Introduction
Uterine leiomyomas, also known as fibroids, are
benign tumors that arise from the smooth muscle tissue of the
uterus (1,2). They are found in 20-30% of women
during their reproductive years and affect 40-50% of women over the
age of 35, making them a prevalent condition (3). Although the exact cause of uterine
fibroids remains unknown, they are believed to originate from a
single abnormal cell within the smooth muscle of the uterine
(4). Clinical symptoms manifest in
20-50% of affected individuals and vary depending on the number,
size, and location of the fibroids (5). Common symptoms include abnormal
uterine bleeding, pelvic pain or pressure, decreased bladder
capacity, constipation, and reproductive dysfunction (6,7).
Treatment options for uterine fibroids include
medication, surgical interventions, and non-surgical approaches
(8,9). Medication aims to regulate hormone
levels to inhibit fibroid growth and alleviate symptoms (2). Surgical treatments are considered
when the fibroids are large, or the symptoms are severe, and
include myomectomy and hysterectomy (10). Non-surgical treatments, such as
uterine artery embolization and high-intensity focused ultrasound,
are also available (11,12). The choice of treatment depends on
factors such as the health condition, age, menopausal status, and
symptom severity of the patient. It is crucial for patients to have
a detailed consultation with a healthcare provider before deciding
on the appropriate treatment approach.
Recent developments in proteasome-targeting drugs
have drawn attention as potential therapeutic agents for
gynecological diseases, however the literature currently provides
limited data on this topic. Proteasome inhibitors, such as
bortezomib, have been explored in combination with chemotherapy for
various cancers, including breast cancer and other solid tumors.
For instance, bortezomib has been tested with agents such as
paclitaxel, capecitabine, and docetaxel for metastatic breast
cancer, but the results have been disappointing in terms of
efficacy (13-15).
Furthermore, bortezomib did not exhibit significant improvements
when combined with standard treatments for ovarian cancer (16). A Phase I trial also investigated
the combination of bortezomib and chemoradiation for cervical
cancer, indicating that while proteasome inhibitors have been
tested in gynecological cancers, the results have largely been
exploratory and have not demonstrated clear clinical benefits
(17). In summary, although there
has been some investigation of proteasome-targeting drugs for
gynecological malignancies, these trials remain limited and have
not provided compelling evidence of significant efficacy thus
far.
Carbobenzoxyl-L-leucyl-L-leucyl-L-leucine (MG132) is
a potent, reversible, and cell-permeable proteasome inhibitor that
interferes with the activity of the 26S proteasome, thereby
blocking the degradation of ubiquitin-conjugated proteins (18). This inhibition prevents the
breakdown of misfolded, damaged, or regulatory proteins, leading to
their accumulation within the cell and disrupting key processes,
such as cell cycle progression, apoptosis, and signal transduction
pathways (19-21).
Notably, MG132 has been reported to affect several types of
cancers, including breast, prostate, and liver cancers (22-24).
By stabilizing ubiquitinated proteins, MG132 allows researchers to
study their roles in cellular functions. Widely used in cancer
research, MG132 aids in studying apoptosis evasion mechanisms
(20) and uncontrolled
proliferation in cancer cells (25). It is also valuable in studying
neurodegenerative diseases, where protein aggregation plays a key
role (26). Overall, MG132 is
essential for investigating protein degradation dynamics and their
implications in various diseases, providing potential avenues for
therapeutic development.
Despite the potential of MG132, its effects on
uterine leiomyoma cells have not been fully investigated. The
present study examined the potential of proteasome inhibition using
MG132 in Eker leiomyoma tumor-3 (ELT3) uterine leiomyoma cells,
focusing on its mechanisms of action, including cell cycle arrest,
induction of apoptosis, and the role of reactive oxygen species
(ROS) in mediating these effects. The findings of the present study
may contribute to the development of more specific and safer
proteasome-targeting drugs for clinical application in uterine
leiomyomas.
Materials and methods
Cell lines and reagents
The rat leiomyoma cell line, ELT3, was obtained from
the American Type Culture Collection. Human uterine smooth muscle
cells (Ut-SMCs) were purchased from PromoCell GmbH. Both cell lines
were cultured in DMEM (cat. no. LM001-05; Welgene, Inc.) with 10%
fetal bovine serum (cat. no. SH30919.03; Hyclone; Cytiva) and 1%
streptomycin-penicillin (cat. no. 15140122; Gibco; Thermo Fisher
Scientific, Inc.), and maintained in a humidified incubator at 37˚C
with 5% CO2. MG132 was sourced from Selleck Chemicals
(cat. no. S2619). N-acetyl-L-cysteine (NAC; cat. no. A7250) was
purchased from MilliporeSigma. NAC was used alone or in combination
with MG132 to determine the role of ROS production in MG132-induced
apoptosis. Dimethyl sulfoxide (cat. no. DMS555.500; BioShop Canada
Inc.) served as the control.
MTT and lactate dehydrogenase (LDH)
release assay
MTT and LDH release assays assessed cell viability
and cytotoxicity, respectively. MTT and LDH release assays were
performed according to previously established methods (27). Briefly, ELT3 cells
(5.0x103 cells per well) were seeded in 96-well plates
for 24 h and then were treated with varying concentrations of MG132
for either 24 or 48 h. In the MTT assay, the resulting formazan
crystals were dissolved by adding 150 µl dimethyl sulfoxide to each
well, and the absorbance was measured at 570 nm. The absorbance
values were normalized to the control group to determine the
relative viability of the treated cells compared with the untreated
controls.
