Introduction
Inflammation is a protective immune response against
bacterial infection or tissue injury. However, excessive
inflammation often leads to diseases, including arthritis and
sepsis (1,2). The principal components of the outer
membrane of gram-negative bacteria are lipopolysaccharides (LPS),
also termed endotoxins, which initiate inflammatory immune
responses (3). LPS induce
inflammatory cytokine release, including interleukin (IL)-6, IL-1β
and tumour necrosis factor (TNF)-α, via toll-like receptor 4
binding, activating the downstream inflammation-associated
signalling pathways (2,4). The signalling pathways of
mitogen-activated protein kinases (MAPKs), including extracellular
signal-related kinase (ERK)-1/2, p38MAPK, c-Jun NH2-terminal kinase
(JNK) and nuclear factor kappa-light-chain-enhancer of activated B
cells (NF-κB), have been demonstrated to be involved in LPS-induced
inflammatory responses (5,6).
The Janus kinase-signal transducer and activator of transcription
(JAK-STAT) signalling pathway is an additional important
inflammatory signalling pathway activated by LPS (7,8).
Following receptor binding, LPS induce JAK phosphorylation,
effecting STAT phosphorylation. The phosphorylated STATs form
homo-or heterodimers that translocate into the nucleus, regulating
the transcription of a number of pro-inflammatory cytokines,
chemokines and regulatory enzymes, including inducible nitric oxide
synthase (iNOS) and cyclooxygenase-2 (COX-2) (9,10).
Increasing evidence has suggested that the inhibition of the
JAK-STAT signalling pathway attenuates LPS-induced inflammation
(10,11).
Attention has been paid to natural active products,
due to their abundance and minimal side effects. The Aloe
vera plant has been widely used in health and nutritional
supplements in Chinese herbal medicine (12). Aloin, a bioactive ingredient
extracted from Aloe vera, has been indicated to induce
anti-inflammatory (13),
antioxidan (12) and antitumour
(14-16) effects, neuroprotection (17) and osteoclastogenesis (18,19). However, the anti-inflammatory
mechanism of aloin remains unknown.
The present study evaluated the effects of aloin on
the LPS-induced inflammatory response and then investigated the
underlying molecular mechanism in RAW264.7 cells. It was determined
that aloin inhibited LPS-induced TNF-α, IL-1β, IL-6 and nitric
oxide (NO) release, attenuating the iNOS expression induced by LPS.
Mechanistically, aloin suppressed reactive oxygen species
(ROS)-mediated JAK1-STAT1/3 signalling pathway activation,
inhibiting the nuclear translocation of STAT1/3.
Materials and methods
Reagents and antibodies
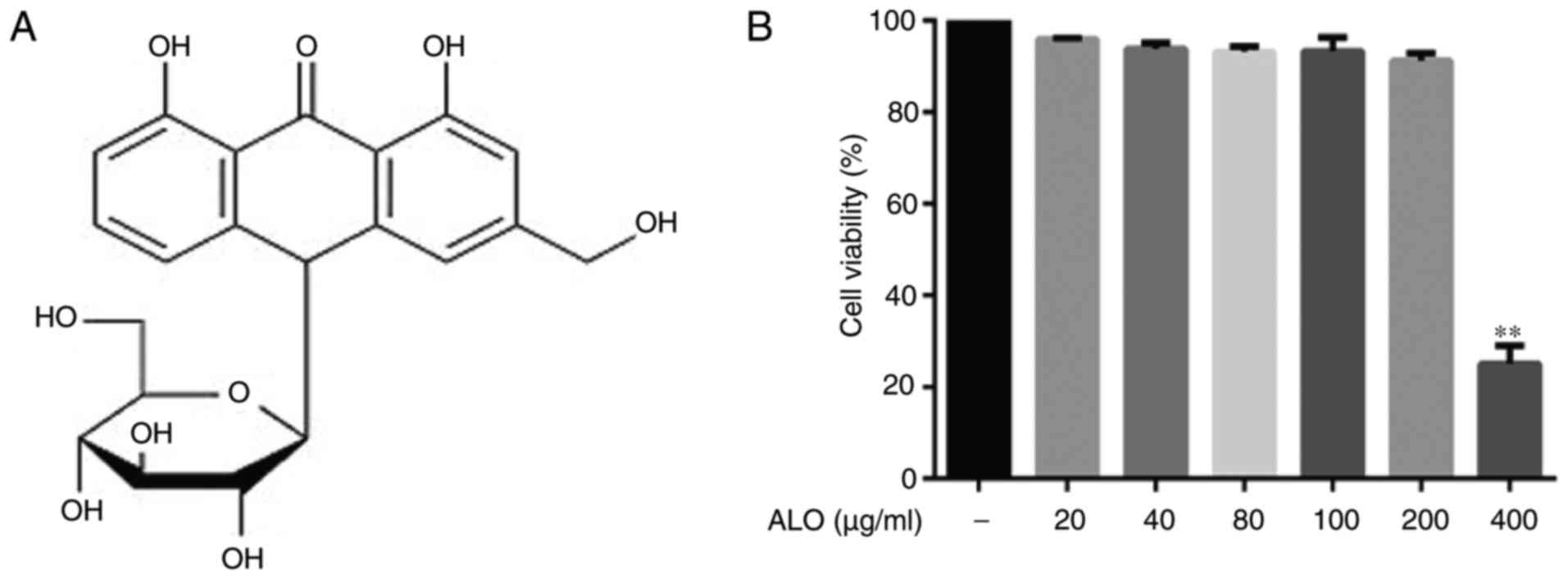
LPS from Escherichia coli and aloin
(purity≥97%; Fig. 1A) were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) and
Aladdin Industrial Corporation (Shanghai, China), respectively. The
aloin was dissolved in dimethyl sulfoxide and diluted with sterile
PBS. DAPI was obtained from Invitrogen; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Anti-phosphorylated (phosphor)-STAT3
(Tyr705, sc7993) and phospho-IκB (B-9, sc8404) antibodies were
obtained from Santa Cruz Biotechnology (Dallas, TX, USA). The
antibodies against phospho-p38 MAPK (p-p38 MAPK; Thr180/Tyr182,
4511S), phospho-ERK (Thr202/Tyr204, 4376S), phospho-JNK
(Thr183/Tyr185, 4668S), p38 MAPK (8690S), ERK (4695S), JNK (9258S),
phospho-JAK1 (Tyr1034/1035, 3331S), phospho-JAK2 (Y1007/1008,
3771S), phospho-STAT1 (Tyr701,9167S), JAK1 (3332S), JAK2 (3230S),
STAT1 (14994S), STAT3 (12640S), COX-2 (4842S), iNOS (13120), GAPDH
(5174S) and β-actin (4970S) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The anti-phospho-IκB (IKK;
S176/177, ab194528) antibody was purchased from Abcam (Cambridge,
UK). Secondary antibodies coupled to IRDye 800 fluorophore used in
the western blot analysis (926-3221 and 926-32210) were obtained
from LI-COR Biosciences (Lincoln, NE, USA). The Alexa
Fluor® 555 goat anti-rabbit IgG secondary antibody used
in the confocal microscopy experiment was obtained from Invitrogen
(Z25305; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell culture and passage
Murine macrophage RAW264.7 cells were purchased from
Kunming Cell Bank of Type Culture Collection, Chinese Academy of
Sciences (Kunming, China) and cultured in high glucose Dulbecco's
modified Eagle's medium supplemented with 10% foetal bovine serum
(both Gibco; Thermo Fisher Scientific, Inc.), 100 µg/m
streptomycin and 100 U/ml penicillin at 37°C in 5% CO2.
