Introduction
Breast cancer has the highest rate of
cancer-associated mortality in women worldwide (1). Typically, patients with breast
cancer are treated with chemotherapy, radiotherapy, surgeries,
targeted therapy, and most importantly, the endocrine therapy,
which may improve their prognosis. The long-term survival is often
hindered by tumor recurrence, which is resistant to original
therapy and causes the tumor treatment strategy to be changed.
Anti-estrogen strategy has been widely used in clinical treatments,
with anti-estrogen drugs or estrogen receptor (ER) antagonists,
including tamoxifen, letrozole and anastrozole, as important breast
cancer treatments (2,3). Unfortunately, many patients are
still either resistant to these drugs, or face serious side effects
(4–6). Reducing drug-resistance and
suppressing cancer malignancy is essential, and effective
strategies to eradicate cancer as required (7).
MicroRNA (miRNA) Let-7a was previously identified to
target stem-like cells in cancer via inhibition of ERα (8), and Let-7b acted as the indicator of
therapy response. Notably, Let-7a may function as the key
downstream activator in the process of upstream anti-oncogenes
induced tumor inhibition (9–12).
The role of Let-7c in cancer stem cells was previously explored,
and the miRNA was demonstrated to reduce the tumor formation
ability of cancer-stem like cells via negative regulation of
estrogen receptor 1 (ESR1)/Wnt1 signaling pathway (13). However, its roles in regulation of
endocrine therapy response have rarely been studied. The current
study aimed to elucidate the potential functions of Let-7 in
mediating the response of breast cancer stem-like cells (CSCs) to
anti-endocrine agents, which may identify strategies to reduce
therapy resistance of cancer cells, and avoid the long-term usage
of endocrine drugs.
Materials and methods
Tissue samples
Sixty NSCLC samples and paired normal tumor-adjacent
samples were obtained from the First Affiliated Hospital of Xi'an
Jiaotong University, Xi'an, China. All samples without
chemotherapy, immunotherapy or radiotherapy were collected between
June 2008 and October 2014. This study was approved by Xi'an
Jiaotong University Ethics Committee and written informed consent
was obtained from all patients. They consisted of 43 males and 17
females with median age of 60 years (age range, 55–81 years). Tumor
tissue and matched normal tumor-adjacent tissue specimens were
obtained at surgery and immediately stored in formalin solution to
make paraffin-embedded specimens for immunohistochemistry.
Cell culture and lentiviral
infection
ER-positive breast cancer cell lines (MCF-7 and
T47-D) and 293T cells were purchased from American Type Culture
Collection (Manassas, VA, USA) and maintained at Center for
Translational Medicine, First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China). Cells culturing and spheres non-adjacent
seeding were performed as previously did (10,13). In brief, cells were cultured in
high glucose Dulbecco's Modified Eagle's Medium (DMEM; HyClone,
Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). For tumor
sphere culture, dissected primary breast tumor cells were plated in
ultra-low attachment dishes (Corning Incorporated, Corning, NY,
USA) without fetal bovine serum to determine their ability to form
primary mammospheres, and the obtained mammospheres were
disaggregated into single cells using Accutase (StemPro; Gibco;
Thermo Fisher Scientific, Inc.) and trypsin-EDTA (0.05%; Gibco;
Thermo Fisher Scientific, Inc.), and then they were re-suspended to
acquire the next generation of spheres. Lentivirus-mediated Let-7c
overexpression or ESR1 short hairpin RNA (shRNA target sequence,
5′-AGGGAGAATGTTGAAGCACAA-3′) mediated knockdown in MCF-7 and T47-D
cells was performed as described and validated in a previous study
(13).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Life
Technologies; Thermo Fisher Scientific, Inc.) from clinical tissues
and cultured cells, as previously described (8,13,14). The first strand cDNA was
synthesised using SuperScript III First-strand synthesis SuperMix
(Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was performed
using Powerup SYBR-Green Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) to detect Let-7c. The small nuclear RNA U6
was used as endogenous control. The primer sequences were as
follows: Let-7c forward, 5′-GCCGCTGAGGTAGTAGGTTGTAT-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′. Expression levels were
calculated and presented following the 2−ΔΔCt method
[ΔΔCt = ΔCt (positive) − ΔCt (control)] (15–17).
Immunohistochemistry (IHC)
Formalin-fixed and paraffin- embedded sections
(5-μm-thick) from mice tumor tissues were deparaffinized in
xylene and rehydrated with descending concentrations of ethanol
(100, 95, 80 and 70%). Antigen retrieval was performed by heating
the sections in 0.01 M citrate buffer at 95°C for 10 min.
Endogenous peroxidase was quenched by treatment with 0.3–3%
H2O2 and 100% methanol for 10 min at room
temperature (RT). The sections were then blocked by applying 1–2
drops of 10% goat serum (ZSGB-BIO, Beijing, China) for 30 min.
After incubation with diluted primary antibody to ESR1 (1:100;
sc-7207) and Wnt1 (1:200; sc-6280; both from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), and β-catenin (1:200; cat.
no. 9562; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C
overnight in a closed incubation chamber, the sections were then
washed in PBS and incubated with orseradish peroxidase
(HRP)-conjugated secondary antibody at RT for 1 h.
Western blot analysis
Total protein were extracted and transferred as
previously described (14,18).
In brief, total protein was extracted using
radioimmunoprecipitation lysis buffer (Bioss, Co., Beijing, China),
with protease and phosphatase inhibitor (×100; cat. no. 5872; Cell
Signaling Technology, Inc.). The concentration of total proteins
was quantified using Protein Quantification kit (BCA; ab102536;
Abcam, Cambridge, UK). The protein lysates were electrophoreted and
resolved in 10% SDS-PAGE, then transferred to nitrocellulose
membranes. After blocking with 5% fat-free milk, the membranes were
incubated at 4°C overnight with specific primary antibodies to ESR1
(1:2,000, sc-87; Santa Cruz Biotechnology, Inc.), t-cell factor 4
(tcf-4; 1:2,000; cat. no. 05-511; EMD Millipore, Billerica, MA,
USA), oct-4 (1:1,000; sc-5279; Santa Cruz Biotechnology, Inc.),
Sox-2 (1:3,000; cat. no. 2748) and vinculin (1:2,000; cat. no.
4650; both from Cell Signaling Technology, Inc.); the secondary
antibodies conjugated with horseradish peroxidase (1:3,000–1:5,000;
cat. nos. 7071 and 7072; Santa Cruz Biotechnology, Inc.) were
incubated for 1 h at room temperature.
