Introduction
Glioblastoma multiforme (GBM) is the most common and
lethal primary malignancy of the central nervous system, and
patients with GBM have a poor prognosis (1). Current standard therapies for GBM
comprise surgical resection, chemotherapy and radiotherapy
(2). Temozolomide (TMZ), a DNA
methylating agent, is the primary chemotherapeutic drug used in the
treatment of malignant gliomas (3). Clinical studies have indicated that
TMZ chemotherapy alone or in combination with radiotherapy
increases the survival rate of patients with GBM (4,5).
Despite advances in treatment strategies, the median survival rate
of patients with GBM is only 12–14 months (6).
Chemoresistance is a major obstacle to effective
cancer chemotherapy. TMZ induces the formation of
O6-methylguanine in DNA, which consequently leads to DNA
replication defects and cell death. A well-established mechanism
for resistance to TMZ is mediated by the DNA repair protein
O6-methylguanine methyltransferase (MGMT), which repairs
TMZ-induced DNA lesions through the removal of the
O6-methyl group (7).
Additionally, the aberrant activation of survival signaling
pathways also contributes to the resistance of GBM cells to TMZ
(8). Signal transducer and
activator of transcription 3 (STAT3) signaling has been implicated
in the development and progression of many types of tumor,
including GBM (9). The
constitutive activation of STAT3 has been shown to contribute to
the resistance of GBM cells to TMZ (10), and thus STAT3 represents an
important therapeutic target for this disease.
MicroRNAs (miRs or miRNAs) are a class of
endogenous, non-coding regulatory RNAs of ~22 nucleotides in
length. They regulate a large number of target genes and thus
affect various biological processes, such as cell proliferation,
differentiation, apoptosis, invasion and tumorigenesis (11,12). miR-31 is dysregulated in many
human malignancies, such as bladder cancer (13), melanoma (14) and colorectal cancer (15). Compared to normal brain tissue,
miR-31 expression is significantly decreased in glioma tissue
(16). miR-31 functions as an
oncogene or a tumor suppressor in different types of cancer
(14–15,17). For instance, miR-31 has been shown
to enhance the proliferation of colon cancer cells (17), whereas in melanoma cells, miR-31
has been shown to inhibit cell migration and invasion (14). Despite these studies, relatively
little is known about the biological role of miR-31 in GBM,
particularly in relation to the regulation of chemosensitivity.
In this study, we investigated the effects of miR-31
overexpression on the proliferation, apoptosis and TMZ sensitivity
of GBM cells, and we also examined the involvement of STAT3
signaling.
Materials and methods
Cell culture
Normal human astrocytes were purchased from
ScienCell Research Laboratories (Carlsbad, CA, USA) and the human
GBM cell lines, U251 and U87, were obtained from the American Type
Culture Collection (ATCC; Rockville, MD, USA). The cells were
maintained in Dulbecco's modified Eagle's medium (DMEM) with 25
mmol/l D-glucose (Invitrogen, Carlsbad, CA, USA) supplemented with
10% heat-inactivated fetal bovine serum, 2 mM glutamine and
penicillin (100 U/ml)-streptomycin (100 μg/ml; all from
Invitrogen).
Measurement of miR-31 expression
miR-31 expression was measured by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), as
previously described (18). In
brief, total RNA was extracted from the cells using TRIzol reagent
(Invitrogen), and cDNA was synthesized with specific stem-loop
reverse transcription primers. miR-31 expression levels were
measured using TaqMan microRNA assays (Taqman® MicroRNA
Reverse Transcription kit; Applied Biosystems, Foster City, CA,
USA) with the following cycling conditions: 95°C for 5 min,
followed by 40 cycles of amplification (95°C for 20 sec, 60°C for
20 sec and 72°C for 30 sec). The relative level of miR-31 following
normalization to U6 small nuclear RNA was analyzed using the
comparative cycle threshold (ΔΔCt) method, as previously described
(19).
Plasmids, miRNA oligonucleotides and cell
transfection
The STAT3-C plasmid expressing the constitutively
active STAT3 mutant was purchased from Addgene (Cambridge, MA,
USA), as previously described (20). The pcDNA 3.1(+) control vector was
purchased from Invitrogen. Human pre-miR-31 and pre-miR negative
control oligonucleotides were purchased from GenePharma Corp.
(Shanghai, China). Cell transfection was carried out using
Lipofectamine 2000 (Invitrogen) according to the manufacturer's
instructions. Untransfected cells were used as the controls. The
final concentration of each miRNA oligonucleotide was 50 nM. To
determine the mediating role of STAT3, 1 μg of STAT3-C or
the control plasmid was co-transfected into the GBM cells. At 24 h
post-transfection, the cells were subjected to cell proliferation,
apoptosis and gene expression analyses.
