Introduction
Intrauterine adhesions (IUAs), characterized by
fibrous scar formation within the uterine cavity, pose a
significant clinical challenge due to their adverse effects on
female fertility (1).
Predominantly associated with post-surgical traumas, especially
following various procedures, such as dilatation and curettage, the
prevalence of IUAs varies based on the extent and frequency of
uterine surgeries (2). These
adhesions contribute to various reproductive dysfunctions,
including menstrual irregularities, reduced fertility, recurrent
miscarriages and placental abnormalities (3). Current treatment strategies mainly
involve surgical interventions, most notably hysteroscopic
adhesiolysis, which is frequently combined with hormonal therapies
to promote endometrial regeneration (1). Although these approaches are able to
restore the uterine anatomy and improve menstrual outcomes to a
certain extent, their long-term efficacy in preserving reproductive
function is limited due to high recurrence rates and adhesion
reformation (4). In addition, the
invasive nature of these treatments predisposes patients to further
risks of adhesion development (3),
highlighting the urgent need to develop novel therapeutic
strategies that are both less invasive and more effective in
restoring complete reproductive potential.
Advances in regenerative medicine have prominently
featured mesenchymal stem cells (MSCs) as potential agents for
tissue repair and functional restoration across a broad spectrum of
organ systems (5,6). Amongst the various MSC sources,
umbilical cord-derived MSCs (UCMSCs) are distinguished by their
superior proliferation rates, robust differentiation capabilities
and reduced immunogenicity compared with MSCs harvested from adult
tissues (7). These attributes
render UCMSCs suitable as clinical tools aimed at repairing organ
damage (8). Previous in
vitro studies have demonstrated that UCMSCs are able to
significantly enhance the proliferation of damaged endometrial
stromal cells and upregulate the expression of vascular
angiogenesis markers (9,10). Furthermore, in animal models,
UCMSCs have been reported to repair endometrial injury, restore
fertility, and promote endometrial cell proliferation and vascular
remodeling (11,12). Other preliminary studies have also
demonstrated that UCMSCs can effectively promote endometrial
regeneration and attenuate fibrosis in rhesus monkeys with
intrauterine adhesion models, indicating their potential
applicability for human therapies (13). However, in reproductive medicine,
the application of UCMSCs for treating IUAs remains in
developmental stages.
Although UCMSCs have demonstrated promise in
regenerative medicine, their use in treating IUAs is confronted
with a number of unresolved issues. The optimal dosage and delivery
route for UCMSCs therapy remain to be determined. Various methods
that have been attempted, such as intraperitoneal, intrauterine and
intravenous injections, have shown mixed results in terms of cell
engraftment and efficacy (14).
Additionally, concerns persist regarding potential immune
responses, insufficient cell retention, and the long-term safety
and durability of treatment efficacy (15). To the best of our knowledge, the
majority of studies of using UCMSCs for the treatment of IUA to
date have been performed on animal models, where the translational
possibility of these findings to the clinic have not been explored.
Therefore, the present study aimed to address these challenges by
systematically evaluating different UCMSC doses and administration
routes in a rat model of IUA, focusing on endometrial regeneration,
fibrosis, and immune responses, to provide insights for the
optimization of stem cell therapies for IUA.
The present study investigated the therapeutic
potential of UCMSCs for treating IUAs using a rat model. Through
systematically comparing different administration routes and
dosages, the aim is to optimize the delivery method for enhancing
endometrial repair and reducing fibrosis.
Materials and methods
Isolation, cultivation and
identification of UCMSCs
UCMSCs were isolated from fresh, full-term umbilical
cords of women who gave birth at term in The Second Hospital of
Hebei Medical University (Shijiazhuang, China) from January 2021 to
December 2022 were collected, and informed consent was obtained
from the six mothers who donated the umbilical cords for research
purposes (n=6; mean age, 35.83±3.061 years), with each mother
providing one umbilical cord. The inclusion criteria were: Healthy
mothers with full-term deliveries. The exclusion criteria were:
Mothers with pregnancy complications or pre-existing medical
conditions, and those whose fetuses were diagnosed with
developmental abnormalities during prenatal screening. The present
study was approved by the Ethical Committee of the Second Hospital
of Hebei Medical University (Shijiazhuang, China; approval no.
2020-R285). The cords were first washed with PBS supplemented with
an antibiotic/antimycotic solution to mitigate microbial
contamination. Subsequently, Wharton's jelly was dissected from the
cords and cut into small explants of 1-2 cm3. These
explants were then placed onto culture dishes pre-coated with 0.1%
gelatin (HapCult™; Precision BioMedicals Co., Ltd.) to enhance cell
adhesion, before the plates were cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) enriched with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 1% L-glutamine (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin and streptomycin solution
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in an atmosphere
containing 95% air/5% CO2. The medium was initially
replaced after 72 h to eliminate non-adherent cells, and then
replaced every 3 days. Once the cells had reached 80-90%
confluence, they were detached using 0.25% trypsin-EDTA (Gibco;
Thermo Fisher Scientific, Inc.) and subsequently passaged. Cells at
passages 3-7 were utilized for further experiments to ensure
consistency and vitality. The morphological features of UCMSCs were
continuously monitored under an inverted light microscope (Carl
Zeiss AG), where they displayed a typical fibroblast-like
morphology.
The UCMSCs were identified as previously described
(16). Cells were first observed
under an inverted light microscope to confirm their morphology.
Subsequently, phenotypic characterization was performed using flow
cytometric (FCM) analysis with a BD FACSCanto™ II flow cytometer
(BD Biosciences). Cells were stained with fluorescently labeled
antibodies to confirm their mesenchymal lineage, whereas
hematopoietic elements were excluded. Additionally, H&E
staining was performed on cellular smears to further authenticate
their morphology.
FCM analysis
FCM analysis was performed to phenotypically
identify the UCMSCs. Cells were detached using 0.25% trypsin-EDTA,
after adding trypsin and incubating at room temperature for 1-3
min, PBS containing 2% FBS was added and stained with fluorescently
labeled antibodies against MSC markers CD90-FITC (1:400; cat. no.
555595), CD44-PE (1:400; cat. no. 566803), CD105-PerCP (1:400; cat.
no. 323215), CD73 PE (1:400; cat. no. 550257) and CD29-APC (1:400;
cat. no. 559883) (all purchased from Biolegend, Inc.) and negative
markers human leukocyte antigen (HLA)-DR-APC (1:400; cat. no.
307609) and CD45-FITC (1:400; cat. no. 555488) (both from
BioLegend, Inc.). The number of cells per aliquot for antibody
staining ranged from 5x106 to 10x106 cells.
Following a 30-min incubation at 4˚C, the cells were washed, fixed
in 1% paraformaldehyde at room temperature for 10-15 min. If prompt
analysis on the machine was not feasible, fixation was required,
followed by storage at 4˚C in the dark. Analysis was conducted
within 24 h. If analysis was performed immediately, the fixation
step was unnecessary (fixed with 1% paraformaldehyde at room
temperature for 10-15 min). After antibody labeling, the cells were
incubated at room temperature in the dark for 15 min. Subsequently,
1 ml of PBS was added to wash the cells. The cells were then
centrifuged at 300 x g for 5 min at 4˚C, and the supernatant was
discarded. Finally, the cells were resuspended in 500 to 1,000 µl
of PBS and analyzed using flow cytometry (CellQuest; BD
Biosciences). Data obtained from ≥10,000 events per sample
confirmed a high expression level of mesenchymal markers and a low
expression level of hematopoietic markers, verifying their MSC
identity.
CM-Dil labeling of UCMSCs
To track the cellular integration of UCMSC
post-transplantation, CellTracker™ CM-DiI dye (cat. no. C7000; Life
Technologies; Thermo Fisher Scientific, Inc.), a lipophilic
fluorescent dye, was used for staining the cell membranes. The
passage 3 UCMSCs were first incubated with the CM-Dil solution (the
CM-Dil working solution was prepared in advance for fluorescence
staining using a ratio of CM-Dil stock solution to medium of
1:1,000). Following an incubation at 37˚C in an atmosphere
containing 5% CO2 for a predetermined duration, the
cells were washed with PBS to remove the excess dye. Successful dye
incorporation was verified under a fluorescence microscope, with
cell nuclei counterstained using DAPI at room temperature 5 min
(cat. no. 0100-20; SouthernBiotech) to enhance visualization.
Animal experiment and construction of
the IUA model
All animals were housed in a pathogen-free facility
at the Animal Center of the Second Hospital of Hebei Medical
University. The rats were kept in standard cages under controlled
conditions (12-h light/dark cycle, temperature of 22-24˚C and
40-60% humidity) with ad libitum access to food and water.
Humane endpoints were predefined to minimize animal suffering. Rats
exhibiting signs of distress, severe illness or immobility were
humanely euthanized using an overdose of pentobarbital sodium (100
mg/kg, intraperitoneal injection). The total duration of the
experiment was 18 weeks, which included a 2-week post-modeling
period for IUA induction, and 4 weeks of treatment and observation
following UCMSC administration, followed by 12 weeks of the
co-housing experiment. A total of 174 rats (149 female rats and 25
male rats) were used in the present study. All animals were
humanely euthanized at the conclusion of the experiment. No animals
reached the humane endpoint and no animals were found dead during
the experiments.
The IUA model was established using female
Sprague-Dawley (SD) rats, aged 6-8 weeks and weighing between
180-220 g, which were procured from the Hebei Medical University
Animal Experiment Center. All animal experiments were conducted
with approval from the Ethical Committee of the Second Hospital of
Hebei Medical University (approval no. 2021-AE261). A total of 12
rats were randomly divided into two groups: A normal control group
(n=6 rats) and a model group (n=6 rats). The use of 6 rats in the
modeling group was based on previous studies and statistical power
analysis that indicated this number to be sufficient for
histological assessments (17). In
similar studies, a sample size of 6 has been shown to provide
reliable and reproducible data for evaluating tissue morphology and
fibrosis (18).
