Introduction
Acute kidney injury (AKI) is one of the most common
clinical syndromes following surgical operations and sepsis; it is
accompanied by a disorder in kidney function (1). AKI occurs in 10–15% of patients
admitted to hospital, whereas its incidence in intensive care has
been reported in >50% of patients. AKI is evaluated by examining
the levels of serum creatinine (SCr) and blood urea nitrogen (BUN)
(2). Among the etiology of AKI,
prerenal etiologies account for 25–60% and renal etiologies account
for 35–70% of AKI cases (3). In
the kidney, proximal tubules are more susceptible to ischemia than
are the inner medulla and deep papillae (4). Ischemia/reperfusion (I/R) injury of
the kidney is the leading cause of AKI (5). It was reported that ischemic injury
or nephrotoxins contribute to 80–90% of the renal etiologies
(3).
Autophagy is a cellular stress response that serves
important roles in cellular homeostasis (6,7).
Autophagy can be caused by stress, including cell starvation,
oxidant injury and endoplasmic reticulum stress, as well as hypoxia
(6,7). Most of these stresses occur in the
pathogenesis of AKI (8).
Accumulating evidence indicates that autophagy may serve a
protective role in cell survival during renal ischemia/reperfusion
injury (9,10).
MicroRNAs (miRNAs) are a family of small (19–22
nucleotides), non-coding RNAs (11). Previous studies have implicated
dysregulated miRNAs in the pathogenesis of kidney injury (5,12,13). miRNA (miR)-30a-5p is a tumor
suppressor in several cancer types (14–16). Previous studies have linked
miR-30a-5p to ischemic heart disease (17) and cerebral ischemic stroke
(18). miR-30a-5p is also
reported to regulate renal cell carcinoma aggressiveness (15). However, the function and
underlying mechanisms of miR-30a-5p in ischemic kidney injury
remain unclear. The present study aimed to explore whether
miR-30a-5p affected ischemic kidney injury and the autophagy of
tubular epithelial cells, as well as to investigate the underlying
mechanism.
Materials and methods
Animal models
Male C57BL/6 mice (age, 6–8 weeks; weight, 18–22 g)
were from Chengdu Dossy Experimental Animals Co., Ltd. The mice
were maintained in a temperature (22±2°C) and humidity-controlled
(55±5%) vivarium with a 12-h light/dark cycle and allowed free
access to food and water. Mice were anesthetized with an
intraperitoneal injection of pentobarbital (70 mg/kg) and kept on a
warm blanket to maintain the body temperature at 37°C during
surgery. To induce renal I/R injury, the bilateral renal pedicles
were clamped for 60 min with a microaneurysm clip followed by 24 h
reperfusion. For miR-30a-5p detection, mice with 6 h reperfusion
after 60 min clamping were also used. Mice were sacrificed by
cervical dislocation, and their kidneys and blood were collected
for examination. Mice in the sham group underwent the same surgical
procedures without clamping. The Ethics Committee of the General
Hospital of Central Theater Command (Wuhan, China) approved this
study, which followed the regulatory standards of the National
Institutes of Health guide for the care and use of laboratory
animals (19).
For the in vivo study of the function of
miR-30a-5p, adenovirus miR-30a-5p mimic (Ad-mimic) and miR-30a-5p
inhibitor (Ad-inhibitor) were purchased from Shanghai GenePharma
Co. Ltd. Mice were intraperitoneally injected with 1×109
pfu Ad-mimic or Ad-inhibitor 24 h before renal I/R injury.
Histopathological evaluation
Freshly harvested kidney tissues from mice were
fixed in 4% paraformaldehyde for 16 h at room temperature. The
tissues were then dehydrated through an ethanol gradient. Tissues
were paraffin embedded and cut into 5 μm sections. The
sections were then deparaffinized with xylene and rehydrated in an
ethanol gradient and distilled water. The sections were stained
with hematoxylin for 5 min, washed with water, differentiated for
30 sec and stained with eosin for 30 sec at room temperature.
Histological damage was quantified in a blinded manner using a
light microscope. Tubular necrosis scores were determined by
assessing the percentage of tubules that displayed epithelial
necrosis or had luminal necrotic debris, tubular dilation and
hemorrhage: 0, none; 1, mild (<25%); 2, moderate (25–50%); 3,
severe (51–75%); and 4, extensive (>75%) damage (20).
Detection of SCr, BUN and kidney injury
molecule 1 (Kim-1)
Serum levels of SCr and BUN were analyzed using
commercially available Creatinine Assay kit (cat. no. C011-1-1) and
Urea Assay kit (cat. no. C013-2-1) from Nanjing Jiancheng
Bioengineering Institute, respectively, according to the
manufacturer's procedure. Urine concentrations of Kim-1 were
analyzed using commercially Kidney Injury Molecule 1 Assay kit
(cat. no. H436-1; Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's procedure.
