Introduction
Gallbladder cancer (GBC) is the most common biliary
tract malignancy and the fifth common gastrointestinal cancer, with
an incidence of 2.5 per 100,000 population globally (1). The majority of GBC cases were
patients who underwent exploration for cholelithiasis (2). Despite the collaboration within
multidisciplinary teams of surgeons, oncologists, and endoscopy
experts to provide the most appropriate treatment strategy for
patients with GBC, the prognosis remains extremely poor (3,4).
Therefore, it is essential to uncover the biological mechanism of
GBC, in order to develop a novel therapeutic strategy for GBC.
The family of eukaryotic initiation factors (eIFs)
includes at least 12 eukaryotic initiation factors, which are
composed of several polypeptides (5). One of those polypeptides, namely
eIF4E, was proposed by numerous previous studies to be a critical
nexus for cancer development (6).
Transcriptional profiling of metastatic human solid tumors revealed
that eIF4E was one of the genes that was consistently up-regulated
(7). However, whether eIF4E is
associated with the human GBC remains unclear.
In present study, the protein level of eIF4E was
notably increased in GBC tissues compared with normal gallbladder
tissues by immunohistochemistry (IHC) analysis. Increased eIF4E
levels were associated with a poorer prognosis. Subsequent results
indicated that knockdown of eIF4E suppressed cell proliferation
in vitro and in vivo.
Materials and methods
Patients and samples
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Anhui Medical
University (Hefei, China), and all patients provided written
informed consent. A total of 76 GBC specimens were obtained from
GBC patients who underwent radical cholecys-tectomy at the General
Surgery Department between November of 2011 and November of 2016.
All patients who were enrolled in the study did not receive any
prior treatment. The control group included 58 normal gallbladder
tissues, of which 21 specimens were obtained from patients who were
diagnosed with hepatic hemangioma and underwent hepatectomy
combined with cholecystectomy, and the other 37 gallbladder adenoma
tissues obtained from patients with gallbladder adenoma who
underwent laparoscopic cholecystectomy (Table I). All tissues were assessed by
tissue microarrays (TMA). Anatomic and histologic grades were
established according to the criteria by American Joint Committee
on Cancer (AJCC) tumor node metastasis (TNM) Staging for
Gallbladder Cancer (8).
 | Table IExpression level of eIF4E in normal
gallbladder, gallbladder adenomas and GBC tissues. |
Table I
Expression level of eIF4E in normal
gallbladder, gallbladder adenomas and GBC tissues.
| Variable | No. of cases
<70/≥70 | No. of cases
(male/female) | Optical density of
eIF4E expression (x±s) | P-value |
|---|
| Normal
gallbladder | 15/6 | 7/14 | 0.264 (0.145,
0.278) | 0.047a |
| Gallbladder
adenomas | 21/16 | 13/24 | 0.341 (0.292,
0.374) | 0.010b |
| GBC | 49/27 | 24/52 | 0.401 (0.348,
0.453) |
3.610×10−7c |
Reagents and antibodies
The following reagents used in the present study
were purchased from the following sources:
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), complete EDTA-free protease inhibitor cocktail
(Roche Applied Science), sea plaque low melting temperature agarose
(Lonza Group, Ltd.), GAPDH antibody (cat. no. sc-166545; 1:5,000;
Santa Cruz Biotechnology, Inc.), eIF4E antibody (cat. no.
sc-271480; 1:1,000; Santa Cruz Biotechnology, Inc.),
cyclin-dependent kinase inhibitor 1B (p27) antibody (cat. no.
P1484; 1:2,000; Sigma-Aldrich; Merck KGaA), cyclinE1 antibody (cat.
no. AF0144; 1:1,000; Affinity Biosciences), cyclinD1 antibody (cat.
no. AF0931; 1:1,000; Affinity Biosciences), Ki67 antibody (cat. no.
AF0198, 1:200; Affinity Biosciences) and horseradish
peroxidase-conjugated secondary antibodies (cat. nos. sc-2005 and
sc-2004; 1:10,000; Santa Cruz Biotechnology, Inc.).
Cell culture
The 2 human GBC cell lines used in the present study
were GBC-SD (Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences) and NOZ (Health Science Research Resources
Bank). The GBC-SD cells were cultured in Dulbecco's modified Eagle
medium (DMEM; Thermo Fisher Scientific, Inc.). The NOZ cells were
cultured in William's medium E (Lonza Group, Ltd.) at 37°C in a
humidified 5% CO2 incubator. Both media were
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.). The NOZ and GBC-SD cells were passaged for 9
times prior to use in the indicated functional experiments. All the
cell lines were routinely examined for mycoplasma contamination
prior to use.
