Introduction
Osteosarcoma (OS) is one of the most common
malignant, aggressive tumors of bone in children and adolescents
which requires a combined approach of surgery and systemic
chemotherapy (1). The prognosis
has improved markedly due to the introduction of aggressive
chemotherapy into the multi-modal treatment regimen of OS. However,
30–50% of these patients with initially localized disease
subsequently develop recurrence, which has an extremely poor
clinical outcome, and 20% of all OS patients succumb to the disease
due to tumor metastasis (2–5).
As a consequence, new therapeutic strategies need to be actively
explored to increase the survival rate of patients with OS.
The processes of tumor growth, invasion, and
metastasis refer to tumor cell proliferation, cell migration and
penetration through the extracellular matrix (ECM) (6). The balance in the activity between
matrix matalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs) is responsible for the proper
maintenance of tissue. Disruption of this balance influences the
metastasis and invasion of cancer and is associated with survival
and prognosis (7,8).
MMPs are a family of zinc-dependent endopeptidases
which have been shown to play a major role in ECM remodeling. MMPs
comprise more than 25 members which are classified based on their
substrate specificities and structural characteristics (9,10).
In particular, the 92-kDa gelatinase B/Type IV (MMP-9) is one of
the well-studied members of the MMP family, which is significantly
involved in tumor invasion and angiogenesis. The ECM and basement
membrane turnover is regulated by a delicate balance between the
molecular factors that are involved in the production, activation,
and inhibition MMPs and the production of natural TIMPs (11,12). Four members of the TIMP family
have been identified (TIMP1–4) (13). TIMPs suppress MMP activity
critical for ECM turnover associated with both physiological and
pathological tissue remodeling. TIMP-1 is a multifunctional protein
which specifically interacts with pro-MMP-9 and is subject to tight
control at the level of transcription (14), whereas it has been shown that the
elevated expression of TIMP-1 closely correlates with a more
aggressive clinical behavior and poor prognosis in human cancer
specimens (15–17). These findings suggest that TIMP-1
plays dual roles in cancer progression. Previous reports have
indicated that MMP-9 and TIMP-1 are involved in the OS progression,
and they have been shown to be associated with poor prognosis in
human OS (18,19).
Gambogic acid (GA)
(C38H44O8), a natural compound
extracted from gamboges, has recently been identified as a potent
anticancer agent. Zhao et al (20) reported that GA can induce
apoptosis and cell cycle arrest of OS cell lines. In recent years,
studies have shown that GA can equally inhibit the growth of a
variety of tumor cells, including hepatoma, gastric and breast
cancer (21–25). Moreover, it was also reported that
GA exhibits low toxicity against normal tissues (26,27). The aim of our study was to examine
the levels of MMP-9 and TIMP-1 in GA-treated OS cells in order to
further investigate the anti-OS effect of GA.
Materials and methods
Reagents
GA was purchased from Sigma-Aldrich (St. Louis, MO,
USA) with a purity of >95%. It was dissolved in dimethyl
sulfoxide (DMSO) (Sigma-Aldrich) and stored at −20°C at a
concentration of 10 mmol/l; the final concentration of DMSO was
<0.1%. The cell counting kit-8 (CCK-8) was purchased from
Dojindo Laboratories (Japan). The primary antibodies against MMP-9,
TIMP-1, GAPDH and secondary antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Saos-2 and MG-63 cell culture
The established human OS cell lines MG-63
(CRL-1427TM, ATCC) and Saos-2 (HTB-85TM, ATCC) were obtained from
the Cell Bank of Shanghai Institute of Biochemistry and Cell
Biology, Chinese Academy of Sciences (Shanghai, China), where they
were tested and authenticated. MG-63 and Saos-2 cells were cultured
in a humid atmosphere of 5% CO2 and 95% air at 37°C in
Dulbecco’s modified Eagle’s medium (DMEM) or McCoy’s 5A Medium
supplemented with 10% heat-inactivated fetal bovine serum (all were
from Gibco-Life Sciences, USA). The medium was changed every 2–3
days, and cells were passaged twice a week. All experiments
described were performed at least six times using cells at the
exponential growth phase.
Effect of GA on cell proliferation by the
cell counting kit-8 (CCK-8) assay
Saos-2 and MG-63 cells were seeded onto a 96-well
culture plate at a density of 0.8–1×104 cells/well. On
the second day of culture, media were replaced with 100 μl of
serum-free medium and GA at concentrations of 0–1 μM. On the third
day, 10 μl of CCK-8 were added to each well and incubated for 1 h.
The absorbance (A) was measured at 450 nm by a Microplate reader
(Bio-Rad 550; Bio-Rad, USA). The percentage of cell viability was
calculated as follows: cell viability (%) = A of experimental
well/A of control well ×100%. The IC50 was the
concentration of GA that caused 50% inhibition of cell
viability.
