Introduction
Apoptosis inhibitor of macrophages (AIM), also known
as Api6, soluble protein α or cluster of differentiation 5 (CD5)
antigen-like, is a 54-kDa glycoprotein secreted by mature tissue
macrophages, and it belongs to the scavenger receptor cysteine-rich
domain superfamily (1–4). AIM was initially found to inhibit
apoptosis of CD4+CD8+ T cells during their
maturation in the thymus (3). The
anti-apoptotic effect of AIM on other cells, such as natural killer
T cells and macrophages, was subsequently reported (3,5–12).
AIM, as a direct gene target of liver X receptors (LXRs), is
induced during LXR activation by oxidized low-density lipoprotein
or microbial infection (3,8,9,11,12).
In turn, the induction of AIM promotes macrophage survival and
accentuates the roles of these cells in the development of
atherosclerosis, immunity against microbial infection and
inflammatory processes (6,8–10,12).
Overexpressing AIM in mice increases the survival rate and
phagocytic activity of macrophages in fulminant hepatitis (6). AIM has a role in atherosclerogenesis
by enhancing macrophage survival within atherosclerotic lesions
(10). Results from reverse
tetracycline-responsive transactivator and Api6 bitransgenic mice
have shown that AIM (Api6) is a pro-inflammatory and oncogenic
molecule that stimulates cell proliferation and lung tumorigenesis
(13,14). Recent evidence has indicated that
AIM is incorporated into adipocytes via CD36-mediated endocytosis,
thereby inactivating cytoplasmic fatty acid synthase (15). Among all the biological functions
associated with AIM, the anti-apoptotic function has been the most
clearly demonstrated; however, the molecular mechanism by which AIM
regulates apoptosis is not understood.
Insulin-like growth factor-binding proteins (IGFBPs)
are an important family of secreted proteins with similar high
binding affinities to IGF-I and IGF-II, and are involved in the
regulation of somatic growth and cellular proliferation (16–19). At least 6 isoforms (IGFBPs 1–6)
have been well characterized. The liver is the major source of
circulating IGFs and IGFBPs. Hepatocytes synthesize IGFBP-1, -2 and
-4, and hepatic Kupffer cells synthesize IGFBP-2 and IGFBP-3
(20,21). IGFBPs regulate IGF signaling by
binding to IGF and partially masking the IGF residues responsible
for type 1 IGF receptor binding (17,22,23). IGFBPs are secreted into the
extracellular matrix, whereby they induce the apoptotic cell death
program and inhibit cell growth through IGF-dependent and
-independent mechanisms (24–26).
Using co-immunoprecipitation (co-IP) and biolayer
interferometry (BLI), the present study uncovered IGFBP-4, along
with IGFBP-2 and -3, as binding partners for AIM. An apoptosis
assay showed that AIM inhibited apoptosis through disrupting
IGFBP-4 binding to IGF-I. These data provide the first evidence for
AIM binding to IGFBPs, suggesting a potential mechanism for
AIM-regulated cell survival.
Materials and methods
Materials
Rabbit anti-HA polyclonal antibody (0906-1) and
mouse anti-His monoclonal antibody (M0812-3) were purchased from
Hangzhou HuaAn Biotechnology Co. (Hangzhou, China). Horseradish
peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG)
(SA00001-1) and anti-rabbit IgG (SA00001-2) were purchased from the
ProteinTech Group (Chicago, IL, USA). HisTrap HP columns and PD-10
desalting columns were purchased from Amersham Pharmacia Biotech,
Inc. (Piscataway, NJ, USA). Anti-FLAG antibody (F3165), mouse
anti-HA monoclonal antibody (H9658), control IgG (M5284) and
Coomassie blue R-250 were purchased from Sigma (St. Louis, MO,
USA).
Cell culture
The 293 cells (ATCC, Manassas, VA, USA) were
maintained in high-glucose Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS)
(Invitrogen, Carlsbad, CA, USA). TAMH cells [a mouse hepatocyte
cell line; provided by Christopher C. Franklin, Department of
Pharmaceutical Sciences, University of Colorado Denver (UCD),
Denver, CO, USA] were grown in serum free DMEM/Ham's F12
(Invitrogen) supplemented with 5 mg/ml insulin, 5 mg/ml
transferrin, 5 ng/ml selenium (Collaborative Biomedical Products,
Bedford, MA, USA), 100 nM dexamethasone, 10 mM nicotinamide and
0.1% (v/v) gentamicin (Invitrogen). Drosophila Schneider
cells (S2 cells) were cultured in Schneider's Drosophila
medium (Sigma) supplemented with 10% (v/v) FBS. Cultures were
maintained in a humidified incubator with 5% carbon dioxide and 95%
air at 37°C.
Protein expression and purification
Eukaryotic expression system
The AIM gene was cloned into a modified
pMT/BiP vector (Invitrogen), which contained 6 histidines and BirA
enzyme substrate peptide (BSP, GGGLNDIFEAQKIEWHE) at the amino
terminus. The recombinant plasmid and pCoHygro (19:1 ratio) were
used to co-transfect S2 cells using the calcium phosphate method.
After 4 weeks of culture in Schneider's Drosophila medium
supplemented with 10% FBS and 300 µg/ml hygromycin-B
(Invitrogen), hygromycin-B resistant cells were selected. For
large-scale production of soluble AIM protein, stably transfected
S2 cells were cultured in EX-CELL 420 medium supplemented with 50
µg/ml gentamycin (Sigma) at 28°C. Expression was induced
with 0.5 mM CuSO4 for 7 days. Cells were removed by
centrifugation at 1,000 × g, 4°C for 10 min. The supernatant was
exchanged with buffer containing 50 mM
NaH2PO4 and 500 mM NaCl (pH 8.0). The
resultant solution was passed through a 0.22-µm filter,
supplemented with 5 mM imidazole, and subsequently purified by
Ni-NTA chromatography. Proteins were stored at −80°C following
determination of the concentration by the Bradford assay.