Treatment with TMZ
To determine the effects of miR-31 on TMZ
cytotoxicity, the cells were transfected with pre-miR-31 or
co-transfected with pre-miR-31 and STAT3-C 24 h prior to exposure
to 100 μM TMZ, as previously described (21). Following incubation for an
additional 72 h, the cells were harvested for cell proliferation
and apoptosis assays.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The cells were seeded in 96-well microplates at a
density of 4×103 cells/well. Follwoing treatment, the
cells were subjected to viability analysis using an MTT assay. MTT
solution (5 mg/ml; Sigma, St. Louis, MO, USA) was added to each
well followed by incubation at 37°C for 4 h. Dimethyl sulfoxide
(DMSO) was added to dissolve the formazan crystals. The absorbance
was then measured at 570 nm using a a spectrophotometer (DU-640;
Beckman Coulter, Hialeah, FL, USA).
Apoptosis assay
Following treatment, the cells were harvested by
trypsinization, and apoptosis was detected using the Annexin V
apoptosis kit (Becton-Dickinson Biosciences, San Diego, CA, USA).
The cells were stained with fluorescein isothiocyanate-conjugated
Annexin V and propidium iodide (PI) solution (20 μg/ml) for
15 min in the dark. Apoptotic cells (Annexin V-positive) were
analyzed by flow cytometry (Becton-Dickinson Biosciences).
Measurement of mitochondrial membrane
potential (ΔΨm)
Changes in the ΔΨm were measured using the JC-1
mitochondrial membrane potential assay kit (Beyotime, Nantong,
China). JC-1 forms monomers that emit green fluorescence and JC-1
aggregates are marked by red fluorescence. The ratio of JC-1 green
to red fluorescence is proportional to the strength of ΔΨm. At 72 h
post-transfection, cells were collected by trypsinization and
pelleted. The cells were resuspended in JC-1 working solution and
incubated at 37°C for 15 min. After washing, the stained cells were
analyzed by flow cytometry (Becton-Dickinson Biosciences).
Measurement of caspase-3 and caspase-9
activity
The activity of caspase-3 and caspase-9 was measured
using the Caspase-3/9 Activity assay kit (Beyotime), according to
the manufacturer's instructions. Briefly,the transfected cells were
lysed and the lysates were incubated with reaction buffer, which
contained the fluorescent substrates,
N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DEVD-pNA) and
N-acetyl-Leu-Glu-His-Asp-p-nitroanilide (Ac-LEHD-pNA), for
caspase-3 and caspase-9, respectively. The fluorescence of the
cleaved substrates was determined using an SLM 8000 fluorometer
(SLM-Aminco, Urbana, IL, USA) at 405 nm.
Western blot analysis
For western blot analysis, the following primary
antibodies were used: rabbit anti-total STAT3 (sc-482),
anti-phosphorylated (p-)STAT3 (sc-8001-R), anti-cyclin D1 (sc-753),
anti-Mcl-1 (sc-819) and anti-survivin (sc-10811) polyclonal
antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
and mouse anti-β-actin (A5316) monoclonal antibody (Sigma).
Horseradish peroxidase (HRP)-conjugated goat anti-rabbit (#31460)
or anti-mouse (#31430) IgGs were purchased from Pierce
Biotechnology (Rockford, IL, USA). Following treatment, the cells
were lysed in RIPA buffer (Sigma) containing 1 mM
phenylmethanesulfonyl fluoride and complete protease inhibitors
(Roche, Mannheim Germany). The protein concentrations in cellular
lysates were measured using a BCA Protein assay kit (Thermo
Scientific, Rockford, IL, USA). The protein samples were separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto polyvinylidene fluoride membranes. The membranes
were then blocked with 5% fat-free milk and incubated with the
primary antibodies at 4°C overnight, followed by incubation with
HRP-conjugated secondary antibodies for 1 h. Immunoreactive bands
were visualized using an enhanced chemiluminescence detection
system (Amersham Biosciences, Piscataway, NJ, USA) and quantified
using Quantity One software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). Differences between groups were examined by one-way analysis
of variance using the SPSS software package v19.0 (SPSS, Inc.,
Chicago, IL, USA). A P-value <0.05 was considered to indicate a
statistically significantly difference.
