Introduction
Liver fibrosis (LF), which results from the
overaccumulation of liver stellate cells and extracellular matrix
by continuous hepatocellular injury and inflammatory reactions, is
a main cause of the incidence and mortality rates of patients with
liver-related diseases (1,2).
Moreover, LF at an advanced stage leads to cirrhosis with
architecture and attendant functional loss and eventually causes
lethal complications (3). LF is
mostly triggered by viral infection, but can also be triggered by
alcoholism, chronic cholangiopathy, autoimmune hepatitis and
obesity (4). Currently, LF is
mainly diagnosed by non-invasive diagnostic methods, such as
magnetic resonance imaging techniques and ultrasonography (5). Additionally, liver biopsy has failed
to be effective for LF staging and diagnosis due to sampling
uncertainty, possible pain and high patient reluctance (6). Moreover, current effective
treatments for LF are limited only to the removal of the potential
etiology and liver transplantation (7). Novel therapeutic strategies for LF
thus are thus urgently required.
Resveratrol (RSV) is associated with different
signaling pathways and immune responses due to its significant
anti-inflammatory effects and oxidation resistance (8). In a recent study, RSV was found to
strengthen liver function and block oxidative stress, thus
alleviating LF (9). RSV relieves
LF by inducing autophagy (10).
Phosphatase and tensin homolog is highly involved in disease
pathogenesis and the intracellular axis, rendering it an
indispensable biomarker in a number of human diseases (11). PTEN has been found to hinder
hepatic stellate cell (HSC) activation during LF (12). The regulation of PTEN in LF is
tightly bound to autophagy (13).
PTEN also affects the downstream PI3K/AKT signaling pathway and
thus plays an essential role in LF (14). It has been previously demonstrated
that RSV hinders HSC activation to obstruct LF by inhibiting the
PI3K/AKT signaling pathway (15,16). The PI3K/PTEN/AKT axis has been
found to be related to the induction of autophagy in
liver-associated disorders (17).
miR-20a expression has been shown to be markedly increased in
patients with LF (18).
Furthermore, a negative correlation between miR-20a and PTEN has
been found in liver diseases (19). It is also evident that RSV can
attenuate the inhibitory effects of miR-20a on PTEN (20). From the above, it is thus
reasonable to hypothesize that RSV, the PTEN/PI3K/AKT signaling
pathway and miR-20a interact in LF. Thus, the present study
conducted a series of experiments to verify this hypothesis.
Materials and methods
Ethics statement
The present study was approved and super-vised by
the Ethics Committee of the Affiliated Baiyun Hospital of Guizhou
Medical University (Approval no. 2019-064). Each step in this
experiment was approved by the laboratory animal ethics
committee.
Model establishment and animal
grouping
A total of 60 healthy adult male Sprague-Dawley rats
[6-8 weeks old, weighing 250±30 g; Guangzhou University of
Traditional Chinese Medicine; SYXK (Guangdong) 2018-0182] were
adaptively raised in a room with 50-60% humidity at 20-24°C with
free access to food and water for 1 week prior to being used in the
following experiments.
The rats were arranged by body weight and randomly
assigned into the following groups of 12 rats each: i) The normal
group; ii) LF (model) group; iii) low-dose RSV (L) group (treated
with 40 mg/kg RSV); iv) medium-dose RSV (M) group (treated with 120
mg/kg RSV; and v) the high-dose RSV (H) group (treated with 200
mg/kg RSV). The doses of RSV used were according to relevant
studies (21-24).
Apart from the rats in the normal group, which were
injected with 1 ml/kg peanut oil, the rats in the other groups were
intraperitoneally injected with a 1:1 mixture of 1 ml/kg carbon
tetrachloride (CCL4) and peanut oil 3 times a week for 6
weeks. Beginning at the end of the 6th week, the rats were injected
once a week for 4 weeks. Beginning at the end of the 6th week, the
rats in the RSV group were treated with RSV (R8350, Beijing
Solarbio Science & Technology Co., Ltd.) by gavage following an
intraperitoneal injection of CCL4 once a day for 4
weeks. During the experiment, the behaviors and health of the
animals were monitored by observing their diet, defecation and
changes in weight every day.
Animal treatment and tissue sample
collection
The experiment was performed based on the ARRIVE
checklist (https://www.nc3rs.org.uk/arrive-guidelines). At the
end of the 10th week, 24 h after the final dose, 1% pentobarbital
sodium (45 mg/kg) was intraperitoneally injected to anaesthetize
the rats. Rat blood (1 ml) was then routinely collected and
centrifuged at 4°C at 3,000 × g for 15 min to separate the serum,
which was saved at −20°C for further use. All 60 rats were then
euthanized by an intraperitoneal injection of 800 mg/kg
pentobarbital sodium (25), and
the death of the rats was confirmed by observing that there was no
fluctuation in the chest cavity (respiratory arrest) and no
breathing and heartbeat sound (cardiac arrest) using a stethoscope.
Subsequently, the rats were placed on ice for the immediate
collection of liver tissues. Liver tissue homogenates were obtained
from 3 rats in each group and stored at −80°C for use in subsequent
experiments. Liver tissues were prepared from another 3 rats from
each group for observation using a transmission electron microscope
(TEM). Tissues from the same liver regions of the remaining 6 rats
were fixed with 10% formaldehyde to prepare paraffin-embedded
sections for tissue staining.
Detection of alanine aminotransferase
(ALT), aspartate aminotransferase (AST) and albumin (ALB)
levels
The previously prepared serum samples stored at
−20°C were removed and allowed to thaw at room temperature. The
supernatant was then retained after the samples were centrifuged at
3,000 × g at 4°C for 10 min. ALT, AST and ALB levels in rat serum
were then detected with a kit in accordance with the manufacturer's
instructions (Nanjing Jiancheng Bioengineering Institute).
Detection of laminin (LN), hyaluronic
acid (HA), procollagen (PC)-III and collagen type 1 (COL-1)
levels
After serum samples and supernatants were obtained
following the treatments described above, the LN, HA, PC-III and
COL-1 levels in the rats were examined in accordance with the
instructions of the ELISA kits (Shanghai ML Biotech Co., Ltd.).
Detection of hydroxyproline (HYP),
malondialdehyde (MDA) and superoxide dismutase (SOD) levels
Following a wash in normal saline, 1 g of fresh
liver tissue was ground into tissue homogenate and centrifuged at
3,000 × g at 4°C for 10 min. The supernatant was then retained, and
HSC-T6 cells were collected. The HYP, MDA and SOD contents in the
liver tissue homogenate and cells were measured using HYP, MDA and
SOD kits (Beijing Solarbio Science & Technology Co., Ltd.) in
compliance with the manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using an RNA extraction kit
(Takara Biotechnology Co., Ltd.), and the extracted RNA
concentration was detected by measuring the absorbance at 260 nm.
Total RNA was reverse transcribed into complementary DNA (cDNA)
using a One StepPrimeScript miRNA cDNA Synthesis kit (Takara
Biotechnology Co., Ltd.). The PrimeScript RT Reagent kit with gDNA
Eraser (Takara Biotechnology Co., Ltd.) was used for reverse
transcription of the mRNA. cDNA was used as the template, and the
expression of each gene was detected by RT-qPCR with the SYBR
Premix Ex Tap™ II kit (Takara Biotechnology Co., Ltd.) according to
the manufacturer's instructions on a Roche LightCycler 480 (F.
