Introduction
Acute leukemia, which is characterised by abnormal
cell infiltration into the bone marrow, can be categorised
according to cell origin and genotypic characteristics, namely as
acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL)
(1). The most frequently observed
subtype in the adult patients is AML, where the mean 5-year
survival rate is 28% for patients aged ≥20 years (2).
When the prognostic factors are examined in patients
with AML, in addition to various patient-related factors, such as
age and comorbidities, there are also the parameters of cytogenic
characteristics, treatment-related AML, whether or not there is a
response to initial treatment, and measurable residual disease
positivity (MRD) (3).
By contrast, ALL is a group formed from T and B cell
progenitors that can be sub-classified according to genetic and
immunophenotypic characteristics (1). When the prognostic characteristics in
the course of ALL are examined, various factors are taken into
consideration, such as the initial white blood cell count, age and
cytogenetic characteristics (4).
Although severe elevations in the liver function
test (LFT) are rarely encountered at the time of diagnosis in
patients with acute leukemia, mild elevations occur more frequently
(5). Leukemic liver involvement
can either emerge as drug-related or associated with a number of
other reasons, such as primary liver disease (5). Liver dysfunction is a parameter that
is easily accessible, can be detected in the first hours after
diagnosis and can predict tissue involvement of the disease outside
of blood circulation. Therefore, liver dysfunction at the time of
diagnosis of AML is a parameter that requires investigation and can
assist the clinician in predicting prognosis. The aim of the
present study was to investigate liver dysfunction at the time of
diagnosis using the associated parameters in patients with AML.
Materials and methods
Patient recruitment and
assessment
The present retrospective study included 90 patients
with a diagnosis of AML who were hospitalised in the Hematology
Clinic of Dışkapı Yıldırım Beyazıt Training and Research Hospital
between April 2020 and August 2022. Patients who were eligible for
full-dose standard induction chemotherapy (3+7) (Daunorubicin, 60
mg/m2/1 to 3 days; Cytarabine (Ara-C), 100
mg/m2/1 to 7 days) were included in the study (6). All the patients included in the
present study received (3+7) standard induction therapy. Since
patients with serious liver dysfunction were not included and liver
dysfunction was attributed to leukemia as a result of the necessary
tests, no dose reduction was made in the treatment and the standard
dose was given. The demographic characteristics and descriptive
parameters of the patients participating in the prsesent study were
analyzed. The patients were then separated into two groups
according to the LFT results, namely into the liver dysfunction
group and those the normal LFT results group, before parameters
were compared between these two groups. The demographic
characteristics of the patients were recorded together with
hemogram results, anemia parameters, MRD and risk category, the
presence of hepatosplenomegaly and infection, neutrophil recovery
time (NRT), platelet recovery time (PRT) and LFT results [Alanine
amino transferase (ALT), aspartate amino transferase (AST),
alkaline phosphatase (ALP), γ-glutamyl transferase (GGT) and
Bilirubin].
Patients would be excluded from the study if they
had a history of continuous drug use (especially painkillers),
regular smoking and alcohol use-including those who had quit
smoking for <1 year, the presence of high plasma cholesterol and
triglyceride levels, heart failure, electrocardiographic changes,
viral and autoimmune hepatitis diagnosis, body mass index >25,
thyroid dysfunction, patients receiving low-intensity therapy and
those with comorbidities [for example, old age (>70 years),
frailty, sarcopenia] that may affect age- and sex-related
prognosis. All the patients underwent routine abdominal
ultrasonography to exclude possible diagnoses and patients with
normal results were included in the study.
Liver dysfunction would be considered if at least
two of the parameters used in the evaluation of liver function
tests were elevated simultaneously. Patients with the isolated
elevation of one parameter were excluded from the study. Patients
with elevated aminotransferases (15X), ALP (4X), GGT (4X) and
bilirubin (>3 mg/dl) were considered as severe group and were
excluded from the study. For the abdominal ultrasonography
measurements, liver size >16 cm was deemed to be hepatomegaly,
whereas spleen size >12 cm would be deemed as splenomegaly.
The risk category was determined according to the
European Leukemia Network (ELN) 2022 AML risk classification based
on cytogenetic results (7).
Cytogenetic analysis of the patients was performed on blood samples
taken from the bone marrow. Genetic abnormalities were reported
using karyotype analysis, fluorescence in situ hybridization
(FISH) and reverse transcription PCR. Favorable risk category
includes the following: t(8;21)/RUNX1:RUNX1T1, inv(16)/CBFB:MYH11, nucleophosmin (NPM1)
mutated without FMS-like tyrosine kinase-3 (FLT3)-internal tandem
duplication (ITD), and in-frame mutated bZIP CCAAT/enhancer-binding
protein α positivity (CEBPA). Intermediate risk category includes
the following: mutated NPM1 with FLT3-ITD, t (9;11)/MLLT3:KMT2A and
cytogenetic and/or molecular abnormalities not classified as
favorable or adverse. Adverse risk category includes the following:
t(6;9)/DEK:NUP214, KMT2A-rearranged, t(9;22)/BCR:ABL1,
t(8;16)/KAT6A:CREBBP, inv(3)/GATA2, MECOM (EVI1)-rearranged,
del(5q) or -5, abn(17p) or -7, Mutated ASXL1, BCOR,
EZH2, RUNX1, SF3B1, SRSF2,
STAG2, U2AF1, ZRSR2 or TP53 and complex
karyotype, and monosomal karyotype.
