Introduction
Salivary gland hypofunction is the inevitable result
of Sjogren syndrome (1) or
radiation therapy for head and neck cancer (2). There are different aspects of
salivary gland hypofunction, including dry mouth, saliva secretion
and saliva composition alterations, which seriously affect
patients' quality of life and oral health (3,4).
In mice, salivary gland development begins with the thickening of
the oral epithelium at embryonic day 11.5, followed by successive
branching and lumenization, with a functional arborized structure
present at eight weeks following birth (5-7).
Salivary gland morphogenesis is precisely controlled by regulatory
genes and growth factors (7).
Bone morphogenic proteins (BMPs) serve important roles in the
morphogenesis of the submandibular gland (SMG) by regulating
extracellular matrix (ECM) synthesis (8-10).
Although the roles of BMPs in the salivary glands have been
revealed, additional upstream regulatory proteins have yet to be
studied.
Family with sequence similarity 20-member C
(FAM20C), also known as dentin matrix protein 4, is a Golgi kinase
that catalyzes the attachment of phosphates to serine in S-x-E/pS
motifs in secretory pathway proteins, including BMP4 (11-15). FAM20C can phosphorylate >100
secreted proteins, including specific ECM and transport proteins,
proteases and protease inhibitors, and biologically active peptide
hormones. The broad substrate spectrum of FAM20C suggests that this
protein kinase participates in a wide range of biological
processes, including cell migration and adhesion, ECM deposition
and wound healing, in addition to biomineralization (16). FAM20C gene mutations in
humans lead to Raine syndrome, which includes osteosclerotic bone
dysplasia (17-20). In mice, Fam20c deletion
results in hypophosphatemic rickets, increased levels of fibroblast
growth factor 23 (FGF23) in the serum, reduced serum phosphorus
levels and severe dentin, and enamel defects (21-24), indicating an essential role of
FAM20C in bone and tooth development. Intriguingly, FAM20C is also
likely to exert biological effects on processes other than
mineralization due to its presence in mineralized tissues and soft
organs, including salivary glands (24,25). Given that teeth and SMGs share
similarities in morphological and molecular features during
development (26), it was
hypothesized that FAM20C may serve a role in regulating the
development and function of salivary glands.
In the present study, Fam20cf/f
mice were bred with mouse mammary tumor virus (Mmtv)-Cre mice that
predominantly express the Cre recombinase in the striated ductal
cells of the salivary glands, the mammary glands and the granular
convoluted tubule (GCT) cells of the SMG (27-29). Fam20cf/f;
Mmtv-Cre mice were generated in which Fam20c was
specifically ablated in the mammary glands and salivary glands to
assess the biological roles of FAM20C in the postnatal development
and function of salivary glands.
Materials and methods
Ethics statement
All animal procedures in this study were approved by
the Institutional Animal Care and Use Committee of Harbin Medical
University (Harbin, China; approved protocol nos. SYDW2018-046) and
performed in strict accordance with the National Institute of
Health Guide for the Care and Use of Laboratory Animals.
Generation of Fam20cf/f;
Mmtv-Cre mice
To generate Fam20c salivary gland conditional
knockout mice, 4 Fam20cf/f mouse (Department of
Biomedical Sciences, Texas A&M University College of Dentistry,
Dallas, TX 75246, USA; age, 1 month) were first mated with 4
Mmtv-Cre mouse (Shanghai Biomodel Organisms Center Co.,
Ltd., Shanghai, China; age 8 weeks) and crossed the offspring of 20
Fam20cf/+; Mmtv-Cre mouse with 20
Fam20cf/f mice to obtain 33
Fam20cf/f; Mmtv-Cre conditional knockout
(cKO) mice, which were salivary gland conditional knockout mice.
Postnatal days 0 mice (1 g) were selected as the starting point of
observation, and the 5-day- (5 g) and 8-week-old (40 g) mice were
selected to evaluate the progression of salivary defects in the cKO
mice. 5 female Fam20cf/f and
Fam20cf/f; Mmtv-Cre mice and 6 male
Fam20cf/f and Fam20cf/f;
Mmtv-Cre mice were analyzed for each age group. The mice
were housed in a specific-pathogen free laboratory animal facility
with 20-23°C, 40-60% humidity and a 12-h light/dark cycle. Standard
laboratory chow and water were supplied ad libitum.
Genotyping was performed by polymerase chain reaction (PCR)
analyses using DNA extracted from mouse tails. The primer sequences
and genotyping protocols used to confirm the presence of
Fam20c null alleles in the salivary glands of the
conditional knockout mice were reported previously (30). Fam20cf/f
littermates of the Fam20cf/f; Mmtv-Cre cKO
mice were used as normal control mice (Ctrl mice); this procedure
not only reduced the number of animals needed but also prevented
the potential confounding effects of individual differences when
comparing mice from different litters. The primer sequences
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) used
are presented in Table I.
 | Table IList of primers for the genotyping
and reverse transcription-polymerase chain reaction. |
Table I
List of primers for the genotyping
and reverse transcription-polymerase chain reaction.
| Gene | Forward
primers | Reverse
primers |
|---|
| Floxed allele | a:
TCCAGCTTGCTAGGGCTCTGACC | b:
CTATGTCCAACGGCCGCAGCTT |
| c:
GTCCTGAGGGCTGACCCAAGACTA | |
| Mmtv-Cre
transgene |
AGCGATGGATTTCCGTCTCTGG |
AGCTTGCATGATCTCCGGTATTGAA |
| Cre-loxP
recombination |
GTGGTCTCTGCCGCTGATGTACC |
TTTGGGAGCCTATGTCCAACGGCC |
| Bmp2 |
TGACTGGATCGTGGCACCTC C |
AGAGTCTGCACTATGGCATGGTTA |
| Bmp4 |
ACAATGTGACACGGTGGGAAAC |
TGTGGGTGATGCTTGGGACTAC |
| Bmp7 |
ACATCCGGGAGCGATTTGAC |
TCCTCAGAAGCCCAGATGGTC |
| β-actin |
AGAGGGAAATCGTGCGTGAC C |
TCGTTGCCAATAGTGATGACC |
Histological analysis and calculation of
the cross-sectional area
Salivary glands were fixed in 4% paraformaldehyde
overnight at 4°C and embedded in paraffin. Sections (4 µm)
were prepared for hematoxylin and eosin (H&E) staining,
periodic acid-Schiff (PAS) staining, and immunohistochemical
analyses. Subsequently, 3 stained sections were randomly selected
for each gland from 5 males and 5 females at each age. Images from
mouse salivary gland sections were captured by a digital camera
installed on an inverted light microscope (Nikon Eclipse Ti-E;
Nikon Corporation, Tokyo, Japan). The ductal cross-sectional area
was calculated using image analysis software (Image-Pro Plus,
version 7.0; Media Cybernetics, Inc., Bethesda, MD, USA). The
insubstantial region of the gland was automatically omitted from
all cross-sectional area calculations and the ducts were manually
encircled. The acinar cross-sectional area was then estimated by
subtracting the duct cross-sectional area from the gland
cross-sectional area.
