1. Introduction
Functional dyspepsia (FD) is regarded as a
functional gastrointestinal disorder (FGID), but it is a
heterogeneous disorder (1).
Although FD is divided into 2 major subgroups, epigastric pain
syndrome (EPS) and postprandial distress syndrome (PDS) (2), up to half of the patients with FD
present with an overlap of both PDS and EPS (3). Multiple pathogenic mechanisms have
been proposed for FD, but its underlying etiology remains unclear,
and its pharmacological therapy is also poorly understood.
It is known that hormones of the brain-gut axis are
involved in the regulation of gut motility. Motilin,
cholecystokinin (CCK), glucose-dependent insulinotropic peptide
(GIP), glucagon like peptide-1 (GLP-1), peptide YY (PYY) and
ghrelin are major gut hormones, and pharmacological therapy with
certain hormones of the brain-gut axis are considered a novel
treatment for FGID. In particular, ghrelin, which exhibits a
variety of bioactivities, has attracted attention as a novel
therapeutic drug.
Ghrelin, a 28-amino acid peptide hormone with
structural resemblance to motilin, was originally discovered in the
endocrine X/A-like cells of the gastric mucosa in rats and humans
(4). The ghrelin gene consists of
4 exons and 3 introns, and ghrelin exists in 2 major molecular
forms: acyl ghrelin, which has an n-octanoylated serine at position
3 (Ser3) and des-acyl ghrelin, which lacks
n-octanoylation (5–7). The third ghrelin gene product,
obestatin, which is a 23-amino acid peptide and was identified in
the rat stomach, was found by comparative genomic analysis
(8,9). Ghrelin is also detectable in the
hypothalamus, intestine, pituitary gland, placenta, salivary gland,
as well as other tissues (4,8,10–12). Acylation at Ser3 is
essential for the binding of ghrelin to growth hormone (GH)
secretagogue-receptor (GHS-R)-1 and is catalyzed by ghrelin
O-acyltransferase (GOAT) (13). Acyl ghrelin plays an important
role in the stimulating the appetite, it regulates energy
homeostasis and affects gastrointestinal motor activity, emptying
(9,14–16) and gastric acid secretion (17,18). It also suppresses inflammation
(8,9,18–21).
On the basis of this wide range of physiological
functions, clinical trials of the efficacy of exogenous acyl
ghrelin as a novel therapy have been performed in several
disorders, including anorexia (22), cachexia (23,24), FD (25) and malnutrition with gastroparesis
(26). The aim of the present
review was to provide an overview of the effects of ghrelin on FD,
as well as to elucidate its pharmacological potential.
2. Functional dyspepsia
FD is characterized by one or more of 4 chronic
symptoms, including epigastric pain or burning, postprandial
fullness and early satiety in the absence of any known organic,
systemic, or metabolic disease. According to the Rome III
consensus, FD has been further subclassified into PDS and EPS
(27). Although multiple
pathogenic mechanisms are involved, patients with FD do not show
evidence of organic disease in the upper digestive tract when
examined by endoscopy or computed tomography (28). According to the Rome III criteria,
FD symptoms must be present for at least 3 months prior to
diagnosis, and a recent study revealed that the prevalence of FD in
Japanese adults according to the Rome III criteria was 6.5%
(29). However, the Rome III
classification of FD is based on patient symptoms and the symptoms
of patients with FD are not stable; thus, biochemical correlates of
symptoms are extremely difficult to establish. According to the
health insurance system in Japan, few dyspepsia patients wait 3
months prior to seeking medical care. Therefore, in the study by
Miwa (28), it was estimated that
the actual prevalence of FD in Japanese adults approaches
20–30%.
FD refers to symptoms centered in the upper
abdominal region. Some putative mechanisms have been suggested,
involving biological interplay along the brain-gut axis according
to the development of symptoms, including genetics, Helicobacter
pylori (H. pylori) infection, visceral hypersensitivity,
abnormal gastric acid secretion, abnormal gastroduodenal motility,
autonomic/central nervous system (CNS) dysfunction, multiple
psychosocial morbidities, depression and anxiety, as well as the
effects of social stress (1,30–33).
It has previously been reported that G-protein β3
subunit gene polymorphism is associated with FD (34). It has also been suggested that the
serotonin transporter gene polymorphism, 5-hydroxytryptamine
transporter gene linked polymorphic region (5-HTTLPR), affects
susceptibility to PDS (35).
These data suggest that the genetic factor may play a significant
role in the development of FD. Residual inflammation following
gastrointestinal infection is also known to increase the likelihood
of developing FD, as demonstrated by a cohort study (36).
Association between H. pylori infection
and FD
As regards the role of acute gastrointestinal (GI)
infection and genetic susceptibility (37), certain studies in Western and
Japanese populations have suggested that H. pylori infection
is unlikely to influence the prevalence of FD (38,39), and other studies have shown that
H. pylori infection does not affect the overall prevalence
of symptoms, gastric sensorimotor function, gastric sensitivity and
the accommodation of the stomach to meals in patients with FD
(40,41). On the other hand, it has been
reported that acid secretion, which is affected by infection with
H. pylori, is associated with dyspeptic symptoms (42). A meta-analysis in China
demonstrated that the association between H. pylori
eradication and FD improvement is significant (43). A Cochrane review reported that the
eradication of H. pylori in some patients with FD had a
small but statistically significant long-term effect on symptom
relief when compared with the placebo (44,45). Moreover, a recent review also
supported that H. pylori eradication improved symptoms in
patients with FD, although certain studies only evaluated patients
at 1 month (46). Thus, as
regards H. pylori infection, opinion remains divided,
although a recent consensus has suggested that if H. pylori
infection is regarded as gastritis, an organic disease, then H.
pylori associated dyspepsia should not be considered a
functional disorder (47).
