Introduction
Epithelial-mesenchymal transition (EMT) is a complex
process through which epithelial cells lose their polarity and
reorganize their cytoskeleton, while also acquiring a mesenchymal
phenotype and increased motility (1,2).
In addition to tissue remodeling, organ development and wound
healing, EMT plays a critical role in cancer progression (3–6).
The loss of a polarized epithelial phenotype and the acquisition of
a mesenchymal phenotype endow cancer cells with the potential to
invade and metastasize.
Epithelial cells are connected by the epithelial
junctional complex, which consists of tight junctions, adherens
junctions and desmosomes. E-cadherin is a component of the adherens
junction and is involved in the formation and maintenance of
epithelial structures (7).
Desmoglein is a desmosome component and is expressed in
desmosome-bearing epithelial cells (8). E-cadherin and desmoglein are members
of the cadherin family of cell-cell adhesion molecules.
A hallmark of EMT is the loss of E-cadherin
expression (9). Several
transcription factors, including Snail, Slug, Twist and zinc finger
E-box-binding homeobox 1 (ZEB1), have been implicated in the
transcriptional repression of E-cadherin and the induction of EMT
(9,10). Snail belongs to the Snail
super-family of zinc finger transcription factors (11). Snail and Slug, a related
superfamily member, are expressed during development in the early
mesoderm and neural crest (12–14). These two zinc finger transcription
factors repress E-cadherin transcription through an interaction of
their C-terminal regions with a 5′-CACCTG-3′ sequence (termed an
E-box) in the cadherin promoter (15,16). Correlative experiments have
demonstrated that there is an inverse correlation between
E-cadherin expression and Snail expression in human samples
(17).
EMT is accompanied by epigenetic modifications,
including DNA methylation (18,19). DNA methylation, which is commonly
associated with gene repression and heterochromatin formation, is
defined by the addition of a methyl group to the cytosine of a CpG
dinucleotide in the promoter region of a gene (20). Transforming growth factor-β
(TGF-β) is a multifunctional cytokine that regulates a broad range
of cellular responses (21).
TGF-β is the major mediator of EMT and induces the expression of
Snail (22) and Slug (23). Recent studies have revealed that
the effects of Snail on epithelial cells include the promotion of
the expression of other EMT-inducing transcriptional factors, such
as ZEB1 (24), and the activation
of the TGF-β signaling pathway (25). Cells exposed to TGF-β undergo EMT,
which includes E-cadherin promoter DNA methylation (26,27).
The ectopic expression of Snail in several
epithelial cells, including Madin-Darby canine kidney (MDCK) cells
and the human epidermoid carcinoma cell line, A431, has been shown
to result in EMT (28,29). The precise molecular events that
initiate the complex EMT process, however, are poorly understood.
In the present study, in an aim to further understand the role of
Snail in EMT, we generated stable Snail transfectants using a
bovine cell line, Madin-Darby bovine kidney (MDBK) cells.
Surprisingly, MDBK cells transfected with the Snail construct
maintained their epithelial morphology and showed no sign of
reduced cell-cell adhesiveness compared to the control cells.
Consistent with these observations, the downregulation of the
epithelial marker proteins, E-cadherin and desmoglein, and the
upregulation of the mesenchymal marker proteins, N-cadherin and
fibronectin, were not detected. Furthermore, the E-cadherin
promoter was not methylated. Therefore, in the MDBK cells, the
ectopic expression of Snail failed to induce EMT. Although Snail
expression in MDCK cells is accompanied by the increased expression
of other EMT-inducing transcription factors, such as Slug and ZEB1,
MDBK cells ectopically expressing Snail did not show an increased
expression of these factors. Thus, it seems that the inability to
upregulate the expression of additional EMT-inducing transcription
factors may explain the failure of ectopic Snail protein expression
to induce EMT in MDBK cells.
Materials and methods
Cell lines and transfection
MDBK cells, a bovine kidney epithelial cell line and
MDCK cells, a canine kidney epithelial cell line, provided by Dr
Rolf Kemler (Max-Planck Institute of Immunobiology and Epigenetics,
Freiburg, Germany) and Dr Satoshi Daikuhara (Kagoshima University),
respectively. They were grown and were transfected as previously
described (28) using the calcium
phosphate method with 10 µg of either plasmid DNA containing
an HA-tagged human Snail construct (pC-SnailHA) or with a control
empty vector containing a neomycin resistance gene.
