Introduction
Rheumatoid arthritis (RA), as a systemic and
autoimmune disease, is characterized by chronic inflammation,
abnormal synovial cell proliferation, joint swelling and tenderness
and can lead to limitations in joint function and the loss of the
ability to work (1,2). Fibroblast-like synoviocytes (FLS)
are an important type of synovial cell and are the main type of
effector cell mediating joint destruction and synovitis by
functioning in tissue shaping in the physiological state and in
matrix remodeling in pathological injury (3-6).
At present, the specific pathogenesis of RA remains elusive, and
may be related to immune factors, environmental factors, heredity,
as well as other factors (7-9).
Therefore, it is of great theoretical and practical significance to
actively explore the pathogenesis of RA and search for potential
diagnostic markers.
Long noncoding RNAs (lncRNAs), which are a recently
discovered class of noncoding RNAs, are transcribed from noncoding
sequences in the genome, are longer than 200 nt and do not have the
ability to be translated into proteins (10,11). LncRNAs have become a novel
research focus since numerous lncRNAs have been discovered that are
closely related to the occurrence of a great number of diseases due
to their extensive regulatory effects on epigenetics (12), transcription (13), and protein translation (14) and modification (15). Therefore, a systematic study of
the regulatory role of lncRNAs in the pathogenesis of RA will help
in more comprehensively understanding the mechanism of RA and
providing new insight and targets for its clinical diagnosis and
treatment. According to previous research by our group, we revealed
that lncRNAS56464.1 is a crucial gene related to the pathogenesis
of experimental arthritis, which can be used as a potential
biomarker for diagnosis and a clinical treatment target (16). However, the specific mechanism of
lncRNAS56464.1 in RA development and progression remains in its
infancy.
In 2011, Salmena et al (17) proposed a new mechanism for
describing RNA interactions: The competitive endogenous RNA (ceRNA)
hypothesis. The hypothesis holds that RNA transcripts such as
lncRNAs and circRNAs regulate the expression level of mRNAs by
competing with miRNAs to bind miRNA response elements (MREs).
miRNAs are important genetic molecules in organisms that
participate in the regulation of posttranscriptional gene
expression. Their aberrant expression is related to the occurrence
and development of numerous diseases. miR-152-3p was revealed
through miRNA screening in our previous study, to be the key gene
associated with RA (16).
miR-152-3p belongs to the miR-152 family and participates in
biological processes such as cell proliferation, differentiation
and apoptosis (18-20). A study has revealed that miR-152
can target Wnt1 to regulate the Wnt signaling pathway and affect
the development of RA (21).
However, the specific relationship between lncRNAS56464.1,
miR-152-3p, the Wnt signaling pathway and the occurrence and
development of RA remains to be studied. Therefore, in the present
study, based on the ceRNA theory, it was investigated whether
lncRNAS56464.1 can target the miR-152-3p/Wnt pathway and affect the
proliferation of FLS in RA.
Materials and methods
Induction of arthritis and the culture
and identification of synovial cells
In the present study, 40 male SPF Sprague-Dawley
(SD) rats (6-8 weeks old; 200±20 g) were purchased from the Anhui
Experimental Animal Center. The rats were housed individually under
a set temperature (18-22°C) and humidity (40-60%) with a 12-h
light/dark cycle, free access to standard laboratory food and
water, and environmental noise kept to a minimum range. All animal
experiments were approved by the Animal Ethics Committee of Anhui
University of Chinese Medicine (Anhui, China).
After one week of adaptive feeding, all rats were
randomly divided into a control group (n=10) and a model group
(n=30). Except for the control group, all rats were
intracutaneously injected with 0.1 ml Freund's complete adjuvant
(FCA) into the right hind metatarsal footpad to induce
adjuvant-induced arthritis (AA) and 0.9% saline was administered to
the control group rats as a placebo. The rats were anesthetized
with 1.0% sodium pentobarbital by a 60 mg/kg intraperitoneal
injection and were sacrificed after blood was collected from the
abdominal aorta on the 20th day after immunization (22,23).
Then, the synovial tissue was obtained and cultured
in complete DMEM medium with 20% fetal bovine serum (lot no.
1715752; cat. no. 10099141C; Gibco; Thermo Fisher Scientific, Inc.)
at 37°C and 5% CO2 by the tissue explant method
(24). After a large number of
cells were dissociated near the synovial tissue, the tissue mass
was discarded and the cells continued to be cultured. When the cell
density reached 80-90%, the cells were digested with 0.25% trypsin
and passaged at 1:2 and FLS used in the experiment were 3-5
generations. The primary and sub-cultured FLS were observed under
an inverted phase contrast microscope (Olympus Corporation), the
sub-cultured synovial cells were identified by immunofluorescence
staining for vimentin, and the specific operation was carried out
according to the instructions of the kit as previously described
(25,26). The cells were fixed 20 min with 4%
paraformaldehyde (PFA), permeabilized 20 min using 0.1% Triton
X-100 and then blocked in 5% BSA for 30 min; all operations
aforementioned were performed at room temperature. The cells were
then incubated with anti-vimentin (1:100; product code ab92547;
Abcam) overnight at 4°C, followed by a further incubation at room
temperature for 1 h with FITC-labeled Goat Anti-Rabbit IgG (1:500;
cat. no. A0562; Beyotime Institute of Biotechnology). Nuclear DNA
was labelled in blue with DAPI. The cell viability was detected by
trypan blue staining as follows: Cell survival rate
(percentage)=nonblue-stained cells/(blue-stained cells +
nonblue-stained cells) ×100%.
Cell transfection
To suppress lncRNAS56464.1, specific small
interfering RNAs (siRNAs) targeting lncRNAS56464.1 were synthesized
and purchased from Shanghai GenePharma Co., Ltd., and were as
follows: siRNA1 forward sequence, 5′-GCU ACU UUG UGG UAU CAA UTT-3′
and reverse sequence, 5′-AUU GAU ACC ACA AAG UAG CUU-3′; siRNA2
forward sequence, 5′-GAG GAG AUA GGU AGA ACC UTT-3′ and reverse
sequence, 5′-AGG UUC UAC CUA UCU CCU CUU-3′; siRNA negative control
(NC) forward sequence, 5′-UUC UUC GAA CGU GUC ACG UTT-3′ and
reverse sequence, 5′-ACG UGA CAC GUU CGG AGA ATT-3′. The cells were
inoculated on a 6-well plate, and the cell density reached 60-80%
confluence before the transfection of siRNA (20, 50, 100 and 200
nM). The mixture of transfection reagents of Lipofectamine 2000
(cat. no. 11668027; Invitrogen; Thermo Fisher Scientific, Inc.),
Opti-MEM (cat. no. 31985070; Gibco; Thermo Fisher Scientific, Inc.)
and siRNA was slowly dripped into the 6-well plate. After being
cultured at 37°C and 5% CO2 for 4-6 h, the mixed
transfection reagents were replaced with complete DMEM (without
antibiotics), and the follow-up experiment was carried out after 24
h of transfection as previously described (27).
Cell proliferation assay
The effect of lncRNAS56464.1 on FLS proliferation
was detected by an MTT assay as previously described (28). The transfected FLS
(1×105/ml) were cultured in complete DMEM medium with
20% fetal bovine serum for 12, 24 and 48 h in 96-well plates. After
culturing, 20 µl MTT (5 mg/ml) (cat. no. ST316; Beyotime
Institute of Biotechnology,) was added into each well and cultured
at 37°C and 5% CO2 for 4 h. Then, 150 µl dimethyl
sulfoxide (DMSO) was utilized to dissolve the formazan after
discarding the liquid in each well as previously described
(29,30). Determination of the absorbance at
490 nm was performed using a microplate reader (BioTek Instruments,
Inc.). The cell proliferation inhibition rate was calculated as
follows: Inhibition rate = [1−(OD siRNA group-OD blank control
group)/(OD control group-OD blank control group)] ×100%. The blank
control group consisted of MTT reagents without FLS and the control
group refers to FLS treated with DEPC water, which is the solvent
of siRNA.
Prediction of miRNA targets of
lncRNAS56464.1 and validation by a luciferase reporter assay
The full-length sequences of lncRNAS56464.1 and
mature miRNAs such as miR-152-3p, miR-20a-3p, miR-17-2-3p as well
as others which were differentially expressed key miRNAs in the
experimental arthritis in our previous study (16), were obtained from NCBI Refseq and
miRbase (31), respectively. We
predicted the miRNA target of lncRNAS56464.1 using OG-local tools
based on the RNAhybrid algorithm (32) and revealed that miR-152-3p to
lncRNAS56464.1 had the highest binding degree and lowest free
energy. Then, the full fragment of lncRNAS56464.1 with the binding
site of miR-152-3p was amplified through PCR, including the
wild-type or mutant lncRNAS56464.1 sequence binding site. Then, the
constructed plasmid and miR-152-3p mimic with the Lipofectamine
2000 reagent were co-transfected into 293T functional cells (cat.
no. FH0244; Shanghai FuHeng Biology). The cells were collected at
48 h post-transfection, and the luciferase activities were measured
using the Dual-Luciferase Reporter Assay System (Promega
Corporation) and normalized to the Renilla luciferase
activity as previously described (33).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from FLS using TRIzol (lot
no. 90802; cat. no. 15596018; Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse transcribed into cDNAs using the Reverse
Transcription kit (K1621; Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions. RT-qPCR was performed to
amplify the cDNA templates by 2X Universal SYBR-Green Fast qPCR Mix
(cat. no. RK21203; ABclonal) and using the PIKOREAL 96 fluorescence
quantitative PCR instrument (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: Pre-denaturation at 95°C
for 3 min, 40 cycles at 95°C for 5 sec and 60°C for 30 sec. The
relative expression of each target gene was quantified and
normalized by calculating (cycle of quantification) the values and
the level of each index as previously described (34,35). The primers were synthesized by
Shanghai Sangon Biotech Co., Ltd., and are presented in Table I.
 | Table ISequences of primers for RT-qPCR. |
Table I
Sequences of primers for RT-qPCR.
| Targeted gene | Forward sequence
and reverse sequence | Product length
(bp) |
|---|
| Wnt1 | F:
5′-AGAAACCGCCGCTGGAACT-3′ | |
| R:
5′-GGAGGTGATTGCGAAGATAAACG-3′ | 105 |
| β-catenin | F:
5′-CCACGACTAGTTCAGCTGCTTGTAC-3′ | |
| R:
5′-ACTGCACAAACAGTGGAATGGTATT-3′ | 225 |
| c-Myc | F:
5′-CTGGAGTGAGAAGGGCTTTG-3′ | |
| R:
5′-CAGCAGCTCGAATTTCTTCC-3′ | 480 |
| Cyclin D1 | F: 5′-TGGAGCCCCTGA
AGAAGAG-3′ | |
| R:
5′-AAGTGCGTTGTGCGGTAGC-3′ | 424 |
| GSK-3β | F:
5′-TACCCATACGATGTTCCAGAT-3′ | |
| R:
5′-ACCCTGCCCAGGAGTTGCCAC-3′ | 120 |
| SFRP4 | F:
5′-AAGTCTTTGTCACCTATCCCTCG-3′ | |
| R:
5′-CGGCTGGCTATCTGCTTCTT-3′ | 162 |
| β-actin | F:
5′-CAGCGGAACCGCTCATTGATGG-3′ | |
| R:
5′-TCACCCACACTGTGCCCAACGA-3′ | 300 |
Western blotting
FLS was extracted using RIPA lysis buffer (product
no. P0013B; Beyotime Institute of Biotechnology) containing PMSF
(100 mM) (product no. ST506; Beyotime Institute of Biotechnology).