Hoffmann-La Roche Ltd.). The PCR thermocycling conditions were as
follows: 95°C for 3 min, followed by 40 cycles at 95°C for 10 sec,
60°C for 20 sec, and 72°C for 40 sec. U6 was used as the internal
reference for miR-20a, and β-actin was used as the internal
reference for mRNA levels. The 2−ΔΔCq method was used to
calculate the expression levels (26). The sequences of the primers used
for each gene are listed in Table
I.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Gene | Primers |
|---|
| miR-20a | F:
5′-TAAAGTGCTTATAGTGCAGGTAG-3′ |
| R:
5′-CTACCTGCACTATAAGCACTTTA-3′ |
| U6 | F:
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| R:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
| PTEN | F:
5′-ACCAGTGGCACTGTTGTTTCAC-3′ |
| R:
5′-TTCCTCTGGTCCTGGTATGAAG-3′ |
| α-SMA | F:
5′-GGCCCTAGCACCCAGCACCATGAA-3′ |
| R:
5′-CCGGCTTCATCGTATTCCTGTTT-3′ |
| TGF-β1 | F:
5′-ATTCCTGGCGTTACCTTGG-3′ |
| R:
5′-AGCCCTGTATTCCGTCTCCT-3′ |
| TIMP-1 | F:
5′-TTCGTGGGGACACCAGAAGTC-3′ |
| R:
5′-TATCTGGGACCGCAGGGACTG-3′ |
| β-actin | F:
5′-GGCATGGGTCAGAAGGATTCC-3′ |
| R:
5′-ATGTCACGCACGATTTCCCGC-3′ |
Western blot analysis
Total protein was extracted from the tissue
homogenate and cells in each group using radioimmunoprecipitation
assay lysis buffer. The protein concentration was determined using
the bicinchoninic acid method, and loading buffer was added for
denaturation in a metal bath for 10 min at 100°C. Samples
containing the same amount of protein (30 g) were added to 10%
sodium dodecyl sulfate-polyacrylamide gels. Following
electrophoresis, the proteins were transferred onto polyvinylidene
fluoride membranes. The membranes were blocked with 5% skim milk
powder for 1 h and incubated with primary antibodies against the
following (all from Abcam) at 4°C overnight: α-smooth muscle actin
(α-SMA; ab32575, 1:1,000), tissue inhibitor of metalloproteinase-1
(TIMP-1; ab38978, 1:1,000), transforming growth factor-β1 (TGF-β1;
ab92486, 1 µg/ml), light chain (LC)3-I (ab51520, 1:3,000),
LC3-II (ab48394, 1:200), Beclin1 (ab210498, 1:1,000),
anti-thymocyte globulin (Atg)7 (ab52472, 1:100,000), p62 (ab91526,
1 µg/ml), COL-1 (ab96723, 1:500), PTEN (ab32199, 1:10,000)
and p-AKT (ab8805, 1:500), PI3K (ab40776, 1:1,000). The membranes
were incubated for 1 h with anti-rabbit immunoglobulin G (IgG;
ab6728, Abcam, 1:2,000) labeled with horseradish peroxidase and
then visualized for observation. β-actin (ab8226, Abcam, 1:1,000)
was applied as the internal reference. Relative expression is
represented by the ratio of the gray values for the target protein
and internal reference. Image Pro Plus 6.0 (Media Cybernetics,
Inc.) was used for densitometry.
Hematoxylin and eosin (H&E)
staining
The paraffin-embedded sections were regularly
dewaxed and stained with hematoxylin (Beijing Solarbio Science
& Technology Co., Ltd.) at room temperature for 3 min. After
washing, the sections were placed in hydrochloric acid and ammonium
hydroxide for 2 sec. The sections were then washed with distilled
water, stained with eosin at room temperature for 5 min and washed
with distilled water again. Subsequently, the sections were
dehydrated with gradient ethyl alcohol and cleared with xylene.
Finally, the membranes were sealed by neutral resin and then tested
by microscopy (BX60, Olympus Corporation). LF staging in rats was
determined by the Knodell histology activity index system (27).
Masson's trichrome staining
The paraffin-emdedded sections were regularly
dewaxed and stained with 100 µl of Masson's solution
(Beijing Solarbio Science & Technology Co., Ltd.) for 5 min.
The sections were then washed and then at room temperature stained
with 100 µl of phosphomolybdic acid for 5 min. When the
sections had dried, 100 µl of aniline blue was used to stain
the sections at room temperature for 5 min. After being washed with
distilled water, the sections were successively differentiated
twice by incubation with 100 µl of tissue differentiation
solution at room temperature for 40 sec each. The sections were
dehydrated with 95% ethyl alcohol and anhydrous alcohol,
permeabilized, sealed with neutral resin and examine under a
microscope (BX60, Olympus Corporation).
Immunohistochemical staining
The paraffin-embedded sections were placed on
anti-off slides and dried in a 60°C oven for 4 h. After regular
dewaxing and dehydration, the sections were incubated with a 3%
H2O2 solution for 15 min at room temperature
and then incubated with normal anti-goat serum for 15 min at room
temperature for antigen retrieval. Primary antibodies (all from
Abcam) against the following were added to the sections followed by
incubation at 4°C overnight: Beclin1 (ab210498, 1:100), Atg7
(ab52472, 1:500), α-SMA (ab5831, 5 µg/ml), COL-1 (ab3453,
1:500) and p-AKT (ab8805, 1:1,000). Following 3 washes in
phosphate-buffered saline (PBS) for 3 min each, secondary antibody
IgG (ab6721, 1:1,000) was mixed with the sections and incubated for
1 h at 37°C before another 3 PBS washes for 3 min each. The
sections were visualized with diaminobenzidine and counterstained
with hematoxylin for 3-5 min at room temperature. Following regular
dehydration, the dried sections were sealed in gum and then
observed by microscopy (BX60, Olympus Corporation). A total of 5
visual fields were randomly selected from each section and used to
calculate the ratio of positive cells among 100 cells.
TEM observation
Liver tissue sections at 1 mm3 were fixed
with 2% glutaraldehyde for 2 h. Following a buffer wash, the
tissues were fixed with osmic acid and dehydrated with a series of
acetone solutions for 5-15 min each. The liver tissues were soaked
in a mixture of acetone and embedding medium for 2-4 h at room
temperature, embedded in resin embedding medium, sliced and then
stained with uranyl acetate and lead citrate for 15-30 min.
Finally, the tissues were observed under a TEM (Philips CM10,
Philips).
Cell culture and treatment
HSC-T6 cells were obtained from the Shanghai Cell
Biochemistry Institute, Chinese Academy of Sciences. The cells were
cultured in Dulbecco's modified Eagle medium supplemented with 10%
fetal bovine serum and 1% penicillin/streptomycin in a 37°C
incubator with 5% CO2. After reaching 70-80% confluency,
the cells were sub-cultured. Cells in the exponential growth phase
were selected for use in subsequent experiments.
The HSC-T6 cells were assigned to the following
groups: i) The control group, ii) platelet-derived growth factor
(PDGF)-BB group [HSC-T6 cells were treated with 10 mg/ml PDGF-BB
(P00031, Beijing Solarbio Science & Technology Co., Ltd.) for
48 h]; iii) PDGF-BB + RSV + L group (HSC-T6 cells were treated with
10 mg/ml RSV prior to the addition of PDGF-BB 48 h later); iv)
PDGF-BB + RSV + M group (HSC-T6 cells were treated with 30 mg/ml
RSV prior to the addition of PDGF-BB 48 h later); v) the PDGF-BB +
RSV + H group (HSC-T6 cells were treated with 50 mg/ml RSV prior to
the addition of PDGF-BB 48 h later). The HSC-T6 cells treated with
30 mg/ml RSV and PDGF-BB were further divided the following
subgroups: i) The mimic negative control (NC) group (HSC-T6 cells
were treated with 30 mg/ml RSV and PDGF-BB and transfected with
mimic NC); ii) miR-20a mimic group (HSC-T6 cells were treated with
30 mg/ml RSV and PDGF-BB and transfected with miR-20a mimic); iii)
miR-20a mimic + pcDNA3.1 group (HSC-T6 cells were treated with 30
mg/ml RSV and PDGF-BB and transfected with miR-20a mimic +
pcDNA3.1); and iv) the miR-20a mimic + pcDNA3.1-PTEN group (HSC-T6
cells were treated with 30 mg/ml RSV and PDGF-BB and transfected
with miR-20a mimic + pcDNA3.1-PTEN). The vectors were provided by
Beyotime Institute of Biotechnology, Inc., miR-20a mimic and its NC
were constructed and synthesized by Shanghai GenePharma Co., Ltd..
pcDNA3.1-PTEN and miR-20a mimic and its NC used for transfection
were 100 nM and transfection was carried out using Lipofectamine™
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The transfection
efficacy was verified by RT-qPCR and western blot analysis 48 h
later.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The HSC-T6 cells in the exponential growth phase
were counted following detachment with trypsin. The cell suspension
was diluted to 3×104 cells/ml, and 200 µl of the
cell suspension were seeded into each well of a 96-well plate.
Subsequently, 10 mg/ml PDGF-BB and 10, 30 or 50 mg/ml RSV solution
were added following cell adherence, with 6 parallel wells in each
group. The control group was treated with the same volume of
culture solution. This was followed by the addition of 20 µl
of 5 mg/ml MTT (Beijing Solarbio Science & Technology Co.,
Ltd.) dissolved in PBS to the wells following 48 h of culture.
Following 4 h of treatment in an incubator at 37°C, the supernatant
was discarded, and 150 µl of dimethyl sulfoxide were added
to each well and shaken for 10 min. The optical density at a
wavelength of 490 nm was measured using a microplate reader
(BIO-RAD680, Bio-Rad Laboratories Inc.).
Dual-luciferase reporter gene assay
miRs targeting PTEN were detected using starBase
(http://starbase.sysu.edu.cn/) and
TargetScan (http://www.targetscan.org/vert_72/) software. The
overlapping results of these 2 databases revealed specific binding
sites between the 3'UTR of PTEN and miR-20a. pmirGLO vector
(Shanghai GenePharma, Co., Ltd.) was utilized to establish
wild-type (WT) PTEN plasmid (PTEN-WT) and mutant (MUT) PTEN plasmid
(PTEN-MUT), which were separately mixed with mimic NC and miR-20a
mimic and then Lipofectamine™ 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) prior to transfection into 293T cells (China
Center for Type Culture Collection). Finally, luciferase activity
was assessed 48 h later. All the experiments were performed 3
times.