Colony formation assay
ELT3 cells were seeded in 6-well plates at 1,000
cells per well and incubated for 24 h at 37˚C and 5%
CO2. Following incubation, the medium was replaced with
fresh medium containing varying concentrations of MG132 (0, 0.25,
0.5, 1 and 2 µM), and the cells were incubated at 37˚C for 5 days
to allow colony formation. Colony growth was assessed using a
crystal violet assay kit (ab232855; Abcam) as per the
manufacturer's instructions. Colonies were stained with 2% crystal
violet solution at room temperature for 20 min, washed with
distilled water, and air-dried. The stained cells were dissolved in
a solubilization solution (included in the aforementioned kit), and
the absorbance was measured at 570 nm using an INNO microplate
reader (LTEK Co., Ltd.) to quantify colony formation and analyze
the effect of MG132.
Flow cytometric analysis of
apoptosis
The apoptotic profiles of ELT3 cells were measured
using a Muse® Annexin V & Dead Cell Kit (cat. no.
MCH100105; Cytek Biosciences) following the protocol provided by
the manufacturer. Briefly, ELT3 cells were treated with MG132 at
various concentrations (0, 0.5, 1 and 2 µM) for 24 or 48 h at 37˚C
in 6-well plates at a density of 5x104 cells per well.
Following treatment, the cells were trypsinized to detach them from
the wells and harvested by centrifugation at 300 x g for 5 min at
room temperature. The cell pellets were then resuspended in fresh
media to prepare them for staining. Subsequently, 100 µl of
Muse® Annexin V & Dead Cell reagent was added to the
cell suspension. The cells were then incubated for 20 min at room
temperature in the dark to allow proper staining of the apoptotic
and dead cells. Following incubation, the stained cells were
analyzed using a Guava® Muse® Cell Analyzer
(cat. no. 0500-3115; Cytek Biosciences).
Flow cytometric evaluation of cell
cycle distribution
A Muse® Cell Cycle Kit (cat. no.
MCH100106; Cytek Biosciences) was used to analyze the cell cycle
phases of the samples. Initially, cells were harvested and
centrifuged at 300 x g for 5 min at room temperature to form a
pellet, which was then fixed in 70% ethanol at -20˚C for 3 h.
Following fixation, the cells were stained at room temperature
using the Muse® Cell Cycle Reagent and incubated in
darkness for 30 min to ensure proper staining. The stained samples
were analyzed using a Guava® Muse® Cell
Analyzer to determine the distribution of cells across the
different phases of the cell cycle.
ROS quantification using flow
cytometry
ROS production was quantified via flow cytometry
using a Muse® Oxidative Stress Kit (cat. no. MCH100111;
Cytek Biosciences), following the manufacturer's guidelines. In
summary, ELT3 cells were seeded in 6-well plates at a density of
5x104 cells per well and cultured at 37˚C for 24 h. The
cells were treated with 0, 1, or 2 µM MG132 in fresh media at 37˚C
for 24 and 48 h. Following treatment, the cells were detached and
resuspended in 1X Assay Buffer, followed by the addition of the
Muse® Oxidative Stress Reagent working solution. The
samples were incubated at 37˚C for 30 min before being analyzed
with a Guava® Muse® Cell Analyzer.
Protein preparation and western blot
analysis
Protein extraction and western blotting were
conducted following a previously published protocol (27). For detection, the following primary
antibodies were used: LC3B (cat. no. 2775S), p27 (cat. no. 3686T),
phosphorylated (p)-p44/42 MAPK (Erk1/2) (cat. no. 9106S), p44/42
MAPK (cat. no. 4695S), poly(ADP-ribose) polymerase 1 (PARP) (cat.
no. 9542S), caspase-3 (cat. no. 9665S), and cleaved caspase-3 (cat.
no. 9664S) all from Cell Signaling Technology, as well as p21 (cat.
no. sc-6246) and heat shock protein 90 (cat. no. sc-13119) from
Santa Cruz Biotechnology, Inc. Heat shock protein 90 was used as a
loading control to verify equal protein loading across the lanes.
The protein signals were visualized using the Immobilon ECL Ultra
Western HRP Substrate (cat. no. WBKLS0100; MilliporeSigma) and
analyzed with an Azure c280 chemiluminescent imaging system (Azure
Biosystems Ins.). Densitometric analysis of the resulting images
was then performed using ImageJ 1.53a software (National Institutes
of Health).
Statistical analyses
Statistical analyses were conducted using GraphPad
Prism software (version 8.0; Dotmatics). Data are presented as the
mean ± standard deviation. A one-way analysis of variance (ANOVA)
was used to compare multiple groups, followed by Tukey's post hoc
test or Dunnett's multiple comparison test as appropriate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MG132 inhibits ELT3 cell viability and
colony formation
The cytotoxic effects of MG132 on ELT3 uterine
leiomyoma cells were investigated first by assessing cell viability
using MTT assays and LDH activity through LDH release assays. The
results revealed that MG132 significantly reduced cell viability
and increased LDH activity at 24 and 48 h. Specifically, cell
viability was decreased to ~44.8% using 2 µM MG132 after 24 h. It
was further decreased to ~10.7% after 48 h (Fig. 1A). LDH activity was significantly
increased, with a 1.63-fold change using 2 µM MG132 after 24 h and
a 2.32-fold change after 48 h (Fig.
1B). To further evaluate the effects of MG132, a colony
formation assay was performed. Crystal violet staining revealed
that colonies treated with MG132 at 0.25, 0.5, 1 and 2 µM for 5
days were decreased compared with the control group (Fig. 1C). Additionally, the MG132
treatment of 1 and 2 µM reduced the absorbance at 595 nm by ~90%
(Fig. 1D). By contrast, compared
with ELT3 cells, treatment of normal Ut-SMCs with MG132 for 24 and
48 h resulted in a lesser reduction in viability (Fig. 1E). These findings indicated that
high concentrations of MG132 decrease cell viability, increase LDH
release, and significantly impair colony formation in ELT3
cells.
