Introduction
Psoriasis is a chronic, relapsing, papulosquamous
dermatitis characterized by abnormal hyperproliferation of the
epidermis. It affects approximately 2% of the population and all
racial groups (1,2). The prominent cutaneous
manifestations of psoriasis present as raised, well-demarcated,
erythematous plaques with adherent silvery scales. The scales
result from the hyperproliferative epidermis, the premature
maturation of keratinocytes, as well as the incomplete
cornification and retention of nuclei in the stratum corneum
(parakeratosis). The mitotic rate of basal keratinocytes in
psoriatic lesions is significantly increased compared with normal
skin (1). Consequently, psoriasis
has long been considered only a disease of the keratinocytes which
involves basal cell hyperproliferation (3,4).
However, substantial advances have been made in terms of
elucidating the molecular mechanisms of psoriasis, and previous
studies have demonstrated that the disease is a disorder resulting
from the dysregulated interplay between keratinocytes and
infiltrating immune cells (5–7).
In previous research, an array of pro-inflammatory
cytokines have been detected in psoriatic skin lesions, which have
been demonstrated to act as major drivers of acanthosis in
psoriasis. Of these cytokines, interleukin (IL)-17A and IL-22
appear to be the most active cytokines in the immunopathogenesis of
psoriasis (8) and also in cases
of imiquimod (IMQ)-induced psoriasiform dermatitis in mice
(3,6). The functional receptors (IL-17RA and
IL-22R) for those cytokines are constitutively expressed on the
surface of keratinocytes (8,9).
Increased production of IL-17A and IL-22, through infiltration of
Th17 cells, has been reported in psoriatic skin lesions. These
cytokines act on keratinocytes by binding to their cognate
receptors, activating the basal keratinocytes from a quiescent
state into a hyperproliferative state, retarding the terminal
differentiation of keratinocytes, and driving the infiltration of
inflammatory cells such as neutrophils into the epidermis (9). Intradermal injection of recombinant
IL-17A and IL-22 in a mouse model of allogeneic skin-humanized
psoriasis resulted in epidermal hyperplasia and mixed inflammatory
cell infiltrates, features which closely resembled the majority of
those of human psoriasis (10).
The epidermis is an avascular and multilayered
epithelium composed of a single layer of proliferative basal cells
and several suprabasal (or spinous) layers of differentiated
keratinocytes (11). It has been
noted that there are two distinct subpopulations of proliferative
keratinocytes in the basal layer: stem cells, which have an
unlimited capacity for self-renewal (but are thought to proliferate
infrequently and to be generally quiescent) and also
transit-amplifying (TA) cells (the descendants of stem cells, which
are destined to withdraw from the cell cycle and terminally
differentiate after a few rounds of division) (12–15). It is tempting to hypothesize that
activated stem cells give rise to the extreme expansion of TA cells
in the psoriatic epidermis, but it remains uncertain whether
hyperproliferative psoriatic keratinocytes causes the exhaustion
and/or reduction of the stem cell pool. Our aims for the present
study were as follows: i) to investigate the asymmetric cell
division of trypsin-dissociated human psoriatic keratinocytes and
the proportion of mitotic basal cells in the mouse model of
dermatitis induced by the immune activator IMQ, using pulse-chase
labeling with bromodeoxyuridine (BrdU) to understand the transition
of stem cells to TA cells as well as post-mitotic (PM) cells; and
ii) to examine the effects of the Th17-derived cytokines IL-17A and
IL-22 on the expression of differentiation-related markers both in
keratinocytes and in passage-one epidermal cell sheets derived from
skin explants (16), which are of
value in determining the role of the maintenance of the stem-cell
pool in the pro-inflammatory cytokine-enriched milieu of psoriatic
epidermis.
Materials and methods
Chemicals, cytokines and antibodies
All chemicals were obtained from Sigma-Aldrich (St.
Louis, MO, USA) unless otherwise stated. Recombinant human IL-17A
and IL-22 were purchased from PeproTech (Rocky Hill, NJ, USA). The
following monoclonal antibodies (mAbs) were used: anti-BrdU
antibody (BU-33; B2531) was obtained from Sigma-Aldrich, anti-BrdU
antibody conjugated with FITC (ab74545) was purchased from Abcam
(Cambridge, MA, USA), antibodies against differentiation-specific
markers, namely K15 (ab52816; Abcam), K10 (SC31770; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), integrin β1/FITC
(ab46920), filaggrin (ab24584), and Ki67 (ab16667) were all
purchased from Abcam. Secondary antibodies conjugated with Alexa
Fluor 488 (green; A11001) or 555 (red; A21432) were purchased from
Molecular Probes (Eugene, OR, USA). Mowiol 4–88 anti-fade mounting
solution was obtained from Polyscience, Inc. (Warrington, PA,
USA).
Patients
Eleven patients suffering from psoriasis vulgaris
and ten healthy controls were enrolled in this study. The clinical
and demographic characteristics of the subjects included in this
study were summarized. Certain patients had a family history of the
disease (data not shown). At the time that skin biopsies were
taken, medications had been discontinued for several months.
