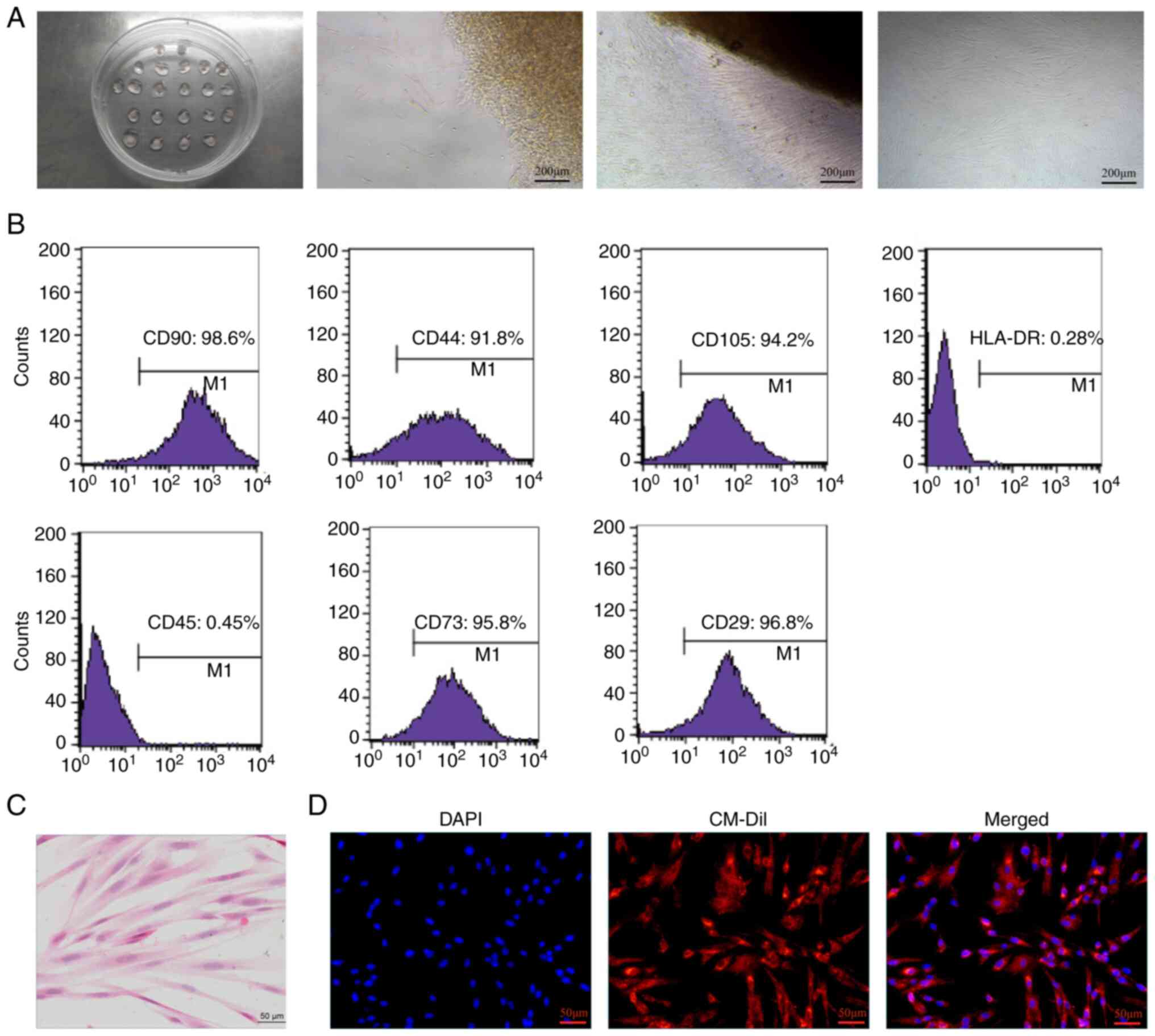
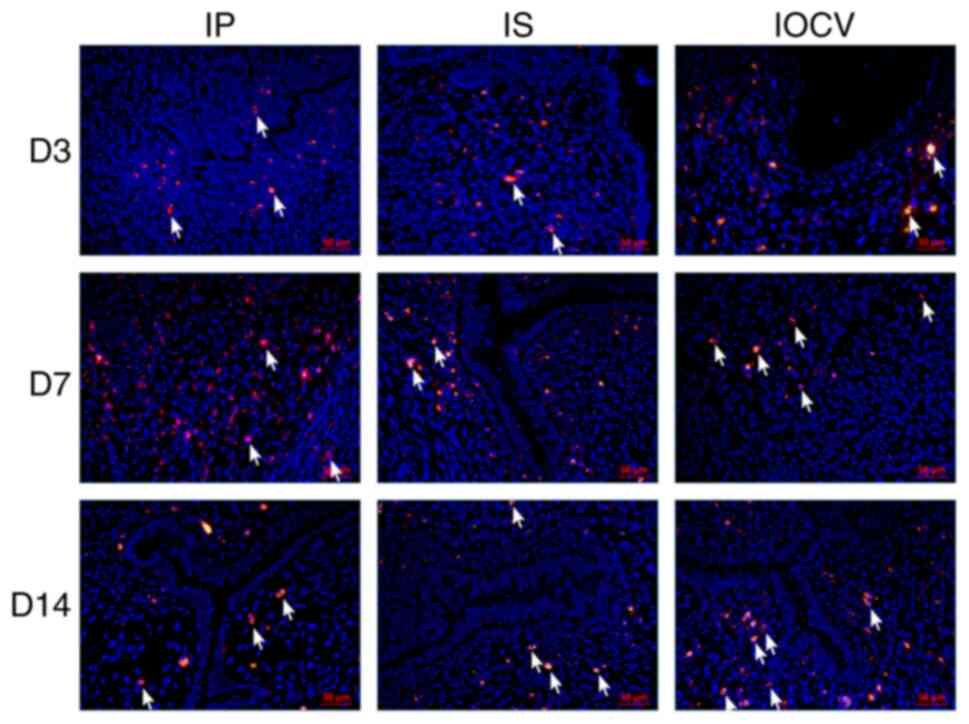
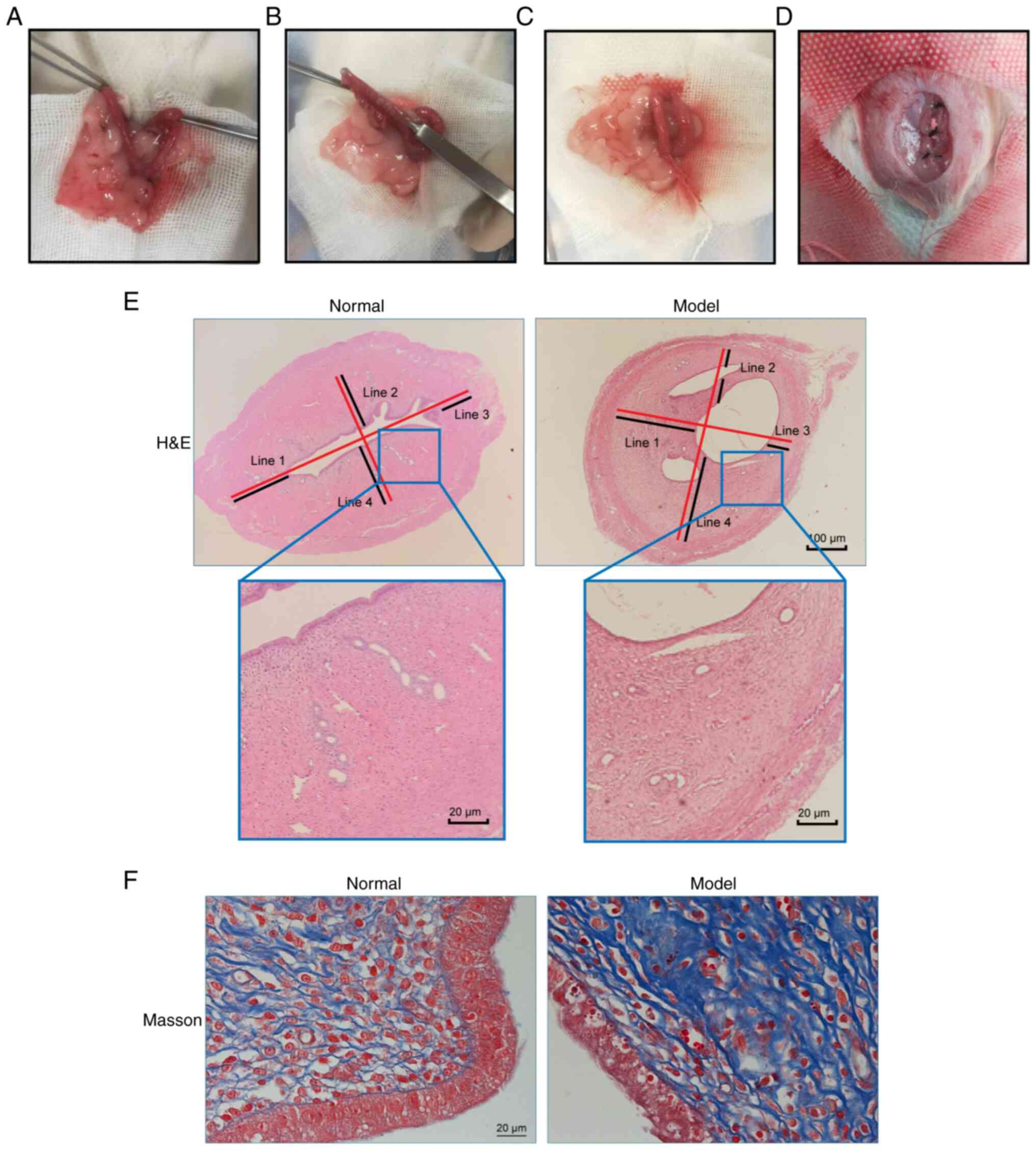
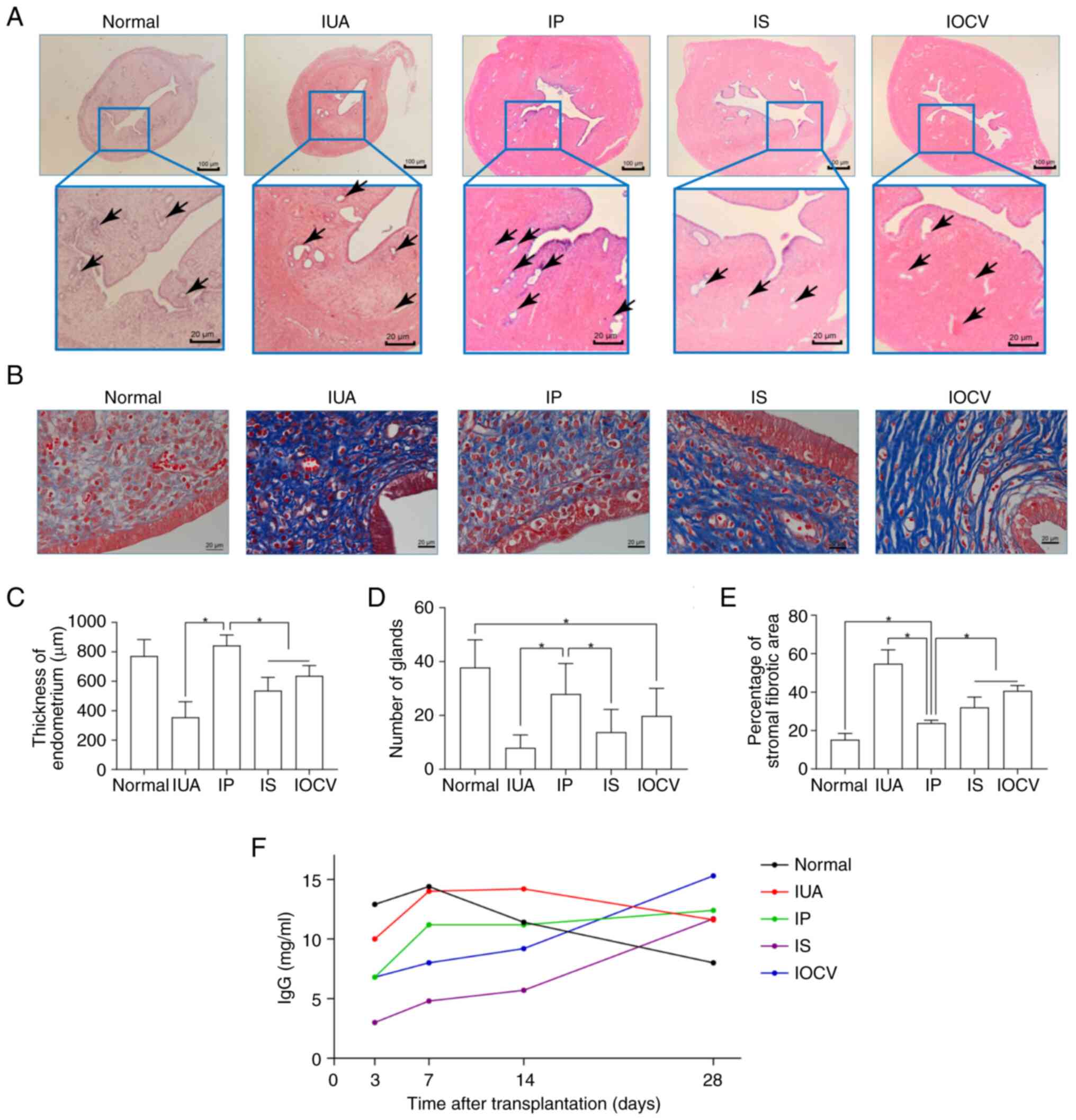
|
1
|
Ma J, Zhan H, Li W, Zhang L, Yun F, Wu R,
Lin J and Li Y: Recent trends in therapeutic strategies for
repairing endometrial tissue in intrauterine adhesion. Biomater
Res. 25(40)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hooker AB, Lemmers M, Thurkow AL, Heymans
MW, Opmeer BC, Brölmann HA, Mol BW and Huirne JA: Systematic review
and meta-analysis of intrauterine adhesions after miscarriage:
prevalence, risk factors and long-term reproductive outcome. Hum
Reprod Update. 20:262–278. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hooker AB, De Leeuw RA, Emanuel MH,
Mijatovic V, Brolmann HAM and Huirne JAF: The link between
intrauterine adhesions and impaired reproductive performance: A
systematic review of the literature. BMC Pregnancy Childbirth.
22(837)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tu CH, Yang XL, Qin XY, Cai LP and Zhang
P: Management of intrauterine adhesions: A novel intrauterine
device. Med Hypotheses. 81:394–396. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Han Y, Li X, Zhang Y, Han Y, Chang F and
Ding J: Mesenchymal Stem cells for regenerative medicine. Cells.