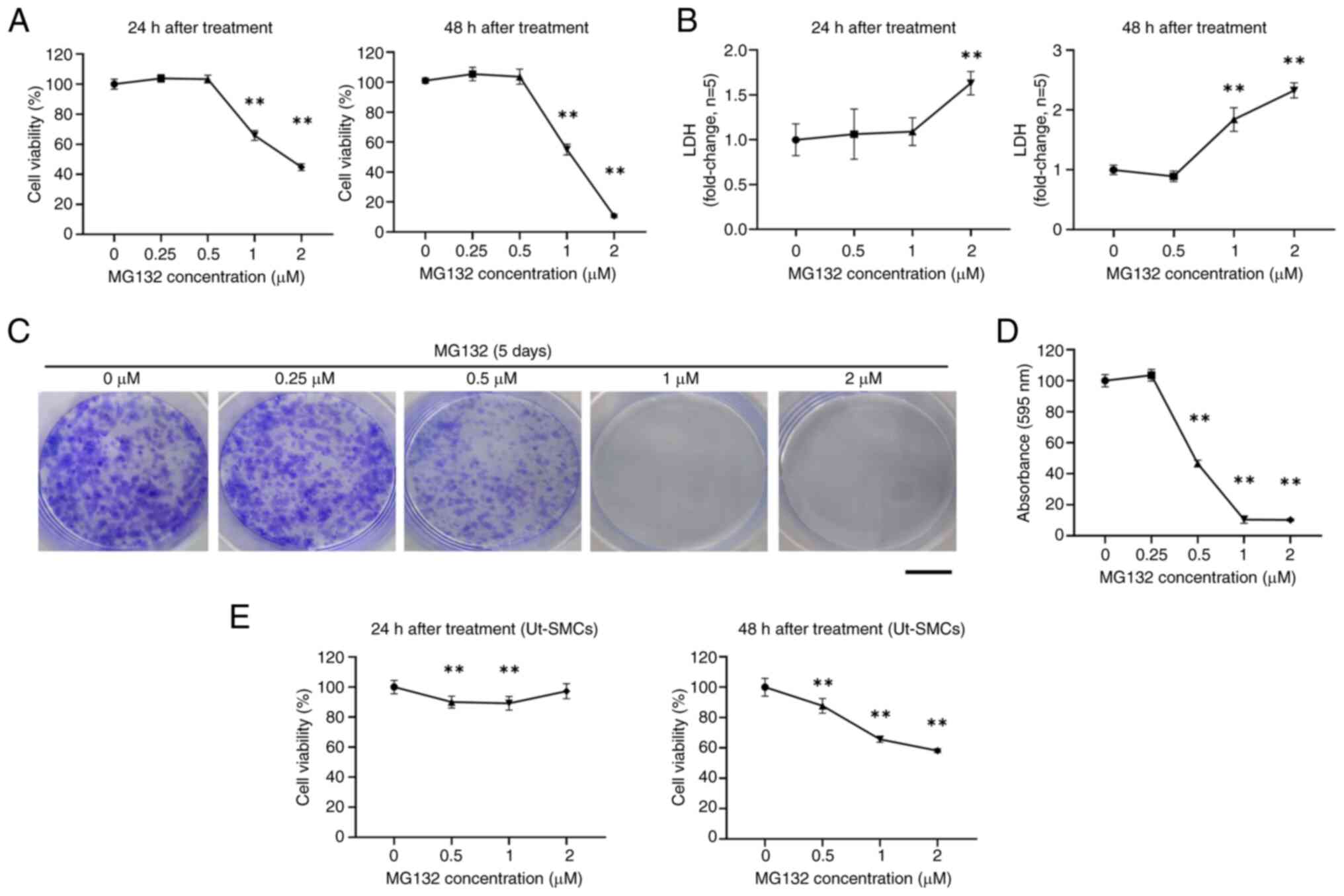 | Figure 1MG132 reduces the cell viability,
increases the release of LDH, and impairs colony formation in ELT3
cells. (A and B) ELT3 cells were treated with MG132 at the
indicated concentrations for 24 and 48 h, followed by MTT cell
viability and LDH release assays. (C) Representative images of the
colonies stained using crystal violet (black scale bar, 10 mm). (D)
The absorbance of the crystal violet-stained cells was measured at
595 nm after lysis. (E) Ut-SMCs were treated with various
concentration (0-2 µM) of MG132 for 24 or 48 h, and cell viability
was measured using the MTT assay. Data are presented as the mean ±
standard deviation. All experiments were conducted in triplicate.