RNA interference
To generate lentiviruses expressing the indicated
shRNA, 293T cells (Americn Type Culture Collection) grown on a 6 cm
dish were transfected with 2 µg shRNA (cloned in PLKO.1,
Sigma-Aldrich; Merck KGaA), 2 µg pREV, 2 µg
pGag/Pol/PRE, and 1 µg pVSVG. A total of 12 h after
transfection using Lipofectamine® 2000, Invitrogen;
Thermo Fisher Scientific, Inc.), the cells were cultured in DMEM
containing 20% FBS for an additional 24 h. The culture medium
containing lentivirus particles was filtered through a 0.45-lm
polyvinylidene difluoride (PVDF) membrane filter (EMD Millipore).
Then, 50% filtered DMEM containing lenti-virus particles with 50%
fresh DMEM or William's medium E was incubated with GBC-SD or NOZ
cells supplemented with 8 µg/ml polybrene (Sigma-Aldrich;
Merck KGaA) for 24 h, followed by selection with 2 µg/ml
puromycin for an additional 24 h. The knockdown efficiency was
evaluated by western blot analysis. Cell flow was executed 96 h
after transfection. The shRNA target sequences used were as
follows: sh-control: CCT AAG GTT AAG TCG CCC TCG, sh-eIF4E-1
(human): CCA CTC TGT AAT AGT TCA GTA, sh-eIF4E-2 (human): CCA AAG
ATA GTG ATT GGT TAT.
Cell proliferation assay
A total of 1×104 GBC-SD or NOZ cells
expressing control shRNA or eIF4E shRNA suspended in 0.5 ml DMEM or
William's medium E containing 10% FBS were plated in 24-well plates
and incubated at 37°C for 7 days. The cells were counted using
Countstar Automated Cell Counter (Simo Biotechnology Co., Ltd.) at
3rd, 5th and 7th days.
Colony formation assay
To measure colony formation on plates,
1×103 GBC-SD or NOZ cells expressing control shRNA or
eIF4E shRNA suspended in 2 ml DMEM or William's medium E containing
10% FBS were plated in 6-well plates and incubated at 37°C for 3
weeks. The colonies were then fixed with 4% paraformaldehyde for 15
min at room temperature, stained with trypan blue (room
temperature, 15 min), and scored using Image J (v1.8.0, National
Institutes of Health). To measure the colony formation in soft
agar, 1×104 GBC-SD or NOZ cells expressing control shRNA
or eIF4E shRNA suspended in 1.5 ml DMEM or William's medium E
containing 10% FBS and 0.3% SeaPlaque low melting temperature
agarose were plated in 1 well of 6-well plates over a 1.5 ml layer
of DMEM or William's medium E containing 10% FBS/0.6% agarose.
Cells were incubated at 37°C for 3 weeks prior to fixing, staining
and scoring, as aforementioned.
IHC
The expression level of eIF4E was detected in TMA
slides of GBC, adenomas and normal gallbladder tissues as described
previously (9). The average
optical density of TMA staining was measured with Image Pro Plus
6.0 software (Media Cybernetics, Inc.).
Western blot analysis
The cells were lysed in radioimmunopre-cipitation
assay buffer (Beyotime of Institute of Biotechnology) in an ice
bath for 30 min, and centrifuged at 12,000 x g for 20 min at 4°C.
The supernatant was stored at −80°C until analysis. The protein
concentration was measured using the bicinchoninic acid (BCA) assay
kit (Beyotime of Institute of Biotechnology). The equal amount (30
µg) of proteins was loaded onto 10% SDS-PAGE and transferred
by electroblotting to a PVDF membrane (EMD Millipore). The membrane
was blocked with 5% bovine serum albumin (Sangon Biotech Co., Ltd.)
for 1 h at room temperature. Primary antibodies were incubated at
4°C overnight. The secondary antibody was added to the membrane at
room temperature for 1 h. Image J (v1.8.0) was used for density
analysis.
Xenograft model
A total of 5×106 NOZ cells expressing
control shRNA or eIF4E shRNA were individually injected into the
dorsal flanks of 4-week-old male athymic nude mice (Nanjing SLAC
Laboratory Animal Co. Ltd.) (n=5 per group). The mice were randomly
assigned to groups in the experiment (sh-control: 5 nude mice,
sh-eIF4E-1: 5 nude mice). A total of 1 week after injection, tumors
were measured per 4 days with Vernier calipers and calculated using
the following equation: volume=length x width2 ×0.52.
Then, 4 weeks after injection, the mice were sacrificed, and the
tumors were excised and weighed. During measurement of the weight
of the tumors, the experimentalists were blinded to the information
of tumor tissues. The excised tumors were homogenized, and proteins
were extracted for western blot analysis, as aforementioned.
Studies on animals were conducted on the basis of approval from the
Animal Research Ethics Committee of the University of Science and
Technology of China (approval no. PXTG-MYD2017102611).
