Introduction
Castleman's disease (CD), a rare condition, is
frequently subject to delayed diagnosis and misidentification due
to gaps in understanding its etiology and characteristics. CD is
categorized as a rare lymphoproliferative disorder impacting lymph
nodes and other immune cell structures within the body.
Predominantly classified based on the abundance of germinal
centers, CD is broadly categorized into multicentric CD (MCD) and
Unicentric CD (UCD), with the latter being exceptionally uncommon
in the Asian population. The incidence of UCD in the USA has been
estimated as 16 per million person-years (PYs); Conversely, in
Japan, the incidence is estimated to be 0.6-4.3 per million PYs
(1). UCD typically manifests in
various anatomical sites such as the mediastinum, lungs, neck,
axilla, pelvis, and retroperitoneum. Among these localized forms,
mesenteric involvement, termed Unicentric Mesenteric CD (UMCD), is
particularly rare.
Due to its deep abdominal cavity location, UMCD
often presents with highly atypical clinical manifestations. Some
patients may only seek medical intervention when the tumor
enlarges, leading to the manifestation of compression symptoms.
This characteristic poses a substantial challenge to both diagnosis
and treatment. Presently, there is a scarcity of literature
detailing UMCD cases. Due to the scarcity of UMCD and its diverse
clinical manifestations, diagnosis is difficult and the optimal
treatment remains controversial. Complete surgical resection is
frequently curative and is therefore the preferred first-line
therapy, if possible. However, addressing unresectable UMCD poses a
more intricate management dilemma.
The present study was a case study of a 29-year-old
male patient with UMCD characterized by a sizable mesenteric mass,
which posed significant challenges in terms of diagnosis and
achieving complete resection.
Case report
Medical history
The patient was hospitalized at Affiliated Huishan
Hospital of Xinglin College (Wuxi, China) in May 2019. A week prior
to admission, an abdominal mass was incidentally detected,
alongside a two-month history of abdominal pain. The pain was
described as dull, intermittent, radiating to the left waist and
back. The patient had no notable past medical or surgical history,
with a personal habit of tobacco chewing and unremarkable family
medical background. Bowel and bladder functions were reported as
normal.
Inspection results
During the physical examination, a palpable 17-cm
mass was identified in the left lower abdominal quadrant, with the
abdomen demonstrating no tenderness. No lymph node enlargement was
noted in other anatomical regions.
