Introduction
The formation of functional skeletal muscles/muscle
tissue utilising the differentiation potential of stem cells is the
intention of skeletal muscle tissue engineering. Due to the
myogenic differentiation potential of human mesenchymal stem cells
(MSCs), these cells are new candidate stem cells for tissue
engineering applications, which bear certain advantages over
previously utilised stem cells, such as satellite cells (1). MSCs can be extracted from various
sources, such as bone marrow (1),
adipose tissue (2), umbilical
cord blood (3) or the placenta
(4), and therefore multiple
harvesting methods are possible. MSCs derived from fat/adipose
tissue (AT-MSCs) or bone marrow (BM-MSCs) are characterised, in
contrast to other stem cells, by their high potential for
replication without losing their differentiation ability (5). Thus, greater amounts of neo-tissue
could be generated by smaller donor cell populations. Moreover,
MSCs can be transplanted autologously and can enhance tissue
regeneration due to immunomodulation (6,7).
These attributes make MSCs ideal stem cells for tissue
engineering.
The myogenic differentiation of MSCs can be
initiated with different growth factors, such as basic fibroblast
growth factor (bFGF), platelet-derived growth factor (PDGF), the
amino acid, L-glutamine, and 5-azacytidine (5-Aza), which can all
be added to the growth medium (5,8).
The exact molecular mechanisms associated with the intracellular
signalling cascades which control the differentiation process
remain unclear (9). The influence
of growth factors on cell differentiation and proliferation varies
according to the type, strain and origin of the MSCs (10). Another important factor is the
degree of activation of the Rho GTPase, which can control the
differentiation in myogenic or adipogenic cell lines (11). Therefore, in this study, we
evaluated the proliferation and myogenic differentiation potential
of human AT-MSCs and BM-MSCs cultured in six different culture
media with different growth factor compositions, in order to detect
possible differences between the two stem cell types. In this
context, we used the following cell culture media: mesenchymal stem
cell growth medium (MSCGM)™ by Lonza® as growth medium,
MSCGM + 5-Aza, skeletal muscle myoblast cell growth medium (SkGM)-2
BulletKit™, and 5, 30 and 50% conditioned cell culture media, i.e.,
supernatant of human satellite cell cultures after three days in
cell culture mixed with MSCGM.
During the transformation from mononucleated
myoblasts into multinucleated myofibers, multiple muscle-specific
genes are switched on and can therefore act as specific markers of
the different stages of myogenesis. As an early marker of
differentiation, we investigated the transcription factor, myogenic
factor 5 (MYF5), as it acts as promoter of multiple muscle-specific
genes, controls the fusion of multinucleated myofibers (12), and is upregulated during the early
stages of myogenesis (13,14).
Genes of the contractile apparatus served as late markers of
differentiation. Myosin heavy chain (MYH) is part of the hexameric
myosin complex, which consists of four light chains and two heavy
chains and constitutes up to 50% of the total protein content of
adult skeletal muscle tissue (14,15). MYH participates in the contraction
processes and exists in multiple isoforms [myosin, heavy chain 8,
skeletal muscle, perinatal (MYH8) and myosin, heavy chain 1,
skeletal muscle, adult (MYH1)], which can serve as markers of
differentiation (14,16). As a marker of the terminal stages
of myogenesis, actin-α1 (ACTA1) is an important protein of the
contractile apparatus in skeletal muscle tissue (14). In order to evaluate the effects of
culture with six different cell culture media on the proliferation
of human AT-MSCs and BM-MSCs, we performed alamarBlue®
proliferation assays from day 0 to day 21. In order to analyse the
effects of the different cell culture media on the myogenic
differentiation of AT-MSCs and BM-MSCs, quantitative reverse
transcription polymerase chain reaction (qRT-PCR) was performed to
determine the gene expression levels of MYF5, MYH8, MYH1 and ACTA1.
As a prerequisite, suitable reference genes for qRT-PCR experiments
needed to be identified. Therefore, we evaluated the expression
stability of β-actin (ACTB), β-2-microglobulin (B2M),
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), peptidylprolyl
isomerase A (PPIA), TATA-box binding protein (TBP) and ribosomal
protein, large, P0 (RPLPO) in human MSCs using two different
software programs, geNorm and NormFinder. Additionally,
immunocytochemistry (ICC) was performed to determine the expression
of desmin (DES), MYF5 and ACTA1, to verify myogenic
differentiation.
Thus, our study presents novel data on the myogenic
differentiation potential of human MSCs derived from bone marrow
and adipose tissue, utilising six different cell culture media with
different growth factor compositions.
Materials and methods
Collection and isolation of human MSCs
derived from bone marrow
Human MSCs were isolated from adult bone marrow as
previously described by Goessler et al (17). Bone marrow was collected from the
femoral shaft of patients undergoing total hip replacement at the
Orthopaedic Department of the University Hospital Mannheim,
Mannheim, Germany. The study protocol was approved by the Ethics
Committee of the Mannheim Medical Faculty of the University of
Heidelberg, Heidelberg, Germany. Bone marrow aspirates were diluted
1:5 with PBS/2 mM EDTA (Nexell, Baxter, Unterschleissheim, Germany
and Merck, Darmstadt, Germany) and carefully loaded into
Ficoll-Hypaque solution (Amersham, Freiburg, Germany). Mononuclear
cells (MNCs) were isolated after density gradient centrifugation at
435 × g for 30 min at room temperature. The MNCs were then removed
from the interphase, washed two to three times with PBS/EDTA and
set in culture at a density of 1×106
cells/cm2. Cell counts were performed using an automated
cell analyser (Cell-Dyn 3200, Abbott, Wiesbaden, Germany). Cells
were grown in MSCGM BulletKit, Cambrex, St. Katharinen, Germany).
Non-adherent cells were removed by the complete exchange of culture
medium every three to four days. The flasks were screened
continuously to access developing colonies of adherent cells.
Fibroblastoid cells were recovered between days seven and ten after
initial plating using 0.04% trypsin/0.03% EDTA (PromoCell,
Heidelberg, Germany) and termed bone marrow-derived fibroblastoid
adherent cells (BM-FACs). Recovered cells were plated at a density
of 4,000–5,000 cells/cm2 as passage 1 cells, as
previously described (17).