NRT and PRT were defined as the time from the
beginning of the chemotherapy protocol until the neutrophil count
was ≥0.5x109/l and the platelet count was
≥20x109/l for 3 consecutive days without transfusion
support, respectively.
MRD assessment by flow cytometric analysis was
performed on bone marrow (BM) samples obtained after standard
induction chemotherapy using a standard stain-lyse-wash procedure
with ammonium chloride lysis. A total of 1x106 cells
were stained per analysis tube. MRD was defined by comparison with
known antigen expression patterns by normal maturing myeloid
precursors and monocytes and then expressed as a percentage of
total leukocytes (8).
In the flow cytometric examination, values of
≤1/1,000 were accepted as MRD (-), whereas values of ≥1/1,000 were
deemed as MRD (+) (9).
Statistical analysis
The study data were analyzed using the SPSS v.27.0
software (IBM Corp). Descriptive statistical methods were used and
the results were stated as the mean ± standard deviation, median,
minimum and maximum values, number (n) and percentage (%). The
distribution of the variables was measured with the
Kolmogorov-Smirnov test and Shapiro-Wilk tests. In the analysis of
independent quantitative data, the Mann Whitney U-test was used.
Independent qualitative data were analyzed using the χ2
test or the Fischer test if χ2 assumptions were not met.
The effect level was investigated using univariate and multivariate
logistic regression. A receiver operating characteristic (ROC)
curve was applied to determine the effect level and cut-off values.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline data
Evaluation was made of 90 patients, comprising of 56
(62.2%) males and 34 (37.8%) females, with a mean age of 52.1±18.3
years. The distribution of the variables was measured using the
Kolmogorov-Smirnov test. The results of the analyses of the
demographic characteristics and descriptive parameters of the
patients in the study are shown in Table I.
 | Table IDescriptive statistics of the data and
distribution of the demographic parameters of the patients. |
Table I
Descriptive statistics of the data and
distribution of the demographic parameters of the patients.
| Parameter | Mean ± standard
deviation or N (%) |
|---|
| Age, years | 52.1±18.3 |
| Sex | |
|
Female | 34 (37.8) |
|
Male | 56 (62.2) |
| White blood cell
count, 103/µl | 30.3±58.9 |
| Hemoglobin, g/dl | 8.7±2.2 |
| Platelet,
103/µl | 71.4±118.0 |
| International
normalized ratio | 1.2±0.2 |
| Albumin, g/dl | 3.5±0.6 |
| Ferritin, ml/ng | 682.7±576.0 |
| B12 Vitamin,
pg/ml | 486.5±451.9 |
| Neutrophil recovery
time, days | 31.7±8.8 |
| Thrombocyte recovery
time, days | 29.0±9.6 |
| Hepatomegaly | |
|
(-) | 56 (62.2) |
|
(+) | 34 (37.8) |
| Splenomegaly | |
|
(-) | 60 (66.7) |
|
(+) | 30 (33.3) |
| Measurable residue
disease | |
|
(-) | 32 (35.6) |
|
(+) | 58 (64.4) |
| Risk Category | |
|
Favorable | 47 (52.2) |
|
İntermediate | 24 (26.7) |
|
Adverse | 19 (21.1) |
| Infection | |
|
(-) | 23 (25.6) |
|
(+) | 67 (74.4) |
The patients were separated into the liver
dysfunction (n=45) and those with normal LFT results (n=45). The
results of statistical analyses between the groups using the
χ2 test and Mann Whitney U-test showed that ΝRΤ and PRT,
MRD positivity, risk category and presence of infection were
statistically significantly higher in the group with liver
dysfunction compared with those in the normal LFT group (P<0.05;
Table II).
 | Table IIComparisons of the parameters between
the groups with and without LFT impairment. |
Table II
Comparisons of the parameters between
the groups with and without LFT impairment.