Statistical analysis
SPSS 21.0 (IBM Corp.) was employed for data
analysis. The Kolmogorov-Smirnov test was used to indicate whether
the data were normally distributed. The results are presented as
the means ± standard deviation. One-way analysis of variance
(ANOVA) or two-way ANOVA were used to compare different groups, and
Tukey's multiple comparisons test was applied for pairwise
comparisons following ANOVA. P-values were attained using a
two-tailed test, and P<0.05 was considered to indicate a
statically significant difference.
Results
RSV mitigates liver injury in rats with
LF
To examine the effects of RSV on LF, a rat model of
LF was established and the ALT, AST and ALB serum levels were
detected in the rats. The results revealed that the ALT and AST
serum levels in the rats with LF were markedly increased, while the
ALB level was markedly decreased. The changes in ALT, AST and ALB
serum levels in rats with LF were normalized by RSV treatment
(P<0.05 and P<0.01; Fig.
1A). The LN, HA and PC-III levels in serum form the model rats,
and the HYP and MDA levels in the liver tissue homogenate were
elevated, while the SOD levels were decreased. Of note, RSV
treatment reversed these outcomes (all P<0.05 and P<0.01;
Fig. 1B and C). The results of
H&E and Masson's staining results revealed that the liver
tissues of rats in the normal group were complete with clearly
visible cells. By contrast, the tissues from the rats in the model
group exhibited loose and extending liver cells, portal necrosis, a
disordered arrangement of the liver lobules, pseudolobuli
formation, loose cytoplasm, massive collagen fiber hyperplasia,
distant fibers and an enlarged collagen volume fraction (CVF).
However, following treatment with low-, medium- and high-dose RSV,
rat liver cell death was gradually alleviated, the liver lobule
structure was normalized, and collagen fiber hyperplasia and CVF
decreased (P<0.05 and P<0.01; Fig. 1D and E). Western blot analysis
revealed that the protein expression of α-SMA, TIMP-1 and TGF-β1 in
the tissues of rats with LF was higher than that in the tissues of
rats in the normal group; however, these effects were reversed by
treatment with RSV (all P<0.05 and P<0.01; Fig. 1F).
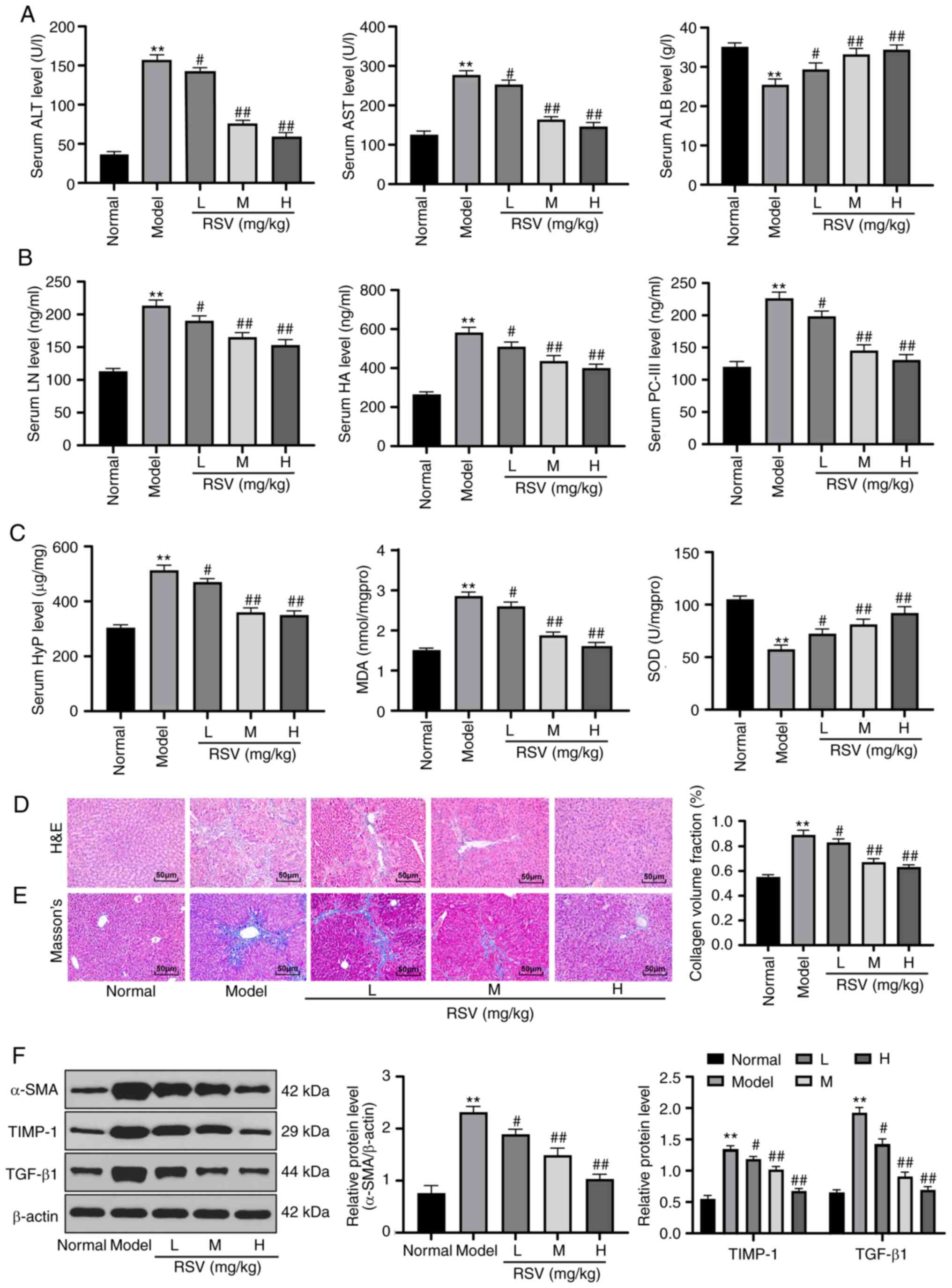 | Figure 1RSV mitigates liver injury in rats
with LF. (A) Detection of ALT, AST and ALB levels in the sera of
rats with LF; n=12 per group. (B) LN, HA and PC-III levels in the
sera of rats with LF were measured with ELISA kits; n=12 per group.
(C) HYP, MDA and SOD levels in liver tissue homogenates were
verified using kits; n=3 rats per group. (D) H&E staining and
(E) Masson's staining were employed to observe pathological changes
in the liver tissues of rats in each group; magnification, ×200,
n=6 rats per group. (F) Western blot analysis was applied to assess
α-SMA, TIMP-1 and TGF-β1 protein expression in the tissues of rats
in each group. Each experiment was independently repeated 3 times.
The data were analyzed by one-way ANOVA or two-way ANOVA, followed
by Tukey's multiple comparisons test; **P<0.01,
compared with the normal group; #P<0.05 and
##P<0.01, compared with the model group. RSV,
resveratrol; LF, liver fibrosis; ALT, alanine aminotransferase;
AST, aspartate aminotransferase; ALB, albumin; LN, laminin; HA,
hyaluronic acid; PC, procollagen; HYP, hydroxyproline; MDA,
malondialdehyde; SOD, superoxide dismutase; ANOVA, analysis of
variance. |
RSV induces hepatocellular autophagy in
rats with LF
A previous study noted that hepatocellular autophagy
plays an important role in LF (28). Thus, the present study used
immunohistochemical staining to evaluate the expression of
autophagy-related proteins in the liver tissues of rats in each
group. It was found that the Beclin1 and Atg7 proteins were mainly
present in the cytoplasm of the liver cells. Compared with that in
the sham-operated rats, Beclin1 and Atg7 expression in the liver
tissues of the model rats was significantly decreased. However,
Beclin1 and Atg7 expression was enhanced following treatment with
RSV at various doses (P<0.05 and P<0.01; Fig. 2A). Subsequently, the expression of
autophagy-related proteins in the liver tissues of rats was
detected by western blot analysis. It was found that compared with
the control rats, the rats in the model group exhibited a decreased
LC3-II/LC3-I ratio, a decreased Beclin1 and Atg7 protein expression
and an increased p62 protein expression. Following treatment with
RSV at various doses, autophagy in the rat liver tissues was
promoted (P<0.05 and P<0.01; Fig. 2B). The autophagic flux is blocked
through the inhibition of the fusion of autophagosomes and
lysosomes, leading to aggravated liver damage (29). In the present study, observation
by TEM revealed that there were fewer autophagosomes in the liver
tissues of the model rats compared with the control rats, while the
number of autophagosomes in the rat liver tissues increased with
the increasing doses of RSV (Fig.
