imaging. 103(109985)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
He M, Liu H and Jiang Y: A case report of
male occult breast cancer first manifesting as axillary lymph node
metastasis with part of metastatic mucinous carcinoma. Medicine
(Baltimore). 94(e1038)2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Rosen PP and Kimmel M: Occult breast
carcinoma presenting with axillary lymph node metastases: A
follow-up study of 48 patients. Hum Pathol. 21:518–523.
1990.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hur SM, Cho DH, Lee SK, Choi MY, Bae SY,
Koo MY, Kim S, Nam SJ, Lee JE and Yang JH: Occult breast cancers
manifesting as axillary lymph node metastasis in men: A two-case
report. J Breast Cancer. 15:359–363. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Matsuoka K, Ohsumi S, Takashima S, Saeki
T, Aogi K and Mandai K: Occult breast carcinoma presenting with
axillary lymph node metastases: Follow-up of eleven patients.
Breast Cancer. 10:330–334. 2003.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zheng H, Gu Y, Qin Y, Huang X, Yang J and
Yang GZ: Small Lesion Classification in Dynamic Contrast
Enhancement MRI for Breast Cancer Early Detection. In: Medical
Image Computing and Computer Assisted Intervention-MICCAI 2018. Vol
11071. Frangi AF, Schnabel JA, Davatzikos C, Alberola-López C and
Fichtinger G (eds). Springer International Publishing, Cham,
pp876-884, 2018.
|
|
71
|
Lei YM, Yin M, Yu MH, Yu J, Zeng SE, Lv
WZ, Li J, Ye HR, Cui XW and Dietrich CF: Artificial intelligence in
medical imaging of the breast. Front Oncol.
11(600557)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Uchida K, Yamashita A, Kawase K and Kamiya
K: Screening ultrasonography revealed 15% of mammographically
occult breast cancers. Breast Cancer. 15:165–168. 2008.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Jacob D, Brombart JC, Muller C, Lefèbvre
C, Massa F and Depoerck A: Analysis of the results of 137
subclinical breast lesions excisions. Value of ultrasonography in
the early diagnosis of breast cancer. J Gynecol Obstet Biol Reprod
(Paris). 26:27–31. 1997.PubMed/NCBI(In French).
|
|
74
|
Asbeutah AM, AlMajran AA, Brindhaban A and
Asbeutah SA: Comparison of radiation doses between diagnostic
full-field digital mammography (FFDM) and digital breast
tomosynthesis (DBT): A clinical study. J Med Radiat Sci.
67:185–192. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Gøtzsche PC and Jørgensen KJ: Screening
for breast cancer with mammography. Cochrane Database Syst Rev.
2013(CD001877)2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Rodríguez-Ruiz A, Krupinski E, Mordang JJ,
Schilling K, Heywang-Köbrunner SH, Sechopoulos I and Mann RM:
Detection of breast cancer with mammography: Effect of an
artificial intelligence support system. Radiology. 290:305–314.
2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
McDonald ES, Hammersley JA, Chou SH,
Rahbar H, Scheel JR, Lee CI, Liu CL, Lehman CD and Partridge SC:
Performance of DWI as a Rapid unenhanced technique for detecting
mammographically occult breast cancer in elevated-risk women with
dense breasts. AJR Am J Roentgenol. 207:205–216. 2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Theunissen CI, Rust EA, Edens MA, Bandel
C, Van't Ooster-van den Berg JG, Jager PL, Noorda EM and Francken
AB: Radioactive seed localization is the preferred technique in
nonpalpable breast cancer compared with wire-guided localization
and radioguided occult lesion localization. Nucl Med Commun.
38:396–401. 2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Morris EA, Liberman L, Ballon DJ, Robson
M, Abramson AF, Heerdt A and Dershaw DD: MRI of Occult Breast
Carcinoma in a High-Risk Population. AJR Am J Roentgenol.
181:619–626. 2003.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Buchanan CL, Morris EA, Dorn PL, Borgen PI
and Van Zee KJ: Utility of breast magnetic resonance imaging in
patients with occult primary breast cancer. Ann Surg Oncol.
12:1045–1053. 2005.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Wecsler JS, Raghavendra A, Mack WJ,
Tripathy D, Yamashita M, Sheth P, Hovanessian-Larsen L, Sener SF,
Russell CA, McDonald H and Lang JE: Abstract P4-02-05: Predictors
of MRI detection of occult lesions in newly diagnosed breast
cancer. Cancer Res. 76 (Suppl 4)(P4-02-05)2016.
|
|
82
|
Zheng B, Hollingsworth AB, Tan MY, Stough
RG and Liu H: Abstract P4-02-06: Improving efficacy of applying
breast MRI to detect mammography-occult breast cancer. Cancer Res.