Matrigel invasion assay
Invasion assay was performed using a transwell
chamber (Neuro Probe, Inc., USA) and 8-μm pore size polycarbonate
membranes (Costar, Cambridge, MA, USA) coated with Matrigel. Saos-2
and MG-63 cells were seeded at a density of 4×104 in 350
μl of serum free-MEM in the upper compartment of the transwell.
Complete MEM was placed in the lower chamber. Following overnight
incubation at 37°C, the medium in the upper chamber was replaced
with serum-free MEM and cells were treated with GA at 0, 0.5, 0.75
and 1 μM for 48 h of incubation at 37°C in a 5% CO2
atmosphere. Non-invaded cells were wiped gently with the Matrigel.
Finally, the invaded cells on the surface of the membranes were
fixed and stained using Hemacolor® (Merck Millipore,
Darmstadt, Germany). The number of invaded cells in six randomly
selected microscopic fields (×200 magnifications) per membrane was
counted.
Western blot analysis
Cells were treated with GA (0, 0.5, 0.75 and 1 μM)
for 48 h, scraped into 1× cell lysis buffer (Cell Signaling
Technology, USA), and incubated for 10 min on ice. Debris from the
lysed cells was pelleted by centrifugation at 6,700 × g at 4°C for
5 min. The protein concentration of each sample was assayed using
the bicinchoninic acid method (BCA kit) (Pierce, Rockford, IL,
USA). Cell lysates, containing same amounts of protein, were mixed
with equal volumes of 4× sample loading buffer, boiled for 5 min,
cooled on ice for 5 min, and then analyzed by 10% Tris-HCl SDS
polyacrylamide gel electrophoresis (SDS-PAGE). Protein was
electrotransferred to a polyvinylidene difluoride membrane, and
then blocked with 5% nonfat dry milk in 20 mM of TBS with 0.1%
Tween-20 for 1 h at room temperature with shaking and incubated
with the indicated primary antibodies followed by HRP-conjugated
secondary antibody. After washing three times, bands were detected
using ECL western blotting detection reagents (Santa Cruz
Biotechnology, Inc.) and were then imaged with LAS-3000 (Life
Science-Fujifilm Global).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Cells were treated with GA (0, 0.5, 0.75 and 1 μM)
for 48 h and washed twice with ice-cold 1× phosphate-buffered
saline (PBS). Total RNA was extracted using TRIzol Reagent
(Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s
instructions. RNA (1 μg) was reverse-transcribed using the
Superscript™ First-Strand Synthesis System for RT-PCR (Invitrogen)
at 37°C. The following primers were used to determine target gene
levels. β-actin, sense, 5′-CTGGAGCATGCC CGTATTTA-3′ and antisense,
5′-TTTGGTCTTGCCACTTT TCC-3′; MMP-9, sense,
5′-AAGTGGCACCACCACAACAT-3′ and antisense,
5′-TTTCCCATCAGCATTGCCGT-3′; and TIMP-1, sense,
5′-TTCCGACCTCGTCATCAGGG-3′ and antisense,
5′-ATTCAGGCTATCTGGGACCGC-3′. All primers were checked against the
GenBank Database to ensure no cross-reactivity with other known
human DNA sequences. PCR cycles were performed using the following
sequence: 94°C for 5 min, then 30 cycles of denaturation at 94°C
for 1 min, annealing at 58°C (for MMP-9) or 60°C (for TIMP-1) for 1
min, and polymerization at 72°C for 1 min, followed by 72°C for 7
min. RT-PCR products were visualized on 1.2% agarose gels
electrophoresed in 0.5 TAE buffer containing 0.5 μg/ml ethidium
bromide.