Collection and isolation of human MSCs
derived from adipose tissue
Lipoaspirates were obtained from patients undergoing
liposuction procedures in accordance with the standard of ethics of
the local ethics committee. The raw lipoaspirates (50–100 ml) were
processed as described previously by Goessler et al
(17). To isolate the stromal
vascular fraction (SVF), lipoaspirates were washed with PBS and
digested with an equal volume of 0.075% collagenase type I
(Sigma-Aldrich, St. Louis, MO, USA) for 30–60 min at 37ºC. The
collagenase activity was blocked with DMEM-lg containing 10% FCS
and the lipoaspirates were centrifuged at 1,200 × g for 10 min to
harvest a SVF pellet. The pellet was re-suspended in MSCGM and
filtered through a 100 μm nylon cell strainer (BD Falcon, Franklin
Lakes, NJ, USA). The filtered cells were centrifuged at 1,200 × g
for 10 min. The SVF cell suspension was plated at a density of
1×106/cm2 into T75 or T175 culture flasks.
Non-adherent cells were removed after 12–18 h by PBS washings. The
resulting fibroblastoid adherent cells were termed adipose
tissue-derived fibroblastoid adherent cells (AT-FACs). AT-FACs were
harvested at approximately 70% confluence using trypsin. On the
second pass, cells were replated at a mean density of
1.8±3.1×103/cm2.
Cultivation of human MSCs derived from
bone marrow and adipose tissue in different culture media
MSCGM by Lonza®
MSCGM (Lonza, Verviers, Belgium) is a growth medium,
which was designed for the cultivation of MSCs. This cell culture
medium is purchased as a kit and contains the amino acid,
L-glutamine, and mesenchymal stem cell growth supplement (MSCGS).
The precise composition is only known to the manufacturer.
MSCGM + 5-Aza
5-Aza (Sigma-Aldrich, Irvine, UK) was added at a
concentration of 10 μmol/l. Additionally, 10 μg/l human,
recombinant fibroblast growth factor (rhFGF) (Lonza) was
appended.
SkGM-2 BulletKit
The SkGM-2 BulletKit (SKM, Lonza) is a commercial
basal medium for MSCs, which includes pro-myogenic factors that
induce myogenic differentiation. The constituents of the medium
are: Skm-medium, FBS, L-glutamine, dexamethasone, rhFGF and GA-1000
(gentamycin/amphotericin 1:1,000).
Conditioned cell culture medium
The conditioned cell culture medium contains the
supernatant of human satellite cells under differentiation
conditions using serum cessation, which were grown in Ham’s F10
culture medium. The supernatant was removed after three days,
filtered for sterility and mixed to 5, 30 and 50% concentrations
with growth medium.
alamarBlue proliferation assay
AT-MSCs and BM-MSCs were cultured in 0.2%
gelatine-coated 96-well plates at a density of 5,000 cells/well in
MSCGM, MSCGM + 5-Aza, SkGM-2 BulletKit, 5, 30 and 50% conditioned
cell culture medium. Proliferation was assessed by measurement of
the fluorescence at wavelength of 540 nm and emission was monitored
at 590 nm on days 0, 4, 8, 11, 16 and 21.
RNA isolation
The RNeasy mini kit (Qiagen, Hilden, Germany) was
used according to the manufacturer’s instructions to isolate total
RNA. RNA purity and concentration were determined by measurements
at A260 and A280 nm (A260/A280, 1.7–2.0) using a NanoDrop 8000
Spectrophotometer (Thermo Scientific, Schwerte, Germany). RNA
integrity was determined using a 2100 Bioanalyzer (Agilent
Technologies, Waldbronn, Germany).
cDNA synthesis and qRT-PCR
cDNA synthesis and qRT-PCR were performed as
previously described (18). An
aliquot of 0.5 μg total RNA was treated with 1 unit DNase
(Fermentas, St. Leon-Rot, Germany) for 30 min at 37ºC. Reverse
transcription of RNA (0.5 μg) was performed using
oligo(dT)12–18 primer and 200 units of SuperScript II
(Invitrogen, Karlsruhe, Germany) and 24 units of RiboLock™ RNase
inhibitor (Fermentas) for 1 h at 42ºC. The cDNA was used for
qRT-PCR analysis. All cDNA probes were analysed for MYF5,
NM_002479; ACTA1, NM_001100; MYH1, NM_005963; and MYH8, NM_002472;
as well as the reference genes: ACTB, NM_001101; B2M, NM_004048;
GAPDH, NM_002046; PPIA (cyclophilin A), NM_203430; RPLPO,
NM_001002; and TBP, NM_003194. The QuantiTect/PrimerAssays were
purchased from Qiagen. cDNA was amplified with
Brilliant® II SYBR®-Green qRT-PCR Master Mix
(Stratagene-Agilent Technologies, Waldbronn, Germany). The thermal
profile consisted of 1 cycle at 50ºC (2 min) followed by 1 cycle at
95ºC (2 min), 45 cycles at 95ºC (15 sec) and 60ºC (1 min).
Amplification was performed using the Mx3005P™ QPCR System
(Stratagene). For relative quantification, a serial dilution of the
cDNA was used to perform a standard curve in every individual run.
The data were analysed using the relative standard curve method.
For each unknown sample, the relative amount was calculated using
linear regression analysis from their respective standard curves.
Data were analysed using Mx3005P analysis software
(Stratagene-Agilent Technologies). The efficiencies of all genes of
interest, including the reference genes were calculated for each
individual run.
Analysis of expression stability
Expression stability was calculated as previously
described (18). The qRT-PCR data
were analysed using the Mx3005P QPCR System (Stratagene). For
stability comparisons of candidate reference genes within sample
groups, the software geNorm (19), version 3.4 (Visual Basic
application tool for Microsoft Excel), was used according to the
developer’s recommendations.
GeNorm uses a gene-stability measurement, M, which
is defined as the average pair wise variation between a particular
gene and all other control genes. It calculates the optimal number
of genes required for the normalisation of a target gene and
combines them into a normalisation factor (NF) (19,20). The expression of the genes of
interest was normalised to the NF.
Immunocytochemistry (ICC)
Immunocytochemical characterisation was carried out
as previously described (18).
The cells were grown on chamber culture slides (BD Falcon). To
indicate the differentiation of the MSCs, the cells were incubated
with antibodies directed against: DES (Dako, Hamburg, Germany),
MYF5 (Santa Cruz, Biotechnology, Heidelberg, Germany) and ACTA1
(Zymed Laboratories, Invitrogen, Karlsruhe, Germany). The
antibodies were used according to the manufacturer’s instructions,
followed by a corresponding biotinylated secondary antibody and
streptavidin, horseradish peroxidase (HRP) conjugate. The
peroxidase reaction was performed using aminoethylcarbazole (Dako)
as a chromogen. Endogenous peroxidase was blocked with 0.3%
hydrogen peroxide (30 min). Sections were washed with PBS and
incubated with normal sheep serum in PBS (3 min, room temperature)
to block non-specific antibody reaction. Nuclei were counterstained
with Harris haematoxylin. Light microscopic investigations were
performed using a Zeiss Axiophot microscope (Carl Zeiss, Jena,
Germany).