The IUA model in the present study was based on a
dual-injury approach: Mechanical injury (scratching) combined with
lipopolysaccharide (LPS) application. The primary focus was on
mechanical injury, with LPS used to represent secondary infection,
which can occur following intrauterine procedures and may
contribute to adhesion formation (17-19).
This method was chosen to reflect clinical situations where
infection may serve a role in adhesion development after surgical
interventions.
The rat model of IUA was established following
previously reported protocols (17-19).
To quantify the migration of CM-Dil-labeled UCMSCs to the
dual-injured uterus in SD rats, the following procedure was
performed. Briefly, SD rats were anesthetized using an
intraperitoneal injection of sodium pentobarbital at a dose of 40
mg/kg body weight and subjected to a midline abdominal incision
(2-2.5 cm). The endometrial lining was then scraped using a 2.5-mm
endometrial curette (RWD Life Science Co., Ltd.) until the uterine
wall became visibly rough. LPS-coated sutures were subsequently
placed into the cavity of one uterine horn, with one end fixed to
the skin through the muscle layer. The sutures were removed after
48 h. Model stability was confirmed after three estrous cycles. Our
previous research conducted observations at 1, 2, 4, 8 and 12 weeks
after IUA modeling. When comparing the 2-week post-modeling period
with 4, 8 and 12 weeks, there were no significant changes in
endometrial thickness, glandular density, and extent of fibrotic
tissue (17). Our research team
(17-19)
used the same IUA modeling method and confirmed that the 2-week IUA
model reached a stable state. CM-Dil-labeled UCMSCs were injected
into the abdominal cavity, tail vein, and uterine myometrium. The
animals were euthanized on days 3, 7 and 14 post-transplantation,
and the uteri were subjected to rapid frozen sectioning followed by
immunofluorescence tracing. In the present study, the time points
for euthanasia varied depending on the experimental purpose. During
the fluorescent tracing experiment, rats were euthanized at D3, D7
and D14 to track the distribution and persistence of the
transplanted cells over time. By contrast, for the stem cell
treatment experiment, rats were euthanized only at D28 to perform
histological analyses and evaluate the therapeutic effects of stem
cell therapy on uterine tissues. Thus, D3, D7 and D14 were
specifically used to investigate the localization of the
transplanted stem cells, while D28 was used to assess the outcomes
of the treatment.
Analysis of the effects of UCMSC
administration routes on IUA treatment
Female SD rats were categorized into five groups,
each comprising 6 animals for histological examination and 5
animals for fertility testing (totaling 11 rats in each group;
Table I), to assess the impact of
different UCMSC administration routes on IUA. The groups included
the following: i) A normal group (Normal), with no modeling or
treatment; ii) a model group (IUA group), in which the rats
underwent IUA modeling without any treatment; iii) an
intraperitoneal injection group (IP group), in which the rats
received UCMSCs through intraperitoneal injection after IUA
modeling; iv) an in-site injection group (IS group), where the rats
were treated with UCMSCs directly into the intrauterine wall
post-modeling; and v) an injection of caudal vein group (IOCV
group), where the rats were administered UCMSCs through the tail
vein following IUA induction.
 | Table IExperimental grouping for UCMSC
administration. |
Table I
Experimental grouping for UCMSC
administration.
| A, Phase 1:
Administration route |
|---|
| Group | Total rats, n | Histological
analysis, n | Fertility testing,
n |
|---|
| Normal group | 11 | 6 | 5 |
| Model group | 11 | 6 | 5 |
| Intraperitoneal
injection group | 11 | 6 | 5 |
| In site injection
group | 11 | 6 | 5 |
| Intravenous
injection group | 11 | 6 | 5 |
| B, Phase 2: Dosage
validation |
| Group | Total rats, n | Histological
analysis, n | Fertility testing,
n |
| Normal group | 11 | 6 | 5 |
| Model group | 11 | 6 | 5 |
| Low dose
(0.5x106 UCMSCs) | 11 | 6 | 5 |
| Medium dose
(1x106 UCMSCs) | 11 | 6 | 5 |
| High dose
(5x106 UCMSCs) | 11 | 6 | 5 |
To ascertain the therapeutic efficacy of UCMSCs on
IUA, UCMSCs were administered to female SD rats through the three
distinct routes (intraperitoneal injection, in-site injection and
caudal vein injection), where each rat received a standardized dose
of 1x106 cells. Over a 4-week post-transplantation period, the
health of the rats was monitored, with particular attention given
to any adverse reactions or complications. At the end of the
observation period (D28), all animals were euthanized via
intraperitoneal injection of an overdose of sodium pentobarbital
(100 mg/kg). Death was confirmed by the cessation of heartbeat and
pupil dilation. The uteri were subsequently collected for
comprehensive histopathological evaluation. Histological
assessments were performed using H&E and Masson's trichrome
staining to evaluate endometrial regeneration and fibrosis.
Quantitative analyses of endometrial thickness, gland density and
fibrotic areas were performed using ImageJ software (Image J 1.53e.
Java 1.8.0_172; National Institutes of Health), and immune
responses were assessed by measuring serum IgG levels using
ELISA.
Intraperitoneal administration of
UCMSCs at various concentrations for treating IUAs
To investigate the effect of different
concentrations of intraperitoneally administered UCMSCs on the
treatment efficacy for IUA, three dosage groups were established
among the model rats: A low-dose group (with the administration of
0.5x106 cells), a medium-dose group (receiving
1x106 cells) and a high-dose group (treated with
5.0x106 cells). The dosage selection was based on
preliminary experiments that were conducted to identify the most
appropriate and effective dosage range for treatment (17), ensuring the doses used were within
the therapeutic window. The normal group underwent a sham operation
(abdominal incision without injury, no IUA induction, and no stem
cell treatment) and served as the baseline control. The model group
received the IUA induction without stem cell treatment, providing a
reference for evaluating the effects of UCMSC therapy. Each group
consisted of 11 rats (6 for histological assessment and 5 for
mating experiments; Table I), all
of which had undergone IUA induction and received UCMSC
administration through the route of intraperitoneal injection.
After 4 weeks of monitoring the rats for adverse effects, they were
euthanized and uterine tissue samples were collected. Histological
assessment was subsequently performed as previously described in
analysis of the effects of UCMSC administration routes on IUA
treatment.
Assessment of fertility restoration
post-treatment with UCMSCs
To assess the efficacy of UCMSCs on fertility after
IUA, a cohabitation study was initiated after the initial 4-week
treatment period. In total, 5 female Sprague-Dawley rats from each
experimental group were randomly selected and paired with fertile
male rats at a 1:1 ratio. The pairs were housed under controlled
environmental conditions (temperature, 20-25˚C; humidity maintained
at 50-60%; and the light/dark cycle of 12 h of light followed by 12
h of darkness) optimal for breeding for a duration of 12 weeks,
with food and water provided ad libitum. The mating pairs
were observed daily to monitor for signs of pregnancy, which were
initially indicated by physical changes in the females and
subsequently confirmed through palpation. Post-gestation, the
number of offspring for each female was recorded. No offspring were
sacrificed in this study. Instead, these offspring were donated to
the Hebei Medical University Animal Experiment Center for use in
other research projects. These data served as a direct indicator of
the fertility potential of UCMSC treatment, providing insights into
the success rate and possible enhancements in reproductive health,
of the rats following the intervention for IUAs.
H&E staining
H&E staining was used to assess morphological
changes in the uterine tissue post-treatment. Samples were fixed in
4% neutral buffered formalin at room temperature for 24 h,
dehydrated in an ascending ethanol gradient, cleared in xylene and
paraffin-embedded. Sections of 5 µm in thickness were
deparaffinized, rehydrated and stained with hematoxylin for nuclei
(at room temperature for 30 min) and counterstained with eosin (at
room temperature for 30 sec) for cytoplasmic and extracellular
components. The slides were subsequently dehydrated, cleared and
mounted in a xylene-based medium. Microscopic examinations at
magnifications of x100, x200 and x400 were undertaken to evaluate
the endometrial thickness, cellular integrity and extent of
fibrosis. Endometrial thickness was measured using H&E-stained
sections imaged under a low-power light microscope. In total, four
perpendicular points were selected within each section and the
thickness was measured at these points. The average of these
measurements was calculated to determine the final endometrial
thickness. Finally, images were taken for comparative analysis
among the experimental groups.
Masson's trichrome staining
Masson's trichrome staining was used to assess the
extent of fibrosis in the uterine tissues post-UCMSC treatment.
Tissue sections, embedded in paraffin and sliced to a thickness of
5 µm, underwent deparaffinization in xylene and rehydration through
a descending graded alcohol series. Staining involved the
sequential application of Weigert's iron hematoxylin for 7 min,
Biebrich scarlet-acid fuchsin for 15 min, and aniline blue for 2
min, and all procedures were completed at room temperature.
Quantitative fibrosis analysis was performed using image processing
software (Image J 1.53e. Java 1.8.0_172; National Institutes of
Health) to measure fibrosis area and intensity in four randomly
selected fields of view.
ELISA
To assess the immunological response following
transplantation of the UCMSCs, the serum IgG levels were measured
using ELISA. Blood samples (1 ml per rat) were collected from the
tail vein immediately prior to euthanasia after the 4-week
treatment period and the serum was separated by centrifugation at
3,018.6 g for 15 min at room temperature, before being stored at
-20˚C for analysis. Using a specific Rat IgG ELISA kit (cat.
nos.866, Meimian Technology Co., Ltd.) for IgG according to the
manufacturer’s instructions, 100 µl serum was added to anti-rat
IgG-coated wells on a 96-well plate. After incubating and washing
the plate, an HRP-conjugated secondary antibody was used to detect
bound IgG. The reaction was developed with tetramethylbenzidine and
stopped by the addition of sulfuric acid. The optical density was
subsequently measured at 450 nm using a microplate reader and the
IgG levels were quantified against a standard curve.
Statistical analysis
Each experiment was performed at least three times.