2C).
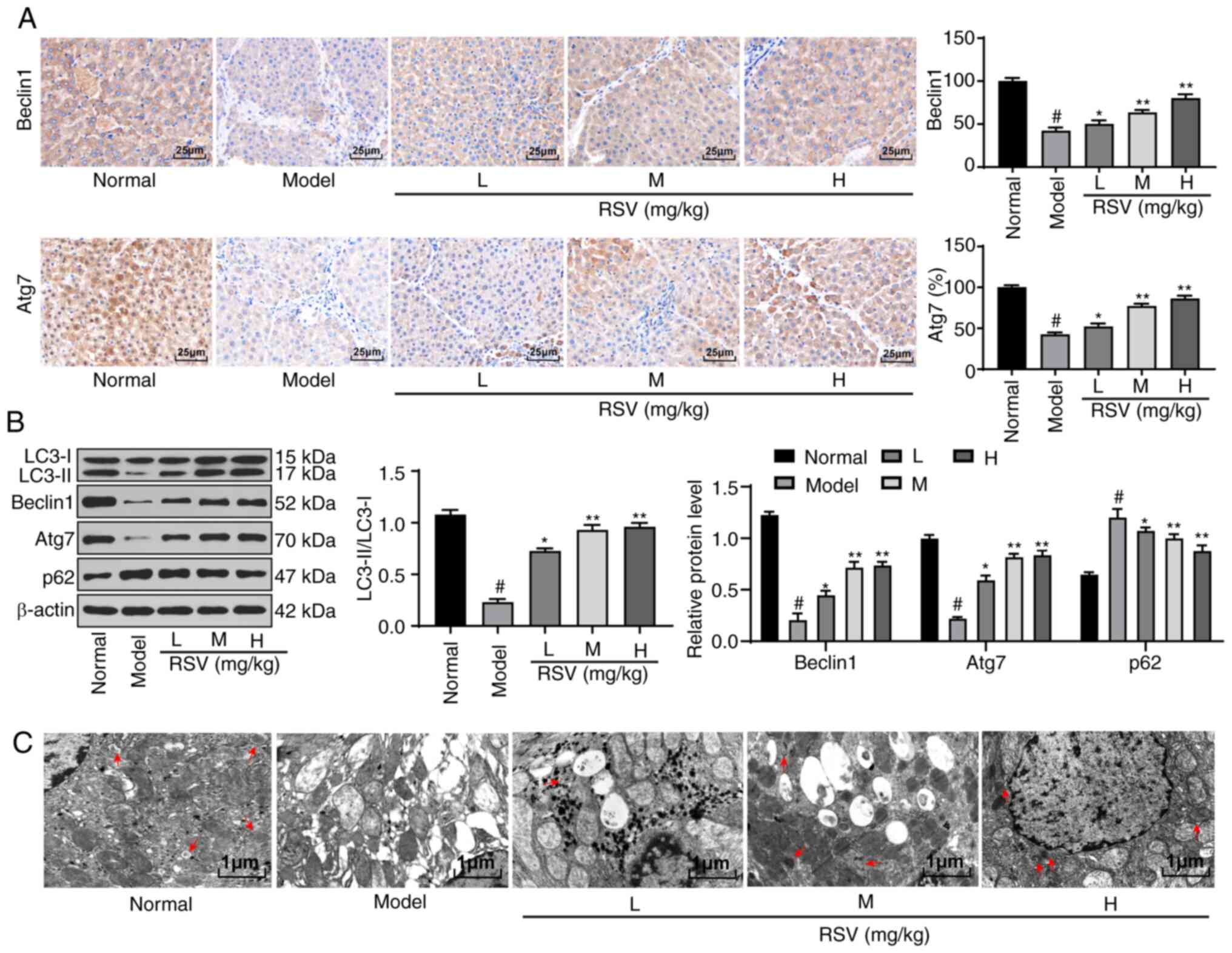 | Figure 2RSV induces autophagy in rats with
LF. (A) Immunohistochemical staining was performed to detect
Beclin1 and Atg7 protein expression in the liver tissues of rats
with LF; magnification, ×400, n=6 rats per group. (B) Western blot
analysis was conducted to assess LC3-II, LC3-I, Beclin1, Atg7 and
p62 protein expression in the liver tissues of rats from each
group; n=3 rats per group. (C) TEM was used to observe
autophagosomes in the liver tissues of rats in every group (as
indicated by the arrow); magnification, 1,000, n=3 rats per group.
Each experiment was independently repeated 3 times. The data were
analyzed by one-way ANOVA or two-way ANOVA, followed by Tukey's
multiple comparisons test; *P<0.05 and
**P<0.01, compared with the model group;
#P<0.05, compared with the normal group. RSV,
resveratrol; LF, liver fibrosis; TEM, transmission electron
microscopy; ANOVA, analysis of variance. |
RSV alleviates LF by activating the
PTEN/PI3K/AKT signaling pathway
A previous study indicated that PTEN affects the
activation and apoptosis of HSCs in LF (30) and that PTEN exerts a specific
effect on LF via the PI3K/Akt/STAT6 axis (31). Hence, it was hypothesized that RSV
plays a role in inhibiting LF via the PTEN/PI3K/AKT axis.
Subsequently, the mRNA and protein expression levels of PTEN in the
liver tissues of rats in each group were detected by RT-qPCR and
western blot analysis, respectively. It was found that PTEN mRNA
and protein expression in rats in the model group was markedly
reduced compared with the rats in the control group. The mRNA and
protein expression levels of PTEN in the rats with LF treated with
RSV at various doses were significantly increased in a
dose-dependent manner (P<0.05 and P<0.01; Fig. 3A and B). Immunohistochemical
staining then revealed few yellow granules in the control group,
but clusters of brownish yellow granules in the model group.
Compared with the model group, the granular brownish yellow
substance in the RSV-treated groups decreased with the increasing
RSV concentration (P<0.05 and P<0.01; Fig. 3C). Compared with those in the
control group, the PI3K and p-AKT protein levels were increased in
the model group, but were reduced in the RSV-treated groups
(P<0.05 and P<0.01; Fig.
3D).
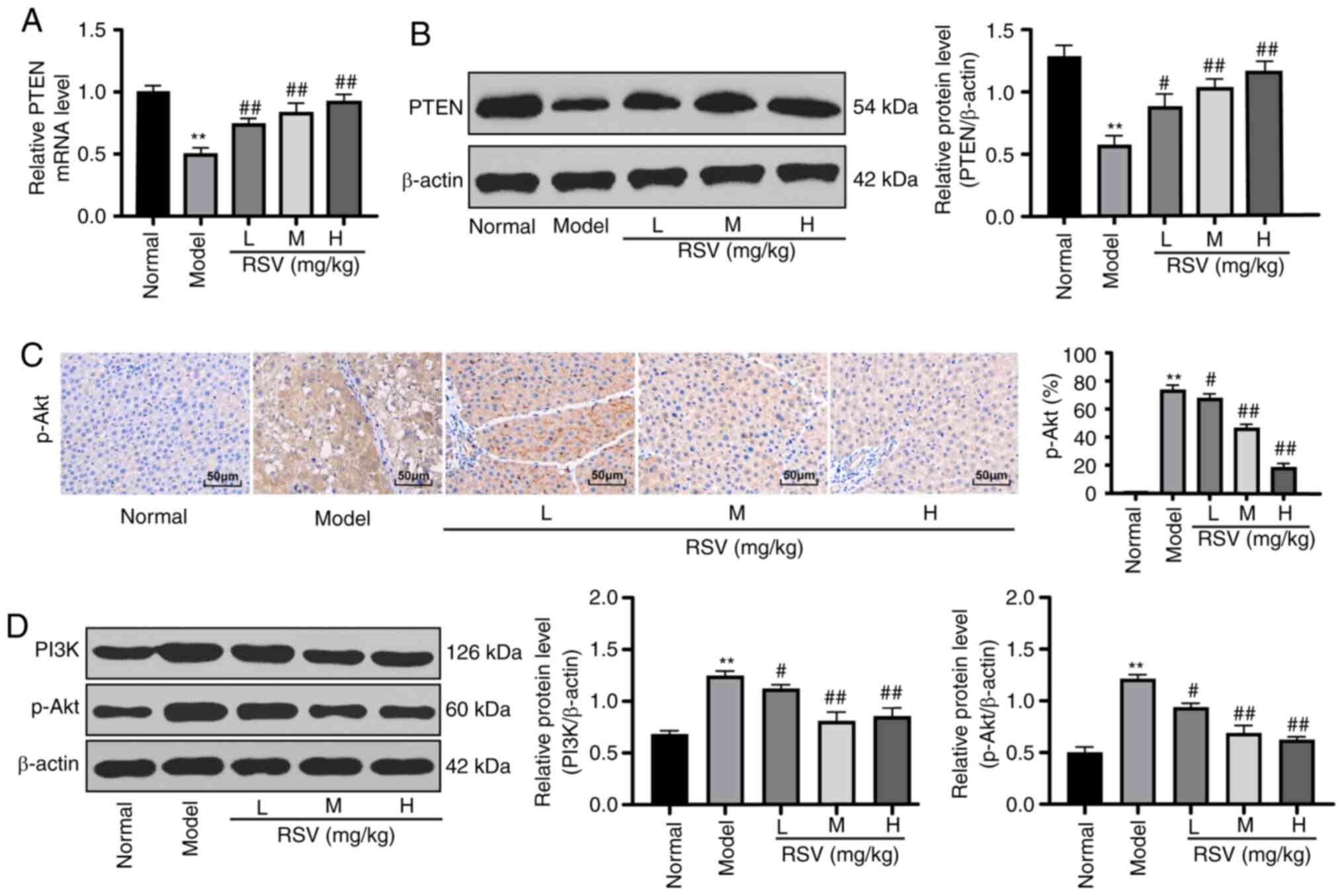 | Figure 3RSV alleviates liver injury in rats
with LF by activating the PTEN/PI3K/AKT signaling pathway. (A and
B) PTEN mRNA and protein expression in the liver tissues of rats
with LF was verified with RT-qPCR and western blot analysis,
respectively; n=3 rats per group. (C) p-AKT protein expression was
measured by immunohistochemical staining; magnification, ×400, n=6
rats per group. (D) PI3K and p-AKT protein expression in the liver
tissues of rats with LF was detected by western blot analysis; n=3
rats per group. Each experiment was independently repeated 3 times.
