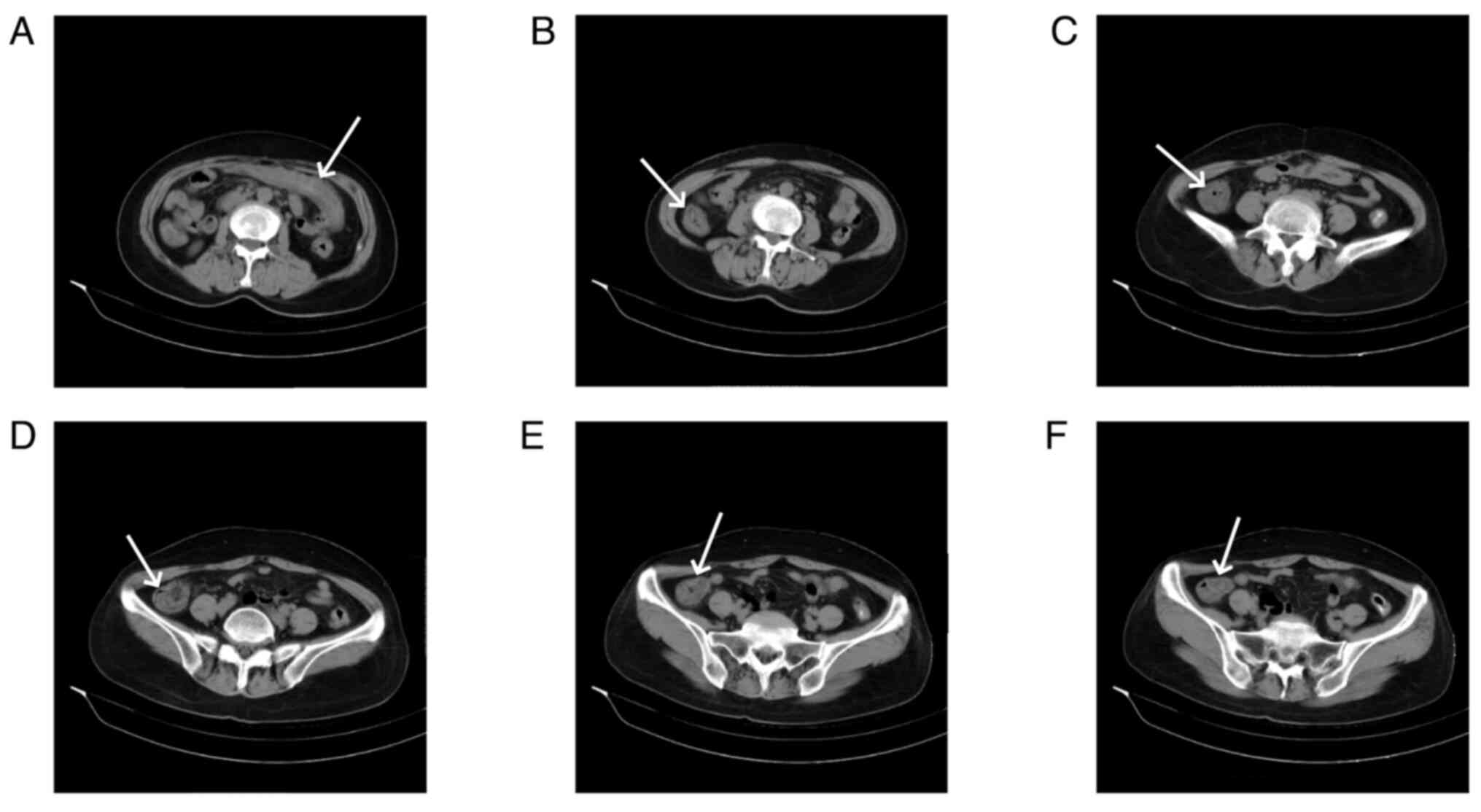
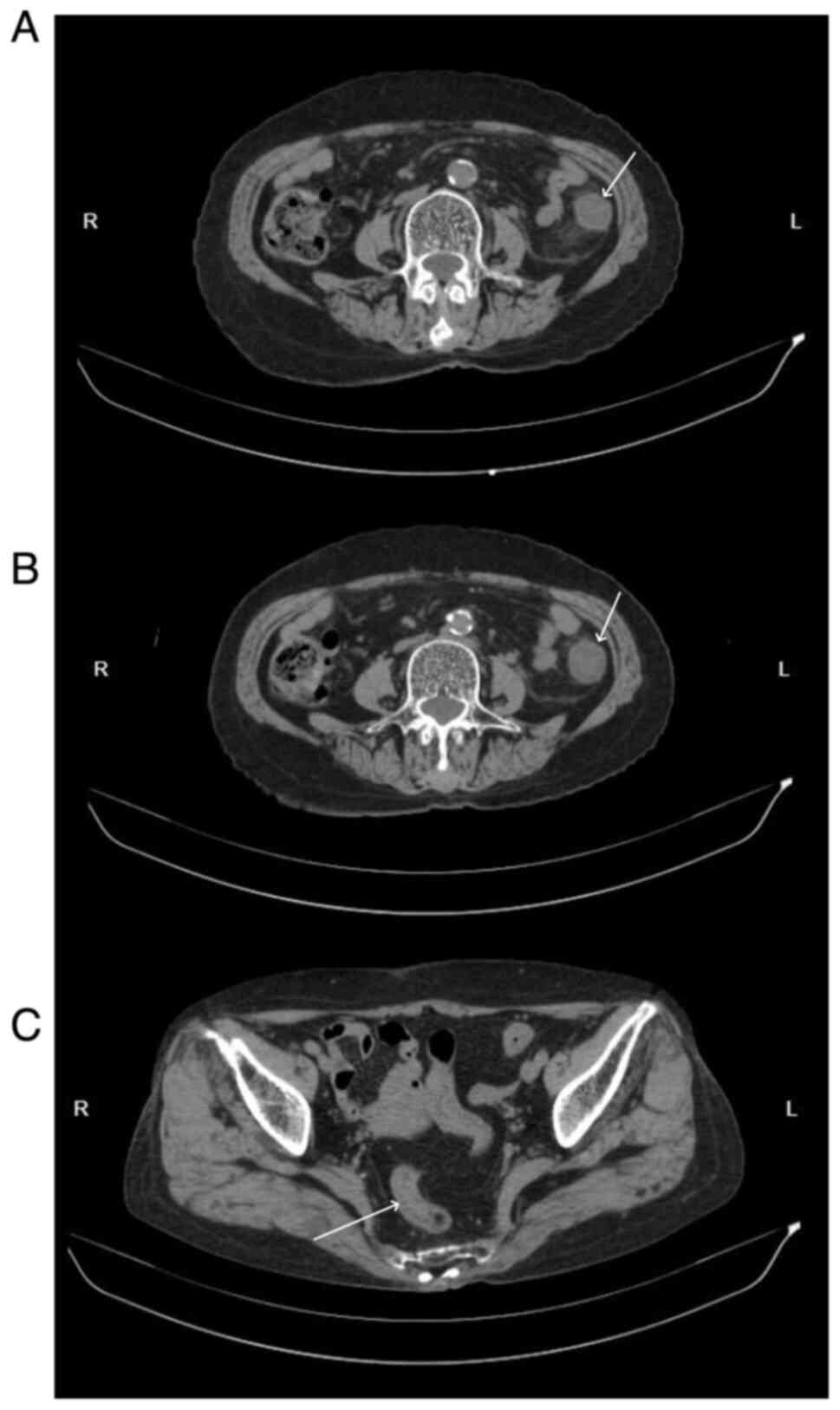
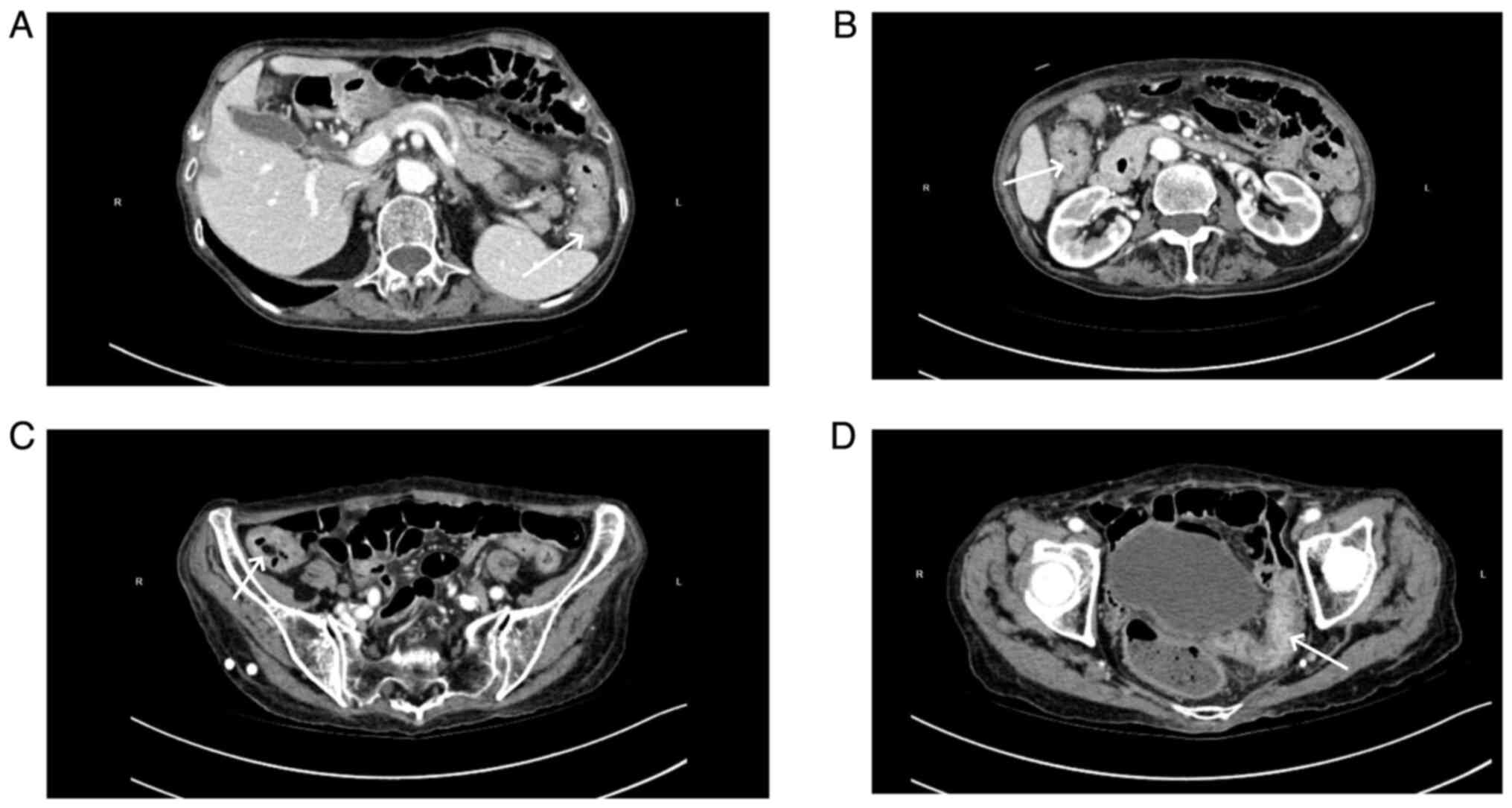
|
1
|
Dahal RH, Kim S, Kim YK, Kim ES and Kim J:
Insight into gut dysbiosis of patients with inflammatory bowel
disease and ischemic colitis. Front Microbiol.
14(1174832)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fairen Oro C and Beresneva O: Diagnosis
and management of ischemic colitis. Dis Colon Rectum. 66:872–875.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Trotter JM, Hunt L and Peter MB: Ischaemic
colitis. BMJ. 355(i6600)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yadav S, Dave M, Edakkanambeth Varayil J,
Harmsen WS, Tremaine WJ, Zinsmeister AR, Sweetser SR, Melton LJ
III, Sandborn WJ and Loftus EV Jr: A population-based study of
incidence, risk factors, clinical spectrum, and outcomes of
ischemic colitis. Clin Gastroenterol Hepatol. 13:731–738.e1-e6,
e41. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ichita C, Goto T, Sasaki A and Shimizu S:
National trends in hospitalizations for gastrointestinal bleeding
in Japan. J Clin Biochem Nutr. 75:60–64. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vodusek Z, Feuerstadt P and Brandt LJ:
Review article: The pharmacological causes of colon ischaemia.
