Introduction
Acute lung injury (ALI) is a critical illness which
plays a pivotal role in the death of patients in the intensive care
unit (1,2). ALI is characterized by an
uncontrolled inflammatory response in the lungs, resulting in
airway dysfunction (3). The most
common cause of ALI is an exposure to the structural component of a
Gram-negative bacterial membrane, lipopolysaccharide (LPS)
(4). In response to bacterial LPS
stimulation, neutrophils migrate across the endothelium and
epithelium into the alveolar space, and are subsequently activated,
causing the excessive production of pro-inflammatory molecules,
such as reactive oxygen species (ROS) and pro-inflammatory
cytokines (5). The
neutrophil-derived overproduction of ROS has been shown to cause
lung tissue damage in animal models of LPS-induced ALI (6,7).
Inducible nitric oxide synthase (iNOS) in neutrophils and alveolar
macrophages (AM) plays a crucial role in the development of ALI by
modulating pulmonary neutrophil infiltration (8,9).
AM regulate neutrophil recruitment in endotoxin-induced lung injury
by controlling monocyte chemoattractant protein-1 (MCP-1) (10). Tumor necrosis factor-α (TNF)-α and
interleukin-6 (IL-6) secreted by AM are early response cytokines
which stimulate neutrophils, and these activated neutrophils
release proteases and oxidants (11). TNF-α and IL-6 also stimulate other
cells locally, such as macrophages, epithelial cells and
endothelial cells to discharge other pro-inflammatory chemokines
involved in the pathogenesis of ALI (12,13). The influx and activation of
neutrophils within the lungs is a hallmark of ALI, and macrophages
are key effector cells that are involved in the process of ALI
(11,14).
Nuclear factor-κB (NF-κB) and mitogen-activated
protein kinases (MAPKs) are involved in regulating pro-inflammatory
mediators and cytokines in LPS-induced ALI (15,16). The activation of the NF-κB and
MAPK pathways in lung tissues is one of the major characteristics
of ALI (17). The inhibition of
the activation of the NF-κB and MAPKs pathways is believed to
alleviate ALI by blocking the induction of inflammatory mediators,
such as iNOS, TNF-α, IL-6 and MCP-1 (13,17).
The expression of heme oxygenase-1 (HO-1), an
antioxidant defense protein, can be induced by stimulants such as
oxidants and inflammatory cytokines (18,19). There is recent evidence to
indicate that the upregulation of HO-1 is involved in the
resolution of inflammation, and attenuates LPS-induced ALI through
the suppression of neutrophil infiltration (20). It has also been reported that the
upregulation of HO-1 attenuates severe lung injury through the
suppression of the migration of macrophages, increasing the
survival of animals (21).
Picrasma quassioides (D. Don) Benn. (PQ) is
widely distributed in most areas of mainland China, and the
branches of this plant are used as a traditional folk medicine for
the treatment of a variety of diseases, such as hypertension
(22), colitis (23), gastroenteritis and cancer
(24). The major active compounds
in PQ are β-carbolines and canthin-6-one alkaloids (24) which have been reported to possess
various activities, such as anti-inflammatory and anti-hypertensive
activities (22,23). 4-Methoxy-5-hydroxycanthin-6-one is
one of the major active constituents of β-carbolines and cathinone
alkaloids isolated from PQ. It has been shown to inhibit the
production of inflammatory mediators, such as iNOS and TNF-α in
LPS-stimulated RAW 264.7 macrophages, and to reduce the development
of carrageenan-induced paw edema and adjuvant-induced chronic
arthritis in rats (25). In our
previous study, we demonstrated that PQ inhibited airway
inflammation in a murine model of allergic asthma (26). However, to the best of our
knowledge, the anti-inflammatory effects of PQ have not yet been
demonstrated in a model of LPS-induced ALI. Therefore, in this
study, we evaluated the effects of PQ in a mouse model of
LPS-induced ALI and in LPS-stimulated RAW 264.7 macrophages.
Materials and methods
Preparation of PQ extract
PQ was collected from Baegunsan Mountain of
Gwangyang-si, Jeollanam-do, Korea. A voucher specimen recorded as
KRIB 0001101 has been deposited at the herbarium of the Korea
Research Institute of Bioscience and Biotechnology (KRIBB). After
drying and grinding the bark of the stem of PQ, the powder (50 g)
was added to 100 liters of methanol. The extraction was performed
using the method of repercolation at room temperature. The extract
was filtered and concentrated using a rotary evaporator
(N-1200AV-W; T.R.K. EYELA, Tokyo, Japan) under reduced pressure,
thereby obtaining 2.07 g of PQ methanolic extract. In the following
experiment, PQ was dissolved in dimethyl sulfoxide (DMSO) at a
concentration of 20 mg/ml, and then diluted to various
concentrations prior to use.
Animal model of LPS-induced ALI
Specific pathogen-free male C57BL/6N mice (6 weeks
old) were obtained from Koatech Co. (Pyeongtaek, Korea) and used
after 2 weeks of quarantine and acclimatization. They were housed
in groups of 7 under standard conditions with food and water.
Briefly, the mice (n=7/group) were divided into 4 groups as
follows: i) the normal control (NC) group; ii) the LPS group; iii)
the dexamethasone (DEX; 3 mg/kg) group (used as a positive
control); and iv) the PQ group (administered 5 and 15 mg/kg PQ). PQ
and DEX were dissolved with 0.5% carboxymethyl cellulose (CMC), and
were administered orally from day 0 to day 2. The mice were exposed
to LPS (10 µg/mouse) intranasally 1 h after the final DEX
and PQ treatment, as previously described (3). All the experimental procedures were
performed in accordance with the procedures approved by the
Institutional Animal Care and Use Committee of the Korea Research
Institute of Bioscience and Biotechnology and performed in
compliance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals and Korean National Laws for
Animal Welfare.
Collection of bronchoalveolar lavage
fluid (BALF), and inflammatory cell counting
To obtain the BALF, ice-cold PBS (0.7 ml) was
infused into the lungs of the mice twice and withdrawn each time
using a tracheal cannula (a total volume of 1.4 ml). The collected
BALF was centrifuged for 5 min at 1,500 rpm and the BALF
differential cell count was determined using Diff-Quik®
staining reagent according to themanufacturer's instructions, and
as previously described (3).
Measurement of ROS and pro-inflammatory
cytokines in BALF
The intracellular levels of ROS were determined
using 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Sigma Aldrich,
St. Louis, MO, USA). Briefly, BALF cells were washed with PBS and
incubated with 20 µM DCFH-DA for 10 min at 37°C. The
activity of intracellular ROS was then detected by measuring the
fluorescence at 488 nm excitation and 525 nm emission on a
fluorescence plate reader (Perkin-Elmer, Waltham, MA, USA). The
levels of pro-inflammatory cytokines (TNF-α and IL-6) in BALF were
measured using ELISA kits according to themanufacturer's
instructions (R&D Systems, Minneapolis, MN, USA). Blood was
collected from the inferior vena cava, and the levels of IL-6 in
serum were determined using ELISA following themanufacturer's
instructions (R&D Systems). The absorbance was measured at 450
nm was determined using an ELISA reader (Molecular Devices,
Sunnyvale, CA, USA).
Western blot analysis
The lung tissue was homoge-n-ized (1/10 w/v) using a
homogenizer with tissue lysis/extraction reagent, containing a
protease inhibitor cocktail (both from Sigma-Aldrich). Equal
amounts of the total cellular protein (30 µg) were resolved
by 12% SDS-polyacrylamide gels and transferred onto a
nitrocellulose membrane. The membrane was blocked by incubation
with 5% skim milk in TBST for 1 h, and incubated overnight at 4°C
with the appropriate primary antibody. Specific antibodies against
Nrf2 (ab137550, 1;1,000; Abcam, Cambridge, MA, USA), HO-1
(ab137749, 1;1,000; Abcam) p-p38 (1:1,000; ADI-KAP-MA022, 1:1,000;
Enzo Life Sciences, Farmingdale, NY, USA), p38 (sc-7149, 1:1,000;
Santa Cruz Biotechnology, Santa Cruz, CA, USA), p-ERK (#9106,
1:1,000; Cell Signaling Technology Inc., Danvers, MA, USA), ERK
(sc-154, 1:1,000; Santa Cruz Biotechnology), p-JNK (KAP-SA011,
1:1,000; Enzo Life Sciences), JNK (sc-474, 1:1,000), IκB (sc-371,
1:1,000), p65 (sc-372, 1,000) (all from Santa Cruz Biotechnology),
p-p65 (#3033, 1:1,000; Cell Signaling Technology Inc.), iNOS
(ADI-905-431, 1:1,000; Enzo Life Sciences) and β-actin (#4967,
1:2,500; Cell Signaling Technology Inc.) were diluted in 5% skim
milk. The blots were washed with TBST and incubated with a 1:2,000
dilution of a horseradish peroxidase (HRP)-conjugated secondary
antibody for 2 h at room temperature. The blots were washed with
TBST and developed using an enhanced chemiluminescence (ECL) kit
(Thermo Fisher Scientific, Inc., Rockford, IL, USA). The protein
bands were visualized using a LAS-4000 luminescent image analyzer
(Fujifilm, Tokyo, Japan) and quantified by densitometry (Fuji Multi
Gauge software version 3.0).