ELISA analysis
Serum levels of TNF-α, IL-1β and IL-6 were
determined by commercially available mouse TNF-α ELISA kit (cat.
no. PT512), mouse IL-1β ELISA kit (cat. no. PI301) and mouse IL-6
ELISA kit (cat. no. PI326) from Beyotime Institute of
Biotechnology, respectively. Absorbance was measured at 450 nM
using a microplate reader (Bio-Rad Laboratories, Inc.). All samples
were analyzed in triplicate.
Cell lines
HK-2 human tubular epithelial and 293T cell lines
were obtained from the American Type Culture Collection (ATCC) and
were cultured according to ATCC protocols. Both cell lines were
cultured in DMEM supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), streptomycin (100 μg/ml),
penicillin (100 U/ml) and 2 mM of L-glutamate. All cells were
cultured at 37°C in a 5% CO2 incubator (Thermo Fisher
Scientific, Inc.). To induce hypoxia/re-oxygenation (H/R) injury,
HK-2 cells were incu- bated in a hypoxic chamber with 0.3%
O2, 5% CO2, and 95% N2 for 16 h,
and then were re-oxygenated in a normoxic chamber with 5%
CO2 and 95% N2 for 4 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from renal cortex tissues
and cells using a RNeasy mini kit (Qiagen Sciences, Inc.) according
to the manufacturer's protocols. cDNA was synthesized using the
SuperScript III First-Strand Synthesis kit (Invitrogen; Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
instructions. qPCR was performed using TaqMan Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and an ABI 7300 Real
Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows: Initial
denaturation at 95°C for 10 min; followed by 40 cycles of
denaturation at 95°C for 10 sec, annealing and extension at 60°C
for 1 min. The following primers were used: miR-30a-5p, forward
5′-TGTAAACATCCTCGACTGGAAG-3′ and reverse 5′-TGCGTGTCGTGGAGTC-3′;
U6, forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse
5′-AACGCTTCACGAATTTGCGT-3′; Beclin-1, forward
5′-AGGTTGAGAAAGGCGAGACA-3′ and reverse 5′-TTTTGATGGAATAGGAGCCG-3′;
and β-actin, forward 5′-GTGGGCCGCCCTAGGCACCA-3′ and reverse
5′-CGGTTGGCCTTAGGGTTCAG-3′. β-actin or U6 were used as the internal
controls. Relative expression levels were analyzed using
2−ΔΔCq method (21).
Western blotting
Proteins were obtained from mouse renal cortex and
HK-2 cells using RIPA lysis buffer (Beijing Solarbio Science &
Technology Co., Ltd.). Protein concentration was measured with a
BCA Protein Assay kit (cat. no. P0011; Beyotime Institute of
Biotechnology). Equal amounts (30 μg) of protein from each
sample were separated by 10% SDS-PAGE. The separated bands were
then electrotransferred onto a polyvinylidene difluoride membrane
(Roche Diagnostics). After being blocked with 5% skim milk on a
rotary shaker at room temperature for 2 h, the blots were incubated
with the following primary antibodies: Rabbit anti-human
microtubule-associated protein light chain 3 (LC3)-I/II monoclonal
antibody (1:2,000; cat. no. 12741; Cell Signaling Technology,
Inc.), rabbit anti-human Beclin-1 monoclonal antibody (1:1,500;
cat. no. 3495; Cell Signaling Technology, Inc.), rabbit anti-human
p62 monoclonal anti-body (1:2,000; cat. no. ab207305; Abcam),
rabbit anti-human autophagy-related gene16 (ATG16) monoclonal
antibody (1:2,000; cat. no. 8089; Cell Signaling Technology, Inc.),
and mouse anti-human β-actin (1:2,500; cat. no. ab8226; Abcam)
overnight at 4°C, followed by horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG (1:10,000; cat. no. ab6789;
Abcam) or HRP-conjugated goat anti-rabbit IgG (1:10,000; cat. no.
ab6721; Abcam) for 30 min at 37°C. Protein bands were visualized
using ECL Western Blotting Substrate kit (cat. no. 32209; Pierce;
Thermo Fisher Scientific, Inc.) and semi-quantified using the
ImageJ software (version 1.47; National Institutes of Health);
β-actin was used for normalization.