Results
Overexpression of miR-31 inhibits the
viability of GBM cells
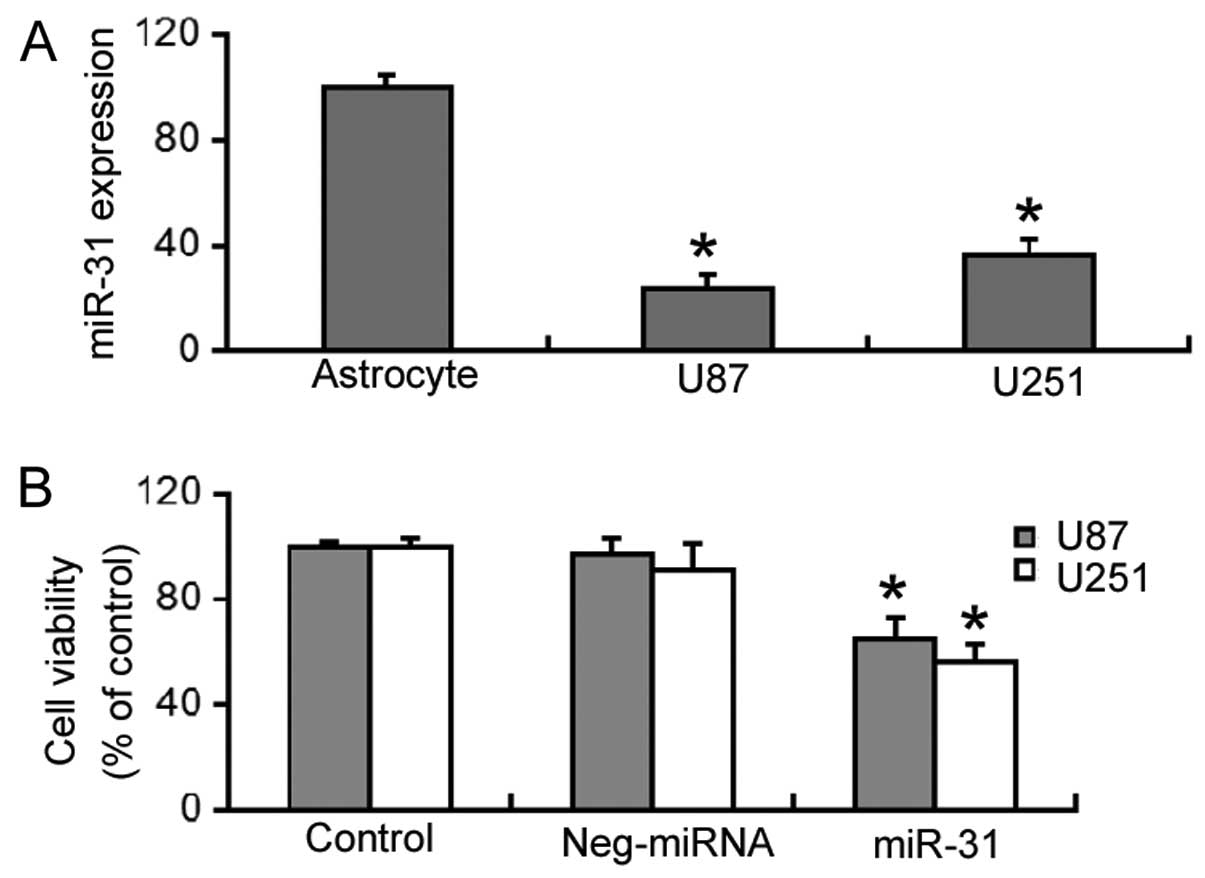
RT-qPCR revealed that miR-31 expression was
significantly decreased (P<0.05) in the U87 and U251 cells,
compared to normal human astrocytes (Fig. 1A). The effects of restoring miR-31
expression on the viability of GBM cells was then investigated. As
shown in Fig. 1B,
miR-31-overexpressing U87 and U251 cells had a significantly lower
viability (P<0.05) compared to the control (untransfected) or
negative miRNA-transfected cells.
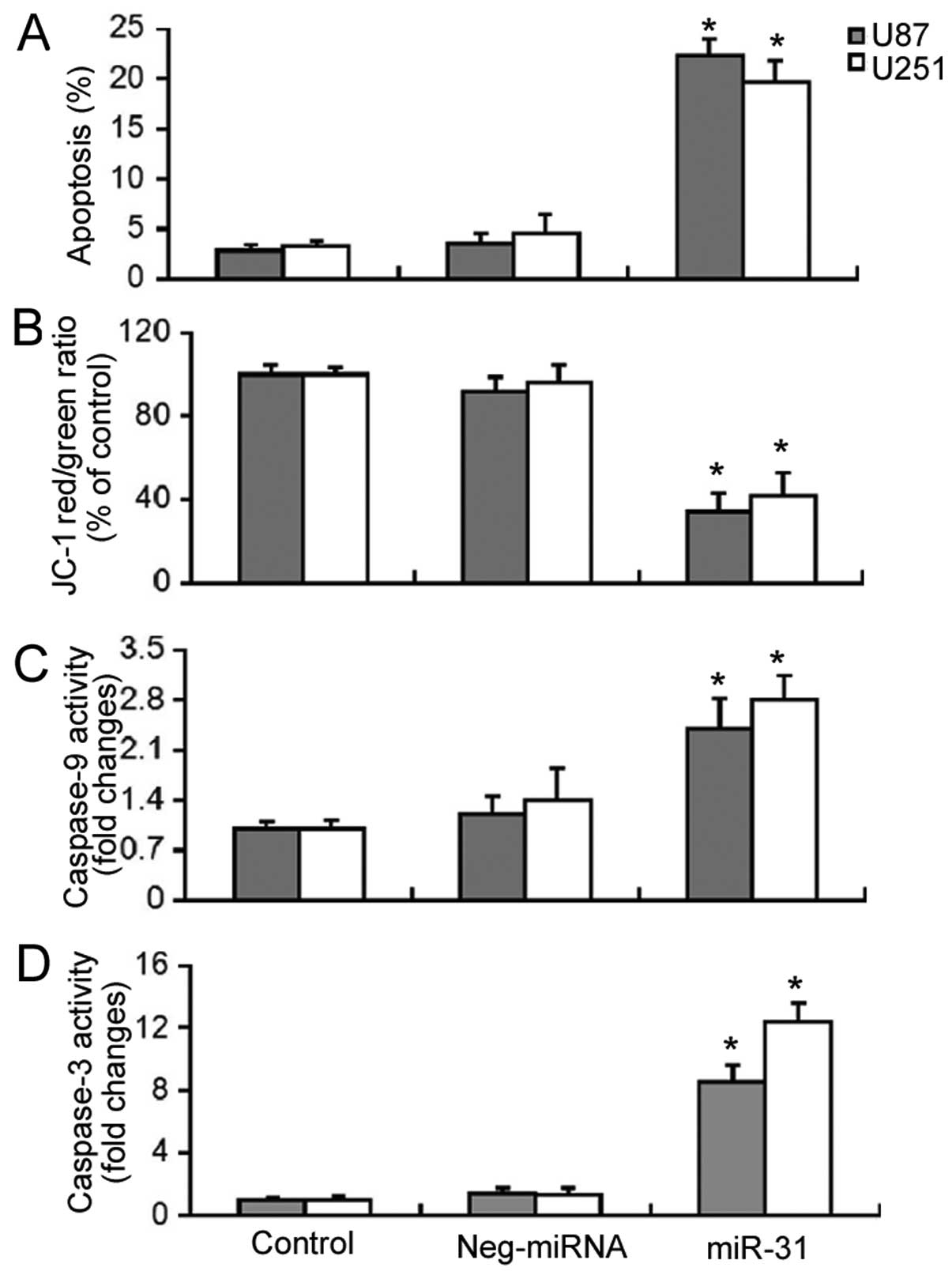
Overexpression of miR-31 significantly
increases the apoptosis of GBM cells through the mitochondrial
pathway
Subsequently, we examined the effects of miR-31
overexpression on the survival of GBM cells. Apoptosis detection by
Annexin V/PI staining demonstrated that the enforced expression of
miR-31 induced a significant increase (P<0.05) in the apoptosis
of the U87 and U251 cells (Fig.
2A). As the depolarization of ΔΨm is an early event in
mitochondrial-related apoptosis, we measured the changes in ΔΨm
induced by miR-31 overexpression using the JC-1 dye. As shown in
Fig. 2B, miR-31 overexpression
resulted in a significant decrease (P<0.05) in the red/green
fluorescence ratio, which was indicative of the loss of ΔΨm. Enzyme
activity assay revealed that there was a 2.4- and 2.8-fold increase
in caspase-9 activity and an 8.6- and 12.4-fold increase in
caspase-3 activity in miR-31-overexpressing U87 and U251 cells,
respectively (Fig. 2C and D).
