Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent malignant human tumors (1,2).
As of aggressive metastasis, recurrence and drug resistance, the
overall 5-year survival rate of patients with HCC remains
unsatisfactory and is <20% (3). Cisplatin (DDP) is one of the most
frequently used anticancer drugs and the front line option for the
treatment of HCC; however, the clinical applications of DDP are
limited largely due to severe side effects and chemoresistance
(4).
Therapeutic ultrasound (US), particularly the use of
low-intensity ultrasound (LIUS), has gained increasing attention in
recent years as numerous studies have reported the synergistic
effects of chemotherapeutic drugs and LIUS in cancer therapy. For
example, Fan et al (5)
revealed that effective low dosages of doxorubicin in combination
with LIUS can inhibit cell proliferation, migration and invasion in
oral squamous cell carcinoma. In a murine lymphoma, Tomizawa et
al (6) reported that the
combination of intraperitoneal bleomycin and US suppressed tumor
growth. Emerging evidence demonstrated that excessive reactive
oxygen species (ROS) production may be the key mechanism of
US-enhanced chemotherapy (5). Hu
et al (7) showed that
combined US and 5-fluorouracil treatment regulated the expression
of apoptosis-associated proteins via ROS in HCC; however, the
mechanisms as to how LIUS enhances the antitumor effects of these
agents are not fully understood.
MicroRNAs (miRNAs/miRs) are a class of endogenous
small noncoding RNAs that regulate gene expression at the
post-transcription level (8).
Previously, the dysregulation of miRNAs and ROS were observed in
human cancers, and extensive research showed that ROS contributed
to the initiation and progression of carcinogenesis via the
regulation of miRNAs (9-11). For example, elevating ROS levels
by ionizing radiation induced profound alterations in global miRNA
expression profiles of normal human fibroblasts following
H2O2 treatment (12). Jajoo et al (10) reported that high ROS levels
contribute to the increased metastatic potential of the prostate
cancer cells via the regulation of miRNA-21. Additionally, US was
observed to increase ROS production to affect tumor cell damage and
apoptosis (13). Taken together,
we proposed that LIUS may affect the sensitivity of HCC to DDP by
regulating the expression of miRNAs via the production of ROS.
In the present study, the synergistic antitumor
effects of DDP combined with LIUS were investigated in HCC cells.
We also explored the potential mechanism of LIUS combined with DDP
that enhances the antitumor effect. This study aimed to provide
novel insight into the use of LIUS in HCC therapy.
Materials and methods
Chemicals and antibodies
Cisplatin and N-acetylcysteine (NAC) were obtained
from Sigma-Aldrich (Merck KGaA). Mouse anti-c-Met (cat. no.
sc-8057) and mouse anti-β-actin (cat. no. sc-47778) were obtained
from Santa Cruz Biotechnology, Inc.
Cell culture
HCC, Huh7, HCCLM3 and 293 cell lines were obtained
from the American Type Culture Collection. All cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo
Fisher Scientific, Inc.) containing with 10% fetal bovine serum
(FBS; Sigma-Aldrich; Merck KGaA), penicillin (100 U/ml) and
streptomycin (100 mg/ml) in an incubator with a humidified
atmosphere and 5% CO2 at 37°C. Cells were treated with
DDP (0, 1, 2, 4, 6, and 8 μg/ml) for 48 h at 37°C; untreated
cells served as a control. In some experiments, the ROS scavenger
NAC (10 mM) was added into cells at 1 h prior to the administration
of cisplatin at 37°C, immediately followed by US exposure.
LIUS
HCC cells were stimulated with rectangular pulse
ultrasound generated by the system SonoPore KTAC-4000 (Nepa Gene,
Co., Ltd.). For the sonication of cell culture, we used the
following US parameters: Frequency 1.1 MHz, provided in a pulse
wave mode with duty cycle 10% and a repetition frequency of 100 Hz;
the Hz intensity was 1.0 W/cm2 and the duration of
sonication was 10 sec. The protocol was conducted as previously
described by Hu et al (7).
Cell viability and the half-maximal
inhibitory concentration (IC50)
Huh7 and HCCLM3 cells transfected with miRNAs and
plasmids (described below) were seeded in 96-well plates at a
density of 5,000 cells/well. Following cellular adhesion, DDP was
added to the cultured cells at a concentration of 1 μg/ml
and incubated for 48 h at 37°C, followed by exposure to LIUS. At 6
h following LIUS treatment, cell viability was monitored using an
MTT assay. Untreated cells served as a blank group. Cells treated
with DDP treatment alone served as the control. MTT solution (15
μl) was added to each well, and the cells were incubated at
37°C for another 4 h. The optical density was measured at 490 nm
using a microplate reader (ELX50; BioTek Instruments, Inc.). The
IC50 was calculated according to the cell viability
curve.
Cell apoptosis detection by flow
cytometry
An Annexin V fluorescein isothiocyanate
(FITC)-propidium iodide (PI) Apoptosis kit (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for apoptosis analysis. The
treated and control cells were collected, and washed twice with PBS
at 4°C. Then, the cells were re-suspended in 490 μl binding
buffer containing 5 μl Annexin V (Bio-Science, Co. Ltd.) and
5 μl PI. After incubation at 4°C in the dark for 30 min, the
cells were sorted using a FACS Aria III (BD Biosciences) and
analyzed with BD FACSDiva (version 6.2; BD Biosciences) software.
Cells were stained simultaneously with Annexin V-FITC (green
fluorescence) and the non-vital dye PI (red fluorescence) allowed
the determination of viable cells (FITC-PI-),
and early apoptotic (FITC+PI-) and late
apoptotic (FITC+PI+), or necrotic cells
(FITC-PI+). The rate of apoptotic cells
(FITC+PI- and FITC+PI+)
was then calculated according to a previous report (14).