Proliferation detection
The detection of cell proliferation was performed as
previously reported (8,10). Briefly, cells of each group were
adjusted to 5×103 cells/well in a volume of 100
μl medium in 96-well plates, and then cultured with 20
μl MTT (5 mg/ml) at 37°C for 4 h, the crystals of formazan
was dissolved in dimethyl sulfoxide. The optical density was
detected by spectrophotometer at 492 nm.
Flow cytometry analysis of cycle
apoptosis and cell cycle
Flow cytometry analysis for apoptosis and cycles was
performed as described previously (8,19,20). For apoptosis ratio analysis, cells
(5×104/ml) in the logarithmic phase were collected and
cultured in 6-well dishes at 1×105 cells/well. The cells
were suspended in 500 μl 1X binding buffer, and then 5
μl Annexin V-fluorescein isothiocyanate and 5 μl
propidium iodide (PI; 20 μg/ml; both from BD Biosciences,
Franklin Lakes, NJ, USA) were added. For cell cycle analysis,
cancer cells were cultured and adjusted to 5×104/ml in
6-well dish, and then fixed with ice-cold 1 ml of 75% ethanol for
24 h at 4°C. Fixed cells were stained with 150 μl PI and 150
μl RNA enzyme inhibitor for 30 min at 37°C. The cells were
analyzed using a flow cytometer within 1 h of incubation and all
analyses were performed in triplicate.
Fluorescence-activated cell sorting
analysis and sphere forming assay
The LSR II flow cytometer (BD Pharmingen, San Diego,
CA, USA) was used to analyze and separate CSCs based on cell
labeling and fluorescence-activated sorting. The activated
ALDEFLUOR™ reagent and diethylaminobenzaldehyde purchased from
Stemcell Technologies, Inc. (Vancouver, BC, Canada) were used to
isolate aldehyde dehydrogenase (ALDH)+ cells (21–23). The ratios of ALDH1+
stem cells in different groups were analyzed. The ratios of aldH 1+
stem cells in different groups were analyzed. For sphere forming
assay, CSCs (1000cells per/ well) were seeded in 6-well ultra-low
cluster plates (Corning Inc., Corning, NY) for one week to obtain
first generation after being given different treatment. Spheres
were cultured in DMEM/F12 serum-free medium (Invitrogen)
supplemented with 2% B27 (BD Biosciences, CA), 20ng/ml epidermal
growth factor (EGF, Sigma), 20ng/ml basic fibroblast growth factor
(bFGF, Sigma), 0.4% BSA and 4 μg/ml insulin (Sigma). The number of
spheres was counted under a microscope.
Luciferase assays and transient
transfection
MCF-7 and T47-D cells were seeded in 24-well plates
with 1×105 cells/well, and only cells growing in the log
phase at a passage number ≤15 were used. Cells were transfected
with Let-7c mimic and Renilla luciferase plasmid containing
a TCF-4-responsive promoter when at 50–70% confluence ratio using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), non-transfected and non-treated cells were set
as control, as described previously (9,11).
Each group included three replicates, and independent triplicate
experiments were performed. Specifically, the cells were collected
with cell lysis buffer after transfection for 24 h, and
Renilla luciferase activity was detected using the
Pierce® Renilla Luciferase Flash Assay kit (no.
16164; Thermo Fisher Scientific, Inc.), following the
manufacturer's instructions. Cell lysate (20 μl) and 50
μl working solution were added to one column, and a Turner
Biosystems TD-20/20 luminometer (Turner Biosystems; Thermo Fisher
Scientific, Inc.) was used to detect the light output (prime the
pump prior to injections with working solution). Firefly luciferase
activity was normalized to Renilla luciferase activity.
Nude mice study
MCF-7 stem cells transfected with Let-7c mimic and
control were pretreated with 10 nM estrogen for 24 h and implanted
subcutaneously into BALB/c mice (n=5; age, 5 weeks) supplied by the
Experimental Animal Center, College of Medicine, Xi'an Jiaotong
University [license no. SCXK (shan) 2007–001] (10,18,24,25). Long-release estradiol pellets
(Innovative Research of America, Sarasota, FL, USA) were implanted
the day before inoculation. Tumor growth was measured by a digital
caliper every 5 days for 5 weeks. Tumor weight was measured after
cell implantation. Animal experimental researches followed the
internationally recognized guidelines. Animal studies were approved
by the Medical College of Xi'an Jiaotong University Animal Ethics
Board. Protocols were conducted in accordance with the guidance for
the Care and Use of Laboratory Animals, established by the Ministry
of Science and Technology of China (Beijing, China).
Statistical analysis
All statistical analyses were performed using SPSS
version 16. All data are presented as mean ± standard error of the
mean (SEM). Statistical analyses were conduced using Student's
t-test and one-way ANOVA. Kruskal-Wallis test was used to analyze
the intensity of ALDH, Wnt1 and TCF-4 in different phases of breast
cancer tissues. P<0.05 was considered to indicate a
statistically significant difference.
Results
Stem-like cells and Wnt signaling
activation are associated with advanced progression of patients
with ER-positive breast cancer
Clinical data of tissues from patients of breast
cancer of ER status were retrospectively analyzed, and ALDH1 was
stained using IHC to determine the ratio of stem-like cells in
cancer specimens. The ratio of ALDH1+ cells increased
significantly with advanced cancer stages (Fig. 1A, left). Increased Wnt signaling
activation, represented by staining for Wnt1 (Fig. 1A, middle panel) and TCF-4
(Fig. 1A, right panel), was
associated with the later phases of breast cancer.