FLS were washed three times with cold PBS and digested with 100
µl protein lysate. The protein concentration was determined
using the BCA method. The lysates (20 µg of protein loaded
per lane) were separated by 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred to a polyvinylidene fluoride membrane (PVDF membrane).
The PVDF membrane was washed with 1X TBST for 2 min, sealed with 5%
skim milk at room temperature for 2 h, and incubated with anti-Wnt1
(1:5,000; product no. ab63934; Abcam), anti-β-catenin (1:500;
ab68183; Abcam), anti-c-Myc (1:500; BM4042; Boster Biological
Technology), anti-cyclin D1 (1:500; ab40754), anti-GSK3β (1:2,000;
product code ab93926), anti-GSK3β (phospho S9) (1:10,000; product
code ab75814), anti-SFRP4 (1:200; ab154167), and anti-β-actin
(1:1,000; ab8226; all from Abcam) antibodies overnight at 4°C.
Then, the membrane was washed three times with TBST and incubated
with a second anti-mouse or anti-rabbit antibody HRP (1:10,000;
cat. nos. AS003 and AS014, respectively; ABclonal) for 2 h at room
temperature. The protein was detected with an enhanced
chemiluminescence kit (EMD Millipore). Each protein band was a
representative picture of three replicates. Western blotting data
were quantified with ImageJ software (version 1.52, National
Institutes of Health) (30).
Immunofluorescence
The cells were fixed in 4% paraformaldehyde for 30
min, then 50-100 µl 0.25% Triton X-100 was added and
incubated for 10 min; all steps aforementioned were performed at
room temperature. The sections were washed with PBS for three
times, and immunostained with primary antibodies, and incubated
overnight in a wet chamber at 4°C. The dilutions of the antibodies
(as aforementioned) were as follows: Wnt1, 1:100; β-catenin, 1:200;
c-Myc, 1:200; cyclin D1, 1:500; p-GSK-3β (Ser9), 1:200; SFRP4,
1:200; and β-actin, 1:200. Then, the sections were stained with the
corresponding secondary antibody (as aforementioned) (1:10,000),
and incubated at room temperature for 50 min. The nucleus was
stained for 5 min with DAPI at room temperature. Six fluorescence
images for semi-quantitative analysis and the average optical
density were used to reflect the fluorescence intensity of immune
response of different indexes as previously described (36).
Statistical analysis
The data were inputted into SPSS17.0 software (SPSS,
Inc.) for statistical processing, and the results were expressed as
the mean ± SD (standard deviation) (x¯±s). The data were analyzed using
one-way ANOVA (one-way analysis of variance) followed by Tukey's
multiple comparison test to detect the differences between groups.
The result was considered statistically significant when
P<0.05.
Results
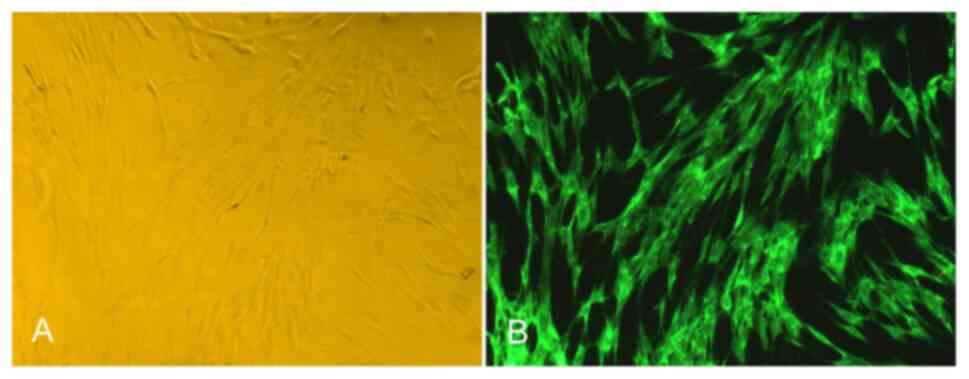
Identification and activity detection of
FLS
After more than two passages, the characteristics of
the FLS were stable, the species were mainly fibroblasts, the shape
was mainly fusiform, and the polar processes were slender, as
revealed in Fig. 1A. Under a
fluorescence microscope, the passage of FLS was positive for
vimentin antibody labeling, the cells were fusiform, and the
nucleus was oval and located in the center of the cells, as
revealed in Fig. 1B. In addition,
the results of trypan blue staining revealed that the survival rate
of the FLS was more than 90% (data not shown).
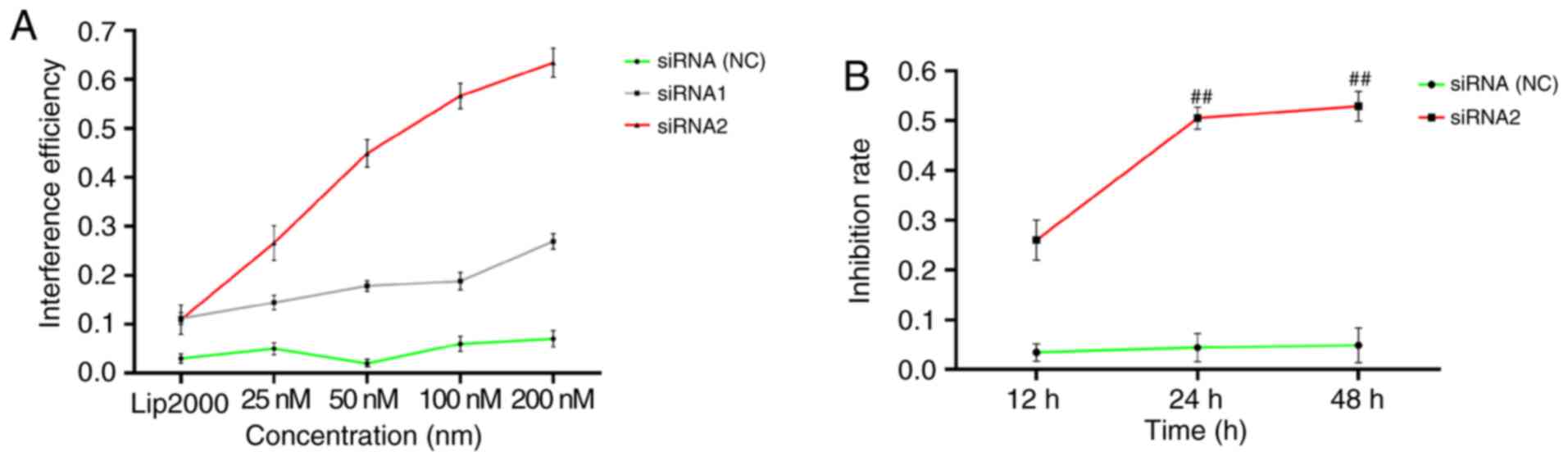
Negative regulation of FLS proliferation
under lncRNAS56464.1 interference
As revealed in Fig.
2A, the highest efficiency of lncRNAS56464.1 siRNA1
interference on FLS was ~30%; the highest efficiency of
lncRNAS56464.1 siRNA2 interference on FLS was >60%, and the
interference efficiency of 100 nM was more than 50%. According to
the interference efficiency, lncRNAS56464.1 siRNA2 (100 nM) was
selected for the following experimental study. As revealed in
Fig. 2B, with the increasing
interference time of the lncRNAS56464.1, the inhibition rate of FLS
increased continuously. The inhibition rate significantly increased
between 12 and 24 h as well as 12 and 48 h. However, there was no
significant increase between 24 and 48 h. Thus, 24 h was selected
as the stimulation time for the follow-up experiment.
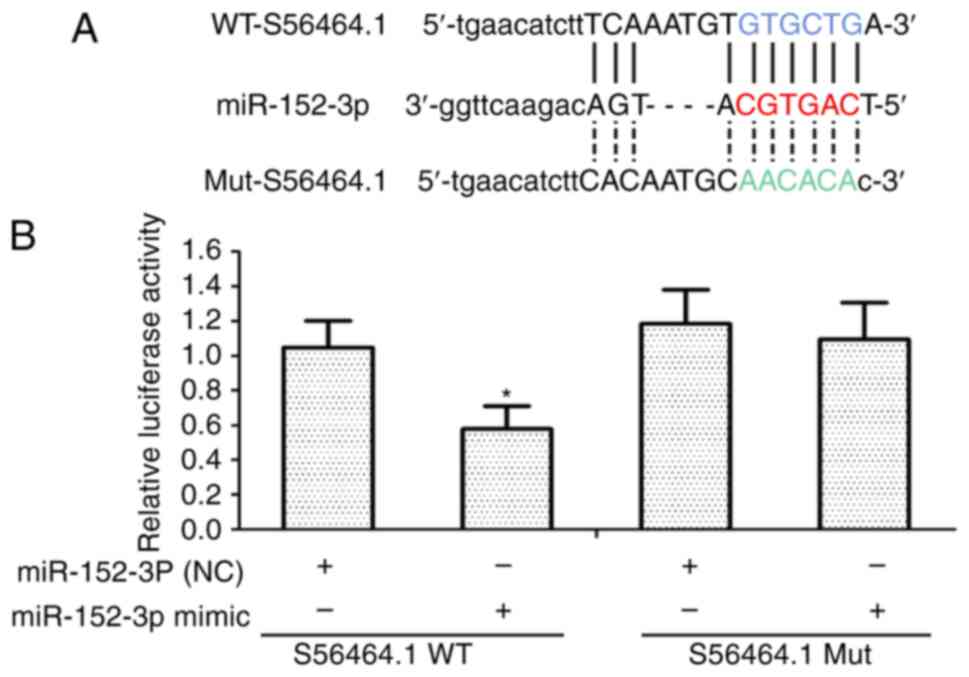
lncRNAS56464.1 can combine with
miR-152-3p
lncRNAs are considered to bind competitively with
miRNAs, resulting in a series of biological effects (37,38). In Fig. 3A, the present results revealed
using RNAhybrid, that miR-152-3p was identified as having a
potential binding site of lncRNAS56464.1. Then, in the subsequent
luciferase experiment, the luciferase constructor containing the
wild-type or mutant lncRNAS56464.1 was co-transfected with
miR-152-3p or control miRNAs to verify whether lncRNAS56464.1 could
target miR-152-3p. As revealed in Fig. 3B, upon overexpressing miR-152-3p
in FLS, the luciferase activity of wild-type lncRNAS56464.1 was
lower than that of mutant lncRNAS56464.1, which also indicated that
lncRNAS56464.1 could directly target miR-152-3p and combine with
it.
