Introduction
Ultraviolet (UV) radiation is a major source of skin
damage, where daylight UV is composed of three different types
classified by wavelengths: UV-A (320–400 nm), UV-B (290–320 nm) and
UV-C (<290 nm) (1–5). Skin inflammation after UV exposure
is caused by UV-B since it easily triggers the cross-linking of
macromolecules, ring-structures and repeated linear molecules
(2,3,5).
UV-induced damage can be measured at the cellular
level through the production of reactive oxygen species (ROS),
leading to genetic mutations, the suppression of gene expression,
and inhibition of peptide repair, resulting in inflammation and
possibly leading to skin cancer (2,3,5–9).
There are three major pathways of UV-induced skin inflammation. The
first pathway involves the COX-2 mechanism whereby UV-B exposure
induces arachidonic acid release from the phospholipid membrane and
the COX-2 enzyme converts it to prostaglandins and free radical
molecules (10–13). It has been reported that COX-2 and
prostaglandin E2 elevations may be responsible for inflammation and
tumorigenesis (11,13,14). The second includes the
mitogen-activated protein kinase (MAPK) signaling pathways. After
exposure to UV-B, phosphorylation of threonine and tyrosine leads
to the activation of the MAPK protein kinase family. SPK/JNK and
p38 kinase are activated (phospho-SPK/JNK and phospho-p38) in
response to cellular stress and play a protective and pro-apoptotic
role (5,15). Thus, UV-B is the main cause of
inflammation in human keratinocytes via the MAPK pathways, JNK and
p38 (16). The third includes the
epidermal growth factor receptor (EGFR) pathway where
UV-B-phosphorylated EGFR induces inflammation and skin
tumorigenesis. Previous studies have demonstrated that EGFR
regulates activation of p38 kinase leading to increased COX-2 and
cytokine expressions (17,18).
The beneficial properties of mucus in UV protection
have been established from research on fish. For example, coral
reef fish can withstand UV due to mycosporine-like amino acids
(MAAs) found in the external epithelial mucus that absorb UV
(19). Relatively little is known
regarding how mollusc mucus-derived compounds may mitigate
UV-induced damage; yet, there is ample knowledge that molluscs,
including abalone, snails, slugs, can survive in UV-exposed
ecological niches. Molluscan mucus has increasingly been found to
contain properties which are being exploited for use in medicines
and cosmetics (20–23). For example, mucus-derived mucin
compounds mixed with a honey gel were found to promote wound
healing (24). Moreover, land
snail (Achatina fulica) mucin promotes wound healing and
provides anti-bacterial protection from Staphylococus aureus
and Staphylococus epidermidis (25). Snail mucus has also been utilized
as a skin cosmetic to promote skin regeneration, and as a treatment
for acne, pigmentation, scarring, wrinkles, and inflammation and
sun protection (23,26–29).
The abalone is a gastropod marine mollusc commonly
recognized as a food source due to an edible foot muscle and
constitutes an important culture industry for many countries
(30,31). On the other hand, its visceral
organs have not been recognized as valuable. Although the visceral
organs do not appear to contain separate highly developed mucous
glands as observed in snails, they do secrete copious amounts of
mucus via numerous organs, including the foot muscle, hypobranchial
gland (HBG) and gills (32,33). This mucus appears to be an
important source for chemosensory cues required for nonspecific
aggregation and larval settlement (34–37), and likely contains protective
properties.
Human keratinocyte HaCaT cells have often been used
for in vitro assays to understand epidermal homeostasis and
processes associated with disease or injury. For example, HaCaT
cells can be used to study molecular mechanisms related to abnormal
human β2-defensin in response to cell cytokines (38). They have also been used to assess
anti-inflammation and apoptosis from extracts derived from natural
products, such as pearls (39) or
pure compounds such as tectroside (40). In the present study, we explored
the cell protective effects of abalone [Haliotis asinina
(H. asinina)] extracts derived from the HBG and gills on
HaCaT cells following UV-B exposure. For the majority of abalone
species, the shell covers the entire animal, however in H.
asinina the shell is reduced, leaving a significant proportion
of the animal's body exposed. Its tropical distribution means that
it is exposed to varying levels of UV. We demonstrated that there
is significant improvement in cell survival following H.
asinina tissue extract application. This is supported by our
analysis of changes in the expression of inflammation-related
proteins.
Materials and methods
Abalone HBG and gill extraction
Twenty adult male and female H. asinina
(1–1.5 years of age) were obtained from the Coastal Aquaculture
Research and Development Centre, Department of Fisheries,
Prachuabkirikhan Province, Thailand. Animals were anesthetized by
immersion in an ice bath for 15 min. The HBG and gills were rapidly
dissected, washed and frozen in liquid nitrogen for storage at
−80°C. Proteins and metabolites were extracted by homogenizing at
4°C in 0.1% trifluoroacetic acid using a Polytron homogenizer
(Brinkmann Instruments, Westbury, NY, USA), followed by sonication.