Results
Proliferation of human AT-MSCs cultured
in different cell culture media
Cell proliferation was measured using the alamarBlue
proliferation assay from day 0 to day 21 (Fig. 1). No cytotoxic effects were
observed during cultivation in any of the cell culture media (data
not shown). AT-MSCs grown in the SkGM-2 BulletKit showed the
highest proliferation rate compared with those grown in the other
cell culture media analysed (Fig.
1). The fluorescence units (FU) increased by approximately
20-fold from 7.27±0.29 on day 0 continuously to the maximum of
138.2±1.66 on day 16. After this time point, the proliferation
almost remained on this high level on day 21 and was approximately
2–2.5-fold higher than that of the AT-MSCs cultured in one of the
other five media (Fig. 1).
The conditioned cell culture media showed,
independent of their concentration (5, 30 and 50%), almost
identical rates of cell proliferation. The FU level began at
7.27±0.29 on day 0 and continuously increased to a maximum level on
day 21 (Fig. 1). After 21 days of
cell culture, the FU values for the conditioned cell culture media
were similar: 70.71±1.88 (5%), 67.78±2.07 (30%) and 64.61±2.26
(50%), and thus, were increased by 9–10-fold in comparison to day 0
(Fig. 1). The stimulation of
AT-MSCs with MSCGM + 5-Aza showed an 8-fold increase in the FU
value from day 0 to day 11, followed by a 35% decrease on day 16
and a renewed increase to a maximum level on day 21 (Fig. 1). After 21 days of cell culture,
the FU values were 46.09±2.39 for the AT-MSCs incubated with MSCGM
and 64.81±5.2 following stimulation with MSCGM + 5-Aza, and were
thus increased by 9-fold in comparison to day 0 (Fig. 1).
Proliferation of human BM-MSCs cultured
in different cell culture media
BM-MSCs incubated with the SkGM-2 BulletKit medium
revealed the highest proliferation rates compared with those grown
in the other cell culture media (Fig.
2). The initial FU value on day 0 was 6.36±0.05 and increased
by approximately 9-fold, reaching the maximum value on day 16 (FU,
57.88±1.26) with the exception of a slight decrease on day 8
(Fig. 2).
Following culture with MSCGM, the BM-MSCs showed a
steep 8.4-fold increase in proliferation, represented by an FU
value of 53.51±1.23 until day 8, followed by a steady decline of
cell turn over on days 11 and 16 until day 21 (−25%), represented
by a final FU value of 40.47±5.43 (Fig. 2).
During the cultivation of BM-MSCs in the different
conditioned (5, 30 and 50%) cell culture media, different cell
proliferation rates were measured, depending on the concentration
of the medium. The FU value of cells grown in 5% conditioned cell
culture medium increased by approximately 7-fold until day 8 to an
FU value of 44.33±0.33, followed by a marginal decline (−12%) of
the FU value to 39.07±0.79 on day 11 and an increase (24%) in
proliferation until day 21 with an FU value value of 48.57±1.64
(Fig. 2). In the BM-MSC cultures
nourished with 30 or 50% conditioned cell culture medium, a similar
time-dependent increase in proliferation was detected (Fig. 2). The proliferation rate increased
steadily by approximately 7-fold from day 0 to day 21. The FU value
was 10.03±0.15 (30% conditioned cell culture medium) and 10.80±0.10
(50% conditioned medium) on day 4 and 19.85±0.25 (30% conditioned
medium) and 20.26±1.19 (50% conditioned medium) on day 8. The FU
value increased further to 46.11±1.23 on day 21 in the cells
cultured in 30% conditioned medium and 42.52±2.16 in those cultured
in 50% conditioned medium (Fig.
2).
Identification of valid reference genes
for qRT-PCR experiments
To identify the most stable reference gene for the
normalisation of gene expression in qRT-PCR measurements, the
following genes were analysed during the cultivation of the human
AT-MSCs/BM-MSCs using the different culture media: ACTB, B2M,
GAPDH, PPIA, TBP and RPLPO. The expression stability was calculated
using the geNorm and NormFinder software.
geNorm
The geNorm program calculated that B2M and ACTB are
the most robust reference genes. The results of the expression
stability measurements are illustrated in Fig. 3.
NormFinder
The NormFinder software identified B2M and ACTB as
the most stable reference genes (Table I).
 | Table IStability measurements of the
candidate reference genes in human MSCs. |
Table I
Stability measurements of the
candidate reference genes in human MSCs.
| Gene name | Stability
value | Standard error |
|---|
| RPLPO | 1.996 | 0.260 |
| TBP | 0.355 | 0.100 |
| GAPDH | 0.496 | 0.104 |
| B2M | 0.307 | 0.101 |
| ACTB | 0.288 | 0.102 |
| PPIA | 0.467 | 0.103 |
Gene expression analyses using
qRT-PCR
In the human AT-MSCs, only the expression of the
myogenic markers, ACTA1, MYH8 and MYH1 could be detected using
qRT-PCR. However, the expression of myogenic markers examined could
not be detected in the human BM-MSCs.
ACTA1
The incubation of AT-MSCs with MSCGM + 5-Aza led on
day 4 and on day 8 to an 1.2- and 2-fold increase in ACTA1 gene
expression, respectively, compared with the cells cultured with
MSCGM alone, followed by a decrease of 50% on day 11 and another
1.6-fold increase on day 16 (Fig.
4). On day 21, ACTA1 expression was almost similar to that
found in the cells incubated with MSCGM alone (Fig. 4). The cells cultured with SkGM-2
medium showed a 2.3-fold increase in ACTA1 expression on day 8 and
a 1.6-fold increase on day 16 compared with the cells cultured with
MSCGM alone. Incubation with 30% conditioned medium led to the
highest relative expression compared to incubation with MSCGM, with
an 3.8- and 1.9-fold increase on day 8 and on day 16, respectively.
In the AT-MSCs nourished with 50% conditioned medium, the maximum
ACTA1 expression was measured on day 4 with a 1.4-fold increase
compared with the MSCGM-cultured cells (Fig. 4).