Isolation of nuclear extract
RAW 264.7 murine macrophages (2×105
cells/ml; ATCC, Manassas, VA, USA) were cultured in 6-well plates,
treated with PQ for 1 h and stimulated with 0.5 µg/ml of
LPS. The cells were harvested and washed twice with ice-cold PBS.
Nuclear and cytoplasmic fractions were prepared using NE-PER
nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL,
USA), according to themanufacturer's instructions. The nuclear
translocation of NF-κB and Nrf2 was determined by western blot
analysis. PARP is a nuclear protein which was used as an internal
control.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The RAW 264.7 macrophages were treated with PQ in
the absence or presence of LPS (0.5 µg/ml) for 6 h. Total
RNA was isolated using TRIzol™ reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the instructions
provided by the manufacturer, and the reverse transcription
reaction was performed using a kit producing cDNA (Qiagen, Hilden,
Germany). Polymerase chain reactions were performed using specific
forward and reverse primers as follows: MCP-1 forward,
5′-AGGTCCCTGTCATGCTTCTG-3′ and reserve, 5′-TCTGGACCCATTCCTTCTTG-3′;
HO-1 forward, 5′-TGAAGGAGGCCACCAAGGAGG-3′ and reverse,
5′-AGAGGTCACCCAGGTAGCGGG-3′; and β-actin forward, 5′-TGTTTG
AGACCTTCAACACC-3′ and reserve, 5′-CGCTCATTGCCGATAGTGAT-3′. Parallel
PCR analysis was run for the housekeeping gene, β-actin, to
normalize the data for differences in mRNA quantity and integrity.
The PCR products were fractionated by 1.5% agarose gel
electrophoresis and stained with 5 µg/ml ethidium bromide.
These experiments were performed in triplicate.
Histological analysis
Lung tissues were obtained to evaluate the effects
of PQ at 24 h after LPS injection. Mice were anesthetized by an
intraperitoneal injection of pentobarbital (50 mg/kg; Hanlim Pharm.
Co., Seoul, Korea). The lung tissues were washed and fixed in 4%
(v/v) paraformaldehyde. The lung tissues were embedded in paraffin,
sectioned at 4 µm thickness, and stained with an H&E
solution (Sigma-Aldrich) to estimate inflammation in peribronchial
and alveolar lesions. Quantitative analysis of inflammation was
performed using an image analyzer (Molecular Devices).
Cell viability
The viability of the RAW 264.7 macrophages was
examined following the treatment of the cells with various
concentrations (0, 2.5, 5, 10 and 20 µg/ml) of PQ and LPS
(0.5 µg/ml). Cell viability was examined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The cells were seeded at 1×104 cells per well of
a96-well plate and incubated with PQ at various concentrations for
24 h at 37°C. After incubation, MTT (0.5 mg/ml in PBS) was added to
each well, and the cells were incubated for 2 h at 37°C and 5%
CO2. The resulting formazan crystals were dissolved in
DMSO. The absorbance was determined at 540 nm. The results were
expressed as a percentage of surviving cells over control
cells.
Statistical analysis
The data are expressed as the means ± standard
deviation (SD). The statistical significance was determined using
analysis of variance (ANOVA) followed by a multiple comparison test
with Dunnett's adjustment. A value of p<;0.05 was considered to
indicate a statistically significant diffence.
Results
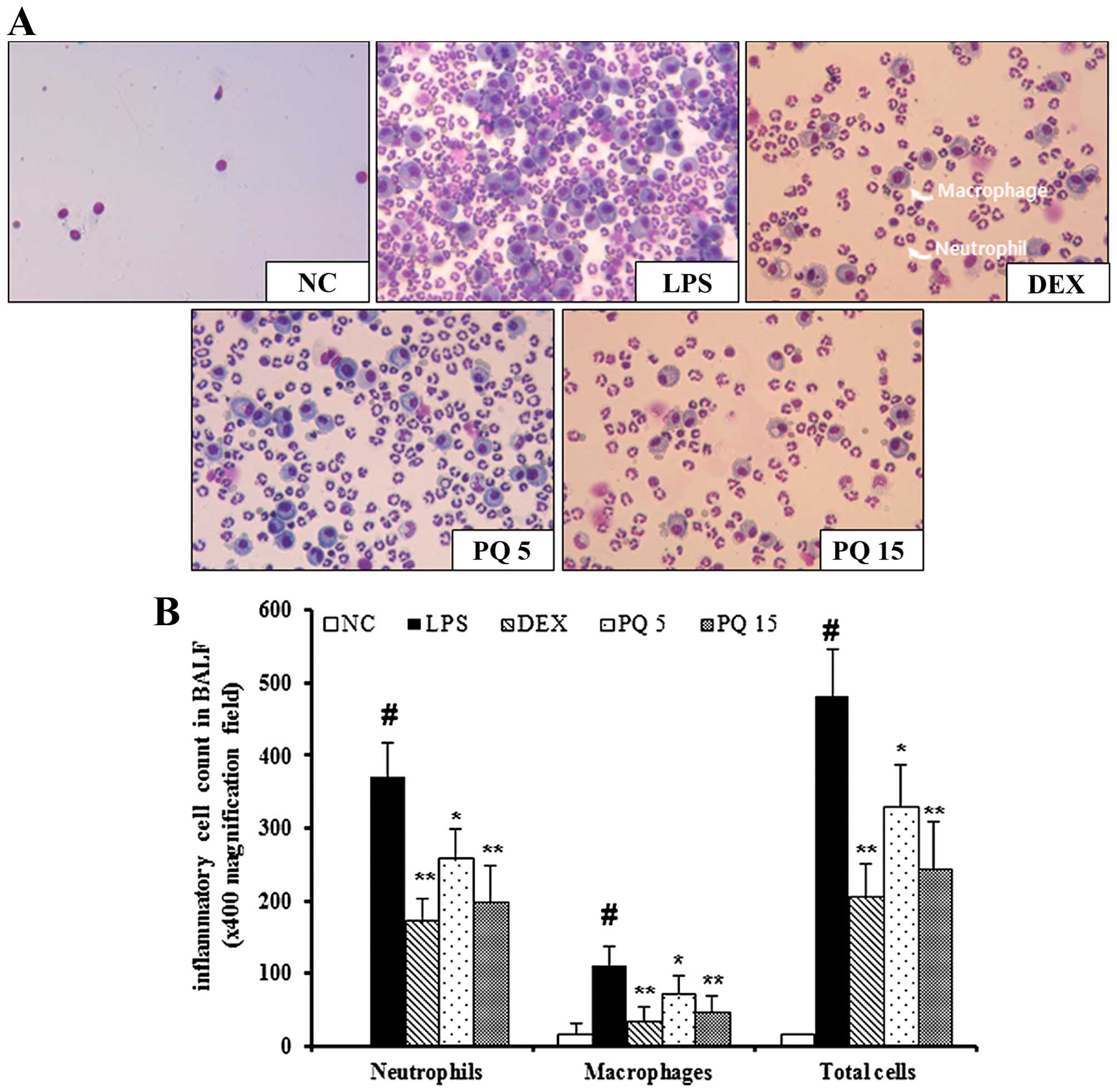
Treatment with PQ suppresses the
infiltration of inflammatory cells in the BALF of mice with
LPS-induced ALI
The mice with LPS-induced ALI exhibited a
significant increase in the number of inflammatory cells, including
neutrophils and macrophages in BALF compared with the normal
control mice (Fig. 1). In
particular, the number of neutrophils and macrophages in BALF was
markedly increased in the mice with LPS-induced ALI. However, the
PQ-treated mice exhibited a marked decrease in the number of
neutrophils and macrophages in BALF compared with the mice with
LPS-induced ALI in a concentration-dependent manner. Treatment with
DEX was used as a positive control. The mice treated with DEX also
exhibited a decrease in the number of neutrophils and macrophages
in BALF compared with the LPS-exposed mice. The effects of DEX were
similar to those of treatment with PQ at 15 mg/kg.