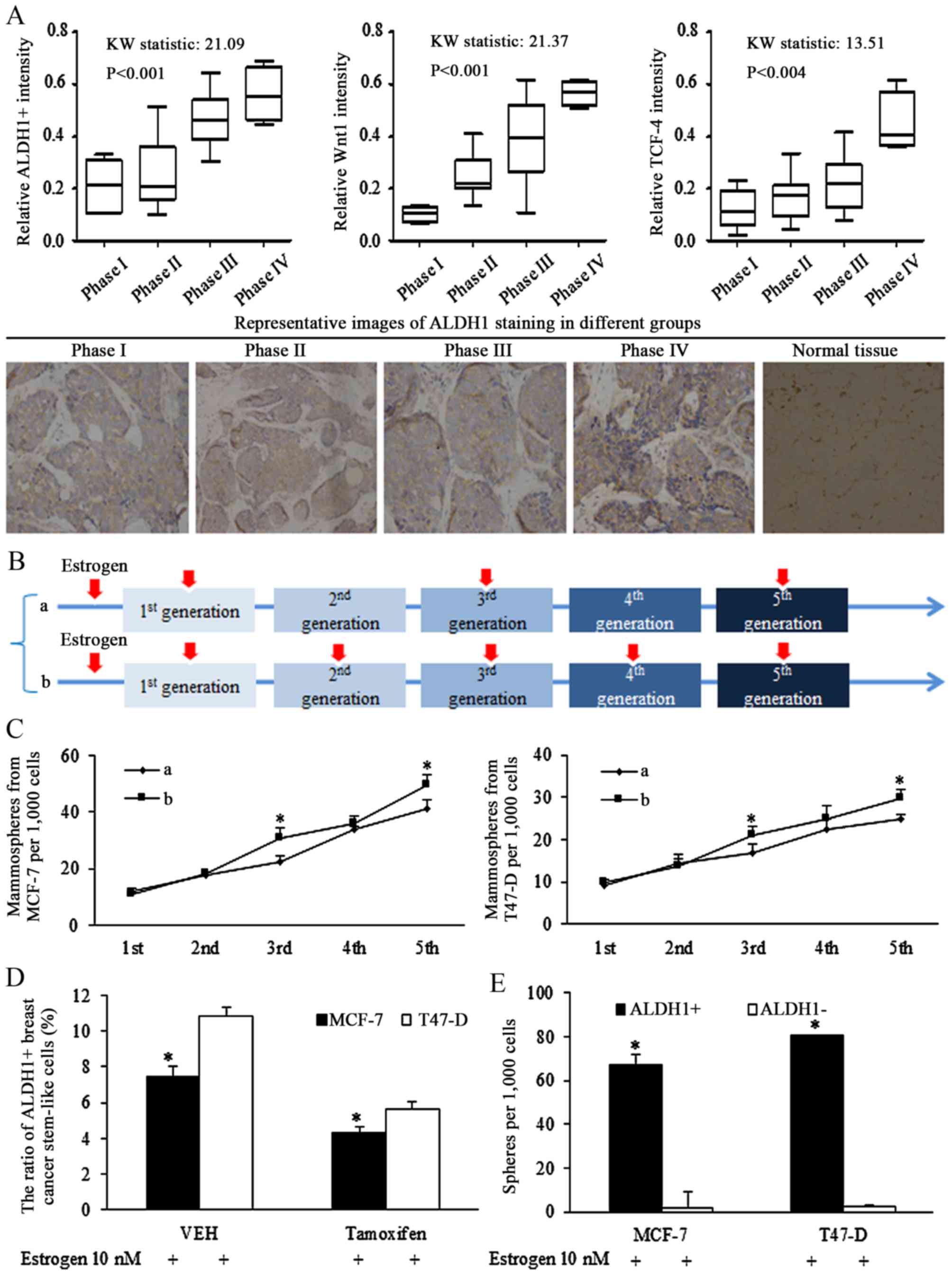 | Figure 1Stem-like cells are associated with
progressed cancer stages and Wnt signaling activation in breast
cancer of estrogen receptor (ER) status. (A) Clinical studies of
tissues from patients of breast cancer of ER status, ALDH1 was
stained for illustrating the ratio of stem-like cells in cancer
specimens, and the ratio of ALDH1-positive cells increased with
advanced cancer stages (left panel). The Wnt signaling activation
was corresponding to the later phase of breast cancer, identified
by Wnt1 (middle panel) and TCF-4 (right panel) immunohistochemical
staining (original magnification, ×400). (B) Description of
estrogen addition strategy in sphere-formation assays, the 10 nM
estrogen was added into the medium continuously in every generation
of spheres culture, or was added in pretreatment medium, and the
1st, 3rd and 5th generation. (C) Comparison of effect of different
estrogen addition strategy on mammosphere formation ability of
breast cancer stem cells, and the existence of estrogen stimulated
the self-renewal ability of stem cells of breast cancer.
*P<0.05 vs. group a. (D) Anti-estrogenic tamoxifen
decreased the ratio of ALDH1-positive cells in MCF-7 and T47-D
cells. *P<0.05. (E) ALDH1-positive cells harbored
increases sphere formation ability, compared with ALDH1-negative
cells. Statistical significance was evaluated by using
Kruskal-Wallis test or analysis of variance. *P<0.05
vs. ALDH−. ALDH, aldehyde dehydrogenase; TCF-4, T-cell
factor 4; VEH, vehicle (n=3 repeats with similar results. Values
are presented as the means ± SEM). |
Estrogen induction of stimulation of
self-renewal ability
To identify the oncogenic role of estrogen in
regulations of isolated stem cells, intermittent addition of
estrogen was used during the culture of non-adjuvant spheres. The
intermittent treatment of estrogen in certain generations assured
its effects on either sphere formation, or sphere cell
proliferation. Estrogen (10 nM) was added into the medium
continuously in every generation of spheres culturing (group b), or
was added on the 1st, 3rd and 5th generation (group a), as
illustrated in Fig. 1B. Estrogen
treatment at the 1st generation increased the ratio of stem cells,
affecting the self-renewal, and no significant difference was
detected between groups a and b. However, the difference was
notified and identified in the 3rd and the 5th generation, which
was resulted from different strategy of estrogen addition in the
2nd and 4th generation (Fig. 1C),
demonstrating the effects of estrogen in controlling the
self-renewal ability of stem-like cells.
Our previous study demonstrated that Let-7c
inhibited the ratio of ALDH1+ cells in breast cancer
cells via suppressing the function of estrogen induction of Wnt
signaling activation (13). The
results of the current study demonstrated that the inhibition of
tamoxifen on self-renewal ability of CSCs became greater when the
concentration increased (data not shown), and 10−5 mol/l
tamoxifen reduced the ratio of ALDH1+ cells (Fig. 1D), and isolated ALDH1+
cells had higher sphere formation capacity than ALDH1-
cells (Fig. 1E).