Statistical analysis was performed using SPSS version 26.0 (IBM
Corp.). Continuous variables were presented as mean ± standard
deviation. One-way ANOVA was used for the homogeneous variance in
≥3 group comparisons followed by the Tukey's test for multiple
comparisons between groups. Welch's ANOVA was applied to the uneven
variance in ≥3 group comparisons followed by the Games-Howell
method between groups. P<0.05 was considered to indicate a
statistically significant difference. Each data analysis included
at least three independent molecular experiments, with a minimum of
three animal samples per group in each experiment.
Results
Isolation, cultivation and
characterization of UCMSCs
After the UCMSCs had been successfully isolated and
cultured within ~2 weeks, spindle-shaped cells began to migrate
from the explanted tissue pieces and reached 80-90% confluence,
displaying a characteristic ‘whirlpool-like’ pattern in their
arrangement (Fig. 1A). Further
phenotypic characterization through FCM analysis confirmed the
mesenchymal identity of the cells. Specifically, the cells were
found to exhibit high expression levels of MSC markers CD90, CD44,
CD105, CD73 and CD29, with minimal expression of the hematopoietic
markers HLA-DR and CD45, thereby validating their mesenchymal
lineage from Wharton's jelly (Fig.
1B). Subsequently, the morphological characteristics of the
UCMSCs were assessed using H&E staining, which revealed
elongated, spindle-shaped cells with well-defined oval nuclei and
light red cytoplasm, exhibiting a uniform fusiform shape, growing
in a vortex-like or parallel pattern, and arranged in a neat and
orderly fashion adherent to the substrate (Fig. 1C). These results confirmed the
successful isolation and characterization of UCMSCs, where the
specific marker profile supported their identity as MSCs.
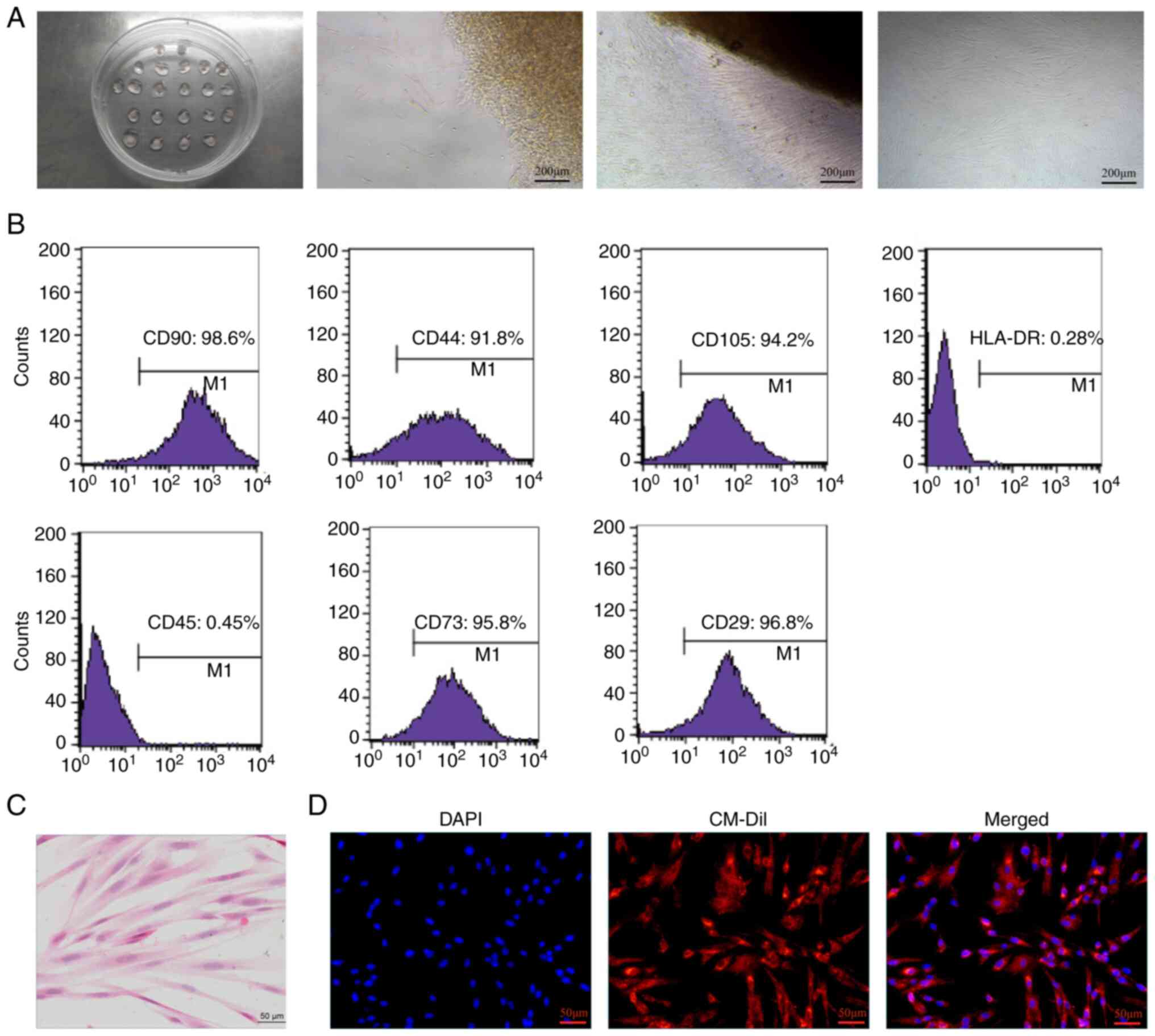 | Figure 1Isolation and characterization of
UCMSCs. (A) Representative images of the culture process, showing
the progression from explanted tissue pieces to 80-90% confluence
of spindle-shaped cells with a characteristic ‘whirlpool-like'
arrangement. From left to right: Tissue-culture plate, initial
tissue adhesion, early cell migration and near-confluent culture.
(B) Flow cytometry histograms, demonstrating the expression of
mesenchymal stem cell markers (CD90, CD44, CD105, CD73 and CD29)
and minimal expression of hematopoietic markers (HLA-DR and CD45).
Each panel shows the percentage of cells expressing the respective
marker, confirming the mesenchymal phenotype. (C) H&E-stained
micrograph of UCMSCs, highlighting elongated, spindle-shaped cells
with well-defined, oval nuclei and light red cytoplasm. (D)
Fluorescence microscopy images of UCMSCs stained with DAPI (blue)
for nuclei, CM-Dil (red) for cell membranes and a merged image
showing the co-localization of DAPI and CM-Dil, illustrating cell
morphology and integrity. The scale bar represents 50 µm. The data
shown are representative of three independent experiments (each
data analysis included at least three independent molecular
experiments, with a minimum of three animal samples per group in
each experiment). UCMSCs, human umbilical cord-derived mesenchymal
stem cells. |
CM-Dil labeling and in vivo tracking
of UCMSCs
Post-transplantation tracking of UCMSCs was achieved
using CM-Dil. After completion of the labeling process, UCMSCs
exhibited strong red fluorescence in the cytoplasm, confirming
successful uptake of the dye. Their nuclei were counterstained with
DAPI, producing a striking blue fluorescence (Fig. 1D). This dual-color imaging process
enabled precise tracking of the UCMSCs in the rat IUA model,
confirming that the CM-Dil-labeled cells both maintained their
structural integrity and were easily identifiable within the target
tissues.
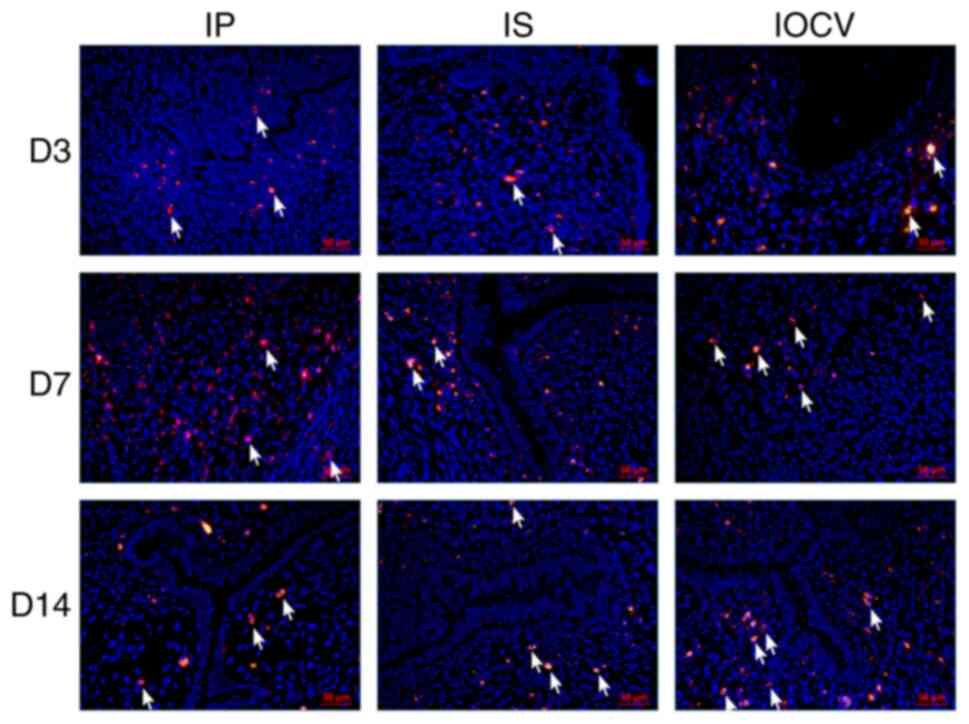
The localization and persistence of UCMSCs within
rat uterine tissues were tracked using fluorescence imaging
following transplantation. Fig. 2
specifically shows the distribution of UCMSCs post-transplantation
and does not include data from IUA-induced rats or a normal control
group. The figure was designed solely to evaluate the in
vivo localization of the transplanted cells. Cells were
pre-labeled with a fluorescent dye for enhanced visualization in
the tissue sections. By day 3, fluorescently labeled UCMSCs were
detectable in all treatment groups, confirming successful
engraftment (Fig. 2). By day 7,
the IP group exhibited widespread fluorescence throughout the
uterine muscular and interstitial tissues, whereas the IS group
exhibited no detectable fluorescence in the muscular layer,
indicating poor retention. The IOCV group displayed localized
fluorescence at the endometrial-myometrial junction, indicating
route-specific migration patterns. By day 14, all experimental
groups exhibited fluorescent markers near the uterine cavity's
inner lining, suggesting retention and potential integration of
UCMSCs into the endometrial layer (Fig. 2).