8(886)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ma J, Wu J, Han L, Jiang X, Yan L, Hao J
and Wang H: Comparative analysis of mesenchymal stem cells derived
from amniotic membrane, umbilical cord, and chorionic plate under
serum-free condition. Stem Cell Res Ther. 10(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
El Omar R, Beroud J, Stoltz JF, Menu P,
Velot E and Decot V: Umbilical cord mesenchymal stem cells: the new
gold standard for mesenchymal stem cell-based therapies? Tissue Eng
Part B Rev. 20:523–544. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang X, Zhang M, Zhang Y, Li W and Yang B:
Mesenchymal stem cells derived from Wharton jelly of the human
umbilical cord ameliorate damage to human endometrial stromal
cells. Fertil Steril. 96:1029–1036. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun B, Shi L, Shi Q, Jiang Y, Su Z, Yang X
and Zhang Y: Circular RNAs are abundantly expressed and upregulated
during repair of the damaged endometrium by Wharton’s jelly-derived
mesenchymal stem cells. Stem Cell Res Ther. 9(314)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang L, Li Y, Guan CY, Tian S, Lv XD, Li
JH, Ma X and Xia HF: Therapeutic effect of human umbilical
cord-derived mesenchymal stem cells on injured rat endometrium
during its chronic phase. Stem Cell Res Ther. 9(36)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun D, Jiang Z, Chen Y, Shang D, Miao P
and Gao J: MiR-455-5p upregulation in umbilical cord mesenchymal
stem cells attenuates endometrial injury and promotes repair of
damaged endometrium via Janus kinase/signal transducer and
activator of transcription 3 signaling. Bioengineered.
12:12891–12904. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang L, Yu C, Chang T, Zhang M, Song S,
Xiong C, Su P and Xiang W: In situ repair abilities of human
umbilical cord-derived mesenchymal stem cells and autocrosslinked
hyaluronic acid gel complex in rhesus monkeys with intrauterine
adhesion. Sci Adv. 6(eaba6357)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Giri J and Galipeau J: Mesenchymal stromal
cell therapeutic potency is dependent upon viability, route of
delivery, and immune match. Blood Adv. 4:1987–1997. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Levy O, Kuai R, Siren EMJ, Bhere D, Milton
Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, et al:
Shattering barriers toward clinically meaningful MSC therapies. Sci
Adv. 6(eaba6884)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zheng JH, Zhang JK, Kong DS, Song YB, Zhao
SD, Qi WB, Li YN, Zhang ML and Huang XH: Quantification of the
CM-Dil-labeled human umbilical cord mesenchymal stem cells migrated
to the dual injured uterus in SD rat. Stem Cell Res Ther.
11(280)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao S, Qi W, Zheng J, Tian Y, Qi X, Kong
D, Zhang J and Huang X: Exosomes derived from adipose mesenchymal
stem cells restore functional endometrium in a rat model of
intrauterine adhesions. Reprod Sci. 27:1266–1275. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kong D, Zhang L, Xu X, Zhang J, Li Y and
Huang X: Small intestine submucosa is a potential material for
intrauterine adhesions treatment in a rat model. Gynecol Obstet
Invest. 83:499–507. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rodríguez-Eguren A, Bueno-Fernandez C,
Gómez-Álvarez M, Francés-Herrero E, Pellicer A, Bellver J, Seli E
and Cervelló I: Evolution of biotechnological advances and
regenerative therapies for endometrial disorders: A systematic
review. Hum Reprod Update. 30:584–613. 2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Azizi R, Aghebati-Maleki L, Nouri M,
Marofi F, Negargar S and Yousefi M: Stem cell therapy in Asherman
syndrome and thin endometrium: Stem cell-based therapy. Biomed
Pharmacother. 102:333–343. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo LP, Chen LM, Chen F, Jiang NH and Sui
L: Smad signaling coincides with epithelial-mesenchymal transition
in a rat model of intrauterine adhesion. Am J Transl Res.