Antibodies
Mouse monoclonal antibodies (mAbs) against
E-cadherin (Cat. no. 610182), p120 (Cat. no. 612537) and
fibronectin (Cat. no. 610077) were purchased from BD Biosciences
(Lexington, KY, USA). A mouse mAb against vimentin (Cat. no.
18-0052) was obtained from Zymed Laboratories (South San Francisco,
CA, USA). Mouse mAbs recognizing Snail (Cat. no. 3895) and Slug
(Cat. no. 9589) were purchased from Cell Signaling Technology
(Danvers, MA, USA). A mAb against desmoglein 1 and 2 (Cat. no.
61002) was purchased from Progen Biotechnik GmbH (Heidelberg,
Germany). A goat antibody recognizing ZEB1 (Cat. no. sc-5711) was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). A mouse mAb recognizing vinculin (Cat. no. V9131) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). A rat mAb
against hemagglutinin (HA; Cat. no. 11867423001) was purchased from
Roche Applied Science (Mannheim, Germany). All secondary antibodies
were obtained from Jackson ImmunoResearch Laboratories (West Grove,
PA, USA).
RT-PCR
Total RNA was extracted and reverse transcribed as
previously described (29). The
resulting cDNA was used as a template for PCR and the PCR
conditions were optimized for each primer pair as previously
described (29). The following
primer combinations were used: E-cadherin sense, 5′-GACA
CCCGATTCAAAGTGCAC-3′ and antisense, 5′-GTCTCTC TTCTGTCTCCTGAG-3′;
Slug sense, 5′-GCGTTCTCCAGA CCCTGGT-3′ and antisense,
5′-GCACAGCAGCCAGACT CCT-3′; Twist1 sense, 5′-GAGTCCGCAGTCCTACGAG-3′
and antisense, 5′-TCTGTAGGACCTGGTAGAGG-3′; ZEB1 sense,
5′-TGGGCAGTGACGGTAGGTAT-3′ and antisense, 5′-GCA
GGTCGAACCTCTTGATC-3′; and β-actin sense, 5′-CAA
GGACCTCTACGCCAACA-3′ and antisense, 5′-CGTACTCC
TGCTTGCTGATC-3′.
Cell aggregation assay
Cell aggregation assays were performed as previously
described (30). In brief, the
cells were incubated for 10 min at 37°C in HEPES-buffered saline
containing 0.01% trypsin (type XI; Sigma-Aldrich) and 2 mM
CaCl2 or 1 mM EGTA. After the addition of soybean
trypsin inhibitor (Sigma-Aldrich), the cells were washed,
resuspended and incubated for 30 min at 37°C with constant rotation
at 70 rpm. The extent of cell aggregation was represented by the
index: (Nc-Np)/Nc, where Np and Nc are the total number of
particles and cells/dish, respectively.
Immunoblot analysis
For immunoblot analysis, proteins were separated by
8% polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes. After blocking, the membranes were
incubated with specific primary antibodies followed by treatment
with peroxidase-conjugated secondary antibodies (Jackson
ImmunoResearch Laboratories). After washing with phosphate-buffered
saline (PBS) containing 0.1% Tween-20, the protein bands were
visualized by enhanced chemiluminescence (ECL; Amersham
International, Little Chalfont, UK) as previously described
(31). ImageJ software (National
Institutes of Health) was used to quantify the protein levels.
α-tubulin was used as a loading control.
Immunofluorescence staining
For immunofluorescence, the cells were grown on
coverslips, fixed with 3% paraformaldehyde in PBS for 20 min at
room temperature, and permeabilized with 0.1% Triton X-100. The
coverslips were immunostained with primary and secondary antibodies
as previously described (31). To
label the nuclei, 4′-6-diamidino-2-phenylindol (DAPI) was used. The
cells were analyzed using an Olympus fluorescence microscope
(Olympus, Tokyo, Japan) or a confocal laser scanning microscope
(LSM 700; Carl Zeiss, Oberkochen, Germany).
DNA methylation analysis
Genomic DNA (~0.75 µg) was treated with
sodium bisulfite using the EpiTect system (Qiagen, Germantown, MD,
USA). The bisulfite-converted DNA (~400 ng) was used as a template
for PCR amplification of the CpG islands in the CDH1 promoter. The
primer pairs were sense, 5′-GAGA TTTGAAGTTTAAAAGATAGAA-3′ and
antisense, 5′-AAC TAAAATCTAACAAAACTTCTAC-3′. PCR products were
purified on a 1.5% agarose gel using a Gel Extraction kit (Qiagen)
and cloned into the pGEM-T easy vector (Promega, Madison, WI, USA).