Statistical analysis
The statistical analysis was conducted by using SPSS
17.0 software (IBM Corp.). Data were presented as the mean ±
standard deviation. The skewness data are expressed as the mean
with 1st and 3rd interquartile ranges, and were determined using a
Mann-Whitney U test. The mortality risks of GBC were analyzed using
Kaplan-Meier estimator for univariate survival analysis followed by
log-rank test. The patient's age, sex, anatomic stage, histological
grade and eIF4E status indicators were analyzed by the Cox
proportional hazards model. The data from the in vitro
experiments were analyzed using one-way analysis of variance
followed by Bonferroni's correction post-hoc test.
Results
Overexpressing eIF4E indicates a poor
prognosis in patients with GBC
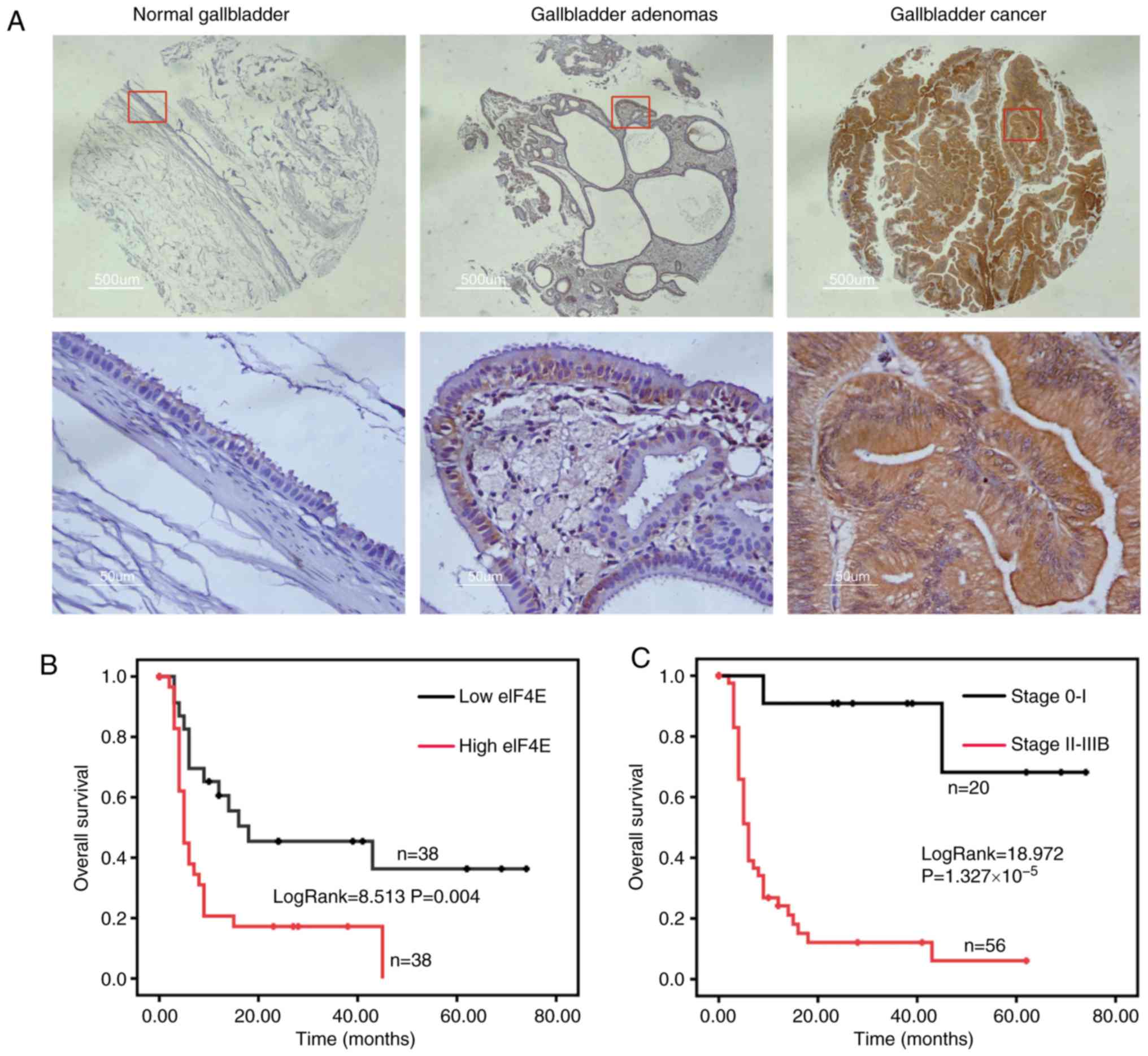
eIF4E expression was assessed in normal gallbladder,
gallbladder adenomas and GBC by IHC. The expression of eIF4E was
significantly increased in GBC compared with gallbladder adenomas,
and normal gallbladder tissue exhibited significantly decreased
eIF4E expression compared with gallbladder adenomas (Fig. 1A; Table I). The expression of eIF4E was
notably increased in advanced stage GBC (anatomic stage III and IV)
compared with early stage GBC (anatomic stage I and II) (P=0.022;
Table II). Furthermore, the
eIF4E expression was markedly associated with histological grade
(P=0.004; Table II).