The extracts were centrifuged (15,000 × g for 30 min at 4°C) and
then the supernatant was lyophilized.
Human keratinocyte culture
HaCaT cells were purchased from Cell Lines Service
(CLS, Eppelheim, Germany). Cells were cultured under standard
conditions of 5% CO2 in air at 37°C with medium renewal
every 2 days. Culturing medium was Dulbecco's modified Eagle's
medium (DMEM) (Gibco BRL, Gaithersburg, MD, USA) containing 5%
fetal bovine serum (FBS), 10 μg/ml of
penicillin/streptomycin. Cultured HaCaT cells were plated at a
density of 5×105 cells/cm2 onto 60×100
cm2 tissue culture-treated Petri dishes (SPL Life
Sciences Co., Ltd., Pochoen, Korea).
Cytotoxicity test of abalone HBG and
gills extracts
HaCaT cells were trypsinized from subconfluent
cultures by adding Trypsin-EDTA (0.25%) solution (Gibco BRL), and
incubated for 10 min with regular gentle shaking. The trypsin
reaction was stopped by adding 10 ml of DMEM (Gibco BRL) containing
10% FBS. The cell suspension was then centrifuged at 1,000 × g for
5 min at 25°C. The cell pellet was re-suspended in 2 ml of
culturing medium with 5% FBS and mixed by vortexing. The cells were
counted with a hemocytometer. Then, 1×105 cells were
equally seeded into each well of a 96-well black plate and
incubated at 37°C for 24 h. After that, the culturing medium was
removed and replaced with 200 μl of serial abalone extracts
in 1% serum-media. Serial concentrations of abalone extracts were
from 0.1 to 50 mg/ml. The cells were then incubated at 37°C for 24
h and 100 μl of a 10% AlamarBlue solution (Invitrogen,
Carlsbad, CA, USA) in serum-free medium was directly added to each
well. The plate was incubated at 37°C for 4 h and the absorbance
was measured at 540 and 630 nm with a fluorescent spectrophotometer
(Epoch; BioTek, Winooski, VT, USA). The percentage of cell
viability was analyzed by GraphPad Prism version 6 (GraphPad
Software, Inc., San Diego, CA, USA) using one-way ANOVA at
P<0.001.
UV-B irradiation of HaCaT cells
BLX-312 ultraviolet radiometer UV-B incubator
emitted UV-B light with a wavelength of 300–320 nm. Irradiance was
measured by UV sensor. To test UV-B irradiation, cultured HaCaT
cells were divided into 4 groups: a control group and 3
experimental groups. In the control group, cells were exposed to
serial dosages of UV-B from 10 to 110 mJ/cm2. In the
experimental groups, cells are incubated with 10, 100 and 200
μg/ml abalone extract prior to and during the serial UV-B
exposure. Then, cell viability, morphological changes and
inflammatory cytokine expression levels were observed and measured
by AlamarBlue, calcein AM-propidium iodide (PI) live-dead staining
and western blot analysis.
AlamarBlue cell viability assay to
compare the UV-B resistance of the control group with abalone
extract groups
HaCaT cells were trypsinized from subconfluent
cultures by adding trypsin solution, incubated for 10 min with
regular gentle shaking. The trypsin reaction was stopped by adding
10 ml of DMEM culture medium containing 10% FBS. The cell
suspension was then centrifuged at 1,000 × g for 5 min at 25°C. The
cell pellet was re-suspended in 2 ml of culturing-medium with 5%
FBS and properly mixed by vortexing. The cells were counted with a
hemocytometer. Then, 1×105 cells were equally seeded
into each well of a 96-well black-plate and incubated at 37°C for
24 h. After that, the culture medium was removed and replaced with
100 μl of serum-free medium. Ready to test cell plate was
covered by a black sticker except one column opened for UV-B
exposure. UV-B was emitted at 10 mJ/cm2, and then the
UV-B exposure was stopped for 5 min and the next column sticker was
removed and the next 10 mJ/cm2 UV-B dose was
administered. The same protocol was repeating and continuing 10
times. Thus, the first column was exposed to 110 mJ/cm2
of UV-B, the 11th column received 1 mJ/cm2 and the last
column was covered and exposed to no UV. Then, 100 μl of 10%
AlamarBlue solution in serum-free medium was added to each well.
The plate was incubated in 37°C for 4 h and the absorbance was
measured at 540 and 630 nm with a fluorescent spectrophotometer
(Epoch; BioTek). The number of viable cell was calculated as
described previously (41). Next,
viability of the cells in the experimental groups was tested in the
same way as the control group but either the abalone HBG or gill
extract was administered. In the experimental groups, the cells
were incubated with abalone peptides at the concentrations of 10,
100, 200 μg/ml for 8 h prior to and during the UV-B
exposure. After that the serial UV-B exposure and viability test
was carried out in the same way as the control group. The
percentage of cell viability was analyzed by GraphPad Prism version
6 (GraphPad Software, Inc.) using one-way ANOVA at P<0.001.
