Introduction
Mycobacterium tuberculosis (MTB) is a leading
cause of morbidity and mortality worldwide. It is estimated that
1.6 million people succumb to tuberculosis (TB) infection and nine
million new cases of active TB are reported each year (1). One third of the world’s population
lives with TB. In developing countries, TB infection remains a
major health problem.
Elimination of TB largely depends on definitive
rapid diagnosis and treatment. A rapid, simple and relatively
inexpensive diagnostic test is crucial for future control efforts.
The most common laboratory-based detection techniques used to
identify MTB are mycobacterial culture and acid-fast bacillus (AFB)
staining (2). Although
mycobacterial culture continues to be a valuable diagnostic tool,
it usually requires several weeks to obtain the results. AFB
staining provides rapid evidence for the presence of mycobacteria
in a clinical specimen, however, 40–60% of patients with pulmonary
TB infection and approximately 75% of patients with extra-pulmonary
TB infection are smear negative (3).
Immunological detection methods provide a useful
approach for the diagnosis of TB. The major advantages of
immunological detection are speed and simplicity, and it is also
useful for the detection of extra-pulmonary TB or smear negative
TB. Although considerable efforts have been made for the
development of immune-based tests for the detection of antibodies,
antigens, and immune complexes, their performance varies widely.
Therefore, the development of rapid and accurate new diagnostic
tools is crucial. Antibody detection is one of the most convenient
forms for the TB immunodiagnostic test, which relies on the
MTB-specific antigen. Despite extensive studies on mycobacterial
antigens over the past 100 years, so far only a few serodiagnostic
kits are available. Due to the common antigen existing in MTB and
M. bovis Bacillus Calmette-Guérin (BCG), selection of
MTB-specific antigens for antibody detection is key in
distinguishing the MTB infection or BCG vaccination.
In the present study, we cloned, expressed and
purified several MTB recombinant proteins including 6-kDa early
secretory antigenic target of MTB (ESAT-6), 10-kDa culture filtrate
protein (CFP-10), ESX-1 substrate protein C (ESPC), 14KD/38KD, and
ESAT-6/14KD/38KD antigens. Based on these antigens, we also set up
the ELISA-based serological test and evaluated the immunoreactivity
of the target proteins with sera from pulmonary TB patients and
healthy controls. We demonstrated that the serological detection of
IgG antibody against the target MTB antigens can successfully
distinguish the active TB-infected patient from the healthy
control. This study may help identify the MTB-specific antigens to
improve the serological detection sensitivity for MTB.
Materials and methods
Study population and serum sample
collection
A total of 98 serum samples were obtained from
patients (65 males and 33 females, aged between 16 to 84 years)
suspected to have active pulmonary TB. All were patients at Nanjing
Chest Hospital and had not received any anti-TB chemotherapy when
the serum samples were collected. No patient identified as having
the HIV-1 infection was included in this study. All subjects
provided informed consent to participate in this study. The study
population of TB patients was divided into the following groups:
Group I included the mycobacterium positive pulmonary TB patients
(n=54); Group II included the mycobacterium negative pulmonary TB
patients (n=44); Group III was the healthy individuals of this
locality, which included 102 healthy control volunteers who had
reported previously receiving BCG vaccination. The TB patients and
healthy control volunteers were confirmed with the following
methods: X-ray, clinical symptoms and patient history. Blood
samples were collected, sera were separated, and serum specimens
were obtained upon admission prior to any therapy and stored at
−80°C until testing.
Cloning, expression and purification of
recombinant MTB antigens
Related gene encoding individual MTB antigen was
amplified from H37RV genomic DNA by using the codon-optimized
primers (Table I). The PCR
product was sub-cloned into the vector pET-30a and pET-32a
(Novagen) using the relevant restriction enzyme recognition sites.
An His6-tag fusion peptide was attached at the C-terminus of the
MTB protein, and a Trx.Tag was fusion expressed on the N-terminus
of the MTB ESAT-6, CFP-10 and ESPC antigens. The correctness of the
sequence was confirmed by DNA sequencing analysis.
 | Table IPrimers and vectors used for cloning
MTB antigen genes. |
Table I
Primers and vectors used for cloning
MTB antigen genes.
