Introduction
Allergic inflammation is characterized by
pathophysiological or medical disabilities, including allergic
asthma, atopic dermatitis, eczema, allergic rhinitis and
anaphylaxis, following exposure to allergens or harmful stimuli,
including pathogens, damaged cells and irritants (1). An allergic reaction is the result of
an inappropriate immune response, for example,
hypersensitivity-triggered inflammation. This inflammation is
associated with pro-inflammatory mediators, including histamine,
cytokines and chemokines, secreted from mast cells (2). Mast cells are traditionally viewed
as effector cells of immediate hypersensitivity reactions. Mast
cells are important in specific immunity through the interaction of
multivalent antigens with IgE bound to the high-affinity IgE
receptor (FcεRI) on these cells. Upon allergen provocation, mast
cells release inflammatory mediators, which trigger the process of
degranulation in activated mast cells (3,4).
The mitogen-activated protein kinase (MAPK)
signaling cascade controls important cellular processes, including
gene expression, cell proliferation, cell survival and death, and
cell mobility (5). The activation
of MAPKs is associated with allergic inflammatory responses via the
translocation of nuclear factor-κB (NF-κB), which causes the
production of pro-inflammatory cytokines and chemokines (6). In mammalian systems, there are three
well-characterized subfamilies of MAPKs. These include the
extracellular signal-regulated kinases (ERKs), the p38 MAPKs and
the c-Jun N-terminal kinases (JNKs) (7). These pathways are linear kinase
cascades in which MAPK kinase (MKK) kinase phosphorylates and
activates MKK, which in turn phosphorylates and activates MAPK. The
MKK family members are unique in that they are dual-specificity
kinases, phosphorylating MAPKs on threonine and tyrosine residues
(8). MKKs are essential
components of the evolutionarily conserved MAPK signaling cascade,
which regulates a variety of cellular activities and innate immune
responses. Numerous studies have been performed to investigate the
role of MKKs in the innate immune system (9).
As several cytokines promote allergic inflammation
through cytokine receptors, the signal transducer and activator of
transcription (STAT) family of proteins have obligate roles in
pro-allergic cytokine-induced gene regulation in multiple cell
types (10). STATs have been
implicated as the key transcription factors in immunity and
inflammatory pathways (11).
However, the role of the STAT pathway in mast cells remains to be
fully elucidated. STAT3, a key cytoplasmic transcription factor
involved in inflammation, becomes activated in response to various
cytokines, chemokines and growth factors. The activation of STAT3
requires the phosphorylation of tyrosine residue 705 (Tyr705),
leading to protein dimerization and translocation from the
cytoplasm to the nucleus (12).
Activated STATs dimerize and translocate to the nucleus, where they
bind to specific promoter sequences and induce the transcription of
several target genes.
Bee venom (BV), which is extracted from honey bees,
is a bitter and colorless liquid, and its active portion contains a
mixture of proteins that cause local inflammation and act as an
anticoagulant (13). It has been
reported that the majority of cases of humans succumbing to
mortality as a result of one or multiple bee stings are due to
allergic reactions, heart failure, or suffocation from swelling
around the neck or the mouth. Compared with other diseases,
accidents and other unusual cases, bee sting-associated mortality
is rare, indicating that BV is safe for treating human diseases
(14). BV therapy is a form of
medicine, which originated from ancient Greece and China (15). Due to its anti-inflammatory
(16), antibacterial (17), antinociceptive (18), hepatocyte-protective (19) and anticancer characteristics
(20), it has a long history of
use in folk medicine to treat various diseases. In Korea, BV has
long been used to relieve pain and to treat several diseases,
including arthritis (21),
rheumatism (22), rhinitis
(23), cancer (24), asthma (25) and skin diseases (26). The collected BV is purified in
aseptic conditions and lyophilized for clinical use at a
concentration suitable for the patient's symptoms and
conditions.
BV contains a variety of different peptides,
including melittin, apamin, adolapin and mast cell degranulating
(MCD) peptide. The two main components of BV, melittin and
adolapin, have anti-inflammatory effects, which involve the
inhibited expression of cyclooxygenase-2 and phospholipase A2, and
decreased levels of tumor necrosis factor-α (TNF-α), interleukin
(IL)-1, IL-6 and nitric oxide (23). These components are known to exert
their pharmacological effects individually or interactively
depending on their concentration or dose. Although several studies
have demonstrated the anti-allergic inflammatory effects of BV and
its components in a number of cell types, the exact molecular
mechanism underlying the effect of BV in mast cells has not been
investigated. In the present study, the inhibitory effects of BV on
the mRNA expression and production of pro-inflammatory cytokines
and the associated molecular signaling pathways were investigated
in phorbol-12-myristate 13-acetate plus calcium ionophore A23187
(PMACI)-stimulated HMC-1 cells and in a compound 48/80-induced
anaphylaxis animal model.
Materials and methods
Chemicals and reagents
For the present study, BV (from Apis
mellifera), phorbol 12-myristate 13-acetate (PMA), calcium
ionophore A23187 (Calcimycin;
C29H37N3O6) and all
other chemicals were purchased from Sigma; EMD Millipore
(Billerica, MA, USA). 3-(4,5-dimethylthiazol-2-yl)-5-(3-carb
oxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was
purchased from Promega Corporation (Madison, WI, USA). Iscove's
modified Dulbecco's medium (IMDM), fetal bovine serum (FBS),
penicillin and streptomycin were obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Primary antibodies against ERK
(cat. no. 9102), phosphorylated (p-)JNK (cat. no. 9251), JNK (cat.
no. 9252), p-p38 (cat. no. 9215), p38 (cat. no. 9212), p-Akt (cat.
no. 9271), p-MKK3/6 (cat. no. 12280), p-MKK4 (cat. no. 9156), MKK4
(cat. no. 9152), p-STAT3 (Tyr705; cat. no. 9145) and p-STAT3
(Ser727; cat. no. 9134) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Primary antibodies for p-ERK
(cat. no. sc-7383), p-MAPK kinase 1/2 (MEK1/2; cat. no. sc-81503),
MEK1/2 (cat. no. sc-81504), MKK3/6 (cat. no. sc-13069), Akt (cat.
no. sc-8312) and STAT3 (cat. no. sc-8019), β-actin (cat. no.
sc-81178) were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Horseradish peroxidase-conjugated secondary
antibodies were purchased from Jackson ImmunoResearch Laboratories,
Inc. (West Grove, PA, USA). The histamine enzyme-linked
immunosorbent assay (ELISA) kit was obtained from Enzo life
Sciences, Inc. (Farmingdale, NY, USA). The ELISA kits for TNF-α,
IL-6, and IL-1β were obtained from R&D Systems, Inc.
(Minneapolis, MN, USA). SYBR Premix Ex Taq was purchased from
Takara Bio, Inc. (Shiga, Japan). TNF-α, IL-6, IL-1β, and GAPDH
oligonucleotide primers were purchased from Bioneer Corporation
(Daejeon, Korea).
Cell culture and sample treatment
HMC-1 cells were provided by Professor Jae-Young Um
(Kyung Hee University, Republic of Korea), and were grown at 37°C
in IMDM supplemented with 10% FBS, penicillin (100 U/ml) and
streptomycin (100 μg/ml) in a humidified atmosphere of 5%
CO2. The BV was dissolved in distilled water and
filtered using Acrodisc® Syringe Filters 0.2-μm
Supor® Membrane (Pall Life Sciences, Port Washington,
NY, USA). HMC-1 cells were seeded at a density of 1×106
cell per well, and then treated with BV at concentrations of 5 and
10 μg/ml for 30 min at 37°C in humidified air with 5%
CO2, and then stimulated with 40 nM of PMA and 1
μM of A23187 (PMACI) at 37°C for 5-30 min, 6 and 12-24 h for
the western blot analysis, reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and ELISA, respectively. The
various concentrations of test compounds dissolved in distilled
water were added together with PMACI. The cells were either treated
with media or vehicle control.
Histamine assay
The HMC-1 cells were pre-treated with BV for 30 min
and then stimulated with 40 nM of PMA and 1 μM of A23187
(PMACI) for 12 h. The conditioned medium was collected and used as
a sample. The release of histamine was measured using an ELISA kit
in accordance with the manufacturer's protocol.