| Parameters | LFT impairment
(-) | LFT impairment
(+) | P-values |
|---|
| Age, years | 51.9±19.5 | 52.3±17.2 | 0.781b |
| Sex | | | 0.192a |
|
Female | 14 (31.1) | 20 (44.4) | |
|
Male | 31 (68.9) | 25 (55.6) | |
| White blood cell
count, 103/µl | 34.2±72.1 | 26.4±42.3 | 0.744b |
| Hemoglobin,
g/dl | 8.8±2.48 | 8.59±1.96 | 0.958b |
| Platelet,
103/µl | 85.3±155.7 | 57.5±59.5 | 0.744b |
| International
normalized ratio | 1.25±0.19 | 1.20±0.20 | 0.085b |
| Albumin, g/dl | 3.51±0.54 | 3.46±0.65 | 0.691b |
| Ferritin,
ml/ng | 629.5±617.0 | 735.9±533.5 | 0.145b |
| B12 Vitamin,
pg/ml | 473.0±444.7 | 500.0±463.6 | 0.878b |
| Neutrophil recovery
time, days | 29.5±8.7 | 33.8±8.4 | 0.022b |
| Thrombocyte
recovery time, days | 26.9±9.1 | 31.2±9.8 | 0.026b |
| Hepatomegaly | | |
>0.999a |
|
(-) | 28 (62.2) | 28 (62.2) | |
|
(+) | 17 (37.8) | 17 (37.8) | |
| Splenomegaly | | | 0.371a |
|
(-) | 32 (71.1) | 28 (62.2) | |
|
(+) | 13 (28.9) | 17 (37.8) | |
| Measurable residue
disease | | | 0.008a |
|
(-) | 22 (48.9) | 10 (22.2) | |
|
(+) | 23 (51.1) | 35 (77.8) | |
| Risk category | | | 0.030a |
|
Favorable | 29 (64.4) | 18 (40.0) | |
|
İntermediate | 11 (24.4) | 13 (28.9) | |
|
Adverse | 5 (11.1) | 14 (31.1) | |
| Infection | | | 0.008a |
|
(-) | 17 (37.8) | 6 (13.3) | |
|
(+) | 28 (62.2) | 39 (86.7) | |
Univariate and multivariate model
results
In the univariate model, when the patient group with
liver dysfunction was compared with the group with normal LFT, the
risk category, MRD positivity, presence of infection, and NRT and
PRT values were observed to be significant variables for the effect
of liver dysfunction on the prognosis of acute myeloid leukemia.
(P<0.05; Table III). In the
multivariate model, when the patient group with liver dysfunction
was compared with the group with normal LFT, the risk category, MRD
positivity, and PRT value were observed to be
significant-independent variables for the effect of liver
dysfunction on the prognosis of acute myeloid leukemia (Table III).
 | Table IIILogistic regression analysis. |
Table III
Logistic regression analysis.
| | Univariate
model | Multivariate