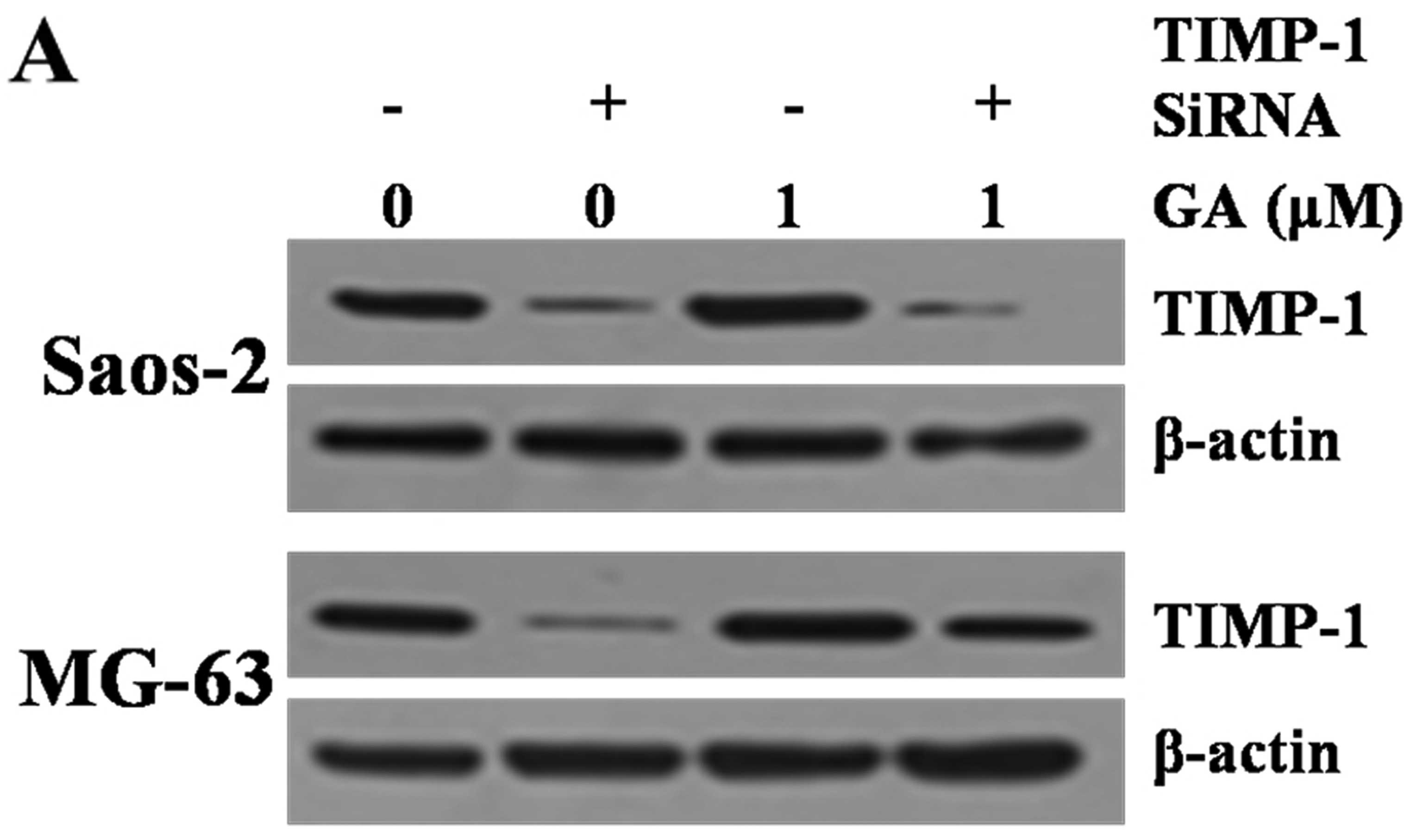
siRNA knockdown of TIMP-1
siRNA knockdown was used to inhibit mammalian
TIMP-1. Cells were transfected with a pre-designed siRNA (100 nM)
against TIMP-1 (sc-29505) using the siRNA Transfection Reagent
(sc-29528). The transfection efficiency was >80% (transfection
efficiency was assessed visually using control siRNA (fluorescein
conjugates) (sc-36869) (all were from Santa Cruz Biotechnology,
Inc.) and the extent of TIMP-1 knockdown was determined by western
blot analysis of protein levels.
Statistical analysis
Intensity of bands was quantified using SPSS 17.0
software. Results are expressed as the means ± standard deviation.
We performed Student’s t-test statistical analysis. P<0.05 was
considered to indicate statistically significant differences.
Asterisks indicate the level of significance.
Results
Growth inhibition of OS cell lines by
GA
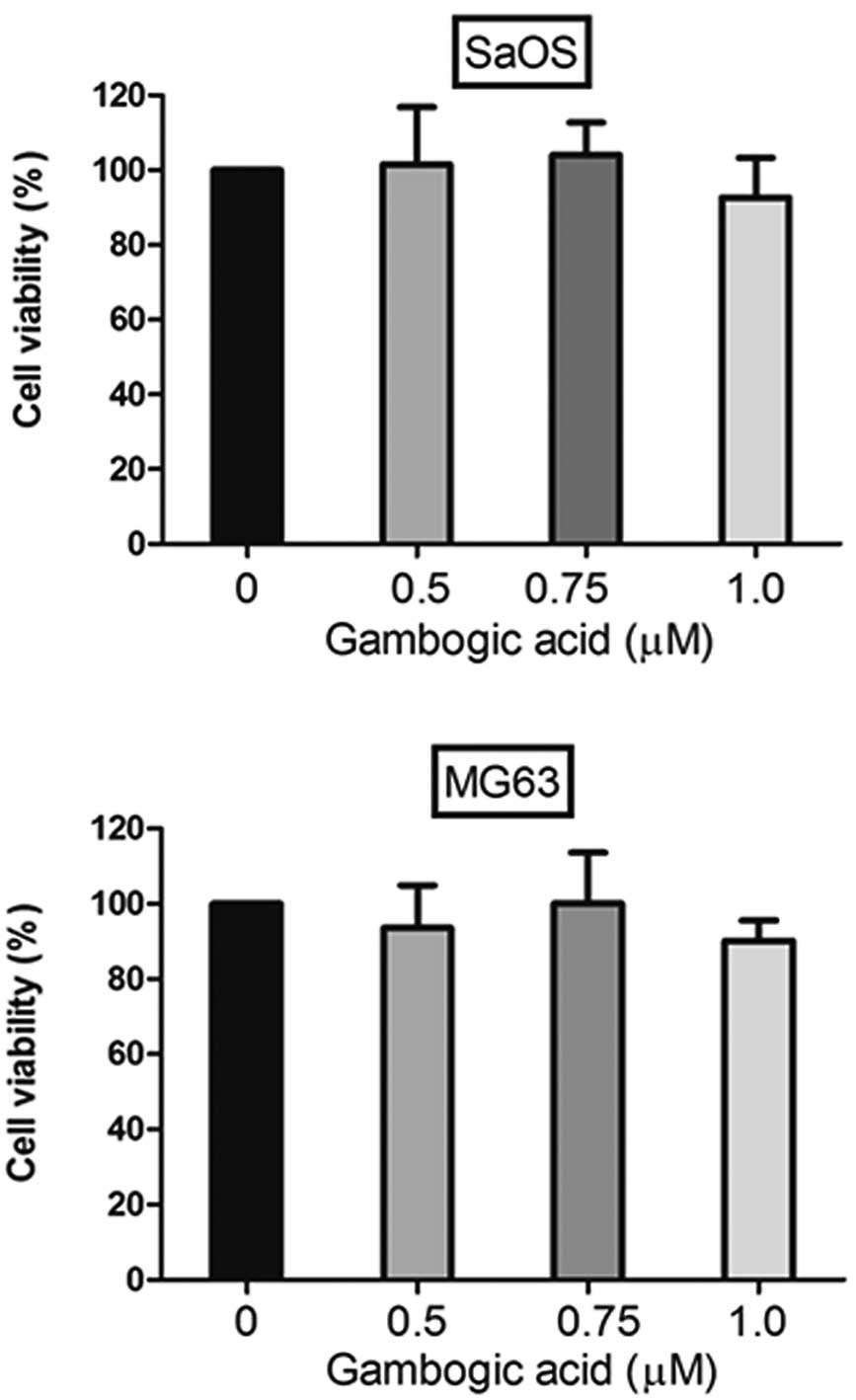
We examined the role of GA on proliferation using a
CCK-8 assay after treating both cell lines with GA. Saos-2 and
MG-63 cells (0.8–1×104 cell/well) were plated in each
well of a 96-well plate. Control and 0.5, 0.75 and 1 μM GA-treated
Saos-2 and MG-63 cells were incubated for 48 h. CCK-8 assay was
performed 2 days after the treatment in order to quantify the
proliferation of cells. GA treatment at 0–1 μM for 48 h did not
significantly inhibit the growth of either cell line, therefore,
