Introduction
Silver (Ag) can be used as a coating for orthopedic
implants due to its antimicrobial properties (1), with applications primarily in
arthroplasty in tumor patients (2)
and in revisions performed due to periprosthetic joint infections
(3). Ionic silver (Ag+)
exerts numerous intracellular effects, including opening of pores
in the bacterial membrane, denaturation of intracellular proteins,
and accumulation of DNA damage caused by the generation of reactive
oxygen species (4). Similarly,
Ag+ poses potential toxicity to osteogenic cells
(5,6). This is why Ag-coated implants are not
used clinically for cementless fixation of arthroplasty implants:
coatings are limited to their extraosseous components only. An
exception to this is the Kyocera® Ag-hydroxyapatite
coating, which is applied to cementless cups, stems, and lumbar
interbody cages (7,8).
Several orthopedic implants equipped with Ag
coatings are currently in clinical practice. These include the
electrochemically Ag-coated MUTARS® prosthesis
(Implantcast), containing 0.7-1.2 grams of Ag per implant, and hip
or knee megaprostheses with a thinner PorAg® coating
deposited by physical vapor deposition (Waldemar Link), containing
up to 0.33 grams per implant. The Agluna® implants
intended for primary arthroplasty are electrochemically coated with
only 0.006 g of Ag per implant (3,9),
whereas the Kyocera® implants have 0.0028 g of Ag per
implant (8). Ag+ levels
in the blood of patients who have received these prostheses range
from 1.4 to 200 ppb (9), and the
reported side effects (e.g., local argyria) are generally mild and
rare (10-12).
Ag-coated intramedullary nails with covalently attached Ag on the
titanium surface of the nail (Bactiguard®) have been
used in open long bone fractures (13), leading to uneventful bone healing
with few infections. Plates for fracture fixation have also been
coated with Ag (14). Although
Ag-coated locking plates are still not commonplace in clinical
practice, evidence from a rabbit humerus osteotomy model showed
uneventful fracture healing when bridged with an Ag-coated plate
containing 60 µg of Ag (15). The
PorAg® coating, commonly used in oncological cases and
periprosthetic joint infections, reduces bacterial counts by 68%
compared to uncoated, grit-blasted titanium. This benefit, however,
is offset by a reduction in osteoblast viability (5).
Ag-mediated toxicity to osteogenic cells is dose
dependent (16). Therefore, below
a specific concentration threshold, the osteoconductivity of the
implant should not be hampered while sufficient antibacterial
activity is maintained. Our previous research showed that
additively manufactured trabecular titanium coated with silver
nitrate (TLSN) containing 7 at% of Ag effectively suppressed S.
aureus biofilm formation. Conversely, the alkaline phosphatase
(ALP) and lactate dehydrogenase (LDH) activity of osteoblasts
cultured on these samples did not differ from that of osteoblasts
cultured on uncoated titanium (6).
The literature concerning the effects of
Ag+ concentrations on the gene expression profile of
osteogenic markers, bone differentiation, and mineralization of
human osteogenic cells and primary human osteoblasts is sparse. The
genes encoding alkaline phosphatase (ALPL) and collagen type I
chains a1 and a2 (COL1A1 and COL1A2) are early markers of
osteogenic differentiation and defects in these genes are
implicated in such diseases as hypophosphatasia and osteogenesis
imperfecta (17,18). Osteocalcin (OCN) is a glycoprotein
synthesized by osteoblasts at their later stages of
differentiation, plays an important role in bone mineralization by
binding calcium and hydroxyapatite (19-21).
Therefore, the expression of the proteins mentioned above is a
prerequisite for a healthy bone matrix, which is essential for
osseointegration and long-term implant stability (22).
Consequently, this study sought to investigate the
effects of elevated Ag+ concentrations on titanium's
osseointegration potential. We examined the impact of different
Ag+ concentrations on the morphology, osteogenic
differentiation, expression of the ALPL, COL1A1, and COL1A2 genes,
and mineralization by human mesenchymal stem cells (hMSCs) and
primary human osteoblasts (hOBs).
Materials and methods
Cells and culture
hMSCs derived from bone marrow were purchased from
Sigma (C-12974) and stored in liquid nitrogen until use. hOBs were
collected from five different patients who underwent hip
arthroplasty at Uppsala University Hospital between Q4 2022 and Q1
2024 (Swedish Ethical Review Authority approval number 2020-04462),
using a previously published protocol (23). Briefly, the retrieved femoral heads
were diced into small fragments, which were rinsed with PBS and
then placed in 25-cm2 flasks containing alpha-modified
minimum essential medium (αMEM; Cytiva SH30265.01, obtained from
Fisher Scientific 10346952), 10% fetal bovine serum (FBS;
Sigma-Aldrich/Merck F9665), 1% penicillin/streptomycin, and 0.5%
amphotericin B (Cytiva HyClone®, Fisher Scientific
11556461 and Gibco® 15290026, Fisher Scientific
11520496). The culture medium (5 ml) was refreshed once weekly
until confluence was reached.
To investigate the effects of Ag+ on cell
viability and differentiation, different concentrations of
AgNO3 (Sigma-Aldrich/Merck S6506) were added to the
culture media. Both hMSCs and hOBs used for immunohistochemistry
and differentiation studies were cultured in αMEM supplemented with
10% FBS, 1% PeSt, and 0.5% amphotericin B for 4 weeks.
AgNO3 was introduced to the growth media at
concentrations of 0 ppm (control), 0.5 ppm, 1 ppm, 1.5 ppm and at
the dynamic concentration range of TLSN (Table I). This addition took place 24 h
after cell seeding (day 0). The Ag+ concentrations in
Table I simulate the temporal
release profile of Ag+ from TLSN implants, as discussed
previously (6). The cells were
seeded in 24-well plates at a density of 35,000 cells per well,
with cell numbers measured using a NucleoCounter®, and
the medium (1 ml per well) was refreshed every other day. After 1
week of culture, the medium was supplemented with 10 mM
β-glycerophosphate (Sigma-Aldrich/Merck G9422), 100 nM
dexamethasone (Sigma-Aldrich/Merck D4902), and 80 µM ascorbic acid
(Sigma-Aldrich/Merck A4544) to stimulate osteoblastic
differentiation. Cell morphology and viability were examined at 3,
7, 14, 21, and 28 days using live-image microscopy (Leica DMi8
Microscope with INCUBATORi8 environmental chamber). Representative
phase contrast images were taken at all time points (10x
magnification, 12 ms exposure time).
 | Table IAgNO3 concentrations in
the cell media for the entirety of the experiment. |
Table I
AgNO3 concentrations in
the cell media for the entirety of the experiment.
| Time-point | Base-medium | Control, ppm | 0.5, ppm | 1.0, ppm | 1.5, ppm | TLSN, ppm |
|---|
| Day 0 | Complete
medium | 0.0 | 0.5 | 1.0 | 1.5 | 0.7 |
| Day 2 | Complete
medium | 0.0 | 0.5 | 1.0 | 1.5 | 0.7 |
| Day 4 | Complete
medium | 0.0 | 0.5 | 1.0 | 1.5 | 0.2 |
| Day 6 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.2 |
| Day 8 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.2 |
| Day 10 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.1 |
| Day 12 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.1 |
| Day 14 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.1 |
| Day 16 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.1 |
| Day 18 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.1 |
| Day 20 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.1 |
| Day 22 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.0 |
| Day 24 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.0 |
| Day 26 | OIM | 0.0 | 0.5 | 1.0 | 1.5 | 0.0 |
Cell experiments with hMSCs and hOBs were performed
in triplicates. For hOBs, four biological replicates (n=4) were
used for ALP and LDH assays, two biological replicates for the gene
experiments after 1 and 2 weeks, five biological replicates for the
gene experiments after 4 weeks, and one biological replicate for
the mineralization assay (Fig.