One-way ANOVA and Tukey's multiple comparisons test were applied
for data analysis; **P<0.01, compared with the normal
group; #P<0.05 and ##P<0.01, compared
with the model group. RSV, resveratrol; LF, liver fibrosis; mRNA,
messenger RNA; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; ANOVA, analysis of variance. |
miR-20a suppresses PTEN expression
The above-mentioned results revealed that the
PTEN/PI3K/AKT axis played a specific role in the rat model of LF.
However, the upstream mechanism of the PTEN/PI3K/AKT axis remains
unclear. Therefore, miRs containing PTEN-binding sites were
predicted through starBase. It was found that a number of miRs,
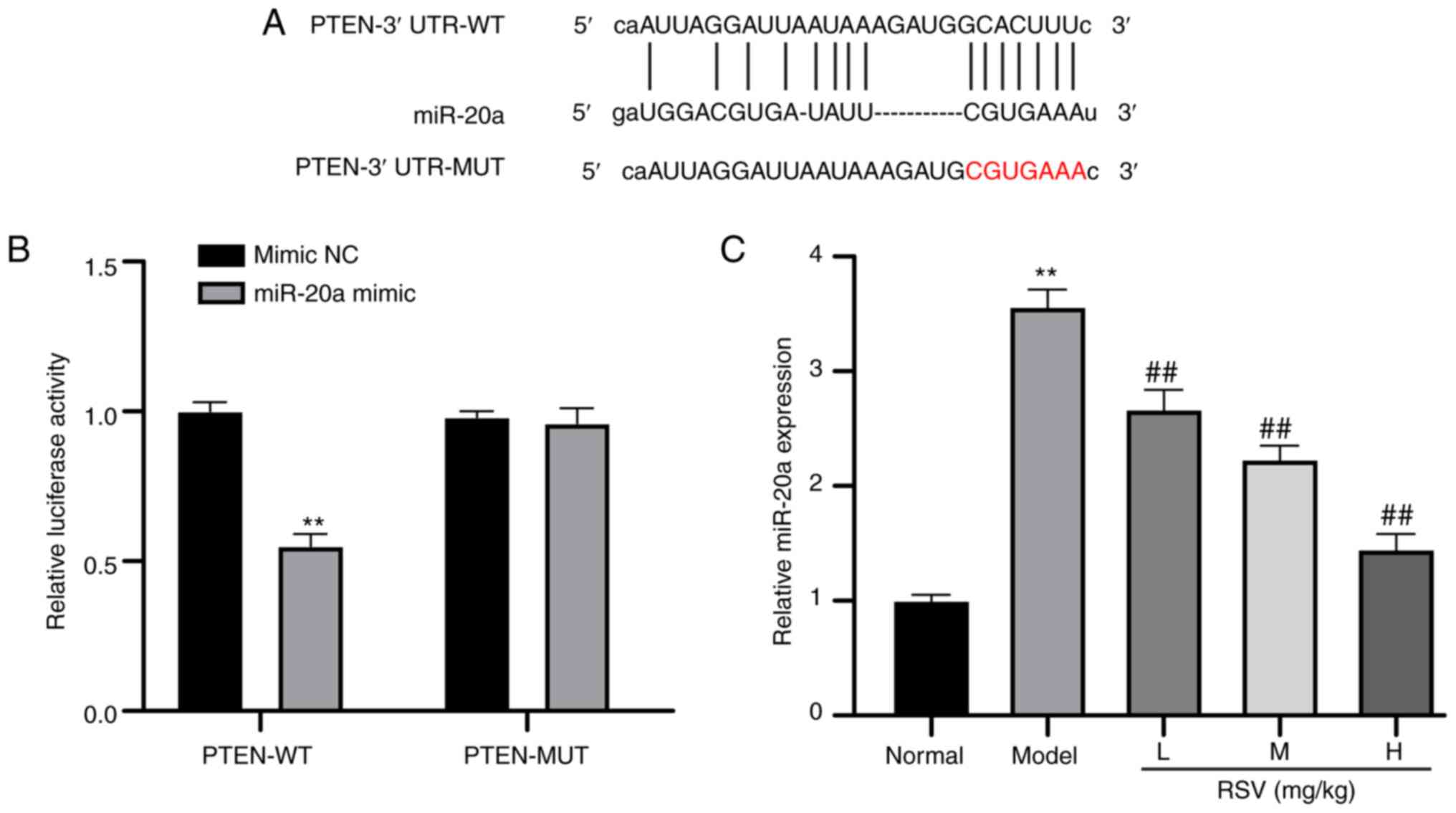
including miR-20a, contain PTEN-binding sites (Fig. 4A). miR-20a is a fibrosis-related
miR (32). Hence, it was
hypothesized that miR-20a may play a role in LF by regulating the
PTEN/PI3K/AKT axis. Through a dual-luciferase reporter gene assay,
it was found that the luciferase activity of the miR-20a mimic in
cells expressing PTEN-WT was markedly decreased (P<0.01;
Fig. 4B). Compared with that in
the control group, miR-20a expression was higher in the model
group, but was significantly decreased in the RSV-treated groups
(P<0.01; Fig. 4C).
RSV promotes cell autophagy and decreases
the inhibitory effects of miR-20a on PTEN
Following treatment with PDGF-BB, HSC-T6 cell
viability was increased, and the mRNA expression of α-SMA and
TGF-β1 was promoted. However, RSV at increasing concentrations
gradually decreased HSC-T6 cell viability and decreased the mRNA
expression of α-SMA and TGF-β1 (all P<0.01; Fig. 5A and B). Western blot analysis was
used to detect autophagy-related indexes. The LC3-II/LC3-I ratio
and Beclin 1 protein level were significantly decreased, and p62
protein expression was evidently increased. However, following
treatment with RSV at increasing concentrations, the LC3-II/LC3-I
ratio and Beclin 1 protein expression markedly increased, and p62
protein expression notably decreased (all P<0.01; Fig. 5C). miR-20a was actively expressed
in HSC-T6 cells treated with PDGF-BB; however, its expression
decreased following treatment with RSV at increasing concentrations
(P<0.01; Fig. 5D). In
activated HSC-T6 cells treated with 30 mg/ml RSV, the mRNA and
protein expression of PTEN was significantly decreased following
its upregulation by miR-20a (P<0.01; Fig. 5E and F).