| Antigen | Forward primer
sequence (5′→3′) | Reverse primer
sequence (5′→3′) | Expression
vector |
|---|
| ESAT-6/14KD/38KD | F1: GGATCCATGGAGGCCGCGGCAAG | R1:
ACCAGAACCTGCGAACATCCCAGTGACGTT | pET-30a |
| F2:
GGTTCTGGTGAGTTTTCTGAGCTGTTCGCGG | R2:
ACCAGAACCGTTGGTGGACCGGATCTGAATGT | |
| F3:
GGTTCTGGTCACGAGAGGTATCCGAACGTCAC | R3: CTCGAGTCAGTCAGACAACTTCACCACCGCG | |
| 14KD+38KD | F1: GGATCCATGGCCACCACCCTTCCCG | R1:
ACCAGAACCCCGGATCTGAATGTGCTTTTCG | pET-30a |
| F2:
GGTTCTGGTAGCACGCTGCTCTACCCGCT | R2: CTCGAGTCACGCGATCAACGCGTCAGAC | |
| ESAT-6 | F: GGAATTCCATATGGCAGAGATGAAGACCGATGC | R:CCCAAGCTTTCAGAAGCCCATTTGCGAGGAC | pET-32a |
| CFP-10 | F: GGAATTCCATATGACAGAGCAGCAGTGGAATTTCG | R:CCCAAGCTTCTATGCGAACATCCCAGTGACGTT | pET-32a |
| ESPC | F: GGAATTCCATATGACGGAAAACTTGACCGTCCAG | R:CCCAAGCTTTCAGGTAAACAACCCGTCGATAGC | pET-32a |
For the production of recombinant protein, the
obtained construct pET-30-MTB and pET-32-MTB coded for the target
recombinant MTB proteins was transformed into Escherichia
coli (E. coli) BL21(DE3) Rosetta 2 (Novagen) cells. The
recombinant cells were grown in 4 liters of 2YT media at 37°C,
shaken at 110 rpm in chicane flasks and induced with 2 mM
isopropyl-β-D-thiogalactopyranoside (IPTG) at an optical density
(OD) 600 1.5 for 4 h at 37°C. Subsequently, the cells were
harvested by a 10-min centrifugation at 6,000 × g, and were
re-suspended in TBS buffer (containing 20 mM HEPES pH 7.5).
To purify the recombinant MTB protein, the lysate
supernatant was applied to a 10 ml Ni-NTA (Qiagen) column
equilibrated with TBS, washed with 10 column volumes (CV) of TBS,
and eluted with 2 CV of 500 mM imidazole in TBS. The eluted protein
was concentrated to 5 ml with a centrifugal filter concentration
apparatus (10 kDa molecular mass cutoff, cellulose membrane;
Millipore) and further purified by size-exclusion chromatography
(Sephacryl S-100 HiPrep 26/60, TBS equilibrated) using an AKTAprime
system (GE Healthcare) at a flow-rate of 1.0 ml/min. Fractions
containing the purified protein of interest were concentrated as
before to 10 mg/ml. The final protein concentration was measured
using a bicinchoninic acid (BCA) kit (Pierce) and a
spectrophotometer. The protein purity was confirmed by the presence
of a single band on 12% sodium dodecyl-sulphate polyacrylamide gel
electrophoresis (SDS-PAGE) stained with Coomassie Brilliant
Blue.
Antibody detection by ELISA
The ELISA procedure was performed as follows:
96-well polystyrene microtiter plates were coated overnight at 4°C
with 100 μl of antigen solution (1 μg/ml for final
concentration) in phosphate-buffered saline (PBS, pH 7.4). The
coated plates were washed twice with PBS-T (PBS; 0.05% Tween-20, pH
7.4), and blocked with 1% bovine serum albumin (BSA) in PBS-T for 2
h at room temperature. Serum dilution of 1:200 was used in the
assay. Serum samples were added and incubated for 2 h at room
temperature. The plates were washed three times with PBS-T and then
incubated for 1 h with rabbit anti-human IgG or rabbit anti-human
IgM antibody conjugated with horseradish peroxidase diluted
1:10,000 in PBS-T. After five washes with PBS-T, the enzyme
activity was assayed by incubation for 15 min at room temperature
with 100 μl of mixtures of tetramethylbenzidine (TMB) (10
μg TMB) per well. Then, 50 μl of 1 N sulfuric acid
was added to stop the reaction and the OD was evaluated in 450 nm.
The serum samples were tested in duplicate wells and at least three
repeat experiments were performed to verify the reproducibility of
results.
Data management and statistical
analysis
The statistical analysis was performed as previously
described (4). Briefly, the means
± standard deviation of the OD of individual groups were determined
in comparison with the mean OD value for the healthy control serum
samples ± standard deviations. The ROC curve and cutoff value was
calculated using the MedCalc statistical software (Broekstraat,
Belgium). The individual samples were scored as positive for the
specific antibody response when the OD value was above the cutoff
value. Sensitivity was determined by dividing the number of
positive cases by the total number of TB patients. Specificity was
determined by dividing the number of negative controls by the total
number of healthy controls. The differences among groups were
analyzed by the Mann-Whitney test using the SPSS for Windows
statistical software (SPSS Inc., Chicago, IL, USA). Differences
were considered statistically significant at P<0.05.
