Introduction
Human pancreatic carcinoma, a highly malignant
cancer with a poor prognosis, is the sixth leading cause of
mortality due to malignant disease in China and the fourth leading
cause of cancer-related mortality in the United States (1,2).
The current literature indicates that the 5-year survival rate of
pancreatic carcinoma patients remains <5% and has not increased
significantly over the past 20 years, partly due to the fact that
pancreatic carcinoma cells are relatively resistant to chemotherapy
and radiotherapy (3,4). It has been suggested that resistance
to Fas-Fas ligand (FasL)-mediated apoptosis may play an important
role in the pathogenesis of pancreatic carcinoma; although human
pancreatic adenocarcinoma cells express Fas and FasL, they are
still resistant to Fas-mediated apoptosis (5).
As previously reported, a soluble decoy receptor 3
(DcR3) binds to FasL and inhibits FasL-mediated apoptosis. DcR3,
also known as Tr6 or M68, is a member of the tumor necrosis factor
receptor (TNFR) superfamily and maps to chromosome position 20q13,
which is associated with gene amplification in various types of
cancer. It shares sequence homology with osteoprotegerin (31%),
TNFR2 (29%) and has relatively less homology with Fas (17%)
(6). It has been reported that
DcR3 has 3 ligands: FasL, TNF-like molecule 1A (TL1A) and
homologous to lymphotoxins, exhibits inducible expression, competes
with herpes simplex virus glycoprotein D for HVEM, expressed by T
lymphocytes (LIGHT) (7–9). DcR3 contributes to tumor growth by
blocking apoptosis, impeding the immune response and inducing
angiogenesis (10,11). There is strong evidence indicating
that DcR3 is overexpressed in a variety of human tumors, including
cancers of lungs (6), colon
(12) and liver (13), as well as gastric carcinoma
(14) and malignant gliomas
(15).
In this study, we examined the expression of DcR3 in
pancreatic carcinoma tissues, serum and cell lines. Moreover, using
small interfering RNA (siRNA) to silence the expression of DcR3, we
investigated the effects of FasL-mediated apoptosis. Our findings
indicated that DcR3 may be a potential target for gene therapy of
pancreatic carcinoma.
Materials and methods
Clinical samples
Tissue samples from 50 pancreatic carcinoma patients
were collected during surgical resections performed at the First
Affiliated Hospital of Soochow University, Suzhou, China, from
January 2008 to June 2012. Tumor tissues and adjacent non-tumor
tissues were frozen immediately after surgical removal in liquid
nitrogen and stored at −80ºC. Serum for ELISA was obtained from
cancer patients prior to surgery and serum from healthy individuals
was used as the control. The patients had not received any
pre-operative chemotherapy, radiotherapy or immunotherapy. All
samples were obtained with patient consent and local ethics
committee approval.
Lentiviral vectors for DcR3 siRNA
Three different siRNAs targeting DcR3 were designed
using the DcR3 gene sequence (GenBank, NM-003823) as a template.
The sequence with the most effective silencing effect was selected
for subsequent experiments (data not shown). The recombinant
lentivirus was synthesized and purified by Genechem Co., Ltd.
(Shanghai, China).
Cell culture and transfection
The human pancreatic carcinoma cells, Panc-1 and
SW1990, from the Shanghai Institute of Cell Biology (Shanghai,
China) were maintained in Dulbecco's modified Eagle's medium (DMEM)
or RPMI-1640 (Gibco, Carlsbad, CA, USA) respectively, supplemented
with 10% fetal bovine serum (Gibco) and 100 μg/ml each of
penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA) in 5%
CO2 at 37ºC.
In 6-well plates, 5×104 cells/well were
cultured overnight, then transfected with 10 μl recombinant
lentivirus of DcR3 siRNA (LV-RNAi) or mock lentivirus (LV-NC) using
5 μg/ml polybrene. Non-transfected cells were used as the blank
controls.
Quantitative reverse transcriptase PCR
(qRT-PCR)
Total RNA from the tissues and cells was extracted
using TRIzol reagent (Invitrogen). Single-stranded cDNA for a PCR
template was synthesized from 10 μg of total RNA using random
primers and M-MLV reverse transcriptase (Takara, Dalian, China).
The relative levels of target gene mRNA transcripts to those of the
control (actin) were detemined by qRT-PCR. The primers used for PCR
were as follows (forward and reverse): 5′-CTCTTCCTCCCATGACAC-3′ and
5′-CTGGAAAGCC ACAAAGTC-3′ for DcR3 (112 bp); 5′-AAGGAGTACACAGA
CAAAGCCC-3′ and 5′-GGTGATATTTACTCAAGTG-3′ for Fas (684 bp);
5′-GCATTGGGCCTGGGGATGTTTCA-3′ and 5′-TTGTGGCTCAGGGGCAGGTTGTTG-3′
for FasL (344 bp); 5′-AGAGGTGGAGAACTGGGATT-3′ and 5′-CCAA
GGAAATGGGACAAA-3′ for Fas-associated death domain (FADD) (119 bp);
5′-TTGGAACAAATGGACCTG-3′ and 5′-ACAAAGCGACTGGATGAA-3′ for caspase-3
(278 bp); 5′-TGAACCCAAGAGGTCAAG-3′ and 5′-AGAAGGCAT AAAGCAAGT-3′
for caspase-8 (192 bp); 5′-AGCGAGC ATCCCCCAAAGTT-3′ and
5′-GGGCACGAAGGCT CATCATT-3′ for actin (256 bp). The RT-PCR
conditions were as follows: 10 min at 95ºC for pre-heating, 40
cycles of 95ºC for 15 min, 60ºC for 1 min, 72ºC for 30 sec,
followed by extension at 72ºC for 10 min. The amplified segments
were verified by electrophoresis on 2% agarose gels with ethidium
bromide. The relative levels of mRNA transcripts to the control,
actin, were calculated using the 2ΔΔCt method.