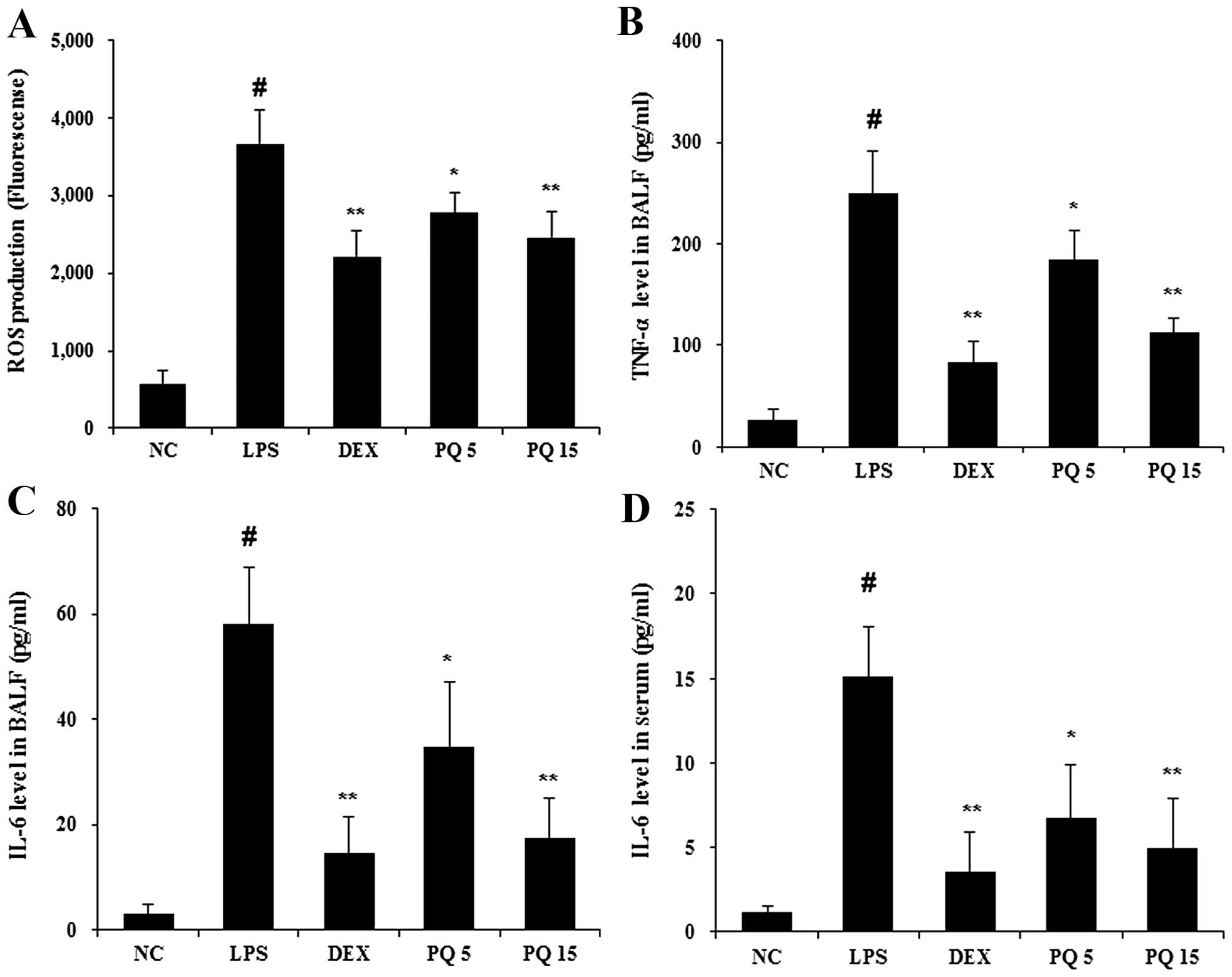
Treatment with PQ reduces the production
of ROS and pro-inflammatory cytokines in BALF
Given the fact that ALI is involved in neutrophil
migration, the release of ROS and cytokine production (12), the levels of ROS and cytokines
were examined in BALF. The levels of ROS were significantly
increased in the LPS-exposed mice compared with the normal control
mice (Fig. 2A). The mice treated
with PQ at a dose of 15 mg/kg exhibited a significant reduction in
ROS production compared with the LPS-exposed mice. Similar to the
results obtained for ROS, the LPS-exposed mice exhibited a marked
increase in the levels of TNF-α and IL-6, compared with the normal
control mice, and the PQ-treated mice exhibited significantly
decreased levels of TNF-α and IL-6 compared with the LPS-exposed
mice (Fig. 2B and C). To further
examine the effects of PQ on the production of pro-inflammatory
cytokines (27), the levels of
IL-6 were detected using ELISA in serum of mice. As shown Fig. 2D, PQ significantly suppressed the
release of IL-6 compared with the LPS-exposed mice. The mice
treated with DEX also exhibited a decrease in the levels of ROS,
TNF-α and IL-6 compared with the LPS-exposed mice. The effects of
DEX were similar to those of treatment with PQ at 15 mg/kg.
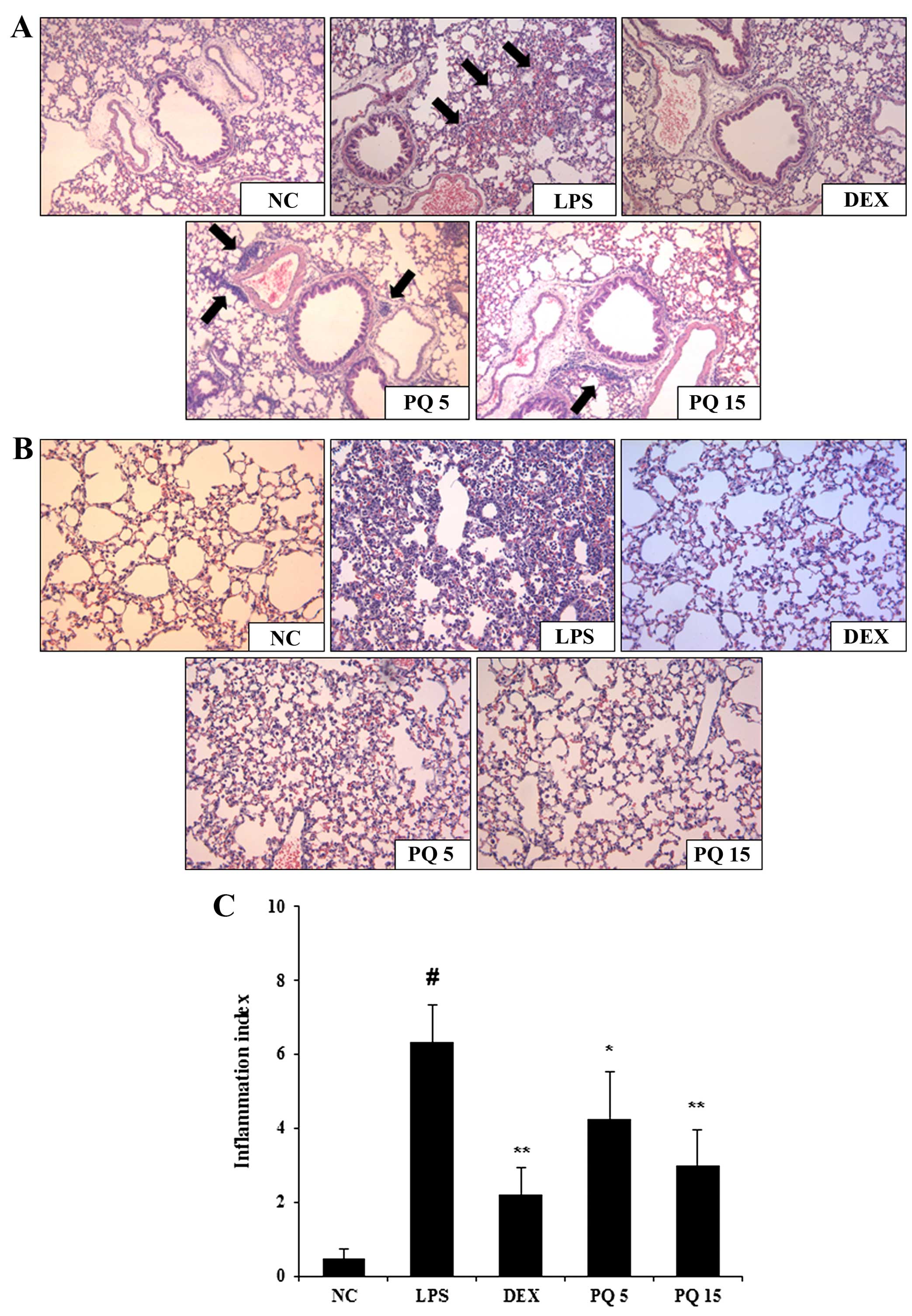
Treatment with PQ decreases inflammatory
cell infiltration into the lungs
The infiltration of inflammatory immune cells into
the lungs is one of the common characteristics of ALI. As shown by
histological analysis, the LPS-exposed mice exhibited extensive
infiltration of inflammatory cells into the lung tissue (Fig. 3A and B). However, the PQ-treated
mice exhibited a marked reduction in inflammatory cell infiltration
induced by the LPS challenge. The mice treated with DEX also
exhibited a decrease in airway inflamamation compared with the
LPS-exposed mice. Again, the effects of DEX were similar to those
of treatment with PQ at 15 mg/kg.
