Introduction
Benign prostatic hyperplasia (BPH), also known as
benign prostatic hypertrophy or benign prostatic obstruction, is a
common condition affecting aging males. It involves the
non-cancerous enlargement of the prostate gland, leading to lower
urinary tract symptoms (LUTS) that may affect the quality of life
of patients (1). The enlarged
prostate presses against and pinches the urethra, causing the
bladder wall to thicken. As a result, the bladder may weaken over
time, leading to incomplete bladder emptying and urine retention
(1). These changes in the urinary
tract cause LUTS associated with BPH, such as urinary frequency
(pollakiuria), urinary urgency, a weak urine stream and an
increased need to urinate at night (nocturia) (2). At present, the specific cause of BPH
is not yet fully understood. Changes in hormonal levels,
particularly a decrease in active testosterone and an increase in
estrogen levels, have been proposed as possible contributors to the
development of BPH (3).
The results of a previous study suggested that
dihydrotestosterone (DHT) may play a role in promoting prostate
cell growth, even when testosterone levels are low (3). Inflammation and oxidative stress have
also been proposed as potential contributors to the development of
BPH (4). BPH is the most common
condition of the prostate in males aged >50 years, and the
prevalence of the disease increases with age. While <50% of
males with BPH experience LUTS, the condition may lead to
complications, such as acute or chronic urinary retention, blood in
the urine, urinary tract infections, bladder and kidney damage, and
bladder stones (5). At present,
the pharmacological treatment of BPH involves medications that aim
to reduce prostate growth or improve urinary symptoms. These
medications include alpha blockers, phosphodiesterase-5 inhibitors,
5-alpha reductase inhibitors and combination therapies (2). Alpha blockers relax the smooth
muscles of the prostate and bladder neck to improve urinary flow,
while 5-alpha reductase inhibitors block the production of DHT,
which may lead to shrinkage of the prostate or prevention of
further growth. The aforementioned medications provide relief of
the urinary symptoms associated with BPH; however, their use may
lead to side-effects, including retrograde ejaculation, excessive
reduction in blood pressure (orthostatic hypotension) and a reduced
libido (6).
Research has focused on the use of alternative and
complementary therapies for the management of BPH, including
natural extracts derived from medicinal plants (7). This has led to the formulation of a
combination of three specific plant extracts; namely, Prunus
africana, Urtica dioica and Epilobium angustifolium.
Traditionally, these individual plant extracts have been used for
the treatment of urinary disorders. Thus, Prunus africana,
Urtica dioica and Epilobium angustifolium may exhibit
potential for use in the management of BPH. Prunus Africana
(Pygeum africanum Hook f., or Prunus Africana Kalk.),
derived from the bark of the African plum tree, is used in
traditional African medicine to alleviate urinary symptoms
associated with BPH (8). The
bioactive compounds, including phytosterols, triterpenes and
ferulic acid esters, exhibit anti-inflammatory and anti-androgenic
properties that may reduce prostate enlargement and improve urinary
flow dynamics (8).
Clinical studies have demonstrated the efficacy of
Pygeum extracts in reducing nocturia, improving the quality
of life of patients and reducing International Prostate Symptom
Score (IPSS) (9,10). Urtica dioica (Urtica
dioica L. and/or Urtica urens L.), also known as
stinging nettle root, is well-established in herbal medicine
(11). Notably, Urtica
dioica contains bioactive compounds, such as phytosterols
(β-sitosterol, daucosterol and associated glucosides) and
scopoletin. This extract also contains tannins, lecithins, mineral
salts, phenylpropanes and lignans (11). Extracts derived from Urtica
dioica exhibit anti-inflammatory and 5-alpha reductase
inhibitory properties, which may improve hormonal symptoms
associated with BPH. The results of clinical studies have
demonstrated that extracts of Urtica dioica significantly
improved LUTS, and these results were observed following short-term
treatment (12,13). Epilobium angustifolium
(Onagraceae), commonly known as small-flowered willowherb,
is a plant used in traditional European medicine (14). Epilobium angustifolium
contains flavonoids, tannins and phenolic acids that exhibit
antioxidant and anti-proliferative properties, which may contribute
to the alleviation of BPH (15).
Enotein B, a main constituent of Epilobium angustifolium,
has demonstrated immunomodulatory, antioxidant and
anti-proliferative properties (16). It was thus hypothesized that a
combination of the three plant extracts may exhibit potential as an
alternative or complementary therapy for the management of BPH.
Their respective bioactive compounds may exert synergistic
anti-inflammatory, anti-androgenic and antioxidant effects, for the
improvement of urinary symptoms and overall prostate health.
At present, research focused on the safety and
efficacy of the combination of Prunus africana, Urtica
dioica and Epilobium angustifolium is limited. Thus, the
present study aimed to determine the effects of this herbal
combination in patients diagnosed with BPH.