Cell viability assay
The effect of soluble FasL (sFasL) on cancer cell
viability was measured using the Cell Counting Kit-8 (CCK-8). After
72 h of transfection, the cells were seeded in 96-well plates at
5×103 cells/well and treated with sFasL (25 ng/ml) for
24 h. A total of 20 μl CCK-8 (Dojindo Laboratories, Kumamoto,
Japan) was added followed by incubation at 37ºC for an additional 2
h. An ultraviolet spectrophotometer was used to measure the
absorbance of each well at 450 nm, and the cell viability index was
calculated as follows: (experimental OD value/control OD value)
×100%.
Colony formation assay
After 72 h of transfection, the cells were cultured
at 500 cells/well in 6-well plates at 37ºC for 20 days. The cells
were then treated with sFasL (25 ng/ml) for 24 h and fixed with
methanol and stained with 1% crystal violet. The colonies
containing >50 cells were counted using a microscope.
Cell proliferation assay
The effects of sFasL on cell proliferation were
measured by EdU assay. After 72 h of transfection, the cells were
seeded in 96-well plates at 5×103 cells/well and treated
with sFasL (25 ng/ml) for 24 h, then 50 μM of EdU for additional 2
h. The cells were fixed with 4% formaldehyde for 15 min and treated
with 0.5% Triton X-100 for permeabilization. Subsequently, each
well was stained with 100 μl Apollo solution for 30 min, then with
100 μl Hoechst 33342 for 15 min and visualized under a fluorescence
microscope. The cell proliferation rate was calculated as follows:
(EdU+ cell number/Hoechst+ cell number)
×100%.
Cell cycle and apoptosis analysis
After 72 h of transfection, the cells were treated
with sFasL (25 ng/ml) for 24 h. For cell cycle analysis, the cells
were collected and fixed in 70% ethanol at 4ºC overnight, followed
by staining with propidium iodide (PI) (BD Biosciences, Franklin
Lakes, NJ, USA) and were kept in the dark at 4ºC for 30 min. The
cell cycle was analyzed by FACSCalibur with CellQuest software. For
apoptosis analysis, a volume of 100 μl of cell suspension
(1×106 cells/ml) was labeled with 10 μl of PI and 5 μl
of Annexin V/FITC (BD Biosciences). The cells were incubated in the
dark for 15 min at room temperature and early apoptotic cells were
assessed by FACSCalibur.
ELISA
Cell culture supernatants and serum from cancer
patients were collected and DcR3 levels were measured using ELISA
kits (R&D Systems, Minneapolis, MN, USA) according to the
manufacturer's instructions.
In vivo tumor model
Male BALB/C nude mice (4–6 weeks old and weighing
16–20 g) were purchased from the Shanghai Experimental Animal
Center (Shanghai, China) and housed in a specific pathogen-free
environment. Animal experiments were carried out in accordance with
the Guide for the Care and Use of Laboratory Animals of Soochow
University. To establish a tumor xenograft model, 2×106
Panc-1 cells in 200 μl saline were inoculated subcutaneously into
the flanks of nude mice. When the tumors reached approximately 5–7
mm in diameter, the mice were randomly divided into groups and
injected intratumorally with PBS, LV-NC or LV-RNAi/DcR3
(1×107 pfu/50 μl) once per week for 4 weeks. The tumor
size was measured every 5 days with calipers, and the tumor volumes
were calculated according to the formula: V = (L × W2)
×0.5 by measuring tumor length (L) and width (W). At the end of
experiment, the tumors were dissected and weighed.