Association between stress and FD
Gastric hypersensitivity has been associated with
symptoms of postprandial pain, belching and weight loss (48), although other studies have failed
to demonstrate these associations (49,50). A recent study suggested that
gastric hypersensitivity is greater in patients with FD with a
traumatic stress in early life than in the control patients with FD
(33). Stress responses are
mediated by stimulation of the hypothalamus-pituitary-adrenal (HPA)
axis (51). In the hypothalamic
paraventricular nucleus (PVN), stress induces the secretion of
corticotropin-releasing factor (CRF), which stimulates the
secretion of adrenocorticotropic hormone (ACTH) through the
corticotrophs of the anterior pituitary (52). Moreover, ACTH induces the
secretion of cortisol (53). The
CRF system consists of 4 ligands: CRF, urocortin (UCN)-1, −2 and
−3, and 2 G-protein-coupled receptors (GPCR), CRF-receptor 1
(CRF-R1) and CRF-R2, as well as a secreted CRF binding protein
(CRF-BP) (54,55). The CRF-R1 system regulates anxiety
via the HPA axis. CRF-R2 is involved in regulation of upper GI
motility and CRF-R1 is involved in regulation of colonic motility
(56–58). A history of abuse, both physical
and sexual, has been associated with alterations in gastric
sensorimotor function and impaired gastric empting in patients with
FD (33). In animal experiments,
it has been shown that maternal separation during the neonatal
period is a powerful stress inducer that results in increased
visceral sensation and plasma corticosterone levels (59). When repeated doses of strong
electric shock were administered to neonatal mice, their
hippocampal glial cells became necrotic and this incuded the
migration of stem cells from the bone marrow to the hippocampus
(60). Thus, traumatic stress and
noxious stimuli in early life may lead to serious neuroplastic
alterations in the HPA and in the descending pathways that modulate
spinal nociceptive transmission, as well as in the CNS (61). Moreover, such neuroplastic changes
can occur in spinal ascending neurons receiving synaptic input from
the gastrointestinal organs (central sensitization) and in the
brain (supraspinal pain modulation), but also in primary sensory
afferent terminals (peripheral sensitization) (61). These alterations in the processing
of sensory information are considered mechanisms of visceral
hypersensitivity (61).
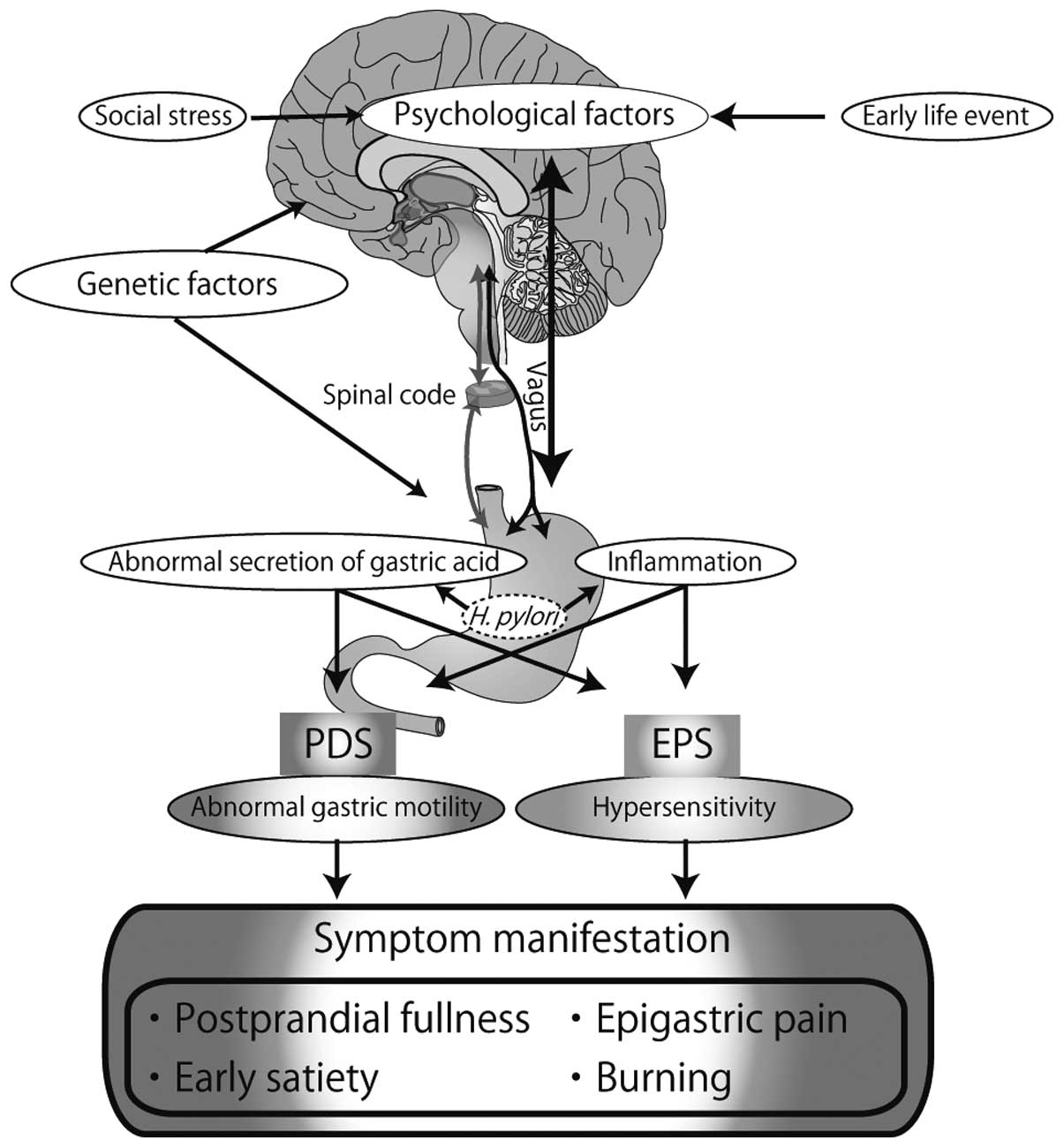
Although several factors have been implicated in the
pathophysiology of FD, the underlying pathogenesis of FD remains
unclear and warrants further investigation. A schematic model
illustrating the suggested pathogeneis of FD is presented in
Fig. 1.
3. Effect of ghrelin on gastrointestinal
motility via the brain-gut axis
The derangement of plasma ghrelin family protein
levels may affect gut motility regulation in patients with FD.
Fasting total ghrelin levels may assist with the evaluation of the
pathophysiology of FD. In addition, acyl ghrelin levels have been
associated with gastric emptying (62,63), and delayed gastric emptying has
been associated with postprandial fullness and severe early satiety
in patients with FD (64). In
this section, we elucidate the neural transmission mechanisms of
each of the 3 ghrelin gene products as they act on gastrointestinal
or gastroduodenal motility.