MYH8
Gene expression analysis detected MYH8 in each of
the six evaluated groups of human AT-MSCs cultured with the
different cell culture media. Measured values showed very
heterogeneous results in each culture medium. Trends were not
observed. The highest gene expression was measured on day 21 with a
concentration-dependent effect of the conditioned medium with a
maximum 200-fold increase using the 50% conditioned cell culture
medium (Fig. 5). The highest MYH
8 gene expression for the cells cultured in MSCGM + 5-Aza was
detected on day 8 with a 2.7-fold increase compared with the
MSCGM-nourished cells (Fig. 5).
The maximum values for cells cultured in SkGM-2 was measured on day
21 (2.7-fold higher compared with MSCGM-cultured cells).
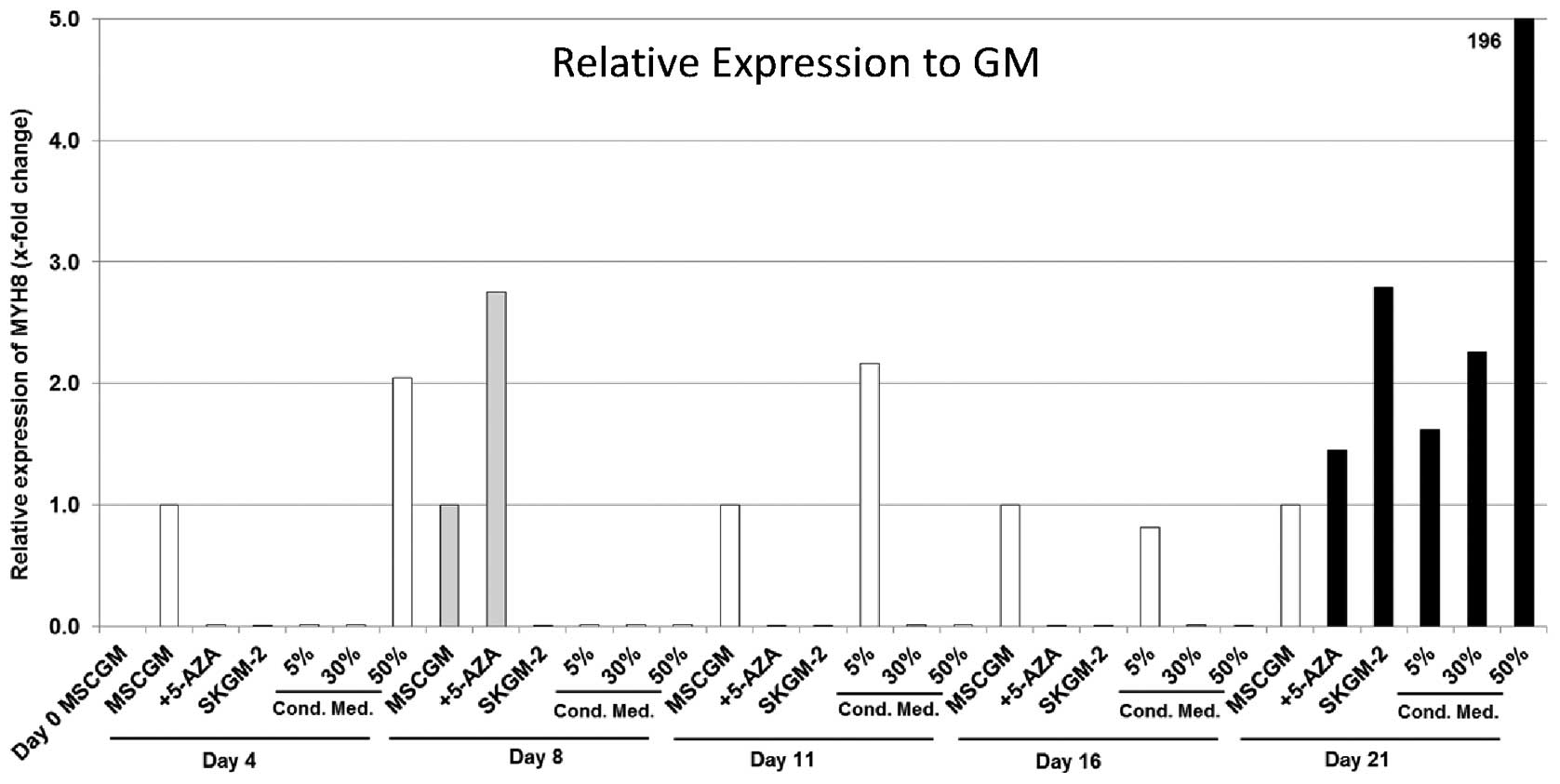 | Figure 5Expression analyses (qRT-PCR) of the
myosin, heavy chain 8, skeletal muscle, perinatal (MYH8) gene in
human mesenchymal stem cells (MSCs) derived from fat/adipose tissue
(AT-MSCs). Gene expression is presented relative to the expression
in human AT-MSCs incubated in MSCGM. The genes were normalised
using normalisation factor (NF) calculated by geNorm software.
MSCGM, MSCGM growth medium (GM); 5-Aza, MSCGM + 5-azacytidine
(5-Aza); SkGM-2, SkGM-2 BulletKit; 5, 30, 50% conditioned cell
culture media, supernatant of human satellite cell cultures after
three days in cell culture mixed with MSCGM. Cond. Med.,
conditioned medium. |
MYH1
The expression of MYH1 in the human AT-MSCs also
showed very diverse results with the different culture media. The
highest levels of expression of MYH1 were detected in the AT-MSCs
nourished with SkGM-2 BulletKit (57-fold increase), as well as in
those cultured with 5, 30 and 50% conditioned medium (7.7-, 115-
and 112-fold increase, respectively) on day 16 (Fig. 6).
