Introduction
Originally derived from trophoblasts, human amniotic
epithelial stem cells (HuAECs) are a specific type of cell that
possess the pluripotency of embryonic stem cells (ESCs) (1–3).
HuAECs can be induced to differentiate into various human tissues
and cells, and have specific therapeutic effects in some human
diseases (1–3). However, it is difficult to maintain
the pluripotency of HuAECs for long periods in culture. Following
primary isolation of HuAECs, pluripotency factors, including
octamer-binding protein 4 (Oct4), SRY-box 2 (Sox2) and Nanog, are
highly expressed; however, by the fifth passage, the expression
levels of these factors are decreased tô10% of the original levels,
and after subsequent passages, HuAECs hardly express these factors.
Furthermore, at this point, HuAECs can no longer be induced to
differentiate into other adult cells, retaining only the phenotype
and partial characteristics of epithelial cells (1). These issues have seriously hindered
the use of HuAECs.
Ubiquitination is a post-translational modification
process by which ubiquitin becomes covalently bound to a substrate
protein by enzymatic action (4–8).
The function of ubiquitination is to mark target proteins so that
they are recognized and degraded by proteasomes (4–8).
Comprising 76 amino acids, ubiquitin is a small polypeptide with a
conserved sequence, which is present universally and ubiquitously
in eukaryotic cells (4–8). The ubiquitination process begins
with an E1 ubiquitin-activating enzyme, which, in an ATP-dependent
reaction, activates a single free ubiquitin molecule by forming a
thioester bond between the Cys residue of the activity center of E1
and the C-terminus of ubiquitin. The activated ubiquitin is
channeled to an E2 ubiquitin-conjugating enzyme, which, together
with the recruited specific substrate protein and an E3 ubiquitin
ligase, mediates the transfer of ubiquitin from E2 to the target
protein. The recognition and degradation of ubiquitinated proteins
by proteasomes is a non-specific process; therefore, the E3 ligase
is a crucial factor in the process of protein degradation,
determining the specificity of the protein degradation reaction. In
most cases, the ubiquitination reaction involves one E1
ubiquitin-activating enzyme, and numerous E2 ubiquitin-conjugating
enzymes and E3 ubiquitin ligases. Quantitatively, one E1 interacts
with several E2 enzymes, whereas one E2 enzyme interacts with many
more E3 ubiquitin ligases. The inverted 'pyramid' structure formed
by E3, E2 and E1 is a large and complex signaling cascade network,
which extensively and accurately regulates cell activities. The
highly specific protein degradation mechanism in the cell is
predominantly determined by E3 ubiquitin ligase, and all E3
ubiquitin ligases are capable of linking a target protein to a
particular E2 enzyme. There are three major categories of E3
ubiquitin ligase: Those containing a homologous to the E6-AP
carboxyl terminus (HECT) domain; those containing a RING domain;
and those containing a U-box protein domain. The HECT
domain-containing E3 ubiquitin ligases act mainly by forming a
thioester bond with ubiquitin, which is essential to catalysis,
whereas the RING domain-containing E3 ubiquitin ligases provide the
binding sites for E2 enzymes and the substrate protein, so that the
E2 enzyme can catalyze direct transfer of ubiquitin to the
substrate (4–8). WW domain containing E3 ubiquitin
protein ligase 2 (WWP2) (also known as atrophin-1-interacting
protein 2) is a novel member of the E3 ubiquitin ligase family,
which specifically induces ubiquitination of Oct4, ultimately
leading to its degradation (9–14).
Following the loss of ligase activity of WWP2 by a point mutation
in the active site, the ubiquitination level of Oct4 is
significantly reduced (9–14). Conversely, once a ubiquitin
molecule is fused to the N-terminus of Oct4, a negative correlation
is observed between Oct4 level and cellular ubiquitination level
(9–14). In addition, when WWP2 is
overexpressed in ESCs, it may inhibit the activity of endogenous
Oct4 (9–14).
Based on these findings, the present study aimed to
investigate whether microRNA (miR)-32 weakens the ubiquitination of
Oct4 by specifically inhibiting WWP2 expression, thus maintaining
the pluripotency of HuAECs and enhancing their capacity for
differentiation into β islet-like cells by directed induction.
Materials and methods
HuAECs isolation and culture
The present study was approved by the ethics
committee of Shanghai Geriatric Institute of Chinese Medicine,
Longhua Hospital, Shanghai University of Traditional Chinese
Medicine (Shanghai, China). From May 2015 to December 2015, human
placentas were obtained with the written informed consent of
pregnant women (age, 28±3), who were negative for human
immunodeficiency virus-I, hepatitis B and hepatitis C. The
institutional ethics committee approved the use of human amnions.
Amnion membranes were peeled mechanically from the chorions of
placentas obtained from women that underwent an uncomplicated
Cesarean section. The epithelial layers, with the basement membrane
attached, were obtained and used to harvest HuAECs, as previously
described, with some modification (15–17). Briefly, the membrane was placed in
a 250-ml flask containing Dulbecco's modified Eagle's medium (DMEM)
and was cut into 0.5–1.0 cm2 segments using a razor. The
segments were digested with 0.25% trypsin-EDTA at 37°C for 45 min.
The resulting cell suspension was seeded in a 6-well plate in DMEM
supplemented with 10% fetal calf serum (GE Healthcare, Chicago, IL,
USA), penicillin (100 U/ml) and glutamine (0.3 mg/ml) at 37°C in a
humidified tissue culture incubator containing 5% CO2.
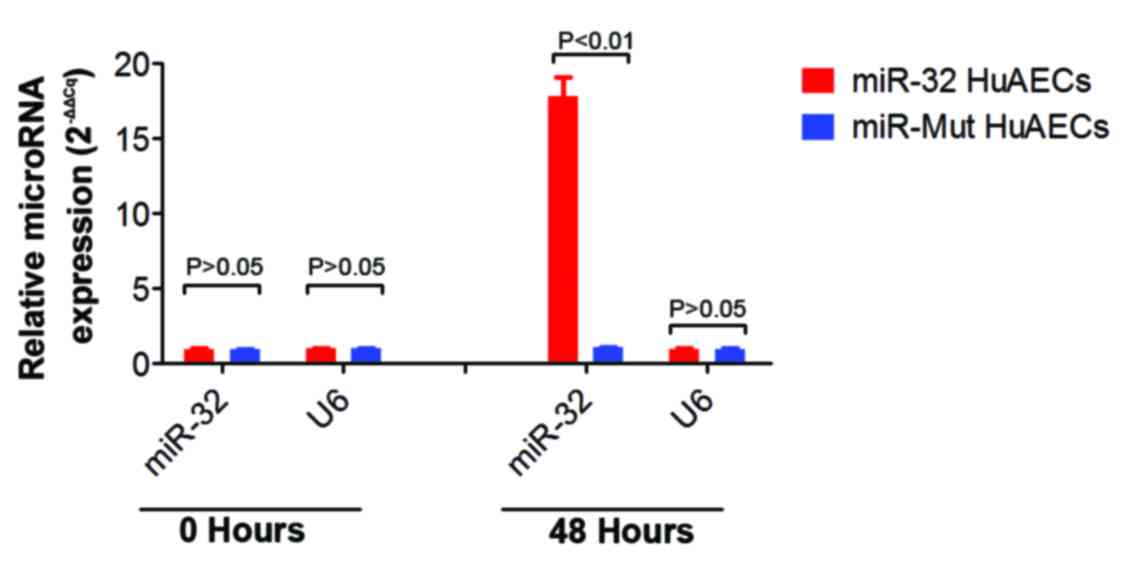
Successful infection of mutated miR (miR-Mut) and miR-32 was
confirmed by RT-qPCR (Fig.
1).