**P<0.01 compared with the control group. MG132,
carbobenzoxyl-L-leucyl-L-leucyl-L-leucine; LDH, Lactate
dehydrogenase; Ut-SMCs, uterine smooth muscle cells. MG132,
carbobenzoxyl-L-leucyl-L-leucyl-L-leucine; LDH, lactate
dehydrogenase; ELT3, Eker leiomyoma tumor-3; Ut-SMCs, uterine
smooth muscle cells. |
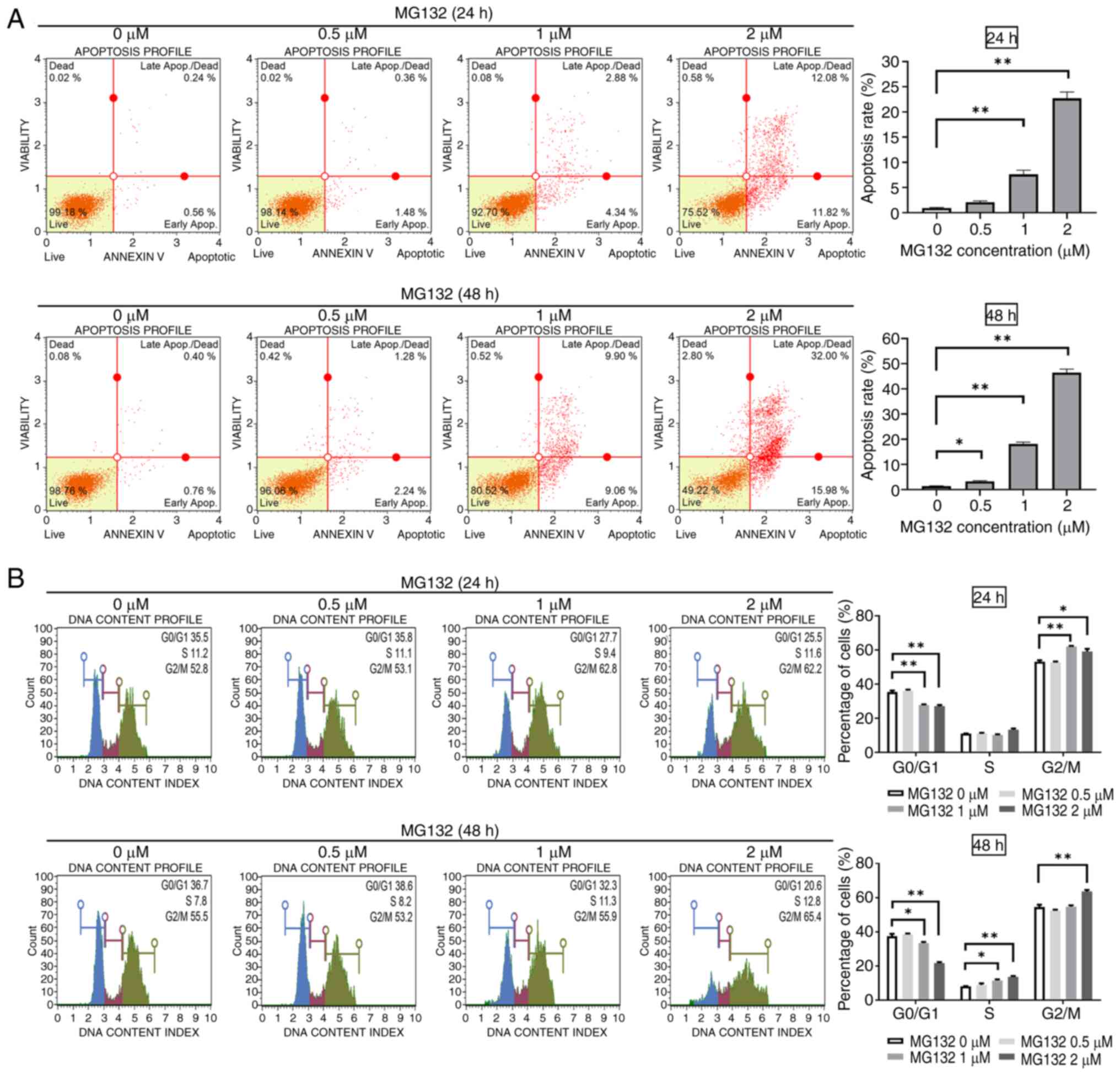
MG132 induces apoptosis and
G2/M phase arrest in ELT3 cells
To examine whether ELT3 cells undergo apoptosis,
cells were treated with MG132 at concentrations of 0.5, 1 or 2 µM
for 24 and 48 h. Apoptosis was measured using Annexin V staining
and analyzed by flow cytometry. Significant increases in apoptosis
were observed at 1 and 2 µM after 24 h of treatment (Fig. 2A, upper images). Similar patterns
were observed after 48 h of treatment (Fig. 2A, lower images). To investigate
whether MG132-mediated inhibition of cell proliferation was related
to cell cycle arrest, ELT3 cells were treated with varying
concentrations of MG132 for 24 and 48 h. Cell cycle profiles were
assessed using a Guava® Muse® Cell Analyzer.
MG132 significantly increased the cell population in the
G2/M phase while reducing the cell population in the
G0/G1 phase after 24 and 48 h of treatment
(Fig. 2B). These results confirmed
that MG132 induces apoptosis in ELT3 cells in a concentration- and
time-dependent manner and causes cell cycle arrest at the
G2/M phase.
MG132 increases ROS production in ELT3
cells
Excessive accumulation of ROS in cells can lead to
oxidative stress, damaging nucleic acids, lipids, proteins,
membranes, and mitochondria (28).
To assess whether MG132 increases ROS levels in ELT3 cells, the
cells were treated with different concentrations of MG132 (0, 1 and
2 µM) for 24 and 48 h, and then analyzed using a Guava®
Muse® Cell Analyzer. ROS levels were 30.68, 42.97 and
47.88% in the control and the MG132-treated ELT3 cells at MG132
concentrations of 0, 1 and 2 µM for 24 h, respectively (Fig. 3A, upper images), and after 48 h of
MG132 treatment, the ROS levels were 16.07, 25.95 and 42.38%,
respectively (Fig. 3A, bottom
images). To further determine whether ROS production was involved
in MG132-induced apoptosis, ELT3 cells were treated with 2 µM MG132
for 48 h in the presence or absence of 5 mM NAC. NAC is a known
antioxidant (29). NAC effectively
reduced MG132-induced apoptosis in ELT3 cells (Fig. 3B). These results indicated that
MG132 induces ROS generation, which leads to ROS-mediated apoptosis
in ELT3 cells.