Prokaryotic expression system
The AIM gene carrying a HA tag sequence at
the 5′-terminus was cloned into a pET28a vector (Novagen, Madison,
WI, USA), containing 6 histidines at the amino terminus. The
IGFBP-4 gene was cloned into a pET28c vector (Novagen),
containing 6 histidines at the amino terminus. The Escherichia
coli (E. coli) strains, BL21 (DE3) transformed with the
expression plasmid, including His-HA-tagged AIM or His-IGFBP-4,
were induced with 1 mM isopropyl β-D-thiogalactopyranoside to
express His-HA-AIM or His-IGFBP-4 protein. The proteins were
purified using Ni-NTA affinity chromatography (Qiagen, Valencia,
CA, USA) and stored at −80°C following determination of
concentration by the Bradford assay.
Western blot assay
Samples were fractionated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and the
separated proteins were electrophoretically transferred onto the
nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Non-specific
binding was blocked with 0.05% Tween-20 in phosphate-buffered
saline (PBST) containing 5% non-fat milk for 1 h at room
temperature. The membranes were subsequently incubated overnight at
4°C with antibodies against His tag or HA tag in PBST containing 1%
non-fat milk at the dilutions specified by the manufacturers.
Following 3 washes with PBST, the membranes were incubated with the
HRP-conjugated secondary antibodies at 1:5,000 dilution in PBST
containing 1% non-fat milk for 1 h at room temperature. The
membranes were subsequently washed 3 times with PBST and the
protein bands were detected with a western blotting detection
system.
Co-IP
The AIM or IGFBP 1–6 genes were cloned
into a cytomegalovirus promoter-based vector-pRK containing a 5′-HA
or 5′-FLAG-tag. The plasmids were transiently transfected into the
293 cells (2×106) using the calcium phosphate method.
After 24 h, the transfected cells were lysed with 1 ml of lysis
buffer [20 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1 mM
ethylenediaminetetraacetic acid, 1 mM phenylmethylsulfonyl
fluoride, 2 mM Na3VO4, 20 mM NaF, 10
µg/ml aprotinin and 10 µg/ml leupeptin]. Cell lysate
(0.4 ml) was incubated with the appropriate monoclonal antibody or
control IgG, as well as 20 µl of a 1:1 slurry of GammaBind
Plus-Sepharose (GE Healthcare, Logan, UT, USA). After an overnight
incubation at 4°C, the sepharose beads were washed 3 times with 1
ml lysis buffer. The precipitates were fractionated by SDS-PAGE,
and western blot analysis was performed. All the
immunoprecipitation experiments were repeated ≥3 times, and similar
data were obtained.
BLI
The interaction between AIM and IGFBP-4 was measured
using Bio-Layer Interferometry on Octet RED (ForteBio, Menlo Park,
CA, USA). All the interaction analyses were conducted at 25°C in
PBS buffer unless stated. IGFBP-4 was purified from E. coli
as described above and subsequently labeled with biotin (Thermo
Fisher Scientific, Waltham, MA, USA), which is optimal for binding
and immobilizing target proteins on superstreptavidin (SA)
biosensors (ForteBio) for studying protein-protein interactions.
Biotinylated IGFBP-4 was separated and loaded onto SA biosensors
for 300 sec to ensure saturation. The 96-well microplates used in
the Octet were filled with 200 µl of sample or buffer per
well and agitated at 800 × g. The loaded biosensors were washed in
buffer for 120 sec and transferred to the wells containing AIM at
concentrations of 400, 200, 100, 50, 25 and 12.5 nM in buffer,
respectively. The association was observed for 240 sec and
dissociation was observed for 300 sec for each protein of interest
in the sample diluent. A parallel set of superstreptavidin
biosensors was prepared with biotinylated streptavidin to act as a
control. Kinetic parameters (Kon and
Koff) and affinities (KD) were
calculated from a non-linear global fit of the data between IGFBP-4
and AIM using the Octet software. Independent measurements were
performed ≥3 times.
Caspase-3/7 activity
The TAMH cells were plated in 96-well plates at a
density of 1×104 cells/well for 24 h. Cells were
subsequently washed with DMEM/F12 medium and serum-starved
overnight. Certain cultures were treated with IGF-I (20 ng/ml) in
the presence or absence of IGFBP-4 (500 ng/ml) and AIM (2
µg/ml) for 6 h before the supernatant and cells were
harvested. Cells were lysed for 20 min on ice, and subsequently
incubated with caspase-3/7 substrate (Ac-DEVE-AMC, 20 µM)
for 1 h at 37°C. Fluorescence intensity was measured using
excitation wavelength of 380 nm and an emission wavelength of 460
nm.