 | Figure 6Expression analyses (qRT-PCR) of the
myosin, heavy chain 1, skeletal muscle, adult (MYH1) gene in human
mesenchymal stem cells (MSCs) derived from fat/adipose tissue
(AT-MSCs). Gene expression is presented relative to the expression
in MSCGM. The genes were normalised using the normalisation factor
(NF) calculated by geNorm software. MSCGM, MSCGM growth medium
(GM); 5-Aza, MSCGM + 5-azacytidine (5-Aza); SkGM-2, SkGM-2
BulletKit; 5, 30, 50% conditioned cell culture media, supernatant
of human satellite cell cultures after three days in cell culture
mixed with MSCGM. Cond. Med., conditioned medium. |
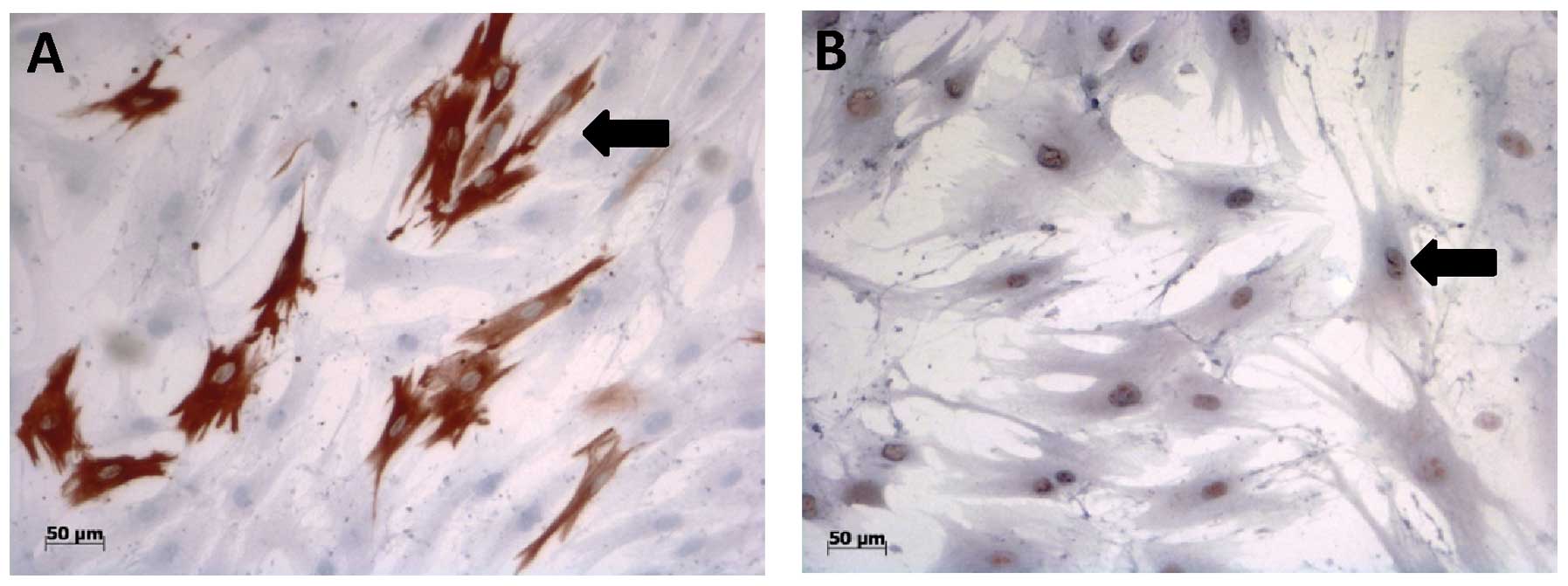
Immunocytochemistry
To evaluate the myogenic differentiation of the
human MSCs, immunocytochemical staining with antibodies directed
against DES, MYF5 or ACTA1 was performed.
First of all, there was no ACTA1 immunoreactivity
found in the human AT-MSCs incubated with each of the six media
examined. However, the human AT-MSCs grown in conditioned cell
culture media showed positive staining for the myogenic markers,
DES and MYF5, after day 4 in cell culture (Fig. 7). The percentage of DES- and
MYF5-positive AT-MSCs (Table II)
increased in a time-dependent manner following culture in the 5%
conditioned cell culture medium. Following culture in the 30%
conditioned cell culture medium, the amount of DES-positive cells
increased to a maximum level on day 8 and remained at this level
during the following days (Table
II). The percentage of MYF5-positive cells, on the other hand,
constantly increased in a time-dependent manner in cell culture.
The human AT-MSCs nourished with 50% conditioned cell culture
medium showed lower percentages of DES- and MYF5-positive cells,
compared with those cultured with 5 and 30% conditioned cell
culture media, and these levels did not increase (Table II). Analyses of the
immunocytochemical staining of the human AT-MSCs cultured in MSCGM,
MSCGM + 5-Aza or SkGM-2 BulletKit media showed immunoreactive
cells, beginning on day 4 of culture. The percentage of
immunoreactive AT-MSCs was similar during culture in the three
different media. However, the percentage of immunoreactive AT-MSCs
was lower than that observed in the cells cultured in 5 and 30%
conditioned cell culture media (Table II).
 | Table IIImmunocytochemical evaluation of the
muscle-specific differentiation markers, DES and MYF5, in human
AT-MSCs and BM-MSCs grown in different cell culture media. |
Table II
Immunocytochemical evaluation of the
muscle-specific differentiation markers, DES and MYF5, in human
AT-MSCs and BM-MSCs grown in different cell culture media.
| Staining
methods | Day 0 | Day 4 | Day 8 | Day 11 | Day 14 |
|---|
| Anti-DES staining
of MSCs |
| MSCGM | − | + | ++ | ++ | ++ |
| SkGM-2
BulletKit | − | −/+ | −/+ | − | − |
| 5-Aza | − | −/+ | ++ | ++ | ++ |
| Contidioned
medium |
| 5% | − | −/+ | +/++ | ++/+++ | ++/+++ |
| 30% | − | + | ++/+++ | ++/+++ | ++/+++ |
| 50% | − | + | ++ | ++ | ++ |
| Anti-MYF5 staining
of MSCs |
| MSCGM | − | +/++ | + | ++ | ++ |
| SkGM-2
BulletKit | − | + | −/++ | ++ | +++/++++ |
| 5-Aza | − | + | −/++ | +++ | ++ |
| Contidioned
medium |
| 5% | − | − | −/+ | ++ | ++/+++ |
| 30% | − | + | −/+ | ++ | ++/+++ |
| 50% | − | + | −/+ | +/++ | ++ |
During the cultivation of BM-MSCs in the different
cell culture media, only MYF5 was sporadically detected.
Discussion
Human MSCs represent a promising alternative stem
cell source for skeletal muscle tissue engineering applications, as
these cells do not lose their differentiation capacity after cell
expansion (5). The myogenic
differentiation potential of MSCs has been demonstrated by several
research groups; however, it remains unclear whether all cells or
only a subpopulation of MSCs can differentiate into skeletal muscle
tissue (21,22). MSCs can be isolated from a variety
of tissues, which differ in phenotype, differentiation potential
and proliferation capacity (10).
