Introduction
Prostate cancer is ranked as the second major cause
of cancer-related mortality in developed countries (1). Although the incidence of latent
prostate cancer appears to be constant worldwide, the incidence of
its clinical forms varies substantially (2). African-American males have long been
known to have the highest rates of prostate cancer worldwide,
whereas native Japanese and Chinese males have the lowest known
prostate cancer rates (3). This
difference is likely due to both environmental and genetic factors.
In addition to the role of age and race on the risk of prostate
cancer, family history appears to be one of the most important risk
factors (4): the incidence of
prostate cancer is positively associated with relevant family
history with a strong genetic dose-effect (5).
Androgen plays an important role in the growth and
functions of both normal and malignant prostate glands and can
affect the carcinogenesis of prostate cancer (3). Androgen function is achieved by the
androgen receptor, which is a ligand-dependent nuclear
transcription factor (6,7). Dihydrotestosterone, transformer of
testosterone, combines with the carboxyl-terminal of an androgen
receptor, which is activated and changed into a form with greater
structural stability. Subsequently, it enters the nucleus to
combine with the androgen response elements (AREs) in the DNA to
induce transcription (8). The
androgen receptor gene is located on chromosome Xq11–12 and is
composed of eight exons. These eight exons each perform in the
transcription of the amino-terminal transcriptional activation
(transactivation) domain, the DNA binding domain (a hinge region)
and the carboxyl-terminal ligand binding domain. Among these three
domains, the transactivation domain has several polymorphisms,
which regulate the manifestation of the target gene. Three
microsatellite trinucleotide repeats exist in this transactivation
domain. In particular, CAG presents with a different length for
each person and exists upstream and downstream of each domain to
encode polyglutamine and polyglycine (9). An experimental study discovered that
the replication of the androgen receptor gene within prostate
epithelial cells was increased with a shorter CAG repeat (10).
The average CAG repeat length has a wide ethnic
variety: Africans possess a slightly shorter CAG repeats than
Caucasians, whereas Asians have a longer CAG repeat than other
races. In general, the CAG repeat length is measured as 19, 22 and
23 for Africans, Caucasians and Asians, respectively (11). Therefore, in this meta-analysis,
we aimed to evaluate the effects of (CAG)n repeat polymorphisms of
the androgen receptor gene in relation to the risk of prostate
cancer, as regards race and the number of CAG repeat polymorphisms
simultaneously, as well as the characteristics of the study
design.
Materials and methods
Search strategy
To collect articles published on the association
between CAG repeats and prostate cancer, publications were
identified from the National Center for Biotechnology Information
(NCBI) database of epidemiological studies. The following keywords
were used: prostate cancer risk, CAG repeat polymorphism, androgen
receptor gene and human. We further examined citations for all
retrieved articles of studies that had not been initially
identified. If more than one geographical or ethnic population was
included, we considered each population or group independently.
We identified studies that fulfilled the following
criteria: i) evaluation of the association between prostate cancer
and CAG repeat polymorphisms; ii) nested case-control, case-control
or cross-sectional study; and iii) sufficient information on CAG
repeat distributions between patients and controls for estimating
the odds ratio (OR) and the 95% confidence interval (95% CI).
Data extraction
The two authors of this article independently
extracted the following information from all available studies.
Original studies were blinded for authors, affiliations, journal
names, publication year and acknowledgments. Each study was
categorized as one of the following items: general information
(publication year and geographical area), population
characteristics (size of the study, population and ethnicity) and
patient and control subject characteristics (number, mean age at
diagnosis and their respective status in terms of to what extent
the prostate cancer of the subject had progressed). To investigate
the potential influence of the timing of diagnosis, all studies
were classified as either prospective (cohort) studies or
retrospective (case-control and cross-sectional) studies. For
stratified analysis by ethnicity, each article was classified into
Caucasians, Asians and Africans based on the respective number of
participants, apart from one study on Hispanics. If the results of
various races were included in one report, the results of each race
were separately used during the subgroup analysis. Finally, 47
studies were included in this meta-analysis (13–18,23–24,30–68).
For each study, we extracted an OR to evaluate the
risk of CAG repeat polymorphisms in relation to the risk of
prostate cancer. If the OR was not presented, but the number of
case and controls were reported, we calculated the OR. We analyzed
47 studies by using shorter/longer repeats presented in each
article regardless of the exact cut-off length of the CAG repeat.
In addition, we focused on two widely evaluated dichotomous
comparisons, viz. ≥23 repeats of the CAG sequence vs. others and
≥22 repeats vs. others. This was done as no studies provided the
specific distributions of the repeat counts.
Statistical analysis
The strength of the association between the cut-off
values of the repeat number and the risk of prostate cancer was
assessed by calculating the OR and the 95% CI. In this
meta-analysis, we used the random-effects model instead of the
fixed-effects model. Estimates were also stratified by study design
(prospective vs. retrospective), the CAG repeat polymorphism
(shorter vs. longer) and ethnicity (Caucasian vs. Asian vs.
African). The effect, standard error and variability were measured
for the heterogeneity test in accordance with the log OR and
calculated through function Meta. In addition, meta-regression was
employed to estimate the covariates which could explain the
heterogeneity.
The heterogeneity between the studies was presented
through the random effects model. The between-study heterogeneity
was assessed by the χ2 test-based Q statistic. A P-value
<0.05 was considered to indicate a statistically significant
difference. A meta-regression was conducted to identify sources of
between-study heterogeneity.
An estimate of potential publication bias was
carried out by the funnel plot and Egger's linear regression test
(69). The potential publication
bias was examined visually in a funnel plot of log (OR) against its
standard error (SE) and the degree of asymmetry was tested by
Egger's test (P<0.05 was considered a significant publication
bias). Begg's test (70) and
Egger's test can detect funnel plot asymmetry by determining
whether the intercept deviates significantly from zero in a
regression of the standardized effect estimates against their
precision. If publication bias existed, the non-parametric ‘trim
and fill’ method was used to adjust for it (71). We predicted the contribution of
the CAG repeat polymorphism to the risk of prostate cancer using
Stata software version 10.0.