 | Table IIExpression of eIF4E and
clinicopathological characteristics of patients with gallbladder
cancer. |
Table II
Expression of eIF4E and
clinicopathological characteristics of patients with gallbladder
cancer.
| Variable | N | Optical density of
eIF4E expression
|
|---|
| Value, mean (1st and
3rd interquartile ranges) | Z-value | P-value |
|---|
| Age | | | −0.613 | 0.540 |
| <70 | 49 | 0.401 (0.334,
0.455) | | |
| ≥70 | 27 | 0.398 (0.357,
0.452) | | |
| Sex | | | −0.995 | 0.320 |
| Male | 24 | 0.383 (0.348,
0.429) | | |
| Female | 52 | 0.408 (0.347,
0.467) | | |
| Primary tumor
(T) | | | −2.194 | 0.028 |
| Tis-T1 | 22 | 0.350 (0.278,
0.440) | | |
| T2-T4 | 54 | 0.394 (0.357,
0.460) | | |
| Regional lymph
nodes (N) | | | −0.235 | 0.814 |
| N0 | 52 | 0.391 (0.342,
0.467) | | |
| N1 | 24 | 0.408 (0.353,
0.447) | | |
| Anatomic stage | | | −2.283 | 0.022 |
| 0-I | 20 | 0.349 (0.288,
0.435) | | |
| II-IIIB | 56 | 0.413 (0.357,
0.457) | | |
| Histologic
grade | | | −2.908 | 0.004 |
| G1-G2
(well-moderately) | 51 | 0.387 (0.326,
0.431) | | |
| G3-G4
(poorly-undifferentiated) | 25 | 0.441 (0.377,
0.521) | | |
Then, the Kaplan-Meier analysis indicated that the
group with increased eIF4E expression (the optical density of TMA
staining was ≥0.3955) was significantly associated with poor
overall survival (OS) in patients with GBC (log-rank=8.513;
P=0.004; Fig. 1B). However, the
OS of the early stage (0-I) group was notably improved compared
with that in the advanced stage (II-IIIB) group (log-rank=18.972;
P=1.327×10−5; Fig.
1C). The median OS for the high eIF4E expression group was 5
months, whilst it was 18 months for the low eIF4E expression group
(Table III).
 | Table IIIMultivariate Cox regression analysis
of the anatomic stage, histologic grade and optical density of
eukaryotic translation initiation factor 4 expression in
gallbladder cancer cells. |
Table III
Multivariate Cox regression analysis
of the anatomic stage, histologic grade and optical density of
eukaryotic translation initiation factor 4 expression in
gallbladder cancer cells.
| Variable | B | SE B | Wald | Relative risk (95%
confidence interval) | P-value |
|---|
| Anatomic stage | 2.532 | 0.762 | 11.041 | 12.575
(2.825-55.986) | 0.001 |
| Histologic
grade | 0.794 | 0.353 | 5.067 | 2.212
(1.108-4.416) | 0.024 |
| Optical density of
eIF4E expression | 1.054 | 0.374 | 7.937 | 2.868
(1.378-5.969) | 0.005 |
Furthermore, multivariate Cox regression analysis
confirmed that overexpression of eIF4E was negatively correlated
with post-operative OS, whereas it was positively associated with
anatomic stage and historical grade, indicating that eIF4E
expression is an independent prognostic factor in patients with
GBC.