model |
|---|
| Parameter | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Risk Category | 2.084 | 1.192-3.646 | 0.010 | 2.542 | 1,344-4.806 | 0.004 |
| Measurable residue
disease | 3.348 | 1.342-8.351 | 0.010 | 4.249 | 1.523-11.857 | 0.006 |
| Infection | 3.946 | 1.381-11.274 | 0.010 | | | |
| Neutrophil recovery
time | 1.060 | 1.008-1.115 | 0.023 | | | |
| Thrombocyte
recovery time | 1.050 | 1.003-1.099 | 0.037 | 1.060 | 1.008-1.115 | 0.024 |
ROC results
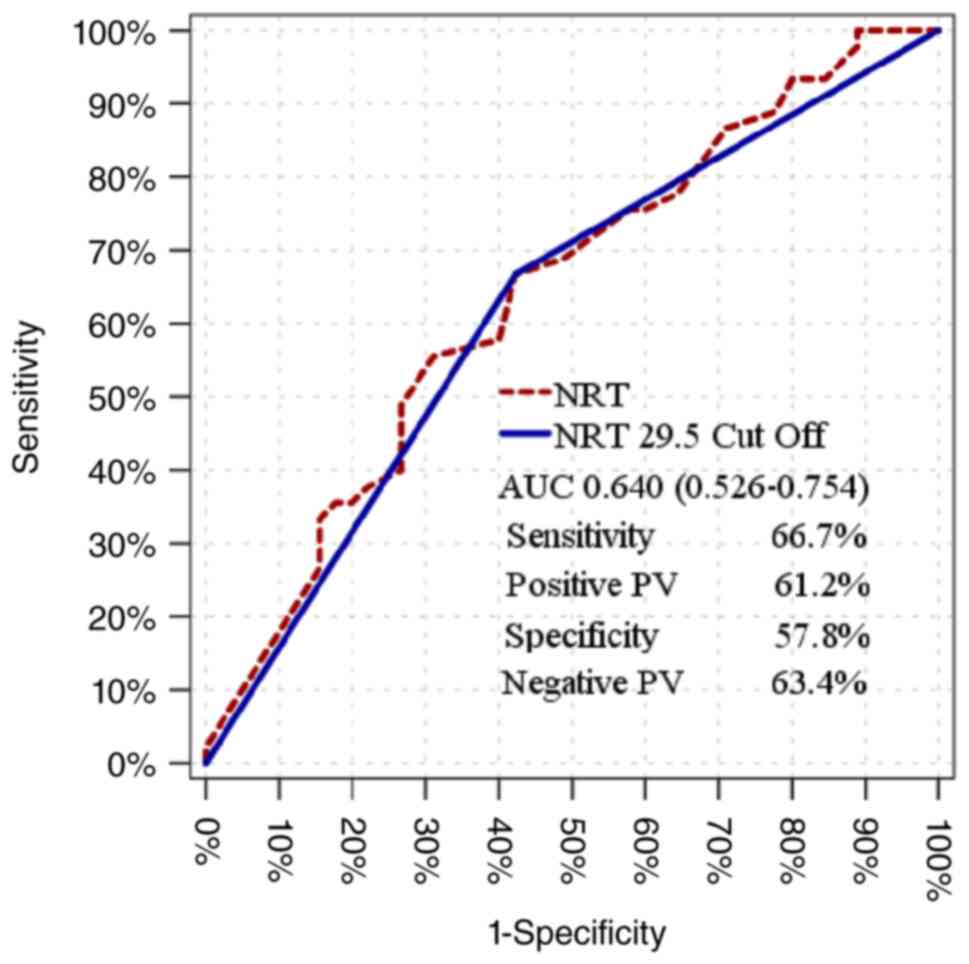
The NRT value was seen to have a significant effect
[Area under the curve (AUC), 0.640 (0.526-0.754)] in distinguishing
patients with and without liver dysfunction (Table IV) The significant effect at the
cut-off value of 29.5 for NRT [AUC, 0.622 (0.506-0.739)] was 57.8%,
where the negative prediction rate was 63.4% in distinguishing
patients with and without liver dysfunction with a sensitivity of
66.7% and the positive prediction rate was 61.2% for specificity
(Fig. 1 and Table V).
 | Table IVReceiver operating characteristic
curve using NRT cut-off. |
Table IV
Receiver operating characteristic
curve using NRT cut-off.
| Parameter | Area under the
curve | 95% CI | P-value |
|---|
| NRT | 0.640 | 0.526-0.754 | 0.022 |
| NRT 29.5
cut-off | 0.622 | 0.506-0.739 | 0.046 |
 | Table VDistribution of patients in the group
at 29.5 NRT cut-off value |
Table V
Distribution of patients in the group
at 29.5 NRT cut-off value
| Neutrophil recovery
timea,b | Liver dysfunction
(-) | Liver dysfunction
(+) |
|---|
| ≤29.5 | 26 | 15 |
| >29.5 | 19 | 30 |
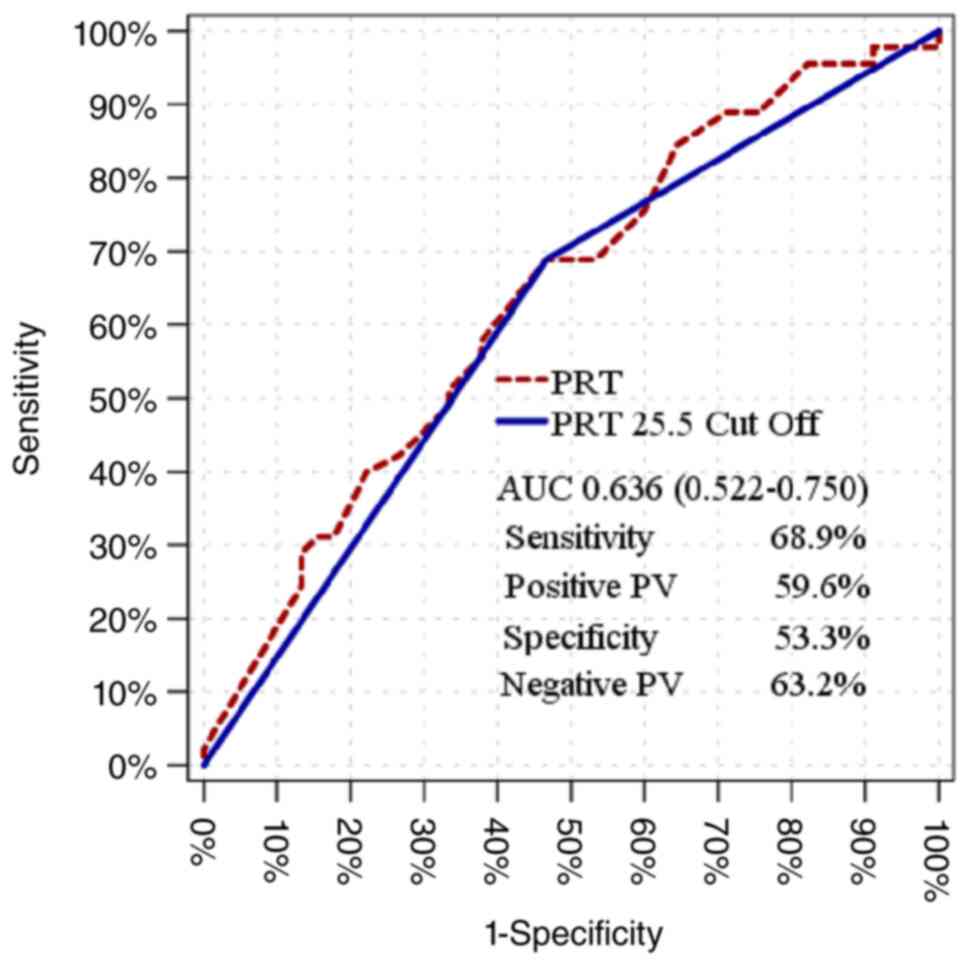
The PRT value was observed to have a significant
effect [AUC, 0.636 (0.522-0.750)] in distinguishing patients with
and without liver dysfunction (Table
VI). Youden index was used to find the cut-off value. The PRT
cut-off value at 25.5 was determined to be statistically
significant [AUC, 0.611 (0.494-0.728)] in distinguishing patients
with and without liver dysfunction, with a sensitivity of 68.9%,
positive prediction of 59.6%, specificity of 53.3% and negative
prediction of 63.2% (Fig. 2 and
Table VII).
