Introduction
Ischemic colitis (IC) is characterized by ischemic
changes in the intestinal mucosa due to non-occlusive damage to
small blood vessels (1-3).
There has been a gradual increase in the prevalence of IC in recent
years. A population-based retrospective cohort study indicated that
the incidence of IC increased by approximately four-fold, from 6.1
cases/100,000 individuals/year in 1976-1980 to 22.9 cases/100,000
individuals/year in 2005-2009(4).
A 2024 Japanese study revealed that the incidence of ischemic
colitis increased from 20.8 to 34.9 per 100,000, representing an
~1.6-fold increase (5). While IC
is more common in older patients (>60 years) with underlying
atherosclerosis, IC is also associated with certain medications,
including psychotropic drugs (such as quetiapine and clozapine),
digoxin, oral contraceptives, immunomodulators [such as
lenalidomide, corticosteroids and tumor necrosis factor (TNF)-α
inhibitors], laxatives and non-steroidal anti-inflammatory drugs
(6). Thus, identifying
drug-induced IC and implementing accurate management strategies is
important for the effective and prompt control of the disease.
Evidence suggests that herbal medicines containing
components of indigo naturalis may contribute to the pathogenesis
of IC (7-10).
Indigo naturalis is a dried powdered mass obtained from processing
leaves or stems of plants such as Baphicacanthus cusia
Bremek, Polygonum tinctorium Ait and Isatis
indigotica Fort (11,12). This dark blue compound has been
used in Traditional Chinese medicines for thousands of years for
its therapeutic properties, and may be used to treat conditions
such as hemoptysis, epistaxis and infantile convulsion as well as
for the management of psoriasis and ulcerative colitis (UC). Indigo
naturalis contains >63 identified biologically active compounds,
including indole alkaloids, terpenoids, organic acids, steroids and
nucleosides. Indirubin, an indole alkaloid, is recognized for its
therapeutic potential, particularly in treating leukemia and as an
anti-inflammatory agent. The chemical diversity of indigo naturalis
contributes to its broad range of pharmacological activities,
including its anti-inflammatory, antimicrobial and antitumor
properties (10,13). Despite these therapeutic
applications, there are concerns regarding the potential adverse
effects associated with its long-term use, which highlights the
need to investigate its safety profile (10,13).
Various formulas of Traditional Chinese medicine contain indigo
naturalis, such as indigo naturalis pills, powder and
Sheng-Xue-Xiao-Ban capsules. Indigo naturalis in compound formula
products, such as Sheng-Xue-Xiao-Ban capsules, is commonly used to
treat immune thrombocytopenic purpura, psoriasis and UC (7,14-16).
Additonally, Sheng-Xue-Xiao-Ban capsules are commonly used to treat
hypersplenism-induced thrombocytopenia and to promote normal bone
marrow cell proliferation in Jiaxing (Zhejiang, China), a region
where schistosomiasis was prevalent with a number of middle-aged
(45-60 years) and elderly patients (>60 years) still suffering
from schistosomiasis hepatic fibrosis (17). As a result, indigo naturalis is
widely prescribed to patients in this region.
Ischemic injury to the colorectal mucosa associated
with indigo naturalis has a manifestation that is similar to
transient IC, characterized by segmental distribution, mucosal
hemorrhage, erosion and ulcer formation (7-9).
From 2004 to June 2012, the China National Center for Adverse Drug
Reaction Monitoring reported 344 cases associated with compound
formulas such as indigo naturalis pills, capsules and tablets.
These patients primarily had digestive system complaints, and
presented with symptoms such as hepatitis, abnormal liver function,
diarrhea, abdominal pain and dizziness. Among the reported cases,
23 were classified as severe due to drug-induced liver injury and
gastrointestinal bleeding (1).
There has been a notable increase in IC morbidity to
date (4,5). Previous studies have identified
patients with atypical ischemic colorectal mucosal injury with a
history of taking indigo naturalis orally (8,9).
Furthermore, the clinical symptoms of these patients were
alleviated when the medication was discontinued (8,9).
Therefore, patients that exhibit ‘ischemic-like changes in the
colorectal mucosa’ during colonoscopy should be investigated
further, especially patients that have historically taken indigo
naturalis medication. Therefore, the present study aimed to
elucidate the clinical characteristics of patients that experienced
ischemic injury to the colorectal mucosa when it was associated
with the consumption of Chinese patent medicines containing indigo
naturalis.
Patients and methods
Study design and population
The present case series study included patients that
developed ischemic colonic mucosal injury after consuming Chinese
patent medicines containing indigo naturalis between March 2013 and
February 2016 at the Second Affiliated Hospital of Jiaxing
University (Jiaxing, China). Patients were included if the
following inclusion criteria were met: i) Gastrointestinal symptoms
occured within 6 months of consuming Sheng-Xue-Xiao-Ban capsules
containing indigo naturalis (oral consumption, 1.8 g per dose,
three times a day); ii) ischemic injury of the colorectal mucosa
was diagnosed according to clinical symptoms and colonoscopic
findings based on the American College of Gastroenterology (ACG)
clinical guidelines (18); and
iii) mucosal recovery to normalcy was confirmed by another
colonoscopy after drug discontinuation. Patients were excluded
based on the following exclusion criteria: i) A previous medical
history of IC; ii) the presence of other conditions, such as portal
hypertensive colopathy, venous sclerosing enteropathy, hernia,
intussusception, volvulus, intestinal obstruction, intestinal
tumors or other diseases known to cause ischemic colonic mucosal
injury; iii) conditions that could not be clinically distinguished
from inflammatory bowel disease, infectious colitis or
pseudomembranous enteritis; iv) the use of other medications known
to cause IC within 6 months, including psychotropic drugs (such as
quetiapine and clozapine), digoxin, oral contraceptives,
immune-modulators (such as lenalidomide, corticosteroids and TNF-α
inhibitors), laxatives and non-steroidal anti-inflammatory drugs;
and v) patients with incomplete clinical data.
Diagnostic criteria for ischemic injury of the
colorectal mucosa were based on ACG clinical guidelines and
included: i) Clinical symptoms, such as abdominal pain, hemafecia
or diarrhea; ii) colonoscopy findings that indicated intestinal
wall ischemia or intraoperative colonic ischemia and necrosis; and
iii) abdominal computed tomography (CT) findings of intestinal wall
thickening, intestinal lumen stenosis, intestinal dilatation or
pneumoperitoneum, with or without additional CT angiography or
abdominal vascular ultrasound suggesting abdominal vascular
stenosis or occlusion. The study protocol was approved by the
Ethics Committee of The Second Affiliated Hospital of Jiaxing
University (Jiaxing, China; approval no. 2023-ZFYJ-048-01). As the
present study was retrospective, informed consent was waived.
Data collection
Clinical data, including sex, age, date of initial
medication, duration of uninterrupted medication, time between
initial medication and onset of gastrointestinal symptoms,
underlying medical conditions, past surgical history, presenting
symptoms, abdominal CT scan results, colonoscopy findings,
histological examination of intestinal mucosa, treatment
modalities, prognosis, and follow-up outcomes, were collected
retrospectively for the period from March 2013 to February 2016
through the medical record system and endoscopy system of The
Second Affiliated Hospital of Jiaxing University.
Staining method
The staining method utilized for histopathological
examination was hematoxylin and eosin staining. Tissue specimens
were subjected to fixation in a 10% neutral buffered formalin
solution for a period ranging from 12 to 24 h, and maintained at
ambient temperature. Following fixation, the tissues were processed
for paraffin embedding and subsequently sectioned at a precise
thickness of 4 µm. The staining protocol entailed the following
steps: Initially, the sections were stained with hematoxylin
solution for a duration of 5 to 10 min at room temperature;
subsequently, they were stained with eosin solution for 2 to 3 min,
under the same temperature conditions. The stained sections were
then meticulously examined and analyzed utilizing a conventional
optical microscope.
Statistical analysis
The distribution of continuous variables was
assessed for normality using the Shapiro-Wilk test, a widely
accepted method for small to moderately-sized samples. Variables
that passed the normality test (P>0.05) were considered normally
distributed and reported as mean ± standard deviation (SD).