Cytokine assays
Culture media were collected at 12, 18, and 24 h
post-treatment with BV and stored at −70°C. The levels of TNF-α,
IL-6 and IL-1β were measured using ELISA kits according to the
manufacturer's protocol.
Western blot analysis
Segments of cells or liver tissue were suspended in
PRO-PREP™ protein extraction solution (Intron Biotechnology, Inc.,
Seoul, Korea) and incubated for 20 min at 4°C. Cell debris was
removed via micro-centrifugation 11,000 × g for 30 min at 4°C,
followed by rapid freezing of the supernatant. The protein
concentration was determined using Bio-Rad protein assay reagent
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's protocol. Cellular proteins from the treated and
untreated cell extracts (10-30 μl) were electroblotted onto
a polyvinylidene fluoride membrane following separation via 10-12%
SDS-PAGE. The membrane was incubated for 1 h with blocking solution
(5% skim milk) at room temperature, followed by overnight
incubation with the primary antibodies (1:1,000) at 4°C. The blots
were washed three times with Tween 20/Tris-buffered saline (T/TBS)
and incubated with horseradish peroxidase-conjugated secondary
antibody (1:2,000) for 2 h at room temperature. The blots were
washed three times with T/TBS, and then developed via enhanced
chemiluminescence (GE Healthcare Life Sciences, Chalfont, UK).
Densitometric analysis was performed using Bio-Rad Quantity One
software version 4.3.0 (Bio-Rad Laboratories, Inc.).
RT-qPCR analysis
Total RNA was isolated from the cells or liver
tissues using an Easy Blue kit (Intron Biotechnology, Inc.)
according to the manufacturer's protocol. Total RNA was quantified
using an Epoch micro-volume spectrophotometer system (BioTek
Instruments, Inc., Winooski, VT, USA). cDNA was obtained using
isolated total RNA (2 μg), d(T)16 primer, and Avian
Myeloblastosis Virus reverse transcriptase with genomic DNA
remover. The relative gene expression was quantified using RT-qPCR
analysis (Real Time PCR System 7500; Applied Biosystems; Thermo
Fisher Scientific, Inc.) with SYBR Premix Ex Taq. Each reaction
tube contained 0.4 μl forward primer, 0.4 μl reverse
primer, 7.2 μl diethyl pyrocarbonate water, 10 μl
SYBR and 2 μl cDNA template (10 ng/μl). The PCR
cycling conditions were as follows: 10 min at 95°C; 40 cycles of 5
sec at 95°C and 45 sec at 60°C; and a final melting curve of 15 sec
at 95°C, 1 min at 60°C, and 15 sec at 95°C. The oligonucleotide
primers were as follows: Human TNF-α, forward
5′-GCTGGAGAAGGGTGACCGAC-3′ and reverse 5′-GTTCGTCCTCCTCACAGGGC-3′;
mouse TNF-α, forward 5′-ATGAGCACAGAAAGCATGAT-3′ and reverse
5′-TACAGGCTTGTCACTCGAAT-3′; human IL-6, forward
5′-ATTCCGGGAACGAAAGAGAA-3′ and reverse 5′-TCTTCTCCTGGGGGTACTGG-3′;
mouse IL-6, forward 5′-TTCCATCCAGTTGCCTTCTTG-3′ and reverse
5′-GGGAGTGGTATCCTCTGTGAAGTC-3′; human IL-1β, forward
5′-TGGACCTCTGCCCTCTGGAT-3′ and reverse 5′-GGCAGGGAACCAGCATCTTC-3′;
for mouse IL-1β, forward 5′-GATCCACACTCTCCAGCTGCA-3′ and reverse
5′-CAACCAACAAGTGATATTCTCCATG-3′; human GAPDH, forward
5′-CTCCTCCACCTTTGACGCTG-3′ and reverse 5′-CTCTTGTGCTCTTGCTGGGG-3′;
mouse GAPDH, forward 5′-GACGGCCGCATCTTCTTGT-3′ and reverse
5′-CACACCGACCTTCACCATTTT-3′. The size of the synthesized cDNAs was
100-150 bp. Fold changes of gene expression were calculated using
the comparative quantification cycle (Cq) method (Applied
Biosystems; Thermo Fisher Scientific, Inc.) (27). The Cq values of target genes
TNF-α, IL-6 and IL-1β were normalized to that of GAPDH using the
ABI gene express 2.0 program (Applied Biosystems; Thermo Fisher
Scientific, Inc.).
Compound 48/80-induced anaphylactic shock
model
A total of 24 ICR male mice (6 weeks old; 20-25 g
body weight) were obtained from Raon Bio (Yongin, Korea) and
maintained under constant conditions at a temperature of 20-25°C,
humidity of 40-60% and a 12-h light/dark cycle. The mice were
randomly assigned to one of four groups (n=6 per group). The ICR
mice were injected intraperitoneally (i.p.) with phosphate-buffered
saline (PBS) or compound 48/80 (8 mg/kg dissolved in PBS) as
described previously (28,29).
BV or disodium cromoglycate (DSCG; Sigma-Aldrich; EMD Millipore) or
PBS were dissolved in saline and injected i.p. at doses of 25 mg/kg
DSCG and 20 mg/kg BV for 1 h prior to the compound 48/80 injection.
Survival was monitored for 1 h following the induction of
anaphylactic shock. Survival data were analyzed using the
Kaplan-Meier method and log-rank test. Following the assessment of
animal survival, blood was collected from the heart of each mouse
to measure serum cytokine production. The blood was allowed to clot
for 1 h at room temperature and then centrifuged for 20 min at
3,000 × g at 4°C to obtain serum. Following collection of blood
samples from the mice, the mice were sacrificed by cervical
dislocation. All procedures were performed in accordance with
university guidelines and approved by the Ethical Committee for
Animal Care and the Use of Laboratory Animals, Korean Medicine,
Sangji University (Wonju, Korea; approval no. 2015-11).
Statistical analysis
The data are expressed as the mean ± standard
deviation of triplicate experiments. Statistically significant
differences were compared using one-way analysis of variance and
Dunnett's post hoc test. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using SPSS statistical analysis software (version 19.0;
IBM SPSS, Armonk, NY, USA).
Results
BV suppresses PMACI-induced histamine
release, and production and mRNA expression of pro-inflammatory
cytokines in HMC-1 cells
The present study evaluated the cytotoxic effect of
various concentrations of BV (0.625, 1.25, 2.5, 5, 10 and 20
μg/ml) on HMC-1 cells. After 4 h, the cells were treated
with BV and incubated for an additional 24 h. Cytotoxicity in the
HMC-1 cell line was determined using the MTS assay. BV did not
cause nonspecific cytotoxicity, as it had no effect on cell
viability at concentrations between 0.625 and 20 μg/ml
(Fig. 1A).