Samples were obtained under local anesthesia from psoriasis skin
plaques using a 6-mm biopsy punch. All patients were recruited from
the Department of Dermatology (Renmin Hospital of Wuhan University,
Wuhan, China), and normal skin samples were collected from patients
who underwent breast reconstruction in the Department of Plastic
Surgery (Renmin Hospital of Wuhan University). This study was
conducted in accordance with the Declaration of Helsinki
Principles, and was approved by the Ethics Committee of Renmin
Hospital of Wuhan University.
Mouse model and BrdU injections
BALB/c male mice (8–11 weeks old) were purchased
from the animal facility of Hubei Provincial Center for Disease
Control and Prevention (CDC; Wuhan, China), raised in pathogen-free
conditions and provided with food and water ad libitum. All
experiments were approved by the Animal Ethics Committee according
to Chinese legislation on animal experiments. Mice were treated
daily with commercially available IMQ cream (5%) (Aldara; 3M
Pharmaceuticals, Minneapolis, MN, USA), which induced psoriasiform
dermatitis, on shaved dorsal skin, or treated with a control cream
on shaved dorsal skin for 6 consecutive days as previously reported
(6). BrdU incorporation was
performed to monitor the mitotic activity of basal cells in the
inflamed skin. A single dose of BrdU at 50 µg/gram body
weight was administered to mice by intraperitoneal injection 2 h
prior to mice being sacrificed by carbon dioxide inhalation. Dorsal
skin was biopsied and fixed in buffered formalin and embedded in
paraffin. Five-micrometer sections were cut on a microtome and
stained with mouse-anti-BrdU (Sigma-Aldrich) and a commercially
available streptavidin-peroxidase (SP) staining kit (Maixin-Bio,
Fuzhou, China) in order to visualize BrdU immunostaining in the
paraffin-embedded sections.
Cell cultures and treatments
As previously described (17), the skin tissue was trimmed to
remove subcutaneous adipose tissue and cut into small strips. These
skin strips were soaked in Dulbecco's modified Eagle's medium
(DMEM) (Gibco, Carlsbad, CA, USA) containing 0.25% dispase
(Sigma-Aldrich) and digested overnight at 4°C. The following day,
the epidermis was peeled off and further dissociated after simple
digestion in 0.25% trypsin. Single cell cultures were obtained by
passing the cell suspension through a nylon cell strainer with
70-µm pore size (Becton-Dickinson, Franklin Lakes, NJ, USA)
and then washing with PBS. Primary cells were maintained in EpiLife
keratinocyte medium with 1% (v/v) human keratinocyte growth
supplement (both from Invitrogen, Camarillo, CA, USA) and 60
µM calcium. Cells at an early passage (≤5) were used for all
experiments. To analyze the expression pattern of
differentiation-related proteins, the calcium concentration was
increased to 1.3 mM in cultures. The primary cells were also
stimulated with recombinant IL-17A (10 ng/ml) and IL-22 (10 ng/ml)
for 24 h before protein extraction (17,18).
Pulse-chase BrdU labeling to assess
asymmetric cell division
For labeling of cells in culture, the cells were
plated at a low density (~10–20 cells/mm2) in 6-well
tissue culture plates which contained a collagen
(Sigma-Aldrich)-coated coverslip in each well, as previously
described (19,20). After cells had attached, 10
µM BrdU (B9285; Sigma-Aldrich) was added for 30 h of
labeling. Subsequently, cell cultures were washed with PBS twice
and replaced with fresh medium containing 5 µM cytochalasin
D (C8273; Sigma-Aldrich) to arrest cytokinesis. After a chase
period of 30 h, cells on the coverslips were fixed with 4%
paraformaldehyde solution for subsequent BrdU immunostaining.
Preparation of passage-one epidermal cell
sheets
Passage one epidermal cell sheets were prepared
following a previously reported protocol (16). Briefly, skin tissue samples were
trimmed to remove subcutaneous tissue and were further cut into
small strips using ophthalmologic scissors. Skin strips were placed
on glass coverslips at the bottom of 6-well plates with the
epidermal side facing upwards. After allowing the skin pieces to
firmly attach to the coverslips, DMEM containing 20% fetal bovine
serum (FBS) and antibiotics (0.625 µg/ml amphotericin B, 100
IU/ml penicillin and 100 µg/ml streptomycin) were carefully
added to the plates, ensuring no tissue pieces floated upwards.
After 3–5 days of cultivation, passage one epidermal cells had
grown out from the edge of each skin explant to form a tongue-like
epidermal sheet, as visualized in Fig. 6A. The epidermal sheet cultures
were treated with recombinant IL-17A (10 ng/ml) and IL-22 (10
ng/ml) for 24 h to further analyze the expression pattern of
differentiation-related proteins. Finally, the skin pieces were
removed gently using an ophthalmologic forceps and fixed with 4%
paraformaldehyde solution for subsequent immunofluorescence
staining.