Categorical data are presented as frequencies and percentages.
Statistical tests were conducted using basic descriptive statistics
suitable for the type and distribution of the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
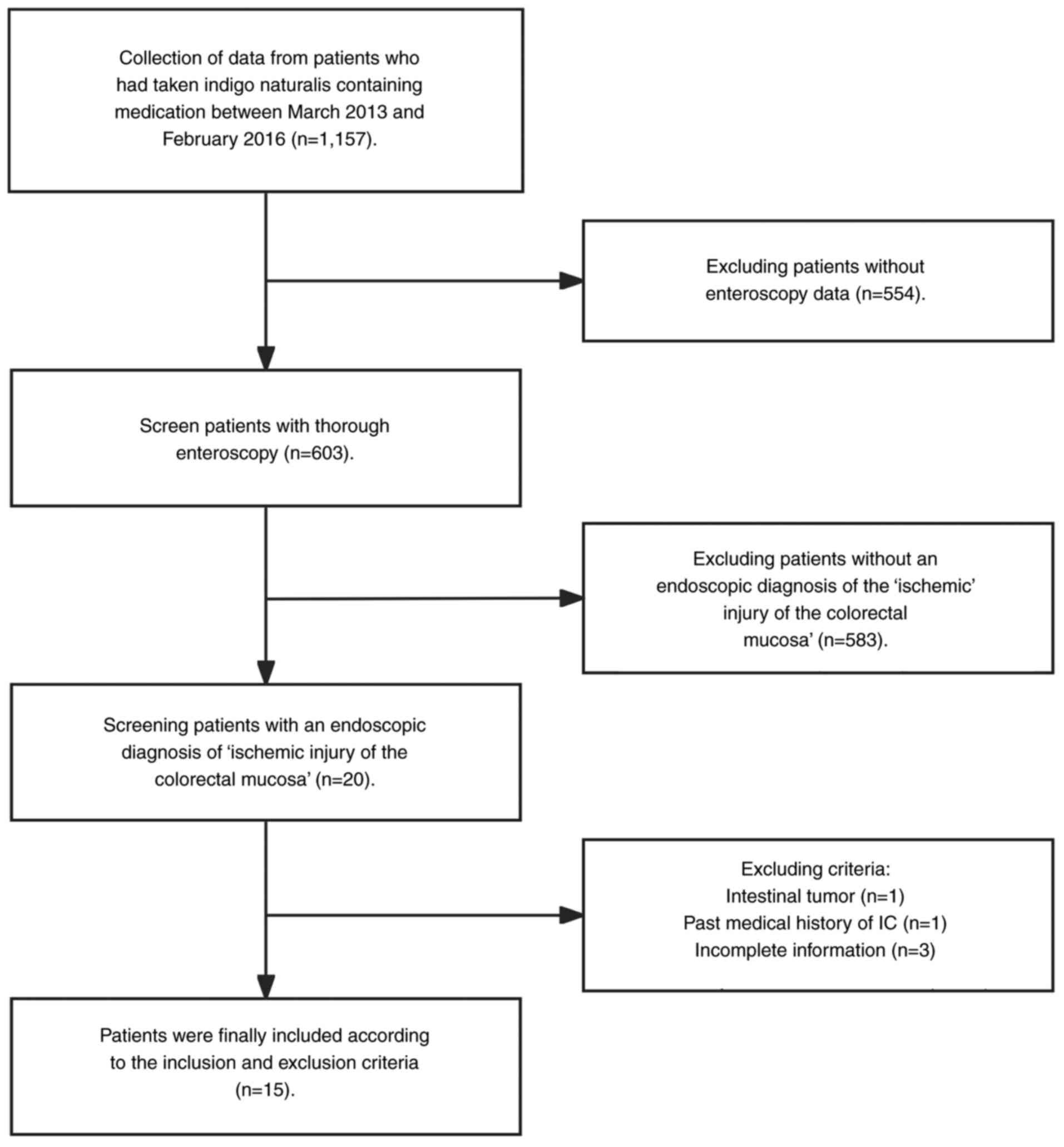
A total of 1,157 patients had consumed indigo
naturalis. However, 554 patients that lacked colonoscopy data, 583
patients without evidence of ischemic colorectal mucosal injury, 1
patient with intestinal tumors, 1 patient with a prior history of
IC and 3 patients without complete data, were excluded. Therefore,
15 patients (mean age, 61.7±15.1 years; 5 males) with ischemic
injury of the colorectal mucosa attributed to indigo naturalis
consumption (Sheng-Xue-Xiao-Ban capsules containing indigo
naturalis; 1.8 g per dose, three times a day) were included in the
present study (Fig. 1).
Therefore, the overall incidence was 1.3%. Of the 15