Statistical analysis
SPSS software version 16.0 was used for statistical
analysis. Data are expressed as the means ± SD. One-way analysis of
variance (one-way ANOVA) or the t-test was performed for
inter-group comparisons. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
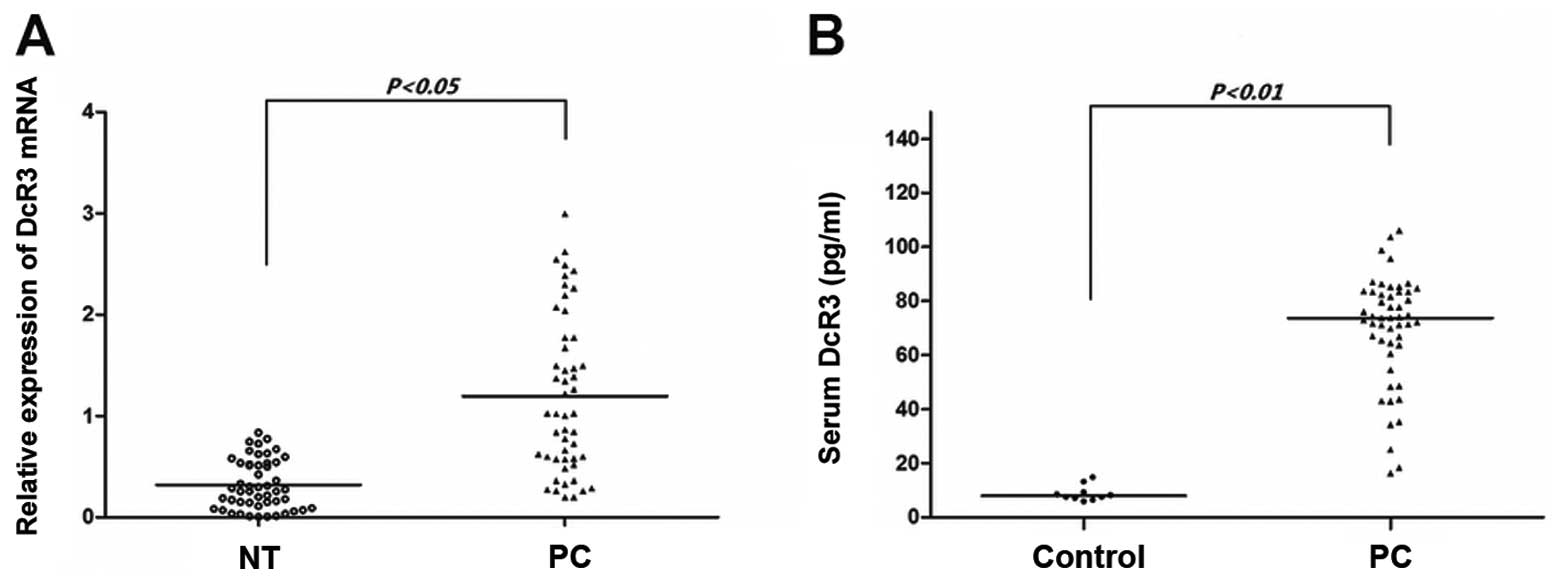
DcR3 is overexpressed in pancreatic
carcinoma
In our cohort of 50 patients recently operated for
pancreatic carcinoma, paired samples of tumor and non-tumor tissues
were subjected to qRT-PCR (Fig.
1A). DcR3 mRNA was overpressed in the pancreatic carcinoma
tissues compared with the non-tumor tissues (P<0.05). As DcR3
lacks a transmembrance sequence and is a soluble protein, we used
ELISA assay to determine the serum levels of DcR3. As shown in
Fig. 1B, the serum from the
cancer patients had significantly higher levels of DcR3 than the
serum from the controls (P<0.01). These results demonstrate that
DcR3 is overexpressed in pancreatic carcinoma and that the protein
expression of DcR3 is compatible with its mRNA expression.
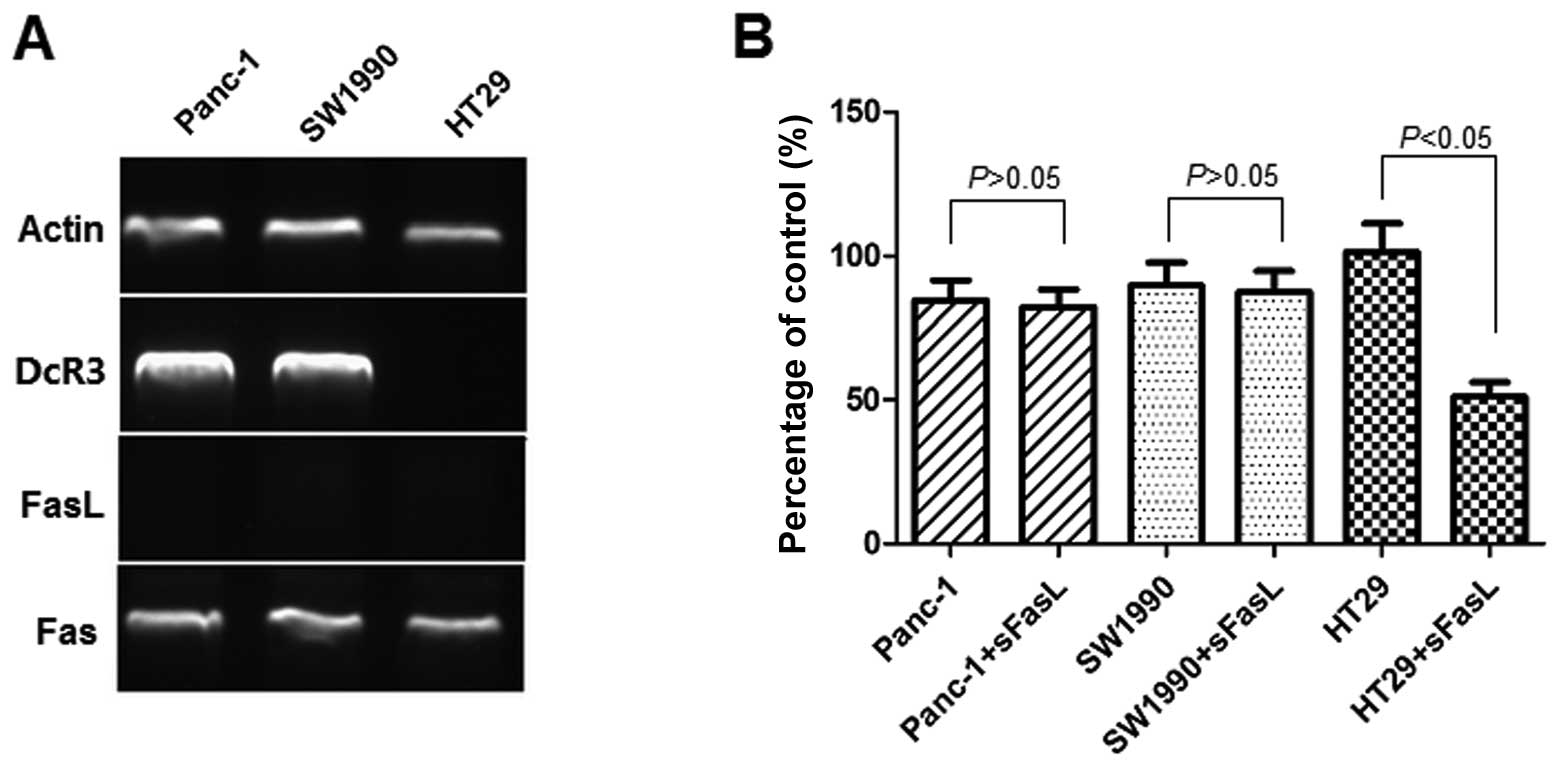
Expression of DcR3 in pancreatic
carcinoma cells and inhibitory effect of sFasL
The expression of FasL and that of its receptor,
Fas, as well as that of DcR3, was assessed by RT-PCR (Fig. 2A). The Panc-1 cells and SW1990
cells expressed Fas and DcR3. The mRNA ratio of DcR3 to actin was
2.48±0.72 for Panc-1 and 2.57±0.81 for SW1990 cells. HT29 cells was