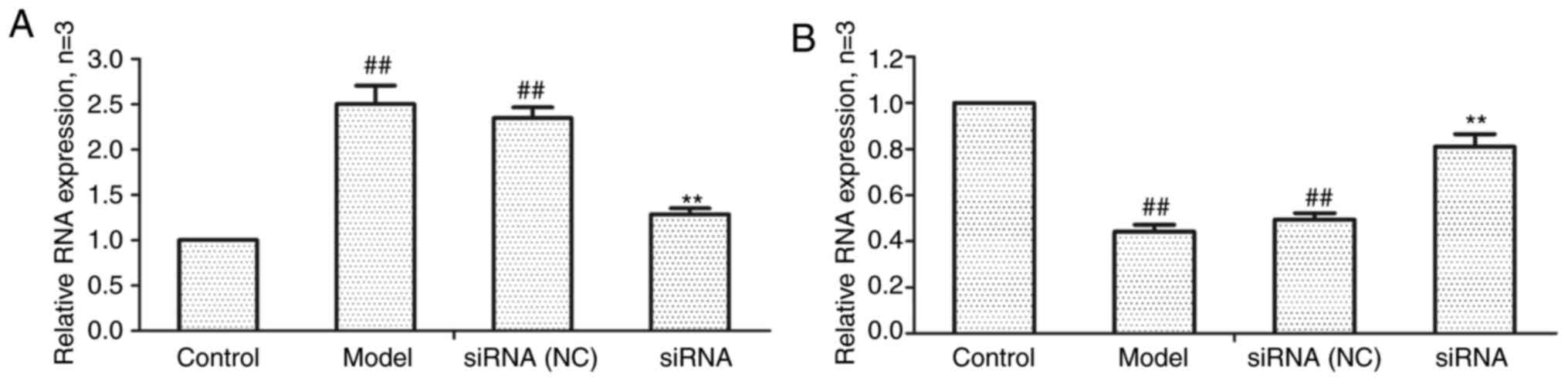
Effect of lncRNAS56464.1 interference on
the expression of lncRNAS56464.1 and miR-152-3p mRNA in FLS
As revealed in Fig.
4, compared with the control group, the expression of
lncRNAS56464.1 was significantly increased (Fig. 4A) and that of miR-152-3p was
significantly decreased (Fig. 4B)
in the model group. Compared with the model group, the expression
of lncRNAS56464.1 was significantly decreased (Fig. 4A) and that of miR-152-3p
significantly increased (Fig. 4B)
in the siRNA group.
Effect of lncRNAS56464.1 interference on
the Wnt signaling pathway
Effect of lncRNAS56464.1 interference
on Wnt1
It has been demonstrated that Wnt1 can directly
target miR-152-3p (39) and thus
Wnt1 was selected as the detection indicator. As revealed in
Fig. 5, compared with the control
group, the mRNA and protein expression of Wnt1 in the model group
and the siRNA (NC) group was significantly increased. Compared with
the model group, Wnt1 expression in the siRNA group was
significantly decreased.
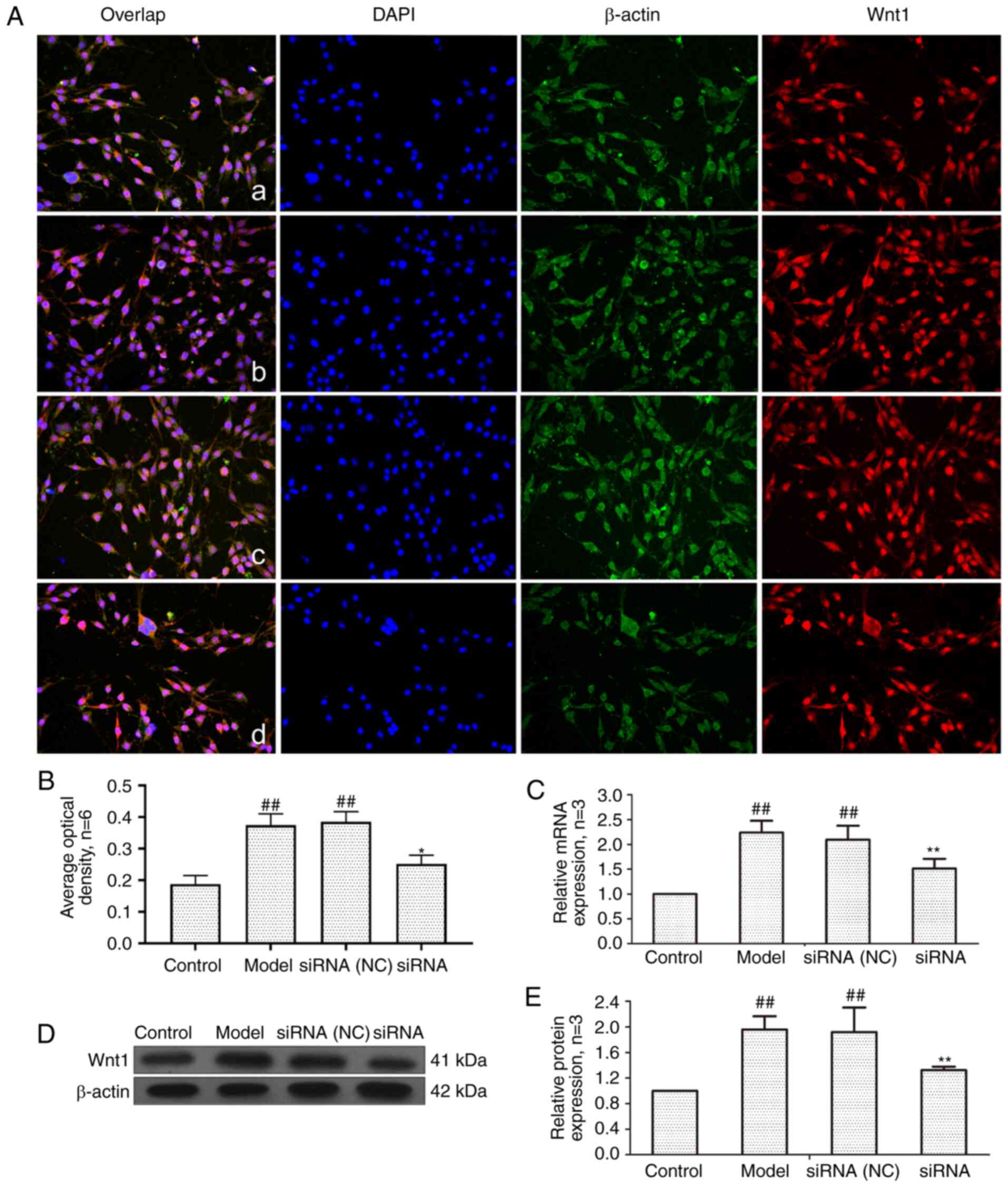 | Figure 5Changes of Wnt1 protein and mRNA
expression in FLS of AA rats after siRNA treatment. (A) The changes
of Wnt1 protein expression were observed by an immunofluorescence
technique (×400); a, control group; b, model group, c, siRNA (NC)
group, d, siRNA group. (B) Semiquantitative analysis of
fluorescence intensity. (C) The changes of Wnt1 mRNA expression
were observed by RT-qPCR. (D) The changes of Wnt1 protein
expression were observed by western blotting. (E) Semiquantitative
analysis of Wnt1 protein. ##P<0.01 compared with the
control group; *P<0.05 and **P<0.01
compared with the model group. Control, FLS of the normal group;
Model, FLS of the AA model group. siRNA (NC), small interfering RNA
negative control; siRNA, small interfering RNA; FLS,
fibroblast-like synoviocytes; AA, adjuvant-induced arthritis;
RT-qPCR, reverse transcription-quantitative PCR. |
Effect of lncRNAS56464.1 interference on
the expression of β-catenin in FLS of AA rats
β-catenin is a key gene in the classical Wnt
signaling pathway, which plays a central role in signal
transduction (40). As revealed
in Fig. 6, the mRNA and protein
expression of β-catenin in the model group and siRNA (NC) group was
significantly higher than that in the control group. The expression
levels of β-catenin at both the protein and mRNA level were
significantly lower in the siRNA group than that in the model
group.
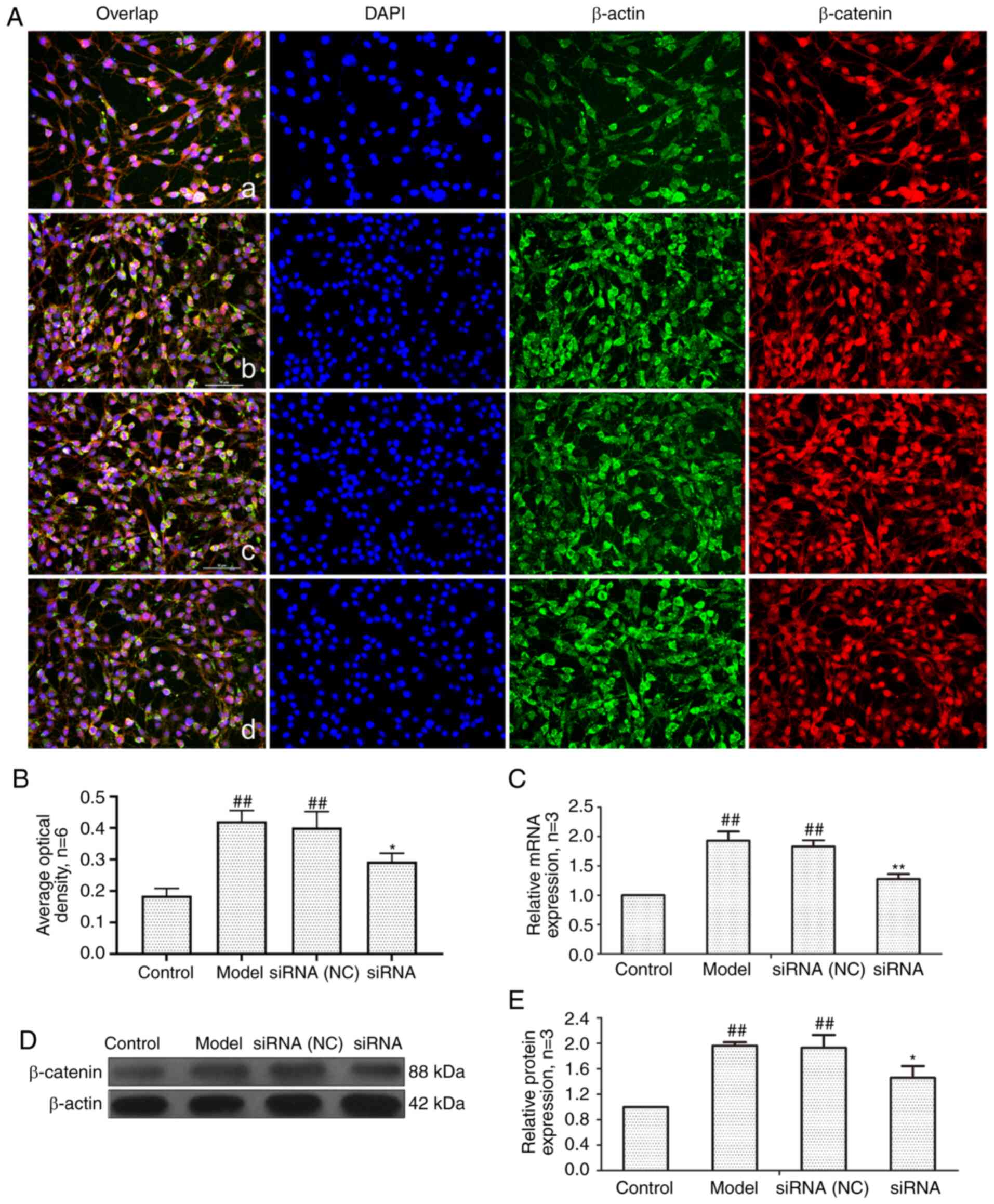 | Figure 6Changes of β-catenin protein and mRNA
expression in FLS of AA rats after siRNA treatment. (A) The changes
of β-catenin protein expression were observed by an
immunofluorescence technique (×400); a, control group; b, model
group, c, siRNA (NC) group, d, siRNA group. (B) Semiquantitative
analysis of fluorescence intensity. (C) The changes of Wnt1 mRNA
expression were observed by RT-qPCR. (D) The changes of β-catenin
protein expression were observed by western blotting. (E)
Semiquantitative analysis of β-catenin protein.