Aliment Pharmacol Ther. 49:51–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Suo BJ, Zhou LY, Ding SG, Lü YM, Gu F, Lin
SR and Zheng YA: The endoscopic and clinical features of indigo
naturalis-associated ischemic lesions of colonic mucosa. Zhonghua
Nei Ke Za Zhi. 50:646–649. 2011.PubMed/NCBI(In Chinese).
|
|
8
|
Cho B, Yoon SM, Son SM, Kim HW, Kim KB and
Youn SJ: Ischemic colitis induced by indigo naturalis in a patient
with ulcerative colitis: A case report. BMC Gastroenterol.
20(154)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kondo S, Araki T, Okita Y, Yamamoto A,
Hamada Y, Katsurahara M, Horiki N, Nakamura M, Shimoyama T,
Yamamoto T, et al: Colitis with wall thickening and edematous
changes during oral administration of the powdered form of Qing-dai
in patients with ulcerative colitis: A report of two cases. Clin J
Gastroenterol. 11:268–272. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun Q, Leng J, Tang L, Wang L and Fu C: A
comprehensive review of the chemistry, pharmacokinetics,
pharmacology, clinical applications, adverse events, and quality
control of indigo naturalis. Front Pharmacol.
12(664022)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hendrick V, Altshuler L and Whybrow P:
Psychoneuroendocrinology of mood disorders. The
hypothalamic-pituitary-thyroid axis. Psychiatr Clin North Am.
21:277–292. 1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang L, Li X, Huang W, Rao X and Lai Y:
Pharmacological properties of indirubin and its derivatives. Biomed
Pharmacother. 151(113112)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu Y, Lin C, Tan HY and Bian ZX: The
double-edged sword effect of indigo naturalis. Food Chem Toxicol.
185(114476)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Matsuno Y, Torisu T, Umeno J, Shibata H,
Hirano A, Fuyuno Y, Okamoto Y, Fujioka S, Kawasaki K, Moriyama T,
et al: One-year clinical efficacy and safety of indigo naturalis
for active ulcerative colitis: A real-world prospective study.
Intest Res. 20:260–268. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Uchiyama K, Takami S, Suzuki H, Umeki K,
Mochizuki S, Kakinoki N, Iwamoto J, Hoshino Y, Omori J, Fujimori S,
et al: Efficacy and safety of short-term therapy with indigo
naturalis for ulcerative colitis: An investigator-initiated
multicenter double-blind clinical trial. PLoS One.
15(e0241337)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Saiki JP, Andreasson JO, Grimes KV,
Frumkin LR, Sanjines E, Davidson MG, Park KT and Limketkai B:
Treatment-refractory ulcerative colitis responsive to indigo
naturalis. BMJ Open Gastroenterol. 8(e000813)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ruan SL, Lu QM, Yang ZH, Yu YW and Tang
LL: Clinical investigation on schistosomiasis after a
16-year-interruption program regarding its transmission, in Jiaxing
region of Zhejiang province. Zhonghua Liu Xing Bing Xue Za Zhi.
34:523–525. 2013.PubMed/NCBI(In Chinese).
|
|
18
|
Brandt LJ, Feuerstadt P, Longstreth GF and
Boley SJ: American College of Gastroenterology. ACG clinical
guideline: Epidemiology, risk factors, patterns of presentation,
diagnosis, and management of colon ischemia (CI). Am J
Gastroenterol. 110:18–45. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou S, Shi Q, Zheng Y, Zhuang Y, Lin Y,
Huang Z and Yu J: Sheng-Xue-Xiao-Ban Capsule-induced ischemic
colitis and pulmonary embolism in an idiopathic thrombocytopenic
purpura patient: A rare case report. Ann Transl Med.
10(1027)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ahmed M: Ischemic bowel disease in 2021.
World J Gastroenterol. 27:4746–4762. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cotter TG, Bledsoe AC and Sweetser S:
Colon ischemia: An update for clinicians. Mayo Clin Proc.