used as the negative controls for DcR3 expression. We could not
detect FasL expression by RT-PCR in the Panc-1 and SW1990 cells. To
investigate whether DcR3 plays a role in the sensitivity to
apoptosis mediated by FasL, we examined the effects of 24 h of
exposure to sFasL (25 ng/ml) by CCK-8 assay (Fig. 2B). The results indicated that
sFasL did not inhibit the cell growth of Panc-1 and SW1990 cells,
but only that of HT29 cells, which do not express DcR3.
Silencing DcR3 expression by
lentivirus-mediated RNA interference (RNAi)
To investigate the role of DcR3 in the cell
apoptosis mediated by FasL, the Panc-1 and SW1990 cells were
transfected with recombinant lentivirus-mediated RNAi targeting
DcR3 and further used for functional analysis. As shown in Fig. 3A and B, the significant decrease
in DcR3 expression was verified by RT-PCR and ELISA in the cells
transfected with LV-RNAi.
Knockdown of DcR3 expression by RNAi
enhances the effects of FasL
To explore the function of DcR3, cell proliferation
was determined by CCK-8 assays, colony formation and EdU assays. As
shown in Fig. 4, there was no
significant effect on cell viability without exposure to sFasL,
whereas when the cells were transfected with DcR3 siRNA and treated
with sFasL for 24 h, cell growth inhibition was observed. An
analysis of clonogenicity indicated that the LV-RNAi-transfected
cells displayed much fewer and smaller colonies than the