Determination of ROS
ROS levels were assessed by using the oxidation
sensitive probe 2′,7′-dichlorofluorescin diacetate (DCFH-DA). The
treated and control cells were incubated with 10 μM DCFH-DA
at 37°C for 30 min, and then the cells were sorted using a FACS
Aria III (BD Biosciences) at an excitation wavelength of 488 nm and
an emission wavelength of 530 nm and analyzed with BD FACSDiva
software.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the treated and control
cells using the miRNeasy mini kit (Qiagen, Inc.) according to the
manufacturer's instructions. Total RNA was converted to cDNA and
added with a universal tag by using a miScript II RT kit (Qiagen,
Inc.) for 60 min at 37°C. qPCR was performed using the Power
SYBR-Green PCR master mix on an ABI 7900HT system (both Applied
Biosystems; Thermo Fisher Scientific, Inc.). The expression of
miRNAs and mRNA were normalized to that of U6 and GAPDH,
respectively. Thermocycling conditions were as following: 95°C for
5 min, followed by 40 cycles of 95°C for 30 sec and 55°C for 25
sec, and extension at 72°C for 10 sec. The primer sequences were
synthesized by the Sangon Biotech Co. Ltd; the sequences were as
follows: miR-34a forward, 5′-GCGGCCAATCAGCAAGTATACT-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; miR-133b forward,
5′-GTCCCCTTCAACCAGCTACA-3′ and reverse, 5′-GAGTGCAAAGGCACAGAACA-3′;
miR-340 forward, 5′-ACACTCCAGCTGGGTTATAAAGCAATGAGA-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′; miR-130a forward,
5′-CTTGCCCCTAAAGAGGGGGA-3′ and reverse, 5′-CGAGTCAAAGGCTCCCCA-3′;
miR-199a-5p forward, 5′-ACACTCCAGCTGGGCCCAGTGTTCAGACTAC-3′ and
reverse, 5′-CTCAACTGGTGTCGTGGAGTCGGCAA-3′; miR-125b forward,
5′-UCCCUGAGACCCUAACUUGUGA-3′ and reverse, 5′-ACAAGUUAGGGUCUCAGGG
AUU-3′; U6 forward, 5′-AAAGACCTGTACGCCAACAC-3′ and reverse,
5′-GTCATACTCCTGCTTGCTGAT-3′; c-Met forward,
5′-CATCTCAGAACGGTTCATGCC-3′ and reverse,
5′-TGCACAATCAGGCTACTGGG-3′; GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse,
5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Cell transfection
Huh7 and HCCLM3 cells (1.0×105 per well)
were seeded and grown overnight in six-well plates into 6-well
plates (2,000,000 cells/well) in DMEM supplemented with 10% FBS for
24 h at 37°C. miR-34a mimics, miR-34a inhibitor and the
corresponding negative controls (NCs) were purchased from Shanghai
GenePharma Co., Ltd. In addition, the coding domain sequences of
c-Met mRNA were amplified by PCR, and inserted into pcDNA 3.0
vector to enhance its expression (Invitrogen; Thermo Fisher
Scientific, Inc.), named as pcDNA-c-Met. pcDNA 3.0 empty vector was
used as the control. A total of 100 nM miR-34a mimics, 100 nM
miR-34a inhibitor or 2 μg pcDNA-c-Met were transfected into
Huh7 and HCCLM3 cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) following
manufacturer's instructions. After 48 h transfection, cells were
harvested for subsequent analysis.
Target gene analyses of miR-34a
Bioinformatics tools, including TargetScan 7.0
(targetscan.org/) and miRanda (microrna.org/), were used to predict the potential
target genes of miR-34a.
Luciferase reporter assay
The 3′-untranslated region (UTR) of c-Met with
wild-type (WT) or mutant (Mut) binding sites for miR-34a, was
amplified and cloned into the pGL3 vector (Promega Corporation) to
generate pGL3-WT-c-Met-3′-UTR or pGL3-Mut-c-Met-3′-UTR,
respectively. For the luciferase reporter assay, cells were
co-transfected with the luciferase reporter vectors and miR-34a
mimics, miR-34a inhibitor or the corresponding NCs. The pRL-TK
plasmid (Promega Corporation) was used as a normalizing control.
After 48 h of incubation, luciferase activity was analyzed using
the Dual-Luciferase Reporter Assay System (Promega
Corporation).
Western blot analysis
Total protein was extracted using
radioimmunoprecipitation assay lysis buffer (cat. no. P0013K;
Beyotime Institute of Biotechnology) and the protein concentration
was measured using a Bicinchoninic Acid assay kit (Pierce; Thermo
Fisher Scientific, Inc.). Total protein (50 μg) was loaded
and separated by 15% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (GE Healthcare) by electroblotting. Primary
antibodies against c-Met (1:1,000) and β-actin (1:3,000) were
applied to the membrane at 4°C overnight. After incubating with
goat anti-mouse IgG-horseradish peroxidase antibody (cat. no.
sc2005; 1:10,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature, the bands were detected using an enhanced
chemiluminescence kit (GE Healthcare). The intensity of the bands
was analyzed by ImageJ software (version 1.46; National Institutes
of Health).
Statistical analysis
Statistical analyses were performed with SPSS 13.0
software (SPSS, Inc.). Data were expressed as the mean ± standard
deviation of three independent experiments. One-way analysis of
variance followed by a Tukey's post-hoc test was applied to compare
differences between multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
LIUS enhances the anticancer activity of
cisplatin in vitro
Extensive investigations have demonstrated that the
use of LIUS in combination with certain chemotherapeutic agents may
be an important novel therapeutic strategy in treating human
cancers (15-17). Thus, we sought to investigate
whether LIUS enhanced the anticancer activity of DDP in
vitro.
The present study determined the effects of DDP on
the growth of HCC cells, Huh7 cells treated with DDP of different
concentrations for 48 h, by an MTT Assay. As shown in Fig. 1A and B, the viability of Huh7 and
HCCLM3 cells was inhibited in a dose-dependent manner. DDP at 1
μg/ml was recommended for the following analysis as a slight
anticancer effect was observed. As shown in Fig. 1C, the IC50 values of
Huh7 cells with or without LIUS treatment were 5.362±0.080 and
3.922±0.095 ng/ml, respectively, and the differences were
statistically significant. Similarly, the IC50 values of
HCCLM3 cells with or without LIUS treatment were 3.781±0.053 and
2.179±0.262 ng/ml, respectively; significant differences were
observed between cells treated with or without LIUS. These results
indicate that LIUS could effectively increase the sensitivity of
HCC cells to DDP. To determine whether the reduction in cell
viability was associated with cell apoptosis, flow cytometry was
applied to detect the apoptotic rate of Huh7 and HCCLM3 cells
following LIUS + DDP treatment. In the DDP group, DDP increased
apoptosis by 9.5±2.4%, in the LIUS group, apoptosis increased by
8.4±1.3%, but in the LIUS + DDP group, the apoptotic rate was
significantly increased by 35.5±2.3% in Huh7 cells compared with
the control and DDP groups (Fig.
1D). In addition, the apoptotic rate in the LIUS + DDP group
was significantly increased by 41.6±2.1% compared with the control
and DDP groups; in the DDP and LIUS groups, apoptosis was increased
by 7.8±2.5 and 10.6±1.5%, respectively in HCCLM3 cells (Fig. 1E). These data suggest that LIUS
may improve the antitumor effects of DDP by increasing DDP
sensitivity and DDP-induced apoptosis.