Induction of HuAECs differentiation into
β islet-like cells and C-peptide assay
According to a previous study (18), to induce their differentiation
into insulin-producing β islet-like cells, HuAECs
(1×106) were seeded into a 10-cm dish and cultured in
serum-free DMEM containing 25 mM D-glucose, N2 supplement (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 1%
antibiotic-antimycotic and 10 mM nicotinamide (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 2 weeks; medium was changed twice a
week. Cell culture supernatants were collected at appropriate time
points, centrifuged to remove cell debris, and the C-peptide levels
were assessed using a human C-peptide radioimmunoassay kit (China
Diagnostics Medical Corporation, Beijing, China).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from each cell sample using
TRΙzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. RNA samples were
digested with DNase I (Sigma-Aldrich; Merck KGaA), quantified and
reverse transcribed into cDNA using a ReverTra Ace-α First Strand
cDNA Synthesis kit (Toyobo Life Science, Osaka, Japan). A RealPlex4
real-time PCR detection system (Eppendorf, Hamburg, Germany) was
used to conduct RT-qPCR, with SYBR-Green Real-time PCR Master mix
(Toyobo Life Science) used as the detection dye. The RT-qPCR
amplification conditions were as follows: 1 cycle of initial
denaturation at 95°C for 2 min; and 40 cycles of denaturation at
95°C for 15 sec and annealing at 58°C for 45 sec and extension at
72°C for 42 sec; Then, 1 cycle of final extension at 72°C for 10
min. Target cDNA was quantified using the relative quantification
method. The quantification cycle (Cq) method was used to determine
gene expression relative to a control (calibrator), and
steady-state mRNA levels were reported as the fold difference
relative to the calibrator. For each sample, the marker gene Cq
values were normalized using the formula ΔCq = Cq_genes − Cq_18s
ribosomal RNA (rRNA). The relative expression levels were
determined according to the following formula: ΔΔCq = ΔCq_sample
group − ΔCq_control group. The 2−ΔΔCq method was used to
calculate the relative expression levels of marker genes (19). The calibrator used was the 18s
rRNA gene. The cDNA of each gene was amplified using primers shown
in Table I.
 | Table IPolymerase chain reaction
primers. |
Table I
Polymerase chain reaction
primers.
| Gene name | Primers
(5′→3′) | Size (bp) |
|---|
| Nanog | F:
GATTTGTGGGCCTGAAGAAA | |
| R
ATGGAGGAGGGAAGAGGAGA | 93 |
| Oct4 | F:
CAGTGCCCGAAACCCACAC | |
| R:
GGAGACCCAGCAGCCTCAAA | 161 |
| Sox2 | F:
AACCCCAAGATGCACAACTC | |
| R:
GCTTAGCCTCGTCGATGAAC | 100 |
| Insulin | F:
TTCTACACACCCAAGACCCG | |
| R:
CAATGCCACGCTTCTGC | 130 |
| Pdx1 | F:
CCTTTCCCATGGATGAAGTC | |
| R:
CGTCCGCTTGTTCTCCTC | 96 |
| Pax6 | F:
GTTGGTATCCGGGGACTTC | |
| R:
TCCGTTGGAACTGATGGAGT | 101 |
| Nkx2.2 | F:
TCTACGACAGCAGCGACAAC | |
| R:
GGAGCTTGAGTCCTGAGGG | 110 |
| Glut2 | F:
TGTGCCACACTCACACAAGA | |
| R:
GACAGTGAAAACCAGGGTCC | 96 |
| GCK | F:
GATGGATGTCACAAGGAGCC | |
| R:
CCTTCTTCAGGTCCTCCTCC | 95 |
| Isl1 | F:
TGTTTGAAATGTGCGGAGTG | 142 |
| R:
TCTTGCTGAAGCCGATGC | |
| 18S rRNA | F:
CAGCCACCCGAGATTGAGCA | |
| R:
TAGTAGCGACGGGCGGTGTG | 253 |
Luciferase reporter assay
The luciferase reporter assay was performed
according to a previously described method (15,20). NIH-3T3 cells were seeded at
3×104/well in 48-well plates and were cotransfected with
400 ng pRNAT-CMV-miR-32, pRNAT-CMV or pRNAT-CMV-miR-32-Mut, and 20
ng pGL-WWP2-3′ untranslated region (UTR)-wild-type (WT) or pGL-WWP2
3′UTR-Mut, and pRL-TK (Promega Corporation, Madison, WI, USA) using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
48 h of transfection, luciferase activity was measured using a
dual-luciferase reporter assay system (Promega Corporation).
Immunofluorescence staining
Cultured cells were washed three times with fetal
calf serum and fixed with 4% paraformaldehyde (Sigma-Aldrich; Merck
KGaA) for 30 min. After blocking, the cells were incubated with
primary antibodies (Table II)
overnight at 4°C, and then with goat anti-rabbit IgG H&L (Alexa
Fluor® 555) pre-adsorbed (1:200; ab150086; Abcam,
Cambridge, UK) and 5 µg/ml 4′,6-diamidino-2-phenylindole
(Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. The cells were then
washed thoroughly with Tris-buffered saline-Tween (TBST) [25 mM
Tris/HCl (pH 8.0), 125 mM NaCl and 0.05% Tween-20] and viewed under
a fluorescence microscope (DMI3000; Leica Microsystems, Inc.,
Buffalo Grove, IL, USA).
 | Table IIAntibodies. |
Table II
Antibodies.
| Antibody | Supplier | Application |
|---|
| Rabbit
anti-human | Cell Signaling | IF (1:200) |
| Oct4 (sc-9081) | Technology,
Inc. | WB (1:1,000) |
| Rabbit
anti-human | Cell Signaling | IF (1:200) |
| Nanog
(sc-33759) | Technology,
Inc. | WB (1:1,000) |
| Rabbit
anti-human | Santa Cruz | IF (1:200) |
| Sox2
(sc-20088) | Biotechnology,
Inc. | WB (1:1,000) |
| Mouse anti-human
insulin (sc-377071) | Santa Cruz | |
| Biotechnology,
Inc. | WB (1:1,000) |
| Rabbit
anti-human | Santa Cruz | |
| PDX1
(sc-25403) | Biotechnology,
Inc. | WB (1:1,000) |
| Mouse
anti-human | Santa Cruz | IF (1:200) |
| WWP2
(sc-398090) | Biotechnology,
Inc. | WB (1:1000) |
| Rabbit
anti-human | Cell Signaling | WB (1:1,000) |
| GADPH
(sc-25778) | Biotechnology,
Inc. | |
Western blot analysis
HuAECs were lysed in 2X loading lysis buffer [50 mM
Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, 10%
β-mercaptoethanol, 10% glycerol and 0.002% bromophenol blue]. The
concentration of total protein was then determined by Enhanced BCA
Protein Assay kit (Beyotime Institute of Biotechnology, Shanghai,
China). The total proteins (15 µl, 25 µg/µl)
from cultured cells were then separated by 12% SDS-PAGE and
transferred onto Hybrid-polyvinylidene fluoride membranes (EMD
Millipore, Bedford, MA, USA). The membranes were blocked with 5%
(w/v) non-fat dried milk in TBST and were washed four times with
TBST at room temperature (15 min/wash) prior to incubation with
primary antibodies (Table II) in
5% (w/v) non-fat dried milk in TBST. After further washing, the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit IgG secondary antibody (1:1,000; sc-2004; Santa
Cruz Biotechnology, Dallas, TX, USA) at 37°C for 1 h. After washing
four times with TBST at room temperature (15 min/wash), the
immunoreactive protein bands were visualized by enhanced
chemiluminescence (ECL) using an ECL kit (PerkinElmer, Inc.,
Waltham, MA, USA).