##P<0.01 compared with the control group;
*P<0.05 and **P<0.01 compared with the
model group. Control, FLS of the normal group; Model, FLS of the AA
model group. siRNA (NC), small interfering RNA negative control;
siRNA, small interfering RNA; FLS, fibroblast-like synoviocytes;
AA, adjuvant-induced arthritis; RT-qPCR, reverse
transcription-quantitative PCR. |
Effect of lncRNAS56464.1 interference on
the expression of c-Myc in FLS of AA rats
c-Myc is a proto-oncogene that is a downstream
target gene of the Wnt signaling pathway (41). As revealed in Fig. 7, compared with the control group,
the mRNA and protein expression of c-Myc in the model group and
siRNA (NC) group was significantly increased. After lncRNAS56464.1
interference, compared with the model group, the mRNA and protein
expression of c-Myc was significantly decreased.
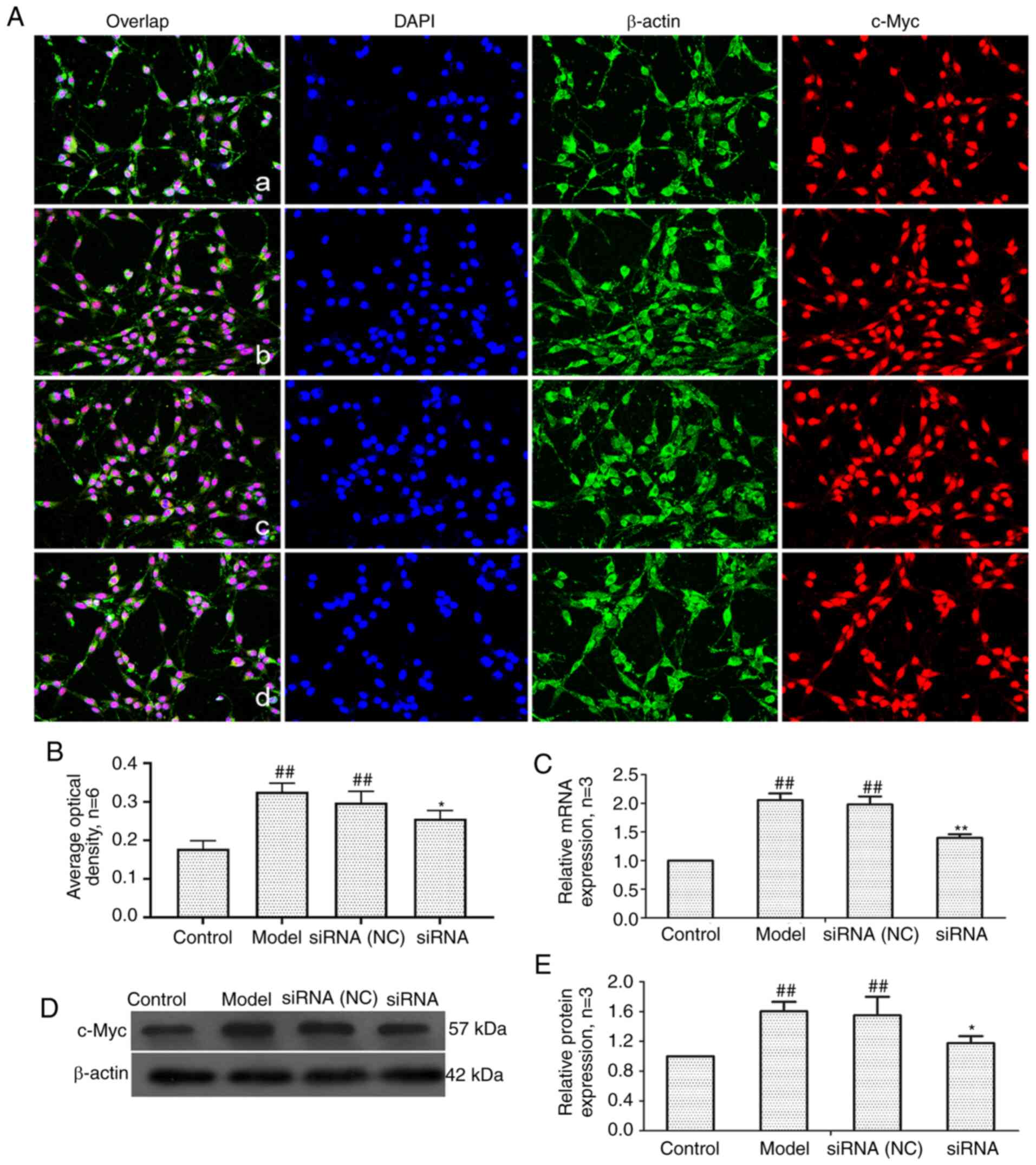 | Figure 7Changes of c-Myc protein and mRNA
expression in FLS of AA rats after siRNA treatment. (A) The changes
of c-Myc protein expression were observed by an immunofluorescence
technique (×400); a, control group; b, model group, c, siRNA (NC)
group, d, siRNA group. (B) Semiquantitative analysis of
fluorescence intensity. (C) The changes of c-Myc mRNA expression
were observed by RT-qPCR. (D) The changes of c-Myc protein
expression were observed by western blotting. (E) Semiquantitative
analysis of c-Myc protein. ##P<0.01 compared with the
control group; *P<0.05 and **P<0.01
compared with the model group. Control, FLS of the normal group;
Model, FLS of the AA model group. siRNA (NC), small interfering RNA
negative control; siRNA, small interfering RNA; FLS,
fibroblast-like synoviocytes; AA, adjuvant-induced arthritis;
RT-qPCR, reverse transcription-quantitative PCR. |
Effect of lncRNAS56464.1 interference on
the expression of cyclinD1 in FLS of AA rats
The main function of cyclin D1 is to promote cell
proliferation, and its overexpression can lead to cell loss of
control (42). To detect the
level of cyclin D1, RT-qPCR and western blotting were employed. As
revealed in Fig. 8, the mRNA and
protein expression of cyclin D1 in the model group and siRNA (NC)
group was significantly higher than that in control group. The
expression of cyclin D1 protein and mRNA in the siRNA group was
significantly lower than that in the model group.
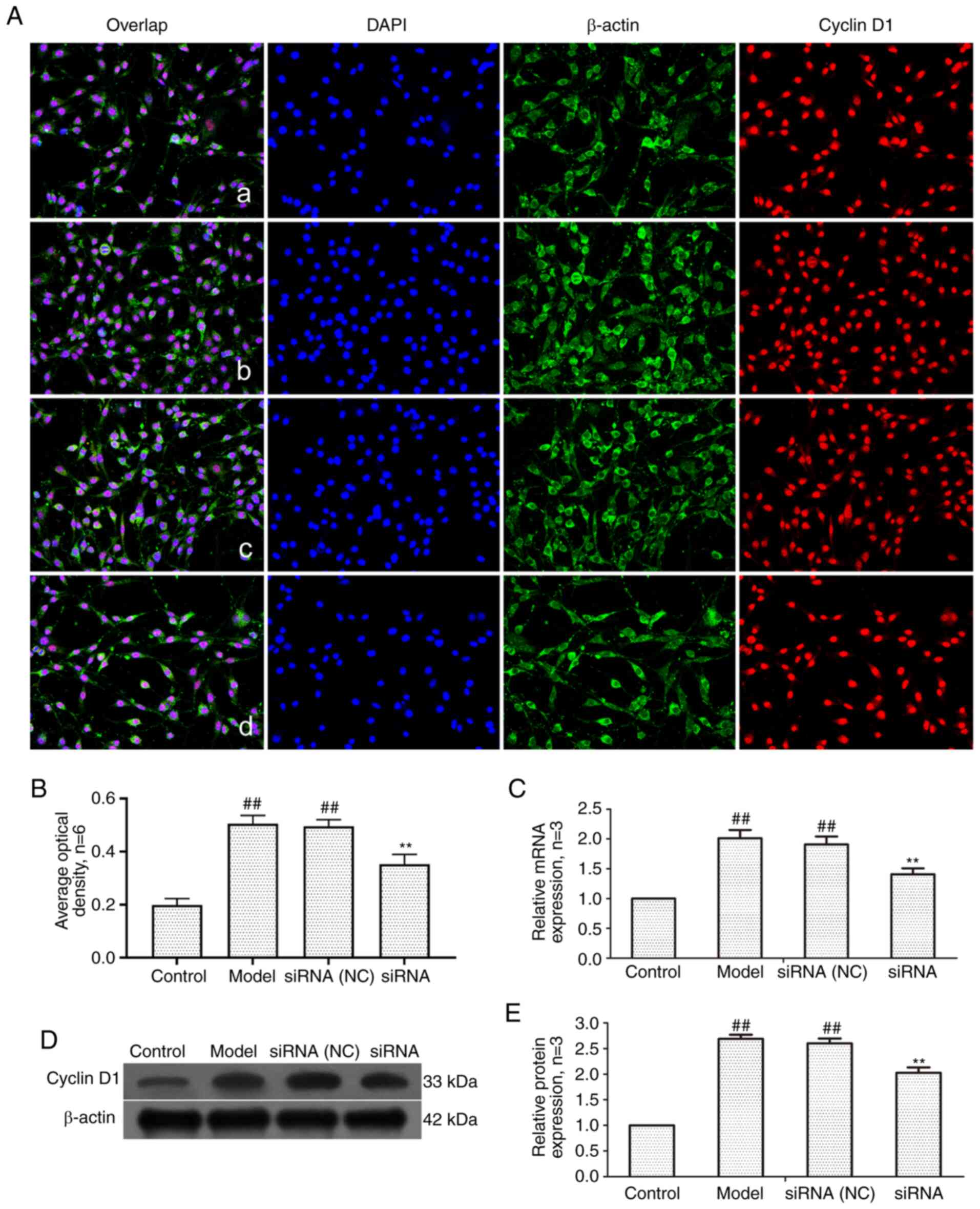 | Figure 8Changes of cyclin D1 protein and mRNA
expression in FLS of AA rats after siRNA treatment. (A) The changes
of cyclin D1 protein expression were observed by an
immunofluorescence technique (×400); a, control group; b, model
group, c, siRNA (NC) group, d, siRNA group. (B) Semiquantitative
analysis of fluorescence intensity. (C) The changes of cyclin D1
mRNA expression were observed by RT-qPCR. (D) The changes of cyclin
D1 protein expression were observed by western blotting. (E)
Semiquantitative analysis of cyclin D1 protein.
