Introduction
The coronavirus disease-19 (COVID-19) pandemic has
been considered as very important health issue between 2020 and
2023. Therefore, several studies have focused on characterizing the
sequences of Severe Acute Respiratory Syndrome Coronavirus 2
(SARS-CoV-2) infection and developing novel therapeutic options
(1-3).
Despite the development, validation, approval and worldwide use of
anti-SARS-CoV-2 vaccines (4,5),
specific anti-viral agents targeting the life cycle of SARS-CoV-2
and improving COVID-19-related clinical manifestations are still
needed (6-9).
In this respect, the majority of patients with COVID-19 show
moderate symptoms; however, a consistent number of patients develop
severe COVID-19, which is generally characterized by a
hyperinflammatory state (10-13).
Additionally, inflammation is considered as a significant feature
of ‘long COVID-19’ (14) and
post-acute sequelae of SARS-CoV-2 infection (15).
It has been clearly shown that following lung
infection, SARS-CoV-2 promotes immune responses, which are
characterized by the high production of inflammatory cytokines.
This event, also known as ‘cytokine storm’ (16,17),
can occur due to the activation of several transcription factors,
such as nuclear factor kappa-B (NF-κB) and signal transducer and
activator of transcription 3 (STAT-3), which in turn regulate the
expression of numerous inflammation-related genes, including
vascular endothelial growth factor (VEGF), monocyte chemoattractant
protein 1 (MCP-1), interleukin-8 (IL-8) and IL-6 (18-21).
Among the proteins which have been proposed to play a key role in
the ‘cytokine storm’ phenomenon in patients with COVID-19, IL-1α,
IL-1β, IL-2, IL-6, IL-7, IL-8, IL-9, IL-10, basic fibroblast growth
factor, granulocyte-macrophage colony-stimulating factor (GM-CSF),
G-CSF, platelet-derived growth factor, VEGF, interferon gamma,
interferon-inducible protein 10, MCP-1, macrophage inflammatory
protein 1-alpha (MIP-1-α), MIP-1-β and tumor necrosis factors
(TNFs) are the most studied ones (22).
Examples of clinical trials on IL-6- and
IL-8-targeting drugs for the treatment of patients with COVID-19
are summarized in Table I. These
trials supported the concept that this approach could be considered
for the development of possible therapeutic strategies against
COVID-19. The above speculation has been also sustained by several
published reports, such as those by Comarmond et al
(23), Ghosn et al
(24), Pomponio et al
(25) and Castelnovo et al
(26). Therefore, Comarmond et
al (23) reported that
patients suffering from inflammatory rheumatic and musculoskeletal
diseases and treated with anti-IL6 therapy based on tocilizumab or
sarilumab, displayed a less severe form of COVID-19. Although the
association between COVID-19 outcomes and the duration of
pre-existing anti-IL6 therapy was not analyzed in depth, the
results of the study suggested that the anti-IL6 therapy was
associated with less frequent hospitalization and need for
respiratory support (23).
Additionally, the pilot study by Pomponio et al (25) indicated that the efficacy of
tocilizumab, an anti-IL6 therapy, could improve the respiratory
function of patients. However, this result warrants further
investigation in randomized trials. Castelnovo et al
(26) collected data from patients
with COVID-19 treated with the anti-IL6 drugs, tocilizumab and
sarilumab. The analysis revealed that the anti-IL6 drugs could be
effective in treating the severe forms of COVID-19. Therefore, it
was hypothesized that these drugs could reduce inflammation and the
risk of multi-organ failure-related mortality (26). Furthermore, a meta-analysis by
Tharmarajah et al (27)
analyzed the outcomes of IL-6 inhibition in the treatment of
COVID-19. In general, the aforementioned studies on anti-IL6
therapies suggested that despite anti-IL6 monotherapy could display
several benefits, this approach could be of great interest if
combined with other anti-inflammatory drugs. Previous large
clinical trials demonstrated that when tocilizumab was used in
combination with dexamethasone to treat severe COVID-19-associated
Acute Respiratory Distress Syndrome, provided significant clinical
improvement. In this respect, Segú-Vergés et al (28) employed artificial intelligence
approaches to evaluate the potential of tocilizumab in combination
with corticosteroid therapy in treating COVID-19. This in
silico study suggested that the administration of tocilizumab
and dexamethasone could induce a synergistic effect, thus strongly
reducing inflammation and associated pathological processes,
eventually supporting the beneficial effects of the combined
therapy in patients with COVID-19 in critical clinical
conditions.
 | Table IExamples of clinical trials for
COVID-19 based on IL-6 and IL-8 targeting. |
Table I
Examples of clinical trials for
COVID-19 based on IL-6 and IL-8 targeting.
| Clinical trial
number | Title | Bioactive
molecule |
|---|
| NCT04381052 | Study for the Use
of the IL-6 Inhibitor Clazakizumab in Patients With
Life-threatening COVID-19 Infection | Clazakizumab |
| NCT04343989 | A Randomized
Placebo-controlled Safety and Dose- finding Study for the Use of
the IL-6 Inhibitor Clazakizumab in Patients With Life-threatening
COVID-19 Infection | Clazakizumab |
| NCT04322773 | Anti-IL6 Treatment
of Serious COVID-19 Disease with Threatening Respiratory
Failure | Two human
monoclonal antibodies against IL-6 receptor, tocilizumab and
sarilumab |
| NCT04486521 | Clinical Outcome of
Anti-IL6 vs. Anti-IL6 Corticosteroid Combination in Patients With
SARS-CoV-2 Cytokine Release Syndrome | Cortocosteroid
combinations |
| NCT04347226 | Anti-Interleukin-8
for patients with COVID-19 | BMS-986253 |
| NCT04878055 | Study on Efficacy
and Safety of Reparixin in the Treatment of Hospitalized Patients
With Severe COVID-19 Pneumonia | Reparixin |
Currently, less studies have been published
regarding the effect of anti-IL-8 therapies against COVID-19. Two
IL-8 inhibitors are being currently evaluated for the treatment of
COVID-19. HuMax-IL-8 (BMS986253), a human monoclonal antibody
targeting IL-8, was studied for the first time on several types of
cancer (29). Reparixin, an
allosteric inhibitor of IL-8 activity, has been investigated for
its safety and efficacy in patients with severe COVID-19 pneumonia
(30). Promising results were
obtained in a phase II trial (REPAVID-19). Reparixin was also found
to effectively improve biochemical and biophysical parameters in a
murine model of liposaccharide (LPS)-induced acute lung injury
(31). A murine model of COVID-19
was also employed and the results demonstrated a clear clinical
improvement following IL-8-like signaling inhibition with reparixin
(32). Taken together, these
studies supported that IL-8 could be considered as a promising
therapeutic target in the treatment of severe COVID-19 and
therefore more anti-IL-8 therapies should be developed in basic and
applied research.
MicroRNAs (miRNAs/miRs) are short, non-coding RNAs,
19-25 nucleotides in length, which are involved in regulating gene
expression at the post-transcriptional level. Commonly, miRNAs act
via specifically binding to the 3'-untraslated region (3'-UTR) of
their target mRNAs, thus promoting mRNA degradation (33-37).
Importantly, a single miRNA can target several mRNAs, while the
3'-UTR of a single mRNA can encompass several sequences recognized
by different miRNAs. It has been reported that >60% of mammalian
mRNAs can be targeted by miRNAs (37). A previous study supported that the
release of key proteins of the COVID-19-related ‘cytokine storm’
could be strongly inhibited via mimicking the biological activity
of miRNAs (38). In the above
study, it was hypothesized that among possible anti-inflammatory
agents, ‘miRNA targeting’ should be carefully considered as a
significant strategy (38).