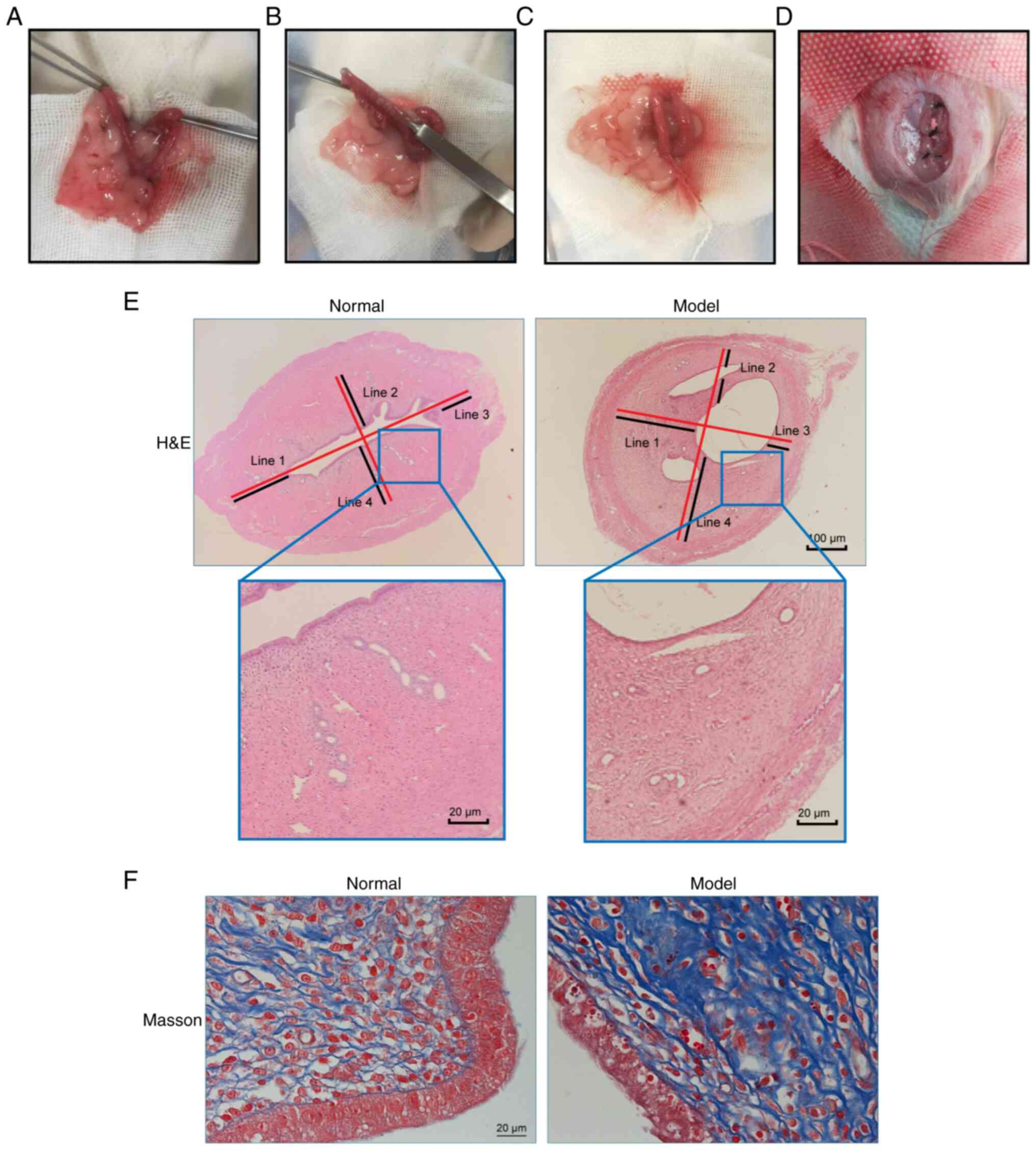
Construction of the rat IUA model
Following the established modeling procedures, a rat
model of IUA was developed (Fig.
3A-D). Histological analysis using H&E staining revealed a
significant reduction in the endometrial thickness in the model
group compared with that in the normal group (P<0.05; Fig. 3E and Table II). The number of endometrial
glands also decreased significantly in the model group compared
with that in the normal group (P<0.05), suggesting the
occurrence of substantial glandular loss due to induced adhesions
(Fig. 3E and Table II). Furthermore, the Masson's
trichrome staining experiments demonstrated a significant increase
in fibrotic areas within the endometrial interstitium of the model
group compared with that in the normal group (P<0.05; Fig. 3E and Table II). Collectively, these data
suggest the efficacy of the combined mechanical and
infection-induced injury methods in replicating IUAs in rats.
 | Table IIAssessment of the stability of the
intrauterine adhesion model. |
Table II
Assessment of the stability of the
intrauterine adhesion model.
| Parameters | Glands, n | Thickness, µm | Fibrotic area,
% |
|---|
| Normal | 25.7±5.3 | 612.1±41.3 | 19.9±1.5 |
| Model | 11.8±5.2 | 413.8±115.2 | 50.5±11.3 |
| P-value | 0.001 | 0.005 | 0.001 |
Effects of different UCMSC
administration routes on IUA treatment
The effectiveness of the different administration
routes of UCMSCs that were investigated in the present study in
terms of treating IUAs was evaluated through various histological
and immunological assessments. Significant differences in
endometrial thickness and gland numbers were observed comparing
among the experimental groups (P<0.001 for thickness; P<0.001
for gland number; Fig. 4A,
C and D). In particular, the IP group
demonstrated an increase in endometrial thickness, which was around
normal levels and was significantly higher compared with that in
the IS and IOCV groups. Additionally, the gland density was also
higher in the IP group (close to normal) compared with that in the
IS group and the baseline IUA model.
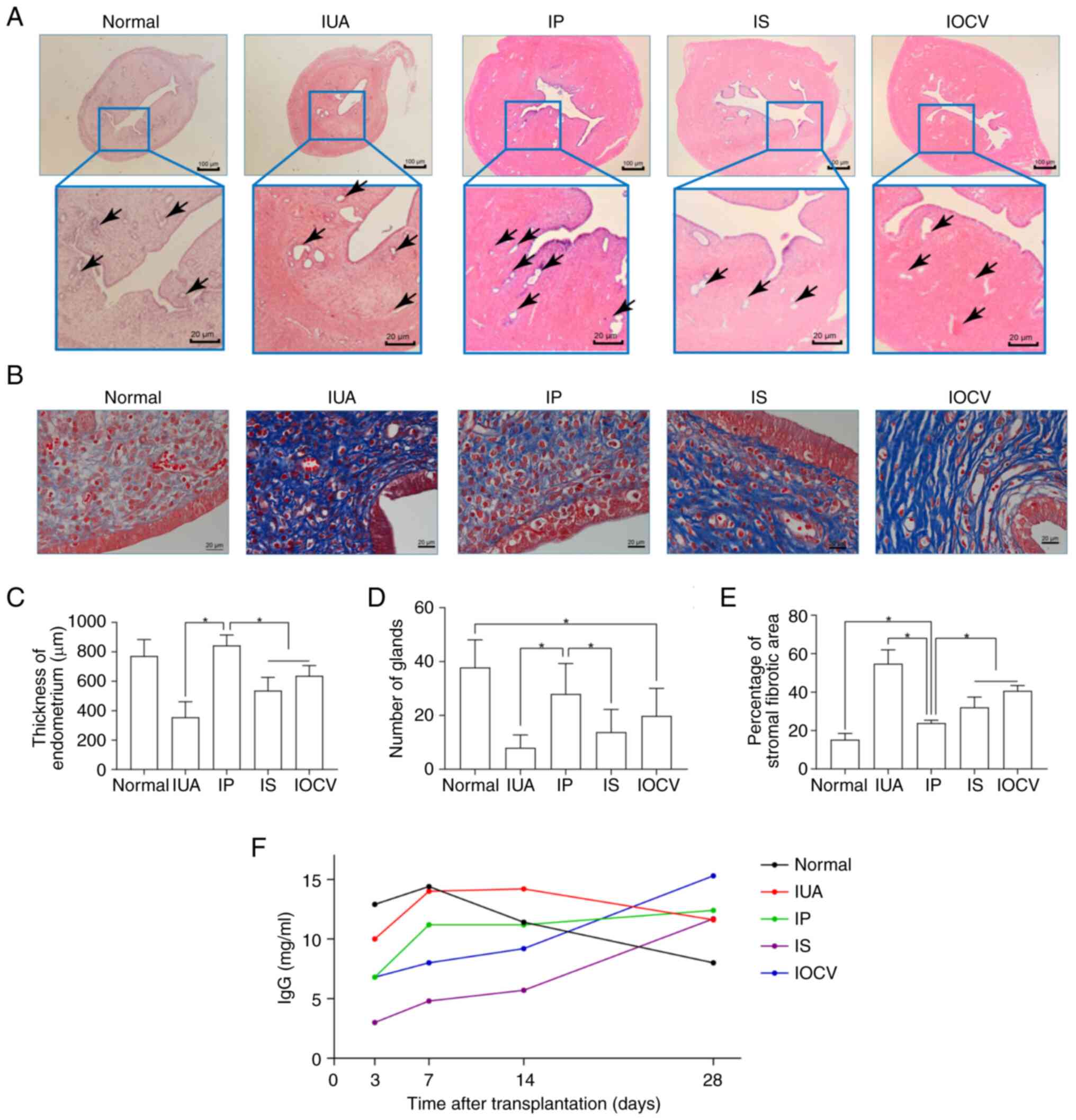 | Figure 4Histological and immunological
evaluation of the effectiveness of human umbilical cord-derived
mesenchymal stem cell treatment in a rat model of IUA. (A)
H&E-stained sections, displaying endometrial thickness among
the different experimental groups, namely the normal, IUA, IP, IS
and IOCV groups (arrows indicate the endometrial glands). Scale
bars represent 100 and 50 µm for the larger and smaller bars,
respectively. (B) Masson's trichrome staining highlighting fibrotic
areas in the same groups, illustrating variations in fibrosis. (C)
A bar graph comparing endometrial thickness among the groups,
showing the significantly thicker endometrium in the IP group
compared with the IS and IOCV group. (D) A bar graph of the
endometrial gland numbers, indicating a higher gland density in the
IP group close to normal levels. (E) The percentages of fibrotic
areas quantified are shown, with the IP group showing the least
fibrosis compared with the other treated and IUA groups. (F) A line
graph of IgG levels over time post-transplantation, with the
distinct immunological responses among groups highlighted. The IP
group's levels initially surged and then stabilized, whereas the IS
and IOCV groups showed gradual increases. IUA, intrauterine
adhesion; IP, intraperitoneal; IS, intrauterine site injection;
IOCV, intravenous ovary-cervix. Data are presented as the mean ±
SD. *P<0.05. |
The extent of fibrosis was also shown to be
significantly different among the experimental groups (P<0.001),
with the IP group showing the lowest percentage of fibrotic areas,
a value that was significantly lower compared with that in the IS,
IOCV and IUA groups (Fig. 4B and
E). IgG levels, measured using
ELISA, showed an initial rise within 3-7 days post-transplantation
in the five groups, although this trend was followed by diverging
trends afterwards. The IP group exhibited a rapid increase in IgG
levels, which were later stabilized, whereas the IS and IOCV groups
exhibited a more gradual increase (Fig. 4F). By day 28, the normal group had
the lowest immune response, whereas the IOCV group showed markedly
higher levels, reflecting the varying immunological impacts of the
different treatments. Taken together, these findings underscored
the distinct impact of different UCMSC administration routes on
both structural restoration and the immunological environment in
the IUA model of rats.