Results
Generation of MTB recombinant
antigens
To generate the MTB recombinant antigens for
immunoassay, we first amplified the individual MTB antigen encoding
gene by PCR. Five MTB related antigen genes including the gene
encoding CFP-10, ESAT-6, ESPC antigen, ESAT-6/14KD/38KD fusion
protein, 14KD/38KD fusion protein, were successfully constructed.
The corresponding genes were inserted into the individual pET
vectors using either BamHI or XhoI for pET-30a
vector, or using EcoRI and HindIII restriction site
for pET-32a vector. The construction of recombinant plasmids which
expressed CFP-10, ESAT-6 and ESPC antigen protein were fusion
expressed with an N-terminal Trx.Tag and a C-terminal His6-tag,
which were confirmed by DNA sequencing. The expression of the
corresponding MTB recombinant proteins was induced by IPTG in E.
Coli BL21(DE3). The expression product was further purified by
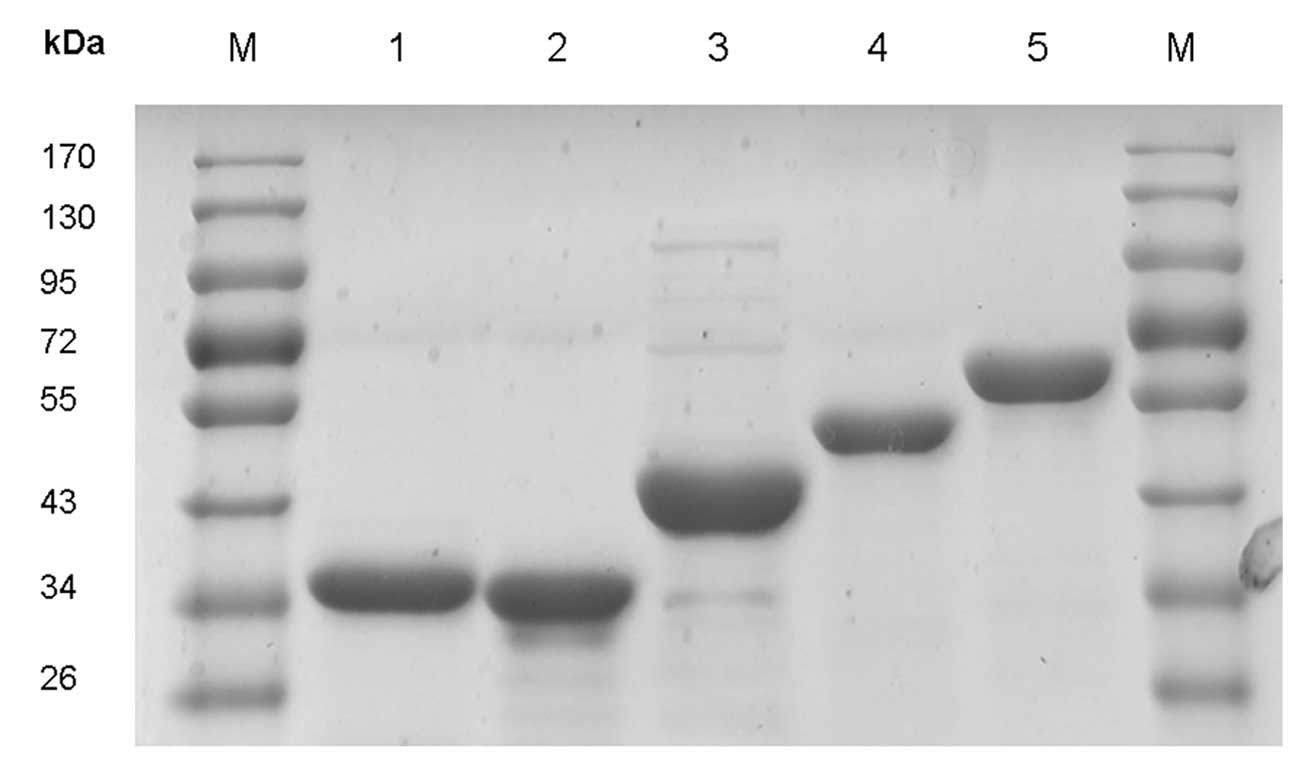
metal chelate affinity chromatography (Fig. 1); >90% purity was achieved,
measured by SDS-PAGE assay.