Cell transfection
miR-30a-5p mimic (sense,
5′-UGUAAACAUCCUCGACUGGAAG-3′; anti-sense,
5′-CUUCCAGUCGAGGAUGUUUACA-3′), miR-30a-5p inhibitor
(5′-CUUCCAGUCGAGGAUGUUUACA-3′), miRNA mimic-negative control (nc)
(sense, 5′-UCACAACCUCCUAGAAAGAGUAGA-3′; anti-sense,
5′-UACUCUUUCUAGGAGGUUGUGAUU-3′) and inhibitor-nc
(5′-CACAUUGTGCUCUCUGCACUGCTC-3′), Beclin-1 small interfering
(si)RNA (sense, 5′-GGAGCCAUUUAUUGAAACUTT-3′; anti-sense,
5′-AGUUUCAAUAAAUGGCUCCTT-3′), siRNA-scramble control (sense,
5′-GUACGCCAAAAGUUAAACC-3′, anti-sense, 5′-GGUUUAACUUUUGGCGUAC-3′)
and ATG16 siRNA (sense, 5′-AGAGCUUGACUCAGACCAAGU-3′; anti-sense,
5′-UUGGUCUGAGUCAAGCUCUGA-3′) were purchased from Shanghai
GenePharma Co. Ltd. Beclin-1 cDNA was amplified from the HK-2 cells
and cloned into the pcDNA3.1 vector (Invitrogen; Thermo Fisher
Scientific, Inc.). For miR-30a-5p function analysis,
3×105 HK-2 cells were transfected with miR-30a-5p mimic
(50 nM), miR-30a-5p inhibitor (100 nM), miRNA-nc (50 nM) or
inhibitor-nc (100 nM) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) for 48 h. To downregulate the expression
of Beclin-1 or ATG16, 3×105 HK-2 cells were transfected
with siRNA-scramble control (50 nM), Beclin-1 siRNA (50 nM) or
ATG16 siRNA (50 nM) using Lipofectamine® 2000 for 48 h. To
overexpress Beclin-1, 3×105 HK-2 cells were transfected
with pcDNA3.1 empty vector (0.5 μg) or pcDNA3.1-Beclin-1
(0.5 μg) using Lipofectamine® 2000 for 48 h.
Flow cytometry
Apoptosis was detected using the Annexin V-FITC
Apoptosis Detection kit (cat. no. C1062L; Beyotime Institute of
Biotechnology) according to previously reported protocols (22). Briefly, cells (1×106)
were re-suspended in 100 μl binding buffer. Annexin V-FITC
(5 μl) and propidium iodide staining solution (10 μl)
were added to the cell suspension and the cells were cultured in
the dark for 15 min at room temperature. The percentage of early +
late stage apoptotic was detected using a BD FACSCalibur flow
cytometer (BD Biosciences), and data were analyzed using FlowJo
7.6.1 (FlowJo LLC).
Immunofluorescence staining
Immunofluorescence staining of LC3 was performed
according to previously described methods (23). Briefly, 3×105 cells
were fixed with 4% paraformaldehyde for 20 min at room temperature,
permeabilized with 0.02% Triton X-100 for 5 min at room
temperature, blocked with 5% bovine serum albumin (Beyotime
Institute of Biotechnology) in PBS for 20 min at 4°C and incubated
with rabbit anti-human LC3 monoclonal antibody (1:1,000; cat. no.
3868; Cell Signaling Technology, Inc.) for 12 h at 4°C. After
washing three times with PBS, cells were incubated with the Alexa
Fluor 488-conjugated goat anti-rabbit IgG secondary antibody
(1:200; cat. no. A-11034; Thermo Fisher Scientific, Inc.) for 2 h.
Images were captured using fluorescence microscopy (Zeiss AG). The
number of LC3 punctae in each cell were counted. A minimum of 50
cells were included for each group.
In silico analysis
The predicted targets of miR-30a-5p were analyzed by
the EIMMo miRNA target prediction server (24), microRNA.org
database (25), and TargetScan
(Release 7.2: March 2018; http://www.targetscan.org) database.
Luciferase reporter assay
The pGLO-Beclin-1-wild-type (WT) plasmids were
constructed by cloning the 3′-untranslated region (3′-UTR) of
Beclin-1 containing the predicted miR-30a-5p binding site
downstream of the firefly luciferase structural gene of the pmirGLO
Dual-Luciferase miRNA Target Expression Vector (Promega
Corporation). Similarly, the mutant (Mut) 3′-UTR of Beclin-1 was
generated using a QuikChange Site-Directed Mutagenesis kit (Agilent
Technologies, Inc.), according to the manufacturer's protocol, and
used to make the pGLO-Beclin-1-Mut plasmids. The 293T cells were
plated into 24-well plates (1.5×105 cells/well) and
incubated at 37°C for 24 h. Subsequently, the cells were
transfected at 37°C with 100 nM miR-30a-5p or a mimic-nc combined
with 100 ng pGLO-Beclin-1-WT or pGLO-Beclin-1-Mut plasmid using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
At 48 h after transfection, luciferase activities were measured
using a Dual-Luciferase Reporter Assay kit (Promega Corporation)
according to the manufacturer's instructions using a GloMax 20/20
luminometer (Promega Corporation). The relative luciferase activity
was normalized by Renilla luciferase activity.
RNA immunoprecipitation (RIP)
RIP was performed using an EZ-Magna RIP RNA-Binding
Protein Immunoprecipitation kit (cat. no. 17-701; MilliporeSigma)
in HK-2 cells transfected with miR-30a-5p mimic or mimic-nc.
5×106 cells were lysed with complete RIP lysis buffer.