LV-NC-transfected cells (Fig.
5A). The relative colony number of LV-RNAi-transfected cells
was reduced by almost 50 and 40% in the Panc-1 and SW1990 cells,
respectively (Fig. 5B).
Similarly, we found that the number of EdU+
LV-RNAi-transfected cells was significantly reduced compared with
the LV-NC-transfected cells (P<0.05). After silencing DcR3
expression, the relative cell proliferation rate (LV-RNAi- to
LV-NC-transfected cells) was 41.46±4.25% for Panc-1 and 48.29±5.16%
for SW1990 cells (Fig. 6).
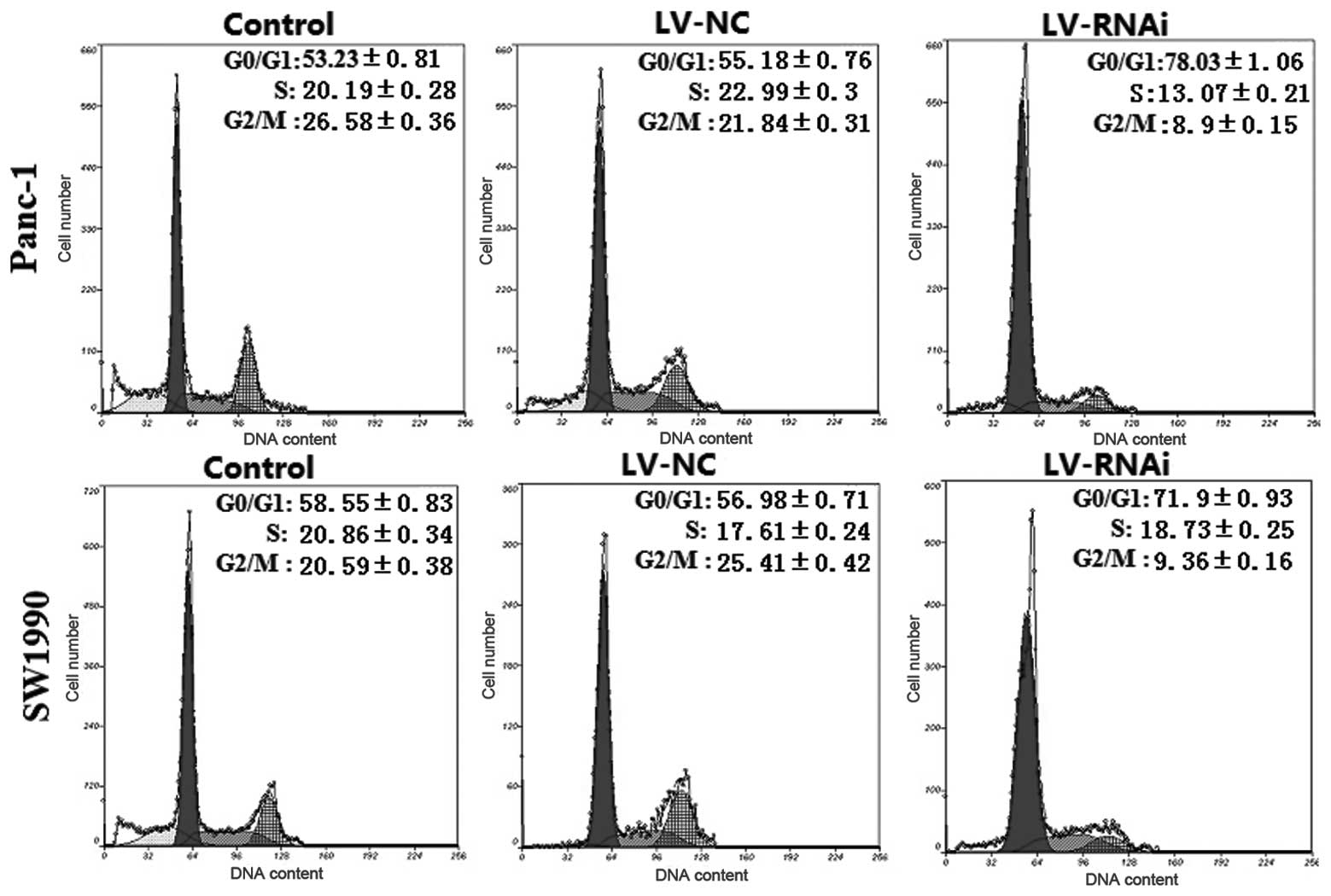
The cell cycle and apoptosis are associated with
tumor cell growth and proliferation. Thus, we examined the effects
of silencing DcR3 expression on the cell cycle and apoptosis by
flow cytometry. As shown in Fig.
7, the percentage of G0/G1 phase LV-RNAi-transfected cells was
78.03±1.06% for Panc-1 and 71.9±0.93% for SW1990 cells, which was
significantly higher than that of the LV-NC-transfected cells
(55.78±0.76% for Panc-1 and 56.98±0.71% for SW1990 cells;
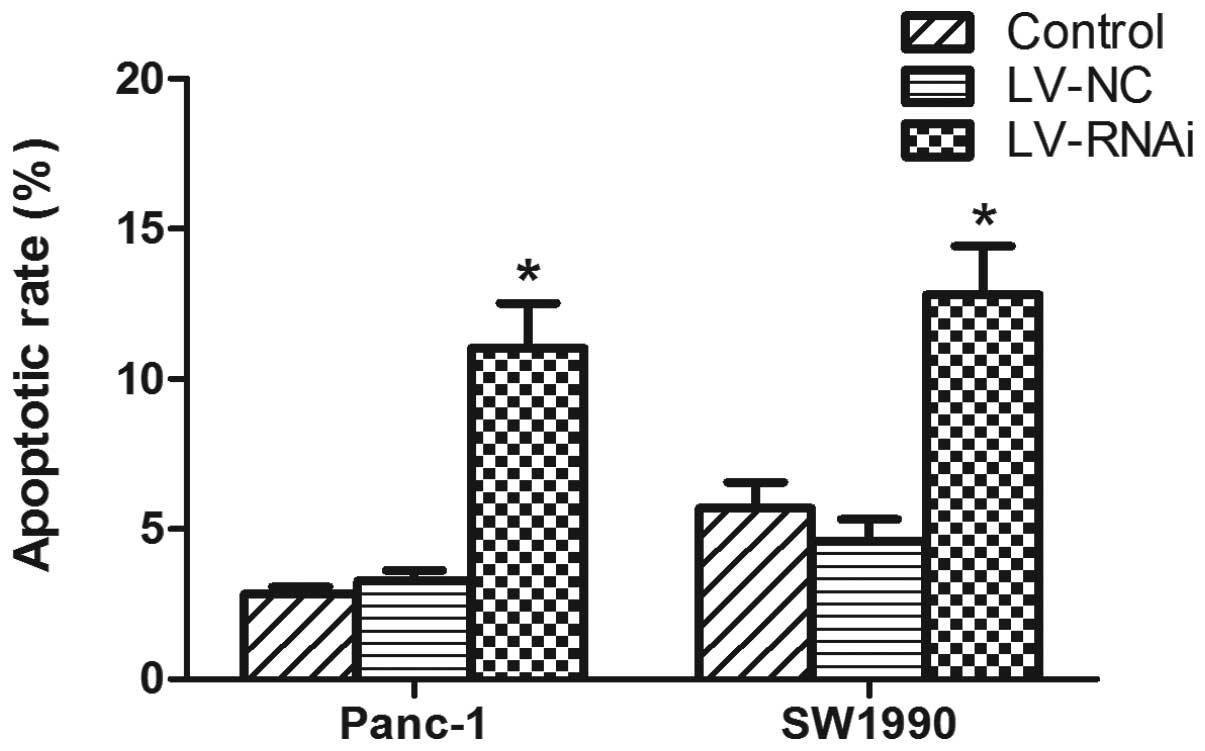
P<0.05, respectively). An analysis of apoptosis revealed that
the number of apoptotic cells was significantly increased in the
LV-RNAi-transfected cells compared with the LV-NC-transfected cells
(P<0.05) (Fig. 8). These
results suggest that the knockdown of DcR3 expression by RNAi
enhances the apoptotic effects of FasL on pancreatic carcinoma
cells.
