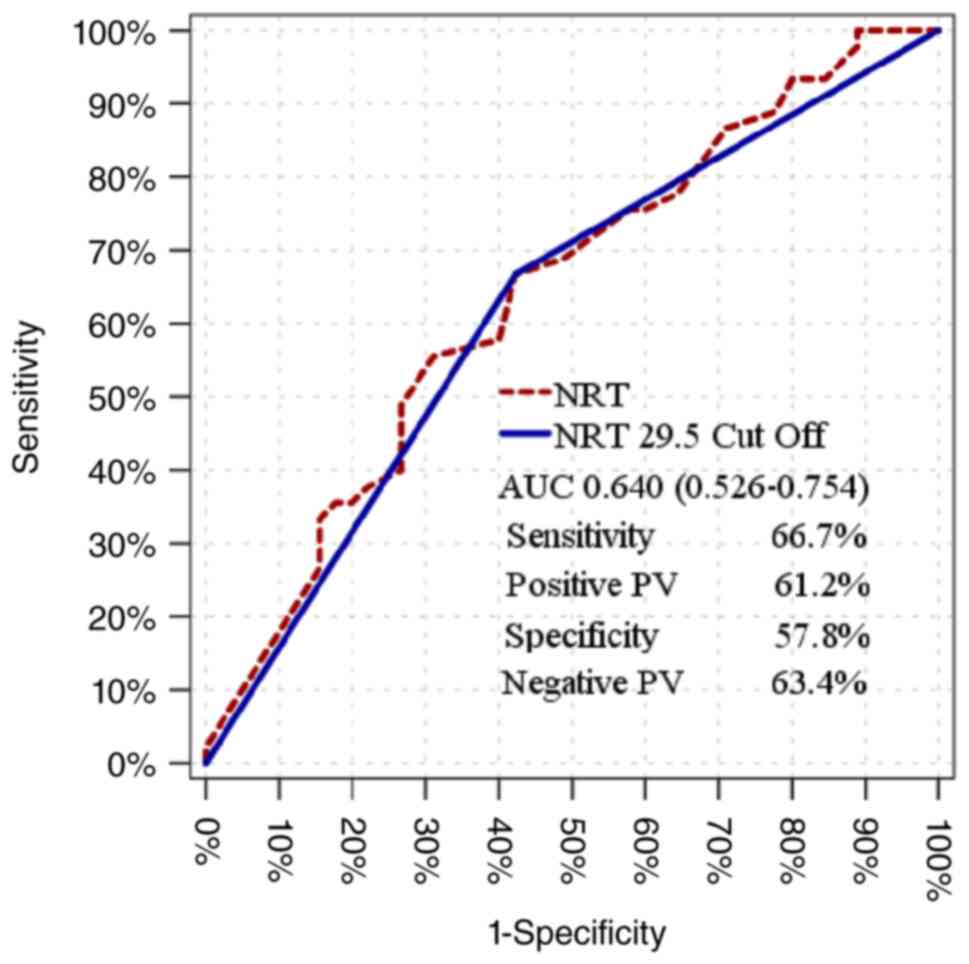
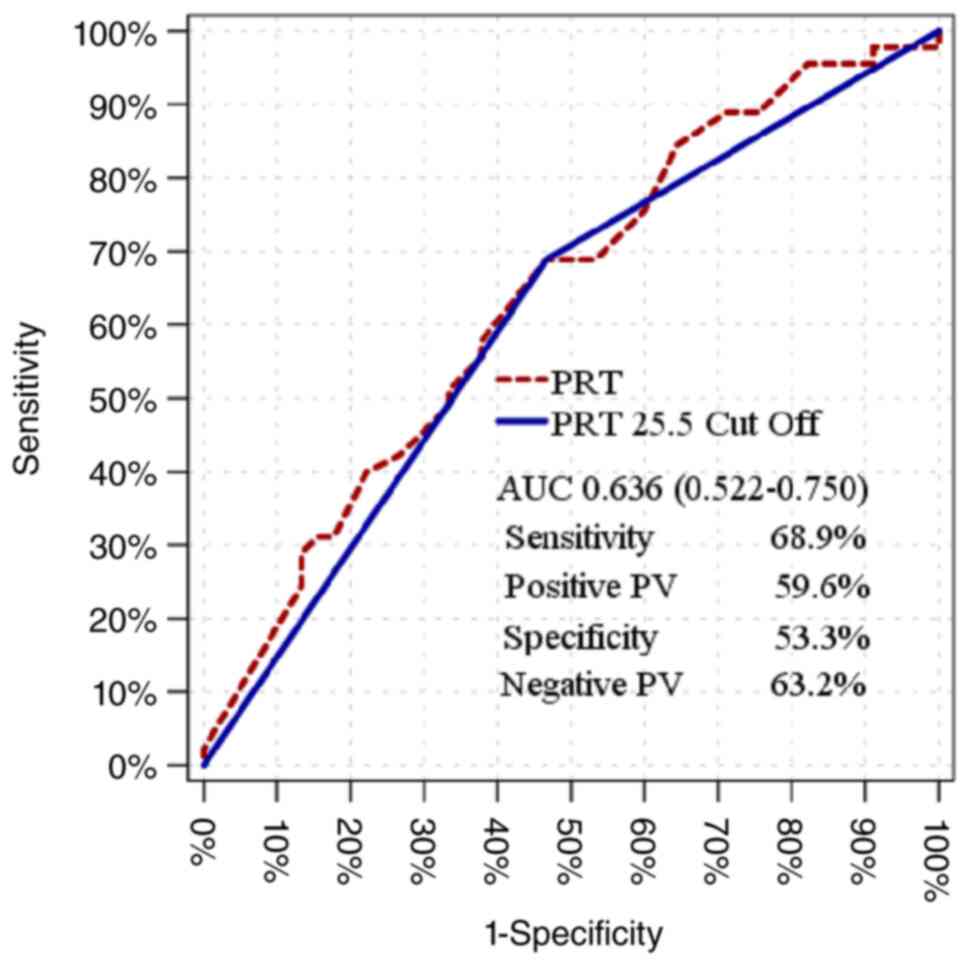
|
1
|
Chennamadhavuni A, Lyengar V, Mukkamalla S
and Shimanovsky A: Leukemia.[Updated 2023 Jan 17]. StatPearls
Publishing, Treasure Island, FL, 2023.
|
|
2
|
Chennamadhavuni A, Lyengar V and
Shimanovsky A: Leukemia. StatPearls. StatPearls Publishing,
Treasure Island, FL, USA, 2022.
|
|
3
|
Stubbins RJ, Francis A, Kuchenbauer F and
Sanford D: Management of acute myeloid leukemia: A review for
general practitioners in oncology. Curr Oncol. 29:6245–6259.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Künz T, Hauswirth AW, Hetzenauer G, Rudzki
J, Nachbaur D and Steiner N: Changing landscape in the treatment of
adult acute lymphoblastic leukemia (ALL). Cancers (Basel).
14(4290)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bruguera M and Miquel R: The Effect of
Haematological and Lymphatic Diseases on the Liver. In: Textbook of
Hepatology: From basic science to clinical practice. Rodés J,
Benhamou JP, Blei AT, Reichen J, Rizzetto M (eds). Wiley, Hoboken,
NJ, 1662-1670, 2007.
|
|
6
|
Döhner H, Estey E, Grimwade D, Amadori S,
Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA,
et al: Diagnosis and management of AML in adults: 2017 ELN
recommendations from an international expert panel. Blood.
