Introduction
Thyroid hemiagenesis (TH), which was first reported
in 1852, is a rare, congenital anatomical abnormality that is
defined as the absence of one thyroid lobe with or without an
isthmus (1). In the majority of
cases, TH is discovered incidentally when performing imaging of the
neck (1). However, to the best of
our knowledge, the clinical significance of this malformation has
not been fully elucidated. Therefore, to date, there are no
clinical recommendations for patients with euthyroidism and TH.
Thyroid cancer is the most common endocrine
malignancy (2) and the cancer with
the fastest increasing incidence rate worldwide (2); it can be classified into papillary
thyroid cancer and follicular thyroid cancer, which are derived
from follicular cells. By contrast, medullary thyroid cancer (MTC),
another type of thyroid cancer, arise from parafollicular C cells
(3). In particular, MTC is a rare
type of thyroid cancer and only accounts for 3% of all thyroid
malignancies (4,5). The 10-year survival rate of MTC is
~50%, while the 10-year survival rate of differentiated thyroid
cancer, including papillary and follicular thyroid cancer, is 94%
(5,6). However, the co-occurrence of TH and
thyroid cancer is rare, where in the majority of cases
co-occurrence presents as synchronous TH and papillary thyroid
cancer (7). To date, to the best
of our knowledge, only one case of TH and MTC co-occurrence of has
been reported (8). In addition,