Acyl ghrelin
Acyl ghrelin has various functions in the
stimulation of GH secretion through GHS-R and in the regulation of
food intake, energy homeostasis, insulin secretion, as well as
glucose and lipid metabolism. Acyl ghrelin functions in the
gastrointestinal system, the cardiovascular system and the immune
system (8). Acyl ghrelin is the
only ghrelin hormone with an orexigenic effect following peripheral
administration (65). Acyl
ghrelin induces the gastric phase III of the migrating motor
complex and the gastric emptying of solids in healthy human
volunteers (15). Studies
conducting experiments on rats in the fed state, have shown that
the intracerebroventricular (ICV) and intravenous (IV)
administration of acyl ghrelin induces phase III-like contractions
of the antrum and the duodenum in the fasted state and increases
the percentage motor index (%MI) of the antrum (66). This fasted motor activity of the
gastrointestinal tract has been considered to be a mechanical
cleansing of the stomach and the intestines in preparation for the
next meal. Truncal vagotomy blocked the effects of the ICV
administration of acyl ghrelin on antral and duodenal motility
(66). Therefore, a vagal pathway
may mediate the influence of centrally administrated ghrelin on
gastroduodenal motility. Acyl ghrelin induces fasted motor activity
in the gastrointestinal tract by the activation of neuropeptide Y
(NPY) neurons in the hypothalamus via both central pathways and a
vago-vagal reflex (12). The
effects of the IV administration of acyl ghrelin were not altered
by the ICV administration of GHS-R antagonist in a vagotomized rat
model. Acyl ghrelin acts via the GHS-R of vagal afferent fibers in
the stomach (14) that transmit
the signal to the nucleus of the solitary tract (NTS). The
information from the NTS is projected to the arcuate nucleus (ARC)
of the hypothalamus, where the NPY neurons are activated. The NPY
Y2 and/or Y4 receptors in the CNS may be involved in upper
gastrointestinal motility as Y2 and Y4 receptor agonists can induce
phase III-like contractions in the duodenum when administered to
animals in the fed state (67,68). The signal from the ARC is finally
transmitted to the dorsal motor nucleus of the vagus nerve [dorsal
vagal complex (DVC)] and via vagal efferent fibers, and fasted
motor activity is induced in the gut (69). Evidence suggests that acyl ghrelin
may potentially be used as an exogenous prokinetic agent for the
treatment of slow transit in the upper gastrointestinal tract
(70).
Des-acyl ghrelin
Des-acyl ghrelin exerts opposite effects to acyl
ghrelin on food intake and gastrointestinal motility (71). Two separate studies have shown
that transgenic mice with an overexpression of des-acyl ghrelin
exhibited a decrease in body weight (71,72). The intracisternal (IC) and
peripheral administration of des-acyl ghrelin have been shown to
significantly decrease food intake in food-deprived mice and delay
gastric emptying (73,74), and des-acyl ghrelin has been shown
to inhibit the orexigenic effect of acyl ghrelin (75). Moreover, des-acyl ghrelin
abolishes the effects of acyl ghrelin on the secretion of
pancreatic hormone (76).
The ICV and IV administration of des-acyl ghrelin
has been shown to disrupt fasted motility in the antrum but does
not alter the fed duodenum in conscious rats, and does not alter
fed motor activity in either the antrum or the duodenum (74). The IV administration of des-acyl
ghrelin increases neuronal c-fos expression in the ARC and in the
PVN of the hypothalamus, but not in the NTS. It has been suggested
that des-acyl ghrelin is involved in the regulation of motor
activity in the stomach and crosses the blood-brain barrier (BBB)
(77), but not through the
activation of vagal afferent pathways (71).
Changes in gastric motility induced by the IV
administration of des-acyl ghrelin are antagonized by the ICV
administration of a selective CRF-R2 antagonist, but are not
affected by CRF-R1 antagonist (74). It has been suggested that des-acyl
ghrelin directly activates neurons in the PVN and exerts inhibitory
effects on antral motility via CRF-R2, but not CRF-R1 (74). CRF and the endogenous CRF receptor
ligand, UCN, are feeding-inhibitory peptides localized at the PVN
in the hypothalamus (78). UCN
binds both CRF-R1 and CRF-R2. CRF-R2 is involved in stress-induced
alterations in GI function (79).
The ICV and IV administration of UCN has been shown to disrupt
fasted motor activity in both antrum and duodenum, which show
fed-like motor patterns (80).
Therefore, des-acyl ghrelin may effect the stress-induced
alteration of GI function.
Obestatin
Although obestatin was initially reported to be an
endogenous ligand for the orphan G protein coupled receptor GPR39
(81), a number of studies have
reported the non-specific binding of obestatin to various types of
GPR39-expressing cells (82–84). In contrast to des-acyl ghrelin,
the effects of obestatin on food intake are controversial (71,74,85,86). The IV administration of obestatin
decreases the %MI in the antrum and prolongs the time before the
return of fasted motility in the duodenum (87); obestatin also inhibits
gastroduodenal motility in the fed state but not in the fasted
state of conscious rats (87).
The IV administration of obestatin has been shown to induce c-fos
expression in the PVN of the hypothalamus, and immunofluorescence
overlap staining has shown that PVN neurons containing CRF and
UCN-2 are activated by the IV administration of obestatin (87). The inhibitory effect of the IV
administration of obestatin on the motor activities in the antrum
and duodenum is blocked by the ICV administration of CRF-R1 and
CRF-R2 antagonists (87).
Treatment with capsaicin has been shown to block the effects of
obestatin on duodenal motility but not on antral motility (87). It has been shown that obestatin
fails to antagonize the ability of acyl ghrelin to stimulate
gastroduodenal motility (87).
These results suggest that obestatin inhibits motor activity in the
antrum and duodenum in the fed state and that both CRF-R1 and
CRF-R2 receptors in the brain may be involved in the effects of
obestatin on gastroduodenal motility. Moreover, vagal afferent
pathways may be partially, but not entirely, involved (87).