The laboratory results, including blood routine,
liver function, kidney function, electrolytes, tumor markers, urine
routine and stool routine tests, were all within the normal
parameters. HIV test was negative. The chest X-ray was normal.
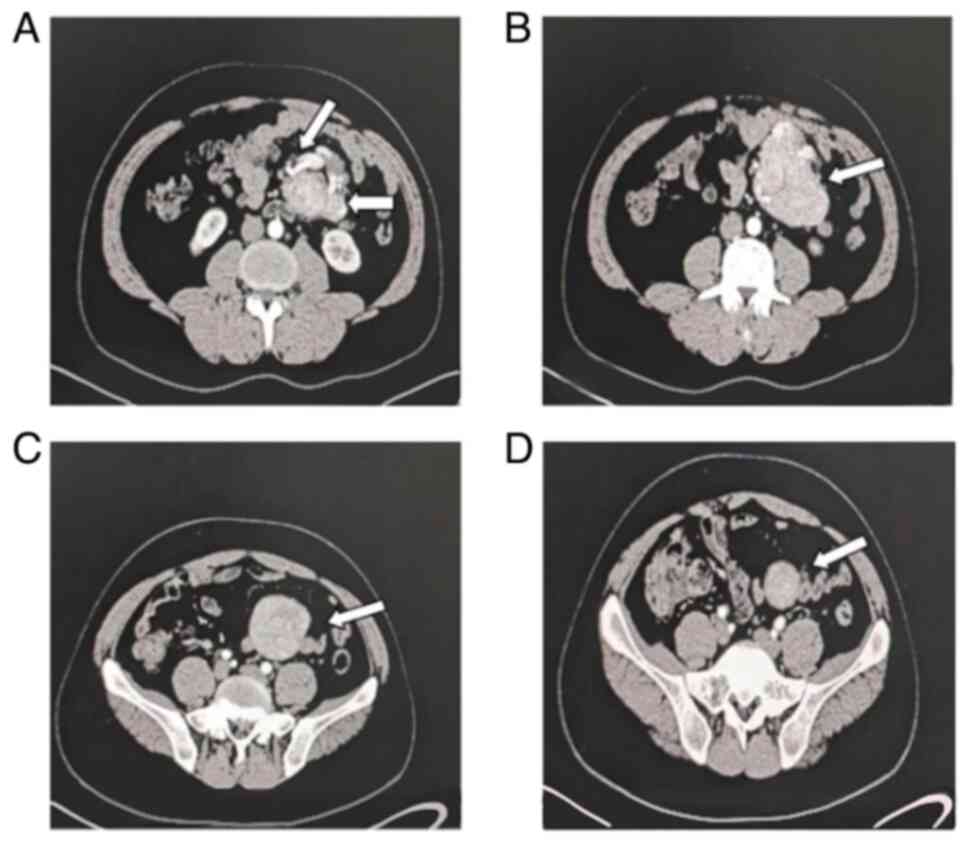
Computerized tomography (CT) scan revealed a well-defined soft
tissue density lesion measuring 16x12x10 cm, encircling large
mesenteric blood vessels within the left mid-abdominal cavity
(Fig. 1). Although CT scan
revealed a clear soft tissue mass with calcification inside the
mesentery, suggestive of Angiosarcoma, differential diagnoses
including Castleman's disease and lymphoma could not be
definitively excluded. At this point, fine needle aspiration was
considered as a viable option for further pathological
clarification; however, the patient declined this procedure. Given
the suspicion of a malignant tumor, surgical intervention was
chosen to facilitate a definitive diagnosis and subsequent
treatment.
Surgical procedure
During the surgical procedure, an irregular circular
mass measuring 15x11x10 cm was identified at the base of the
jejunum mesentery, exhibiting an indistinct boundary with the
mesenteric blood vessels. The mass displayed a firm consistency and
was enveloped by a thin fibrous capsule. A thorough resection of
the tumor was executed, navigating along the apparent space between
the tumor capsule and surrounding tissues. Despite the difficulty,
a successful tumor excision was accomplished, including intestinal
resection and anastomosis. The surgical intervention, lasting 125
min, proceeded smoothly without any complications. Intraoperative
blood loss was estimated at ~100 ml. Following surgery, the patient
experienced an uneventful recovery, with resolution of abdominal
pain and prompt restoration of gastrointestinal function.
Postoperative pathological
results
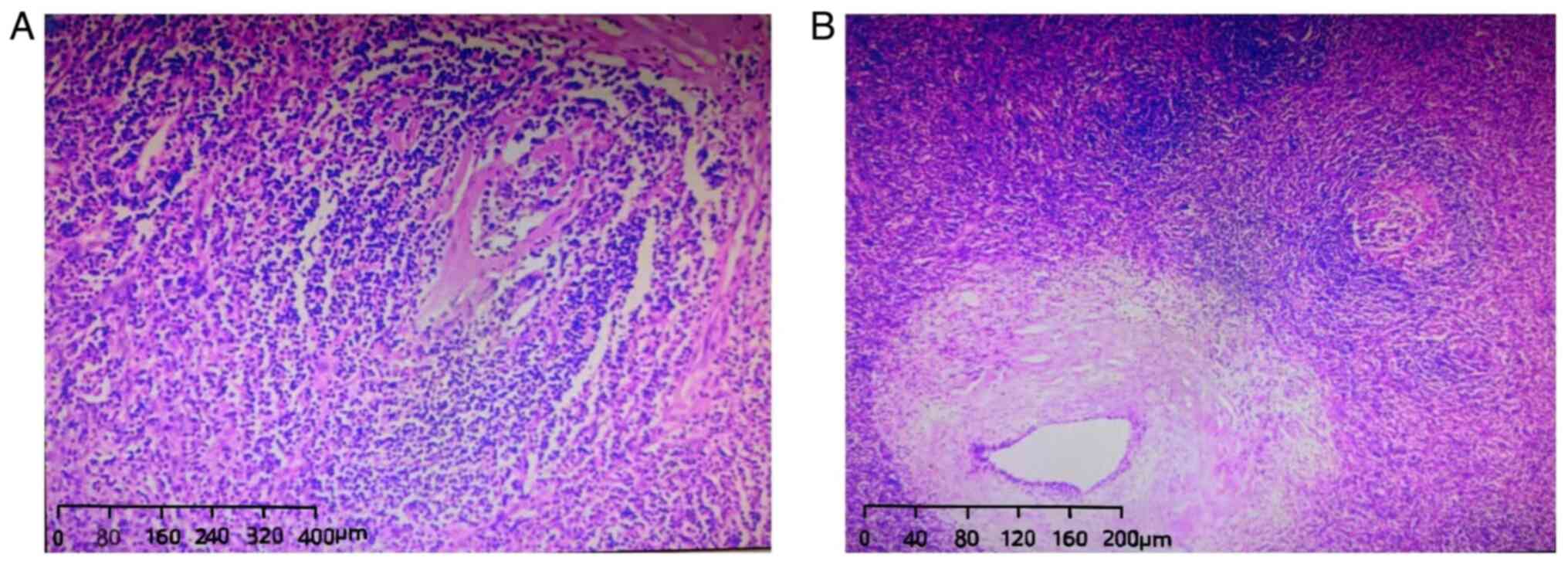
In the postoperative sectioning, a homogenous grey
mass with a medium texture and localized calcifications was
observed, displaying well-defined characteristics. Histopathologic
analysis revealed the mass to be situated within the mesentery
without involvement of the intestinal wall. The specimen exhibited
lymphoid follicles segregated by connective tissue bands containing
thickened blood vessels at the center, consistent with the hyaline
vascular type of Castleman disease (Fig. 2). Additionally, a total of eight
lymph nodes in the second and third stations demonstrated reactive
hyperplasia upon examination. Through comprehensive postoperative
pathological examination, differential diagnoses including
angiosarcoma and lymphoma were successfully ruled out.