Therefore, the general working hypothesis was that targeting the
miRNA network could serve a significant role for the development of
therapeutic approaches against the SARS-CoV-2-induced
pro-inflammatory responses. This hypothesis was based on several
publications, showing that miRNA overexpression could inhibit the
expression of ‘cytokine storm’-related proteins in
COVID-19(38). The above study
demonstrated that a molecule mimicking miR-93-5p could modulate the
expression of IL-8 gene via targeting its RNA transcript. The first
evidence of the effect of miR-93-5p on regulating IL-8 gene
expression was reported by Fabbri et al (39). This study demonstrated that
miR-93-5p could bind to the IL-8 3'-UTR. In addition, it was found
that the binding sites of miR-93-5p on IL-8 3'-UTR were conserved
within different species, thus supporting its molecular evolution
(39). Accordingly, Gasparello
et al (40) showed that the
treatment of bronchial epithelial IB3-1 cells with the SARS-CoV-2
spike protein enhanced the secretion of IL-8. In addition, IL-8
synthesis and extracellular release could be strongly reduced by a
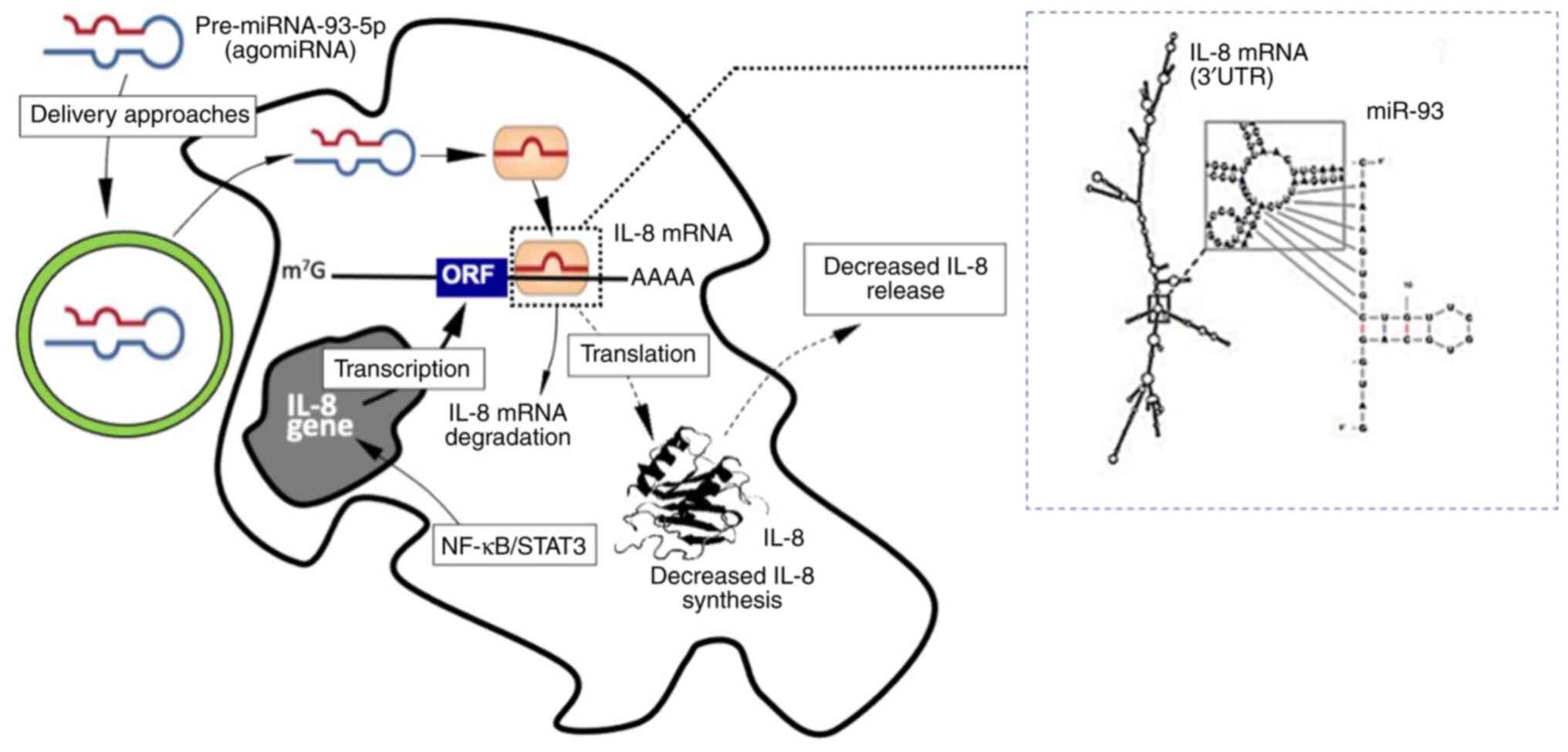
molecule mimicking miR-93-5p. The effect of miR-93-5p on directly
affecting IL-8 expression is presented in Fig. 1. This figure illustrates the
possible interaction between miR-93-5p and the 3'-UTR of IL-8 RNA,
and the expected (experimentally validated) effects of miR-93-5p on
IB3-1 cells (39,40). This study also revealed that other
genes coding for proteins involved in the COVID-19 ‘cytokine storm’
are regulated by miR-93-5p, including IL-6, G-CSF, GM-CSF and IL-1β
(40). More particularly, the
results of the above study demonstrated that the expression of
these genes was upregulated and downregulated following cell
transfection with anti-miR-93-5p and pre-miR-93-5p, respectively,
thus highlighting the key role of miR-93-5p in regulating the
expression of these genes (40).
Fabbri et al (39) showed that the inhibitory effect of
ago-miRNAs on the expression of pro-inflammatory genes was mediated
by the interaction of these ago-miRNAs with the 3'-UTR of the
target pro-inflammatory mRNAs. This finding was also verified in
the miR-93-5p/IL-8 axis. In addition, the ago-miRNA molecule could
interact and down-regulate the expression of other target
pro-inflammatory genes. Furthermore, all IL-1α, IL-6 and IL-8 genes
are upregulated by NF-κB. Eventually, all ago-miRNAs mimicking the
biological activity of miRNAs on downregulating NF-κB, such as that
of miR-130a, miR-146a/b, miR-218 and miR-451, could also inhibit
the expression levels of NF-κB and other NF-κB-regulated genes
(41). The focus of applied
research on miRNA therapeutics has been discussed in several review
articles, describing the results of both pre-clinical and clinical
studies (42-45).
However, a general and clear consensus on the potential biomedical
applications of this approach is still missing (44,45).
In addition, clinical studies using both PROOFS-FIG2 anti-miRNA
(46) and miRNA mimicking
(47) molecules are ongoing.
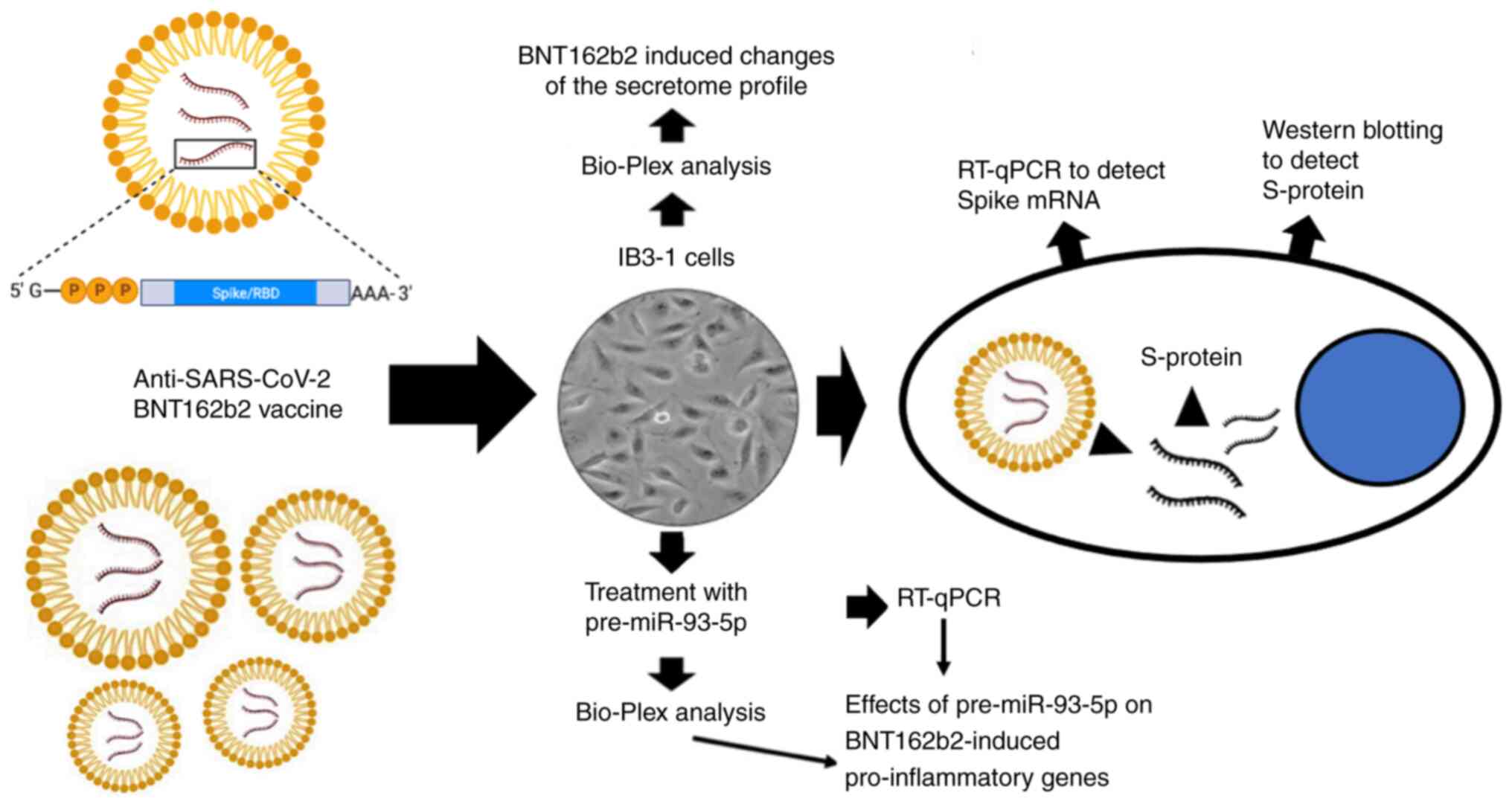
The experimental system, which could be employed to
study the effects of potential inhibitors on the expression of
pro-inflammatory genes is described in Fig. 2. According to the above
experimental system, the upregulation of pro-inflammatory genes
could be achieved following treatment of target cell lines, and
particularly bronchial epithelial IB3-1 cells, with the spike
RNA-based BNT162b2 vaccine. The approach was the same with that
described in other experimental model systems, which aimed to
uncover the effects of the BNT162b2 vaccine on the gene expression
and functions of eukaryotic cells (48,49).