##P<0.01 compared with the control group;
**P<0.01 compared with the model group. Control, FLS
of the normal group; Model, FLS of the AA model group. siRNA (NC),
small interfering RNA negative control; siRNA, small interfering
RNA; FLS, fibroblast-like synoviocytes; AA, adjuvant-induced
arthritis; RT-qPCR, reverse transcription-quantitative PCR. |
Effect of lncRNAS56464.1 interference on
the expression of GSK-3β in synovial cells of AA rats
GSK-3β is a serine/threonine kinase that can
regulate cell proliferation and differentiation (43). As revealed in Fig. 9, compared with the control group,
the mRNA and protein expression of GSK-3β in the model group and
siRNA (NC) group had no significant change, but the protein level
of p-GSK-3β (Ser9)/GSK-3β was significantly increased. Compared
with the model group, the mRNA and protein expression of GSK-3β in
the siRNA group had no significant change, but the protein level of
p-GSK-3β (Ser9)/GSK-3β was significantly decreased.
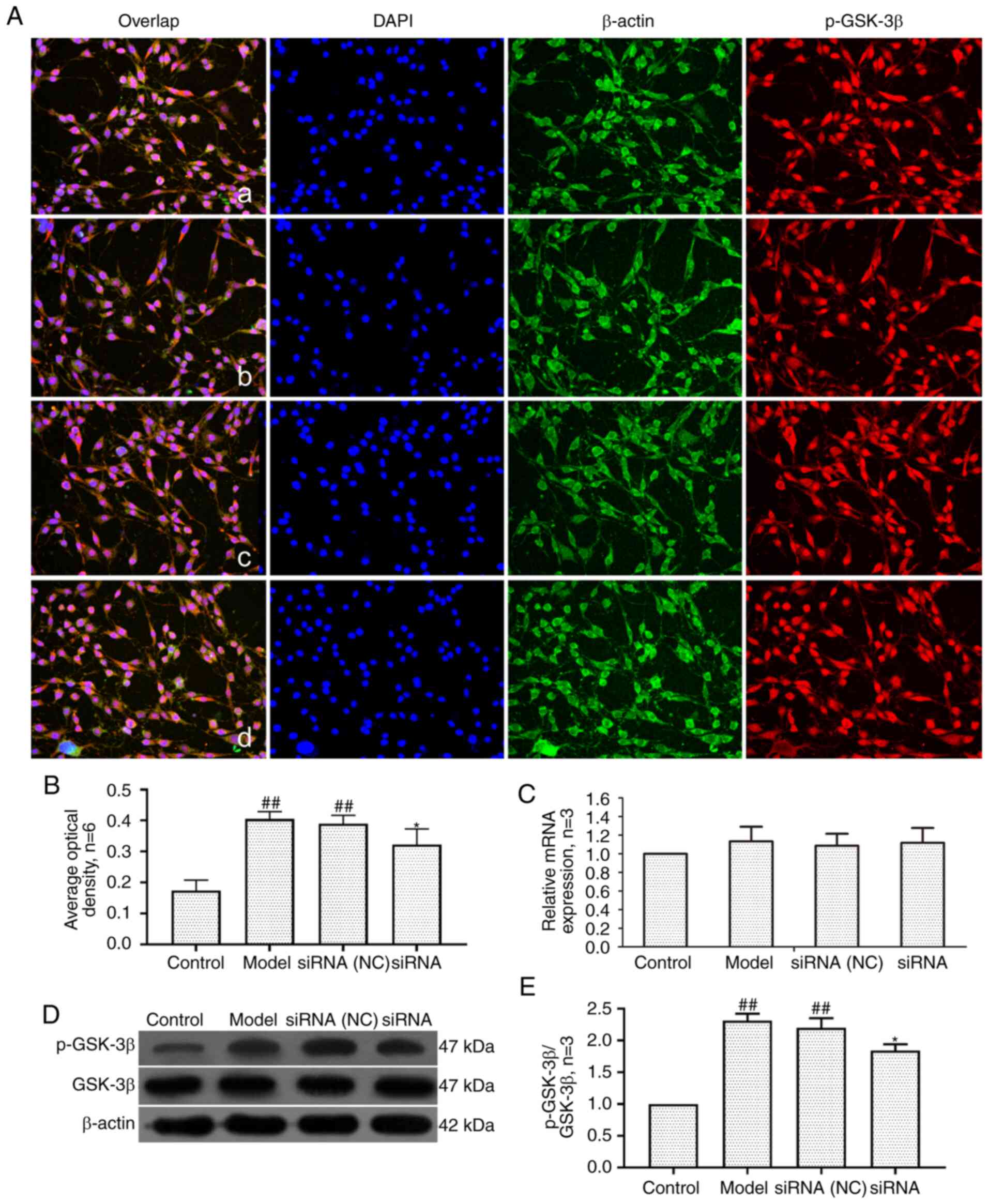 | Figure 9Changes of GSK-3β and p-GSK-3β (Ser9)
protein and mRNA expression in FLS of AA rats after siRNA
treatment. (A) The changes of p-GSK-3β(Ser9) protein expression
were observed by an immunofluorescence technique (×400); a, control
group; b, model group, c, siRNA (NC) group, d, siRNA group. (B)
Semiquantitative analysis of fluorescence intensity. (C) The
changes of p-GSK-3β(Ser9) mRNA expression were observed by RT-qPCR.
(D) The changes of GSK-3β and p-GSK-3β(Ser9) protein expression
were observed by western blotting. (E) Semiquantitative analysis of
p-GSK-3β(Ser9)/GSK-3β protein. ##P<0.01 compared with
the control group; *P<0.05 compared with the model
group. Control, FLS of the normal group; Model, FLS of the AA model
group. siRNA (NC), small interfering RNA negative control; siRNA,
small interfering RNA; FLS, fibroblast-like synoviocytes; AA,
adjuvant-induced arthritis; RT-qPCR, reverse
transcription-quantitative PCR. |
Effect of lncRNAS56464.1 interference on
the expression of SFRP4 in FLS of AA rats
SFRP4 is an antagonist of the Wnt signaling pathway,
which is closely related to cell proliferation and differentiation
(44). As revealed in Fig. 10, the mRNA and protein expression
of SFRP4 in the model group and the siRNA (NC) group was
significantly lower than that in the control group. After
lncRNAS56464.1 interference, the expression of SFRP4 at both
protein and mRNA levels was significantly higher than that in the
model group.
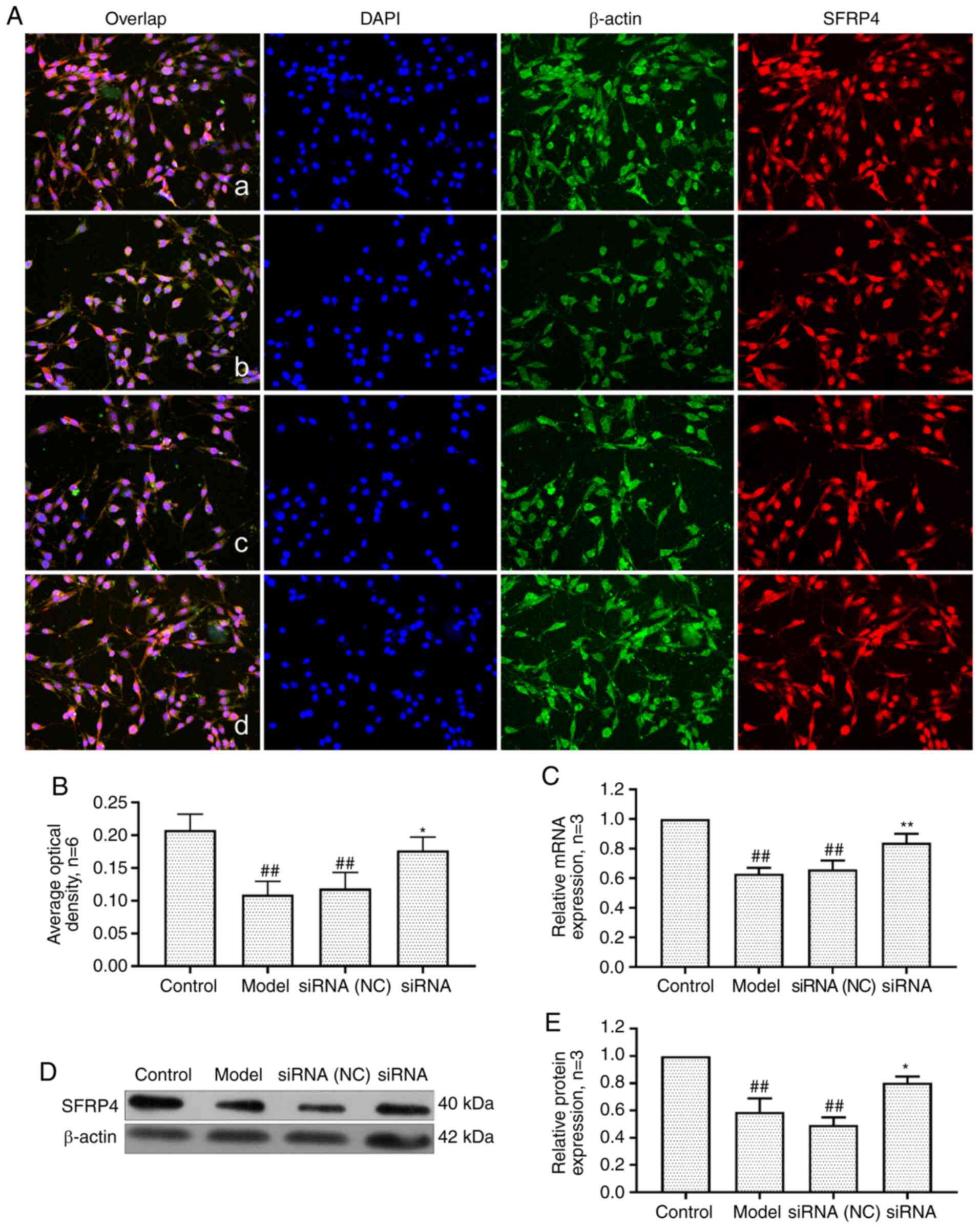 | Figure 10Changes of SFRP4 protein and mRNA
expression in FLS of AA rats after siRNA treatment. (A) The changes
of SFRP4 protein expression were observed by an immunofluorescence
technique (×400); a, control group; b, model group, c, siRNA (NC)
group, d, siRNA group. (B) Semiquantitative analysis of
fluorescence intensity. (C) The changes of SFRP4 mRNA expression
were observed by RT-qPCR. (D) The changes of SFRP4 protein
expression were observed by western blotting. (E) Semiquantitative
analysis of SFRP4 protein. ##P<0.01 compared with the
control group; *P<0.05 and **P<0.01
compared with the model group. Control, FLS of the normal group;
Model, FLS of the AA model group. siRNA (NC), small interfering RNA
negative control; siRNA, small interfering RNA; FLS,
fibroblast-like synoviocytes; AA, adjuvant-induced arthritis;
RT-qPCR, reverse transcription-quantitative PCR. |
Discussion
RA is a type of autoimmune disease characterized by
synovial hyperplasia and bone erosion. Patients exhibit some joint
swelling at the beginning of the course of the disease, which can
develop into systemic joint deformities at the later stage of the
course, causing serious damage to the body and psychology of a
patient. The pathological process of RA involves numerous types of
cells, among which FLS are the most important (45-47). FLS contain a large amount of
endoplasmic reticulum and have the main characteristics of a
protein secretory phase. Moreover, FLS can be activated
continuously in both an inflammatory and noninflammatory
environment, exhibiting tumor-like proliferation and high invasive
ability, which are closely related to the destruction of articular
cartilage and joint injury in RA (48-50).
lncRNAs, play a regulatory role in epigenetic,
pre-transcriptional and post-transcriptional aspects and widely
participate in the biological functions of the body and are closely
related to the progression of numerous diseases (51,52). In our previous study, we confirmed
that lncRNAS56464.1, as an antisense noncoding RNA, was found to be
located on chromosome 4 and exhibited increased expression in RA.