the treatment strategy of concurrent TH and MTC has not been
well-illustrated. The present report chronicles a rare case of
synchronous TH and MTC and summarizes the epidemiology of the
condition, thyroid gland embryology and the treatment strategy for
synchronous TH and MTC.
Case report
A 33-year-old man was referred to the thyroid clinic
of Weifang People's Hospital (Weifang, China) in May 2022, as a
lesion on the left thyroid lobe had been incidentally discovered
during a routine health examination 1 month previously. The patient
had no personal or family history of endocrine disorders. The
patient's physical examination was unremarkable. Laboratory tests
(Table I) showed a
thyroid-stimulating hormone (TSH) level of 3.4 µU/ml (normal range:
0.5-5.5 µU/ml), serum calcitonin level of 115.2 pg/l (normal range:
0-6.4 pg/l) and serum carcinoembryonic antigen (CEA) level of 30.7
ng/l (normal range: 0-5 ng/l). Laboratory tests results showed that
the patient might suffer from MTC.
 | Table ISummary of patient's laboratory test
results. |
Table I
Summary of patient's laboratory test
results.
| Test | Pre-surgery | 1-Week
post-surgery | 1-Month
post-surgery |
|---|
| Thyroid-stimulating
hormone, µU/ml | 3.4 | 5.6 | 2.7 |
| Carcinoembryonic
antigen, ng/l | 30.7 | 4.2 | 2.1 |
| Calcitonin, pg/l | 115.2 | 2.6 | 3.4 |
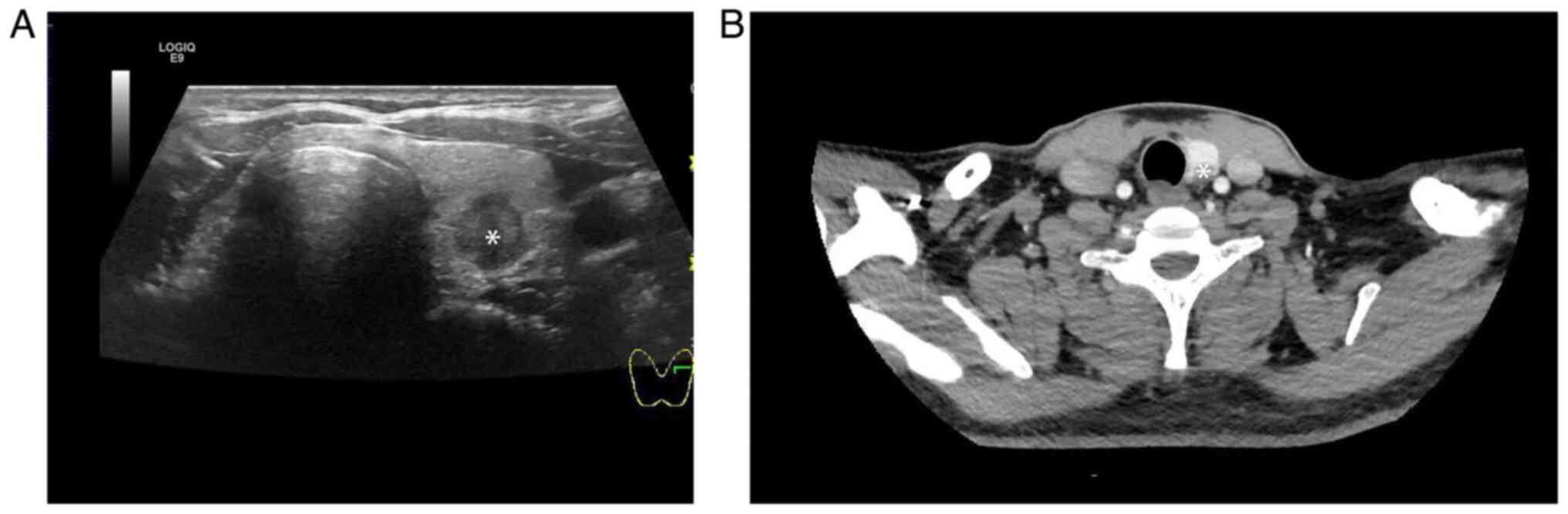
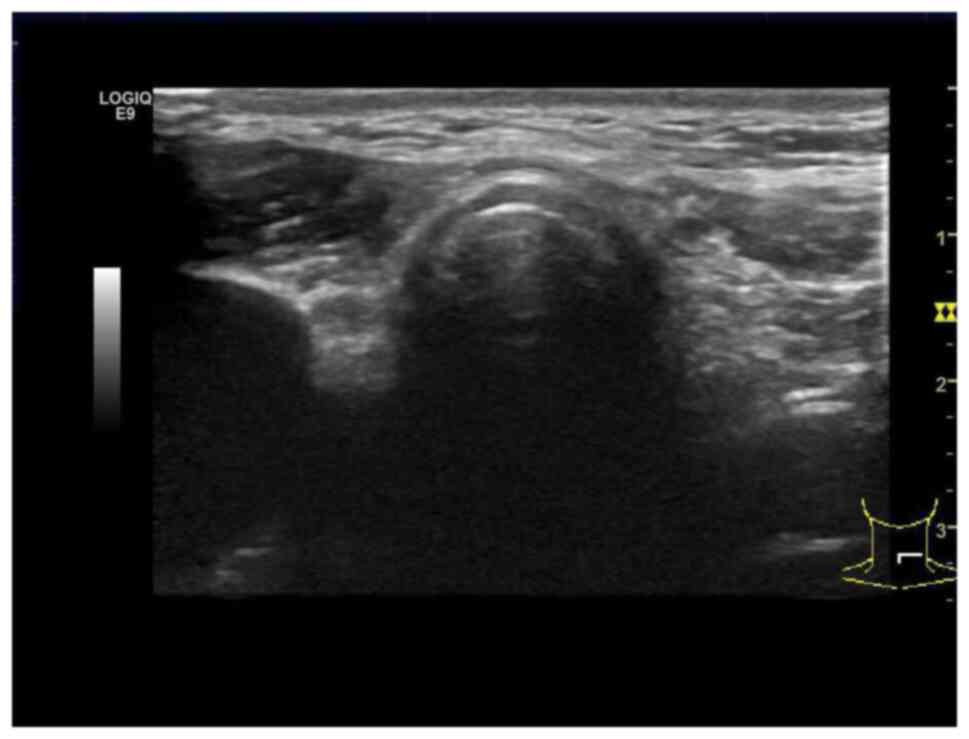
Ultrasound of the neck revealed that the left