The most significant characteristics of this approach are the
following: i) The cellular uptake of spike mRNA could upregulate
and promote the release of S-protein; and ii) the resulted NF-κB
upregulation, could be also associated with the increased
expression of other NF-κB-regulated genes, including those coding
proteins associated with ‘cytokine storm’ and those involved in the
hyperinflammation state of other human diseases, such as cystic
fibrosis. Regarding the roles of the BNT162b2 vaccine in the
expression of NF-κB and NF-κB-dependent genes, it should be noted
that according to the study by Gasparello et al (50) on the effects of purified SARS-CoV-2
S-protein on IB3-1 cells, these should be expected. In this
respect, BNT162b2 vaccine is composed of a liposomal vaccine vector
which is used to carry the spike mRNA. Since lipid-based
nanoparticles are known to stimulate inflammatory responses through
endosomal Toll-like receptor (TLR)-7 and TLR-8 pathways (51-53),
it was suggested that both lipid formulation and spike mRNA are
involved in the activity of BNT162b2 on regulating the expression
of pro-inflammatory genes. In any case, in the experiments based on
the use of the BNT162b2 vaccine on IB3-1 cells, the effects of the
anti-inflammatory compounds could be studied. Interestingly, in the
majority of cases, the inducing effects of BNT162b2 on IB3-1 cells
were more potent compared with those of other control stimuli, such
as SARS-CoV-2 spike (40,50), Pseudomonas aeruginosa
infection (39), TNF-a (54,55)
and LPS (56). Although the
experimental condition may vary in these studies, the data
available strongly suggest that the BNT162b2 treatment of IB3-1
cells is an efficient strategy to induce the expression of
pro-inflammatory genes.
A previous study by Gao et al (57) indicated that miR-93-5p could
suppress inflammation in an LPS-induced acute lung injury mouse
model via targeting the TLR4/myeloid differentiation factor 88
(MyD88)/NF-κB signaling pathway. In the above study, a luciferase
construct, composed of a partial sequence of the TLR4 3'-UTR,
encompassing the binding sites of miR-93, and sub-cloned into a
dual-luciferase reporter vector (pmirGLO), was used to verify that
TLR4 was a direct target of miR-93-5p (57). The results demonstrated that
RAW264.7 cell transfection with miR-93 mimics inhibited the
luciferase activity directed by the TLR4-3'UTR reporter plasmid. By
contrast, cell co-transfection with a miR-93 inhibitor enhanced
luciferase activity. These findings were consistent with those
reported by Fabbri et al (39). This study investigated the effects
of miR-93-5p on luciferase activity using a vector carrying the
3'-UTR of human IL-8 mRNA. In both studies, the control experiments
demonstrated that there were no changes in luciferase activity in
cells transfected with mutant TLR4(57) and IL-8(39) 3'-UTRs, lacking the miR-93 binding
sites, thus indicating that there was a direct interaction between
miR-93 and the 3'-UTR of IL-8 and TLR4 (39,57).
The possible effects of miR-93-5p on TLR4 are of
great significance in applied research, regarding its possible role
as inhibitor of SARS-CoV-2 infection and SARS-CoV-2-associated
pro-inflammatory gene expression. In fact, several previous studies
showed that the SARS-CoV-2 spike protein could activate TLR4, which
in turn promoted the synthesis of proinflammatory cytokines
(58-60).
In this context, the molecular effect of TLR4 in COVID-19 should be
considered as a significant regulatory factor of SARS-CoV-2
infection (61). The interaction
between SARS-CoV-2 spike protein and TLR4, and its activation, was
also reported by Zhao et al (62) and Patra et al (63). The above studies suggested that the
binding of SARS-CoV-2 spike protein to TLR4, resulting in its
activation, upregulates angiotensin-converting enzyme 2, thus
promoting virus entry and hyperinflammation. These studies
supported that targeting human TLRs could be a strategy to combat
COVID-19. Accordingly, Das et al (64) performed in silico
experiments to identify novel anti-SARS-CoV-2 agents from bioactive
phytocompounds targeting the viral spike glycoprotein and human
TLR4. The above study analyzed a group of 30 phytocompounds,
previously demonstrated to retain antiviral activity. These
compounds were theoretically screened, using molecular docking, MD
simulations and ADME analysis, for their binding efficacy to
SARS-CoV-2 spike protein and TLR4. The in silico results
showed that cajaninstilbene acid and papaverine could block human
TLR4, thus suggesting that these phytocompounds could mitigate
SARS-CoV-2-induced proinflammatory responses (64). The significance of this approach
was also verified by Sahanic et al (65), reporting that SARS-CoV-2 could
activate the TLR4/MyD88 pathway in human macrophages, thus
indicating that this effect could be associated with strong
pro-inflammatory responses in severe COVID-19. Accordingly,
Nakazawa et al (66) found
that SARS-CoV-2 could directly impair renal cells via promoting
pro-inflammatory responses, while inhibition of TLR4 and IL-1R
could prevent SARS-CoV-2 mediated kidney injury. The interplay
between TLR4 and SARS-CoV-2, which is involved in the complex
mechanisms of inflammation and severity in COVID-19 infections, has
been recently reviewed by Asaba et al (67). This study provided novel insights
into the unique role of TLR4 in SARS-CoV-2 infection, thus
highlighting its potential in the development of novel treatment
strategies for COVID-19.
Materials and methods
Materials
All chemicals and reagents were of analytical grade.
Aged Garlic Extract (AGE) was obtained by Wakunaga Pharmaceutical
Co., Ltd. (Hiroshima, Japan). The Pre-miR™ miRNA
Precursor (PM10951, hsa-miR-93-5p), and the Pre-miR™
miRNA Precursor Negative Control #1 were both purchased from
Ambion-ThermoFisher (cat no. AM17100 and cat no. AM17110,
respectively).
Cell culture
Human bronchial epithelial IB3-1 cells (40,50)
were cultured as previously described (39). Cells were transfected with 100 nM
hsa-miR-93-5p precursor and pre-miR-93 negative control (NEG93)
(40) using the Lipofectamine
RNAiMAX Transfection Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The AGE powder used in our experiments was prepared by
lyophilization. The concentration of AGE employed in this study was
0.5 mg/ml, in agreement with the study by Gasparello et al
(68). The AGE powder was freshly
dissolved in complete cell culture medium prior to each
experiment.
Treatment of IB3-1 cells with the
BNT162b2 vaccine
The BNT162b2 vaccine (Comirnaty™; Lot.
no. FP8191) was obtained from the Hospital Pharmacy of the
University of Padova. For treatment with the BNT162b2 vaccine,
IB3-1 cells were seeded at a density of 200,000 cells/ml and were
then treated with 0.5 µg/ml of the vaccine for 24 h (48,49).
When needed, cells were transfected with pre-miR-93-5p and/or
co-treated with AGE.
mRNA quantitative analyses
For the quantification of the relative mRNA
expression levels, total RNA was reverse transcribed into cDNA, as
described by Gasparello et al (40). Quantitative (q)PCR analysis for
IL-6 (assay ID, Hs.PT.58.40226675), IL-8 (assay ID,
Hs.PT.58.38869678.g) and IL-1β (assay ID, Hs.PT.58.1518186) was
performed using the corresponding assay kits, as previously
described (40). PCR amplification
was carried out as previously described using the CFX96 Touch
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.)
(40,69). RT-qPCR analysis for BNT162b2 Spike
mRNA was performed according to the study by Fertig et al
(70) (as reported in Data S1). Relative IL-8 gene expression
was calculated using the comparative cycle threshold method
(ΔΔCq method) and the endogenous control human β-actin
was used as normalizer, described previously (39).
Statistical analysis
The differences among different groups were compared
using one-way ANOVA (analyses of variance between groups,
http://vassarstats.net/anova1u.html).
Prism (v. 9.02) by GraphPad software was also employed (followed by
Bonferroni's post-hoc tests). Differences were considered
statistically significant when P<0.05 and highly significant
when P<0.01 (1,4,68,70).
Results
Effect of BNT162b2 vaccine on NF-κB
expression
The BNT162b2-treated IB3-1 bronchial cell model is
characterized by the BNT162b2-induced expression of
pro-inflammatory genes with a higher efficiency compared with
SARS-CoV-2 spike protein (40). As
a control, in this model spike protein is produced following cell
treatment with the BNT162b2 vaccine. The intracellular content of
SARS-CoV-2 spike protein should be increased based on the
concentrations of BNT162b2 used to treat IB3-1 cells. Herein, a
significant increase (P<0.01) in the content of intracellular
BNT162b2 Spike mRNA was observed following cell treatment with 1
µg/ml BNT162b2 vaccine for 24 h (Fig.