The cells were passaged every 2 days.
Cell viability detection
Cell viability was detected using a Cell Counting
Kit-8 (CCK-8; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
according to the manufacturer's protocol. Briefly, RAW264.7 cells
were treated at 37°C with different concentrations of aloin (20,
40, 80, 100, 200 and 400 µg/ml) for 24 h and then incubated
at 37°C with 10 µl CCK-8 working solution for 2 h. The
absorbance was detected using a Multiskan™ GO spectrophotometer
(Thermo Fisher Scientific, Inc.) at 450 nm. All experiments were
repeated in triplicate, and the data are presented as mean ±
standard deviation (SD).
Pro-inflammatory cytokine detection
Following a 2 h pre-treatmentat 37°C with different
aloin concentrations (100, 150 and 200 µg/ml), RAW264.7
cells were seeded at a density of 1 ×106/well in 12-well
cell culture plates and stimulated with LPS (100 ng/ml) for 16 h at
37°C. The levels of TNF-α, IL-1β and IL-6 in the cell culture
supernatants were detected using TNF-α (P06804), IL-1β (P10749) or
IL-6 (P08505) ELISA kits (RayBiotech, Inc., Norcross, GA, USA)
according to the manufacturer's protocol. The experiments were
repeated in triplicate for all aloin concentrations. The results
are presented as mean ± SD.
Nitric oxide detection
RAW264.7 cells were pre-treated at 37°C with aloin
(100, 150 and 200 µg/ml) for 2 h followed by a 16 h LPS
treatment. NO production in the cell culture medium was determined
using a Total Nitric Oxide Assay kit (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol. The absorbance at 540 nm was measured using a Multiskan™
GO spectrophotometer (Thermo Fisher Scientific, Inc.). Each
experiment was repeated in triplicate for all aloin
concentrations.
ROS determination
RAW264.7 cells were seeded at a density of 2
×105/well in 12-well cell culture plates. Following
aloin (100,150 and 200 µg/ml) pre-treatment at 37°C, the
cells were stimulated with LPS for 30 min, and intracellular total
ROS was detected using a Reactive Oxygen Species Assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Briefly, following treatment, the cell
culture medium was removed, and dichloro-dihydro-fluorescein
diacetate (DCFH-DA) was added to a final concentration of 10
µM. Then, the cells were incubated in a CO2
incubator for 20 min at 37°C and washed 3 times with PBS to
completely remove the DCFH-DA from the cells. ROS production was
observed by inverted fluorescence microscopy (magnification, x 100;
Olympus Corporation, Tokyo, Japan) and quantified using ImageJ
software version 1.46 (National Institutes of Health, Bethesda, MD,
USA). The experiments were repeated in triplicate.
Nuclear and cytoplasmic protein
separation and western blot analysis
The nuclear and cytoplasmic proteins were extracted
using a Nuclear and Cytoplasmic Protein Extraction kit (P0028;
Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The extraction of total intracellular
protein was as follows: Cells were pretreated with aloin (100, 150,
200 µg/ml) for 2 h, then stimulated with 100 ng/ml LPS for
different times (30 min, 4 or 16 h) at 37°C. Pre-treated RAW264.7
cells were washed twice with ice-cold PBS and lysed in ice-cold
cell lysis buffer (P0013; Beyotime Institute of Biotechnology)
including 20 mM Tris (pH7.5), 150 mM NaCl, 1% Triton X-100, sodium
pyrophosphate, β-glycerophosphate, EDTA,
Na3VO4 and leupeptin. Following lysis on ice
for 30 min, the lysates were centrifuged (14,300 × g) for 15 min at
4°C, and the protein samples were quantified using a BCA/Bradford
assay. Then, equal amounts (50 µg) of protein were denatured
in SDS and electrophoresed on a 12% SDS-PAGE prior to transferring
to a nitrocellulose membrane (Pall Corporation, Port Washington,
NY, USA). The membrane was blocked in 5% skimmed milk dissolved in
TBST for 1 h at room temperature. Following washing with TBS and
0.1% Tween®-20 3 times, the membrane was incubated
overnight at 4°C with primary antibodies diluted 1:500 with TBST,
followed by incubation with IRDye 800-conjugated IgG secondary
antibodies (1:5,000; LI-COR Biosciences) at room temperature for 1
h. The antigen-antibody complex was visualised using an
Odyssey® infrared imaging system (LI-COR Biosciences).
ImageJ version 1.46 software (National Institutes of Health) was
used for the densitometry analysis.
