Introduction
The optimization of immune functions is a lifelong
task for an individual's well-being and is possibly the ultimate
solution for longevity in addition to an individual's genetic
contribution as regards lifespan. There is evidence to indicate
that a group of suppressive T cells
[CD4+CD25+Forkhead box P3 (FoxP3)+
T cells, namely natural regulatory T cells (Tregs); and
CD4+CD25+interleukin (IL)-10+,
CD4+CD25+transforming growth factor
(TGF)-β+ T cells, namely inductive Tregs] is critical in
maintaining immunohomeostasis in elderly subjects (1,2).
During advanced aging, autoantigens are frequently generated from
aged organs and tissues, caused by the decline in the antioxidant
capacity to scavenge harmful free radicals. Progressive
pro-inflammatory conditions or related disease symptoms often have
severe health consequences to aged individuals. To compensate the
overwhelming oxidative stress and inflammation, the immune system
undergoes a profound adaptation by diverting its T effector cell
responses toward CD4+CD25+ Treg-mediated
immunity. The Treg population increases with age and is
significantly higher in centenarians (1,3).
Naturally, Tregs composed 5–10% of CD4 T helper cell populations
possess immunosuppressive functions by secreting surface CD25,
cytotoxic T lymphocyte-associated antigen 4 (CTLA-4; CD152),
glucocorticoid-induced tumor necrosis factor receptor (GITR) and
also producing anti-inflammatory cytokine, such as IL-10 and TGF-β
(4,5). The specific nuclear transcription
factor, FoxP3, is tightly associated with Treg development and
regulatory functions. Tregs can be further divided into
FoxP3-expressing natural Tregs developed in the thymus, and
peripheral-induced Tregs which express low levels of FoxP3, but
mainly generate IL-10 or TGF-β (6,7).
Estrogen synthesized from cholesterol through
steroidogenesis with no gender bias tightly modulates immune cells
of both myeloid and lymphoid origin, thus assisting reproductive
immune tolerance. Estrogen controls the production of
pro-inflammatory mediators, chemokines and chemokine receptor
expression, as well as the regulation of TNF-α and inducible nitric
oxide synthase(iNOS) expression (8,9),
suggesting that estrogen has an anti-inflammatory property. Indeed,
in pregnancy, high levels of estrogen and progesterone levels have
been shown to have ameliorative effects on autoimmune diseases,
whereas pathogenic inflammatory conditions may be exacerbated
during the menstrual cycle due to the decline in estrogen levels.
Moreover, post-menopausal women deficient in estrogen, or estrogen
receptor (ER) knockout or ovariectomized animals are all prone to
developing autoimmune-related diseases (10,11). Although discrepancies in the
lymphoid tissue distributions of the ERα and β isoforms still exist
in different animal species, the ER is expressed in the thymus and
peripheral blood mononuclear cells in response to fluctuating serum
estradiol levels during the estrous cycle.
Due to structural similarities, stilbenes exhibit
phytoestrogenic activities. Among the family of stilbenoids,
resveratrol (Res; trans-3,5,4′-trihydroxystilbene) is most
well-studied for its biological activities. The phytoestrogenic
activity of Res has been well documented and discussed (12–14). Res is abundantly found in grapes,
blueberries, peanuts and in the TCM herb Rhizoma polygoni Cuspidati
(15). Stilbenes are secondary
metabolites produced in peanuts during infection with
Aspergillus flavus (16),
and stilbenes, including Res, isopentadienylresveratrol,
arachidin-1 (Ara-1) and arachidin-3 are available through the
enhanced biosynthesis of floating peanut sprout cultivation
(17). Res is able to compete
with estrogen on ER binding (18)
and activates the ER-mediated phosphoinositide-3 kinase signaling
pathway (19). Ara-1 of the major
peanut stilbenoids possesses anti-inflammatory activity by
inhibiting lipopolysaccharide (LPS)-induced iNOS activity,
CCAAT-enhancer-binding protein (C/EBP)δ expression and nuclear
factor-κB (NF-κB) activation in macrophages (20). Gao et al (21) reported that Res suppressed
cell-mediated cytotoxicity, interferon-γ (IFN-γ) and IL-2
production, as well as concanavalin A (ConA)-induced lymphocyte
proliferation through the nhibition of NF-κB activation.
Immunomodulatory evidence is still lacking to
demonstrate that the stilbenes, Res and Ara-1, may have similar
effects to those of estrogen by altering the Treg ratio and
functional activities. Moreover, the question of whether Ara-1, a
major stilbene derived from peanut sprouts extract would have
better biological activity than Res in immunomodulation remains to
be determined. In order to shed light on this matter, in the
present study, the immunomodulatory activities of the stilbenes,
Res and Ara-1, were assessed. The mechanisms associated with their
phytoestrogenic properties in modulating aging-associated
CD4+CD25+ Treg functions were examined in
both in vitro and in vivo experiments.
Materials and methods
Animals and immune cell culture
Forty healthy female Balb/c mice aged 6–8 weeks were
purchased from the National Laboratory Animal Center and all animal
experimentations met the regulations of the Institution of Animal
Care and Utilization Committee, National Chiayi University, Chiayi,
Taiwan. The animals were raised on standard laboratory rodent chow
(LabDiet, Richmond, VA, USA) were euthanized by an overdose of
CO2, and splenocytes were subsequently harvested by the
Ficoll Histopaque (Sigma Co., Ltd., St. Louis, MO, USA) gradient
separation technique as previously described by Weng et al
(22). Spleno-lymphocytes
suspended in 10% fetal bovine serum (FBS) with RPMI-1640 medium
(phenol red free) (Sigma Co., Ltd.) were automatically counted to
determine the number of live or dead cells by Trypan blue exclusion
assay (Cellometer® Auto T4; Nexcelom Bioscience,
Lawrence, MA, USA) and adjusted live 5×105 cells were
then seeded onto 96-well plates for further assays. All materials,
unless otherwise indicated, were purchased from Sigma Co., Ltd.
Plastic ware was obtained from Corning Co., Ltd. (Corning, NY,
USA). Cell cultures were performed in a humidified, 37°C, 5%
CO2 incubator.
Cytotoxicity of stilbenes to
lymphocytes
MTT assay was used to determine lymphocyte viability
following incubation with Res or Ara-1 [Res and Ara-1 were
biosynthesized and purified by floating peanut sprout cultivation
in our laboratory previously described by Chang et al
(17). A commercial Res was
purchased and used as standard for comparative checking purposes].
Res and Ara-1 (1, 5, 10 and 25 μM) were prepared by
dissolving with dimethyl sulfoxide (DMSO). The final concentration
of DMSO was controlled under 0.1% (v/v). MTT solution (0.5 mg/ml)
was added to each well followed by incubation for 4 h. The MTT
formazan is reduced to purplish formazan crystals, indicating
proliferating cells. This was then dissolved by the addition of a
95% EtOH and DMSO mixture. The absorbance was measured at OD = 570
nm using an ELISA reader (Biotek Instruments, Inc., Winooski, VT,
USA).
ConA-induced lymphocyte proliferation
assay
The T cell mitogen, ConA, was used to
non-specifically activate T cell polyclonal proliferations at a
predetermined sub-optimal concentration of 5 μg/ml. An
aliquot of 100 μl, 5×105 cells purified
lymphocytes from either splenocytes or thymocytes was seeded into
96-well round-bottom plates of a phenol-red free basal medium in
the presence of estrogenic treatments for 24 h prior to mitogen
stimulation. The cells were pre-incubated with either E2
(17-β-estradiol, 160 pM; Cat. no. 3301; Merck, Darmstadt, Germany),
Res at concentrations of 5, 10 and 25 μM, or Ara-1 at
concentrations of 0.5, 1 and 5 μM, and an additional 20
μl ConA was then added for an extended 48 h of incubation at
37°C, 5% CO2 in a humidified incubator. During the
incubation period, 20 μl Alamar Blue Dye™ (Accumed
International Inc., Chicago, IL, USA) were added to each well 24 h
before the end of incubation. The dye is in a blue oxidized form
(resazurin) and is reduced to resorufin with a red color when cells
are proliferating. The level of lymphocyte proliferation was
determined by the change in absorbency when the fluorescent dye was
reduced. The difference between the mean values of absorbence of
duplicates from the unstimulated cell medium and the
ConA-stimulated cell medium was detected using a microplate reader
(FLx800; BioTek, Winooski, VT, USA) to measure the reduced form at
an excitation of 528 nm and the oxidized form at an emission of 590
nm, as previously described (22). The percentage proliferation index
was calculated as follows: the value of the treatment group divided
by that of the control group.