Subsequently, 100 μl of cell lysate was incubated with RIP
buffer containing magnetic beads conjugated with mouse anti-human
ago2 antibody (1:10; cat. no. MA5-23515; Thermo Fisher Scientific,
Inc.) or negative control normal mouse IgG (1:10; cat. no.
ab188776; Abcam). Samples were incubated with Proteinase K buffer,
washed with RIP Wash Buffer, and then the bound RNA was extracted
with phenol:chloroform:isoamyl alcohol (25:24:1, v/v) for RT-qPCR
assay, aforementioned.
Statistical analysis
All statistical analyses were conducted using SPSS
20.0 software (IBM Corp.). Data are expressed as the mean ±
standard deviation. Differences between two groups were assessed
using an independent samples t-test. For multiple-group
comparisons, a significant one-way analysis of variance F-test was
used, followed by the Tukey-Kramer test. P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-30a-5p expression is decreased in
mouse models of renal I/R injury and in H/R-exposed HK-2 cells
To induce renal I/R injury, the bilateral renal
pedicles were clamped for 60 min with a microaneurysm clip followed
by 24 h reperfusion (model group). Mice were subsequently
euthanized, and their kidneys and blood were collected for
examination. Mice in the sham group underwent the same surgical
procedures without clamping. Compared with the sham surgery group,
the mouse models of renal I/R injury exhibited significantly
increased serum levels of SCr, BUN and urine concentrations of
Kim-1 (Fig. 1A-C, respectively).
To assess the levels of inflammation during injury, the
concentrations of TNF-α, IL-1β and IL-6 were also examined using
ELISA kit. The result showed that serum levels of TNF-α, IL-1β and
IL-6 were significantly elevated after renal I/R compared with the
sham group (Fig. 1D).
Histopathological examination revealed tubular epithelial cell
necrosis in renal I/R injury model group, including abscission of
tubular epithelial cells, glomerular hypertrophy and loss of
epithelial cell nuclei (Fig. 1E and
F). These results confirmed the successful establishment of the
mouse models of kidney injury.
 | Figure 1miR-30a-5p expression is decreased in
in vivo and in vitro models of renal I/R injury. Mice
were intraperitoneally injected with Ad-mimic or Ad-inhibitor 24 h
before renal ischemia/reperfusion injury. ELISA analysis for serum
levels of (A) SCr and (B) BUN, and (C) urine concentration of Kim-1
in mice (n=6). (D) ELISA analysis for serum levels of TNF-α, IL-1β
and IL-6. (E) Renal histological changes in mice were observed by
H&E staining; scale bar, 40 μm. Black arrows indicate
the loss of epithelial cell nuclei; white arrows point to
abscission of tubular epithelial cells; and black arrowheads
indicate glomerular hypertrophy. (F) The histological tubular
necrosis scores were evaluated. (G) miR-30a-5p expression levels in
the cortex region of mouse kidneys following intraperitoneal
injection with Ad-mimic or Ad-inhibitor were detected by RT-qPCR.
(H) miR-30a-5p expression levels in the renal cortex region of mice
was detected using RT-qPCR at 6 and 24 h post-reperfusion (n=6).
(I) miR-30a-5p expression in H/R-exposed HK-2 cells was assessed by
RT-qPCR (n=3). *P<0.05 vs. sham or control;
#P<0.05 vs. model. Ad, adenovirus; H/R,
hypoxia/reoxygenation; Kim-1, kidney injury molecule 1; miR,
microRNA; RT-qPCR, reverse transcription-quantitative PCR; BUN,
blood urea nitrogen; SCr, serum creatinine. |
For the in vivo study of the function of
miR-30a-5p, model mice were intraperitoneally injected with
Ad-mimic or Ad-inhibitor 24 h before renal I/R injury. miR-30a-5p
expression levels in the cortex region of were detected by RT-qPCR,
which demonstrated that miR-30a-5p was significantly upregulated by
Ad-mimic injection and was significantly downregulated by
Ad-inhibitor injection compared with control group (Fig. 1G). The result showed that
miR-30a-5p overexpression partially relieved renal I/R injury in
vivo, whereas miR-30a-5p knockdown exhibited less effect on
serum levels of SCr, BUN, TNF-α, IL-1β and IL-6, urine
concentrations of Kim-1 and tubular necrosis score in the renal I/R
injury mouse models compared with the untreated model group
(Fig. 1A-F). In the cortex
region of mice with renal I/R injury, miR-30a-5p expression
decreased compared with the sham group (Fig. 1H). Consistent with the results of
the in vivo experiments, miR-30a-5p expression was also
significantly decreased in the H/R-exposed HK-2 cells as compared
with the control cells (Fig.