Immunohistochemical and
immunofluorescence assays
In the present study, formalin-fixed paraffin
sections (5 µM) were dewaxed by melting for 30–60 min at
60°C, and they were cleared in xylene 3 times for 5 min each and
then rehydrated in water bath solutions containing decreasing
percentages of ethanol. The sections were then treated to inhibit
endogenous peroxidases, blocked and incubated overnight at 4°C with
specific primary antibodies against proliferation and/or
differentiation-related protein markers: anti-BrdU (dilution
1:100), anti-K15 (1:100), anti-K10 (1:100), anti-integrin β1
(1:50), anti-filaggrin (1:100) and anti-Ki67 (1:100). After
incubation with the primary antibodies, sections were rinsed with
Tris-buffered solution (TBS) and were then incubated with a
biotinylated secondary antibody (dilution 1:2,000; Maixin-Bio,
Fuzhou, China) for 1 h. The immune signals were detected using a
commercially available SP staining kit (Maixin-Bio). Sections were
counterstained with hematoxylin and eosin and mounted. The number
of positively immunostained cells in the basal layers (aside from
K10 and Ki67 in the suprabasal layer) was counted from at least 6
randomly selected fields under a magnification of x400 and
expressed as a percentage of the total cells counted. For BrdU
immunofluorescence staining, cell cultures were fixed in 70%
ethanol, washed with PBS, and incubated with 4 M HCl for 10 min at
room temperature, and then washed 3 times with PBS containing 1%
FBS and 0.01% sodium azide. They were then incubated with
anti-BrdU/FITC antibody for 45 min at 37°C. The sections were
counterstained with 4′,6-diamidino-2-phenylindole (DAPI)
(Invitrogen) or propidium iodide (Sigma-Aldrich) to identify the
nuclei. The routine protocol was also carried out for the detection
of intracellular and extracellular epitopes, as detailed in the
figure legends. Double immunofluorescence was performed with
anti-BrdU/FITC (dilution 1:100) and anti-K15 (1:50). Slides were
mounted with Mowiol 4–88 anti-fade mounting solution.
Immunostaining was subsequently observed using an Olympus IX71
fluorescence microscope.
Western blotting
Cells were washed in PBS and lysed in extraction
buffer containing 1% Nonidet P-40, 0.01% SDS and a protease
inhibitor cocktail (Roche, Indianapolis, IN, USA). Protein content
was determined with a BCA assay kit (Pierce, Rockford, IL, USA).
Equal amounts of each protein extract (30 µg/lane) were then
resolved by using 6% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). Following blotting onto Immobilon-P
membranes (Millipore, Bedford, MA, USA) and blocking with 5%
non-fat milk in saline buffer, the membranes were incubated with
anti-K15, anti-K10, anti-integrin, anti-filaggrin, and anti-Ki67,
each at a 1:2,000 dilution, or with an anti-GAPDH antibody (Santa
Cruz Biotechnology, Inc.) at a dilution of 1:1,000, for 1 h at room
temperature. The membranes were then washed and incubated with
horseradish-peroxidase-conjugated anti-rabbit IgG (Pierce) at a
dilution of 1:2,000 for 1 h at room temperature. Membranes were
then washed again, and specific bands were visualized using
enhanced chemiluminescence (ECL) reaction (Amersham, Piscataway,
NJ, USA).
Statistical analysis
The Student's t-test was used to compare the average
band intensities resulting from western blotting, the average
epidermal thickness and the average cell-pair number of asymmetric
cell divisions. Epidermal thickness of the dorsal skin was
quantifed using ImageJ densitometry software (NIH, Bethesda, MD,
USA) (21). Groups were compared
using ANOVA. In the present study, a P-value <0.05 (5%) was
considered to indicate a statistically significant difference and
was denoted with asterisks. SPSS software for Windows version 11.0
was used to analyze the results.
Results
Aberrant activation of basal stem cells
causes excessive expansion of the TA cell compartment in the
psoriatic epidermis
To fully characterize the histological alterations
of psoriatic TA cell expansion, we first analyzed the expression of
phenotypic markers relevant to proliferation and differentiation in
psoriatic and normal skin samples. We used immunohistochemical
analysis and a panel of specific antibodies against putative stem
cells (K15 and integrin β1), TA cells (integrin β1) and PM cells
(K10) as well as a cellular proliferative marker (Ki67) (Fig. 1). The immunostaining results
listed in Table I indicate the
percentage of positive cells in the psoriatic lesions and the
normal epidermis. K15 expression was significantly decreased in the
psoriatic epidermis (11.7±3.2%) compared with normal skin
(43.3±12.6%, P<0.05). Immunostaining for Ki67 revealed a
markedly increased proportion of stained cells in the suprabasal
layer, but the emergence of K10-positive keratinocytes was
postponed until they reached the granular layer. By contrast to the
immunostaining pattern of K10 in the psoriatic epidermis, intensive
staining for K10 was clear in the entire suprabasal layer in the
normal epidermis, aside from the single basal cell layer that did
not express K10. These findings suggest that excessive expansion of
the TA cell population existed in the psoriatic epidermis, which
may have exhausted the stem cell pool to some extent since
K15-positive cells were barely detectable in the psoriatic basal
layer. Considering that in vivo lineage tracing to directly
monitor changes of the stem cell pool is not feasible in humans
(12), we thus measured the
number of mitotic basal cells using BrdU labeling in the mouse
model of IMQ-induced dermatitis (Fig.