Statistical analysis
All the conditions were performed in triplicate, and
the reported values are representative of 3 independent
experiments. All the values are expressed as the mean ± standard
deviation of 3 parallel measurements. Data were analyzed by
Student's t-test and P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of binding partners of
AIM
Our yeast two hybrid screening for binding partners
of AIM identified IGFBP-4 as a potential candidate. To further
confirm that AIM binds to IGFBP-4, the proteins His-HA-AIM and
His-IGFBP-4 were purified from transformed E. coli cells,
respectively, and subsequently, the binding activity of AIM to
IGFBP-4 was assessed using the in vitro co-IP assay. The
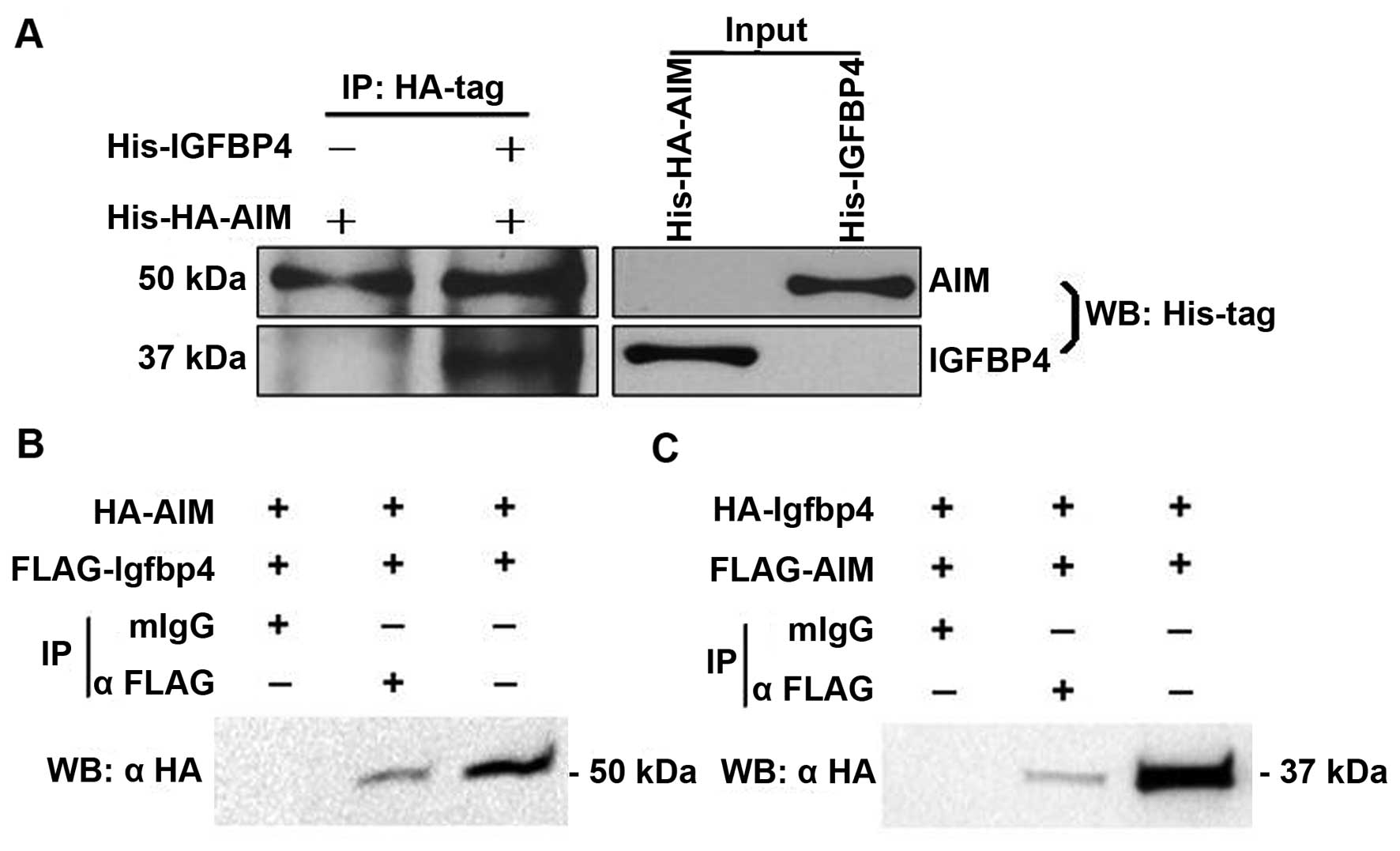
results showed that His-IGFBP-4 appeared in immunoprecipitates of
His-HA-AIM bound to beads with anti-HA tag antibody (Fig. 1A), demonstrating that the
recombinant AIM and IGFBP-4 purified from E. coli could
interact with each other in vitro.
In order to identify the interaction between AIM and
IGFBP-4 in mammalian cells, 293 cells were co-transfected with
expression vectors encoding HA-AIM, FLAG-AIM, HA-IGFBP-4 and
FLAG-IGFBP-4. The interaction between AIM and IGFBP-4 was
determined by the co-IP assay. As shown in Fig. 1, HA-AIM was clearly detected in
the immunoprecipitates of Flag-IGFBP-4 bound to beads with
anti-Flag antibody (Fig. 1B).
Reciprocally, HA-IGFBP-4 was also readily precipitated with
Flag-AIM on beads with anti-Flag antibody (Fig. 1C).