1I).
miR-30a-5p suppresses autophagy and
apoptosis in renal I/R injury
Compared with those of the sham group, the cortex
region of mice with renal I/R injury exhibited an upregulation in
the protein expression levels of autophagy-related proteins LC3-II
and Beclin-1, and downregulated expression of p62 (Fig. 2A). Ad-mimic infection reduced the
expression of LC3-II and Beclin-1 but increased the expression of
p62 compared with the model group (Fig. 2A). Ad-inhibitor infection
increased the expression of LC3-II and decreased the expression of
p62 compared with the model group (Fig. 2A); however, no significant
difference was detected for the expression levels of Beclin-1. In
the HK-2 cells, miR-30a-5p mimic transfection significantly
increased and miR-30a-5p inhibitor transfection significantly
decreased the expression levels of miR-30a-5p (Fig. S1). Moreover, HK-2 cells in the
H/R condition showed significantly increased LC3-II and Beclin-1
levels, and decreased p62 level, compared with the control group
(Fig. 2B). To confirm the
effects of miR-30a-5p on the pathogenesis of renal I/R injury, HK-2
cells were transfected with miR-30a-5p mimic or miR-30a-5p
inhibitor and were then exposed to H/R. Compared with H/R-injured
cells, miR-30a-5p overexpression decreased LC3-II and Beclin-1
protein expression levels but increased p62 expression in HK-2
cells (Fig. 2B). miR-30a-5p
knockdown increased LC3-II and decreased p62 expression but had no
significant effect on Beclin-1 expression compared with the H/R
group. LC3 punctae analysis was performed using immunofluorescence
staining; the data showed that the number of LC3 punctae was
significantly elevated in H/R-exposed HK-2 cells compared with the
control cells (Fig. 2C).
Transfection of miR-30a-5p mimic significantly decreased the number
of punctae compared with the H/R group, whereas miR-30a-5p
inhibitor exhibited no significant effect of number of LC3 punctae
under H/R condition (Fig. 2C).
Following H/R exposure, apoptotic rates significantly increased
compared with the control group (Fig. 2D); miR-30a-5p mimic transfection
significantly decreased cell apoptosis compared with the H/R group,
whereas the miR-30a-5p inhibitor had little effect on cell
apoptosis. These results suggested that miR-30a-5p may suppress
autophagy and apoptosis under H/R injury.
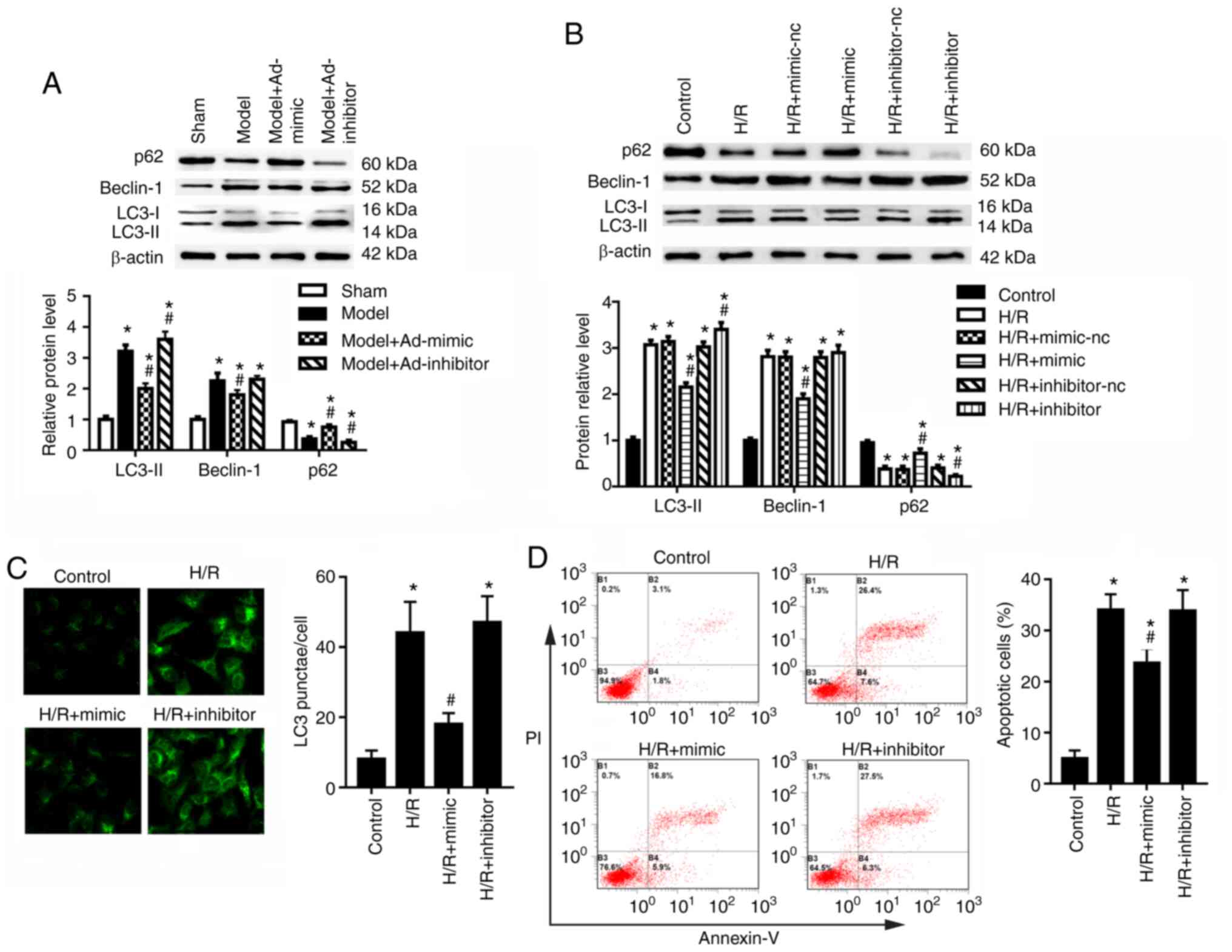 | Figure 2miR-30a-5p regulates autophagy in
HK-2 cells under H/R injury. (A) I/R model mice were
intraperitoneally injected with Ad-mimic or Ad-inhibitor 24 h
before renal I/R injury. Western blot analysis was used to
determine the protein expression levels of LC3-I-II, Beclin-1 and
p62 in the renal cortex (n=6). *P<0.05 vs. sham;
#P<0.05 vs. model. (B) HK-2 cells were transfected
with miR-30a-5p mimic or miR-30a-5p inhibitor, and the protein
expression levels of LC3, Beclin-1 and p62 were measured in HK-2
cells (n=3). *P<0.05 vs. control;
#P<0.05 vs. H/R. (C) Immunofluorescence staining was
performed to detect LC3 punctae in HK-2 cells under H/R injury with
the use of miR-30a-5p mimic or miR-30a-5p inhibitor (n=3;
magnification, x40). *P<0.05 vs. control;
#P<0.05 vs. H/R. (D) Apoptosis was analyzed by flow
cytometry following staining for Annexin V-FITC/PI (n=3).
*P<0.05 vs. control, #P<0.05 vs. H/R.
Ad, adenovirus; H/R, hypoxia/reoxygenation; I/R,