Electron microscope analysis
The dissected tissues were fixed in 2.5%
glutaraldehyde overnight at 4°C and post-fixed with 1% osmium
tetroxide at 4°C for 3 h. Following dehydration and embedding in
Spurr resin (Shanghai GenMed, Co., Ltd., Shanghai, China), the
samples were cut into 70-nm-thick sections using an ultramicrotome
(Leica Ultracut R; Leica Microsystems GmbH, Wetzlar, Germany).
Ultrathin sections were double-stained with uranyl acetate and lead
citrate at room temperature for 10 min each. The samples were
examined with a transmission electron microscope (HITACHI H-7650;
Hitachi, Ltd., Tokyo, Japan).
Immunohistochemical staining
For immunohistochemical staining, paraffin sections
were treated with 3% H2O2 to block endogenous
peroxidase activity. Sodium citrate heat-induced (about 120°C)
antigen retrieval was used for all specimens except cytokeratin 7
antigen, which was retrieved by EDTA buffer. The sections were
washed three times with PBS plus 0.1% Tween, (Beyotime Institute of
Biotechnology, Shanghai, China) for 5 min each. To avoid
nonspecific immunoreactions, the sections were incubated with 10%
normal goat or rabbit serum (Beyotime Institute of Biotechnology)
and 3% bovine serum albumin (BSA; Beyotime Institute of
Biotechnology), followed by overnight incubation at 4°C with
primary antibodies and incubation at room temperature for 1 h with
biotinylated secondary antibodies. All antibodies are presented in
Table II. Immunopositive
reactions were visualized using 3,3′-diaminobenzidine
tetrahydrochloride solution. Sections were counterstained with
hematoxylin at room temperature for 1 min and the expression of
target proteins was detected by the antibodies presented in
Table II. Negative controls were
included for all target proteins. The negative control group in
which the primary antibody was replaced with PBS did not
demonstrate any positive reactions. Images were captured using an
inverted light microscope and analyzed using image analysis
software (Image-Pro Plus, version 7.0; Media Cybernetics, Inc.,
Bethesda, MD, USA). The insubstantial region of the gland was
automatically omitted. The integrated option density (IOD) values
were counted and statistically analyzed using Prism 3.0 (GraphPad
Software, Inc., La Jolla, CA, USA).
 | Table IIList of antibodies. |
Table II
List of antibodies.
| Antibody | Cat. no.,
manufacturer | Source | Dilution (IHC) | Dilution (WB) | Dilution (IF) |
|---|
| FAM20C | ab107079,
Abcam | Rabbit | 1:400 | | 1:200 |
| FAM20C | AV49490,
Sigma-Aldrich | Rabbit | | 1:1,000 | |
| AQP5 | ab78486, Abcam | Rabbit | 1:200 | | |
| Cytokeratin 7 | ab181598,
Abcam | Rabbit | 1:8,000 | | |
| α-Amylase | 3796s, CST | Rabbit | | 1:1,000 | |
| β-NGF | ab6199, Abcam | Rabbit | 1:500 | | |
| Bmp2 | 18933,
Proteintech | Rabbit | | 1:500 | |
| Bmp4 | ab39973, Abcam | Rabbit | 1:200 | 1:1,000 | |
| Bmp7 | ab56023, Abcam | Rabbit | | 1:1,000 | |
| panSmad1/5/9 | ab66737, Abcam | Rabbit | 1:200 | 1:1,000 | |
| p-Smad1/5/9 | 13820, CST | Rabbit | 1:50 | 1:500 | |
| pan-Erk1/2 | 4695, CST | Rabbit | 1:200 | 1:1,000 | |
| p-Erk1/2 | 4370, CST | Rabbit | 1:50 | 1:500 | |
| pan-P38 | 8690, CST | Rabbit | 1:200 | 1:1,000 | |
| p-P38 | 4511, CST | Rabbit | 1:50 | 1:500 | |
| β-actin | 4970T, CST | Rabbit | | 1:1,000 | |
Immunofluorescence (IF) microscopy
Frozen salivary gland tissue sections were fixed
with cold acetone at 4°C for 10 min and blocked in PBS (pH 7.4)
containing 5% BSA for 20 min at room temperature. The sections were
incubated with a primary antibody specific for FAM20C (cat. no.
ab107079; Abcam, Cambridge, UK) overnight at 4°C, followed by
incubation with tetramethylrhodamine-conjugated secondary
antibodies (Zhongshan Jinqiao Biological Technology, Co., Ltd.,
Beijing, China) at room temperature for 1.5 h and the nuclei were
stained with 4′,6-diamidino-2-phenylindole (Invitrogen; Thermo
Fisher Scientific, Inc.) at room temperature for 5 min. The slides
were examined and photographed with a fluorescence microscope
(Nikon E800; Nikon Corporation) equipped with a digital camera
(1200F; Nikon Corporation) and image acquisition software (ACT-1;
Nikon Corporation). Antibody details can be found in Table II.
PCR and primers
Quantitative PCR (qPCR) was performed to evaluate
the alterations in gene expression in Fam20c conditional
knockout mice. Total RNA was extracted from the salivary glands
with TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. The RNA concentrations were
measured by a Nanovue spectrophotometer (GE Healthcare Life
Sciences, Marlborough, MA, USA) and converted into cDNA using a
real-time SYBR Premix Ex Taq™ kit (Takara Bio Inc., Otsu, Japan) on
an MxPro-Mx3000P real-time PCR system (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA). The reverse
transcription conditions were as follows: 37°C for 15 min followed
by 85°C for 5 sec. qPCR conditions were as follows: 95°C for 2 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 30 sec. The
primer sequences (Invitrogen; Thermo Fisher Scientific, Inc.) for
the genes used in this study are listed in Table I. β-actin was used as an internal
standard to calculate relative gene expression levels with the
2−ΔΔcq method (31).
Western immunoblotting (WB)
Total protein was extracted from the salivary glands
by using cold radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology), that contained Benzonase nuclease,
phenylmethylsulfonyl fluoride, a protease inhibitor cocktail and a
phosphatase inhibitor. The protein concentration was determined
with a bicinchoninic acid (BCA) protein assay (Beyotime Institute
of Biotechnology). A total of 40 µg of sample from mouse
salivary glands was separated by SDS-PAGE (10-12%) and transferred
to polyvinylidene difluoride membranes (EMD Millipore, Bedford, MA,
USA). Following blocking with 5% nonfat dry milk (Beyotime
Institute of Biotechnology) at room temperature for 1 h, the
membranes containing significant proteins were incubated with
primary antibodies at 4°C overnight. All antibodies used in this
study are listed in Table II.
Subsequently, the membranes were incubated with a horseradish
peroxidase-conjugated antibody (Zhongshan Jinqiao Biological
Technology, Co., Ltd., Beijing, China) for 1.5 h at room
temperature, followed by detection with an enhanced
chemiluminescence kit (Biosharp, Hefei, China) according to the
manufacturer's protocol. The immunoreactive bands were captured
with SmartChemi™ I (Beijing Sage Creation Science, Co., Beijing,
China). The band density was determined using ImageJ 1.46r
(National Institutes of Health, Bethesda, MA, USA) and normalized
to β-actin. Each experiment was repeated three times.