Reverse transcription polymerase chain
reaction (RT-PCR)
Intracellular total RNA was extracted from RAW264.7
cells using TRIzol® reagent (Life Technologies; Thermo
Fisher Scientific, Inc.), and a RevertAid™ First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) was used to
synthesise cDNA. The PCR primers were: 5′-GGGTCTTGTTCACTCCACGG-3′
(iNOS forward), and 5′-GCTCAGAACAGCACAAGGGG-3′ (iNOS reverse);
5′-GGAGAGTGTTTCCTCGTCCC-3′ (GAPDH forward), and
5′-ACTGTGCCGTTGAATTTGCC-3′ (GAPDH reverse). The thermocycling
conditions were as follows: 94°C for 3 min, followed by 30 cycles
of 94°C for 30 sec, 55°C for 30 sec, 72°C for 1 min and 72°C for 1
min with a final extension step at 72°C for 10 min. The PCR
products were detected by agarose gel (1.5%) electrophoresis and
visualized with GoldView (Service Biological Technology Co., Ltd.,
Wuhan, China). The image was captured by the Gel Doc™ EZ imager
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). ImageJ version
1.46 software (National Institutes of Health) was used for
densitometry analysis.
Confocal laser microscopy
RAW264.7 cells were seeded in a small confocal laser
dish at 500 cells/well. RAW264.7 cells were pre-treated with 200
µg/ml aloin for 2 h, and then stimulated with LPS for 4 h at
37°C. Following treatment, the cells were washed with PBS, fixed
with 4% paraformaldehyde for 30 min at room temperature,
permeabilised with 0.2% Triton X-100, blocked with 3% bovine serum
albumin in PBS for 1 h at room temperature and incubated with STAT1
and STAT3 primary antibodies diluted 1:100 with PBS overnight at
4°C. Following rinsing with PBS, the cells were incubated with a
goat anti-rabbit IgG Alexa Fluor® 555 conjugated
fluorescent secondary antibody diluted 1:200 with PBS for 1 h in
the dark at room temperature. Finally, the cells were stained at
room temperature with 0.1 µg/ml DAPI for 3 min in the dark.
Images were captured using a TCS SP8 confocal microscope
(magnification, ×200; Leica Microsystems GmbH, Wetzlar,
Germany).
Statistical analysis
All data were presented as mean ± standard
deviation. Statistical analyses were performed using Prism 6.0
software (GraphPad Software, Inc., La Jolla, CA, USA). The results
were analyzed by one-way analysis of variance followed by a post
hoc Tukey's test for multiple comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
Aloin inhibits LPS-induced iNOS
expression in RAW264.7 cells
The present study investigated aloin cytotoxicity
using a CCK-8 cell viability assay. Aloin did not affect cell
viability, even at a high dose of 200 µg/ml (Fig. 1B). As iNOS and COX-2 are
considered to be associated with LPS stimulation (7), the effects of aloin on LPS-induced
iNOS and COX-2 expression were investigated. RAW264.7 cells were
pre-treated with different doses of aloin for 2 h, then treated
with LPS for 16 h, and the iNOS and COX-2 levels were determined by
western blot analysis. Aloin treatment suppressed iNOS expression
dose-dependently but did not affect COX-2 expression (Fig. 2A). According to these results,
aloin concentrations of 100, 150 and 200 µg/ml were selected
for subsequent experiments. RT-PCR and western blot analyses were
used to detect iNOS expression levels in RAW264.7 cells pre-treated
with different aloin doses (100, 150 and 200 µg/ml) for 2 h
and then LPS for 6 or 16 h. Aloin markedly attenuated iNOS
expression at mRNA and protein levels in a dose-dependent manner
(Fig. 2B and C).
 | Figure 2ALO inhibits LPS-induced expression
of iNOS, but not COX-2. RAW264.7 cells were pre-treated with (A)
different doses of ALO (10, 20, 50, 75, 100, 150 and 200
µg/ml) for 2 h or (B) 100, 150 and 200 µg/ml of ALO
for 2 h, then stimulated with LPS (100 ng/ml) for 16 h. Following
total protein extraction, iNOS and COX-2 levels were determined by
western blot analysis. GAPDH or β-actin was used as a control. (C)
RAW264.7 cells were pre-treated with ALO for 2 h, then treated with
LPS for 6 h. Total cellular RNA was extracted using
TRIzol® reagent, and reverse transcription-polymerase
chain reaction was used to quantify the iNOS transcripts. GAPDH was
used as a control. Data are presented as mean ± standard deviation.
*P<0.05 and **P<0.01 vs. LPS-stimulated
cells. ALO, aloin; LPS, lipopolysaccharide; iNOS, inducible nitric
oxide synthase; COX-2, cyclooxygenase-2. |
Aloin inhibits the release of
pro-inflammatory cytokines and mediators induced by LPS
To investigate the anti-inflammatory effect of
aloin, the levels of pro-inflammatory cytokines and mediators,
including TNF-α, IL-1β, IL-6 and NO in LPS-stimulated RAW264.7
cells were first determined. The obvious increasement of the levels
of TNF-α, IL-1β, IL-6 and NO resulting from the LPS stimulation
were inhibited by aloin in a dose-dependent manner (Fig. 3A–D). Collectively, these results
suggest that aloin inhibited pro-inflammatory cytokine and mediator
release, exhibiting an anti-inflammatory effect.
Aloin suppresses the LPS-activated
JAK1-STAT1/3 pathway, but not the MAPK signalling pathway
It has been suggested that the JAK-STAT, MAPK and
NF-κB signalling pathways are involved in LPS-triggered
inflammatory responses (7,10,20).
Therefore, to investigate the underlying molecular mechanism of
aloin-based inhibition of LPS-induced inflammatory reactions, the
effects of aloin on LPS-activated STAT and MAPK signalling pathways
were determined. Firstly, the phosphorylation levels of MAPKs and
STATs following LPS stimulation of RAW264.7 cells were assessed.
LPS stimulation was performed for different time periods, and
western blot analysis was used to detect MAPK and STAT activation.
At 10 min of LPS stimulation, p38 MAPK, ERK and JNK were activated,
peaking at ~30 min visually (Fig.