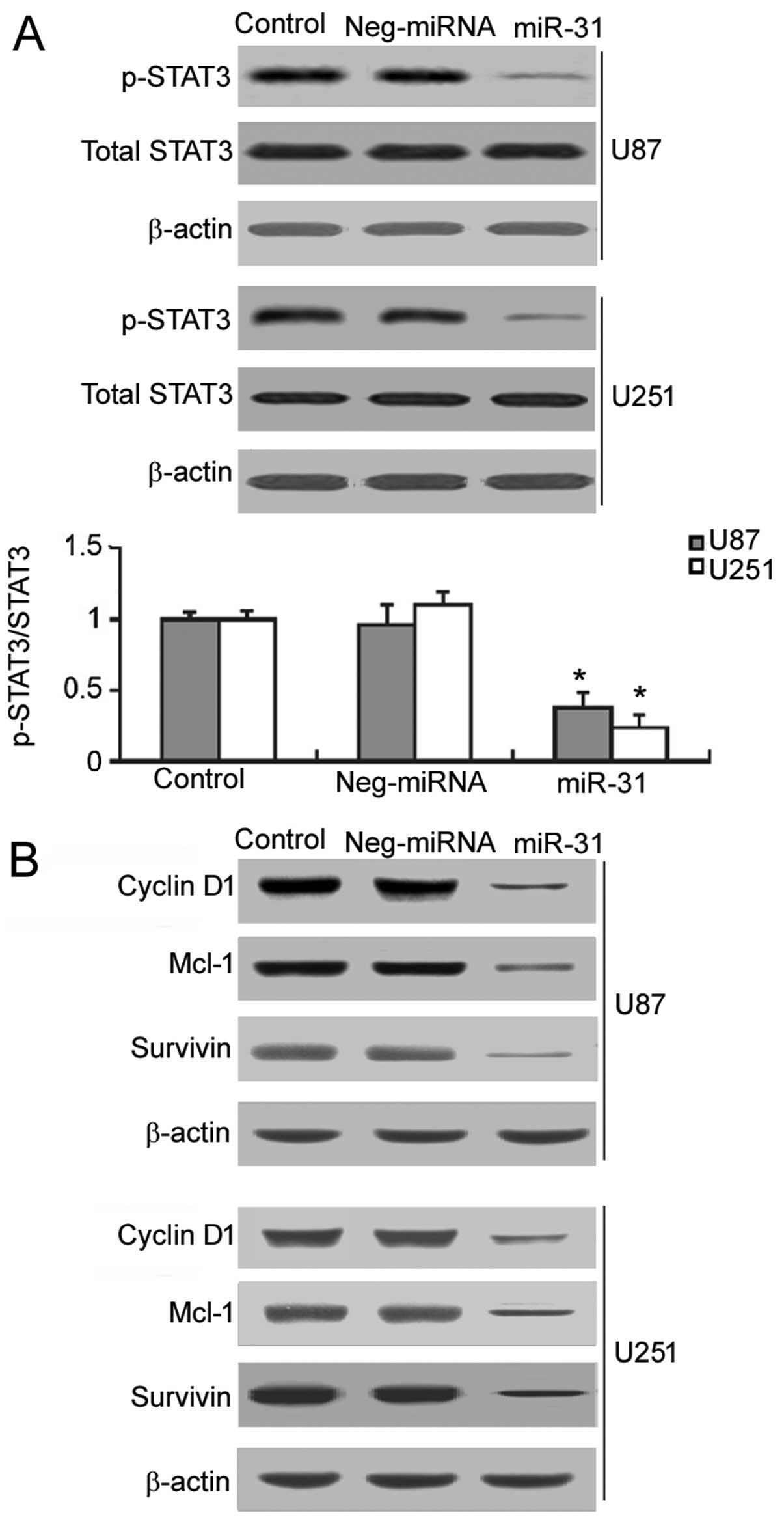
Restoration of miR-31 interferes with the
activation of STAT3 signaling in GBM cells
It is known that the constitutive activation of
STAT3 signaling contributes to GBM growth and survival (9). Thus, we examined the effects of
restoring miR-31 expression on the activation of STAT3 signaling.
As shown in Fig. 3A, in the
miR-31-overexpressing U87 and U251 cells, a significant decrease
(P<0.05) in the phosphorylation of STAT3 was noted, compared to
the controls and negative miRNA-transfected cells. We also examined
the effects of restoring miR-31expression on the expression of
target genes of STAT3, including cyclin D1, Mcl-1 and survivin.
Western blot analysis demonstrated that miR-31 overexpression
markedly inhibited the expression of cyclin D1, Mcl-1 and survivin
in the U87 and U251 cells (Fig.
3B).
miR-31 enhances the efficacy of TMZ in
GBM cells
We then investigated the effects of miR-31
overexpression on the sensitivity of GBM cells to TMZ. MTT assay
revealed that TMZ at 100 μM induced ~20% decrease in the
viability of the U87 and U251 cells after a 72-h incubation
(Fig. 4A). Notably, the delivery
of miR-31 significantly enhanced (P<0.05) the cytotoxic effects
of TMZ on GBM cells, leading to a 60–70% decrease in cell viability
(Fig. 4A). Similarly, the
overexpression of miR-31 significantly increased (P<0.05) the
apoptosis of the TMZ-treated GBM cells (Fig. 4B).
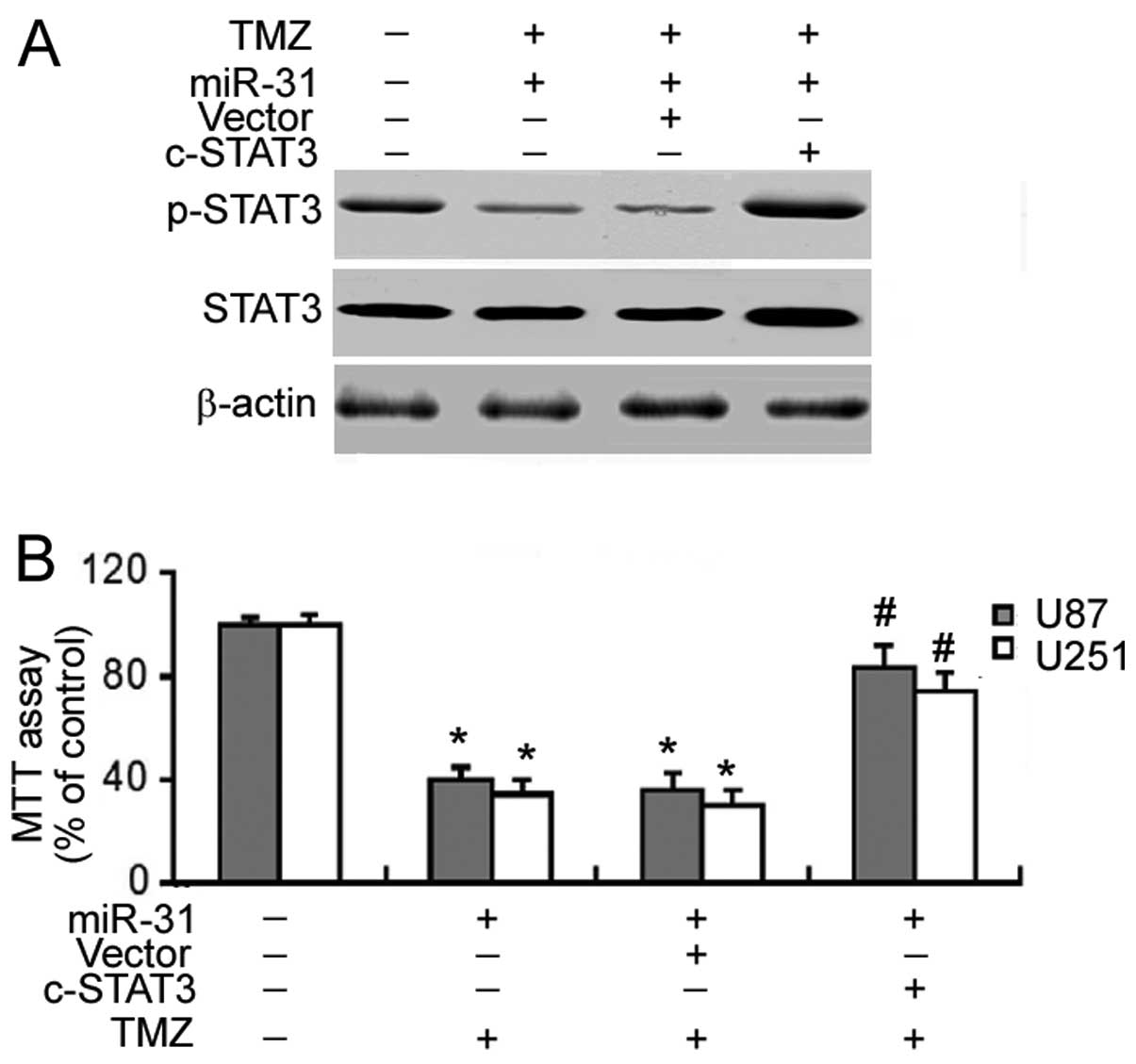
Chemosensitizing effect of miR-31 is
mediated through the inactivation of STAT3