Collection and compositional analysis of
saliva
The mice were weighed and injected intraperitoneally
with carbachol (0.25 mg/kg of body weight) and were then injected
intraperitoneally with pilocarpine (10 mg/kg of body weight)
(Aladdin Shanghai Biochemical Technology Co., Ltd., Shanghai,
China; P129614) as previously described (32). A total of 2 min following
pilocarpine injection, the total saliva was collected in calibrated
glass capillary tubes on ice at intervals of 5, 10 and 15 min and
injected into pre-weighed tubes on ice; the collected saliva was
then placed into pre-weighed tubes and stored at −80°C. The flow
rate was calculated as µg of saliva per normalized body
weight. The total protein concentration of saliva was determined by
a BCA assay (Beyotime Institute of Biotechnology). The
concentration of Na+ and K+ in the saliva was
analyzed by an ion chromatography system (DIONEX, ICS-3000; Thermo
Fisher Scientific, Inc.), and Cl− activity was measured
with an optical emission spectrometer (Optima, 5300DV; Optima,
Inc., Tokyo, Japan).
Measurement of circulating androgen
levels
Serum testosterone was measured by the ELISA method
with a testosterone parameter assay kit (R&D Systems,
Minneapolis, MN, USA; cat. no. SKGE010) according to the
manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical testing was performed
using Prism 3.0 (GraphPad Software, Inc., La Jolla, CA, USA). All
statistical analyses were conducted by one-way analysis of variance
followed by Tukey's test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Verification of FAM20C expression and
inactivation in mouse salivary glands
Genotyping PCR was performed with genomic DNA
extracted from mouse tails. Using forward primer a and reverse
primer b (Table I), the wild-type
allele gave rise to a fragment of 500 bp, while a 400 bp fragment
was generated from the floxed allele. The Mmtv-Cre
recombinase gene gave rise to a 272 bp band; the conditional cKO
mouse contained the Mmtv-Cre allele (reflected by the
presence of the 272 bp PCR product) and the recombined allele that
gave rise to a PCR fragment of 260 bp when forward primer a and
reverse primer c (Table I) were
employed for PCR analyses (Fig.
1A).
 | Figure 1Verification of FAM20C expression and
inactivation. (A) Genomic DNA was extracted from the tails of mice
of each genotype and genotyping was performed with specific primers
for the floxed Fam20c allele, the recombined Fam20c
allele and the Mmtv-Cre allele. (B) Immunofluorescence assay
of FAM20C in the SMG. The SMG from control littermates exhibited
strong staining. FAM20C was expressed in the cytoplasm of the ID,
SD and ED cells (long arrows) and the GCT cells (short arrows); in
contrast, the cells from cKO mice did not stain. (C)
Immunohistochemistry of FAM20C in the SMG, PG and SLG. In the SMG
of control mice, FAM20C was expressed in the cytoplasm of the ID,
SD and ED cells (long arrows) and the GCT cells (short arrows). In
the PG and SLG of control mice, the immunostaining signal for
FAM20C was also observed in the ductal cells, whereas the cKO mice
did not stain. For images of Fam20cf/f or
Fam20cf/f; Mmtv-cre mice, the second panel
presents magnified images of the outlined areas from the
corresponding panel. Fam20C, family with sequence similarity
20-member C; PG, parotid gland; SLG, sublingual gland; SMG,
submandibular gland; GCT, granular convoluted tubule; ID,
intercalated duct; SD, striated duct; ED, excretory duct; cKO,
Fam20cf/f; Mmtv-Cre conditional
knockout. |
Immunohistochemistry (IHC) and IF staining were used
to determine the expression and distribution of FAM20C in the
salivary glands of normal control mice and to assess whether FAM20C
was absent in cKO mice (Fig. 1B and
C). Previous studies (12,17,30,33) demonstrated that FAM20C was
expressed in mineralized tissues. However, the expression and
distribution of FAM20C in salivary glands have not been studied. In
the present study the expression of FAM20C in the submandibular
gland (SMG), parotid gland (PG) and sublingual gland (SLG) was
identified. In the SMG of the control mice, FAM20C was expressed in
the cytoplasm of cells of the intercalated duct (ID), striated duct
(SD) and excretory duct (ED), but granular convoluted tubule (GCT)
cells, which are situated between the striated and IDs, expressed a
much higher level of FAM20C. In the PG and SLG, the immunostaining
signal for FAM20C was also identified in the ductal cells.
Furthermore, the signal for FAM20C was not observed in the
corresponding components in the cKO mice. The negative staining
demonstrated that FAM20C was effectively nullified in the salivary
glands of the cKO mice (Fig.
1C).
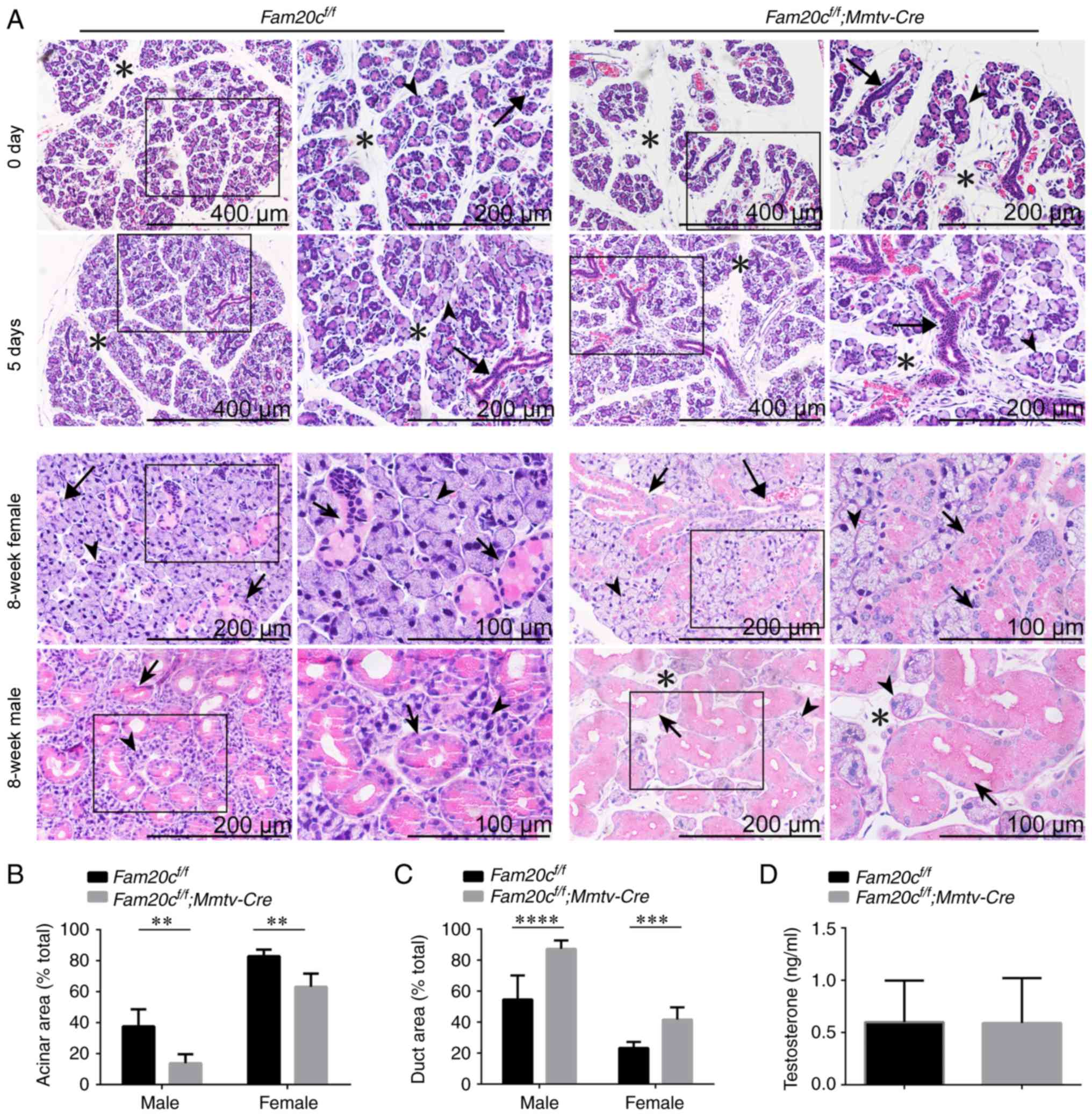
Conditional inactivation of Fam20c leads
to morphological and structural alterations in the salivary
glands
H&E staining was performed to determine whether
the conditional inactivation of Fam20c leads to
morphological and structural alterations in the salivary glands.