LIUS enhances intracellular ROS levels
and miR-34a expression
It is widely accepted that the biological effects
elicited by LIUS are predominantly due to the generation of ROS
(18,19). Providing that ROS can alter the
expression of certain miRNAs (20,21), we investigated whether the
synergistic antitumor effects of LIUS occurs by altering the
expression of miRNAs. Thus, we selected six miRNAs that have been
reported to be regulated by ROS for further validation, including
miR-34a (22,23), miR-133b (24), miR-340 (25), miR-130a (26), miR-199a-5p (27) and miR-125b (28). The results of RT-qPCR showed that
LIUS significantly increased the expression of miR-34a in
DDP-treated Huh7 and HCCLM3 cells compared with the control;
however, no significant changes in expression were observed for
miR-133b, miR-340, miR-130a, miR-199a-5p and miR-125b (Fig. 2A-F). In addition, the accumulation
of ROS was detected. The results demonstrated that compared with
the control group, the fluorescence intensity of ROS significantly
increased in the DDP group compared with untreated cells; however,
a stronger increase was observed in the LIUS + DDP group (Fig. 2G). Of note, the upregulation of
miR-34a expression induced by LIUS was reversed in Huh7 and HCCLM3
cells that were pre-treated with the ROS scavenger NAC, suggesting
that ROS modulated the expression of miR-34a (Fig. 2H). These findings indicated that
the effects of LIUS may be associated with the induction of miR-34a
mediated by ROS.
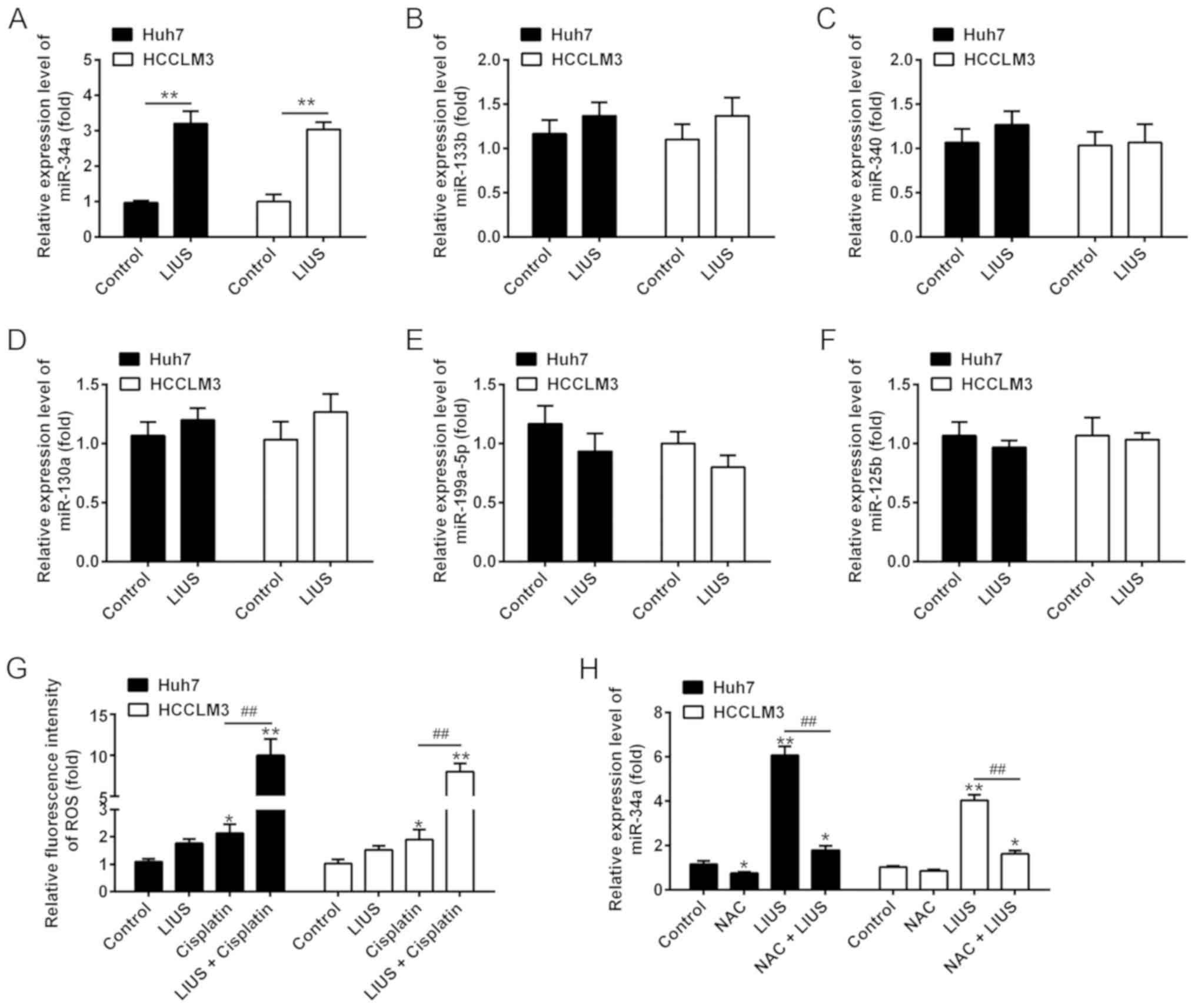 | Figure 2LIUS enhances the intracellular ROS
levels and miR-34a expression. The cells were treated with
cisplatin (1 μg/ml) and/or LIUS for 48 h. (A-F) Expression
levels of miR-34a, miR-133b, miR-340, miR-130a, miR-199a-5p and
miR-125b were measured by RT-qPCR. (G) The relative levels of
intracellular ROS were quantified using 2′,7′-dichlorofluorescin
diacetate. n=3. *P<0.05, **P<0.01 vs.