Several research groups have demonstrated that BM-MSCs, regardless
of whether they are injected intravenously or directly into muscle,
participate in the regeneration of damaged skeletal muscle in
vivo (23,24). However, it should be noted that
the detected cell number in the damaged muscle was extremely low
(25). These results suggest that
a fraction of MSCs are involved in muscle regeneration in
vivo and thus, appear promising for in vitro generation
of functional skeletal muscle (25). Otherwise, characterised by their
high number of pluripotent stem cells, AT-MSCs represent an
alternative source of stem cells (26). In addition, AT-MSCs have been
detected in skeletal muscle tissue after transplantation into
damaged muscle tissue (27),
supporting the hypthesis that AT-MSCs contribute to muscle
regeneration. The determination of which particular cell line MSCs
differentiate into is controlled by multiple factors and
extracellular signals, which can be influenced by culture
conditions and growth factor compositions. Growth factors and
chemical signals that can induce myogenic differentiation in MSCs
are bFGF, PDGF, the amino acid, glutamine, and 5-Aza (5,10,28). The underlying mechanisms and
pathways have not yet been fully deciphered and are still the
subject of investigations. MSCs isolated from different donor sites
show similar characteristics in terms of phenotype; however, the
ability to differentiate in response to growth factor stimulation
differs between MSCs (8,10). De la Garza-Rodea et al
demonstrated that myogenic differentiation varied between human
MSCs depending on their origin (10). Therefore, in this study, we
investigated the myogenic differentiation potential of AT-MSCs and
BM-MSCs cultured in different cell culture media with different
growth factor compositions in order to detect possible differences
in their response to the different cell culture media.
The induction of myogenic differentiation can be
initiated using different methods. One method consists of the
stimulation of MSCs with 5-Aza, a DNA methyltransferase inhibitor,
which leads to the expression of the muscle-specific protein, DES,
via the stochastic hypomethylation of random DNA residues (29). However, the myogenic effect of
5-Aza on MSCs remains in question due to findings of other studies
(30) and it appears that the
effect of 5-Aza depends on cell origin and species (10). The stimulation of myogenic
differentiation was achieved in MSCs derived from bone marrow in
rats (29), human menstrual blood
(31) and the synovial membrane
(32). It should be noted that
the mode of action and the effect of 5-Aza has not yet been fully
elucidated.
Another method of initiation of the myogenic
differentiation is the exposure of MSCs to a myogenic environment
(33). Due to paracrine secreted
cytokines and/or the extracellular matrix, differentiation can be
induced in MSCs, although the underlying mechanisms are not yet
fully understood. Myogenic differentiation can also be initiated by
co-cultivation with myoblasts and the utilisation of conditioned
cell culture media in vitro (5). For this reason, we evaluated the
effect of the conditioned medium obtained by cultures of human
satellite cells grown for three days in MSCGM on the expression of
myogenic differentiation markers in human AT-MSCs and BM-MSCs.
Furthermore, we evaluated the commercially available MSC culture
medium, SkGM-2 BulletKit, which is able to induce myogenic
differentiation, which contains the myogenic additives,
dexamethasone, FGF and L-glutamine. The exact composition of the
medium is known to the manufacturer. MSCGM is a basal growth medium
which contains MSCGS and L-glutamine.
To determine the effects of growth factors and media
supplements on cell proliferation, an alamarBlue proliferation
assay was performed on the human AT-MSC and BM-MSC cultures. In the
AT-MSCs, the highest proliferation rates were obtained using the
SkGM-2 BulletKit (Fig. 1). The
proliferation rate was 3-fold higher compared with the other cell
culture media examined. One reason for this effect may be the
higher concentration of the epidermal growth factor (rhEGF) in the
culture medium, which is known to exert a proliferative effect on
cells of mesodermal and ectodermal origin (34). This effect was also detected in
the human BM-MSCs, where SkGM-2 BulletKit-treated cells had the
highest proliferation rates from day 11 (Fig. 2). Higher rates of cell
proliferation were detected in the human AT-MSCs (maximum, 138.2±
1.66 FU on day 16) compared with the BM-MSCs (maximum, 57.88±1.26
FU on day 16) following culture in SkGM-2 BulletKit medium. These
results support the data of De la Garza-Rodea et al, who
demonstrated that AT-MSCs have higher proliferation rates than
BM-MSCs (10). This result
appears to be consistent in other species, since Nakanishi et
al also demonstrated a higher proliferation capacity of AT-MSCs
compared with BM-MSCs in rats (35). It should be mentioned that other
growth factor compositions were utilised in our experiments. The
addition of the DNA methyltransferase inhibitor, 5-Aza, to MSCGM
led only to a marginal increase in the proliferation rate of
AT-MSCs in comparison to the cells that were cultured in MSCGM.
This effect is surprising, since 5-Aza has a cytostatic effect by
the demethylation of DNA and RNA. We hypothesised that the
cytostatic effect occurs at higher concentrations in human MSCs
than the 10 μmol/l that was used, since no apoptotic cells were
detected at any time in our preliminary investigations. This result
could also not be detected in the BM-MSC. In our study, 5-Aza led
to a slight decrease in the proliferation rate. Cytotoxic effects,
i.e., cell death, were not detected at any time point. This finding
supports the data of Yang et al who demonstrated higher
proliferation rates of AT-MSCs compared with BM-MSCs following
stimulation with 5-Aza (36) and
supports the higher proliferation capacity of human AT-MSCs
compared with BM-MSCs.
Beier et al demonstrated the possible
myogenic differentiation of MSCs by co-cultivation with myoblasts
in rats, verifying the possible paracrine influences on myogenesis
(5). Therefore, in this study, we
evaluated the effects of conditioned cell culture media, which
induced a steady increase in the proliferation rate of AT-MSCs; the
concentration (5, 30 and 50%) of the conditioned medium had no
effect on the cell proliferation rate. Thus, it can be deduced that
the proliferative effects of the growth factors in the conditioned
media cannot be enhanced by increasing the concentration. Similar
results were obtained in human BM-MSCs, which also showed no
significant difference in proliferation with the different
conditioned cell culture media, with the exception of the rate on
day 8. These data present the first results on the proliferation
capacity of human AT-MSCs and BM-MSCs, investigating the paracrine
influences of human myoblasts by different concentrations of
conditioned cell culture media.