2), which is an animal model simulating some clinical features
of human psoriasis (6).
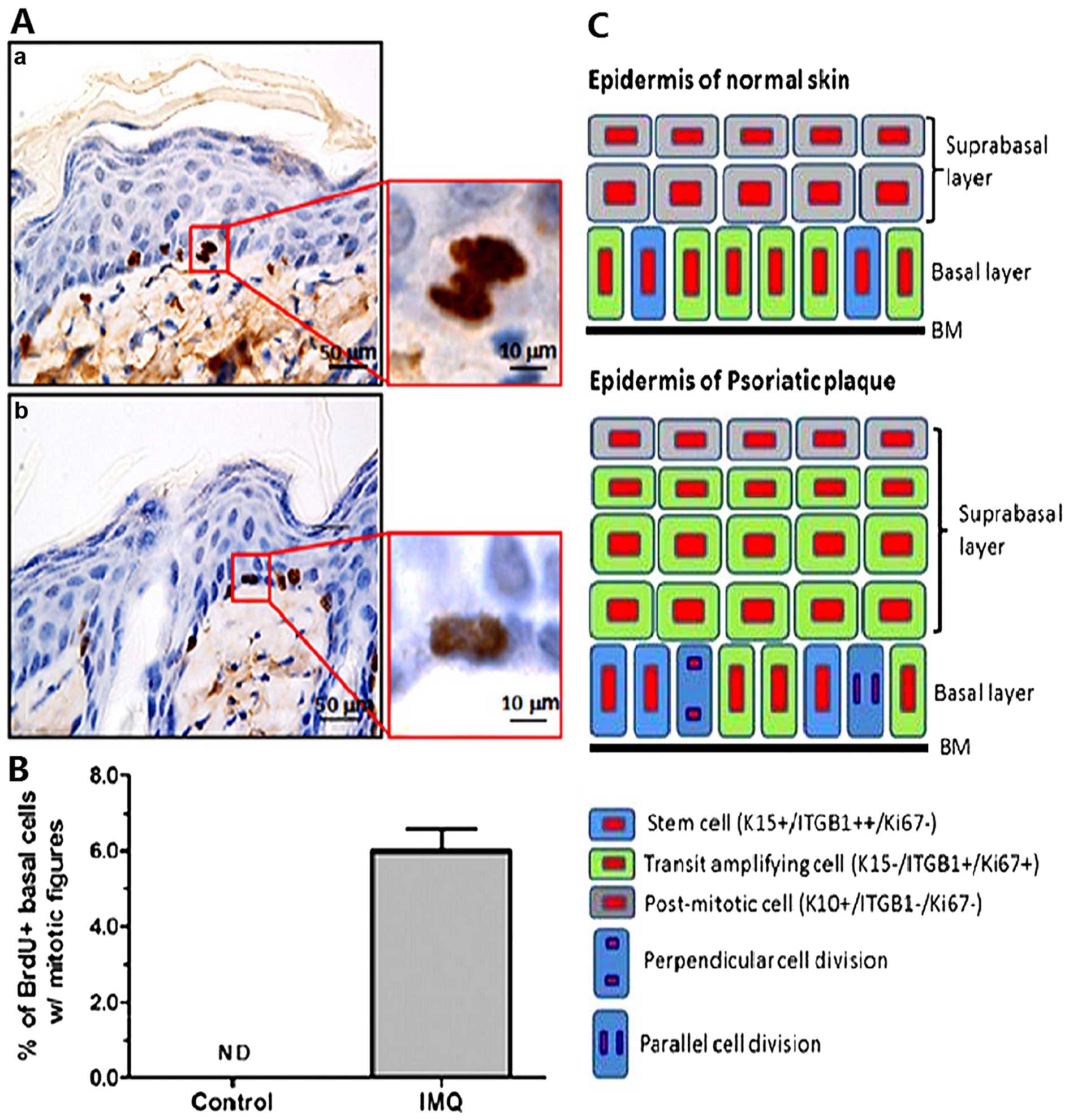
Representative stained images of BrdU-labeled basal cells are shown
in Fig. 3. A proportion (6%) of
BrdU-labeled mitotic basal cells was easily detected in the
inflamed skin of the mice, but those cells were negligible in the
control mice. Interestingly, two types of asymmetric cell division,
perpendicular and parallel (17),
were clearly discerned in BrdU-labeled basal cells (Fig. 3). These data indicate that the
quiescent basal cells become activated to undergo cell division,
which may serve as a prelude to epidermal hyperplasia in this model
of psoriasis.
 | Table IImmunostaining analysis of
proliferation and differentiation of keratinocytes in the psoriatic
and normal epidermis (means ± SEM). |
Table I
Immunostaining analysis of
proliferation and differentiation of keratinocytes in the psoriatic
and normal epidermis (means ± SEM).