Cytokine concentrations in culture
supernatants
The TGF-β, and IL-10 levels in the supernatant of
ConA-activated lymphocyte repertories of spleno-lymphocytes or
thymocytes were assessed after 48 h of culture. The supernatants
from each assay were stored in −80°C freezer until final analysis.
Specific cytokines were detected by standard enzyme-linked
immunosorbent assay (ELISA) using assay techniques as described in
the manufacturer kit instructions (TGF-β, Cat. no. DY1679; R&D
Systems Inc., USA; and IL-10, Ready-Set-Go kit, Cat. no. 88-7904;
eBioscience, San Diego, CA, USA). Duplicates of each sample were
measured for their cytokine production using a microplate ELISA
reader.
Lymphocyte phenotyping
ConA-stimulated lymphocyte populations of
splenocytes or thymocytes were subjected to flow cytometric
analysis to determine the number of CD25+CD4+
T cell populations. CD3+ cells were firstly gated as the
T lymphocyte compartment using an anti-CD3 antibody conjugated with
FITC anti-CD3 antibody conjugated with FITC (Cat. no. 561798).
Anti-CD4-conjugated PE antibody (Cat. no. 558642) and
anti-CD25-conjugated PerCP-Cy5.5 antibody (Cat. no. 551071) (all
from BD Pharmingen, San Diego, CA, USA) were used to stain the
cells at 4°C for 45 min followed by washing with fresh
phosphate-buffered saline (PBS) with sodium azide. Following
forward and side scatter adjustment on a flow cytometer (FACScan;
Becton-Dickinson, San Jose, CA, USA), the fluorescence and
frequency of FL-1a, FL-2 and FL-3 channels were examined for the
pan CD3 population and two color analysis of
CD25+CD4+ T cells. Values are presented as
the mean percentage of the total cell population of 3 independent
experiments. Three-color fluorescence flow cytometric analysis was
performed to determine CTLA-4 (anti-CD152 conjugated FITC antibody;
BD Pharmingen) expression. Logical gated FL-2- and FL-3-positive
populations were then analyzed for CTLA-4 expression (FL-1). Data
were analyzed using WinMDI® (Free software, Flow
Cytometry Core Facility, Scripps Institute, La Jolla, CA, USA).
Semi-quantitative determination of
specific mRNA expression by RT-PCR
For RNA extraction, 1×106 cells were
collected at designated experimental phases. Total RNA was
extracted using TRIzol reagent following the manufacturer's
instructions (Protech Technology Enterprise Co., Ltd, Taipei,
Taiwan). RNA was reverse transcribed using a cDNA synthesis kit
(Promega, Madison, WI, USA). Synthesized cDNA was then amplified
with Taq polymerase and specific primers for β-actin, FoxP3, CTLA-4
and TGF-β in a thermal cycler. The optimum conditions for RT-PCR
were 58°C for 0.5 min, 60°C for 0.5 min, and 55°C for 0.5 min for
30 cycles for β-actin, 30 cycles for FoxP3, 35 cycles for CTLA-4,
and 30 cycles for TGF-β, respectively, and a final extension at
72°C for 7 min. The primers used in this study and the product size
were 5′-TCTACGAGGGCTATGCTCTCC-3′ (sense) and
5′-GGATGCCACAGGATTCCATAC-3′ (antisense) for β-actin (340 bp);
5′-TTCACCTATGCCACCCTTATCC-3′ (sense) and
5′-GGCTCCTCTTCTTGCGAAACTC-3′ (antisense) for FoxP3 (216 bp);
5′-ATTCACCATCACACAACACT-3′ (sense) and 5′-GGGGCATTTTCACATAGACC-3′
(antisense) for CTLA-4 (483 bp); 5′-CTGTCCAAACTAAGGCTCGC-3′ (sense)
and 5′-CGTCAAAAGACAGCCACTCA-3′ (antisense) for TGF-β (440 bp). A
total of 25 μl of final PCR products was analyzed by
electrophoresis on 1.2% agarose gel in TBE buffer. The bands were
visualized using ethidium bromide and each band was measured and
analyzed using Gel-Pro Analyzer® software (Media
Cybernetics, Silver Spring, MD, USA).
Effect of estrogen receptor blocker on
the stilbene-mediated inhibition of lymphocyte proliferation
4-Hydroxytamoxifen (TAM, Cat. no. H7904; Sigma Co.,
Ltd.) is a known ERα antagonist which mimics endogenous estrogen.
The cells were pre-incubated with 1 μM TAM and then
subjected to co-incubation with the stilbenes for 24 h.
Subsequently, ConA was added for an additional 48 h of incubation
prior to the measurement of lymphocyte proliferation. The same
methodology was used as described above in the lymphocyte
proliferation assay.
Dietary intervention with a
stilbene-fortified diet on sorted Treg functions in aged ICR
mice
Stilbenes were extracted and purified from peanut
sprouts as previously described by Lin et al (23) and Huang et al (24) and fortified into a rodent basal
diet at levels of 15 mg (L-S-PNT; low) and 75 mg (H-S-PNT; high)
per kilogram body weight per day. Peanut sprout powder deficient in
stilbenes following extraction was used as a vehicle control (PNT)
at the same quantity as the treatment groups. The control group
(negative control, NC) was fed basal rodent diet only. Animals were
purchased from the National Laboratory Animal Center, Taiwan, and
all animal experimentations met the regulations of the Institution
of Animal Care and Utilization Committee, National Chiayi
University, Chiayi, Taiwan. A total of 40 12-week-old male ICR mice
were equally assigned into 4 dietary treatment groups as follows:
the control group, the PNT group, the L-S-PNT group and the H-S-PNT
group. The mice were fed on an ad libitum basis for an
entire 48 weeks until they were euthanized by an overdose of
CO2 to determine the ratio of Tregs. For monitoring
purposes, measurements of body weight and blood biochemicals, and
hematological analysis were performed, and the results did not
reveal any statistically significant differences among the
treatment groups (data not shown). Spleno-lymphocyte Treg subset
analysis was performed as described above. The spleens of 3 mice
from each group were aseptically removed and forced through a
stainless steel wire mesh. Spleno-lymphocytes were then harvested
by Ficoll gradient separation. The spleno-lymphocytes of 3 mice
were pooled for CD4+CD25+ Treg enrichment by
magnetic bead separation. The CD4+CD25+ Treg
cell enrichment protocol was performed according to the
manufacturer's recommendations (regulatory T cell isolation kit,
Cat. no. 130-091-041; Miltenyi Biotec Inc., Bergisch Gladbach,
Germany). Briefly, non-CD4+ cells were removed
magnetically with a cocktail of biotin-labeled antibodies and
anti-biotin microbeads using a large column. Subsequently,
CD25-positive selection was performed using specific
microbead-labeled antibody using a small column. Enriched Tregs
were then subjected to RNA extraction for the determination of
CTLA-4 and FoxP3 expression as described above.
Statistical analysis
All data were conducted using the GLM model
procedure (SAS Institute, 1996) for statistical analysis.
Significant differences among the treatment groups were determined
using Fisher's LSD test. Values represented in the bar graphs are
the means ± standard deviation (SD). For the in vitro
experiments, the Student's t-test was used for paired comparisons
among experimental groups.