S1).
Osteogenic differentiation
Osteogenic differentiation was expressed as the
ratio between alkaline phosphatase (ALP) and lactate dehydrogenase
(LDH) ALP/LDH, and was assessed after 1, 2, and 4 weeks of exposure
to Ag+. The medium was discarded, and the cells were
rinsed with PBS, followed by enzymatic lysis with 400 µl of lysis
buffer (CelLytic® M, Sigma-Aldrich/Merck C2978) per well
for 15 min on a shaker at room temperature (RT). 50 µl of the
lysate was mixed with the In Vitro Toxicology Assay Kit
(LDH, TOX7; Sigma-Aldrich/Merck) and the ALP substrate
(p-nitrophenyl phosphate; Sigma-Aldrich/Merck P7998) in 96-well
plates, as per the manufacturer's protocol. The 96-well plates were
incubated at 37˚C for 30 min, and the absorbance was measured in a
spectrophotometer at 690 and 492 nm (Multiscan Ascent, ThermoFisher
Scientific, Waltham, MA, USA) for LDH and at 405 nm for ALP. ALP
absorbance values were converted to concentrations (in mM) using a
standard calibration curve of nitrophenol dilutions (4-Nitrophenol
solution, Sigma-Aldrich/Merck N7660) ranging from 0 to 2.5 mM.
COL1A1, COL1A2, and ALPL gene
expression
The messenger RNA (mRNA) levels of
osteogenic-related genes in hMSCs and hOBs were analyzed through
real-time quantitative polymerase chain reaction (RT-qPCR) at 1, 2,
and 4 weeks. The cells were cultured as previously described, lysed
at the endpoints using 400 µl of TRIzol®
(Invitrogen®, bought from ThermoFisher 15596026) and
stored at -20˚C until use. Ribonucleic acid (RNA) extraction was
performed according to the manufacturer's protocol, and the total
RNA yield was determined using a Nanodrop (ND-1000
Spectrophotometer, ThermoFisher Scientific Inc., Waltham, MA, USA).
Subsequently, RNA was reverse transcribed into complementary DNA
(cDNA) (High Capacity RNA to c-DNA® kit, Applied
Biosystems®, bought from ThermoFisher, 4387406), and RT
measured the expression of osteogenic-related genes-qPCR (7500 Fast
RT-PCR System, Applied Biosystems®, ThermoFisher
Scientific Inc. 4387406). The primers listed in Table II were used for quantification,
and GAPDH (FAM®/MGB probe, non-primer limited) (Applied
Biosystems®, bought from ThermoFisher, 4333764F) was
used as a housekeeping gene. All primers were purchased from
ThermoFisher Scientific [TaqMan® Fast Universal PCR
Master Mix (2X), catalogue number 4331182, no AmpEras®
UNG, product code 4352042]. The melting curves' cycle threshold
(CT) values were calculated and expressed using the 2DDΔCT
method.
 | Table IITaqMan probes for the primers used in
gene expression quantification. |
Table II
TaqMan probes for the primers used in
gene expression quantification.
| Acronym | Name | TaqMan assay no.
ID |
|---|
| COL1A1 | Collagen type I
alpha 1 | Hs00164004_m1 |
| COL1A2 | Collagen type I
alpha 2 | Hs01028970_m1 |
| ALPL | Alkaline
phosphatase | Hs01029144_m1 |
Histochemistry, immunofluorescence,
and mineralization assays
Following 4 weeks of culture, the cell nuclei and
cytoplasm were stained and visualized with an inverted Leica
microscope (Leica DMi8, Microsystem CMS, Wetzlar, Germany) after
staining. The cell nuclei were stained with
4',6-diamidino-2-phenylindole (DAPI; Invitrogen, Waltham, MA, USA),
and the cytoplasm was stained with carboxyfluorescein diacetate
(CFDA; Merck KGaA, Darmstadt, Germany). Intracellular osteocalcin
(OCN) was detected by immunofluorescence. In detail, cells were
fixed with 4% v/v paraformaldehyde at RT for 20 min and then
permeabilized with 0.1% Triton X-100 (Merck KGaA) for 15 min. The
cytoplasm was stained with 500 nM CFDA for 15 min. Blocking of
unspecific epitopes was performed with a normal 10% goat serum
(s-1000; Sigma-Aldrich, Sweden) in a washing solution consisting of
PBS with 2% bovine serum albumin (BSA) and 0.3% Triton X-100 for 30
min. The plates were incubated with a solution containing the
anti-OCN antibody (20 µg/ml monoclonal mouse anti-human OCN
MAB1419; R&D Systems, Abingdon, UK) overnight at 4˚C. The wells
were rinsed four times with PBS/1% Triton X-100, and the secondary
antibody (1:200, goat anti-mouse, Biotin Novus NB7537; Bio-Techne,
Abingdon, UK) was added. The wells were left under agitation at RT.
Following the rinsing procedure, the cells were stained with DAPI
(300 nM) and Dylight Streptavidin Red (Vector sa-5549,
concentration 20 µg/ml), both dissolved in PBS, for a duration of
30 min at RT. Subsequently, the wells were rinsed four times with
PBS/1% Triton X-100.
Calcium deposits in the culture wells were measured
with Alizarin red staining (AR; Sigma-Aldrich/Merck A5533) as a
proxy for mineralization after 4 weeks of culture. The cells were
rinsed with PBS and fixed with 70% ice-cold ethanol for 1 h. The
wells were then rinsed with distilled water and stained with 40 mM
AR at pH 4.2 for 10 min at RT. Next, the dye was removed, and the
cells were rinsed with distilled water until the supernatant became
transparent, followed by rinsing with PBS on a shaker for 15 min at
300 rpm. The AR dye was finally eluted from the cells with 10 wt%
cetylpyridinium chloride (Sigma-Aldrich/Merck C9002) in 10 mM
sodium phosphate solution for 20 min at 300 rpm at RT. The
absorbance of this supernatant was measured at 562 nm and converted
to concentration values ranging from 0.05 to 0.8 mM using an
alizarin red calibration curve.
Statistics
All statistical tests were performed using R
software version 4.3.3(24), with
the level of statistical significance set at P<0.05. To evaluate
the difference of the means of the treatment groups at each time
point, Levene's test (leveneTest) was used to assess whether the
assumption of homogeneity of variance was met before performing an
ANOVA (aov). Dunnett's (dunn.test) or Tukey's (TukeyHSD) tests were
used for post hoc pairwise comparisons, depending on the
presence of a control group. If the condition of homogeneity of
variance was not met, the nonparametric Kruskal-Wallis test
(kruskal.test) was used with Dunn's test (dunn.test) for pairwise
comparisons. Graphs were created with GraphPad Prism, the bar
height corresponding to the mean value and the error bars to the
standard deviation.