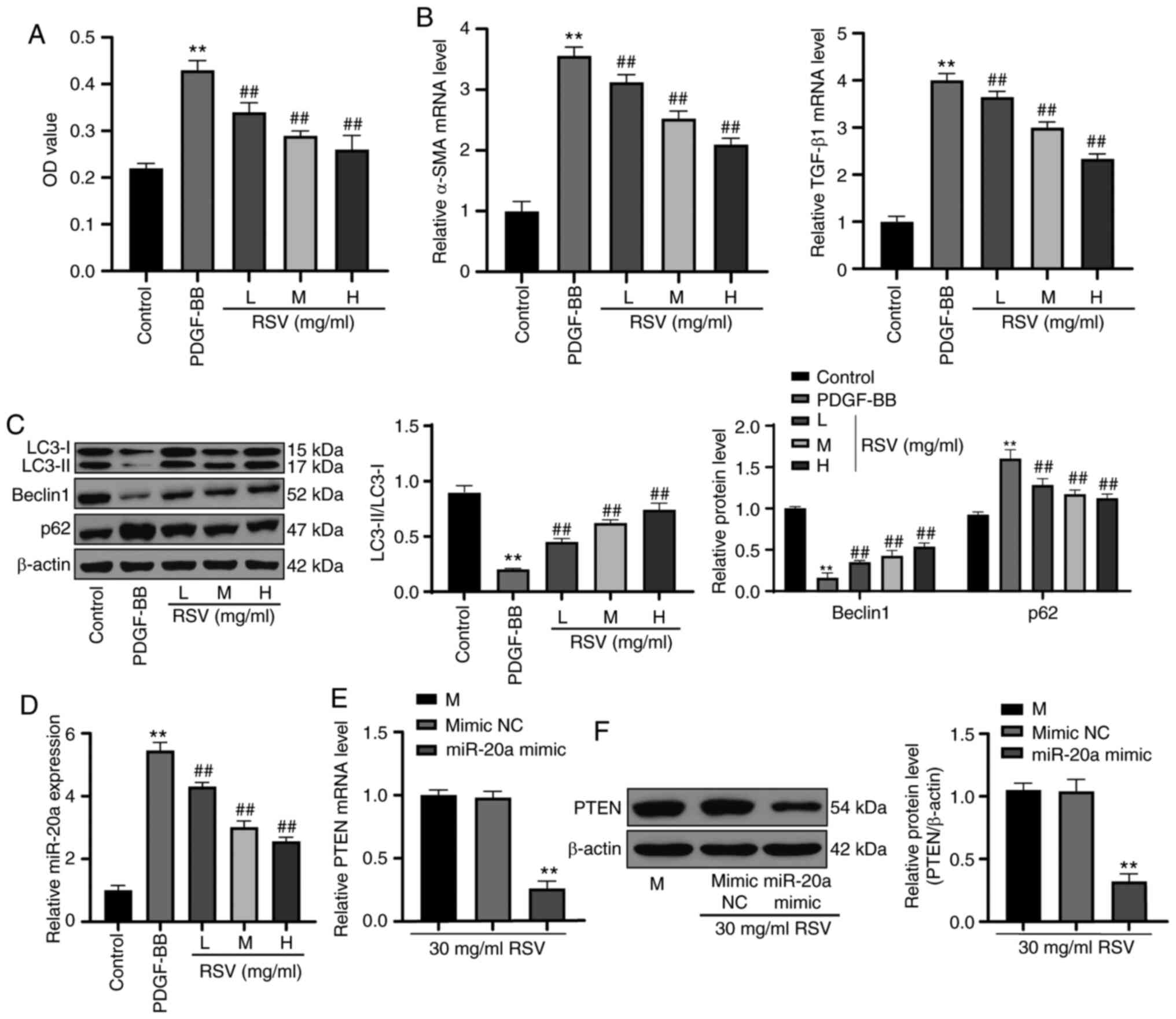 | Figure 5RSV promotes cell autophagy and
decreases the inhibitory effect of miR-20a on PTEN. (A) HSC-T6 cell
proliferation following RSV treatment was verified by MTT assay.
(B) mRNA expression of α-SMA and TGF-β1 in HSC-T6 cells treated
with RSV was detected by RT-qPCR. (C) Western blot analysis was
used to detect autophagy-related protein expression. (D) miR-20a
expression in HSC-T6 cells following treatment with RSV was
measured by RT-qPCR. (E and F) After miR-20a was overexpressed,
RT-qPCR and western blot analysis were performed to determine PTEN
mRNA and protein expression, respectively in HSC-T6 cells treated
with RSV. Each experiment was independently repeated 3 times.
One-way ANOVA and Tukey's multiple comparisons test were applied to
determine the statistical significance of the data;
**P<0.01, compared with the control group and the
mimic NC group; ##P<0.01, compared with the PDGF-BB
group. RSV, resveratrol; miR, microRNA; MTT,
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; mRNA,
messenger RNA; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; ANOVA, analysis of variance; NC,
negative control; PDGF, platelet-derived growth factor. |
RSV inhibited LF by downregulating
miR-20a and modulating the PTEN/PI3K/AKT signaling pathway
When both miR-20a and PTEN were overexpressed in
activated HSC-T6 cells treated with RSV, the mRNA and protein
expression of PTEN, as well as the protein expression of PI3K and
p-AKT were decreased (all P<0.01; Fig. 6A and B). The HYP and MDA contents
were decreased, while the SOD content was increased (Fig. 6C), and the COL-1 content was
decreased (Fig. 6D). Moreover,
the mRNA and protein expression of α-SMA and TIMP-1 also decreased
(Fig. 6E and F; all
P<0.01).
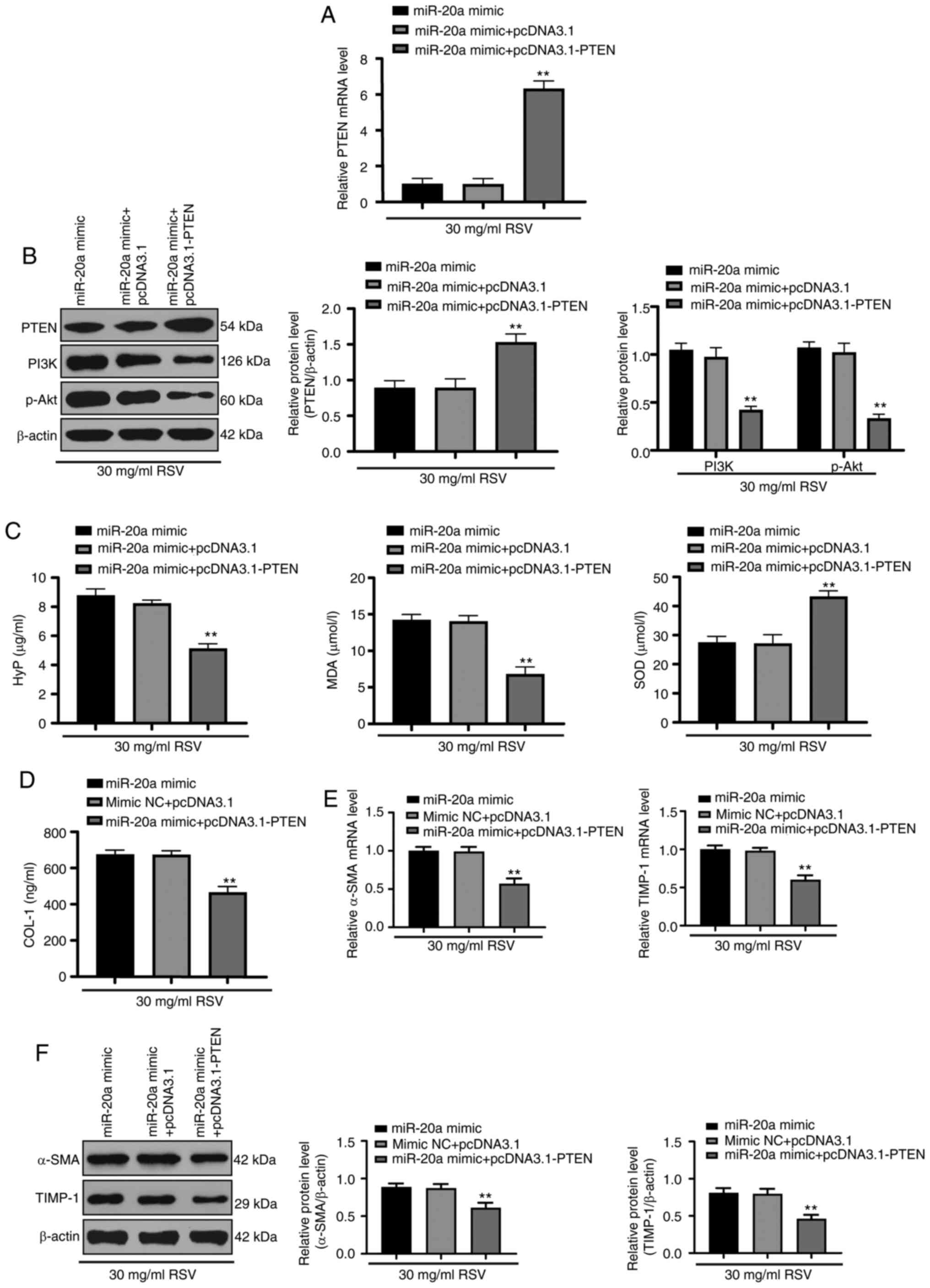 | Figure 6miR-20a promotes LF by activating the
PTEN/PI3K/AKT signaling pathway. (A and B) RT-qPCR and western blot
analysis were performed to determine PTEN mRNA and protein
expression, respectively, in HSC-T6 cells and western blot analysis
was performed to determine PI3K and p-AKT protein expression. (C)
HYP, MDA and SOD levels in HSC-T6 cells were detected with a kit.