Subsequent treatment and
follow-up
Given the challenges posed by the tumor's
substantial size and invasion of mesenteric blood vessels,
achieving a complete resection was notably complex. To reduce the
risk of tumor recurrence, we referred to previous treatment
experience and administered on postoperative chemotherapy of
cyclophosphamide, epirubicin, vincristine and prednisolone (CHOP)
regimen at weeks 1, 4 and 7. The regimen included intravenous
administration of cyclophosphamide at 750 mg/m2,
epirubicin at 90 mg/m2, vincristine at 1.4
mg/m2 (capped at 2 mg dose) and oral prednisone at 100
mg for a 5-day period. Encouragingly, the patient did not encounter
any adverse effects or toxicities associated with the chemotherapy
agents. Due to family reasons, the patient declined further
treatment, including the orphan targeted drug cetuximab for
treating CD. Despite this decision, the patient was confirmed to
have achieved complete recovery, with no evidence of tumor
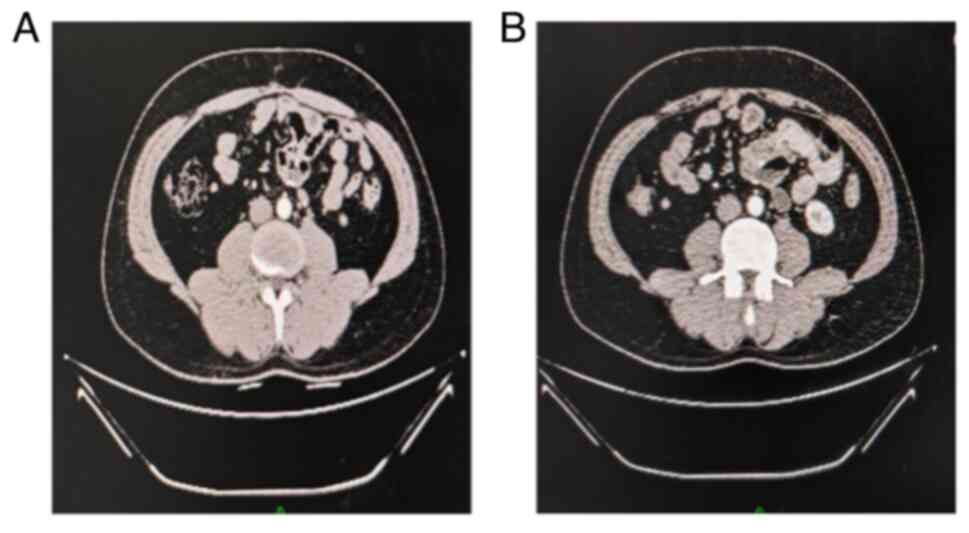
recurrence observed during the 5-year follow-up period (Fig. 3). At the 65th month since the last
follow-up visit, complete remission persists, leading us to posit
that the patient has achieved clinical cure.
Discussion
Diagnostic challenges. Despite the passage of
decades since the initial documentation of CD (2), it continues to represent a rare
clinical entity characterized by limited public awareness,
rendering it prone to misdiagnosis and underdiagnosis, including
UMCD. Initially characterized as hyaline-vascular type, plasma cell
type, and mixed cellularity type, CD encompasses a spectrum of
histopathological presentations (3). In clinical practice, CD is further
categorized based on the origin or quantity of affected lymph nodes
into MCD and UCD.