4A). To additionally explore the effects of aloin on MAPK
phosphorylation induced by LPS, the cells were pre-treated with
aloin for 2 h and LPS for 30 min. Activation was detected by
western blot analysis. LPS-induced phosphorylation of p38 MAPK, ERK
and JNK were not affected by aloin treatment (Fig. 4B). A previous study demonstrated
that NF-κB became activated following the activation of its
upstream kinase IKK and IκB regions (21,22). To explore the effects of aloin on
the LPS-induced NF-κB signaling pathway, levels of IκB kinase (IKK)
and IκB phosphorylation were determined by western blot analysis.
The results of the present study demonstrated that aloin exhibited
a minimal effect on the phosphorylation levels of upstream IKK and
IκB (Fig. 4C). Therefore, the
transcriptional activity of downstream transcription factor NF-κB
was not examined. STAT1 and STAT3 phosphorylation increased at 0.5
h, peaked at 4 h and was sustained until 6 h following LPS
stimulation as observed visually (Fig. 4D). The increased levels of STAT1
and STAT3 phosphorylation were markedly decreased by aloin in a
dose-dependent manner (Fig. 4E).
Collectively, these data suggested that aloin attenuated the
LPS-triggered inflammatory response by suppressing STAT1 and STAT3
activation, but not MAPKs, IKK or IκB.
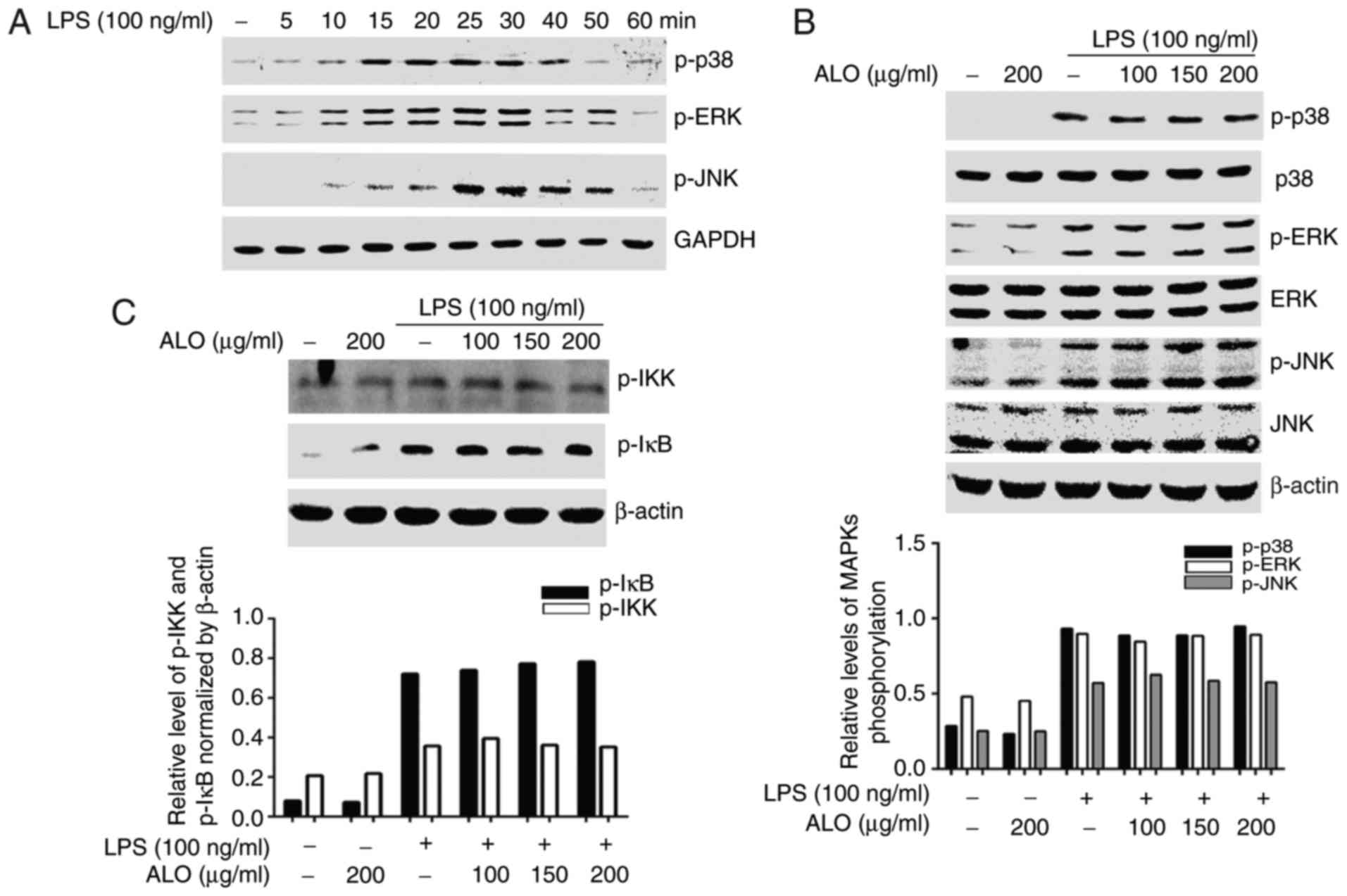 | Figure 4ALO inhibits LPS-activated STAT
signalling pathways but does not affect MAPKs, IKK and IκB
activation. (A) RAW264.7 cells were stimulated with LPS for
different time intervals. (B) RAW264.7 cells were pre-treated with
the indicated ALO doses for 2 h and then stimulated with LPS for 30
min. Proteins were extracted, and p-p38, p-ERK, p-JNK, p-IKK and
p-IκB expression levels were detected by western blot analysis
using target-specific antibodies. (C) Densitometric analysis of
western blot analysis data from (B). The histogram indicates the
relative expression of p-p38, p-ERK and p-JNK normalized to total
p38, ERK and JNK respectively. (D) STAT1 and STAT3 phosphorylation
in RAW264.7 cells stimulated with LPS for different time intervals.