thyroid lobe contained an irregular, hypoechoic, hypervascular
tumor with a long diameter of 1.5 cm (Fig. 1A). Cervical CT imaging showed a
thyroid tumor located in the left thyroid (Fig. 1B). The right thyroid lobe could not
be visualized in the ultrasound and CT images. The patient
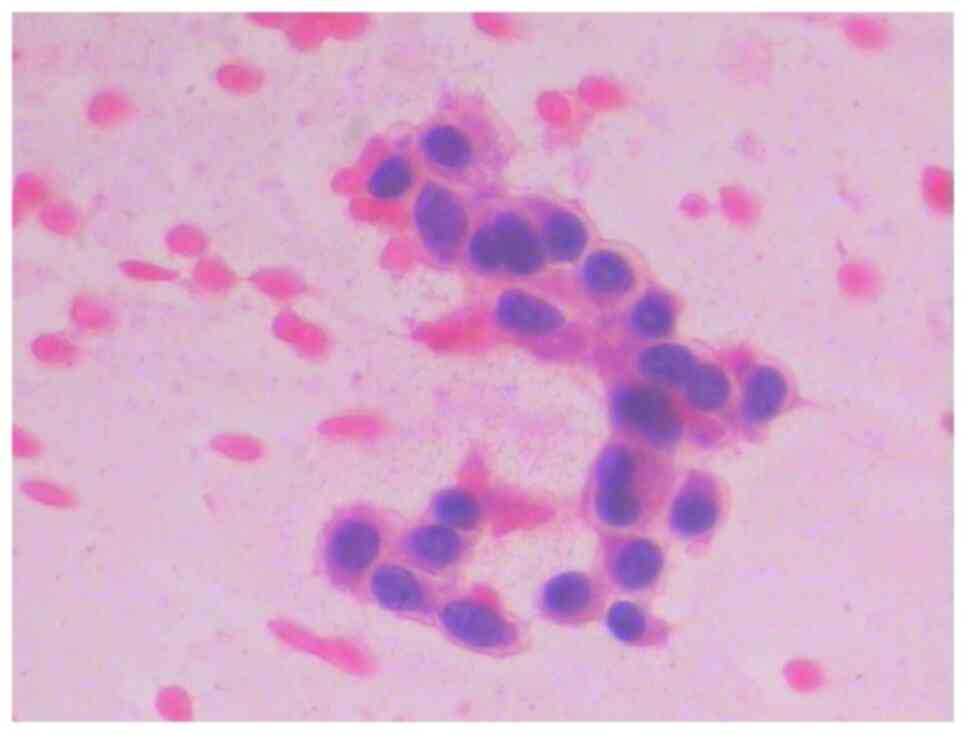
underwent fine-needle aspiration cytology. The result showed that
the morphology of the cells was spindle shaped (Fig. 2) and MTC was suspected. However,
ultrasound and CT showed that no suspicious lymph nodes were
apparent.
The patient underwent total thyroidectomy, bilateral
central lymph node dissection and left cervical compartment
dissection in May 2022. The intraoperative findings indicated
right-sided TH and a suspicious lesion (13x12 mm.) in the left
thyroid lobe with an isthmus. Tissue specimens were fixed with 4%
formalin at room temperature for 12 h, embedded in paraffin at 60˚C
for 15 min, cut into 4-µm sections, stained for 5 min at room
temperature with hematoxylin and eosin, and observed under a light
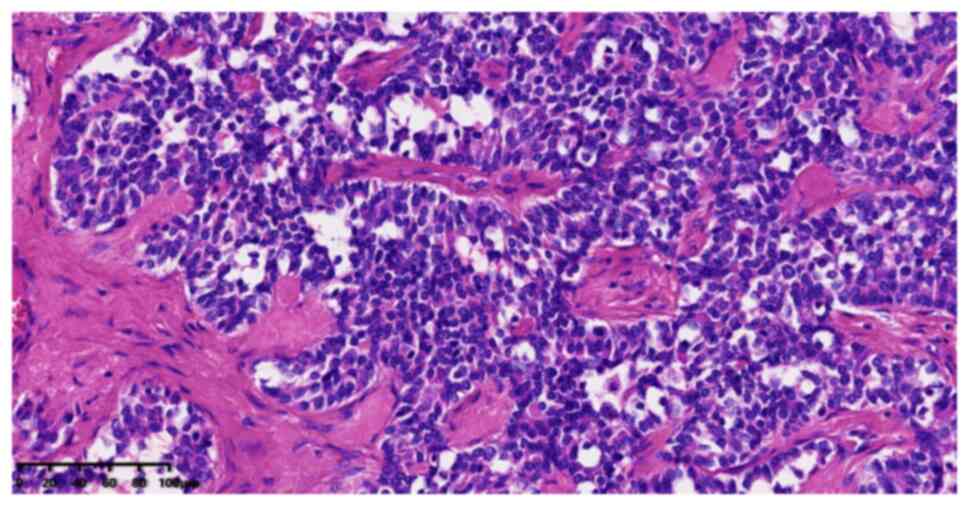
microscope (Nikon Corporation). On their histopathology under the
microscope, the lesion cells were observed to have round nuclei and
clumped chromatin with scant amphophilic cytoplasm (Fig. 3). Immunohistochemical analysis of
the tissue was performed. Tumor tissue was fixed with 4% neutral
formalin at room temperature for 12 h, embedded in paraffin at 60˚C