Results
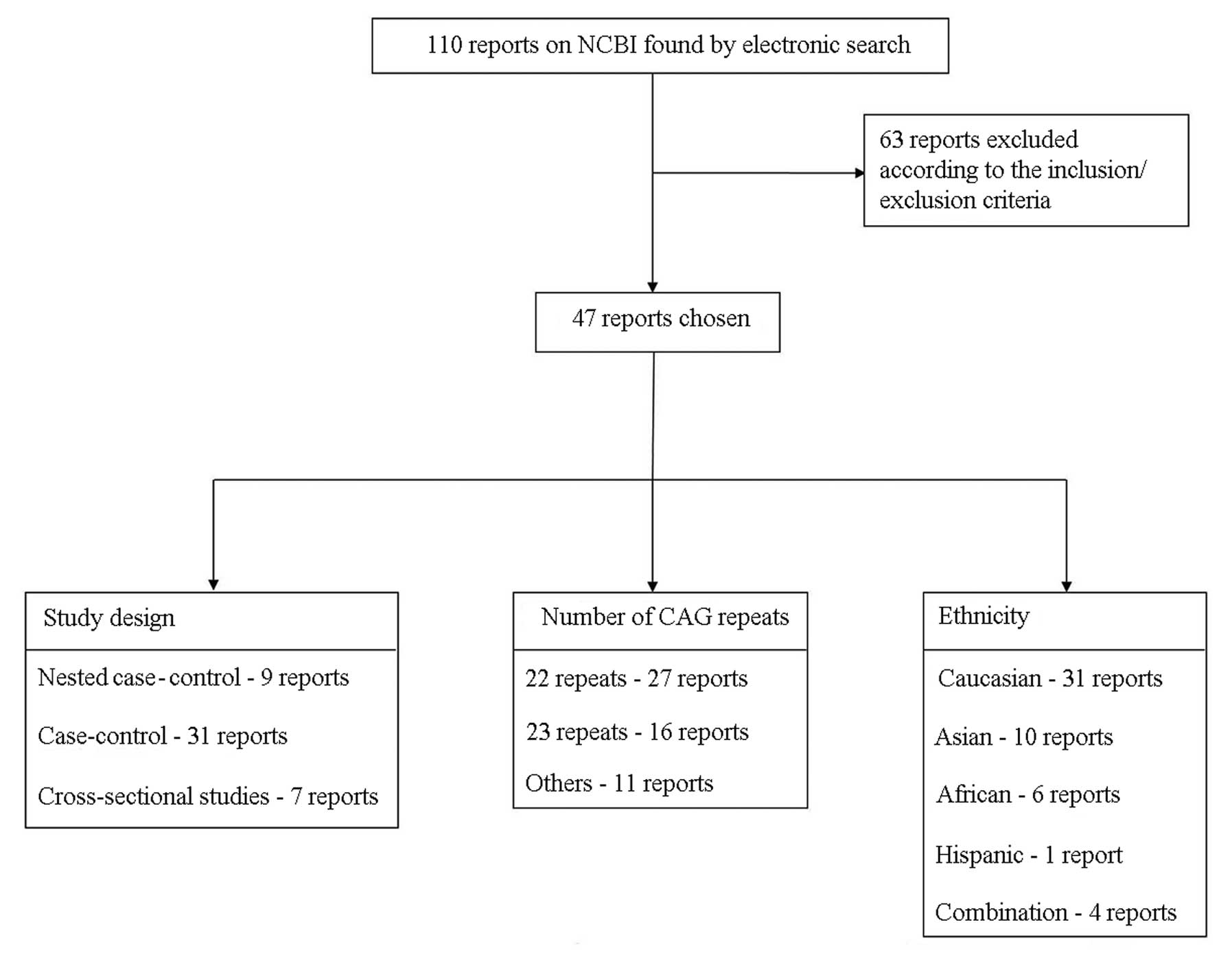
After an extensive literature search, we finally
identified 47 reports that satisfied our inclusion/exclusion
criteria and conducted at least one of the aforementioned
comparisons. Our search and selection process is described in
Fig. 1. The selected literature
included nine nested case-control studies, 31 case-control studies
and seven cross-sectional studies. We focused on two widely
evaluated dichotomous comparisons: as considering overlap, 16 and
27 studies reported the comparison of ≥23 repeats of CAG sequences
vs. others and ≥22 repeats vs. others, respectively. Studies were
classified according to race: 31 reports on Caucasians, ten on
Asians, six on Africans, one on Hispanics and four on mixed race
subjects.
In total, there were 47 reports with 13,346 patients
and 15,172 controls. A total of 11 studies were selected from seven
Asian countries (including Japan, China, Singapore, India, Taiwan,
Iran and Israel), 36 studies were selected from nine Western
countries (including the USA, Austria, Israel, France, Sweden,
Finland, Germany, England and Italy) and seven studies were
selected from Brazil, South Africa, Nigeria and Colombia. The age
range was from 45 to 76.2 years for the patient group and from 45
to 75 years for the control group. The pathological stage of
prostate cancer was presented in 21 reports among the selected
literature (Table I).
 | Table ICharacteristics of published
epidemiological studies concerning the association between the
length of CAG repeat polymorphisms and the risk of prostate
cancer. |
Table I
Characteristics of published
epidemiological studies concerning the association between the
length of CAG repeat polymorphisms and the risk of prostate
cancer.
| Authors
(Refs.) | Population | No. of subject
case/control | Age | Case Ad. (%) | Cut-off point of
repeat no.a |
|---|
|
|
|---|
| Ethnicity | Country | Case | Control |
|---|
| Prospective
studies |
| Lange et al
(26) | Afr | USA | 131/340 | 67.0 | 62.1 | - | 22 | 23 | |
| Platz et al
(27) | C | USA | 448/448 | 69.8 | - | - | 22 | | |
| Freedman et
al (28) | A + Afr + C +
L | USA | 2036/2160 | 60.0 | 60.0 | - | 22 | | 23 |
| Visvanathan et
al (29) | C | USA | 164/324 | 66.1 | 66.0 | - | 22 | | |
| Chen et al
(30) | C | USA | 300/300 | 61.2 | 60.8 | 11.5 | 22 | | |
| Latil et al
(31) | C | France | 226/156 | 70.5 | 71.7 | 69.8 | | 23 | |
| Platz et al
(32) | C | USA | 582/794 | 62.0 | - | 46.6 | | | 20 |
| Giovannucci et
al (18) | C | USA | 587/588 | - | - | 30.7 | 22 | | |
| Price et al
(33) | Afr | USA | 116/149 | 63.4 | 63.6 | - | | | 19 |
| C | USA | 1076/1047 | 63.4 | 63.6 | - | | | |
| Retrospective
studies |
| Nicolaiew et
al (34) | C | French | 1045/814 | 67.0 | 63.0 | - | | | 17 |
| Silva et al
(35) | C + U | Brazil | 49/51 | 64.0 | 59.3 | - | 22 | | |
| Das et al
(36) | A | Singapore | 52/46 | 66.0 | 69.0 | - | | | 23 |
| Andersson et
al (24) | C | Sweden | 137/125 | 76.2 | 60.0 | - | | 23 | |
| Lindström et
al (37) | C | Sweden | 1461/796 | - | - | 48.0 | | 23 | |
| Okugi et al
(38) | A | Japan | 102/117 | 69.9 | 71.0 | 55.8 | | 23 | |
| Krishnaswamy et
al (39) | A | india | 87/120 | 67.5 | 66.5 | - | | | 20 |