Results and Discussion
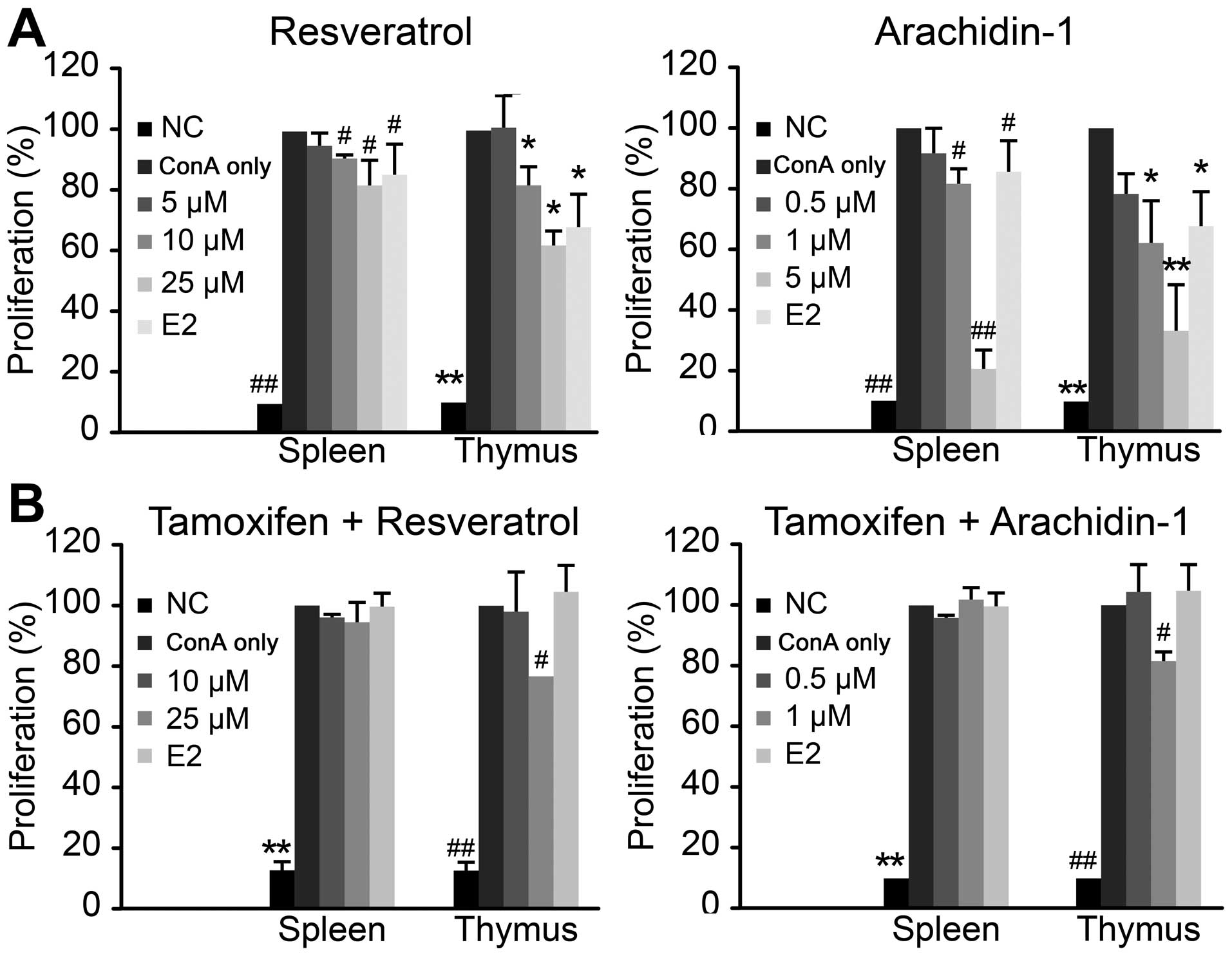
Immunotoxicity of stilbenes and their
inhibitory effects on ConA-induced lymphoblastogenesis
The silbenes, Res or Ara-1, extracted from peanut
sprout powder were purified (17,24) and assessed for their cytotoxicity
to murine lymphocytes (Fig. 1).
The IC50 value of Ara-1 was approximately 25 μM, while
little or no toxicity was observed with Res at the same
concentration. Ara-1 has a chemical structure similar to Res, but
an additional hydroxyl and isopentenyl moiety, which possibly
facilitates a better biological potency. It is not chemically
synthesized and has been shown to inhibit LPS-induced nitric oxide
production in murine macrophages by reducing the expression of
C/EBPδ and NF-κB signaling at concentrations <15 μM
(20). Hence, the concentrations
of Res and Ara-1 used in the ex vivo experiments were
maximum at 25 and 5 μM, respectively.
To reduce progressive T cell responses to
aging-associated self-antigens, Tregs are largely expanded in
successfully aged individuals to offset the pathogenic autoimmune
diseases resulting from overreactive T cells. The non-specific
polyclonal activation of T cell blastogenesis by ConA was setup as
a 72-h culture period with an initial 24 h of priming with
different concentrations of Res, Ara-1, E2 or medium only (ConA
only) prior to 48 h of stimulation with ConA. T cell proliferation
was presented as an index of proliferation (%) as a percentage of
the proliferation of ConA-stimulated lymphocytes. Pre-culture with
160 pM E2 exerted an approximately 20 and 40% inhibitory effect on
lymphoblastogenesis in the spleen and thymus respectively. All
levels of Res, apart from the lowest level, exerted a significant
(P<0.05) inhibitory effect on ConA-stimulated T cell
proliferation. At the concentration of 25 μM, Res exerted a
similar effect as E2, and the inhibitory effects were more
prominent on the lymphocytes from the thymus than those from the
spleen (Fig. 2A). Similar to the
inhibitory effects of E2 and Res, Ara-1 exerted a more prominent
(P<0.001) inhibitory effect on lymphoblastogenesis at the
concentration of 5 μM, whereas Res did not affect
ConA-stimulated lymphoblastogen-esis at the same concentration. In
general, the inhibitory effects were more prominent on the thymus
lymphocytes in all estrogenic treatments. Both stilbenes did not
exert any cytotoxic effects on the lymphocytes at the concentration
range tested in the present experiment; thus, the inhibition of
cell proliferation due to cytotoxicity was devoid. It has been
reported that E2 and diethylstilbestrol (DES) exert suppressive
modulatory effects on lymphocyte activation and blastogenic
responses, and alter the peripheral T cell repertoire by altering
thymic selection (25,26). In addition, a previous study using
20 μM Res demonstrated that ConA-induced lymphocyte
proliferation was suppressed (21), which may be attributed to the
decreased expression of the T cell co-stimulatory molecules, CD28,
and CD80 of antigen-presenting cells (3,21).
CTLA-4 is known to compete with CD28 in their ligands and mediates
immunosuppression that is often observed with upregulated
regulatory T cell activity (31).
Whether CTLA-4 expression causes the unresponsiveness to
ConA-induced lymphoblastogenesis shall be firstly tested if the
inhibitory efffects of stilbenes on ConA-activated
lymphoblastogenesis is attributed to their estrogenic
properties.