Breast cancer stem-like cell are
sensitized to tamoxifen-induced apoptosis and self-renewal
inhibition by Let-7c overexpression
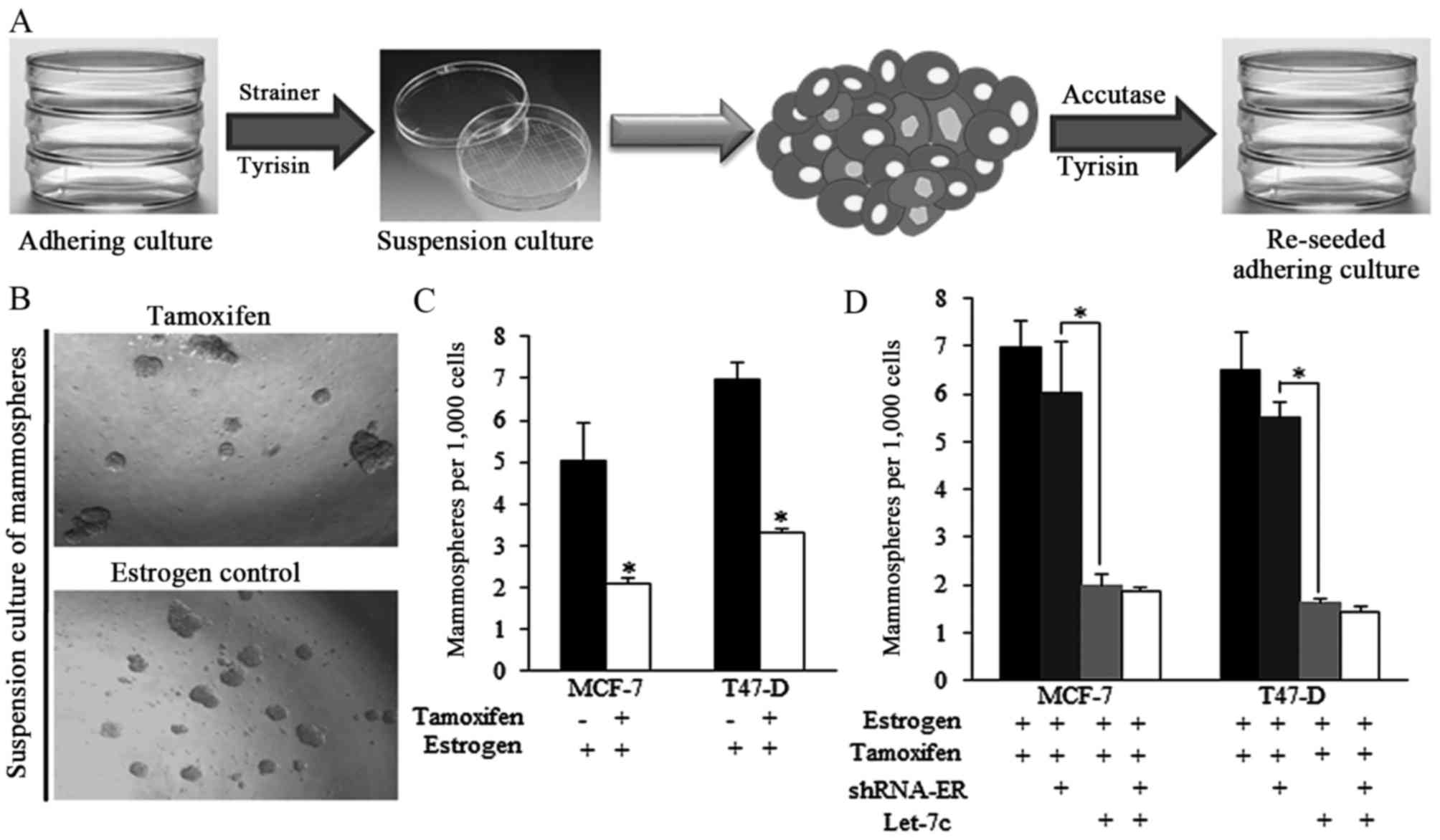
The strategy of acquiring mammospheres and the
representative images of spheres from different groups are
presented in Fig. 2A and B.
Tamoxifen decreased the ratio of mammospheres produced from
estrogen-treated breast cancer cells (Fig. 2B and C). However, the effect of
tamoxifen was reduced when shRNA-ER was introduced (Fig. 2D), indicating that the expression
of ER is critical for tamoxifen-induced effects on self-renewal
regulation. Let-7c greatly facilitated the tamoxifen functions,
however the ER inhibition by shRNA did not exhibit improved
anticancer effects of combined application of tamoxifen and Let-7c
(Fig. 2D), suggesting the way
Let-7c sensitized tamoxifen was dependent on ER.
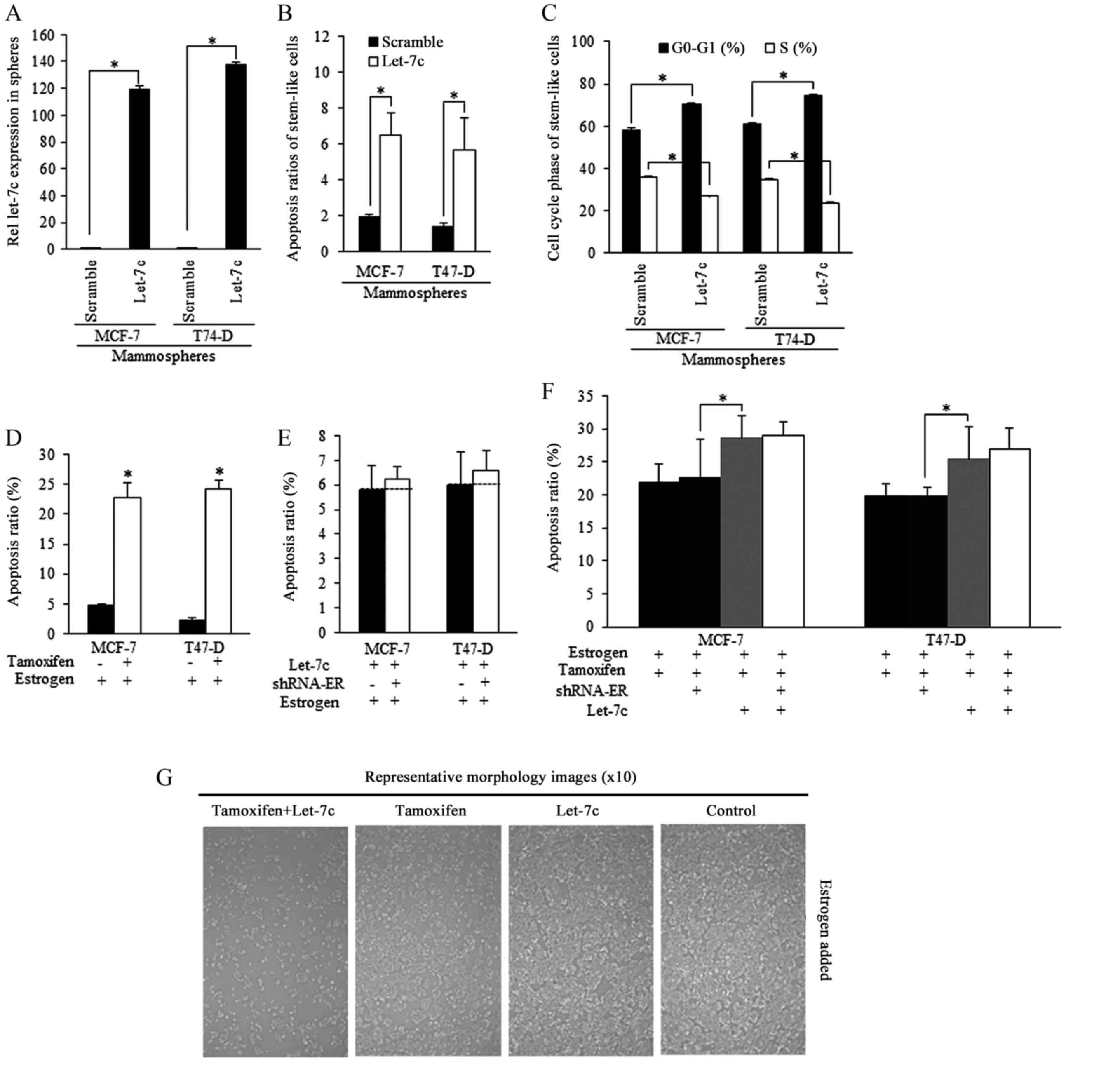
Tamoxifen induced apoptosis could be
strengthened by Let-7c and required ER
Mammospheres isolated from breast cancer cells were
successfully infected by Let-7c lentiviral plasmid [lentiviral