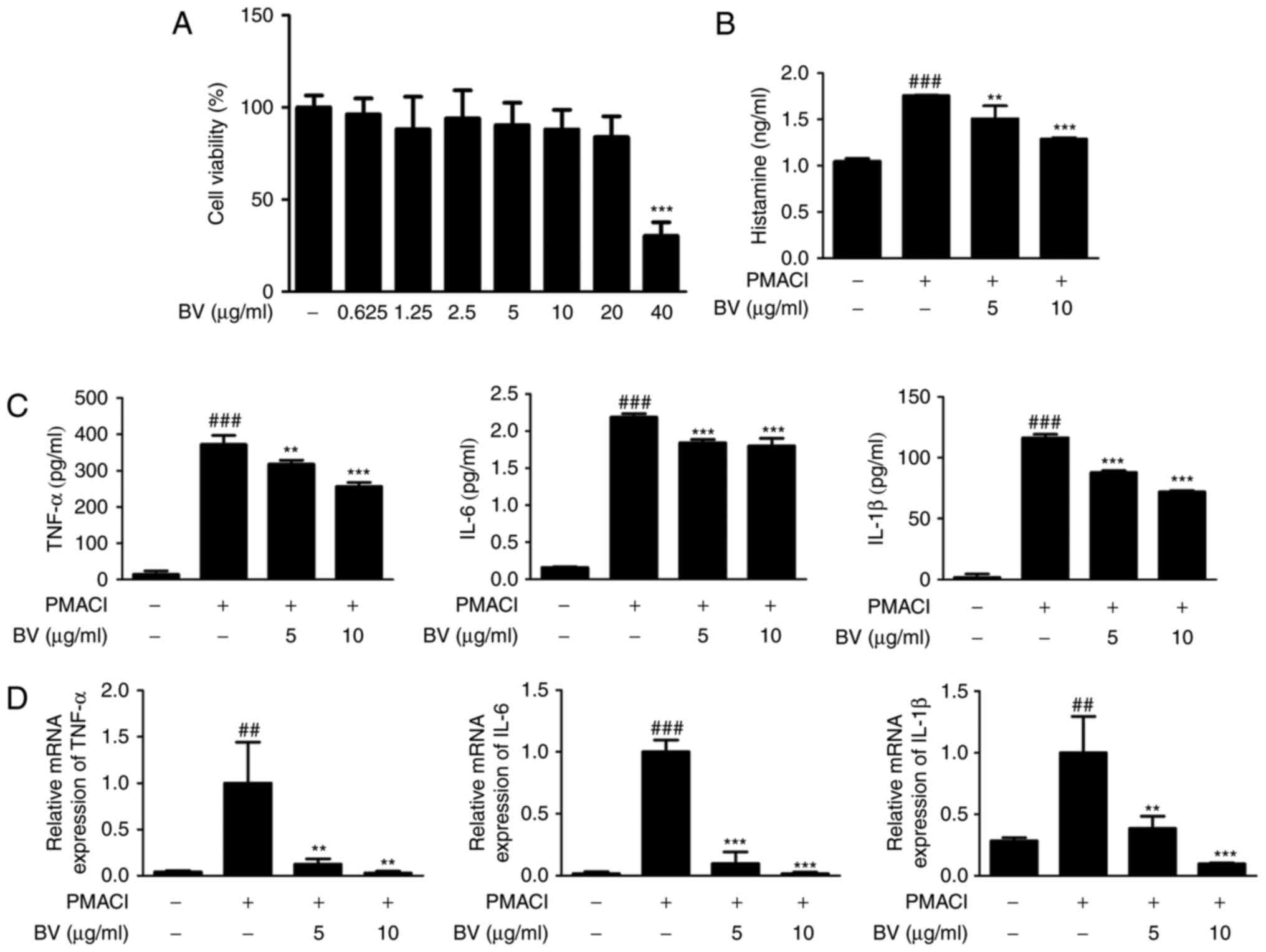 | Figure 1Effect of BV on histamine release and
pro-inflammatory cytokines in PMACI-stimulated HMC-1 cells. (A)
HMC-1 cells were treated with different concentrations of BV for 24
h, and their viability was determined using a
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tet-razolium
assay. (B) Cells were pre-treated with BV for 30 min prior to the
addition of 40 nM PMA + 1 μM PMACI, and the cells were
incubated for 12 h. Histamine release in the culture medium was
measured using an ELISA kit. (C) HMC-1 cells were treated with 5
and 10 μg/ml of BV for 30 min prior to the addition of 40 nM
PMA + 1 μM PMACI and the cells were incubated for 12, 18 and
24 h for the determination of TNF-α, IL-6 and IL-1β production,
respectively. Cytokine production was measured using an ELISA kit.
(D) Cells were pre-treated with BV for 30 min prior to the addition
of 40 nM PMA + 1 μM PMACI for 6 h. The mRNA levels of TNF-α,
IL-6 and IL-1β were determined using reverse
transcription-quantitative polymerase chain reaction analysis.
Values are presented as the mean ± standard deviation of three
independent experiments. ##P<0.01 and
###P<0.001, vs. control group; **P<0.01
and ***P<0.001, vs. PMACI-treated group. BV, bee
venom; PMA, phorbol 12-myristate 13-acetate; PMACI,
phorbol-12-myristate 13-acetate plus calcium ionophore A23187;
ELISA, enzyme-linked immunesorbent assay; TNF-α, tumor necrosis
factor-α; IL, interleukin. |
Among the inflammatory mediators released from mast
cells, histamine is known to be the most well-characterized
mediator implicated in the acute phase of hypersensitivity,
including anaphylactic shock (30). To determine whether BV inhibits
histamine release in the culture medium from mast cells, the
PMACI-induced histamine release was measured. BV at a dose of 5 and
10 μg/ml decreased the PMACI-induced histamine levels
(Fig. 1B). To determine the
inhibitory effect of BV on pro-inflammatory cytokine production,
its effect on the PMACI-induced production and mRNA expression of
TNF-α, IL-6 and IL-1β and were investigated using ELISA and RT-qPCR
analysis, respectively (Fig. 1C and
D). Pre-treatment of cells with BV downregulated the
PMACI-induced production and mRNA expression of TNF-α, IL-6 and
IL-1β in a concentration-dependent manner. These results indicated
that BV exerted potential protection via the inhibition of
histamine release during allergic reaction and regulates the
PMACI-induced expression of TNF-α, IL-6 and IL-1β through
transcriptional inhibition.
BV suppresses the activation of
PMACI-induced MAPKs and MKKs in HMC-1 cells
The MAPK cascade is one of the important signaling
pathways in immune responses, and is activated in response to
diverse extracellular stimuli, leading to the activation of mast
cells during allergic inflammation (31). To investigate the effect of BV on
MAPK signaling pathways in PMACI-stimulated HMC-1 cells, the
phosphorylation of the three MAPK signaling molecules, ERK, JNK and
p38, were analyzed using western blot analysis. As shown in
Fig. 2A, PMACI significantly
induced the phosphorylation of ERK, JNK and p38, whereas BV
suppressed the PMACI-induced activation of these MAPKs. BV did not
affect the total levels of MAPKs.