76 (Suppl 4)(P4-02-06)2016.
|
|
83
|
Amornsiripanitch N, Rahbar H, Kitsch AE,
Lam DL, Weitzel B and Partridge SC: Visibility of mammographically
occult breast cancer on diffusion-weighted MRI versus ultrasound.
Clin Imaging. 49:37–43. 2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Gao Y, Bagadiya NR, Jardon ML, Heller SL,
Melsaether AN, Toth HB and Moy L: Outcomes of Preoperative
MRI-Guided needle localization of nonpalpable mammographically
occult breast lesions. AJR Am J Roentgenol. 207:676–684.
2016.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Smith LF, Henry-Tillman R, Mancino AT,
Johnson A, Price Jones M, Westbrook KC, Harms S and Klimberg VS:
Magnetic resonance imaging-guided core needle biopsy and needle
localized excision of occult breast lesions. Am J Surg.
182:414–418. 2001.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ryu JK, Rhee SJ, Song JY, Cho SH and Jahng
GH: Characteristics of quantitative perfusion parameters on dynamic
contrast-enhanced MRI in mammographically occult breast cancer. J
Appl Clin Med Phys. 17:377–390. 2016.PubMed/NCBI View Article : Google Scholar
|
|
87
|
McCartan DP, Zabor EC, Morrow M, Van Zee
KJ and El-Tamer MB: Oncologic outcomes after treatment for MRI
occult breast cancer (pT0N+). Ann Surg Oncol. 24:3141–3147.
2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Ramírez Huaranga MA, Salas Manzanedo V,
Huertas MP, Torres Sousa Y and Ramos Rodríguez CC: Lumbar pain as
the single manifestation of an occult breast cancer. Usefulness of
positron emission tomography. Reumatol Clin. 11:118–120.
2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Takabatake D, Taira N, Aogi K, Ohsumi S,
Takashima S, Inoue T and Nishimura R: Two cases of occult breast
cancer in which PET-CT was helpful in identifying primary tumors.
Breast Cancer. 15:181–184. 2008.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Brem RF, Ruda RC, Yang JL, Coffey CM and
Rapelyea JA: Breast-Specific γ-Imaging for the detection of
mammographically occult breast cancer in women at increased risk. J
Nucl Med. 57:678–684. 2016.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Brem RF, Shahan C, Rapleyea JA, Donnelly
CA, Rechtman LR, Kidwell AB, Teal CB, McSwain A and Torrente J:
Detection of occult foci of breast cancer using breast-specific
gamma imaging in women with one mammographic or clinically
suspicious breast lesion. Acad Radiol. 17:735–743. 2010.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zand S and Abdolali A: Radioguided Occult
Lesion Localisation (ROLL) for Excision of Non-Palpable Breast
Lesions, a Personal Experience in a Patient with Multifocal Breast
Cancer. Arch Breast Cancer. 3:139–143. 2016.
|
|
93
|
Ong JSL, The J, Saunders C, Bourke AG,
Lizama C, Newton J, Phillips M and Taylor DB: Patient satisfaction
with Radioguided Occult Lesion Localisation using iodine-125 seeds
(‘ROLLIS’) versus conventional hookwire localisation. Eur J Surg
Oncol. 43:2261–2269. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Li H, Liu Z, Yuan L, Fan K, Zhang Y, Cai W
and Lan X: Radionuclide-Based imaging of breast cancer: State of
the art. Cancers (Basel). 13(5459)2021.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Altıparmak Güleç B and Yurt F: Treatment
with radiopharmaceuticals and radionuclides in breast cancer:
Current options. Eur J Breast Health. 17:214–219. 2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Tolmachev V and Vorobyeva A: Radionuclides
in diagnostics and therapy of malignant tumors: New development.
Cancers (Basel). 14(297)2022.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Amir E, Bedard PL, Ocaña A and Seruga B:
Benefits and harms of detecting clinically occult breast cancer. J
Natl Cancer Inst. 104:1542–1547. 2012.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Health Commission Of The People's Republic
Of China N. National guidelines for diagnosis and treatment of
breast cancer 2022 in China (English version). Chin J Cancer Res.