| Groups | K15 (%) | ITGB1 (%) | K10 (%) | Ki67 (%) |
|---|
| Normal skin | 43.3±12.6 | 94.6±6.6 | 93.3±4.1 | 22.3±8.0 |
| Psoriatic
lesion | 11.7±3.2 | 33.6±9.7 | 60.6±15.5 | 53.6±8.7 |
| P-value | 0.025 | 0.0008 | 0.036 | 0.029 |
Both symmetric and asymmetric cell
division is increased in psoriatic epidermal cells
Based on the above observation that K15
immunostaining was significantly reduced in psoriatic basal cells,
we further examined whether there was a reduction of the stem cell
pool in cases of psoriasis. It should be noted, however, that one
may speculate that decreased K15 expression contributes to the
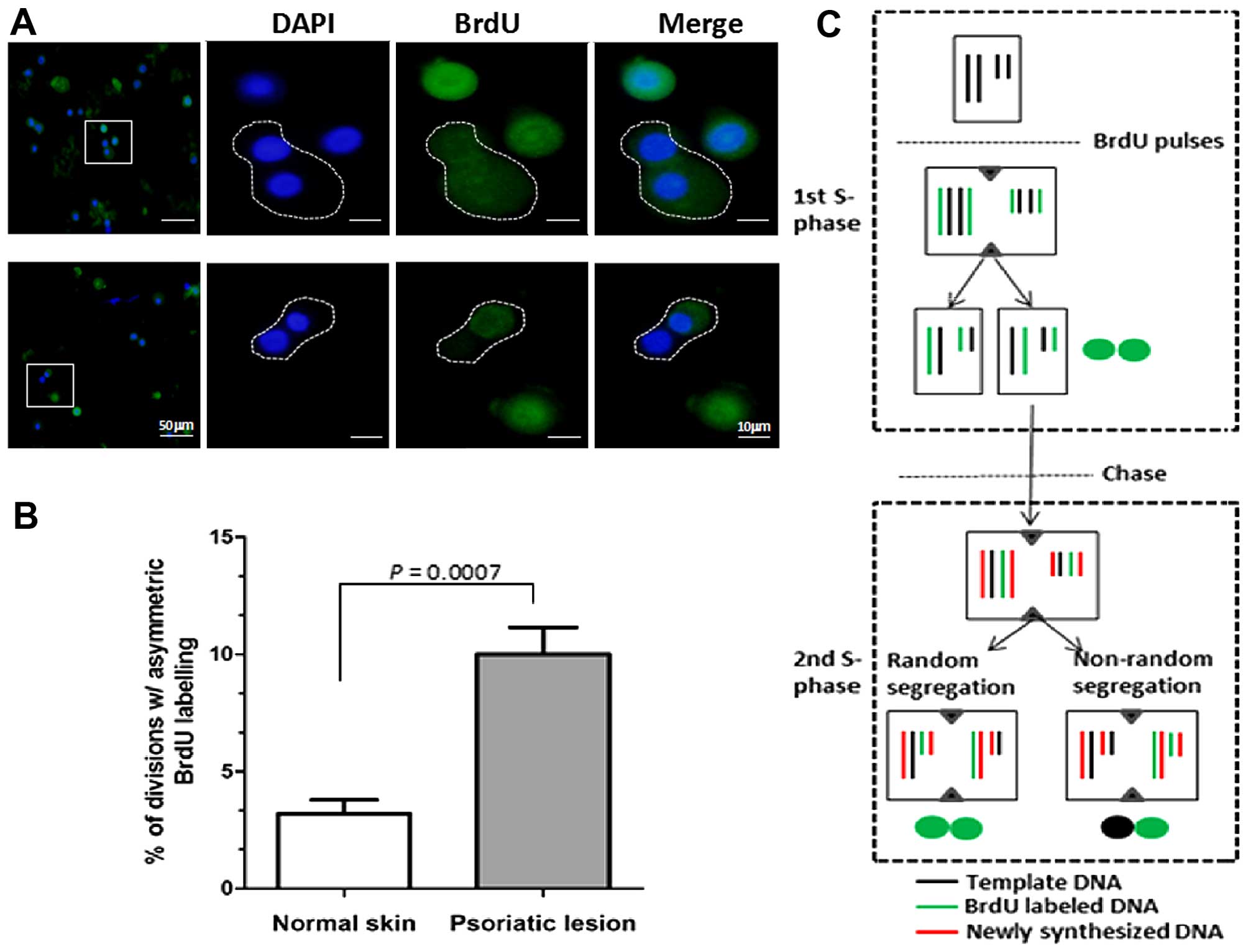
active states of stem cells (22,23). First, the BrdU pulse-chase
labeling protocol was employed to identify template-DNA strand
segregation during mitosis in primary cultures of psoriatic
epidermal cells. In general, daughter cells inheriting the older
template DNA are considered stem cells according to the immortal
strand hypothesis (19,20). The data demonstrated that an
increased proportion (~10%) of asymmetric segregation of BrdU was
noted in the cell pairs of psoriatic epidermal cells, whereas only
3% was noted in normal epidermal cells (P<0.001) (Fig. 4). This suggests that hyperactive
asymmetric stem cell division exists in cultured psoriatic
keratinocytes. Secondly, the correlation between asymmetric cell
division and the transition from stem cells to TA cells was also
investigated by examination of BrdU pulse-chased anaphase cells
using dual immunostaining for both BrdU segregation and K15
expression. The results showed that primary keratinocytes underwent
asymmetric division in which the template DNA (unlabeled) always
segregated to the daughter cell that retained K15, as shown in
Fig. 5A. The percentage (~13%) of
cells expressing K15 that were asymmetrically labeled with BrdU
(BrdU−/K15+;
BrdU+/K15−; putative asymmetric stem cell
division in the second round) was increased in psoriatic
keratinocytes compared with normal cells (P<0.05), as shown in
Fig. 5B (bottom row). The
percentage (~32%) of cells expressing K15 that were symmetrically
labeled with BrdU (BrdU+/K15+;
BrdU+/+; putative stem cell self-renewal in the first
round) was also increased in psoriatic keratinocytes compared with
normal cells (P<0.01), as shown in Fig. 5B (top row). It seems safe to
conclude that the increased proportion of both symmetric and
asymmetric cell division may provide a mechanism by which the
psoriatic epidermis maintains persistent hyperplastic plaques.