Four or five randomly selected clones from each sample were
selected for sequencing. As a positive control for methylated DNA,
genomic DNA was methylated in vitro using CpG
methyltransferase (M.SssI; New England BioLabs, Inc., Ipswich, MA,
USA).
Results
The ectopic expression of Snail does not
induce morphological changes or change the adhesiveness of MDBK
cells
MDBK cells, a cell line derived from bovine kidney,
display epithelial properties, including a brickstone morphology.
We introduced a control empty vector containing a neomycin
resistance gene or an expression vector encoding HA-tagged Snail
protein into the MDBK cells and isolated stable transfectants,
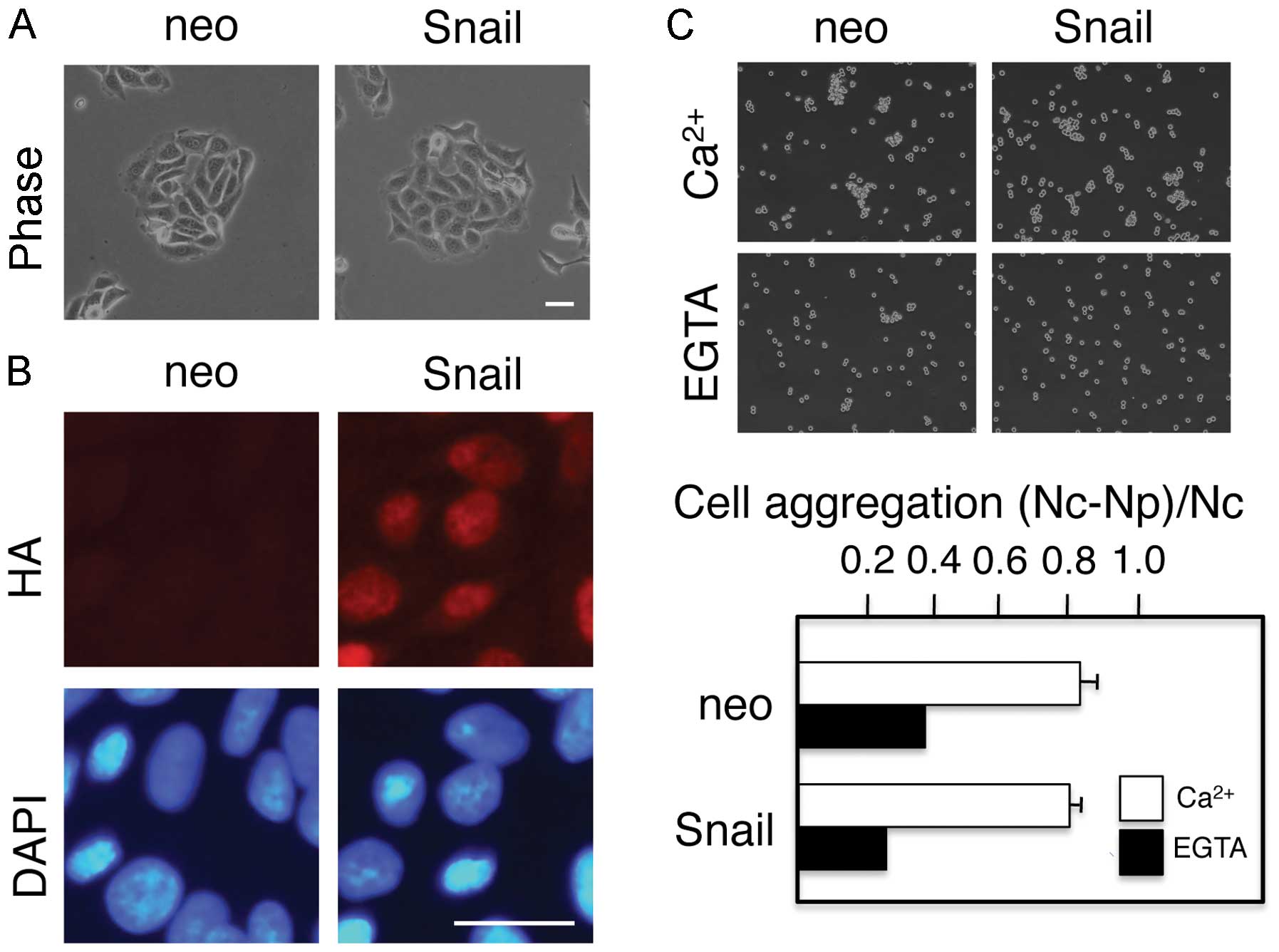
designated as neo or Snail cells, respectively. The Snail cells
retained the same epithelial morphology as the control neo cells
(Fig. 1), despite the clear
nuclear localization of Snail protein, as revealed by staining with
an anti-HA antibody (Fig. 1B).