Calcein AM-PI live-dead assay and
morphological change
Live-dead solution was prepared by adding: i) 250
μl of calcein AM (1 mg/ml stock solution); ii) 100 μl
PI (1 mg/ml stock solution; iii) 3 ml of 5X binding buffer into
serum-free medium to make 15 ml of calcein AM-PI live-dead staining
solution (Molecular Probes Life Technologies, Carlsbad, CA, USA).
The ready-to-use solution was maintained at 4°C. The fully grown
HaCaT cells in 24-well Petri dishes were divided into two groups:
i) control group; and ii) experimental group. In the control group,
each dish was exposed to the serial UV-B doses started from 0, 10,
20, 30, 40, 50, 60, 70, 80, 90, 100, 110 mJ/cm2. After
every 10 mJ/cm2, the culture dish was taken out of an
incubator and incubated at room temperature for 5 min and then put
back in and given the next dose of 10 mJ of UV-B. In the
experimental group, 100 μg of abalone HBG and gill extracts
(1:1) was incubated with the cultured cells for 8 h prior and
during UV-B exposure. Images were captured using phase contrast and
fluorescence modes, with an inverted digital microscope (EVOS FLC,
Life Technologies, Grand Island, NY, USA).
Protein determination and western blot
analysis
Fully grown HaCaT cells in Petri dishes were divided
into three groups: i) control group in which cells were cultured
normally; ii) UV-B group in which cells were exposed to UV-B at
3×10 and 5×10 mJ/cm2; and iii) abalone peptide-treated
group in which cells were incubated with 100 μg/ml peptides
prior and during UV-B exposure and the cells were exposed to UV-B
at 3×10 and 5×10 mJ/cm2. After that, confluent cells
were scraped and sonicated in 5X lysis buffer containing Protease
Inhibitor Cocktail (Merck Millipore, Darmstadt, Germany). Then, the
lysates were centrifuged at 15,000 × g at 4°C for 30 min and the
supernatants were frozen in liquid nitrogen. The frozen protein
samples were lyophilized in a freeze dryer (Scanvac CoolSafe 110-4
PRO). Approximately 25 μg of extract was re-suspended in 0.1
M phosphate-buffered saline (PBS) and mixed with loading dye
containing 50 mM Tris-HCl, 10% glycerol, 2% SDS, 100 mM DTT and
0.1% bromophenol blue. Samples were separated using 15% sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Proteins were then transferred to 0.45-μm polyvinylidene
fluoride membrane. The membranes were blocked for 1 h by blocking
solution contained 4% non-fat dry milk, 2% bovine serum albumin
(BSA) and 0.1% Tween-20 in Tris-buffered saline (TBS) pH 7.6.
Polyclonal antibodies for COX-2 (ab52237), SPK/JNK (ab112501),
phospho-SPK/JNK (ab4821), p38 (ab31828) and phospho-p38 (ab4822)
were purchased from Abcam (Cambridge, UK) and were used at a
1:1,000 dilution to detect expression levels of the above proteins.
The membranes were washed and incubated for 45 min with the
secondary antibody (anti-rabbit HRP conjugated; Amersham plc,
Amersham, UK) at a 1:5,000 dilution. Then, the protein bands were
visualized by enhanced chemiluminescence (Amersham plc) under GENE
GNOME (Syngene Bioimaging Private Ltd., Gurgaon, India). The
western blot analyses were repeated in triplicate and band density
was quantified using densitometry UN-SCAN-IT software (Silk
Scientific, Inc., Orem, UT, USA). Protein expression levels were
analyzed by GraphPad Prism version 6 (GraphPad Software, Inc.)
using Dunnett's multiple comparison test.
Results
Cytotoxicity of extracts from abalone HBG
and gills
Optimal doses of abalone HBG and gill extracts were
determined using an AlamarBlue cytotoxicity assay (Fig. 1A and B). For extracts derived from
HBG, the maximal concentration that enabled cell survival of
>50% was at 12.5 μg/ml (Fig. 1A and C). For extracts derived from
gills, the concentration was at 12.5 μg/ml (Fig. 1B and D). Based on these results,
subsequent experiments were performed using concentrations of 0.5,
2.5, 5.0 μg/ml of abalone tissue extracts.
Comparison of cell viability between UV-B
control group and extract-treated groups
HaCaT cells were exposed to serial doses of UV-B,
starting from 1×10 to 11×10 mJ/cm2, and then cell
viability was compared between the four groups treated with the HBG
and gill extracts (0, 0.1, 2.5 and 5.0 μg/ml with UV-B). At
1×10 to 3×10 mJ/cm2 UV-B, there was no difference in
cell viability among the four groups. At 4×10 mJ/cm2
UV-B, the extract groups showed a significant increase in cell
viability compared to the control group (Fig. 2A). In the control group, the cell
viability was <50% at 6×10 mJ/cm2 UV-B, while in the
extract groups it remained at >80%. Moreover, HaCaT cell
survival was at >50% following 9×10 mJ/cm2 UV-B.