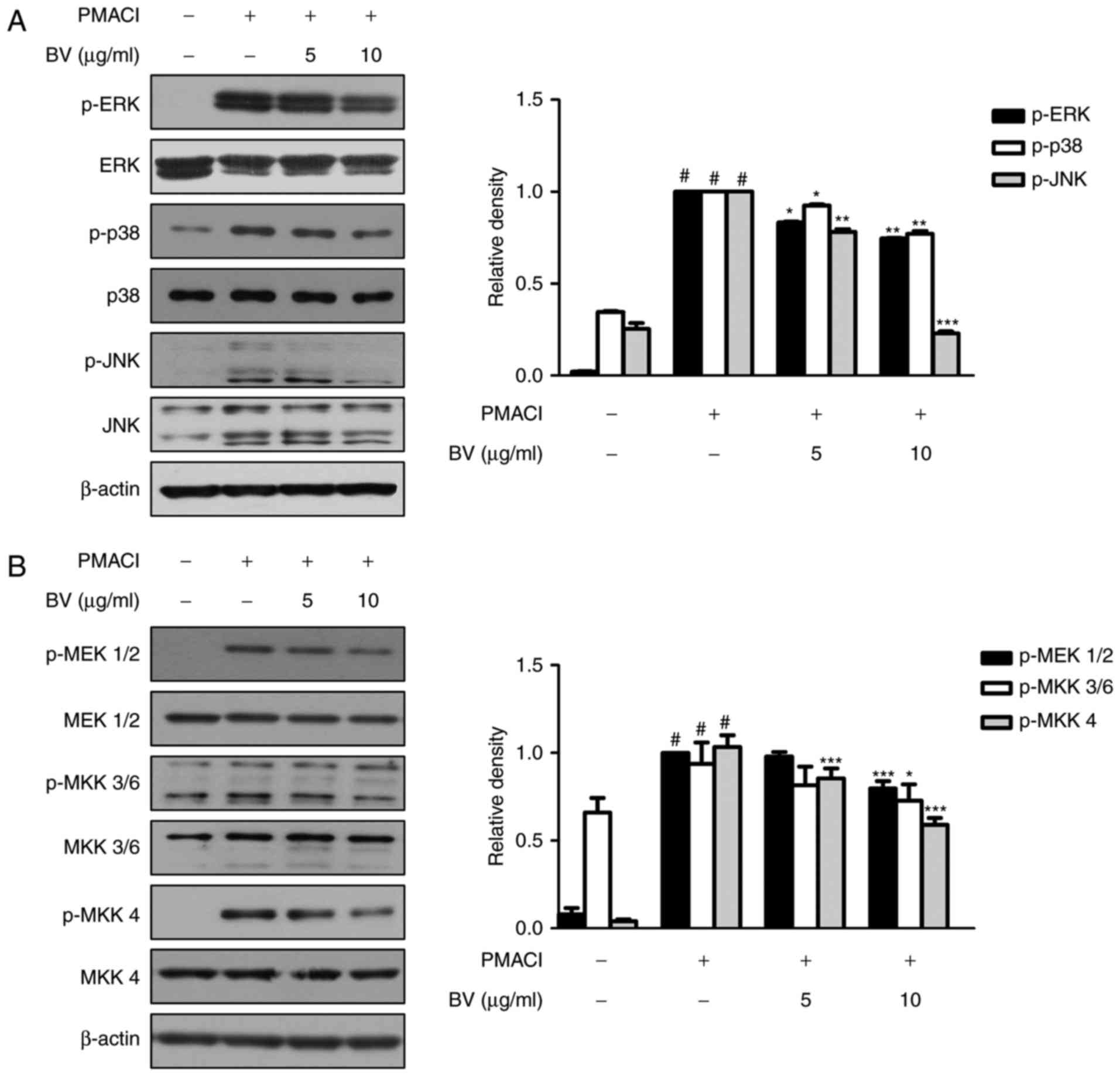 | Figure 2Effect of BV on PMACI-induced
activation of mitogen-activated protein kinase and MKKs in HMC-1
cells. (A) HMC-1 cells were pre-treated with 5 and 10 μg/ml
of BV for 30 min prior to the addition of 40 nM PMA + 1 μM
of PMACI for 30 min. (B) HMC-1 cells were pre-treated with 5 and 10
μg/ml of BV for 30 min prior to the addition of 40 nM PMA +
1 μM PMACI for 5 min (p-MEK1/2) or for 10 min (p-MKK3/6 and
p-MKK4). Total proteins were prepared, and western blot analysis
was performed using specific antibodies. β-actin was used as an
internal control. Proteins were prepared, and western blot analysis
was performed using specific antibodies. #P<0.05, vs.
control group; *P<0.05, **P<0.01 and
***P<0.001, vs. PMACI-treated group. BV, bee venom;
PMA, phorbol 12-myristate 13-acetate; PMACI, phorbol-12-myristate
13-acetate plus calcium ionophore A23187; MEK1/2, MAPK kinase 1/2;
MKK, MAPK kinase; ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; p-, phosphorylated. |
The MAPK isoforms are activated by the dual
phosphory-lation of threonine and tyrosine residues. MKKs
phosphorylate and activate MAPKs (32). To investigate the upstream targets
of MAPKs, the present study examined whether BV prevents the
PMACI-induced phosphorylation of MEK1/2, MKK3/6 and MKK4. It was
found that cells pre-treated with BV significantly suppressed the
phosphorylation of MEK1/2, MKK3/6 and MKK4, compared with those
treated with PMACI alone; however, the total levels of MKKs were
not affected (Fig. 2B).
BV suppresses the PMACI-induced
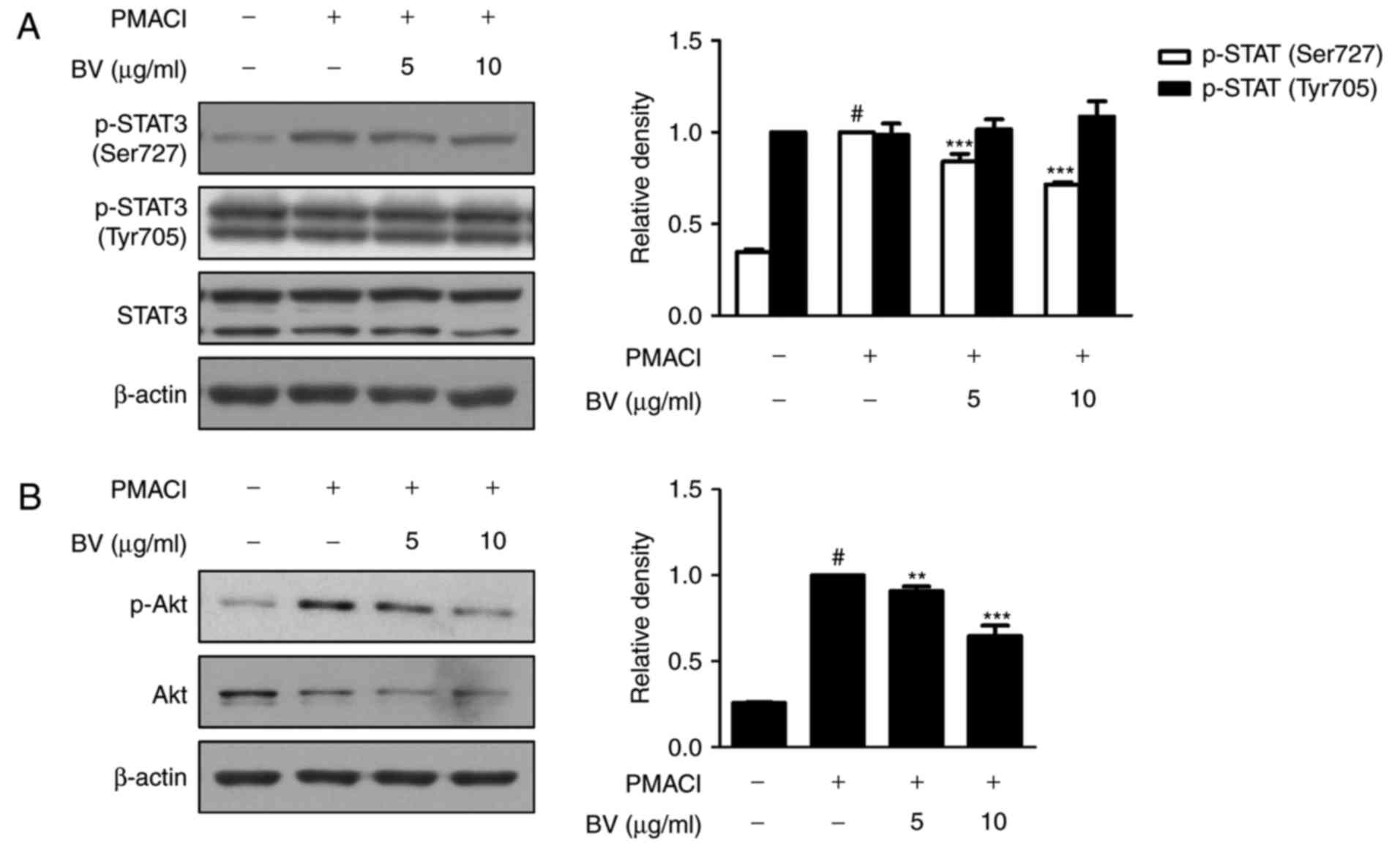
activation of STAT3 and Akt in HMC-1 cells
STAT3 has been implicated as a key transcription
factor in inflammatory pathways (11). In addition, Akt is a
multifunctional mediator of the activation of phosphoinositide
3-kinase (PI3K) in various cell types, and STAT3 is interconnected
with PI3K (33,34). Therefore, the present study
examined the effect of BV on the PMACI-stimulated phosphorylation
of STAT3 and Akt. As shown in Fig.
3A, PMACI induced the phosphorylation of STAT3 on Ser727,
whereas BV pre-treatment suppressed the PMACI-induced activation of
STAT3 on Ser727, but did not affect the phosphorylation of STAT3 on
Tyr705. Pre-treatment with BV also significantly suppressed the
phosphorylation of Akt, compared with that in cells treated with
PMACI alone (Fig. 3B).