Results
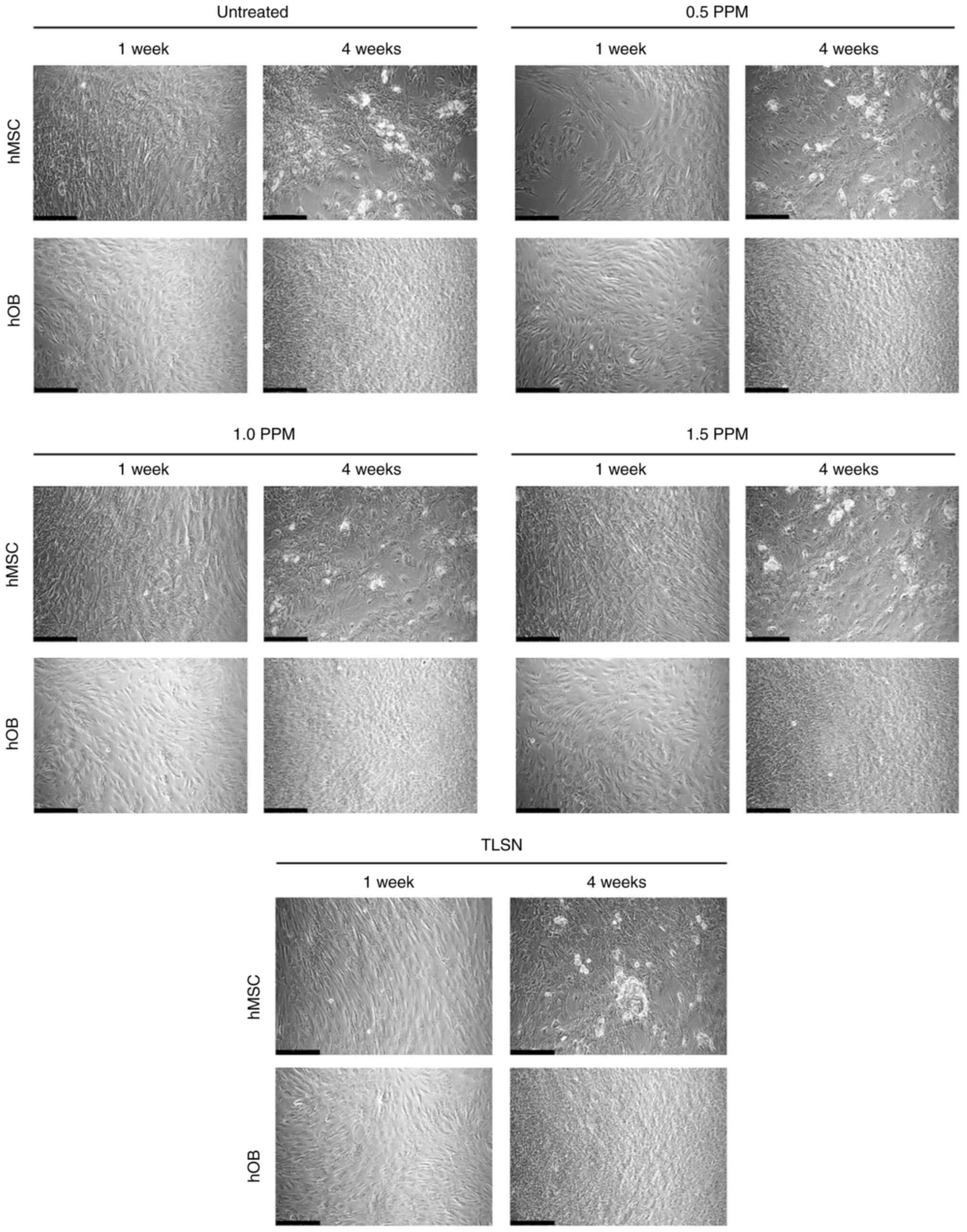
Cell microscopy and
immunofluorescence
Both hMSCs and hOBs in the control wells covered the
entire area of the well plate and exhibited an elongated fibrillary
shape. When visualized with bright-field microscopy after 1 or 4
weeks of culture, no obvious differences were evident in the cell
shape or confluence of hMSCs or hOBs after exposure to different
Ag+ concentrations (Fig.
1).
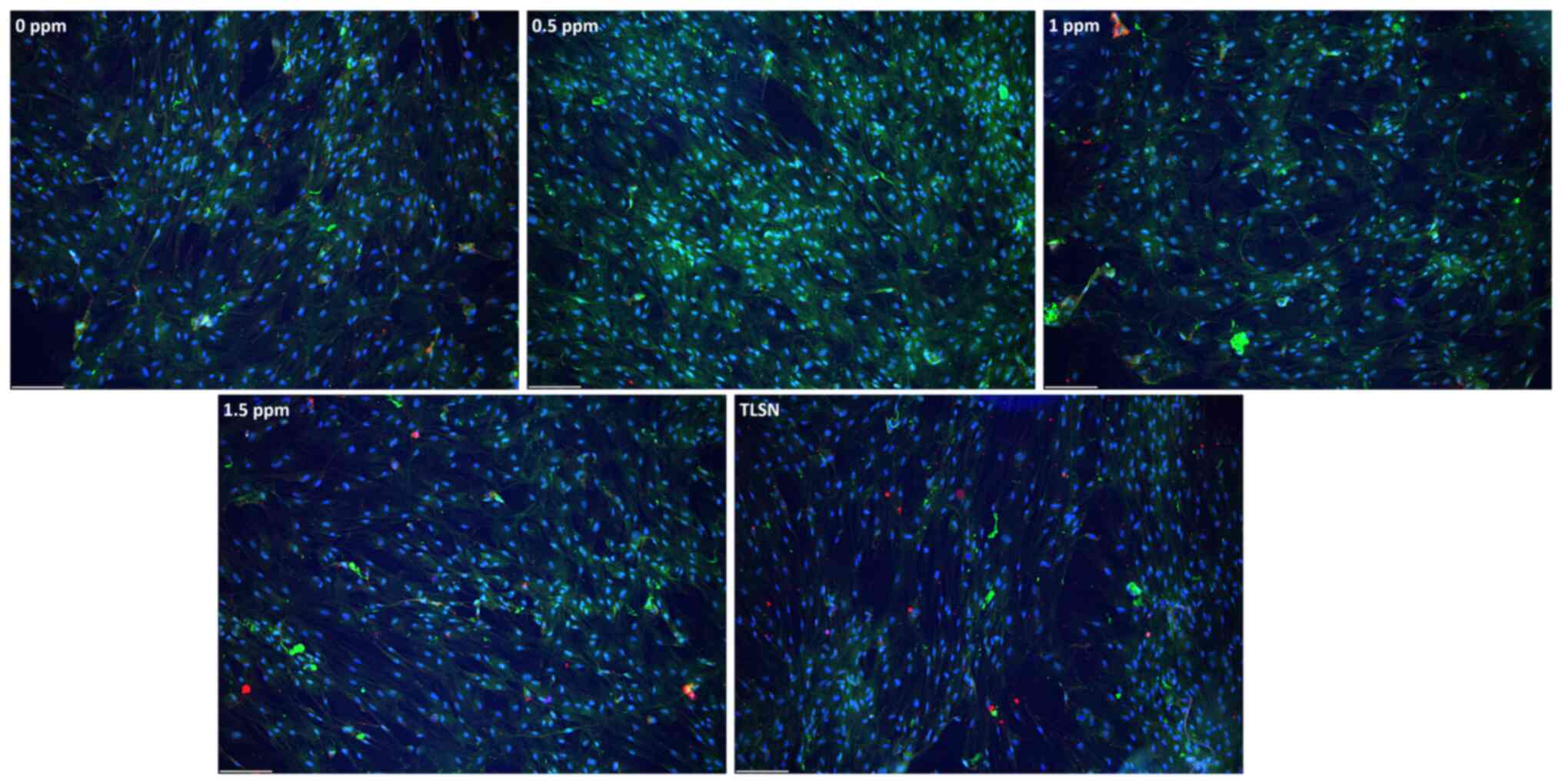
Fluorescence microscopy of fixed and stained cells
indicated that hMSCs had a homogenous distribution across the
surface of the wells after 4 weeks of culture (Figs. 2, S2), and the cells generally maintained
an elongated shape with a prominent nucleus. Cell growth was slower
at an Ag+ concentration of 1.5 ppm, as evidenced by the
sparser cells (Fig. 2). No
differences in the OCN staining pattern were detected between cells
treated with different Ag+ concentrations and control
cells not exposed to Ag+ (Figs. 2, S3).