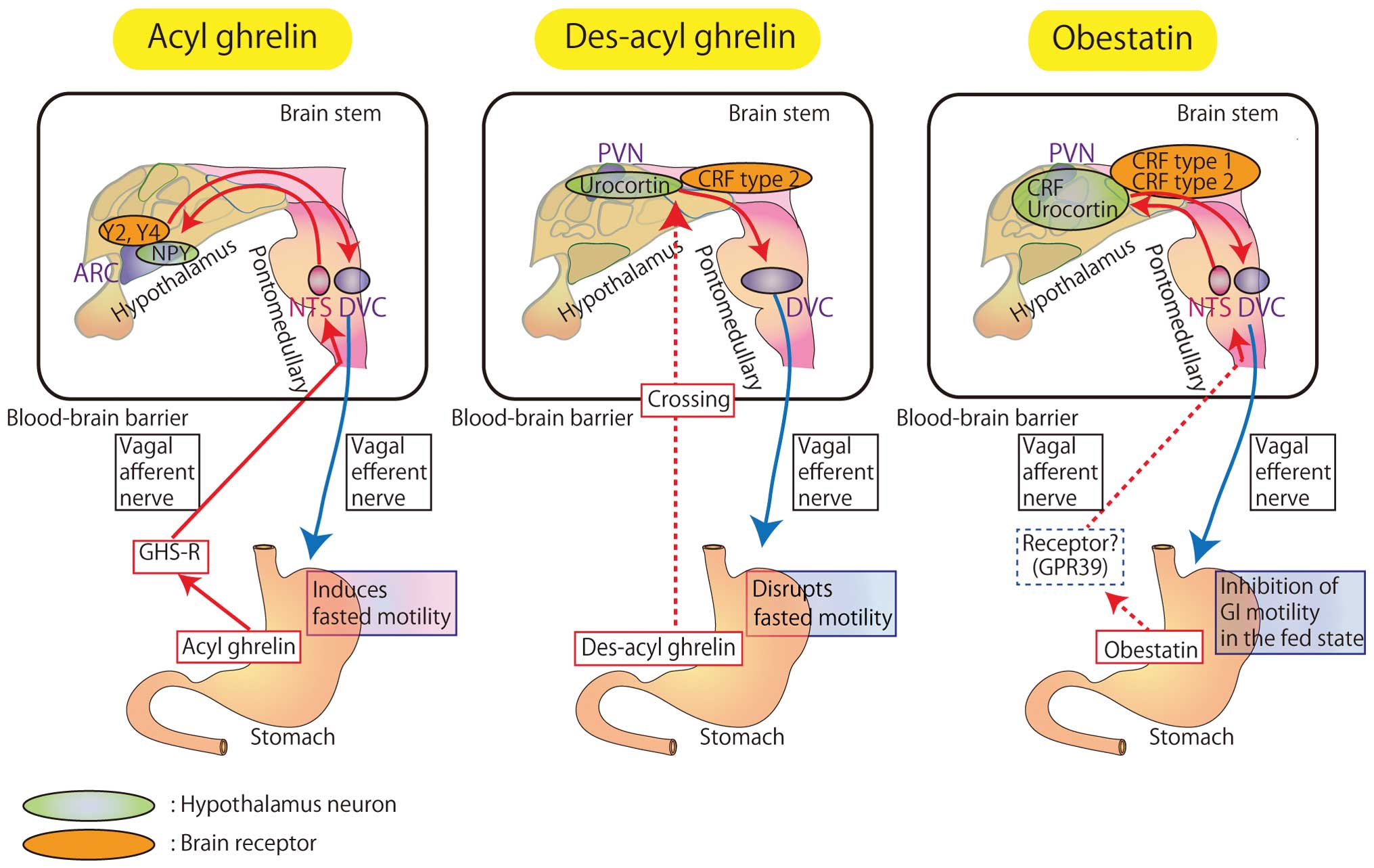
The distinct effects of each of the 3 ghrelin gene
products on gastrointestinal motility via the brain-gut axis are
summarized in Fig. 2.
4. Alterations in plasma ghrelin levels of
patients with FD
Two studies found that fasting total ghrelin levels
were significantly lower in patients with FD compared with healthy
volunteers (63,88). Furthermore, these studies showed a
significant decrease in the total ghrelin concentration that was
evident postprandially in the controls but was absent in the
patients with FD (63,88). Nishizawa et al (89) showed that the total ghrelin levels
in patients with FD categorized by the gastrointestinal symptom
rating scale (GSRS) score positively correlated with the
indigestion scores.
Two independent studies also demonstrated that
plasma acyl ghrelin levels correlated with a subjective symptom
score in female patients with FD (89,90), and Nishizawa et al
(89) further demonstrated that
plasma acyl ghrelin levels were significantly higher in patients
with FD compared with healthy volunteers. However, the study by
Shinomiya et al (90)
indicated that plasma acyl ghrelin levels were not significantly
different between patients with FD and the controls, and another
recent study indicated that plasma acyl ghrelin levels were
significantly lower in patients with PDS compared with healthy
volunteers, and the Tmax value, which is a marker of
gastric emptying using a 13C acetate breath test, was
significantly higher in patients with PDS compared with healthy
volunteers (62). Moreover, this
study demonstrated that only patients with PDS showed a significant
inverse correlation between plasma acyl ghrelin levels and
Tmax values (62).
Although the results presented in the study by Kim
et al (91) leave room for
doubt as they focused only on 2 patients, the fasting plasma acyl
ghrelin levels of the 2 patients with EPS were lower than the
postprandial plasma acyl ghrelin levels. It is important to gather
clinical data on patients with EPS as no general statement can be
obtained from this study.
Takamori et al (88) reported that fasting des-acyl
ghrelin levels were significantly lower in patients with FD
compared with the controls, but Shinomiya et al (90) showed that des-acyl ghrelin levels
were not significantly different between patients with FD and the
controls.
It has been reported that the ratio of acyl ghrelin
to des-acyl ghrelin (A/D ratio) also positively correlates with
acyl ghrelin levels between patients with FD and the controls
(90). Takamori et al
(88) also suggested that A/D
ghrelin ratios were significantly higher in patients with FD
compared with the controls. Therefore, the A/D ratio may be an
important signal for the evaluation of the pathophysiology of FD.
However, the role of this ratio in clinical studies has not yet
been established. Further mechanistic studies are required.
Studies that have assessed total ghrelin, acyl
ghrelin and des-acyl ghrelin levels in patients with FD have
produced conflicting results, as patients with FD present with a
variety of symptoms. This variability may contribute to the
different results in each study. As a result, there may be little
evidence of a clearly defined role of ghrelin in FD. However, since
acyl ghrelin affects gastrointestinal motor activity and emptying
(9,16), acyl ghrelin may be more closely
associated with PDS than EPS. Similarly, other forms of ghrelin may
also be associated with FD. Therefore, it is important that
patients are divided into groups based on their symptoms when
evaluating the role of ghrelin in FD. The association between
ghrelin and FD is summarized in Table
I.
 | Table ITotal ghrelin, acyl ghrelin and
des-acyl ghrelin levels in patients with functional dyspepsia. |
Table I
Total ghrelin, acyl ghrelin and
des-acyl ghrelin levels in patients with functional dyspepsia.
|
Authors/(Refs.) | Criteria | Patients | Control | Acyl ghrelin | Des-acyl
ghrelin | Total ghrelin | Correlation |
|---|
| Kim et al
(91) | (Rome III) | EPS; n=9
PDS; n=13 | n=12 | No
difference
No difference | | | Fasting plasma acyl
ghrelin levels negatively correlated with the sum score of
epigastric pain.
The postprandial/fasting plasma acyl ghrelin ratio positively
correlated with the sum score of early satiety.