Effects of different concentrations of
UCMSCs administered intraperitoneally on IUA treatment
This series of experiments assessed the therapeutic
efficacy of administering different concentrations of UCMSCs
intraperitoneally in a rat model of IUA. Specifically, significant
differences in endometrial thickness were observed among the five
groups (P<0.001; Fig. 5A and
C). The group treated with
1.0x106 UCMSCs (the middle dose) exhibited a substantial
increase in thickness, surpassing the low-dose (0.5x106
cells) and high-dose (5.0x106 cells) groups, where the
thickness was comparable with that of the normal group, indicating
effective endometrial regeneration. Gland numbers also varied
significantly (P<0.001; Fig. 5A
and D). The 5.0x106
cells treatment group exhibited a significantly higher gland count
compared with that in the 0.5x106 cells treatment group
and the baseline IUA model, which was comparable with the normal
and 1.0x106 cells groups, suggesting a dose-dependent
effect on glandular restoration. The differences in fibrotic area
were also found to be statistically significant (P<0.001;
Fig. 5B and E). The 1.0x106 cells (middle
dose) group exhibited the lowest fibrotic percentage, which was
significantly lower compared with that in the high dose, low dose
and the baseline IUA model groups, highlighting the optimal dose
for minimizing fibrosis. Taken together, these findings suggested
that the concentration of UCMSCs that is administered
intraperitoneally critically influences both endometrial tissue
regeneration and fibrosis reduction.
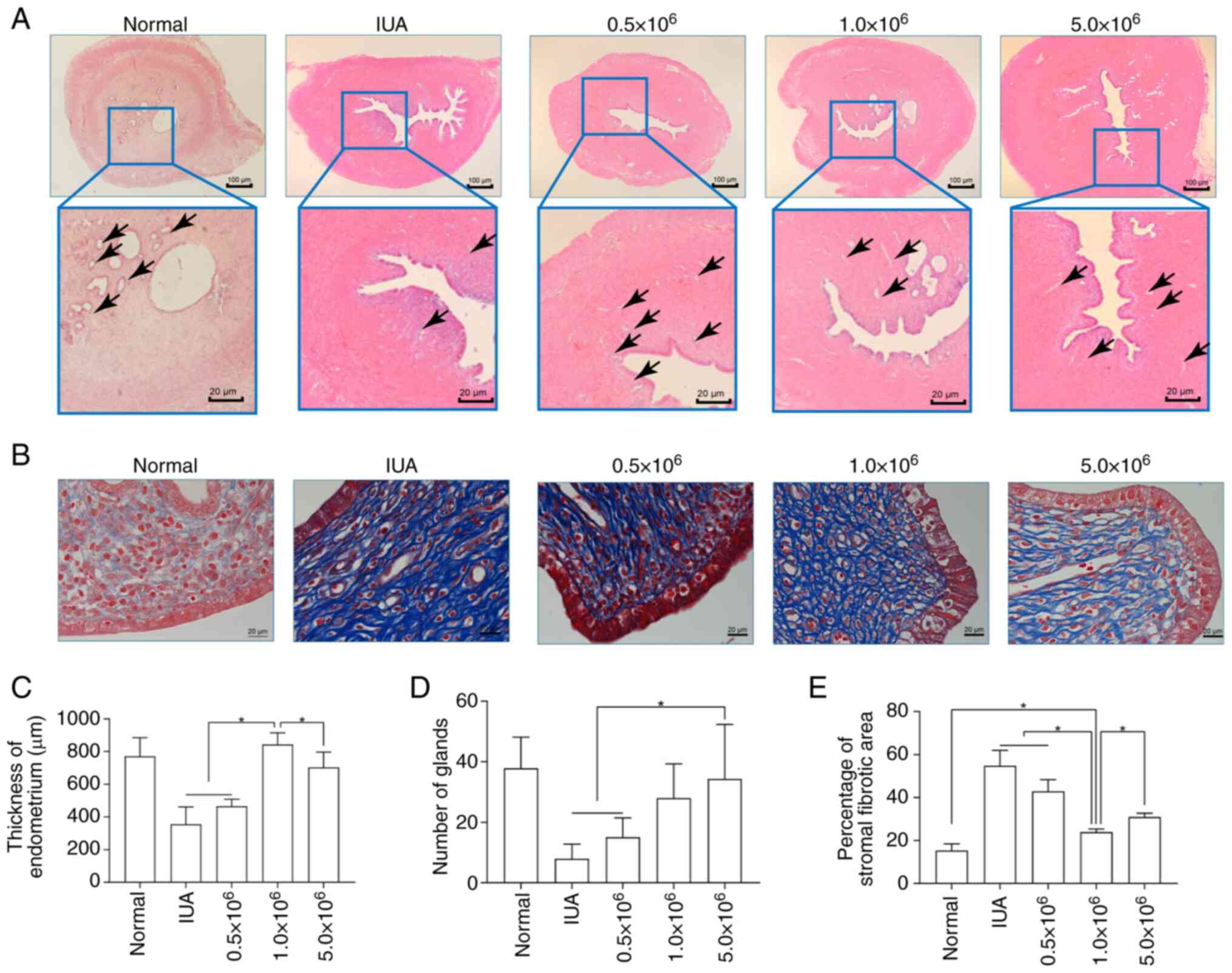 | Figure 5Evaluation of UCMSC therapy in an IUA
rat model using various concentrations of UCMSCs. (A)
H&E-stained sections, showing endometrial thickness across the
treatment groups, namely the Normal, IUA and three UCMSC-dosage
(0.5x106, 1.0x106 and 5.0x106)
groups (arrows indicate the glands). Scale bars, 100 and 20 µm. (B)
Masson's trichrome staining highlighting differences in the
fibrotic area among the same groups. (C) A graph of endometrial
thickness, illustrating the significant thickness recovery in the
1.0x106 group, which was approaching that of the normal
levels. (D) A bar graph depicting the number of glands, with the
5.0x106 group showing enhanced glandular recovery
compared with the other treatment groups. (E) Percentages of the
quantified fibrotic areas are shown, indicating the lowest level of
fibrosis in the 1.0x106 group, which significantly
outperformed the other groups. UCMSCs, human umbilical cord-derived
mesenchymal stem cells; IUA, intrauterine adhesion. Data are
presented as the mean ± SD. *P<0.05. |
Assessment of fertility post-UCMSC
treatment
Subsequently, the fertility restoration capabilities
of various concentrations and administration routes of UCMSCs were
next systematically evaluated. A 12-week co-housing experiment was
performed to assess the reproductive outcomes by counting the
offspring from different treatment and control groups (Fig. 6A). No pregnancies were observed in
the IUA model group (Fig. 6B) or
the low-dose UCMSC (0.5x106 cells) group (Fig. 6C). Among the administration
methods, intraperitoneal injection markedly outperformed the
in-site injection and intravenous tail-vein injection groups in
terms of offspring count (P<0.05). However, these numbers
remained significantly lower compared with those of the normal
group (Fig. 6B). The high-dose
(5.0x106 cells) and the middle-dose (1.0x106
cells) groups produced similar reproductive outcomes, but were both
significantly lower compared with the average number of pups in the
normal group (P<0.05; Fig. 6C).
Collectively, these results suggest that the treatment with UCMSCs
could partly restore fertility in rats afflicted with IUA.