Using qRT-PCR, we detected the expression of the
myogenic marker genes, MYH8, MYH1 and ACTA1, in human AT-MSCs. MYH8
and MYH1 expression was heterogeneous in all the analysed cell
culture media with the highest expression values observed in the
cells nourished with conditioned media. These results can be
explained by the fact that the AT-MSCs represent a heterogeneous
group of cells, and not all subpopulations can be driven into the
myogenic cell line by medium stimulation (26). Moreover, we detected ACTA1
expression in human AT-MSCs following incubation with each of the
six cell culture media examined. The highest expression was
measured following culture in 30% conditioned medium on day 8. An
expression tendency could not be deduced from the expression
levels. These results are in accordance with the findings of De la
Garza-Rodea et al who also detected a large variety of gene
expression of myogenic markers in AT-MSCs (10), supporting the fact that the level
of myogenic differentiation is low.
Immunocytochemical staining revealed that the
muscle-specific intermediate filament, DES, had the highest
expression in human AT-MSCs cultured in conditioned media. The
highest expression rate was measured following culture in 30%
conditioned medium. Interestingly, DES could only be detected by
ICC at very low concentrations in the cells nourished with the
commercially available cell culture medium, SkGM-2 BulletKit, which
indicates the low degree of myogenic differentiation. This finding
underlines the antagonism between proliferation and myogenic
differentiation, even in MSCs. Thus, the high proliferation rates
seem to be responsible for the lower myogenic differentiation of
MSCs.
Moreover, in human BM-MSCs, none of the myogenic
differentiation markers were detected, underscoring the low degree
of myogenic differentiation of the cells. Immunocytochemical
staining of human BM-MSCs revealed no expression of myogenic
markers on the protein level and support the gene expression
results. These results are in accordance with data of others who
also did not detect myogenic differentiation in human adult BM-MSCs
following stimulation with 5-Aza (30). The pro-myogenic effect of 5-Aza
was achieved in MSCs of rodents and rabbits (29,37) and in human synovial-membrane
derived MSCs (32), which
provides evidence that MSCs from other species and cell origins
respond differently to chemical stimuli, leading to differences in
the myogenic differentiation capacity. Chan et al also
demonstrated that only in human fetal MSCs, but not in adult
BM-MSCs, myogenic differentiation was initiated by cultivation with
conditioned cell culture media (30). These data are in accordance with
our findings, concerning the absence of myogenic differentiation in
BM-MSCs following stimulation with different concentrations of
conditioned cell culture media from human satellite cell cultures.
According to the data of others, BM-MSCs have been shown to
differentiate into functional skeletal muscle only by fusing with
pre-determined muscle cells; these data are supported by our
results, since in our experiments, no co-cultivation with satellite
cells was performed (38).
Moreover, it has been shown that adult BM-MSCs cannot form myotubes
following implantation into blastocysts, which indicates that these
cells are not capable of tissue formation without reprogramming
(38).
The induction of myogenic differentiation in human
AT-MSCs succeeds only to a certain degree by stimulation with
culture media. This conclusion can be derived from our results of
gene expression analyses and immunocytochemical investigations,
which revealed that the myogenic markers, DES, MYF5, MYH1/8 and
ACTA1, were detected in human AT-MSC cultures. The strongest
expression of myogenic markers was found in the cells nourished
with 30% conditioned medium. However, it should be noted that the
expression of each marker examined could not be detected and no
formation of myofibrils was observed.
Our results concerning human AT-MSCs may be
explained by the findings of others, who observed that only a small
subpopulation of MSCs can spontaneously differentiate into skeletal
muscle (26), which explains why
such heterogeneous results were detected in terms of
differentiation marker analysis. According to data of Di Rocco
et al, the myogenic phenotype of AT-MSCs can be increased by
co-culture with satellite cells (26), providing evidence that the
secretion of paracrine growth factors and signalling by muscle
precursor cells is a prerequisite for the myogenic differentiation
of MSCs. This conclusion is supported by our observation that the
highest percentage of cells positive for myogenic markers was found
in human AT-MSCs nourished with conditioned cell culture media.
Chan et al also observed myogenic differentiation in human
fetal MSCs cultured with conditioned cell media in a small
population of cells (1–2%) (30).
Moreover, Di Rocco et al also observed that no formation of
myofibrils was detected if no cell-cell contacts between muscle
cells and MSCs are present (26).
Thus, functional skeletal muscle in the sense of multinucleated
myofibrils can only be generated by AT-MSCs by fusion with already
differentiated myofibrils (26).
In conclusion, it can be stated that SkGM-2
BulletKit led to the highest proliferation rates in AT-MSCs and
BM-MSCs and the different concentration of the conditioned cell
culture medium had no significant effect on the proliferation of
MSCs, regardless of their origin. Furthermore, the evaluation of
the effects of six different cell culture media on the myogenic
differentiation of human AT-MSCs and BM-MSCs led to the following
findings: the myogenic differentiation of human BM-MSCs failed,
despite stimulation with different cell culture media and
therefore, no myogenic markers (gene expression/protein expression)
could be detected. In the human AT-MSCs, myogenic differentiation
was initiated to a lower extent following culture in conditioned
cell culture media, where the highest expression of the analysed
myogenic markers was detected following culture in 30% conditioned
cell culture medium.
Acknowledgements
The authors would like to thank Michael Collins for
assisting with the manuscript, as well as Petra Prohaska and Ulrike
Traut for their excellent technical assistance. This study contains
part of the doctoral thesis of Stephanie Juritz.
References
|
1
|
Belema Bedada F, Technau A, Ebelt H,
Schulze M and Braun T: Activation of myogenic differentiation
pathways in adult bone marrow-derived stem cells. Mol Cell Biol.
25:9509–9519. 2005.PubMed/NCBI
|
|
2
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bieback K, Kern S, Kluter H and Eichler H:
Critical parameters for the isolation of mesenchymal stem cells
from umbilical cord blood. Stem Cells. 22:625–634. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Igura K, Zhang X, Takahashi K, Mitsuru A,
Yamaguchi S and Takashi TA: Isolation and characterization of
mesenchymal progenitor cells from chorionic villi of human
placenta. Cytotherapy. 6:543–553. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beier JP, Bitto FF, Lange C, et al:
Myogenic differentiation of mesenchymal stem cells co-cultured with
primary myoblasts. Cell Biol Int. 35:397–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garcia-Castro J, Trigueros C, Madrenas J,
Perez-Simon JA, Rodriguez R and Menendez P: Mesenchymal stem cells
and their use as cell replacement therapy and disease modelling
tool. J Cell Mol Med. 12:2552–2565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Circ Res.
95:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meligy FY, Shigemura K, Behnsawy HM,
Fujisawa M, Kawabata M and Shirakawa T: The efficiency of in vitro
isolation and myogenic differentiation of MSCs derived from adipose
connective tissue, bone marrow, and skeletal muscle tissue. In
Vitro Cell Dev Biol Anim. 48:203–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vidane AS, Zomer HD, Oliveira BM, et al:
Reproductive stem cell differentiation: extracellular matrix,
tissue microenvironment, and growth factors direct the mesenchymal
stem cell lineage commitment. Reprod Sci. 20:1137–1143. 2013.