| Sieh et al
(40) | Afr + C | USA | 193/391 | 76.7 | 72.9 | 34.2 | 22 | | |
| Salinas et
al (41) | C | USA | 591/538 | 57.3 | 56.8 | - | 22 | | |
| Forrest et
al (42) | C | UK | 50/76 | 51.1 | - | - | | | 23 |
| Mishra et al
(43) | A | india | 113/133 | 65.6 | 63.7 | - | | 23 | 20 |
| Cicek et al
(44) | C | USA | 397/397 | 63.0 | 63.0 | - | 22 | | |
| Afr | USA | 38/38 | 62.0 | 63.0 | - | | | |
| Gilligan et
al (45) | Afr | Columbia | 118/567 | 66.7 | 55.5 | 24.5 | 22 | | |
| Huang et al
(46) | A | Taiwan | 66/104 | 71.5 | 71.7 | 40.9 | | 23 | |
| Gsur et al
(47) | C | Austria | 190/190 | 65.9 | 66.5 | - | | 23 | |
| Mononen et
al (48) | C | Finland | 461/574 | 68.1 | - | 48.1 | | | 19,25 |
| Balic et al
(49) | H | USA | 82/145 | 64.0 | 57.0 | | | | 19 |
| Modugno et
al (50) | C | USA | 88/241 | 68.9 | 73.6 | - | | 23 | |
| Xue et al
(51) | C | USA | 57/156 | 57.8 | - | - | | | 20 |
| Lange et al
(52) | C | USA | 133/305 | 64.0 | | - | 22 | | |
| Hsing et al
(53) | A | China | 191/304 | 72.2 | 71.9 | 62.6 | 22 | 23 | |
| Correa-Cerro et
al (54) | C | Fra./Ger. | 85/46 | 68.2 | 71.2 | - | 22 | | |
| Edwards et
al (55) | C | UK | 178/195 | 68.1 | - | 75.3 | 22 | | |
| Ekman et al
(56) | C | Sweden | 59/38 | 69.0 | 72.0 | - | 22 | 23 | |
| A | Japan | 34/33 | 71.0 | 60.0 | - | | | |
| Ingles et al
(57) | C | USA | 57/169 | 57.8 | 58.2 | 46.0 | 22 | | |
| Stanford et
al (58) | C | USA | 301/277 | 54.9 | 54.0 | 45.9 | 22 | | |
| Hakimi et al
(19) | C | USA | 59/370 | 62.1 | - | 42.4 | | | 18 |
| Li et al
(59) | A | Japan | 33/43 | 33.0 | - | 75.0 | 22 | 23 | |
| C | Sweden | 59/98 | 59.0 | - | 50.4 | | | |
| Kuasne et al
(60) | C | Brazil | 160/160 | 65.4 | 63.9 | - | | | 21 |
| Ashtiani et
al (61) | A | Iran | 110/67 | 69.5 | 60.4 | - | 22 | | |
| Akinloye et
al (62) | Afr | Nigeria | 70/73 | 63.5 | 62.3 | - | 22 | | |
| Chang et al
(63) | C | USA | 245/222 | 60.9 | 58.0 | - | 22 | | |
| Miller et al
(64) | C | USA | 137/69 | 65.7 | 66.2 | - | 22 | | |
| Irvine et al
(65) | C | USA | 57/39 | 57.7 | 35.0 | 47.0 | 22 | | |
| Panz et al
(66) | C | USA | 20/20 | 68.0 | - | 30.0 | 22 | 23 | |
| Afr | Israel/ South
Africa | 20/20 | 76.0 | - | 30.0 | | | |
| Mittal et al
(67) | A | India | 135/142 | 66.2 | 64.1 | - | 22 | | |
| Santos et al
(68) | A + Afr + C | Brazil | 133/279 | 65.0 | 58.0 | - | 22 | | |
| Risio et al
(12) | C | Italy | 69/234 | 65 | 62.5 | 24.2 | | | 21 |
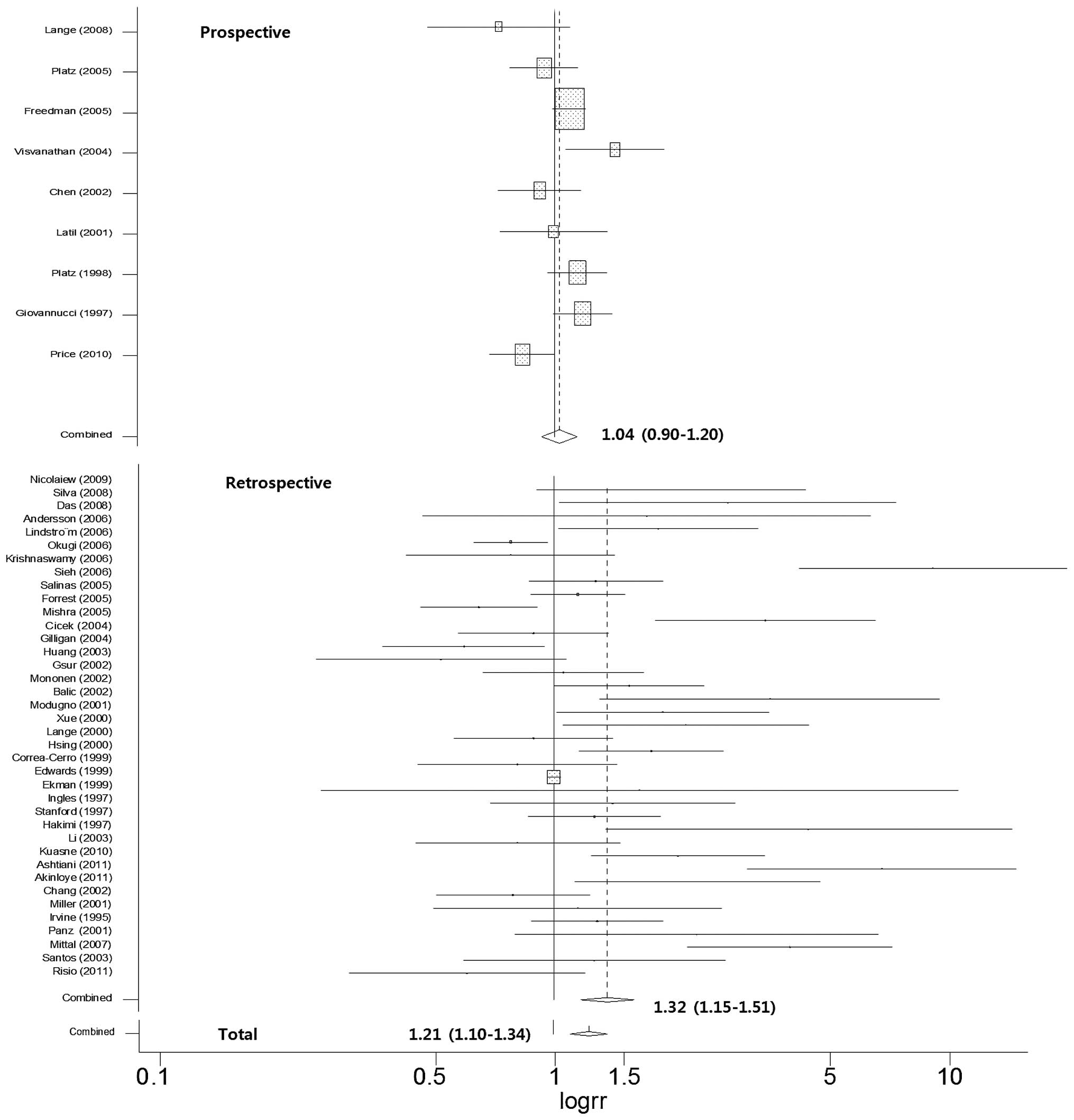
The carriers of a shorter CAG repeat had an
increased risk of prostate cancer (OR 1.21, 95% CI 1.10–1.34)
compared with those of a longer CAG repeat based on the presented
criteria taken from the original study whatever the exact length of
the CAG repeat (Fig. 2). Most
prospective studies showed no significant differences between
shorter and longer repeats, apart from one report. Therefore, the
results of the meta-analysis in relation to the prospective studies
did not indicate the association of shorter CAG repeats with the
risk of prostate cancer risk (OR 1.04, 95% CI 0.90–1.20). In terms
of retrospective studies, 14 studies out of 31 reports presented a
higher risk of shorter CAG repeats compared with longer CAG repeats
(OR 1.32, 95% CI 1.15–1.51).