Northern blot analysis
Northern blotting was performed according to a
previously described method (15). For all groups, 20 µg good
quality total RNA was analyzed on a 7.5 M urea, 12% polyacrylamide
denaturing gel and was transferred to Hybond N+ nylon
membrane (GE Healthcare). Ultraviolet light, for 30 sec at 1,200
mjoule/cm2, was used to crosslink the membranes. The
membranes were hybridized with the miR-32 antisense starfire probe,
5′-ATAACGTGTAATGATTCAACGT-3′ (Integrated DNA Technologies,
Coralville, IA, USA), to detect the 22-nt miR-32 fragments,
according to the manufacturer's protocol. After washing by Northern
washing buffer (25 mM
NaH2PO4·2H2O, 25 mM
Na2HPO4·12H2O, 1 mM EDTA, 1% SDS,
5% formamide deionized), the membranes were exposed to Kodak XAR-5
films for 20–40 h (Sigma-Aldrich; Merck KGaA). All membranes were
hybridized with a human U6 snRNA probe as a positive control,
5′-GCAGGGGCCATGCTAATCTTCTCTGTATCG-3′. The exposure times for the U6
control probe varied between 15 and 30 min.
Co-immunoprecipitation (co-IP) assay
Cells were seeded at 3×105/well in 6-well
plates and were cultured until they reached 85% confluence. The
cells were then lysed in 500 µl modified cell lysis buffer
[20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA,
sodium pyrophosphate, β-glycerophosphate,
Na3VO4 and leupeptin) (Beyotime Institute of
Biotechnology) for western blotting and co-IP. After lysis, each
sample was clarified by centrifugation to remove insoluble debris
and the supernatant was preincubated with 20 µg protein A
agarose beads (Beyotime Institute of Biotechnology) for 30 min at
4°C with agitation. Subsequently, the samples were centrifuged and
transferred to a fresh 1.5 ml tube. The samples were incubated with
antibodies (Table II) for 90 min
prior to the addition of 20 µg protein A agarose beads to
capture immune complexes. The pelleted beads were washed three
times with 500 µl cell lysis buffer, dissolved in 4X
SDS-PAGE sample loading buffer and heated for 10 min at 95°C.
KCl and tolbutamide stimulation
experiments
According to the protocol of C-peptide RIA kit
(China Diagnostics Medical Corporation), briefly, the
1×106 cells/well were washed twice in DMEM (Gibco;
Thermo Fisher Scientific, Inc.) containing 2 mM glucose
(Sigma-Aldrich; Merck KGaA) and cultured in this medium for 1 h.
The medium was then replaced by 2 ml DMEM containing glucose (0, 1,
5, 25 mM, respectively, for each well) or KCl (0, 10, 20, 40 mM,
respectively, for each well; Sigma-Aldrich) or tolbutamide (0, 50,
100 mM, respectively, for each well; Sigma-Aldrich) for 60 min. The
culture supernatants were collected after 12 h of incubation,
centrifuged to remove debris, and concentration of C-peptide was
tested by human C-peptide RIA kit.
Statistical analysis
Each experiment was performed at least three times.
Data are presented as the means ± standard error. Student's t-test
was used to evaluate differences between two groups. One-way
analysis of variance was used to compare multiple groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
With increasing passage number,
endogenous miR-32 expression and WWP2 expression are negatively
associated in HuAECs
The results of the RT-qPCR analysis suggested that
the mRNA expression levels of WWP2 were gradually increased in the
HuAECs with continued passage (Fig.
2). Northern blot analysis confirmed that with continued
passage of HuAECs, the hybridization signal of endogenous miR-32
became increasingly weaker (Fig.
2). These results indicated that endogenous miR-32 expression
and WWP2 expression in HuAECs were negatively associated.
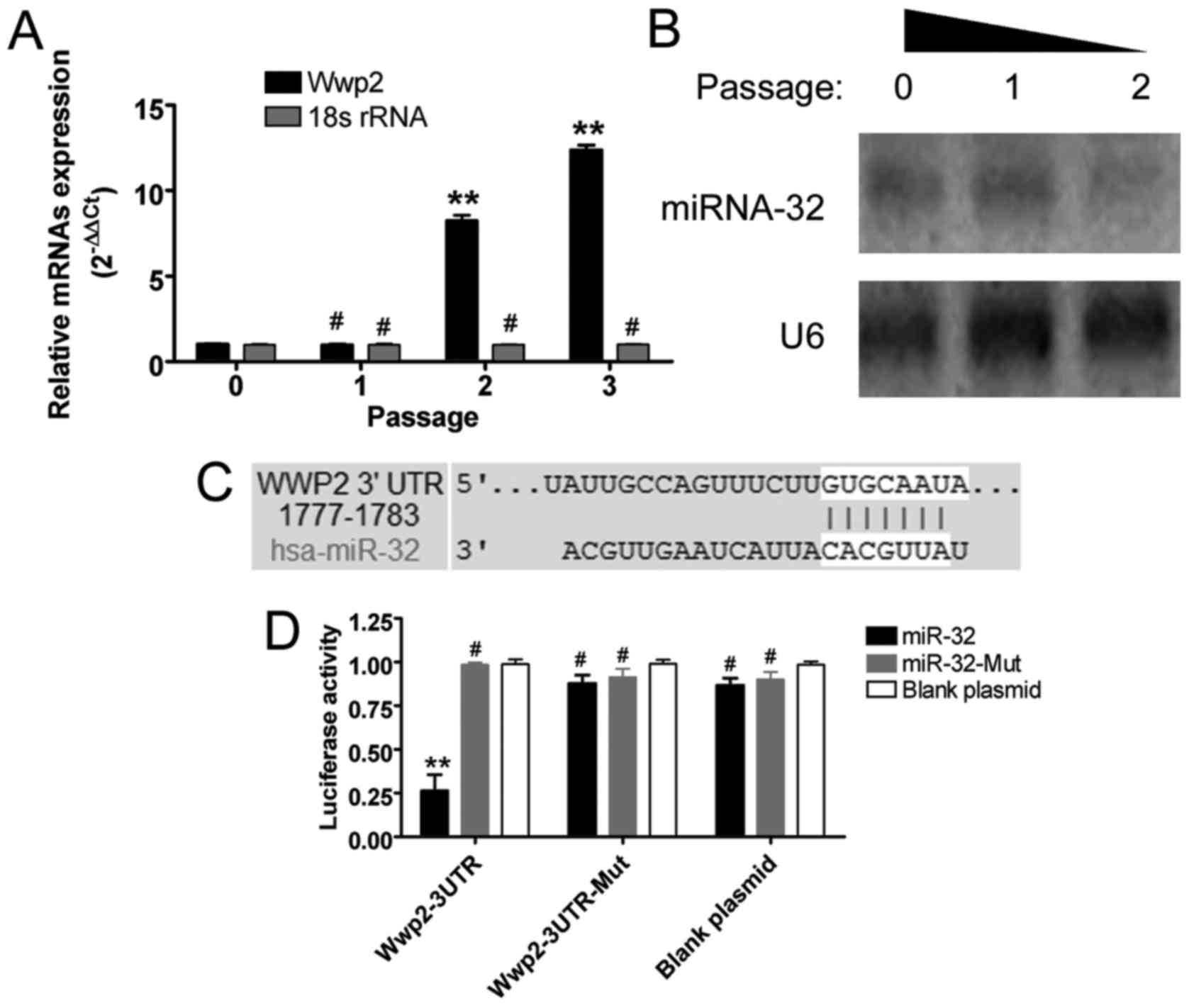 | Figure 2Expression of miR-32 and WWP2 in
HuAECs and target validation. (A) Reverse
transcription-quantitative polymerase chain reaction indicated that
the expression levels of endogenous WWP2 were gradually increased
in HuAECs with passage (**P<0.01 vs. passage 0;
#P>0.05 vs. passage 0; one-way ANOVA, n=3). (B)
Northern blotting demonstrated that, with increasing passage of
HuAECs, the hybridization signal of endogenous miR-32 became
increasingly weaker. (C) Sequence alignment identified a
complementary binding site for mature miR-32 in the 3′UTR of WWP2
mRNA (between 1777 and 1783 bp). (D) Luciferase reporter assays
demonstrated that following overexpression of miR-32 in NIH-3T3
cells containing the wild-type 3′UTR of WWP2, luciferase activity
was significantly decreased (**P<0.01 vs. blank
plasmid; #P>0.05 vs. blank plasmid; one-way ANOVA,
n=3). 3′UTR, 3′ untranslated region; ANOVA, analysis of variance;
HuAECs, human amniotic epithelial stem cells; miR/miR-32,
microRNA-32; Mut, mutated; WWP, WW domain containing E3 ubiquitin
protein ligase 2. |
Targeted binding of miR-32 to the 3′UTR
of WWP2 mRNA silences its expression
Sequence alignment, using bioinformatics analysis
software (microRNA.org - Targets and Expression tools, http://34.236.212.39/microrna/home.do),
identified a complementary binding site for mature miR-32 in the
3′UTR (between 1777 and 1783 bp) of WWP2 mRNA (Fig. 2). Luciferase reporter assays
indicated that following overexpression of miR-32 in the NIH-3T3
cell line, the luciferase activity was significantly decreased when
cells were cotransfected with WWP2 containing WT 3′UTR (Fig. 2). These results suggested that
miR-32 could silence the mRNA expression of WWP2.