S1). Quantitative RT-qPCR analysis for BNT162b2 Spike mRNA was
performed according to the study by Fertig et al (70). In this study, primers were designed
to be specific to the publicly available, codon-optimised vaccine
mRNA sequence (70). Fully in
agreement with the RT-qPCR data shown in Supplementary Fig. S1, full-length S-protein was
detected by western blot analysis (data not shown). Since previous
studies demonstrated that S-protein could promote pro-inflammatory
gene expression via activating the NF-κB pathway in several
cellular systems (69,71,72),
in the present study NF-κB was upregulated in IB3-1 cells treated
with the BNT162b2 vaccine (data not shown).
AGE as a possible anti-inflammatory
agent for tackling ‘cytokine storm’ in COVID-19
The AGE powder used in our experiments was prepared
by lyophilization. The concentration of AGE employed in this study
was 0.5 mg/ml, in agreement with the study by Gasparello et
al, who found that this concentration is optimal to obtain
inhibition of pro-inflammatory genes in bronchial epithelial IB3-1
cells stimulated with either the SARS-CoV-2 S-protein or the
BNT162b2 vaccine (68). The AGE
powder was freshly dissolved in complete cell culture medium prior
to each experiment. The analysis of the organosulfur compound
content in the AGE powder was performed using a GC-MS
chromatography and has been reported by Gasparello et al
(68). The relative expression
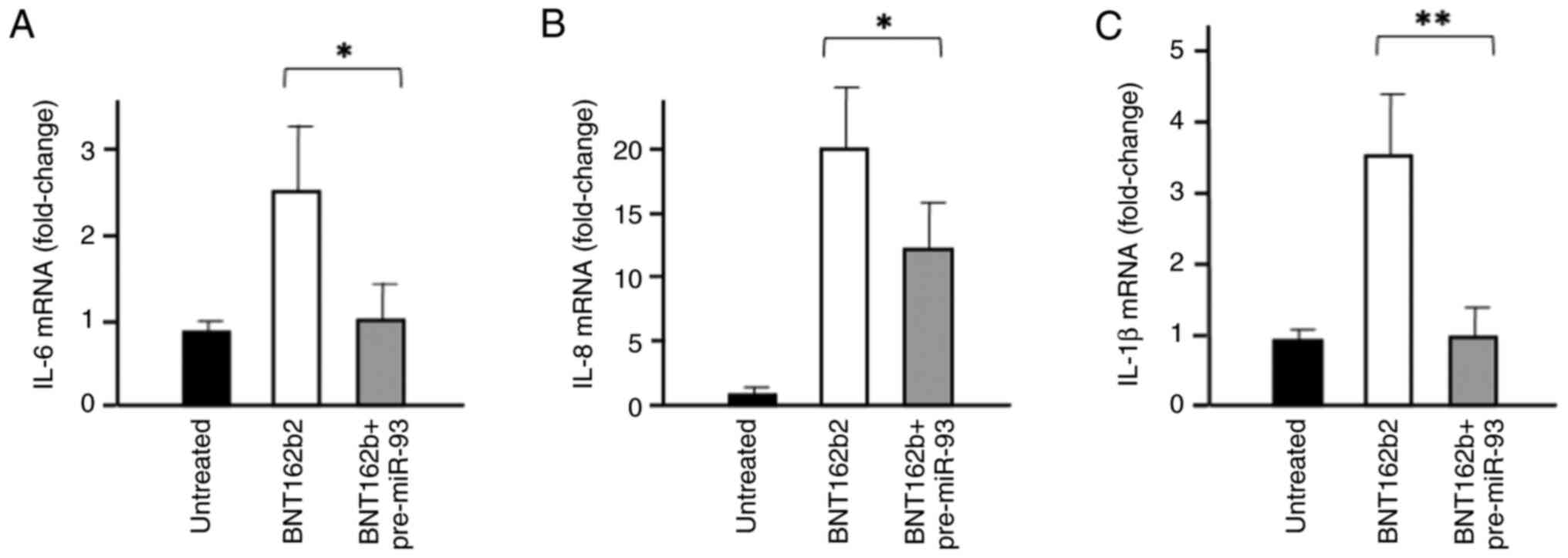
levels of the pro-inflammatory genes, IL-6, IL-8 and IL-1β, were
detected after 3 days treatment by reverse transcription-qPCR. As
shown in Fig. 3, the mRNA
expression levels of the aforementioned cytokines were increased
following IB3-1 cell treatment with 0.5 µg/ml BNT162b2 (white
bars). However, cell transfection with 100 nM pre-miR-93-5p
displayed the opposite effect (grey bars).
AGE as a potential anti-inflammatory
agent for tackling ‘cytokine storm’ in COVID-19: A pilot study
exploring the effects of a combined treatment with
pre-miR-93-5p
The present study aimed to explore the possible
effects of the combination of pre-miR-93 and AGE in mitigating
‘cytokine storm’ in COVID-19, since a previous study demonstrated
that the AGE constituent S-allyl-cysteine (SAC) could interact with
TLR4(71). For example, docking
analysis performed by Gasparello et al (68), predicted that the SAC/TLR4
interaction was based on hydrogen bonds, involving His685, Tyr657,
Ser659 and Arg722. This effect could affect, at least in principle,
TLR4 activity, since TIR domain is essential for NF-κB activation
(73-76).
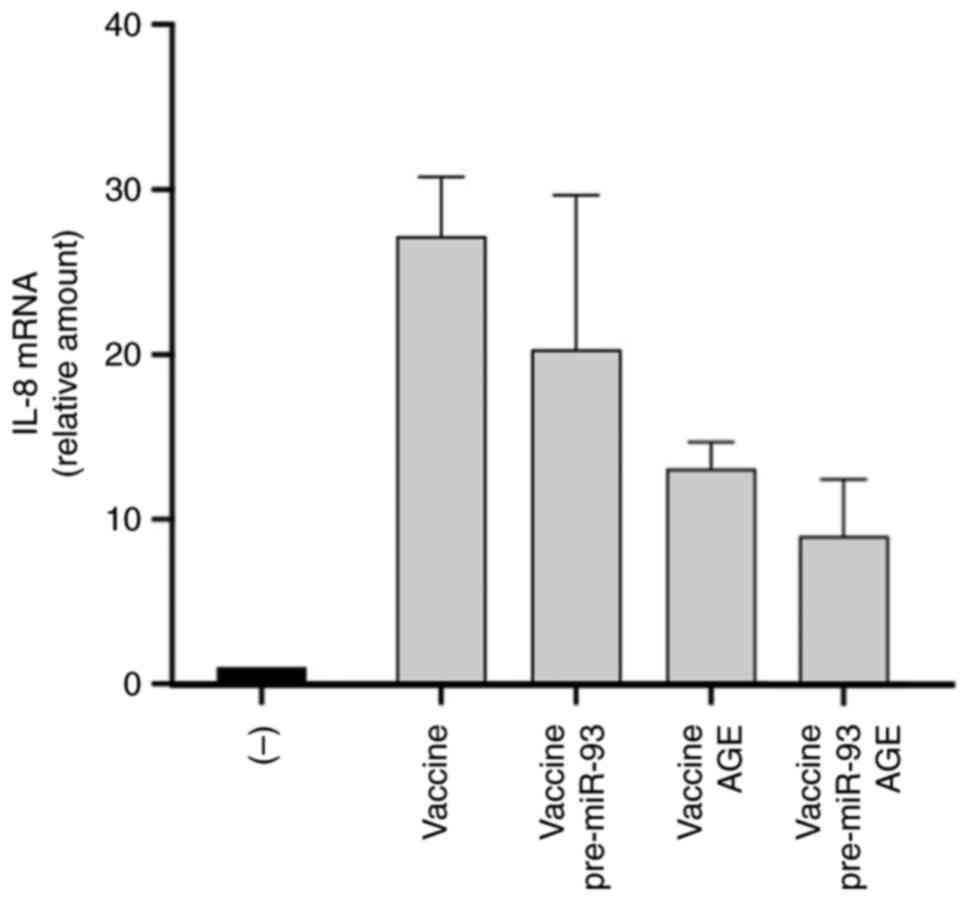
The results of the pilot experiments, where cells were co-treated
with AGE and pre-miR-93-5p, are shown in Fig. 4. In preparing this experiment, we
first verified the transfection efficiency of
Lipofectamine-delivered pre-miR-93-5p, according with the procedure
described by Gasparello et al (40); this validation was performed by
RT-qPCR analysis of the miR-93-5p sequences accumulated in
pre-miR-93-5p transfected IB3-1 cells. The list of the assays
employed for miRNA detection is reported in Table SI. A representative experiment is
shown in Supplementary Fig. S2.
We always found high transfection efficiency similar to that shown
in Fig. S2, with no exception.