Serological test of active TB infection
using recombinant antigens
We generated and identified the serodiagnostic
potential of various MTB-specific antigens including ESAT-6,
CFP-10, ESPC, ESAT-6/14KD/38KD and 14KD/38KD recombinant proteins.
Using ELISA, we characterized the humoral immune response during TB
infection by measuring serum IgG and IgM antibodies to
mycobacterial antigen in 98 patients with active TB compared to 102
healthy control.
Mean ± SD values of IgG and IgM antibody titers to
the individual mycobacterial antigens in active TB patients were
determined. Sensitivity and specificity for a particular antigen
were determined using ROC analysis. For all the antigens, the
cutoff was selected at the point which showed the best accuracy,
sensitivity and specificity by ROC. A predictive value (PV) to
define the probability of a disease was also analyzed and included
the positive predictive value (PPV) to characterize a patient for
the particular disease from the patient’s population, and a
negative predictive value (NPV) to exclude the disease. The OD
value of TB patients and control subjects with IgG antibodies to
the individual mycobacterial antigen are summarized in Table II. The areas of under the curves
and 95% CI were also calculated (Table II). The OD value of IgM antibody
detection results is not shown, as there were no significant
differences between the healthy control subjects and active TB
patients.
 | Table IIIgG reactivity against various MTB
antigens. |
Table II
IgG reactivity against various MTB
antigens.
| Antigen | Cutoff | Sensitivity (%) | Specificity (%) | PPV | NPV | +LR | −LR | ROC area |
|---|
| ESAT-6/14KD/38KD | 0.365 | 77.6 | 84.3 | 82.6 | 79.6 | 4.94 | 0.27 | 0.867 |
| 14+38 kDa | 0.378 | 74.5 | 90.2 | 88.1 | 78.6 | 7.6 | 0.28 | 0.873 |
| ESAT-6 | 0.268 | 69.4 | 89.2 | 86.1 | 75.2 | 6.43 | 0.34 | 0. 842 |
| CFP-10 | 0.241 | 72.5 | 78.4 | 76.3 | 74.8 | 3.36 | 0.35 | 0.804 |
| ESPC | 0.245 | 75.5 | 84.3 | 82.2 | 78.2 | 4.81 | 0.29 | 0.855 |
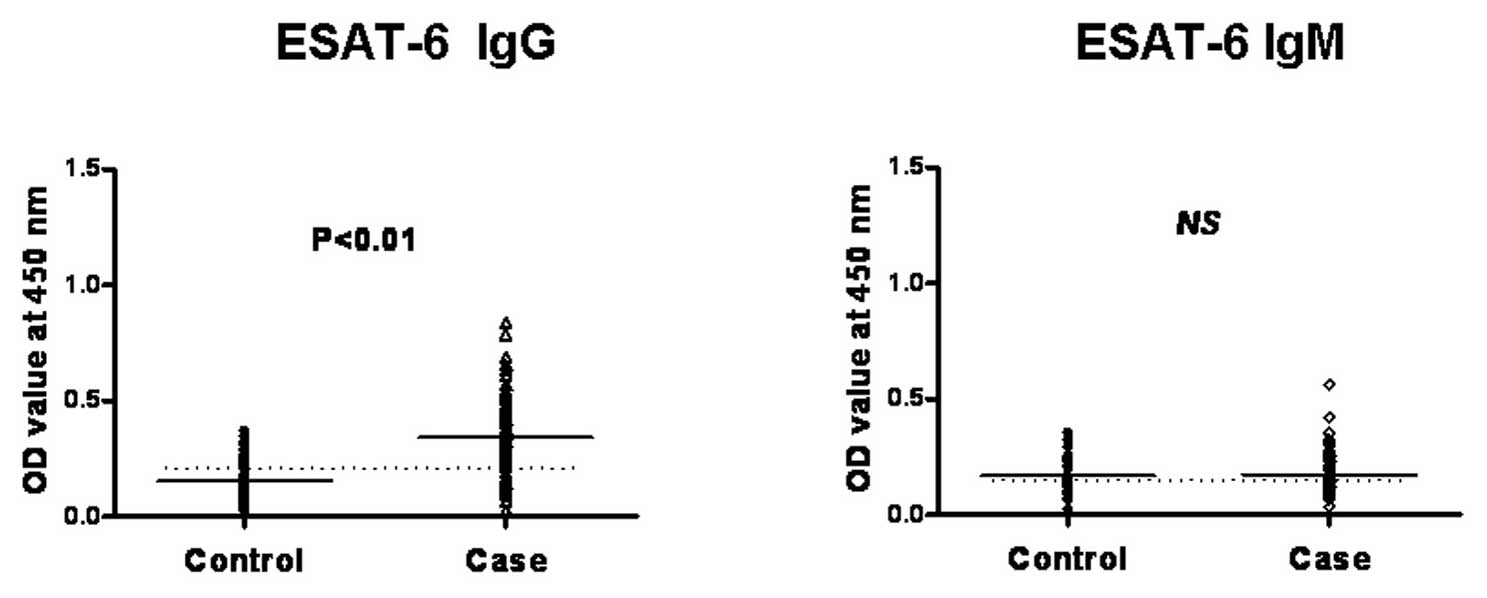
We found the levels of IgG antibodies against each
antigen in TB patients were significantly higher than those that in
the healthy controls (P<0.01) (Fig. 2). However, regarding the IgM
antibody detection, we found that the IgM level did not differ
between the examined active TB and the healthy control group; a
relatively low OD value was obtained in both assays (Fig. 2). Therefore, this result indicated
that characterization of IgG antibodies against these antigens can
effectively distinguish between TB patients and healthy
controls.