Exogenous overexpression of miR-32
effectively inhibits WWP2 expression in HuAECs
miR-32 was overexpressed in one group of HuAEC cells
following infection with a lentivirus, whereas the negative control
group of HuAECs were infected with miR-Mut. The two groups were
cultured under the same conditions and passaged five consecutive
times. Subsequently, RT-qPCR analysis indicated that, in HuAECs
infected with miR-32, the mRNA expression levels of WWP2 were
reduced at different passage numbers (passages 1, 2, 3, 4 and 5)
compared with in the HuAECs infected with miR-Mut (Fig. 3). Furthermore, western blotting
confirmed that at the 5th passage, miR-32-infected HuAECs exhibited
a reduced abundance of WWP2 protein expression compared with in the
miR-Mut-infected HuAECs (Fig.
3).
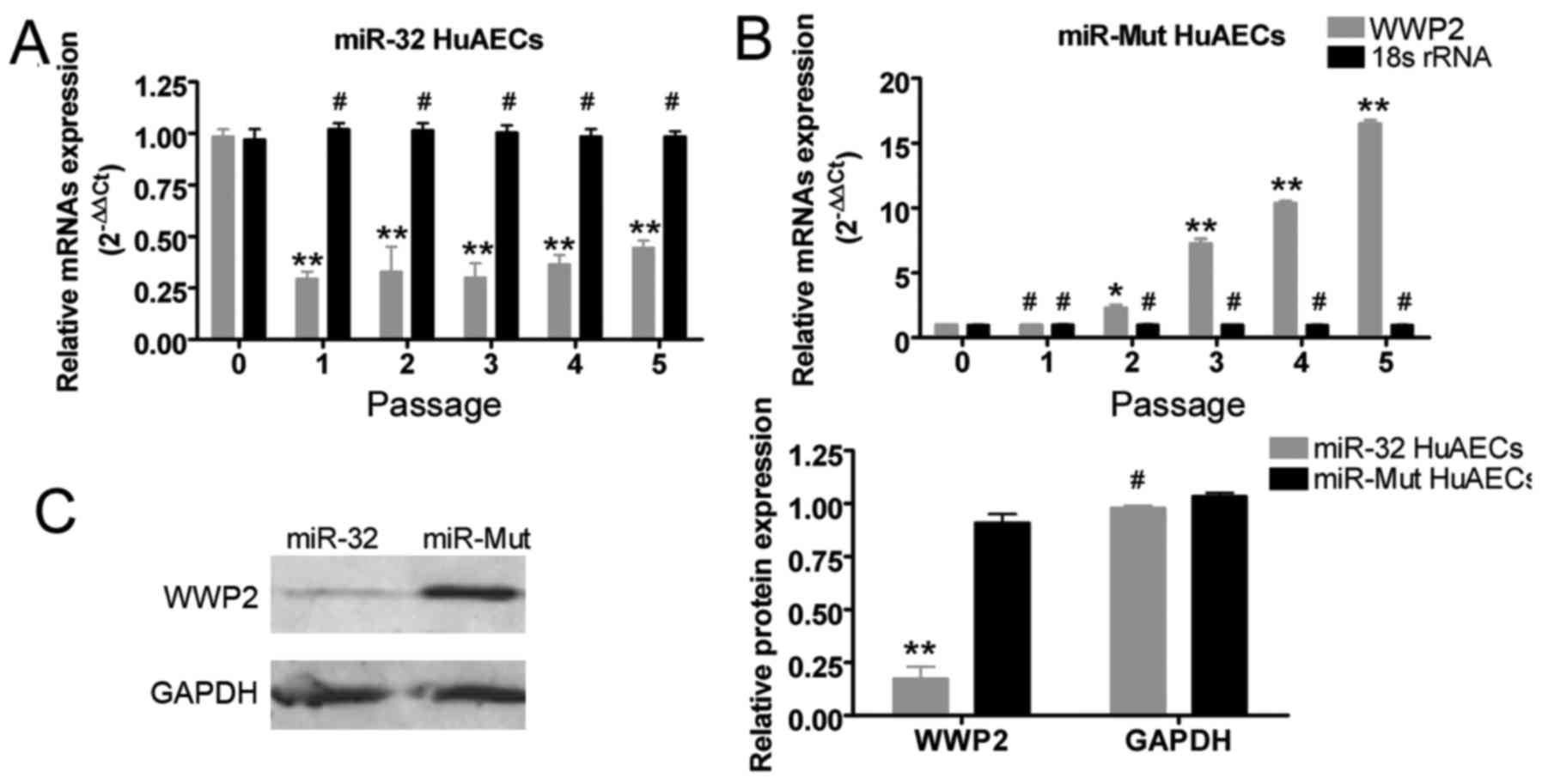 | Figure 3miR-32 inhibits WWP2 expression. (A
and B) Reverse transcription-quantitative polymerase chain reaction
demonstrated that, at different passages, the mRNA expression
levels of WWP2 were reduced in miR-32-infected HuAECs compared with
in miR-Mut-infected HuAECs (**P<0.01 vs. passage 0;
*P<0.05 vs. passage 0; #P>0.05 vs. passage 0;
one-way analysis of variance, n=3). (C) Western blot analysis
confirmed, that at the 5th passage, the protein expression levels
of WWP2 were reduced in miR-32-infected HuAECs compared with in
miR-Mut-infected HuAECs (**P<0.01 vs. miR-Mut HuAECs;
#P>0.05 vs. miR-Mut HuAECs; Student's t-test, n=3).
HuAECs, human amniotic epithelial stem cells; miR-32, microRNA-32;
Mut, mutated; WWP, WW domain containing E3 ubiquitin protein ligase
2. |
Exogenous overexpression of miR-32
inhibits Oct4 ubiquitination by WWP2 in HuAECs
RT-qPCR demonstrated that, at the 5th passage,
miR-32-infected HuAECs exhibited significantly increased expression
levels of mRNAs encoding embryonic stem cell markers (Oct4, Sox2
and Nanog) compared with in the miR-Mut-infected HuAECs (Fig. 4). Furthermore, western blotting
confirmed that, at the 5th passage, miR-32-infected HuAECs
exhibited significantly increased protein expression of embryonic
stem cell markers (Oct4, Sox2 and Nanog) compared with in the
miR-Mut-infected HuAECs (Fig. 4).
In addition, western blotting of the co-IP samples indicated that,
at the 5th passage, in miR-32-infected HuAECs Oct4 protein
ubiquitination was reduced compared with in miR-Mut-infected HuAECs
(Fig. 4). Immunofluorescence
analysis revealed that the expression levels of Oct4, Sox2, Nanog
and WWP2 were consistent with the western blotting results
(Fig. 4). These findings
suggested that exogenous overexpression of miR-32 effectively
inhibited Oct4 ubiquitination by WWP2 in HuAECs, and maintained the
expression of transcription factors associated with ESC
pluripotency.