 | Table VIReceiver operating characteristic
curve using PRT cut-off. |
Table VI
Receiver operating characteristic
curve using PRT cut-off.
| Parameter | Area under the
curve | 95% CI | P-value |
|---|
| PRT | 0.636 | 0.522-0.750 | 0.026 |
| PRTa,b 25.5 cut-off | 0.611 | 0.494-0.728 | 0.069 |
 | Table VIIDistribution of patients in the group
at 25.5 PRT cut-off value |
Table VII
Distribution of patients in the group
at 25.5 PRT cut-off value
| Platelet recovery
timea,b | Liver dysfunction
(-) | Liver dysfunction
(+) |
|---|
| ≤25.5 | 24 | 14 |
| >25.5 | 21 | 31 |
Discussion
Hemolytic anemias, disorders in clotting factors,
myeloproliferative neoplasm, multiple myeloma, leukemia and
lymphomas are the main hematological diseases affecting the liver
(10).
AML is a hematological neoplasm that is
characterized by the clonal proliferation of immature cells of
myeloid origin, called blasts, in the peripheral blood and/or bone
marrow (11). Generally a certain
blast ratio (>20%) is required for AML diagnosis (12). The annual incidence of AML is 4.3
per 100,000, with the median age of onset of 68 years in the United
States (13). With developing
technology, the possibility of early diagnosis and treatment
increases, which results in an extended life expectancy. As life
expectancy increases, malignancies that increase in frequency with
age may also increase the likelihood of being seen in the general
population. In addition, with advancements in technology and an
increase in genetic studies, the speed, ease and frequency of
diagnosis have also increased (14). For the prognosis of patients,
although there have been developments in various prognotic
evaluation parameters, especially those of the genetic variety (for
example NUP98 rearrangement, KMT2A rearrangement), the process
remains time-consuming (6,7). Therefore, simple parameters that can
provide results within a short period of time at the time of
diagnosis would be of benefit for the clinician. LFT are parameters
that can be easily accessed and provide results rapidly (within the
first 4 h after the patient is admitted) at the time of diagnosis.
In the present study, it was investigated whether LFT can be of
benefit in terms of overall survival at the time of diagnosis in
patients with AML.
Although the main site of hematopoiesis in adult
life is the bone marrow, the liver, which is the main site of
hematopoiesis in fetal life, also continues this function (15). Therefore, liver dysfunction is one
of the most common conditions encountered in hematological
disorders (15). Gastrointestinal
symptoms are present in ~25% cases of acute leukemias at the time
of diagnosis. By contrast, liver and spleen involvement is less
common in acute leukemias compared with in chronic leukemia cases
(16).
Liver involvement in hematological malignancies and
other rheumatological and oncological systemic diseases can be
explained by four main mechanisms, namely vascular, toxic, immune
and hormonal (17). As a result of
such mechanisms, damage is frequently observed in hepatocytes,
cholangiocytes and endothelial cells (17). In acute leukemia, these mechanisms
underlie hepatocellular necrosis due to infiltration by leukemic
cells and lymphocytes (18).
Therefore, it is of high importantance to distinguish whether the
initial liver dysfunction is due to leukemic infiltration or if it
is unrelated to the acute leukosis. Liver dysfunction due to
leukemic infiltration improves after induction chemotherapy.
Therefore, it is difficult to distinguish at the time of diagnosis
(18,19).
By contrast, in cases of chronic lymphocytc
leukemia, leukemic cell infiltration, primary and secondary hepatic
malignancies, drug-induced hepatotoxicity, immunological effects,
infections and Richter transformation are possible causes of LFT
disorders (20).