Fasting plasma acyl ghrelin levels negatively correlated with
fasting normogastriaa. |
| Shindo et al
(62) | (Rome III) | EPS; n=36
PDS; n=76 | n=20 | Lower in PDS | No difference | | Acyl ghrelin levels
negatively correlated with Tmax value. |
| Lee et al
(63) | (Rome II) | Dysmotility-like
dyspepsia; n=42 | n=14 | | | Lower in FD | |
| Shinomiya et
al (90) | (Rome II) | Dysmotility-like
dyspepsia; n=14
Ulcer-like; dyspepsia; n=4 | n=18 | No difference | No difference | | Acyl ghrelin levels
positively correlated with the subjective symptom score. |
| Takamori et
al (88) | (Rome II) | Dysmotility-like;
dyspepsia; n=16 | n=19 | No difference | Lower in FD | Lower in FD | Plasma ghrelin
levels, including total, acyl and des-acyl ghrelin did not
correlate with Tmax value. |
| Nishizawa et
al (89) | (Rome II) | Dysmotility-like;
dyspepsia; n=16
Ulcer-like; dyspepsia; n=12
Non-specific type; dyspepsia; n=19 | n=17 | Higher in FD | | Higher in FD | Acyl ghrelin and
total ghrelin levels positively correlated with indigestion
score. |
5. Clinical potential of acyl ghrelin as a
novel therapy for FD
Although few clinical reports are available on the
administration of acyl ghrelin to patients with FD (25), it is anticipated that acyl ghrelin
may be effective in relieving the symptoms of FD. As previously
reported, repeated IV infusions of acyl ghrelin (3 μg/kg) twice a
day to patients with FD for 2 weeks tends to increase daily food
intake by approximately 30%, and hunger sensation has been shown to
be significantly enhanced at the end of this course of therapy
(25). Increased food intake was
maintained even 1 week following the termination of treatment. In
addition, ulimorelin (TZP-101), which is an acyl ghrelin receptor
agonist, has been shown to relieve upper gastrointestinal symptoms
and accelerate gastric emptying in diabetic patients with
gastroparesis in a randomized clinical trial (26). These results demonstrate the
therapeutic potential of acyl ghrelin, including its agonists or
antagonists.
Similarly, serotonin [5-hydroxytryptamine (5-HT)]
released from enterochromaffin cells of the duodenal mucosa
mediates intestinal phase III-like contractions via 5-HT4 receptors
in conscious rats (92). A
randomized, double-blinded, placebo-controlled, crossover study
revealed that the 5-HT1A receptor agonist, buspirone, significantly
reduced the overall severity of symptoms of FD and the individual
symptoms of postprandial fullness, early satiation and upper
abdominal bloating. A 5-HT1A receptor agonist did not alter the
rate of gastric emptying of solids or the sensitivity to gastric
distention, but significantly increased gastric accommodation,
whereas gastric emptying of liquids was delayed (93). An experimental study on conscious
rats showed that mosapride citrate (mosapride), which is a
prokinetic agent with 5-HT4 receptor agonistic activity, inhibited
gastric distension-induced visceromotor response (VMR) along with
blockage of 5-HT3 receptors by a mosapride metabolite (94,95). In addition, a recent clinical
trial showed that the orexigenic effect of rikkunshito, a
traditional Japanese medicine, is involved in the stimulation of
acyl ghrelin secretion by blocking the 5-HT2B/2C receptor pathway
and enhancing GHS-R activity (96); another recent randomized
controlled trial showed that the administration of rikkunshito to
patients with FD for 4 weeks improved upper gastrointestinal
symptoms, which was accompanied by an increase in the levels of
acyl ghrelin (97). Thus,
rikkunshito may be another therapeutic agent, leading to the
stimulation of acyl ghrelin secretion by inhibiting the effects of
5-HT in patients with FD. It has been shown that the oral
administration of rikkunshito enhances the plasma levels of
acyl-ghrelin without exerting significant effects on the plasma
levels of des-acyl ghrelin, leading to an increase in the A/D ratio
in rats (98). Furthermore, in a
study on humans, the plasma levels of acyl-ghrelin and the A/D
ratio significantly increased following the administration of
rikkunshito, whereas the plasma levels of des-acyl ghrelin showed a
decreasing trend (99).
These results support the therapeutic potential of
acyl ghrelin in patients with FD. However, the long-term effects
and side-effects of peptide hormone therapies have not yet been
elucidated. Thus, further mechanistic studies are required to
confirm the therapeutic potential of acyl ghrelin in the treatment
of FD.
6. Conclusion
In conclusion, in our review, we summarize the
association between ghrelin and FD. FD involves abnormal gastric
motility and visceral hypersensitivity, as well as multiple
psychiatric and individual factors. Ghrelin is a novel gastric
hormone that is recognized as a mediator of GH release. Our review
presents the various physiological functions of ghrelin, that have
been discovered over the past decade. In addition, the data
presented in this review indicate that acyl ghrelin plays a role in
the regulation of food intake and gut motility. Therefore, acyl
ghrelin may contribute to the improvement of FD symptoms. Future
studies on the clinical application of acyl ghrelin in patients
with FD are required to fully elucidate its role in FD.
Acknowledgements
This review was supported by the Japan Society for
the Promotion of Science (JSPS) KAKENHI grant no. 24593103 and
24390464.
References
|
1
|
Tack J, Talley NJ, Camilleri M, et al:
Functional gastroduodenal disorders. Gastroenterology.
130:1466–1479. 2006. View Article : Google Scholar
|
|
2
|
Geeraerts B and Tack J: Functional
dyspepsia: past, present, and future. J Gastroenterol. 43:251–255.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tack J and Talley NJ: Gastroduodenal
disorders. Am J Gastroenterol. 105:757–763. 2010. View Article : Google Scholar
|
|
4
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsumoto M, Kitajima Y, Iwanami T, et al:
Structural similarity of ghrelin derivatives to peptidyl growth
hormone secretagogues. Biochem Biophys Res Commun. 284:655–659.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka M, Hayashida Y, Iguchi T, Nakao N,
Nakai N and Nakashima K: Organization of the mouse ghrelin gene and
promoter: occurrence of a short noncoding first exon.
Endocrinology. 142:3697–3700. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hosoda H, Kojima M, Matsuo H and Kangawa
K: Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin
peptide in gastrointestinal tissue. Biochem Biophys Res Commun.
279:909–913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen CY, Asakawa A, Fujimiya M, Lee SD and
Inui A: Ghrelin gene products and the regulation of food intake and
gut motility. Pharmacol Rev. 61:430–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asakawa A, Inui A, Kaga T, et al: Ghrelin
is an appetite-stimulatory signal from stomach with structural
resemblance to motilin. Gastroenterology. 120:337–345. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakazato M, Murakami N, Date Y, et al: A
role for ghrelin in the central regulation of feeding. Nature.