34:151–175. 2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Zhang S, Yu YH, Qu W, Zhang Y and Li J:
Diagnosis and treatment of accessory breast cancer in 11 patients.
Oncol Lett. 10:1783–1788. 2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Pesapane F, Downey K, Rotili A, Cassano E
and Koh DM: Imaging diagnosis of metastatic breast cancer. Insights
Imaging. 11(79)2020.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Feuerman L, Attie JN and Rosenberg B:
Carcinoma in axillary lymph nodes as an indicator of breast cancer.
Surg Gynecol Obstet. 114:5–8. 1962.PubMed/NCBI
|
|
102
|
Larsen RR, Sawyer KC, Sawyer RB and Torres
RC: Occult carcinoma of the breast. Am J Surg. 107:553–555.
1964.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Osteen RT, Kopf G and Wilson RE: In
pursuit of the unknown primary. Am J Surg. 135:494–497.
1978.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Chen QX, Wang XX, Lin PY, Zhang J, Li JJ,
Song CG and Shao ZM: The different outcomes between
breast-conserving surgery and mastectomy in triple-negative breast
cancer: A population-based study from the SEER 18 database.
Oncotarget. 8:4773–4780. 2017.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Masinghe SP, Faluyi OO, Kerr GR and
Kunkler IH: Breast radiotherapy for occult breast cancer with
axillary nodal metastases-does it reduce the local recurrence rate
and increase overall survival? Clin Oncol (R Coll Radiol).
23:95–100. 2011.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Macedo FI, Eid JJ, Flynn J, Jacobs MJ and
Mittal VK: Optimal surgical management for occult breast carcinoma:
A meta-analysis. Ann Surg Oncol. 23:1838–1844. 2016.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Foroudi F and Tiver KW: Occult breast
carcinoma presenting as axillary metastases. Int J Radiat Oncol
Biol Phys. 47:143–147. 2000.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Shannon C, Walsh G, Sapunar F, A'Hern R
and Smith I: Occult primary breast carcinoma presenting as axillary
lymphadenopathy. Breast. 11:414–418. 2002.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Buisman FE, van Gelder L, Menke-Pluijmers
MB, Bisschops BH, Plaisier PW and Westenend PJ: Non-primary breast
malignancies: A single institution's experience of a diagnostic
challenge with important therapeutic consequences-a retrospective
study. World J Surg Oncol. 14(166)2016.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Kemeny MM: Mastectomy: Is it necessary for
occult breast cancer? N Y State J Med. 92:516–517. 1992.PubMed/NCBI
|
|
111
|
Copeland EM and Mcbride CM: Axillary
metastases from unknown primary sites. Ann Surg. 178:21–27.
1973.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Kim BH, Kwon J and Kim K: Evaluation of
the benefit of radiotherapy in patients with occult breast cancer:
A population-based analysis of the SEER database. Cancer Res Treat.
50:551–561. 2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Rubovszky G, Kocsis J, Boér K,
Chilingirova N, Dank M, Kahán Z, Kaidarova D, Kövér E, Krakovská
BV, Máhr K, et al: Systemic treatment of breast cancer. 1st
Central-Eastern European professional consensus statement on breast
cancer. Pathol Oncol Res. 28(1610383)2022.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Song MW, Ki SY, Lim HS, Lee HJ, Lee JS and
Yoon JH: Axillary metastasis from occult breast cancer and
synchronous contralateral breast cancer initially suspected to be
cancer with contralateral axillary metastasis: A case report. BMC
Womens Health. 21(418)2021.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Wu SG, Zhang WW, Sun JY, Li FY, Lin HX,
Chen YX and He ZY: Comparable survival between additional
radiotherapy and local surgery in occult breast cancer after
axillary lymph node dissection: A population-based analysis. J
Cancer. 8:3849–3855. 2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Neal L, Sookhan N and Reynolds C: Occult
breast carcinoma presenting as gastrointestinal metastases. Case
Rep Med. 2009(564756)2009.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Ciulla A, Castronovo G, Tomasello G,
Maiorana AM, Russo L, Daniele E and Genova G: Gastric metastases
originating from occult breast lobular carcinoma: Diagnostic and
therapeutic problems. World J Surg Oncol. 6(78)2008.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Khan I, Malik R, Khan A, Assad S, Zahid M,
Sohail MS, Yasin F and Qavi AH: Breast cancer metastases to the
gastrointestinal tract presenting with Anemia and intra-abdominal
bleed. Cureus. 9(e1429)2017.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Rodrigues MV, Tercioti-Junior V, Lopes LR,
Coelho-Neto Jde S and Andreollo NA: Breast cancer metastasis in the
stomach: When the gastrectomy is indicated? Arq Bras Cir Dig.