there was no significant effect of GA on Saos-2 or MG-63 survival
even at the concentration of 1 μM. We performed all subsequent
experiments using GA concentrations ranging from 0 to 1 μM
(Fig. 1).
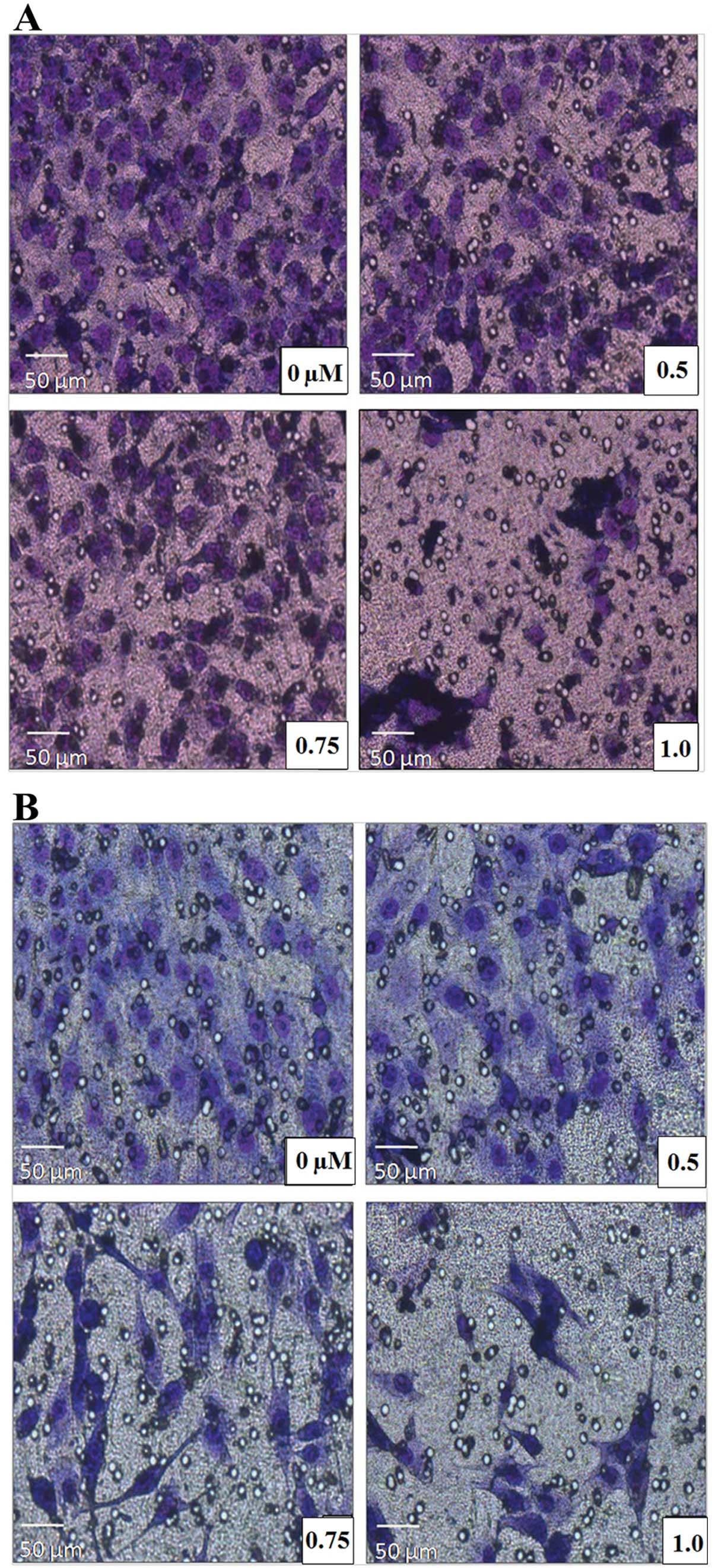
GA suppresses the invasive ability of
Saos-2 and MG-63 cells
We carried out Matrigel invasion assays to ascertain
whether GA affects the invasive behavior of Saos-2 and MG-63 cells.
We treated both cell lines with either control or GA (0.5, 0.75 and
1 μM). After 48 h of incubation, we removed transwells and stained
invaded cells with Hemacolor® (Merck Millipore) and
removed the non-invaded cells. The number of invaded Saos-2 and
MG-63 cells treated by GA was significantly reduced, compared to
the control, in a dose-dependent manner, indicating that GA
inhibited the basal invasion capacity of Saos-2 and MG-63 cells
(P<0.05) (Fig. 2).
GA suppresses MMP-9 and enhances TIMP-1
protein and mRNA levels in both cells
To elucidate the mechanism of invasive suppression
of Saos-2 and MG-63 cells, we investigated the effect of GA on
MMP-9 and TIMP-1 protein expression, which are key effectors for
tissue invasion. The expression of TIMP was evaluated since the
expression of TIMP suppresses the effect of MMP-9. Saos-2 and MG-63
cells were treated with GA and MMP-9 and TIMP-1 protein levels were
observed by western blotting. Western blotting revealed that GA
attenuated MMP-9 and increased TIMP-1 protein levels of both cell