Thus, contrary to our previous experiments with MDCK or A431 cells
(28,29), the ectopic expression of Snail did
not induce morphological changes that were characteristic of
EMT.
Cells undergoing EMT lose cell-cell adhesion. It is
well known that cadherins at the cell surface resist tryptic
digestion in the presence of Ca2+, but not in the
absence of Ca2+ (7).
Therefore, cell aggregation assays following the tryptic digestion
of cells in the presence of either 2 mM Ca2+ or 1 mM
EGTA can be used to distinguish between cadherin-mediated and
cadherin-independent cell-cell adhesion. Cell aggregation assays
revealed Ca2+-dependent, cadherin-mediated cell-cell
adhesion in both the neo cells and Snail cells; no significant
differences in cell-cell adhesion were observed between these two
cell populations (Fig. 1C) These
results are consistent with the morphological observation that the
Snail cells were not undergoing EMT.
The ectopic expression of Snail in MDBK
cells does not alter the expression levels of epithelial and
mesenchymal markers
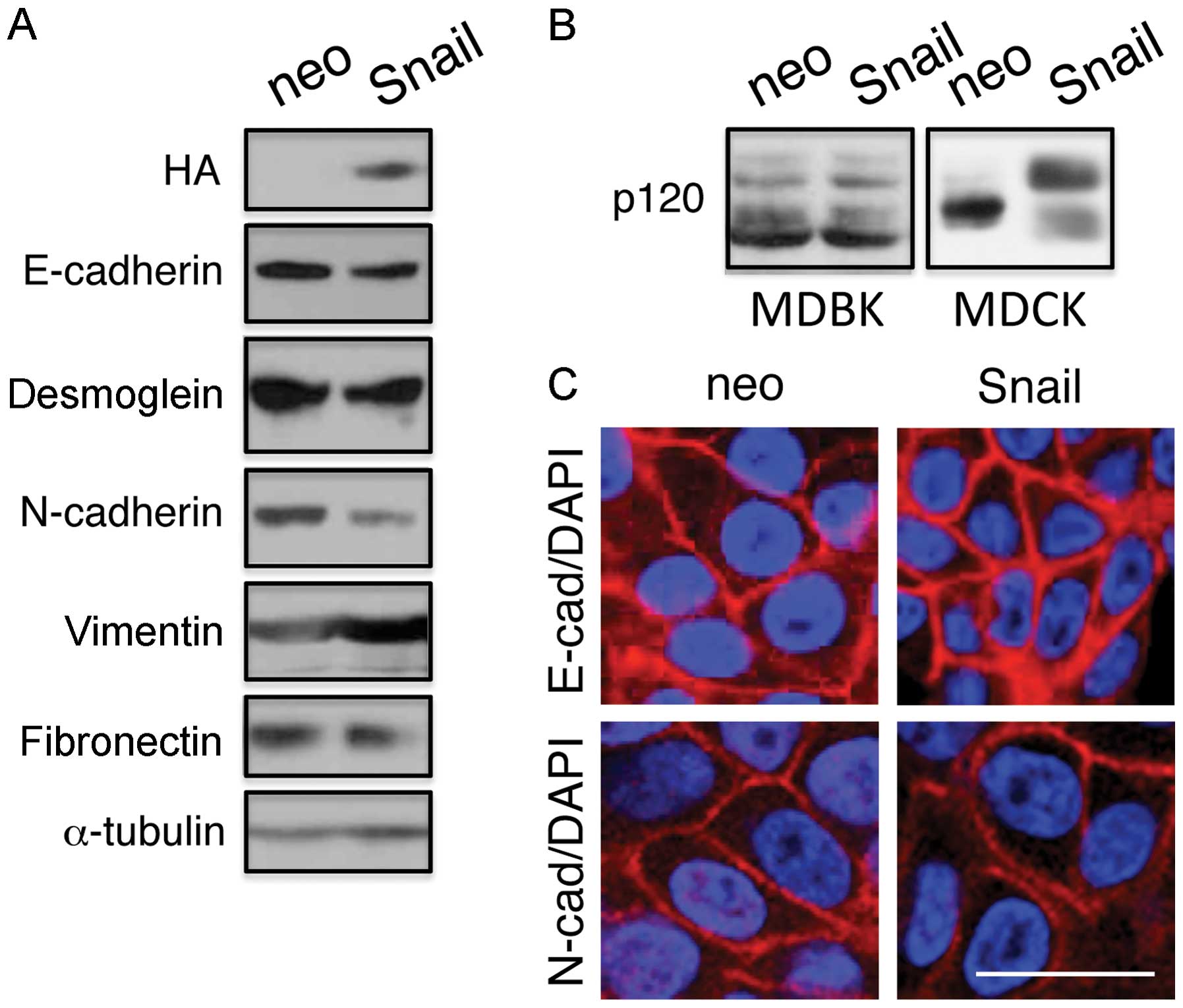
Next, we determined the expression levels of
epithelial markers, E-cadherin and desmoglein, using immunoblot
analysis (Fig. 2). Although the
Snail cells expressed exogenous Snail protein as detected by
anti-HA antibodies, they showed essentially the same expression
levels of E-cadherin and desmoglein as the control neo cells. The
control neo cells also expressed the mesenchymal markers,
N-cadherin, vimentin and fibronectin, and the expression levels of
these proteins did not increase in the Snail cells (Fig. 2 and Table I). Thus, the ectopic expression of
Snail in the MDBK cells did not lead to the downregulation of
E-cadherin or desmoglein expression or to the upregulation of
N-cadherin, vimentin or fibronectin expression. Furthermore, as
previously reported (28), the
expression of Snail altered the splicing patterns of p120 in the
MDCK cells, but not in the MDBK cells (Fig. 2B).
 | Table IRelative expression levels of
epithelial and mesenchymal markers in MDBK cells ectopically
expressing Snail protein. |
Table I
Relative expression levels of