The tissues were processed at almost the same level. At postnatal
days 0 and 5, when there was no sex difference in the SMG and the
GCTs did not appear, the SMG of normal control mice was composed of
multiple lobules separated by thin septa and consisted of acinar
cells and few ductal structures. However, the SMG of cKO mice
contained smaller lobules, prominent ductal structures and more
mesenchymal tissue. The number of ducts was also increased in the
cKO mice (Fig. 2A). At 8 weeks
following birth, the GCTs in the SMG of male mice were more
abundant than the GCTs in female mice due to the effects of
androgens (34). In the SMG of
8-week-old mice, the cross-sectional area occupied by acinar cells
in cKO mice was significantly decreased (P<0.01) and the
mesenchyme was increased. The SMG of normal male mice was composed
of ~40% acinar areas; however, the acinar cross-sectional area in
the SMG of male cKO mice covered ~20% of the gland. The SMG of
normal female mice was composed of ~80% acinar areas; however, the
acinar cross-sectional area in the SMG of female cKO mice covered
~60% of the gland (Fig. 2A and
B). In contrast, ductal structures were more prominent and
ductal cross-sectional areas were increased in the SMGs of male and
female cKO mice. The SMG of normal male mice was composed of ~30%
duct areas; however, the ductal cross-sectional area in the SMG of
male cKO mice covered ~85% of the gland. The SMG of normal female
mice was composed of ~20% duct areas; however, the ductal
cross-sectional area in the SMG of female cKO mice covered ~40% of
the gland (Fig. 2A and C). In
addition to the alterations in the ratio of ductal cells to acinar
cells, the diameter of the GCTs became markedly larger and the
cells were swollen with acidophilic secretion products in the SMG
of cKO mice. The testosterone levels in the serum were examined to
demonstrate that these morphological alterations could not be
attributed to androgen changes. There were no significant
alterations in serum testosterone levels between the cKO and
control mice, indicating that the expanded GCTs are not associated
with altered androgens (Fig.
2D).
There was also increased mesenchymal tissue present
within the mutant PG, but the density of the ducts did not change
appreciably (Fig. 3A). While the
SMG exhibited apparent morphological altertions in the cKO mice,
the SLG was histologically normal (Fig. 3A). PAS staining was used to
observe whether FAM20C affects mucin synthesis in the salivary
glands. Mucin production by the SLG and PG was not markedly
different between the cKO and control mice (Fig. 3B).
To further confirm the morphological alterations in
the cKO mice, the protein expression of several functional markers
of acinar cells and duct cells in the salivary tissues was
examined. To prove that acinar differentiation was reduced in the
mutant SMG, immunohistochemical staining was performed for
aquaporin 5 (AQP5), a functional marker of acinar cells. In the SMG
of the control mice, AQP5 was expressed in the apical pole of
acinar cells and the localization of this expression was normal in
the mutant SMG (Fig. 4A). The
protein expression of AQP5 was significantly decreased due to the
reduced number of acinar cells in cKO mice (P<0.001; Fig. 4A and B). Cytokeratin 7 (KRT7) is a
major marker of ductal cells. The expression of KRT7 was examined
to assess the differentiation of ducts. In the SMG of the control
mice and cKO mice, KRT7 was expressed in the membrane and
cytoplasmic of GCT cells (Fig.
4A). In accordance with the increased ducts in cKO mice, the
expression of KRT7 were significantly increased in the SMG of cKO
mice (P<0.001; Fig. 4A and
C).
 | Figure 4Expression of AQP5, KER7, β-NGF and
SAA in the SMG. (A) Immunohistochemistry of AQP5, KER7 and β-NGF
(duct cells: Arrows; acinar cells: Arrowheads). Semiquantitative
immunohistochemical analysis of (B) AQP5, (C) KER7 and (D) β-NGF.
In cKO mice, the expression of AQP5 and β-NGF was decreased
significantly compared with the control mice.
***P<0.001. The expression of KER7 in cKO mice
increased compared with the control mice. ***P<0.001.
(E) The level of SAA protein was analyzed by western blotting. The
expression of SAA was decreased in cKO mice compared with control
mice. (F) Densitometry analysis of the western blots is presented.
*P<0.05. Error bars represent the standard deviation
(n=3). SAA, α-amylase; AQP5, aquaporin 5, KER7, cytokeratin 7;
β-NGF, β nerve growth factor; cKO, Fam20cf/f;
Mmtv-Cre conditional knockout. |
Fam20c deficiency resulted in defective
GCT maturation
Nerve growth factor (β-NGF) is a highly specific
marker of GCT cells. The expression of β-NGF was examined to assess
the development of GCTs. In the Fam20cf/f mice,
high-level expression of β-NGF was identified in the cytoplasm of
GCT cells (Fig. 4A). However, the
expression of β-NGF was noticeably reduced in cKO mice (Fig. 4A and D).
Amylase is another highly specific marker of GCT
cells. The expression of α-amylase (SAA) was examined in the SMG to
assess the secretion function of GCT cells. The expression of SAA
was noticeably reduced in the salivary gland tissues of cKO mice
compared to that in the normal control mice. Representative bands,
and histograms of the relative protein expression level are
displayed in Fig. 4E and F.
To further determine whether Fam20c
deficiency affected the secretory function of GCT cells, the cell
structure of the GCT cells was examined by transmission electron
microscopy (TEM). The apical region of the GCT cells was filled
with rounded and uniformly dense secretory granules. The TEM images
demonstrated that very small secretory granules with aberrant
morphology accumulated in the GCT cells of cKO mice; in comparison,
the granules in the GCT cells of the control mice were of normal
size, indicating that the GCT cells were at an immature stage of
development in the cKO mice (Fig.