Control group; ##P<0.01 vs. Cisplatin. (H) Expression
of miR-34a was measured by RT-qPCR after cisplatin and/or LIUS
treatment in the presence or absence of the ROS scavenger NAC. Data
are presented as the mean ± standard deviation. n=3,
*P<0.05, **P<0.01 vs. Control group;
##P<0.01 vs. LIUS group. LIUS, low-intensity
ultrasound; miR, microRNA; NAC, N-acetylcysteine; ROS, reactive
oxygen species; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
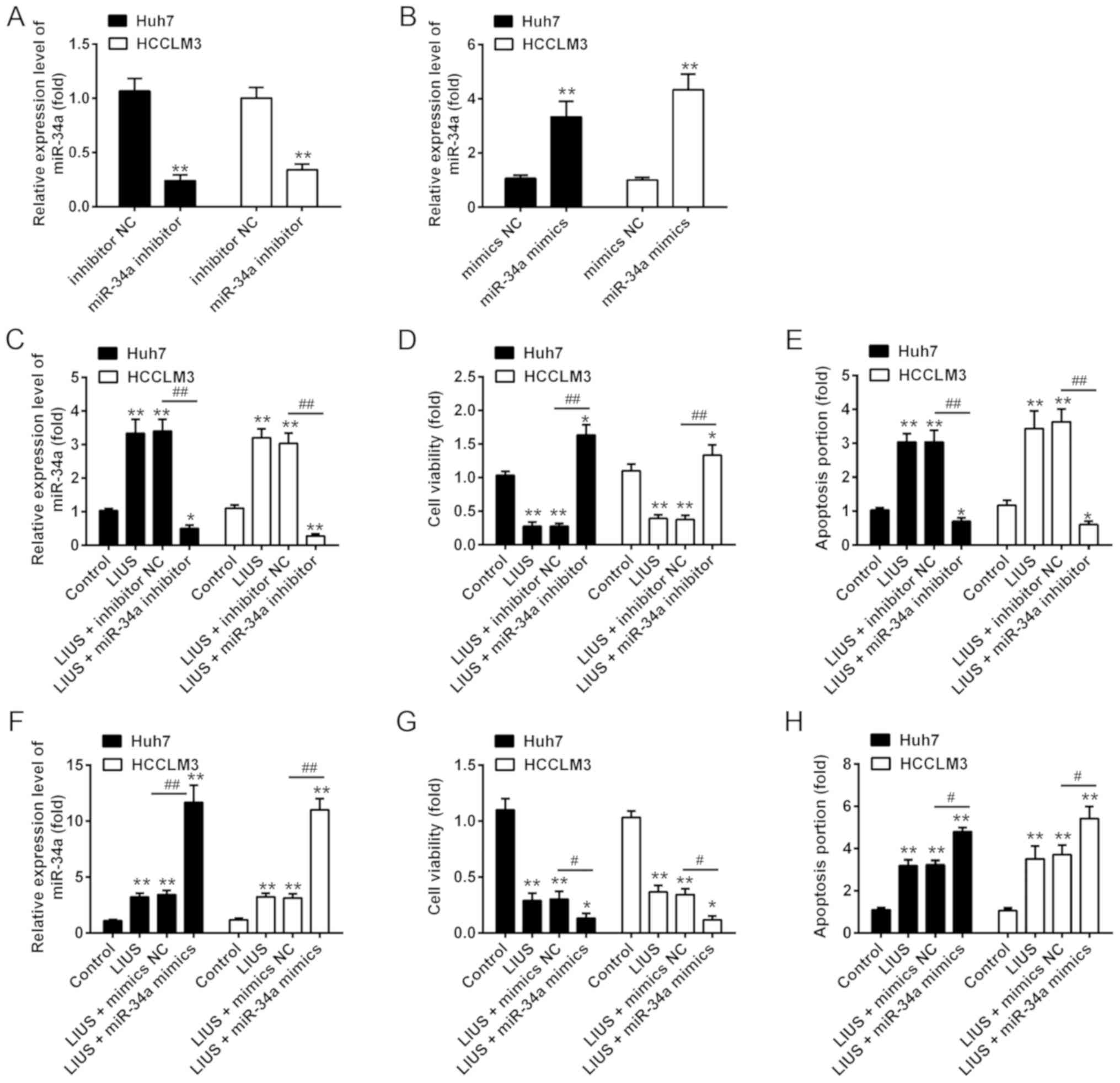
miR-34a expression is mediated the
synergistic antitumor effects of LIUS and DDP
We then examined whether miR-34a participates in the
synergistic antitumor effects of LIUS with DDP. The miRNA
transfection efficiency of miR-34a in cultured Huh7 and HCCLM3 were
determined; the results of RT-qPCR showed that the expression of
miR-34a was significantly increased/decreased after miR-34a
mimics/inhibitor transfection, respectively, compared with the
corresponding NC (Fig. 3A and B).
Subsequently, Huh7 and HCCLM3 cells were pre-treated with miR-34a
inhibitor or inhibitor NC, followed by LIUS and DDP treatment. DDP
treatment was used as control group. As anticipated, the expression
of miR-34a was significantly increased in Huh7 and HCCLM3 cells
compared to that in the control group; however, this promotive
effect was inhibited when miR-34a was knocked down (Fig. 3C). In addition, cell viability and
apoptosis were assessed by an MTT assay and flow cytometry,
respectively. The results showed that LIUS + DDP treatment
inhibited cell viability compared with the control group, whereas
this inhibitory effect was reversed by miR-34a knock down (Fig. 3D). The increased apoptosis ratio
induced by LIUS + DDP was also attenuated by miR-34a inhibitor
(Fig. 3E). Additionally, Huh7 and
HCCLM3 cells were transfected with miR-34a mimics, followed by LIUS
and DDP treatment. We reported that miR-34a was overexpressed
following miR-34a mimics transfection in Huh7 and HCCLM3 cells
under LIUS + DDP treatment compared with the control (Fig. 3F). Furthermore, overexpression of
miR-34a enhanced the synergistic antitumor effects of LIUS with
DDP, as observed by a significant reduction in cell viability and
increased apoptosis (Fig. 3G and
H). These results suggested that the synergistic antitumor
effects of LIUS and DDP may be mediated by miR-34a.
c-Met is a direct target of miR-34a
To further investigate the mechanisms by which
miR-34a mediated the synergistic antitumor effects of LIUS and DDP
in HCC cells, we determined the potential target genes of miR-34a
using the miRanda and TargetScan 6.1 databases. As presented in
Fig. 4A, miR-34a contained a
sequence complementary to c-Met. In addition, c-Met reported as a
target of miR-34a in colorectal cancer cell and human mesothelial
cells (29,30). To further validate these findings,
the c-Met-3′UTR wt luciferase reporter systems with the putative
binding sites and c-Met-3′UTR mut reporter systems with the
mutation sites were constructed in 293 cells. The results of the
luciferase reporter assays showed that introduction of miR-34a
mimics significantly inhibited, while miR-34a inhibitor increased
the relative luciferase activity of constructs containing the
c-Met-3′UTR compared with the respective NC group. However, the
luciferase activity of the reporters containing the mutant binding
site exhibited marked alterations (Fig. 4B). To further confirm that c-Met
is negatively regulated by miR-34a, western blot analysis was
conducted to determine the protein expression levels of c-Met. The
results revealed that c-Met was significantly downregulated
following the overexpression of miR-34a, but was upregulated after
knockdown in Huh7 and HCCLM3 cells compared with the corresponding
NC group (Fig. 4C and D).
Subsequently, the effects of LIUS and DDP on the expression of
c-Met were detected in Huh7 and HCCLM3 cells by western blot
analysis. As presented in Fig. 4E and
F, DDP significantly reduced the expression of c-Met compared
with untreated cells, whereas LIUS + DDP significantly inhibited
expression in Huh7 and HCCLM3 cells. Conversely, c-Met expression
was significantly increased following treatment with the ROS
scavenger NAC. Our results suggested that the combined treatment of
LIUS and DDP could upregulate the expression of miR-34a via ROS
production and reduce the expression of c-Met.