Knockdown of eIF4E inhibits the
proliferation and colony formation of GBC and NOZ cells in
vitro
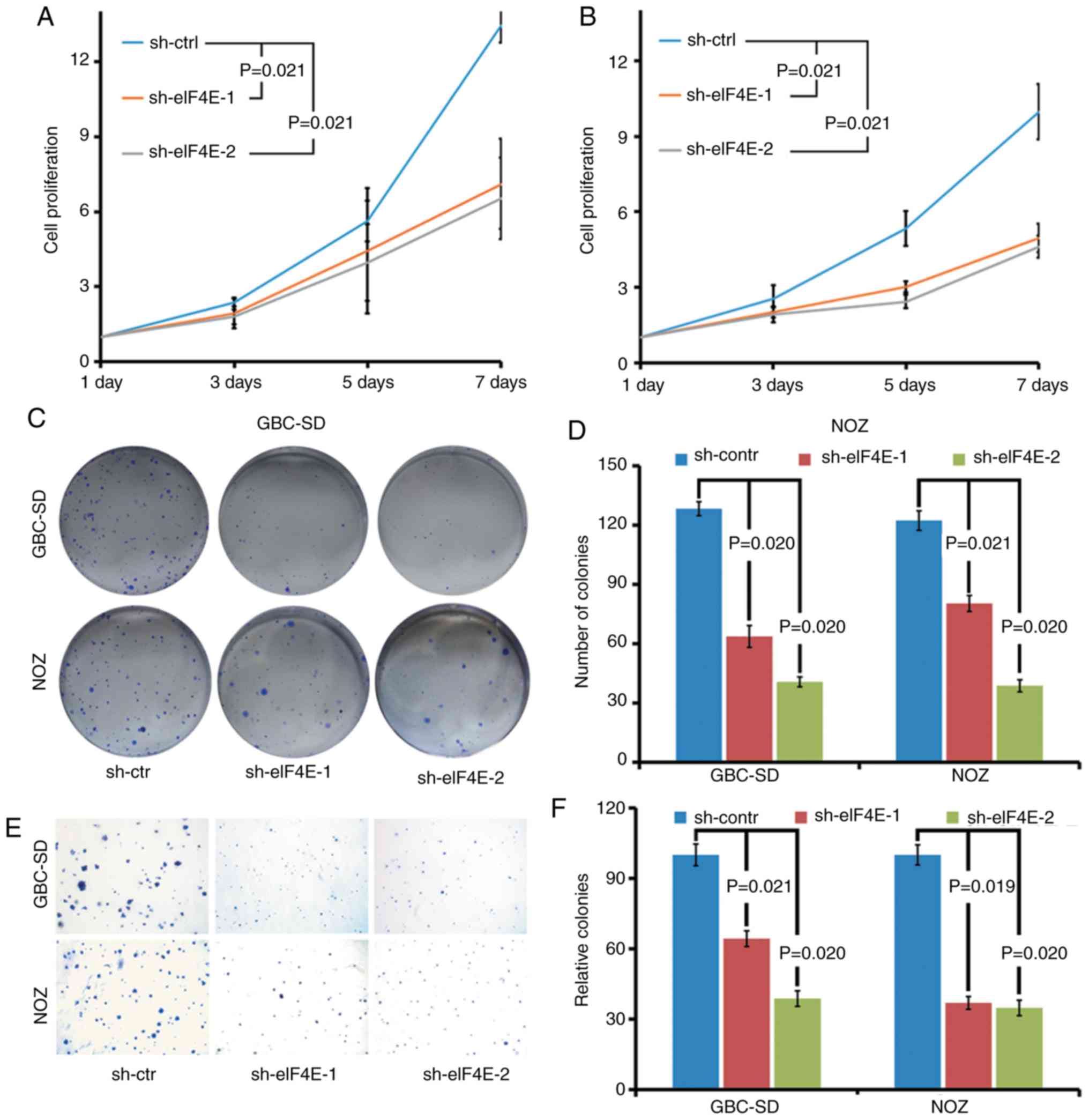
The biological function of eIF4E in GBC was
investigated using eIF4E-specific shRNA knockdown cells. The
effects of eIF4E on proliferation of GBC cells were determined by
cell growth curve and colony formation assays. As demonstrated in
Fig. 2A and B, the cell growth
curve indicated that the knockdown of eIF4E significantly inhibited
the proliferation of GBC-SD and NOZ cells. Consistent with the
observations of the cell growth curve, the colony forming ability
of GBC-SD and NOZ cells was markedly decreased in eIF4E shRNA
transfected cells (sh-eIF4E group; Fig. 2C and D). Furthermore, the number
of the colonies in the sh-eIF4E group was significantly decreased
compared with that of the sh-control group in soft agar (Fig. 2E and F).