129:424–447. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Döhner H, Wei AH, Appelbaum FR, Craddock
C, DiNardo CD, Dombret H, Ebert BL, Fenaux P, Godley LA, Hasserjian
RP, et al: Diagnosis and management of AML in adults: 2022
recommendations from an international expert panel on behalf of the
ELN. Blood. 140:1345–1377. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ravandi F, Jorgensen J, Borthakur G,
Jabbour E, Kadia T, Pierce S, Brandt M, Wang S, Konoplev S, Wang X,
et al: Persistence of minimal residual disease assessed by
multiparameter flow cytometry is highly prognostic in younger
patients with acute myeloid leukemia. Cancer. 123:426–435.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
San Miguel JF, Vidriales MB, López-Berges
C, Dıaz-Mediavilla J, Gutiérrez N, Canizo C, Ramos F, Calmuntia MJ,
Pérez JJ, González M and Orfao A: Early immunophenotypical
evaluation of minimal residual disease in acute myeloid leukemia
identifies different patient risk groups and may contribute to
postinduction treatment stratification. Blood. 98:1746–1751.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shan S, Zhao XY and Jia JD: Hepatic
manifestations of hematological diseases. Zhonghua Gan Zang Bing Za
Zhi. 30:347–351. 2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
11
|
Vakiti A and Mewawalla P: Acute myeloid
leukemia, 2018.