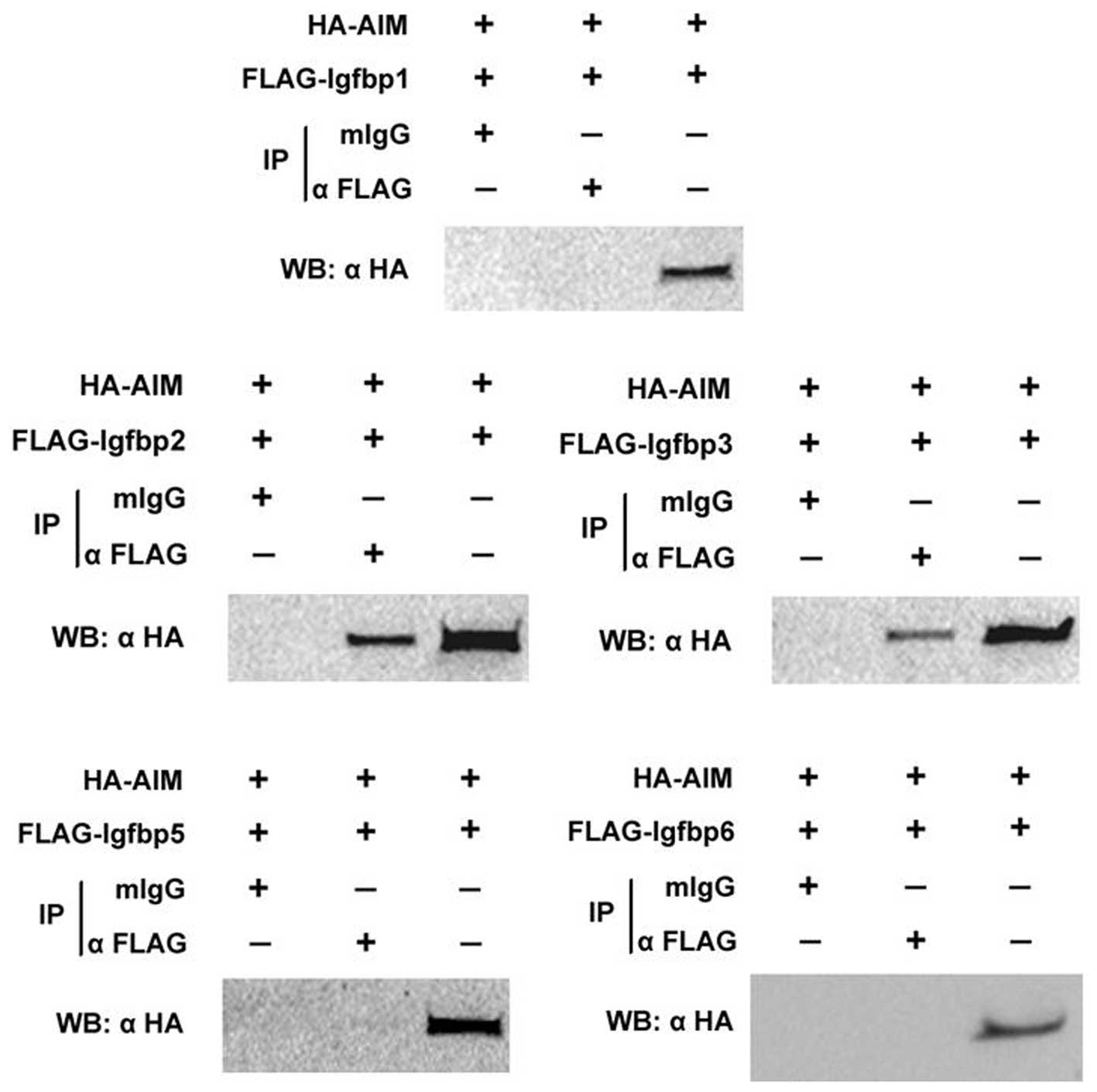
The family of IGFBPs consists of ≥6 isoforms that
have been well characterized (17). Subsequently, whether AIM could
interact with other IGFBP family members was investigated. The 293
cells were co-transfected with HA-AIM and Flag-IGFBPs (1, 2, 3, 5
or 6) for 24 h. Cell extracts from the 293 cells were
immunoprecipitated with anti-Flag antibody or control IgG. Western
blot analysis revealed that AIM can interact with IGFBP-2 and
IGFBP-3, but not IGFBP-1, IGFBP-5 or IGFBP-6 (Fig. 2).
Evaluation of binding activity between
AIM and IGFBP-4
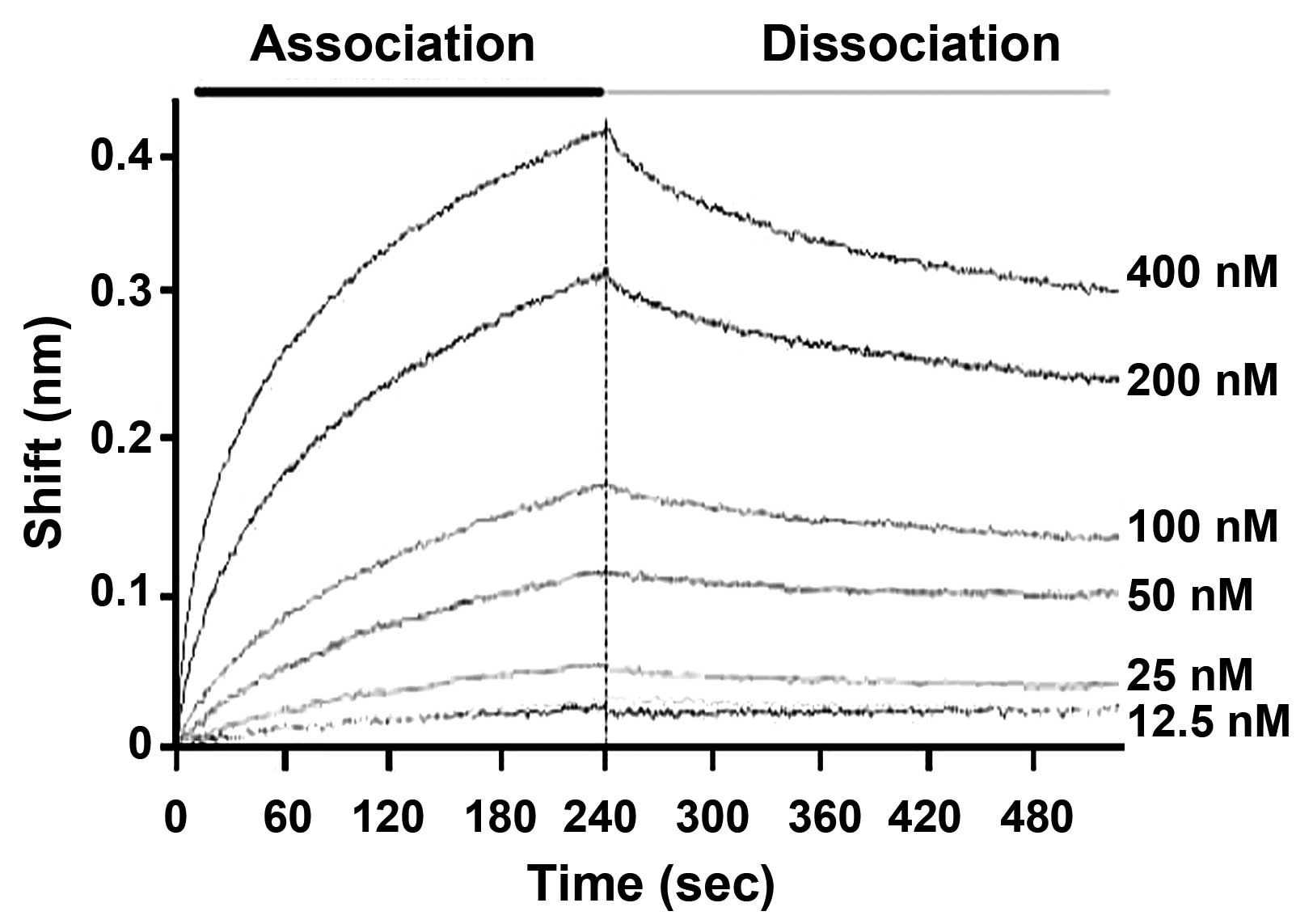
To obtain the kinetic parameters of the interaction
between AIM and IGFBP-4, BLI was employed to assess the rates of
association and dissociation. IGFBP-4 was biotin-labeled and
captured on the SA sensor chips, and kinetic parameters were
determined for AIM as analytes (Fig.
3). The results show that AIM rapidly associates with
biotinylated IGFBP-4 bound to SA-biosensors through the fast
association rate constant (Kon) of
3.96±0.04×104 Ms−1. By contrast, the
dissociation of AIM from biotinylated IGFBP-4 on SA biosensors
appeared to be slow, evident from the dissociation rate constant
(Koff) of 9.99±0.11×10−4
s−1. The equilibrium dissociation constant