patients included, 11 patients were admitted to an inpatient
setting and 4 patients attended outpatient care. Abdominal pain or
discomfort was reported by 14 patients (93.3%), diarrhea was
reported by 9 patients (60.0%) and hemafecia was reported by 8
patients (53.3%). Additionally, 9 patients had idiopathic
thrombocytopenic purpura and 6 patients indicated schistosomal
liver fibrosis associated with hypersplenism and normal
myeloproliferative thrombocytopenia. In addition, 5 patients had a
history of hypertension and 4 patients had type 2 diabetes
mellitus. The patients continuously consumed Sheng-Xue-Xiao-Ban
capsules for 12-330 days, with a mean of 98.0±52.3 days. Symptom
onset occurred between 6 and 90 days from initial drug therapy,
with a mean of 28.9±22.2 days.
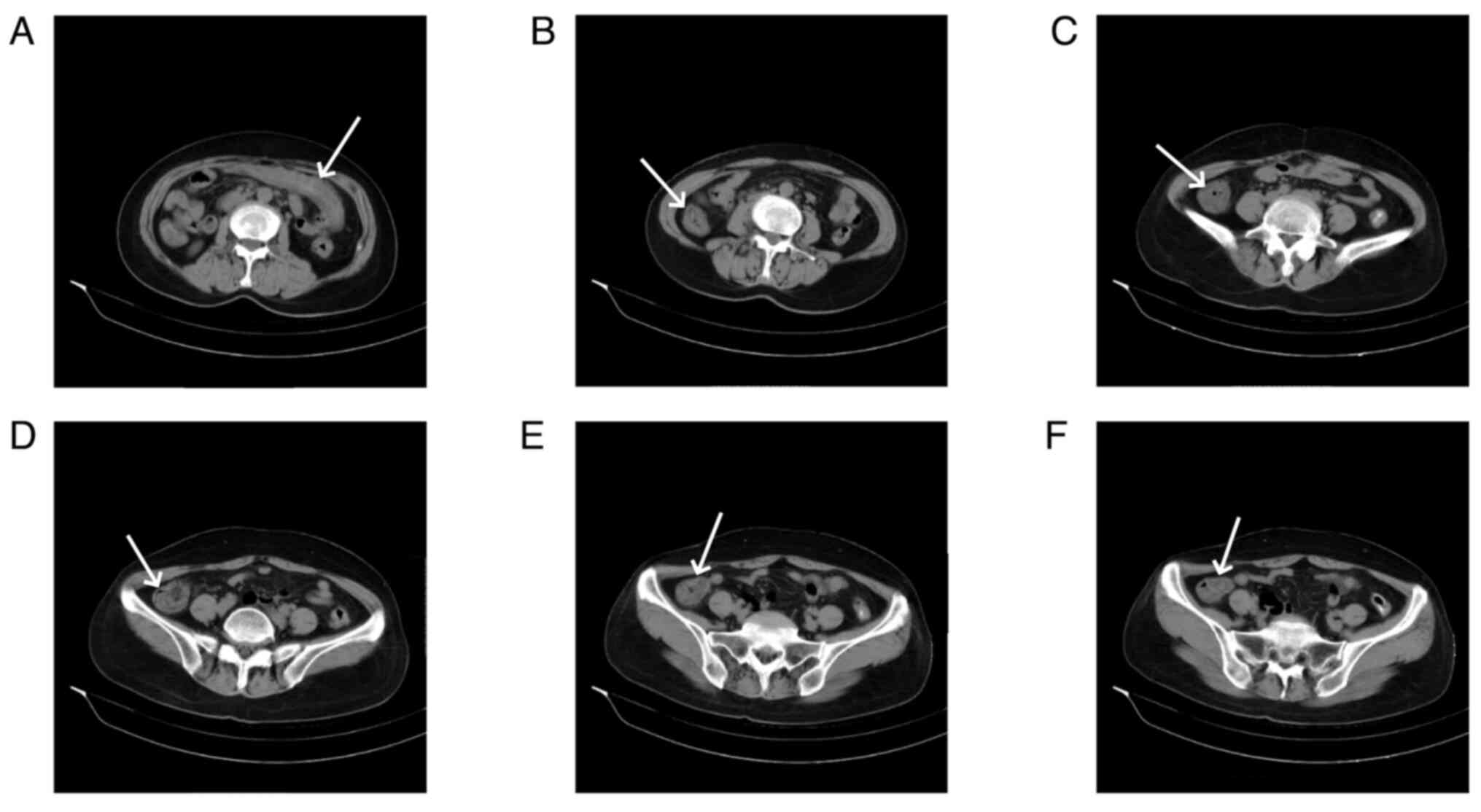
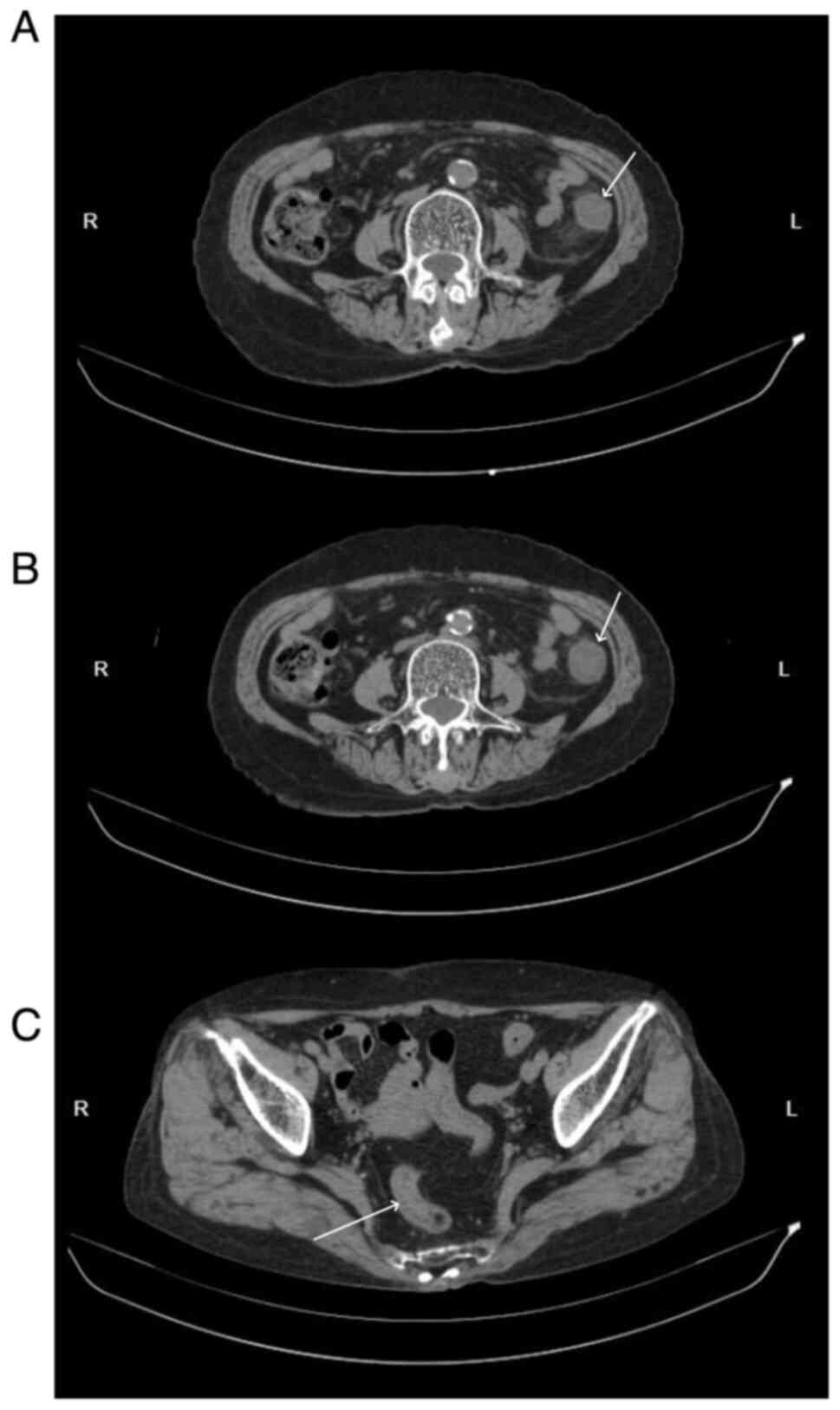
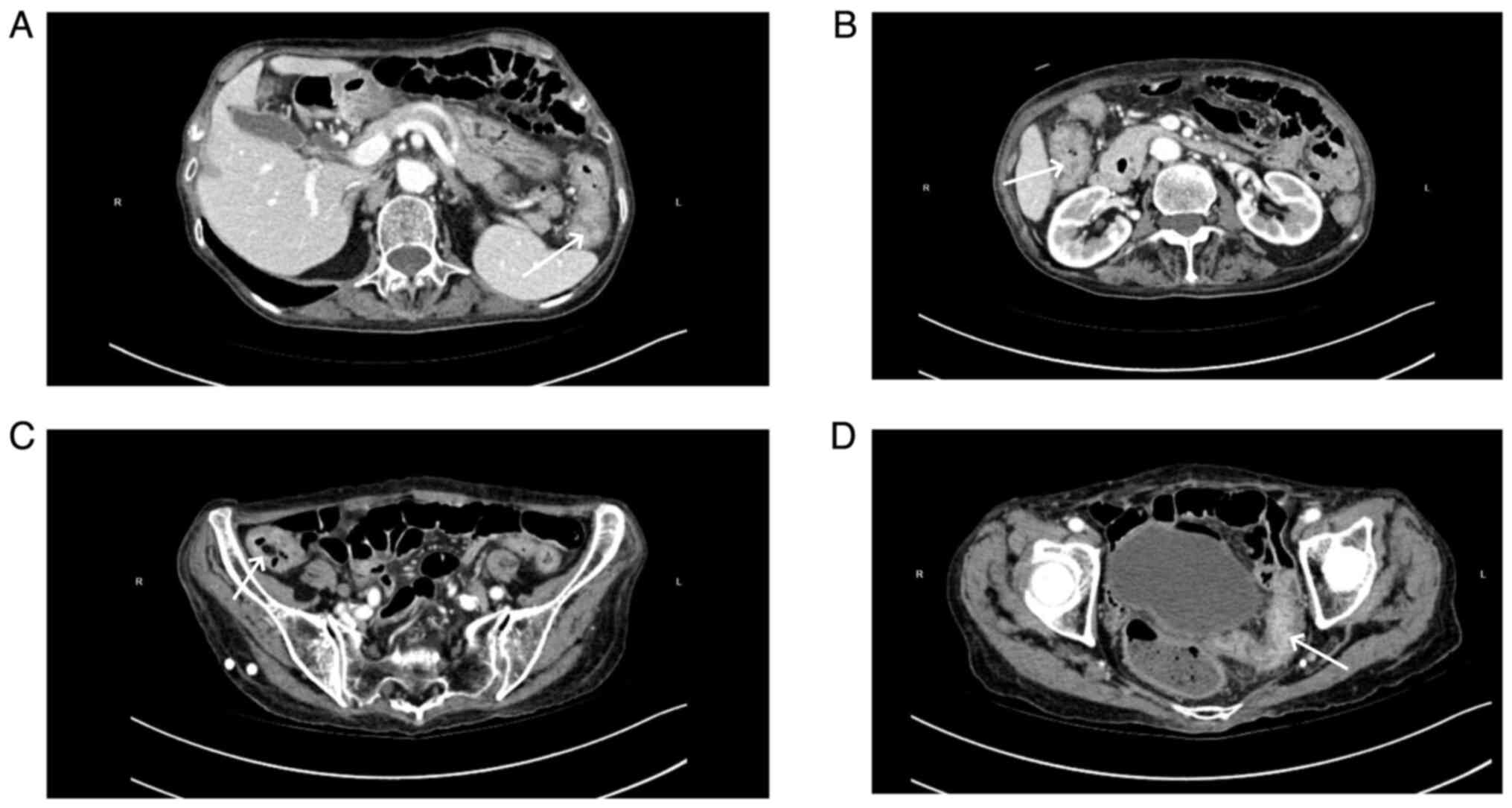