for 15 min, cut into 4-µm sections and sealed with 3% hydrogen
peroxide at room temperature for 10 min. Antigen retrieval was
performed with EDTA at 100˚C for 2.5 min followed by washing with
PBS. Primary antibody incubation was performed at 37˚C for 60 min
and secondary antibody incubation at 37˚C for 20 min. The Anti-CEA
antibody was purchased from Santa Cruz Biotechnology. The other
primary antibodies were purchased from Beyotime Institute of
Biotechnology. The following primary antibodies were used:
Calcitonin (cat. no. AG8159; 1:100), CEA (sc-48364; cat. no.
AF6480; 1:100), thyroid transcription factor 1 (TTF-1; cat. no.
AG8751; 1:100) and thyroglobulin (TG; cat. no. AG3385; 1:100).
Biotinylated Goat anti-Mouse and Rabbit secondary antibodies were
obtained from OriGene Technologies, Inc. (cat. no. PV-6000; 1:500).
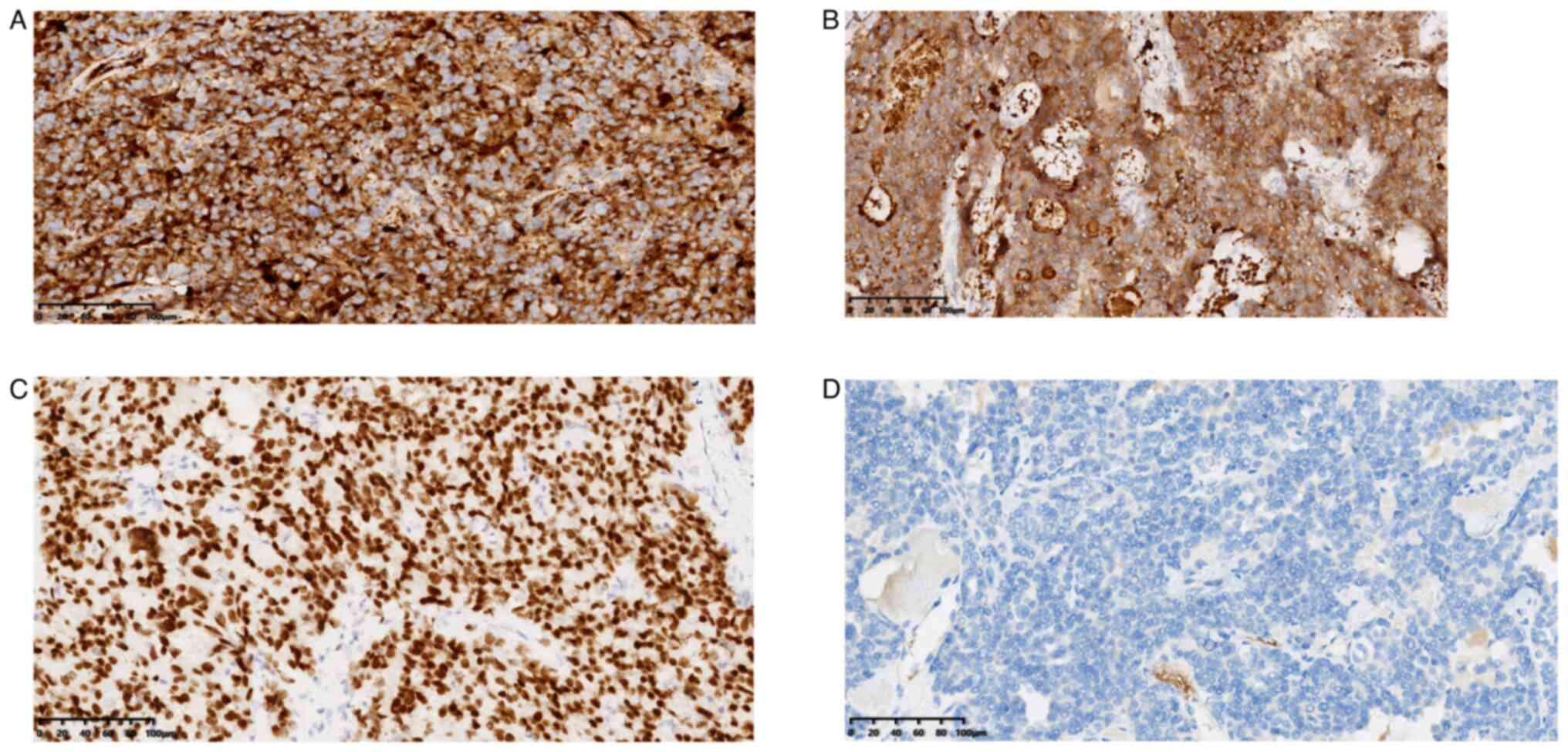
Immunohistochemical staining results revealed positivity for
calcitonin (Fig. 4A), CEA
(Fig. 4B) and TTF-1 (Fig. 4C) and negativity forTG) (Fig. 4D). The positivity of calcitonin,
CEA and TTF-1 enhanced the diagnosis of MTC, while negative results
for TG excluded the diagnosis of differentiated thyroid carcinoma.
The lymphatic adipose tissue of the central compartment and the
left cervical compartment was dissected. A total of 32 lymph nodes
were discovered. Lymph node metastasis was detected in 4 of the 32
lymph node samples harvested.
Serum calcitonin and CEA levels dropped to normal 1