HaCaT cell survival rates were further analyzed using a calcein
AM-PI live-dead staining assay (Fig.
2B–I). In the 0 and 0.5 μg/ml extract-treated groups,
5×10 mJ/cm2 UV-B resulted in almost 50% cell death
(Fig. 2B and C), while the
survival rate was >90% in the 2.5 and 5 μg/ml
extract-treated groups at the same UV-B level (Fig. 2D and E). At 10×10
mJ/cm2 UV-B, there was almost complete cell death (0.84%
survival) in the control groups (Fig.
2F and G), while there was >30% cell survival when cells
were incubated with 2.5 μg/ml abalone extract at the same
UV-B level (Fig. 2H and I).
Changes in cell morphology in response to
UV-B and abalone extracts
To monitor changes in cell morphology by calcein
AM-PI live-dead staining, phase contrast and fluorescence
microscopy was used (Fig. 3A–J).
HaCaT cell morphological changes and viability following UV-B
exposure (6×10 mJ/cm2) were assessed in two groups: i)
control group, where HaCaT cells were exposed to UV-B alone; and
ii) the experimental group where HaCaT cells received 2.5
μg/ml abalone extracts (1:1 HBG and gill extract) before
UV-B exposure. UV-B exposure resulted in three obvious changes in
cell morphology: i) cytoplasmic condensation; ii) cell shrinkage
and membrane budding; and iii) condensation of chromatin. Fig. 3A–D shows representative
micrographs of HaCaT cells with morphological changes after
exposure to 6×10 mJ/cm2 UV-B alone and with the abalone
extract co-treatment. In the presence of 2.5 μg/ml of
abalone extract, most cells were viable with only minor
morphological changes being observed. At 8×10 mJ/cm2
UV-B, most cells in the control group were dead (Fig. 3E and F) while in the experimental
group there was >50% cell survival (Fig. 3G and H). This was also observed in
the abalone extract-treated groups exposed to a higher UV-B dose
(10×10 mJ/cm2). In the negative control (no UV-B
exposure) (Fig. 3I and J), HaCaT
cells appeared as round or rectangular in shape with an ~40–80
μm diameter. In addition, the nucleus occupied almost half
of the total area (with 10–20 μm nucleolus), and the plasma
membranes were clear, often attached to neighboring cells.
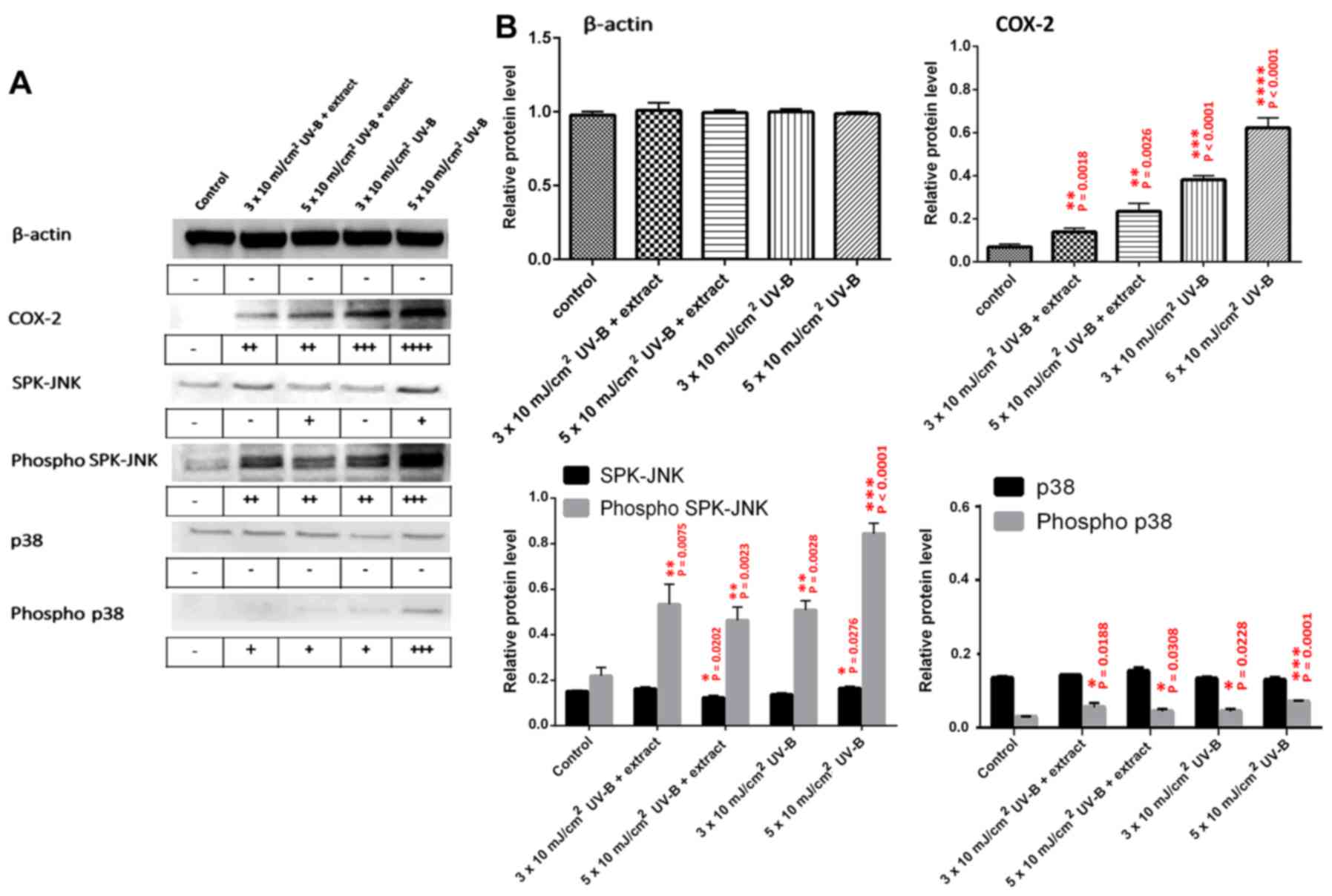
Measurement of inflammatory proteins
following UV-B irradiation of HaCaT cells
Expression of inflammation-related proteins (COX-2,
SPK/JNK, phospho-SPK/JNK, p38, and phospho-p38) was analyzed by
western blot analysis of HaCaT cell extracts following UV-B
exposure, with or without treatment with the abalone extracts
(Fig. 4A and B). The control
represents HaCaT cell extracts with no UV-B and no abalone extract.
HaCaT cells were also exposed to UV-B (3×10 and 5×10
mJ/cm2) with or without 2.5 μg/ml abalone
extract. No significant change was observed in β-actin protein
levels for any treatment. Abalone extract did show more significant
inhibition of COX-2 expression when compared to the levels observed
at 3×10 and 5×10 mJ/cm2 UV-B. The extract also resulted
in a more significant inhibition of the inflammatory-related active
forms of SPK-JNK and p38, namely phospho-SPK-JNK and phospho-p-38,