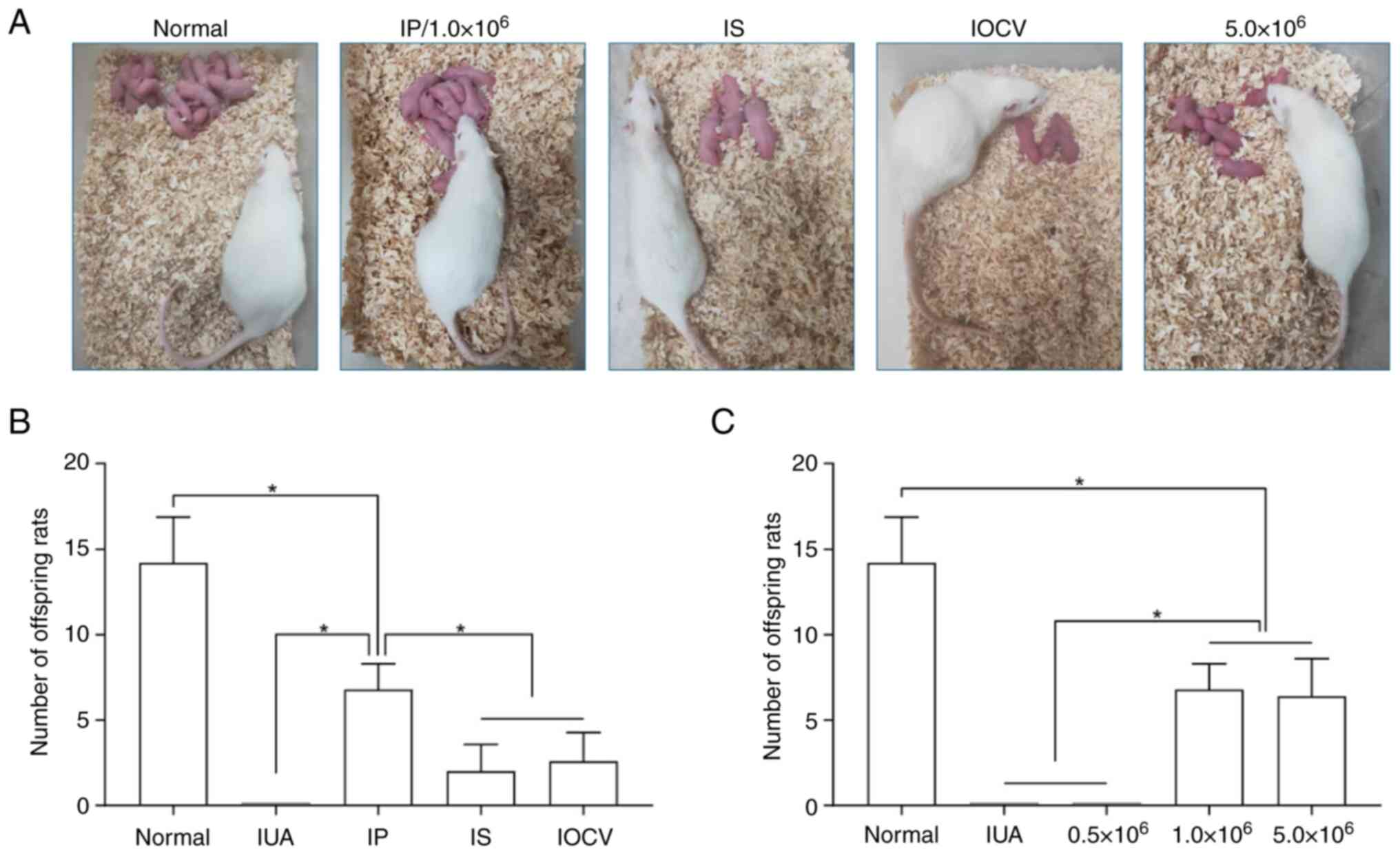 | Figure 6Fertility restoration in an IUA rat
model using UCMSC therapy. (A) Visual representation of co-housing
experiments with the Normal, IP/1.0x106 (intraperitoneal
injection of 1.0x106 cells), IS, IOCV and
5.0x106 cells groups is shown. (B) The numbers of
offspring in the Normal, IUA model and different administration
route (IP, IS and IOCV) groups are shown. The IP route showed the
highest offspring count among the various treatment groups,
although it remained lower compared with that in the Normal group.
(C) Comparison of offspring numbers among the cell dosage groups
(0.5x106, 1.0x106 and 5.0x106
cells), demonstrating that a higher dose does not significantly
increase fertility restoration compared with the medium
(1.0x106 cells) dose, while all were below the Normal
group outcomes. The data are shown as the mean ± SD
(*P<0.05). The data shown are representative of five
independent experiments in each group. IUA, intrauterine adhesion;
IP, intraperitoneal; IS, intrauterine site injection; IOCV,
intravenous ovary-cervix. |
Discussion
The limited efficacy of surgical and pharmacological
therapies that are currently in practice for the treatment of
moderate-to-severe IUAs has necessitated the exploration of
regenerative medicine, with stem cell therapy emerging as a
promising alternative (20). Among
the various stem cell sources, human UCMSCs have been increasingly
utilized in clinical trials due to their potent regenerative
capabilities and low immunogenicity (8). The present study has highlighted the
therapeutic potential of UCMSCs for treating IUAs, with particular
focus on both the effectiveness of injecting different
concentrations of UCMSCs and the type of delivery route applied.
The findings obtained have suggest that UCMSCs can significantly
enhance endometrial repair, especially at the optimal dose (found
to be 1x106 cells), which consistently outperformed both
the lower and the higher doses of UCMSCs in terms of endometrial
thickness restoration and fibrosis reduction.
The specific underlying mechanism through which MSCs
can promote endometrial repair remains controversial. However,
discussions are ongoing regarding whether their regenerative
potential stems primarily from their differentiation capabilities
or from their secretory functions (21). Evidence supports the hypothesis
that the paracrine effects of MSCs serve a predominant role in
tissue repair (21). The potential
mechanisms through which MSCs facilitate endometrial regeneration
in IUA include the following: i) Homing of MSCs to the injury site,
followed by differentiation into new endometrial cells; ii)
inhibition of epithelial-mesenchymal transition, thereby
suppressing the progression of fibrosis; iii) regulation of local
MSC proliferation and migration to promote endometrial
regeneration; iv) release of immunomodulatory factors that can
influence angiogenesis; and v) modulation of the immune response
through the upregulation of anti-inflammatory cytokines and
downregulation of pro-inflammatory cytokines (21,22).
However, at present, the clinical translational potential of UCMSCs
for IUA treatment remains unclear due to the significant
variability in outcomes reported. This was reportedly attributable
to various factors, such as cell source, timing of implantation,
route of administration and dosage (20). In addition, previous studies have
indicated that optimizing these parameters will likely enhance the
efficacy of stem cell therapies in restoring uterine function
(23,24). The present study has therefore
systematically evaluated the impact of cell dosage and
administration routes on the therapeutic outcomes in a rat model of
IUA, emphasizing the importance of precise standardization in
preclinical studies to optimize UCMSC treatment protocols for
future clinical applications in reproductive disorders, such as
IUAs.
UCMSCs have emerged as a promising MSC type for the
treatment of endometrial disorders. In vitro studies have
previously demonstrated that UCMSCs can significantly enhance the
proliferation of endometrial stromal cells and promote the
expression of vascular markers, contributing to tissue
regeneration. Sun et al (10) demonstrated that circular RNA
(hsa_circRNA_0111659) is upregulated during endometrial repair by
UCMSCs, providing a novel perspective on the role of non-coding
RNAs in regulating stem cell-mediated repair mechanisms. Similarly,
Shi et al (25) showed that
treatment with UCMSCs led to a significant improvement in the
proliferation and angiogenesis of endometrial stromal cells,
further highlighting their therapeutic potential. In terms of in
vivo animal models of IUA, a previous study showed that the
administration of UCMSCs during the chronic phase of endometrial
injury both facilitated endometrial regeneration and restored
fertility in rats (11).
Furthermore, another group reported that UCMSCs, through the
upregulation of microRNA-455-5p, could enhance endometrial
regeneration by modulating the JAK/STAT signaling pathway (12). Collectively, these studies
underscored the potential of UCMSC-based therapy for treating IUAs,
highlighting the need for further research into optimizing the mode
of delivery.
Additionally, the route of UCMSC administration
appears to occupy a crucial role in therapeutic outcomes. In animal
studies, the local administration of UCMSCs through collagen
scaffolds or hydrogels has been shown to improve endometrial
regeneration and decrease the extent of fibrosis, whilst collagen
scaffolds loaded with UCMSCs have also demonstrated significant
benefits in terms of fertility restoration (26,27).
Similarly, intraperitoneal, intrauterine and intravenous injections
of UCMSCs have all been evaluated, with varying results, depending
on the delivery method. Sabry et al (28) previously showed that combining MSC
therapy with neupogen, a granulocyte colony-stimulating factor,
resulted in significant improvements in endometrial fibrosis and
tissue regeneration, especially when the MSCs were administered
intraperitoneally. In another study, the combination of intravenous
and intrauterine UCMSC transplantation resulted in superior
outcomes compared with intravenous administration alone (29). Although the optimal delivery route
for UCMSC therapy remains controversial, several studies have
highlighted the need to tailor the approach to the specific
characteristics of the target tissue. A previous study on MSC
administration for treating type 1 diabetes suggested that the
intraperitoneal delivery route was more effective compared with
systemic administration in animal models (30). Similarly, studies comparing the
efficacy of different administration routes for the MSC-based
treatment of colitis found that intravenous administration yielded
improved outcomes compared with intraperitoneal delivery in terms
of reducing inflammation (31,32).
In addition, Zhao et al (18) demonstrated that MSC treatment
through the intraperitoneal and intrauterine routes was more
effective in treating recurrent spontaneous abortion in mice
compared with the route of intravenous administration. In clinical
settings, the application of UCMSCs through various routes,
including the intrauterine and intravenous routes, has shown
promise in improving outcomes in patients with Asherman's syndrome
or IUAs (11,33-35).