On the other hand, the effect of shorter CAG repeats
on the incidence of prostate cancer was predominant among Asians
(OR 1.83, 95% CI 1.04–3.22, Table
II); the results for Caucasians indicated borderline
significance (OR 1.12, 95% CI 0.99–1.26) and there was no
significant difference between the CAG repeat polymorphisms and
prostate cancer risk among Africans (OR 0.87, 95% CI 0.35–2.17).
Based on the specific repeat number of CAG polymorphisms, we
carried out an advanced analysis following stratification by race
and the number of CAG repeats (Table
II). Based on the meta-analysis of 27 studies, which presented
the association between the ≥22 CAG repeat polymorphisms and the
risk of prostate cancer, we observed a positive association of
<22 CAG repeat polymorphisms with the risk of cancer (OR= 1.16,
95% CI 1.04–1.29). In particular, the risk increased by 2.06-fold
in Asians (OR 2.06, 95% CI 1.00–4.24), but not in Caucasians (OR
1.07, 95% CI 0.98–1.18) and Africans (OR 0.95, 95% CI 0.53–1.70).
On the other hand, there was no association of the <23 CAG
repeat polymorphisms with the risk of prostate cancer using 16
studies compared with ≥23 CAG repeats. In the analysis conducted
according to race, no difference was presented between longer CAG
repeats and shorter CAG repeats in terms of the risk of prostate
cancer (Table II).
 | Table IIAssociation of CAG repeat
polymorphisms with the risk of prostate cancer following
stratification by study design, cut-off point for repeat number and
ethnicity. |
Table II
Association of CAG repeat
polymorphisms with the risk of prostate cancer following
stratification by study design, cut-off point for repeat number and
ethnicity.
| Refs. | Meta-analysis
(OR) |
|---|
| Overall | | |
| Shorter/longer | (47) | 1.21
(1.10–1.34) |
| Subgroup | | |
| Study design | | |
| Prospective | | |
| All | (9) | 1.04
(0.90–1.20) |
| Caucasian | (7) | 1.09
(0.95–1.25) |
| African | (2) | 0.75
(0.49–1.15) |
| Retrospective | | |
| All | (38) | 1.32
(1.15–1.51) |
| Caucasian | (24) | 1.12
(0.99–1.26) |
| Asian | (10) | 1.83
(1.04–3.22) |
| African | (4) | 0.87
(0.35–2.17) |
| Length of CAG
repeat no. | | |
| <22/≥22 | (27) | 1.16
(1.04–1.29) |
| <23/≥23 | (16) | 1.09
(0.90–1.33) |
| Ethnicity | | |
| Caucasian | | |
| All | (31) | 1.10
(1.00–1.21) |
| <22/≥22 | (17) | 1.07
(0.98–1.18) |
| <23/≥23 | (9) | 1.07
(0.83–1.39) |
| Asian | | |
| All | (10) | 1.83
(1.04–3.22) |
| <22/≥22 | (5) | 2.06
(1.00–4.24) |
| <23/≥23 | (7) | 1.16
(0.65–2.00) |
| African | | |
| All | (6) | 0.86
(0.52–1.43) |
| <22/≥22 | (5) | 0.95
(0.53–1.70) |
| <23/≥23 | (2) | 0.71
(0.43–1.19) |
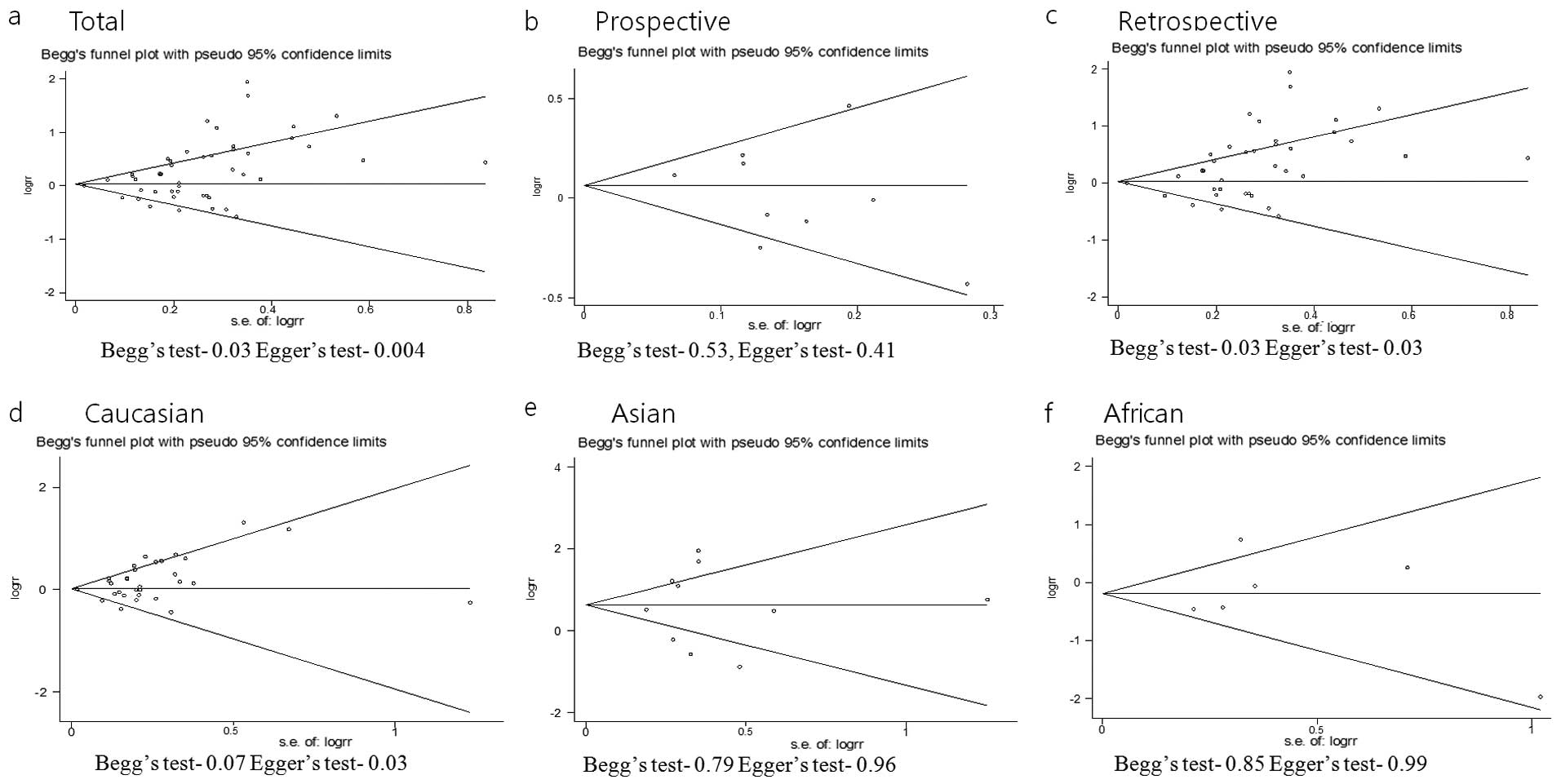
Publication bias was analyzed according to the study
design (Fig. 3). For the
prospective studies, the P-value was 0.53 for Begg's test and 0.41
for Egger's test, and no publication bias was identified. For the
retrospective studies, we found a publication bias (both Begg's and
Egger's test was 0.03). Five retrospective studies contributed to
the publication bias based on the deviability from the standard
(19,39,43,49,67). Publication bias was observed
according to race. For Caucasians, the P-value was 0.07 for Begg's
test and 0.03 for Egger's test. For Asians, the P-value was 0.79
for Begg's test and 0.96 for Egger's test. For Africans, the
P-value was 0.85 for Begg's test and 0.99 for Egger's test. A
statistical significance in terms of the heterogeneity between the
47 studies was observed (Q=196.18; P=0.00).
Discussion
The results of this meta-analysis of 13,346 patients
and 15,172 controls from 47 reports suggest that shorter CAG repeat
polymorphisms of the androgen receptors are associated with the
increased risk of prostate cancer compared with longer CAG repeats