Moreover, bioinformatics analysis indicated that lncRNAS56464.1 is
a key gene in RA (16). In this
study, it was revealed that lncRNAS56464.1 interference inhibited
the proliferation of FLS, indicating that lncRNAS56464.1 can
promote the proliferation of FLS.
Since lncRNAS56464.1 is newly discovered as revealed
in our pervious study (16),
determination of the specific mechanism of lncRNAS56464.1 in RA
development and progression remains in its infancy. At present, it
is widely accepted that lncRNAs can regulate the abundance of
miRNAs by binding and sequestering them, acting as the so-called
miRNA sponges, and then regulating the expression of target mRNAs
(53,54). Thus, it has been revealed to be
efficient to infer the potential functions of lncRNAs by studying
the related miRNAs and mRNAs, whose functions have been annotated
(55-57). Through bioinformatics prediction,
it was revealed that there was a high degree of binding between
miR-152-3p and lncRNAS56464.1. Thereafter, through luciferase
reporter assays, it was also revealed that the luciferase activity
of lncRNAS56464.1-WT was lower than that of lncRNAS56464.1-Mut,
indicating that miR-152-3p was the downstream target of
lncRNAS56464.1 and can be combined with it.
miR-152 originates from chromosome 17 (17q21.32) of
chr10:84719319-84719403 (58).
Miao et al (59) revealed
that the expression of miR-152 was significantly decreased in rats
with adjuvant arthritis, and the upregulation of miR-152 expression
could inhibit the activation of the Wnt signaling pathway, thus
reducing the excessive proliferation of FLS. A recent study
reported that miR-152-3p could directly target Wnt1 and activate
the Wnt signaling pathway (60).
Large amounts of evidence indicate that the Wnt
signaling pathway can participate in the occurrence and development
of RA by regulating inflammation, proliferation, apoptosis,
articular cartilage maturation and bone destruction (61,62). Wnt1 plays a regulatory role in the
Wnt pathway, participates in biological processes such as cell
differentiation, proliferation and migration and is closely related
to the pathogenesis of RA (63,64). Sen et al and Nakamura et
al (65,66) revealed that the expression levels
of Wnt and fibronectin in the synovium of patients with RA were
significantly increased. The present study revealed that under the
condition of lncRNAS56464.1 interference, the expression of Wnt1
was significantly decreased, suggesting that lncRNAS56464.1 could
affect the proliferation of FLS by affecting the Wnt pathway.
β-catenin is the key gene of the classical Wnt
signaling pathway, which plays a central role in signal
transduction and is directly related to the abnormal proliferation
of RA synovial cells (67).
Yoshioka et al (68)
demonstrated that β-catenin siRNAs could significantly inhibit the
proliferation of FLS stimulated by IL-1β. c-Myc and cyclin D1 are
proto-oncogenes, which participate in cell proliferation,
differentiation and apoptosis and are closely related to the
occurrence and development of numerous diseases (69,70). Recent studies have revealed that
c-Myc and cyclin D1, as important target genes downstream of the
Wnt signaling pathway, are highly expressed in FLS and involved in
the pathogenesis of RA (71,72). GSK-3β is a widely expressed
serine/threonine family kinase, in which the abnormal activity of
the GSK-3β subtype has been demonstrated to be involved in the
occurrence and development of numerous diseases (73,74). Studies have revealed that the
stability and nuclear translocation of intracellular β-catenin are
landmark molecular events in the activation of the Wnt signaling
pathway. β-catenin, is the classical phosphorylation substrate of
GSK-3β, and its degradation is regulated by GSK-3β. When GSK-3β is
phosphorylated to p-GSK-3β (Ser9), degradation of β-catenin by
GSK-3β is inhibited, and then affects the signal transduction of
the Wnt signaling pathway (75).
The present study revealed that after lncRNAS56464.1 interference,
the expression levels of β-catenin, c-Myc, cyclin D1 and p-GSK-3β
(Ser9)/GSK-3β in FLS were significantly decreased.
SFRP4 is a secretory protein with a size of 40 kDa,
consisting of more than 300 amino acids (76). It can inhibit the activation of
the Wnt signaling pathway by competitively binding with receptors
related to the Wnt signaling pathway. The present data indicated
that the expression levels of SFRP4 were decreased at both the mRNA
and protein levels in FLS, which was in accordance with findings
from other relevant research studies (77,78). Notably, after lncRNAS56464.1
interference, the expression of SFRP4 was significantly
increased.
Based on the ceRNA theory (17), the effects of lncRNAS56464.1 on
targeting the miR-152-3p/Wnt pathway in FLS were studied, and the
relationship between RA and lncRNAs, miRNAs, and the Wnt pathway
was elucidated. However, the targeting relationship between
lncRNAS56464.1 and miR-152-3p in AA was only verified, thus in
future studies we will explore the relationship between miR-152-3p
and the Wnt signaling pathway in experimental arthritis.
In conclusion, as revealed in Fig. 11, the present study revealed that
lncRNAS56464.1 could target the miR-152-3p/Wnt pathway, promote the
proliferation of FLS, and then lead to the occurrence and
development of RA. This study deepens our understanding of the
pathogenesis of RA from the perspective of lncRNAs and provides a
new direction and target for the clinical diagnosis and treatment
of RA.
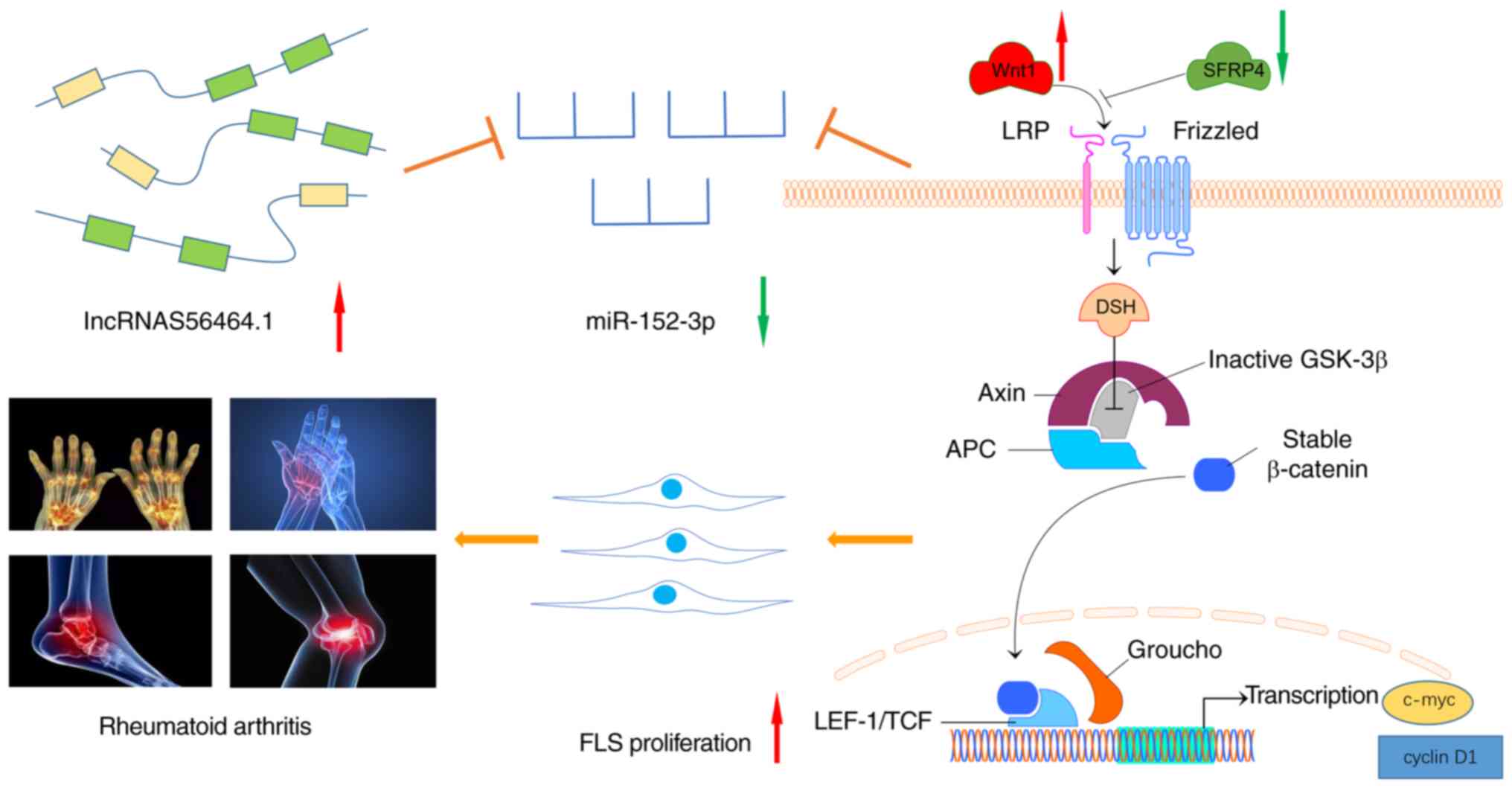 | Figure 11lncRNAS56464.1 as a ceRNA promotes
the proliferation of fibroblast-like synoviocytes in experimental
arthritis via the Wnt signaling pathway by sponging miR-152-3p.
When the body is stimulated by external damage factors, the
expression of lncRNAS56464.1 increases, and it competitively binds
to miRNA and decreases the expression of miR-152-3p. Therefore, it
increases the expression of Wnt1, β-catenin, c-Myc, and cyclin D1
in the Wnt signaling pathway, further promoting the phosphorylation
of GSK-3β, reducing the expression of SFRP4, and activating the Wnt
signaling pathway and thus promoting the proliferation of FLS and
affecting the occurrence and development of RA. FLS,
fibroblast-like synoviocytes; RA, rheumatoid arthritis. |
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant no. 81873139),
the Anhui Provincial Laboratory of Applied Basis and Development of
Internal Medicine of Modern Traditional Chinese Medicine (grant no.