According to studies, the incidence rates of MUD and
UCD varies greatly in different regions. For example, in the United
States, an estimated 6,500-7,700 cases of Castleman's disease are
reported annually, with 75% attributed UCD. Conversely, in Japan,
while a comparable overall incidence of Castleman's disease has
been documented, UCD represents 30% of cases, with MCD comprising
the remaining 70%. The underlying rationale for this significant
inter-regional variation remains unclear (4,5). One
possible reason is that there are very few case series at present.
These data have been provided by guidelines, including the
diagnostic criteria and classification collaborative network
developed for Castleman's disease since 2017, which have
statistical biases. Another possible reason is that the evaluation
of CD was limited by the lack of a specific ICD code or
evidence-based consensus diagnostic criteria until a specific
ICD-10 code for CD (ICD-10-CM D47.Z2) was created in 2016, and
diagnostic criteria for iMCD were established in 2017(6).
Significant disparities exist in the incidence rate,
clinical manifestations and treatment methods between MCD and UCD.
In the United States, the estimated incidence of UCD is ~16 cases
per million PYs. UCD occurs in individuals of all ages, with the
median age of onset occurring in the fourth decade, and no
discernible sex predilection (7).
Conversely, in Japan, the estimated incidence ranges between
0.6-4.3 cases per million PYs (1).
The regional difference in the cause of MCD in Japan is
hypothesized to be potentially associated with the relatively low
prevalence of Kaposi's sarcoma-associated herpesvirus in Japan
compared to other nations.
According to the study by Wojtyś et al
(8) UCD predominantly manifests in
the mediastinum, whereas MCD typically presents as generalized
lymphadenopathy. In fact, UCD is generally characterized by a
uniform phenotype, often appearing as asymptomatic isolated lymph
nodes or symptomatic manifestations related to mass effect
(3). Symptoms in UCD patients are
sparsely reported, with a minority experiencing pale complexion,
abdominal discomfort, chest pain, fatigue, anorexia and growth
retardation, while the majority are asymptomatic. Furthermore, UCD
predominantly manifests in anatomical sites such as the
mediastinum, lungs, neck, axilla, pelvis and retroperitoneum, with
mesenteric involvement being exceptionally rare. Overall,
peripherally located masses are readily discernible, facilitating
prompt medical attention, reducing diagnostic delays and decreasing
the likelihood of symptomatic presentation (9,10).
Therefore, the diagnosis of UCD poses increased challenges, with
higher rates of misdiagnosis and delayed diagnosis.
In a study by Hu et al (11), the median diagnostic delay for
patients with UCD was reported as 6 months for asymptomatic
individuals and 4.5 months for symptomatic cases. In the case of
our patient, who presented with abdominal pain and bloating
persisting for two months without a definitive diagnosis, diligent
evaluation ultimately revealed a space-occupying lesion in the
mesentery. Subsequent postoperative histopathological analysis
confirmed the diagnosis of UMCD.
One reason for misdiagnosis is the patient's
relative obesity, which can obscure the identification of abdominal
masses. Additionally, insufficient attention to abdominal findings
by both patients and healthcare providers during evaluations can
lead to diagnostic oversights. In the present case, meticulous
physical examination and use of CT imaging proved instrumental in
promptly identifying the scope of lesion in the patient, although
the etiology cannot be fully determined. Therefore, emphasizing
physical examination and necessary auxiliary examinations can
greatly reduce the misdiagnosis rate of UMCD.
Treatment considerations
Given the rarity and complexity of CD, treatment
strategies remain a subject of ongoing debate, particularly in the
case of UMCD. Individualized treatment approaches for CD are
contingent upon the specific disease subtype. The prevailing
consensus among experts advocates curative surgery as the primary
therapeutic modality for UCD, whereas MCD necessitates monoclonal
antibody-based immunotherapy (10,11).
Patients with MCD had diagnostic partial surgical excision of the
lesions, followed by a range of treatment modalities such as
corticosteroids, chemotherapy, radiotherapy and immunomodulatory
agents, or adopt a ‘watch and wait’ approach (12,13).
Notably, anti-interleukin-6 therapy with cetuximab is an important
treatment option for MCD, although its availability may be limited
and efficacy is observed in less than half of patients (14). Conversely, for UCD, surgical
intervention alone has demonstrated favorable outcomes without the
need for adjuvant therapies (15-17).