Having concluded that STAT3 signaling was suppressed
by miR-31 overexpression, we examined the involvement of STAT3
signaling in miR-31-induced chemosensitization. Co-transfection
with a constitutively active STAT3 mutant markedly enhanced the
phosphorylation of STAT3 in the miR-31-transfected GBM cells
(Fig. 5A). Of note, the
constitutive activation of STAT3 significantly reversed (P<0.05)
the enhancement of TMZ cytotoxicity which was achieved by miR-31
overexpression (Fig. 5B).
Discussion
Certain miRs are deregulated in GBM and have been
implicated in tumor growth and survival (22,23). Yue et al (23) demonstrated that miR-205 was
significantly downregulated in human glioblastoma cells and that
the restoration of its expression induced apoptosis and cell cycle
arrest in glioma cells. Zhang et al (24) reported that miR-195 plays a
tumor-suppressor role in human glioblastoma cells, impairing cell
cycle progression and cellular invasion. Similarly, our data
demonstrated that miR-31 functions as a tumor suppressor in GBM, as
evidenced by the observation that the ectopic expression of miR-31
significantly suppressed GBM cell proliferation and induced
apoptosis. Our data further demonstrated that miR-31 induced the
apoptosis of GBM cells through the mitochondrial cascade, which was
manifested by the loss of ΔΨm and the activation of caspase-3 and
capsase-9. The tumor-suppressive effects of miR-31 have also been
documented in several other types of cancer, such as melanoma
(14) and breast cancer (25). However, there is also evidence