ischemia/reperfusion; LC3, microtubule-associated protein light
chain 3; miR, microRNA; nc, negative control. |
miR-30a-5p directly interacts with
Beclin-1
Beclin-1 was reported to be closely related to cell
autophagy (26). Bioinformatics
analysis demonstrated Beclin-1 to be a potential target of
miR-30a-5p (Fig. 3A). The
dual-luciferase reporter assay demonstrated that, compared with the
miR-30a-5p mimic-nc, the miR-30a-5p mimic resulted in an ~60%
reduction in the luciferase activity of 293T cells transfected with
the pGLO-Beclin-1-WT plasmid (Fig.
3B); however, the miR-30a-5p mimic did not affect the
luciferase activity of 293T cells that were transfected with the
pGLO-Beclin-1-Mut plasmid. Western blot analysis showed that the
miR-30a-5p mimic transfection significantly reduced the protein
expression level of Beclin-1, whereas miR-30a-5p inhibitor
transfection significantly upregulated Beclin-1 protein levels
compared with the control group (Fig. 3C). However, neither miR-30a-5p
mimic nor inhibitor transfection affected the mRNA expression
levels of Beclin-1 significantly compared with the control group
(Fig. 3D). To confirm the direct
interaction between miR-30a-5p and Beclin-1, RIP assays were
conducted. As shown in Fig. 3E,
expression levels of miR-30a-5p and 3′UTR of Beclin-1 mRNA in the
Ago2-RIP products of miR-30a-5p mimic transfected HK-2 cells were
both significantly increased compared to the mimic-nc group.
Beclin-1 serves a crucial role in HK-2
cell autophagy
To investigate the role of Beclin-1 in autophagy of
HK-2 cells under H/R condition, cells were transfected with
miR-30a-5p mimic, Beclin-1 siRNA, pcDNA3.1-Beclin-1 or a
combination of the miR-30a-5p mimic with pcDNA3.1-Beclin-1 for 48 h
and then exposed to H/R. Beclin-1 siRNA transfection significantly
decreased and pcDNA3.1-Beclin-1 transfection significantly
increased the mRNA and protein expression levels of Beclin-1
compared with the control group (Fig. S2A and B). In HK-2 cells under
H/R condition, Beclin-1 siRNA significantly decreased and
pcDNA3.1-Beclin-1 significantly increased Beclin-1 expression
compared with the H/R group (Fig.
4A). Compared with cells in the H/R group, Beclin-1 siRNA
significantly inhibited expression of LC3-II and increased p62
expression. Conversely, pcDNA3.1-Beclin-1 transfection led to
increased LC3-II and decreased p62 expression (Fig. 4A). miR-30a-5p mimic transfection
partly reversed the effects of pcDNA3.1-Beclin-1 on the expression
of LC3-II, Beclin-1 and p62. LC3 puncta analysis using
immunofluorescence staining showed that the number of LC3 punctae
was significantly decreased by Beclin-1 knockdown and increased by
Beclin-1 overexpression compared with the H/R group (Fig. 4B). Co-transfection of miR-30a-5p
mimic combined with Beclin-1 overexpression decreased the number of
LC3 puncta compared with the Beclin-1 overexpression group
(Fig. 4B). Beclin-1 siRNA
transfection also significantly decreased cell apoptosis, whereas
Beclin-1 overexpression significantly increased cell apoptosis,
compared with cells in the H/R group (Fig. 4C). Co-transfection of miR-30a-5p
mimic and pcDNA3.1-Beclin-1 significantly reduced cell apoptosis
compared with the Beclin-1 overexpression group. These data
indicate that Beclin-1 may serve a crucial role in autophagy of
HK-2 cells.