Treatment with PQ inhibits the expression
of iNOS and increases the expression of HO-1 in the lungs
iNOS has been shown to be involved in the
pathogenesis of ALI (28). HO-1
inhibits the LPS-induced production of iNOS and pro-inflammatory
cytokines, such as TNF-α and IL-6 (17). In this study, to examine the
effects of PQ on LPS-induced ALI in mice, the expression of iNOS
and HO-1 was detected using western blot analysis. As shown in
Fig. 4A, the expression of iNOS
was significantly increased in the lungs of mice with LPS-induced
ALI. However, treatment with PQ significantly decreased iNOS
expression compared with the LPS-exposed mice. In addition, PQ
significantly increased the expression of HO-1 in the lungs of mice
with LPS-induced ALI (Fig. 4B).
Treatment with DEX also decreased the expression of iNOS and
increased the expression of HO-1 in the lungs of mice compared with
the LPS-exposed mice. The effects of DEX were similar to those of
treatment with PQ (15 mg/kg).
Treatment with PQ suppresses MAPK
activation in the lungs of mice with LPS-induced ALI
MAPKs are involved in the inflammatory response in
ALI (2). It is also well known
that LPS administration leads to the increased phosphorylation of
p38, ERK and JNK in the lungs (17). In the present study, exposure to
LPS significantly increased the phosphorylation of p38, ERK and JNK
in the lungs of mice. However, treatment with PQ significantly
decreased the LPS-induced MAPK phosphorylation compared with the
mice with LPS-induced ALI (Fig.
5). Treatment with DEX also suppressed the activation of MAPKs
in the lungs of mice compared with the LPS-exposed mice. Treatment
with DEX was slightly more effective in reducing MAPK
phosphorylation than treatment with PQ (p<;0.01 as opposed to
p<;0.05).
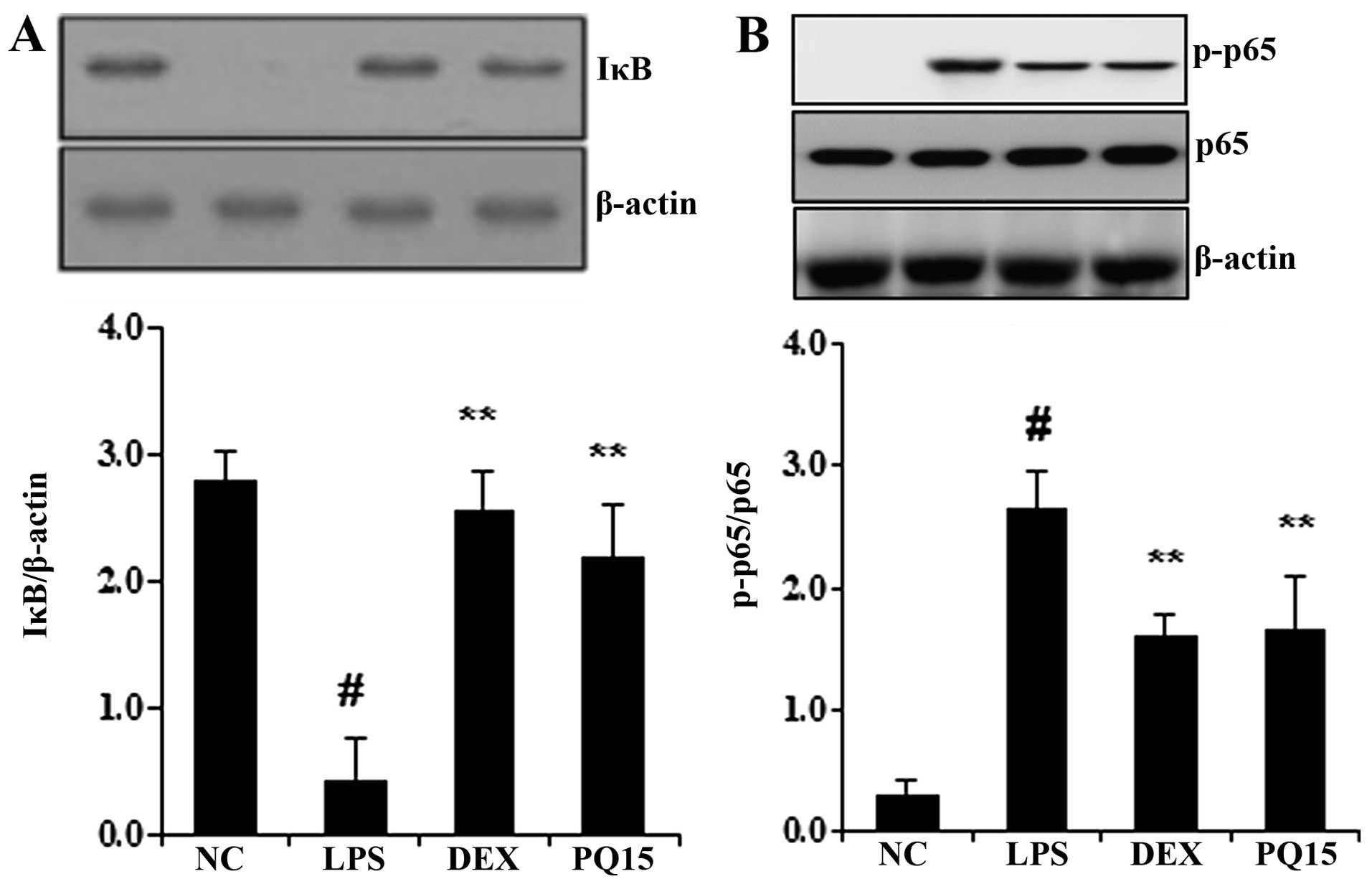
Treatment with PQ decreases IκB
degradation and NF-κB p65 phosphorylation in the lungs of
mice with LPS-induced ALI
It is well known that the degradation of IκB and the
phosphorylation of NF-κB p65 induces the transcription of most
pro-inflammatory cytokines, including TNF-α and IL-6, thus playing
a pivotal role in the pathogenesis of ALI (17). Thus, in the present study, to
determine whether PQ affects the LPS-induced degradation of IκB and
the phosphorylation of NF-κB p65, the levels of IκB and the
phosphorylation of NF-κB were examined by western blot analysis. As
shown in Fig. 6, the LPS
administration induced the degradation of IκB and the
phosphorylation of NF-κB p65. However, treatment with PQ
significantly decreased the LPS-induced IκB degradation. In
addition, PQ significantly decreased the phosphorylation of NF-κB
p65 in the lungs of mice with LPS-induced ALI. Treatment with DEX
also significantly suppressed the degradation of IκB and decreased
the phosphorylation of NF-κB p65 in the lungs of mice compared to
the LPS-exposed mice. The effects of DEX were similar to those of
treatment with PQ (15 mg/kg).