that miR-31 plays an oncogenic role in certain human tumors. For
instance, Mitamura et al (26) reported that miR-31 facilitates the
anchorage-independent growth and tumorigenesis of endometrial
cancer cells. In non-small cell lung cancer cells, it was
demonstrated that miR-31 has the ability to inhibit
cisplatin-induced apoptosis (27). Therefore, these data suggest that
miR-31 regulates tumor growth and survival in a cancer
type-dependent manner.
The constitutive activation of Stat3STAT3 plays an
important role in the development and progression of GBM (9). It has been reported that the nuclear
factor-κB (NF-κB)-induced STAT3 activation contributes to
aggressive phenotypes in GBM and that the inhibition of STAT3
signaling retards the growth of human GBM xenografts (28). The pharmacological inhibition of
STAT3 signaling has been found to induce the apoptosis of human GBM
cells (29). Of note, our data
revealed that the overexpression of miR-31 impaired the activation
of STAT3. Moreover, the ectopic expression of miR-31 markedly
downregulated multiple STAT3 target genes, including cyclin D1,
Mcl-1 and survivin in GBM cells. The downregulation of cyclin D1 is
linked to the reduced proliferation and enhanced apoptosis of
glioblastoma cells (30). Mcl-1
and survivin are two key pro-survival proteins, and their
inhibition contributes to the apoptosis of GBM cells through the
mitochondrial death pathway (31). These studies, combined with our
findings, suggest that STAT3 signaling is involved in the
tumor-suppressive activity of miR-31 in GBM.