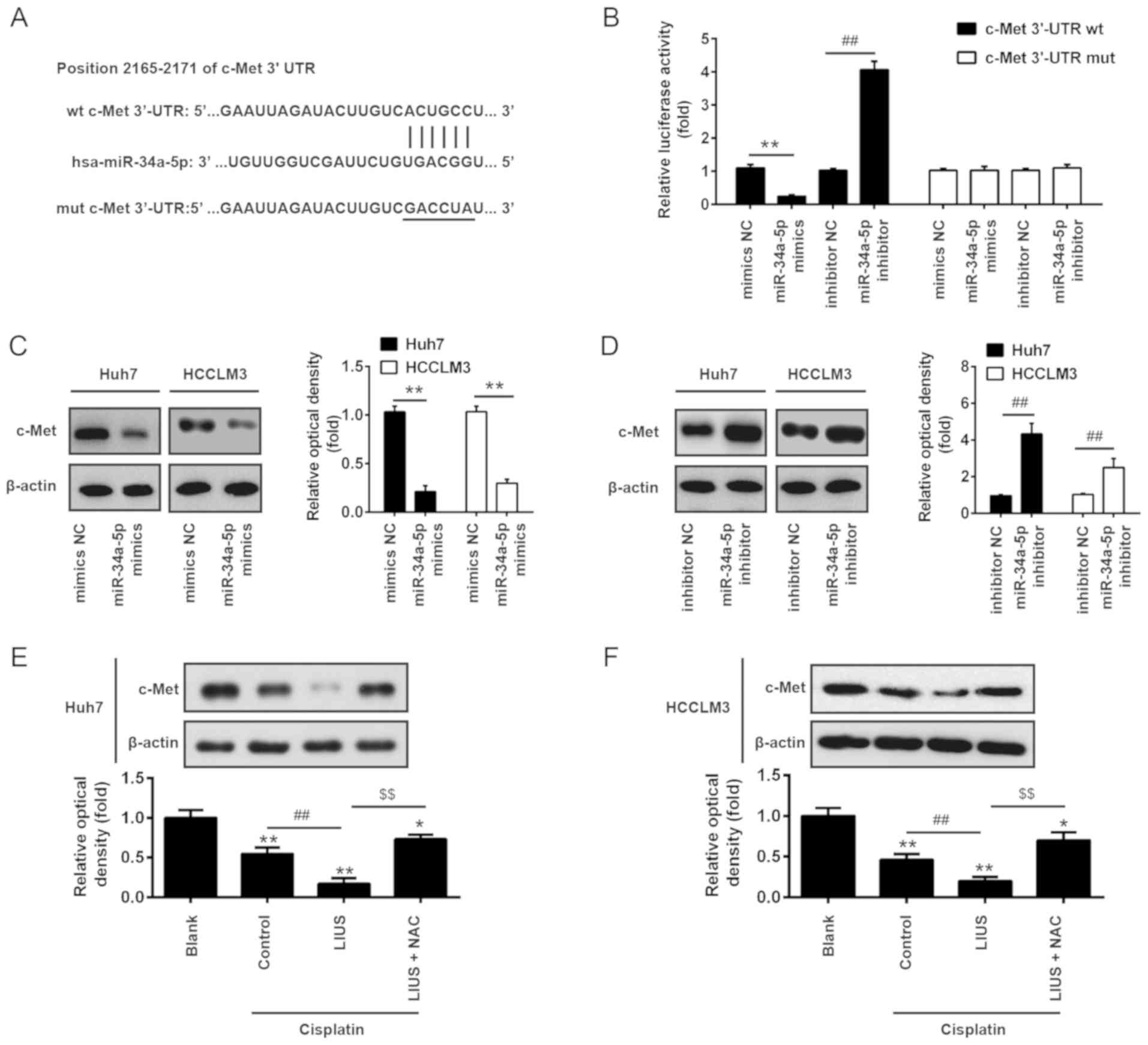 | Figure 4c-Met is a direct target of miR-34a.
(A) The predicted binding sites for miR-34a on c-Met. (B)
Luciferase activity in 293 cells co-transfected with miR-34a
mimics, miR-34a inhibitor and luciferase reporters containing c-Met
or MUT 3′-UTR. Histogram indicates the values of luciferase
measured 48 h after transfection. Data are presented as the mean ±
standard deviation. n=3, **P<0.01 vs. mimic NC;
##P<0.01 vs. inhibitor NC group. (C and D) miR-34a
mimics, miR-34a inhibitor and controls were transfected into Huh7
and HCCLM3 cells, then after 48 h transfection, the protein
expression levels of c-Met were detected by western blotting. Data
are presented as the mean ± standard deviation. n=3,
**P<0.01 vs. mimics NC group; ##P<0.01
vs. inhibitor NC group. (E and F) The expression of c-Met was
measured by western blotting after cisplatin and/or LIUS treatment
in the presence or absence of the ROS scavenger NAC. Untreated
cells served as Blank group. Cells treated with DDP treatment alone
served as the control. Data are presented as the mean ± standard
deviation. n=3, *P<0.05, **P<0.01 vs.
Blank group; ##P<0.01 vs. Cisplatin group;
$$P<0.01 vs. Cisplatin + LIUS group. LIUS,
low-intensity ultrasound; miR, microRNA; Mut, mutant; NAC,
N-acetylcysteine; NC, negative control; Wt, wild type. |
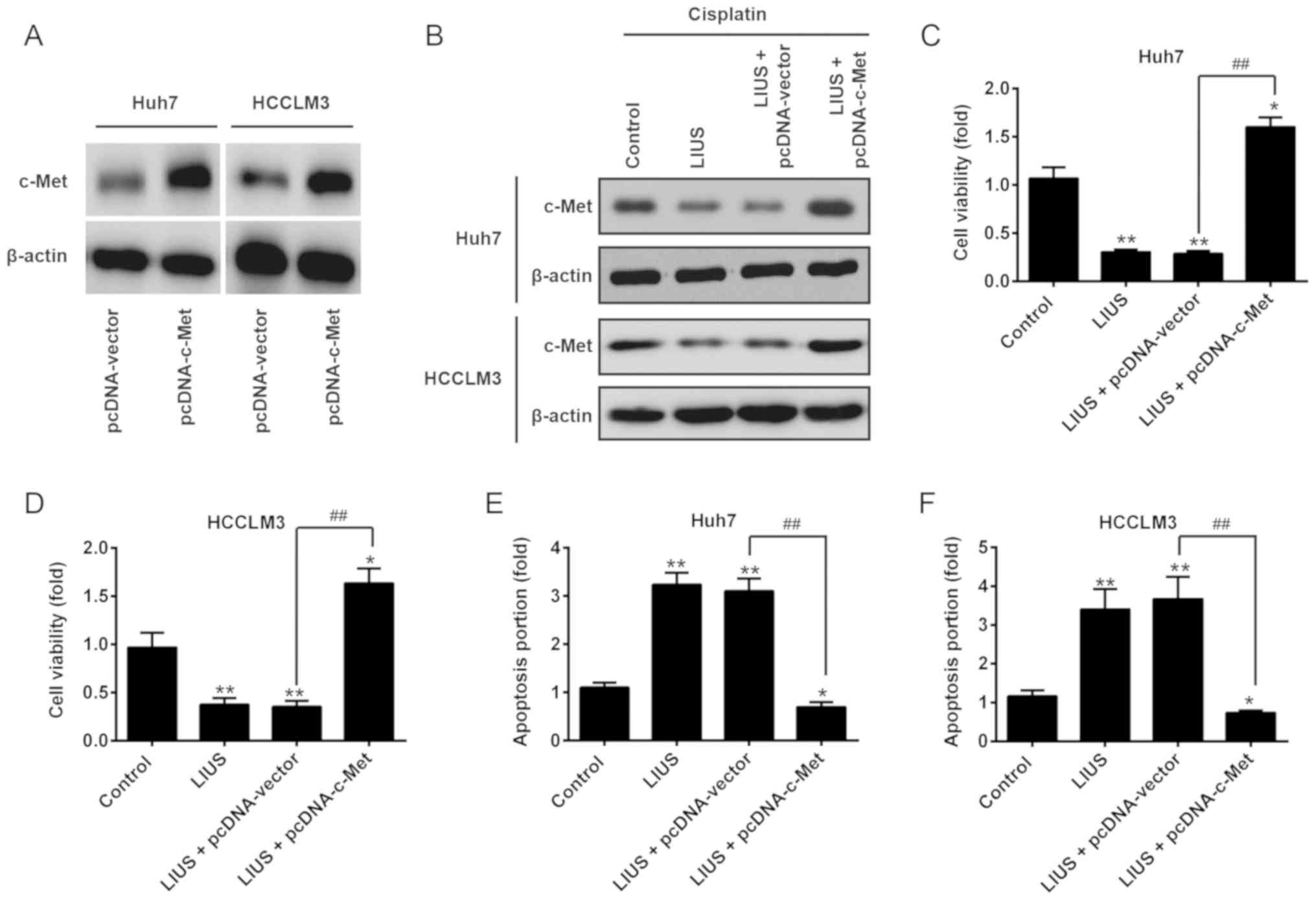
Restoration of c-Met reverses the
antitumor effects of LIUS combined with DDP
After confirming c-Met was a direct target of
miR-34a in HCC cells, we further investigated whether the antitumor
effects of LIUS combined with DDP is mediated via the
downregulation of c-Met. Huh7 and HCCLM3 cells were transfected
with pcDNA-c-Met plasmids, followed by LIUS and DDP treatment.
Western blot analysis revealed the upregulation of c-Met by the
pcDNA-c-Met plasmids, compared with that in the pcDNA-vector group
(Fig. 5A). It was also observed
that LIUS suppressed the endogenous expression of c-Met in
DDP-treated Huh7 and HCCLM3 cells, which was reversed by
pcDNA-c-Met (Fig. 5B). Then, cell
viability and apoptosis were evaluated by an MTT assay and flow
cytometry, respectively. As presented in Fig. 5C and D, the reduction in cell
viability induced by LIUS + DDP was reversed by pcDNA-c-Met
overexpression. Furthermore, flow cytometry indicated that the
promotion of apoptosis induced by LIUS + DDP was attenuated when
c-Met was overexpressed (Fig. 5E and
F). These findings suggest that miR-34a may have mediated the
synergistic antitumor effects of LIUS combined with DDP in HCC
cells by targeting c-Met.
Discussion
In the present study, we first revealed that LIUS
could enhance the antitumor activity of DDP in HCC cells. Based on
in vitro explorations, it was proposed that miR-34a and the
regulation of c-Met were key mechanisms involved in the synergistic
antitumor effects of LIUS and DDP combination treatment.
In recent years, the role of LIUS in cancer therapy
has been implicated in vitro and in vivo (13,31,32). Few investigations demonstrated
that LIUS enhanced the antitumor effects of several
chemotherapeutic agents, including doxorubicin, cyclophosphamide,