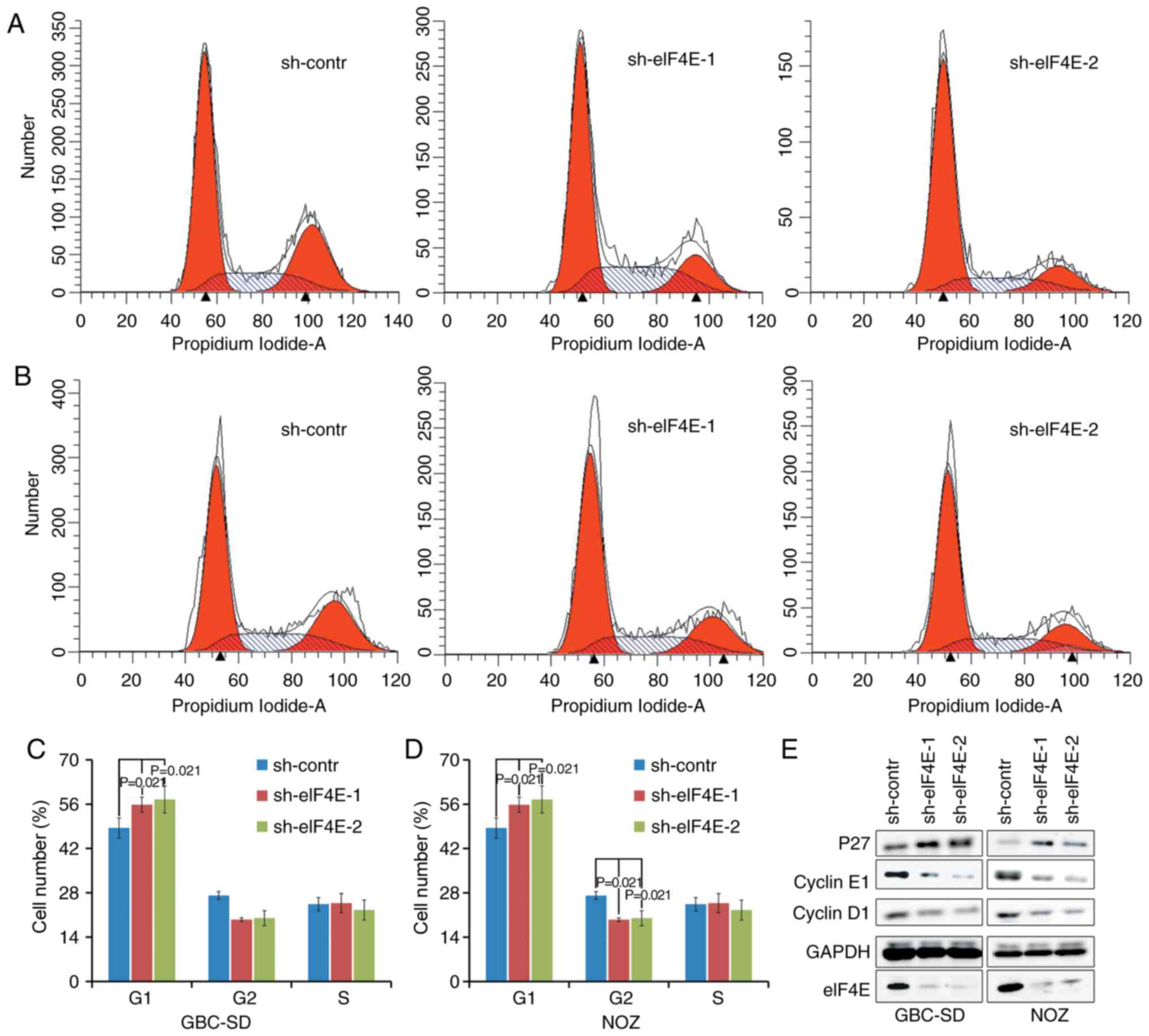
Knockdown of eIF4E inhibits the cell
cycle of GBC and NOZ cells in vitro
Subsequent analyses were conducted to evaluate the
effects of eIF4E on cell cycle using flow cytometry. A total of 96
h after lentivirus infection, an increase in the fraction of cells
in the G0/G1 phase was observed in sh-eIF4E
group compared with that in the sh-control group in GBC-SD and NOZ
cells. The fraction of cells in the G2/M phase decreased
in knocking down eIF4E shRNA-transfected NOZ cells (Fig. 3A-D). In addition, whether eIF4E
was able to regulate the expression levels of cell cycle-associated
proteins was investigated using western blot analysis. As indicated
in Fig. 3E, knockdown of eIF4E
resulted in a decrease in the expression levels of cyclin D and
cyclin E, in addition to an increase in the expression level of p27
in GBC-SD and NOZ cells compared with those in the control group.
Collectively, these data demonstrated that eIF4E serves a pivotal
role in regulating proliferation of GBC cells by modulating the
expression of cell cycle-associated proteins.
Knockdown of eIF4E inhibits the GBC tumor
growth in vivo
To additionally confirm the function of eIF4E in GBC
development, subcutaneous xenograft models were established in
BALB/C nude mice using NOZ cells. As demonstrated in Fig. 4A-D, the tumor growth was
significantly suppressed in sh-eIF4E group compared with that in
the sh-control group, as evidenced by decrease of volume and weight
of xenografts.
Additionally, the expression levels of p27, cyclin
D1 and cyclin E1 in harvested subcutaneous xenografts was detected
using western blot analysis, and the expression levels of P27,
cyclin D1, cyclin E1 and Ki-67 by IHC assays. As demonstrated in
Fig. 4E and F, knockdown of eIF4E
resulted in the decreased expression levels of cyclin D1, cyclin E1
and Ki-67, while increased expression of p27. Taken together, these
results indicated that knockdown of eIF4E inhibits the GBC tumor
growth in vivo.