epithelial and mesenchymal markers in MDBK cells ectopically
expressing Snail protein.
| E-cadherin | Desmoglein | N-cadherin | Fibronectin | Vimentin |
|---|
| Ratios | 0.76±0.09 | 0.87±0.06 | 0.74±0.13 | 0.74±0.13 | 1.16±0.12 |
Consistent with the observations that Snail
expression did not alter cadherin-mediated cell-cell adhesion
(Fig. 1) or the expression levels
of E- or N-cadherin (Fig. 2),
immunofluores-cence staining revealed that E- and N-cadherin were
detected at the plasma membrane of both the neo and Snail cells
(Fig. 2C).
The E-cadherin promoter is not methylated
in MDBK cells ectopically expressing Snail protein
Previous analysis of the E-cadherin gene revealed
that its proximal promoter contains CpG islands, which are targets
for methylation during TGF-β-induced EMT (26,27). Therefore, in this study, we
examined the methylation status of the E-cadherin promoter. No
significant de novo DNA methylation was detected at the
E-cadherin promoter in the Snail cells as compared to the control
neo cells, as measured by bisulfite sequencing (Fig. 3). These results were consistent
with the observation that no significant downregulation of
E-cadherin expression was detected in the Snail cells.
The ectopic expression of Snail protein
in MDBK cells does not increase the production of EMT-related
transcription factors
As previously reported, the expression of lymphoid
enhancer-binding factor 1 (LEF-1), an EMT-inducer, in MDCK cells
resulted in the significantly increased expression of other
EMT-inducing transcription factors, including Slug and ZEB1
(31). Using an Agilent Whole
Canine Genome microarray, we found that the ectopic expression of
Snail in MDCK cells resulted in the increased expression of Slug
and ZEB1 [Ozawa et al, (32)]. The upregulation of Twist and ZEB1
expression and the induction of EMT in human mammary epithelial
HMLE cells upon Snail overexpression have been previously reported
(33). Therefore, in this study,
we used RT-PCR to compare the mRNA expression levels of Slug, Twist
and ZEB1 in the neo cells and Snail cells. We observed no
significant changes in the mRNA levels of these factors upon the
ectopic expression of Snail (Fig.