docetaxel and DDP (33-36). Yoshida et al (15) had demonstrated that LIUS could
increase the uptake of doxorubicin, and caused a synergistic
enhancement in cell killing and the induction of apoptosis in human
lymphoma U937 cells. The synergistic efficacy of chemotherapy and
US has also been studied in mouse tumor models. For example, Li
et al (37) found the
treatment of scutellarin and ultrasound significantly delayed human
tongue carcinoma xenograft growth, inhibited tumor angiogenesis and
lymphanigoenesis in tumor-bearing Balb/c mice. Although
applications of LIUS are still in the process of investigation,
LIUS has distinct potential as a technique for cancer treatment,
particular in cases of DDP resistance (16). In the present study, it was
revealed that LIUS effectively enhanced HCC cell sensitivity to a
low concentration of DDP, indicating that LIUS could enhance the
antitumor effects of DDP in HCC. However, the mechanisms underlying
the synergistic effects of LIUS combined with DDP in HCC are yet to
be elucidated.
Increasing experimental evidence has indicated that
the cell damage induced by the synergistic effects of US and drugs
may contribute to the generation ROS (7,37).
For example, LIUS combined with 5-aminolevulinic acid significantly
suppressed the growth of human tongue squamous carcinoma in
vitro and in vivo via the production of ROS (38). Huang et al (39) showed that new quinolone
antibiotics could exhibit notable antitumor activity under US
irradiation, and that generation of ROS is involved in this
process. In our study, it was observed that LIUS stimulation
increased DDP-mediated ROS generation in HCC cells, suggesting that
LIUS enhanced the antitumor effects of DDP via ROS generation.
Recently, it was reported that ROS is involved in the initiation of
cancer metastasis through the regulation of miRNA expression
(11), including miR-21 in
prostate cancer (10) and
miR-125b in breast cancer (40).
Therefore, we proposed that novel miRNAs regulated by ROS may serve
an important role in the synergistic antitumor effects of LIUS
combined with DDP. In this study, we selected six miRNAs regulated
by ROS including miR-34a (22,23), miR-133b (24), miR-34a (25), miR-130a (26), miR-199a-5p (27) and miR-125b (28), and found that miR-34a was
significantly increased following combination treatment.
Additionally, the upregulation of miR-34a could be abrogated when
ROS were scavenged, indicating that LIUS induced the expression of
miR-34a via ROS production. Furthermore, several studies have
reported that miR-34a functions as a tumor suppressor in a variety
of human cancers, such as pancreatic cancer (41), colorectal cancer (42), breast cancer (43) and lung cancer (44). Subsequently, we investigated the
function of miR-34a in the synergistic antitumor effects of LIUS
combined with DDP. The results demonstrated that knockdown of
miR-34a inhibited the synergistic antitumor effects of LIUS
combined with DDP, whereas overexpression of miR-34a enhanced these
synergistic antitumor effects. These results suggested that the
synergistic antitumor effects of LIUS and DDP combination occurred
via the ROS-mediated upregulation of miR-34a.