week after the surgery (Table I).
Replacement therapy with L-thyroxine (100 µg/per day; lifetime) was
performed at a TSH level of 0.5-5.5 µU/ml. TSH, calcitonin and CEA
were detected every 3 months and follow-up ultrasound was performed
every 3 months. The latest follow-up was performed in May 2024.
Ultrasound showed no suspicious lymph nodes or recurrent lesions
(Fig. 5). Laboratory tests
illustrated a TSH level of 3.1 µU/ml, a serum calcitonin level of
2.9 pg/l and a CEA level of 2.5 ng/l.
Discussion
TH is a rare, congenital abnormality that is defined
as the absence of one thyroid lobe with or without an isthmus
(1). MTC is a rare malignancy that
is derived from neuroendocrine parafollicular C-cells of the
thyroid gland (3). To the best of
our knowledge, only one case of the co-occurrence of TH and MTC has
so far been reported (8).
In the majority of cases, TH is accidentally
discovered in patients with other thyroid conditions (9). Previous studies conducted in Northern
Poland and Sicily have demonstrated that the prevalence of TH in
adolescents is 0.05% (10,11), whereas its prevalence in Belgium in
the population with similar demographics is ~0.2% (12). However, children with congenital
hypothyroidism in Isfahan (Iran), where goiter and thyroid nodules
are prevalent, tend to have a higher TH prevalence at ≤3.7%
(13,14). In addition, TH is more common in
females, with a female-to-male ratio of 4.3-7:1 (15,16).