4). Furthermore, immunoblot analysis revealed that MDCK cells
expressing Snail presented with an increased Slug and ZEB1
production at the protein level, whereas the MDBK cells expressing
Snail did not. Thus, our data suggest that the Snail-mediated
upregulation of Slug and ZEB1 is required for the downregulation of
E-cadherin expression and the induction of EMT.
Discussion
In this study, we demonstrated that the ectopic
expression of Snail in MDBK cells, a bovine kidney epithelial cell
line, failed to induce changes that were characteristic of EMT.
None of the following events were observed: i) epithelial to
fibroblastic morphological changes; ii) reduced cell-cell adhesion;
iii) the downregulation of the epithelial markers, E-cadherin and
desmoglein; or iv) the upregulation of the mesenchymal markers,
N-cadherin, vimentin and fibronectin. Although the downregulation
of E-cadherin and desmoglein in human squamous cell carcinoma HSC-4
cells is not extensive (34), the
transfection of cells with the Snail construct used in the present
study has been shown to induce EMT in a number of cell lines of
different origin, including canine kidney epithelial MDCK cells
(28,29), the human epidermoid carcinoma cell
line, A431 (8,29), the human squamous cell carcinoma
cell line, HSC5 (35) and the
murine embryonal carcinoma cell, P19 (Izawa et al,
unpublished data).
The exogenous expression of Snail has been reported
to suppress the activity of an E-cadherin promoter-reporter
construct in MDCK cells, but not in mouse mammary epithelial NMuMG
cells (36). In that study, the
reason behind the cell context-dependent Snail activity was not
analyzed. Snail protein undergoes post-translational modifications,
including glycogen synthase kinase-3 (GSK3β)-mediated
phosphorylation (37), and
protein kinase D1 (PKD1)-mediated phosphorylation (38), followed by ubiquitination, which
leads to Snail protein degradation. In a previous study, although
wild-type Snail protein could not induce EMT in MCF7 cells, mutant
Snail protein, in which serine residues that are targets for GSK3β
phosphorylation were substituted with alanine residues, was
stabilized and did induce EMT (37). Therefore, the failure of Snail
protein to induce EMT in MCF7 cells was explained by its rapid
turnover rate and low protein expression in this cell line
(37). Since the protein levels
of Snail in MDBK cells were very similar/comparable (>70%) to
those in MDCK cells (Fig. 4B), it
seems less likely that rapid turnover and low protein levels were
responsible for the failure of Snail protein to induce EMT in MDBK
cells. Consistent with this hypothesis, the addition of the GSK3β
inhibitor, 6-bromoindirubin-3′-oxime (BIO), did not induce EMT in
MDBK cells ectopically expressing Snail (Izawa et al,
unpublished data). Phosphorylation regulates the subcellular
localization of Snail protein (39). In this study, the immunostaining
of Snail, however, revealed that a significant portion of Snail is
present in the nucleus (Fig.
1B).
The levels of EMT-inducing transcription factors are
under the control of microRNAs, which are regulated by wild-type
p53 (40,41). Therefore, the presence of
wild-type p53 has been proposed to be responsible for the failure
of overexpressed Snail protein to induce EMT in MCF7 cells
(33). MDBK cells seem to express
wild-type p53 (42). Thus, the
same mechanism could be operating in MDBK cells to suppress Snail
activity. However, MDCK cells, in which the overexpression of Snail
does induce EMT, also express wild-type p53 (43). Therefore, the presence of
wild-type p53 alone cannot explain the failure of Snail to induce
EMT in some cell lines.
As previously reported, the expression of LEF-1, an
EMT-inducer, in MDCK cells resulted in the significantly increased
expression of other EMT-inducing transcription factors, e.g., Slug
and ZEB1 (31). The upregulation
of Twist and ZEB1 expression and the induction of EMT in HMLE cells
upon Snail overexpression have also been reported (33). Therefore, the expression of
multiple EMT-inducing factors seems to be necessary to complete the
EMT process. As demonstrated in the present study, ectopic Snail
expression increased Slug and ZEB1 production at the protein level
in MDCK cells, but not in the MDBK cells. Double transfectants of
MDBK cells expressing Snail and Slug showed no sign of EMT (Izawa
et al, unpublished data). Thus, the failure to upregulate
multiple EMT-inducing factors may underlie the inability of the
ectopic expression of Snail to induce EMT in MDBK cells.