Silencing DcR3 expression modulates the
expression of cell apoptotic regulators
FADD, caspase-3 and caspase-8 are the regulators of
the FasL-mediated apoptotic pathway. Following treatment with sFasL
at 25 ng/ml for 24 h, the relative mRNA levels of FADD, caspase-3
and caspase-8 to the control, actin, were determined by RT-PCR
(Fig. 9). The results revealed
that the cells transfected with LV-RNAi had an upregulated
expression of FADD, caspase-3 and caspase-8. These results further
support the hypothesis that silencing the expression of DcR3
modulates cell apoptotic regulators, thus triggering cell
apoptosis.
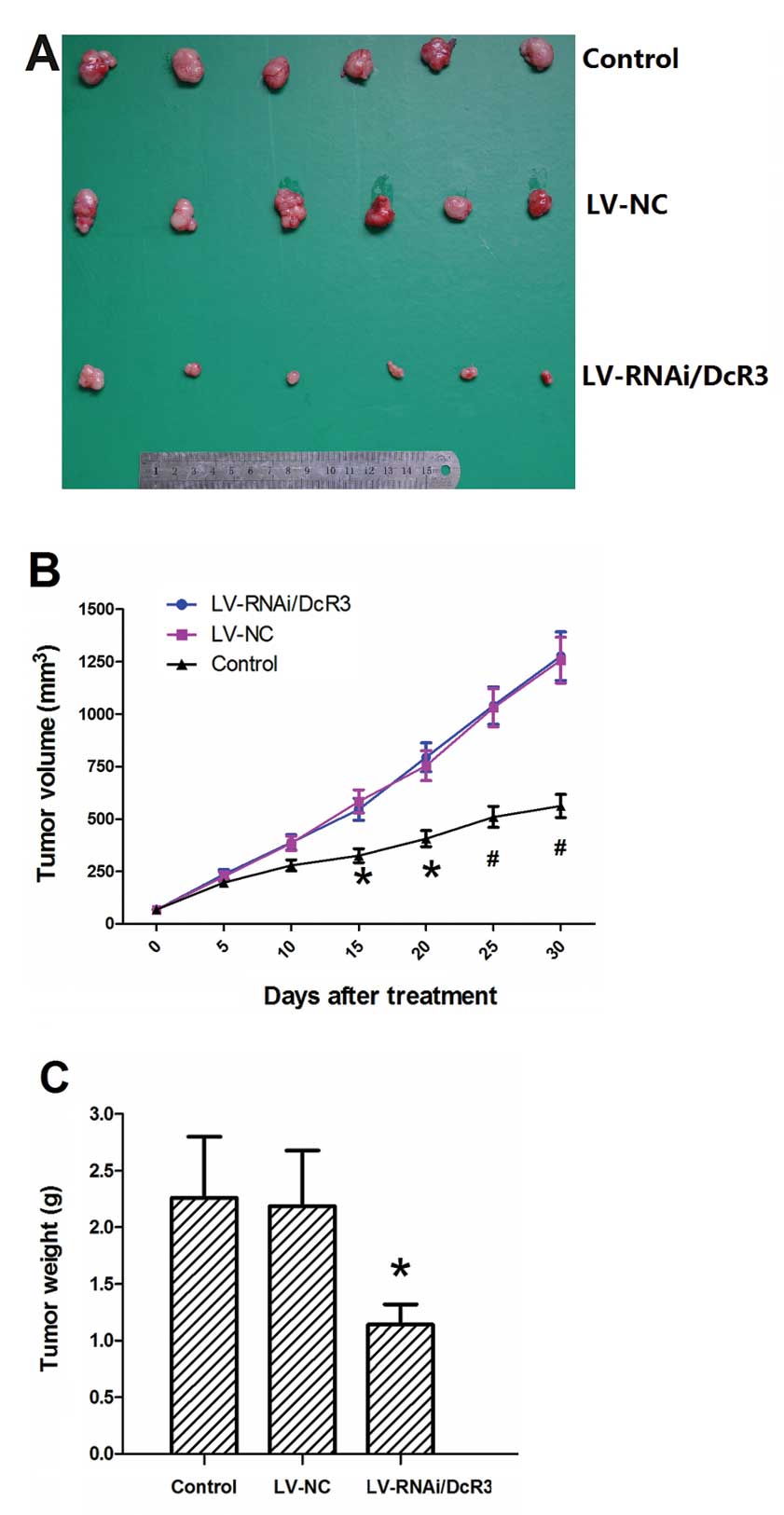
Treatment with LV-RNAi/DcR3 inhibits the
growth of pancreatic carcinoma in vivo
As DcR3 plays a crucial role in pancreatic
carcinoma, it may be used as a potential therapeutic target for the
treatment of pancreatic carcinoma. BALB/C nude mice were inoculated
with Panc-1 cells to establish subcutaneous tumor xenografts. The
intratumoral injection of LV-RNAi/DcR3 significantly inhibited the
growth of the tumors (Fig. 10A).