Recovery of stilbene-mediated suppression
of lymphoblastogenesis by tamoxifen
Mixed lymphocyte reaction (MLR) resembles the
lymphocyte proliferation reaction and is downregulated by elevated
estrogen concentrations, and is known to be essential in minimizing
allograft rejection for successful pregnancy. Res acts as a
phytoestrogen, exerting agonistic effects with ER in lymphocytes to
modulate mitogen-induced T cell proliferation (21). Similarly, our results demonstrated
that the ConA-induced lymphoblastogenesis of spleno- or thymic
lymphocytes was suppressed by Res and Ara-1 in a dose-dependent
manner, and the inhibitory effects were more promiment with Ara-1
at a concentration range of 0.5 to 5 μM (Fig. 2A). When the lymphocytes were
pre-incubated with tamoxifen, a known ERα blocker, the suppressive
effects of Res and Ara-1 on ConA-induced lymphoblastogenesis were
attenuated, and lymphoblastogenesis returned to levels similar to
those of the ConA only group treated with ConA alone (Fig. 2B). Moreover, partial recovery was
observed in the thymic lymphocytes treated with high levels of Res
(25 μM) or Ara-1 (5 μM), which indicated that the
thymus is more prone to estrogenic modulation. The recovery of
lymphoblastogenesis by pre-treatment with tamoxifen indicated that
the immunosuppressive effects of the stilbenes was similar to those
of E2 and that they are mediated through the ER-mediated cellular
signaling pathway. ER has two isoforms, ERα and ERβ, which are
differentially distributed in various tissues. In the thymus, ERβ
expression is high and that of ERα is low (28). Res binds to ERα and ERβ with
similar affinity. In a previous study, at concentrations of 100
μM, Res was shown to exert an inhibitory effect on the
proliferation of CHO-K1 cells transfected with either ERα or ERβ
(12). Moreover, tamoxifen and
the non-steroidal estrogen, DES, are known to be ligands exclusive
to the ERα receptor containing the C-terminal ligand binding domain
(27). In immune organs, the
spleen and thymus have higher a mRNA expression of ERβ than ERα
(28); however, epithelial cells
of these tissues primarily express ERβ, whereas ERα is mainly
expressed in CD4 and CD8 T cells (29). In the present study,
pre-incubation with tamoxifen effectively repressed the subsequent
stilbene modulation of T lymphocyte functions. Additional pathways
through other targeted receptors or cellular protein molecules of
Res (those that are beyond ER-mediated activities), such as the
inhibition of tyrosine kinase in cell signal transduction occur
particularly during high concentration of stilbenes (13) should also be considered.
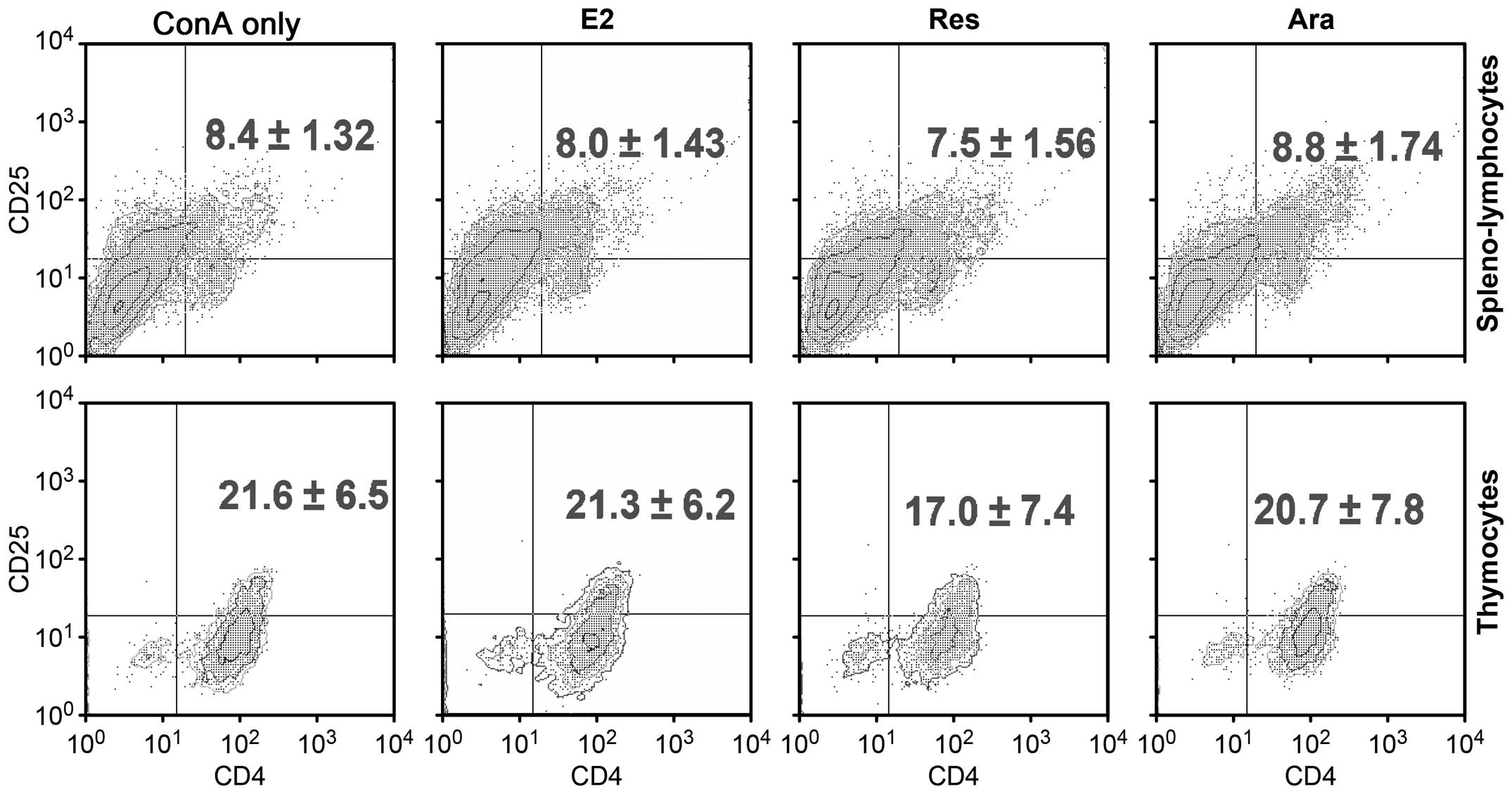
Stilbenes do not affect the
CD4+CD25+ regulatory T cell population in the
ConA-activated T lymphocyte repertoire
It has been reported that the expansion of Tregs
during the late menstrual cycle is tightly correlated with plasma
estradiol levels (30). To
determine whether phytoestrogenic stilbenes alter the Treg ratio,
we analyzed the repertoire of Tregs in the ConA-stimulated thymic
or spleno- lymphocytes pre-treated with E2, Res or Ara-1. Flow
cytometry detected an approximately 10% increase in the
CD4+CD25+ T cell population following
stimulation with ConA (ConA only group) when compared with the
unstimulated lymphocyte population (data not shown). Following
stimulation with ConA, the CD25+ T cell population was
markedly increased (data not shown). Anti-CD25 antibody is specific
for capturing the IL-2α subunit. IL-2 receptor expression increases
in proliferating T cells in an IL-2 autocrine manner (6). In this study, to obtain stably
expanded T cell colonies at a resting state, samples were replaced
with fresh medium for a 12-h extention of culture prior to flow
cytometric analysis. As shwon in Fig.
3, no significant changes were observed in the
CD4+CD25+ T cell ratio among the E2-, Res- or
Ara-1-treated cells as compared to the ConA only group
(ConA-activated lymphoblast repertoire of either spleno- or thymic
lymphocytes). It has been suggested that
CD4+CD25+ cells remain at a fixed ratio
relative to CD4−CD25+ cells, even though the
absolute number of a particular T cell is clonally expanded
(31). In addition, it has been
reported that follicular or gestational high-level E2 may expand
Treg compartments to maintain an immunological tolerance (30). In the present study, lymphocytes
pre-treated with E2 at a physiological gestation level of 160 pM,
or with Res or Ara-1 had an overall lower proliferative index than
the ConA only group. This may be attributed to the inhibitory
effects of estrogenic stilbenes on the
CD4+CD25+ Treg cell populations. For better
elucidation, we further characterized Treg cell functional activity
by the unique nuclear transcription factor, FoxP3, and their
essentially expressed inhibitory molecule, CTLA-4 as affected by
phytoestrogenic stilbenes.