Effect of MG132 on the expression of
proteins related to cell proliferation and apoptosis in ELT3
cells
To study the mechanism of MG132, western blotting
was used to detect the cell cycle-regulatory proteins p21 and p27,
as well as the phosphorylation of ERK, which is essential for its
activation. MG132 significantly increased the p21 protein
expression at 1 and 2 µM at 24 h, but decreased the expression at 2
µM at 48 h (Fig. 4A; lanes 2 and 3
at 24 h and lanes 5 and 6 at 48 h). In addition, untreated ELT3
cells had elevated levels of p27 and ERK phosphorylation at 48 h,
whereas MG132 treatment decreased p27 expression and ERK
phosphorylation in a dose-dependent manner at 48 h (Fig. 4A; lanes 4 to 6). To understand how
MG132 triggers apoptosis in ELT3 cells, caspase-3, cleaved
caspase-3, and PARP expression levels were analyzed. MG132
increased the cleaved caspase-3 to total caspase-3 ratio and the
cleaved PARP to total PARP ratio at 2 µM for 24 and 48 h compared
to the control (Fig. 4B). These
findings indicated that MG132 modulates cell cycle- and
apoptosis-related proteins, leading to reduced proliferation and
increased apoptosis in ELT3 cells.
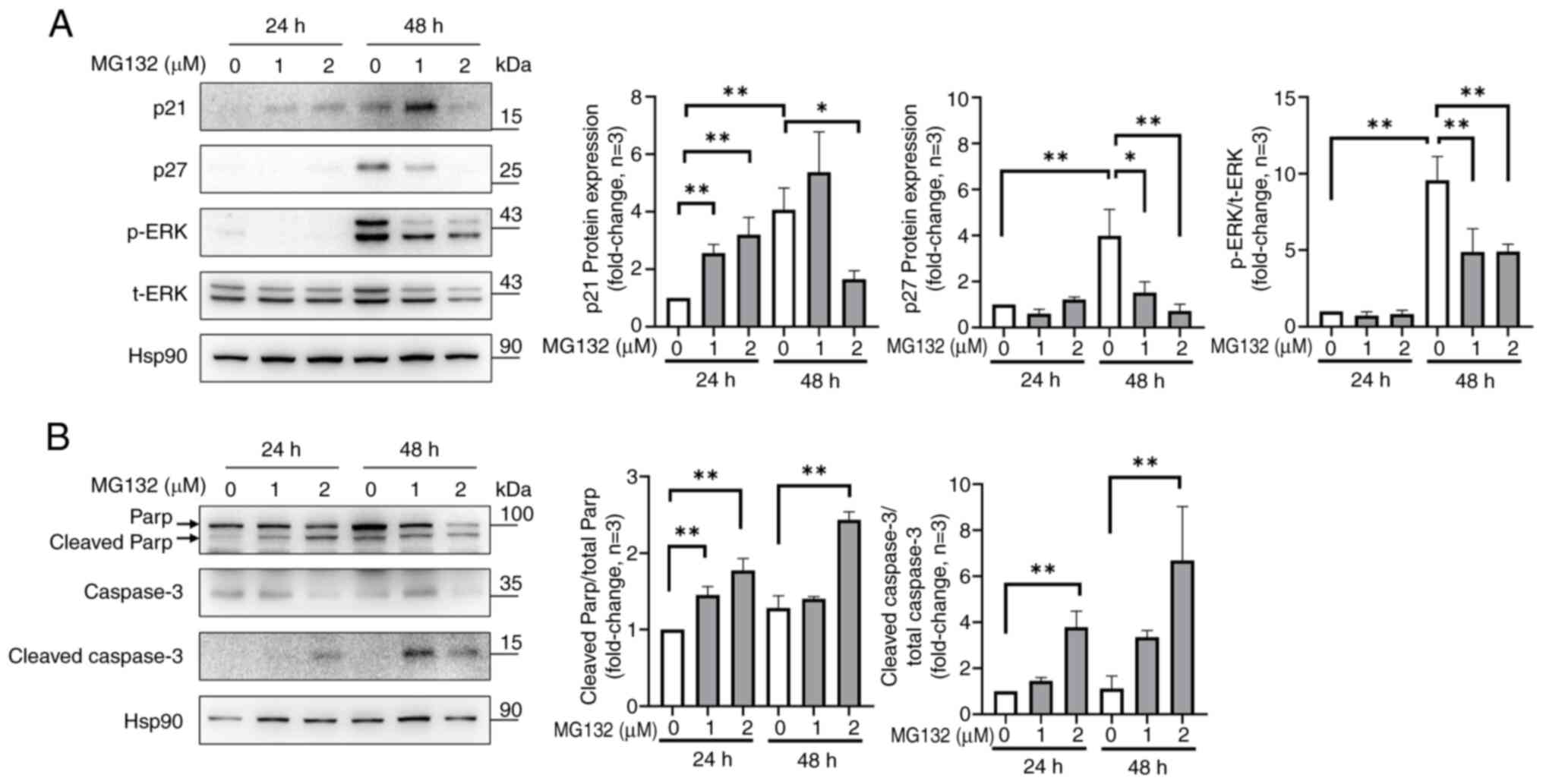 | Figure 4Effects of MG132 on cell
proliferation- and apoptosis-related proteins in ELT3 cells. (A)
Western blot analysis of p21, p27, p-ERK, and ERK, with bar graphs
revealing the p21 and p27 ratios relative to Hsp90. (B) The effect
of MG132 on caspase-3 activation and PARP cleavage was also
assessed. Data are presented as the mean ± standard deviation from
three independent experiments. *P<0.05 and
**P<0.01 compared with each group as indicated in the
graphs. MG132, carbobenzoxyl-L-leucyl-L-leucyl-L-leucine; ELT3,
Eker leiomyoma tumor-3; ERK, extracellular signal-regulated kinase;
Hsp90, heat shock protein 90; PARP, poly(ADP-ribose) polymerase 1;
p, phosphorylated; t, total. |
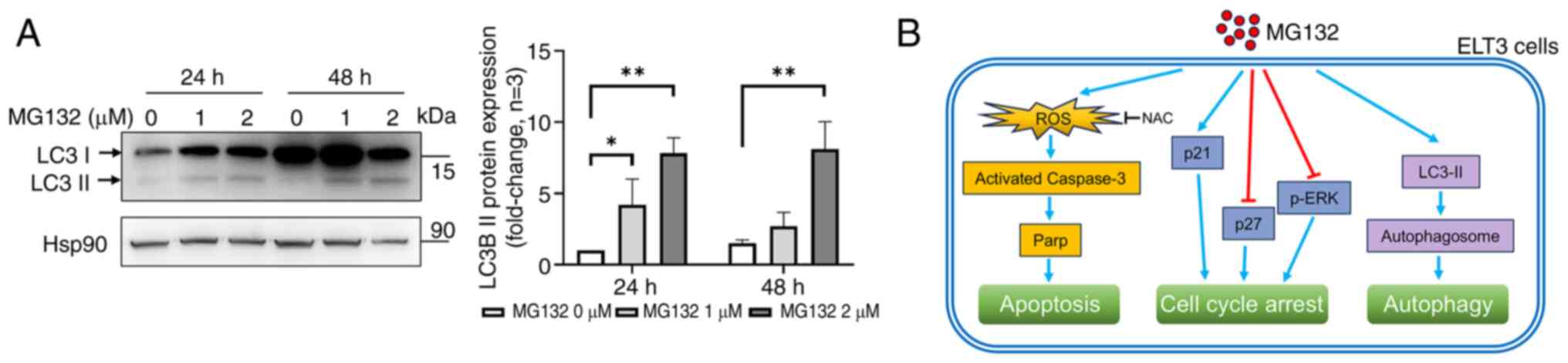
MG132 induces autophagy in ELT3 cells
by activating LC3
Autophagy is a crucial process that maintains
cellular homeostasis under normal and stress conditions (30). To evaluate autophagy induction,
western blotting was used to measure LC3 protein expression, a key
protein involved in autophagosome formation (30). The conversion of LC3 I to LC3 II, a
marker of autophagy, was observed after treating ELT3 cells with 1
and 2 µM MG132 for 24 and 48 h, as shown by the increased LC3 II
levels (Fig. 5A). These findings
indicated that MG132 triggers autophagy or a form of programmed
cell death in ELT3 cells.
Discussion
The findings of the present study are summarized and
illustrated in Fig. 5B. The
present study offered important insights into the potential use of
MG132 to treat ELT3 uterine leiomyoma cells, which serve as a model
for a common and often troubling gynecological condition. These
results demonstrated that MG132 significantly reduced cell growth
and colony formation, caused cell death, stopped cells from
progressing through the G2/M phase of the cell cycle,
and increased ROS production. These results suggest that MG132 and
similar proteasome inhibitors could effectively target leiomyoma
cells. Furthermore, the present research improved the understanding
of how MG132 impacts important proteins involved in the cell cycle
and cell death, including p21, p27, ERK, and caspase-3.