lines (P<0.05) (Fig. 3).
RT-PCR was used to investigate the effects of GA on MMP-9 and
TIMP-1 at the transcriptional levels. GA was found to clearly
attenuate MMP-9 mRNA levels and the mRNA transcripts of TIMP-1 in
both Saos-2 and MG-63 cell lines (P<0.05) (Fig. 4).
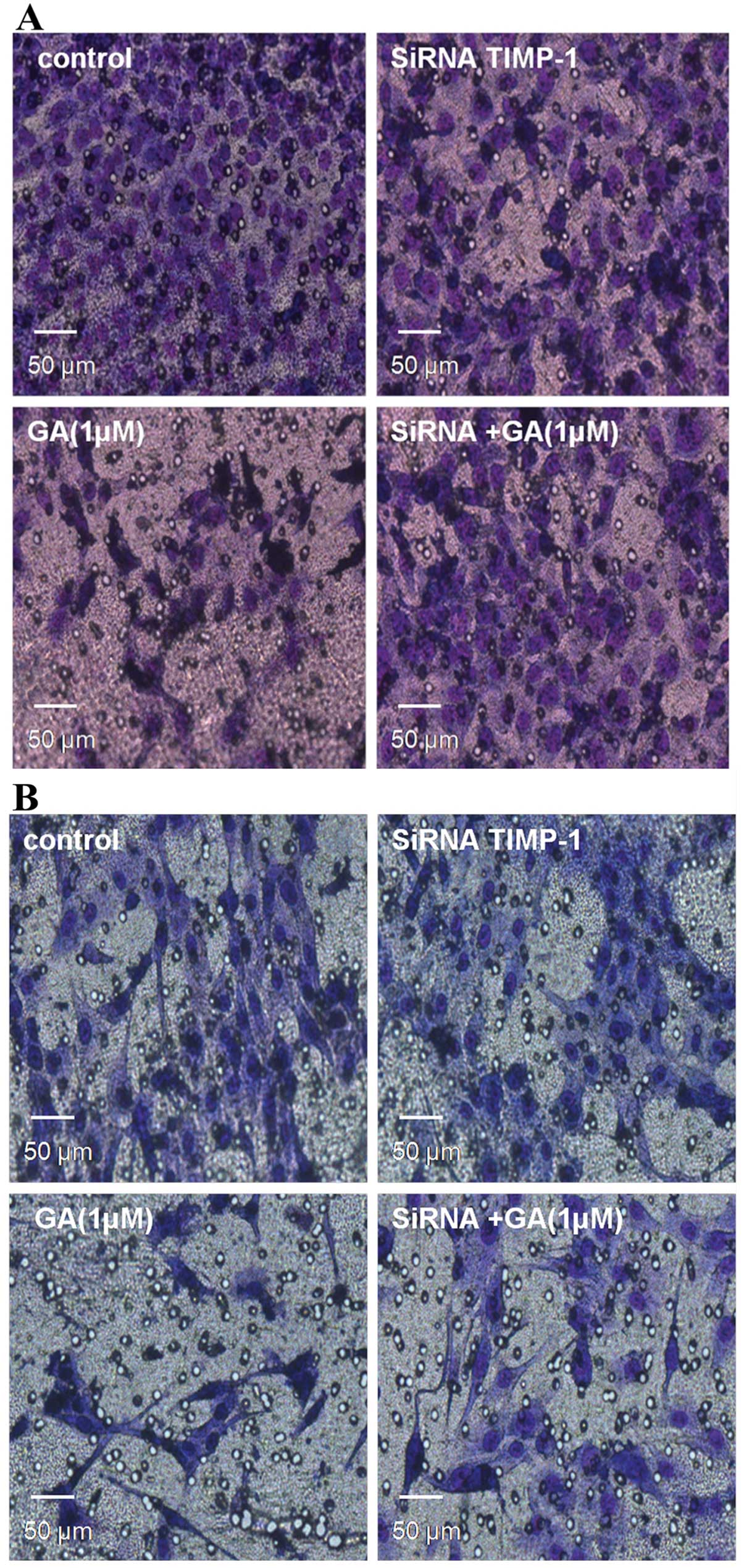
Knockdown of TIMP-1 restrains the
invasion ability of GA in both cell types
We carried out Matrigel invasion assays again to
examine whether TIMP-1 affects the invasive behavior of Saos-2 and
MG-63 cells. After transfection with siRNA TIMP-1 for 24 h, we
treated both cell lines with either control or GA (1 μM). After 48
h of incubation, we removed transwells and stained invaded cells
with Hemacolor® (Merck Millipore) and removed the
non-invaded cells. The number of invaded Saos-2 and MG-63 cells
treated by GA and siRNA TIMP-1 was significantly increased compared
to the cells treated by siRNA TIMP-1 alone, demonstrating that
TIMP-1 plays an important role in GA inhibiting the basal invasion
capacity of Saos-2 and MG-63 cells (P<0.05) (Fig. 5).
GA specifically enhances TIMP-1 protein
and mRNA levels in both cells
To further elucidate the mechanism of invasive
suppression of Saos-2 and MG-63 cells, we investigated the effect
of GA on TIMP-1 protein expression after transfection with siRNA
TIMP-1, which are key effectors for tissue invasion. Saos-2 and
MG-63 cells were treated with GA and/or siRNA TIMP-1. TIMP-1