vector lentilox3.7 (pLL3.7)], and exhibited higher Let-7c
expression level than the scramble group (Fig. 3A). Enforced Let-7c induced
increased apoptosis (Fig. 3B) and
cell cycle arrest compared with the scramble control (Fig. 3C). Tamoxifen stimulated apoptosis
of estrogen-treated stem-like breast cancer cells (Fig. 3D). Enforced Let-7 did not increase
the apoptosis when ER level was inhibited, and did not sensitize
stem-like cells to tamoxifen induction of apoptosis, indicating
that Let-7c functions was relying on ER inhibition (Fig. 3E and F), as were illustrated in
Fig. 3G.
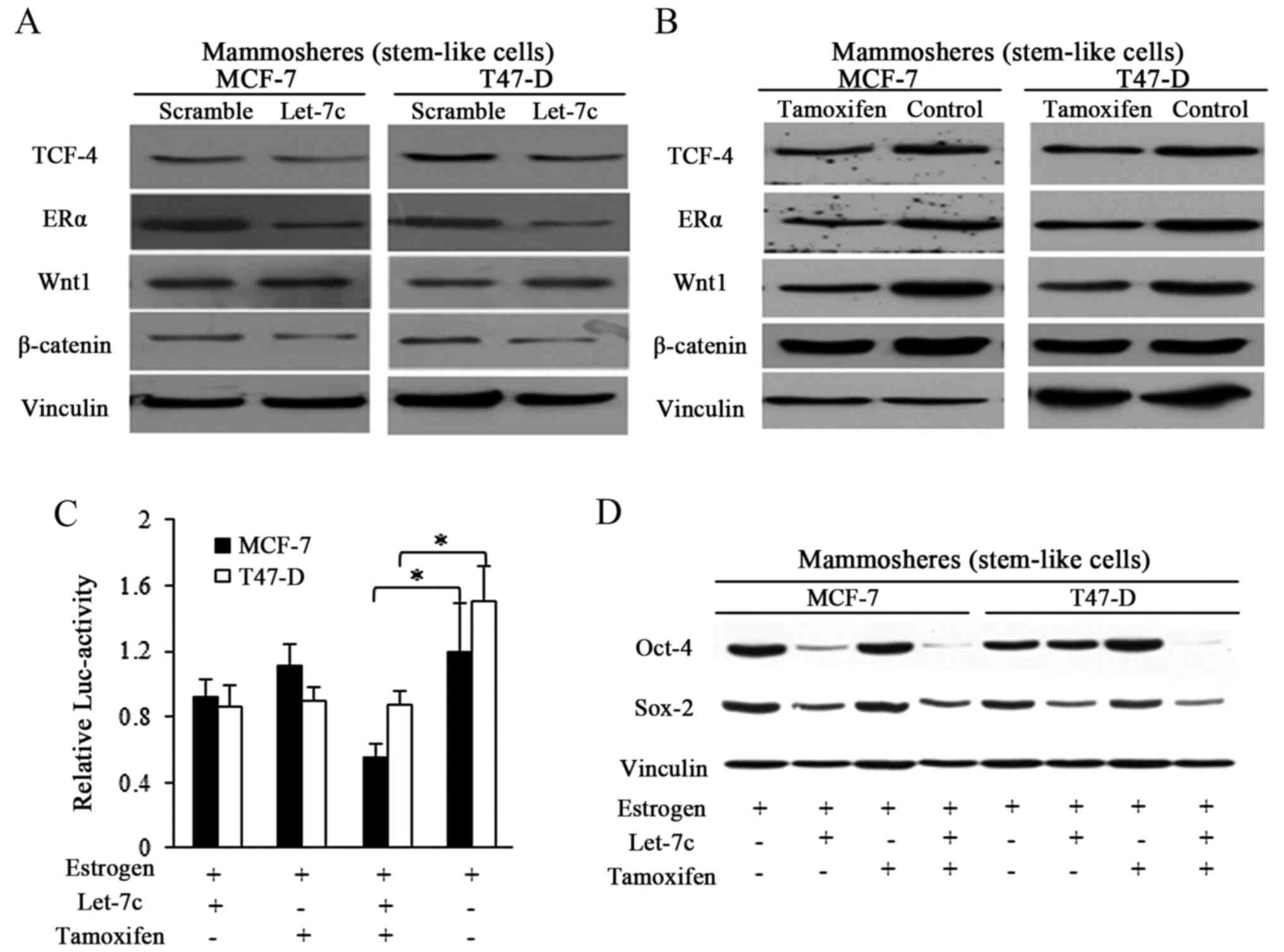
Let-7c sensitized the breast CSCs through
ER-dependent Wnt signaling inhibition
Let-7 family was previously reported to function
through post-translational regulation of ER (8,13),
and Let-7c decreased ER expression by binding to the 3′untranslated
region of its mRNA (13). In the
current study, enforced Let-7c expression exhibited suppressive
effects on ER and its downstream Wnt signaling activity in spheres
from breast cancer (Fig. 4A).