MTC is a rare type of thyroid cancer that accounts
for 3% of all thyroid malignancies (4,5).
Despite its relative rarity, MTC is associated with a significantly
higher death rate compared with other types of thyroid cancer, such
as papillary and follicular thyroid cancer (5,6).
A previous study has revealed that TH can co-occur
with certain benign thyroid conditions, such as Hashimoto's
thyroiditis, simple goiters and subacute thyroiditis (8). In addition, TH can co-occurs with
other types of thyroid cancer, including papillary and follicular
thyroid cancer (8). However, to
the best of our knowledge, there is only one reported case of TH
and MTC co-occurrence (8). In that
report, a 49-year-old woman with MTC and TH underwent a
thyroidectomy plus ipsilateral central and lateral neck dissection,
and achieved long-term disease-free survival (8). The present study reported another
rare case of TH and MTC co-occurrence.
Thyroid embryogenesis is a complex process that
remains largely unknown. In addition, to the best of our knowledge,
the mechanism underlying the lobulation of the thyroid primordium
and controlling the descent of the thyroid gland has not been fully
elucidated.
The thyroid gland is the first endocrine organ to
develop during gestation. Derived from a medial anlage, it develops
between the 3rd and 11th week of gestation (17). Thyroid anlage cells develop because
of the proliferation of the endodermal epithelium that lines the
floor of the primitive pharynx between the first and second
pharyngeal arch (17).
Subsequently, this midline structure undergoes numerous
transformations, such as enlargement, bifurcation, lobulations and
detachment from the pharynx (17).
Accompanying the enlargement, the median thyroid anlage is
initially hollow and spherical, where then it solidifies, forming
follicular elements of the thyroid gland, after which it becomes a
bilobed structure (18). During
the 5th week of gestation, the lateral thyroid primordia are
derived from the ventral part of pharyngeal pouches in the
ultimobranchial bodies, where they are attached to the posterior
part of the thyroid gland (19).
The lateral primordia originate from neural crest cells and provide
parafollicular C cells, which produce calcitonin (19). At the end of the 7th week of
gestation, the thyroid gland descends in front of the hyoid bone
and laryngeal cartilage and then settles in its final position
anterior to the trachea (17).
Between the 8th and 12th weeks of gestation, the thyroid follicular
cells arise from the median thyroid anlage and incorporate iodine
(17).
At present, TH is considered to be idiopathic, where
theories of its etiology include genetic aberrations, defects in
lobulation and failure of descent. A number of transcription
factors, such as Forkhead Box Protein E1 (FOXE1), have been shown
to be crucial in the proliferation and descent of the thyroid
primordial (20-22).
In addition, the mutation of these transcription factors (FOXE1,
NKX2-1 and PAX8) may induce TH occurrence (20-22).
Regarding the diagnosis, in the majority of cases,
TH is typically discovered incidentally during neck imaging. The
diagnosis of TH can be confirmed if the contralateral thyroid
tissue is revealed by ultrasonography, CT or thyroid scintigraphy
(7,23). The combination of ultrasound and
scintigraphy or CT is sufficient to confirm the diagnosis of TH
(7,23). However, thyroid scintigraphy alone
cannot confirm the diagnosis of TH because of the presence of a
non-functional hypoplastic lobe, such as in unilateral thyroiditis
(7,23). Ultrasound is considered the gold
standard imaging modality for diagnosing TH due to its
non-invasiveness, low cost and wide availability (7,23).
In the present study, ultrasonography and CT showed that the right
lobe of thyroid was absence. Therefore, the diagnosis of TH was
confirmed.
In general, a cytological smear of a thyroid lesion
is the first step in MTC diagnosis (4,5). MTC
cells are usually discohesive or weakly cohesive (4,5). The
morphology of MTC cells may be spindle-shaped or epithelioid
(4,5). However, the diagnosis of MTC via
cytological smear is difficult due to a variety of cellular
morphologies, non-typical cell shapes and low cellularity (4,5).