|
|
12
|
Park HS: What is new in acute myeloid
leukemia classification? Blood Res. 59(15)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shallis RM, Wang R, Davidoff A, Ma X and
Zeidan AM: Epidemiology of acute myeloid leukemia: Recent progress
and enduring challenges. Blood Rev. 36:70–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pulumati A, Pulumati A, Dwarakanath BS,
Verma A and Papineni RV: Technological advancements in cancer
diagnostics: Improvements and limitations. Cancer Rep (Hoboken).
6(e1764)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Medvinsky A, Rybtsov S and Taoudi S:
Embryonic origin of the adult hematopoietic system: Advances and
questions. Development. 138:1017–1031. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ebert EC and Hagspiel KD: Gastrointestinal
manifestations of leukemia. J Gastroenterol Hepatol. 27:458–463.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Edwards L and Wanless IR: Mechanisms of
liver involvement in systemic disease. Best Pract Res Clin
Gastroenterol. 27:471–483. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gu RL, Xiang M, Suo J and Yuan J: Acute
lymphoblastic leukemia in an adolescent presenting with acute
hepatic failure: A case report. Mol Clin Oncol. 11:135–138.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Walsh LR, Yuan C, Boothe JT, Conway HE,
Mindiola-Romero AE, Barrett-Campbell OO, Yerrabothala S and
Lansigan F: Acute myeloid leukemia with hepatic infiltration
presenting as obstructive jaundice. Leuk Res Rep.
15(100251)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kreiniz N, Katz OB, Polliack A and Tadmor
T: The clinical spectrum of hepatic manifestations in chronic
lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 17:863–869.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Segal I, Rassekh SR, Bond MC, Senger C and
Schreiber RA: Abnormal liver transaminases and conjugated
hyperbilirubinemia at presentation of acute lymphoblastic leukemia.
Pediatr Blood Cancer. 55:434–439. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sandart A, Harila-Saari A, Arnell H,
Fischler B and Vakkila J: Pattern and prevalence of liver
involvement in pediatric acute lymphoblastic and myeloid leukemia
at diagnosis. J Pediatr Gastroenterol Nutr. 73:630–635.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Choi JH, Shukla M and Abdul-Hay M: Acute
myeloid leukemia treatment in the elderly: A comprehensive review
of the present and future. Acta Haematol. 146:431–457.
2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tazi Y, Arango-Ossa JE, Zhou Y, Bernard E,
Thomas I, Gilkes A, Freeman S, Pradat Y, Johnson SJ, Hills R, et
al: Unified classification and risk-stratification in acute myeloid
leukemia. Nat Commun. 13(4622)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hourigan C, Gale R, Gormley N,
Ossenkoppele G and Walter R: Measurable residual disease testing in
acute myeloid leukaemia. Leukemia. 31:1482–1490. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lustberg MB: Management of neutropenia in
cancer patients. Clin Adv Hematol Oncol. 10(825)2012.PubMed/NCBI
|
|
27
|
Gao A, Zhang L and Zhong D:
Chemotherapy-induced thrombocytopenia: Literature review. Discov
Oncol. 14(10)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamazaki E, Kanamori H, Itabashi M, Ogusa
E, Numata A, Yamamoto W, Ito S, Tachibana T, Hagihara M, Matsumoto
K, et al: Hyper-recovery of platelets after induction therapy is a
predictor of relapse-free survival in acute myeloid leukemia. Leuk
Lymphoma. 58:104–109. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Løhmann DJ, Asdahl PH, Abrahamsson J, Ha
SY, Jónsson ÓG, Kaspers GJL, Koskenvuo M, Lausen B, De Moerloose B,
Palle J, et al: Associations between neutrophil recovery time,
infections and relapse in pediatric acute myeloid leukemia. Pediatr
Blood Cancer. 65(e27231)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Van de Louw A, Twomey K, Habecker N and
Rakszawski K: Prevalence of acute liver dysfunction and impact on
outcome in critically ill patients with hematological malignancies:
A single-center retrospective cohort study. Ann Hematol.
100:229–237. 2021.PubMed/NCBI View Article : Google Scholar
|