The mesenchymal-epithelial transition factor c-Met
is a well-known oncogene, and its aberrant activation is
responsible for the growth, progression, invasion and poor
prognosis of various solid tumors, including HCC (45,46). Importantly, overexpression of
c-Met is frequently observed in patients with HCC, and is closely
associated with the clinicopathological characteristics of HCC
(47). Of note, targeting c-Met
is emerging as an anticancer therapeutic option for HCC (48). Interestingly, c-Met has been
reported to be a direct target of miR-34a in colorectal cancer and
human mesothelial cells (29,30). In the present study, c-Met was
also identified as a target of miR-34a. Additionally, LIUS-DDP
significantly decreased the expression of c-Met, but this
inhibitory effect was reversed when ROS were scavenged.
Furthermore, we observed that the synergistic antitumor effects of
LIUS-DDP were abolished when c-Met was overexpressed. These results
suggested that LIUS combined with DDP exerts the synergistic
antitumor effects via downregulation of c-Met by the ROS-mediated
upregulation of miR-34a.
In conclusion, we demonstrated that LIUS enhanced
the anticancer effects of DDP by increasing ROS production, which
induced the expression of miR-34a and reduced that of c-Met, a
well-known oncogene, to suppress cell viability and induce
apoptosis (Fig. 6). Our findings
indicated that LIUS combined with DDP may be a promising novel
therapy, particularly for drug-resistant patients with HCC.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PL, JZ, FL and YY performed the experiments,
contributed to data analysis and wrote the paper. PL, JZ, FL and YY
analyzed the data. YC made substantial contributions to the concept
of the study, contributed to data analysis and acquired
experimental materials. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Huaihe
Hospital of Henan University Ethics Committees.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Sapisochin G, de Sevilla EF, Echeverri J
and Charco R: Management of 'very early' hepatocellular carcinoma
on cirrhotic patients. World J Hepatol. 6:766–775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Faloppi L, Scartozzi M, Maccaroni E, Di
Pietro Paolo M, Berardi R, Del Prete M and Cascinu S: Evolving
strategies for the treatment of hepatocellular carcinoma: From
clinical-guided to molecularly-tailored therapeutic options. Cancer
Treat Rev. 37:169–177. 2011. View Article : Google Scholar
|
|
3
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: CONCORD Working Group: Global surveillance of
trends in cancer survival 2000-14 (CONCORD-3): Analysis of
individual records for 37 513 025 patients diagnosed with one of 18
cancers from 322 population-based registries in 71 countries.
Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas MB, O'Beirne JP, Furuse J, Chan AT,
Abou-Alfa G and Johnson P: Systemic therapy for hepatocellular
carcinoma: Cytotoxic chemotherapy, targeted therapy and
immunotherapy. Ann Surg Oncol. 15:1008–1014. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan H, Li H, Liu G, Cong W, Zhao H, Cao W
and Zheng J: Doxorubicin combined with low intensity ultrasound
suppresses the growth of oral squamous cell carcinoma in culture
and in xenografts. J Exp Clin Cancer Res. 36:1632017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomizawa M, Ebara M, Saisho H, Sakiyama S
and Tagawa M: Irradiation with ultrasound of low output intensity
increased chemosensitivity of subcutaneous solid tumors to an
anti-cancer agent. Cancer Lett. 173:31–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu Z, Lv G, Li Y, Li E, Li H, Zhou Q, Yang
B and Cao W: Enhancement of anti-tumor effects of 5-fluorouracil on
hepatocellular carcinoma by low-intensity ultrasound. J Exp Clin
Cancer Res. 35:712016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Melnik BC: miR-21: An environmental driver
of malignant melanoma? J Transl Med. 13:2022015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jajoo S, Mukherjea D, Kaur T, Sheehan KE,
Sheth S, Borse V, Rybak LP and Ramkumar V: Essential role of NADPH
oxidase-dependent reactive oxygen species generation in regulating
microRNA-21 expression and function in prostate cancer. Antioxid
Redox Signal. 19:1863–1876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He J and Jiang BH: Interplay between
Reactive oxygen species and microRNAs in cancer. Curr Pharmacol
Rep. 2:82–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simone NL, Soule BP, Ly D, Saleh AD,
Savage JE, Degraff W, Cook J, Harris CC, Gius D and Mitchell JB:
Ionizing radiation-induced oxidative stress alters miRNA
expression. PLoS One. 4:e63772009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu T, Wang Z and Mason TJ: A review of
research into the uses of low level ultrasound in cancer therapy.
Ultrason Sonochem. 11:95–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi XF, Kim DH, Yoon YS, Kim SK, Cai DQ,
Teng YC, Shim KY and Lee KJ: Involvement of oxidative stress in
simvastatin-induced apoptosis of murine CT26 colon carcinoma cells.
Toxicol Lett. 199:277–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida T, Kondo T, Ogawa R, Feril LB Jr,
Zhao QL, Watanabe A and Tsukada K: Combination of doxorubicin and
low-intensity ultrasound causes a synergistic enhancement in cell
killing and an additive enhancement in apoptosis induction in human
lymphoma U937 cells. Cancer Chemother Pharmacol. 61:559–567. 2008.
View Article : Google Scholar
|
|
16
|
Watanabe Y, Aoi A, Horie S, Tomita N, Mori
S, Morikawa H, Matsumura Y, Vassaux G and Kodama T: Low-intensity
ultrasound and microbubbles enhance the antitumor effect of
cisplatin. Cancer Sci. 99:2525–2531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Masui T, Ota I, Kanno M, Yane K and Hosoi
H: Low-intensity ultrasound enhances the anticancer activity of
cetuximab in human head and neck cancer cells. Exp Ther Med.
5:11–16. 2013. View Article : Google Scholar
|
|
18
|
McHale AP, Callan JF, Nomikou N, Fowley C
and Callan B: Sonodynamic therapy: Concept, mechanism and
application to cancer treatment. Adv Exp Med Biol. 880:429–450.
2016. View Article : Google Scholar
|
|
19
|
Wan GY, Liu Y, Chen BW, Liu YY, Wang YS
and Zhang N: Recent advances of sonodynamic therapy in cancer
treatment. Cancer Biol Med. 13:325–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin J, Chuang CC and Zuo L: Potential
roles of microRNAs and ROS in colorectal cancer: Diagnostic
biomarkers and therapeutic targets. Oncotarget. 8:17328–17346.
2017.PubMed/NCBI
|
|
21
|
Dando I, Cordani M, Dalla Pozza E,
Biondani G, Donadelli M and Palmieri M: Antioxidant mechanisms and
ROS-related microRNAs in cancer stem cells. Oxid Med Cell Longev.
2015:4257082015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li QC, Xu H, Wang X, Wang T and Wu J:
miR-34a increases cisplatin sensitivity of osteosarcoma cells in
vitro through up-regulation of c-Myc and Bim signal. Cancer
Biomark. 21:135–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song C, Lu P, Sun G, Yang L and Wang Z and
Wang Z: miR-34a sensitizes lung cancer cells to cisplatin via
p53/miR-34a/MYCN axis. Biochem Biophys Res Commun. 482:22–27. 2017.