91:671–677. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen MS and Du LY: Effect of Compound
Qingdai Granula on the expression of TGF-β1 and VEGF in the colon
tissue of rats with ulcerative colitis. Chin J Integr Tradit West
Med Dig. 21:393–396. 2013.
|
|
23
|
Wang Y, Liu L, Guo Y, Mao T, Shi R and Li
J: Effects of indigo naturalis on colonic mucosal injuries and
inflammation in rats with dextran sodium sulphate-induced
ulcerative colitis. Exp Ther Med. 14:1327–1336. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sugimoto S, Naganuma M and Kanai T: Indole
compounds may be promising medicines for ulcerative colitis. J
Gastroenterol. 51:853–861. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kudo T, Jimbo K, Shimizu H, Iwama I,
Ishige T, Mizuochi T, Arai K, Kumagai H, Uchida K, Abukawa D and
Shimizu T: Qing-dai for pediatric ulcerative colitis multicenter
survey and systematic review. Pediatr Int.
64(e15113)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Naganuma M, Sugimoto S, Suzuki H, Matsuno
Y, Araki T, Shimizu H, Hayashi R, Fukuda T, Nakamoto N, Iijima H,
et al: Adverse events in patients with ulcerative colitis treated
with indigo naturalis: A Japanese nationwide survey. J
Gastroenterol. 54:891–896. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xiao HT, Peng J, Hu DD, Lin CY, Du B,
Tsang SW, Lin ZS, Zhang XJ, Lueng FP, Han QB and Bian ZX: Qing-dai
powder promotes recovery of colitis by inhibiting inflammatory
responses of colonic macrophages in dextran sulfate sodium-treated
mice. Chin Med. 10(29)2015.
|
|
28
|
Gu S, Xue Y, Gao Y, Shen S, Zhang Y, Chen
K, Xue S, Pan J, Tang Y, Zhu H, et al: Mechanisms of indigo
naturalis on treating ulcerative colitis explored by GEO gene chips
combined with network pharmacology and molecular docking. Sci Rep.
10(15204)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Adachi S, Hoshi N, Inoue J, Yasutomi E,
Otsuka T, Dhakhwa R, Wang Z, Koo Y, Takamatsu T, Matsumura Y, et
al: Indigo naturalis ameliorates oxazolone-induced dermatitis but
aggravates colitis by changing the composition of gut microflora.
Int Arch Allergy Immunol. 173:23–33. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang QY, Ma LL, Zhang C, Lin JZ, Han L, He
YN and Xie CG: Exploring the mechanism of indigo naturalis in the
treatment of ulcerative colitis based on TLR4/MyD88/NF-κB signaling
pathway and gut microbiota. Front Pharmacol.
12(674416)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liang YN, Yu JG, Zhang DB, Zhang Z, Ren
LL, Li LH, Wang Z and Tang ZS: Indigo naturalis ameliorates dextran
sulfate sodium-induced colitis in mice by modulating the intestinal
microbiota community. Molecules. 24(4086)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Luo L, Li C, Huang N, Wang Q and Zhang Z,
Song C, Yang H, Yuan M, Xu Z, Sun J and Zhang Z: Traditional
mineral medicine realgar and realgar-indigo naturalis formula
potentially exerted therapeutic effects by altering the gut
microbiota. Front Microbiol. 14(1143173)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sun Z, Li J, Dai Y, Wang W, Shi R, Wang Z,
Ding P, Lu Q, Jiang H, Pei W, et al: Indigo naturalis alleviates
dextran sulfate sodium-induced colitis in rats via altering gut
microbiota. Front Microbiol. 11(731)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Maimone A, De Ceglie A, Siersema PD, Baron
TH and Conio M: Colon ischemia: A comprehensive review. Clin Res
Hepatol Gastroenterol. 45(101592)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee JM and Lee KM: Endoscopic diagnosis
and differentiation of inflammatory bowel disease. Clin Endosc.
49:370–375. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li Z, Liping D, Weihong Y, et al: The
clinical feature and possible pathogenesis of natural indigo
(Qingdai) induced hematochezia. Chin J Gastroenterol Hepatol.
13:161–164. 2004.(In Chinese).
|
|
37
|
Wang CL, Si ZK, Liu GH, Chen C, Zhao H and
Li L: Ischemic colitis induced by a platelet-raising capsule: A
case report. World J Clin Cases. 12:607–615. 2024.PubMed/NCBI View Article : Google Scholar
|