Previous research corroborates the importance of
STAT3 signaling in the development of resistance to TMZ in GBM
(32). Kohsaka et al
(10) reported that the
inhibition of STAT3 signaling can helpt overcome resistance to TMZ
in glioblastoma. Given our knowledge of the regulation of STAT3
activation by miR-31, in this study, we examined the effects of
miR-31 on TMZ chemosensitivity in GBM cells. Notably, we found that
miR-31 overexpression significantly enhanced TMZ cytotoxicity to
GBM cells. Moreover, the enforced expression of miR-31 enhanced the
apoptosis of GBM cells in the presence of TMZ. The miR-31-mediated
chemosensitization to TMZ was reversed by co-transfection with a
constitutively active STAT3 mutant. Taken together, these data
suggest that the restoration of miR-31 expression sensitizes GBM
cells to TMZ largely through the suppression of STAT3 activation.
The chemosensitizing activity of miR-31 has also been described in
relation to ovarian cancer cells, where the re-introduction of
miR31 was shwn to re-sensitize paclitaxel-resistant cells to this
agent (33).
In conclusion, our data confirm that miR-31
functions as a tumor suppressor gene in GBM. The restoration of
miR-31 expression induced the apoptosis of and enhanced TMZ
cytotoxicity in human GBM cells, which was mediated through the
suppression of STAT3 activation. The re-expression of miR-31 may
thus represent a promising strategy for improving the efficacy of
TMZ in GBM.
References
|
1
|
Wilson TA, Karajannis MA and Harter DH:
Glioblastoma multiforme: State of the art and future therapeutics.
Surg Neurol Int. 5:642014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alifieris C and Trafalis DT: Glioblastoma
multiforme: Pathogenesis and treatment. Pharmacol Ther. 152:63–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hart MG, Garside R, Rogers G, Stein K and
Grant R: Temozolomide for high grade glioma. Cochrane Database Syst
Rev. 4:CD0074152013.PubMed/NCBI
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hau P, Koch D, Hundsberger T, Marg E,
Bauer B, Rudolph R, Rauch M, Brenner A, Rieckmann P, Schuth J, et
al: Safety and feasibility of long-term temozolomide treatment in
patients with high-grade glioma. Neurology. 68:688–690. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
do Carmo A, Balça-Silva J, Matias D and
Lopes MC: PKC signaling in glioblastoma. Cancer Biol Ther.
14:287–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan QW and Weiss WA: Targeting the
RTK-PI3K-mTOR axis in malignant glioma: overcoming resistance. Curr
Top Microbiol Immunol. 347:279–296. 2010.PubMed/NCBI
|
|
9
|
Luwor RB, Stylli SS and Kaye AH: The role
of Stat3 in glioblastoma multiforme. J Clin Neurosci. 20:907–911.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kohsaka S, Wang L, Yachi K, Mahabir R,
Narita T, Itoh T, Tanino M, Kimura T, Nishihara H and Tanaka S:
STAT3 inhibition overcomes temozolomide resistance in glioblastoma
by downregulating MGMT expression. Mol Cancer Ther. 11:1289–1299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fabbri M: MicroRNAs and cancer: towards a
personalized medicine. Curr Mol Med. 13:751–756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Williams AE: Functional aspects of animal
microRNAs. Cell Mol Life Sci. 65:545–562. 2008. View Article : Google Scholar
|
|
13
|
Wang S, Li Q, Wang K, Dai Y, Yang J, Xue
S, Han F, Zhang Q, Liu J and Wu W: Decreased expression of
microRNA-31 associates with aggressive tumor progression and poor
prognosis in patients with bladder cancer. Clin Transl Oncol.
15:849–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asangani IA, Harms PW, Dodson L, Pandhi M,
Kunju LP, Maher CA, Fullen DR, Johnson TM, Giordano TJ, Palanisamy
N and Chinnaiyan AM: Genetic and epigenetic loss of microRNA-31
leads to feed-forward expression of EZH2 in melanoma. Oncotarget.
3:1011–1025. 2012.PubMed/NCBI
|
|
15
|
Nosho K, Igarashi H, Nojima M, Ito M,
Maruyama R, Yoshii S, Naito T, Sukawa Y, Mikami M, Sumioka W, et
al: Association of microRNA-31 with BRAF mutation, colorectal
cancer survival and serrated pathway. Carcinogenesis. 35:776–783.
2014. View Article : Google Scholar
|
|
16
|
Wang S, Jiao B, Geng S, Song J, Liang Z
and Lu S: Concomitant microRNA-31 downregulation and radixin
upregulation predicts advanced tumor progression and unfavorable
prognosis in patients with gliomas. J Neurol Sci. 338:71–76. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li T, Luo W, Liu K, Lv X and Xi T: miR-31
promotes proliferation of colon cancer cells by targeting E2F2.