Abdominal CT scans, both plain and enhanced, were
available for 11 patients, and had all been carried out within 1
week of the onset of abdominal symptoms. The right hemi-colon
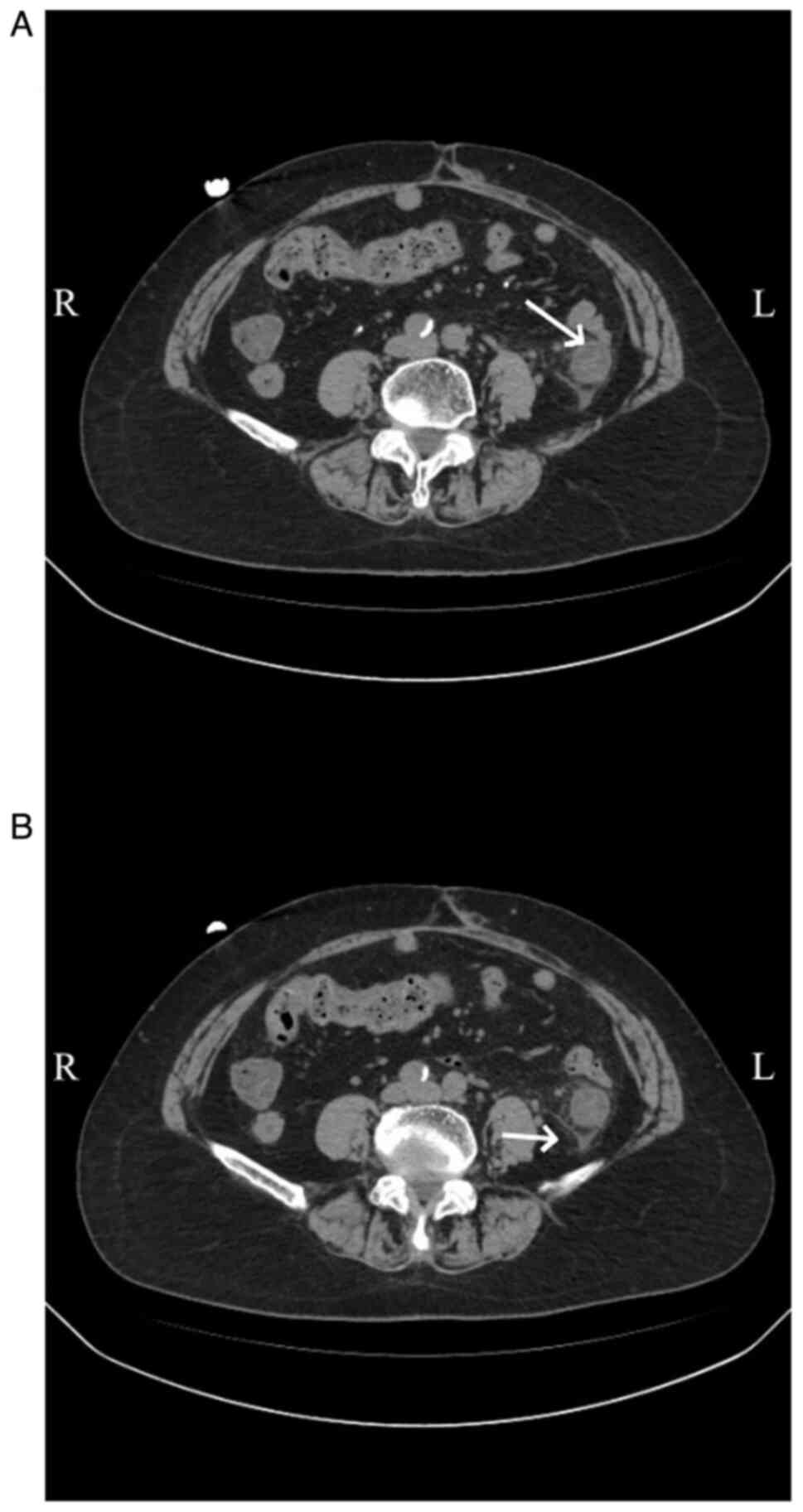
(Fig. 2) was affected in 4
patients (36.36%), the left hemi-colon (Fig. 3) in 2 patients (18.18%) and the
total colon (Fig. 4) in 4 patients
(36.36%). Additionally, 1 patient had periintestinal exudation
(Fig. 5), while 4 patients had
abdominopelvic effusion. The abdominal CT scan of 1 patient showed
no abnormalities. Abdominal CT plain scans involving the transverse
and ascending colon of a patient are presented in Fig. 2. CT images of a patient with the
descending colon and sigmoid colon involved are presented in
Fig. 3. Enhanced CT images of a
patient with the total colon involved are presented in Fig. 4. CT images of a patient with