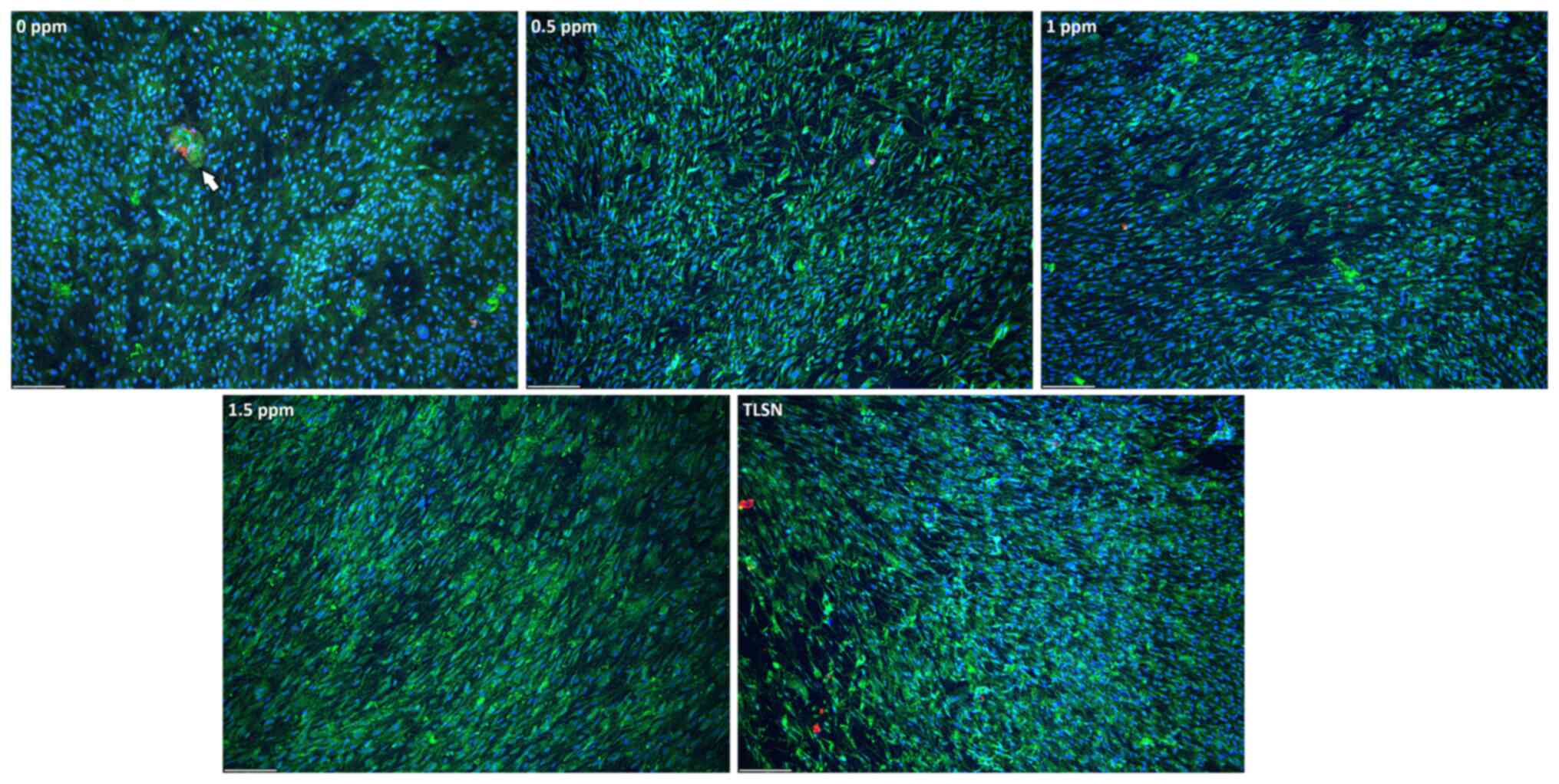
In the untreated hOB cultures, the cells developed a
confluent monolayer, exhibiting rapid migration in diverse
directions (Fig. 3).
Agglomerations of cells, identified by OCN staining, were observed
within the untreated group (Figs.
3, S3). With the introduction
of Ag+, no major differences in cell confluence were
observed. However, the cellular cytoskeleton of hOB cells exposed
to Ag+ exhibited a thinner profile and a more
leaf-shaped morphology. HOBs featured a higher CFDA signal
intensity, with a tendency to form aggregates in both the untreated
and the Ag+-exposed groups (Fig. 3). The pattern was similar for all
Ag+ concentrations tested, with no evident OCN staining
compared to the untreated group (Fig.
S3).
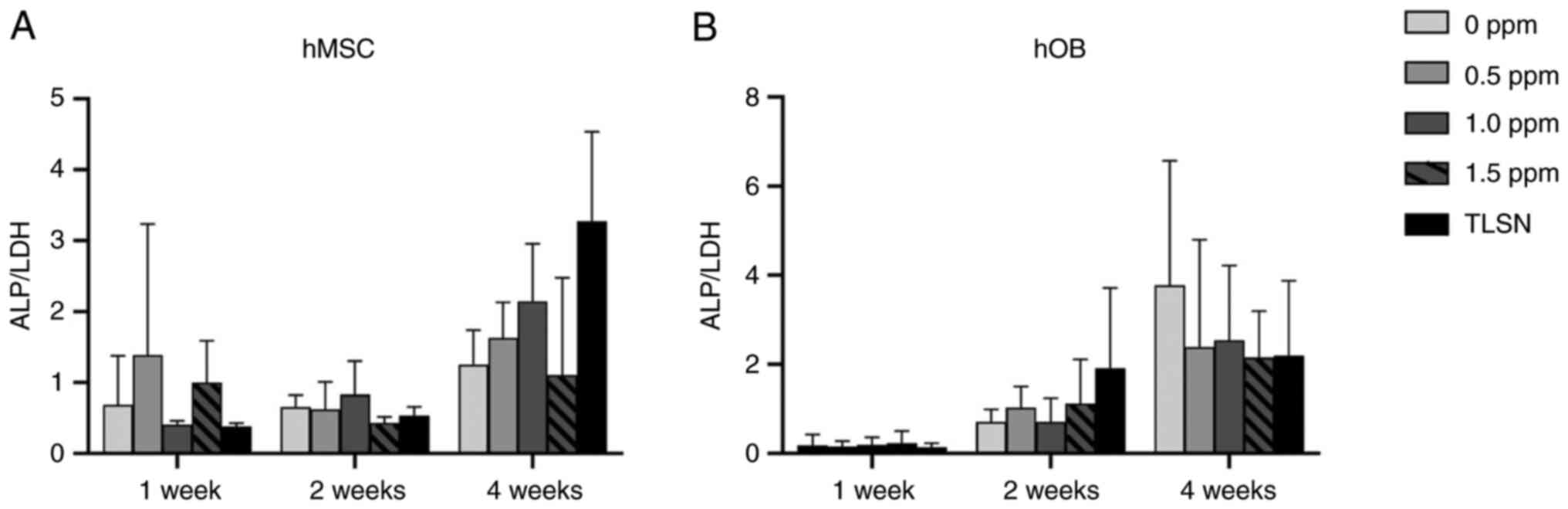
Osteogenic differentiation
In the untreated and Ag-treated hMSC cultures, the
ALP/LDH ratio increased with time (Fig. 4), and no statistically significant
differences were found between the different Ag+
concentrations and the control group not exposed to Ag+
at any time. Overall, the osteogenic activity of the hMSCs was not
affected, even at higher concentrations of Ag+. For the
hOBs, the ALP/LDH ratio displayed a more consistent rise,
demonstrating a progressive trend over the 4-week period. The
highest ratios were observed in the control group not exposed to
Ag+ at the final time point after 4 weeks. In contrast
to hMSCs, hOBs were inclined to display decreased osteogenic
activity at concentrations of Ag+ of 0.5 ppm or higher
at 4 weeks. However, similar to hMSCs, no statistically significant
differences were seen between groups at any time.