(KD) of AIM was 2.53±0.06×10−8 M,
which was calculated from the ratio of the rate constant,
Koff/Kon (Table I). These data reveal a strong
association between AIM and IGFBP-4.
 | Table IAffinity and rate constants for the
interaction between AIM and IGFBP-4. |
Table I
Affinity and rate constants for the
interaction between AIM and IGFBP-4.
| Interaction
proteins |
KD (×10−8
M) | Rate constants
| R2 |
|---|
|
Kon (×104
Ms−1) |
Koff (×10−4
s−1) |
|---|
| AIM IGFBP-4 | 2.53±0.06 | 3.96±0.04 | 9.99±0.11 | 0.988695 |
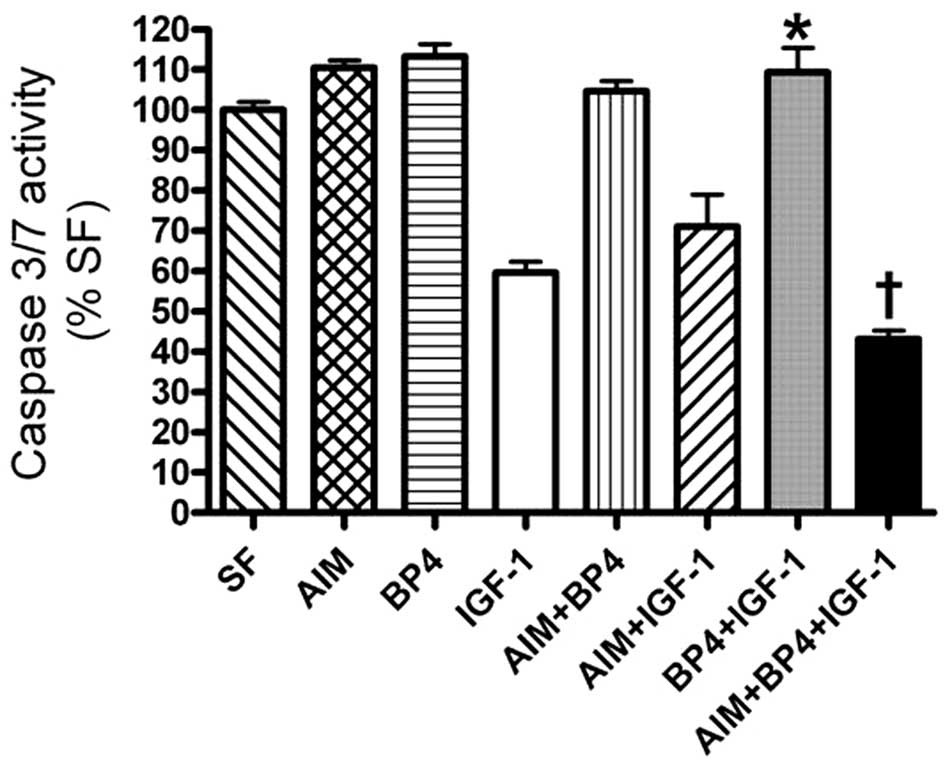
AIM inhibits IGFBP-4-mediated
apoptosis
Previous studies have demonstrated that IGFBPs
modulate the biological activity of IGF-I to inhibit cell growth
and proliferation (24,27–29). Thus, we hypothesized that AIM
inhibited apoptosis by binding to IGFBP-4, thus increasing the
binding of IGF-I to IGF receptors. To examine this hypothesis, TAMH
cells were treated with IGF-I in the presence and absence of
IGFBP-4 and AIM, and apoptosis was examined by measuring the
caspase-3/7 activities. The caspase-3/7 activity induced by serum
starvation in TAMH cells was significantly decreased when the cells
were treated with IGF-I, indicating an anti-apoptotic and
pro-survival effect of IGF-I (Fig.