exudation around the descending colon accompanied by an
abdominopelvic effusion are presented in Fig. 5.
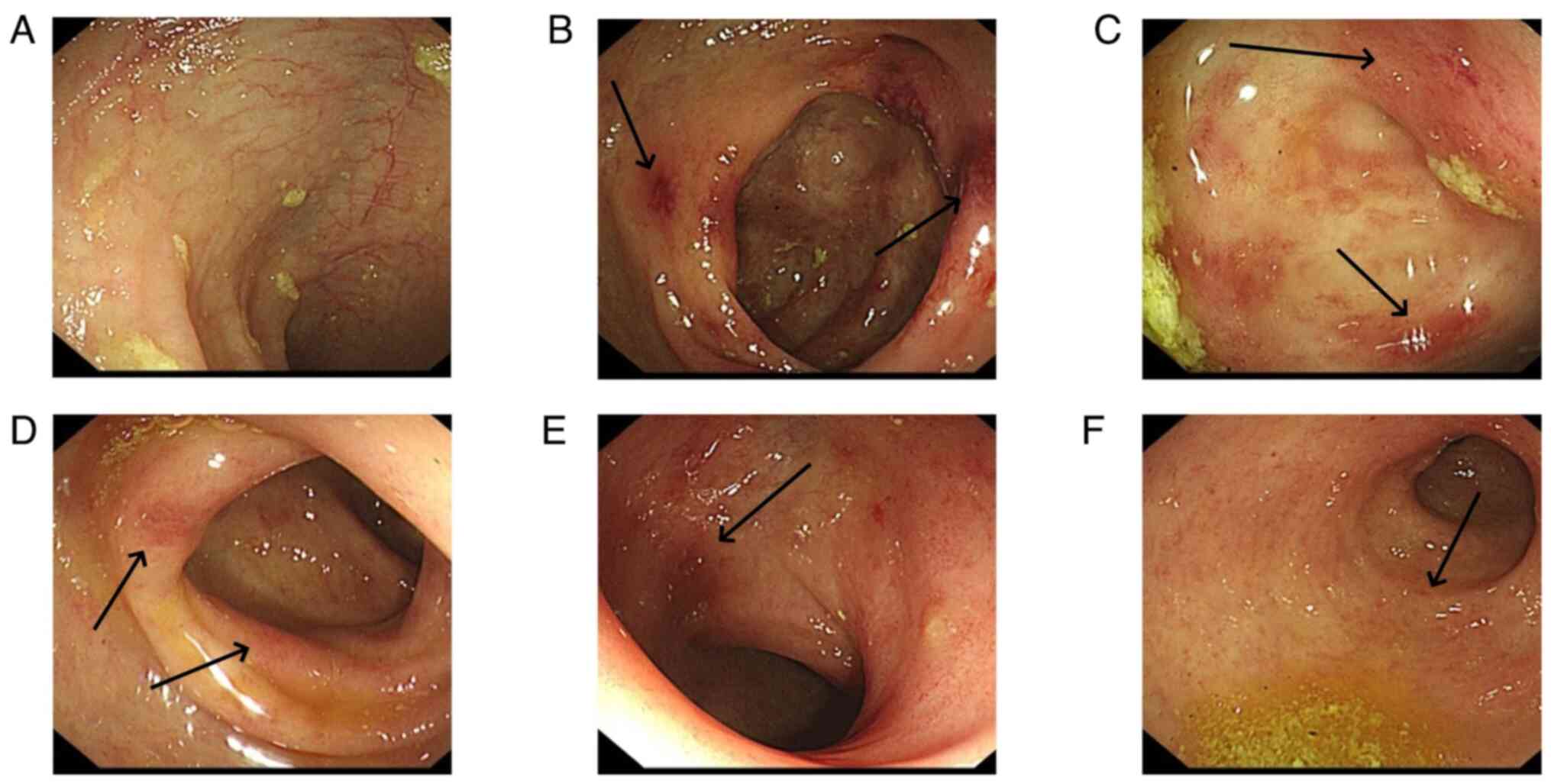
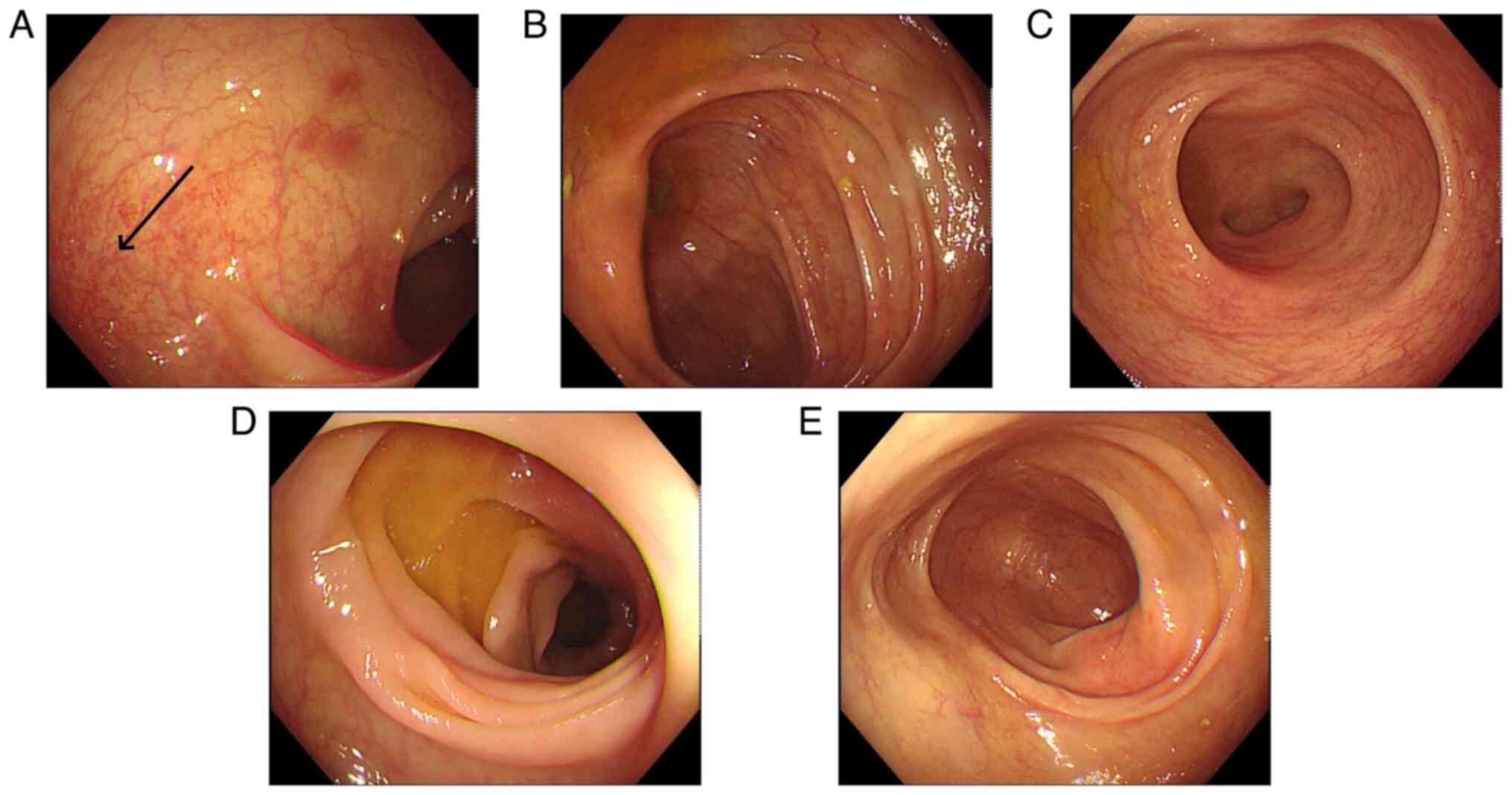
Colonoscopy revealed that the total colon was
affected in 9 patients (60.0%), the left hemi-colon in 5 patients
(33.3%) and the right hemi-colon in 1 patient (6.7%). Rectal
involvement was observed in 12 patients (80.0%). In 6 patients, the
end of the ileum was reached during the colonoscopy, but it
revealed that the small bowel was not involved. The typical
presentation of the total colon and rectal involvement is presented
in Fig. 6. Fig. 7 demonstrates 1 case that is
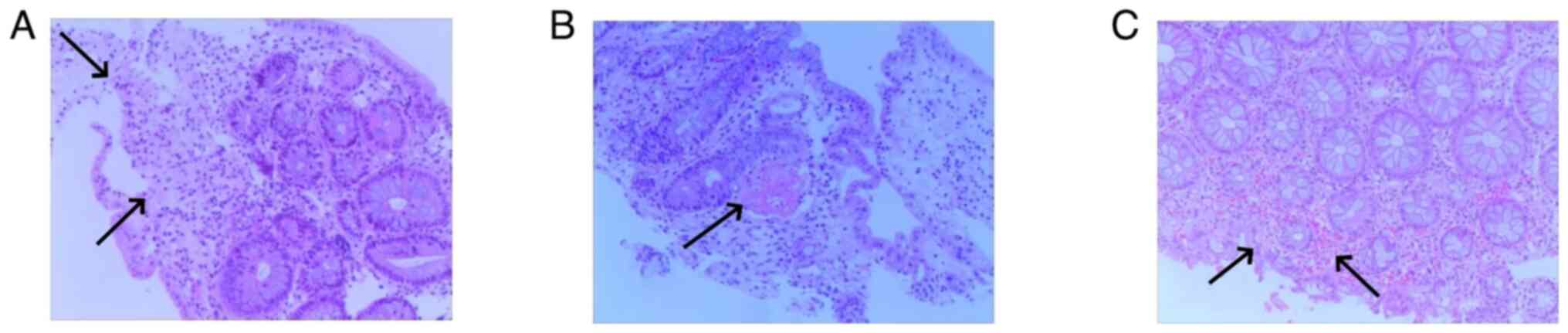
primarily characterized by ulceration. Pathological examination was
carried out for 7 patients and revealed mucosal superficial
epithelial detachment and necrosis, fibrinoid exudation and
inflammatory cell infiltration (Fig.