5A). Additionally, in the cKO mice, certain GCT cells appeared
to have entered the apoptotic process, as reflected by the presence
of endoplasmic reticulum expansion and nuclear condensation. In
accordance with these morphological alterations, the mesenchyme was
increased in the SMG of cKO mice compared with that in the normal
control mice (Fig. 5B).
Saliva from Fam20c-deficient mice
exhibits an abnormal flow rate and composition
To determine whether the morphological alterations
observed in the cKO mice resulted in dysfunction of the salivary
gland, the flow rate and electrolyte composition of saliva were
measured. Saliva is mainly produced in the acini and the
electrolytes secreted by the acini are resorbed in the duct
(35). In accordance with the
morphological changes that indicated the presence of fewer acinar
cells, the volume of saliva obtained from the male and female cKO
mice was significantly decreased compared with that obtained from
the control mice (P<0.01; Fig.
6A). In addition, the concentration of total protein in the
saliva from cKO mice was significantly increased compared with in
the saliva from the control mice (P<0.001; Fig. 6B). Analyses of the ion
concentrations in the saliva demonstrated that the Na+,
Cl− and K+ concentrations in the saliva were
significantly increased in the cKO mice compared with in the
control mice (P<0.01; Fig.
6C-E). These results indicated that knockout of Fam20c
influenced the secretory function of the salivary glands.
Conditional knockout of Fam20c leads to
an altered BMP4 distribution pattern and attenuates BMP
signaling
As a family of secreted proteins, BMPs, especially
BMP4, have a typical S-x-E/S motif and are predicted to be
substrates of FAM20C (16). IHC
and WB were performed to determine whether the expression of BMP2,
BMP4, and BMP7 and the activity of canonical and noncanonical
signaling pathways were altered in the salivary glands of the cKO
mice. In Fam20cf/f mice, BMP4 was localized to
the cytoplasm of ID, SD and ED cells, particularly GCT cells, and
was widely distributed in the ECM in the SMG. However, in cKO mice,
BMP4 accumulated in the GCT cells but was nearly undetectable in
the ECM (Fig. 7A). These results
demonstrated that the conditional inactivation of Fam20c
resulted in an altered BMP4 distribution pattern. In the SMG of the
normal control and cKO mice, pan-mothers against decapentaplegic
homolog 9 (Smad)1/5/9, p-Smad1/5/9, pan-extracellular signal
regulated kinase (ERK) and pan-p38 were mainly localized to the
cytoplasm of the duct cells, and phosphorylated (p)-Erk and p-p38
was expressed in the nucleus of the duct cells (Fig. 7B and C). In the PG and SLG of the
normal control and cKO mice, BMP4 was also localized to the
cytoplasm of duct cells, and the expression was downregulated in
the cKO mice (Fig. 8A and B). The
expression and location of BMP signaling pathway members in the PG
and SLG were the same as in the SMG (Fig. 8C-F). In addition, WB revealed that
Fam20c deficiency significantly increased BMP2 and BMP7
expression and decreased BMP4 expression (P<0.05; Fig. 9A). Notably, it was observed that
although the transcription of Bmp4 did not change, the
transcript levels of Bmp2 and Bmp7 were significantly
increased (P<0.05; Fig. 9B),
which implied that phosphorylation may be important for the
activity of BMP ligands. The results of WB were consistent with
IHC. The expression of both p-Smad1/5/9 and pan-Smad1/5/9
significantly decreased in cKO mice (P<0.05), but the ratio of
p-Smad1/5/9 to pan-Smad1/5/9 did not alter significantly between
Fam20cf/f mice and Fam20cf/f;
Mmtv-Cre mice (Fig. 9C).
The expression of pan-ERK was not different between the control
mice and cKO mice, but the p-Erk/pan-Erk ratio was significantly
decreased in the cKO mice due to the reduction in p-Erk (P<0.05;
Fig. 9D). Despite the
downregulation of both pan-p38 and p-p38, the p-p38/pan-p38 ratio
increased markedly in the cKO mice (Fig. 9E). These results implied that
Fam20c deficiency altered the activity of the canonical and
noncanonical BMP signaling pathways.
 | Figure 7Conditional knockout of Fam20c
leads to an altered BMP4 distribution pattern and altered BMP
expression in the SMG. (A) IHC of BMP4 in the SMG. In control mice,
BMP4 was localized in the cytoplasm of ducts cells (black arrows)
and ECM (arrowheads). In the cKO mice, BMP4 was restricted to ducts
(black arrows). For images of Fam20cf/f or
Fam20cf/f; Mmtv-cre mice, the second panel
presents magnified images of the outlined areas from the
corresponding panel. IHC of BMP signaling pathways components in
the SMG. IHC reaction to (B) pan-Smad1/5/9, (C) p-Smad1/5/9,
pan-ERK and pan-p38 were detected in the cytoplasm of duct cells
(black arrows) while the signals against p-Erk and p-p38 were
observed in the nucleus (red arrows). IHC, immunohistochemistry;
BMP, bone morphorgenic protein; Smad, mothers against
decapentaplegic homolog 9; p-, phosphorylated; ERK, extracellular
signal regulated kinase; Fam20C, cKO, Fam20cf/f;
Mmtv-Cre conditional knockout; family with sequence
similarity 20-member C. |
 | Figure 8Conditional knockout of Fam20c
leads to alterations in the distribution pattern of BMP4 and the
expression of BMP signaling pathway components in the PG and SLG
IHC of BMP4 in the (A) PG and the (B) SLG. In control mice, BMP4
was localized to the cytoplasm of duct cells (black arrows). For
images of Fam20cf/f or
Fam20cf/f; Mmtv-cre mice, the second panel
exhibits magnified images of the outlined areas from the
corresponding panel. IHC of BMP signaling pathways in the PG and
the SLG. (C) IHC of pan-ERK and pan-p38 in the PG. (D) IHC of p-ERK
and p-p38 in the PG. (E) IHC of pan-ERK and pan-p38 in the SLG. (F)
IHC of p-ERK and p-p38 in the SLG. IHC reaction to pan-ERK and
pan-p38 were detected to the cytoplasm of duct cells (black
arrows), however the p-Erk and p-p38 were observed in the PG (red
arrows) but not in the SLG (red arrows). IHC, immunohistochemistry;
BMP, bone morphorgenic protein; p-, phosphorylated; ERK,
extracellular signal regulated kinase; PG, parotid gland; SLG,
sublingual gland; Fam20C, family with sequence similarity 20-member
C. |
 | Figure 9The canonical and noncanonical BMP
signaling pathways are attenuated in the SMG of cKO mice. (A)
Canonical and noncanonical BMP signaling pathways components were
examined by western blotting with antibodies against BMP2, BMP4 and
BMP7. (B) Comparison of the relative mRNA expression levels of
Bmp2, Bmp4 and Bmp7 between normal control
mice and cKO mice. *P<0.05. (C) Canonical and
noncanonical BMP signaling pathways components were examined by
western blotting, with antibodies against pan-Smad1/5/9 and
p-Smad1/5/9, with antibodies against (D) pan-Erk and (E) p-Erk, and
with antibodies against pan-p38 and p-p38. β-actin was used as the
internal control. *P<0.05 vs. the
Fam20cf/f mice and the error bars represent the
standard deviation (n=3). BMP, bone morphogenic protein; Smad,
mothers against decapentaplegic homolog 9; p-, phosphorylated; ERK,
extracellular signal regulated kinase; cKO,
Fam20cf/f; Mmtv-Cre conditional
knockout. |
Discussion
FAM20C is expressed in multiple tissues, including
mineralized and nonmineralized tissues and bodily fluids (21,22,33). FAM20C can phosphorylate >100
secreted proteins; the broad substrate spectrum and ubiquitous
distribution of FAM20C indicate that in addition to its role in
biomineralization, this kinase may serve roles in a number of other
biological functions (16).