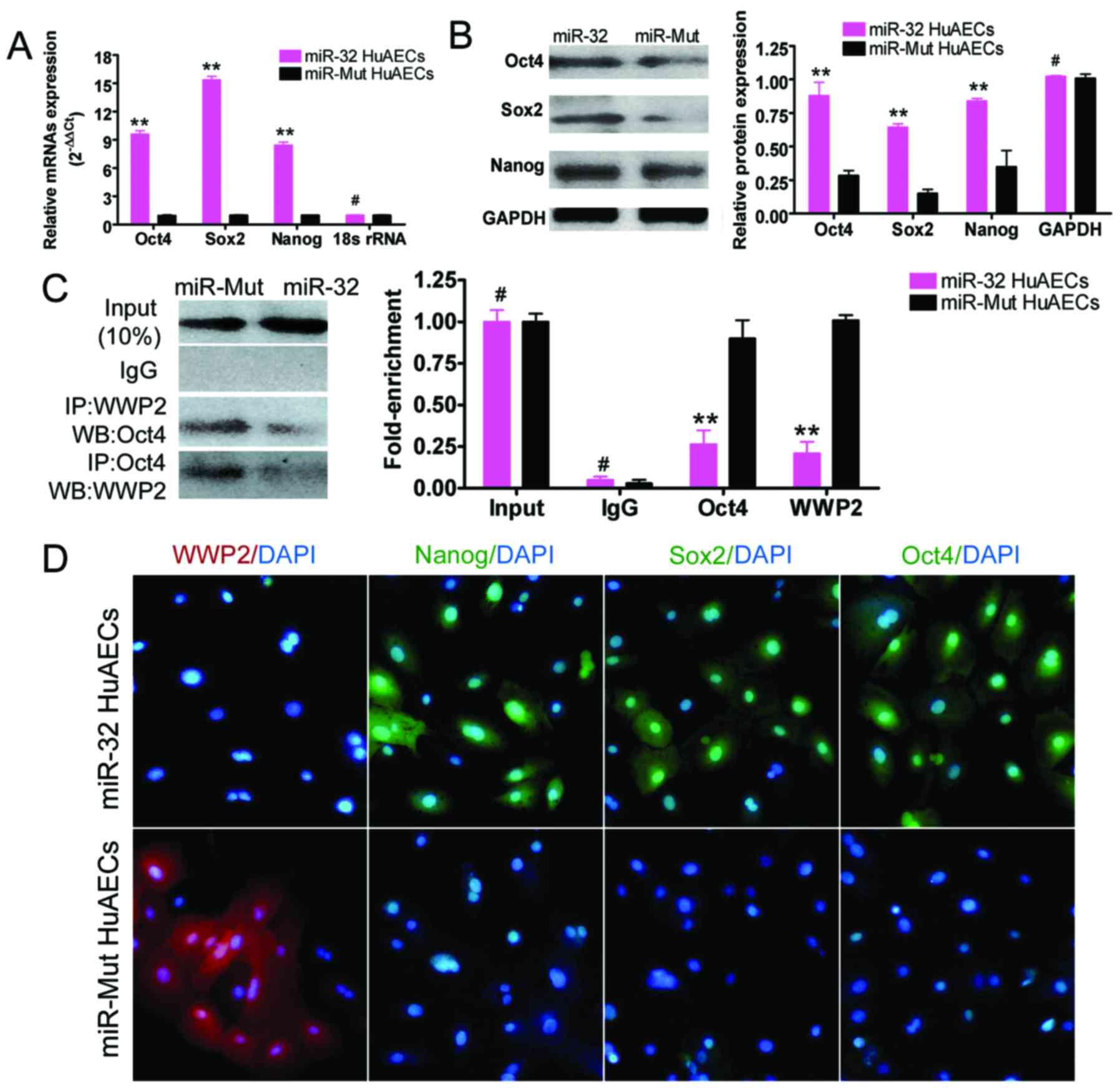 | Figure 4miR-32 effectively inhibits
ubiquitination of Oct4 by WWP2. (A) Reverse
transcription-quantitative polymerase chain reaction indicated that
the mRNA expression levels of embryonic stem cell markers were
increased in miR-32-infected HuAECs compared with in
miR-Mut-infected HuAECs (**P<0.01 vs. miR-Mut HuAECs;
#P>0.05 vs. miR-Mut HuAECs; Student's t-test, n=3).
(B) Western blot analysis confirmed that the protein expression
levels of embryonic stem cell markers were increased in
miR-32-infected HuAECs compared with in miR-Mut-infected HuAECs
(**P<0.01 vs. miR-Mut HuAECs; #P>0.05
vs. miR-Mut HuAECs; Student's t-test, n=3). (C)
Co-immunoprecipitation western blotting results indicated that
ubiquitination of Oct4 protein was significantly reduced in
miR-32-infected HuAECs compared with in miR-Mut-infected HuAECs
(**P<0.01 vs. miR-Mut HuAECs; #P>0.05
vs. miR-Mut HuAECs; Student's t-test, n=3). (D) Immunofluorescence
staining demonstrated that the expression of embryonic stem cell
markers in miR-32-infected HuAECs was significantly increased
compared with in miR-Mut-infected HuAECs, whereas WWP2 expression
was decreased (magnification, ×200). DAPI,
4′,6-diamidino-2-phenylindole; HuAECs, human amniotic epithelial
stem cells; IgG, immunoglobulin G; IP, immunoprecipitation; miR-32,
microRNA-32; Mut, mutated; Oct4, octamer-binding protein 4; Sox2,
SRY-box 2; WB, western blotting; WWP, WW domain containing E3
ubiquitin protein ligase 2. |
Exogenous overexpression of miR-32
effectively maintains the capability of HuAECs to differentiate
into β islet-like cells and express β islet-like cell markers
Under specific cell culture conditions, at the 5th
passage, the two HuAEC cultures (infected with miR-32 or miR-Mut)
were simultaneously induced to differentiate into β islet-like
cells in vitro. After 21 days of induction, the results of
RT-qPCR suggested that, in the induced cell cultures, the mRNA
expression levels of β islet-like cell biomarkers [insulin,
pancreatic duodenal homeobox-1 (Pdx1), ISL LIM homeobox 1, paired
box 6, NK2 homeobox 2, glucose transporter 2 and glucokinase) were
significantly increased in miR-32-infected HuAECs compared with in
miR-Mut-infected HuAECs (Fig. 5).
Western blotting confirmed that, after induction, the protein
expression levels of insulin and PDX1 were significantly increased
in miR-32-infected HuAECs compared with in miR-Mut-infected HuAECs
(Fig. 5). These results suggested
that exogenous overexpression of miR-32 may effectively maintain
the ability of HuAECs to differentiate into β islet-like cells and
to express β islet-like cell markers.