4). The effect was abrogated by IGFBP-4, as the caspase-3/7
activity returned to the levels of serum-starved cells. This result
is consistent with a previous report that IGFBP-4 inhibited the
pro-survival activity of IGF-I (29). Compared with the caspase-3/7
activity in cells treated with IGFBP-4 + IGF-I, the caspase
activity in cells treated with the combination of AIM, IGFBP-4 and
IGF-I was markedly reduced. These results revealed that the
mechanism of AIM inhibiting apoptosis was partly through disrupting
IGFBP-4 binding to IGF-I.
Discussion
AIM, produced and secreted by tissue macrophages,
has been indicated in a broad spectrum of biological functions,
attributable to its anti-apoptotic effects on macrophages and other
cell types (3,5). However, the molecular mechanism by
which AIM regulates apoptosis is not clear. The present data
demonstrate that AIM binds to IGFBP-4, which may explain the
underlying mechanism of AIM-mediated anti-apoptotic function.
AIM acts as an immune regulator by inhibiting immune
cell apoptosis. AIM inhibits apoptosis of monocytes, T cells, NKT
cells and CD4/CD8 double-positive thymocytes undergoing maturation
in the thymus, and the loss of AIM promotes cell apoptosis at the
inflammatory sites (3,5). Recent investigations from animal
models have shown that AIM appears to be multifunctional and is
effective in cell types other than immune cells, including
adipocytes and epithelial cells (13–15,30). Increasing evidence has shown that
AIM has key roles in the pathogenesis of multiple diseases,
including atherosclerosis, metabolic diseases, inflammation,
infection and cancer by supporting the survival of macrophages
(6,8,10–13,31). Thus, AIM may be a therapeutic
target in these diseases (30).
With regard to the anti-apoptosis function of AIM,
it has been suggested that Stat3, Erk1/2 and p38 signaling pathways
may be involved (14). However,
the underlying molecular mechanism accounting for the action of AIM
is not understood, as the binding partners of AIM are unknown.
Previous data have shown that the circulating level of AIM is
dependent on its association with the IgM pentamer to increase the
stabilization in blood (32). The
present data demonstrate that AIM binds to IGFBP-4 and that this
interaction may have an important role in mediating the
anti-apoptotic function of AIM. All IGFBPs share a highly conserved
structure that is generally described as consisting of three
distinct domains of approximate lengths as follows: Highly
conserved cysteine-rich N and C domains and a central linker
domain, unique to each IGFBP species. IGFBPs exist in the
circulation in the free form or in complexes with IGFs, thereby
prolonging their half-lives and modulating their biological
functions in target cells (17,23). The present results revealed that
AIM can interact with IGFBP-2, -3 and -4, but not IGFBP-1, -5 or -6
(Fig. 2). According to the
evolution assay of the IGFBPs family, they can be divided into 2
main subgroups. One cluster includes IGFBP-1, -2 and -4 as they are
more closely associated with each other, the other includes
IGFBP-3, -5 and -6 (33).
Furthermore, there are significant similarities between the
IGFBP-2-NMR crystal structure and IGFBP-4-X-ray crystal structure
(23,34,35). The majority of the residues
involved in binding to IGF-I were conserved between N-BP-2 and
N-BP-4; these two residues show that the N- and C-termini are in
close contact. Due to the structural similarities, AIM may interact
with IGFBP-2 and -4 in a similar manner. Although IGFBP-1 belongs
to the same group as IGFBP-2 and -4, it is not able to bind to AIM.
By contrast, IGFBP-3 can interact with AIM despite belonging to a
different group. Further detailed investigations are warranted to
identify the critical domains in the IGFBP proteins that are
critical in binding to AIM.
Among all the IGFBPs, IGFBP-4 has been predominately
associated with counteracting the pro-survival and
pro-proliferative effects of IGF. IGFBP-4 decreases cell
proliferation and DNA synthesis, as well as induces apoptosis in a
cell type- and tissue-specific manner (36,37). Sitar et al (23) reported the high-resolution X-ray
structure of a complex of the N- and C-terminal domains of IGFBP-4
bound to IGF-I, which provided the structural basis for the
inhibition of IGFs by IGFBP-4. The N-terminal domain of IGFBP-4
contains pivotal IGF-binding residues, rendering high-affinity
binding to IGF and partially masking the IGF residues responsible
for the type 1 IGF receptor binding. The C-terminal domain also
contributes to blocking of the IGF-I receptor-binding region of
IGF-I. The central domain of the IGFBP-4 contains proteolytic
cleavage sites. On these sites, the IGFBP-4 protease specifically
cleaves IGFBP-4 into fragments with low affinity for IGF-I, leading
to IGF release (17). The present
data demonstrate that AIM can directly bind to IGFBP-4 and reverse
the pro-apoptotic effect of IGFBP-4. We hypothesize that the
binding of AIM to IGFBP-4 may reduce the affinity of its N- and/or
C-terminal domains that bind with IGF. The competition for IGFBP-4
binding by AIM releases IGF, thereby promoting IGF binding to IGF
receptors and thus IGF signaling.