when compared to these levels following exposure to 5×10
mJ/cm2 UV-B without the extract.
Discussion
In the present study, we investigated the effects of
extracts derived from abalone tissues well known for secreting
mucus, to ascertain whether they attenuate UV-based cell damage. We
found that abalone tissue extracts attenuated the effects of UV-B
on HaCaT cells, as determined by both morphological and molecular
analyses of inflammatory-related proteins.
Having established that HBG and gill extract doses
less than 10 μg/ml were not detrimental to HaCaT cell
survival, we then tested cell survival in the presence of various
concentrations of extracts with UV-B exposure. We demonstrated the
capacity for abalone extracts to inhibit the process of
inflammation at 4–8 mJ/cm2 UV-B, and morphological
analysis and protein expression profiling supported this. In the
absence of extract (control) HaCaT cells showed cytoplasmic
condensation, cell shrinkage, membrane budding and accumulation of
the condensation of chromatin after being exposed to UV-B. In
support of the protective effects of the abalone extracts, COX-2
and phosphorylated forms of SPK-JNK and p38, were relatively less
highly expressed when the extracts were applied to HaCaT cells
exposed to 5×10 mJ/cm2 UV-B
The UV absorbent properties of animal mucus have
been well studied in fish and corals. In corals, the algal
symbionts help determine the mucus composition, including the
release of MAAs which protect against UV (42). Other biomolecules that may act
against photochemical damage to UV-B include cytosolic
water-soluble reductants and membrane-bound lipid-soluble
antioxidants.
In tropical fish species, MAAs present within the
epithelial mucus are critical for absorbing UV-A and UV-B, with
strong absorbance peaks between 290 and 400 nm (19). The requirement for UV-absorbent
compounds in tropical water is essential, since this water is often
low in UV-absorbing particulate and dissolved organic matter
(43). H. asinina is
distributed throughout many tropical regions worldwide, including
the Great Barrier Reef (44). To
date, analysis of its mucus composition has primarily focused on
the existence of water-soluble peptides that may be required for
non-specific communication, showing that there are three major
water-soluble peptides that are released in large amounts from
secretory cells and diffuse into the surrounding seawater (36). Future studies should explore the
existence of MAAs, or other known and novel UV-absorbing
biomolecules.
Besides the role of mucus in UV protection, other
medically relevant applications should be explored. Of most
interest are the bioactive compounds secreted by marine snails in
the family Muricidae, and in particular their brominated indoles
released from the HBG that appear to have anti-inflammatory,
anticancer and steriodogenic activity (45). A recent study of the mosquito
Aedes aegypti analyzed salivary gland extracts,
demonstrating that semi-purified preparations can ameliorate
inflammatory bowel disease (46).