We observed the IgG antibody reactivity to the
individual antigens, ESAT-6, CFP-10, ESPC, ESAT-6/14KD/38KD and
14KD/38KD components was 69.4, 72.5, 75.5, 77.6 and 74.5%;
specificity was 89.2, 78.4, 84.3, 84.3 and 90.2%, respectively. The
overall positive rates of the detection of MTB IgG antibodies
against each recombinant antigen by ELISA were calculated. The
positive rates in active TB patients were significantly higher than
those in healthy controls (P<0.01). Using the cutoff established
by ROC, we determined that the positive rate for ESAT-6, CFP-10,
ESPC, 14KD/38KD and ESAT-6/14KD/38KD antigens was 65, 73, 77, 80
and 82%, respectively. We noted that the positive rates for
antibody responses to multiple antigens such as ESAT-6/14KD/38KD
and 14KD/38KD antigens were slightly increased and at the same time
the specificity was at approximately the comparable level.
Therefore, detection of serum antibody responses to multiple
antigens may be valuable for the diagnosis of MTB infection.
Moreover, we did not observe any difference regarding to the
detection rate, the sensitivity and specificity by comparing the
mycobacterium positive and negative group (data not shown).
Discussion
Currently, the test used for the diagnosis of MTB
infection includes sputum examination for the presence of MTB,
culture of sputum or other body fluid, the tuberculin skin test and
radiology, which is either insensitive or time consuming. In search
of rapid and cost-effective diagnostic methods for MTB infection,
immunodiagnosis, which uses the specific humoral and cellular
immune responses of the host to identify the presence of infection
or disease, is considered an attractive option. During the MTB
infection, although the cellular immune responses are more
important than the humoral immune responses in mediated immune
protection, the antibody-mediated immune response can be used to
set up serodiagnosis of MTB infection (5). MTB serodiagnosis is considerably
simpler and less expensive and it has additional advantages in
situations when the patient is unable to produce adequate sputum or
when sputum smear results are negative and TB is
extra-pulmonary.
For the establishment of effective immunoassays,
extensive studies have been carried out for the identification of
MTB-specific immunodominant antigens (6). Purification of these immunogenic
antigens directly from MTB is challenging due to low cell yields,
slow growth rates and the virulent nature of the bacteria (7). A potential solution to this problem
is the production of recombinant antigens in organisms such as
E. coli. In this study, we successfully cloned, expressed
and purified several MTB antigens including ESAT-6, CFP-10, ESPC,
ESAT-6/14KD/38KD, 14KD/38KD recombinant proteins and evaluated
their diagnostic potential by ELISA for the detection of
MTB-specific antibodies. By comparing the healthy individuals with
confirmed TB patients, we found higher levels of MTB-specific IgG
antibodies can be detected in the sera of TB patients. The levels
of MTB antigen-specific IgG antibodies in TB patients are much
higher than those in healthy controls, indicating that serological
tests for MTB IgG antibodies against these MTB antigens are
beneficial for the diagnosis of MTB infection.