Materials and methods
Study design
The present study was an observational, controlled,
mono-centric trial aimed at evaluating the efficacy of a
combination of natural extracts (Progamet; USP-Union of
Pharmaceutical Sciences S.r.l.; https://www.usponline.it/catalogo-prodotti/) derived
from Prunus africana, Urtica dioica and Epilobium
angustifolium, in addition to standard therapy (alpha
blockers), for the treatment of symptoms associated with BPH. The
present study aimed to assess whether the combination of these
plant-based extracts improves the efficacy of the standard
monotherapy in male patients diagnosed with clinical BPH, who were
at an increased risk of clinical progression. The patients included
in the present study were >50 years of age. The included
patients presented with moderate-to-severe symptoms, an enlarged
prostate (>30 cc) and prostate specific antigen (PSA) levels
≤1.5 ng/ml. All patients had visited the Department of Urology,
Umberto I Hospital, Salerno, Italy. The present study was conducted
following the Declaration of Helsinki, and was approved by the
Campania SUD Ethics Committee (ethics approval no. 0152029). All
patients provided written informed consent, and were divided into
two groups as follows: i) The treatment group and ii) the control
group. The patients in the treatment group received a daily dose of
the natural extract combination in addition to the standard
pharmacological therapy for 90 days. The control group received the
standard therapy alone. Standard therapy had been prescribed to the
patients before the commencement of the study and consisted in the
use of alpha blockers, namely tamsulosin at 0.4 mg once daily,
alfuzosin at 10 mg once daily and doxazosin at 4 mg once daily.
Patients were consistently maintained on the same alpha blocker
throughout the whole study period. All patients attended a
screening visit (visit 1) and a baseline visit (visit 2). The
patients included in the study presented with a total IPSS of ≥12
at visits 1 and 2. Following these initial visits, follow-up visits
were scheduled 90 days after the baseline visit.
Patients were excluded from the present study
according to the following criteria: i) Moderate or severe renal
impairment, measured as creatinine clearance <50 ml/min, as
estimated by the Cockcroft Gault formula; ii) severe hepatic
impairment; iii) the concurrent use of other herbal or natural
products that exert effects on LUTS (for example, Serenoa
serrulata/repens); iv) a previous diagnosis of prostate cancer;
v) an active urinary tract infection; vi) acute or recurrent
prostatitis (>3 episodes over the past year); vii) a history of
neurological disease that may influence bladder function; viii) a
history of substance abuse, including alcohol or drugs over the
past 12 months; ix) participation in a study involving the
administration of an investigational compound over the past 30
days; and x) any other condition that, in the investigator's
assessment, increased patient's risk, impeded optimal participation
in achieving the study objectives, or highlighted that the patient
was unable to complete the study.
Clinical investigation endpoints
The primary endpoint of the present study was a
change in IPSS following 3 months of treatment, compared with the
baseline. Secondary endpoints included the percentage of patients
with an improved IPSS, an increase in maximum urinary flow rate
(Q-max) from baseline, the percentage of patients able to reduce or
discontinue standard pharmacological therapy, and a change in the
International Index of Erectile Function (IIEF) score from
baseline. The safety endpoint focused on monitoring any adverse
events or complications associated with the use of the herbal
extracts, as reported by patients.
IPSS
IPSS is a seven-item tool designed to quantify
urinary symptoms in patients with BPH, with an additional eighth
question regarding quality of life (17). IPSS was scored at Visit 1, Visit 2
and at the follow-up visit. The participants were instructed to
refrain from using any alpha-adrenergic agonists, cholinergic or
anticholinergic agents, including antihistamines or decongestants,
within 48 h prior to each IPSS assessment. In addition, the
patients were recommended to stop the use of selective
beta-3-adrenoceptor agonists (mirabegron) for the treatment of
overactive bladder syndrome 2 weeks prior to evaluation. Patients
completed questionnaires as per the study schedule. The patients
were instructed to complete the questionnaires in a quiet
environment, in the same location each time, and consistently
during study visits. Patients were encouraged to complete
questionnaires in a thorough and accurate manner, without being
influenced by test results or other external factors.
Prostate volume measurement
Prostate volume was measured at visit 2 (baseline)
and after 90 days of treatment. The anteroposterior, cranio-caudal
and transverse diameters of the prostate were obtained through
suprapubic and/or transrectal ultrasound, to calculate prostate
volume using the following formula: π/6 (anteroposterior width x
cranio-caudal width x transverse width). Prostate volume measured
with pre-programmed equipment was accepted if all three dimensions
were emitted and recorded in the electronic case report form.
Urinary flow measurement
Urinary flow measurements were conducted using a
uroflowmeter (Laborie Medical Technologies Corporation) at visits 1
and 2, and the follow-up visit. Maximal urinary flow (Q-max) with a
voided volume ≥125 ml and residual urinary volume were recorded.
The results are expressed as ml/sec (standard deviation; SD).
IIEF questionnaire
The IIEF questionnaire is a widely-used and
validated self-administered tool designed to assess the presence
and severity of erectile dysfunction in males (18). This questionnaire consists of five
items that cover five domains associated with erectile function.