In conclusion, to the best of our knowledge, this
study provides the first evidence that AIM binds to IGFBP-2, -3 and
-4. The data suggest that this interaction between AIM and IGFBP-4
may contribute to the mechanism of AIM-mediated anti-apoptosis
effect. These findings may provide valuable information regarding
the mechanism of apoptosis regulation by AIM.
Acknowledgments
The present study was partly supported by US
National Institutes of Health (grant No. U01 AA021723 to C.J.), the
ALSAM Foundation Skaggs Scholars Program Award (to C.J.), the
National Natural Science Foundation of China (grant No. 31170882 to
G.L. and grant No. 81428006 to C.J.) and the S&T Development
Planning Program of Jilin Province (grant Nos. 20111806 and
20150414027GH to G.L.).
Abbreviations:
|
AIM
|
apoptosis inhibitor of macrophage
|
|
LXR
|
liver X receptor
|
|
IGF
|
insulin-like growth factor
|
|
IGFBP
|
insulin-like growth factor binding
protein
|
References
|
1
|
Gebe JA, Kiener PA, Ring HZ, Li X, Francke
U and Aruffo A: Molecular cloning, mapping to human chromosome 1
q21–q23, and cell binding characteristics of Spalpha, a new member
of the scavenger receptor cysteine-rich (SRCR) family of proteins.
J Biol Chem. 272:6151–6158. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sarrias MR, Roselló S, Sánchez-Barbero F,
Sierra JM, Vila J, Yélamos J, Vives J, Casals C and Lozano F: A
role for human Spα as a pattern recognition receptor. J Biol Chem.
280:35391–35398. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miyazaki T, Hirokami Y, Matsuhashi N,
Takatsuka H and Naito M: Increased susceptibility of thymocytes to
apoptosis in mice lacking AIM, a novel murine macrophage-derived
soluble factor belonging to the scavenger receptor cysteine-rich
domain superfamily. J Exp Med. 189:413–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gebe JA, Llewellyn M, Hoggatt H and Aruffo
A: Molecular cloning, genomic organization and cell-binding
characteristics of mouse Spalpha. Immunology. 99:78–86. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuwata K, Watanabe H, Jiang SY, Yamamoto
T, Tomiyama-Miyaji C, Abo T, Miyazaki T and Naito M: AIM inhibits
apoptosis of T cells and NKT cells in Corynebacterium-induced
granuloma formation in mice. Am J Pathol. 162:837–847. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haruta I, Kato Y, Hashimoto E, Minjares C,
Kennedy S, Uto H, Yamauchi K, Kobayashi M, Yusa S, Müller U, et al:
Association of AIM, a novel apoptosis inhibitory factor, with
hepatitis via supporting macrophage survival and enhancing
phagocytotic function of macrophages. J Biol Chem. 276:22910–22914.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bensinger SJ and Tontonoz P: Integration
of metabolism and inflammation by lipid-activated nuclear
receptors. Nature. 454:470–477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joseph SB, Bradley MN, Castrillo A, Bruhn
KW, Mak PA, Pei L, Hogenesch J, O'Connell RM, Cheng G, Saez E, et
al: LXR-dependent gene expression is important for macrophage
survival and the innate immune response. Cell. 119:299–309. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valledor AF, Hsu LC, Ogawa S,
Sawka-Verhelle D, Karin M and Glass CK: Activation of liver X
receptors and retinoid X receptors prevents bacterial-induced
macrophage apoptosis. Proc Natl Acad Sci USA. 101:17813–17818.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arai S, Shelton JM, Chen M, Bradley MN,
Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ,
Tontonoz P, et al: A role for the apoptosis inhibitory factor
AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab.
1:201–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amézaga N, Sanjurjo L, Julve J, Aran G,
Pérez-Cabezas B, Bastos-Amador P, Armengol C, Vilella R, Escolà-Gil
JC, Blanco-Vaca F, et al: Human scavenger protein AIM increases
foam cell formation and CD36-mediated oxLDL uptake. J Leukoc Biol.
95:509–520. 2014. View Article : Google Scholar
|
|
12
|
Martinez VG, Escoda-Ferran C, Tadeu Simões
I, Arai S, Orta Mascaró M, Carreras E, Martínez-Florensa M, Yelamos
J, Miyazaki T and Lozano F: The macrophage soluble receptor
AIM/Api6/CD5L displays a broad pathogen recognition spectrum and is
involved in early response to microbial aggression. Cell Mol
Immunol. 11:343–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Qu P, Wu L, Li B, Du H and Yan C:
Api6/AIM/Spα/CD5L overexpression in alveolar type II epithelial
cells induces spontaneous lung adenocarcinoma. Cancer Res.
71:5488–5499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qu P, Du H, Li Y and Yan C:
Myeloid-specific expression of Api6/AIM/Sp alpha induces systemic
inflammation and adenocarcinoma in the lung. J Immunol.