BV has anti-allergic inflammatory effects
on compound 48/80-induced hypersensitivity in an animal model of
anaphylaxis
To assess the anti-allergic inflammatory effect of
BV in vivo, the present study investigated its effect on the
survival rate of mice with compound 48/80-induced hypersensitive
anaphylaxis. In this experiment, 8 mg/kg compound 48/80 was used,
which was considered a suitable concentration for investigating the
anaphylactic response in previous studies (35,36). Following i.p. injection of
compound 48/80, all mice were monitored for 1 h and their survival
rates were determined. When the mice were pre-treated with BV at a
dose of 20 mg/kg for 1 h prior to the administration of compound
40/80, their mortality rates were reduced (Fig. 4A).
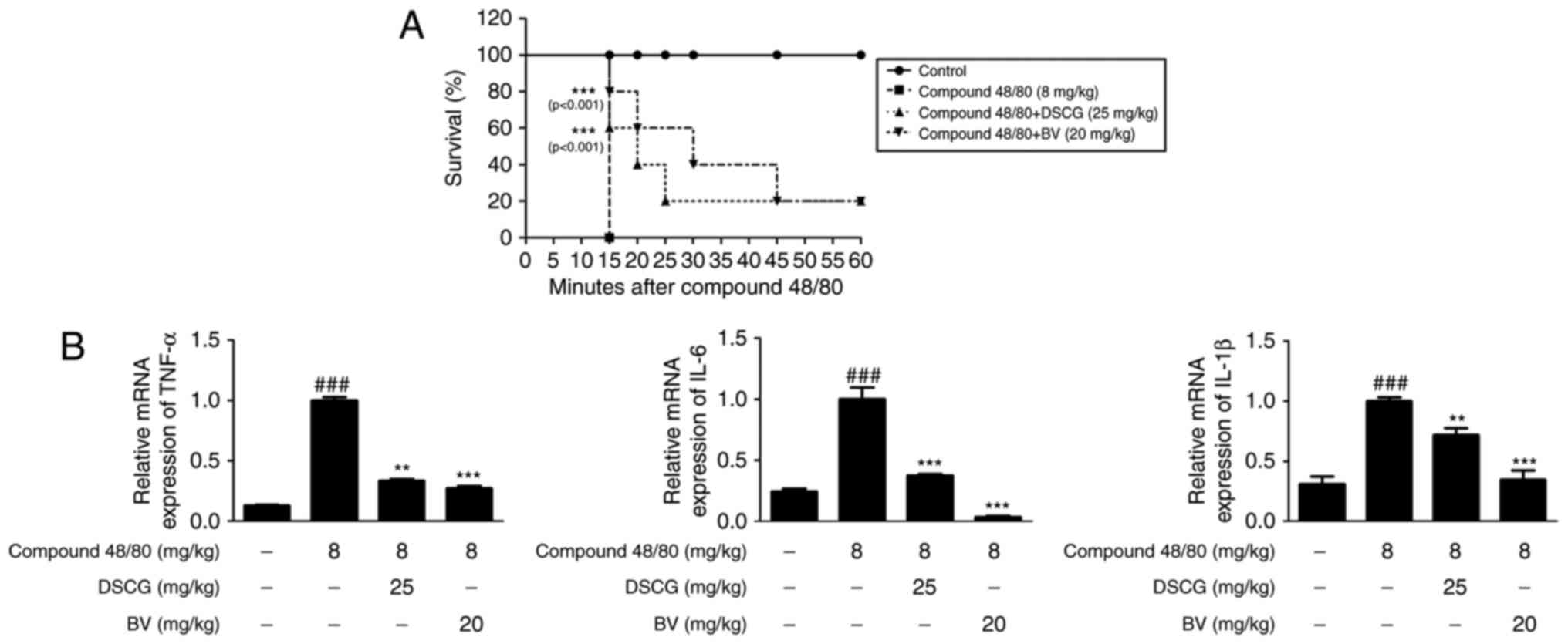 | Figure 4Effects of BV on compound
48/80-induced mortality and inflammatory cytokines in an
anaphylactic shock animal model. Mice were injected with BV, DSCG
and PBS as a vehicle (n=6 per group or total) for 1 h prior to
compound 48/80 injection (8 mg/kg i.p.). (A) Survival rates of the
mice were monitored for 1 h. (B) Total RNA was prepared from the
liver tissue, and the levels of TNF-α, IL-6 and IL-1β were
determined using reverse transcription-quantitative polymerase
chain reaction analysis. Densitometric analysis was performed using
Bio-Rad Quantity One® software. The data shown are
presented as the mean ± standard deviation of three independent
experiments. ###P<0.001, vs. control group;
**P<0.01 and ***P<0.001, vs. compound
48/80-treated group. BV, bee venom; DSCG, disodium cromoglycate;
PBS, phosphate-buffered saline; TNF-α, tumor necrosis factor-α; IL,
interleukin. |
To evaluate cytokine levels in response to the
allergic reaction, the mRNA levels of cytokines in the liver of
anaphylactic mice were examined. As shown in Fig. 4B, compound 48/80 administration
markedly increased the mRNA levels of TNF-α, IL-6 and IL-1β,
whereas pre-treatment with BV (20 mg/kg, i.p.) for 1 h prior to
compound 48/80 administration significantly decreased the
expression levels of these pro-inflammatory cytokines. These
results indicated that BV provides protection via the inhibition of
cytokine release during a systemic anaphylactic reaction.
BV suppresses the compound 48/80-induced
activation of MAPKs and STAT3 in an animal model of
anaphylaxis
To investigate the role of BV in the activation of
MAPK and STAT3 in animal model of anaphylaxis, the present study
determined the protein levels of MAPKs and STAT3 using western blot
analysis. As shown in Fig. 5A and
B, the administration of BV inhibited the compound
48/80-induced phosphorylation of MAPKs. BV inhibited the compound
48/80-induced phosphorylation of STAT3 on Tyr705, but did not
affect the phosphorylation of STAT3 on Ser727. These results
demonstrated that BV exerted suppressive effects on allergic
inflammation via the regulation of MAPK and STAT3 activation in
this model of anaphylactic shock.
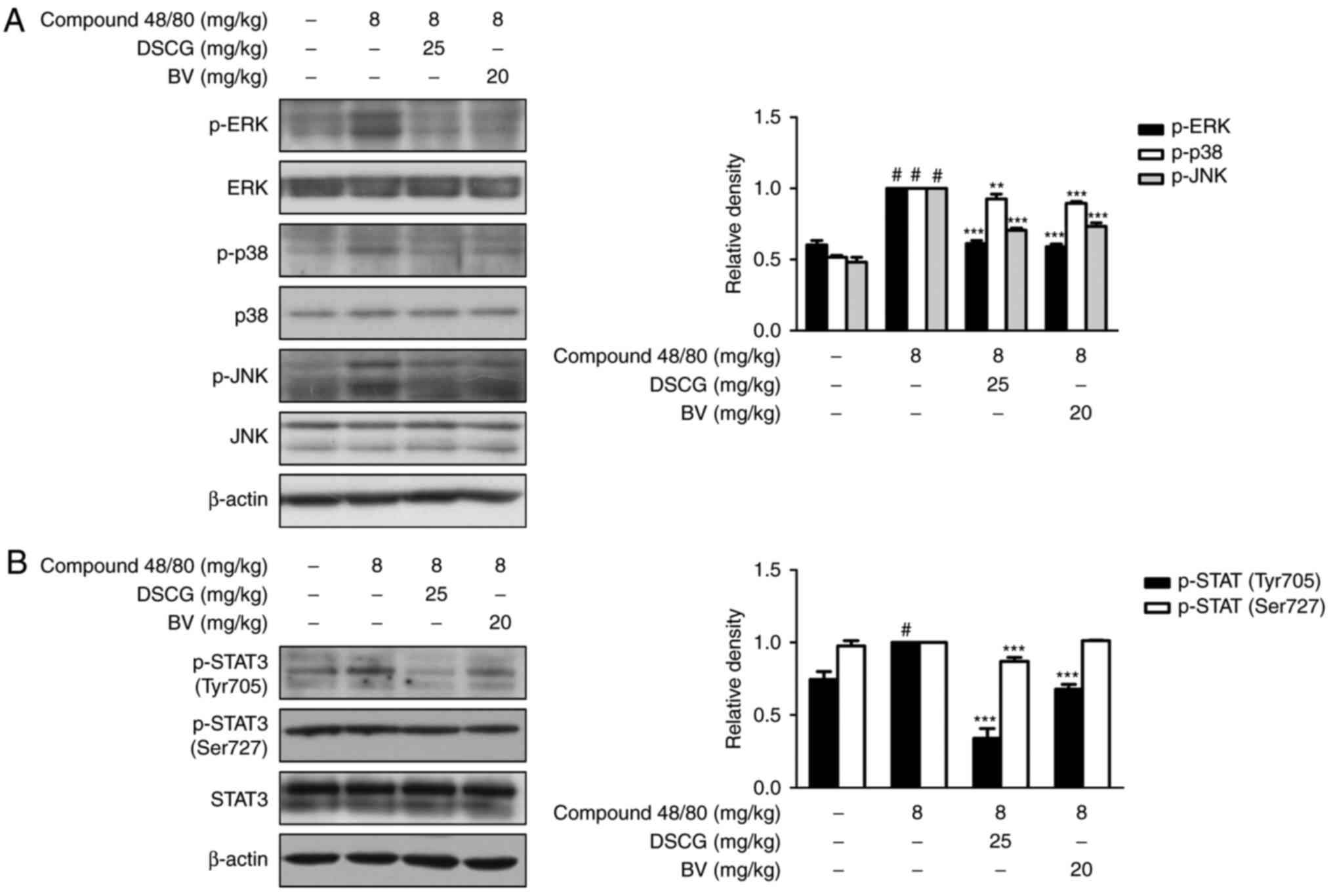 | Figure 5Effects of BV on compound
48/80-induced activation of MAPKs and STAT3 in an anaphylactic
shock animal model. Mice were injected with BV, DSCG and PBS as a
vehicle (n=6 per group or total) for 1 h prior to compound 48/80
injection (8 mg/kg i.p.). Expression levels of (A) MAPKs and (B)
STAT3 were determined by western blot analysis using specific
antibodies. Densitometric analysis was performed using Bio-Rad
Quantity One® software. The data shown are presented as
the mean ± standard deviation of three independent experiments.