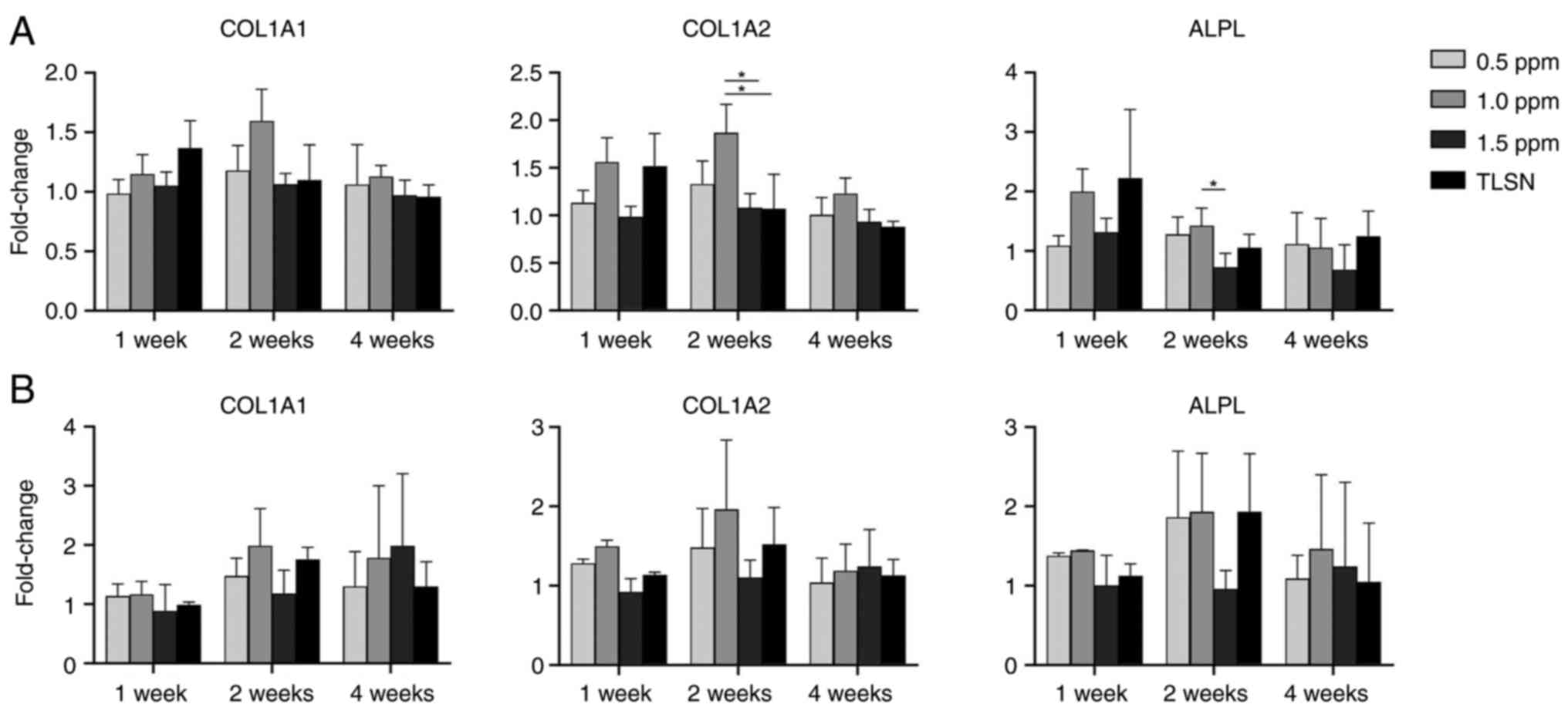
Gene expression
In hMSC cultures (Fig.
5A), exposure to Ag+ concentrations of ≥ 1 ppm
significantly suppressed COL1A2 and ALPL gene transcription after 2
weeks of culture. Gene expression showed an upward trend during the
first week of culture but then gradually decreased over time. In
hOB cultures, no clear concentration-dependent effect was detected
(Fig. 5B). In hOB cells at 4
weeks, the genes did not generally display a clear pattern of
change with increasing concentrations of Ag+. Gene
expression trended upward over time without significant differences
between Ag+ concentrations.
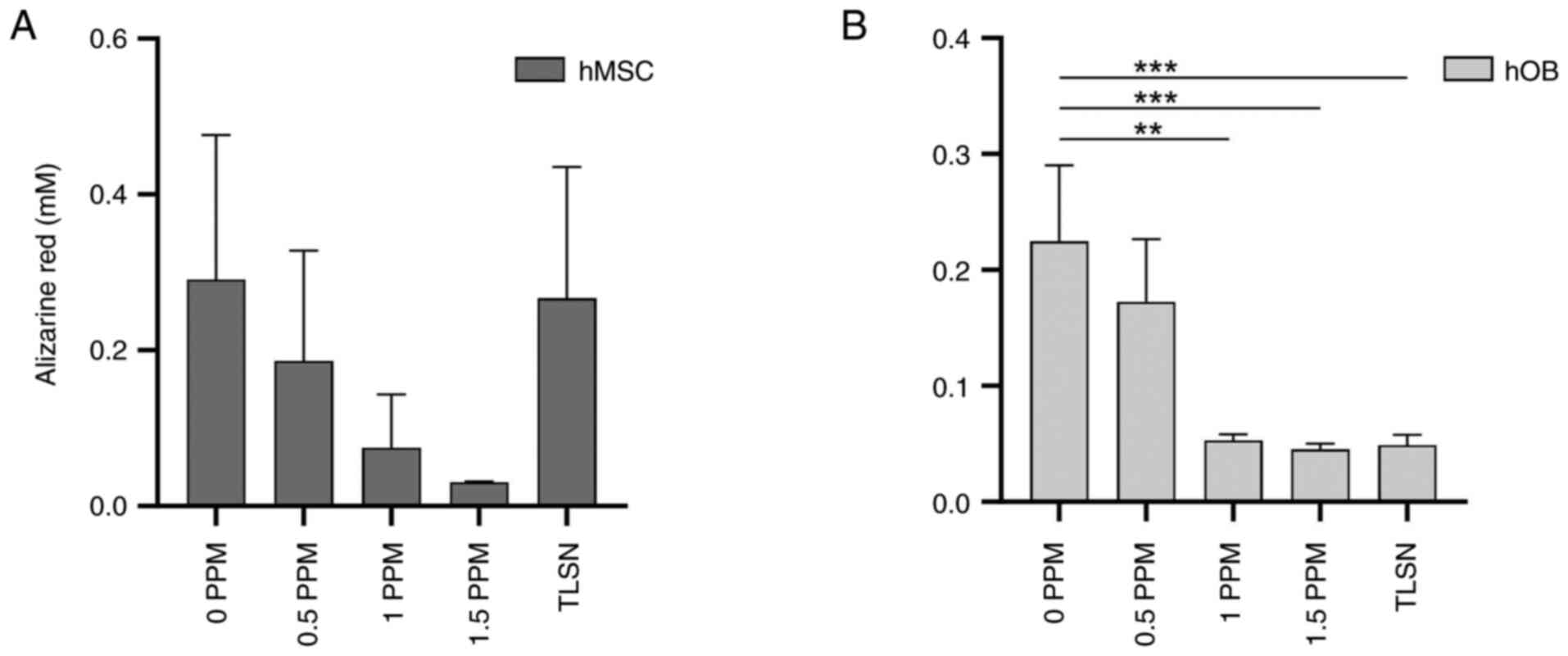
Cell mineralization
Robust mineralization was detected in hMSCs cultured
in control media after 4 weeks of culture (Figs. 6A, S1). However, the alizarin red staining
intensity showed a decreasing trend with increasing Ag+
concentration, and the lowest signal was found at 1.5 ppm; however,
no statistically significant differences were found. In hOB, on the
other hand (Fig. 6B), a
statistically significant reduction in mineralization was observed
for Ag+ concentrations ≥1 ppm compared to that in the
control group not exposed to Ag+. These findings
indicated that osteoid deposition in hOB was severely hampered at
concentrations of ≥1 ppm.