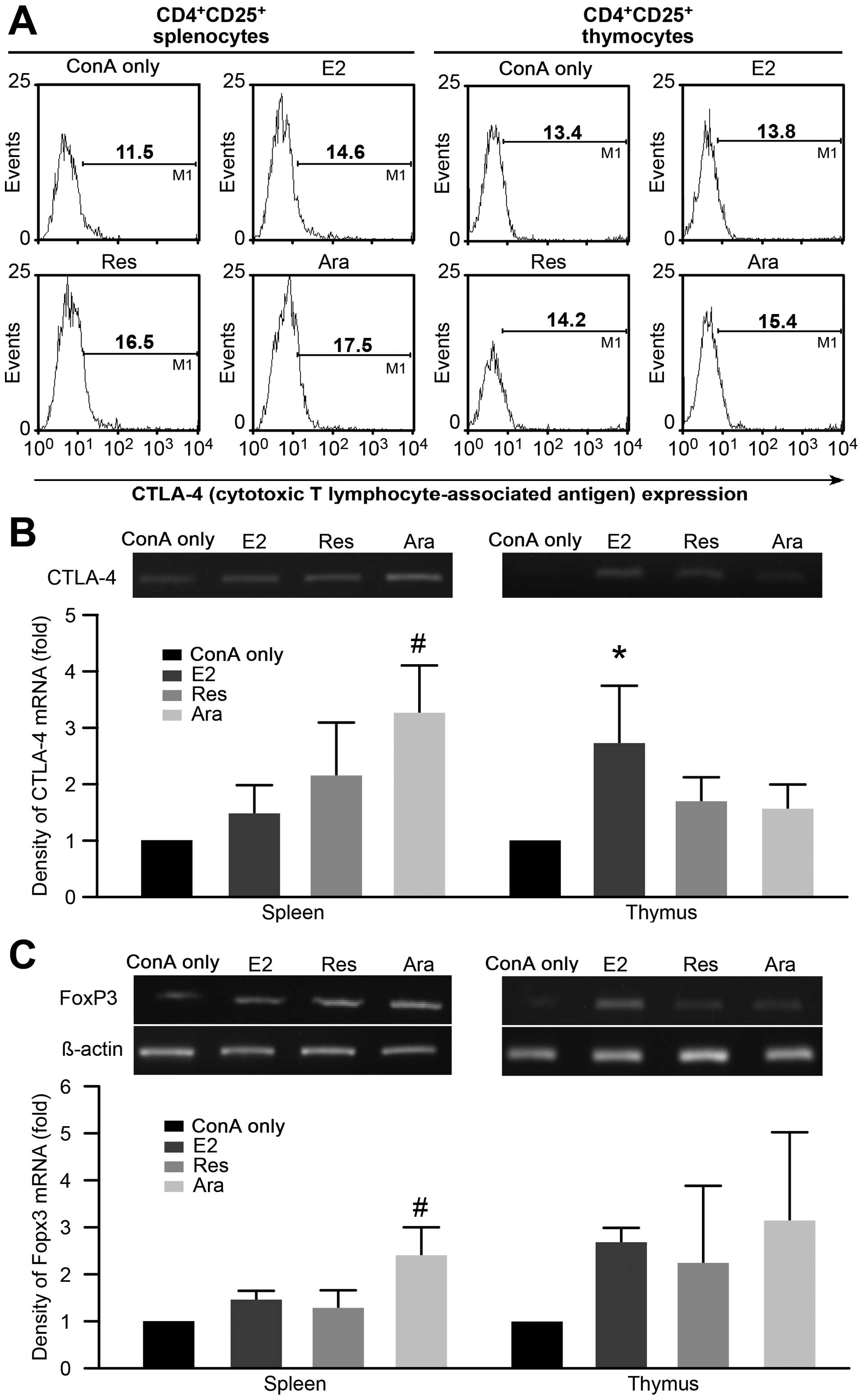
Functional activity of Tregs treated with
stilbenes in the ConA-activated T lymphocyte repertoire
CTLA-4 is well documented as a contact inhibitory
molecule expressed in Tregs. CTLA-4 expression in the
CD4+CD25+ T cells from the ConA-stimulated
lymphocytes from the E2-, Res-, or Ara-1-treated groups was
examined and the results are shown in Fig. 4A. Flow cytometric histograms
demonstrated that the lymphocytes pre-cultured with E2, Res or
Ara-1 had an increased the mean ratio of CTLA-4-expressing
populations following stimulation with ConA. It should be noted
that the T cells analyzed in the present study were pre-cultured
with E2, Res or Ara-1 for 24 h prior to a 48 h of stimulation with
ConA, followed by an additional 12 h of culture in fresh medium by
replacing mitogen containing medium with fresh RPMI-1640 basal
medium (without FBS). This is to allow mitogen-activated T cells to
return to a resting state for better estimation of the
CTLA-4-expressing population. Moreover, the semi-quantitative
analysis of the gene expression of CTLA-4 was also carried out and
results are shown in Fig. 4B. The
results of the density of CTLA-4 mRNA expression exhibited a
>50% increase (P<0.05) in the Ara-1 pre-treated
spleno-lymphocytes as compared to the ConA only group. Although the
Res-treated cells exhibited a similar enhancement as the
Ara-1-treated cells, the effective concentration of Res (25
μM) was much higher than that of Ara-1 (5 μM). In
general, thymocytes were less responsive to stilbene modulation,
while E2 exerted more prominent (P<0.05) promoting effects on
CTLA-4 gene expression. It has been reported that CTLA-4 gene
expression protects mice from experimental autoimmune
encephalomyelitis (32). CTLA-4
expression has also been shown tobe upregulated in the
CD4+CD25+ Tregs of pregnant women (33). Using concentrations of 1 to 20
μM of Res, Sharma et al (34) did not demonstrate changes in
CTLA-4 expression in ConA-stimulated T cells, but repressed
lymphocyte proliferation by the downregulation of the expression of
CD28 and CD80. The discrepancy in the modulatory effects of Res on
CTLA-4 expression require further investigation. Ara-1 belongs to
the family of stilbenoids and is structurally similar to Res, but
with an additional hydroxyl group and terpene tail; it exhibited
greater potency than Res in stimulating CTLA-4 expression.
Moreover, we also demonstrated that FoxP3 gene activity was
upregulated by E2, as well as by Res and Ara-1 in both the
ConA-activated spleno- and thymic lymphocytes (Fig. 4C). The FoxP3-transduced Tregs
(natural Tregs) are found in high numbers in mice positive for
estrogen (17-β-estradiol, E2), and FoxP3 is downregulated in
ERα-deficient animals (35). In
this study, we demonstrated that the stilbenes, Res and Ara-1,
acted in a manner similar to E2 in promoting FoxP3 activity, and
this effect was more prominent in thymocytes. Natural Tregs
developed in the thymus known to express FoxP3 compared to
peripheral polarized Tregs, which mainly produce the cytokines,
IL-10 and TGF-β. Hence, we further investigated whether the
production of IL-10 and TGF-β by Tregs is also affected by
treatment with stilbenes, which may contribute to peripheral
tolerance in leading to successful aging.