Serum calcitonin levels of >100 pg/m may further confirm the
diagnosis of MTC in ambiguous cases (4,5). In
addition, CEA is another reliable tumor marker of MTC (4,5).
Pathology is considered to be the gold standard for MTC diagnosis
(4,5). On immunohistochemistry, calcitonin,
CEA will typically yield positive results, whereas TG should be
negative (4,5). In the present case, the serum level
of calcitonin and CEA was markedly higher compared with the normal
level. MTC markers, including calcitonin and CEA, were positive,
confirming the diagnosis of TH and MTC.
Regarding the treatment strategy and follow-up,
certain considerations should be made. In general, TH is associated
with normal thyroid function, where clinically euthyroid patients
should not be treated (7,23). However, TH can be found in
association with other thyroid diseases, such as Hashimoto's
thyroiditis, Graves' disease and hyperthyroidism (7,23).
TH treatment should be performed in accordance with the co-existing
thyroid disease. In addition, TH with anatomic abnormalities,
including the presence of a thyroglossal cyst or cervical thymic
cysts, should be treated in accordance with other congenital
diseases (7,23).
For patients with TH and MTC co-occurrence,
treatment should be performed in accordance with the MTC treatment
guideline (24). Surgery is the
first step in MTC treatment and the scope of surgery should include
the thyroid gland, bilateral central compartment lymph nodes and
lateral neck lymph nodes in accordance with the serum calcitonin
level. During follow-up, measuring calcitonin and CEA levels every
3 months is important to determine the chemical relapse of the
disease. In addition, neck ultrasonography should be performed
every 3 months to check for MTC relapse. In the present case, the
patient remains healthy with no evidence of disease recurrence.
Notably, the present case has certain limitations.
The pathology and clinical significance of TH were not completely
illustrated. Therefore, gene mutation and epigenetic changes of TH
require further investigation. Furthermore, the association between
TH and other thyroid diseases has not been well-studied. In future
studies, high-throughput technologies may discover the cause of TH
in thyroid embryogenesis and the relationship between TH and other
thyroid diseases.
In conclusion, TH is a rare congenital anomaly that
is typically asymptomatic. MTC occurrence in the remnant lobe is
uncommon. Knowledge of this rare type of thyroid disorder and
immunohistochemical markers are key to making a correct diagnosis.
The treatment strategy for TH and MTC co-occurrence should follow
the MTC guideline.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CL, YW and QD contributed to the drafting of the
manuscript and design of the study. CL, CZ, CM and YW contributed
to the conceptualization and design of the study, as well as
performing the surgery. CL, CZ and CM collected clinical
information and assisted with drafting the manuscript. YW and QD
critically revised the intellectual content, confirm the
authenticity of all the raw data and gave final approval of the
version to be published. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this paper and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang YS and Hong KH: Case of thyroid
hemiagenesis and ectopic lingual thyroid presenting as goitre. J
Laryngol Otol. 122(e17)2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang Y, Yin D, Ren G, Wang Z and Kong F:
Mixed medullary-follicular thyroid carcinoma: A case report and
literature review. Oncol Lett. 26(429)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim M and Kim BH: Current guidelines for
management of medullary thyroid carcinoma. Endocrinol Metab
(Seoul). 36:514–524. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jaber T, Dadu R and Hu MI: Medullary
thyroid carcinoma. Curr Opin Endocrinol Diabetes Obes. 28:540–546.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pelizzo MR, Mazza EI, Mian C and Merante
Boschin I: Medullary thyroid carcinoma. Expert Rev Anticancer Ther.
23:943–957. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lesi OK, Thapar A, Appaiah NNB, Iqbal MR,
Kumar S, Maharaj D, Saad Abdalla Al-Zawi A and Dindyal S: Thyroid
hemiagenesis: Narrative review and clinical implications. Cureus.
14(e22401)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alqahtani SM, Alanesi S and Alalawi Y:
Thyroid hemiagenesis with primary hyperparathyroidism or papillary
thyroid carcinoma: A report of two cases and literature review.