protein levels were observed by western blotting. Western blotting
and RT-PCR revealed that GA increased only slightly the TIMP-1
protein and mRNA levels of both cell lines treated by siRNA TIMP-1
(P>0.05) (Fig. 6). However,
upregulation of TIMP-1 was significant in siRNA TIMP-1 and GA
treated cell lines compared to siRNA TIMP-1 treated cell lines
(P>0.05).
Discussion
Tumor metastasis involves a series of mechanisms
including vessel formation, cell attachment, invasion, migration
and cell proliferation, whose regulation is complex. Numerous in
vitro studies have demonstrated that GA exerts its antitumor
effects by impeding tumor cell proliferation, inhibiting invasion,
inducing tumor cell apoptosis and suppressing MMPs (20,28). In addition, Matrigel is used for
in vitro invasion assays. It contains a number of proteins
such as laminin, collagen IV, heparan sulfate proteoglycan,
entactin, nidogen and growth factors (e.g. TGF-β, fibroblast growth
factor, tissue plasminogen activator). It has been used as a model
in quantitative analysis. In the present study, we investigated the
anti-metastatic effect of GA on the invasion and migration of human
OS cell lines using Matrigel migration assay. We found that GA
inhibited the in vitro migration and invasion ability of
both cell lines (Fig. 2). Our
results support the potential use of GA as a new strategy for
anticancer therapy against migration and invasion of both cell
lines.