Additionally, the present study demonstrated that MG132 induces
autophagy, as indicated by the increased conversion of LC3 I to LC3
II (Fig. 5). This is crucial as it
highlights the potential of MG132 for regulating the survival of
uterine leiomyomas by demonstrating the possibility of targeting
the proteasome.
In several studies, MG132 has been revealed to play
a crucial role in inducing apoptosis. MG132 disrupts protein
degradation within cells by affecting the ubiquitin-proteasome
pathway, leading to increased cellular stress and modulating
inflammatory responses (31,32).
Specifically, MG132 can induce apoptosis through the mitochondrial
pathway and activate cell death pathways by causing an accumulation
of ROS within the cells (25).
Additionally, MG132 has been demonstrated to suppress the
expression of anti-apoptotic proteins, such as Bcl-2, and to
promote apoptosis by activating apoptosis-related proteins, such as
caspase-3(33). Moreover, numerous
studies have revealed that MG132 can cause cells to arrest in the
G2/M phase of the cell cycle (34,35).
During this phase, cells undergo DNA damage checks and repair
processes, and MG132 interferes with the degradation of proteins
essential for this process, leading to cell cycle arrest. For
example, cyclin-dependent kinase inhibitors (CDK inhibitors), such
as p21, are stabilized by MG132, causing cells to halt at the
G2/M phase (35).
The western blot analysis revealed that MG132
increased p21 expression at 24 h but decreased the expression at 48
h, indicating a complex regulatory effect on cell cycle
progression. In addition, MG132 treatment decreased p27 expression
and ERK phosphorylation, both of which are associated with cell
proliferation. These findings suggest that the anti-proliferative
effects of MG132 are more likely related to changes in p21 and ERK
phosphorylation rather than changes in p27. Additionally, the
activation of caspase-3 and the cleavage of PARP further confirmed
the pro-apoptotic effects of MG132 in ELT3 cells. This similarity
between the results of the present study and previous studies
suggests that MG132 and other proteasome inhibitors could be
effective in targeting leiomyoma cells, highlighting their
potential as promising treatments in cancer therapy (33,35).
Notably, MG132 was revealed to induce autophagy in
ELT3 cells, as evidenced by the increased conversion of LC3 I to
LC3 II. Autophagy is a cellular process that can either promote
survival or lead to cell death, depending on the context (36,37).
In MCF-7 breast cancer cells, MG132-induced autophagy was linked to
endoplasmic reticulum stress (38). Similarly, in A549 human lung
adenocarcinoma cells, MG132 treatment enhanced autophagy, marked by
the upregulation of LC3B and the conversion of LC3B-I to lipidated
LC3B-II, aiding the degradation of the polyubiquitinated AGR2
protein (39). In N2a cells and
primary neurons, MG132 also triggered a time-dependent increase in
LC3-II levels (40). While
autophagy initially acts as a protective mechanism against
MG132-induced proteotoxic stress, its persistence may lead to
autophagic cell death, as suggested by the significant rise in LC3
II levels observed in the present study. This dual role of
autophagy highlights the complex balance between survival and death
mechanisms in response to MG132.