The growth curves of LV-RNAi/DcR3 and LV-NC became divergent after
15 days of treatment (Fig. 10B).
The tumor weight at sacrifice in the LV-RNAi/DcR3 group was lower
than that of the LV-NC group (Fig.
10C). These results indicated that LV-RNAi/DcR3 attenuated
tumor growth and reduced tumor weight.
Discussion
In this study, we demonstrate that DcR3 is
overexpressed in pancreatic carcinoma tissues, serum and cell
lines. In our previous study, we demonstrated that the expression
of DcR3 was associated with clinicopathological features, such as
lymph node metastasis, tumor size and clinical stage (16). In this study, we also found that
silencing DcR3 expression enhanced the apoptotic effects mediated
by FasL in vitro and inhibited the tumor growth in
vivo. DcR3 is a member of the TNFR superfamily and is regarded
as a secreted molecule, as it lacks a transmenbrance sequence. Wu
et al reported that 55% of patients with liver, gastric and
colon carcinoma were serum DcR3-positive (17). The detection of DcR3 in serum
offers an easy-to-access method for tumor diagnosis.
Apoptosis is a cell suicide mechanism which
maintains a stable internal environment. The imbalance between cell
proliferation and apoptosis plays an important role in the
occurrence and progression of malignant tumors. FasL is mainly
expressed in activated T cells and natural killer (NK) cells, and
it induces apoptosis in target cells through the death receptor,
Fas. The most common function of the FasL/Fas system is to mediate
the killing of tumor cells by cytotoxic T cells. However, studies
have indicated that many tumors, including pancreatic carcinoma,
are resistant to FasL/Fas-mediated growth inhibition signals,
despite expressing Fas (5,18).
Similar results were obtained in this study using Panc-1 and SW1990
cells. Several mechanisms have been suggested to play a role in
this phenomena, such as the downregulation of Fas (19), the upregulation of FasL (20) and the expression of soluble Fas
(21). DcR3, which binds to FasL
and inhibits FasL-induced apoptosis, may also play a role in the
resistance to FasL/Fas-mediated growth inhibition signals.
DcR3 is a soluble decoy receptor belonging to the
TNFR superfamily, which binds to FasL, LIGHT and TL1A. DcR3 can
block the effects of FasL, TL1A and LIGHT by inhibiting the
FasL-Fas, TL1A-death receptor 3 (DR3) and LIGHT-HVEM interaction
(22–24). Evidence has shown that DcR3 not
only protects tumor cells from apoptosis induced by FasL, LIGHT and
TL1A, but also suppresses immune surveillance by blocking T cell
costimulation mediated by TL1A and LIGHT (25,26). Previous studies have demonstrated
that DcR3 neutralizes the FasL-mediated apoptotic signal;
therefore, we wished to determine whether the silencing of DcR3
expression enhances the apoptotic effects mediated by FasL.
In this study, we succeeded in silencing DcR3 using
lentivirus-mediated RNAi targeting DcR3. We found that the
knockdown of DcR3 expression in Panc-1 and SW1990 cells treated
with sFasL inhibited cell viability, proliferation and
clonogenicity. Flow cytometric analysis revealed that silencing
DcR3 expression induced G0/G1 phase arrest in the cells transfected
with LV-RNAi and induced apoptosis, compared with the cells
transfected with LV-NC. These results suggest that the
downregulation of DcR3 expression enhances the effects of FasL in
Panc-1 and SW1990 cells. FADD, caspase-3 and caspase-8 are the
major regulators of the death receptor apoptotic pathway. FADD is a
central adaptor molecule for Fas and forms a complex with Fas and
pro-caspase-8 (27). Once
caspase-8 is released from the complex in an active form to
transmit death signals to downstream caspase family members, such
as caspase-3, apoptosis is induced. We found that the knockdown of
DcR3 expression upregulated the expression of FADD, caspase-3 and
caspase-8. These results further demonstrate that silencing DcR3
expression modulates cell apoptotic regulators, thus triggering
cell apoptosis.
Pancreatic carcinoma is insensitive to radiotherapy
and chemotherpy and anticancer therapy remains a major clinical
challenge. Gene therapy has provided us with an alternative
approach for the treatment of human cancer. The data presented in
this study suggest the potential use of DcR3 as a therapeutic
target. To the best of our knowledge, the anticancer effects of
silencing DcR3 expression in vivo have not been reported to
date. Our experiments on animals indicated that LV-RNAi/DcR3
suppressed the growth of pancreatic carcinoma xenografts in nude
mice. The blocking of DcR3 may prove to be an effective therapeutic
strategy, by supporting T lymphocytes and NK cells, which express
FasL, to kill tumor cells.
In conclusion, this study demonstrates that DcR3 is
overexpressed in pancreatic carcinoma tissues and serum. Silencing
DcR3 by lentivirus-mediated DcR3 RNAi enhances the effects of FasL
and inhibits tumor growth in vitro and in vivo. These
findings indicate that DcR3 may be a potential therapeutic for the
gene therapy of pancreatic carcinoma.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81201905), and the
Post-Graduate Scientific Research Innovation Project of the
Education Department of Jiangsu Province (no. CXLX11_0088), and
Suzhou Science and Education Youth Health Foundation (no.
8WKQ0802), China.