Therefore, it is necessary to eliminate Fam20c specifically
in the salivary glands to investigate the biological effects of
this enzyme on the development and function of the salivary gland.
In the present study, Mmtv-Cre mice were used, in which Cre
activity was restricted to the ductal cells of the salivary gland
at an early embryonic stage (29), to prevent the expression of
Fam20c in the salivary gland.
In this study, the distribution of FAM20C was
assessed in the salivary glands and demonstrated that FAM20C serves
an important role in the formation and maturation of the salivary
gland ducts. Phenotypic analysis by histological staining
demonstrated that more mesenchymal tissue and smaller lobules were
present within the mutant salivary glands, and the proportion of
duct to acinar cells was altered by the inactivation of
Fam20c at 0, 5 days and 8 weeks following birth. This result
is highly suggestive of a branching defect during embryogenesis
that leads to the formation of fewer epithelial end buds and
ultimately fewer secretory acini. The induction of duct
differentiation and inhibition of acinar differentiation were
further supported by the expression alterations in AQP5 and KRT7.
AQP5 is a water channel protein, which serves a major role in
regulating the saliva fluid secretion. The production level of AQP5
indicates defective acinar cell function (35,36). The reduced expression of AQP5 in
cKO mice was the result of inhibition of acinar differentiation.
KRT7 is expressed strongly in the ducts of the SMG and a number of
other glandular tissues from E14 (37,38). Fam20c deficiency in
salivary glands increased the expression of KRT7 indicated that
FAM20C promoted induction of duct differentiation. Theoretically,
the development of GCT cells depends on androgen signaling and the
GCTs are much larger in the SMG in males (34). However, in the present study,
although the GCT duct cells were larger in the cKO mice, the
concentration of serum testosterone was not different between the
control and cKO mice. In male and female adult cKO mice, along with
the morphological alterations of GCTs, the cross-sectional areas of
the duct cells were increased, without sex-associated differences.
Therefore, the morphological alterations were not attributed to
androgen signaling but rather to the role of FAM20C.
Although FAM20C was expressed only in the ducts of
salivary glands in adults, knockout of FAM20C promoted ductal
differentiation and restricted acinar differentiation. In the
salivary glands, morphological differentiation of most acinar and
ductal cells occurred at E17 and continued following birth until
puberty (39-41). The expression of
Mmtv-Cre can already be observed prior to E11.5 and
is clearly visible at E13.5 and E15.5 (27). The mRNA and protein of
Fam20c were detected in the teeth and at ossification sites
in the head at E14.5 and were also expressed in the cerebral cortex
and cranial nerve ganglia in E15.5 and E16.5 embryos (21). All these previous studies provided
clues that FAM20C may modulate salivary acinar and ductal
differentiation during early embryonic development. Therefore,
although FAM20C was expressed only in the ducts following birth,
the proportion of duct cells to acinar cells was affected by the
knockout of FAM20C in the salivary gland. The PG and SLG were
histologically normal in the cKO mice, implying that the SMG may
simply be more sensitive to Fam20c deficiency during
morphogenesis than the PG and SLG.
GCT epithelial cells have numerous secretory
granules containing various bioactive peptides, including β-NGF
(42), whose expression level
parallels the maturation of GCTs (34,37,43). Amylase is an important digestive
enzyme for polysaccharides and it is produced by acinar cells of
the PG, serous demilune cells of the SLG, and GCT cells of the male
SMG (44-46). Previous studies (47,48) demonstrated that SAA activity in
mouse SMG homogenates increased following puberty, paralleling the
development of GCT cells, which leads to sex-biased levels in
adulthood. In the present study, although the number and
cross-sectional area of ducts increased in the Fam20c-null
mice, the expression of β-NGF and SAA was noticeably reduced
suggesting the abnormal function of the GCT duct cells. In
addition, TEM revealed the accumulation of very small secretory
granules with aberrant morphology in the Fam20c-deficient
GCT cells, suggesting that FAM20C may participate in regulating the
biogenesis and secretion of secretory granules and may be involved
in the highly specific steps of secretory organelle maturation
(49), considering that the Golgi
participates in the biogenesis of secretory granules (50). FAM20C phosphorylates secretory
proteins within the consensus sequence S-x-E/pS, which is present
in BMP2, BMP4 and BMP7; therefore, BMPs may be the substrates for
FAM20C. In the present study, the loss of Fam20c resulted in
the abnormal expression of BMP4; BMP4 was not observed in the
mesenchyme. Secreted proteins are stored at high concentrations in
dense-core secretory granules, which can be released in response to
external signals (51) and are
important for the transport of secreted proteins. Whether
abnormally expressed BMP4 accumulated in the cytoplasm of GCT cells
due to dysregulated transport of the immature secretory granules or
to structural alterations in proteins that cannot be phosphorylated
is unclear. Therefore, future studies are needed to demonstrate the
specific effect of FAM20C on the transport of secretory
proteins.
The normal function of salivary glands requires an
adequate area of salivary gland acini that function
effectively.
The abnormal morphology of the cKO salivary glands,
with reduced acinar cell differentiation, was confirmed by the
reduced expression of AQP5. However, these acinar cells appeared to
be perfectly formed and not atrophic. This finding suggests that
the secretory function of acinar cells is normal in the cKO mice,
but owing to the smaller number of acinar cells, less saliva is
secreted. The alterations in the electrolyte composition of the
saliva suggest that the altered physiological function of the ducts
may result from ductal immaturity.
Past studies (6,10,52) on the salivary glands focused
mostly on branching development and studies on the regulation of
the acini to duct ratio were limited. FAM20C is a phosphorylated
protein kinase that specifically recognizes S-x-E/pS motifs. BMPs
contain multiple phosphorylated S-x-E/S motifs, including BMP2
(46SDE48, 117SLE119 and
147SAE149), BMP4
(50SHE52, 91SGE93 and
155SAE157), and BMP7
(48SQE50, 219SEE221 and
248SVE250), implying that FAM20C may
phosphorylate BMPs. Analysis of gene expression associated with
salivary gland morphogenesis indicated that BMPs serve important
roles during embryonic SMG morphogenesis. It was reported that
compared with SMGs from Bmp7+/+ mice, SMGs from
Bmp7−/− mice exhibit a disordered mesenchyme and
markedly fewer ducts; the effect of BMP4 on the salivary glands is
opposite to that of BMP7; BMP4 inhibits the size and number of
buds, as well as further branching (9). In the present study, it was
demonstrated that FAM20C deficiency altered the protein level
and/or distribution of BMP2, BMP4 and BMP7 but did not alter the
mRNA level of Bmp4. Furthermore, BMP signaling pathways were
attenuated in the salivary gland of cKO mice. FAM20C deficiency
resulted in the attenuated phosphorylation of Smad1/5/9, Erk and
p38 in the salivary glands, implying that FAM20C positively
regulates BMP signaling via the phosphorylation of BMP ligands.