#P<0.05, vs. control group; **P<0.01
and ***P<0.001, vs. compound 48/80-treated group. BV,
bee venom; PBS, phosphate-buffered saline; DSCG, disodium
cromoglycate; MAPK, mitogen-activated protein kinase; ERK,
extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; STAT3, signal transducer and activator of transcription 3;
p-, phosphorylated. |
Discussion
Allergic inflammation is classified into two phases,
early-phase (or type immediate hypersensitivity) and late-phase
reactions, which result in subsequent chronic allergic inflammation
(1). Early-phase immediate
hypersensitivity occurs within minutes of allergen exposure and is
induced by the release of preformed mediators, including histamine
and tryptases, chemotactic factors from activated mast cells
(37). In particular, histamine
is chemically classified as an amine, and is the most potent
mediator with various biological roles, including in anaphylactic
shock, inflammation and neurotransmission (38). By contrast, late-phase reactions
are the result of pro-inflammatory cytokine production and the
recruitment of immune cells, including neutrophils, basophils,
eosinophils, macrophages and mast cells, to sites of inflammation.
In accordance with these reports, the mast cell number increases in
atopic dermatitis, allergic rhinitis and asthma, and the
pro-inflammatory cytokines, TNF-α, IL-6, IL-8 and IL-1β, released
by mast cells enhance the inflammatory process (39). These cytokines are associated with
biological functions, including regulation of cell proliferation,
differentiation and immunity, with recruitment of additional immune
cells to inflammatory sites being the main function (40,41).
The present study showed that the level of histamine
increased by PMACI was significantly lowered by treatment with BV.
As calcium is a crucial secondary messenger in mast cell signaling,
the regulation of intracellular calcium is critical to histamine
release by mast cells. Intracellular calcium level correlates with
mast cell degranulation, exocytosis from mast cells and the
expression of inflammatory cytokines (42). Therefore, reduced intracellular
calcium may be involved in the inhibitory effect of BV on histamine
release. In addition, BV significantly inhibited the production and
mRNA expression of TNF-α, IL-6 and IL-1β in the PMACI-stimulated
HMC-1 cells and in the compound 48/80-induced anaphylaxis model in
mice. These data suggested that the effect of BV on
pro-inflammatory cytokines may assist in preventing and treating
mast cell-mediated inflammatory diseases. Among the results of the
mRNA expression of pro-inflammatory cytokines in the anaphylactic
shock animal model, the high concentration of BV effectively
reduced the level of IL-6 expressed, even in a normal state. As
IL-6 is a multifunctional cytokine involved in a broad spectrum of
biological events, and increased levels of IL-6 are observed in
several human inflammatory diseases, it may be that BV has a potent
suppressive effect on inflammatory responses. However, further
clarification of the molecular mechanisms underlying the function
if IL-6 and the inhibition of IL-6 signaling is required.
MAPKs are present in numerous cells and tissues, and
consist of three major protein kinase families: ERK, p38 and JNK.
The MAPK signaling cascade regulates important cellular processes,
which transduce extracellular stimuli into intracellular responses,
including gene expression, cell proliferation, cell survival and
death, and cell mobility (5). It
has been reported that MAPK signal transduction pathways control
inflammatory responses and cytokine production (43). The prototypical MAPK
phosphorylation cascade consists of an MAPK kinase kinase (MAPKKK
or MEKK), an MKK and an MAPK. MAPKKK phosphorylates and activates
MKK, which in turn phosphorylates MAPK. MKKs in the MAPK cascade
act as dual-specificity kinases and activate MAPKs through double
phosphorylation of the threonine-X-tyrosine motif in the activation
loop. During this phosphorylation relay, the input signal can be
amplified through the MAPK cascade and the activated MAPKs
eventually modify the phosphorylation of a specific set of
downstream target proteins, including transcription factors and
other signaling components, leading to the activation of downstream
genes (44). In the present
study, the data showed that cells pre-treated with BV suppressed
the PMACI-induced phosphorylation of MAPKs and MKKs, compared with
the cells treated with PMACI alone, however, the total levels of
MAPK and MKK were unaffected. In the case of MKK7, an upstream
factor of JNK, PMACI did not induce its phosphorylation and BV
pre-treatment had no effect (data not shown). These data revealed
that the effect of BV on mast cell-mediated inflammatory reactions
may be mediated through MAPK pathways, result in cytokine
production.
Allergic inflammation is associated with an
increased expression of multiple inflammatory proteins, which are
regulated by STAT transcription factors that are activated by Janus
kinases and a large number of cytokines present in the pro-allergic
environment (45). STAT3 is
important in the signaling involved in mast cells and mediates mast
cell degranulation (46). STAT3
acquires DNA-binding activity through dimerization and then
translocates to the nucleus, where it binds to gene promoters and
activates transcription. Tyrosine phosphorylation is required for
STAT3 dimerization, nuclear translocation and DNA binding. In
addition, phosphorylation of a conserved carboxy-terminal serine
residue (Ser727) has been shown to enhance STAT3 transcriptional
activation (47,48). The Ser727 phosphorylation of STAT3
either inhibits tyrosine phosphorylation or increases tyrosine
dephosphorylation (49). These
reports indicate that each residue of STAT3 has a different role
and activates different targets. In the present study, BV had no
effect on the activation of NF-κB, which is crucial in the
regulation of allergic inflammatory responses (50). As BV inhibited the
PMACI-stimulated phosphorylation of MAPKs, which contribute to the
transmission of extracellular signals that can result in the
phosphorylation of various transcription factors and alterations in
gene expression (51), the
present study focused on examining the effects of BV on STAT3 as it
is a critical component in multiple aspects of allergic disease.
The resulting data indicated that STAT3 was activated on Ser727 in
PMACI-induced HMC-1 cells, whereas it was activated on Tyr705 in
the compound 48/80-induced anaphylactic shock animal model. Based
on these data, it was hypothesized that the inhibitory effects of
BV on STAT3 signaling depend on tissue specificity in the mast
cell-mediated allergic inflammatory response.