2016080503B041), and the 12th Batch of '115' Innovation Team of
Anhui Province [Anhui Talent Office (2019) no. 1].
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL made substantial contributions to the conception
and design of the study. HJ, CF and JW performed the experiments.
HJ contributed to data acquisition, and data analysis and
interpretation. JL revised the manuscript critically for important
intellectual content. All authors agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of Anhui University of Chinese Medicine (Hefei,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
We are grateful to Mr Qiang Fan (Ao Ji Bio-tech Co.,
Ltd. Shanghai, China) for providing help with data analysis.
References
|
1
|
Taylor PC: Aetiopathology of rheumatoid
arthritis. Medicine. 42:227–230. 2014. View Article : Google Scholar
|
|
2
|
Gutierrez MJ, Desiderio SV, Wang NY,
Darrah E, Cappelli L, Nino G, Jones M and Bingham CO III: Soluble
markers of antibody secreting cell function as predictors of
infection risk in rheumatoid arthritis. J Immunol Res.
2019:36582152019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Onuora S: Rheumatoid arthritis: RA
synovium harbours distinct fibroblast subsets. Nat Rev Rheumatol.
14:2502018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collison J: Rheumatoid arthritis: Features
of synovium in RA remission revealed. Nat Rev Rheumatol.
12:3162016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu R, Zhao P, Zhang Q, Che N, Xu L, Qian
J, Tan W and Zhang M: Adiponectin promotes fibroblast-like
synoviocytes producing IL-6 to enhance T follicular helper cells
response in rheumatoid arthritis. Clin Exp Rheumatol. 38:11–18.
2020.
|
|
6
|
Hanlon MM, Rakovich T, Cunningham CC,
Ansboro S, Veale DJ, Fearon U and McGarry T: STAT3 mediates the
differential effects of oncostatin M and TNFα on RA synovial
fibroblast and endothelial cell function. Front Immunol.
10:20562019. View Article : Google Scholar
|
|
7
|
Adawi M, Firas S and Blum A: Rheumatoid
arthritis and atherosclerosis. Isr Med Assoc J. 21:460–463.
2019.PubMed/NCBI
|
|
8
|
Ferreira CC, Campi-Azevedo AC,
Peruhype-Magalhāes V, Coelho-Dos-Reis JG, Antonelli LRDV, Torres K,
Freire LC, da Costa-Rocha IA, Oliveira ACV, Maia MLS, et al: Impact
of synthetic and biological immunomodulatory therapy on the
duration of 17DD yellow fever vaccine-induced immunity in
rheumatoid arthritis. Arthritis Res Ther. 21:752019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu M, Hou J, Zheng M, Cao Y, Alike Y, Mi Y
and Zhu J: IL-21 gene rs6822844 polymorphism and rheumatoid
arthritis susceptibility. Biosci Rep. 40:BSR201914492020.
View Article : Google Scholar :
|
|
10
|
Bi X, Guo XH, Mo BY, Wang ML, Luo XQ, Chen
YX, Liu F, Olsen N, Pan YF and Zheng SG: lncRNA PICSAR promotes
cell proliferation, migration and invasion of fibroblast-like
synoviocytes by sponging miRNA-4701-5p in rheumatoid arthritis.
EBioMedicine. 50:408–420. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang J, Chen W and Lin J: LncRNA: An
all-rounder in rheumatoid arthritis. J Transl Int Med. 7:3–9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fok ET, Davignon L, Fanucchi S and Mhlanga
MM: The lncRNA connection between cellular metabolism and
epigenetics in trained immunity. Front Immunol. 9:31842019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng H, Sun M, Wang ZL, Wu Q, Yao J, Ren
G and Sun XL: lncRNA RMST-mediated miR-107 transcription promotes
OGD-induced neuronal apoptosis via interacting with hnRNPK.
Neurochem Int. 133:1046442020. View Article : Google Scholar
|
|
14
|
Guo M, Liu T, Zhang S and Yang L:
RASSF1-AS1 an antisense lncRNA of RASSF1A, inhibits the translation
of RASSF1A to exacerbate cardiac fibrosis in mice. Cell Biol Int.
43:1163–1173. 2019. View Article : Google Scholar
|
|
15
|
Liu J and Du W: lncRNA FENDRR attenuates
colon cancer progression by repression of SOX4 protein. Onco
Targets Ther. 12:4287–4295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang H, Ma R, Zou S, Wang Y, Li Z and Li
W: Reconstruction and analysis of the lncRNA-miRNA-mRNA network
based on competitive endogenous RNA reveal functional lncRNAs in
rheumatoid arthritis. Mol Biosyst. 13:1182–1192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Huang A, Li T, Su X, Ding H, Li H,
Qin X, Hou L, Zhao Q, Ge X, et al: miR-152 reduces human umbilical
vein endothelial cell proliferation and migration by targeting
ADAM17. FEBS Lett. 588:2063–2069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dang YW, Zeng J, He RQ, Rong MH, Luo DZ
and Chen G: Effects of miR-152 on cell growth inhibition, motility
suppression and apoptosis induction in hepatocellular carcinoma
cells. Asian Pac J Cancer Prev. 15:4969–4976. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang H, Hu M, Li P, Lu C and Li M:
miR-152 inhibits cell proliferation and colony formation of
CD133(+) liver cancer stem cells by targeting KIT. Tumour Biol.
36:921–928. 2015. View Article : Google Scholar
|
|
21
|
Miao CG, Qin D, Du CL, Ye H, Shi WJ, Xiong
YY, Zhang XL, Yu H, Dou JF, Ma ST, et al: DNMT1 activates the
canonical Wnt signaling in rheumatoid arthritis model rats via a
crucial functional crosstalk between miR-152 and the DNMT1, MeCP2.
Int Immunopharmacol. 28:344–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang H, Liu J, Wang T, Gao JR, Sun Y,
Huang CB, Meng M and Qin XJ: Urinary metabolite profiling provides
potential differentiation to explore the mechanisms of
adjuvant-induced arthritis in rats. Biomed Chromatogr.
30:1397–1405. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu L, Li J, Guo L, Yu X, Wu D, Luo L, Zhu
L, Chen W, Chen C, Ye C and Zhang D: Activation of NALP1
inflammasomes in rats with adjuvant arthritis; a novel therapeutic
target of carboxyami-dotriazole in a model of rheumatoid arthritis.
Br J Pharmacol. 172:3446–3459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang Y, Jia X, Sun X, Xu S, Wu Y, Zhang L
and Wei W: APRIL promotes proliferation, secretion and invasion of
fibroblast-like synoviocyte from rats with adjuvant induced
arthritis. Mol Immunol. 64:90–98. 2015. View Article : Google Scholar
|
|
25
|
Luo C, Liang JS, Gong J, Zhang HL, Feng
ZJ, Yang HT, Zhang HB and Kong QH: miRNA-31 over-expression improve
synovial cells apoptosis induced by RA. Bratisl Lek Listy.
119:355–360. 2018.PubMed/NCBI
|
|
26
|
Kragstrup TW, Sohn DH, Lepus CM, Onuma K,
Wang Q, Robinson WH and Sokolove J: Fibroblast-like synovial cell
production of extra domain A fibronectin associates with
inflammation in osteoarthritis. BMC Rheumatol. 3:462019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen X, Wang Z, Tong F, Dong X, Wu G and
Zhang R: lncRNA UCA1 promotes gefitinib resistance as a ceRNA to
target FOSL2 by sponging miR-143 in non-small cell lung cancer. Mol
Ther Nucleic Acids. 19:643–653. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Präbst K, Engelhardt H, Ringgeler S and
Hübner H: Basic colorimetric proliferation assays: MTT, WST, and
Resazurin. Methods Mol Biol. 1601:1–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng H, Zheng M, Hu Z, Zeng X, Kuang N and
Fu Y: Effects of daphnetin on the autophagy signaling pathway of
fibroblast-like synoviocytes in rats with collagen-induced
arthritis (CIA) induced by TNF-α. Cytokine. 127:1549522020.
View Article : Google Scholar
|
|
30
|
Wang Y, Feng T, Duan S, Shi Y, Li S, Zhang
X and Zhang L: miR-155 promotes fibroblast-like synoviocyte
proliferation and inflammatory cytokine secretion in rheumatoid
arthritis by targeting FOXO3a. Exp Ther Med. 19:1288–1296.
2020.PubMed/NCBI
|
|
31
|
Kozomara A, Birgaoanu M and
Griffiths-Jones S: miRBase: From microRNA sequences to function.
Nucleic Acids Res. 47(D1): D155–D162. 2019. View Article : Google Scholar :
|
|
32
|
Rehmsmeier M, Steffen P, Hochsmann M and
Giegerich R: Fast and effective prediction of microRNA/target
duplexes. RNA. 10:1507–1517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J and Liu S: LncRNA GAS5 suppresses
inflammatory responses and apoptosis of alveolar epithelial cells
by targeting miR-429/DUSP1. Exp Mol Pathol. 113:1043572020.
View Article : Google Scholar
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
35
|
Zhu W, Xu J, Jiang C, Wang B, Geng M, Wu
X, Hussain N, Gao N, Han Y, Li D, et al: Pristane induces autophagy
in macrophages, promoting a STAT1-IRF1-TLR3 pathway and arthritis.
Clin Immunol. 175:56–68. 2017. View Article : Google Scholar
|
|
36
|
Kikuta S, Nakamura Y, Yamamura Y, Tamura
A, Homma N, Yanagawa Y, Tamura H, Kasahara J and Osanai M:
Quantitative activation-induced manganese-enhanced MRI reveals
severity of Parkinson's disease in mice. Sci Rep. 5:128002015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ye Y, Gao X and Yang N: lncRNA ZFAS1
promotes cell migration and invasion of fibroblast-like
synoviocytes by suppression of miR-27a in rheumatoid arthritis. Hum
Cell. 31:14–21. 2018. View Article : Google Scholar
|
|
38
|
Javanmard AR, Dokanehiifard S, Bohlooli M
and Soltani BM: LOC646329 long non-coding RNA sponges miR-29b-1 and
regulates TGFβ signaling in colorectal cancer. J Cancer Res Clin
Oncol. 146:1205–1215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao X, Sun S, Xu J, Luo Y, Xin Y and Wang
Y: MicroRNA-152 inhibits cell proliferation of osteosarcoma by
directly targeting Wnt/β-catenin signaling pathway in a
DKK1-dependent manner. Oncol Rep. 40:767–774. 2018.PubMed/NCBI
|
|
40
|
Sun S, Xie F, Xu X, Cai Q, Zhang Q, Cui Z,
Zheng Y and Zhou J: Advanced oxidation protein products induce
S-phase arrest of hepatocytes via the ROS-dependent,
β-catenin-CDK2-mediated pathway. Redox Biol. 14:338–353. 2018.