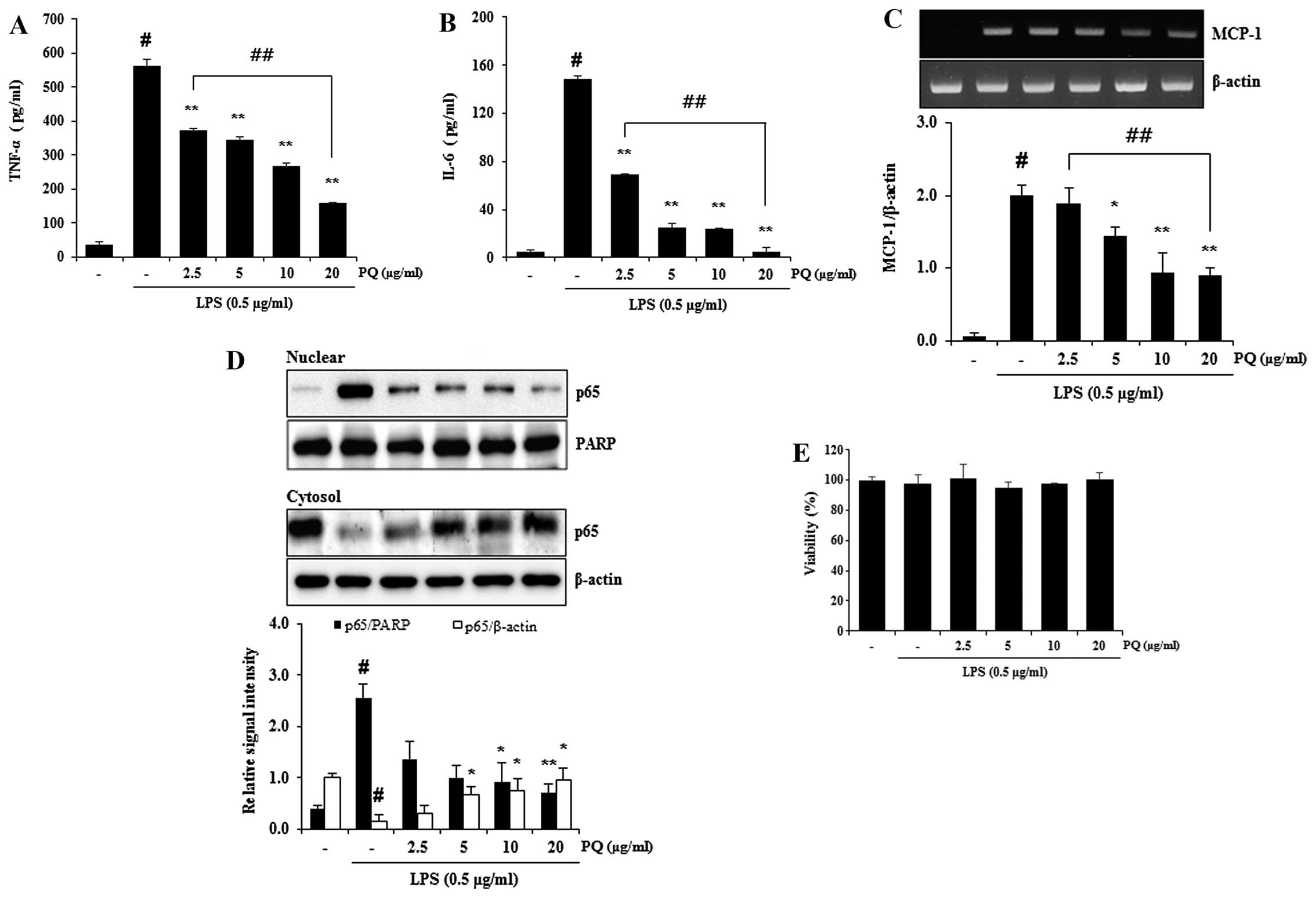
Treatment with PQ inhibits the release of
TNF-α and IL-6, and decreases the mRNA expression of MCP-1 and the
nuclear translocation of NF-κB in LPS-stimulated RAW 264.7
macrophages
TNF-α and IL-6 are major pro-inflammatory cytokines
involved in the recruitment of neutrophils into the lungs of mice
with LPS-induced ALI (17). MCP-1
is one of the key chemokines (29) that contributes to the recruitment
of monocytes/macrophage into sites of immune response (30). NF-κB is a major transcription
factor that is a predominant regulator of numerous pro-inflammatory
cytokines and mediators, such as TNF-α, IL-6, iNOS and MCP-1
(31). We previously demonstrated
that PQ attenuated the increase in iNOS protein expression in
LPS-stimulated RAW 264.7 macrophages (26). In the present study, treatment
with PQ inhibited the release of TNF-α and IL-6 from LPS-stimulated
RAW 264.7 macrophages (Fig. 7A and
B). Treatment with PQ also resulted in the suppression of NF-κB
nuclear translocation, as well as in a decrease in MCP-1 expression
in LPS-stimulated RAW 264.7 cells in a concentration-dependent
manner (Fig. 7C and D). No
noticeable cell death was observed following treatment with PQ at
the concentration of up to 20 µg/ml (Fig. 7E).
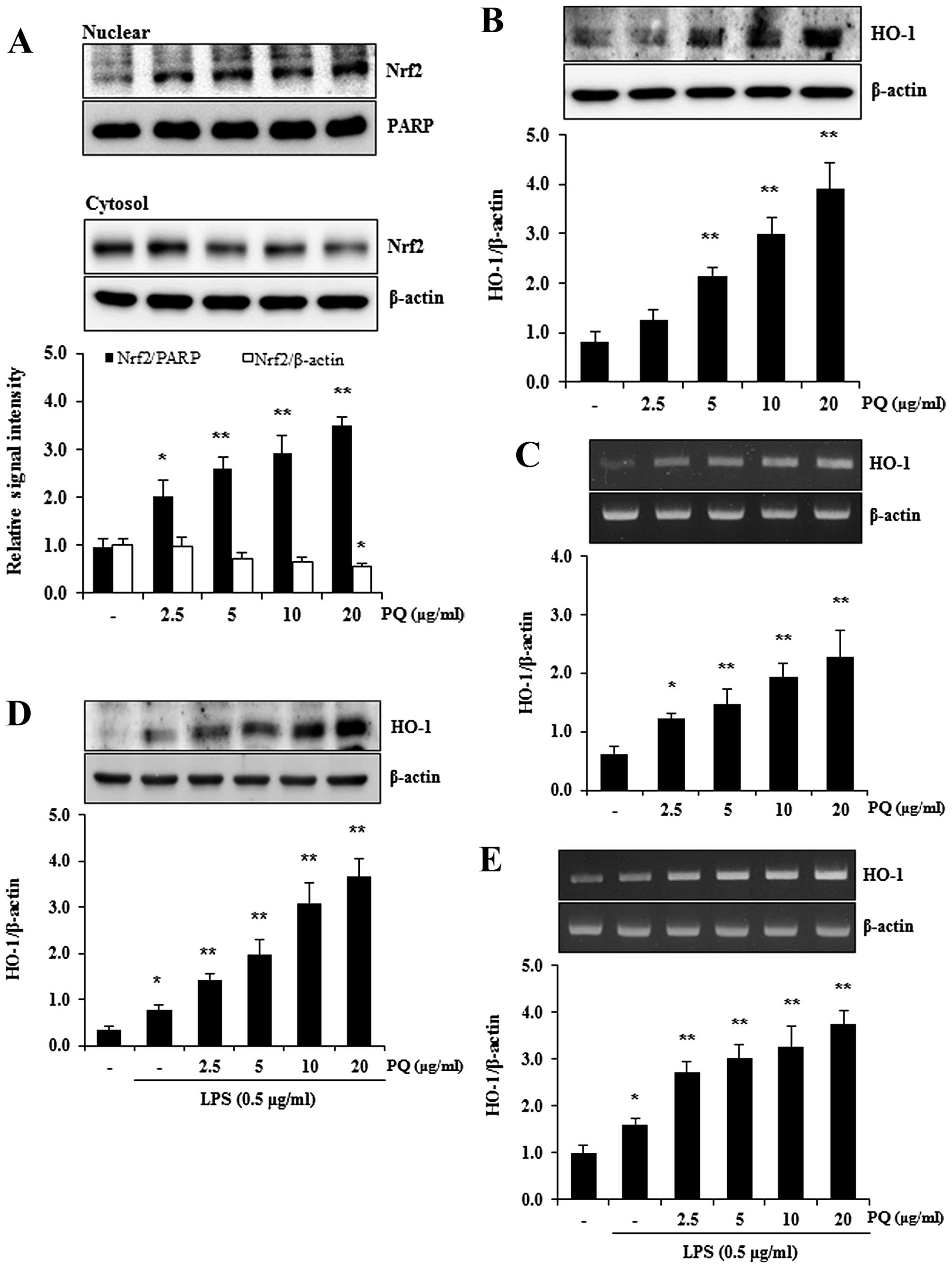
Treatment with PQ promotes the nuclear
translocation of nuclear factor erythroid-2-related factor 2 (Nrf2)
and the expression of HO-1 in RAW 264.7 cells
Nrf2 is a pivotal transcription factor that
regulates a variety of cytoprotective enzymes, including HO-1
(32). HO-1 is an antioxidant
enzyme that can be induced by stimulants, such as inflammatory
oxidants and cytokines, and the induction of HO-1 attenuates ALI
(33). Therefore, in this study,
we used western blot analysis to determine whether PQ promotes the
nuclear translocation of Nrf2 in RAW 264.7 macrophages. As shown in
Fig. 8A, treatment with PQ
significantly increased the nuclear translocation of Nrf2 in the
RAW 264.7 cells in a concentration-dependent manner. To examine
whether PQ upregulates HO-1 expression, the protein and mRNA levels
of HO-1 were examined by western blot analysis and RT-PCR.