regardless of the exact number of CAG repeat polymorphisms. The
association was not shown in the meta-analysis using prospective
studies, but was observed using retrospective studies. In
particular, while the risk of prostate cancer increased
predominately among Asians, this was not the case among Africans
and Caucasians. Considering the number of CAG repeat polymorphisms,
the association was only presented after stratification by
dichotomous comparison viz. <22 CAG repeats of CAG polymorphisms
vs. others.
Although the majority of studies report a positive
association between shorter CAG repeat polymorphisms and the risk
of prostate cancer, the cut-off point for the length of CAG repeats
differed from each race and study population. The length of a CAG
repeat was usually longer in Asians than in Caucasians (12). In general, the majority of studies
may have taken the average CAG repeat as the cut-off point for the
CAG repeats. Therefore, it is difficult to take a unique and
standard cut-off point of the CAG repeat polymorphisms for a
meta-analysis. In our meta-analysis, the effect of the shorter CAG
repeat polymorphisms on the increased risk of prostate cancer was
evaluated using shorter and longer CAG repeats whatever the exact
cut-off point of the CAG repeat length in each of the 47 studies. A
meta-analysis with 23 studies published in 2004 (13) suggested that prostate cancer
patients have a short CAG repeat length (the average difference
between cases and controls was 0.26) and reported that the
incidence rate of prostate cancer decreased to 1.02 with the
increase of one CAG repeat. Another meta-analysis conducted in 2012
(14) was based on 27 studies and
reported that the risk decreased by 0.79-fold among subjects aged
45 years and above when the cut-off point of the CAG repeats was
taken as <21 vs. ≥21 CAG repeat polymorphisms. On the other
hand, a recent and major prospective study in the USA (15) reported no association between the
CAG repeat and the risk of prostate cancer based on a continuous
model (OR 0.96, 95% CI 0.88–1.08, P(trend) = 0.46, per 10 CAG
repeat increment). However, this study did not examine the effect
of the CAG repeat polymorphisms in terms of shorter vs. longer.
Testosterone combines with androgen receptors to
stabilize the structure and promote the replication of androgen
receptors associated with prostate cancer. The testosterone level
of African-Americans was 15% higher than that of
European-Americans, which may explain the difference in the
incidence of prostate cancer between the two ethnic groups
(16). An experimental study
discovered that the increased transcription of the androgen
receptor gene within prostate epithelial cells was associated with
shorter CAG repeats (17).
Certain studies have suggested that short CAG repeats constantly
stimulate androgen, which gives rise to the proliferation of
prostate cells and finally induces somatic mutation (6,18).
Furthermore, short CAG repeats have been associated with the
aggressive forms of prostate cancer, as well as the incidence of
other androgen-related diseases (18,19). The decreased formation and
fertilization of sperm cased by longer CAG repeats (20), balding (21) and prostatic hypertrophy (22,23) have been associated with shorter
CAG repeats. Andersson et al suggested two possible
mechanisms to explain the association between the length of CAG
repeat polymorphisms and androgen receptor transcription. First,
the trinucleotide CAG repeat may act as a suppressor of
transactivation, where the longer length of the triple region acts
as a more effective suppressor. Second, a receptor with a shorter
CAG repeat has a more stable structure (24).