Discussion
This study examined the effects of different
Ag+ concentrations on the viability, osteogenic
differentiation, osteogenic gene expression, and mineralization of
hMSC and hOB cultures. We found that matrix mineralization in hOBs
was notably reduced at higher concentrations of Ag+,
particularly at concentrations >1 ppm, indicating a negative
impact on mineralization by these cells. However, after 4 weeks of
culture, no cell shape or confluence differences were discernible
between media containing varying Ag+ concentrations when
examined using bright field microscopy. HOBs, however, which is a
slower proliferative cell type than hMSCs, featured a higher CFDA
signal intensity. No significant differences were observed in
ALP/LDH ratios between hMSC and hOB cultures exposed to varying
Ag+ concentrations over time. A trend was observed in
the hOB cultures at 4 weeks, showing a lower ALP/LDH ratio with an
increasing Ag+ concentration. The osteogenic genes ALPL,
COL1A1, and COL1A2 expression tended to decrease with
Ag+ concentrations >1 ppm in both hMSCs and hOBs.
However, no clear concentration-dependent response was detected,
with statistically significant effects only being detected in
hMSCs.
The impact of Ag exposure on hMSC cultures is
variable and contingent upon culture conditions and Ag
concentrations (25-27).
For example, Ag+ exposure of hMSCs (approximately 3 ppm)
for up to 3 weeks in a spheroid culture has been shown to increase
osteogenic gene expression through WNT and MAPK signaling without
impacting matrix mineralization (26). Alternatively, Ag-induced oxidative
stress might induce adipogenesis in hMSCs (27). Our study demonstrated statistically
significant differences in the expression of osteogenic genes in
hMSCs treated with increasing Ag+ concentrations, as
well as a trend toward decreased mineralization. It has been
suggested that Ag+ in the range of 0.1 to 1.6 ppm
preserves the antibacterial properties of Ag and prevents toxicity
to osteogenic cells (28,29). A previous study conducted by our
research group (6), showed that
hOBs cultured on 3D-printed, Ag-coated trabecular titanium discs
(TLSN) and exposed to a cumulative release of 3.5 ppm after 4 weeks
did not express significant differences in ALP production or
overall viability compared to cells cultured on uncoated control
discs. While hMSC cultures exhibited an increasing trend in
osteogenic marker expression over the first week, followed by a
gradual decrease, hOB cultures tended to increase osteogenic marker
expression. This increase may be attributable to the maturity of
the hOB expressing these markers. However, compared to the uncoated
controls, we noted a trend for downregulating the COL1A1 and COL1A2
genes in hOB at 4 weeks and an upregulation of ALPL. The decreased
gene expression observed at 4 weeks relative to 2 weeks could be
attributed to contact inhibition among the cells. Previous research
conducted by our group, involving the culture of hOB on TLSN
implants, revealed a tendency towards decreased mineralization.
However, statistically significant differences were not observed
compared to cells cultured on non-silver-coated implants (6). Nevertheless, it is noteworthy that
the TLSN implants used as substrates for the cell cultures in those
experiments had an average roughness of Ra=5.0 µm. This factor may
have improved the osteogenic cell response despite exposure to
Ag+ (6,30). Ag-coated implants possessing
distinct physicochemical properties conducive to osteogenic cell
adhesion, proliferation, and differentiation might mitigate the
cytotoxic effects of Ag. This cytotoxic effect would be more
pronounced when cells are solely exposed to Ag+ and
cultured on a smooth, less supportive substrate compared to
trabecular titanium. Examples of modifications supporting
osteogenic cells include specific surface geometries (31,32)
and the use of composite materials, such as those containing Sr
(33,34), Ga (35) or hydroxyapatite (8,36).
Another parameter contributing to the discrepancy between our
findings and those documented in previous works is that
Ag+ in nanoparticle form may be less cytotoxic than
Ag+ in an aqueous solution, as in the AgNO3
solutions used in our current experiments (37).
Our study did not observe any differences in the
gene expression of hOB for the examined range of Ag+
concentrations. However, osteoid deposition was adversely affected
at concentrations ≥1 ppm, suggesting that these levels could
compromise implant stability when employed in a press-fit
application. This disparity in the mineralization data in
comparison to our previous study (6) might be attributed to the inherent
variability that hOBs exhibit from patient to patient.
One of the strengths of our study is the use of not
only commercially available hMSCs but also patient-derived primary
hOBs. In contrast, many other studies in Ag and osseointegration
use only commercially accessible cells or cell lines (38). HMSCs are a widely employed and
relevant cell type; however, the less frequently used hOB can more
closely mimic clinical conditions (39). A limitation of hOBs is their
greater biological variation, as they differ from patient to
patient. For this study's experiments, hOBs from up to five
patients were used. An additional strength of our study lies in its
use of diverse modalities for evaluating osteogenic
differentiation, encompassing morphological cell analysis, ALP
measurements, LDH enzymatic activity, a comprehensive panel of
osteogenic genes, and a mineralization assay. Long-term cultures,
lasting up to 4 weeks, were used and are preferable to short-term
experiments (25-28)
in discerning the effects of chronic Ag+ exposure and
facilitating investigations into the slower mineralization
processes. Future research should explore the effects of a more
restricted range of Ag+ concentrations on a broader
spectrum of osteogenic genes, encompassing Runx2, osteonectin,
osteopontin, and osteocalcin.
Although the future looks promising for some
Ag-coated implants, such as plates (15), nails (13), and cemented megaprostheses
(2), the question of Ag-coated
press-fit hip stems persists. Stability is achieved in the
press-fit concept when the implant transitions from primary
stability to secondary stability by osseointegration, which occurs
through mineralization and bone remodeling, eliminating
micromotions. Reduced mineralization can lead to long-term implant
loosening due to sustained micromotion. For press-fit implants
featuring antibacterial coatings, minimizing toxicity towards
osteoblasts is paramount (5,40).