29:86–89. 2016.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
120
|
Yang H, Li L, Zhang M, Zhang S, Xu S and
Ma X: Application of neoadjuvant chemotherapy in occult breast
cancer: Five case reports. Medicine (Baltimore).
96(e8200)2017.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Asaoka M, Gandhi S, Ishikawa T and Takabe
K: Neoadjuvant chemotherapy for breast cancer: Past, present, and
future. Breast Cancer (Auckl). 14(1178223420980377)2020.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Hessler LK, Molitoris JK, Rosenblatt PY,
Bellavance EC, Nichols EM, Tkaczuk KHR, Feigenberg SJ, Bentzen SM
and Kesmodel SB: Factors influencing management and outcome in
patients with occult breast cancer with axillary lymph node
involvement: Analysis of the National cancer database. Ann Surg
Oncol. 24:2907–2914. 2017.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Gosset M, Hamy AS, Mallon P, Delomenie M,
Mouttet D, Pierga JY, Lae M, Fourquet A, Rouzier R, Reyal F and
Feron JG: Prognostic impact of time to ipsilateral breast tumor
recurrence after breast conserving surgery. PLoS One.
11(e0159888)2016.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Liu YM, Ge JY, Chen YF, Liu T, Chen L, Liu
CC, Ma D, Chen YY, Cai YW, Xu YY, et al: Combined single-cell and
spatial transcriptomics reveal the metabolic evolvement of breast
cancer during early dissemination. Adv Sci (Weinh).
10(2205395)2023.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Chagpar AB, Cicek AF and Harigopal M: Can
tumor biology predict occult multifocal disease in breast cancer
patients? Am Surg. 83:704–708. 2017.PubMed/NCBI
|
|
126
|
Merkkola-von Schantz PA, Jahkola TA,
Krogerus LA and Kauhanen SMC: Reduction mammaplasty in patients
with history of breast cancer: The incidence of occult cancer and
high-risk lesions. Breast. 35:157–161. 2017.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Risk-reducing Salpingo-Oophorectomy in
Women at Higher Risk of Ovarian and Breast Cancer: A Single
Institution Prospective Series. AR 37, 2017.
|
|
128
|
Abe H, Naitoh H, Umeda T, Shiomi H, Tani
T, Kodama M and Okabe H: Occult breast cancer presenting axillary
nodal metastasis: A case report. Jpn J Clin Oncol. 30:185–187.
2000.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Montagna E, Bagnardi V, Rotmensz N, Viale
G, Cancello G, Mazza M, Cardillo A, Ghisini R, Galimberti V,
Veronesi P, et al: Immunohistochemically defined subtypes and
outcome in occult breast carcinoma with axillary presentation.
Breast Cancer Res Treat. 129:867–875. 2011.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Aydoğan F, Taşçı Y and Sagara Y: Phyllodes
Tumors of the Breast. In: Breast Disease. Aydiner A, İgci A and
Soran A (eds). Springer International Publishing, Cham, pp421-427,
2016.
|
|
131
|
Li L, Zhang D, Wen T, Wu Y, Lv D, Zhai J
and Ma F: Axillary lymph node dissection plus radiotherapy may be
an optimal strategy for patients with occult breast cancer. J
National Cancer Center. 2:198–204. 2022.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Schwab FD, Burger H, Isenschmid M, Kuhn A,
Mueller MD and Günthert AR: Suspicious axillary lymph nodes in
patients with unremarkable imaging of the breast. Eur J Obstet
Gynecol Reprod Biol. 150:88–91. 2010.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Shetty MK and Carpenter WS: Sonographic
evaluation of isolated abnormal axillary lymph nodes identified on
mammograms. J Ultrasound Med. 23:63–71. 2004.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Christiansen P, Carstensen SL, Ejlertsen
B, Kroman N, Offersen B, Bodilsen A and Jensen MB: Breast
conserving surgery versus mastectomy: Overall and relative
survival-a population based study by the Danish Breast Cancer
Cooperative Group (DBCG). Acta Oncol. 57:19–25. 2018.PubMed/NCBI View Article : Google Scholar
|
|
135
|
de Boniface J, Szulkin R and Johansson
ALV: Survival after breast conservation vs mastectomy adjusted for
comorbidity and socioeconomic status: A Swedish National 6-year
follow-up of 48 986 women. JAMA Surg. 156:628–637. 2021.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Keelan S, Flanagan M and Hill ADK:
Evolving trends in surgical management of breast cancer: An
analysis of 30 years of practice changing papers. Front Oncol.