The average CAG repeat length has a wide ethnic
variety (11). Africans possess a
slightly shorter CAG repeat than Caucasians, whereas Asians have a
longer CAG repeat than subjects from other races. In general, the
length of the CAG repeat was measured respectively as 19, 22 and 23
for Africans, Caucasian and Asians (11). The risk of prostate cancer
generally increases in Western countries but decreases in Asian
countries (3). This suggests that
there is a clear association of the CAG repeat polymorphisms with
the risk of prostate cancer (25). Our meta-analysis also presents the
protective effect of longer CAG repeat polymorphisms on prostate
cancer in Caucasians and Asians; the cut-off point of criteria for
the meta-analysis was 22 and 23 for Caucasians and Asians,
respectively. Although shorter CAG repeats were associated with a
higher risk of prostate cancer than longer CAG repeats in
case-control studies, no significant results were observed in
nested case-control studies. The results of Caucasians and Asians
in case-control studies were identical. Otherwise, the significance
was still not observed in the analysis of nested case-control
studies following stratification by race. Therefore, we should
carefully explain the difference by race, as the association may
not be independent of study design or the number of CAG
repeats.
The strength of this meta-analysis is its large
number of subjects, including 13,346 cases and 15,172 controls
based on 47 studies (nine nested case-control studies, 31
case-control studies and seven cross-sectional studies). A
meta-analysis conducted in 2004 composed of 4,274 cases and 5,275
controls and quoted a total of 23 studies: five nested case-control
studies and 19 case-control studies (13). Another meta-analysis (14), which reported the association
between androgen receptor CAG repeat polymorphisms with ≥22 repeats
and the risk of prostate cancer among subjects aged 45 years or
older, was composed of 3,835 cases and 4,774 controls and only
quoted a total of 27 studies. Furthermore, in the present
meta-analysis, an advanced analysis was conducted according to
study design, race and the number of CAG repeat polymorphisms, in
contrast to previous meta-analysis reports.
However, this meta-analysis also had certain
limitations. Confounding factors could not be used as we were
unable to retrieve common information from all these original
publications for a variety of confounding factors, such as smoking
history or other lifestyle factors. Therefore, the findings from
our meta-analysis require further confirmation or validation. On
the other hand, the association between the risk of prostate cancer
and androgen receptor CAG repeat polymorphisms was not observed in
all races or studies. It is insufficient to explain the incidence
of prostate cancer based on genetic factors only, as it does not
correspond with the results of the genetic variation of previously
presented studies. Individual differences in the sensitivity of
prostate cancer cells must be explained in relation to other
reasons, such as lifestyle (including smoking history and other
environmental factors).
In conclusion, in this meta-analysis, it was
verified that shorter CAG repeats increase the risk of prostate
cancer compared with longer CAG repeats, whatever the exact length
of the CAG repeat. In particular, race and the length of the CAG
repeat polymorphisms may influence the association between the CAG
repeat polymorphisms and the risk of prostate cancer.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar
|
|
2
|
Parkin DM, Whelan SL, Ferlay J, Raymond
and Young J: Cancer incidence in five continents. VIIIARC Sci Publ;
(143): pp. i–xxxiv. pp. 1–1240. Lyon: 1997
|
|
3
|
Ross RK, Pike MC, Coetzee GA, Reichardt
JK, Yu MC, Feigelson H, et al: Androgen metabolism and prostate
cancer: establishing a model of genetic susceptibility. Cancer Res.
58:4497–4504. 1998.PubMed/NCBI
|
|
4
|
Zeegers MP, Jellema A and Ostrer H:
Empiric risk of prostate carcinoma for relatives of patients with
prostate carcinoma: a meta-analysis. Cancer. 97:1894–1903. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
|
|
6
|
Nelson KA and Witte JS: Androgen receptor
CAG repeats and prostate cancer. Am J Epidemiol. 155:883–890. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilding G: The importance of steroid
hormones in prostate cancer. Cancer Surv. 14:113–130.
1992.PubMed/NCBI
|
|
8
|
Crawford ED: Understanding the
epidemiology, natural history, and key pathways involved in
prostate cancer. Urology. 73(Suppl 5): S4–S10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montgomery JS, Price DK and Figg WD: The
androgen receptor gene and its influence on the development and
progression of prostate cancer. J Pathol. 195:138–146. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chamberlain NL, Driver ED and Miesfeld RL:
The length and location of CAG trinucleotide repeats in the
androgen receptor N-terminal domain affect transactivation
function. Nucleic Acids Res. 22:3181–3186. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buchanan G, Yang M, Cheong A, Harris JM,
Irvine RA, Lambert PF, et al: Structural and functional
consequences of glutamine tract variation in the androgen receptor.
Hum Mol Genet. 13:1677–1692. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Risio M, Venesio T, Kolomoets E, Armaroli
P, Gallo F, Balsamo A, et al: Genetic polymorphisms of CYP17A1,
vitamin D receptor and androgen receptor in Italian heredo-familial
and sporadic prostate cancers. Cancer Epidemiol. 35:e18–e24. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeegers MP, Kiemeney LA, Nieder AM and
Ostrer H: How strong is the association between CAG and GGN repeat
length polymorphisms in the androgen receptor gene and prostate
cancer risk? Cancer Epidemiol Biomarkers Prev. 13:1765–1771.
2004.PubMed/NCBI
|
|
14
|
Gu M, Dong X, Zhang X and Niu W: The CAG
repeat polymorphism of androgen receptor gene and prostate cancer:
a meta-analysis. Mol Biol Rep. 39:2615–2624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lindstrom S, Ma J, Altshuler D,
Giovannucci E, Riboli E, Albanes D, et al: A large study of
androgen receptor germline variants and their relation to sex
hormone levels and prostate cancer risk. Results from the National
Cancer Institute Breast and Prostate Cancer Cohort Consortium. J
Clin Endocrinol Metab. 95:E121–E127. 2010. View Article : Google Scholar
|
|
16
|
Ahluwalia B, Jackson MA, Jones GW,
Williams AO, Rao MS and Rajguru S: Blood hormone profiles in
prostate cancer patients in high-risk and low-risk populations.
Cancer. 48:2267–2273. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kazemi-Esfarjani P, Trifiro MA and Pinsky
L: Evidence for a repressive function of the long polyglutamine
tract in the human androgen receptor: possible pathogenetic
relevance for the (CAG)n-expanded neuronopathies. Hum Mol Genet.
4:523–527. 1995. View Article : Google Scholar
|
|
18
|
Giovannucci E, Stampfer MJ, Krithivas K,
Brown M, Dahl D, Brufsky A, et al: The CAG repeat within the
androgen receptor gene and its relationship to prostate cancer.
Proc Natl Acad Sci USA. 94:3320–3323. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hakimi JM, Schoenberg MP, Rondinelli RH,
Piantadosi S and Barrack ER: Androgen receptor variants with short
glutamine or glycine repeats may identify unique subpopulations of
men with prostate cancer. Clin Cancer Res. 3:1599–1608.