Each item is scored on a five-point Likert scale, with responses
ranging from 0 to 5. The total IIEF score was calculated following
the addition of the scores of all five items, resulting in a score
range of 0 to 25. Higher scores indicated improved erectile
function and sexual satisfaction.
Clinical patient information, ECG and
physical examination
The detailed medical histories of all patients were
obtained at visit 1, including information on LUTS onset, time
since BPH diagnosis, previous use of alpha-1-adrenoreceptor
antagonists and/or herbal therapies, overall health, previous or
current medical conditions, medication use, allergies, family
history of cardiovascular diseases and sexual activity/sexual
dysfunction. ECG and a complete physical examination were performed
at visit 2.
Extracts
Natural extracts derived from Pygeum
africanum, Urtica dioica and Epilobium
angustifolium were contained in a supplement known by its
commercial name, Progamet (USP-Union of Pharmaceutical Sciences
S.r.l.; https://www.usponline.it/catalogo-prodotti/). Progamet
was administered once daily the to patients in the treatment group,
in addition to the standard pharmacological therapy for a duration
of 90 days. Details regarding the standardized formulation and
dosage were provided to the study participants in the form of an
informational leaflet. The composition of Progamet includes the
following standardized extracts: Epilobium angustifolium L.
(Willowherb) standardized to 15% Oenothein B (600 mg), Urtica
dioica L. (Nettle root) standardized to 0.8% Sterols (300 mg)
and Prunus africana (Hook. f.) Kalkman (Pygeum bark)
standardized to 2.5% beta-sitosterols (100 mg).
Statistical analysis
Sample size was calculated based on the estimated
improvement in IPSS compared with the control, using information
obtained from the CombAT study (19). Sample size estimates were made
considering a continuous response variable with a normal
distribution. The expected change in IPSS (mean difference between
treatment groups) was estimated at 3.5 (SD, 6). With 80% power, the
minimum required sample size per arm was calculated to be 46
subjects. Statistical analysis was performed using a independent
sample two-tailed Student t-test, to determine whether the
combination treatment was superior to standard therapy after 90
days. For the within-group analysis a paired sample two-tailed
Student's t-test was used. A value of P<0.05 was considered to
indicate a statistically significant difference. Descriptive
statistics were used to summarize baseline characteristics of the
study population. Continuous variables are presented as the mean ±
SD, while categorical variables are presented as frequencies and
percentages. Secondary endpoints were analyzed using appropriate
statistical methods.
Results
Patients
A total of 92 patients were enrolled in the present
study, with 46 patients assigned to the treatment group and 46
patients assigned to the control group. There were no clinically
meaningful differences in demographic or clinical baseline
characteristics between groups (Table
I). The patients in the treatment group had a mean age of 65.09
(±10.9) years, and the patients in the control group had a mean age
of 66.2 (±12.4) years. There was no notable difference in the mean
body mass index (BMI) between the treatment and control groups.
Notably, the mean BMI was 25.1 (±3.3) in the treatment group, and
26.0 (±1.5) in the control group. The mean prostate volume at
baseline was 50.5 (±17.7) cc in the treatment group, compared with
48.2 (±8.5) cc in the control group. In the treatment group (n=46),
comorbidities, such as hypertension (n=8, 17.4%), diabetes mellitus
(n=6, 13.0%) and dyslipidemia (n=4, 8.6%) were documented alongside
BPH. Similarly, in the control group (n=46), hypertension (n=4,
8.7%), diabetes mellitus (n=3, 6.5%) and dyslipidemia (n=2, 4.3%)
were observed as the most common comorbidities. In the treatment
group, 12 patients (26.0%) had prostates with characteristics of
congestion, and 8 patients (17.4%) had prostates with inflammation
(acute, subacute, or chronic). These observations indicate a
heterogeneous presentation of BPH, similarly to the control group,
where 7 patients (15.2%) had prostates with characters of
congestion, while 6 patients (13.0%) had prostates showing signs of
inflammation.
 | Table IComparison of baseline
characteristics and comorbidities between the treatment and control
groups. |
Table I
Comparison of baseline
characteristics and comorbidities between the treatment and control
groups.