Biotechnol Lett. 37:523–532. 2015. View Article : Google Scholar
|
|
18
|
Huang S, Wu B, Li D, Zhou W, Deng G, Zhang
K and Li Y: Knockdown of astrocyte elevated gene-1 inhibits tumor
growth and modifies microRNAs expression profiles in human
colorectal cancer cells. Biochem Biophys Res Commun. 444:338–345.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
20
|
Pilati C, Amessou M, Bihl MP, Balabaud C,
Nhieu JT, Paradis V, Nault JC, Izard T, Bioulac-Sage P, Couchy G,
et al: Somatic mutations activating STAT3 in human inflammatory
hepatocellular adenomas. J Exp Med. 208:1359–1366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Filippi-Chiela EC, Thomé MP, Bueno e Silva
MM, Pelegrini AL, Ledur PF, Garicochea B, Zamin LL and Lenz G:
Resveratrol abrogates the temozolomide-induced G2 arrest leading to
mitotic catastrophe and reinforces the temozolomide-induced
senescence in glioma cells. BMC Cancer. 13:1472013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Wang X, Wang H, Li Y, Yan W, Han
L, Zhang K, Zhang J, Wang Y, Feng Y, et al: miR-137 is frequently
downregulated in glioblastoma and is a negative regulator of Cox-2.
Eur J Cancer. 48:3104–3111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yue X, Wang P, Xu J, Zhu Y, Sun G, Pang Q
and Tao R: MicroRNA-205 functions as a tumor suppressor in human
glioblastoma cells by targeting VEGF-A. Oncol Rep. 27:1200–1206.
2012.
|
|
24
|
Zhang QQ, Xu H, Huang MB, Ma LM, Huang QJ,
Yao Q, Zhou H and Qu LH: MicroRNA-195 plays a tumor-suppressor role
in human glioblastoma cells by targeting signaling pathways
involved in cellular proliferation and invasion. Neuro Oncol.
14:278–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Körner C, Keklikoglou I, Bender C, Wörner
A, Münstermann E and Wiemann S: MicroRNA-31 sensitizes human breast
cells to apoptosis by direct targeting of protein kinase C epsilon
(PKCepsilon). J Biol Chem. 288:8750–8761. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mitamura T, Watari H, Wang L, Kanno H,
Kitagawa M, Hassan MK, Kimura T, Tanino M, Nishihara H, Tanaka S
and Sakuragi N: microRNA 31 functions as an endometrial cancer
oncogene by suppressing Hippo tumor suppressor pathway. Mol Cancer.
13:972014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong Z, Zhong Z, Yang L, Wang S and Gong
Z: MicroRNA-31 inhibits cisplatin-induced apoptosis in non-small
cell lung cancer cells by regulating the drug transporter ABCB9.
Cancer Lett. 343:249–257. 2014. View Article : Google Scholar
|
|
28
|
McFarland BC, Hong SW, Rajbhandari R,
Twitty GB Jr, Gray GK, Yu H, Benveniste EN and Nozell SE:
NF-κB-induced IL-6 ensures STAT3 activation and tumor
aggressiveness in glioblastoma. PLoS One. 8:e787282013. View Article : Google Scholar
|
|
29
|
Swiatek-Machado K, Mieczkowski J,
Ellert-Miklaszewska A, Swierk P, Fokt I, Szymanski S, Skora S,
Szeja W, Grynkiewicz G, Lesyng B, et al: Novel small molecular
inhibitors disrupt the JAK/STAT3 and FAK signaling pathways and
exhibit a potent antitumor activity in glioma cells. Cancer Biol
Ther. 13:657–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Wang Q, Cui Y, Liu ZY, Zhao W,
Wang CL, Dong Y, Hou L, Hu G, Luo C, et al: Knockdown of cyclin D1
inhibits proliferation, induces apoptosis, and attenuates the
invasive capacity of human glioblastoma cells. J Neurooncol.
106:473–484. 2012. View Article : Google Scholar
|
|
31
|
Premkumar DR, Jane EP, Foster KA and
Pollack IF: Survivin inhibitor YM-155 sensitizes tumor necrosis
factor- related apoptosis-inducing ligand-resistant glioma cells to
apoptosis through Mcl-1 downregulation and by engaging the
mitochondrial death pathway. J Pharmacol Exp Ther. 346:201–210.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lo HW, Cao X, Zhu H and Ali-Osman F:
Constitutively activated STAT3 frequently coexpresses with
epidermal growth factor receptor in high-grade gliomas and
targeting STAT3 sensitizes them to Iressa and alkylators. Clin
Cancer Res. 14:6042–6054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mitamura T, Watari H, Wang L, Kanno H,
Hassan MK, Miyazaki M, Katoh Y, Kimura T, Tanino M, Nishihara H, et
al: Downregulation of miRNA-31 induces taxane resistance in ovarian
cancer cells through increase of receptor tyrosine kinase MET.
Oncogenesis. 2:e402013. View Article : Google Scholar : PubMed/NCBI
|