182:1648–1659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kurokawa J, Arai S, Nakashima K, Nagano H,
Nishijima A, Miyata K, Ose R, Mori M, Kubota N, Kadowaki T, et al:
Macrophage-derived AIM is endocytosed into adipocytes and decreases
lipid droplets via inhibition of fatty acid synthase activity. Cell
Metab. 11:479–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwa V, Oh Y and Rosenfeld RG: The
insulin-like growth factor-binding protein (IGFBP) superfamily.
Endocr Rev. 20:761–787. 1999.PubMed/NCBI
|
|
17
|
Firth SM and Baxter RC: Cellular actions
of the insulin-like growth factor binding proteins. Endocr Rev.
23:824–854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clemmons DR: Use of mutagenesis to probe
IGF-binding protein structure/function relationships. Endocr Rev.
22:800–817. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bach LA, Headey SJ and Norton RS:
IGF-binding proteins - the pieces are falling into place. Trends
Endocrinol Metab. 16:228–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zimmermann EM, Li L, Hoyt EC, Pucilowska
JB, Lichtman S and Lund PK: Cell-specific localization of
insulin-like growth factor binding protein mRNAs in rat liver. Am J
Physiol Gastrointest Liver Physiol. 278:G447–G457. 2000.PubMed/NCBI
|
|
21
|
Kuemmerle JF: Insulin-like growth factors
in the gastrointestinal tract and liver. Endocrinol Metab Clin
North Am. 41:409–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bunn RC and Fowlkes JL: Insulin-like
growth factor binding protein proteolysis. Trends Endocrinol Metab.
14:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sitar T, Popowicz GM, Siwanowicz I, Huber
R and Holak TA: Structural basis for the inhibition of insulin-like
growth factors by insulin-like growth factor-binding proteins. Proc
Natl Acad Sci USA. 103:13028–13033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohan S and Baylink DJ: IGF-binding
proteins are multifunctional and act via IGF-dependent and
-independent mechanisms. J Endocrinol. 175:19–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moreno MJ, Ball M, Rukhlova M, Slinn J,
L'abbe D, Iqbal U, Monette R, Hagedorn M, O'Connor-McCourt MD,
Durocher Y, et al: IGFBP-4 anti-angiogenic and anti-tumorigenic
effects are associated with anti-cathepsin B activity. Neoplasia.
15:554–567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ryan AJ, Napoletano S, Fitzpatrick PA,
Currid CA, O'Sullivan NC and Harmey JH: Expression of a
protease-resistant insulin-like growth factor-binding protein-4
inhibits tumour growth in a murine model of breast cancer. Br J
Cancer. 101:278–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sueoka N, Lee HY, Wiehle S, Cristiano RJ,
Fang B, Ji L, Roth JA, Hong WK, Cohen P and Kurie JM: Insulin-like
growth factor binding protein-6 activates programmed cell death in
non-small cell lung cancer cells. Oncogene. 19:4432–4436. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perks CM, Newcomb PV, Norman MR and Holly
JM: Effect of insulin-like growth factor binding protein-1 on
integrin signalling and the induction of apoptosis in human breast
cancer cells. J Mol Endocrinol. 22:141–150. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Durai R, Davies M, Yang W, Yang SY,
Seifalian A, Goldspink G and Winslet M: Biology of insulin-like
growth factor binding protein-4 and its role in cancer (Review).
Int J Oncol. 28:1317–1325. 2006.PubMed/NCBI
|
|
30
|
Arai S and Miyazaki T: Impacts of the
apoptosis inhibitor of macrophage (AIM) on obesity-associated
inflammatory diseases. Semin Immunopathol. 36:3–12. 2014.
View Article : Google Scholar :
|
|
31
|
Sanjurjo L, Amézaga N, Vilaplana C,
Cáceres N, Marzo E, Valeri M, Cardona PJ and Sarrias MR: The
scavenger protein apoptosis inhibitor of macrophages (AIM)
potentiates the antimicrobial response against Mycobacterium
tuberculosis by enhancing autophagy. PLoS One. 8:e796702013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kai T, Yamazaki T, Arai S and Miyazaki T:
Stabilization and augmentation of circulating AIM in mice by
synthesized IgM-Fc. PLoS One. 9:e970372014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daza DO, Sundström G, Bergqvist CA, Duan C
and Larhammar D: Evolution of the insulin-like growth factor
binding protein (IGFBP) family. Endocrinology. 152:2278–2289. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galea CA, Mobli M, McNeil KA, Mulhern TD,
Wallace JC, King GF, Forbes BE and Norton RS: Insulin-like growth
factor binding protein-2: NMR analysis and structural
characterization of the N-terminal domain. Biochimie. 94:608–616.
2012. View Article : Google Scholar
|
|
35
|
Forbes BE, McCarthy P and Norton RS:
Insulin-like growth factor binding proteins: A structural
perspective. Front Endocrinol (Lausanne). 3:382012.
|
|
36
|
Damon SE, Maddison L, Ware JL and Plymate
SR: Overexpression of an inhibitory insulin-like growth factor
binding protein (IGFBP), IGFBP-4, delays onset of prostate tumor
formation. Endocrinology. 139:3456–3464. 1998.PubMed/NCBI
|
|
37
|
Zhou R, Flaswinkel H, Schneider MR, Lahm
H, Hoeflich A, Wanke R and Wolf E: Insulin-like growth
factor-binding protein-4 inhibits growth of the thymus in
transgenic mice. J Mol Endocrinol. 32:349–364. 2004. View Article : Google Scholar : PubMed/NCBI
|