| Parameters | Control | Treatment |
|---|
| Age, years
(±SD) | 66.2 (±12.4) | 65.09 (±10.9) |
| BMI (±SD) | 26.0 (±1.5) | 25.1 (±3.3) |
| Prostate volume, cc
(±SD) | 48.2 (±8.5) | 50.5 (±17.7) |
| Comorbidities | Hypertension (n=4,
8.7%), diabetes mellitus (n=3, 6.5%), dyslipidemia (n=2, 4.3%) | Hypertension (n=8,
17.4%), diabetes mellitus (n=6, 13.0%), dyslipidemia (n=4,
8.6%) |
| BHP
presentation | Congestion (n=7,
15.2%) | Congestion (n=12,
26.0%) |
| | Inflammation (n=6,
13.0%) | Inflammation (n=8,
17.4%) |
IPSS
IPSS was utilized as a primary outcome measure to
assess the efficacy of Progamet in the management of LUTS
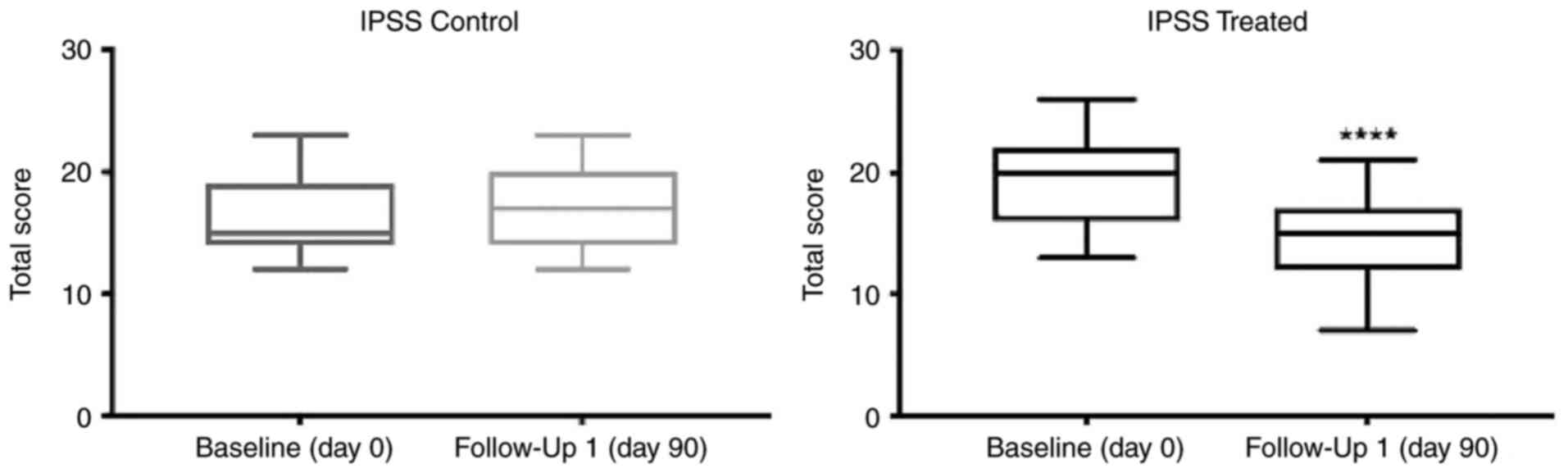
associated with BPH. The results obtained are presented in Table II. In the treatment group, the
mean baseline IPSS was 19 (±3.3). Following 90 days of Progamet
supplementation, the follow-up IPSS was significantly decreased to
14 (±3.7; Table II and Fig. 1). Notably, the baseline IPSS in the
control group was 16.3 (±3.3), and there was no significant change
in follow-up IPSS (17±3.2; Table
II; Fig. 1). The comparison of
IPSS at follow-up demonstrated a notable difference in treatment
efficacy. Thus, treatment with Progamet led to a clinically and
statistically significant improvement in LUTS in the treatment
group, compared with the control group. Collectively, these results
demonstrated that treatment with Progamet led to a significant
reduction in IPSS and improvements in LUTS in the treatment group,
compared with the control group. The difference in treatment
efficacy between the two groups was statistically significant.
 | Table IIIPSS scores in the control and
treatment groups. |
Table II
IPSS scores in the control and
treatment groups.
| | Control group | Treatment
group |
|---|
| Baseline IPSS | 16.3 (±3.3) | 19 (±3.3) |
| Follow-up IPSS | 17 (±3.2) | 14 (±3.7) |
| Change (Δ
IPSS) | +0.7 (±3.2) | -5 (±3.7) |
| P-value | NS |
<0.0001a |
| Between-group
comparison | |
<0.0001a |
| P-value | | |
Urinary flow measurement
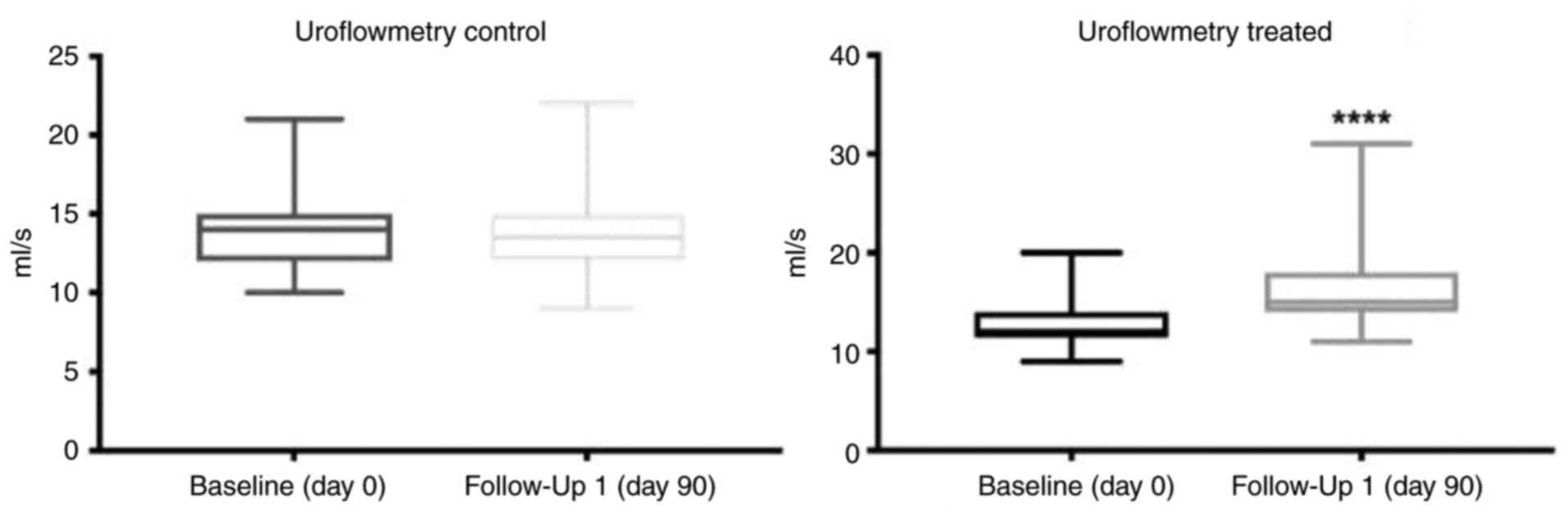
The results of urinary flow measurement are
presented in Table III. In the
treatment group, the mean Q-max at baseline was 13 ml/sec (±2.4;
Table III and Fig. 2). Following 90 days of treatment
with Progamet, mean Q-max significantly increased to 16 ml/s
(±3.8), indicating a statistically significant improvement in
urinary flow (Table III and
Fig. 2). In the control group, the
mean Q-max at baseline was 14 ml/sec (±2.7), and this remained
stable for 90 days [mean Q-max, 14 ml/sec (±2.1)]. These results
highlighted that there was no significant change in mean Q-max
(Table III and Fig. 2). Between-group comparisons
revealed that following 90 days of treatment, the mean urinary flow
was significantly higher in the treatment group [16 ml/sec (±3.8);
Table III] compared with the
control group (14 ml/sec ±2.1). Collectively, these results