Another study from the authors' group (not yet published)
demonstrated that the number of salivary gland ducts increased
significantly in
Bmp2f/f;Bmp4f/f;K14-Cre mice. The
similar morphological changes in the salivary glands from
Fam20C cKO mice and Bmp2;Bmp4 double-cKO mice
indicates that FAM20C may affect the development of salivary glands
via the BMP signaling pathway. Future studies are warranted to
validate the hypothesis that FAM20C inhibits duct formation by
regulating BMP signaling.
In conclusion, the results of the present study
demonstrated that FAM20C may be a key regulator of acinar and duct
structure and duct maturation; therefore, the results augment
existing information about the biological roles of FAM20C,
establish a possible link between FAM20C and the transport of
secreted proteins and provide a novel avenue for investigating new
therapeutic targets for oral diseases including xerostomia.
Funding
The present study was supported by the Postgraduate
Research Innovation Fund of Harbin Medical University (grant no.
YJSCX2017-61HYD), the National Natural Science Foundation of China
(grant nos. 81870736, 81801040 and 81600848), the Special
Foundation for Sino-Russian Translational Medicine Research Center
of Harbin Medical University (grant nos. CR201412 and CR201504),
and the Natural Science Foundation of Heilongjiang Province of
China (grant no. H2015103), Science Foundation of the Second
Affiliated Hospital of Harbin Medical University (grant no.
CX2016-20).
Availability of data and materials
All data generated or analyzed during this study
are included in the published article.
Authors' contributions
BZ, YL and CQ conceived and designed the
experiments. NM, YZ, YX, HY, XX and SG performed the experiments.
NM, SM, FL, HM and MY analyzed the data. NM and CQ wrote the paper.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures used in this study were
approved by the Institutional Animal Care and Use Committee of
Harbin Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Fox PC: Autoimmune diseases and Sjogren's
syndrome: An autoimmune exocrinopathy. Ann NY Acad Sci. 1098:15–21.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shiboski CH, Hodgson TA, Ship JA and
Schiødt M: Management of salivary hypofunction during and after
radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
103(Suppl): S66.e61–e19. 2007. View Article : Google Scholar
|
|
3
|
Nederfors T: Xerostomia and
hyposalivation. Adv Dent Res. 14:48–56. 2000. View Article : Google Scholar
|
|
4
|
Napeñas JJ, Brennan MT and Fox PC:
Diagnosis and treatment of xerostomia (dry mouth). Odontology.
97:76–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tucker AS: Salivary gland development.
Semin Cell Dev Biol. 18:237–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patel VN, Rebustini IT and Hoffman MP:
Salivary gland branching morphogenesis. Differentiation.
74:349–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harunaga J, Hsu JC and Yamada KM: Dynamics
of salivary gland morphogenesis. J Dent Res. 90:1070–1077. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heine U, Munoz EF, Flanders KC,
Ellingsworth LR, Lam HY, Thompson NL, Roberts AB and Sporn MB: Role
of transforming growth factor-beta in the development of the mouse
embryo. J Cell Biol. 105:2861–2876. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoffman MP, Kidder BL, Steinberg ZL,
Lakhani S, Ho S, Kleinman HK and Larsen M: Gene expression profiles
of mouse submandibular gland development: FGFR1 regulates branching
morphogenesis in vitro through BMP- and FGF-dependent mechanisms.
Development. 129:5767–5778. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jaskoll T, Zhou YM, Chai Y, Makarenkova
HP, Collinson JM, West JD, Hajihosseini MK, Lee J and Melnick M:
Embryonic submandibular gland morphogenesis: Stage-specific protein
localization of FGFs, BMPs, Pax6 and Pax9 in normal mice and
abnormal SMG phenotypes in FgfR2-IIIc(+/Delta), BMP7(−/−) and
Pax6(−/−) mice. Cells Tissues Organs. 170:83–98. 2002. View Article : Google Scholar
|
|
11
|
Tagliabracci VS, Engel JL, Wen J, Wiley
SE, Worby CA, Kinch LN, Xiao J, Grishin NV and Dixon JE: Secreted
kinase phosphorylates extracellular proteins that regulate
biomineralization. Science. 336:1150–1153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hao J, Narayanan K, Muni T, Ramachandran A
and George A: Dentin matrix protein 4, a novel secretory
calcium-binding protein that modulates odontoblast differentiation.
J Biol Chem. 282:15357–15365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishikawa HO, Xu A, Ogura E, Manning G and
Irvine KD: The Raine syndrome protein FAM20C is a Golgi kinase that
phosphorylates bio-mineralization proteins. PLoS One. 7:e429882012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
George A and Veis A: Phosphorylated
proteins and control over apatite nucleation, crystal growth, and
inhibition. Chem Rev. 108:4670–4693. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tagliabracci VS, Xiao J and Dixon JE:
Phosphorylation of substrates destined for secretion by the Fam20
kinases. Biochem Soc Trans. 41:1061–1065. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tagliabracci VS, Wiley SE, Guo X, Kinch
LN, Durrant E, Wen J, Xiao J, Cui J, Nguyen KB, Engel JL, et al: A
Single Kinase Generates the Majority of the Secreted
Phosphoproteome. Cell. 161:1619–1632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simpson MA, Hsu R, Keir LS, Hao J,
Sivapalan G, Ernst LM, Zackai EH, Al-Gazali LI, Hulskamp G,
Kingston HM, et al: Mutations in FAM20C are associated with lethal
osteosclerotic bone dysplasia (Raine syndrome), highlighting a
crucial molecule in bone development. Am J Hum Genet. 81:906–912.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Acevedo AC, Poulter JA, Alves PG, de Lima
CL, Castro LC, Yamaguti PM, Paula LM, Parry DA, Logan CV, Smith CE,
et al: Variability of systemic and orodental phenotype in two
families with non-lethal Raine syndrome with FAM20C mutations. BMC
Med Genet. 16:82015. View Article : Google Scholar
|
|
19
|
Seidahmed MZ, Alazami AM, Abdelbasit OB,
Al Hussein K, Miqdad AM, Abu-Sa'da O, Mustafa T, Bahjat S and
Alkuraya FS: Report of a case of Raine syndrome and literature
review. Am J Med Genet A. 167A:2394–2398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raine J, Winter RM, Davey A and Tucker SM:
Unknown syndrome: Microcephaly, hypoplastic nose, exophthalmos, gum
hyperplasia, cleft palate, low set ears, and osteosclerosis. J Med
Genet. 26:786–788. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Hao J, Xie Y, Sun Y, Hernandez B,
Yamoah AK, Prasad M, Zhu Q, Feng JQ and Qin C: Expression of FAM20C
in the osteogenesis and odontogenesis of mouse. J Histochem
Cytochem. 58:957–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du EX, Wang XF, Yang WC, Kaback D, Yee SP,
Qin CL, George A and Hao JJ: Characterization of Fam20C expression
in odontogenesis and osteogenesis using transgenic mice. Int J Oral
Sci. 7:89–94. 2015. View Article : Google Scholar
|
|
23
|
Wang X, Wang S, Lu Y, Gibson MP, Liu Y,
Yuan B, Feng JQ and Qin C: FAM20C plays an essential role in the
formation of murine teeth. J Biol Chem. 287:35934–35942. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vogel P, Hansen GM, Read RW, Vance RB,
Thiel M, Liu J, Wronski TJ, Smith DD, Jeter-Jones S and Brommage R:
Amelogenesis imperfecta and other biomineralization defects in
Fam20a and Fam20c null mice. Vet Pathol. 49:998–1017. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tibaldi E, Arrigoni G, Brunati AM, James P
and Pinna LA: Analysis of a sub-proteome which co-purifies with and
is phosphorylated by the Golgi casein kinase. Cell Mol Life Sci.