Treatment with PQ resulted in a significant increase in the protein
and mRNA expression of HO-1 in the RAW 264.7 macrophages in a
concentration-dependent manner (Fig.
8B and C). In accordance with the increased expression of HO-1,
PQ also significantly upregulated the protein and mRNA expression
of HO-1 in the LPS-stimulated RAW 264.7 macrophages in a
concentration-dependent manner (Fig.
8D and E).
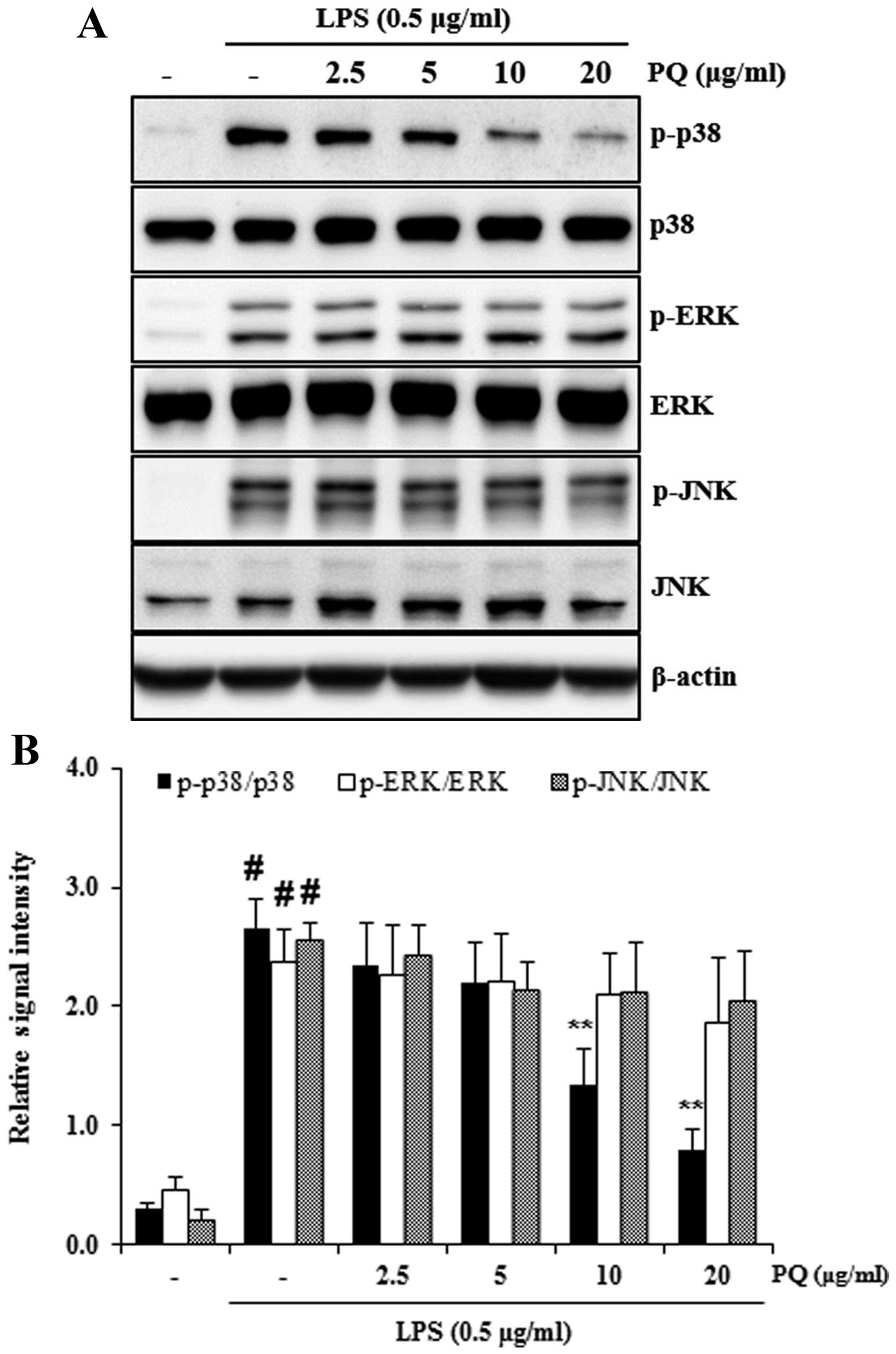
Treatment with PQ attenuates the
activation of p38 MAPK in LPS-stimulated RAW 264.7 cells
To determine whether PQ affects the activation of
MAPKs in LPS-stimulated RAW 264.7 cells, the phosphorylation levels
of MAPKs were measured by western blot analysis. Exposure to LPS
markedly increased the phosphorylation of MAPKs in the RAW 264.7
cells. However, treatment with PQ significantly decreased the
phosphorylation of p38 MAPK in the LPS-stimulated RAW 264.7 cells
(Fig. 9).
Discussion
ALI is a major cause of acute respiratory failure
(34) and remains a significant
cause of morbidity and mortality in critically ill patients
(35,36). Infection is the most common cause
of ALI (4). The intranasal
administration of LPS in mice has been reported to induce
neutrophil influx and lung damage (37), and has gained wide acceptance as a
model of ALI and severe lung injury (38). The recruitment and activation of
neutrophils (12) and macrophages
(10) can lead to lung damage by
promoting the generation of ROS (7) and pro-inflammatory mediators
(39). In this study, the
intranasal administration of LPS increased the infiltration of
neutrophils and macrophages in the BALF and in the lungs of mice
with ALI. The administration of LPS also increased the production
of ROS in the BALF of mice with ALI. However, treatment with PQ
attenuated the recruitment of neutrophils and macrophages, as well
as the production of ROS (Figs.
1, 2A and 3A).
Pro-inflammatory cytokines have been reported to
play an important role in the pathogenesis of ALI (40). Increased levels of TNF-α and IL-6
can eventually increase iNOS expression in LPS-induced ALI
(3,41). Increased levels of NO produced by
iNOS are believed to be involved in inflammatory disorders,
including ALI (42). It has been
reported that the inhibition of iNOS attenuates ALI (43). It is also well known that
iNOS-knockout mice have less lung inflammation compared with
wild-type mice (44,45). In the present study, treatment
with PQ reduced the production of pro-inflammatory cytokines, such
as TNF-α and IL-6 in the BALF of mice with LPS-induced ALI and iin
LPS-stimulated RAW 264.7 macrophages (Figs. 2B–D, and 7A and B). PQ also decreased the
expression of iNOS in the lungs of mice with LPS-induced ALI
(Fig. 4A). Therefore, these
results indicate that PQ protects against LPS-induce ALI by
decreasing the production of pro-inflammatory cytokines and
mediators, such as TNF-α, IL-6 and iNOS.
Nrf2 is an antioxidant transcription factor that is
essential for protection against acute pulmonary injury (46). HO-1 is an inducible stress protein
that is induced by Nrf2 and exerts anti-inflammatory effects in ALI
(47). Previous studies have
demonstrated that the induction of HO-1 inhibits the infiltration
of neutrophils and macrophages (48), and the production of
pro-inflammatory cytokines, including TNF-α in response to LPS
(17,20). It is also well known that the
upregulation of HO-1 expression is involved in the inhibitory
effects against LPS-induced iNOS expression (2). The present data demonstrated that
HO-1 expression was significantly increased by treatment with PQ in
the lungs of mice with LPS-induced ALI (Fig. 4B) and in RAW 264.7 macrophages
(Fig. 8B–D). Treatment with PQ
also promoted the Nrf2 nuclear translocation in RAW 264.7
macrophages in a concentration-dependent manner (Fig. 8A). These findings are in
accordance with those of previous studies that demonstrated the
protective role of HO-1 in ALI (49,50).
The MAPK signaling pathways play a crucial role in
the production of pro-inflammatory cytokines and mediators induced
by LPS (13). MAPK
phosphorylation is regarded as a critical step in the expression of
iNOS in LPS-induced ALI in mice (51). MAPKs also play an important role
in the regulation of pro-inflammatory cytokines, such as TNF-α and
IL-6 in ALI induced by LPS in mice (52). Furthermore, the inhibition of MAPK
activation is related to the suppression of MCP-1 expression in
LPS-stimulated RAW 264.7 cells (53). In the present study, PQ suppressed
the phosphorylation of MAPKs in the lungs of mice with LPS-induced
ALI (Fig. 5A and B). PQ also
reduced the activation of p38 MAPK in LPS-stimulated RAW 264.7
macrophages (Fig. 9A).