It has been demonstrated that shRNA-mediated
knockdown of E-cadherin induces EMT (44). Thus, the knockdown of E-cadherin
expression seems to be an essential step for the induction of EMT.
Although the suppression of E-cadherin expression during EMT is
commonly associated with CpG island methylation within its
promoter, our bisulfite sequencing analysis revealed that the
E-cadherin promoter was not methylated in MDBK cells ectopically
expressing Snail protein, and immunoblot analysis revealed that
E-cadherin expression was maintained in those cells. Therefore, the
failure to downregulate E-cadherin expression may also explain why
Snail-expressing MDBK cells did not undergo EMT.
Acknowledgments
We would like thank Dr M. Sato (Kagoshima
University) for his helpful discussion. This study was supported by
the following grants from the Ministry of Education, Culture,
Sports, Science and Technology of Japan. We would also like to
thank the Joint Research Laboratory at Kagoshima University
Graduate School of Medical and Dental Sciences for the use of their
facilities.
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
MDBK
|
Madin-Darby bovine kidney
|
|
MDCK
|
Madin-Darby canine kidney
|
References
|
1
|
Duband JL, Monier F, Delannet M and
Newgreen D: Epithelium-mesenchyme transition during neural crest
development. Acta Anat (Basel). 154:63–78. 1995. View Article : Google Scholar
|
|
2
|
Hay ED: An overview of
epithelio-mesenchymal transformation. Acta Anat (Basel). 154:8–20.
1995. View Article : Google Scholar
|
|
3
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nawshad A, Lagamba D, Polad A and Hay ED:
Transforming growth factor-beta signaling during
epithelial-mesenchymal transformation: Implications for
embryogenesis and tumor metastasis. Cells Tissues Organs.
179:11–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeichi M: The cadherins: Cell-cell
adhesion molecules controlling animal morphogenesis. Development.
102:639–655. 1988.PubMed/NCBI
|
|
8
|
Koch PJ, Walsh MJ, Schmelz M, Goldschmidt
MD, Zimbelmann R and Franke WW: Identification of desmoglein, a
constitutive desmosomal glycoprotein, as a member of the cadherin
family of cell adhesion molecules. Eur J Cell Biol. 53:1–12.
1990.PubMed/NCBI
|
|
9
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barrallo-Gimeno A and Nieto MA: The Snail
genes as inducers of cell movement and survival: Implications in
development and cancer. Development. 132:3151–3161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cobaleda C, Pérez-Caro M, Vicente-Dueñas C
and Sánchez-García I: Function of the zinc-finger transcription
factor SNAI2 in cancer and development. Annu Rev Genet. 41:41–61.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Côme C, Magnino F, Bibeau F, De Santa
Barbara P, Becker KF, Theillet C and Savagner P: Snail and slug
play distinct roles during breast carcinoma progression. Clin
Cancer Res. 12:5395–5402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cedar H and Bergman Y: Linking DNA
methylation and histone modification: Patterns and paradigms. Nat
Rev Genet. 10:295–304. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reik W: Stability and flexibility of
epigenetic gene regulation in mammalian development. Nature.
447:425–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCabe MT, Brandes JC and Vertino PM:
Cancer DNA methylation: Molecular mechanisms and clinical
implications. Clin Cancer Res. 15:3927–3937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-β family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peinado H, Quintanilla M and Cano A:
Transforming growth factor β-1 induces snail transcription factor
in epithelial cell lines: Mechanisms for epithelial mesenchymal
transitions. J Biol Chem. 278:21113–21123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Romano LA and Runyan RB: Slug is an
essential target of TGFbeta2 signaling in the developing chicken
heart. Dev Biol. 223:91–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dave N, Guaita-Esteruelas S, Gutarra S,
Frias À, Beltran M, Peiró S and de Herreros AG: Functional
cooperation between Snail1 and twist in the regulation of ZEB1
expression during epithelial to mesenchymal transition. J Biol
Chem. 286:12024–12032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dhasarathy A, Phadke D, Mav D, Shah RR and
Wade PA: The transcription factors Snail and Slug activate the
transforming growth factor-beta signaling pathway in breast cancer.