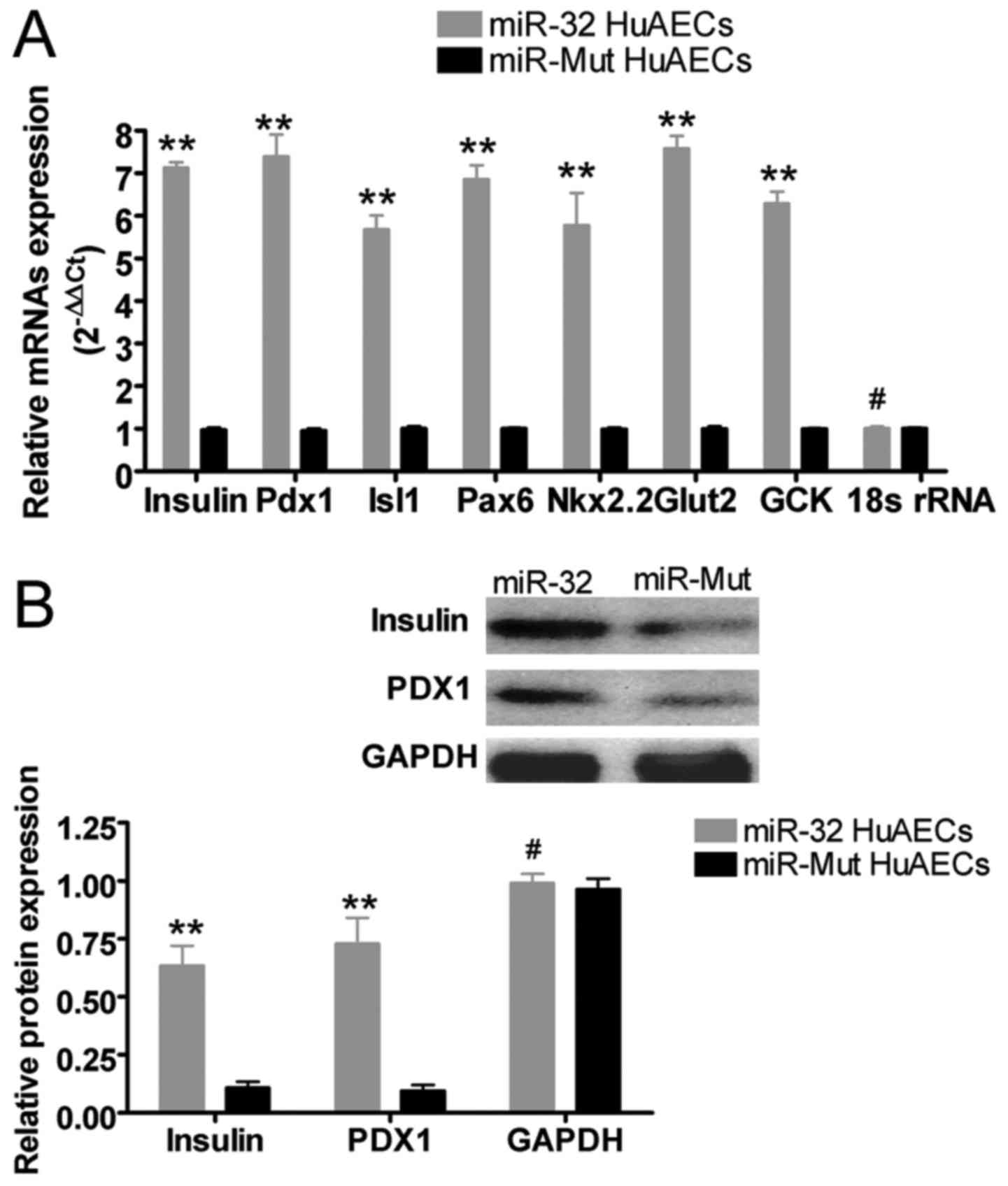 | Figure 5miR-32 maintains the ability of HuAECs
to differentiate into β islet-like cells. (A) Reverse
transcription-quantitative polymerase chain reaction analysis
suggested that, after induction, the mRNA expression levels of β
islet-like cell biomarkers were increased in miR-32-infected HuAECs
compared with in miR-Mut-infected HuAECs (**P<0.01
vs. miR-Mut HuAECs; #P>0.05 vs. miR-Mut HuAECs;
Student's t-test, n=3). (B) Western blot analysis demonstrated
that, after induction, the protein expression levels of insulin and
PDX1 were increased in miR-32-infected HuAECs compared with in
miR-Mut-infected HuAECs (**P<0.01 vs. miR-Mut HuAECs;
#P>0.05 vs. miR-Mut HuAECs; Student's t-test, n=3).
GCK, glucokinase; Glut2, glucose transporter 2; HuAECs, human
amniotic epithelial stem cells; Isl1, ISL LIM homeobox 1; miR-32,
microRNA-32; Mut, mutated; Nkx2.2, NK2 homeobox 2; Pax1, paired box
6; Pdx1, pancreatic duodenal homeobox-1. |
Exogenous overexpression of miR-32
effectively enhances C-peptide secretion from β islet-like cells
derived from HuAECs
C-peptide is secreted into the cell culture medium
in amounts equal to those of insulin secreted by the pancreas;
therefore, the C-peptide content of the supernatant of cell culture
medium was determined, in order to investigate the synthetic
insulin-secreting capability of β islet-like cells derived from the
two HuAEC groups. Firstly, after directed induction of
differentiation of HuAECs into β islet-like cells, the levels of
C-peptide secreted by the two cell groups were analyzed at
different time-points (1, 2 and 3 weeks). The results demonstrated
that, with increasing time after induction, the levels of C-peptide
secreted by miR-32-infected HuAECs were much greater compared with
those secreted by miR-Mut-infected HuAECs (Fig. 6 and Table III). Secondly, the levels of
C-peptide secreted following stimulation of β islet-like cells with
various concentrations of glucose were compared. The results
indicated that with increasing glucose concentration, the amount of
C-peptide secreted by β islet-like cells derived from
miR-32-infected HuAECs was significantly increased compared with
that secreted by cells derived from miR-Mut-infected HuAECs
(Fig. 6 and Table III). In addition, following
supplementation of the culture medium with various concentrations
of KCl, the results indicated that, with increasing KCl
concentration, the amount of C-peptide secreted by β islet-like
cells derived from miR-32-infected HuAECs was much greater than
that secreted by cells derived from miR-Mut-infected HuAECs
(Fig. 6 and Table III). Furthermore, since blockage
of the functionally sensitive intracellular KATP ion
channels may induce an increase in C-peptide secretion,
tolbutamide, an ATP-sensitive KATP ion channel
inhibitor, was added to the culture medium of each cell group. The
results indicated that with increasing tolbutamide concentration,
C-peptide secretion of β islet-like cells derived from
miR-32-infected HuAECs was much higher than that of cells derived
from miR-Mut-infected HuAECs (Fig.
6 and Table III). These
findings confirmed that exogenous overexpression of miR-32 could
effectively enhance the C-peptide secretion of β islet-like
cells.
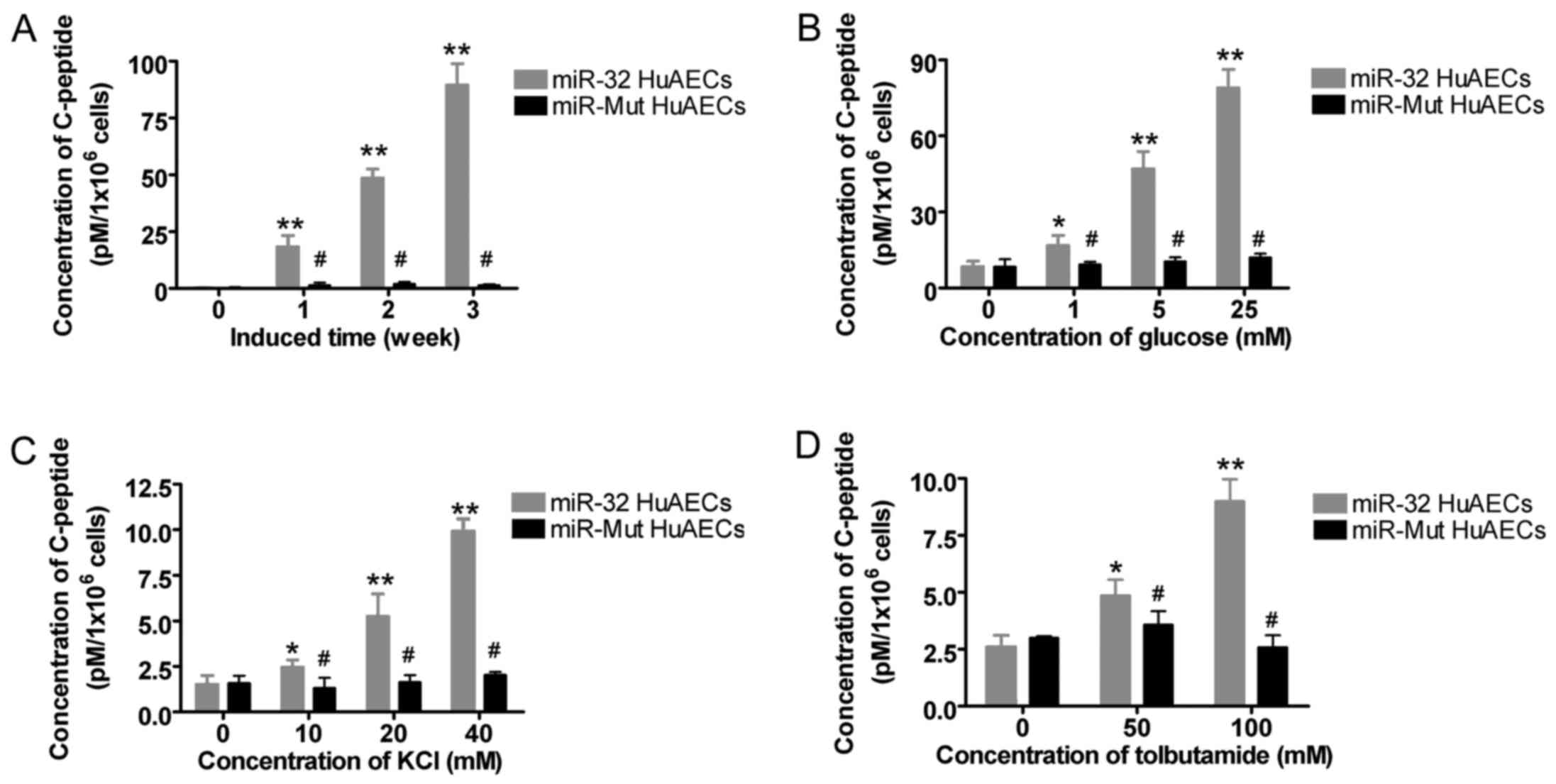 | Figure 6miR-32 enhances the secretion of
C-peptide from β islet-like cells derived from HuAECs. (A) With
increasing time after induction, C-peptide secretion from β
islet-like cells derived from miR-32-infected HuAECs was
significantly increased compared with that from cells derived from
miR-Mut-infected HuAECs (**P<0.01 vs. miR-Mut HuAECs
at 0 week; #P>0.05 vs. miR-Mut HuAECs at 0 week;
one-way ANOVA, n=3). (B) With increasing glucose concentration in
the medium, C-peptide secretion from β islet-like cells derived
from miR-32-infected HuAECs was significantly increased compared
with that from cells derived from miR-Mut-infected HuAECs
(**P<0.01 vs. 0 mM glucose-treated miR-Mut HuAECs;
*P<0.05 vs. 0 mM glucose-treated miR-Mut HuAECs;
#P>0.05 vs. 0 mM glucose-treated miR-Mut HuAECs,
one-way ANOVA; n=3). (C) With increasing concentration of KCl in
the medium, C-peptide secretion from β islet-like cells derived
from miR-32-infected HuAECs was significantly increased compared
with that from cells derived from miR-Mut-infected HuAECs
(**P<0.01 vs. 0 mM KCl-treated miR-Mut HuAECs;
*P<0.05 vs. 0 mM KCl-treated miR-Mut HuAECs;
#P>0.05 vs. 0 mM KCl-treated miR-Mut HuAECs; one-way
ANOVA, n=3). (D) With increasing concentration of tolbutamide in