Beclin-1 contributes to HK-2 cell
autophagy by modulating ATG16 expression
A previous study demonstrated that ATG16 serves a
crucial role in ischemic kidney injury (27). We hypothesized that miR-30a-5p
may regulate HK-2 cell autophagy and proliferation by suppressing
Beclin-1 expression and ATG16 expression. As expected, in the
ischemic kidney injury mouse models and in the H/R-exposed HK-2
cells, ATG16 expression was significantly upregulated (Fig. 5A and B). In the H/R-exposed HK-2
cells, it was revealed that Beclin-1 siRNA decreased and
pcDNA3.1-Beclin-1 increased the expression levels of ATG16,
compared with the H/R group (Fig.
5C). In addition, miR-30a-5p mimic transfection significantly
down- regulated, whereas miR-30a-5p inhibitor increased ATG16
expression in H/R-injured HK-2 cells.
The effects of ATG16 on autophagy and apoptosis of
HK-2 cells were examined following transfection with ATG16 siRNA,
which was used to downregulate ATG16 expression (Fig. S3). In HK-2 cells under H/R
condition, ATG16 knockdown significantly decreased ATG16 and LC3-II
protein expression levels, and increased p62 expression in HK-2
cells compared with the H/R group (Fig. 6A). miR-30a-5p mimic transfection
enhanced, whereas miR-30a-5p inhibitor transfection partly
reversed, the effect of ATG16 knockdown on the expression of LC3-II
(Fig. 6A). Compared with cells
in the H/R group, ATG16 knockdown significantly decreased the
number of LC3 punctae and decreased cell apoptosis (Fig. 6B and C). miR-30a-5p mimic
enhanced, but miR-30a-5p inhibitor reversed, the effect of ATG16
knockdown on the number of LC3 punctae and apoptosis compared with
the H/R + ATG16 siRNA group (Fig. 6B
and C). Taken together, these results indicate that miR-30a-5p
may mediate HK-2 cell autophagy in renal I/R injury by directly
targeting Beclin-1 to modulate ATG16 expression.
Discussion
AKI is one of the most common complications in
hospitalized patients. It is defined as a sudden decline in renal
function, including cell death and inflammation in the proximal
tubule (1). The underlying
mechanism of AKI has not been fully elucidated (28). Kidney I/R injury is a major cause
of AKI (1,29). Data from the present study
demonstrated a significant decrease of miR-30a-5p expression, along
with upregulated expression of autophagy-related proteins LC3-II
and Beclin-1 and decreased p62 expression, in mice with renal I/R
injury and in HK-2 cells exposed to hypoxic conditions in
vitro. It was also revealed that miR-30a-5p directly interacted
with the 3′-UTR of Beclin-1, and that Beclin-1 effectively promoted
cell autophagy by enhancing ATG16 levels. These data suggested that
Beclin-1/ATG16 signaling may be a novel and promising downstream
target of miR-30a-5p for therapeutic intervention in ischemic
kidney injury. Autophagy may serve as a cellular homeostatic
mechanism under stress conditions, including H/R injury (27). Thus, in the current study,
autophagy was examined by detecting the expression of LC3-II,
Beclin-1 and p62, as well as by the LC3 punctae analysis. The
I/R-injured mice showed increased LC3-II and Beclin-1, and
decreased p62 protein expression levels. Similar results were
observed in H/R-exposed HK-2 cells in vitro.
miRNAs are important in the maintenance of normal
cellular functions, and dysregulation of miRNAs can result in a
number of pathological states, including cellular differentiation,
proliferation and apoptosis (30). Several studies have reported that
miRNAs regulate tubular epithelial cell growth, apoptosis and renal
injury (31,32). Studies have also demonstrated
that miR-30a-5p may regulate mitochondrial dynamics and autophagy
(33), and it may also promote
replication of porcine circovirus type 2 by enhancing autophagy
(34). The present study data
demonstrated that administration of miR-30a-5p mimic markedly
decreased HK-2 cell apoptosis, downregulated expression of LC3-II
and Beclin-1, and enhanced p62 expression in H/R-exposed HK2 cells.
Conversely, transfection with a miR-30a-5p inhibitor enhanced
autophagy of H/R exposed HK-2 cells; the miR-30a-5p inhibitor had
little effect on cell apoptosis. Several studies have associated
dysregulated miRNAs not only to the pathogenesis of kidney injury
but also to renal fibrosis (35–37). Li et al (38) reported that miR-30 expression was
significantly inhibited by TGF-β1 in the kidney fibroblast, and
miR-30 was downregulated in renal fibrosis. Adenovirus-mediated
upregulation of miR-30 in the kidney fibroblast significantly
reduced unilateral ureteral obstruction-induced renal fibrosis by
targeting SRY-box transcription factor 9 in mouse models (38). Unfortunately, the role of
miR-30a-5p in renal fibrosis was not examined in the present study;
we hypothesized that miR-30a-5p may affect renal fibrosis in the
I/R model.
miRNAs conduct their biological function by binding
to their target molecules (39).