BV is the venom stored by bees within their venom
sacs for self-defense, and has traditionally been used in oriental
medicine to relieve pain and treat inflammatory diseases (52). BV is composed of various peptides,
enzymes and non-peptide components. The peptides are mainly
composed of apamin, melittin, MCD peptide and adolapinm, and the
enzymes include phospholipase A2, hyaluronidase, acid
phosphomonoesterase, α-d-glucosidase and lypophospholipase. The
non-peptide components consist of histamine, dopamine and
noradrenaline. Although it has been reported that melittin, a major
component of BV, induces paw edema in mice, and that the
administration of BV into the hind paw produces local inflammation,
BV components have been the subject of several investigations using
diverse methodologies in an effort to determine their
anti-inflammatory effects (53,54). BV and its components have been
used to treat various conditions, including arthritis, rheumatism,
back pain and skin diseases, by regulating inflammatory responses
(55). In the present study,
although the BV complex was used, future investigations aim to
investigate the use of major active components of BV to overcome
the limitations of complexity and to identify which components act
to cause these effects. In addition, further investigations are
required to identify the effect of each component of BV on the
regulation of STATs during an allergic inflammatory response, with
the present study contributing to this further understanding.
In the present study, it was shown that BV
suppressed the phosphorylation of MAPKs, MKKs and STAT3 in
PMACI-stimulated HMC-1 cells and in an anaphylactic shock animal
model. Furthermore, in addition to the inhibition of histamine
release in PMACI-stimulated HMC-1 cells, BV inhibited the
production and mRNA expression of pro-inflammatory cytokines in the
cells and animal model. Therefore, the results of the present study
suggested that BV has an anti-allergic inflammatory effect and that
this effect of BV may be an effective modulator of mast
cell-mediated allergic inflammatory responses.
Acknowledgments
The authors would like to thank Professor Jae-Young
Um (Kyung Hee University, Republic of Korea) for providing the
HMC-1 cells.
Abbreviations:
|
HMC-1
|
human mast cell
|
|
BV
|
bee venom
|
|
PMACI
|
phorbol-12-myristate 13-acetate plus
calcium ionophore A23187
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MKK
|
MAPK kinase
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
Notes
[1]
Funding
This study was supported by the Basic Science
Program through the National Research Foundation of Korea funded by
the Ministry of Education (grant no. NRF-2017R1C1B2008617).
[2] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
YMK, KSC, ML, and HJA conceived and designed the
experiments. YMK and IHK performed the experiments and analyzed the
data with KSC and HJA. HB contributed samples. ML and YBK
contributed reagents, materials and analytical tools. YMK and KSC
wrote the manuscript. All authors read and approved the final
manuscript.
[4] Ethics
approval and consent to participate
All procedures were performed in accordance with
university guidelines and approved by the Ethical Committee for
Animal Care and the Use of Laboratory Animals, Korean Medicine,
Sangji University (approval no. 2015-11).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Hong MH, Kim JH, Bae H, Lee NY, Shin YC,
Kim SH and Ko SG: Atractylodes japonica Koidzumi inhibits the
production of proinflammatory cytokines through inhibition of the
NF-κB/IκB signal pathway in HMC-1 human mast cells. Arch Pharm Res.
33:843–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sohn Y, Han NY, Lee MJ, Cho HJ and Jung
HS: [6]-Shogaol inhibits the production of proinflammatory
cytokines via regulation of NF-κB and phosphorylation of JNK in
HMC-1 cells. Immunopharmacol Immunotoxicol. 35:462–470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JY and Ro JY: Signal pathway of
cytokines produced by reactive oxygen species generated from
phorbol myristate acetate-stimulated HMC-1 cells. Scand J Immunol.
62:25–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeong HJ, Hong SH, Park RK, An NH and Kim
HM: Ethanol induces the production of cytokines via the
Ca2+, MAP kinase, HIF-1α, and NF-κB pathway. Life Sci.
77:2179–2192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ko YJ, Kim HH, Kim EJ, Katakura Y, Lee WS,
Kim GS and Ryu CH: Piceatannol inhibits mast cell-mediated allergic
inflammation. Int J Mol Med. 31:951–958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeon YD, Kee JY, Kim DS, Han YH, Kim SH,
Kim SJ, Um JY and Hong SH: Effects of Ixeris dentata water extract
and caffeic acid on allergic inflammation in vivo and in vitro. BMC
Complement Altern Med. 15:1962015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ichijo H: From receptors to
stress-activated MAP kinases. Oncogene. 18:6087–6093. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Favata MF, Horiuchi KY, Manos EJ, Daulerio
AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F,
et al: Identification of a novel inhibitor of mitogen-activated
protein kinase kinase. J Biol Chem. 273:18623–18632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zou J, Wang R, Li R, Kong Y, Wang J, Ning
X, Zhang L, Wang S, Hu X and Bao Z: The genome-wide identification
of mitogen-activated protein kinase kinase (MKK) genes in Yesso
scallop Patinopecten yessoensis and their expression responses to
bacteria challenges. Fish Shellfish Immunol. 45:901–911. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Glosson NL, Bruns HA and Kaplan MH:
Wheezing and itching: The requirement for STAT proteins in allergic
inflammation. JAKSTAT. 1:3–12. 2012.PubMed/NCBI
|
|
11
|
Samavati L, Rastogi R, Du W, Hüttemann M,
Fite A and Franchi L: STAT3 tyrosine phosphorylation is critical
for interleukin 1 beta and interleukin-6 production in response to
lipopolysaccharide and live bacteria. Mol Immunol. 46:1867–1877.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michaud-Levesque J, Bousquet-Gagnon N and
Béliveau R: Quercetin abrogates IL-6/STAT3 signaling and inhibits
glioblastoma cell line growth and migration. Exp Cell Res.
318:925–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zolfagharian H, Mohajeri M and Babaie M:
Honey bee venom (Apis mellifera) contains anticoagulation factors
and increases the blood-clotting time. J Pharmacopuncture. 18:7–11.
2015. View Article : Google Scholar
|
|
14
|
Ali MAA-SM: Studies on bee venom and its
medical uses. Int J Adv Res Technol. 1:69–83. 2012.
|
|
15
|
Hwang DS, Kim SK and Bae H: Therapeutic
effects of Bee Venom on immunological and neurological diseases.
Toxins. 7:2413–2421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim WH, An HJ, Kim JY, Gwon MG, Gu H, Park
JB, Sung WJ, Kwon YC, Park KD, Han SM and Park KK: Bee venom
inhibits porphyromonas gingivalis lipopolysaccharides-induced
pro-inflammatory cytokines through suppression of NF-κB and AP-1
signaling pathways. Molecules. 21:E15082016. View Article : Google Scholar
|
|
17
|
Zolfagharian H, Mohajeri M and Babaie M:
Bee venom (Apis Mellifera) an effective potential alternative to
gentamicin for specific bacteria strains: Bee venom an effective
potential for bacteria. J Pharmacopuncture. 19:225–230. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nipate SS, Hurali PB and Ghaisas MM:
Evaluation of anti-inflammatory, anti-nociceptive, and
anti-arthritic activities of Indian Apis dorsata bee venom in
experimental animals: Biochemical, histological, and radiological
assessment. Immunopharmacol Immunotoxicol. 37:171–184. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim KH, Kum YS, Park YY, Park JH, Kim SJ,
Lee WR, Lee KG, Han SM and Park KK: The protective effect of bee
venom against ethanol-induced hepatic injury via regulation of the
mitochondria-related apoptotic pathway. Basic Clin Pharmacol
Toxicol. 107:619–624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Russell PJ, Hewish D, Carter T,
Sterling-Levis K, Ow K, Hattarki M, Doughty L, Guthrie R, Shapira
D, Molloy PL, et al: Cytotoxic properties of immunoconjugates
containing melittin-like peptide 101 against prostate cancer: In
vitro and in vivo studies. Cancer Immunol Immunother. 53:411–421.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Son DJ, Lee JW, Lee YH, Song HS, Lee CK
and Hong JT: Therapeutic application of anti-arthritis,
pain-releasing, and anti-cancer effects of bee venom and its
constituent compounds. Pharmacol Ther. 115:246–270. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park HJ, Lee SH, Son DJ, Oh KW, Kim KH,
Song HS, Kim GJ, Oh GT, Yoon DY and Hong JT: Antiarthritic effect
of bee venom: Inhibition of inflammation mediator generation by
suppression of NF-kappaB through interaction with the p50 subunit.