409:194–198. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wren AM, Small CJ, Ward HL, et al: The
novel hypothalamic peptide ghrelin stimulates food intake and
growth hormone secretion. Endocrinology. 141:4325–4328. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inui A: Ghrelin: an orexigenic and
somatotrophic signal from the stomach. Nat Rev Neurosci. 2:551–560.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
An W, Li Y, Xu G, et al: Modulation of
ghrelin O-acyltransferase expression in pancreatic islets.
Cell Physiol Biochem. 26:707–716. 2010.
|
|
14
|
Murray CD, Martin NM, Patterson M, et al:
Ghrelin enhances gastric emptying in diabetic gastroparesis: a
double blind, placebo controlled, crossover study. Gut.
54:1693–1698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tack J, Depoortere I, Bisschops R, Verbeke
K, Janssens J and Peeters T: Influence of ghrelin on gastric
emptying and meal-related symptoms in idiopathic gastroparesis.
Aliment Pharmacol Ther. 22:847–853. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tack J, Depoortere I, Bisschops R, et al:
Influence of ghrelin on interdigestive gastrointestinal motility in
humans. Gut. 55:327–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mori M, Suzuki H, Masaoka T, et al:
Intravenous ghrelin administration enhances gastric acid secretion
- evaluation using wireless pH capsule. Aliment Pharmacol Ther.
24:96–103. 2006. View Article : Google Scholar
|
|
18
|
Masuda Y, Tanaka T, Inomata N, et al:
Ghrelin stimulates gastric acid secretion and motility in rats.
Biochem Biophys Res Commun. 276:905–908. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castaneda TR, Tong J, Datta R, Culler M
and Tschop MH: Ghrelin in the regulation of body weight and
metabolism. Front Neuroendocrinol. 31:44–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fetissov SO, Laviano A, Kalra S and Inui
A: Update on ghrelin. Int J Pept. 2010:9635012010. View Article : Google Scholar
|
|
21
|
Asakawa A, Inui A, Kaga T, et al: A role
of ghrelin in neuroendocrine and behavioral responses to stress in
mice. Neuroendocrinology. 74:143–147. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hotta M, Ohwada R, Akamizu T, Shibasaki T,
Takano K and Kangawa K: Ghrelin increases hunger and food intake in
patients with restricting-type anorexia nervosa: a pilot study.
Endocr J. 56:1119–1128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akamizu T, Iwakura H, Ariyasu H, et al:
Effects of ghrelin treatment on patients undergoing total hip
replacement for osteoarthritis: different outcomes from studies in
patients with cardiac and pulmonary cachexia. J Am Geriatr Soc.
56:2363–2365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miki K, Maekura R, Nagaya N, et al:
Ghrelin treatment of cachectic patients with chronic obstructive
pulmonary disease: a multicenter, randomized, double-blind,
placebo-controlled trial. PloS One. 7:e357082012. View Article : Google Scholar
|
|
25
|
Akamizu T, Iwakura H, Ariyasu H, et al:
Repeated administration of ghrelin to patients with functional
dyspepsia: its effects on food intake and appetite. Eur J
Endocrinol. 158:491–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wo JM, Ejskjaer N, Hellstrom PM, et al:
Randomised clinical trial: ghrelin agonist TZP-101 relieves
gastroparesis associated with severe nausea and vomiting -
randomised clinical study subset data. Aliment Pharmacol Ther.
33:679–688. 2011. View Article : Google Scholar
|
|
27
|
Drossman DA and Dumitrascu DL: Rome III:
New standard for functional gastrointestinal disorders. J
Gastrointestin Liver Dis. 15:237–241. 2006.PubMed/NCBI
|
|
28
|
Miwa H: Why dyspepsia can occur without
organic disease: pathogenesis and management of functional
dyspepsia. J Gastroenterol. 47:862–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miwa H: Life style in persons with
functional gastrointestinal disorders - large-scale internet survey
of lifestyle in Japan. Neurogastroenterol Motil. 24:464–471.
e2172012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Norton GR, Norton PJ, Asmundson GJ,
Thompson LA and Larsen DK: Neurotic butterflies in my stomach: the
role of anxiety, anxiety sensitivity and depression in functional
gastrointestinal disorders. J Psychosom Res. 47:233–240. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khoo J, Rayner CK, Feinle-Bisset C, Jones
KL and Horowitz M: Gastrointestinal hormonal dysfunction in
gastroparesis and functional dyspepsia. Neurogastroenterol Motil.
22:1270–1278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Quartero AO, de Wit NJ, Lodder AC, Numans
ME, Smout AJ and Hoes AW: Disturbed solid-phase gastric emptying in
functional dyspepsia: a meta-analysis. Dig Dis Sci. 43:2028–2033.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Geeraerts B, Van Oudenhove L, Fischler B,
et al: Influence of abuse history on gastric sensorimotor function
in functional dyspepsia. Neurogastroenterol Motil. 21:33–41. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Holtmann G, Siffert W, Haag S, et al:
G-protein beta 3 subunit 825 CC genotype is associated with
unexplained (functional) dyspepsia. Gastroenterology. 126:971–979.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Toyoshima F, Oshima T, Nakajima S, et al:
Serotonin transporter gene polymorphism may be associated with
functional dyspepsia in a Japanese population. BMC Med Genet.
12:882011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mearin F, Perez-Oliveras M, Perello A, et
al: Dyspepsia and irritable bowel syndrome after a Salmonella
gastroenteritis outbreak: one-year follow-up cohort study.
Gastroenterology. 129:98–104. 2005.PubMed/NCBI
|
|
37
|
Drossman DA: The functional
gastrointestinal disorders and the Rome III process.
Gastroenterology. 130:1377–1390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Danesh J, Lawrence M, Murphy M, Roberts S
and Collins R: Systematic review of the epidemiological evidence on
Helicobacter pylori infection and nonulcer or uninvestigated
dyspepsia. Arch Intern Med. 160:1192–1198. 2000.PubMed/NCBI
|
|
39
|
Kawamura A, Adachi K, Takashima T, et al:
Prevalence of functional dyspepsia and its relationship with
Helicobacter pylori infection in a Japanese population. J
Gastroenterol Hepatol. 16:384–388. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thumshirn M, Camilleri M, Saslow SB,
Williams DE, Burton DD and Hanson RB: Gastric accommodation in
non-ulcer dyspepsia and the roles of Helicobacter pylori
infection and vagal function. Gut. 44:55–64. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sarnelli G, Cuomo R, Janssens J and Tack
J: Symptom patterns and pathophysiological mechanisms in dyspeptic
patients with and without Helicobacter pylori. Dig Dis Sci.