Another important control was the formal demonstration of the
specificity of the effects of transfection of IB3-1 cells with the
pre-miR-93-5p. This was demonstrated by comparing the effects of
pre-miR93-5p to those of the pre-miR93 negative control, as
reported in the representative experiments shown in Fig. S3 and in Table SII. The results concerning the
effects of a combined treatment based on AGE and pre-miR-93-5p is
shown in Fig. 4. The results
obtained revealed a sharp increase in IL-8 gene expression levels
following IB3-1 cell treatment with the BNT162b2 vaccine for three
days. However, the BNT162b2-induced IL-8 gene expression was
reduced after treatment of cells with pre-miR-93-5p, AGE or
pre-miR-93-5p plus AGE. Interestingly, the inhibition of IL-8 gene
expression was more potent in cells treated with the combination of
pre-miR-93-5p and AGE.
Effects of miRNA therapeutics combined
with approaches based on the use of natural products: Proposed
mechanism of action of miR-93-5p and future perspectives
The extent of complementarity between miR-93-5p and
the 3'-UTRs of TLR4 and IL-8 mRNAs are shown in Fig. 5A (39,57).
The number of hydrogen bonds generated between miR-93-5p and the
3'-UTRs of TLR4 and IL-8 mRNAs were very similar, thus supporting
that both TLR4 and IL-8 mRNAs could be direct targets of miR-93-5p.
In addition, miR-93-5p could significantly affect NF-κB signaling
via directly interacting with IL-1 receptor-associated kinase 4
(IRAK4), a kinase known to activate NF-κB in the TLR signaling
pathways (77,78). Accordingly, the possible mechanism
of action of miR-93-5p is depicted in Fig. 5B. In this theoretical model, the
mRNA expression levels of IL-8 could be directly inhibited by
miR-93-5p, as suggested by Fabbri et al (39). Additionally, IL-8 expression could
be indirectly suppressed via the inhibition of the TLR4/NF-κB
pathway. This finding could be due to the direct
miR-93-5p/TLR-4-3'-UTR-mediated TLR4 downregulation (57) and the direct interaction (and
consequent inhibition) with IRAK4(77). In this case, NF-κB inhibition
resulted in the inhibitory effects of miR-93-5p on NF-κB-regulated
genes, such as IL-1β and IL-6. Data sustaining the direct
interaction between miR-93-5p and the 3'-UTRs of IL-8, TLR4 and
IRAK4 have been also reported in the studies by Fabbri et al
(39), Gao et al (57) and Xu et al (77).
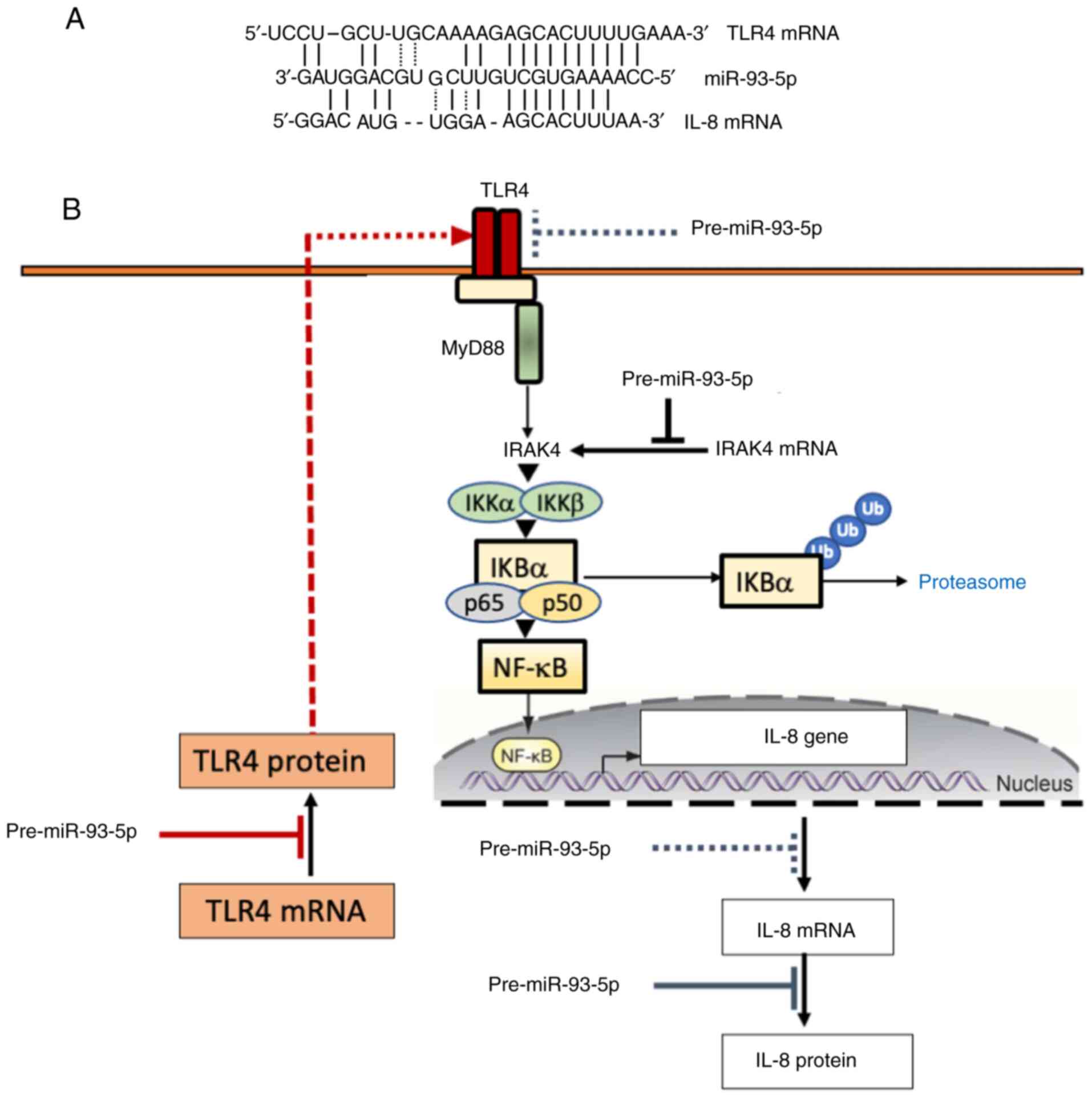 | Figure 5Binding of miR-93-5p to the 3'-UTR of
TLR4 and IL-8 mRNAs. (A) Interactions of miR-93-5p sequences with
TLR4 and IL-8 mRNAs (39,45). (B) Proposed mechanism of action of
miR-93-5p, leading to inhibition of IL-8 gene expression. The
proposed mechanism of action of miR-93-5p on TLR4 and IL-8 mRNAs is
based on the studies published by Gao et al (57) and Fabbri et al (39). In both cases, a luciferase
construct was used to demonstrate functional binding of miR-93-5p
to the target RNA sequences; the constructs were constituted by a
partial sequence of TLR4 3'-UTR or IL-8 3'UTR (both containing
miR-93 binding sites) cloned into the dual-luciferase reporter
vector pmirGLO (38,45). Control constructs containing
mutated miR-93 binding sites were employed, to demonstrate that the
effects found on luciferase activity were related to functional
miR-93 binding sites. The proposed mechanism of action of miR-93-5p
on IRAK4 mRNA is based on the studies published by Xu et al
(77) and Tian et al
(78). IL, interleukin; miR,
microRNA; TLR4, Toll-like receptor 4; UTR, untranslated region;
IRAK4, IL-1 receptor-associated kinase 4; NF-κB, nuclear
factor-κB. |
Discussion
The present study reported preliminary evidence
suggesting that the miR-93-5p based ‘miRNA therapeutics’ combined
with AGE could be considered as an efficient approach for
inhibiting IL-8 expression. This could be of significant interest,
since the TLR4/NF-κB-related inhibitory agents, such as miR-93-5p,
could be applied in the treatment of human pathologies associated
with a hyperinflammatory state, such as COVID-19 (45-49)
and cystic fibrosis (62,63), which are characterized by IL-8
upregulation.
Emerging evidence has suggested that IL-8, TLR4 and
IRAK4 can be directly targeted by miR-93-5p (39,57,77).
Accordingly, the proposed mechanism of action of miR-93-5p
(Fig. 5B), indicated that the
direct targeting of the IL-8 3'-UTR by miR-93-5p could inhibit IL-8
expression. At the same time, the indirect suppression of IL-8
expression could be achieved via blocking the TLR4/NF-κB pathway,
possibly through the direct interaction between miR-93-5p and IRAK4
or TLR4. Although several studies have supported the direct
interaction between miR-93-5p and the 3'-UTR of IL-8, TLR4 and
IRAK4 (39,57,77),
future studies are still needed to verify the possible interactions
of miR-93-5p with TLR4 and IRAK4 in the BNT162b2 based experimental
model system, particularly in cells also treated with pre-miR-93-5p
and AGE.