8). The clinical and pathological data for all 15 patients is
presented in Table I.
 | Table IClinical and pathological data of the
patients. |
Table I
Clinical and pathological data of the
patients.
| Patient no. | Age, years | Sex | Section of colon
affected | Pathological features
revealed |
|---|
| 1 | 27 | Female | Sigmoid colon and
descending colon | The superficial
epithelium of the mucosa had shed and was necrotic, with an
aggregation of red blood cells |
| 2 | 60 | Female | Rectum and total
colon | Pathology not
assessed |
| 3 | 67 | Male | Rectum, sigmoid
colon and descending colon | Pathology not
assessed |
| 4 | 76 | Female | Rectum and total
colon | Pathology not
assessed |
| 5 | 52 | Male | Rectum and total
colon | The superficial
epithelium of the mucosa had shed, with an infiltration of red
blood cells |
| 6 | 78 | Female | Ascending colon and
hepatic flexure | Pathology not
assessed |
| 7 | 65 | Female | Rectum and total
colon | Intestinal mucosal
bleeding, epithelial shedding, mucus secretion and neutrophil
exudation |
| 8 | 75 | Female | Total colon | Pathology not
assessed |
| 9 | 58 | Female | Rectum and total
colon | Thrombus formation
within the submucosal blood vessels |
| 10 | 55 | Male | Rectum, sigmoid
colon and descending colon | Chronic mucosal
inflammation with vascular congestion and bleeding, and submucosal
edema |
| 11 | 64 | Female | Rectum and sigmoid
colon | The superficial
epithelium of the mucosa had shed and was necrotic, with an
infiltration of inflammatory cells |
| 12 | 81 | Female | Rectum and total
colon | Pathology not
assessed |
| 13 | 35 | Male | Rectum, sigmoid
colon and descending colon | Pathology not
assessed |
| 14 | 73 | Female | Rectum and total
colon | The superficial
epithelium of the mucosa had shed and was necrotic, with an
infiltration of inflammatory cells and glandular atrophy |
| 15 | 59 | Male | Rectum and total
colon | Pathology not
assessed |
In 4 cases, gastroenterological symptoms and
intestinal lesions resolved ~1 week after discontinuing
Sheng-Xue-Xiao-Ban capsules without additional treatment. Due to
symptoms such as abdominal pain, diarrhea and hemafecia, 6 patients
were hospitalized in the Department of Gastroenterology of the
Second Affiliated Hospital of Jiaxing University, but were
discharged after their symptoms abated. A total of 3 patients were
hospitalized in the Department of Hematology of the Second
Affiliated Hospital of Jiaxing University due to thrombocytopenia.
During their hospital stay, they developed gastrointestinal
symptoms and were subsequently transferred to the Department of
Gastroenterology, where they discontinued the Sheng-Xue-Xiao-Ban
capsules, resulting in symptom improvement. Due to abdominal pain,
diarrhea and mucus stools, 1 patient was readmitted to the
Department of Gastroenterology of the Second Affiliated Hospital of
Jiaxing University after discontinuation of Sheng-Xue-Xiao-Ban
capsules; however, complete symptom resolution occurred 40 days
after drug withdrawal. Another patient had been diagnosed with UC
in the outpatient clinic of the Second Affiliated Hospital of
Jiaxing University and had orally consumed indigo naturalis and
mesalazine (0.5 g per dose, three times a day) for 1 year before
discontinuing the drugs. Subsequently, symptoms disappeared 3
months after discontinuing the drug. The mean time from drug
withdrawal to the resolution of symptoms was 17.3±12.4 days. The
therapeutic measures for all inpatients were mainly symptomatic
treatments such as fasting and fluid infusion. Following the
resolution of symptoms, all patients underwent colonoscopy, which
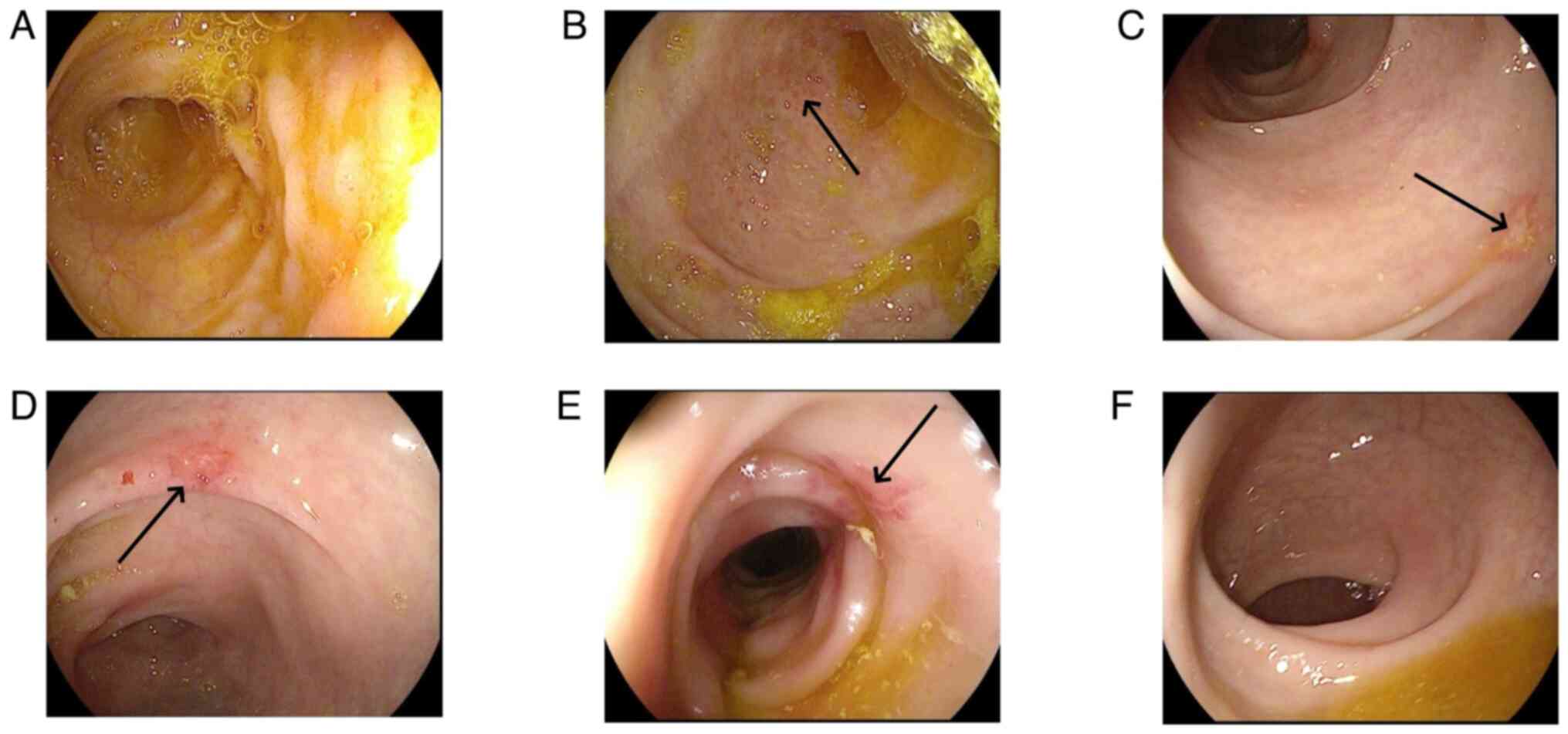
revealed recovery of the intestinal mucosa. Fig. 9 presents the recovery phase images
of the same patient as presented in Fig. 6. These images indicated hyperemia
and edema in the descending colon, while the mucosa of the
remaining colonic regions had no notable abnormalities. To date,
none of the patients have had surgical intervention for intestinal
necrosis or perforation.
Discussion
The present study reported ischemic injury to the
intestinal mucosa, which was associated with the consumption of
indigo naturalis. Abdominal CT scans and colonoscopy revealed that
lesions were predominantly located in the right hemi-colon and the
total colon, with rectal involvement in a number of cases. After
discontinuing medication, gastrointestinal symptoms resolved, and
patients generally exhibited a favorable prognosis with no
recurrence. Therefore, it is suggested that clinicians assess for
ischemic injury to the intestinal mucosa in patients consuming
indigo naturalis.
A total of 53.3% of the patients that were enrolled
in the present study were elderly individuals (>60 years) who
presented with comorbidities such as hypertension and diabetes
mellitus. Female patients had an increased prevalence, consistent
with previously reported findings (7). This suggested that older age,
underlying comorbidities and being female may increase an
individuals susceptibility to indigo naturalis-associated ischemic
lesions in the colorectal mucosa. Clinical examination of the
patients in the present study did not reveal notable physical
signs, and were predominantly manifested as pressure discomfort in
the left lower abdominal region or the lower abdomen, occasionally
accompanied by mild muscular tension. Vital signs revealed minimal
alterations. The interval between drug initiation and symptom onset
ranged from 6-90 days, which was shorter compared with that
reported in a previous study (7).