(D) A kit was used to determine COL-1 protein levels in HSC-T6
cells. (E) The mRNA expression of α-SMA and TGF-β1 in HSC-T6 cells
was assessed by RT-qPCR. (F) Western blot analysis was conducted to
measure α-SMA and TIMP-1 protein expression in HSC-T6 cells. Each
experiment was independently repeated 3 times. The data were
analyzed by one-way ANOVA or two-way ANOVA, followed by Tukey's
multiple comparisons test; **P<0.01, compared with
the miR-20a mimic + pcDNA3.1 group. miR, microRNA; LF, liver
fibrosis; RSV, resveratrol; mRNA, messenger RNA; ALT, alanine
aminotransferase; AST, aspartate aminotransferase; HYP,
hydroxyproline; MDA, malondialdehyde; SOD, superoxide dismutase;
COL-1, collagen type 1; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; ANOVA, analysis of variance. |
Discussion
LF represents a protective response against chronic
liver injury; however, incessant LF due to dysregulation results in
liver cirrhosis and even hepatocellular carcinoma (33). Hepatocytes, immune and endothelial
cells, macrophages and activated liver stellate cells jointly
participate in the genesis of LF, which ultimately leads to chronic
liver injury (3). RSV has been
discovered to affect various biological features; for instance, it
discourages inflammatory reactions, oxidative stress, apoptosis and
mitochondrial function failure and promotes angiogenesis (34). In a previous study, RSV assisted
in remedying liver diseases, including its effects on depressing
necrosis, apoptosis and fat deposition caused by ischemia after
liver transplantation; preventing liver injury from alcohol,
cholestatic liver disease and chemicals; and modulating lipid and
glucose metabolism to fight against liver steatosis and LF
(35). In the present study, it
was thus hypothesized that RSV may affect LF by inducing autophagy
and activating the miR-20a-mediated PTEN/PI3K/AKT signaling
pathway. Consequently, the data demonstrated that RSV suppressed LF
progression.
First, the results of H&E and Masson's staining
revealed that RSV attenuated rat LF. RSV, a type of dietary
poly-phenol found in grapes and red wine, exhibits the ability to
protect the liver (36). It has
been reported that RSV inhibits reactive oxygen species that are
active players in the initiation of LF, thereby ameliorating LF
malignancy (37). The present
study revealed the ongoing development of autophagy in rats with LF
resulting from RSV. Autophagy substantially participates in
eliminating misfolded, aggregated and long-lived proteins, and
impaired organelles and modulating growth, as it is closely related
to the responses to a number of types of stress, including hypoxia,
inflammation and endoplasmic reticulum stress (38). Autophagy induced by RSV is an
essential step in the beneficial effect of RSV on inhibiting
inflammation (39). As an
essential protective factor, autophagy carries out an
anti-inflammatory mechanism by phagocytizing pathogens, modulating
T cell activation and controlling immune cell differentiation to
hinder lipid peroxidation and oxidative stress to mitigate LF
(40). In summary, RSV markedly
alleviated LF.
Additionally, the present study revealed that RSV
upregulated the PTEN/PI3K/AKT signaling pathway to suppress LF. As
a variable phosphatase in human cancer, PTEN is an important
participant in protein synthesis deregulation, cell growth and DNA
activity (41). A previous study
demonstrated that the loss of PTEN caused the overexpression of
liver insulin and development of liver diseases, suggesting a
positive role for PTEN in LF treatment (42). Evidence has indicated that the
PI3K/AKT signaling pathway promotes T cell activation and
inflammatory reactions to exacerbate ulcerative colitis (43). The deregulation of the PI3K/AKT
signaling pathway is positively associated with metabolic function
failure in liver diseases (44),
which suggests that the PI3K/AKT signaling pathway may have a
detrimental influence on LF treatment. The results from a previous
study demonstrated that increased PTEN expression serves as a
negative regulator of the PI3K/AKT signaling pathway through a
series of biological activities (45).
The present study found that miR-20a suppressed PTEN
expression. In lung cancer, the upregulated expression of miR-20a
has emerged as an enhancer of cell growth, encouraging tumor
development (46). In multiple
myeloma, miR-20a directly targets PTEN, suppressing PTEN activity
by reducing proliferation and promoting apoptosis (47). Notably, the present study found
that RSV reversed the suppressive effects of miR-20a on PTEN.
Functional assays revealed that RSV markedly decreased miR-20a
expression and thus activated PTEN mRNA and protein expression,
which was suppressed by miR-20a (20). Finally, RSV inhibited LF by
down-regulating miR-20a and upregulating the PTEN/PI3K/AKT
signaling pathway. A previous study revealed a significant increase
in miR-20a in liver-related diseases, suggesting that miR-20a
functions as a convenient target in predicting LF (18). Generally, RSV was effective in
blocking LF progression.
In conclusion, the present study supports the notion
that RSV inhibits LF by inducing autophagy and activating the
miR-20a-mediated PTEN/PI3K/AKT signaling pathway. These results
reveal a novel strategy for the treatment of LF. Due to limitations
in research funding and experimental conditions, the present study
did not perform immunostaining to detectα-SMA. Furthermore, further
investigations to determine whether cells in the exponential growth
phase replicate physiological conditions in the liver are required.
In the future, the authors aim to explore this aspect and further
explore the mechanism of other targets of RSV via α-SMA
immunostaining and focus more on seeking reliable therapeutic
targets in LF. Nevertheless, although the findings of the present
preclinical study have implications for the treatment of LF, these
experimental results and the effective applications of RSV in
clinical practice warrant further validation.
Acknowledgments
Not applicable.
Funding
The present study was financially supported by the
Qian Ke He Platform Talent [grant no. (2018) 5779-26]; the Training
Program of the National Natural Science Foundation of Affiliated
Hospital of Guizhou Medical University (grant no. I-2020-20) and
Doctoral Fund of Affiliated Hospital of Guizhou Medical University
(grant no. I-20190-11).
Availability of data and materials
All the data generated or analyzed during this study
are included in this published article.
Authors' contributions
All authors guarantee the integrity of the entire
study. LZ, QM and MC conceived and designed the study. YW performed
the experiments and acquired the data. ZZ analyzed and interpreted
the data. All authors revised the manuscript for important
intellectual content and edited the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved and supervised by the
Ethics Committee of the Affiliated Baiyun Hospital of Guizhou
Medical University. Every step in this experiment was approved by
the laboratory animal ethics committee.
Patient consent for publication
Not applicable.
Competing interests
Not applicable.
References
|
1
|
Schuppan D, Ashfaq-Khan M, Yang AT and Kim
YO: Liver fibrosis: Direct antifibrotic agents and targeted
therapies. Matrix Biol. 68-69:435–451. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zoubek ME, Trautwein C and Strnad P:
Reversal of liver fibrosis: From fiction to reality. Best Pract Res
Clin Gastroenterol. 31:129–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campana L and Iredale JP: Regression of
liver fibrosis. Semin Liver Dis. 37:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Altamirano-Barrera A, Barranco-Fragoso B
and Mendez- Sanchez N: Management strategies for liver fibrosis.
Ann Hepatol. 16:48–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Poilil Surendran S, George Thomas R, Moon
MJ and Jeong YY: Nanoparticles for the treatment of liver fibrosis.
Int J Nanomedicine. 12:6997–7006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petitclerc L, Gilbert G, Nguyen BN and
Tang A: Liver fibrosis quantification by magnetic resonance
imaging. Top Magn Reson Imaging. 26:229–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun M and Kisseleva T: Reversibility of
liver fibrosis. Clin Res Hepatol Gastroenterol. 39(Suppl 1):
S60–S63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi Y, Zhou J, Jiang B and Miao M:
Resveratrol and inflamma-tory bowel disease. Ann N Y Acad Sci.
1403:38–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hessin AF, Hegazy RR, Hassan AA, Yassin NZ
and Kenawy SA: Resveratrol prevents liver fibrosis via two possible
pathways: Modulation of alpha fetoprotein transcriptional levels
and normalization of protein kinase C responses. Indian J
Pharmacol. 49:282–289. 2017. View Article : Google Scholar
|
|
10
|
Liu C, Liao JZ and Li PY: Traditional
Chinese herbal extracts inducing autophagy as a novel approach in
therapy of nonalcoholic fatty liver disease. World J Gastroenterol.
23:1964–1973. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Zhang T, Guo L and Huang L:
Regulation of PTEN expression by noncoding RNAs. J Exp Clin Cancer
Res. 37:2232018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang H, Wang X, Si C, Duan Y, Chen B,
Liang H and Yang D: Downregulation of miR141 deactivates hepatic
stellate cells by targeting the PTEN/AKT/mTOR pathway. Int J Mol
Med. 46:406–414. 2020.PubMed/NCBI
|
|
13
|
Petersen DR, Saba LM, Sayin VI,
Papagiannakopoulos T, Schmidt EE, Merrill GF, Orlicky DJ and Shearn
CT: Elevated Nrf-2 responses are insufficient to mitigate protein
carbonylation in hepatospecific PTEN deletion mice. PLoS One.