Complete surgical excision is the optimal therapy for resectable
UCD, yielding a 5-year overall survival rate >90% (18,19).
However, managing unresectable UCD poses significant
challenges. In rare instances where UCD is deemed unresectable due
to size and location constraints, initial therapeutic interventions
may render the lesion amenable to surgical resection through
medical approaches such as rituximab, steroids, radiotherapy, or
embolization (18,20,21).
In the present case, the presence of numerous indistinct mesenteric
blood vessels encircling a sizable lesion rendered complete
resection surgery technically challenging. As per existing
literature, therapy using rituximab, steroids and chemotherapy
emerged as a potentially more optimal strategy for addressing the
treatment needs of the present patient. However, the patient
declined a pathology-determining puncture procedure for the mass,
impeding the implementation of the aforementioned therapeutic
interventions. Moreover, the challenging nature of complete
excision was exacerbated by the unique location of the tumor.
Furthermore, the absence of published systematic studies assessing
the optimal management of unresectable UCD further complicates the
treatment decision-making process in such cases. Nevertheless, due
to the compression of adjacent structures causing symptoms in the
patient, surgery emerged as the preferred treatment modality. After
thorough deliberation and obtaining informed consent from the
patient and their family, a relatively comprehensive resection
surgery was performed. Subsequent to the postoperative pathological
findings, further treatment was recommended to mitigate the risk of
recurrence. Despite the patient's decision to undergo only three
cycles of CHOP chemotherapy (22,23)
and refusal of additional interventions, the 5-year follow-up
revealed complete recovery without any recurrence or metastasis.
Therefore, it may be asserted that surgery remains the primary
treatment choice for similar conditions, with chemotherapy and
immunotherapy serving as beneficial adjunctive options for patients
with specific indications.
Further research is warranted in UMCD patients,
particularly those who have undergone complete excision and exhibit
normal laboratory markers. We aim for our experience to contribute
to the breadth of UMCD literature, aiding healthcare providers in
determining the optimal therapeutic approach for their patients
(18).
Due to the special location of UMCD, its
preoperative diagnosis is difficult and the optimal treatment is
still controversial. The prevalent instances of delayed diagnosis
and misdiagnosis underscore a deficiency in comprehending the
disease's etiology and characteristics, essential for the
development of novel therapies. Diagnostic modalities such as CT
imaging and pathological examination are pivotal in confirming the
diagnosis of UMCD. The present study supported surgery as the
preferred treatment modality for UMCD, with chemotherapy and
immunotherapy offering additional benefits for patients with
indications.
Acknowledgements
The authors would like to thank Professor Zhong
Ming, Department of Pathology, Qingdao University Affiliated Tai'an
Central Hospital, China, for his comments and suggestions.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LH was responsible for conceptualization, data
curation, investigation, validation, writing of the original draft,
reviewing and editing. ZY was responsible for writing, reviewing
and editing. RY was responsible for conceptualization, writing,
reviewing and editing. LH and RY confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted following the
Declaration of Helsinki. All treatment plans applied in the present
study were conducted in accordance with the standards of the Ethics
Committee of Huishan District People's Hospital of Wuxi City
(approval no. HYLL20240607001), China.
Patient consent for publication
The diagnosis and treatment process, as well as the
disclosure of data, have received fully informed oral consent from
the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Masaki Y, Kawabata H, Fujimoto S, Kawano
M, Iwaki N, Kotani T, Nakashima A, Kurose N, Takai K, Suzuki R and
Aoki S: Epidemiological analysis of multicentric and unicentric
Castleman disease and TAFRO syndrome in Japan. J Clin Exp Hematop.
59:175–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Castleman B, Iverson L and Menendez VP:
Localized mediastinal lymphnode hyperplasia resembling thymoma.