11(622621)2021.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Saha A, Mukhopadhyay M, Das C, Sarkar K,
Saha AK and Sarkar DK: FNAC Versus core needle biopsy: A
comparative study in evaluation of palpable breast lump. J Clin
Diagn Res. 10:EC05–EC08. 2016.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Lieske B, Ravichandran D and Wright D:
Role of fine-needle aspiration cytology and core biopsy in the
preoperative diagnosis of screen-detected breast carcinoma. Br J
Cancer. 95:62–66. 2006.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Willems SM, Van Deurzen CHM and Van Diest
PJ: Diagnosis of breast lesions: Fine-needle aspiration cytology or
core needle biopsy? A review. J Clin Pathol. 65:287–292.
2012.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Calhoun BC and Collins LC: Predictive
markers in breast cancer: An update on ER and HER2 testing and
reporting. Semin Diagn Pathol. 32:362–369. 2015.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Onitilo AA, Engel JM, Greenlee RT and
Mukesh BN: Breast cancer subtypes based on ER/PR and Her2
expression: Comparison of clinicopathologic features and survival.
Clin Med Res. 7:4–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Wang R, Yang HX, Chen J, Huang JJ and Lv
Q: Best treatment options for occult breast cancer: A
meta-analysis. Front Oncol. 13(1051232)2023.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Tsai C, Zhao B, Chan T and Blair SL:
Treatment for occult breast cancer: A propensity score analysis of
the National Cancer Database. Am J Surg. 220:153–160.
2020.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Mariscotti G, Houssami N, Durando M,
Bergamasco L, Campanino PP, Ruggieri C, Regini E, Luparia A,
Bussone R, Sapino A, et al: Accuracy of mammography, digital breast
tomosynthesis, ultrasound and MR imaging in preoperative assessment
of breast cancer. Anticancer Res. 34:1219–1225. 2014.PubMed/NCBI
|
|
145
|
Kim WH, Chang JM, Moon HG, Yi A, Koo HR,
Gweon HM and Moon WK: Comparison of the diagnostic performance of
digital breast tomosynthesis and magnetic resonance imaging added
to digital mammography in women with known breast cancers. Eur
Radiol. 26:1556–1564. 2016.PubMed/NCBI View Article : Google Scholar
|
|
146
|
An YY, Kim SH and Kang BJ: Characteristic
features and usefulness of MRI in breast cancer in patients under
40 years old: Correlations with conventional imaging and prognostic
factors. Breast Cancer. 21:302–315. 2014.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Van Goethem M, Tjalma W, Schelfout K,
Verslegers I, Biltjes I and Parizel P: Magnetic resonance imaging
in breast cancer. Eur J Surg Oncol. 32:901–910. 2006.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Zeeshan M, Salam B, Khalid QSB, Alam S and
Sayani R: Diagnostic accuracy of digital mammography in the
detection of breast cancer. Cureus. 10(e2448)2018.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Li H, Mendel KR, Lan L, Sheth D and Giger
ML: Digital mammography in breast cancer: Additive value of
radiomics of breast parenchyma. Radiology. 291:15–20.
2019.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Seeram E: Full-Field Digital Mammography.
In: Digital Radiography. Springer Singapore, Singapore, pp111-123,
2019.
|
|
151
|
Jadvar H and Colletti PM: Competitive
advantage of PET/MRI. Eur J Radiol. 83:84–94. 2014.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Koolen BB, Vidal-Sicart S, Benlloch
Baviera JM and Valdés Olmos RA: Evaluating heterogeneity of primary
tumor (18)F-FDG uptake in breast cancer with a dedicated breast PET
(MAMMI): A feasibility study based on correlation with PET/CT. Nucl
Med Commun. 35:446–452. 2014.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Singh BK, Verma K and Thoke AS: Fuzzy
cluster based neural network classifier for classifying breast
tumors in ultrasound images. Exp Sys Appl. 66:114–123. 2016.
|
|
154
|
Gómez-Flores W and Ruiz-Ortega BA: New
fully automated method for segmentation of breast lesions on
ultrasound based on texture analysis. Ultrasound Med Biol.