the medium, C-peptide secretion from β islet-like cells derived
from miR-32-infected HuAECs was significantly increased compared
with that of cells derived from miR-Mut-infected HuAECs
(**P<0.01 vs. 0 mM tolbutamine-treated miR-Mut
HuAECs; *P<0.05 vs. 0 mM tolbutamine-treated miR-Mut
HuAECs; #P>0.05 vs. 0 mM tolbutamine-treated miR-Mut
HuAECs; one-way ANOVA, n=3). ANOVA, analysis of variance; HuAECs,
human amniotic epithelial stem cells; miR-32, microRNA-32 Mut,
mutated; |
 | Table IIIC-peptide release. |
Table III
C-peptide release.
| Variable | miR-32 HuAECs
(pM) | miR-Mut HuAECs
(pM) |
|---|
| Induced time
(weeks) | | |
| 0 | 0.445±0.215 | 0.270±0.270 |
| 1 |
18.285±4.995a | 1.335±1.225 |
| 2 |
48.505±4.065a | 1.950±0.930 |
| 3 |
89.475±9.285a | 1.370±0.490 |
| Glucose
concentration (mM) | | |
| 0 | 8.400±2.150 | 8.385±2.905 |
| 1 |
16.650±4.110b | 9.225±1.065 |
| 5 |
47.015±6.825a | 10.375±1.705 |
| 25 |
78.865±7.375a | 12.065±1.485 |
| KCl concentration
(mM) | | |
| 0 | 1.515±0.495 | 1.590±0.400 |
| 10 | 2.455±0.405b | 1.315±0.565 |
| 20 | 5.245±1.235a | 1.640±0.390 |
| 40 | 9.920±0.670a | 2.035±0.155 |
| Tolbutamide
concentration (mM) | | |
| 0 | 2.595±0.515 | 3.005±0.065 |
| 50 | 4.860±0.690b | 3.580±0.590 |
| 100 | 8.990±0.970a | 2.590±0.520 |
Discussion
HuAECs are derived from trophoblasts, but retain the
characteristics of ESCs (1–3).
Although HuAECs are difficult to grow in vitro (they can be
grown for a maximum of five passages), they have pluripotent
capabilities, which are similar to stem cells (1–3).
HuAECs are able to differentiate into numerous human tissues and
cells that belong to the three human germ layers, under various
induction conditions (1–3). In addition, they possess specific
physiological and biochemical functions of adult cells; therefore,
they are considered promising candidates for cell therapy (1–3).
However, it is difficult to maintain the pluripotency of HuAECs
in vitro (1–3). The present study demonstrated that
the expression levels of Oct4, WWP2 and Nanog, which are
transcription factors associated with stem cell pluripotency, were
markedly decreased with increasing passage number, leading directly
to the loss of pluripotency of HuAECs and an inability to induce
differentiation into certain adult cells. Therefore, investigating
the mechanism underlying the maintenance of stem cell pluripotency
may help to improve the culture efficiency of HuAECs and maintain
their 'stemness'. Previous studies have indicated that the
transcription factors associated with pluripotent stem cells serve
important regulatory roles in in vitro and in vivo
proliferation, the maintenance of pluripotency, and the directed
differentiation of stem cells. The present study aimed to determine
why the expression level of WWP2 was slowly increased in HuAECs
alongside passage number. It has previously been reported that Oct4
activity is regulated by numerous factors (9,10,13,14). At the gene expression level, there
are two regulatory pathways: Transcriptional modification and
post-transcriptional modification. Generally, in adult cells, the
Oct4 gene is inactivated, and epigenetic analyses indicated that
the CpG islands in the gene promoter are highly methylated
(9,10,13). In addition, binding sites in the
promoter and in histones, including H3K27 and H3K9, are modified by
methylation and deacetylation, which cause direct downregulation of
gene transcription, ultimately affecting gene expression (9,10,13). These modifications are at the
transcriptional level (9,10,13). At the post-transcriptional level,
some preliminary studies have suggested that endogenous Oct4
protein is degraded in ESCs following prolonged culture in
vitro via the main degradation pathway of protein
ubiquitination (9,10,13,14). With continued passage of ESCs,
WWP2 may be activated and bind to the Oct4 protein, thereby
triggering subsequent ubiquitination and degradation, thus leading
to loss of Oct4 protein expression and reduced pluripotency of ESCs
(9,10,13,14). These results suggested that, in
order to maintain Oct4 expression, blocking the expression and
activity of WWP2 is crucial (9,10,13,14).
Based on these findings, the present study focused
on the regulatory mechanism of WWP2 ubiquitin ligase in HuAECs, in
order to provide a novel hypothesis regarding maintenance of the
pluripotency of stem cells in vitro. The results confirmed
that WWP2 is regulated by endogenous miR-32, particularly in the
primary culture stage when miR-32 expression is relatively high.