Furthermore, it is well known that MMPs and TIMPs
play key roles in tumor invasion and metastasis; as a result, the
prognostic risk factors of recent studies in OS are focusing on the
MMPs and TIMPs. Overall, studies on MMPs and TIMPs in cancer
provide the rationale for developing anticancer drugs which target
TIMP and MMP activities. TIMP-1 was described to specifically
regulate proMMP-9 activation (15). Zhang et al (29) demonstrated that tumor invasion and
metastasis were more frequent in patients with tumors which
secreted MMP-9 positively whereas they were significantly reduced
if TIMP-1 was also expressed; thus, MMP-9 mainly functions to
accelerate cancer invasion and metastasis, but TIMP-1 independently
exerts an inhibitory function on cancer invasion and metastasis.
However, Seo et al (30)
suggested that the expression of TIMP-1, as well as the balance
between the expression of MMP-9 and TIMP-1, indicates the
progressed state of the tumor. It has recently been reported that
MMP-2 and MMP-9 are present in human OS cells (31,32). In this study, GA inhibited MMP-9
expression and enhanced TIMP-1 at the protein levels; on the other
hand, the mRNA expression of MMP-9 and TIMP-1 was also suppressed
by GA.
As we described above, the results of the
experiments of molecular biology were based on the assumption of
survival rate of the cells greater than 80%. As a consequence, the
effect of GA on OS cell invasion is probably not due to cell death.
In terms of variation of MMP-9, TIMP-1 and previous theories, we
concluded that GA promotes the expression of TIMP-1, probably
further repressing the expression of MMP-9. Therefore, the balance
between MMP-9 and TIMP-1 may be one of the mechanisms which lead to
the inhibition of the OS cell invasion by GA. Of note, in this
study, the pharmaceutical effect of GA was reduced when the
expression of TIMP-1 was restrained by siRNA. This result revealed
that TIMP-1 plays an important role in the reduction of the
invasive potential of the OS cells which were treated by GA. The
specific pathway of GA-induced TIMP-1 and MMP-9 expression requires
further study to be elucidated.
In conclusion, our findings suggest that GA affects
the expression and balance of MMP-9 and TIMP-1 in OS, and that this
is responsible for its effect on OS cell invasiveness. This report
provides the first evidence that GA interferes with the expression
and balance of MMP-9 and TIMP-1 in OS cells in vitro.
Furthermore, TIMP-1 plays an important role in the reduction of the
invasive potential of the OS cells which were treated by GA.
Although the precise molecular mechanism of cancer invasiveness and
related targeted chemotherapy requires further clarification, these
data indicate that GA may be a potential candidate for targeted
drug therapy preventing recurrence and metastasis.
Acknowledgements
This study was supported by a grant (N20100757) from
the Department of Health of Zhejiang Province.
Abbreviations:
|
GA
|
gambogic acid
|
|
MMPs
|
matrix metalloproteinases
|
|
OS
|
osteosarcoma
|
|
TIMPs
|
tissue inhibitors of matrix
metalloproteinases
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
References
|
1
|
Bacci G, Longhi A, Fagioli F, Briccoli A,
Versari M and Picci P: Adjuvant and neoadjuvant chemotherapy for
osteosarcoma of the extremities: 27 year experience at Rizzoli
Institute, Italy. Eur J Cancer. 41:2836–2845. 2005.PubMed/NCBI
|
|
2
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU and Flege S: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: an analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar
|
|
3
|
Ferrari S, Briccoli A, Mercuri M, Bertoni
F and Picci P: Postrelapse survival in osteosarcoma of the
extremities: prognostic factors for long-term survival. J Clin
Oncol. 21:710–715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hauben EI, Arends J, Vandenbroucke JP, van
Asperen CJ, van Marck E and Hogendoorn PC: Multiple primary
malignancies in osteosarcoma patients. Incidence and predictive
value of osteosarcoma subtype for cancer syndromes related with
osteosarcoma. Eur J Human Genetics. 11:611–618. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Link MP: Preoperative and adjuvant
chemotherapy in osteosarcoma. Frontiers of Osteosarcoma Research:
Interdisciplinary Survey of Clinical and Research Advances. Novak
JF and Mcmaster JH: Hogrefe and Huber; Seattle: pp. 41–49. 1993
|
|
6
|
Wolf K and Friedl P: Mapping proteolytic
cancer cell-extracellular matrix interfaces. Clin Exp Metastasis.
26:289–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clark IM, Swingler TE, Sampieri CL and
Edwards DR: The regulation of matrix metalloproteinases and their
inhibitors. Int J Biochem Cell Biol. 40:1362–1378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: a tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hidalgo M and Eckhardt SG: Development of
matrix metalloproteinase inhibitors in cancer therapy. J Natl
Cancer Inst. 93:178–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Denhardt DT, Feng B, Edwards DR, Cocuzzi
ET and Malyankar UM: Tissue inhibitor of metalloproteinases (TIMP,
aka EPA): structure, control of expression and biological
functions. Pharmacol Ther. 59:329–341. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Phillips BW, Sharma R, Leco PA and Edwards
DR: A sequence-selective single-strand DNA-binding protein
regulates basal transcription of the murine tissue inhibitor of
metalloproteinases-1 (Timp-1) gene. J Biol Chem. 274:22197–22207.