Discussion
At present, the biological mechanism of eIF4E in GBC
is not well understood. In the present study, it was revealed that
the expression of eIF4E was associated with the prognosis of GBC
and its role in the proliferation of GBC cells.
Several previous studies have verified that eIF4E is
overexpressed or deregulated in different types of cancer,
including breast cancer (10),
melanoma (11), and prostate
(12), lung (13) and colorectal (14) cancer. It also has been
demonstrated that a subset of mRNAs encoding cancer-associated
proteins, such as c-MYC and cyclin D1, were identified to be
sensitive to the activity of the eIF4F complex (15) and may serve as a convergence point
for hyperactive signaling pathways to promote tumorigenesis
(16). The present study
confirmed that knockdown of eIF4E in GBC cells inhibited cell
proliferation, oncogenic potential and cell cycle arrest, which is
consistent with the previously described data (17,18).
Whether the knockdown of eIF4F is an appropriate
strategy for cancer treatment is an important avenue of study. By
generating an eIF4F haploinsufficient mouse model, Truitt et
al (19) identified that
decreasing the level of eIF4E inhibited the oncogenic potential of
human KRAS-driven lung cancer cells, and that this was not
detrimental to normal mammalian physiological processes. In
addition, the eIF4E must be phosphorylated to promote tumor
development (20). The androgen
receptor is a negative regulator of phosphorylation of serine 209
in eIF4F, indicating a potential therapeutic target in prostate
cancer (21). Based on
preclinical data demonstrating that eIF4F regulates the translation
of mRNAs involved in cell survival and chemotherapy resistance, a
clinical trial (clinical trial no. 01675128) that combined ISIS
183750, a second-generation antisense oligonucleotide designed to
inhibit the production of the eIF4E protein, and irinotecan in
patients with irinotecan-refractory metastatic colorectal cancer
and did not result in objective clinical responses (22). However, Robichaud et al
(23) attempted to provide a
rationale for targeting eIF4E phosphorylation in cancer cells and
cells that comprise the tumor microenvironment to halt metastasis,
demonstrating the efficacy of this strategy using merestinib,
representing a therapeutic strategy for cancer.
The present study identified that the expression of
eIF4E was markedly upregulated in GBC tissues compared with
gallbladder adenomas samples or normal gallbladder tissues, and
that high eIF4E expression levels in GBC tissues were associated
with advanced stage, higher histologic grade and poorer
prognosis.
In summary, the results from the present study
demonstrated that eIF4E served a critical role in the regulation of
cell proliferation, and may function as an independent prognostic
maker for GBC.
Acknowledgments
The authors would like to thank Dr Chengfeng Wang
from the Chinese Academy of Science Key Laboratory of Innate
Immunity and Chronic Disease, School of Life Sciences and Medical
Center, University of Science and Technology of China for his
scrupulous and skillful assistance in generating the subcutaneous
xenograft models in BALB/C nude mice.
Funding
The present study was financially supported by
Natural Science Research Project of Anhui Medical University (grant
no. 2018xkj057).
Availability of data and materials
All data generated during this study are included in
this published article.
Authors' contributions
XG and DF made substantial contributions to the
design of the present study; DF, JP, GW, and DZ performed the
experiments; DF and JP analyzed the data; DF wrote the manuscript.
All authors approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Anhui Medical
University (Hefei, China), and all patients provided written
informed consent. Studies on animals were conducted on the basis of
approval from the Animal Research Ethics Committee of the
University of Science and Technology of China (approval no.
PXTG-MYD2017102611).
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Randi G, Franceschi S and La Vecchia C:
Gallbladder cancer worldwide: Geographical distribution and risk
factors. Int J Cancer. 118:1591–1602. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pitt SC, Jin LX, Hall BL, Strasberg SM and
Pitt HA: Incidental gallbladder cancer at cholecystectomy: When
should the surgeon be suspicious? Ann Surg. 260:128–133. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elmasry M, Lindop D, Dunne DF, Malik H,
Poston GJ and Fenwick SW: The risk of malignancy in ultrasound
detected gallbladder polyps: A systematic review. Int J Surg. 33(Pt
A): 28–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dasari BVM, Ionescu MI, Pawlik TM, Hodson
J, Sutcliffe RP, Roberts KJ, Muiesan P, Isaac J, Marudanayagam R
and Mirza DF: Outcomes of surgical resection of gallbladder cancer
in patients presenting with jaundice: A systematic review and
meta-analysis. J Surg Oncol. 118:477–485. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aitken CE and Lorsch JR: A mechanistic
overview of translation initiation in eukaryotes. Nat Struct Mol
Biol. 19:568–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pelletier J, Graff J, Ruggero D and
Sonenberg N: Targeting the eIF4F translation initiation complex: A
critical nexus for cancer development. Cancer Res. 75:250–263.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramaswamy S, Ross KN, Lander ES and Golub
TR: A molecular signature of metastasis in primary solid tumors.