PLoS One. 6:e265142011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang X, Pursell B, Lu S, Chang TK and
Mercurio AM: Regulation of β4-integrin expression by epigenetic
modifications in the mammary gland and during the
epithelial-to-mesenchymal transition. J Cell Sci. 122:2473–2480.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong C, Wu Y, Yao J, Wang Y, Yu Y,
Rychahou PG, Evers BM and Zhou BP: G9a interacts with Snail and is
critical for Snail-mediated E-cadherin repression in human breast
cancer. J Clin Invest. 122:1469–1486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohkubo T and Ozawa M: The transcription
factor Snail downregulates the tight junction components
independently of E-cadherin downregulation. J Cell Sci.
117:1675–1685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haraguchi M, Okubo T, Miyashita Y,
Miyamoto Y, Hayashi M, Crotti TN, McHugh KP and Ozawa M: Snail
regulates cell-matrix adhesion by regulation of the expression of
integrins and basement membrane proteins. J Biol Chem.
283:23514–23523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ozawa M, Ringwald M and Kemler R:
Uvomorulincatenin complex formation is regulated by a specific
domain in the cytoplasmic region of the cell adhesion molecule.
Proc Natl Acad Sci USA. 87:4246–4250. 1990. View Article : Google Scholar
|
|
31
|
Kobayashi W and Ozawa M: The transcription
factor LEF-1 induces an epithelial-mesenchymal transition in MDCK
cells independent of β-catenin. Biochem Biophys Res Commun.
442:133–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ozawa M and Kobayashi W: Reversibility of
the Snail-induced epithelial-mesenchymal transition revealed by the
CreloxP system. Biochem Biophys Res Commun. 458:608–613. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang P, Wei Y, Wang L, Debeb BG, Yuan Y,
Zhang J, Yuan J, Wang M, Chen D, Sun Y, et al: ATM-mediated
stabilization of ZEB1 promotes DNA damage response and
radioresistance through CHK1. Nat Cell Biol. 16:864–875. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kume K, Haraguchi M, Hijioka H, Ishida T,
Miyawaki A, Nakamura N and Ozawa M: The transcription factor Snail
enhanced the degradation of E-cadherin and desmoglein 2 in oral
squamous cell carcinoma cells. Biochem Biophys Res Commun.
430:889–894. 2013. View Article : Google Scholar
|
|
35
|
Shimokawa M, Haraguchi M, Kobayashi W,
Higashi Y, Matsushita S, Kawai K, Kanekura T and Ozawa M: The
transcription factor Snail expressed in cutaneous squamous cell
carcinoma induces epithelial-mesenchymal transition and
down-regulates COX-2. Biochem Biophys Res Commun. 430:1078–1082.
2013. View Article : Google Scholar
|
|
36
|
Shirakihara T, Saitoh M and Miyazono K:
Differential regulation of epithelial and mesenchymal markers by
deltaEF1 proteins in epithelial mesenchymal transition induced by
TGF-β. Mol Biol Cell. 18:3533–3544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by GSK-3β-mediated
phosphorylation in control of epithelial-mesenchymal transition.
Nat Cell Biol. 6:931–940. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng H, Shen M, Zha YL, Li W, Wei Y,
Blanco MA, Ren G, Zhou T, Storz P, Wang HY, et al: PKD1
phosphorylation-dependent degradation of SNAIL by SCF-FBXO11
regulates epithelial-mesenchymal transition and metastasis. Cancer
Cell. 26:358–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Domínguez D, Montserrat-Sentís B,
Virgós-Soler A, Guaita S, Grueso J, Porta M, Puig I, Baulida J,
Francí C and García de Herreros A: Phosphorylation regulates the
subcellular location and activity of the snail transcriptional
repressor. Mol Cell Biol. 23:5078–5089. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang CJ, Chao CH, Xia W, Yang JY, Xiong
Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, et al: p53 regulates
epithelial-mesenchymal transition and stem cell properties through
modulating miRNAs. Nat Cell Biol. 13:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim T, Veronese A, Pichiorri F, Lee TJ,
Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, et al:
p53 regulates epithelial-mesenchymal transition through microRNAs
targeting ZEB1 and ZEB2. J Exp Med. 208:875–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Devireddy LR and Jones CJ: Activation of
caspases and p53 by bovine herpesvirus 1 infection results in
programmed cell death and efficient virus release. J Virol.
73:3778–3788. 1999.PubMed/NCBI
|
|
43
|
Zhang Y, Yan W and Chen X: Mutant p53
cooperates with knockdown of endogenous wild-type p53 to disrupt
tubulogenesis in Madin-Darby canine kidney cells. PLoS One.
8:e856242013. View Article : Google Scholar
|
|
44
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|