Tamoxifen induced the same effects in a similar manner to Let-7c,
however with weaker suppressive effects (Fig. 4B). TCF-4 responsive luciferase
promoter reporter assay was performed by using pCMV-Green
Renilla luciferase assay and Pierce Luciferase Cell Lysis
Buffer, and the luciferase activity was normalized to the
Renilla luciferase activity. Wnt signaling activity was
detected by using a β-catenin/wild-type TCF-4 responsive luciferase
promoter reporter, and results showed the suppressive effects of
both Let-7c and tamoxifen exerted on Wnt activity (Fig. 4C). When in combination, stronger
inhibition was detected in the group treated with combined Let-7
and tamoxifen, suggesting the assistant roles of Let-7 in tamoxifen
induced Wnt blocking (Fig. 4C).
To further explore the suppressive Let-7 functioning in estrogen
and tamoxifen regulation of stem cells potency, Sox-2 and Oct-4
were detected, and the results demonstrated that Let-7c affected
the protein expression of the pluripotency stimulators, and the
combined usage of Let-7c and tamoxifen reduced the expression of
these marker more efficiently than either alone (Fig. 4D).
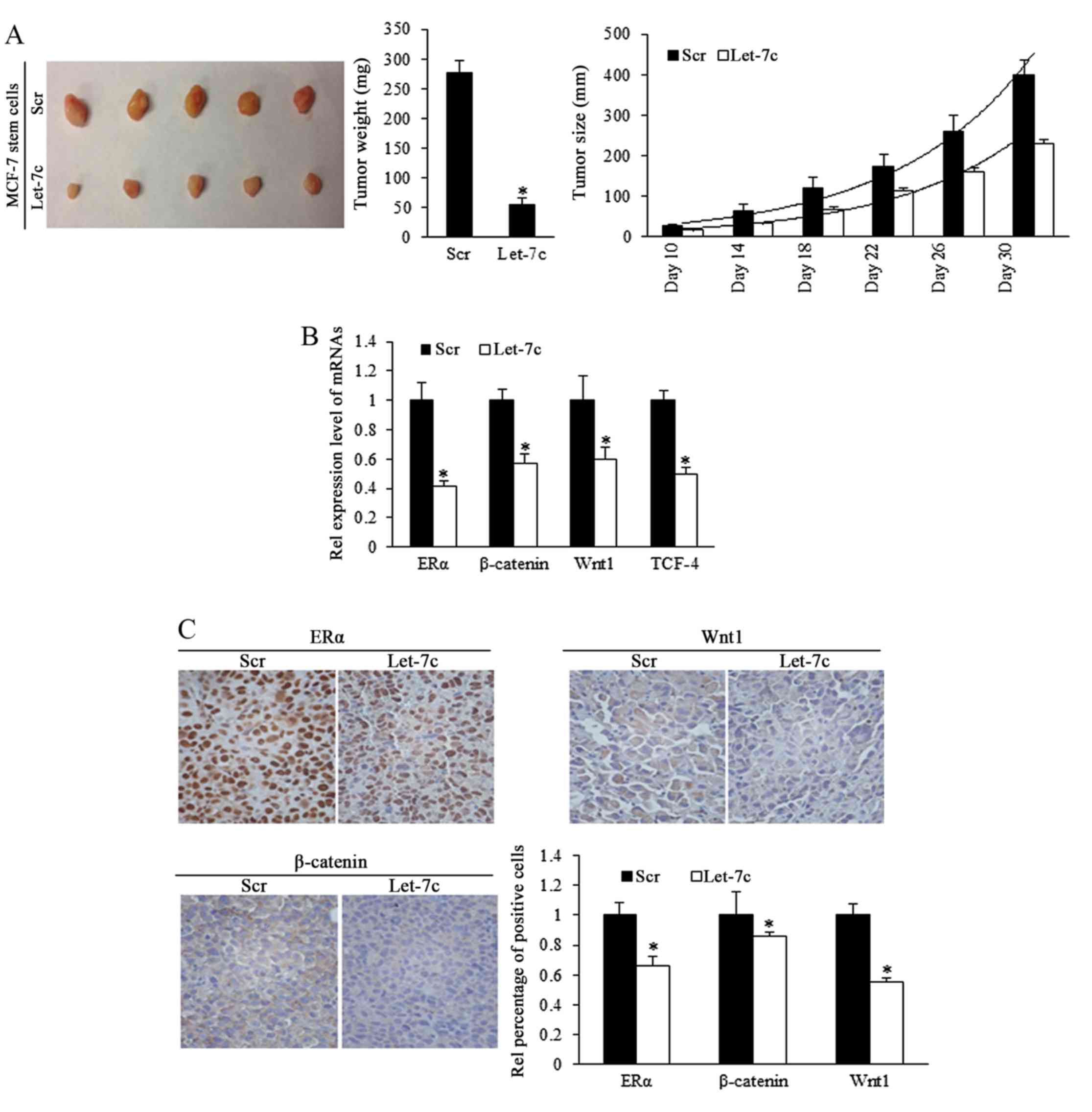
Let-7c inhibits tumor formation and
growth of breast CSCs in vivo
To investigate the role of Let-7c in inhibiting the
growth of ER-positive breast CSCs in vivo, estrogen-treated
MCF-7 stem cells that stably overexpressed Let-7c were
subcutaneously implanted into immune-deficient mice to form tumors.
Tumor size was measured every 5 days. Results demonstrated that
overexpressed Let-7c inhibited tumor growth and weight (Fig. 5A; Table I). To identify the effects of
Let-7c on MCF-7 stem-like cells in tumor formation, a cell dilution
assay was performed in vivo, and Let-7c-induced inhibition
of self-renewal was assessed. The end point of observation was
tumor emergence within 30 days after implantation at any
time-point. The number of tumor occurrence in nude mice was
recorded and orresponding to stem cells' renewal, but the tumor
growth was not included in the observation. Using the mouse models,
Let-7c was demonstrated to decrease tumor formation ability by
reducing the self-renewal ability of MCF-7 stem-like cells
(Table II). Let-7c inhibits the
self-renewal ability following the estrogen treatment, increasing
the number of stem cells required for tumor formation in mice with
the comparison being 1:398.2 with 1:4,832.6.
 | Table IStatistical analysis of tumor growth
of stem cells from different groups in immune compromised mice
(mm). |
Table I
Statistical analysis of tumor growth
of stem cells from different groups in immune compromised mice
(mm).
| Day | Mean ± SE (mm)
| F-value | P-value |
|---|
| Scramble | Let-7c |
|---|
| 10 | 27.08±4.72 | 16.28±1.27 | 0.0263 | 0.1374 |
| 14 | 62.66±16.89 | 32.87±2.10 | 0.001 | 0.1705 |
| 18 | 121.08±26.62 | 67.96±5.55 | 0.010 | 0.1049 |
| 22 | 173.93±30.24 | 114.12±6.02 | 0.009 | 0.0996 |
| 26 | 261.53±37.04 | 160.19±8.93 | 0.017 | 0.0477 |
| 30 | 398.34±38.88 | 228.56±11.24 | 0.034 | 0.0110 |
 | Table IIFrequency of different stem cell
suspensions in forming tumors in the mice. |
Table II
Frequency of different stem cell
suspensions in forming tumors in the mice.