Clin Case Rep. 9:1615–1620. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sato H, Tsukahara K, Motohashi R, Wakiya
M, Serizawa H and Kurata A: Thyroid carcinoma on the side of the
absent lobe in a patient with thyroid hemiagenesis. Case Rep
Otolaryngol. 2017(4592783)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Korpal-Szczyrska M, Kosiak W and Swieton
D: Prevalence of thyroid hemiagenesis in an asymptomatic
schoolchildren population. Thyroid. 18:637–639. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maiorana R, Carta A, Floriddia G, Leonardi
D, Buscema M, Sava L, Calaciura F and Vigneri R: Thyroid
hemiagenesis: Prevalence in normal children and effect on thyroid
function. J Clin Endocrinol Metab. 88:1534–1536. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shabana W, Delange F, Freson M, Osteaux M
and De Schepper J: Prevalence of thyroid hemiagenesis: Ultrasound
screening in normal children. Eur J Pediatr. 159:456–458.
2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ayaz ÜY, Ayaz S, Döğen ME and Api A:
Ultrasonographic and scintigraphic findings of thyroid hemiagenesis
in a child: Report of a rare male case. Case Rep Radiol.
2015(917504)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hashemipour M, Ghasemi M, Hovsepian S,
Heiydari K, Sajadi A, Hadian R, Mansourian M, Mirshahzadeh N,
Kelishadi R and Dalvi M: Etiology of congenital hypothyroidism in
Isfahan: Does it different? Adv Biomed Res. 3(21)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mikosch P, Gallowitsch HJ, Kresnik E,
Molnar M, Gomez I and Lind P: Thyroid hemiagenesis in an endemic
goiter area diagnosed by ultrasonography: Report of sixteen
patients. Thyroid. 9:1075–1084. 1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ruchala M, Szczepanek E, Szaflarski W,
Moczko J, Czarnywojtek A, Pietz L, Nowicki M, Niedziela M, Zabel M,
Köhrle J and Sowinski J: Increased risk of thyroid pathology in
patients with thyroid hemiagenesis: Results of a large cohort
case-control study. Eur J Endocrinol. 162:153–160. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mohebati A and Shaha AR: Anatomy of
thyroid and parathyroid glands and neurovascular relations. Clin
Anat. 25:19–31. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Policeni BA, Smoker WR and Reede DL:
Anatomy and embryology of the thyroid and parathyroid glands. Semin
Ultrasound CT MR. 33:104–114. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Organ GM and Organ CH Jr: Thyroid gland
and surgery of the thyroglossal duct: Exercise in applied
embryology. World J Surg. 24:886–890. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Campennì A, Giovinazzo S, Curtò L,
Giordano E, Trovato M, Ruggeri RM and Baldari S: Thyroid
hemiagenesis, Graves' disease and differentiated thyroid cancer: A
very rare association: Case report and review of literature.
Hormones (Athens). 14:451–458. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Macchia PE, Lapi P, Krude H, Pirro MT,
Missero C, Chiovato L, Souabni A, Baserga M, Tassi V, Pinchera A,
et al: PAX8 mutations associated with congenital hypothyroidism
caused by thyroid dysgenesis. Nat Genet. 19:83–86. 1998.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Szczepanek E, Ruchala M, Szaflarski W,
Budny B, Kilinska L, Jaroniec M, Niedziela M, Zabel M and Sowinski
J: FOXE1 polyalanine tract length polymorphism in patients with
thyroid hemiagenesis and subjects with normal thyroid. Horm Res
Paediatr. 75:329–334. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Szczepanek-Parulska E, Zybek-Kocik A,
Wartofsky L and Ruchala M: Thyroid hemiagenesis: Incidence,
clinical significance, and genetic background. J Clin Endocrinol
Metab. 102:3124–3137. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Boucai L, Zafereo M and Cabanillas ME:
Thyroid cancer: A review. JAMA. 331:425–435. 2024.PubMed/NCBI View Article : Google Scholar
|