highlighted that 90 days of Progamet treatment led to a significant
improvement in urinary flow. In addition, Progamet supplementation
resulted in a significant increase in Q-max in the treatment group
(ΔQ-max, +3 ml/sec), compared with no significant change in the
control group (ΔQ-max, 0 ml/s). Notably, the difference in Q-max
between treatment and control groups was statistically
significant.
 | Table IIIComparison of urinary flow (Q-max)
between the treatment and control groups. |
Table III
Comparison of urinary flow (Q-max)
between the treatment and control groups.
| | Control group | Treatment
group |
|---|
| Baseline (SD) | 14 (±2.7) | 13 (±2.4) |
| Follow-up (SD) | 14 (±2.1) | 16 (±3.8) |
| Change (Δ
Q-max) | 0 (±2.0) | +3 (±2.6) |
| P-value | NS |
<0.0001a |
| Between-group
comparison | |
<0.05a |
| P-value | | |
IIEF questionnaire
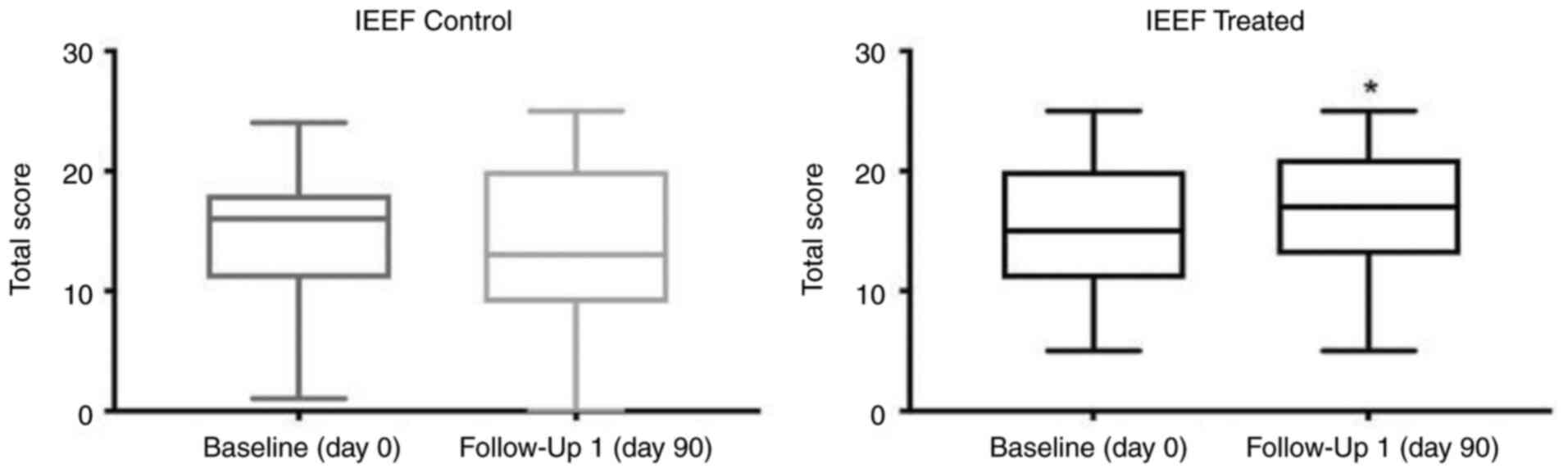
In the treatment group, the mean IIEF score at
baseline was 15 (±5.5), indicating a moderate level of erectile
function (Table IV; Fig. 3). At the 3-month follow-up, the
mean IIEF score significantly increased to 16.5 (±5.6), indicative
of an improvement in erectile function (Table IV; Fig. 3). In the control group, the mean
IIEF score at baseline was 15 (±6.3), which was comparable with the
treatment group (Table IV;
Fig. 3). At the 3-month follow-up,
the mean IIEF score markedly decreased to 14 (±7), suggesting a
minor decline in erectile function. However, this change was not
statistically significant (Table
IV; Fig. 3). When comparing
the change in the IIEF score the between treatment and control
groups, the observed improvement in erectile function in the
treatment group was statistically significant (Table IV). Collectively, these findings
suggested that treatment with Progamet exerted a positive impact on
sexual function. In addition, a significant increase in IIEF score
(ΔIIEF score, +1.5) was observed in the treatment group following
90 days of Progamet supplementation, indicating an improvement in
erectile function. By contrast, a notable decrease in IIEF score
(ΔIIEF score, -1) was observed in the control group, suggesting a
minor decline in erectile function. However, this result was not
statistically significant.
 | Table IVComparison of the IIEF score between
the treatment and control groups. |
Table IV
Comparison of the IIEF score between
the treatment and control groups.
| | Control group | Treatment
group |
|---|
| Baseline IIEF
Score | 15 (±6.3) | 15 (±5.5) |
| Follow-up IIEF
Score | 14 (±7) | 16.5 (±5.6) |
| Change (Δ IIEF
Score) | -1 (±1.5) | +1.5 (±1.5) |
| P-value | NS |
<0.05a |
| Between-group
comparison | |
<0.05a |
| P-value | | |
Percentage of patients able to reduce
or discontinue standard pharmacological therapy
Patients responding well to treatment were
instructed to reduce or discontinue the standard pharmacological