The antigens selected for the current study have
been reported to be strong targets for humoral and cell-mediated
immune responses. These target antigen genes are conserved in MTB
and M. Bovis isolates but are partially deleted or absent in
M. bovis BCG as well as in most nontuberculous mycobacteria
(NTM) (8). Therefore, these
antigens are potential candidate antigens for the serodiagnosis of
TB. Previous comparative genomic studies using subtractive DNA
hybridization and DNA microarray identified several MTB-specific
antigens. In the MTB genome, the region of difference (RD) 1, which
encodes several MTB-specific antigens, is present in virulent
strains of MTB but is missing from most NTM and M. bovis
BCG. The significance of this gene to virulence has been
demonstrated experimentally; deletion of the 9.5 Kb RD1 region from
MTB results in attenuation similar to BCG in cultured macrophages
and mice (9). The RD1 region has
been reported to encode several immunodominant proteins, RV3875,
also known as the 6-kDa early secretory antigenic target of MTB
(ESAT-6), RV3874 also known as the 10-kDa culture filtrate antigen
(CFP-10). ESAT-6 and CFP-10 are both linked with virulent MTB
(10). The MTB strains and
complements that were positive for ESAT-6 were virulent, whereas
all strains negative for ESAT-6 were avirulent. Similarly, all
strains shown to export CFP-10 at high levels were virulent,
whereas strains with less CFP-10 in supernatants were avirulent.
ESAT-6 and CFP-10 have been reported to be strong targets for
humoral and cell-mediated immune response (11). RV3615, also known as ESX-1
substrate protein C (ESPC), is a highly immunodominant
RD1-dependent secreted antigen specific for MTB infection (12). Although the ESPC encoding gene is
located outside RD1, the expression and secretion of this antigen
were RD1-dependent. It has been reported that the ESPC antigen can
be detected in the MTB H37RV culture filtrate, but it is absent
from the MTB H37RvΔRD1 culture filtrate. Therefore, ESPC can be
recognized specifically in MTB infection but not in BCG-vaccinated
persons. ESPC contained multiple T cell recognize peptides, which
have been used for T cell-based immunoassay such as immunospot or
cytokine secretion assay. Whether it can be used for antibody
detection has yet to be investigated. In this study, we
demonstrated that the ESPC antigen also elicits strong humoral
immune responses, which can be used for the serodiagnostic assay
for MTB infection.
Distinguishing active TB from non TB diseases in
clinical practice using ELISA IgG against mycobacterial ESAT-6 and
CFP-10 antigen has been proven to be useful. In a study by Kumar
et al (11) that was based
on the ELISA method, high sensitivity (64.9/66%) and specificity
(88.9/85.2%) were detected using either ESAT-6 or CFP-10 antigen.
Another report detected 60.4% sensitivity and 73.8% specificity
using the ESAT-6/CFP-10 recombinant antigen (13). Similar to these previous studies,
we found that the sensitivities of ELISA for detecting IgG
antibodies against ESAT-6, CFP-10 and ESPC antigen were 69.4, 72.5
and 75.5%, respectively, with specificities of 89.2, 78.4 and
84.3%, respectively. The positive rates in active TB patients were
significantly higher than those in healthy controls
(P<0.01).
In addition to the individual MTB antigens, we also
generated two recombinant fusion proteins by combined expression of
several individual MTB antigens. These included ESAT-6/14KD/38KD
and 14KD/38KD MTB antigens. The 38-kDa extracellular lipoprotein of
MTB is a phosphate-binding protein (PBP) with features very similar
to those of the well characterized periplasmic phosphate-binding
protein of E. coli, which serves as an initial receptor for
active transport. It is one of the most important immunogenic
antigens of MTB-inducing B- and T-cell responses with high
specificity for TB. Using the immunoblot assay, this antigen was
the first to be specifically associated with TB infection (14), and it is also considered a prime
candidate for the development of new diagnostic reagents for the
diagnosis of active TB (15).
The 14KD molecular weight antigen of MTB is also
known as 16-kDa antigen, belonging to the α crystalline family of
low molecular weight heat shock proteins (HSP) (3). This antigen is one of the prominent
antigens of MTB defined by anti TB monoclonal antibodies (MAbs). It
was originally identified by three MAbs generated in two separate
laboratories and it demonstrated potential as a serodiagnostic
target in assay protocols based on MAb competition and direct ELISA
(16). This antigen contains B
cell epitopes specific for the MTB complex, and it has been
suggested that this antigen is immunogenic in the early stages of
infection with MTB and in primary TB infection (17).
Compared with the individual ESAT-6, CFP-10 and ESPC
antigen, in this study, we noted that the combination expression of
the above target antigens ESAT-6/14KD/38KD and 14KD/38KD can
improve the sensitivity at the same time without affecting
specificity; 81 and 81.6% sensitivity for ESAT-6/14KD/38KD and
14KD/38KD antigens was determined in patients with active pulmonary
TB. This result indicated that the antibody response against TB
infection was heterogeneous (18). Therefore, investigation of the
antibody profile as well as the antibody detection against multiple
MTB antigens is crucial for improving the detection accuracy of
TB.