In addition, anti-inflammatory proteins have been discovered within
the salivary gland extracts of the tick, Hyalomma
anatolicumanatolicum (47)
and horsefly Tabanusyao (48). Moreover, researchers discovered a
free amino acid from Haliotis discus water named taurine,
which has anti-inflammatory and antioxidant potentials in zebrafish
(49). These types of discoveries
clearly indicate that numerous other animal mucus-associated
biomolecules could be used for human medicine. Moreover, numerous
unpublished reports suggest the benefits of land snail mucus for
skin anti-aging and wound healing. Abalone, also a gastropod, could
possibly have similar, yet unexplored advantageous attributes.
In conclusion, the present study demonstrated the
ability of biomolecules derived from tropical abalone gland
extracts to attenuate UV-B damage. Without extract, HaCaT cell
viability was significantly reduced upon exposure to UV-B at 5
mJ/cm2. This was determined based on morphological
changes, live-dead staining assay and analysis of changes in the
abundance of inflammatory-related proteins. Subsequent research
will be carried out to determine the exact factors in the abalone
extracts that are responsible for these property.
Acknowledgments
We sincerely thank the Strategic Wisdom and Research
Institute (Srinakharinwirot University, Thailand) and Research and
Foreign Relation Department, Faculty of Medicine (Srinakharinwirot
University, Thailand) for the financial support and professional
cooperation.
References
|
1
|
de Gruijl FR and Van der Leun JC: Estimate
of the wavelength dependency of ultraviolet carcinogenesis in
humans and its relevance to the risk assessment of a stratospheric
ozone depletion. Health Phys. 67:319–325. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Svobodova A, Walterova D and Vostalova J:
Ultraviolet light induced alteration to the skin. Biomed Pap Med
Fac Univ Palacky Olomouc Czech Repub. 150:25–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nichols JA and Katiyar SK: Skin
photoprotection by natural polyphenols: Anti-inflammatory,
antioxidant and DNA repair mechanisms. Arch Dermatol Res.
302:71–83. 2010. View Article : Google Scholar
|
|
4
|
Afaq F: Natural agents: Cellular and
molecular mechanisms of photoprotection. Arch Biochem Biophys.
508:144–151. 2011. View Article : Google Scholar :
|
|
5
|
López-Camarillo C, Ocampo EA, Casamichana
ML, Pérez- Plasencia C, Alvarez-Sánchez E and Marchat LA: Protein
kinases and transcription factors activation in response to
UV-radiation of skin: Implications for carcinogenesis. Int J Mol
Sci. 13:142–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bender K, Göttlicher M, Whiteside S,
Rahmsdorf HJ and Herrlich P: Sequential DNA damage-independent and
-dependent activation of NF-kappaB by UV. EMBO J. 17:5170–5181.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hernandez-Pigeon H, Jean C, Charruyer A,
Haure MJ, Titeux M, Tonasso L, Quillet-Mary A, Baudouin C,
Charveron M and Laurent G: Human keratinocytes acquire cellular
cytotoxicity under UV-B irradiation. Implication of granzyme B and
perforin. J Biol Chem. 281:13525–13532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ali D, Verma A, Mujtaba F, Dwivedi A, Hans
RK and Ray RS: UV-B-induced apoptosis and DNA damaging potential of
chrysene via reactive oxygen species in human keratinocytes.
Toxicol Lett. 204:199–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu MS, Sun DS, Lin YC, Cheng CL, Hung SC,
Chen PK, Yang JH and Chang HH: Nanodiamonds protect skin from
ultraviolet B-induced damage in mice. J Nanobiotechnology.
13:352015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Devary Y, Rosette C, DiDonato JA and Karin
M: NF-kappa B activation by ultraviolet light not dependent on a
nuclear signal. Science. 261:1442–1445. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buckman SY, Gresham A, Hale P, Hruza G,
Anast J, Masferrer J and Pentland AP: COX-2 expression is induced
by UV-B exposure in human skin: Implications for the development of
skin cancer. Carcinogenesis. 19:723–729. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muller-Decker K, Neufang G, Berger I,
Neumann M, Marks F and Furstenberger G: Transgenic cyclooxygenase-2
overexpression sensitizes mouse skin for carcinogenesis. Proc Natl
Acad Sci USA. 99:12483–12488. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibata A, Nakagawa K, Yamanoi H, Tsuduki
T, Sookwong P, Higuchi O, Kimura F and Miyazawa T: Sulforaphane
suppresses ultraviolet B-induced inflammation in HaCaT
keratinocytes and HR-1 hairless mice. J Nutr Biochem. 21:702–709.