(E) STAT1 and STAT3 phosphorylation in RAW264.7 cells pre-treated
with indicated doses of ALO for 2 h and then stimulated with LPS
for 4 h. The levels of p-STAT1/3 and total STAT1/3 were measured by
western blot analysis. Data arepresented as mean ± standard
deviation. **P<0.01 vs. LPS-stimulated cells. LPS,
lipopolysaccharide; STAT, signal transducer and activator of
transcription; p, phosphorylated; MAPK, mitogen-activated protein
kinase; p38, p38 MAPK; ERK, extracellular signal-regulated kinase;
JNK, c-Jun NH2-terminal kinase; ALO, aloin; IKK, IκB kinase. |
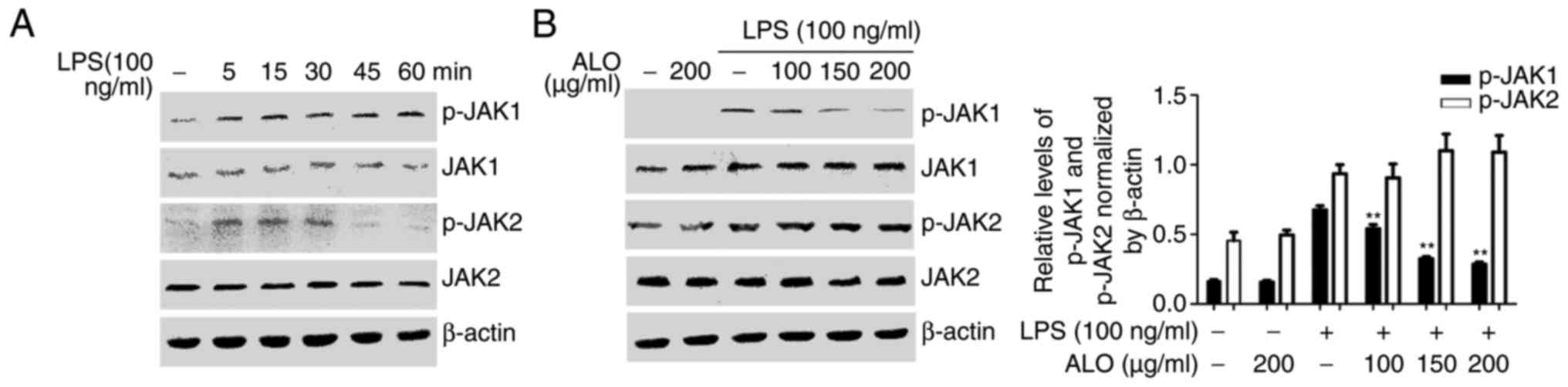
As STAT transcription factors have been determined
to be activated by the JAKs (23), the effects of aloin on JAK signals
were investigated. RAW264.7 cells were stimulated with LPS for
different time intervals, and JAK1 and JAK2 phosphorylation was
determined by western blot analysis. JAK1 and JAK2 phosphorylation
increased at 5 min and peaked at ~15 min following LPS treatment
(Fig. 5A). RAW264.7 cells were
also incubated with aloin for 2 h and treated with LPS for 15 min
to detect JAK1 and JAK2 activation by western blot analysis. Aloin
pre-treatment suppressed LPS-induced JAK1 phosphorylation, whereas
JAK2 phosphorylation was not affected (Fig. 5B). Collectively, these results
indicated that aloin inhibited STAT1 and STAT3 phosphorylation,
potentially via the inhibition of JAK1 in LPS-induced inflammatory
responses.
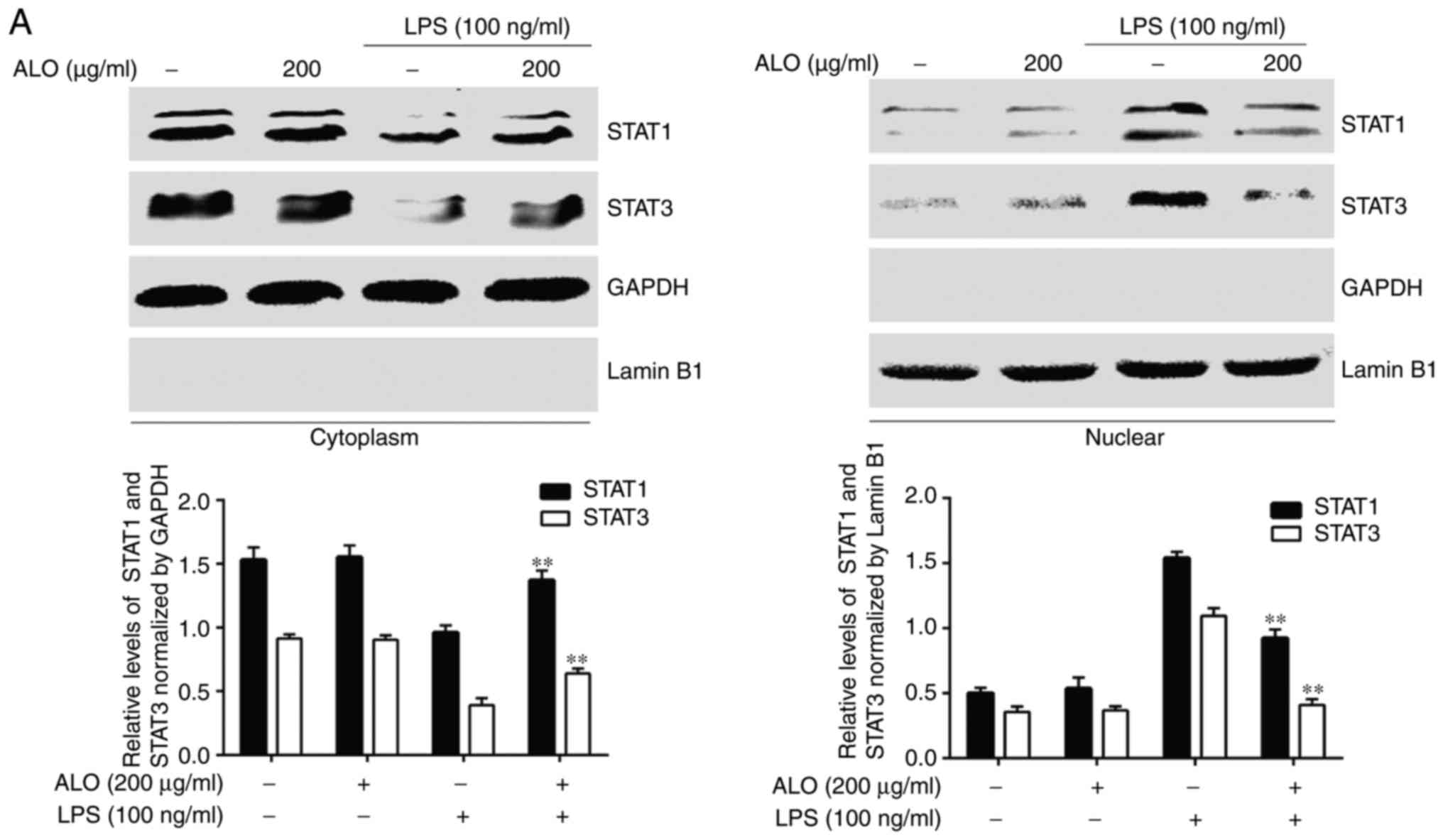
Aloin suppresses LPS-induced STAT1 and
STAT3 nucleo- cytoplasmic translocation
Activated STATs undergo dimerisation and translocate
into the nucleus to initiate transcription. Therefore, the present
study investigated whether aloin may inhibit the nuclear
translocation of phosphorylated STAT1 and STAT3. The cytoplasmic
and nuclear proteins of RAW264.7 cells pre-treated with aloin for 2
h and stimulated with LPS for 4 h were extracted to determine
cytoplasmic and nuclear STAT1 and STAT3 levels. LPS stimulation
induced the nuclear translocation of STAT1 and STAT3 and aloin
pre-treatment significantly inhibited their nuclear translocation
(Fig. 6A). A confocal microscopy
experiment was performed to detect the location of STAT1 and STAT3.