63:378–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jernvall J and Thesleff I: Reiterative
signaling and patterning during mammalian tooth morphogenesis. Mech
Dev. 92:19–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wagner KU, McAllister K, Ward T, Davis B,
Wiseman R and Hennighausen L: Spatial and temporal expression of
the Cre gene under the control of the MMTV-LTR in different lines
of transgenic mice. Transgenic Res. 10:545–553. 2001. View Article : Google Scholar
|
|
28
|
Ewald D, Li M, Efrat S, Auer G, Wall RJ,
Furth PA and Hennighausen L: Time-sensitive reversal of hyperplasia
in transgenic mice expressing SV40 T antigen. Science.
273:1384–1386. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wagner KU, Wall RJ, St-Onge L, Gruss P,
Wynshaw-Boris A, Garrett L, Li M, Furth PA and Hennighausen L:
Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res.
25:4323–4330. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Wang S, Li C, Gao T, Liu Y,
Rangiani A, Sun Y, Hao J, George A, Lu Y, et al: Inactivation of a
novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in
mice. PLoS Genet. 8:e10027082012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Romanenko VG, Nakamoto T, Srivastava A,
Begenisich T and Melvin JE: Regulation of membrane potential and
fluid secretion by Ca2+-activated K+ channels
in mouse submandibular glands. J Physiol. 581:801–817. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nalbant D, Youn H, Nalbant SI, Sharma S,
Cobos E, Beale EG, Du Y and Williams SC: FAM20: An evolutionarily
conserved family of secreted proteins expressed in hematopoietic
cells. BMC Genomics. 6:112005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gresik EW: The granular convoluted tubule
(GCT) cell of rodent submandibular glands. Microsc Res Tech.
27:1–24. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma T, Song Y, Gillespie A, Carlson EJ,
Epstein CJ and Verkman AS: Defective secretion of saliva in
transgenic mice lacking aquaporin-5 water channels. J Biol Chem.
274:20071–20074. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krane CM, Melvin JE, Nguyen HV, Richardson
L, Towne JE, Doetschman T and Menon AG: Salivary acinar cells from
aquaporin 5-deficient mice have decreased membrane water
permeability and altered cell volume regulation. J Biol Chem.
276:23413–23420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Penschow JD, Drinkwater CC, Haralambidis J
and Coghlan JP: Sites of expression and induction of glandular
kallikrein gene expression in mice. Mol Cell Endocrinol.
81:135–146. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smith FJ, Porter RM, Corden LD, Lunny DP,
Lane EB and McLean WH: Cloning of human, murine, and marsupial
keratin 7 and a survey of K7 expression in the mouse. Biochem
Biophys Res Commun. 297:818–827. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Melnick M and Jaskoll T: Mouse
submandibular gland morphogenesis: A paradigm for embryonic signal
processing. Crit Rev Oral Biol Med. 11:199–215. 2000. View Article : Google Scholar
|
|
40
|
Jaskoll T, Chen H, Min Zhou Y, Wu D and
Melnick M: Developmental expression of survivin during embryonic
submandibular salivary gland development. BMC Dev Biol. 1:52001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fiaschi M, Kolterud A, Nilsson M, Toftgård
R and Rozell B: Targeted expression of GLI1 in the salivary glands
results in an altered differentiation program and hyperplasia. Am J
Pathol. 179:2569–2579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barka T: Biologically active polypeptides
in submandibular glands. J Histochem Cytochem. 28:836–859. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshida S, Ohbo K, Takakura A, Takebayashi
H, Okada T, Abe K and Nabeshima Y: Sgn1, a basic helix-loop-helix
transcription factor delineates the salivary gland duct cell
lineage in mice. Dev Biol. 240:517–530. 2001. View Article : Google Scholar
|
|
44
|
Yamagishi R, Wakayama T, Nakata H,
Adthapanyawanich K, Kumchantuek T, Yamamoto M and Iseki S:
Expression and localization of alpha-amylase in the submandibular
and sublingual glands of mice. Acta Histochem Cytochem. 47:95–102.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Marchetti L, Gabrielli MG, Materazzi G and
Menghi G: Cellular compartmentation of lysozyme and alpha-amylase
in the mouse salivary glands. Immunogold approaches at light and
electron microscopy level. Histol Histopathol. 15:337–346.
2000.PubMed/NCBI
|
|
46
|
Menghi G, Marchetti L, Bondi AM, Accili D,
Sabbieti MG and Materazzi G: Double-sided staining with a gold
probe and silver enhancement to detect alpha-amylase and sugar
moieties in the mouse salivary glands. Histol Histopathol.
14:687–695. 1999.PubMed/NCBI
|
|
47
|
Smith RJ and Frommer J: Effects of
prepubertal castration on development of granular tubules and
amylase activity in the male mouse submandibular gland. Arch Oral
Biol. 17:1561–1571. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gresik EW: The postnatal development of
the sexually dimorphic duct system and of amylase activity in the
submandibular glands of mice. Cell Tissue Res. 157:411–422. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Morgan-Bathke M, Lin HH, Chibly AM, Zhang
W, Sun X, Chen CH, Flodby P, Borok Z, Wu R, Arnett D, et al:
Deletion of ATG5 shows a role of autophagy in salivary homeostatic
control. J Dent Res. 92:911–917. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Deretic V, Jiang S and Dupont N: Autophagy
intersections with conventional and unconventional secretion in
tissue development, remodeling and inflammation. Trends Cell Biol.
22:397–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dikeakos JD and Reudelhuber TL: Sending
proteins to dense core secretory granules: Still a lot to sort out.
J Cell Biol. 177:191–196. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Musselmann K, Green JA, Sone K, Hsu JC,
Bothwell IR, Johnson SA, Harunaga JS, Wei Z and Yamada KM: Salivary
gland gene expression atlas identifies a new regulator of branching
morphogenesis. J Dent Res. 90:1078–1084. 2011. View Article : Google Scholar : PubMed/NCBI
|