IL-17A and IL-22 inhibit keratinocyte
differentiation to maintain the stem cell phenotype
To explore the effects of IL-17A and IL-22 on basal
cells expressing stem cell markers characteristic for K15 and the
integrin β1, we first established a short-term culture system that
allowed passage-one epidermal cells migrating from skin explants to
form an epidermal cell sheet, as shown in Fig. 6A. We then treated these
tongue-like epithelial sheets with 10 ng/ml recombinant IL-17A plus
10 ng/ml IL-22 for 24 h in vitro. The pattern of K15 and
integrin β1 expression was then examined in those epithelial sheets
by immunofluorescence staining. The results showed that cells
expressing K15 and/or integrin β1 were restricted to the
leadingedge zone of the epithelial sheet in the absence of cytokine
stimulation. However, following exposure to IL-17A and IL-22, both
markers were expressed by cells throughout the epithelial tongue,
and integrin β1 was very brightly labeled in the inner cells close
to the explant (Fig. 6C).
Likewise, western blotting revealed that the expression of K15 and
integrin β1 was significantly upregulated in cells stimulated with
both cytokines under low (0.06 mM) and high (1.3 mM) calcium
conditions compared with untreated controls, and those 2 cytokines
also exhibited a mild synergistic action (Fig. 7). These findings demonstrate that
IL-17A and IL-22 play critical roles in maintaining an
undifferentiated stem-cell like phenotype in basal
keratinocytes.
Discussion
The emerging view is that psoriasis is a T
cell-driven inflammatory skin disease characterized by keratinocyte
hyperproliferation and aberrant terminal differentiation in sync
with lymphocytic infiltrates (1,10).
In spite of persistent keratinocyte hyperproliferation, the
constant maintenance of a single intact basal-cell layer has been
noted as a distinct morphological characteristic of psoriatic
epidermal hyperplasia (4,24). Within the limited space of the
basal layer, two types of proliferative keratinocytes, stem cells
and TA cells, are aligned together and are attached to the
underlying basement membrane. It has been proposed that the
integrin β1 expression level on the cell surface of basal
keratinocytes is a key determinant which controls the transition of
cells from the basal layer to the suprabasal layer (25,26). Stem cells expressing higher levels
of integrin β1 than TA cells firmly adhere to the basement
membrane, whereas TA cells have downregulated expression of
integrin β1 and thus lose their adherent ability. These integrin
β1-deficient TA cells then exit the basal layer and enter the
suprabasal layer, and the terminal differentiation program is
executed simultaneously, turning K15 transcription off and K10
transcription on (27). It has
further been proposed that the proliferation taking place in the
basal stem cells is a prerequisite for replenishing the enlarged
compartment of TA cells in psoriatic epidermis (28,29). The immunohistochemical data in the
present study indicate that excessive expansion of the TA cell
(K15-negative/integrin β1-low/Ki67-positive) population indeed
existed in the lesional psoriatic skin sections. BrdU-labeled
mitotic basal cells in the mouse model of IMQ-induced
psoriasis-like dermatitis were significantly increased compared
with those treated with the control cream (P<0.05), and two
types (perpendicular and parallel) of asymmetric cell division were
also discerned in the inflamed skin of the mice (Fig. 3). In addition, the results of our
in vitro analyses for template-DNA strand segregation in
trypsin-dissociated epidermal cells using BrdU pulse-chase labeling
are compatible with our assumption that stem cell division exists
in the psoriatic epidermis (30).
An increased percentage of asymmetric segregation of BrdU was noted
in the cell pairs of psoriatic epidermal cells, whereas only a
small proportion was noted in normal epidermal cells (P<0.001).
The template DNA (BrdU-unlabeled strand) always segregated to the
daughter cell, which retains K15 expression, indicating that
psoriatic stem cell division also complies with the immortal strand
hypothesis prediction that the cell inheriting the older template
is the more undifferentiated cell, as reflected by the expression
of K15. The percentage of cells expressing K15 and asymmetrically
labeled with BrdU (BrdU−/K15+;
BrdU+/K15−) is increased in psoriatic
keratinocytes compared with normal cells (P<0.05). The
percentage of cells expressing K15 that were symmetrically labeled
with BrdU (BrdU+/K15+;
BrdU+/K15+) was also increased in psoriatic
keratinocytes compared with normal cells (P<0.01). These data
reconfirm that both symmetric and asymmetric cell division
contribute to the excessive expansion of the TA cell compartment in
psoriatic epidermis (Fig. 5).