In the control and aloin groups, STAT1 and STAT3 were primarily
localised in the cytoplasm, indicated by red staining. In the
LPS-treated cells STAT1 and STAT3 transferred into the nuclei, as
indicated by blue staining patterns (Fig. 6B and C). However, the levels of
nucleocytoplasmic translocation of STAT1 and STAT3 induced by LPS
were markedly decreased by aloin. Collectively, the results
indicated that aloin inhibited LPS-induced inflammatory responses
by suppressing JAK1-STAT1/3 activation and the nuclear
translocation of STAT1 and STAT3, at least partially.
Aloin inhibits LPS-induced ROS
release
ROS are crucial to LPS-induced inflammation in
RAW264.7 cells through the activation of STAT transcription factors
(23), and Simon et al
(24) suggested that ROS
production contributing to JAK-STATs activation. Furthermore, a
previous study has revealed that aloin exhibits an antioxidan
effect (25). Therefore, the
present study investigated whether the anti-inflammatory effect of
aloin was due in part to its inhibition of ROS accumulation.
RAW264.7 cells were pre-treated with aloin for 2 h and stimulated
with LPS for 30 min. A ROS detection kit was used to assess ROS
accumulation. Aloin significantly decreased LPS-induced ROS
production in a dose-dependent manner (Fig. 7). The data of the present study
demonstrated that aloin may function as an antioxidan. The
anti-inflammatory mechanism of aloin may involve the inhibition of
ROS-mediated JAK1-STAT1/3 signalling pathway activation.
Discussion
Inflammation is a protective response. However, the
excessive release of pro-inflammatory cytokines from activated
macrophages and monocytes causes systemic inflammation (26). As LPS increase the release of
pro-inflammatory cytokines, they have been used for several years
in the study of this process (27).
Increasing evidence has revealed that a number of
bioactive products may antagonise the inflammatory response induced
by LPS, having little or no side effects on the human body
(28,29). The Aloe vera plant has been
widely used in Chinese herbal medicine and extracts have been
suggested to possesses anti-inflammatory properties (30). Aloin, the bioactive compound
obtained from the leaf exudates of Aloe vera, has been
demonstrated to exhibit anti-inflammatory activity (25,31). However, the detailed molecular
mechanisms remain unknown. Therefore, the present study
investigated the effects of aloin on LPS-stimulated inflammation
responses and additionally explored the potential molecular
mechanisms.
To identify aloin cytotoxicity in RAW264.7 cells,
the cells were treated for 24 h with different aloin doses prior to
determining cell viability. Aloin did not affect cell viability in
RAW264.7 cells at 200 µg/ml, suggesting that aloin
cytotoxicity from 20–200 µg/ml could be rejected in
subsequent experiments.
iNOS and COX-2 are 2 key inflammatory factors, and
TNF-α, IL-1β, IL-6 and NO are important pro-inflammatory cytokines
and mediators in inflammatory disease pathogenesis. The data from
the present study revealed that aloin decreased LPS-enhanced iNOS
expression at protein and transcript levels in a dose-dependent
manner. The release of LPS-induced pro-inflammatory cytokines and
mediators were markedly inhibited in a dose-dependent manner. A
number of studies have demonstrated the interaction between COX-2
and iNOS, and that NO or its product may activate COX enzymes
(32). However, the results of
the present study indicated that aloin inhibited iNOS expression
and NO release but had no inhibitory effect on COX-2 expression. A
potential cause of this discrepancy maybe due to the purity of the
aloin used.
To investigate the molecular mechanism, the effects
of aloin on LPS-induced inflammatory signalling pathway activation
were explored. The JAK-STAT, NF-κB and MAPK signalling pathways
have been demonstrated to be involved in inflammation (33). Therefore, the present study aimed
to determine whether aloin suppressed the activation of JAK-STAT,
NF-κB and MAPK signalling pathways. It was identified that aloin
inhibited JAK1, STAT1 and STAT3 phosphorylation in a dose-dependent
manner. As STAT phosphorylation is required for their nuclear
translocation and transcriptional activity (34), the effect of aloin on the nuclear
distributions of STAT1 and STAT3 in RAW264.7 cells was determined.
The nucleocytoplasmic separation experiment and confocal microscopy
analysis of the present study revealed that aloin markedly
suppressed STAT1 and STAT3 nuclear translocation. Unexpectedly,
aloin treatment did not affect the phosphorylation of MAPKs, IKK
and IκB induced by LPS. Collectively, the results of the present
study suggested that aloin may inhibit the LPS-induced inflammatory
response by suppressing the activation of the JAK1-STAT1/3
signalling pathway and the nuclear translocation of STAT1 and
STAT3.