treatment. Among these, 8 patients (17%) were able to discontinue
the standard pharmacological treatment after ~2 months of using
Progamet. After terminating the standard pharmacological treatment,
patients did not report a worsening of symptoms. At follow-up, all
patients demonstrated an improvement of all scores compared with
baseline. Notably, no patients in the control group were able to
discontinue the standard pharmacological treatment. Data were
analyzed using the Chi-squared test, and the results demonstrated
that the percentage of patients who discontinued the standard
pharmacological treatment was statistically significant
(P<0,05). (Table V).
 | Table VContingency table demonstrating the
number of patients who discontinued the pharmacological treatment
(Yes). |
Table V
Contingency table demonstrating the
number of patients who discontinued the pharmacological treatment
(Yes).
| Data analyzed | Yes | No | Total |
|---|
| Progamet | 8a | 38 | 46 |
| CTRL | 0 | 46 | 46 |
| Total | 8 | 84 | 92 |
Safety evaluation
Throughout the study period, patients responded well
to treatment. Side-effects were observed in a small group of
patients, and the most common side-effects included
gastrointestinal problems, such as diarrhea and nausea. The safety
profile of Progamet remained consistent and comparable across all
study participants.
Discussion
Conventional pharmacological treatments offer
symptomatic relief for BHP; however, such treatments may induce
potential side-effects that are not well-tolerated by some patients
(6). This has led to an increased
interest in the use of natural alternatives, such as plant
extracts, which may possess therapeutic properties that are
beneficial for BHP. Herbal products are frequently administered to
male patients in Western Europe who are experiencing LUTS (20). The results of a previous study
demonstrated positive outcomes in patients with BPH, following
treatment with a combination of extracts derived from Urtica
dioica and Prunus Africana (9). Extracts derived from Epilobium
angustifolium have also demonstrated favorable results in
patients with BHP (15). Notably,
Epilobium angustifolium contains enotein B, a polyphenol
with immunomodulatory properties (21). The aforementioned results led to
the formulation of Progamet, a combination of three specific plant
extracts; namely, Prunus africana, Urtica dioica and
Epilobium angustifolium. In the present study, significant
improvements in IPSS were observed in the treatment group compared
with the control group, and these results were indicative of
reduced LUTS. An improvement in LUTS following 90 days of treatment
highlighted the potential of Progamet as a complementary therapy to
standard treatment with alpha blockers. In addition, patients in
the treatment group demonstrated notable improvements in urinary
flow.