42:1637–1650. 2016.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Kozegar E, Soryani M, Behnam H, Salamati M
and Tan T: Breast cancer detection in automated 3D breast
ultrasound using iso-contours and cascaded RUSBoosts. Ultrasonics.
79:68–80. 2017.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Mohammed MA, Al-Khateeb B, Rashid AN,
Ibrahim DA, Abd Ghani MK and Mostafa SA: Neural network and
multi-fractal dimension features for breast cancer classification
from ultrasound images. Comp Elect Eng. 70:871–882. 2018.
|
|
157
|
Moon WK, Chen IL, Yi A, Bae MS, Shin SU
and Chang RF: Computer-aided prediction model for axillary lymph
node metastasis in breast cancer using tumor morphological and
textural features on ultrasound. Comput Methods Programs Biomed.
162:129–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Kim SA, Chang JM, Cho N, Yi A and Moon WK:
Characterization of breast lesions: Comparison of digital breast
tomosynthesis and ultrasonography. Korean J Radiol. 16:229–238.
2015.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Cai SQ, Yan JX, Chen QS, Huang ML and Cai
DL: Significance and application of digital breast tomosynthesis
for the BI-RADS classification of breast cancer. Asian Pac J Cancer
Prev. 16:4109–4114. 2015.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Nakashima K, Uematsu T, Itoh T, Takahashi
K, Nishimura S, Hayashi T and Sugino T: Comparison of visibility of
circumscribed masses on Digital Breast Tomosynthesis (DBT) and 2D
mammography: Are circumscribed masses better visualized and assured
of being benign on DBT? Eur Radiol. 27:570–577. 2017.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Mercier J, Kwiatkowski F, Abrial C,
Boussion V, Dieu-de Fraissinette V, Marraoui W, Petitcolin-Bidet V
and Lemery S: The role of tomosynthesis in breast cancer staging in
75 patients. Diagn Interv Imaging. 96:27–35. 2015.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Roganovic D, Djilas D, Vujnovic S, Pavic D
and Stojanov D: Breast MRI, digital mammography and breast
tomosynthesis: Comparison of three methods for early detection of
breast cancer. Bosn J Basic Med Sci. 15:64–68. 2015.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Das BK, Biswal BM and Bhavaraju M: Role of
scintimammography in the diagnosis of breast cancer. Malays J Med
Sci. 13:52–57. 2006.PubMed/NCBI
|
|
164
|
Simanek M and Koranda P: SPECT/CT imaging
in breast cancer-current status and challenges. Biomed Pap Med Fac
Univ Palacky Olomouc Czech Repub. 160:474–483. 2016.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Berrington de González A, Mahesh M, Kim
KP, Bhargavan M, Lewis R, Mettler F and Land C: Projected cancer
risks from computed tomographic scans performed in the United
States in 2007. Arch Intern Med. 169:2071–2077. 2009.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Brem RF, Floerke AC, Rapelyea JA, Teal C,
Kelly T and Mathur V: Breast-specific gamma imaging as an adjunct
imaging modality for the diagnosis of breast cancer. Radiology.
247:651–657. 2008.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Yoon HJ, Kim Y, Chang KT and Kim BS:
Prognostic value of semi-quantitative tumor uptake on Tc-99m
sestamibi breast-specific gamma imaging in invasive ductal breast
cancer. Ann Nucl Med. 29:553–560. 2015.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Tan H, Zhang H, Yang W, Fu Y, Gu Y, Du M,
Cheng D and Shi H: Breast-specific gamma imaging with
Tc-99m-sestamibi in the diagnosis of breast cancer and its
semiquantitative index correlation with tumor biologic markers,
subtypes, and clinicopathologic characteristics. Nucl Med Commun.
37:792–799. 2016.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Yu X, Hu G, Zhang Z, Qiu F, Shao X, Wang
X, Zhan H, Chen Y, Deng Y and Huang J: Retrospective and
comparative analysis of (99m)Tc-Sestamibi breast specific gamma
imaging versus mammography, ultrasound, and magnetic resonance
imaging for the detection of breast cancer in Chinese women. BMC
Cancer. 16(450)2016.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Cho MJ, Yang JH, Yu YB, Park KS, Chung HW,
So Y, Choi N and Kim MY: Validity of breast-specific gamma imaging
for Breast Imaging Reporting and Data System 4 lesions on
mammography and/or ultrasound. Ann Surg Treat Res. 90:194–200.
2016.PubMed/NCBI View Article : Google Scholar
|