11:4726–4737. 2019.PubMed/NCBI
|
|
23
|
Arikan G, Turan V, Kurekeken M, Goksoy HS
and Dogusan Z: Autologous bone marrow-derived nucleated cell
(aBMNC) transplantation improves endometrial function in patients
with refractory Asherman's syndrome or with thin and dysfunctional
endome-trium. J Assist Reprod Genet. 40:1163–1171. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
de Miguel-Gómez L, López-Martínez S, Campo
H, Francés-Herrero E, Faus A, Díaz A, Pellicer A, Domínguez F and
Cervelló I: Comparison of different sources of platelet-rich plasma
as treatment option for infertility-causing endometrial
pathologies. Fertil Steril. 115:490–500. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shi Q, Sun B, Wang D, Zhu Y, Zhao X, Yang
X and Zhang Y: Circ6401, a novel circular RNA, is implicated in
repair of the damaged endometrium by Wharton's jelly-derived
mesenchymal stem cells through regulation of the miR-29b-1-5p/RAP1B
axis. Stem Cell Res Ther. 11(520)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xin L, Lin X, Pan Y, Zheng X, Shi L, Zhang
Y, Ma L, Gao C and Zhang S: A collagen scaffold loaded with human
umbilical cord-derived mesenchymal stem cells facilitates
endometrial regeneration and restores fertility. Acta Biomater.
92:160–171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu Y, Cai J, Luo X, Wen H and Luo Y:
Collagen scaffold with human umbilical cord mesenchymal stem cells
remarkably improves intrauterine adhesions in a rat model. Gynecol
Obstet Invest. 85:267–276. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sabry D, Mostafa A, Marzouk S, Ibrahim W,
Ali HHM, Hassan A and Shamaa A: Neupogen and mesenchymal stem cells
are the novel therapeutic agents in regeneration of induced
endometrial fibrosis in experimental rats. Biosci Rep.
37(BSR20170794)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhuang M, Zhang W, Cheng N, Zhou L, Liu D,
Yan H, Fang G, Heng BC, Sun Y and Tong G: Human umbilical cord
mesenchymal stromal cells promote the regeneration of severe
endometrial damage in a rat model. Acta Biochim Biophys Sin
(Shanghai). 54:148–151. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hashemi SM, Hassan ZM, Hossein-Khannazer
N, Pourfathollah AA and Soudi S: Investigating the route of
administration and efficacy of adipose tissue-derived mesenchymal
stem cells and conditioned medium in type 1 diabetic mice.
Inflammopharmacology. 28:585–601. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Goncalves Fda C, Schneider N, Pinto FO,
Meyer FS, Visioli F, Pfaffenseller B, Lopez PL, Passos EP,
Cirne-Lima EO, Meurer L and Paz AH: Intravenous vs intraperitoneal
mesenchymal stem cells administration: What is the best route for
treating experimental colitis? World J Gastroenterol.
20:18228–18239. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huldani H, Margiana R, Ahmad F, Opulencia
MJC, Ansari MJ, Bokov DO, Abdullaeva NN and Siahmansouri H:
Immunotherapy of inflammatory bowel disease (IBD) through
mesenchymal stem cells. Int Immunopharmacol.
107(108698)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou S, Lei Y, Wang P, Chen J, Zeng L, Qu
T, Maldonado M, Huang J, Han T, Wen Z, et al: Human umbilical cord
mesenchymal stem cells encapsulated with pluronic F-127 enhance the
regeneration and angiogenesis of thin endometrium in rat via local
IL-1β stimulation. Stem Cells Int. 2022(7819234)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang H, Yang X, Chen X, Xie H, Wang J and
Zhang Y: Identify the role of human Wharton's jelly mesenchymal
stem cells in repairing injured uterine of rat. J Obstet Gynaecol
Res. 47:320–328. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang L, Li Y, Dong YC, Guan CY, Tian S,
Lv XD, Li JH, Su X, Xia HF and Ma X: Transplantation of umbilical
cord-derived mesenchymal stem cells promotes the recovery of thin
endometrium in rats. Sci Rep. 12(412)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G,
Wang J, Bai D, Wang J, Wang L, et al: Allogeneic cell therapy using
umbilical cord MSCs on collagen scaffolds for patients with
recurrent uterine adhesion: A phase I clinical trial. Stem Cell Res
Ther. 9(192)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kaczynski JB and Rzepka JK: Endometrial
regeneration in Asherman's syndrome and endometrial atrophy using
Wharton's jelly-derived mesenchymal stem cells. Ginekol Pol.