Clinical trials have demonstrated that delivering UCMSCs into the
uterine cavity, especially when loaded onto a collagen scaffold, is
a safe and effective method for improving endometrial regeneration
and fertility in patients with recurrent IUAs following
adhesiolysis surgery (36,37). In summary, optimizing the delivery
route, dosage and integration of biomaterials in UCMSC therapy has
been shown to be key in terms of enhancing MSC homing and retention
in the endometrium, thereby improving the treatment efficacy for
IUAs and advancing the clinical application of UCMSC therapy.
The homing mechanism of MSCs exerts a critical role
in tissue regeneration by enabling the recruitment and targeting of
stem cells to damaged tissues in need of repair (38). MSC homing can occur through both
systemic and site-specific pathways. Systemic homing involves the
migration of cells through the bloodstream, extravasation near the
lesion and interstitial migration towards the injury site (39). By contrast, non-systemic homing
involves local recruitment or the transplantation of MSCs close to
the target tissue, where the cells migrate in response to
chemokines released from injured tissues. However, the efficiency
of MSC homing is typically poor, with <10% of the cells reaching
the target site, especially when the MSCs are administered
intravenously, since they may become trapped in the lungs (40). Therefore, optimizing the route of
administration, whether systemic or non-systemic, has become a
crucial factor in improving therapeutic outcomes.
The simplest and most intuitive methods to enhance
MSC homing has been proposed to be to deliver the cells directly to
or near the target tissue, rather than relying on traditional
intravenous infusion (41). By
applying the method of localized (non-systemic) administration, MSC
retention in the target tissue is likely to improve compared with
systemic delivery methods (42).
Nevertheless, recent evidence has suggested that the route of
administration serves a crucial role in the effectiveness of the
treatment, which this is likely to be due to enhanced homing
efficiency (41,43). However, to date, to the best of our
knowledge, few studies have directly compared targeted with
systemic administration, where the majority of the evidence
currently available is from meta-analyses (44,45).
In the limited comparative studies available, it was observed that
the optimal route of MSC administration varies depending on the
disease being treated and the characteristics of the target tissue
(46-49).
Therefore, it is imperative to select the most appropriate MSC
delivery method based on the specific attributes of the disease and
the target tissue for effective treatment.
In the present study, systemic homing (through tail
vein injection) was systematically compared with non-systemic
homing (through intraperitoneal and intrauterine wall injections).
The results obtained revealed that intraperitoneal injection as the
administration route provided the optimal therapeutic outcomes for
treating IUAs, most likely due to reduced MSC clearance in the
lungs compared with tail-vein injection, which resulted in lower
retention of MSCs at the target site. Although intrauterine wall
injection represents a targeted approach, it may cause local trauma
to the endometrial tissue, thereby hindering the repair process. By
contrast, intraperitoneal injection may enhance the homing of
UCMSCs to the injured endometrial stroma through the gradient
induction of multiple chemotactic factors. These factors include
growth factors such as platelet-derived growth factor-AB,
insulin-like growth factor-1 and to a lesser extent, chemokines,
such as RANTES (also known as chemokine ligand 5),
macrophage-derived chemokine and stromal cell-derived factor 1
[also known as C-X-C motif ligand 12 (CXCL12)] (50). Data from a previous study showed
that the CM-Dil-labeled cells in the stroma region are
significantly higher compared with those in the superficial
myometrium, in the seroma and in the epithelium. Along with the
CM-Dil-labeled UCMSC tracking experiments in the present study,
confirmed that intraperitoneally administered UCMSCs can home to
the endometrial stroma (17).
The present study also focused on investigating the
routes of administration and dosages of UCMSC therapy for treating
IUAs, with a preliminary exploration of the therapeutic effects of
systemic compared with non-systemic homing. Future clinical
practices could enhance MSC homing efficiency through a number of
possible methods, such as genetic modification, cell surface
modulation, in vitro priming of MSCs, reducing intravascular
trapping in the lungs, and utilizing chemokine- or
cytokine-impregnated hydrogel scaffolds, nanoparticle-based
chemokine release (such as using CXCL12) or pulsed ultrasound
targeting injured tissues. To avoid the influence of scaffold
materials on the effect of UCMSCs, the present study did not
include intrauterine infusion, a commonly used administration
route. However, intrauterine infusion or placement of biomaterials
loaded with MSCs may represent the optimal future approach for
UCMSC therapy in IUA treatment. Although MSCs may be potentially
applied in reproductive medicine, their procurement can require
invasive procedures, especially in the case of bone marrow MScs
(BMMSCs), which has higher risk of tumorigenesis and
immunoreactivity (51). The
paradigm of biomedical research is shifting towards developing
personalized therapies. Future therapeutic strategies may consist
of combinations of stem cell-based therapies that promote cell
renewal and differentiation, and acellular therapies that modulate
inflammation and promote tissue repair, coupled with biomaterials
that concentrate these actions at the target site. These
synergistic approaches could hold the key to restoring endometrial
function, ultimately improving reproductive outcomes for patients
with uterine-factor infertility. On the basis of the existing
evidence concerning the cost, accessibility and availability of the
therapies discussed herein, a ‘triple-hit’ regenerative strategy
has been proposed, which would combine high-yield MSCs (such as
BMMSCs or UCMSCs) with acellular treatments, possibly integrated
into extracellular matrix hydrogels (20). Considered individually, these
approaches have demonstrated efficacy, but their combined impact
may yet significantly transform the clinical management of
endometrial disorders once their synergistic effects have been
verified. Finally, multi-center randomized controlled trials are
essential to further evaluate the safety and efficacy of these
biotechnological treatments.
In conclusion, the present study has demonstrated
the significant therapeutic potential of UCMSCs for treating IUAs,
especially emphasizing the superior efficacy of an optimal dose of
1x106 cells and the intraperitoneal delivery route in
terms of enhancing endometrial repair by restoring endometrial
thickness and reducing fibrosis. These findings underscore the
importance of dosage and delivery route optimization for maximizing
the therapeutic benefits of UCMSCs in IUA treatment.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Innovative
Capacity Improvement Plan of Hebei Province (S&T Program of
Hebei; grant no. 20577705D).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MLZ, ZKL and XHH provided the concept and design for
this study. HG, JHZ and YPT conducted the experiments and wrote the
manuscript. YLX, QL and YFD were responsible for the identification
of mesenchymal stem cells. MLZ and HG were responsible for data
analysis and preparing the figures. ZKL, MLZ and XHH discussed and
revised the manuscript. XHH and ZKL reviewed the manuscript. All
authors agree to the submission and publication of the final
manuscript. MLZ and HG confirm the authenticity of all the raw
data. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The design and implementation steps of the animal
experiments, as well as the acquisition of human umbilical cords,
were approved by the Institution Animal Ethics Committee of the
Second Hospital of Hebei Medical University (Shijiazhuang, China;
approval no. 2020-R285; approval no. for animal study: 2021-AE261).
Written informed consent was obtained from the mothers who donated
the umbilical cords for research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma J, Zhan H, Li W, Zhang L, Yun F, Wu R,
Lin J and Li Y: Recent trends in therapeutic strategies for
repairing endometrial tissue in intrauterine adhesion. Biomater
Res. 25(40)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hooker AB, Lemmers M, Thurkow AL, Heymans
MW, Opmeer BC, Brölmann HA, Mol BW and Huirne JA: Systematic review
and meta-analysis of intrauterine adhesions after miscarriage:
prevalence, risk factors and long-term reproductive outcome. Hum
Reprod Update. 20:262–278. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hooker AB, De Leeuw RA, Emanuel MH,
Mijatovic V, Brolmann HAM and Huirne JAF: The link between
intrauterine adhesions and impaired reproductive performance: A
systematic review of the literature. BMC Pregnancy Childbirth.
22(837)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tu CH, Yang XL, Qin XY, Cai LP and Zhang
P: Management of intrauterine adhesions: A novel intrauterine
device. Med Hypotheses. 81:394–396. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Han Y, Li X, Zhang Y, Han Y, Chang F and
Ding J: Mesenchymal Stem cells for regenerative medicine. Cells.
8(886)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ma J, Wu J, Han L, Jiang X, Yan L, Hao J
and Wang H: Comparative analysis of mesenchymal stem cells derived
from amniotic membrane, umbilical cord, and chorionic plate under
serum-free condition. Stem Cell Res Ther. 10(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
El Omar R, Beroud J, Stoltz JF, Menu P,
Velot E and Decot V: Umbilical cord mesenchymal stem cells: the new
gold standard for mesenchymal stem cell-based therapies? Tissue Eng
Part B Rev. 20:523–544. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang X, Zhang M, Zhang Y, Li W and Yang B:
Mesenchymal stem cells derived from Wharton jelly of the human
umbilical cord ameliorate damage to human endometrial stromal
cells. Fertil Steril. 96:1029–1036. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun B, Shi L, Shi Q, Jiang Y, Su Z, Yang X
and Zhang Y: Circular RNAs are abundantly expressed and upregulated
during repair of the damaged endometrium by Wharton’s jelly-derived
mesenchymal stem cells. Stem Cell Res Ther. 9(314)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang L, Li Y, Guan CY, Tian S, Lv XD, Li
JH, Ma X and Xia HF: Therapeutic effect of human umbilical
cord-derived mesenchymal stem cells on injured rat endometrium
during its chronic phase. Stem Cell Res Ther. 9(36)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun D, Jiang Z, Chen Y, Shang D, Miao P
and Gao J: MiR-455-5p upregulation in umbilical cord mesenchymal
stem cells attenuates endometrial injury and promotes repair of
damaged endometrium via Janus kinase/signal transducer and
activator of transcription 3 signaling. Bioengineered.