1999. View Article : Google Scholar
|
|
15
|
Kossakowska AE, Urbanski SJ and Edwards
DR: Tissue inhibitor of metalloproteinases-1 (TIMP-1) RNA is
expressed at elevated levels in malignant non-Hodgkin’s lymphomas.
Blood. 77:2475–2481. 1991.
|
|
16
|
Yoshiji H, Gomez DE and Thorgeirsson UP:
Enhanced RNA expression of tissue inhibitor of metalloproteinases-1
(TIMP-1) in human breast cancer. Int J Cancer. 69:131–134. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fong KM, Kida Y, Zimmerman PV and Smith
PJ: TIMP1 and adverse prognosis in non-small cell lung cancer. Clin
Cancer Res. 2:1369–1372. 1996.PubMed/NCBI
|
|
18
|
Bjørnland K, Flatmark K, Pettersen S,
Aaasen AO, Fodstad O and Maelandsmo GM: Matrix metalloproteinases
participate in osteosarcoma invasion. J Surg Res. 127:151–156.
2005.PubMed/NCBI
|
|
19
|
Korpi JT, Hagström J, Lehtonen N, et al:
Expression of matrix metalloproteinases-2, -8, -13, -26, and tissue
inhibitors of metalloproteinase-1 in human osteosarcoma. Surg
Oncol. 20:e18–e22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao W, Zhou SF, Zhang ZP, Xu GP, Li XB
and Yan JL: Gambogic acid inhibits the growth of osteosarcoma cells
in vitro by inducing apoptosis and cell cycle arrest. Oncol
Rep. 25:1289–1295. 2011.
|
|
21
|
Guo QL, You QD, Wu ZQ, Yuan ST and Zhao L:
General gambogic acids inhibited growth of human hepatoma SMMC-7721
cells in vitro and in nude mice. Acta Pharmacol Sin. 25:769–774.
2004.PubMed/NCBI
|
|
22
|
Yu J, Guo QL, You QD, et al: Gambogic
acid-induced G(2)/M phase cell-cycle arrest via disturbing
CDK7-mediated phosphorylation of CDC2/p34 in human gastric
carcinoma BGC-823 cells. Carcinogenesis. 28:632–638. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kasibhatla S, Jessen KA, Maliartchouk S,
et al: A role for transferrin receptor in triggering apoptosis when
targeted with gambogic acid. Proc Natl Acad Sci USA.
102:12095–12100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Gu HY, Lu N, et al: Microtubule
depolymerization and phosphorylation of c-Jun n-terminal kinase-1
and p38 were involved in gambogic acid induced cell cycle arrest
and apoptosis in human breast carcinoma McF-7 cells. Life Sci.
83:103–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu ZQ, Guo QL, You QD, Zhao L and Gu HY:
Gambogic acid inhibits proliferation of human lung carcinoma SPC-A1
cells in vivo and in vitro and represses telomerase activity and
telomerase reverse transcriptase mRNA expression in the cells. Biol
Pharm Bull. 27:1769–1774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo Q, Qi Q, You Q, Gu H, Zhao L and Wu Z:
Toxicological studies of gambogic acid and its potential targets in
experimental animals. Basic Clin Pharmacol Toxicol. 99:178–184.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi Q, You Q, Gu H, et al: Studies on the
toxicity of gambogic acid in rats. J Ethnopharmacol. 117:433–438.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi Q, Lu N, Wang XT, et al: Anti-invasive
effect of gambogic acid in MDA-MB-231 human breast carcinoma cells.
Biochem Cell Biol. 86:386–395. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang S, Li L, Lin JY and Lin H: Imbalance
between expression of matrix metalloproteinase-9 and tissue
inhibitor of metalloproteinase-1 in invasiveness and metastasis of
human gastric carcinoma. World J Gastroenterol. 9:899–904.
2003.PubMed/NCBI
|
|
30
|
Seo YS, Park JJ, Kim JH, et al: Usefulness
of MMP-9/TIMP-1 in predicting tumor recurrence in patients
undergoing curative surgical resection for gastric carcinoma. Dig
Dis Sci. 52:753–759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guruvayoorappan C and Kuttan G:
Amentoflavone inhibits experimental tumor metastasis through a
regulatory mechanism involving MMP-2, MMP-9, prolyl hydroxylase,
lysyl oxidase, VEGF, ERK-1, ERK-2, STAT-1, NM23 and cytokines in
lung tissues of C57BL/6 mice. Immunopharmacol Immunotoxicol.
30:711–727. 2008. View Article : Google Scholar
|
|
32
|
Waas ET, Wobbes T, Lomme RM, DeGroot J,
Ruers T and Hendriks T: Matrix metalloproteinase 2 and 9 activity
in patients with colorectal cancer liver metastasis. Br J Surg.
90:1556–1564. 2003. View
Article : Google Scholar : PubMed/NCBI
|