| MCF-7 cell
suspensions | Stem cell number
| SC frequency
|
|---|
| 106 | 105 | 104 | 103 | 102 | 50 | Estimate L | imits |
|---|
| Scramble-E2 | 7/7 | 10/10 | 7/9 | 4/11 | 0/6 | 0/7 | 1:398.2 | 1:250a |
| Let-7c-E2 | 9/9 | 8/9 | 5/13 | 1/10 | 0/9 | 0/7 | 1:4,832.6 | 1:1,000 |
ER/Wnt signaling activity is involved in
Let-7c-induced functions
To further identify the different expression levels
of ER and Wnt signaling pathway downstream components in the murine
tumors, mRNA levels by RT-qPCR. The results demonstrated that ER
and signaling factors of Wnt were downregulated in the Let-7 group
(Fig. 5B). IHC staining confirmed
the downregulation of ER, β-catenin and Wnt1 proteins (Fig. 5C), suggesting that Let-7 may
inhibit the self-renewal of stem-like cells and decrease the tumor
formation efficiency of stem cells by suppressing ER and Wnt
signaling.
Discussion
Breast cancer is the leading cause of
cancer-associated mortality in women worldwide, due to its natural
malignancy and long-term recurrence with acquired drug resistance
(26). Improved and complex
strategies of therapeutics aiming at eliminating breast cancer
presented a promising condition using any or all of the following
in patients of different clinical/pathological phages: Surgeries,
chemotherapies, radiotherapies, targeted agents, hormonal therapies
and biotherapies (8). Tamoxifen
is an anti-estrogen drug that binds to ERs and blocks estrogen
signaling, acting as a competitive antagonist (27,28). Although it has been applied in
clinical treatment, it is only effective in 49% of patients with
ER-positive breast cancer. The association tamoxifen and miRNAs has
been reported in certain previous studies (29–31), in addition to in-depth research
into the role of miRNAs and other non-coding RNAs in sensitization
to therapeutics (32–34). Cancer stem cells within a tumor
prefer to stay at silent mitosis phase, and therefore the lower
proliferation index, resulted in resistance to therapies aiming at
eliminating the proliferative cells (7). Let-7 exerted inhibitory effects on
stem cell renewal, which may control the ratio of stem-like cells
in a tumor, which are involved in tumor emergence and recurrence.
The mechanisms through which Let-7 functions were reviewed
previously (12); however, with
the full functions of Let-7 are not established. The current study
explored the potential role of Let-7 in sensitization to
therapeutic agents, based on previous studies on cancer malignancy
and stem cell features. The results will increase our understanding
of the potential practical applications of Let-7. Tamoxifen was
demonstrated to inhibit cancer proliferation, blocking the
estrogen-stimulated stem cell renewal, and Let-7c sensitized CSCs
to the effects of tamoxifen via co-regulation of ESR1, in an ESR1
dependent manner. The addition of Let-7c strengthened the
suppressive effect of tamoxifen on cancer and renewal activity.
In vivo, Let-7 was demonstrated to act as suppressor via
Let-7c/ESR1/Wnt signaling, as previously identified in
vitro. Additionally, Let-7c suppressed the renewal ability of
stem cells, illustrated in the cells dilution assay in vivo,
particularly demonstrating the association of stem cell
self-renewal with tumor formation. The finding of the current study
and previous research indicate that Let-7 has important functions
in breast cancer occurrence, recurrence and stem cell renewal,
which may provide a solid research foundation for further clinical
applications of ncRNA in breast cancer, and expand the interactions
among non-coding RNAs in functional biology, yielding comprehensive
understandings of the intracellular genes regulation.
Acknowledgments
The authors acknowledge assistants in the Key
Laboratory (Ministry of Education) of Environment and Genes Related
to Disease, Medical College of Xi'an Jiaotong University, the
Center for Translational Medicine, the First Affiliated Hospital of
Xi'an Jiaotong University (Xi'an, China), and the Institute of
Urinary Surgery (the Key Laboratory, Ministry of Education), the
First Affiliated Hospital of Xi'an Jiaotong University. Professor
Peijun Liu (Center for Translational Medicine, the First Affiliated
Hospital of Xi'an Jiaotong University) has exerted his utmost
effort in conducting and performing the studies, and his
contribution is appreciated. This study was supported by National
Science Foundation for Young Scientists of China (grant no.
81602597) referred to Dr Xin Sun, National Natural Science
Foundation of China, (grant no. 81272418) referred to Professor
Hong Ren. This study was also supported in part by National Science
Foundation for Young Scientists of China (grant no. 81402506)
referred to Dr Sida Qin.
Notes
[1] Competing
interests
The authors declare there is no competing
interest.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roy V and Perez EA: Biologic therapy of
breast cancer: focus on co-inhibition of endocrine and angiogenesis
pathways. Breast Cancer Res Treat. 116:31–38. 2009. View Article : Google Scholar
|
|
3
|
Geisler J: Differences between the
non-steroidal aromatase inhibitors anastrozole and letrozole - of
clinical importance. Br J Cancer. 104:1059–1066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brodie A, Sabnis G and Macedo L: Xenograft
models for aromatase inhibitor studies. J Steroid Biochem Mol Biol.
106:119–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nabholtz JM, Mouret-Reynier MA, Durando X,
Van Praagh I, Al-Sukhun S, Ferriere JP and Chollet P: Comparative
review of anastrozole, letrozole and exemestane in the management
of early breast cancer. Expert Opin Pharmacother. 10:1435–1447.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miller WR and Larionov AA: Understanding
the mechanisms of aromatase inhibitor resistance. Breast Cancer
Res. 14:2012012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun X, Jiao X, Pestell TG, Fan C, Qin S,
Mirabelli E, Ren H and Pestell RG: MicroRNAs and cancer stem cells:
the sword and the shield. Oncogene. 33:4967–4977. 2014. View Article : Google Scholar
|
|
8
|
Sun X, Qin S, Fan C, Xu C, Du N and Ren H:
Let-7: a regulator of the ERα signaling pathway in human breast
tumors and breast cancer stem cells. Oncol Rep. 29:2079–2087. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun X, Tang S-C, Xu C, Wang C, Qin S, Du
N, Liu J, Zhang Y, Li X and Luo G: DICER1 regulated let-7
expression levels in p53-induced cancer repression requires cyclin
D1. J Cell Mol Med. 19:1357–1365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Xu C, Qin X, Wang S, Zheng H, Xu Z,
Luo S, Liu G, Liu P, Du JN, et al: Let-7a regulates mammosphere
formation capacity through Ras/NF-κB and Ras/MAPK/ERK pathway in
breast cancer stem cells. Cell Cycle. 14:1686–1697. 2015.