The most significant limitation of the current study
was that the expression levels of only a few pro-inflammatory genes
(IL-1β, IL-6 and IL-8) were detected in BNT162b2-treated IB3-1
cells and cells co-treated with both miR-93-5p mimics and AGE
(IL-8). Therefore, the present study should be considered as an
exploratory pilot study based on a proof-of-principle, suggesting
that a combined treatment with pre-miR-93-5p and AGE could be most
effective in inhibiting the expression of pro-inflammatory genes.
Future studies, focusing on more proteins involved in the ‘cytokine
storm’ in COVID-19 and on the hyperinflammation state of other
human pathologies, such as cystic fibrosis and chronic obstructive
pulmonary disease, should be performed. In addition, the current
preliminary study could promote the design of other experimental
studies on the effects of pre-miR-93-5p in combination with other
extracts from medicinal plants, including the bioactive
constituents of AGE (SAC and S1PC), on regulating IL-8 expression.
Although the BNT162b2 treated cells are not a stringent model
system for viral infection, the results of this study are expected
to stimulate experimental efforts to determine whether the
combination of pre-miR-93-5p and AGE exerts the same effects in
SARS-CoV-2-infected cells (68).
The preliminary data of the present study suggested
that pre-miR-93-5p could be considered as a double-acting agent,
since it could directly inhibit IL-8 mRNA translation and
indirectly block NF-κB signaling via inhibiting TRL4 and IRAK4.
Further studies on other pro-inflammatory genes could determine
whether the inhibitory effects of the pre-miR-93/AGE combined
treatment on IL-8 expression could be also generalized to other
genes involved in the COVID-19-related hyper-inflammatory state.
This experimental plan could also verify the association between
the inhibitory effects on pro-inflammatory genes and corresponding
alterations on the NF-κB pathway. Another significant limitation of
the current study was that only one ago-miRNA molecule, namely
pre-miR-93 ago-miRNA, was employed. However, it has been reported
that other miRNAs can interact with TLRs, such as miR-145-5p, and
NF-κB, such as miR-130a, miR-146a/b, miR-218 and miR-451 (79-82).
Studies based on molecules mimicking the function(s) of these
miRNAs should be also considered to fully understand the interplay
between miRNAs, NF-κB, TLR4 and ‘cytokine storm’-related
pro-inflammatory genes. In addition, these future studies could
allow the identification of novel pre-miRNA molecules, which could
be useful for verifying the hypothesis that pre-miRNA molecules in
combination with natural products, such as AGE and other compounds
isolated from AGE, could possibly enhance their inhibitory activity
on inflammation.
Supplementary Material
Data S1
(A) Representative examples showing
the RT-qPCR analysis of SARS-CoV-2 S-protein mRNA. (B) Content of
the SARS-CoV-2 mRNA (fold increase with respect to trace amount of
hybridizable material found in control cells, taken as 1). The
content of SARS-CoV-2 S-protein mRNA has been evaluated by RT-qPCR
using as described by Fertig et al (70) and further detailed in Data S1. Endogenous β-actin was used as
internal control. Results represent the average ± standard
deviation of three independent experiments. RT-qPCR, reverse
transcription-quantitative PCR; SARS-CoV-2, severe acute
respiratory syndrome coronavirus 2.
Representative examples showing the
increase of the content of miR-93-5p (fold increase with respect to
endogenous miR-93-5p) in IB3-1 cells transfected with the
pre-miR-93-5p. The content of miR-93-5p has been evaluated by
RT-qPCR using the assays described in Table SI. The relative expression of
miR-93-5p was calculated as elsewhere described (39) using human Let-7c-5p (white) and U6
(grey) RNAs as normalizers. Results represent the average ±
standard deviation of five independent experiments. RT-qPCR,
reverse transcription-quantitative PCR; miR, microRNA; n.s., not
significant.
Representative examples showing the
increase of the expression of pro-inflammatory genes upon treatment
of IB3-1 cells with 5 nm SARS-CoV2 S-protein and the effect of
transfection with 200 nM pre-miR-93-5p (agomiR-93) and miR-93
negative control using (A) Bio-plex analysis and (B) RT-qPCR. The
relative expression of IL-8 mRNA was calculated as elsewhere
described (1,4) using human β-actin as normalizer.
Results represent the average ± standard deviation of four
independent experiments. Modified from the study reported by
Gasparello et al (1).
Copyright can be found at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8492649/. IL,
interleukin; SARS-CoV-2, severe acute respiratory syndrome
coronavirus 2. Further information is included in Table SII.
List of assays employed for miRNA
detection.
Effects of pre-miR-93-5p on IB3-1
exposed to SARS-CoV-2 S-protein.
Acknowledgements
The authors wish to thank Dr. Takahiro Ogawa and Dr.
Toshiaki Matsutomo (Wakunaga Pharmaceutical Co., Ltd.) for
organizing the shipment of the AGE extract.
Funding
Funding: This work was funded by the Italian Ministery of
University and Research (MUR)-FISR COVID-miRNA Peptide Nucleic Acid
Project (grant no. FISR2020IP_04128), by FIRD 2024 funds from the
University of Ferrara. (grant no. FIRD-Finotti 2024) and by the
Interuniversity Consortium for Biotechnologies, Italy (grant nos.
CIB-Unife-2020 and CIB-Unife-2021).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AF, EA and RG designed the experimental plan. JG,
CP, AF were involved in the methodology development. JG and CP
performed the experiments. AF, CP, JG and RG curated the data and
analysed the results. AF and RG were responsible for funding
acquisition. AF and RG supervised the investigation. RG wrote the
original draft. AF, RG and EA reviewed and edited the draft. All
authors read and approved the final version of the manuscript. RG
and AF confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The company Wakunaga Pharmaceutical Co., Ltd. both
provided the aged garlic extract used in this study and also paid
for the publication charges.
References
|
1
|
Killerby ME, Biggs HM, Midgley CM, Gerber
SI and Watson JT: Middle east respiratory syndrome coronavirus
transmission. Emerg Infect Dis. 26:191–198. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Murakami N, Hayden R, Hills T, Al-Samkari
H, Casey J, Del Sorbo L, Lawler PR, Sise ME and Leaf DE:
Therapeutic advances in COVID-19. Nat Rev Nephrol. 19:38–52.
2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Narayanan SA, Jamison DA Jr, Guarnieri JW,
Zaksas V, Topper M, Koutnik AP, Park J, Clark KB, Enguita FJ,
Leitão AL, et al: A comprehensive SARS-CoV-2 and COVID-19 review,
Part 2: Host extracellular to systemic effects of SARS-CoV-2
infection. Eur J Hum Genet. 32:10–20. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tulimilli SV, Dallavalasa S, Basavaraju
CG, Kumar Rao V, Chikkahonnaiah P, Madhunapantula SV and Veeranna
RP: Variants of severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) and vaccine effectiveness. Vaccines (Basel).
10(1751)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Watson OJ, Barnsley G, Toor J, Hogan AB,
Winskill P and Ghani AC: Global impact of the first year of
COVID-19 vaccination: A mathematical modelling study. Lancet Infect
Dis. 22:1293–1302. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Walker PGT, Whittaker C, Watson OJ,
Baguelin M, Winskill P, Hamlet A, Djafaara BA, Cucunubá Z, Olivera
Mesa D, Green W, et al: The impact of COVID-19 and strategies for
mitigation and suppression in low- and middle-income countries.
Science. 369:413–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Villamagna AH, Gore SJ, Lewis JS and
Doggett JS: The need for antiviral drugs for pandemic coronaviruses
from a global health perspective. Front Med (Lausanne).
7(596587)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mikulska M, Sepulcri C, Dentone C, Magne
F, Balletto E, Baldi F, Labate L, Russo C, Mirabella M, Magnasco L,
et al: Triple combination therapy with 2 antivirals and monoclonal
antibodies for persistent or relapsed severe acute respiratory
syndrome coronavirus 2 infection in immunocompromised patients.
Clin Infect Dis. 77:280–286. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Meyerowitz EA and Li Y: Review: The
landscape of antiviral therapy for COVID-19 in the era of
widespread population immunity and omicron-lineage viruses. Clin
Infect Dis. 78:908–917. 2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Selickman J, Vrettou CS, Mentzelopoulos SD
and Marini JJ: COVID-19-related ARDS: Key mechanistic features and
treatments. J Clin Med. 11(4896)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Silva MJA, Ribeiro LR, Gouveia MIM,
Marcelino BDR, Santos CSD, Lima KVB and Lima LNGC:
Hyperinflammatory response in COVID-19: A systematic review.
Viruses. 15(553)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Conti P, Caraffa A, Gallenga CE, Ross R,
Kritas SK, Frydas I, Younes A and Ronconi G: Coronavirus-19
(SARS-CoV-2) induces acute severe lung inflammation via IL-1
causing cytokine storm in COVID-19: A promising inhibitory
strategy. J Biol Regul Homeost Agents. 34:1971–1975.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zawawi A, Naser AY, Alwafi H and Minshawi
F: Profile of circulatory cytokines and chemokines in human
coronaviruses: A systematic review and meta-analysis. Front
Immunol. 12(666223)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Altmann DM, Whettlock EM, Liu S,
Arachchillage DJ and Boyton RJ: The immunology of long COVID. Nat
Rev Immunol. 23:618–634. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ghorra N, Popotas A, Besse-Hammer T,
Rogiers A, Corazza F and Nagant C: Cytokine profile in patients
with postacute sequelae of COVID-19. Viral Immunol. 37:346–354.