However, with consecutive passages of HuAECs, miR-32 expression was
significantly reduced, whereas endogenous WWP2 expression was
increased. HuAECs were then induced to overexpress exogenous miR-32
and were compared with HuAECs infected with miR-Mut. The results
demonstrated that WWP2 expression in miR-32-infected cells was
significantly decreased, whereas the expression of transcription
factors associated with pluripotency (Oct4, Sox2 and Nanog) were
significantly increased, thus suggesting that miR-32 may
significantly inhibit WWP2 expression and promote the expression of
pluripotency-associated transcription factors. Furthermore, the
results of co-IP demonstrated that, cross-linking between WWP2 and
Oct4 proteins was significantly increased in miR-Mut expressed
HuAECs but not in miR-32 expressed HuAECs. These findings suggested
that ubiquitination of Oct4 was enhanced, whereas miR-32
overexpression in HuAECs significantly inhibited endogenous WWP2
protein expression, and crosslinking between Oct4 and WWP2 was also
decreased.
In the present study, HuAECs infected with either
miR-32 or miR-Mut were induced to differentiate into β islet-like
cells. Molecular analyses indicated that following directed
induction, the expression levels of β islet-like cell markers were
significantly increased in miR-32-infected HuAECs compared with in
miR-Mut-infected HuAECs. Furthermore, with increasing induction
time, C-peptide secretion was also increased from miR-32-infected
HuAECs compared with in miR-Mut-infected HuAECs. In addition,
C-peptide secretion of the two HuAEC groups were investigated
following stimulation with various concentrations of glucose. With
increasing glucose concentration in the medium, the amount of
C-peptide secreted by β islet-like cells derived from
miR-32-infected HuAECs was much greater than that secreted by cells
derived from miR-Mut-infected HuAECs. In addition, with increasing
concentrations of KCl in the medium, C-peptide secretion from β
islet-like cells derived from miR-32-infected HuAECs was
significantly increased compared with that from cells derived from
miR-Mut-infected HuAECs. Furthermore, increasing concentrations of
tolbutamide (a hypoglycemic drug) were added to the HuAEC groups,
which increased C-peptide secretion from β islet-like cells derived
from miR-32-infected HuAECs compared with that secreted from β
islet-like cells derived from miR-Mut-infected HuAECs. These
results confirmed that after WWP2 was silenced by miR-32
overexpression in miR-32-infected HuAECs, the responsiveness to
sugar concentrations in the culture medium and C-peptide secretion
from β islet-like cells derived from them were significantly
increased compared with in cells derived from miR-Mut-infected
HuAECs.
In conclusion, miR-32 may prevent the ubiquitination
and degradation of Oct4 by inhibiting the expression of WWP2, thus
promoting the maintenance of stem cell pluripotency during
consecutive passages of HuAECs in vitro.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81471461
and 81202811), the China Postdoctoral Science Foundation (grant
nos. 2014M550250 and 2015T80455), the Shanghai Municipal Health
Bureau Fund (grant nos. 14XD1403200, SHDC12013114, 201420 06,
PWZxq2014 - 02 and 20124320), the Shanghai Natural Science
Foundation (grant no. 16ZR1434000), the projects sponsored by the
Development Fund for Shanghai Talents (grant no. 2017054) and the
Fund for Xinglin Talents of Shanghai University of TCM (grant no.
201707081).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu T, Wu J, Huang Q, Hou Y, Jiang Z, Zang
S and Guo L: Human amniotic epithelial cells ameliorate behavioral
dysfunction and reduce infarct size in the rat middle cerebral
artery occlusion model. Shock. 29:603–611. 2008.PubMed/NCBI
|
|
2
|
Liu YH, Chan J, Vaghjiani V, Murthi P,
Manuelpillai U and Toh BH: Human amniotic epithelial cells suppress
relapse of corticosteroid-remitted experimental autoimmune disease.
Cytotherapy. 16:535–544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu XY, Chen J, Zhou Q, Wu J, Zhang XL,
Wang L and Qin XY: In vitro tissue engineering of lamellar cornea
using human amniotic epithelial cells and rabbit cornea stroma. Int
J Ophthalmol. 6:425–429. 2013.PubMed/NCBI
|
|
4
|
Verma R, Oania R, Graumann J and Deshaies
RJ: Multiubiquitin chain receptors define a layer of substrate
selectivity in the ubiquitin-proteasome system. Cell. 118:99–110.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jennissen HP: Ubiquitin and the enigma of
intracellular protein degradation. Eur J Biochem. 231:1–30. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guardavaccaro D and Pagano M: Oncogenic
aberrations of cullin-dependent ubiquitin ligases. Oncogene.
23:2037–2049. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao B, Bhuripanyo K, Schneider J, Zhang
K, Schindelin H, Boone D and Yin J: Specificity of the E1-E2-E3
enzymatic cascade for ubiquitin C-terminal sequences identified by
phage display. ACS Chem Biol. 7:2027–2035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Z and Lu W: Roles of ubiquitination
and SUMOylation on prostate cancer: Mechanisms and clinical
implications. Int J Mol Sci. 16:4560–4580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liao B and Jin Y: Wwp2 mediates Oct4
ubiquitination and its own auto-ubiquitination in a
dosage-dependent manner. Cell Res. 20:332–344. 2010. View Article : Google Scholar
|
|
10
|
Xu H, Wang W, Li C, Yu H, Yang A, Wang B
and Jin Y: WWP2 promotes degradation of transcription factor OCT4
in human embryonic stem cells. Cell Res. 19:561–573. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen W, Jiang X and Luo Z: WWP2: A
multifunctional ubiquitin ligase gene. Pathol Oncol Res.
20:799–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zou W, Chen X, Shim JH, Huang Z, Brady N,
Hu D, Drapp R, Sigrist K, Glimcher LH and Jones D: The E3 ubiquitin
ligase Wwp2 regulates craniofacial development through
mono-ubiquitylation of Goosecoid. Nat Cell Biol. 13:59–65. 2011.
View Article : Google Scholar
|
|
13
|
Xu HM, Liao B, Zhang QJ, Wang BB, Li H,
Zhong XM, Sheng HZ, Zhao YX, Zhao YM and Jin Y: Wwp2, an E3
ubiquitin ligase that targets transcription factor Oct-4 for
ubiquitination. J Biol Chem. 279:23495–23503. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Zhang Z, Wang B, Zhang J, Zhao Y and
Jin Y: Wwp2-mediated ubiquitination of the RNA polymerase II large
subunit in mouse embryonic pluripotent stem cells. Mol Cell Biol.
27:5296–5305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu T, Chen Q, Huang Y, Huang Q, Jiang L
and Guo L: Low microRNA-199a expression in human amniotic
epithelial cell feeder layers maintains human-induced pluripotent
stem cell pluripotency via increased leukemia inhibitory factor
expression. Acta Biochim Biophys Sin (Shanghai). 44:197–206. 2012.
View Article : Google Scholar
|
|
16
|
Liu T, Cheng W, Huang Y, Huang Q, Jiang L
and Guo L: Human amniotic epithelial cell feeder layers maintain
human iPS cell pluripotency via inhibited endogenous microRNA-145
and increased Sox2 expression. Exp Cell Res. 318:424–434. 2012.
View Article : Google Scholar
|
|
17
|
Liu T, Cheng W, Liu T, Guo L, Huang Q,
Jiang L, Du X, Xu F, Liu Z and Lai D: Human amniotic epithelial
cell feeder layers maintain mouse embryonic stem cell pluripotency
via epigenetic regulation of the c-Myc promoter. Acta Biochim
Biophys Sin (Shanghai). 42:109–115. 2010. View Article : Google Scholar
|
|
18
|
Zou G, Liu T, Zhang L, Liu Y, Li M, Du X,
Xu F, Guo L and Liu Z: Induction of pancreatic β-cell-like cells
from CD44+/CD105+ human amniotic fluids via epigenetic regulation
of the pancreatic and duodenal homeobox factor 1 promoter. DNA Cell
Biol. 30:739–748. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Liu T, Qin W, Hou L and Huang Y:
MicroRNA-17 promotes normal ovarian cancer cells to cancer stem
cells development via suppression of the LKB1-53-21/WAF1 pathway.
Tumour Biol. 36:1881–1893. 2015. View Article : Google Scholar
|