Cance. 9:822–830. 1956.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dispenzieri A and Fajgenbaum DC: Overview
of Castleman disease. Blood. 135:1353–1364. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Simpson D: Epidemiology of Castleman
disease. Hematol Oncol Clin North Am. 32:1–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Oksenhendler E, Boutboul D, Fajgenbaum D,
Mirouse A, Fieschi C, Malphettes M, Vercellino L, Meignin V, Gérard
L and Galicier L: The full spectrum of Castleman disease: 273
Patients studied over 20 years. Br J Haematol. 180:206–221.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cohen AB, Swaminathan A, Wang X, Zamora S,
Repucci MA, Kelly J, Hannan MD, Sepler D, Sridharma S, Orlando A,
et al: Clinical characteristics, treatment patterns, and overall
survival of real-world patients with idiopathic multicentric
Castleman disease. J Clin Oncol. 39 (Suppl 15)(S7048)2021.
|
|
7
|
Boutboul D, Fadlallah J, Chawki S, Fieschi
C, Malphettes M, Dossier A, Gérard L, Mordant P, Meignin V,
Oksenhendler E and Galicier L: Treatment and outcome of Unicentric
Castleman disease: A retrospective analysis of 71 cases. Br J
Haematol. 186:269–273. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wojtyś M, Piekarska A, Kunc M, Ptaszyński
K, Biernat W, Zaucha JM, Waloszczyk P, Lisowski P, Kubisa B and
Grodzki T: Clinicopathological comparison and therapeutic approach
to Castleman disease-a case-based review. J Thorac Dis.
11:4859–4874. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Muhammad T, Alkheder A, Mazloum A, Almooay
A, Naziha L and Shaheen M: Unicentric Castleman disease: A case
report of an atypical presentation and successful management. Int J
Surg Case Rep. 118(109688)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Talat N, Belgaumkar AP and Schulte KM:
Surgery in Castleman's disease: A systematic review of 404
published cases. Ann Surg. 255:677–684. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu S, Li Z, Wang H, Chen L, Ma Y, Zhu X,
Li J, Dong R, Yao W, Dong C, et al: Clinical features and treatment
outcomes of Castleman disease in children: A retrospective cohort
in China. Eur J Pediatr. 182:5519–5530. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van Rhee F, Wong RS, Munshi N, Rossi JF,
Ke XY, Fosså A, Simpson D, Capra M, Liu T, Hsieh RK, et al:
Siltuximab for multicentric Castleman's disease: A randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 15:966–974.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang L, Zhao AL, Duan MH, Li ZY, Cao XX,
Feng J, Zhou DB, Zhong DR, Fajgenbaum DC and Li J: Phase 2 study
using oral thalidomide-cyclophosphamide-prednisone for idiopathic
multicentric Castleman disease. Blood. 133:1720–1728.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Carbone A, Borok M, Damania B, Gloghini A,
Polizzotto MN, Jayanthan RK, Fajgenbaum DC and Bower M: Castleman
disease. Nat Rev Dis Primers. 7(84)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mitsos S, Stamatopoulos A, Patrini D,
George RS, Lawrence DR and Panagiotopoulos N: The role of surgical
resection in Unicentric Castleman's disease: A systematic review.
Adv Respir Med. 86:36–43. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang MY, Jia MN, Chen J, Feng J, Cao XX,
Zhou DB, Fajgenbaum DC, Zhang L and Li J: UCD with MCD-like
inflammatory state: Surgical excision is highly effective. Blood
Adv. 5:122–128. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
van Rhee F, Oksenhendler E, Srkalovic G,
Voorhees P, Lim M, Dispenzieri A, Ide M, Parente S, Schey S,
Streetly M, et al: International evidence-based consensus
diagnostic and treatment guidelines for unicentric Castleman
disease. Blood Adv. 4:6039–6050. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Abraham SS, Narayanan G, Thambi SM,
Vasudevan JA, Joy Philip DS, Purushothaman PN, Nair SG and Nair R:
Castleman disease: Experience from a single institution. Med Int
(Lond). 3(56)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mohan M, Meek JC, Meek ME, Broadwater R,
Alapat D and van Rhee F: Combinatorial treatment for unresectable
unicentric Castleman disease. Eur J Haematol. 107:484–488.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhu SH, Yu YH, Zhang Y, Sun JJ, Han DL and
Li J: Clinical features and outcome of patients with HIV-negative
multicentric Castleman's disease treated with combination
chemotherapy: A report on 10 patients. Med Oncol.
30(492)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Seo HY, Kim EB, Kim JW, Shin BK, Kim SJ
and Kim BS: Complete remission in a patient with human herpes
virus-8 negative multicentric Castleman disease using CHOP
chemotherapy. Cancer Res Treat. 41:104–107. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
van Rhee F and Stone K: Storming the
Castle with TCP. Blood. 133:1697–1698. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kaneko H, Ohkawara T, Aragane H, Ohkawara
Y and Taniwaki M: Clinicopathological analysis of a case with
mesenteric solitary Castleman's disease: Diagnostic value of
radiological findings. Int Surg. 92:272–275. 2007.PubMed/NCBI
|