View Article : Google Scholar
|
|
41
|
Tögel L, Nightingale R, Chueh AC,
Jayachandran A, Tran H, Phesse T, Wu R, Sieber OM, Arango D,
Dhillon AS, et al: Dual targeting of bromodomain and extraterminal
domain proteins, and WNT or MAPK signaling, inhibits c-MYC
expression and proliferation of colorectal cancer cells. Mol Cancer
Ther. 15:1217–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tetsuo F, Arioka M, Miura K, Kai M, Kubo
M, Igawa K, Tomooka K, Takahashi-Yanaga F, Nishimura F and Sasaguri
T: Differentiation-inducing factor-1 suppresses cyclin D1-induced
cell proliferation of MCF-7 breast cancer cells by inhibiting
S6K-mediated signal transducer and activator of transcription 3
synthesis. Cancer Sci. 110:3761–3772. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong ZC, Zhang D, Wang SB and Lin ZQ:
Target inhibition on GSK-3beta by miR-9 to modulate proliferation
and apoptosis of bladder cancer cells. Differentiation-inducing
factor-1 suppresses cyclin D1-induced cell proliferation of MCF-7
breast cancer cells by inhibiting S6K-mediated signal transducer
and activator of transcription 3 synthesis. Eur Rev Med Pharmacol
Sci. 22:3018–3026. 2018.PubMed/NCBI
|
|
44
|
Perumal V, Dharmarajan AM and Fox SA: The
Wnt regulator SFRP4 inhibits mesothelioma cell proliferation,
migration, and antagonizes Wnt3a via its netrin-like domain. Int J
Oncol. 51:362–368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Madav Y, Barve K and Prabhakar B: Current
trends in theranostics for rheumatoid arthritis. Eur J Pharm Sci.
145:1052402020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
DeMizio DJ and Geraldino-Pardilla LB:
Autoimmunity and inflammation link to cardiovascular disease risk
in rheumatoid arthritis. Rheumatol Ther. 7:19–33. 2020. View Article : Google Scholar :
|
|
47
|
Yang CA, Li JP, Yen JC, Lai IL, Ho YC,
Chen YC, Lan JL and Chang JG: lncRNA NTT/PBOV1 axis promotes
monocyte differentiation and is elevated in rheumatoid arthritis.
Int J Mol Sci. 19:28062018. View Article : Google Scholar :
|
|
48
|
Samarpita S, Ganesan R and Rasool M:
Cyanidin prevents the hyperproliferative potential of
fibroblast-like synoviocytes and disease progression via targeting
IL-17A cytokine signalling in rheumatoid arthritis. Toxicol Appl
Pharmacol. 391:1149172020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu Q, Yin S, Yao Y, Li X, Song B, Yang Y,
Liu Y, Chen R, Li J, Ma T, et al: MAST3 modulates the inflammatory
response and proliferation of fibroblast-like synoviocytes in
rheumatoid arthritis. Int Immunopharmacol. 77:1059002019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Jiao T, Fu W, Zhao S, Yang L, Xu N
and Zhang N: miR-410-3p regulates proliferation and apoptosis of
fibroblast-like synoviocytes by targeting YY1 in rheumatoid
arthritis. Biomed Pharmacother. 119:1094262019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang X, Wang W, Zhu W, Dong J, Cheng Y,
Yin Z and Shen F: Mechanisms and functions of long non-coding RNAs
at multiple regulatory levels. Int J Mol Sci. 20:55732019.
View Article : Google Scholar :
|
|
52
|
Jiang Z, Li L, Hou Z, Liu W, Wang H, Zhou
T, Li Y and Chen S: lncRNA HAND2-AS1 inhibits 5-fluorouracil
resistance by modulating miR-20a/PDCD4 axis in colorectal cancer.
Cell Signal. 66:1094832020. View Article : Google Scholar
|
|
53
|
Zhao CC, Jiao Y, Zhang YY, Ning J, Zhang
YR, Xu J, Wei W and Kang-Sheng G: lnc SMAD5-AS1 as ceRNA inhibit
proliferation of diffuse large B cell lymphoma via Wnt/β-catenin
pathway by sponging miR-135b-5p to elevate expression of APC. Cell
Death Dis. 10:2522019. View Article : Google Scholar
|
|
54
|
Wang H, Wang G, Gao Y, Zhao C, Li X, Zhang
F, Jiang C and Wu B: lnc-SNHG1 activates the TGFBR2/SMAD3 and
RAB11A/Wnt/β-catenin pathway by sponging MiR-302/372/373/520 in
invasive pituitary tumors. Cell Physiol Biochem. 48:1291–1303.
2018. View Article : Google Scholar
|
|
55
|
Li Y, Zou L, Yang X, Chu L, Ni J, Chu X,
Guo T and Zhu Z: Identification of lncRNA, microRNA, and
mRNA-associated CeRNA network of radiation-induced lung injury in a
mice model. Dose-Response. 17:15593258198910122019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu J, Li H, Zheng B, Sun L, Yuan Y and
Xing C: Competitive endogenous RNA (ceRNA) regulation network of
lncRNA-miRNA-mRNA in colorectal carcinogenesis. Dig Dis Sci.
64:1868–1877. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang J, Yin J, Wang X, Liu H, Hu Y, Yan X,
Zhuang B, Yu Z and Han S: Changing expression profiles of mRNA,
lncRNA, circRNA, and miRNA in lung tissue reveal the
pathophysiological of bronchopulmonary dysplasia (BPD) in mouse
model. J Cell Biochem. 120:9369–9380. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Friedrich M, Pracht K, Mashreghi MF, Jäck
HM, Radbruch A and Seliger B: The role of the miR-148/-152 family
in physiology and disease. Eur J Immunol. 47:2026–2038. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Miao CG, Yang YY, He X, Huang C, Huang Y,
Qin D, Du CL and Li J: MicroRNA-152 modulates the canonical Wnt
pathway activation by targeting DNA methyltransferase 1 in
arthritic rat model. Biochimie. 106:149–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun LB, Zhao SF, Zhu JJ, Han Y and Shan
TD: Long noncoding RNA UCID sponges miR-152-3p to promote
colorectal cancer cell migration and invasion via the Wnt/β-catenin
signaling pathway. Oncol Rep. 44:1194–1205. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Miao CG, Yang YY, He X, Li XF, Huang C,
Huang Y, Zhang L, Lv XW, Jin Y and Li J: Wnt signaling pathway in
rheumatoid arthritis, with special emphasis on the different roles
in synovial inflammation and bone remodeling. Cell Signal.
25:2069–2078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu J, Fan W, Ma L and Geng X: miR-708-5p
promotes fibroblast-like synoviocytes' cell apoptosis and
ameliorates rheumatoid arthritis by the inhibition of
Wnt3a/β-catenin pathway. Drug Des Devel Ther. 12:3439–3447. 2018.
View Article : Google Scholar :
|
|
63
|
Zhang LH, Wang Y, Fan QQ, Liu YK, Li LH,
Qi XW, Mao Y and Hua D: Up-regulated Wnt1-inducible signaling
pathway protein 1 correlates with poor prognosis and drug
resistance by reducing DNA repair in gastric cancer. World J
Gastroenterol. 25:5814–5825. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Malysheva K, de Rooij K, Lowik CW, Baeten
DL, Rose-John S, Stoika R and Korchynskyi O: Interleukin 6/Wnt
interactions in rheumatoid arthritis: Interleukin 6 inhibits Wnt
signaling in synovial fibroblasts and osteoblasts. Croat Med J.
57:89–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sen M, Reifert J, Lauterbach K, Wolf V,
Rubin JS, Corr M and Carson DA: Regulation of fibronectin and
metalloproteinase expression by Wnt signaling in rheumatoid
arthritis synoviocytes. Arthritis Rheum. 46:2867–2877. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nakamura Y, Nawata M and Wakitani S:
Expression profiles and functional analyses of Wnt-related genes in
human joint disorders. Am J Pathol. 167:97–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang W, Guo P, Chen M, Chen D, Cheng Y and
He L: FOXM1/LINC00152 feedback loop regulates proliferation and
apoptosis in rheumatoid arthritis fibroblast-like synoviocytes via
Wnt/β-catenin signaling pathway. Biosci Rep. 40:BSR201919002020.
View Article : Google Scholar
|
|
68
|
Yoshioka R, Kita Y, Nagahira A, Manno A,
Makita N, Tomita U and Murakawa M: Quantitative analysis of
cadherin-11 and β-catenin signalling during proliferation of
rheumatoid arthritis-derived synovial fibroblast cells. J Pharm
Pharmacol. 67:1075–1082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ramanan M, Pilli VS, Aradhyam GK and Doble
M: Transcriptional regulation of microsomal prostaglandin E
synthase 1 by the proto-oncogene, c-myc, in the pathogenesis of
inflammation and cancer. Biochem Biophys Res Commun. 482:556–562.
2017. View Article : Google Scholar
|
|
70
|
Liu Q, Loo WT, Sze SC and Tong Y: Curcumin
inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer
cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1
transcription. Phytomedicine. 16:916–922. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang H, Jia XZ, Sui CJ, Zhao YP, Mei YF,
Zheng YN and Zhang ZY: Effects of thapsigargin on the proliferation
and survival of human rheumatoid arthritis synovial cells.
ScientificWorldJournal. 2014:6054162014.PubMed/NCBI
|
|
72
|
Huang XY, Zhang XM, Chen FH, Zhou LL, Deng
XF, Liu YJ and Li XJ: Anti-proliferative effect of recombinant
human endostatin on synovial fibroblasts in rats with adjuvant
arthritis. Eur J Pharmacol. 723:7–14. 2014. View Article : Google Scholar
|
|
73
|
Zhu B, Zhang S, Meng N, Zhang H, Yuan S
and Zhang J: Long non-coding RNA RNCR3 promotes glioma progression
involving the Akt/GSK-3β pathway. Oncol Lett. 18:6315–6322.
2019.PubMed/NCBI
|
|
74
|
Li S, Oh YT, Yue P, Khuri FR and Sun SY:
Inhibition of mTOR complex 2 induces GSK3/FBXW7-dependent
degradation of sterol regulatory element-binding protein 1 (SREBP1)
and suppresses lipogenesis in cancer cells. Oncogene. 35:642–650.
2016. View Article : Google Scholar
|
|
75
|
Pronobis MI, Rusan NM and Peifer M: A
novel GSK3-regulated APC: Axin interaction regulates Wnt signaling
by driving a catalytic cycle of efficient βcatenin destruction.
Elife. 4:e080222015. View Article : Google Scholar
|
|
76
|
Guan H, Zhang Y, Gao S, Bai L, Zhao S,
Cheng XW, Fan J and Liu E: Differential patterns of secreted
frizzled-related protein 4 (SFRP4) in adipocyte differentiation:
Adipose depot specificity. Cell Physiol Biochem. 46:2149–2164.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ghoshal A and Ghosh SS: Antagonizing
canonical Wnt signaling pathway by recombinant human sFRP4 purified
from E. coli and its implications in cancer therapy. Mol Cell
Biochem. 418:119–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Haraguchi R, Kitazawa R, Mori K, Tachibana
R, Kiyonari H, Imai Y, Abe T and Kitazawa S: sFRP4-dependent Wnt
signal modulation is critical for bone remodeling during postnatal
development and age-related bone loss. Sci Rep. 6:251982016.
View Article : Google Scholar : PubMed/NCBI
|