View Article : Google Scholar
|
|
24
|
Zhuang Q, Zhou T, He C, Zhang S, Qiu Y,
Luo B, Zhao R, Liu H, Lin Y and Lin Z: Protein phosphatase 2A-B55δ
enhances chemotherapy sensitivity of human hepatocellular carcinoma
under the regulation of microRNA-133b. J Exp Clin Cancer Res.
35:672016. View Article : Google Scholar
|
|
25
|
Shi L, Chen ZG, Wu LL, Zheng JJ, Yang JR,
Chen XF, Chen ZQ, Liu CL, Chi SY, Zheng JY, et al: miR-340 reverses
cisplatin resistance of hepatocellular carcinoma cell lines by
targeting Nrf2-dependent antioxidant pathway. Asian Pac J Cancer
Prev. 15:10439–10444. 2014. View Article : Google Scholar
|
|
26
|
Xu N, Shen C, Luo Y, Xia L, Xue F, Xia Q
and Zhang J: Upregulated miR-130a increases drug resistance by
regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell.
Biochem Biophys Res Commun. 425:468–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Jiang W, Hu Y, Da Z, Zeng C, Tu M,
Deng Z and Xiao W: MicroRNA-199a-5p inhibits cisplatin-induced drug
resistance via inhibition of autophagy in osteosarcoma cells. Oncol
Lett. 12:4203–4208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Yao J, Guo K, Huang H, Huai S, Ye
R, Niu B, Ji T, Han W and Li J: The functional mechanism of
miR-125b in gastric cancer and its effect on the chemosensitivity
of cisplatin. Oncotarget. 9:2105–2119. 2017.
|
|
29
|
Luo Y, Ouyang J, Zhou D, Zhong S, Wen M,
Ou W, Yu H, Jia L and Huang Y: Long noncoding RNA GAPLINC promotes
cells migration and invasion in colorectal cancer cell by
regulating miR-34a/c-MET signal pathway. Dig Dis Sci. 63:890–899.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tanaka N, Toyooka S, Soh J, Tsukuda K,
Shien K, Furukawa M, Muraoka T, Maki Y, Ueno T, Yamamoto H, et al:
Downregulation of microRNA-34 induces cell proliferation and
invasion of human mesothelial cells. Oncol Rep. 29:2169–2174. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Misík V and Riesz P: Free radical
intermediates in sonodynamic therapy. Ann N Y Acad Sci.
899:335–348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosenthal I, Sostaric JZ and Riesz P:
Sonodynamic therapy-a review of the synergistic effects of drugs
and ultrasound. Ultrason Sonochem. 11:349–363. 2004.PubMed/NCBI
|
|
33
|
Tinkov S, Coester C, Serba S, Geis NA,
Katus HA, Winter G and Bekeredjian R: New doxorubicin-loaded
phospholipid micro-bubbles for targeted tumor therapy: in-vivo
characterization. J Control Release. 148:368–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Todorova M, Agache V, Mortazavi O, Chen B,
Karshafian R, Hynynen K, Man S, Kerbel RS and Goertz DE: Antitumor
effects of combining metronomic chemotherapy with the antivascular
action of ultrasound stimulated microbubbles. Int J Cancer.
132:2956–2966. 2013. View Article : Google Scholar
|
|
35
|
Goertz DE, Todorova M, Mortazavi O, Agache
V, Chen B, Karshafian R and Hynynen K: Antitumor effects of
combining docetaxel (taxotere) with the antivascular action of
ultrasound stimulated microbubbles. PLoS One. 7:e523072012.
View Article : Google Scholar
|
|
36
|
Yu T, Yang Y, Liu S and Yu H: Ultrasound
increases DNA damage attributable to cisplatin in
cisplatin-resistant human ovarian cancer cells. Ultrasound Obstet
Gynecol. 33:355–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Fan H, Wang Z, Zheng J and Cao W:
Potentiation of scutellarin on human tongue carcinoma xenograft by
low-intensity ultrasound. PLoS One. 8:e594732013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv Y, Fang M, Zheng J, Yang B, Li H,
Xiuzigao Z, Song W, Chen Y and Cao W: Low-intensity ultrasound
combined with 5-aminolevulinic acid administration in the treatment
of human tongue squamous carcinoma. Cell Physiol Biochem.
30:321–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang D, Okada K, Komori C, Itoi E and
Suzuki T: Enhanced antitumor activity of ultrasonic irradiation in
the presence of new quinolone antibiotics in vitro. Cancer Sci.
95:845–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu G, Zhao X, Wang J, Lv L, Wang C, Feng
L, Shen L and Ren W: miR-125b regulates the drug-resistance of
breast cancer cells to doxorubicin by targeting HAX-1. Oncol Lett.
15:1621–1629. 2018.PubMed/NCBI
|
|
41
|
Zhang QA, Xu HY, Chen D, et al: miR-34
increases in vitro PANC-1 cell sensitivity to gemcitabine via
targeting Slug/PUMA. Cancer Biomark. 21:755–762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Toyota M, Suzuki H, Sasaki Y, Maruyama R,
Imai K, Shinomura Y and Tokino T: Epigenetic silencing of
microRNA-34b/c and B-cell translocation gene 4 is associated with
CpG island methylation in colorectal cancer. Cancer Res.
68:4123–4132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Achari C, Winslow S, Ceder Y and Larsson
C: Expression of miR-34c induces G2/M cell cycle arrest in breast
cancer cells. BMC Cancer. 14:5382014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Garofalo M, Jeon YJ, Nuovo GJ, Middleton
J, Secchiero P, Joshi P, Alder H, Nazaryan N, Di Leva G, Romano G,
et al: MiR-34a/c-dependent PDGFR-α/β downregulation inhibits
tumorigenesis and enhances TRAIL-induced apoptosis in lung cancer.
PLoS One. 8:e675812013. View Article : Google Scholar
|
|
45
|
Gherardi E, Birchmeier W, Birchmeier C and
Vande Woude G: Targeting MET in cancer: Rationale and progress. Nat
Rev Cancer. 12:89–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Awad MM, Oxnard GR, Jackman DM, Savukoski
DO, Hall D, Shivdasani P, Heng JC, Dahlberg SE, Jänne PA, Verma S,
et al: MET exon 14 mutations in non-small-cell lung cancer are
associated with advanced age and stage-dependent MET genomic
amplification and c-met overexpression. J Clin Oncol. 34:721–730.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han P, Li H, Jiang X, Zhai B, Tan G, Zhao
D, Qiao H, Liu B, Jiang H and Sun X: Dual inhibition of Akt and
c-Met as a second-line therapy following acquired resistance to
sorafenib in hepatocellular carcinoma cells. Mol Oncol. 11:320–334.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
de Rosamel L and Blanc JF: Emerging
tyrosine kinase inhibitors for the treatment of hepatocellular
carcinoma. Expert Opin Emerg Drugs. 22:175–190. 2017. View Article : Google Scholar : PubMed/NCBI
|