In vitro experiments offer a rough estimate of how cells
react to specific antibacterial coatings. Still, in vivo
experiments are mandatory because they can offer biomechanical
(41) and histological data on
osseointegration and bone formation around coated implants. Our
study lacks these important data (40). Overall, Ag coating is an effective
strategy for reducing infection rates (2). However, providing clinical
recommendations requires robust, randomized, controlled trials. For
example, the BASICS (42), a
multicenter randomized trial, showed that Ag-coated
ventriculoperitoneal shunts did not perform better than uncoated
shunts in reducing infection rates. However, such studies do not
exist in orthopedic surgery. The hOB experiments constitute a more
valid model than those using hMSCs, as hOBs are sourced from the
femoral head of human donors and more closely replicate the cell
types that would interact with an Ag+-coated implant.
Chronic Ag+ release from the implant into the
periprosthetic space, and consequently through tissue continuity to
the bone, potentially hinders osteoblast activity and bone
formation, as seen in our hOB experiments. Over time, this process
may reduce bone apposition around the implant. Therefore, we
recommend limiting Ag+ release from the implant both in
its concentration and duration of exposure.
The supplementation of Ag+ in the growth
medium of hMSC and hOB cultures over 4 weeks did not confer any
differences in cell viability and differentiation. Still,
statistically significant differences were noted in the expression
of the osteogenic genes COL1A2 and ALPL in hMSC cultures. The
presence of Ag+ in the hMSC culture medium, mimicking
the release profile of TLSN implants, did not impact
mineralization. However, mineralization was profoundly compromised
in hOB cultures. This observation underscores the importance of
accounting for cell type-specific responses when evaluating the
biocompatibility of implant compounds, such as Ag, given that the
effects on hOBs are likely more representative of the actual in
vivo scenario. If the implant is designed for uncemented
fixation within the host bone, a thorough investigation of the
Ag+ release profile from the implant and its associated
cellular responses is crucial.
Supplementary Material
Mineralization of hMSC and hOB
cultures after 4 weeks. The mineralized matrix is stained with
alizarin red. hMSC, human mesenchymal stem cells; hOB, human
osteoblasts; TLSN, simulated Ag+ release from
TrabecuLink with silver nitrate coating.
Higher magnification images of hMSC
and hOB cultures. Blue is DAPI, green is CFDA and red is
osteocalcin (scDDale bar, 140 μm). hMSC, human mesenchymal
stem cells; hOB, human osteoblasts; TLSN, simulated Ag+
release from TrabecuLink with silver nitrate coating.
OCN staining of hMSC and hOB cultures
after 4 weeks (scale bar, 140 μm). hMSC, human mesenchymal
stem cells; hOB, human osteoblasts; TLSN, simulated Ag+
release from TrabecuLink with silver nitrate coating.
Acknowledgements
Not applicable.
Funding
Funding: The study was funded by grants from the Swedish
Research Council (grant no. VR 2021-00980) and Stiftelsen
Promobilia (grant no. A23003).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MGK analyzed and curated the data, performed the
experiments, wrote, reviewed and edited the original draft, and
created the figures. EC performed the experiments, curated the data
and reviewed and editing the manuscript. CPN validated the
reproducibility of the experiments, contributed to the analysis and
interpretation of data, supervised the study and reviewed and
edited the manuscript. NPH conceptualized and supervised the study,
designed the methodology, validated the reproducibility of the
experiments, reviewed and edited the manuscript and acquired
funding. MGK, EC and CPN confirm the authenticity of all raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Swedish Ethical
Review Authority (approval no. 2020-04462).
Patient consent for publication
Patient consent was waived for this study based on
section 4, subsection 3 of the relevant law (2003:460) which
specifies that the requirement for informed consent applies only
when biological material collected from patients can be traced back
to the individual patient. This was confirmed by the above ethics
committee, as the hOBs used in this study are not traceable to
specific patients.
Competing interests
Michael G. Kontakis, Elin Carlsson and Carlos
Palo-Nieto declare that they have no competing interests. Nils P.
Hailer reports receiving institutional support and lecturer
honoraria from two hip implant manufacturers, Waldemar Link GmbH
& Co. KG and Zimmer Biomet, as well as lecturer honoraria from
Heraeus, a bone cement manufacturer.
References
|
1
|
Wyatt MC, Foxall-Smith M, Roberton A,
Beswick A, Kieser DC and Whitehouse MR: The use of silver coating
in hip megaprostheses: A systematic review. HIP Int. 29:7–20.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hardes J, Henrichs MP, Hauschild G,
Nottrott M, Guder W and Streitbuerger A: Silver-Coated
megaprosthesis of the proximal tibia in patients with sarcoma. J
Arthroplasty. 32:2208–2213. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fiore M, Sambri A, Zucchini R, Giannini C,
Donati DM and De Paolis M: Silver-coated megaprosthesis in
prevention and treatment of peri-prosthetic infections: A
systematic review and meta-analysis about efficacy and toxicity in
primary and revision surgery. Eur J Orthop Surg Traumatol.
31:201–220. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sportelli MC, Izzi M, Volpe A, Clemente M,
Picca RA, Ancona A, Lugarà PM, Palazzo G and Cioffi N: The pros and
cons of the use of laser ablation synthesis for the production of
silver nano-antimicrobials. Antibiotics (Basel).
7(67)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kontakis MG, Diez-Escudero A, Hariri H,
Andersson B, Jarhult JD and Hailer NP: Antimicrobial and
osteoconductive properties of two different types of titanium
silver coating. Eur Cell Mater. 41:694–706. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Diez-Escudero A, Andersson B, Carlsson E,
Recker B, Link H, Jarhult JD and Hailer NP: 3D-printed porous
Ti6Al4V alloys with silver coating combine osteocompatibility and
antimicrobial properties. Biomater Adv. 133(112629)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Morimoto T, Hirata H, Eto S, Hashimoto A,
Kii S, Kobayashi T, Tsukamoto M, Yoshihara T, Toda Y and Mawatari
M: Development of silver-containing hydroxyapatite-coated
antimicrobial implants for orthopaedic and spinal surgery. Medicina
(Kaunas). 58(519)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Eto S, Kawano S, Someya S, Miyamoto H,
Sonohata M and Mawatari M: First clinical experience with
thermal-sprayed silver oxide-containing hydroxyapatite coating
implant. J Arthroplasty. 31:1498–1503. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Diez-Escudero A and Hailer NP: The role of
silver coating for arthroplasty components. Bone Joint J.
103-B:423–429. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Glehr M, Leithner A, Friesenbichler J,
Goessler W, Avian A, Andreou D, Maurer-Ertl W, Windhager R and Tunn
PU: Argyria following the use of silver-coated megaprostheses. Bone
Joint J. 95-B:988–992. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hardes J, Ahrens H, Gebert C,
Streitbuerger A, Buerger H, Erren M, Gunsel A, Wedemeyer C, Saxler
G, Winkelmann W and Gosheger G: Lack of toxicological side-effects
in silver-coated megaprostheses in humans. Biomaterials.
28:2869–2875. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hussmann B, Johann I, Kauther MD,
Landgraeber S, Jäger M and Lendemans S: Measurement of the silver
ion concentration in wound fluids after implantation of
silver-coated megaprostheses: Correlation with the clinical
outcome. Biomed Res Int. 2013:1–11. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Karupiah T, Yong AP, Ong ZW, Tan HK, Tang
WC and Salam HB: Use of a novel anti-infective noble metal
alloy-coated titanium orthopedic nail in patients with open
fractures: A case series from Malaysia. Antibiotics (Basel).