48:2229–2236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Savarino E, Zentilin P, Dulbecco P,
Malesci A and Savarino V: The role of Acid in functional dyspepsia.
Am J Gastroenterol. 106:1168–1169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin X and Li YM: Systematic review and
meta-analysis from Chinese literature: the association between
Helicobacter pylori eradication and improvement of
functional dyspepsia. Helicobacter. 12:541–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moayyedi P, Soo S, Deeks J, et al:
Eradication of Helicobacter pylori for non-ulcer dyspepsia.
Cochrane Database Syst Rev. 2006:CD0020962006.PubMed/NCBI
|
|
45
|
Moayyedi P: Helicobacter pylori
eradication for functional dyspepsia: what are we treating?:
comment on ‘Helicobacter pylori eradication in functional
dyspepsia’. Arch Intern Med. 171:1936–1937. 2011. View Article : Google Scholar
|
|
46
|
Suzuki H and Moayyedi P: Helicobacter
pylori infection in functional dyspepsia. Nat Rev Gastroenterol
Hepatol. 10:168–174. 2013. View Article : Google Scholar
|
|
47
|
Sugano K: Should we still subcategorize
Helicobacter pylori-associated dyspepsia as functional
disease? J Neurogastroenterol Motil. 17:366–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tack J, Caenepeel P, Fischler B,
Piessevaux H and Janssens J: Symptoms associated with
hypersensitivity to gastric distention in functional dyspepsia.
Gastroenterology. 121:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Boeckxstaens GE, Rumessen JJ, de Wit L,
Tytgat GN and Vanderwinden JM: Abnormal distribution of the
interstitial cells of cajal in an adult patient with
pseudo-obstruction and megaduodenum. Am J Gastroenterol.
97:2120–2126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rhee PL, Kim YH, Son HJ, et al: Evaluation
of individual symptoms cannot predict presence of gastric
hypersensitivity in functional dyspepsia. Dig Dis Sci.
45:1680–1684. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chrousos GP: Regulation and dysregulation
of the hypothalamic-pituitary-adrenal axis. The
corticotropin-releasing hormone perspective. Endocrinol Metab Clin
North Am. 21:833–858. 1992.PubMed/NCBI
|
|
52
|
Lamberts SW, Verleun T, Oosterom R, de
Jong F and Hackeng WH: Corticotropin-releasing factor (ovine) and
vasopressin exert a synergistic effect on adrenocorticotropin
release in man. J Clin Endocrinol Metab. 58:298–303. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vale W, Spiess J, Rivier C and Rivier J:
Characterization of a 41-residue ovine hypothalamic peptide that
stimulates secretion of corticotropin and beta-endorphin. Science.
213:1394–1397. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vaughan J, Donaldson C, Bittencourt J, et
al: Urocortin, a mammalian neuropeptide related to fish urotensin I
and to corticotropin-releasing factor. Nature. 378:287–292. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bale TL and Vale WW: CRF and CRF
receptors: role in stress responsivity and other behaviors. Annu
Rev Pharmacol Toxicol. 44:525–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ataka K, Kuge T, Fujino K, Takahashi T and
Fujimiya M: Wood creosote prevents CRF-induced motility via 5-HT3
receptors in proximal and 5-HT4 receptors in distal colon in rats.
Auton Neurosci. 133:136–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Martinez V, Rivier J, Wang L and Tache Y:
Central injection of a new corticotropin-releasing factor (CRF)
antagonist, astressin, blocks CRF- and stress-related alterations
of gastric and colonic motor function. J Pharmacol Exp Ther.
280:754–760. 1997.
|
|
58
|
Tache Y, Martinez V, Million M and Wang L:
Stress and the gastrointestinal tract III. Stress-related
alterations of gut motor function: role of brain
corticotropin-releasing factor receptors. Am J Physiol Gastrointest
Liver Physiol. 280:G173–G177. 2001.PubMed/NCBI
|
|
59
|
O'Mahony SM, Marchesi JR, Scully P, et al:
Early life stress alters behavior, immunity, and microbiota in
rats: implications for irritable bowel syndrome and psychiatric
illnesses. Biol Psychiatry. 65:263–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Brevet M, Kojima H, Asakawa A, et al:
Chronic foot-shock stress potentiates the influx of bone
marrow-derived microglia into hippocampus. J Neurosci Res.
88:1890–1897. 2010.PubMed/NCBI
|
|
61
|
Sengupta JN: Visceral pain: the
neurophysiological mechanism. Handb Exp Pharmacol. 194:31–74. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shindo T, Futagami S, Hiratsuka T, et al:
Comparison of gastric emptying and plasma ghrelin levels in
patients with functional dyspepsia and non-erosive reflux disease.
Digestion. 79:65–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lee KJ, Cha DY, Cheon SJ, Yeo M and Cho
SW: Plasma ghrelin levels and their relationship with gastric
emptying in patients with dysmotility-like functional dyspepsia.
Digestion. 80:58–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sarnelli G, Caenepeel P, Geypens B,
Janssens J and Tack J: Symptoms associated with impaired gastric
emptying of solids and liquids in functional dyspepsia. Am J
Gastroenterol. 98:783–788. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Asakawa A, Inui A, Ueno N, Makino S,
Fujino MA and Kasuga M: Urocortin reduces food intake and gastric
emptying in lean and ob/ob obese mice. Gastroenterology.
116:1287–1292. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fujino K, Inui A, Asakawa A, Kihara N,
Fujimura M and Fujimiya M: Ghrelin induces fasted motor activity of
the gastrointestinal tract in conscious fed rats. J Physiol.