1997.PubMed/NCBI
|
|
20
|
Yoshida KI, Yano M, Chiba K, Honda M and
Kitahara S: CAG repeat length in the androgen receptor gene is
enhanced in patients with idiopathic azoospermia. Urology.
54:1078–1081. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sawaya ME and Shalita AR: Androgen
receptor polymorphisms (CAG repeat lengths) in androgenetic
alopecia, hirsutism, and acne. J Cutan Med Surg. 3:9–15.
1998.PubMed/NCBI
|
|
22
|
Mitsumori K, Terai A, Oka H, Segawa T,
Ogura K, Yoshida O and Ogawa O: Androgen receptor CAG repeat length
polymorphism in benign prostatic hyperplasia (BPH): correlation
with adenoma growth. Prostate. 41:253–257. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shibata A, Stamey TA, McNeal JE, Cheng I
and Peehl DM: Genetic polymorphisms in the androgen receptor and
type II 5 alpha-reductase genes in prostate enlargement. J Urol.
166:1560–1564. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andersson P, Varenhorst E and Soderkvist
P: Androgen receptor and vitamin D receptor gene polymorphisms and
prostate cancer risk. Eur J Cancer. 42:2833–2837. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sartor O, Zheng Q and Eastham JA: Androgen
receptor gene CAG repeat length varies in a race-specific fashion
in men without prostate cancer. Urology. 53:378–380. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lange EM, Sarma AV, Ray A, Wang Y, Ho LA,
Anderson SA, et al: The androgen receptor CAG and GGN repeat
polymorphisms and prostate cancer susceptibility in
African-American men: results from the Flint Men's Health Study. J
Hum Genet. 53:220–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Platz EA, Leitzmann MF, Rifai N, Kantoff
PW, Chen YC, Stampfer MJ, et al: Sex steroid hormones and the
androgen receptor gene CAG repeat and subsequent risk of prostate
cancer in the prostate-specific antigen era. Cancer Epidemiol
Biomarkers Prev. 14:1262–1269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freedman ML, Pearce CL, Penney KL,
Hirschhorn JN, Kolonel LN, Henderson BE and Altshuler D: Systematic
evaluation of genetic variation at the androgen receptor locus and
risk of prostate cancer in a multiethnic cohort study. Am J Hum
Genet. 76:82–90. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Visvanathan K, Helzlsouer KJ, Boorman DW,
Strickland PT, Hoffman SC, Comstock GW, et al: Association among an
ornithine decarboxylase polymorphism, androgen receptor gene (CAG)
repeat length and prostate cancer risk. J Urol. 171:652–655. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen C, Lamharzi N, Weiss NS, Etzioni R,
Dightman DA, Barnett M, et al: Androgen receptor polymorphisms and
the incidence of prostate cancer. Cancer Epidemiol Biomarkers Prev.
11:1033–1040. 2002.PubMed/NCBI
|
|
31
|
Latil AG, Azzouzi R, Cancel GS, Guillaume
EC, Cochan-Priollet B, Berthon PL and Cussenot O: Prostate
carcinoma risk and allelic variants of genes involved in androgen
biosynthesis and metabolism pathways. Cancer. 92:1130–1137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Platz EA, Giovannucci E, Dahl DM,
Krithivas K, Hennekens CH, Brown M, et al: The androgen receptor
gene GGN microsatellite and prostate cancer risk. Cancer Epidemiol
Biomarkers Prev. 7:379–384. 1998.PubMed/NCBI
|
|
33
|
Price DK, Chau CH, Till C, Goodman PJ,
Baum CE, Ockers SB, et al: Androgen receptor CAG repeat length and
association with prostate cancer risk: results from the prostate
cancer prevention trial. J Urol. 184:2297–2302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nicolaiew N, Cancel-Tassin G, Azzouzi AR,
Grand BL, Mangin P, Cormier L, et al: Association between estrogen
and androgen receptor genes and prostate cancer risk. Eur J
Endocrinol. 160:101–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Silva Neto B, Koff WJ, Biolchi V, Brenner
C, Biolo KD, Spritzer PM and Brum IS: Polymorphic CAG and GGC
repeat lengths in the androgen receptor gene and prostate cancer
risk: analysis of a Brazilian population. Cancer Invest. 26:74–80.
2008.PubMed/NCBI
|
|
36
|
Das K, Cheah PY, Lim PL, Zain YB,
Stephanie FC, Zhao Y, et al: Shorter CAG repeats in androgen
receptor and non-GG genotypes in prostate-specific antigen loci are
associated with decreased risk of benign prostatic hyperplasia and
prostate cancer. Cancer Lett. 268:340–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lindström S, Zheng SL, Wiklund F, Jonsson
BA, Adami HO, Balter KA, et al: Systematic replication study of
reported genetic associations in prostate cancer: Strong support
for genetic variation in the androgen pathway. Prostate.
66:1729–1743. 2006.PubMed/NCBI
|
|
38
|
Okugi H, Nakazato H, Matsui H, Ohtake N,
Nakata S and Suzuki K: Association of the polymorphisms of genes
involved in androgen metabolism and signaling pathways with
familial prostate cancer risk in a Japanese population. Cancer
Detect Prev. 30:262–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krishnaswamy V, Kumarasamy T, Venkatesan
V, Shroff S, Jayanth VR and Paul SF: South Indian men with reduced
CAG repeat length in the androgen receptor gene have an increased
risk of prostate cancer. J Hum Genet. 51:254–257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sieh W, Edwards KL, Fitzpatrick AL,
Srinouanprachanh SL, Farin FM, Monks SA, et al: Prostate-specific
antigen and its interaction with the androgen receptor (United
States). Cancer Causes Control. 17:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Salinas CA, Austin MA, Ostrander EO and
Stanford JL: Polymorphisms in the androgen receptor and the
prostate-specific antigen genes and prostate cancer risk. Prostate.
65:58–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Forrest MS, Edwards SM, Houlston R,
Kote-Jarai Z, Key T, Allen N, et al: Association between hormonal
genetic polymorphisms and early-onset prostate cancer. Prostate
Cancer Prostatic Dis. 8:95–102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mishra D, Thangaraj K, Mandhani A, Kumar A
and Mittal R: Is reduced CAG repeat length in androgen receptor
gene associated with risk of prostate cancer in Indian population?