We also found that the sensitivities and
specificities of the IgG test were better than those of the IgM
test; the overall IgM measurement in TB patients had a poor
diagnostic sensitivity compared with the healthy control, which is
in agreement with previous reports that the sensitivities of the
IgM test raised against most of the MTB antigens such as 38KD
antigen, were significantly lower than those of the IgG assays
(19,20). Another report showed that IgM
measurement using different antigens does not correlate with the
presence of TB in children (21).
We consider that this result is likely due to the
heterogeneity of the rates of immunochromatographic IgG and IgM
antibodies to the target MTB antigens, which depends on the phase
of TB infection. In primary pulmonary TB infection, IgM titers were
initially high but IgG titers were very low, resulting in low
positivity of IgG. In post primary pulmonary TB, low titers of IgM
and high titers of IgG could explain high rates of positivity of
IgG. Healing of post-primary TB was accompanied by substantial
lowering of the IgG positivity rate and suppression of IgM
positivity (22). We speculated
the relatively low titers of IgM antibodies is likely due to the
fact that the majority of the patients involved in this study were
post-primary pulmonary TB, or, in another case, it may be due to
the chronic infection with prolonged exposure to MTB in our
setting.
In summary, the present study demonstrated that
diagnosis of active TB with a simple multi-antigen ELISA test is
feasible. This approach is less expensive and involves minimal
technical requirements for testing. Using the recombinant ESAT-6,
CFP-10, ESPC, ESAT-6/14KD/38KD, 14KD/38KD antigens, we detected
elevated levels of IgG antibody against the target mycobacterial
antigens, indicating that this test is of significant value for the
rapid diagnosis of MTB infection. A better understanding of the
dynamic repertoire of antibody responses in patients with MTB
infection as well as other mycobacterial infections may facilitate
the development of more sensitive and specific antibody-based
methods for the diagnosis of active pulmonary TB infection. Further
studies with larger cohorts of patients are required to confirm our
results.
Acknowledgements
This study was supported by the grants
from the Natural Science Foundation of Jiangsu Province China (no.
BK2010245) and the National Natural Science Foundation of China
(no. 81071298 and 81030013), the grant from National Basic Research
Program of China (Grant no. 2009CB918704).
References
|
1
|
WHO. Global Tuberculosis Control. WHO;
Geneva: 2011
|
|
2
|
Ryan K and Ray CG: Sherris Medical
Microbiology. 4th edition. McGraw Hill; New York, NY: 2004
|
|
3
|
Ben-Selma W, Harizi H and Boukadida J:
Immunochromatographic IgG/IgM test for rapid diagnosis of active
tuberculosis. Clin Vaccine Immunol. 18:2090–2094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang MM, Zhao JW, Sun ZQ, Liu J, Guo XK,
Liu WD and Zhang SL: Identification of RD5-encoded Mycobacterium
tuberculosis proteins as B-cell antigens used for serodiagnosis
of tuberculosis. Clin Dev Immunol. 2012:7380432012.PubMed/NCBI
|
|
5
|
Welch RJ, Lawless KM and Litwin CM:
Antituberculosis IgG antibodies as a marker of active
Mycobacterium tuberculosis disease. Clin Vaccine Immunol.
19:522–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujita Y, Doi T, Sato K and Yano I:
Diverse humoral immune responses and changes in IgG antibody levels
against mycobacterial lipid antigens in active tuberculosis.
Microbiology. 151:2065–2074. 2005. View Article : Google Scholar
|
|
7
|
Imaz MS, Comini MA, Zerbini E, Sequeira
MD, Spoletti MJ, Etchart AA, Pagano HJ, Bonifasich E, Diaz N, Claus
JD and Singh M: Evaluation of the diagnostic value of measuring
IgG, IgM and IgA antibodies to the recombinant 16-kilodalton
antigen of Mycobacterium tuberculosis in childhood
tuberculosis. Int J Tuberc Lung Dis. 5:1036–1043. 2001.PubMed/NCBI
|
|
8
|
Raja A, Ranganathan UD and Bethunaickan R:
Improved diagnosis of pulmonary tuberculosis by detection of
antibodies against multiple Mycobacterium tuberculosis
antigens. Diagn Microbiol Infect Dis. 60:361–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis KN, Liao R, Guinn KM, Hickey MJ,
Smith S, Behr MA and Sherman DR: Deletion of RD1 from
Mycobacterium tuberculosis mimics bacille Calmette-Guérin
attenuation. J Infect Dis. 187:117–123. 2003.PubMed/NCBI
|
|
10
|
Hsu T, Hingley-Wilson SM, Chen B, Chen M,
Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M,
Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH
and Jacobs WR Jr: The primary mechanism of attenuation of bacillus
Calmette-Guerin is a loss of secreted lytic function required for
invasion of lung interstitial tissue. Proc Natl Acad Sci USA.