An increase in urinary flow observed after 90 days
of Progamet supplementation highlighted the potential of this
treatment in reducing urinary obstruction resulting from prostate
enlargement. Improvements in urinary dynamics observed in the
present study are consistent with those observed following
treatment with each individual plant extract, indicating a
potential synergistic effect within the Progamet formulation
(8,9,15).
Sexual function, often adversely affected by BPH, was assessed
using the IIEF questionnaire. The results of the present study
revealed a statistically significant improvement in erectile
function within the treatment group, which was not observed in the
control group. This result suggested that Progamet supplementation
may positively affect sexual function, offering additional benefits
to patients with BPH. In addition, Progamet demonstrated a positive
safety profile. Mild and transient gastrointestinal side-effects,
such as diarrhea and nausea, were reported in a small group of
patients in the treatment group. Such effects are consistent with
those observed in previous studies involving the individual plant
extracts, further verifying the overall safety of the formulation
(9,10,12,13).
In conclusion, the results of the present study highlighted the
potential use of Progament in the management of symptoms in
patients with BPH, including improved LUTS, urinary flow and
erectile function.
While the mechanisms of action of the single
phytotherapeutic agents are yet to be fully understood, the plant
extracts in Progamet may function synergistically in the treatment
of BHP. Each component of the exerts an effect that may be benefic
to treat BPH. In particular, Prunus africana has been shown
to inhibit the binding of DHT to androgen receptors in the prostate
(22). Since DHT plays a key role
in BPH, reducing its effect on the prostate helps in managing
symptoms. Urtica dioica root is believed to bind to the sex
hormone binding globulin, thereby reducing the amount of free
testosterone converted into DHT, which is a major contributor to
prostate enlargement (23).
Epilobium angustifolium inhibits the enzyme
5-alpha-reductase and helps to protect prostate cells from
oxidative stress and inflammation (24,25).
The formulation used, by combining these three extracts, may likely
function by reducing DHT levels in the prostate through the
inhibition of 5-alpha-reductase, alleviating inflammation in
prostate tissue, thereby reducing swelling and improving urinary
function and supporting improved bladder contractility and
relieving urinary retention symptoms often associated with BPH.
However, the present study exhibits limitations.
Notably, the study duration was brief, with treatment being
administered for 90 days. In addition, the present study had a
moderate sample size and lacked a placebo group for further
comparison. Thus, further investigations with increased sample
sizes are required, to determine the specific mechanisms of action
and long-term safety profile of this treatment combination. Further
investigations may provide a more comprehensive understanding of
the potential benefits observed in patients with BPH following
treatment with Progamet.
The results of the present study highlighted the
potential of Progamet, a composite herbal formulation of Prunus
africana, Urtica dioica and Epilobium
angustifolium extracts, in addition to standard alpha blocker
therapy, in addressing the symptomatic burden of BPH. Based on the
safety profile of the treatment observed in the present study, this
formulation may exhibit potential as an alternative or
complementary treatment in patients with BPH. Notably, Progamet may
provide patients with an alternative to conventional medications.
As the population ages and the prevalence of BPH increases, further
investigations into the use of natural remedies are required. The
combination of plant extracts included in Progamet, each with
established medicinal properties, may provide effective treatment
for BPH. In conclusion, results of the present study demonstrated
the efficacy of Progamet in patients with BPH. Notably, a diverse
range of treatment options are available for patients, which may
lead to improved quality of life and an increased choice of
holistic healthcare approaches.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
UP, RG and RB made substantial contributions to the
conception and design of the study. RS, UDM and OI interpreted the
patient data. UP and RB confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted following the
Declaration of Helsinki, and was approved by the Campania SUD
Ethics Committee (ethics approval no. 0152029). All patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Madersbacher S, Sampson N and Culig Z:
Pathophysiology of benign prostatic hyperplasia and benign
prostatic enlargement: A mini-review. Gerontology. 65:458–464.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mobley D, Feibus A and Baum N: Benign
prostatic hyperplasia and urinary symptoms: Evaluation and
treatment. Postgrad Med. 127:301–307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Welén K and Damber JE: Androgens, aging,
and prostate health. Rev Endocr Metab Disord. 23:1221–1231.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Paulis G: Inflammatory mechanisms and
oxidative stress in prostatitis: The possible role of antioxidant
therapy. Res Rep Urol. 10:75–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cannarella R, Condorelli RA, Barbagallo F,
La Vignera S and Calogero AE: Endocrinology of the aging prostate:
Current concepts. Front Endocrinol (Lausanne).
12(554078)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yu ZJ, Yan HL, Xu FH, Chao HC, Deng LH, Xu
XD, Huang JB and Zeng T: Efficacy and side effects of drugs
commonly used for the treatment of lower urinary tract symptoms
associated with benign prostatic hyperplasia. Front Pharmacol.