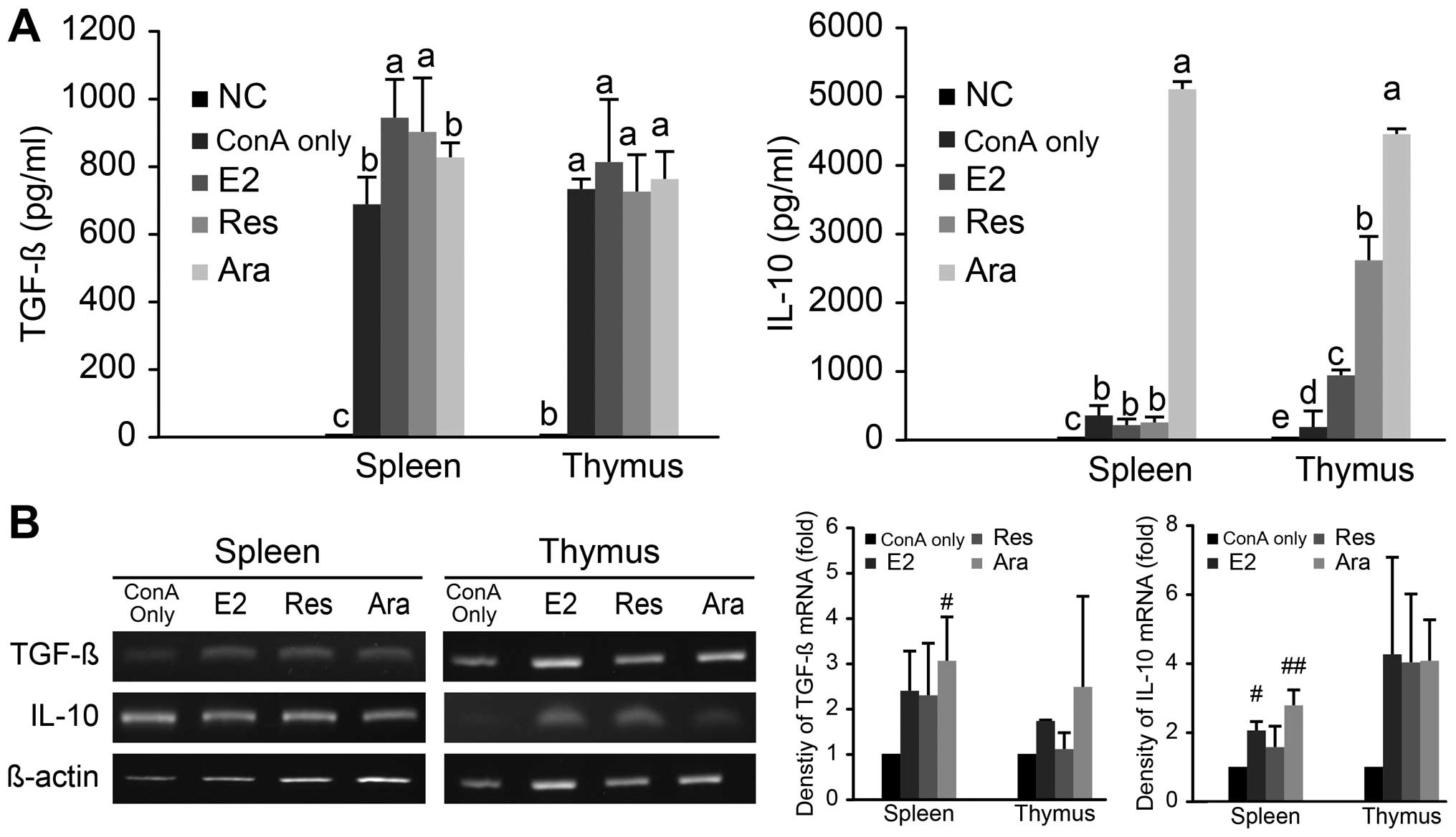
Ara-1 promotes the production of TGF-β
and IL-10 in the ConA-activated T lymphocyte repertoire
TGF-β and IL-10 are anti-inflammatory cytokines and
control pathogenic, as well as aging-associated inflammatory
conditions. They are selectively produced by peripheral Treg
subsets as distant mediators of immunosuppression. The productions
of TGF-β and IL-10 in ConA-activated spleno- and thymic
lymphocytes, as affected by E2, Res, or Ara-1 is shown in Fig. 5A. TGF-β production by the
ConA-stimulated spleno-lymphocytes was significantly (P<0.05)
elevated following treatment with E2 (160 pM) or Res (25
μM), but not with Ara-1 (5 μM). On the other hand,
Ara-1 significantly stimulated IL-10 production in both the
ConA-activated spleno- and thymic lymphocytes as compared to
treatment with E2, Res and ConA alone. In the ConA-activated thymic
lymphocytes, IL-10 was upregulated in an order of significance
which was Ara-1 > Res > E2 > ConA only treatment. The
effects of E2 and the stilbenes on TGF-β and IL-10 mRNA levels were
similar, and were exerted in an elevating manner (Fig 5B). Previous findings have indicated
that physiological levels of estrogen can induce the expansion of
CD4+CD25+ Tregs and the upregulation of FoxP3
and IL-10 genes expression in in vitro setting, which is
attributed to the suppression of T cell proliferation in a mixed
lymphocyte reaction (36). Our
result was in agreement with that study, in that IL-10 production
was upregulated by E2; nevertheless, both phytoestrogenic Res and
Ara-1 exhibited even more superior biological activity as regards
IL-10 production in ConA-activated T lymphocytes. In particular,
Ara-1 used at a relatively lower concentration of 5 μM than
that of Res at 25 μM led to a >5-fold greater increase in
the IL-10 level. It has been reported that Res binds ERα and ERβ
with comparable affinity, but with a 7,000-fold lower affinity than
E2 (12). Whether the estrogen
receptor binding affinity of Ara-1 is higher requires further
investigation. Moreover, DES is a synthetic compound that is
chemically similar to stilbenes. It has potent estrogenic activity
via ERα, and has been reported to alter the T cell repertoire in
the peripheral blood by influencing thymic selection of T cell
development (26). Taken
together, Res and Ara-1 of the stilbenoid family exhibited similar
properties as those of E2 in modulating the production of TGF-β and
IL-10, and their immunomodulatory effects were exerted at the mRNA
level. Ara-1 seems to be more potent than Res in promoting the
immunosuppressive cytokines, TGF-β and particularly IL-10
production in lymphocytes. The phytoestrogenic properties of
stilbenes hold immunological promises in complying with the
successful aging phenomenon of Treg dominant immunity.
Long-term dietary supplementation of
peanut sprout powder rich in stilbenes boosts Treg activity in aged
ICR mice
Stilbenes are naturally abundant in a wide variety
of plant species, and are produced as phytoalexin via the
phenylpropanoid synthesis pathway to protect against microbial
infections (15). In previous
studies, we demonstrated that Res, Ara-1, arachidin-2 and
archidin-3 are abundantly found in peanut sprouts and possess
antioxidant properties (15,17). In this study, dietary
supplementation experiments, by providing either a basal diet,
peanut sprout only (PNT) diet or a peanut sprout diet fortified
with additional 15 mg/kg body weight/day (L-S-PNT) or 75 mg/kg body
weight/day (H-S-PNT) of stilbenes extracts, were conducted. Adult
male ICR mice fed the experimental diets for 48 weeks were at their
last trimester of a known 2-year estimated lifespan of murine
species. CD4+CD25+ T cell subset phenotyping
of lymphocytes harvested from the spleen was carried out as shown
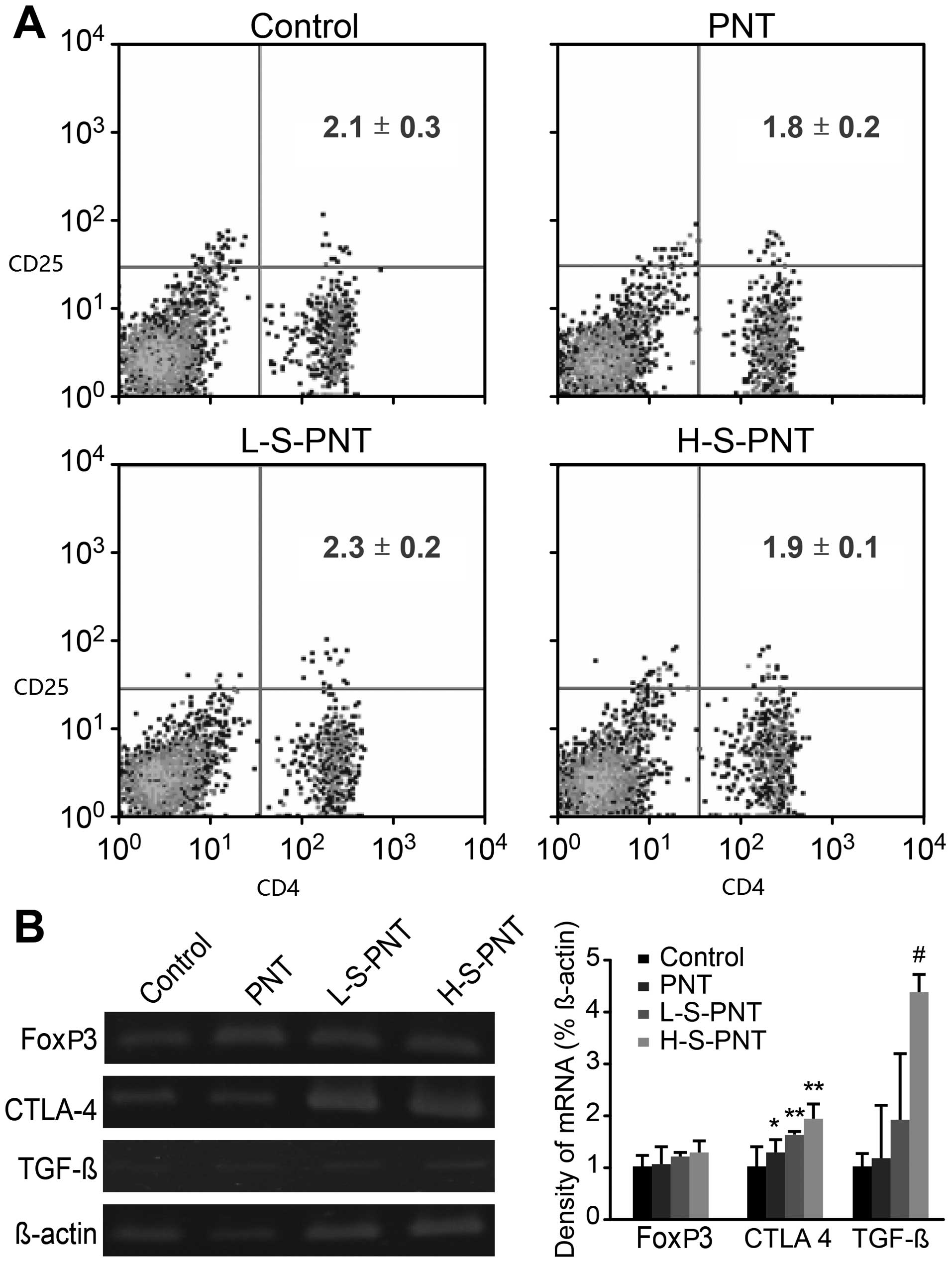
in Fig. 6A. In general, the aging
mice fed either the low or high stilbene-rich diet had a similar
ratio of the CD4+CD25+ T cell population as
those of the control or PNT groups. Moreover, the inhibitory
molecules, CTLA-4 and TGF-β, are crucial to Treg suppressive
functions. TGF-β is required in the development of both natural and
induced Tregs of thymic or peripheral origins (37). In this study, both the L-S-PNT and
H-S-PNT groups had Tregs which highly expressed TGF-β mRNA; the
H-S-PNT group exhibited significantly (P<0.05) higher levels
than the control group (Fig. 6B).
Although FoxP3 gene expression did not differ among the groups, the
expression of CTLA-4, which is known to be constitutively expressed
in Tregs, was significantly upregulated in the aged ICR mice fed
either PNT (P<0.05), L-S-PNT (P<0.001) or H-S-PNT
(P<0.001). Since the stilbenes were removed in the PNT group,
some minor factors may exist in peanut sprouts that may increase
CTLA-4 expression. Tregs may interact with CD80 or CD86 in
dendritic cells in a CTLA-4 dependent manner, which renders