2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Song P, Li W, Xie J, Hou Y and You C:
Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 509:280–287.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sefik E, Qu R, Junqueira C, Kaffe E, Mirza
H, Zhao J, Brewer JR, Han A, Steach HR, Israelow B, et al:
Inflammasome activation in infected macrophages drives COVID-19
pathology. Nature. 606:585–593. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Majidpoor J and Mortezaee K: Interleukin-6
in SARS-CoV-2 induced disease: Interactions and therapeutic
applications. Biomed Pharmacother. 145(112419)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Santa Cruz A, Mendes-Frias A, Oliveira AI,
Dias L, Matos AR, Carvalho A, Capela C, Pedrosa J, Castro AG and
Silvestre R: Interleukin-6 is a biomarker for the development of
fatal severe acute respiratory syndrome coronavirus 2 pneumonia.
Front Immunol. 12(613422)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zizzo G, Tamburello A, Castelnovo L, Laria
A, Mumoli N, Faggioli PM, Stefani I and Mazzone A: Immunotherapy of
COVID-19: Inside and beyond IL-6 signalling. Front Immunol.
13(795315)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li L, Li J, Gao M, Fan H, Wang Y, Xu X,
Chen C, Liu J, Kim J, Aliyari R, et al: Interleukin-8 as a
biomarker for disease prognosis of coronavirus disease-2019
patients. Front Immunol. 11(602395)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu Q, Zhou X, Kapini R, Arsecularatne A,
Song W, Li C, Liu Y, Ren J, Münch G, Liu J and Chang D: Cytokine
storm in COVID-19: Insight into pathological mechanisms and
therapeutic benefits of chinese herbal medicines. Medicines
(Basel). 11(14)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Comarmond C, Drumez E, Labreuche J,
Hachulla E, Thomas T, Flipo RM, Seror R, Avouac J, Balandraud N,
Desbarbieux R, et al: COVID-19 presentation and outcomes in
patients with inflammatory rheumatic and musculoskeletal diseases
receiving IL6-receptor antagonists prior to SARS-CoV-2 infection. J
Transl Autoimmun. 6(100190)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ghosn L, Chaimani A, Evrenoglou T,
Davidson M, Graña C, Schmucker C, Bollig C, Henschke N, Sguassero
Y, Nejstgaard CH, et al: Interleukin-6 blocking agents for treating
COVID-19: A living systematic review. Cochrane Database Syst Rev.
3(CD013881)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pomponio G, Ferrarini A, Bonifazi M,
Moretti M, Salvi A, Giacometti A, Tavio M, Titolo G, Morbidoni L,
Frausini G, et al: Tocilizumab in COVID-19 interstitial pneumonia.
J Intern Med. 289:738–746. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Castelnovo L, Tamburello A, Lurati A,
Zaccara E, Marrazza MG, Olivetti M, Mumoli N, Mastroiacovo D,
Colombo D, Ricchiuti E, et al: Anti-IL6 treatment of serious
COVID-19 disease: A monocentric retrospective experience. Medicine
(Baltimore). 100(e23582)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tharmarajah E, Buazon A, Patel V, Hannah
JR, Adas M, Allen VB, Bechman K, Clarke BD, Nagra D, Norton S, et
al: IL-6 inhibition in the treatment of COVID-19: A meta-analysis
and meta-regression. J Infect. 82:178–185. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Segú-Vergés C, Artigas L, Coma M and Peck
RW: Artificial intelligence assessment of the potential of
tocilizumab along with corticosteroids therapy for the management
of COVID-19 evoked acute respiratory distress syndrome. PLoS One.
18(e0280677)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dominguez C, McCampbell KK, David JM and
Palena C: Neutralization of IL-8 decreases tumor PMN-MDSCs and
reduces mesenchymalization of claudin-low triple-negative breast
cancer. JCI Insight. 2(e9496)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Piemonti L, Landoni G, Voza A, Puoti M,
Gentile I, Coppola N, Nava S, Mattei A, Marinangeli F, Marchetti G,
et al: Efficacy and safety of reparixin in patients with severe
COVID-19 pneumonia: A phase 3, randomized, double-blind
placebo-controlled study. Infect Dis Ther. 12:2437–2456.
2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zarbock A, Allegretti M and Ley K:
Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung
injury in mice. Br J Pharmacol. 155:357–364. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kaiser R, Leunig A, Pekayvaz K, Popp O,
Joppich M, Polewka V, Escaig R, Anjum A, Hoffknecht ML, Gold C, et
al: Self-sustaining IL-8 loops drive a prothrombotic neutrophil
phenotype in severe COVID-19. JCI Insight.
6(e150862)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shang R, Lee S, Senavirathne G and Lai EC:
microRNAs in action: Biogenesis, function and regulation. Nat Rev
Genet. 24:816–833. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fazi and Nervi C: MicroRNA: Basic
mechanisms and transcriptional regulatory networks for cell fate
determination. Cardiovasc Res. 79:553–561. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ivey KN and Srivastava D: microRNAs as
developmental regulators. Cold Spring Harb Perspect Biol.
7(a008144)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chaudhuri K and Chatterjee R: MicroRNA
detection and target prediction: Integration of computational and
experimental approaches. DNA Cell Biol. 26:321–337. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Doench JG and Sharp PA: Specificity of
microRNA target selection in translational repression. Genes Dev.
18:504–511. 2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gasparello J, Finotti A and Gambari R:
Tackling the COVID-19 ‘cytokine storm’ with microRNA mimics
directly targeting the 3'UTR of pro-inflammatory mRNAs. Med
Hypotheses. 146(110415)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fabbri E, Borgatti M, Montagner G, Bianchi
N, Finotti A, Lampronti I, Bezzerri V, Dechecchi MC, Cabrini G and
Gambari R: Expression of microRNA-93 and interleukin-8 during
pseudomonas aeruginosa-mediated induction of proinflammatory
responses. Am J Respir Cell Mol Biol. 50:1144–1155. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gasparello J, d'Aversa E, Breveglieri G,
Borgatti M, Finotti A and Gambari R: In vitro induction of
interleukin-8 by SARS-CoV-2 spike protein is inhibited in bronchial
epithelial IB3-1 cells by a miR-93-5p agomiR. Int Immunopharmacol.
101(108201)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wu J, Ding J, Yang J, Guo X and Zheng Y:
MicroRNA roles in the nuclear factor kappa B signaling pathway in
cancer. Front Immunol. 9(546)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hanna J, Hossain G and Kocerha J: The
potential for microRNA therapeutics and clinical research. Front
Genet. 10(478)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Momin MY, Gaddam RR, Kravitz M, Gupta A
and Vikram A: The challenges and opportunities in the development
of MicroRNA therapeutics: A multidisciplinary viewpoint. Cells.
10(3097)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Diener C, Keller A and Meese E: Emerging
concepts of miRNA therapeutics: From cells to clinic. Trends Genet.
38:613–626. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee EC, Valencia T, Allerson C, Schairer
A, Flaten A, Yheskel M, Kersjes K, Li J, Gatto S, Takhar M, et al:
Discovery and preclinical evaluation of anti-miR-17 oligonucleotide
RGLS4326 for the treatment of polycystic kidney disease. Nat
Commun. 10(4148)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Reid G, Kao SC, Pavlakis N, Brahmbhatt H,
MacDiarmid J, Clarke S, Boyer M and van Zandwijk N: Clinical
development of TargomiRs, a miRNA mimic-based treatment for
patients with recurrent thoracic cancer. Epigenomics. 8:1079–1085.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zurlo M, Gasparello J, Verona M, Papi C,
Cosenza LC, Finotti A, Marzaro G and Gambari R: The anti-SARS-CoV-2
BNT162b2 vaccine suppresses mithramycin-induced erythroid
differentiation and expression of embryo-fetal globin genes in
human erythroleukemia K562 cells. Exp Cell Res.
433(113853)2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cosenza LC, Marzaro G, Zurlo M, Gasparello
J, Zuccato C, Finotti A and Gambari R: Inhibitory effects of
SARS-CoV-2 spike protein and BNT162b2 vaccine on
erythropoietin-induced globin gene expression in erythroid
precursor cells from patients with β-thalassemia. Exp Hematol.
129(104128)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gasparello J, D'Aversa E, Papi C, Gambari
L, Grigolo B, Borgatti M, Finotti A and Gambari R: Sulforaphane
inhibits the expression of interleukin-6 and interleukin-8 induced
in bronchial epithelial IB3-1 cells by exposure to the SARS-CoV-2
spike protein. Phytomedicine. 87(153583)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Verbeke R, Hogan MJ, Loré K and Pardi N:
Innate immune mechanisms of mRNA vaccines. Immunity. 55:1993–2005.