11(658)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ullah R, Wazir J, Hossain MA, Diallo MT,
Khan FU, Ihsan AU and Zhou X: A glimpse into the efficacy of
alternative therapies in the management of benign prostatic
hyperplasia. Wien Klin Wochenschr. 133:153–162. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schleich S, Papaioannou M, Baniahmad A and
Matusch R: Extracts from Pygeum africanum and other
ethnobotanical species with antiandrogenic activity. Planta Med.
72:807–813. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Krzeski T, Kazoń M, Borkowski A, Witeska A
and Kuczera J: Combined extracts of Urtica dioica and
Pygeum africanum in the treatment of benign prostatic
hyperplasia: Double-blind comparison of two doses. Clin Ther.
15:1011–1020. 1993.PubMed/NCBI
|
|
10
|
Breza J, Dzurny O, Borowka A, Hanus T,
Petrik R, Blane G and Chadha-Boreham H: Efficacy and acceptability
of tadenan (Pygeum africanum extract) in the treatment of
benign prostatic hyperplasia (BPH): A multicentre trial in central
Europe. Curr Med Res Opin. 14:127–139. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Taheri Y, Quispe C, Herrera-Bravo J,
Sharifi-Rad J, Ezzat SM, Merghany RM, Shaheen S, Azmi L, Prakash
Mishra A, Sener B, et al: Urtica dioica-derived
phytochemicals for pharmacological and therapeutic applications.
Evid Based Complement Alternat Med. 2022(4024331)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sökeland J: Combined sabal and urtica
extract compared with finasteride in men with benign prostatic
hyperplasia: Analysis of prostate volume and therapeutic outcome.
BJU Int. 86:439–442. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schneider T and Rübben H: Stinging nettle
root extract (Bazoton-uno) in long term treatment of benign
prostatic syndrome (BPS). Results of a randomized, double-blind,
placebo controlled multicenter study after 12 months. Urologe A.
43:302–306. 2004.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
14
|
Granica S, Piwowarski JP, Czerwińska ME
and Kiss AK: Phytochemistry, pharmacology and traditional uses of
different Epilobium species (Onagraceae): A review. J
Ethnopharmacol. 156:316–346. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schepetkin IA, Ramstead AG, Kirpotina LN,
Voyich JM, Jutila MA and Quinn MT: Therapeutic potential of
polyphenols from Epilobium angustifolium (Fireweed).
Phytother Res. 30:1287–1297. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Schepetkin IA, Kirpotina LN, Jakiw L,
Khlebnikov AI, Blaskovich CL, Jutila MA and Quinn MT:
Immunomodulatory activity of oenothein B isolated from Epilobium
angustifolium. J Immunol. 183:6754–6766. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yao MW and Green JSA: How international is
the international prostate symptom score? A literature review of
validated translations of the IPSS, the most widely used
self-administered patient questionnaire for male lower urinary
tract symptoms. Low Urin Tract Symptoms. 14:92–101. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rosen RC, Riley A, Wagner G, Osterloh IH,
Kirkpatrick J and Mishra A: The international index of erectile
function (IIEF): A multidimensional scale for assessment of
erectile dysfunction. Urology. 49:822–830. 1997.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Roehrborn CG, Siami P, Barkin J, Damião R,
Major-Walker K, Nandy I, Morrill BB, Gagnier RP and Montorsi F:
CombAT Study Group. The effects of combination therapy with
dutasteride and tamsulosin on clinical outcomes in men with
symptomatic benign prostatic hyperplasia: 4-year results from the
CombAT study. Eur Urol. 57:123–131. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Popov E, Georgieva R and Slavov C:
Phytotherapeutica in common urological conditions in Western
integrative medicine: A narrative review. Longhua Chin Med.
5(33)2022.
|
|
21
|
Ramstead AG, Schepetkin IA, Quinn MT and
Jutila MA: Oenothein B, a cyclic dimeric ellagitannin isolated from
Epilobium angustifolium, enhances IFNγ production by
lymphocytes. PLoS One. 7(e50546)2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ishani A, MacDonald R, Nelson D, Rutks I
and Wilt TJ: Pygeum africanum for the treatment of patients
with benign prostatic hyperplasia: A systematic review and
quantitative meta-analysis. Am J Med. 109:654–664. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chrubasik JE, Roufogalis BD, Wagner H and
Chrubasik S: A comprehensive review on the stinging nettle effect
and efficacy profiles. part II: Urticae radix. Phytomedicine.
14:568–579. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ducrey B, Marston A, Göhring S, Hartmann
RW and Hostettmann K: Inhibition of 5 alpha-reductase and aromatase
by the ellagitannins oenothein A and oenothein B from
Epilobium species. Planta Med. 63:111–114. 1997.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kiss AK, Bazylko A, Filipek A, Granica S,
Jaszewska E, Kiarszys U, Kośmider A and Piwowarski J: Oenothein B's
contribution to the anti-inflammatory and antioxidant activity of
Epilobium sp. Phytomedicine. 18:557–560. 2011.PubMed/NCBI View Article : Google Scholar
|