12:12891–12904. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang L, Yu C, Chang T, Zhang M, Song S,
Xiong C, Su P and Xiang W: In situ repair abilities of human
umbilical cord-derived mesenchymal stem cells and autocrosslinked
hyaluronic acid gel complex in rhesus monkeys with intrauterine
adhesion. Sci Adv. 6(eaba6357)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Giri J and Galipeau J: Mesenchymal stromal
cell therapeutic potency is dependent upon viability, route of
delivery, and immune match. Blood Adv. 4:1987–1997. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Levy O, Kuai R, Siren EMJ, Bhere D, Milton
Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, et al:
Shattering barriers toward clinically meaningful MSC therapies. Sci
Adv. 6(eaba6884)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zheng JH, Zhang JK, Kong DS, Song YB, Zhao
SD, Qi WB, Li YN, Zhang ML and Huang XH: Quantification of the
CM-Dil-labeled human umbilical cord mesenchymal stem cells migrated
to the dual injured uterus in SD rat. Stem Cell Res Ther.
11(280)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhao S, Qi W, Zheng J, Tian Y, Qi X, Kong
D, Zhang J and Huang X: Exosomes derived from adipose mesenchymal
stem cells restore functional endometrium in a rat model of
intrauterine adhesions. Reprod Sci. 27:1266–1275. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kong D, Zhang L, Xu X, Zhang J, Li Y and
Huang X: Small intestine submucosa is a potential material for
intrauterine adhesions treatment in a rat model. Gynecol Obstet
Invest. 83:499–507. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rodríguez-Eguren A, Bueno-Fernandez C,
Gómez-Álvarez M, Francés-Herrero E, Pellicer A, Bellver J, Seli E
and Cervelló I: Evolution of biotechnological advances and
regenerative therapies for endometrial disorders: A systematic
review. Hum Reprod Update. 30:584–613. 2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Azizi R, Aghebati-Maleki L, Nouri M,
Marofi F, Negargar S and Yousefi M: Stem cell therapy in Asherman
syndrome and thin endometrium: Stem cell-based therapy. Biomed
Pharmacother. 102:333–343. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo LP, Chen LM, Chen F, Jiang NH and Sui
L: Smad signaling coincides with epithelial-mesenchymal transition
in a rat model of intrauterine adhesion. Am J Transl Res.
11:4726–4737. 2019.PubMed/NCBI
|
|
23
|
Arikan G, Turan V, Kurekeken M, Goksoy HS
and Dogusan Z: Autologous bone marrow-derived nucleated cell
(aBMNC) transplantation improves endometrial function in patients
with refractory Asherman's syndrome or with thin and dysfunctional
endome-trium. J Assist Reprod Genet. 40:1163–1171. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
de Miguel-Gómez L, López-Martínez S, Campo
H, Francés-Herrero E, Faus A, Díaz A, Pellicer A, Domínguez F and
Cervelló I: Comparison of different sources of platelet-rich plasma
as treatment option for infertility-causing endometrial
pathologies. Fertil Steril. 115:490–500. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shi Q, Sun B, Wang D, Zhu Y, Zhao X, Yang
X and Zhang Y: Circ6401, a novel circular RNA, is implicated in
repair of the damaged endometrium by Wharton's jelly-derived
mesenchymal stem cells through regulation of the miR-29b-1-5p/RAP1B
axis. Stem Cell Res Ther. 11(520)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xin L, Lin X, Pan Y, Zheng X, Shi L, Zhang
Y, Ma L, Gao C and Zhang S: A collagen scaffold loaded with human
umbilical cord-derived mesenchymal stem cells facilitates
endometrial regeneration and restores fertility. Acta Biomater.
92:160–171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu Y, Cai J, Luo X, Wen H and Luo Y:
Collagen scaffold with human umbilical cord mesenchymal stem cells
remarkably improves intrauterine adhesions in a rat model. Gynecol
Obstet Invest. 85:267–276. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sabry D, Mostafa A, Marzouk S, Ibrahim W,
Ali HHM, Hassan A and Shamaa A: Neupogen and mesenchymal stem cells
are the novel therapeutic agents in regeneration of induced
endometrial fibrosis in experimental rats. Biosci Rep.
37(BSR20170794)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhuang M, Zhang W, Cheng N, Zhou L, Liu D,
Yan H, Fang G, Heng BC, Sun Y and Tong G: Human umbilical cord
mesenchymal stromal cells promote the regeneration of severe
endometrial damage in a rat model. Acta Biochim Biophys Sin
(Shanghai). 54:148–151. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hashemi SM, Hassan ZM, Hossein-Khannazer
N, Pourfathollah AA and Soudi S: Investigating the route of
administration and efficacy of adipose tissue-derived mesenchymal
stem cells and conditioned medium in type 1 diabetic mice.
Inflammopharmacology. 28:585–601. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Goncalves Fda C, Schneider N, Pinto FO,
Meyer FS, Visioli F, Pfaffenseller B, Lopez PL, Passos EP,
Cirne-Lima EO, Meurer L and Paz AH: Intravenous vs intraperitoneal
mesenchymal stem cells administration: What is the best route for
treating experimental colitis? World J Gastroenterol.
20:18228–18239. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huldani H, Margiana R, Ahmad F, Opulencia
MJC, Ansari MJ, Bokov DO, Abdullaeva NN and Siahmansouri H:
Immunotherapy of inflammatory bowel disease (IBD) through
mesenchymal stem cells. Int Immunopharmacol.
107(108698)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou S, Lei Y, Wang P, Chen J, Zeng L, Qu
T, Maldonado M, Huang J, Han T, Wen Z, et al: Human umbilical cord
mesenchymal stem cells encapsulated with pluronic F-127 enhance the
regeneration and angiogenesis of thin endometrium in rat via local
IL-1β stimulation. Stem Cells Int. 2022(7819234)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang H, Yang X, Chen X, Xie H, Wang J and
Zhang Y: Identify the role of human Wharton's jelly mesenchymal
stem cells in repairing injured uterine of rat. J Obstet Gynaecol
Res. 47:320–328. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang L, Li Y, Dong YC, Guan CY, Tian S,
Lv XD, Li JH, Su X, Xia HF and Ma X: Transplantation of umbilical
cord-derived mesenchymal stem cells promotes the recovery of thin
endometrium in rats. Sci Rep. 12(412)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G,
Wang J, Bai D, Wang J, Wang L, et al: Allogeneic cell therapy using
umbilical cord MSCs on collagen scaffolds for patients with
recurrent uterine adhesion: A phase I clinical trial. Stem Cell Res
Ther. 9(192)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kaczynski JB and Rzepka JK: Endometrial
regeneration in Asherman's syndrome and endometrial atrophy using
Wharton's jelly-derived mesenchymal stem cells. Ginekol Pol.
93:904–909. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liesveld JL, Sharma N and Aljitawi OS:
Stem cell homing: From physiology to therapeutics. Stem Cells.
38:1241–1253. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nitzsche F, Muller C, Lukomska B,
Jolkkonen J, Deten A and Boltze J: Concise review: MSC adhesion
cascade-insights into homing and transendothelial migration. Stem
Cells. 35:1446–1460. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Krueger TEG, Thorek DLJ, Denmeade SR,
Isaacs JT and Brennen WN: Concise review: Mesenchymal stem
cell-based drug delivery: The good, the bad, the ugly, and the
promise. Stem Cells Transl Med. 7:651–663. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yuan M, Hu X, Yao L, Jiang Y and Li L:
Mesenchymal stem cell homing to improve therapeutic efficacy in
liver disease. Stem Cell Res Ther. 13(179)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ullah M, Liu DD and Thakor AS: Mesenchymal
stromal cell homing: Mechanisms and strategies for improvement.
iScience. 15:421–438. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Maric DM, Velikic G, Maric DL, Supic G,
Vojvodic D, Petric V and Abazovic D: Stem cell homing in
intrathecal applications and inspirations for improvement paths.
Int J Mol Sci. 23(4290)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jiang Z, Chen L, Huang L, Yu S, Lin J, Li
M, Gao Y and Yang L: Bioactive materials that promote the homing of
endogenous mesenchymal stem cells to improve wound healing. Int J
Nanomedicine. 19:7751–7773. 2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu Z, Mikrani R, Zubair HM, Taleb A,
Naveed M, Baig MMFA, Zhang Q, Li C, Habib M, Cui X, et al: Systemic
and local delivery of mesenchymal stem cells for heart renovation:
Challenges and innovations. Eur J Pharmacol.
876(173049)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kanelidis AJ, Premer C, Lopez J, Balkan W
and Hare JM: Route of delivery modulates the efficacy of
mesenchymal stem cell therapy for myocardial infarction: A
meta-analysis of preclinical studies and clinical trials. Circ Res.
120:1139–1150. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jeong H, Yim HW, Park HJ, Cho Y, Hong H,
Kim NJ and Oh IH: Mesenchymal stem cell therapy for ischemic heart
disease: Systematic review and meta-analysis. Int J Stem Cells.
11:1–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Peng W, Sun J, Sheng C, Wang Z, Wang Y,
Zhang C and Fan R: Systematic review and meta-analysis of efficacy
of mesenchymal stem cells on locomotor recovery in animal models of
traumatic brain injury. Stem Cell Res Ther. 6(47)2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Augustine S, Avey MT, Harrison B, Locke T,
Ghannad M, Moher D and Thébaud B: Mesenchymal stromal cell therapy
in bronchopulmonary dysplasia: Systematic review and meta-analysis
of preclinical studies. Stem Cells Transl Med. 6:2079–2093.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ponte AL, Marais E, Gallay N, Langonné A,
Delorme B, Hérault O, Charbord P and Domenech J: The in vitro
migration capacity of human bone marrow mesenchymal stem cells:
Comparison of chemokine and growth factor chemotactic activities.
Stem Cells. 25:1737–1745. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ramaswamy Reddy SH, Reddy R, Babu NC and
Ashok GN: Stem-cell therapy and platelet-rich plasma in
regenerative medicines: A review on pros and cons of the
technologies. J Oral Maxillofac Pathol. 22:367–374. 2018.PubMed/NCBI View Article : Google Scholar
|