Despite the promising results, the present study has
several limitations. First, while MG132 significantly affected ELT3
cells in vitro, its efficacy and safety in vivo
remain untested. Using a single cell line limits the
generalizability of these findings, given the heterogeneity of
uterine leiomyomas. Although MG132 increased ROS production and
induced apoptosis, the present study did not fully elucidate the
specific pathways linking ROS to cell death. Additionally, the
present study did not entirely rule out the involvement of other
forms of programmed cell death. Prolonged treatment with MG132 can
also decrease the viability of normal Ut-SMCs, indicating potential
non-specific effects on normal tissues. Thus, further studies are
required to determine the optimal concentration and exposure time
of MG132, as well as strategies to minimize off-target effects and
ensure selective toxicity toward leiomyoma cells. These additional
experiments, including in vivo studies, are essential to
assess the therapeutic potential and safety of MG132 as a treatment
for uterine leiomyomas.
In conclusion, the findings of the present study
suggest that MG132 exerts its anti-proliferative effects on ELT3
cells through multiple mechanisms, including the induction of
apoptosis, cell cycle arrest, ROS production, and autophagy. The
results highlight the potential of MG132 in controlling the
survival of uterine leiomyomas by demonstrating the ability to
target the proteasome. However, further in vivo studies are
necessary to fully evaluate the therapeutic efficacy and safety of
MG132 in treating uterine leiomyomas.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a research fund from
Chosun university (grant no. K208554002).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HJ and SRY designed the experiments and revised the
manuscript. HL and SBL conducted the experiments and wrote the
manuscript. HJ and SRY performed the experiments and data analyses.
All authors read and approved the final manuscript. HL and HJ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stewart EA, Laughlin-Tommaso SK, Catherino
WH, Lalitkumar S, Gupta D and Vollenhoven B: Uterine fibroids. Nat
Rev Dis Primers. 2(16043)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sohn GS, Cho S, Kim YM, Cho CH, Kim MR and
Lee SR: Working Group of Society of Uterine Leiomyoma. Current
medical treatment of uterine fibroids. Obstet Gynecol Sci.
61:192–201. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cermik D, Arici A and Taylor HS:
Coordinated regulation of HOX gene expression in myometrium and
uterine leiomyoma. Fertil Steril. 78:979–984. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Okoro CC, Ikpeze OC, Eleje GU, Udigwe GO,
Ezeama CO, Ugboaja JO, Enechukwu CI, Umeononihu OS, Ogabido CA,
Oguejiofor CB, et al: Association between serum vitamin D status
and uterine leiomyomas: A case-control study. Obstet Gynecol Sci.
67:101–111. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
De La Cruz MS and Buchanan EM: Uterine
fibroids: Diagnosis and treatment. Am Fam Physician. 95:100–107.
2017.PubMed/NCBI
|
|
6
|
Lee MJ, Yun BS, Seong SJ, Kim ML, Jung YW,
Kim MK, Bae HS, Kim DH and Hwang JY: Uterine fibroid shrinkage
after short-term use of selective progesterone receptor modulator
or gonadotropin-releasing hormone agonist. Obstet Gynecol Sci.
60:69–73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Navarro A, Bariani MV, Yang Q and Al-Hendy
A: Understanding the impact of uterine fibroids on human
endometrium function. Front Cell Dev Biol. 9(633180)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Angioni S, D'Alterio MN and Daniilidis A:
Highlights on medical treatment of uterine fibroids. Curr Pharm
Des. 27:3821–3832. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee S and Stewart EA: New treatment
options for nonsurgical management of uterine fibroids. Curr Opin
Obstet Gynecol. 35:288–293. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Duhan N: Current and emerging treatments
for uterine myoma-an update. Int J Womens Health. 3:231–241.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Choe YS, Lee WM, Choi JS, Bae J, Eom JM
and Choi E: Clinical characteristics of patients with leiomyoma who
undergo surgery after high intensity focused ultrasound (HIFU).
Obstet Gynecol Sci. 62:258–263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yerezhepbayeva M, Terzic M, Aimagambetova
G and Crape B: Comparison of two invasive non-surgical treatment
options for uterine myomas: Uterine artery embolization and
magnetic resonance guided high intensity focused
ultrasound-systematic review. BMC Womens Health.
22(55)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang CH, Gonzalez-Angulo AM, Reuben JM,
Booser DJ, Pusztai L, Krishnamurthy S, Esseltine D, Stec J, Broglio
KR, Islam R, et al: Bortezomib (VELCADE) in metastatic breast
cancer: Pharmacodynamics, biological effects, and prediction of
clinical benefits. Ann Oncol. 17:813–817. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Engel RH, Brown JA, Von Roenn JH, O'Regan
RM, Bergan R, Badve S, Rademaker A and Gradishar WJ: A phase II
study of single agent bortezomib in patients with metastatic breast
cancer: A single institution experience. Cancer Invest. 25:733–737.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cresta S, Sessa C, Catapano CV, Gallerani
E, Passalacqua D, Rinaldi A, Bertoni F, Viganò L, Maur M, Capri G,
et al: Phase I study of bortezomib with weekly paclitaxel in
patients with advanced solid tumours. Eur J Cancer. 44:1829–1834.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ramirez PT, Landen CN Jr, Coleman RL,
Milam MR, Levenback C, Johnston TA and Gershenson DM: Phase I trial
of the proteasome inhibitor bortezomib in combination with
carboplatin in patients with platinum- and taxane-resistant ovarian
cancer. Gynecol Oncol. 108:68–71. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang H, Zonder JA and Dou QP: Clinical
development of novel proteasome inhibitors for cancer treatment.