This discrepancy may be attributed to inter-individual variability
in drug sensitivity and may be associated with the small sample
size used in the present study. In the present study, symptoms of
diarrhea manifested earlier, with an increased intensity and
duration compared with previous research (7). This observation was likely associated
with the prokinetic effect of indigo naturalis on intestinal
peristalsis (19).
Abdominal CT, coupled with oral and intravenous
contrast agents (such as iohexol and iopromide), can serve as a
promising imaging modality for assessing IC (20). The patients in the present study
had a CT presentation similar to patients with IC, with the main
manifestation being bowel wall edema and thickening (21); however, the lesions in the present
patients were more extensive and frequently involved the right side
of the colon.
Previous studies have demonstrated the therapeutic
efficacy of indigo naturalis in experimental rat models of UC
(22,23). A previous study summarized the
therapeutic benefits of indigo naturalis in patients with UC,
suggesting its potential to promote mucosal healing (24). Numerous medical institutions have
incorporated indigo naturalis into their treatment protocols of UC
(14,25); however, there are associated
adverse events (25,26). In a national survey conducted in
Japan, 877 of 49,320 patients with UC (1.8%) received indigo
naturalis treatment. Of these 877 patients, 91 pateints experienced
adverse events (liver dysfunction, gastrointestinal symptoms,
headache, pulmonary arterial hypertension and intussusception),
including 21 patients who reported gastrointestinal symptoms
(nausea and epigastralgia). Furthermore, 8 patients developed
colitis that was unrelated to UC (26). Cho et al (8) reported the case of a middle-aged
female patient with a 2-year history of UC, who developed IC after
discontinuing mesalamine by themselves and taking indigo naturalis
(daily dose, 2 g) for 3 months. However, the patient did not have
any pre-existing risk factors for IC, such as hypertension,
diabetes mellitus, cardiovascular disease or a history of abdominal
surgery (8). Additionally, a study
by Kondo et al (9) also
reported 2 patients with UC who developed intestinal wall
thickening and edema while receiving indigo naturalis treatment.
Symptoms reported by both patients included abdominal pain and
bloody diarrhea (9). Furthermore,
evidence suggests that indigo naturalis can influence the immune
system (27). The study by Gu
et al (28) demonstrated
that the therapeutic efficacy of indigo naturalis in UC likely
involves the activation of systemic immunity. This immune
activation modulates multiple biological processes, including
nuclear transcription, protein phosphorylation, cytokine activity,
reactive oxygen species metabolism, epithelial cell proliferation
and apoptosis. These effects are mediated through diverse
mechanisms, such as Th17 cell differentiation, the Jak-STAT and
IL-17 signaling pathways, and Toll-like and NOD-like receptors, as
well as other cellular and innate immune signaling pathways.
Nevertheless, further in-depth experimental studies are required to
fully elucidate the underlying mechanisms. A number of studies also
hypothesize that indigo naturalis can exacerbate colitis by
disturbing the normal composition of the intestinal flora (29-33).
Additionally, it has been hypothesized that indigo naturalis can
aggravate IC through several mechanisms: i) Inducing diarrhea in
susceptible individuals, which leads to a decreased blood volume
and an increased intraluminal pressure and vascular spasms. This
compromises the blood supply to the intestinal wall and results in
degenerative and necrotic changes to the mucosal tissue with
inflammatory cell infiltration. ii) The irritant affects the
colonic mucosa, which causes damage to the intestinal mucosal
lining. iii) Indigo naturalis has multi-target biological
regulatory process effects, involving mechanisms such as
anti-inflammatory action, immune modulation, antioxidant activity
and stopping bleeding, and it exhibits a significant procoagulant
effect. This procoagulant activity can lead to the formation of
fibrin thrombi within blood vessels, resulting in vascular
occlusion and subsequent ischemic necrosis of the colonic mucosa
(19). Based on the findings of
the present study, we hypothesize that indigo naturalis potentially
has a key role in the development of ischemic injury and ulcer
formation within the colorectal mucosa, although the precise
underlying mechanism remains unclear.
Ischemic injury to the colorectal mucosa from indigo
naturalis consumption closely resembles transient IC, which is
characterized by segmental mucosal hemorrhage, erosion and
ulceration (34). Manifestations
include shallow ulcers similar to those in UC (35). However, UC typically presents as a
continuous lesion, distinct from ischemic injury, which is
frequently extensive and predominantly affects the entire colon,
especially the right side and rectum (36). The diagnostic challenges arise due
to the lack of awareness among endoscopists of the potential
colorectal mucosal damage caused by indigo naturalis, which is
attributable to the lack of access to the medication histories of
patients. Consequently, the diagnosis frequently defaults to IC or
UC. Endoscopists should proactively inquire about the medication
histories of the patient when encountering atypical ischemic
alterations in the colorectal mucosa. Pathological examination can
reveal abnormalities such as superficial epithelial detachment,
mucosal necrosis, fibrinoid exudation, hemosiderin granule
deposition and submucosal vascular thrombosis, which aid in
diagnosing ischemic lesions but may not indicate the specific
etiology (7,19,37).
Due to the retrospective nature of the present study and the
unavailability of multiple colonoscopic reviews for all cases,
precise data regarding the timing of colonic mucosal repair were
not obtained.
The present study has several limitations. Firstly,
it was conducted at a single center. Future research would benefit
from a multicenter approach for a more comprehensive perspective.
Secondly, all patients in the present study received the same dose
of indigo naturalis (Sheng-Xue-Xiao-Ban capsules containing indigo
naturalis, 1.8 g per dose, three times a day). Analyzing patients
treated with varying doses could provide more robust data. Thirdly,
expanding the cohort population would yield further valuable
insights into the safety of the clinical application of indigo
naturalis.
In conclusion, indigo naturalis may be associated
with a notable incidence of ischemic injury to the colorectal
mucosa. Any suspected mucosal ischemic injury during a colonoscopy
should serve as a warning sign to proactively inquire about the
medication history of the patient in order to make a precise
diagnosis. Additionally, such injuries may resolve following drug
discontinuation and may have a favorable prognosis.
Acknowledgements
Not applicable.
Funding
Funding: This project was supported by Jiaxing Science and
Technology Plan Project (grant no. 2021AD30101).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YK and LX were responsible for the conception of the
manuscript, collected the data, and drafted the manuscript. QT and
SR performed the statistical analysis and participated in its
design. ZR and JL participated in the acquisition, analysis or
interpretation of data and drafted the manuscript. All authors read
and approved the final version of the manuscript. YK and LX confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
This work has been carried out in accordance with
the Declaration of Helsinki (2000) of the World Medical
Association. This study has been approved by the Medical Ethics
Committee of the Second Affiliated Hospital of Jiaxing University
(Jiaxing, China; approval no. 2023-ZFYJ-048-01). Given the
retrospective nature of the study design, the requirement for
informed consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dahal RH, Kim S, Kim YK, Kim ES and Kim J:
Insight into gut dysbiosis of patients with inflammatory bowel
disease and ischemic colitis. Front Microbiol.
14(1174832)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fairen Oro C and Beresneva O: Diagnosis
and management of ischemic colitis. Dis Colon Rectum. 66:872–875.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Trotter JM, Hunt L and Peter MB: Ischaemic
colitis. BMJ. 355(i6600)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yadav S, Dave M, Edakkanambeth Varayil J,
Harmsen WS, Tremaine WJ, Zinsmeister AR, Sweetser SR, Melton LJ
III, Sandborn WJ and Loftus EV Jr: A population-based study of
incidence, risk factors, clinical spectrum, and outcomes of
ischemic colitis. Clin Gastroenterol Hepatol. 13:731–738.e1-e6,
e41. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ichita C, Goto T, Sasaki A and Shimizu S:
National trends in hospitalizations for gastrointestinal bleeding
in Japan. J Clin Biochem Nutr. 75:60–64. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vodusek Z, Feuerstadt P and Brandt LJ:
Review article: The pharmacological causes of colon ischaemia.