Luo et al (35) demonstrated that aloin attenuated
LPS-induced NF-κB transcriptional activity by inhibiting its
upstream kinase p38 MAPK and mitogen- and stress-activated protein
kinase-1. However, the results of the present study demonstrated
that aloin pretreatment had no effect on LPS-induced p38
activation. This result was different from that of Luo et al
(35). In that study, the
inhibitory effect of aloin on p38 MAPK activation was detected 2 h
following LPS stimuli. However, the present study detected the
inhibitory effect at 30 min. Therefore, it was hypothesized that
the potential reason for the discrepancy is due to the different
detection times. Additionally, the present study revealed a novel
signal pathway for the anti-inflammatory mechanism of aloin.
It has been demonstrated that LPS stimulation
promotes ROS production in macrophages (36), and that ROS serve as secondary
messengers capable of regulating pro-inflammatory gene expression
(37). Previous studies have
indicated the antioxidan properties of aloin (12,25). In the present study, it was
identified that aloin decreased ROS accumulation in LPS-stimulated
RAW264.7 cells. Furthermore, ROS are potent inducers of various
signalling pathways, including MAPK and JAK-STAT pathways (38). Our previous studies demonstrated
that N-acetyl-L-cysteine, a ROS inhibitor, suppressed the
phosphorylation of JAK-STATs and the expression of iNOS (6,8).
These data led us to hypothesize that the inhibitory effect of
JAK1-STAT1/3 by aloin may beattributed to its antioxidan activity
towards ROS in RAW264.7 cells.
In summary, the present study demonstrated that
aloin may partly exert its anti-inflammatory activities through the
inhibition of ROS-mediated JAK1-STAT1/3 signalling pathway
activation in RAW264.7 macrophages. These results provide novel
insight into the anti-inflammatory molecular mechanisms of aloin
and provide experimental basis for the clinical application of
aloin.
Acknowledgments
Not applicable.
Funding
This work was financially supported by grants from
the Natural Science Foundation of China (grant no. 81601380),
Natural Science Research Project of Anhui Colleges and Universities
(grant no. KJ2016SD59); Outstanding Young Talent Support Programme
Key Projects of Anhui Colleges and Universities (grant no.
gxyqZD2016173), Active Biological Macromolecules Research
Provincial Key Laboratory Project (grant no. 1306C083008), Research
funding project for college students of Wannan Medical College
(grant no. WK2016S24) and College Students' innovation and
Entrepreneurship training program project (grant nos. 201710368002
and 201710368166).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZQ and YZ designed the experiments, YM, TT, QZ and
LS performed this study. ZW and HT analyzed the data. ZQ and YM
wrote the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Na HS, Song YR, Kim S, Heo JY, Chung HY
and Chung J: Aloin inhibits interleukin (IL)-1β-stimulated IL-8
production in KB cells. J Periodontol. 87:e108–115. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu Q, Zeng KW, Ma XL, Jiang Y, Tu PF and
Wang XM: Ginsenoside Rk1 suppresses pro-inflammatory responses in
lipopolysaccharide-stimulated RAW264.7 cells by inhibiting the
Jak2/Stat3 pathway. Chin J Nat Med. 15:751–757. 2017.PubMed/NCBI
|
|
3
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cochet F and Peri F: The role of
carbohydrates in the lipopolysaccharide (LPS)/toll-like receptor 4
(TLR4) signalling. Int J Mol Sci. 18:E23182017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan ED and Riches DW: IFN-γ + LPS
induction of iNOS is modulated by ERK, JNK/SAPK, and
p38mapk in a mouse macrophage cell line. Am J Physiol
Cell Physiol. 280:C441–C450. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi Z, Yin F, Lu L, Shen L, Qi S, Lan L,
Luo L and Yin Z: Baicalein reduces lipopolysaccharide-induced
inflammation via suppressing JAK/STATs activation and ROS
production. Inflamm Res. 62:845–855. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee SB, Lee WS, Shin JS, Jang DS and Lee
KT: Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2,
TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW
264.7 macrophages. Int Immunopharmacol. 49:21–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi S, Feng Z, Li Q, Qi Z and Zhang Y:
Myricitrin modulates NADPH oxidase-dependent ROS production to
inhibit endotoxin-mediated inflammation by blocking the JAK/STAT1
and NOX2/p47phox pathways. Oxid Med Cell Longev.
2017:97387452017.
|
|
9
|
Ganster RW, Taylor BS, Shao L and Geller
DA: Complex regulation of human inducible nitric oxide synthase
gene transcription by Stat 1 and NF-kappa B. Proc Natl Acad Sci
USA. 98:8638–8643. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Wang J, Yang W, Qi X, Lan L, Luo L
and Yin Z: Bergapten prevents lipopolysaccharide-induced
inflammation in RAW264.7 cells through suppressing JAK/STAT
activation and ROS production and increases the survival rate of
mice after LPS challenge. Int Immunopharmacol. 48:159–168. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi Z, Qi S, Ling L, Lv J and Feng Z:
Salidroside attenuates inflammatory response via suppressing
JAK2-STAT3 pathway activation and preventing STAT3 transfer into
nucleus. Int Immunopharmacol. 35:265–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu FW, Liu FC, Wang YR, Tsai HI and Yu
HP: Aloin protects skin fibroblasts from heat stress-induced
oxidative stress damage by regulating the oxidative defense system.
PLoS One. 10:e01435282015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park MY, Kwon HJ and Sung MK: Evaluation
of aloin and aloe-emodin as anti-inflammatory agents in aloe by
using murine macrophages. Biosci Biotechnol Biochem. 73:828–832.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Esmat AY, Said MM and Khalil SA: Aloin: A
natural antitumor anthraquinone glycoside with iron chelating and
non-atherogenic activities. Pharm Biol. 53:138–146. 2015.
View Article : Google Scholar
|
|
15
|
Buenz EJ: Aloin induces apoptosis in
Jurkat cells. Toxicol In Vitro. 22:422–429. 2008. View Article : Google Scholar
|
|
16
|
Wan L, Zhang L, Fan K and Wang J: Aloin
promotes A549 cell apoptosis via the reactive oxygen speciesmitogen
activated protein kinase signaling pathway and p53 phosphorylation.