View Article : Google Scholar
|
|
10
|
de la Garza-Rodea AS, van der Velde-van
Dijke I, Boersma H, et al: Myogenic properties of human mesenchymal
stem cells derived from three different sources. Cell Transplant.
21:153–173. 2012.PubMed/NCBI
|
|
11
|
Sordella R, Jiang W, Chen GC, Curto M and
Settleman J: Modulation of Rho GTPase signaling regulates a switch
between adipogenesis and myogenesis. Cell. 113:147–158. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tapscott SJ and Weintraub H: MyoD and the
regulation of myogenesis by helix-loop-helix proteins. J Clin
Invest. 87:1133–1138. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Christ B and Brand-Saberi B: Limb muscle
development. Int J Dev Biol. 46:905–914. 2002.
|
|
14
|
Stern-Straeter J, Bonaterra GA, Kassner
SS, et al: Characterization of human myoblast differentiation for
tissue-engineering purposes by quantitative gene expression
analysis. J Tissue Eng Regen Med. 5:e197–e206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wright C, Haddad F, Qin AX and Baldwin KM:
Analysis of myosin heavy chain mRNA expression by RT-PCR. J Appl
Physiol (1985). 83:1389–1396. 1997.PubMed/NCBI
|
|
16
|
Wehrle U, Dusterhoft S and Pette D:
Effects of chronic electrical stimulation on myosin heavy chain
expression in satellite cell cultures derived from rat muscles of
different fiber-type composition. Differentiation. 58:37–46. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goessler UR, Bugert P, Bieback K, et al:
Integrin expression in stem cells from bone marrow and adipose
tissue during chondrogenic differentiation. Int J Mol Med.
21:271–279. 2008.PubMed/NCBI
|
|
18
|
Stern-Straeter J, Bonaterra GA, Kassner
SS, et al: Impact of static magnetic fields on human myoblast cell
cultures. Int J Mol Med. 28:907–917. 2011.
|
|
19
|
Vandesompele J, De Preter K, Pattyn F, et
al: Accurate normalization of real-time quantitative RT-PCR data by
geometric averaging of multiple internal control genes. Genome
Biol. 3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stern-Straeter J, Bonaterra GA, Hormann K,
Kinscherf R and Goessler UR: Identification of valid reference
genes during the differentiation of human myoblasts. BMC Mol Biol.
10:662009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dezawa M, Ishikawa H, Itokazu Y, et al:
Bone marrow stromal cells generate muscle cells and repair muscle
degeneration. Science. 309:314–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Corti S, Strazzer S, Del Bo R, et al: A
subpopulation of murine bone marrow cells fully differentiates
along the myogenic pathway and participates in muscle repair in the
mdx dystrophic mouse. Exp Cell Res. 277:74–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
LaBarge MA and Blau HM: Biological
progression from adult bone marrow to mononucleate muscle stem cell
to multinucleate muscle fiber in response to injury. Cell.
111:589–601. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrari G and Mavilio F: Myogenic stem
cells from the bone marrow: a therapeutic alternative for muscular
dystrophy? Neuromuscul Disord. 12(Suppl 1): S7–S10. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferrari G, Cusella-De Angelis G, Coletta
M, et al: Muscle regeneration by bone marrow-derived myogenic
progenitors. Science. 279:1528–1530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Di Rocco G, Iachininoto MG, Tritarelli A,
et al: Myogenic potential of adipose-tissue-derived cells. J Cell
Sci. 119:2945–2952. 2006.PubMed/NCBI
|
|
27
|
Miranville A, Heeschen C, Sengenes C,
Curat CA, Busse R and Bouloumie A: Improvement of postnatal
neovascularization by human adipose tissue-derived stem cells.
Circulation. 110:349–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gang EJ, Jeong JA, Hong SH, et al:
Skeletal myogenic differentiation of mesenchymal stem cells
isolated from human umbilical cord blood. Stem Cells. 22:617–624.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wakitani S, Saito T and Caplan AI:
Myogenic cells derived from rat bone marrow mesenchymal stem cells
exposed to 5-azacytidine. Muscle Nerve. 18:1417–1426. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chan J, O’Donoghue K, Gavina M, et al:
Galectin-1 induces skeletal muscle differentiation in human fetal
mesenchymal stem cells and increases muscle regeneration. Stem
Cells. 24:1879–1891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui CH, Uyama T, Miyado K, et al:
Menstrual blood-derived cells confer human dystrophin expression in
the murine model of Duchenne muscular dystrophy via cell fusion and
myogenic transdifferentiation. Mol Biol Cell. 18:1586–1594. 2007.
View Article : Google Scholar
|
|
32
|
De Bari C, Dell’Accio F, Tylzanowski P and
Luyten FP: Multipotent mesenchymal stem cells from adult human
synovial membrane. Arthritis Rheum. 44:1928–1942. 2001.
|
|
33
|
Lee JH, Kosinski PA and Kemp DM:
Contribution of human bone marrow stem cells to individual skeletal
myotubes followed by myogenic gene activation. Exp Cell Res.
307:174–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tamama K, Kawasaki H and Wells A:
Epidermal growth factor (EGF) treatment on multipotential stromal
cells (MSCs). Possible enhancement of therapeutic potential of MSC.
J Biomed Biotechnol. 2010:7953852010. View Article : Google Scholar
|
|
35
|
Nakanishi C, Nagaya N, Ohnishi S, et al:
Gene and protein expression analysis of mesenchymal stem cells
derived from rat adipose tissue and bone marrow. Circ J.
75:2260–2268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang J, Song T, Wu P, et al:
Differentiation potential of human mesenchymal stem cells derived
from adipose tissue and bone marrow to sinus node-like cells. Mol
Med Rep. 5:108–113. 2012.PubMed/NCBI
|
|
37
|
Rangappa S, Fen C, Lee EH, Bongso A and
Sim EK: Transformation of adult mesenchymal stem cells isolated
from the fatty tissue into cardiomyocytes. Ann Thorac Surg.
75:775–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schulze M, Belema-Bedada F, Technau A and
Braun T: Mesenchymal stem cells are recruited to striated muscle by
NFAT/IL-4-mediated cell fusion. Genes Dev. 19:1787–1798. 2005.
View Article : Google Scholar : PubMed/NCBI
|