Previous studies have examined the role of Th17
cells in psoriatic epidermal hyperplasia (9,10,18). Th17 cells have been reported to
co-synthesize large amounts of IL-17A and IL-22, which disrupt
keratinocyte terminal differentiation and enhance immune cell
infiltration in psoriasis (9,10).
Several lines of evidence indicate that IL-22 (with or without
IL-17) exerts an inhibitory effect on keratinocyte differentiation
(31,32). Our data demonstrate that upon
stimulation with IL-17A and IL-22, the immunostaining pattern of
K15 and integrin β1 is changed in cultured epithelial cell sheets
from the leading-edge zone to the entire sheet, as illustrated in
Fig. 6. Similar results were
obtained by western blotting, i.e., that undifferentiated markers
(K15 and integrin β1) are upregulated while differentiation markers
(K10 and filaggrin) are downregulated in cultured keratinocytes
stimulated by IL-17A and IL-22, respectively. As previously noted,
even under high calcium (1.2 mM CaCl2) conditions, the
differentiated marker pattern of keratinocytes is also suppressed
by both cytokines (33).
Surprisingly, the expression of the proliferative marker Ki67 was
not upregulated in cells treated with IL-17A and IL-22 (Fig. 7), indicating that no
proliferation-stimulating effect of IL-17A and IL-22 was achieved
in cultured monolayers of keratinocytes, which is inconsistent with
a previous report that IL-17A and IL-22 induced keratinocyte
hyperproliferation in a three-dimensional skin equivalent model
(10).
Taken together, these data provide unequivocal
evidence that asymmetric basal stem-cell division contributes to
psoriatic epidermal hyperplasia, which complies with the immortal
strand hypothesis for the purpose of minimizing potential lethal
mutations during the increased proliferation of psoriatic
keratinocytes. We suggest that IL-17 and IL-22 cytokines play
critical roles in regulating the expression of stem cell markers
and in controlling the transition rate of cells from the basal
layer to the suprabasal layer. Inhibition of hyperactive stem cells
represents an intriguing potential therapeutic target to combat
recalcitrant epidermal hyperplasia in psoriasis.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 8107138 and 81371717).
The authors wish to thank Dr Vincent J. Hearing of the National
Institutes of Health, Bethesda, MD, USA, for manuscript
editing.
References
|
1
|
Nestle FO, Kaplan DH and Barker J:
Psoriasis. N Engl J Med. 361:496–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Capon F, Burden AD, Trembath RC and Barker
JN: Psoriasis and other complex trait dermatoses: from loci to
functional pathways. J Invest Dermatol. 132:915–922. 2012.
View Article : Google Scholar :
|
|
3
|
Di Cesare A, Di Meglio P and Nestle FO:
The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J
Invest Dermatol. 129:1339–1350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leigh IM, Pulford KA, Ramaekers FC and
Lane EB: Psoriasis: maintenance of an intact monolayer basal cell
differentiation compartment in spite of hyperproliferation. Br J
Dermatol. 113:53–64. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma HL, Liang S, Li J, Napierata L, Brown
T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M,
et al: IL-22 is required for Th17 cell-mediated pathology in a
mouse model of psoriasis-like skin inflammation. J Clin Invest.
118:597–607. 2008.PubMed/NCBI
|
|
6
|
van der Fits L, Mourits S, Voerman JS,
Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens
EP and Lubberts E: Imiquimod-induced psoriasis-like skin
inflammation in mice is mediated via the IL-23/IL-17 axis. J
Immunol. 182:5836–5845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nickoloff BJ: Keratinocytes regain
momentum as instigators of cutaneous inflammation. Trends Mol Med.
12:102–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eyerich S, Eyerich K, Cavani A and
Schmidt-Weber C: IL-17 and IL-22: siblings, not twins. Trends
Immunol. 31:354–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nograles KE, Zaba LC, Guttman-Yassky E,
Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, Khatcherian A,
Gonzalez J, Pierson KC, White TR, et al: Th17 cytokines interleukin
(IL)-17 and IL-22 modulate distinct inflammatory and
keratinocyte-response pathways. Br J Dermatol. 159:1092–1102.
2008.PubMed/NCBI
|
|
10
|
Guerrero-Aspizua S, García M, Murillas R,
Retamosa L, Illera N, Duarte B, Holguín A, Puig S, Hernández MI,
Meana A, et al: Development of a bioengineered skin-humanized mouse
model for psoriasis: dissecting epidermal-lymphocyte interacting
pathways. Am J Pathol. 177:3112–3124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beck B and Blanpain C: Mechanisms
regulating epidermal stem cells. EMBO J. 31:2067–2075. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blanpain C and Fuchs E: Epidermal
homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol
Cell Biol. 10:207–217. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dazard JE, Piette J, Basset-Seguin N,
Blanchard JM and Gandarillas A: Switch from p53 to MDM2 as
differentiating human keratinocytes lose their proliferative
potential and increase in cellular size. Oncogene. 19:3693–3705.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arwert EN, Lal R, Quist S, Rosewell I, van
Rooijen N and Watt FM: Tumor formation initiated by nondividing
epidermal cells via an inflammatory infiltrate. Proc Natl Acad Sci
USA. 107:19903–19908. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Janich P, Pascual G, Merlos-Suárez A,
Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L
and Benitah SA: The circadian molecular clock creates epidermal
stem cell heterogeneity. Nature. 480:209–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo A and Jahoda CA: An improved method of
human keratinocyte culture from skin explants: cell expansion is
linked to markers of activated progenitor cells. Exp Dermatol.