100:12420–12425. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar G, Dagur P, Singh P, Shankar H,
Yadav V, Katoch V, Bajaj B, Gupta R, Sengupta U and Joshi B:
Serodiagnostic efficacy of Mycobacterium tuberculosis
30/32-kDa mycolyl transferase complex, ESAT-6, and CFP-10 in
patients with active tuberculosis. Arch Immunol Ther Exp. 58:57–65.
2010.
|
|
12
|
Millington KA, Fortune SM, Low J, Garces
A, Hingley-Wilson SM, Wickremasinghe M, Kon OM and Lalvani A:
Rv3615c is a highly immunodominant RD1 (region of difference
1)-dependent secreted antigen specific for Mycobacterium
tuberculosis infection. Proc Natl Acad Sci USA. 108:5730–5735.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu X, Yang Y, Zhang J, Li B, Liang Y,
Zhang C, Dong M, Cheng H and He J: Humoral immune responses against
the Mycobacterium tuberculosis 38-kilodalton, MTB48, and
CFP-10/ESAT-6 antigens in tuberculosis. Clin Vaccine Immunol.
17:372–375. 2010.PubMed/NCBI
|
|
14
|
Espitia C, Cervera I, González R and
Mancilla R: A 38-kD Mycobacterium tuberculosis antigen
associated with infection. Its isolation and serologic evaluation
Clin Exp Immunol. 77:373–377. 1989.
|
|
15
|
Bothamley GH and Rudd RM: Clinical
evaluation of a sero-logical assay using a monoclonal antibody
(TB72) to the 38 kDa antigen of Mycobacterium tuberculosis.
Eur Respir J. 7:240–246. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Verbon A, Hartskeerl RA, Schuitema A, Kolk
AH, Young DB and Lathigra R: The 14,000-molecular-weight antigen of
Mycobacterium tuberculosis is related to the
alpha-crystallin family of low-molecular-weight heat shock
proteins. J Bacteriol. 174:1352–1359. 1992.PubMed/NCBI
|
|
17
|
Bothamley GH, Beck JS, Potts RC, Grange
JM, Kardjito T and Ivanyi J: Specificity of antibodies and
tuberculin response after occupational exposure to tuberculosis. J
Infect Dis. 166:182–186. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lyashchenko K, Colangeli R, Houde M, Al
Jahdali H, Menzies D and Gennaro ML: Heterogeneous antibody
responses in tuberculosis. Infect Immun. 66:3936–3940.
1998.PubMed/NCBI
|
|
19
|
Demkow U, Filewska M, Białas B,
Szturmowicz M, Zielonka T, Wesołowski S, Kuś J, Ziołkowski J,
Augustynowicz-Kopeć E, Zwolska Z, Skopińiska-Rábewska E and
Rowińska-Zakrzewska E: Antimycobacterial antibody level in pleural,
pericardial and cerebrospinal fluid of patients with tuberculosis.
Pneumonol Alergol Pol. 72:105–110. 2004.PubMed/NCBI
|
|
20
|
Wilkinson RJ, Hasløv K, Rappuoli R,
Giovannoni F, Narayanan PR, Desai CR, Vordermeier HM, Paulsen J,
Pasvol G, Ivanyi J and Singh M: Evaluation of the recombinant
38-kilo-dalton antigen of Mycobacterium tuberculosis as a
potential immunodiagnostic reagent. J Clin Microbiol. 35:553–557.
1997.PubMed/NCBI
|
|
21
|
Demkow U, Ziołkowski J, Białas-Chromiec B,
Filewska M, Zielonka T, Wasik M and Rowińska-Zakrzewska E: Humoral
immune response against mycobacterial antigens in children with
tuberculosis. J Physiol Pharmacol. 57(Suppl 4): S63–S73. 2006.
|
|
22
|
Zou YL, Zhang JD, Chen MH, Shi GQ, Prignot
J and Cocito C: Serological analysis of pulmonary and
extrapulmonary tuberculosis with enzyme-linked immunosorbent assays
for anti-A60 immunoglobulins. Clin Infect Dis. 19:1084–1091. 1994.
View Article : Google Scholar : PubMed/NCBI
|