2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sartorius R, Trovato M, Manco R, D'Apice L
and De Berardinis P: Exploiting viral sensing mediated by Toll-like
receptors to design innovative vaccines. NPJ Vaccines.
6(127)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Delehedde C, Even L, Midoux P, Pichon C
and Perche F: Intracellular routing and recognition of lipid-based
mRNA nanoparticles. Pharmaceutics. 13(945)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dechecchi MC, Nicolis E, Norez C, Bezzerri
V, Borgatti M, Mancini I, Rizzotti P, Ribeiro CM, Gambari R, Becq F
and Cabrini G: Anti-inflammatory effect of miglustat in bronchial
epithelial cells. J Cyst Fibros. 7:555–565. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Gambari R, Borgatti M, Lampronti I, Fabbri
E, Brognara E, Bianchi N, Piccagli L, Yuen MCW, Kan CW, Hau DKP, et
al: Corilagin is a potent inhibitor of NF-kappaB activity and
downregulates TNF-alpha induced expression of IL-8 gene in cystic
fibrosis IB3-1 cells. Int Immunopharmacol. 13:308–315.
2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
De Stefano D, Ungaro F, Giovino C,
Polimeno A, Quaglia F and Carnuccio R: Sustained inhibition of IL-6
and IL-8 expression by decoy ODN to NF-κB delivered through
respirable large porous particles in LPS-stimulated cystic fibrosis
bronchial cells. J Gene Med. 13:200–208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Gao H, Xiao D, Gao L and Li :
MicroRNA-93 contributes to the suppression of lung inflammatory
responses in LPS-induced acute lung injury in mice via the
TLR4/MyD88/NF-κB signaling pathway. Int J Mol Med. 46:561–570.
2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Silva-Aguiar RP, Teixeira DE, Peruchetti
DB, Peres RAS, Alves SAS, Calil PT, Arruda LB, Costa LJ, Silva PL,
Schmaier AH, et al: Toll like receptor 4 mediates the inhibitory
effect of SARS-CoV-2 spike protein on proximal tubule albumin
endocytosis. Biochim Biophys Acta Mol Basis Dis.
1870(167155)2024.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Chakraborty C, Mallick B, Bhattacharya M
and Byrareddy S: SARS-CoV-2 omicron spike shows strong binding
affinity and favourable interaction landscape with the TLR4/MD2
compared to other variants. J Genet Eng Biotechnol.
22(100347)2024.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Fontes-Dantas FL, Fernandes GG, Gutman EG,
De Lima EV, Antonio LS, Hammerle MB, Mota-Araujo HP, Colodeti LC,
Araújo SMB, Froz GM, et al: SARS-CoV-2 spike protein induces
TLR4-mediated long-term cognitive dysfunction recapitulating
post-COVID-19 syndrome in mice. Cell Rep. 42(112189)2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Choudhury A and Mukherjee S: In silico
studies on the comparative characterization of the interactions of
SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and
human TLRs. J Med Virol. 92:2105–2113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhao Y, Kuang M, Li J, Zhu L, Jia Z, Guo
X, Hu Y, Kong J, Yin H, Wang X and You F: SARS-CoV-2 spike protein
interacts with and activates TLR41. Cell Res. 31:818–820.
2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Patra R, Chandra Das N and Mukherjee S:
Targeting human TLRs to combat COVID-19: A solution? J Med Virol.
93:615–617. 2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Das NC, Labala R, Patra R, Chattoraj A and
Mukherjee S: In silico identification of new anti-SARS-CoV-2 agents
from bioactive phytocompounds targeting the viral spike
glycoprotein and human TLR4. Lettr Drug Des Discov. 19:175–191.
2022.
|
|
65
|
Sahanic S, Hilbe R, Dünser C, Tymoszuk P,
Löffler-Ragg J, Rieder D, Trajanoski Z, Krogsdam A, Demetz E,
Yurchenko M, et al: SARS-CoV-2 activates the TLR4/MyD88 pathway in
human macrophages: A possible correlation with strong
pro-inflammatory responses in severe COVID-19. Heliyon.
9(e21893)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Nakazawa D, Takeda Y, Kanda M, Tomaru U,
Ogawa H, Kudo T, Shiratori-Aso S, Watanabe-Kusunoki K, Ueda Y,
Miyoshi A, et al: Inhibition of Toll-like receptor 4 and
Interleukin-1 receptor prevent SARS-CoV-2 mediated kidney injury.
Cell Death Discov. 9(293)2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Asaba CN, Ekabe CJ, Ayuk HS, Gwanyama BN,
Bitazar R and Bukong TN: Interplay of TLR4 and SARS-CoV-2:
Unveiling the complex mechanisms of inflammation and severity in
COVID-19 infections. J Inflamm Res. 17:5077–5091. 2024.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Gasparello J, Papi C, Marzaro G, Macone A,
Zurlo M, Finotti A, Agostinelli E and Gambari R: Aged garlic
extract (AGE) and its constituents S-allyl-cysteine (SAC) inhibit
the expression of pro-inflammatory genes induced in bronchial
epithelial IB3-1 cells by exposure to the SARS-CoV-2 spike protein
and the BNT162b2 vaccine. Molecules. 29(5938)2024.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gasparello J, Marzaro G, Papi C, Gentili
V, Rizzo R, Zurlo M, Scapoli C, Finotti A and Gambari R: Effects of
Sulforaphane on SARS-CoV-2 infection and NF-κB dependent expression
of genes involved in the COVID-19 ‘cytokine storm’. Int J Mol Med.
52(76)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Fertig TE, Chitoiu L, Marta DS, Ionescu
VS, Cismasiu VB, Radu E, Angheluta G, Dobre M, Serbanescu A,
Hinescu ME and Gherghiceanu M: Vaccine mRNA can be detected in
blood at 15 days post-vaccination. Biomedicines.
10(1538)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhou Q, Zhang L, Dong Y, Wang Y, Zhang B,
Zhou S, Huang Q, Wu T and Chen G: The role of SARS-CoV-2-mediated
NF-κB activation in COVID-19 patients. Hypertens Res. 47:375–384.
2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Forsyth CB, Zhang L, Bhushan A, Swanson B,
Zhang L, Mamede JI, Voigt RM, Shaikh M, Engen PA and Keshavarzian
A: The SARS-CoV-2 S1 spike protein promotes MAPK and NF-κB
activation in human lung cells and inflammatory cytokine production
in human lung and intestinal epithelial cells. Microorganisms.
10(1996)2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Zhang G and Ghosh S: Toll-like
receptor-mediated NF-kappaB activation: A phylogenetically
conserved paradigm in innate immunity. J Clin Invest. 107:13–19.
2001.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gargiulo S, Gamba P, Testa G, Rossin D,
Biasi F, Poli G and Leonarduzzi G: Relation between TLR4/NF-κB
signaling pathway activation by 27-hydroxycholesterol and
4-hydroxynonenal, and atherosclerotic plaque instability. Aging
Cell. 14:569–581. 2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Muir A, Soong G, Sokol S, Reddy B, Gomez
MI, Van Heeckeren A and Prince A: Toll-like receptors in normal and
cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol.
30:777–783. 2004.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Greene CM, Carroll TP, Smith SGJ, Taggart
CC, Devaney J, Griffin S, O'neill SJ and McElvaney NG: TLR-induced
inflammation in cystic fibrosis and non-cystic fibrosis airway
epithelial cells. J Immunol. 174:1638–1646. 2005.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Xu Y, Jin H, Yang X, Wang L, Su L, Liu K,
Gu Q and Xu X: MicroRNA-93 inhibits inflammatory cytokine
production in LPS-stimulated murine macrophages by targeting IRAK4.
FEBS Lett. 588:1692–1698. 2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Tian F, Yuan C, Hu L and Shan S:
MicroRNA-93 inhibits inflammatory responses and cell apoptosis
after cerebral ischemia reperfusion by targeting interleukin-1
receptor-associated kinase 4. Exp Ther Med. 14:2903–2910.
2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wei L and Zhao D: M2 macrophage-derived
exosomal miR-145-5p protects against the
hypoxia/reoxygenation-induced pyroptosis of cardiomyocytes by
inhibiting TLR4 expression. Ann Transl Med. 10(1376)2022.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Jin C, Wang A, Liu L, Wang G, Li G and Han
Z: miR-145-5p inhibits tumor occurrence and metastasis through the
NF-κB signaling pathway by targeting TLR4 in malignant melanoma. J
Cell Biochem. 120:11115–11126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ma X, Becker Buscaglia LE, Barker JR and
Li Y: MicroRNAs in NF-kappaB signaling. J Mol Cell Biol. 3:159–166.
2011.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ghafouri-Fard S, Abak A, Fattahi F, Hussen
BM, Bahroudi Z, Shoorei H and Taheri M: . The interaction between
miRNAs/lncRNAs and nuclear factor-κB (NF-κB) in human disorders.
Biomed Pharmacother. 138(111519)2021.PubMed/NCBI View Article : Google Scholar
|