11(1763)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schoder S, Lafuente M and Alt V:
Silver-coated versus uncoated locking plates in subjects with
fractures of the distal tibia: A randomized, subject and
observer-blinded, multi-center non-inferiority study. Trials.
23(968)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Arens D, Zeiter S, Nehrbass D, Ranjan N,
Paulin T and Alt V: Antimicrobial silver-coating for locking plates
shows uneventful osteotomy healing and good biocompatibility
results of an experimental study in rabbits. Injury. 51:830–839.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shimabukuro M, Tsutsumi Y, Yamada R,
Ashida M, Chen P, Doi H, Nozaki K, Nagai A and Hanawa T:
Investigation of realizing both antibacterial property and
osteogenic cell compatibility on titanium surface by simple
electrochemical treatment. ACS Biomater Sci Eng. 5:5623–5630.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Komori T: Regulation of bone development
and extracellular matrix protein genes by RUNX2. Cell Tissue Res.
339:189–195. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Choi KY, Lee SW, Park MH, Bae YC, Shin HI,
Nam S, Kim YJ, Kim HJ and Ryoo HM: Spatio-temporal expression
patterns of Runx2 isoforms in early skeletogenesis. Exp Mol Med.
34:426–433. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hauschka PV, Lian JB, Cole DE and Gundberg
CM: Osteocalcin and matrix Gla protein: Vitamin K-dependent
proteins in bone. Physiol Rev. 69:990–1047. 1989.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jayakumar P and Di Silvio L: Osteoblasts
in bone tissue engineering. Proc Inst Mech Eng H. 224:1415–1440.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mestres G, Carter SD, Hailer NP and
Diez-Escudero A: A practical guide for evaluating the
osteoimmunomodulatory properties of biomaterials. Acta Biomater.
130:115–137. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Grzeskowiak RM, Schumacher J, Dhar MS,
Harper DP, Mulon PY and Anderson DE: Bone and cartilage interfaces
with orthopedic implants: A literature review. Front Surg.
7(601244)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dillon JP, Waring-Green VJ, Taylor AM,
Wilson PJM, Birch M, Gartland A and Gallagher JA: Primary human
osteoblast cultures. Methods Mol Biol. 816:3–18. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
R Core Team (2021). R: A language and
environment for statistical computing. R Foundation for Statistical
Computing, Vienna, Austria. URL https://www.R-project.org/.
|
|
25
|
Liu X, He W, Fang Z, Kienzle A and Feng Q:
Influence of silver nanoparticles on osteogenic differentiation of
human mesenchymal stem cells. J Biomed Nanotechnol. 10:1277–1285.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kersey AL, Singh I and Gaharwar AK:
Inorganic ions activate lineage-specific gene regulatory networks.
Acta Biomater. 183:371–386. 2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
He W, Elkhooly TA, Liu X, Cavallaro A,
Taheri S, Vasilev K and Feng Q: Silver nanoparticle-based coatings
enhance adipogenesis compared to osteogenesis in human mesenchymal
stem cells through oxidative stress. J Mater Chem B. 4:1466–1479.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Saravanapavan P, Gough JE, Jones JR and
Hench LL: Antimicrobial macroporous gel-glasses: Dissolution and
cytotoxicity. Key Engineering Materials. 254-256:1087–1090.
2003.
|
|
29
|
Panacek A, Kvitek L, Prucek R, Kolar M,
Vecerova R, Pizurova N, Sharma VK, Nevecna T and Zboril R: Silver
colloid nanoparticles: Synthesis, characterization, and their
antibacterial activity. J Phys Chem B. 110:16248–16253.
2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Feller L, Jadwat Y, Khammissa RAG, Meyerov
R, Schechter I and Lemmer J: Cellular responses evoked by different
surface characteristics of intraosseous titanium implants. Biomed
Res Int. 2015(171945)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Diez-Escudero A, Andersson B, Persson C
and Hailer NP: Hexagonal pore geometry and the presence of
hydroxyapatite enhance deposition of mineralized bone matrix on
additively manufactured polylactic acid scaffolds. Mater Sci Eng C
Mater Biol Appl. 125(112091)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sanchez-Salcedo S, Garcia A,
Gonzalez-Jimenez A and Vallet-Regi M: Antibacterial effect of 3D
printed mesoporous bioactive glass scaffolds doped with metallic
silver nanoparticles. Acta Biomater. 155:654–666. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Parizi MK, Doll K, Rahim MI, Mikolai C,
Winkel A and Stiesch M: Antibacterial and cytocompatible: Combining
silver nitrate with strontium acetate increases the therapeutic
window. Int J Mol Sci. 23(8058)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cheng H, Xiong W, Fang Z, Guan H, Wu W, Li
Y, Zhang Y, Alvarez MM, Gao B, Huo K, et al: Strontium (Sr) and
silver (Ag) loaded nanotubular structures with combined
osteoinductive and antimicrobial activities. Acta Biomater.
31:388–400. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pinera-Avellaneda D, Buxadera-Palomero J,
Delint RC, Dalby MJ, Burgess KV, Ginebra MP, Rupérez E and Manero
JM: Gallium and silver-doped titanium surfaces provide enhanced
osteogenesis, reduce bone resorption and prevent bacterial
infection in co-culture. Acta Biomater. 180:154–170.
2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Eto S, Miyamoto H, Shobuike T, Noda I,
Akiyama T, Tsukamoto M, Ueno M, Someya S, Kawano S, Sonohata M and
Mawatari M: Silver oxide-containing hydroxyapatite coating supports
osteoblast function and enhances implant anchorage strength in rat
femur. J Orthop Res. 33:1391–1397. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yusuf A and Casey A: Liposomal
encapsulation of silver nanoparticles (AgNP) improved nanoparticle
uptake and induced redox imbalance to activate caspase-dependent
apoptosis. Apoptosis. 25:120–134. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Spriano S, Yamaguchi S, Baino F and
Ferraris S: A critical review of multifunctional titanium surfaces:
New frontiers for improving osseointegration and host response,
avoiding bacteria contamination. Acta Biomater. 79:1–22.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Czekanska EM, Stoddart MJ, Richards RG and
Hayes JS: In search of an osteoblast cell model for in vitro
research. Eur Cells Mater. 24:1–17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bretschneider H, Mettelsiefen J, Rentsch
C, Gelinsky M, Link HD, Gunther KP, Lode A and Hofbauer C:
Evaluation of topographical and chemical modified TiAl6V4 implant
surfaces in a weight-bearing intramedullary femur model in rabbit.
J Biomed Mater Res B Appl Biomater. 108:1117–1128. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hauschild G, Hardes J, Gosheger G,
Stoeppeler S, Ahrens H, Blaske F, Wehe C, Karst U and Höll S:
Evaluation of osseous integration of PVD-silver-coated hip
prostheses in a canine model. Biomed Res Int.
2015(292406)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mallucci CL, Jenkinson MD, Conroy EJ,
Hartley JC, Brown M, Dalton J, Kearns T, Moitt T, Griffiths MJ,
Culeddu G, et al: Antibiotic or silver versus standard
ventriculoperitoneal shunts (BASICS): A multicentre,
single-blinded, randomised trial and economic evaluation. Lancet.
394:1530–1539. 2019.PubMed/NCBI View Article : Google Scholar
|