It has been previously shown that mild-to-moderate
liver dysfunction can be encountered in patients with newly
diagnosed acute leukemia. In paediatric patients diagnosed with
ALL, liver dysfunction at the time of diagnosis has been determined
at the rate of 34% in Canada (21). In a study by Sandart et al
(22) on paediatric patients
diagnosed with AML, liver involvement at the time of diagnosis was
reported at the rate of 29.5% (22).
The present study included a total of 90 patients
with newly diagnosed AML who met the study criteria, who were then
separated into two groups, namely those with and without liver
dysfunction at the time of diagnosis. Since those with age and
sex-dependent diseases [for example, old age (>70 years),
frailty, sarcopenia] that can potentially confound prognosis were
excluded from the study, there was no statistically significant
difference in age distribution between the two groups. Age is a
parameter that can affect prognosis and causes restrictions in AML
treatment protocols. Depending on age, it may be preferable to
reduce the dose or apply less intense treatments (23). In the multivariate model, the
independent variables of risk category, MRD positivity and PRT
value were determined to be significant in the patient group with
liver dysfunction compared to the group without.
In patients diagnosed with acute leukemia, there is
a number of prognostic factors, such as patient-related (age, lack
of physical capacity, etc.) disease-related (WBC >200,000 at
diagnosis) and cytogenetic characteristics (adverse risc category:
tp53, RUNX etc.). Risk category is one of the most important
parameters for determining the prognosis and treatment regimen of
patients with acute leukemia (6).
In the present study, the ELN 2022 AML risk classification was used
to determine the risk category of each patient.
Determining the risk category by performing genetic
testing at the time of AML diagnosis, performing diagnostic blast
percentage and MRD monitoring with flow cytometry are parameters
used in routine practice, which can be used to predict prognosis.
In addition, determining the relationship between liver dysfunction
at the time of diagnosis and cytogenetic and MRD monitoring can
contribute to commenting on prognosis (24). In the present study, patients in
the favorable risk category were determined at the rate of 40.0% in
the group with liver dysfunction and 64.4% in the group without
liver dysfunction, whilst the frequency of patients in the adverse
risk category was found to be 31.1% in the group with liver
dysfunction and 11.1% in the group without liver dysfunction.
According to these results, the probability of finding patients
with adverse risk category is higher in patients with liver
dysfunction at the time of diagnosis. These data can also be
interpreted as the number of patients who will be given intensive
treatment and indications for transplantation may be higher in
patients with liver dysfunction, at the time of diagnosis.
MRD is an independent risk factor that can be used
to determine the deeper (post-treatment blast rate <5% and MRD
negative) remission status, risk classification and treatment plan
after diagnosis. It can be measured using flow cytometry, PCR and
next-generation sequencing methods (25). In a study by Sandart et al
(22) of paediatric patients with
acute leukemia, liver involvement at the time of diagnosis was
reported to be associated with subtype, leukocyte count and patient
age. In addition, liver involvement was determined to be associated
with minimal residual disease, overall survival and time to relapse
(22).
In the present study, there was a high rate of MRD
positivity (77.8%) in the group with liver dysfunction. Since MRD
positivity is an independent risk factor for prognosis, it may be
more likely that MRD positivity will be detected after standard
induction chemotherapy in patients with liver dysfunction at the
time of diagnosis. Liver dysfunction at the time of diagnosis can
be considered to indicate the presence/infiltration of the disease
of AML in a solid organ tissue other than the bone marrow. It can
be hypothesed that treating abnormal cell death in the liver is
more difficult than abnormal cell death in the bloodstream, such
that treatment will be less effective in cases of solid organ
involvement. As a result, liver dysfunction at the time of
diagnosis can be of guidance for assessing prognosis.
In the present study, a statistically significant
association was determined between liver dysfunction and MRD
positivity. This parameter was also a significant, independent
variable in the multivariate model for the effect of liver
dysfunction on the prognosis of acute myeloid leukemia. Based on
these results, the detection of liver dysfunction at the time of
diagnosis can likely be used to predict a high recurrence risk of
leukemia, poor prognosis and overall survival.
In patients diagnosed with acute leukemia, the
longer the duration of neutropenia and thrombocytopenia after
chemotherapy, the greater the risk of complications (26,27).
Certain patients will also succumb to various infections due to
this prolonged neutropenic period (26). Increasing the duration of
thrombocytopenia increases the risk of mild-to-severe bleeding in
patients, resulting in the risk of mortality (27). Yamazaki et al (28) reported that the recovery of
hematopoiesis after induction chemotherapy, especially rapid
platelet recovery, was an important determinant of relapse-free
survival in patients with AML (28). In another previous study, it was
reported that prolonged NRT was associated with grade 3-4 infection
and relapse (29). In the present
study, NRT and PRT were statistically significantly higher in
patients with liver dysfunction at diagnosis, where subsequent
multivariate analysis found PRT to be a significant, independent
variable together with MRD positivity and risk category for the
effect of liver dysfunction on the prognosis of acute myeloid
leukemia.