Mol Med Rep. 16:5759–5768. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang R, Zhou R, Qi X, Wang J, Wu F, Yang
W, Zhang W, Sun T, Li Y and Yu J: Protective effects of aloin on
oxygen and glucose deprivation-induced injury in PC12 cells. Brain
Res Bull. 121:75–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pengjam Y, Madhyastha H, Madhyastha R,
Yamaguchi Y, Nakajima Y and Maruyama M: NF-κB pathway inhibition by
anthrocyclic glycoside aloin is key event in preventing
osteoclastogenesis in RAW264.7 cells. Phytomedicine. 23:417–428.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pengjam Y, Madhyastha H, Madhyastha R,
Yamaguchi Y, Nakajima Y and Maruyama M: Anthraquinone glycoside
aloin induces osteogenic initiation of MC3T3-E1 cells: Involvement
of MAPK mediated wnt and bmp signaling. Biomol Ther. 24:123–131.
2016. View Article : Google Scholar
|
|
20
|
Han BH, Lee YJ, Yoon JJ, Choi ES, Namgung
S, Jin XJ, Jeong DH, Kang DG and Lee HS: Hwangryunhaedoktang exerts
anti-inflammation on LPS-induced NO production by suppressing MAPK
and NF-κB activation in RAW264.7 macrophages. J Integr Med.
15:326–336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Achoui M, Appleton D, Abdulla MA, Awang K,
Mohd MA and Mustafa MR: In vitro and in vivo anti-inflammatory
activity of 17-Oacetylacuminolide through the inhibition of
cytokines, NF-κB translocation and IKK β activity. PLoS One. 5. pp.
e151052010, View Article : Google Scholar
|
|
22
|
Qi S, Xin Y, Guo Y, Diao Y, Kou X, Luo L
and Yin Z: Ampelopsin reduces endotoxic inflammation via repressing
ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int
Immunopharmacol. 12:278–287. 2012. View Article : Google Scholar
|
|
23
|
Pan X, Cao X, Li N, Xu Y, Wu Q, Bai J, Yin
Z, Luo L and Lan L: Forsythin inhibits lipopolysaccharide-induced
inflammation by suppressing JAK-STAT and p38 MAPK signalings and
ROS production. Inflamm Res. 63:597–608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simon AR, Rai U, Fanburg BL and Cochran
BH: Activation of the JAK-STAT pathway by reactive oxygen species.
Am J Physiol. 275:C1640–C1652. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui Y, Ye Q, Wang H, Li Y, Xia X, Yao W
and Qian H: Aloin protects against chronic alcoholic liver injury
via attenuating lipid accumulation, oxidative stress and
inflammation in mice. Arch Pharm Res. 37:1624–1633. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balk RA: Systemic inflammatory response
syndrome (SIRS): Where did it come from and is it still relevant
today? Virulence. 5:20–26. 2014. View Article : Google Scholar :
|
|
27
|
Mueller M, Hobiger S and Jungbauer A: Red
clover extract: A source for substances that activate peroxisome
proliferator-activated receptor alpha and ameliorate the cytokine
secretion profile of lipopolysaccharide-stimulated macrophages.
Menopause. 17:379–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fylaktakidou KC, Hadjipavlou-Litina DJ,
Litinas KE and Nicolaides DN: Natural and synthetic coumarin
derivatives with anti-inflammatory/antioxidant activities. Curr
Pharm Des. 10:3813–3833. 2004. View Article : Google Scholar
|
|
29
|
Mueller M, Hobiger S and Jungbauer A:
Anti-inflammatory activity of extracts from fruits, herbs and
spices. Food Chem. 122:987–996. 2010. View Article : Google Scholar
|
|
30
|
Akaberi M, Sobhani Z, Javadi B, Sahebkar A
and Emami SA: Therapeutic effects of Aloe spp. in traditional and
modern medicine: A review. Biomed Pharmacother. 84:759–772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patel DK, Patel K and Tahilyani V:
Barbaloin: A concise report of its pharmacological and analytical
aspects. Asian Pac J Trop Biomed. 2:835–838. 2012. View Article : Google Scholar
|
|
32
|
Swierkosz TA, Mitchell JA, Warner TD,
Botting RM and Vane JR: Co-induction of nitric oxide synthase and
cyclo-oxygenase: Interactions between nitric oxide and prostanoids.
Br J Pharmacol. 114:1335–1342. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aparicio-Soto M, Sanchez-Hidalgo M,
Cardeno A, Rosillo MÁ, Sánchez-Fidalgo S, Utrilla J, Martín-Lacave
I and Alarcón-de-la-Lastra C: Dietary extra virgin olive oil
attenuates kidney injury in pristane-induced SLE model via
activation of HO-1/Nrf-2 antioxidant pathway and suppression of
JAK/STAT, NF-κB and MAPK activation. J Nutr Biochem. 27:278–288.
2016. View Article : Google Scholar
|
|
34
|
Schindler C, Levy DE and Decker T:
JAK-STAT signaling: From interferons to cytokines. J Biol Chem.
282:20059–20063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo X, Zhang H, Wei X, Shi M, Fan P, Xie
W, Zhang Y and Xu N: Aloin suppresses lipopolysaccharide-induced
inflammatory response and apoptosis by inhibiting the activation of
NF-κB. Molecules. 23:E5172018. View Article : Google Scholar
|
|
36
|
Han W, Li H, Cai J, Gleaves LA, Polosukhin
VV, Segal BH, Yull FE and Blackwell TS: NADPH oxidase limits
lipopolysaccharide-induced lung inflammation and injury in mice
through reduction-oxidation regulation of NF-κB activity. J
Immunol. 190:4786–4794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan JS, Hong MZ and Ren JL: Reactive
oxygen species: A double-edged sword in oncogenesis. World J
Gastroenterol. 15:1702–1707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SK, Dahmer MK and Quasney MW: MAPK
and JAK-STAT signaling pathways are involved in the oxidative
stress-induced decrease in expression of surfactant protein genes.
Cell Physiol Biochem. 30:334–346. 2012. View Article : Google Scholar : PubMed/NCBI
|