Arthritis Rheum. 50:3504–3515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin SH, Kim YH, Kim JK and Park KK:
Anti-allergic effect of bee venom in an allergic rhinitis mouse
model. Biol Pharm Bull. 37:1295–1300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huh JE, Baek YH, Lee MH, Choi DY, Park DS
and Lee JD: Bee venom inhibits tumor angiogenesis and metastasis by
inhibiting tyrosine phosphorylation of VEGFR-2 in LLC-tumor-bearing
mice. Cancer Lett. 292:98–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi MS, Park S, Choi T, Lee G, Haam KK,
Hong MC, Min BI and Bae H: Bee venom ameliorates ovalbumin induced
allergic asthma via modulating CD4+CD25+
regulatory T cells in mice. Cytokine. 61:256–265. 2013. View Article : Google Scholar
|
|
26
|
An HJ, Lee WR, Kim KH, Kim JY, Lee SJ, Han
SM, Lee KG, Lee CK and Park KK: Inhibitory effects of bee venom on
Propionibacterium acnes-induced inflammatory skin disease in an
animal model. Int J Mol Med. 34:1341–1348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Choi YH, Chai OH, Han EH, Choi SY, Kim HT
and Song CH: Lipoic acid suppresses compound 48/80-induced
anaphy-laxis-like reaction. Anat Cell Biol. 43:317–324. 2010.
View Article : Google Scholar
|
|
29
|
Chai OH, Shon DH, Han EH, Kim HT and Song
CH: Effects of Anemarrhena asphodeloides on IgE-mediated passive
cutaneous anaphylaxis, compound 48/80-induced systemic anaphylaxis
and mast cell activation. Exp Toxicol Pathol. 65:419–426. 2013.
View Article : Google Scholar
|
|
30
|
Cho MS, Park WS, Jung WK, Qian ZJ, Lee DS,
Choi JS, Lee DY, Park SG, Seo SK, Kim HJ, et al: Caffeic acid
phenethyl ester promotes anti-inflammatory effects by inhibiting
MAPK and NF-κB signaling in activated HMC-1 human mast cells. Pharm
Biol. 52:926–932. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Jin G, Jiang J, Zheng M, Jin Y, Lin
Z, Li G, Choi Y and Yan G: Cornuside inhibits mast cell-mediated
allergic response by down-regulating MAPK and NF-κB signaling
pathways. Biochem Biophys Res Commun. 473:408–414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dérijard B, Raingeaud J, Barrett T, Wu IH,
Han J, Ulevitch RJ and Davis RJ: Independent human MAP-kinase
signal transduction pathways defined by MEK and MKK isoforms.
Science. 267:682–685. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chae HS, Kim YM and Chin YW: Atractylodin
inhibits interleukin-6 by blocking NPM-ALK activation and MAPKs in
HMC-1. Molecules. 21:E11692016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Granato M, Rizzello C, Gilardini Montani
MS, Cuomo L, Vitillo M, Santarelli R, Gonnella R, D'Orazi G,
Faggioni A and Cirone M: Quercetin induces apoptosis and autophagy
in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and
STAT3 signaling pathways. J Nutr Biochem. 41:124–136. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi YH, Yan GH, Chai OH, Lim JM, Sung SY,
Zhang X, Kim JH, Choi SH, Lee MS, Han EH, et al: Inhibition of
anaphylaxis-like reaction and mast cell activation by water extract
from the fruiting body of Phellinus linteus. Biol Pharm Bull.
29:1360–1365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li GZ, Chai OH, Lee MS, Han EH, Kim HT and
Song CH: Inhibitory effects of Houttuynia cordata water extracts on
anaphylactic reaction and mast cell activation. Biol Pharm Bull.
28:1864–1868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Minai-Fleminger Y and Levi-Schaffer F:
Mast cells and eosinophils: The two key effector cells in allergic
inflammation. Inflamm Res. 58:631–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zampeli E and Tiligada E: The role of
histamine H4 receptor in immune and inflammatory disorders. Br J
Pharmacol. 157:24–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim MH, Seo JH, Kim HM and Jeong HJ: Zinc
oxide nanoparticles, a novel candidate for the treatment of
allergic inflammatory diseases. Eur J Pharmacol. 738:31–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Foster JR: The functions of cytokines and
their uses in toxicology. Int J Exp Pathol. 82:171–192. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saukkonen K, Sande S, Cioffe C, Wolpe S,
Sherry B, Cerami A and Tuomanen E: The role of cytokines in the
generation of inflammation and tissue damage in experimental
gram-positive meningitis. J Exp Med. 171:439–448. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ye J, Piao H, Jiang J, Jin G, Zheng M,
Yang J, Jin X, Sun T, Choi YH, Li L and Yan G: Polydatin inhibits
mast cell-mediated allergic inflammation by targeting I3K/Akt,
MAPK, NF-κB and Nrf2/HO-1 pathways. Sci Rep. 7:118952017.
View Article : Google Scholar
|
|
43
|
Nam SY, Kim HY, Yoou MS, Kim AH, Park BJ,
Jeong HJ and Kim HM: Anti-inflammatory effects of isoacteoside from
Abeliophyllum distichum. Immunopharmacol Immunotoxicol. 37:258–264.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song Q, Li D, Dai Y, Liu S, Huang L, Hong
Y, Zhang H and Song F: Characterization, expression patterns and
functional analysis of the MAPK and MAPKK genes in watermelon
(Citrullus lanatus). BMC Plant Biol. 15:2982015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Barnes PJ: Transcription factors in airway
diseases. Lab Invest. 86:867–872. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Siegel AM, Stone KD, Cruse G, Lawrence MG,
Olivera A, Jung MY, Barber JS, Freeman AF, Holland SM, O'Brien M,
et al: Diminished allergic disease in patients with STAT3 mutations
reveals a role for STAT3 signaling in mast cell degranulation. J
Allergy Clin Immunol. 132:1388–1396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ivashkiv LB: Jak-STAT signaling pathways
in cells of the immune system. Rev Immunogenet. 2:220–230.
2000.
|
|
48
|
Schuringa JJ, Jonk LJ, Dokter WH, Vellenga
E and Kruijer W: Interleukin-6-induced STAT3 transactivation and
Ser727 phos-phorylation involves Vav, Rac-1 and the kinase
SEK-1/MKK-4 as signal transduction components. Biochem J.
347:89–96. 2000. View Article : Google Scholar
|
|
49
|
Decker T and Kovarik P: Serine
phosphorylation of STATs. Oncogene. 19:2628–2637. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Krishnamurthy P and Kaplan MH: STAT6 and
PARP family members in the development of T cell-dependent allergic
inflammation. Immune Netw. 16:201–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vanden Berghe W, Plaisance S, Boone E, De
Bosscher K, Schmitz ML, Fiers W and Haegeman G: p38 and
extracellular signal-regulated kinase mitogen-activated protein
kinase pathways are required for nuclear factor-kappaB p65
transactivation mediated by tumor necrosis factor. J Biol Chem.
273:3285–3290. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jang HS, Kim SK, Han JB, Ahn HJ, Bae H and
Min BI: Effects of bee venom on the pro-inflammatory responses in
RAW264.7 macrophage cell line. J Ethnopharmacol. 99:157–160. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hartman DA, Tomchek LA, Lugay JR, Lewin
AC, Chau TT and Carlson RP: Comparison of antiinflammatory and
antiallergic drugs in the melittin- and D49 PLA2-induced mouse paw
edema models. Agents Actions. 34:84–88. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lariviere WR and Melzack R: The bee venom
test: A new tonic-pain test. Pain. 66:271–277. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chung KS, An HJ, Cheon SY, Kwon KR and Lee
KH: Bee venom suppresses testosterone-induced benign prostatic
hyperplasia by regulating the inflammatory response and apoptosis.
Exp Biol Med. 240:1656–1663. 2015. View Article : Google Scholar
|