18:720–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gutowska-Owsiak D, Schaupp AL, Salimi M,
Selvakumar TA, McPherson T, Taylor S and Ogg GS: IL-17
downregulates filaggrin and affects keratinocyte expression of
genes associated with cellular adhesion. Exp Dermatol. 21:104–110.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guilloteau K, Paris I, Pedretti N,
Boniface K, Juchaux F, Huguier V, Guillet G, Bernard FX, Lecron JC
and Morel F: Skin inflammation induced by the synergistic action of
IL-17A, IL-22, oncostatin M, IL-1α, and TNF-α recapitulates some
features of psoriasis. J Immunol. 184:5263–5270. 2010. View Article : Google Scholar
|
|
19
|
Shinin V, Gayraud-Morel B, Gomès D and
Tajbakhsh S: Asymmetric division and cosegregation of template DNA
strands in adult muscle satellite cells. Nat Cell Biol. 8:677–687.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Conboy MJ, Karasov AO and Rando TA: High
incidence of non-random template strand segregation and asymmetric
fate determination in dividing stem cells and their progeny. PLoS
Biol. 5:e1022007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo LF, Shi Y, Zhou Q, Xu SZ and Lei TC:
Insufficient expression of the melanocortin-1 receptor by human
dermal fibroblasts contributes to excess collagen synthesis in
keloid scars. Exp Dermatol. 22:764–766. 2013. View Article : Google Scholar
|
|
22
|
Waseem A, Dogan B, Tidman N, Alam Y,
Purkis P, Jackson S, Lalli A, Machesney M and Leigh IM: Keratin 15
expression in stratified epithelia: downregulation in activated
keratinocytes. J Invest Dermatol. 112:362–369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Troy TC, Arabzadeh A and Turksen K:
Re-assessing K15 as an epidermal stem cell marker. Stem Cell Rev.
7:927–934. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Körver JE, Vissers WH, van Rens DW, Pasch
MC, van Erp PE, Boezeman JB and van De Kerkhof PC: A double-blind,
randomized quantitative comparison of calcitriol ointment and
calcipotriol ointment on epidermal cell populations, proliferation
and differentiation. Br J Dermatol. 156:130–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watt FM: Epidermal stem cells: markers,
patterning and the control of stem cell fate. Philos Trans R Soc
Lond B Biol Sci. 353:831–837. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grabe N and Neuber K: Simulating psoriasis
by altering transit amplifying cells. Bioinformatics. 23:1309–1312.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones P and Simons BD: Epidermal
homeostasis: do committed progenitors work while stem cells sleep?
Nat Rev Mol Cell Biol. 9:82–88. 2008. View
Article : Google Scholar
|
|
28
|
Truzzi F, Marconi A, Atzei P, Panza MC,
Lotti R, Dallaglio K, Tiberio R, Palazzo E, Vaschieri C and
Pincelli C: p75 neurotrophin receptor mediates apoptosis in
transit-amplifying cells and its overexpression restores cell death
in psoriatic keratinocytes. Cell Death Differ. 18:948–958. 2011.
View Article : Google Scholar :
|
|
29
|
Kawashima K, Doi H, Ito Y, Shibata MA,
Yoshinaka R and Otsuki Y: Evaluation of cell death and
proliferation in psoriatic epidermis. J Dermatol Sci. 35:207–214.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gündüz K, Demireli P, Vatansever S and
Inanir I: Examination of bcl-2 and p53 expressions and apoptotic
index by TUNEL method in psoriasis. J Cutan Pathol. 33:788–792.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boniface K, Bernard FX, Garcia M, Gurney
AL, Lecron JC and Morel F: IL-22 inhibits epidermal differentiation
and induces proinflammatory gene expression and migration of human
keratinocytes. J Immunol. 174:3695–3702. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rabeony H, Petit-Paris I, Garnier J,
Barrault C, Pedretti N, Guilloteau K, Jegou JF, Guillet G, Huguier
V, Lecron JC, et al: Inhibition of keratinocyte differentiation by
the synergistic effect of IL-17A, IL-22, IL-1α, TNFα and oncostatin
M. PLoS One. 9:e1019372014. View Article : Google Scholar
|
|
33
|
Xie Z, Singleton PA, Bourguignon LY and
Bikle DD: Calcium-induced human keratinocyte differentiation
requires src- and fyn-mediated phosphatidylinositol
3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol
Cell. 16:3236–3246. 2005. View Article : Google Scholar : PubMed/NCBI
|