In patients with liver dysfunction at the time of
diagnosis, PRT was found to be longer. This lengthy period causes
an increase in the patient's bleeding risk, replacement number and
duration. The increase in bleeding risk correspondingly increases
the risk of morbidity and mortality. The increase in replacement
number may cause transfusion reactions during the later stages of
treatment and adverse reactions during transplantation. In
addition, NRT was found to be longer in patients with liver
dysfunction at the time of diagnosis. This prolonged period
increases the patient's exposure to infections, the duration of
antibiotic use and the risk of encountering resistant bacteria
emergence during the treatment regimen. When all of the
aforementioned conditions are taken into consideration, detection
of liver dysfunction at the time of diagnosis may be predictive in
terms of prognosis. Previous studies have established a
relationship between the degree of liver dysfunction and prognosis
(10,22,30).
Liver dysfunction was detected in 20% patients with hematological
malignancies who required intensive care and was found to be an
independent risk factor for mortality, where a relationship was
found between the degree of liver dysfunction and poor prognosis
(30).
LFTs are simple, rapid and potentially beneficial
evaluation tools for the first evaluation of patients with newly
diagnosed acute leukemia. Liver dysfunction at the time of
diagnosis can be a clinical guide for patient follow-up. In a
patient with liver dysfunction at the time of diagnosis, the risk
of infection, bleeding and the possibility of recurrence should be
kept in mind during follow-up.
For the accurate evaluation of the parameters,
patients with severe liver dysfunction values were excluded from
the study design. Due to the exclusion of such severe cases, the
relationship between the degree of liver dysfunction and prognosis
in newly diagnosed patients with AML was not evaluated in the
present study. Additional comprehensive studies including patients
with severe liver dysfunction are needed for this evaluation in the
future.
Acknowledgements
The authors would like to thank Mr. Ertan Koç and Dr
Guner Kilic (Etlik City Hospital, Ankara, Turkey) for their
valuable assistance with statistical analysis.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Data collection was performed by DIS and FY.
Statistical analysis was performed by BS and MRA. FY, HBAÖ, BS and
MRA conducted the literature search, writing of the article and
confirm the authenticity of all the raw data. FY, HBAÖ, DIS, AKG
and MA analyzed the results and contributed to the final
manuscript. The original draft was written by FY, AKG and MA. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. The present study was approved by the Ethics Committee
of Etlik City Hospital (date, 03.05.2023; approval no.
AEŞH-EK1-2023-142; Ankara, Turkey). Patients of Dışkapı Training
and Research Hospital Hematology Clinic were transferred to Etlik
City Hospital after the closure of Dışkapı Training and Research
Hospital Hematology Clinic. The study plan was explained in detail
both verbally and in writing to the patients included in the
present study. All patients provided a signed informed consent
form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chennamadhavuni A, Lyengar V, Mukkamalla S
and Shimanovsky A: Leukemia.[Updated 2023 Jan 17]. StatPearls
Publishing, Treasure Island, FL, 2023.
|
|
2
|
Chennamadhavuni A, Lyengar V and
Shimanovsky A: Leukemia. StatPearls. StatPearls Publishing,
Treasure Island, FL, USA, 2022.
|
|
3
|
Stubbins RJ, Francis A, Kuchenbauer F and
Sanford D: Management of acute myeloid leukemia: A review for
general practitioners in oncology. Curr Oncol. 29:6245–6259.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Künz T, Hauswirth AW, Hetzenauer G, Rudzki
J, Nachbaur D and Steiner N: Changing landscape in the treatment of
adult acute lymphoblastic leukemia (ALL). Cancers (Basel).
14(4290)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bruguera M and Miquel R: The Effect of
Haematological and Lymphatic Diseases on the Liver. In: Textbook of
Hepatology: From basic science to clinical practice. Rodés J,
Benhamou JP, Blei AT, Reichen J, Rizzetto M (eds). Wiley, Hoboken,
NJ, 1662-1670, 2007.
|
|
6
|
Döhner H, Estey E, Grimwade D, Amadori S,
Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA,
et al: Diagnosis and management of AML in adults: 2017 ELN
recommendations from an international expert panel. Blood.