Aliment Pharmacol Ther. 49:51–63. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Suo BJ, Zhou LY, Ding SG, Lü YM, Gu F, Lin
SR and Zheng YA: The endoscopic and clinical features of indigo
naturalis-associated ischemic lesions of colonic mucosa. Zhonghua
Nei Ke Za Zhi. 50:646–649. 2011.PubMed/NCBI(In Chinese).
|
|
8
|
Cho B, Yoon SM, Son SM, Kim HW, Kim KB and
Youn SJ: Ischemic colitis induced by indigo naturalis in a patient
with ulcerative colitis: A case report. BMC Gastroenterol.
20(154)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kondo S, Araki T, Okita Y, Yamamoto A,
Hamada Y, Katsurahara M, Horiki N, Nakamura M, Shimoyama T,
Yamamoto T, et al: Colitis with wall thickening and edematous
changes during oral administration of the powdered form of Qing-dai
in patients with ulcerative colitis: A report of two cases. Clin J
Gastroenterol. 11:268–272. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun Q, Leng J, Tang L, Wang L and Fu C: A
comprehensive review of the chemistry, pharmacokinetics,
pharmacology, clinical applications, adverse events, and quality
control of indigo naturalis. Front Pharmacol.
12(664022)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hendrick V, Altshuler L and Whybrow P:
Psychoneuroendocrinology of mood disorders. The
hypothalamic-pituitary-thyroid axis. Psychiatr Clin North Am.
21:277–292. 1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang L, Li X, Huang W, Rao X and Lai Y:
Pharmacological properties of indirubin and its derivatives. Biomed
Pharmacother. 151(113112)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu Y, Lin C, Tan HY and Bian ZX: The
double-edged sword effect of indigo naturalis. Food Chem Toxicol.
185(114476)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Matsuno Y, Torisu T, Umeno J, Shibata H,
Hirano A, Fuyuno Y, Okamoto Y, Fujioka S, Kawasaki K, Moriyama T,
et al: One-year clinical efficacy and safety of indigo naturalis
for active ulcerative colitis: A real-world prospective study.
Intest Res. 20:260–268. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Uchiyama K, Takami S, Suzuki H, Umeki K,
Mochizuki S, Kakinoki N, Iwamoto J, Hoshino Y, Omori J, Fujimori S,
et al: Efficacy and safety of short-term therapy with indigo
naturalis for ulcerative colitis: An investigator-initiated
multicenter double-blind clinical trial. PLoS One.
15(e0241337)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Saiki JP, Andreasson JO, Grimes KV,
Frumkin LR, Sanjines E, Davidson MG, Park KT and Limketkai B:
Treatment-refractory ulcerative colitis responsive to indigo
naturalis. BMJ Open Gastroenterol. 8(e000813)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ruan SL, Lu QM, Yang ZH, Yu YW and Tang
LL: Clinical investigation on schistosomiasis after a
16-year-interruption program regarding its transmission, in Jiaxing
region of Zhejiang province. Zhonghua Liu Xing Bing Xue Za Zhi.
34:523–525. 2013.PubMed/NCBI(In Chinese).
|
|
18
|
Brandt LJ, Feuerstadt P, Longstreth GF and
Boley SJ: American College of Gastroenterology. ACG clinical
guideline: Epidemiology, risk factors, patterns of presentation,
diagnosis, and management of colon ischemia (CI). Am J
Gastroenterol. 110:18–45. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhou S, Shi Q, Zheng Y, Zhuang Y, Lin Y,
Huang Z and Yu J: Sheng-Xue-Xiao-Ban Capsule-induced ischemic
colitis and pulmonary embolism in an idiopathic thrombocytopenic
purpura patient: A rare case report. Ann Transl Med.
10(1027)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ahmed M: Ischemic bowel disease in 2021.
World J Gastroenterol. 27:4746–4762. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cotter TG, Bledsoe AC and Sweetser S:
Colon ischemia: An update for clinicians. Mayo Clin Proc.
91:671–677. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen MS and Du LY: Effect of Compound
Qingdai Granula on the expression of TGF-β1 and VEGF in the colon
tissue of rats with ulcerative colitis. Chin J Integr Tradit West
Med Dig. 21:393–396. 2013.
|
|
23
|
Wang Y, Liu L, Guo Y, Mao T, Shi R and Li
J: Effects of indigo naturalis on colonic mucosal injuries and
inflammation in rats with dextran sodium sulphate-induced
ulcerative colitis. Exp Ther Med. 14:1327–1336. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sugimoto S, Naganuma M and Kanai T: Indole
compounds may be promising medicines for ulcerative colitis. J
Gastroenterol. 51:853–861. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kudo T, Jimbo K, Shimizu H, Iwama I,
Ishige T, Mizuochi T, Arai K, Kumagai H, Uchida K, Abukawa D and
Shimizu T: Qing-dai for pediatric ulcerative colitis multicenter
survey and systematic review. Pediatr Int.
64(e15113)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Naganuma M, Sugimoto S, Suzuki H, Matsuno
Y, Araki T, Shimizu H, Hayashi R, Fukuda T, Nakamoto N, Iijima H,
et al: Adverse events in patients with ulcerative colitis treated
with indigo naturalis: A Japanese nationwide survey. J
Gastroenterol. 54:891–896. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xiao HT, Peng J, Hu DD, Lin CY, Du B,
Tsang SW, Lin ZS, Zhang XJ, Lueng FP, Han QB and Bian ZX: Qing-dai
powder promotes recovery of colitis by inhibiting inflammatory
responses of colonic macrophages in dextran sulfate sodium-treated
mice. Chin Med. 10(29)2015.
|
|
28
|
Gu S, Xue Y, Gao Y, Shen S, Zhang Y, Chen
K, Xue S, Pan J, Tang Y, Zhu H, et al: Mechanisms of indigo
naturalis on treating ulcerative colitis explored by GEO gene chips
combined with network pharmacology and molecular docking. Sci Rep.
10(15204)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Adachi S, Hoshi N, Inoue J, Yasutomi E,
Otsuka T, Dhakhwa R, Wang Z, Koo Y, Takamatsu T, Matsumura Y, et
al: Indigo naturalis ameliorates oxazolone-induced dermatitis but
aggravates colitis by changing the composition of gut microflora.
Int Arch Allergy Immunol. 173:23–33. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang QY, Ma LL, Zhang C, Lin JZ, Han L, He
YN and Xie CG: Exploring the mechanism of indigo naturalis in the
treatment of ulcerative colitis based on TLR4/MyD88/NF-κB signaling
pathway and gut microbiota. Front Pharmacol.
12(674416)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liang YN, Yu JG, Zhang DB, Zhang Z, Ren
LL, Li LH, Wang Z and Tang ZS: Indigo naturalis ameliorates dextran
sulfate sodium-induced colitis in mice by modulating the intestinal
microbiota community. Molecules. 24(4086)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Luo L, Li C, Huang N, Wang Q and Zhang Z,
Song C, Yang H, Yuan M, Xu Z, Sun J and Zhang Z: Traditional
mineral medicine realgar and realgar-indigo naturalis formula
potentially exerted therapeutic effects by altering the gut
microbiota. Front Microbiol. 14(1143173)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sun Z, Li J, Dai Y, Wang W, Shi R, Wang Z,
Ding P, Lu Q, Jiang H, Pei W, et al: Indigo naturalis alleviates
dextran sulfate sodium-induced colitis in rats via altering gut
microbiota. Front Microbiol. 11(731)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Maimone A, De Ceglie A, Siersema PD, Baron
TH and Conio M: Colon ischemia: A comprehensive review. Clin Res
Hepatol Gastroenterol. 45(101592)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee JM and Lee KM: Endoscopic diagnosis
and differentiation of inflammatory bowel disease. Clin Endosc.
49:370–375. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li Z, Liping D, Weihong Y, et al: The
clinical feature and possible pathogenesis of natural indigo
(Qingdai) induced hematochezia. Chin J Gastroenterol Hepatol.
13:161–164. 2004.(In Chinese).
|
|
37
|
Wang CL, Si ZK, Liu GH, Chen C, Zhao H and
Li L: Ischemic colitis induced by a platelet-raising capsule: A
case report. World J Clin Cases. 12:607–615. 2024.PubMed/NCBI View Article : Google Scholar
|