View Article : Google Scholar
|
|
11
|
Sun X, Jiang S, Liu J, Wang H, Zhang Y,
Tang SC, Wang J, Du N, Xu C, Wang C, et al: miR-208a stimulates the
cocktail of SOX2 and β-catenin to inhibit the let-7 induction of
self-renewal repression of breast cancer stem cells and formed
miR208a/let-7 feedback loop via LIN28 and DICER1. Oncotarget.
6:32944–32954. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun X, Liu J, Xu C, Tang SC and Ren H: The
insights of Let-7 miRNAs in oncogenesis and stem cell potency. J
Cell Mol Med. 20:1779–1788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun X, Xu C, Tang SC, Wang J, Wang H, Wang
P, Du N, Qin S, Li G, Xu S, et al: Let-7c blocks estrogen-activated
Wnt signaling in induction of self-renewal of breast cancer stem
cells. Cancer Gene Ther. 23:83–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang T, Liu Z, Guo S, Wu L, Li M, Yang J,
Chen R, Xu H, Cai S, Chen H, et al: The tumor suppressive role of
CAMK2N1 in castration-resistant prostate cancer. Oncotarget.
15:3611–3621. 2014.
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Liu H, Liang Y, Li Y, Li Y, Wang J, Wu H,
Wang Y, Tang SC, Chen J and Zhou Q: Gene silencing of BAG-1
modulates apoptotic genes and sensitizes lung cancer cell lines to
cisplatin-induced apoptosis. Cancer Biol Ther. 9:832–840. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He J, Wang M, Jiang Y, Chen Q, Xu S, Xu Q,
Jiang BH and Liu LZ: Chronic arsenic exposure and angiogenesis in
human bronchial epithelial cells via the
ROS/miR-199a5p/HIF-1α/COX-2 pathway. Environ Health Perspect.
122:255–261. 2014.PubMed/NCBI
|
|
18
|
Wang T, Guo S, Liu Z, Wu L, Li M, Yang J,
Chen R, Liu X, Xu H, Cai S, et al: CAMK2N1 inhibits prostate cancer
progression through androgen receptor-dependent signaling.
Oncotarget. 5:10293–10306. 2014.PubMed/NCBI
|
|
19
|
Qin S, Yang C, Zhang B, Li X, Sun X, Li G,
Zhang J, Xiao G, Gao X, Huang G, et al: XIAP inhibits mature
Smac-induced apoptosis by degrading it through ubiquitination in
NSCLC. Int J Oncol. 49:1289–1296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin S, Xu C, Li S, Wang X, Sun X, Wang P,
Zhang B and Ren H: Hyperthermia induces apoptosis by targeting
survivin in esophageal cancer. Oncol Rep. 34:2656–2664. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Wang Y, Fan C, Gao P, Wang X, Wei G
and Wei J: Estrogen promotes stemness and invasiveness of
ER-positive breast cancer cells through Gli1 activation. Mol
Cancer. 13:1372014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
23
|
Liu SY and Zheng PS: High aldehyde
dehydrogenase activity identifies cancer stem cells in human
cervical cancer. Oncotarget. 4:2462–2475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang T, Liu Z, Guo S, Wu L, Li M, Yang J,
Chen R, Xu H, Cai S, Chen H, et al: The tumor suppressive role of
CAMK2N1 in castration-resistant prostate cancer. Oncotarget.
5:3611–3621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang T, Guo S, Liu Z, Wu L, Li M, Yang J,
Chen R, Liu X, Xu H, Cai S, et al: CAMK2N1 inhibits prostate cancer
progression through androgen receptor-dependent signaling.
Oncotarget. 5:10293–10306. 2014.PubMed/NCBI
|
|
26
|
Dhami GK, Liu H, Galka M, Voss C, Wei R,
Muranko K, Kaneko T, Cregan SP, Li L and Li SS: Dynamic methylation
of Numb by Set8 regulates its binding to p53 and apoptosis. Mol
Cell. 50:565–576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Renoir J-M, Marsaud V and Lazennec G:
Estrogen receptor signaling as a target for novel breast cancer
therapeutics. Biochem Pharmacol. 85:449–465. 2013. View Article : Google Scholar
|
|
28
|
Maczis M, Milstien S and Spiegel S:
Sphingosine-1-phosphate and estrogen signaling in breast cancer.
Adv Biol Regul. 60:160–165. 2016. View Article : Google Scholar
|
|
29
|
Ward A, Shukla K, Balwierz A, Soons Z,
König R, Sahin O and Wiemann S: MicroRNA-519a is a novel oncomir
conferring tamoxifen resistance by targeting a network of
tumour-suppressor genes in ER+ breast cancer. J Pathol.
233:368–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ward A, Balwierz A, Zhang JD, Küblbeck M,
Pawitan Y, Hielscher T, Wiemann S and Sahin Ö: Re-expression of
micro RNA-375 reverses both tamoxifen resistance and accompanying
EMT-like properties in breast cancer. Oncogene. 32:1173–1182. 2013.
View Article : Google Scholar
|
|
31
|
Miller TE, Ghoshal K, Ramaswamy B, Roy S,
Datta J, Shapiro CL, Jacob S and Majumder S: MicroRNA-221/222
confers tamoxifen resistance in breast cancer by targeting
P27Kip1. J Biol Chem. 283:29897–29903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han Li C and Chen Y: Small and long
non-coding RNAs: novel targets in perspective cancer therapy. Curr
Genomics. 16:319–326. 2015. View Article : Google Scholar
|
|
33
|
Crea F, Watahiki A, Quagliata L, Xue H,
Pikor L, Parolia A, Wang Y, Lin D, Lam WL, Farrar WL, et al:
Identification of a long non-coding RNA as a novel biomarker and
potential therapeutic target for metastatic prostate cancer.
Oncotarget. 5:764–774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen ZZ, Huang L, Wu YH, Zhai WJ, Zhu PP
and Gao YF: LncSox4 promotes the self-renewal of liver
tumour-initiating cells through Stat3-mediated Sox4 expression. Nat
Commun. 7:125982016. View Article : Google Scholar : PubMed/NCBI
|