Nat Genet. 33:49–54. 2003. View
Article : Google Scholar
|
|
8
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th ed.
Springer; New York, NY: 2010
|
|
9
|
Lu Li M, Zhang J, Li F, Zhang H, Wu B, Tan
X, Zhang Z, Gao L, Mu GJ, et al: Yes-associated protein 1 (YAP1)
promotes human gallbladder tumor growth via activation of the
AXL/MAPK pathway. Cancer Lett. 355:201–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin X, Kim RH, Sun G, Miller JK and Li BD:
Overexpression of eukaryotic initiation factor 4E is correlated
with increased risk for systemic dissemination in node-positive
breast cancer patients. J Am Coll Surg. 218:663–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khosravi S, Tam KJ, Ardekani GS, Martinka
M, McElwee KJ and Ong CJ: eIF4E is an adverse prognostic marker of
melanoma patient survival by increasing melanoma cell invasion. J
Invest Dermatol. 135:1358–1367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwegyir-Afful AK, Bruno RD,
Purushottamachar P, Murigi FN and Njar VC: Galeterone and VNPT55
disrupt Mnk-eIF4E to inhibit prostate cancer cell migration and
invasion. FEBS J. 283:3898–3918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu F, Wang X, Li J, Gu K, Lv L, Zhang S,
Che D, Cao J, Jin S and Yu Y: miR-34c-3p functions as a tumour
suppressor by inhibiting eIF4E expression in non-small cell lung
cancer. Cell Prolif. 48:582–592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wiegering A, Uthe FW, Jamieson T, Ruoss Y,
Hüttenrauch M, Küspert M, Pfann C, Nixon C, Herold S, Walz S, et
al: Targeting translation initiation bypasses signaling crosstalk
mechanisms that maintain high MYC levels in colorectal cancer.
Cancer Discov. 5:768–781. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sonenberg N and Hinnebusch AG: Regulation
of translation initiation in eukaryotes: Mechanisms and biological
targets. Cell. 136:731–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siddiqui N and Sonenberg N: Signalling to
eIF4E in cancer. Biochem Soc Trans. 43:763–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wan J, Shi F, Xu Z and Zhao M: Knockdown
of eIF4E suppresses cell proliferation, invasion and enhances
cisplatin cytotoxicity in human ovarian cancer cells. Int J Oncol.
47:2217–2225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen B, Zhang B, Xia L, Zhang J, Chen Y,
Hu Q and Zhu C: Knockdown of eukaryotic translation initiation
factor 4E suppresses cell growth and invasion, and induces
apoptosis and cell cycle arrest in a human lung adenocarcinoma cell
line. Mol Med Rep. 13:33842016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Truitt ML, Conn CS, Shi Z, Pang X,
Tokuyasu T, Coady AM, Seo Y, Barna M and Ruggero D: Differential
requirements for eIF4E dose in normal development and cancer. Cell.
162:59–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robichaud N, del Rincon SV, Huor B, Alain
T, Petruccelli LA, Hearnden J, Goncalves C, Grotegut S, Spruck CH,
Furic L, et al: Phosphorylation of eIF4E promotes EMT and
metastasis via translational control of SNAIL and MMP-3. Oncogene.
34:2032–2042. 2015. View Article : Google Scholar
|
|
21
|
D'Abronzo LS, Bose S, Crapuchettes ME,
Beggs RE, Vinall RL, Tepper CG, Siddiqui S, Mudryj M, Melgoza FU,
Durbin-Johnson BP, et al: The androgen receptor is a negative
regulator of eIF4E phosphorylation at S209: Implications for the
use of mTOR inhibitors in advanced prostate cancer. Oncogene.
36:6359–6373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duffy AG, Makarova-Rusher OV, Ulahannan
SV, Rahma OE, Fioravanti S, Walker M, Abdullah S, Raffeld M,
Anderson V, Abi-Jaoudeh N, et al: Modulation of tumor eIF4E by
antisense inhibition: A phase I/II translational clinical trial of
ISIS 183750-an antisense oligonucleotide against eIF4E-in
combination with irinotecan in solid tumors and
irinotecan-refractory colorectal cancer. Int J Cancer.
139:1648–1657. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robichaud N, Hsu BE, Istomine R, Alvarez
F, Blagih J, Ma EH, Morales SV, Dai DL, Li G, Souleimanova M, et
al: Translational control in the tumor microenvironment promotes
lung metastasis: Phosphorylation of eIF4E in neutrophils. Proc Natl
Acad Sci USA. 115:E2202–E2209. 2018. View Article : Google Scholar : PubMed/NCBI
|