indoleadmine 2, 3-dioxygenase production in dendritic cells to
induce the catabolism of tryptophan into pro-apoptotic metabolites,
resulting in T effector cell apoptosis (38). Baeza et al (39) suggested that estrogen or
phytoestrogen treatments are beneficial to aging or overiectomzied
rats by affecting lymphocyte proliferation and NK cell activity.
Immunological benefits to aging individuals were not associated
with Treg populations, but emphasized their antioxidant abilities.
Recently, Wang et al (40)
reported that the peripheral blood Treg number and CTLA-4 gene
expression were increased in mice with obesity due to a high-fat
diet by Res supplementation. In this study, in our ex vivo
experiments, Ara-1 was shown to be much more potent than Res in
promoting IL-10 and CTLA-4 expression, but not TGF-β. Unlike immune
organs, the spleen and thymus have a higher ERβ mRNA expression
than ERα (28), whereas ERα is
mainly expressed in CD4 and CD8 T cells (29). In the present study, due to thymus
involution in aging mice, thymic samples were not sufficient for
analysis, which also indirectly explained why FoxP3 gene expression
was low and there was no difference in FoxP3 expression among the
treatment groups. Dietary peanut sprout powder rich in stilbenes
exerted potent effects on peripheral induced-Treg functions in aged
mice, and the upregulation of CTLA-4 and TGF-β in Tregs is
essential for controling chronic inflammation.
In conclusion, the data demonstrate that
Treg-mediated immunosuppression in aged individuals is crucial in
managing increased pro-inflammatory conditions resulting from
higher ratios of memory T cell and effector T cell activities
reacting to excessive autoantigens in aged individuals. Res of the
stilbene family has been reported to extend lifespan in several
species, particularly in mice fed a high-calorie diet (41). Although some controversial aspects
of the effects of Res on lifespan were raised against dietary
calorie-restriction in the elderly (40,42) the results of the present study
provided an alternative mechanism by its potent phytoestrogenic
activity on Treg cell activity. Upregulated Tregs are dominant in
centenarians and are associated with longevity. Arachidin-1 is
abundantly found in peanut sprouts and has a chemical structure
similar to tht of Res, but with an additional hydroxyl and
isopentenyl groups. It exhibited more potent phytoestrogenic
property in the modulation Treg functions than Res, as demonstrated
in this study. Thus, Res and Ara-1 may be beneficial in successful
aging and longevity through their phytoestrogenic activity. They
are potent in modulating regulatory T cell inhibitory functions by
upregulating CTLA-4, TGF-β and IL-10 expression.
Acknowledgments
The present study was supported by the NSC
97-2321-B-415-00 grant, the Ministry of Science and Technology
(former name National Science Council), Taiwan, R.O.C.
References
|
1
|
Gregg R, Smith CM, Clark FJ, Dunnion D,
Khan N, Chakraverty R, Nayak L and Moss PA: The number of human
peripheral blood CD4+ CD25high regulatory T
cells increases with age. Clin Exp Immunol. 140:540–546. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao L, Sun L, Wang H, Ma H, Liu G and
Zhao Y: Changes of CD4+CD25+Foxp3+
regulatory T cells in aged Balb/c mice. J Leukoc Biol.
81:1386–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma S, Dominguez AL and Lustgarten J:
High accumulation of T regulatory cells prevents the activation of
immune responses in aged animals. J Immunol. 177:8348–8355. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakaguchi S: Naturally arising
CD4+ regulatory T cells for immunologic self-tolerance
and negative control of immune responses. Annu Rev Immunol.
22:531–562. 2004. View Article : Google Scholar
|
|
5
|
Miyara M and Sakaguchi S: Natural
regulatory T cells: Mechanisms of suppression. Trends Mol Med.
13:108–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wan YY and Flavell RA: The roles for
cytokines in the generation and maintenance of regulatory T cells.
Immunol Rev. 212:114–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dario AA: Vignali, Lauren W. Collision,
and Creg J. Workman: How regulatory T cells work. Nat Rev Immunol.
8:523–532. 2008. View
Article : Google Scholar
|
|
8
|
Hunt JS, Miller L, Roby KF, Huang J, Platt
JS and DeBrot BL: Female steroid hormones regulate production of
pro-inflammatory molecules in uterine leukocytes. J Reprod Immunol.
35:87–99. 1997. View Article : Google Scholar
|
|
9
|
Lengi AJ, Phillips RA, Karpuzoglu E and
Ahmed SA: Estrogen selectively regulates chemokines in murine
splenocytes. J Leukoc Biol. 81:1065–1074. 2007. View Article : Google Scholar
|
|
10
|
Pacifici R: Estrogen deficiency, T cells
and bone loss. Cell Immunol. 252:68–80. 2008. View Article : Google Scholar
|
|
11
|
Cunningham M and Gilkeson G: Estrogen
receptors in immunity and autoimmunity. Clin Rev Allergy Immunol.