References
|
1
|
Chen X, Ma S and Zhang Z: Analysis of
clinical characteristics of pancreatic carcinoma in northern China.
Pancreas. 39:1116–1118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Auriemma WS, Berger AC, Bar-Ad V, et al:
Locally advanced pancreatic cancer. Semin Oncol. 39:e9–e22. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar
|
|
4
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar
|
|
5
|
Ungefroren H, Voss M, Jansen M, et al:
Human pancreatic adenocarcinomas express Fas and Fas ligand yet are
resistant to Fas-mediated apoptosis. Cancer Res. 58:1741–1749.
1998.PubMed/NCBI
|
|
6
|
Pitti RM, Marsters SA, Lawrence DA, et al:
Genomic amplification of a decoy receptor for Fas ligand in lung
and colon cancer. Nature. 396:699–703. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Zhang C, Chen C and Zhuang G:
Correlation between expression of DcR3 on tumor cells and
sensitivity to FasL. Cell Mol Immunol. 4:455–460. 2007.PubMed/NCBI
|
|
8
|
Bamias G, Siakavellas SI, Stamatelopoulos
KS, Chryssochoou E, Papamichael C and Sfikakis PP: Circulating
levels of TNF-like cytokine 1A (TL1A) and its decoy receptor 3
(DcR3) in rheumatoid arthritis. Clin Immunol. 129:249–255. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gill RM and Hunt JS: Soluble receptor
(DcR3) and cellular inhibitor of apoptosis-2 (cIAP-2) protect human
cytotrophoblast cells against LIGHT-mediated apoptosis. Am J
Pathol. 165:309–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
You RI, Chang YC, Chen PM, et al:
Apoptosis of dendritic cells induced by decoy receptor 3 (DcR3).
Blood. 111:1480–1488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang CR, Hsieh SL, Teng CM, Ho FM, Su WL
and Lin WW: Soluble decoy receptor 3 induces angiogenesis by
neutralization of TL1A, a cytokine belonging to tumor necrosis
factor superfamily and exhibiting angiostatic action. Cancer Res.
64:1122–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang QL, Wang BR and Li GH: DcR3 and
survivin are highly expressed in colorectal carcinoma and closely
correlated to its clinicopathologic parameters. J Zhejiang Univ Sci
B. 10:675–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen C, Zhang C, Zhuang G, et al: Decoy
receptor 3 overexpression and immunologic tolerance in
hepatocellular carcinoma (HCC) development. Cancer Invest.
26:965–974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Guo E, Yu J and Xie Q: High DcR3
expression predicts stage pN2–3 in gastric cancer. Am J Clin Oncol.
31:79–83. 2008.
|
|
15
|
Arakawa Y, Tachibana O, Hasegawa M,
Miyamori T, Yamashita J and Hayashi Y: Frequent gene amplification
and overexpression of decoy receptor 3 in glioblastoma. Acta
Neuropathol. 109:294–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Song SD, Li DC, Zhu DM and Zheng
SY: Clinical significance of expression and amplification of the
DcR3 gene in pancreatic carcinomas. Asian Pac J Cancer Prev.
13:719–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Y, Han B, Sheng H, et al: Clinical
significance of detecting elevated serum DcR3/TR6/M68 in malignant
tumor patients. Int J Cancer. 105:724–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song JH, Bellail A, Tse MC, Yong VW and
Hao C: Human astrocytes are resistant to Fas ligand and tumor
necrosis factor-related apoptosis-inducing ligand-induced
apoptosis. J Neurosci. 26:3299–3308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gratas C, Tohma Y, Barnas C, Taniere P,
Hainaut P and Ohgaki H: Up-regulation of Fas (APO-1/CD95) ligand
and down-regulation of Fas expression in human esophageal cancer.
Cancer Res. 58:2057–2062. 1998.PubMed/NCBI
|
|
20
|
Meng Y, Graves L, Do TV, So J and Fishman
DA: Upregulation of FasL by LPA on ovarian cancer cell surface
leads to apoptosis of activated lymphocytes. Gynecol Oncol.
95:488–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abbasova SG, Vysotskii MM, Ovchinnikova
LK, et al: Cancer and soluble FAS. Bull Exp Biol Med. 148:638–642.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morishige T, Yoshioka Y, Inakura H, et al:
Creation of a LIGHT mutant with the capacity to evade the decoy
receptor for cancer therapy. Biomaterials. 31:3357–3363. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takahashi M, Miura Y, Hayashi S, Tateishi
K, Fukuda K and Kurosaka M: DcR3-TL1A signalling inhibits
cytokine-induced proliferation of rheumatoid synovial fibroblasts.
Int J Mol Med. 28:423–427. 2011.PubMed/NCBI
|
|
24
|
Tsuji S, Hosotani R, Yonehara S, et al:
Endogenous decoy receptor 3 blocks the growth inhibition signals
mediated by Fas ligand in human pancreatic adenocarcinoma. Int J
Cancer. 106:17–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Migone TS, Zhang J, Luo X, et al: TL1A is
a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell
costimulator. Immunity. 16:479–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan X, Zhang J, Luo H, et al: A TNF family
member LIGHT transduces costimulatory signals into human T cells. J
Immunol. 169:6813–6821. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tourneur L, Buzyn A and Chiocchia G: FADD
adaptor in cancer. Med Immunol. 4:12005. View Article : Google Scholar : PubMed/NCBI
|