Expert Opin Investig Drugs. 18:957–971. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee DH and Goldberg AL: Proteasome
inhibitors: Valuable new tools for cell biologists. Trends Cell
Biol. 8:397–403. 1998.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Han YH, Moon HJ, You BR and Park WH: The
effect of MG132, a proteasome inhibitor on HeLa cells in relation
to cell growth, reactive oxygen species and GSH. Oncol Rep.
22:215–221. 2009.PubMed/NCBI
|
|
20
|
Guo N and Peng Z: MG132, a proteasome
inhibitor, induces apoptosis in tumor cells. Asia Pac J Clin Oncol.
9:6–11. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tarjányi O, Haerer J, Vecsernyés M, Berta
G, Stayer-Harci A, Balogh B, Farkas K, Boldizsár F, Szeberényi J
and Sétáló G Jr: Prolonged treatment with the proteasome inhibitor
MG-132 induces apoptosis in PC12 rat pheochromocytoma cells. Sci
Rep. 12(5808)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang W, Monroe J, Zhang Y, George D,
Bremer E and Li H: Proteasome inhibition induces both pro- and
anti-cell death pathways in prostate cancer cells. Cancer Lett.
243:217–227. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan H, Ma YL, Gui YZ, Wang SM, Wang XB,
Gao F and Wang YP: MG132, a proteasome inhibitor, enhances LDL
uptake in HepG2 cells in vitro by regulating LDLR and PCSK9
expression. Acta Pharmacol Sin. 35:994–1004. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Yang B, Zhao J, Li X, Zhang L and
Zhai Z: Proteasome inhibitor
carbobenzoxy-L-Leucyl-L-leucyl-L-leucinal (MG132) enhances
therapeutic effect of paclitaxel on breast cancer by inhibiting
nuclear factor (NF)-κB signaling. Med Sci Monit. 24:294–304.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han YH and Park WH: MG132 as a proteasome
inhibitor induces cell growth inhibition and cell death in A549
lung cancer cells via influencing reactive oxygen species and GSH
level. Hum Exp Toxicol. 29:607–614. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Duan W, Guo Y, Jiang H, Yu X and Li C:
MG132 enhances neurite outgrowth in neurons overexpressing mutant
TAR DNA-binding protein-43 via increase of HO-1. Brain Res.
1397:1–9. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Joung H and Liu H: 2-D08 mediates notable
anticancer effects through multiple cellular pathways in uterine
leiomyosarcoma cells. Oncol Rep. 52(97)2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cenini G, Lloret A and Cascella R:
Oxidative stress and mitochondrial damage in neurodegenerative
diseases: From molecular mechanisms to targeted therapies. Oxid Med
Cell Longev. 2020(1270256)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhitkovich A: N-acetylcysteine:
Antioxidant, aldehyde scavenger, and more. Chem Res Toxicol.
32:1318–1319. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Aman Y, Schmauck-Medina T, Hansen M,
Morimoto RI, Simon AK, Bjedov I, Palikaras K, Simonsen A, Johansen
T, Tavernarakis N, et al: Autophagy in healthy aging and disease.
Nat Aging. 1:634–650. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ortiz-Lazareno PC, Hernandez-Flores G,
Dominguez-Rodriguez JR, Lerma-Diaz JM, Jave-Suarez LF,
Aguilar-Lemarroy A, Gomez-Contreras PC, Scott-Algara D and
Bravo-Cuellar A: MG132 proteasome inhibitor modulates
proinflammatory cytokines production and expression of their
receptors in U937 cells: Involvement of nuclear factor-kappaB and
activator protein-1. Immunology. 124:534–541. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Park HS, Jun do Y, Han CR, Woo HJ and Kim
YH: Proteasome inhibitor MG132-induced apoptosis via ER
stress-mediated apoptotic pathway and its potentiation by protein
tyrosine kinase p56lck in human Jurkat T cells. Biochem Pharmacol.
82:1110–1125. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Westerberg CM, Hägglund H and Nilsson G:
Proteasome inhibition upregulates Bim and induces
caspase-3-dependent apoptosis in human mast cells expressing the
Kit D816V mutation. Cell Death Dis. 3(e417)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim OH, Lim JH, Woo KJ, Kim YH, Jin IN,
Han ST, Park JW and Kwon TK: Influence of p53 and p21Waf1
expression on G2/M phase arrest of colorectal carcinoma HCT116
cells to proteasome inhibitors. Int J Oncol. 24:935–941.
2004.PubMed/NCBI
|
|
35
|
Zanotto-Filho A, Braganhol E, Battastini
AM and Moreira JC: Proteasome inhibitor MG132 induces selective
apoptosis in glioblastoma cells through inhibition of PI3K/Akt and
NFkappaB pathways, mitochondrial dysfunction, and activation of
p38-JNK1/2 signaling. Invest New Drugs. 30:2252–2262.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jung S, Jeong H and Yu SW: Autophagy as a
decisive process for cell death. Exp Mol Med. 52:921–930.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu S, Yao S, Yang H, Liu S and Wang Y:
Autophagy: Regulator of cell death. Cell Death Dis.
14(648)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bao W, Gu Y, Ta L, Wang K and Xu Z:
Induction of autophagy by the MG-132 proteasome inhibitor is
associated with endoplasmic reticulum stress in MCF-7 cells. Mol
Med Rep. 13:796–804. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang D, Xu Q, Yuan Q, Jia M, Niu H, Liu X,
Zhang J, Young CY and Yuan H: Proteasome inhibition boosts
autophagic degradation of ubiquitinated-AGR2 and enhances the
antitumor efficiency of bevacizumab. Oncogene. 38:3458–3474.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Guo F, He XB, Li S and Le W: A central
role for phosphorylated p38α in linking proteasome
inhibition-induced apoptosis and autophagy. Mol Neurobiol.
54:7597–7609. 2017.PubMed/NCBI View Article : Google Scholar
|