40:66–73. 2011. View Article : Google Scholar
|
|
12
|
Bowers JL, Tyulmenkov VV, Jernigan SC and
Klinge CM: Resveratrol acts as a mixed agonist/antagonist for
estrogen receptors alpha and beta. Endocrinology. 141:3657–3667.
2000.PubMed/NCBI
|
|
13
|
Roupe KA, Remsberg CM, Yáñez JA and Davies
NM: Pharmacometrics of stilbenes: Seguing towards the clinic. Curr
Clin Pharmacol. 1:81–101. 2006. View Article : Google Scholar
|
|
14
|
Michel T, Halabalaki M and Skaltsounis AL:
New concepts, experimental approaches, and dereplication strategies
for the discovery of novel phytoestrogens from natural sources.
Planta Med. 79:514–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiou RY: Review: Resveratrol, a promising
phytochemical in grapes, grape juices, wines and peanuts. Food Sci
Agric Chem. 4:8–14. 2002.
|
|
16
|
Wotton HR and Strange RN: Increased
susceptibility and reduced phytoalexin accumulation in
drought-stressed peanut kernels challenged with Aspergillus flavus.
Appl Environ Microbiol. 53:270–273. 1987.PubMed/NCBI
|
|
17
|
Chang JC, Lai YH, Djoko B, Wu PL, Liu CD,
Liu YW and Chiou RY: Biosynthesis enhancement and antioxidant and
anti-inflammatory activities of peanut (Arachis hypogaea L.)
arachidin-1, arachidin-3, and isopentadienylresveratrol. J Agric
Food Chem. 54:10281–10287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gehm BD, McAndrews JM, Chien PY and
Jameson JL: Resveratrol, a polyphenolic compound found in grapes
and wine, is an agonist for the estrogen receptor. Proc Natl Acad
Sci USA. 94:14138–14143. 1997. View Article : Google Scholar
|
|
19
|
Pozo-Guisado E, Lorenzo-Benayas MJ and
Fernández-Salguero PM: Resveratrol modulates the phosphoinositide
3-kinase pathway through an estrogen receptor alpha-dependent
mechanism: Relevance in cell proliferation. Int J Cancer.
109:167–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Djoko B, Chiou RYY, Shee JJ and Liu YW:
Characterization of immunological activities of peanut stilbenoids,
arachidin-1, piceatannol, and resveratrol on
lipopolysaccharide-induced inflammation of RAW 264.7 macrophages. J
Agric Food Chem. 55:2376–2383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao X, Xu YX, Janakiraman N, Chapman RA
and Gautam SC: Immunomodulatory activity of resveratrol:
Suppression of lymphocyte proliferation, development of
cell-mediated cytotoxicity, and cytokine production. Biochem
Pharmacol. 62:1299–1308. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weng BB, Lin YC, Hu CW, Kao MY, Wang SH,
Lo DY, Lai TY, Kan LS and Chiou RYY: Toxicological and
immunomodulatory assessments of botryosphaeran (β-glucan) produced
by Botryosphaeria rhodina RCYU 30101. Food Chem Toxicol.
49:910–916. 2011. View Article : Google Scholar
|
|
23
|
Lin BS, Lien TF, Chao MR, Lai YH, Chang
JC, Chou SJ, Liao HF and Chiou RYY: Toxicological and nutraceutical
assessments of peanut sprouts as daily supplments to feed
Sprague-Dawley rats for 18 weeks. J Sci Food Agric. 88:2201–2207.
2008. View Article : Google Scholar
|
|
24
|
Huang CP, Au LC, Chiou RYY, Chung PC, Chen
SY, Tang WC, Chang CL, Fang WH and Lin SB: Arachidin-1, a peanut
stilbenoid, induces programmed cell death in human leukemia HL-60
cells. J Agric Food Chem. 58:12123–12129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pfeifer RW and Patterson RM: Modulation of
lectin-stimulated lymphocyte agglutination and mitogenesis by
estrogen metabolites: Effects on early events of lymphocyte
activation. Arch Toxicol. 58:157–164. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown N, Nagarkatti M and Nagarkatti PS:
Diethylstilbestrol alters positive and negative selection of T
cells in the thymus and modulates T-cell repertoire in the
periphery. Toxicol Appl Pharmacol. 212:119–126. 2006. View Article : Google Scholar
|
|
27
|
Shiau AK, Barstad D, Loria PM, Cheng L,
Kushner PJ, Agard DA and Greene GL: The structural basis of
estrogen receptor/coactivator recognition and the antagonism of
this interaction by tamoxifen. Cell. 95:927–937. 1998. View Article : Google Scholar
|
|
28
|
Brandenberger AW, Tee MK, Lee JY, Chao V
and Jaffe RB: Tissue distribution of estrogen receptors alpha
(ER-alpha) and beta (ER-beta) mRNA in the midgestational human
fetus. J Clin Endocrinol Metab. 82:3509–3512. 1997.PubMed/NCBI
|
|
29
|
Adori M, Kiss E, Barad Z, Barabás K,
Kiszely E, Schneider A, Kövesdi D, Sziksz E, Abrahám IM, Matkó J,
et al: Estrogen augments the T cell-dependent but not the
T-independent immune response. Cell Mol Life Sci. 67:1661–1674.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arruvito L, Sanz M, Banham AH and Fainboim
L: Expansion of CD4+CD25+and
FOXP3+ regulatory T cells during the follicular phase of
the menstrual cycle: Implications for human reproduction. J
Immunol. 178:2572–2578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walker LS: CD4+
CD25+ Treg: Divide and rule? Immunology. 111:129–137.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matejuk A, Dwyer J, Zamora A, Vandenbark
AA and Offner H: Evaluation of the effects of 17beta-estradiol
(17beta-e2) on gene expression in experimental autoimmune
encephalomyelitis using DNA microarray. Endocrinology. 143:313–319.
2002.
|
|
33
|
Mjösberg J, Svensson J, Johansson E,
Hellström L, Casas R, Jenmalm MC, Boij R, Matthiesen L, Jönsson JI,
Berg G and Ernerudh J: Systemic reduction of functionally
suppressive CD4dimCD25highFoxp3+
Tregs in human second trimester pregnancy is induced by
progesterone and 17beta-estradiol. J Immunol. 183:759–769. 2009.
View Article : Google Scholar
|
|
34
|
Sharma S, Chopra K, Kulkarni SK and
Agrewala JN: Resveratrol and curcumin suppress immune response
through CD28/CTLA-4 and CD80 co-stimulatory pathway. Clin Exp
Immunol. 147:155–163. 2007.
|
|
35
|
Polanczyk MJ, Carson BD, Subramanian S,
Afentoulis M, Vandenbark AA, Ziegler SF and Offner H: Cutting edge:
Estrogen drives expansion of the CD4+CD25+
regulatory T cell compartment. J Immunol. 173:2227–2230. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tai P, Wang J, Jin H, Song X, Yan J, Kang
Y, Zhao L, An X, Du X, Chen X, et al: Induction of regulatory T
cells by physiological level estrogen. J Cell Physiol. 214:456–464.
2008. View Article : Google Scholar
|
|
37
|
Vignali DAA, Collison LW and Workman CJ:
How regulatory T cells work. Nat Rev Immunol. 8:523–532. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mellor AL and Munn DH: IDO expression by
dendritic cells: Tolerance and tryptophan catabolism. Nat Rev
Immunol. 4:762–774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baeza I, Alvarado C, Alvarez P, Salazar V,
Castillo C, Ariznavarreta C, Fdez-Tresguerres JA and De la Fuente
M: Improvement of leucocyte functions in ovariectomised aged rats
after treatment with growth hormone, melatonin, oestrogens or
phyto-oestrogens. J Reprod Immunol. 80:70–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang B, Sun J, Li X, Zhou Q, Bai J, Shi Y
and Le G: Resveratrol prevents suppression of regulatory T-cell
production, oxidative stress, and inflammation of mice prone or
resistant to high-fat diet-induced obesity. Nutr Res. 33:971–981.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
et al: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Austad S: Recent advances in vertebrate
aging research 2009. Aging Cell. 9:297–303. 2010. View Article : Google Scholar : PubMed/NCBI
|