93:904–909. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liesveld JL, Sharma N and Aljitawi OS:
Stem cell homing: From physiology to therapeutics. Stem Cells.
38:1241–1253. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nitzsche F, Muller C, Lukomska B,
Jolkkonen J, Deten A and Boltze J: Concise review: MSC adhesion
cascade-insights into homing and transendothelial migration. Stem
Cells. 35:1446–1460. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Krueger TEG, Thorek DLJ, Denmeade SR,
Isaacs JT and Brennen WN: Concise review: Mesenchymal stem
cell-based drug delivery: The good, the bad, the ugly, and the
promise. Stem Cells Transl Med. 7:651–663. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yuan M, Hu X, Yao L, Jiang Y and Li L:
Mesenchymal stem cell homing to improve therapeutic efficacy in
liver disease. Stem Cell Res Ther. 13(179)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ullah M, Liu DD and Thakor AS: Mesenchymal
stromal cell homing: Mechanisms and strategies for improvement.
iScience. 15:421–438. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Maric DM, Velikic G, Maric DL, Supic G,
Vojvodic D, Petric V and Abazovic D: Stem cell homing in
intrathecal applications and inspirations for improvement paths.
Int J Mol Sci. 23(4290)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jiang Z, Chen L, Huang L, Yu S, Lin J, Li
M, Gao Y and Yang L: Bioactive materials that promote the homing of
endogenous mesenchymal stem cells to improve wound healing. Int J
Nanomedicine. 19:7751–7773. 2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu Z, Mikrani R, Zubair HM, Taleb A,
Naveed M, Baig MMFA, Zhang Q, Li C, Habib M, Cui X, et al: Systemic
and local delivery of mesenchymal stem cells for heart renovation:
Challenges and innovations. Eur J Pharmacol.
876(173049)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kanelidis AJ, Premer C, Lopez J, Balkan W
and Hare JM: Route of delivery modulates the efficacy of
mesenchymal stem cell therapy for myocardial infarction: A
meta-analysis of preclinical studies and clinical trials. Circ Res.
120:1139–1150. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jeong H, Yim HW, Park HJ, Cho Y, Hong H,
Kim NJ and Oh IH: Mesenchymal stem cell therapy for ischemic heart
disease: Systematic review and meta-analysis. Int J Stem Cells.
11:1–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Peng W, Sun J, Sheng C, Wang Z, Wang Y,
Zhang C and Fan R: Systematic review and meta-analysis of efficacy
of mesenchymal stem cells on locomotor recovery in animal models of
traumatic brain injury. Stem Cell Res Ther. 6(47)2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Augustine S, Avey MT, Harrison B, Locke T,
Ghannad M, Moher D and Thébaud B: Mesenchymal stromal cell therapy
in bronchopulmonary dysplasia: Systematic review and meta-analysis
of preclinical studies. Stem Cells Transl Med. 6:2079–2093.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ponte AL, Marais E, Gallay N, Langonné A,
Delorme B, Hérault O, Charbord P and Domenech J: The in vitro
migration capacity of human bone marrow mesenchymal stem cells:
Comparison of chemokine and growth factor chemotactic activities.
Stem Cells. 25:1737–1745. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ramaswamy Reddy SH, Reddy R, Babu NC and
Ashok GN: Stem-cell therapy and platelet-rich plasma in
regenerative medicines: A review on pros and cons of the
technologies. J Oral Maxillofac Pathol. 22:367–374. 2018.PubMed/NCBI View Article : Google Scholar
|