2010. View Article : Google Scholar
|
|
14
|
Liu S, Mizu H and Yamauchi H:
Photoinflammatory responses to UV-irradiated ketoprofen mediated by
the induction of ROS generation, enhancement of cyclooxygenase-2
expression, and regulation of multiple signaling pathways. Free
Radic Biol Med. 48:772–780. 2010. View Article : Google Scholar
|
|
15
|
Kim JE, Kwon JY, Seo SK, Son JE, Jung SK,
Min SY, Hwang MK, Heo YS, Lee KW and Lee HJ: Cyanidin suppresses
ultraviolet B-induced COX-2 expression in epidermal cells by
targeting MKK4, MEK1, and Raf-1. Biochem Pharmacol. 79:1473–1482.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Englaro W, Dérijard B, Ortonne JP and
Ballotti R: Solar ultraviolet light activates extracellular
signal-regulated kinases and the ternary complex factor in human
normal keratinocytes. Oncogene. 16:661–664. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ashida M, Bito T, Budiyanto A, Ichihashi M
and Ueda M: Involvement of EGF receptor activation in the induction
of cyclooxygenase-2 in HaCaT keratinocytes after UV-B. Exp
Dermatol. 12:445–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Abaseri TB, Hammiller B, Repertinger SK
and Hansen LA: The epidermal growth factor receptor increases
cytokine production and cutaneous inflammation in response to
ultraviolet irradiation. ISRN Dermatol. 2013:8487052013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zamzow JP and Losey GS: Ultraviolet
radiation absorbance by coral reef fish mucus: Photo-protection and
visual communication. Environ Biol Fishes. 63:41–47. 2002.
View Article : Google Scholar
|
|
20
|
Jacobson PB and Jacobs RS: Fuscoside: An
anti-inflammatory marine natural product which selectively inhibits
5-lipoxygenase Part I: Physiological and biochemical studies in
murine inflammatory models. J Pharmacol Exp Ther. 262:866–873.
1992.PubMed/NCBI
|
|
21
|
Ojika M, Kigoshi H, Yoshida Y, Ishigaki T,
Nisiwaki M, Tsukada I, Arakawa M, Ekimoto H and Yamada K:
Aplyronine A, a potent antitumor macrolide of marine origin, and
the congeners aplyronines B and C: Isolation, structures, and
bioactivities. Tetrahedron. 63:3138–3167. 2007. View Article : Google Scholar
|
|
22
|
Uddin MH, Otsuka M, Muroi T, Ono A, Hanif
N, Matsuda S, Higa T and Tanaka J: Deoxymanoalides from the
nudibranch Chromodoris willani. Chem Pharm Bull (Tokyo).
57:885–887. 2009. View Article : Google Scholar
|
|
23
|
Yamada K, Ojika M, Kigoshi H and Suenaga
K: Aplyronine A, a potent antitumor macrolide of marine origin, and
the congeners aplyronines B-H: Chemistry and biology. Nat Prod Rep.
26:27–43. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adikwu MU and Alozie BU: Application of
snail mucin dispersed in detarium gum gel in wound healing. Sci Res
Essays. 2:195–198. 2007.
|
|
25
|
Santana WA, Melo CM, Cardoso JC,
Pereira-Filho RN, Rabelo AS, Reis FP and Albuquerque-Júnior RLC:
Assessment of antimicrobial activity and healing potential of
mucous secretion of Achatina fulica. Int J Morphol. 30:365–373.
2012. View Article : Google Scholar
|
|
26
|
Shimidzu N, Goto M and Miki W: Carotenoids
as singlet oxygen quenchers in marine organisms. Fish Sci.
62:134–137. 1996.
|
|
27
|
Bonnemain B: Helix and drugs: Snails for
western health care from antiquity to the present. Evid Based
Complement Alternat Med : eCAM. 2:25–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Orazio N, Gammone MA, Gemello E, De
Girolamo M, Cusenza S and Riccioni G: Marine bioactives:
Pharmacological properties and potential applications against
inflammatory diseases. Mar Drugs. 10:812–833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cruz MC, Sanz-Rodríguez F, Zamarrón A,
Reyes E, Carrasco E, González S and Juarranz A: A secretion of the
mollusc Cryptomphalus aspersa promotes proliferation, migration and
survival of keratinocytes and dermal fibroblasts in vitro. Int J
Cosmet Sci. 34:183–189. 2012. View Article : Google Scholar
|
|
30
|
Chalermwat K, Szuster BW and Flaherty M:
Shellfish aquaculture in Thailand. Aquac Econ Manag. 7:249–261.
2003. View Article : Google Scholar
|
|
31
|
Cook PA and Roy Gordon H: World abalone
supply, markets, and pricing. J Shellfish Res. 29:569–571. 2010.
View Article : Google Scholar
|
|
32
|
Wanichanon C, Laimek P, Linthong V,
Sretarugsa P, Kruatrachue M, Upatham ES, Poomtong T and Sobhon P:
Histology of hypobranchial glands and gills of Haliotis asinina
Linnaeus. J Shellfish Res. 23:1107–1112. 2004.
|
|
33
|
Naegel LCA and Aguilar-Cruz CA: The
hypobranchial gland from the purple snail Plicopurpura pansa
(Gould, 1853) (Prosobranchia: Muricidae). J Shellfish Res.