129:424–447. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Döhner H, Wei AH, Appelbaum FR, Craddock
C, DiNardo CD, Dombret H, Ebert BL, Fenaux P, Godley LA, Hasserjian
RP, et al: Diagnosis and management of AML in adults: 2022
recommendations from an international expert panel on behalf of the
ELN. Blood. 140:1345–1377. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ravandi F, Jorgensen J, Borthakur G,
Jabbour E, Kadia T, Pierce S, Brandt M, Wang S, Konoplev S, Wang X,
et al: Persistence of minimal residual disease assessed by
multiparameter flow cytometry is highly prognostic in younger
patients with acute myeloid leukemia. Cancer. 123:426–435.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
San Miguel JF, Vidriales MB, López-Berges
C, Dıaz-Mediavilla J, Gutiérrez N, Canizo C, Ramos F, Calmuntia MJ,
Pérez JJ, González M and Orfao A: Early immunophenotypical
evaluation of minimal residual disease in acute myeloid leukemia
identifies different patient risk groups and may contribute to
postinduction treatment stratification. Blood. 98:1746–1751.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shan S, Zhao XY and Jia JD: Hepatic
manifestations of hematological diseases. Zhonghua Gan Zang Bing Za
Zhi. 30:347–351. 2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
11
|
Vakiti A and Mewawalla P: Acute myeloid
leukemia, 2018.
|
|
12
|
Park HS: What is new in acute myeloid
leukemia classification? Blood Res. 59(15)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shallis RM, Wang R, Davidoff A, Ma X and
Zeidan AM: Epidemiology of acute myeloid leukemia: Recent progress
and enduring challenges. Blood Rev. 36:70–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pulumati A, Pulumati A, Dwarakanath BS,
Verma A and Papineni RV: Technological advancements in cancer
diagnostics: Improvements and limitations. Cancer Rep (Hoboken).
6(e1764)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Medvinsky A, Rybtsov S and Taoudi S:
Embryonic origin of the adult hematopoietic system: Advances and
questions. Development. 138:1017–1031. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ebert EC and Hagspiel KD: Gastrointestinal
manifestations of leukemia. J Gastroenterol Hepatol. 27:458–463.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Edwards L and Wanless IR: Mechanisms of
liver involvement in systemic disease. Best Pract Res Clin
Gastroenterol. 27:471–483. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gu RL, Xiang M, Suo J and Yuan J: Acute
lymphoblastic leukemia in an adolescent presenting with acute
hepatic failure: A case report. Mol Clin Oncol. 11:135–138.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Walsh LR, Yuan C, Boothe JT, Conway HE,
Mindiola-Romero AE, Barrett-Campbell OO, Yerrabothala S and
Lansigan F: Acute myeloid leukemia with hepatic infiltration
presenting as obstructive jaundice. Leuk Res Rep.
15(100251)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kreiniz N, Katz OB, Polliack A and Tadmor
T: The clinical spectrum of hepatic manifestations in chronic
lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 17:863–869.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Segal I, Rassekh SR, Bond MC, Senger C and
Schreiber RA: Abnormal liver transaminases and conjugated
hyperbilirubinemia at presentation of acute lymphoblastic leukemia.
Pediatr Blood Cancer. 55:434–439. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sandart A, Harila-Saari A, Arnell H,
Fischler B and Vakkila J: Pattern and prevalence of liver
involvement in pediatric acute lymphoblastic and myeloid leukemia
at diagnosis. J Pediatr Gastroenterol Nutr. 73:630–635.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Choi JH, Shukla M and Abdul-Hay M: Acute
myeloid leukemia treatment in the elderly: A comprehensive review
of the present and future. Acta Haematol. 146:431–457.
2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tazi Y, Arango-Ossa JE, Zhou Y, Bernard E,
Thomas I, Gilkes A, Freeman S, Pradat Y, Johnson SJ, Hills R, et
al: Unified classification and risk-stratification in acute myeloid
leukemia. Nat Commun. 13(4622)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hourigan C, Gale R, Gormley N,
Ossenkoppele G and Walter R: Measurable residual disease testing in
acute myeloid leukaemia. Leukemia. 31:1482–1490. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lustberg MB: Management of neutropenia in
cancer patients. Clin Adv Hematol Oncol. 10(825)2012.PubMed/NCBI
|
|
27
|
Gao A, Zhang L and Zhong D:
Chemotherapy-induced thrombocytopenia: Literature review. Discov
Oncol. 14(10)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamazaki E, Kanamori H, Itabashi M, Ogusa
E, Numata A, Yamamoto W, Ito S, Tachibana T, Hagihara M, Matsumoto
K, et al: Hyper-recovery of platelets after induction therapy is a
predictor of relapse-free survival in acute myeloid leukemia. Leuk
Lymphoma. 58:104–109. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Løhmann DJ, Asdahl PH, Abrahamsson J, Ha
SY, Jónsson ÓG, Kaspers GJL, Koskenvuo M, Lausen B, De Moerloose B,
Palle J, et al: Associations between neutrophil recovery time,
infections and relapse in pediatric acute myeloid leukemia. Pediatr
Blood Cancer. 65(e27231)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Van de Louw A, Twomey K, Habecker N and
Rakszawski K: Prevalence of acute liver dysfunction and impact on
outcome in critically ill patients with hematological malignancies:
A single-center retrospective cohort study. Ann Hematol.
100:229–237. 2021.PubMed/NCBI View Article : Google Scholar
|