13:e01981392018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Z, Li S, Wang X, Si L, Ma R, Bao L
and Bo A: lncRNA GAS5 restrains CCl4-induced hepatic
fibrosis by targeting miR-23a through the PTEN/PI3K/Akt signaling
pathway. Am J Physiol Gastrointest Liver Physiol. 316:G539–G550.
2019. View Article : Google Scholar
|
|
15
|
Zhang DQ, Sun P, Jin Q, Li X, Zhang Y,
Zhang YJ, Wu YL, Nan JX and Lian LH: Resveratrol regulates
activated hepatic stellate cells by modulating NF-κB and the
PI3K/Akt signaling pathway. J Food Sci. 81:H240–H245. 2016.
View Article : Google Scholar
|
|
16
|
Chai R, Fu H, Zheng Z, Liu T, Ji S and Li
G: Resveratrol inhibits proliferation and migration through SIRT1
mediated posttranslational modification of PI3K/AKT signaling in
hepatocellular carcinoma cells. Mol Med Rep. 16:8037–8044. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Q, Luo M, Zhou C, Chen Z, Huang W,
Huang J, Zhao S and Yu X: Effect of danusertib on cell cycle,
apoptosis and autophagy of hepatocellular carcinoma HepG2 cells in
vitro. Nan Fang Yi Ke Da Xue Xue Bao. 38:1476–1484. 2018.In
Chinese.
|
|
18
|
Shrivastava S, Petrone J, Steele R, Lauer
GM, Di Bisceglie AM and Ray RB: Up-regulation of circulating
miR-20a is correlated with hepatitis C virus-mediated liver disease
progression. Hepatology. 58:863–871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Zheng L, Ding Y, Li Q, Wang R,
Liu T, Sun Q, Yang H, Peng S, Wang W and Chen L: MiR-20a induces
cell radiore-sistance by activating the PTEN/PI3K/Akt signaling
pathway in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys.
92:1132–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhar S, Kumar A, Rimando AM, Zhang X and
Levenson AS: Resveratrol and pterostilbene epigenetically restore
PTEN expression by targeting oncomiRs of the miR-17 family in
pros-tate cancer. Oncotarget. 6:27214–27226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang W, Li G, Qiu J, Gonzalez P and
Challa P: Protective effects of resveratrol in experimental retinal
detachment. PLoS One. 8:e757352013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saha L and Chakrabarti A: Understanding
the anti-kindling role and its mechanism of Resveratrol in
Pentylenetetrazole induced-kindling in a rat model. Pharmacol
Biochem Behav. 120:57–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhan YY, Liang BQ, Li XY, Gu EM, Dai DP,
Cai JP and Hu GX: The effect of resveratrol on pharmacokinetics of
aripiprazole in vivo and in vitro. Xenobiotica. 46:439–444. 2016.
View Article : Google Scholar
|
|
24
|
Zhang Y, Dong R, Yang Q, Zhang L, Li J and
Zhao H: Resveratrol upregulates the gene and protein expressions of
N-methyl-D-aspartate receptor 1 and protein kinase C in the
hippocampus in Alzheimer's disease rats. Wei Sheng Yan Jiu.
48:269–278. 2019.In Chinese. PubMed/NCBI
|
|
25
|
Zatroch KK, Knight CG, Reimer JN and Pang
DS: Refinement of intraperitoneal injection of sodium pentobarbital
for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet Res.
13:602017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Brunt EM: Grading and staging the
histopathological lesions of chronic hepatitis: The Knodell
histology activity index and beyond. Hepatology. 31:241–246. 2000.
View Article : Google Scholar
|
|
28
|
Zhang XW, Mi S, Li Z, Zhou JC, Xie J, Hua
F, Li K, Cui B, Lv XX, Yu JJ and Hu ZW: Antagonism of
Interleukin-17A ameliorates experimental hepatic fibrosis by
restoring the IL-10/STAT3-suppressed autophagy in hepatocytes.
Oncotarget. 8:9922–9934. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zou H, Wang T, Yuan J, Sun J, Yuan Y, Gu
J, Liu X, Bian J and Liu Z: Cadmium-induced cytotoxicity in mouse
liver cells is associated with the disruption of autophagic flux
via inhibiting the fusion of autophagosomes and lysosomes. Toxicol
Lett. 321:32–43. 2020. View Article : Google Scholar
|
|
30
|
Niu X, Fu N, Du J, Wang R, Wang Y, Zhao S,
Du H, Wang B, Zhang Y, Sun D and Nan Y: MiR-1273g-3p modulates
activation and apoptosis of hepatic stellate cells by directly
targeting PTEN in HCV-related liver fibrosis. FEBS Lett.
590:2709–2724. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng Y, Tian Y, Xia J, Wu X, Yang Y, Li
X, Huang C, Meng X, Ma T and Li J: The role of PTEN in regulation
of hepatic macro-phages activation and function in progression and
reversal of liver fibrosis. Toxicol Appl Pharmacol. 317:51–62.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Appourchaux K, Dokmak S, Resche-Rigon M,
Treton X, Lapalus M, Gattolliat CH, Porchet E, Martinot-Peignoux M,
Boyer N, Vidaud M, et al: MicroRNA-based diagnostic tools for
advanced fibrosis and cirrhosis in patients with chronic hepatitis
B and C. Sci Rep. 6:349352016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bae M, Park YK and Lee JY: Food components
with antifibrotic activity and implications in prevention of liver
disease. J Nutr Biochem. 55:1–11. 2018. View Article : Google Scholar
|
|
34
|
Abu-Amero KK, Kondkar AA and Chalam KV:
Resveratrol and ophthalmic diseases. Nutrients. 8:2002016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Faghihzadeh F, Hekmatdoost A and Adibi P:
Resveratrol and liver: A systematic review. J Res Med Sci.
20:797–810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang L, Yang F, Fang Z and Hu C:
Resveratrol ameliorates alcoholic fatty liver by inducing
autophagy. Am J Chin Med. 44:1207–1220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao X, Li R, Liu Y, Zhang X, Zhang M,
Zeng Z, Wu L, Gao X, Lan T and Wang Y: Polydatin protects against
carbon tetrachloride-induced liver fibrosis in mice. Arch Biochem
Biophys. 629:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ravanan P, Srikumar IF and Talwar P:
Autophagy: The spotlight for cellular stress responses. Life Sci.
188:53–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park D, Jeong H, Lee MN, Koh A, Kwon O,
Yang YR, Noh J, Suh PG, Park H and Ryu SH: Resveratrol induces
autophagy by directly inhibiting mTOR through ATP competition. Sci
Rep. 6:217722016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ge MX, He HW, Shao RG and Liu H: Recent
progression in the utilization of autophagy-regulating nature
compound as anti-liver fibrosis agents. J Asian Nat Prod Res.
19:109–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hopkins BD, Hodakoski C, Barrows D, Mense
SM and Parsons RE: PTEN function: The long and the short of it.
Trends Biochem Sci. 39:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He L, Gubbins J, Peng Z, Medina V, Fei F,
Asahina K, Wang J, Kahn M, Rountree CB and Stiles BL: Activation of
hepatic stellate cell in Pten null liver injury model. Fibrogenesis
Tissue Repair. 9:82016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Q, Duan X, Fan H, Xu M, Tang Q, Zhang
L, Shou Z, Liu X, Zuo D, Yang J, et al: Oxymatrine protects against
DSS-induced colitis via inhibiting the PI3K/AKT signaling pathway.
Int Immunopharmacol. 53:149–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsuda S, Kobayashi M and Kitagishi Y:
Roles for PI3K/AKT/PTEN pathway in cell signaling of nonalcoholic
fatty liver disease. ISRN Endocrinol. 2013:4724322013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tsai CY, Wu JCC, Fang C and Chang AYW:
PTEN, a negative regulator of PI3K/Akt signaling, sustains brain
stem cardiovascular regulation during mevinphos intoxication.
Neuropharmacology. 123:175–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Babu KR and Muckenthaler MU: MiR-20a
regulates expression of the iron exporter ferroportin in lung
cancer. J Mol Med (Berl). 94:347–359. 2016. View Article : Google Scholar
|
|
47
|
Yuan J, Su Z, Gu W, Shen X, Zhao Q, Shi L,
Jin C, Wang X, Cong H and Ju S: MiR-19b and miR-20a suppress
apoptosis, promote proliferation and induce tumorigenicity of
multiple myeloma cells by targeting PTEN. Cancer Biomark.
24:279–289. 2019. View Article : Google Scholar : PubMed/NCBI
|