25:391–394. 2006. View Article : Google Scholar
|
|
34
|
Laimek P, Clark S, Stewart M, Pfeffer F,
Wanichanon C, Hanna P and Sobhon P: The presence of GABA in
gastropod mucus and its role in inducing larval settlement. J Exp
Mar Biol Ecol. 354:182–191. 2008. View Article : Google Scholar
|
|
35
|
Kuanpradit C, Cummins SF, Degnan BM,
Sretarugsa P, Hanna PJ, Sobhon P and Chavadej J: Identification of
an attractin-like pheromone in the mucus-secreting hypobranchial
gland of the abalone Haliotis asinina linnaeus. J Shellfish Res.
29:699–704. 2010. View Article : Google Scholar
|
|
36
|
Kuanpradit C, Stewart MJ, York PS, Degnan
BM, Sobhon P, Hanna PJ, Chavadej J and Cummins SF: Characterization
of mucus-associated proteins from abalone (Haliotis) - candidates
for chemical signaling. FEBS J. 279:437–450. 2012. View Article : Google Scholar
|
|
37
|
Stewart P, Williams EA, Stewart MJ,
Soonklang N, Degnan SM, Cummins SF, Hanna PJ and Sobhon P:
Characterization of a GABAA receptor β subunit in the abalone
Haliotis asinina that is upregulated during larval development. J
Exp Mar Biol Ecol. 410:53–60. 2011. View Article : Google Scholar
|
|
38
|
Seo MD, Kang TJ, Lee CH, Lee AY and Noh M:
HaCaT keratinocytes and primary epidermal keratinocytes have
different transcriptional profiles of cornified envelope-associated
genes to T helper cell cytokines. Biomol Ther (Seoul). 20:171–176.
2012. View Article : Google Scholar
|
|
39
|
Yang YL, Chang CH, Huang CC and Liu HW:
Anti- inflammation and anti-apoptosis effects of pearl extract gel
on UV-B irradiation HaCaT cells. Biomed Mater Eng. 26(Suppl 1):
S139–S145. 2015.
|
|
40
|
Kim SB, Kang OH, Joung DK, Mun SH, Seo YS,
Cha MR, Ryu SY, Shin DW and Kwon DY: Anti-inflammatory effects of
tectroside on UV-B-induced HaCaT cells. Int J Mol Med.
31:1471–1476. 2013.PubMed/NCBI
|
|
41
|
Al-Nasiry S, Geusens N, Hanssens M, Luyten
C and Pijnenborg R: The use of Alamar Blue assay for quantitative
analysis of viability, migration and invasion of choriocarcinoma
cells. Hum Reprod. 22:1304–1309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shick JM, Lesser MP, Dunlap WC, Stochaj
WR, Chalker BE and Won JW: Depth dependent responses to solar
ultraviolet radiation and oxidative stress in the zooxanthellate
coral Acropora microphthalma. Mar Biol. 122:41–51. 1995. View Article : Google Scholar
|
|
43
|
Baker KS, Smith RC and Green AES: Middle
ultraviolet radiation reaching the ocean surface. Photochem
Photobiol. 32:367–374. 1980. View Article : Google Scholar
|
|
44
|
Geiger DL: Distribution and biogeography
of the recent Haliotidae (Gastropoda: Vetigastropoda) world-wide.
Bollettino Malacologico. 35:57–120. 2000.
|
|
45
|
Benkendorff K, Rudd D, Nongmaithem BD, Liu
L, Young F, Edwards V, Avila C and Abbott CA: Are the traditional
medical uses of muricidae molluscs substantiated by their
pharmacological properties and bioactive compounds? Mar Drugs.
13:5237–5275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sales-Campos H, de Souza PR, Basso PJ,
Ramos AD, Nardini V, Chica JE, Capurro ML, Sá-Nunes A and de Barros
Cardoso CR: Aedes aegypti salivary gland extract ameliorates
experimental inflammatory bowel disease. Int Immunopharmacol.
26:13–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ghosh M, Sangwan N and Sangwan AK: Partial
characterization of a novel anti-inflammatory protein from salivary
gland extract of Hyalomma anatolicum anatolicum (77Acari: Ixodidae)
ticks. Vet World. 8:772–776. 2015. View Article : Google Scholar
|
|
48
|
Wei L, Huang C, Yang H, Li M, Yang J, Qiao
X, Mu L, Xiong F, Wu J and Xu W: A potent anti-inflammatory peptide
from the salivary glands of horsefly. Parasit Vectors. 8:5562015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheong SH, Hwang JW, Lee SH, Kim YS, Sim
EJ, You BI, Lee SH, Park DJ, Ahn CB, Kim EK, et al: In vitro and in
vivo antioxidant and anti-inflammatory activities of abalone
(Haliotis discus) water extract. Adv Exp Med Biol. 803:833–849.
2015. View Article : Google Scholar : PubMed/NCBI
|