NF-κB is an important transcription factor
responsible for the expression of a variety of pro-inflammatory
mediators, including iNOS, TNF-α, IL-6 and MCP-1 (54) and its downstream genes have been
associated with various pathological conditions, including ALI
(3). It is well known that LPS
causes the nuclear transcription of the p65 subunit of NF-κB
through IκB degradation (17).
The present data demonstrated that treatment with PQ significantly
suppressed of p65 phosphorylation, as well as IκB degradation in
the lungs of mice with LPS-induced ALI (Fig. 6A). Furthermore, treatment with PQ
inhibited mRNA expression of MCP-1 and the nuclear translocation of
NF-κB in LPS-stimulated RAW 264.7 macrophages in a
concentration-dependent manner (Fig.
7C and D).
In conclusion, the data from the present study
clearly demonstrated that PQ attenuated the infiltration of
neutrophils and macrophages, and reduced the production of
inflammatory mediators, such as ROS, TNF-α, IL-6 and iNOS in an
animal model of LPS-induced ALI. PQ also elevated the expression of
HO-1, and suppressed the activation of NF-κB and MAPKs in the lungs
of mice with ALI. In LPS-stimulated RAW 264.7 macrophages, PQ
suppressed the release of TNF-α and IL-6, and the expression of
MCP-1. PQ also inhibited the nuclear translocation of NF-κB, and
promoted the nuclear translocation of Nrf2 and increased the
expression of HO-1 in RAW 264.7 cells. Furthermore, PQ inhibited
the activation of p38 MAPK in LPS-stimulated RAW 264.7 cells. These
results suggest that PQ may be a valuable therapeutic agent for use
in the treatment of ALI.
Acknowledgments
This study was supported by the KRIBB Research
Initiative Program (KGM 1221521) of the Republic of Korea.
Abbreviations:
|
ALI
|
acute lung injury
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
HO-1
|
heme oxygenase-1
|
|
IL-6
|
interleukin-6
|
|
iNOS
|
inducible nitric oxide synthase
|
|
LPS
|
lipopolysaccharide
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
NF-κB
|
nuclear factor-κB
|
|
PQ
|
Picrasma quassiodes (D. Don)
Benn.
|
|
ROS
|
reactive oxygen species
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Liang D, Dong L, Ge X, Xu F, Chen
W, Dai Y, Li H, Zou P, Yang S and Liang G: Anti-inflammatory
effects of novel curcumin analogs in experimental acute lung
injury. Respir Res. 16:432015. View Article : Google Scholar :
|
|
3
|
Shin NR, Shin IS, Song HH, Hong JM, Kwon
OK, Jeon CM, Kim JH, Lee SW, Lee JK, Jin H, et al: Callicarpa
japonica Thunb. reduces inflammatory responses: a mouse model of
lipopolysaccharide-induced acute lung injury. Int Immunopharmacol.
26:174–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun XJ, Li XQ, Wang XL, Tan WF and Wang
JK: Sevoflurane inhibits nuclear factor-κB activation in
lipopolysaccharide-induced acute inflammatory lung injury via
toll-like receptor 4 signaling. PLoS One. 10:e01227522015.
View Article : Google Scholar
|
|
5
|
Huang X, Tang J, Cai H, Pan Y, He Y, Dai
C, Chen A, Yu X, Chen M, Zou L and Wang L: Anti-inflammatory
effects of monoammonium glycyrrhizinate on
lipopolysaccharide-induced acute lung injury in mice through
regulating nuclear factor-kappa B signaling pathway. Evid Based
Complement Alternat Med. 2015:2724742015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Auten RL, Whorton MH and Nicholas Mason S:
Blocking neutrophil influx reduces DNA damage in hyperoxia-exposed
newborn rat lung. Am J Respir Cell Mol Biol. 26:391–397. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grommes J, Vijayan S, Drechsler M, Hartwig
H, Mörgelin M, Dembinski R, Jacobs M, Koeppel TA, Binnebösel M,
Weber C and Soehnlein O: Simvastatin reduces endotoxin-induced
acute lung injury by decreasing neutrophil recruitment and radical
formation. PLoS One. 7:e389172012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farley KS, Wang LF, Law C and Mehta S:
Alveolar macrophage inducible nitric oxide synthase-dependent
pulmonary microvascular endothelial cell septic barrier
dysfunction. Microvasc Res. 76:208–216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Taneja R, Razavi HM, Law C, Gillis
C and Mehta S: Specific role of neutrophil inducible nitric oxide
synthase in murine sepsis-induced lung injury in vivo. Shock.
37:539–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beck-Schimmer B, Schwendener R, Pasch T,
Reyes L, Booy C and Schimmer RC: Alveolar macrophages regulate
neutrophil recruitment in endotoxin-induced lung injury. Respir
Res. 6:612005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takashima K, Matsushima M, Hashimoto K,
Nose H, Sato M, Hashimoto N, Hasegawa Y and Kawabe T: Protective
effects of intratracheally administered quercetin on
lipopolysaccharide-induced acute lung injury. Respir Res.
15:1502014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grommes J and Soehnlein O: Contribution of
neutrophils to acute lung injury. Mol Med. 17:293–307. 2011.
View Article : Google Scholar :
|
|
13
|
Yang H, Li Y, Huo P, Li XO, Kong D, Mu W,
Fang W, Li L, Liu N, Fang L, et al: Protective effect of
Jolkinolide B on LPS-induced mouse acute lung injury. Int
Immunopharmacol. 26:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akbarshahi H, Rosendahl AH,
Westergren-Thorson G and Anderson R: Acute lung injury in acute
pancreatitis - awaiting the big leap. Respir Med. 106:1199–1210.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chi G, Wei M, Xie X, Soromou LW, Liu F and
Zhao S: Suppression of MAPK and NF-κB pathways by limonene
contributes to attenuation of lipopolysaccharide-induced
inflammatory responses in acute lung injury. Inflammation.
36:501–511. 2013. View Article : Google Scholar
|
|
16
|
Huang GJ, Deng JS, Chen CC, Huang CJ, Sung
PJ, Huang SS and Kuo YH: Methanol extract of Antrodia camphorata
protects against lipopolysaccharide-induced acute lung injury by
suppressing NF-κB and MAPK pathways in mice. J Agric Food Chem.
62:5321–5329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeh CH, Yang JJ, Yang ML, Li YC and Kuan
YH: Rutin decreases lipopolysaccharide-induced acute lung injury
via inhibition of oxidative stress and the MAPK-NF-κB pathway. Free
Radic Biol Med. 69:249–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JW, Kwon JH, Lim MS, Lee HJ, Kim SS,
Lim SY and Chun W: 3,4,5-Trihydroxycinnamic acid increases
heme-oxygenase-1 (HO-1) and decreases macrophage infiltration in
LPS-induced septic kidney. Mol Cell Biochem. 397:109–116. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JW, Bae CJ, Choi YJ, Kim SI, Kwon YS,
Lee HJ, Kim SS and Chun W: 3,4,5-Trihydroxycinnamic acid inhibits
lipopolysaccharide (LPS)-induced inflammation by Nrf2 activation in
vitro and improves survival of mice in LPS-induced endotoxemia
model in vivo. Mol Cell Biochem. 390:143–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yin H, Li X, Gong Q, Jin X, Gu H, Yuan B,
Zhang B, Zheng F, Gong F and Zhu J: Heme oxygenase-1 upregulation
improves lipopolysaccharide-induced acute lung injury involving
suppression of macrophage migration inhibitory factor. Mol Immunol.
47:2443–2449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hashiba T, Suzuki M, Nagashima Y, Suzuki
S, Inoue S, Tsuburai T, Matsuse T and Ishigatubo Y:
Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates
severe lung injury induced by the influenza virus in mice. Gene
Ther. 8:1499–1507. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao W, Yu J, Su Q, Liang J, Zhao L, Zhang
Y and Sun W: Antihypertensive effects of extract from Picrasma
quassiodes (D. Don) Benn. in spontaneously hypertensive rats. J
Ethnopharmacol. 145:187–192. 2013. View Article : Google Scholar
|
|
23
|
Zhao W, Sun C, He J, Chen L, Zhang Y and
Sun W: The possible mechanisms of Picrasma quassiodes (D. Don)
Benn. in the treatment of colitis induced by 2,4,6-trinitrobenzene
sulfonic acid in mice. J Ethnopharmacol. 145:424–430. 2013.