Clin Genet. 68:55–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cicek MS, Conti DV, Curran A, Neville PJ,
Paris PL, Casey G and Witte JS: Association of prostate cancer risk
and aggressiveness to androgen pathway genes: SRD5A2, CYP17, and
the AR. Prostate. 59:69–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gilligan T, Manola J, Sartor O, Weinrich
SP, Moul JW and Kantoff PW: Absence of a correlation of androgen
receptor gene CAG repeat length and prostate cancer risk in an
African-American population. Clin Prostate Cancer. 3:98–103. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang SP, Chou YH, Chang WS, Wu MT, Yu CC,
Wu T, et al: Androgen receptor gene polymorphism and prostate
cancer in Taiwan. J Formos Med Assoc. 102:680–686. 2003.PubMed/NCBI
|
|
47
|
Gsur A, Preyer M, Haidinger G, Zidek T,
Madersbacher S, Schatzl G, et al: Polymorphic CAG repeats in the
androgen receptor gene, prostate-specific antigen polymorphism and
prostate cancer risk. Carcinogenesis. 23:1647–1651. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mononen N, Ikonen T, Autio V, Rokman A,
Matikainen MP, Tammela TL, et al: Androgen receptor CAG
polymorphism and prostate cancer risk. Hum Genet. 111:166–171.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Balic I, Graham ST, Troyer DA, Higgins BA,
Pollock BH, Johnson-Pais TL, et al: Androgen receptor length
polymorphism associated with prostate cancer risk in Hispanic men.
J Urol. 168:2245–2248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Modugno F, Weissfeld JL, Trump DL, Zmuda
JM, Shea P, Cauley JA and Ferrell RE: Allelic variants of aromatase
and the androgen and estrogen receptors: toward a multigenic model
of prostate cancer risk. Clin Cancer Res. 7:3092–3096.
2001.PubMed/NCBI
|
|
51
|
Xue W, Irvine RA, Yu MC, Ross RK, Coetzee
GA and Ingles SA: Susceptibility to prostate cancer: interaction
between genotypes at the androgen receptor and prostate-specific
antigen loci. Cancer Res. 60:839–841. 2000.PubMed/NCBI
|
|
52
|
Lange EM, Chen H, Brierley K, Livermore H,
Wojno KJ, Langefeld CD, et al: The polymorphic exon 1 androgen
receptor CAG repeat in men with a potential inherited
predisposition to prostate cancer. Cancer Epidemiol Biomarkers
Prev. 9:439–442. 2000.PubMed/NCBI
|
|
53
|
Hsing AW, Gao YT, Wu G, Wang X, Deng J,
Chen YL, et al: Polymorphic CAG and GGN repeat lengths in the
androgen receptor gene and prostate cancer risk: a population-based
case-control study in China. Cancer Res. 60:5111–5116. 2000.
|
|
54
|
Correa-Cerro L, Wohr G, Haussler J,
Berthon P, Drelon E, Mangin P, et al: (CAG)nCAA and GGN repeats in
the human androgen receptor gene are not associated with prostate
cancer in a French-German population. Eur J Hum Genet. 7:357–362.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Edwards SM, Badzioch MD, Minter R, Hamoudi
R, Collins N, Ardern-Jones A, et al: Androgen receptor
polymorphisms: association with prostate cancer risk, relapse and
overall survival. Int J Cancer. 84:458–465. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ekman P, Gronberg H, Matsuyama H, Kivineva
M, Bergerheim US and Li C: Links between genetic and environmental
factors and prostate cancer risk. Prostate. 39:262–268. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ingles SA, Ross RK, Yu MC, Irvine RA, La
Pera G, Haile RW and Coetzee GA: Association of prostate cancer
risk with genetic polymorphisms in vitamin D receptor and androgen
receptor. J Natl Cancer Inst. 89:166–170. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Stanford JL, Just JJ, Gibbs M, Wicklund
KG, Neal CL, Blumenstein BA and Ostrander EA: Polymorphic repeats
in the androgen receptor gene: molecular markers of prostate cancer
risk. Cancer Res. 57:1194–1198. 1997.PubMed/NCBI
|
|
59
|
Li C, Gronberg H, Matsuyama H, Weber G,
Nordenskjold M, Naito K, et al: Difference between Swedish and
Japanese men in the association between AR CAG repeats and prostate
cancer suggesting a susceptibility-modifying locus overlapping the
androgen receptor gene. Int J Mol Med. 11:529–533. 2003.PubMed/NCBI
|
|
60
|
Kuasne H, Rodrigues IS, Fuganti PE,
Losi-Guembarovski R, Ito K, Kishima MO, et al: Polymorphisms in the
AR and PSA genes as markers of susceptibility and aggressiveness in
prostate cancer. Cancer Invest. 28:917–924. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ashtiani ZO, Hasheminasab SM, Ayati M,
Goulian BS and Modarressi MH: Are GSTM1, GSTT1 and CAG repeat
length of androgen receptor gene polymorphisms associated with risk
of prostate cancer in Iranian patients? Pathol Oncol Res.
17:269–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Akinloye O, Gromoll J and Simoni M:
Variation in CAG and GGN repeat lengths and CAG/GGN haplotype in
androgen receptor gene polymorphism and prostate carcinoma in
Nigerians. Br J Biomed Sci. 68:138–142. 2011.PubMed/NCBI
|
|
63
|
Chang BL, Zheng SL, Hawkins GA, Isaacs SD,
Wiley KE, Turner A, et al: Polymorphic GGC repeats in the androgen
receptor gene are associated with hereditary and sporadic prostate
cancer risk. Hum Genet. 110:122–129. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Miller EA, Stanford JL, Hsu L, Noonan E
and Ostrander EA: Polymorphic repeats in the androgen receptor gene
in high-risk sibships. Prostate. 48:200–205. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Irvine RA, Yu MC, Ross RK and Coetzee GA:
The CAG and GGC microsatellites of the androgen receptor gene are
in linkage disequilibrium in men with prostate cancer. Cancer Res.
55:1937–1940. 1995.PubMed/NCBI
|
|
66
|
Panz VR, Joffe BI, Spitz I, Lindenberg T,
Farkas A and Haffejee M: Tandem CAG repeats of the androgen
receptor gene and prostate cancer risk in black and white men.
Endocrine. 15:213–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mittal RD, Mishra DK, Thangaraj K, Singh R
and Mandhani A: Is there an inter-relationship between prostate
specific antigen, kallikrein-2 and androgen receptor gene
polymorphisms with risk of prostate cancer in north Indian
population? Steroids. 72:335–341. 2007. View Article : Google Scholar
|
|
68
|
Santos ML, Sarkis AS, Nishimoto IN and
Nagai MA: Androgen receptor CAG repeat polymorphism in prostate
cancer from a Brazilian population. Cancer Detect Prev. 27:321–326.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Duval S and Tweedie R: Trim and fill: A
simple funnel-plot-based method of testing and adjusting for
publication bias in meta-analysis. Biometrics. 56:455–463. 2000.
View Article : Google Scholar : PubMed/NCBI
|