550:227–240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fujimiya M, Itoh E, Kihara N, Yamamoto I,
Fujimura M and Inui A: Neuropeptide Y induces fasted pattern of
duodenal motility via Y(2) receptors in conscious fed rats. Am J
Physiol Gastrointest Liver Physiol. 278:G32–G38. 2000.PubMed/NCBI
|
|
68
|
Fujimiya M, Asakawa A, Ataka K, Kato I and
Inui A: Different effects of ghrelin, des-acyl ghrelin and
obestatin on gastroduodenal motility in conscious rats. World J
Gastroenterol. 14:6318–6326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Peeters TL: Central and peripheral
mechanisms by which ghrelin regulates gut motility. J Physiol
Pharmacol. 54(Suppl 4): S95–S103. 2003.PubMed/NCBI
|
|
70
|
Venkova K and Greenwood-Van Meerveld B:
Application of ghrelin to gastrointestinal diseases. Curr Opin
Investig Drugs. 9:1103–1107. 2008.PubMed/NCBI
|
|
71
|
Asakawa A, Inui A, Fujimiya M, et al:
Stomach regulates energy balance via acylated ghrelin and desacyl
ghrelin. Gut. 54:18–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ariyasu H, Takaya K, Iwakura H, et al:
Transgenic mice overexpressing des-acyl ghrelin show small
phenotype. Endocrinology. 146:355–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen CY, Chao Y, Chang FY, Chien EJ, Lee
SD and Doong ML: Intracisternal des-acyl ghrelin inhibits food
intake and non-nutrient gastric emptying in conscious rats. Int J
Mol Med. 16:695–699. 2005.PubMed/NCBI
|
|
74
|
Chen CY, Inui A, Asakawa A, et al:
Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted
stomach motility in conscious rats. Gastroenterology. 129:8–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Inhoff T, Monnikes H, Noetzel S, et al:
Desacyl ghrelin inhibits the orexigenic effect of peripherally
injected ghrelin in rats. Peptides. 29:2159–2168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Qader SS, Hakanson R, Rehfeld JF,
Lundquist I and Salehi A: Proghrelin-derived peptides influence the
secretion of insulin, glucagon, pancreatic polypeptide and
somatostatin: a study on isolated islets from mouse and rat
pancreas. Regul Pept. 146:230–237. 2008. View Article : Google Scholar
|
|
77
|
Banks WA, Tschop M, Robinson SM and Heiman
ML: Extent and direction of ghrelin transport across the
blood-brain barrier is determined by its unique primary structure.
J Pharmacol Exp Ther. 302:822–827. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Martinez V, Barquist E, Rivier J and Tache
Y: Central CRF inhibits gastric emptying of a nutrient solid meal
in rats: the role of CRF2 receptors. Am J Physiol. 274:G965–G970.
1998.PubMed/NCBI
|
|
79
|
Million M, Maillot C, Saunders P, Rivier
J, Vale W and Tache Y: Human urocortin II, a new CRF-related
peptide, displays selective CRF(2)-mediated action on gastric
transit in rats. Am J Physiol Gastrointest Liver Physiol.
282:G34–G40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kihara N, Fujimura M, Yamamoto I, Itoh E,
Inui A and Fujimiya M: Effects of central and peripheral urocortin
on fed and fasted gastroduodenal motor activity in conscious rats.
Am J Physiol Gastrointest Liver Physiol. 280:G406–G419.
2001.PubMed/NCBI
|
|
81
|
Zhang JV, Ren PG, Avsian-Kretchmer O, et
al: Obestatin, a peptide encoded by the ghrelin gene, opposes
ghrelin's effects on food intake. Science. 310:996–999. 2005.
View Article : Google Scholar
|
|
82
|
Chartrel N, Alvear-Perez R, Leprince J, et
al: Comment on ‘Obestatin, a peptide encoded by the ghrelin gene,
opposes ghrelin's effects on food intake’. Science.
315:7662007.
|
|
83
|
Holst B, Egerod KL, Schild E, et al: GPR39
signaling is stimulated by zinc ions but not by obestatin.
Endocrinology. 148:13–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tremblay F, Perreault M, Klaman LD, Tobin
JF, Smith E and Gimeno RE: Normal food intake and body weight in
mice lacking the G protein-coupled receptor GPR39. Endocrinology.
148:501–506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bassil AK, Haglund Y, Brown J, et al:
Little or no ability of obestatin to interact with ghrelin or
modify motility in the rat gastrointestinal tract. Br J Pharmacol.
150:58–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bresciani E, Rapetti D, Dona F, et al:
Obestatin inhibits feeding but does not modulate GH and
corticosterone secretion in the rat. J Endocrinol Invest.
29:RC16–RC18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ataka K, Inui A, Asakawa A, Kato I and
Fujimiya M: Obestatin inhibits motor activity in the antrum and
duodenum in the fed state of conscious rats. Am J Physiol
Gastrointest Liver Physiol. 294:G1210–G1218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Takamori K, Mizuta Y, Takeshima F, et al:
Relation among plasma ghrelin level, gastric emptying, and
psychologic condition in patients with functional dyspepsia. J Clin
Gastroenterol. 41:477–483. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Nishizawa T, Suzuki H, Nomoto Y, et al:
Enhanced plasma ghrelin levels in patients with functional
dyspepsia. Aliment Pharmacol Ther. 24(Suppl 4): S104–S110. 2006.
View Article : Google Scholar
|
|
90
|
Shinomiya T, Fukunaga M, Akamizu T, et al:
Plasma acylated ghrelin levels correlate with subjective symptoms
of functional dyspepsia in female patients. Scand J Gastroenterol.
40:648–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kim YS, Lee JS, Lee TH, et al: Plasma
levels of acylated ghrelin in patients with functional dyspepsia.
World J Gastroenterol. 18:2231–2237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Taniguchi H, Ariga H, Zheng J, et al:
Endogenous ghrelin and 5-HT regulate interdigestive
gastrointestinal contractions in conscious rats. Am J Physiol
Gastrointest Liver Physiol. 295:G403–G411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tack J, Janssen P, Masaoka T, Farre R and
Van Oudenhove L: Efficacy of buspirone, a fundus-relaxing drug, in
patients with functional dyspepsia. Clin Gastroenterol Hepatol.
10:1239–1245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Seto Y, Yoshida N and Kaneko H: Effects of
mosapride citrate, a 5-HT4-receptor agonist, on gastric
distension-induced visceromotor response in conscious rats. J
Pharmacol Sci. 116:47–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lee JW, Sung KW, Lee OY, Lee SE and Sohn
CI: The effects of 5-HT4 receptor agonist, mosapride citrate, on
visceral hypersensitivity in a rat model. Dig Dis Sci.
57:1517–1524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fujitsuka N, Asakawa A, Amitani H, Hattori
T and Inui A: Efficacy of ghrelin in cancer cachexia: clinical
trials and a novel treatment by rikkunshito. Crit Rev Oncog.
17:277–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Arai M, Matsumura T, Tsuchiya N, et al:
Rikkunshito improves the symptoms in patients with functional
dyspepsia, accompanied by an increase in the level of plasma
ghrelin. Hepatogastroenterology. 59:62–66. 2012.PubMed/NCBI
|
|
98
|
Takeda H, Sadakane C, Hattori T, et al:
Rikkunshito, an herbal medicine, suppresses cisplatin-induced
anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology.
134:2004–2013. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Matsumura T, Arai M, Yonemitsu Y, et al:
The traditional Japanese medicine Rikkunshito increases the plasma
level of ghrelin in humans and mice. J Gastroenterol. 45:300–307.
2010. View Article : Google Scholar : PubMed/NCBI
|