View Article : Google Scholar
|
|
24
|
Zhang Q, Shu X, Jing F, Wang X, Lin C and
Luo A: Preparative separation of alkaloids from Picrasma
quassioides (D. Don) Benn. by conventional and pH-zone-refining
countercurrent chromatography. Molecules. 19:8752–8761. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan H, Qi D, Yang M, Fang H, Liu K and
Zhao F: In vitro and in vivo anti-inflammatory effects of
4-methoxy-5-hydroxycanthin-6-one, a natural alkaloid from Picrasma
quassioides. Phytomedicine. 20:319–323. 2013. View Article : Google Scholar
|
|
26
|
Shin NR, Shin IS, Jeon CM, Hong JM, Oh SR,
Hahn KW and Ahn KS: Inhibitory effects of Picrasma quassioides (D.
Don) Benn. on airway inflammation in a murine model of allergic
asthma. Mol Med Rep. 10:1495–1500. 2014.PubMed/NCBI
|
|
27
|
Liu YL, Liu YJ, Liu Y, Li XS, Liu SH, Pan
YG, Zhang J, Liu Q and Hao YY: Hydroxysafflor yellow A ameliorates
lipopolysaccharide-induced acute lung injury in mice via modulating
toll-like receptor 4 signaling pathways. Int Immunopharmacol.
23:649–657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Farley KS, Wang LF, Razavi HM, Law C,
Rohan M, McCormack DG and Mehta S: Effects of macrophage inducible
nitric oxide synthase in murine septic lung injury. Am J Physiol
Lung Cell Mol Physiol. 290:L1164–L1172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): an overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi M, Galligan C, Tessarollo L and
Yoshimura T: Monocyte chemoattractant protein-1 (MCP-1), not MCP-3,
is the primary chemokine required for monocyte recruitment in mouse
peritonitis induced with thioglycollate or zymosan A. J Immunol.
183:3463–3471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheung DW, Koon CM, Wat E, Ko CH, Chan JY,
Yew DT, Leung PC, Chan WY, Lau CB and Fung KP: A herbal formula
containing roots of Salvia miltiorrhiza (Danshen) and Pueraria
lobata (Gegen) inhibits inflammatory mediators in LPS-stimulated
RAW 264.7 macrophages through inhibition of nuclear factor κB
(NFκB) pathway. J Ethnopharmacol. 145:776–783. 2013. View Article : Google Scholar
|
|
32
|
Kim KH, Kwun MJ, Han CW, Ha KT, Choi JY
and Joo M: Suppression of lung inflammation in an LPS-induced acute
lung injury model by the fruit hull of Gleditsia sinensis. BMC
Complement Altern Med. 14:4022014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang F, Meng Y, Zhang Y, Zhao G, Zheng X,
Xiao Q and Yu Y: Ketamine reduces lipopolysaccharide-induced
high-mobility group box-1 through heme oxygenase-1 and nuclear
factor erythroid 2-related factor 2/p38 mitogen-activated protein
kinase. J Surg Res. 194:599–613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu KD and Matthay MA: Advances in
critical care for the nephrologist: acute lung injury/ARDS. Clin J
Am Soc Nephrol. 3:578–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin S, Merchant ML, Ritzenthaler JD,
McLeish KR, Lederer ED, Torres-Gonzalez E, Fraig M, Barati MT,
Lentsch AB, Roman J, et al: Baclofen, a GABABR agonist, ameliorates
immune-complex mediated acute lung injury by modulating
pro-inflammatory mediators. PLoS One. 10:e01216372015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johnson ER and Matthay MA: Acute lung
injury: epidemiology, pathogenesis, and treatment. J Aerosol Med
Pulm Drug Deliv. 23:243–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Corteling R, Wyss D and Trifilieff A: In
vivo models of lung neutrophil activation. Comparison of mice and
hamsters. BMC Pharmacol. 2:12002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu XX, Yu DD, Chen MJ, Sun T, Li G, Huang
WJ, Nie H, Wang C, Zhang YX, Gong Q and Ren BX: Hesperidin
ameliorates lipopolysaccharide-induced acute lung injury in mice by
inhibiting HMGB1 release. Int Immunopharmacol. 25:370–376. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo L, Li S, Zhao Y, Qian P, Ji F, Qian L,
Wu X and Qian G: Silencing angiopoietin-like protein 4 (ANGPTL4)
protects against lipopolysaccharide-induced acute lung injury via
regulating SIRT1/NF-kB pathway. J Cell Physiol. 230:2390–2402.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu K, Piao T, Wang M, Zhang J, Jiang J,
Wang X and Liu H: Protective effect of catalpol on
lipopolysaccharide-induced acute lung injury in mice. Int
Immunopharmacol. 23:400–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang SY, Xu LT, Li AX and Wang SM:
Effects of ergosterol, isolated from Scleroderma Polyrhizum Pers.,
on lipopolysaccharide-induced inflammatory responses in acute lung
injury. Inflammation. 38:1979–1985. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen T, Mou Y, Tan J, Wei L, Qiao Y, Wei
T, Xiang P, Peng S, Zhang Y, Huang Z and Ji H: The protective
effect of CDDO-Me on lipopolysaccharide-induced acute lung injury
in mice. Int Immunopharmacol. 25:55–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang WZ, Jiang ZK, He BX and Liu XB:
Arctigenin protects against lipopolysaccharide-induced pulmonary
oxidative stress and inflammation in a mouse model via suppression
of MAPK, HO-1, and iNOS signaling. Inflammation. 38:1406–1414.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Harkin DW, Rubin BB, Romaschin A and
Lindsay TF: Selective inducible nitric oxide synthase (iNOS)
inhibition attenuates remote acute lung injury in a model of
ruptured abdominal aortic aneurysm. J Surg Res. 120:230–241. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Speyer CL, Neff TA, Warner RL, Guo RF,
Sarma JV, Riedemann NC, Murphy ME, Murphy HS and Ward PA:
Regulatory effects of iNOS on acute lung inflammatory responses in
mice. Am J Pathol. 163:2319–2328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang T, Huang Z, Chan JY and Zhang DD:
Nrf2 protects against As(III)-induced damage in mouse liver and
bladder. Toxicol Appl Pharmacol. 240:8–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shin IS, Hong J, Jeon CM, Shin NR, Kwon
OK, Kim HS, Kim JC, Oh SR and Ahn KS: Diallyl-disulfide, an
organosulfur compound of garlic, attenuates airway inflammation via
activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression.
Food Chem Toxicol. 62:506–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hualin C, Wenli X, Dapeng L, Xijing L,
Xiuhua P and Qingfeng P: The anti-inflammatory mechanism of heme
oxygenase-1 induced by hemin in primary rat alveolar macrophages.
Inflammation. 35:1087–1093. 2012. View Article : Google Scholar
|
|
49
|
Han CW, Kwun MJ, Kim KH, Choi JY, Oh SR,
Ahn KS, Lee JH and Joo M: Ethanol extract of Alismatis Rhizoma
reduces acute lung inflammation by suppressing NF-κB and activating
Nrf2. J Ethnopharmacol. 146:402–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kung CW, Lee YM, Cheng PY, Peng YJ and Yen
MH: Ethyl pyruvate reduces acute lung injury via regulation of iNOS
and HO-1 expression in endotoxemic rats. J Surg Res. 167:e323–e331.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li KC, Ho YL, Hsieh WT, Huang SS, Chang YS
and Huang GJ: Apigenin-7-glycoside prevents LPS-induced acute lung
injury via downregulation of oxidative enzyme expression and
protein activation through inhibition of MAPK phosphorylation. Int
J Mol Sci. 16:1736–1754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
San Z, Fu Y, Li W, Zhou E, Li Y, Song X,
Wang T, Tian Y, Wei Z, Yao M, et al: Protective effect of
taraxasterol on acute lung injury induced by lipopolysaccharide in
mice. Int Immunopharmacol. 19:342–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sogo T, Terahara N, Hisanaga A, Kumamoto
T, Yamashiro T, Wu S, Sakao K and Hou DX: Anti-inflammatory
activity and molecular mechanism of delphinidin 3-sambubioside, a
Hibiscus anthocyanin. Biofactors. 41:58–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jing W, Chunhua M and Shumin W: Effects of
acteoside on lipopolysaccharide-induced inflammation in acute lung
injury via regulation of NF-κB pathway in vivo and in vitro.
Toxicol Appl Pharmacol. 285:128–135. 2015. View Article : Google Scholar : PubMed/NCBI
|