The mechanism of miR-30a-5p in ischemic kidney injury is unclear,
and bioinformatic tools [microRNA.org
(25), EIMMo miRNA target
prediction (24) and TargetScan]
were used in the present study to search for potential targets of
miR-30a-5p. The results from many tools identified Beclin-1 to be
an important element of autophagy that may be associated with the
development of ischemic kidney injury (40). In a previous study, Beclin-1,
which was identified as a target gene of miR-30a-5p, was shown to
serve a crucial role in the drug-resistance of small cell lung
cancer (16). In the present
study, miR-30a-5p was shown to regulate autophagy of I/R injury
renal by targeting Beclin-1.
A previous study revealed that Beclin-1 and the
ATG5-ATG12-ATG16 complex serve important roles in autophagy of
thyroid papillary carcinoma cells (41) and are involved in initial
autophagosome formation (42).
To clarify the mechanism underlying the positive effects of
Beclin-1 on cell apoptosis and autophagy, the expression of ATG16
was examined in the present study. In accordance with our
hypothesis, ATG16 expression was significantly upregulated in the
renal I/R injury mouse models and in the H/R-exposed HK-2 cells.
Beclin-1 positively regulated and miR-30a-5p negatively modulated
ATG16 expression. Further analysis of the mechanism revealed that
ATG16 siRNA significantly decreased HK-2 cell apoptosis,
downregulated LC3-II expression and the number of LC3 punctae, and
promoted p62 expression. These results demonstrated that Beclin-1
may regulate autophagy in H/R injured HK2 cells by regulating ATG16
expression.
In conclusion, the present data indicated that
miR-30a-5p may moderate renal I/R injury by inhibiting autophagy
and apoptosis. Furthermore, mechanistic analysis demonstrated that
miR-30a-5p directly interacted with Beclin-1, and ATG16 was
involved in this process. Collectively, our data suggested that
miR-30a-5p may regulate autophagy after renal I/R injury via the
Beclin-1/ATG16 pathway. These results may provide a novel
therapeutic approach to combat ischemic kidney injury.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF conceived and designed the study, performed the
cell experiments and was a major contributor to the writing of the
manuscript. LZ performed the animal experiments and helped write
the manuscript. WH designed the study, performed the cell
experiments and reviewed the manuscript. YF, LZ and WH confirmed
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the General Hospital of
Central Theater Command (Wuhan, China) approved this study, which
followed the regulatory standards of the National Institutes of
Health guide for the care and use of laboratory animals.
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of
interest.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Ronco C, Bellomo R and Kellum JA: Acute
kidney injury. Lancet. 394:1949–1964. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu X, Li W and Li H: miR-214 ameliorates
acute kidney injury via targeting DKK3 and activating of
Wnt/β-catenin signaling pathway. Biol Res. 51:312018. View Article : Google Scholar
|
|
3
|
Basile DP, Anderson MD and Sutton TA:
Pathophysiology of acute kidney injury. Compr Physiol. 2:1303–1353.
2012.PubMed/NCBI
|
|
4
|
Chun N, Coca SG and He JC: A protective
role for microRNA-688 in acute kidney injury. J Clin Invest.
128:5216–5218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Xu Y, Xue S, Wang X, Dai H, Qian
J, Ni Z and Yan Y: Implications of dynamic changes in miR-192
expression in ischemic acute kidney injury. Int Urol Nephrol.
49:541–550. 2017. View Article : Google Scholar :
|
|
6
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim KH and Lee MS: Autophagy-a key player
in cellular and body metabolism. Nat Rev Endocrinol. 10:322–337.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaushal GP and Shah SV: Autophagy in acute
kidney injury. Kidney Int. 89:779–791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian R, Wang P, Huang L, Li C, Lu Z, Lu Z,
Wu A, Bao K, Mao W, Huang Q and Xu P: Sanqi oral solution
ameliorates renal ischemia/reperfusion injury via reducing
apoptosis and enhancing autophagy: Involvement of ERK/mTOR
pathways. Front Pharmacol. 11:5371472020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui J, Bai X and Chen X: Autophagy and
acute kidney injury. Adv Exp Med Biol. 1207:469–480. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ham O, Lee SY, Lee CY, Park JH, Lee J, Seo
HH, Cha MJ, Choi E, Kim S and Hwang KC: Let-7b suppresses apoptosis
and autophagy of human mesenchymal stem cells transplanted into
ischemia/reperfusion injured heart 7by targeting caspase-3. Stem
Cell Res Ther. 6:1472015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilflingseder J, Jelencsics K, Bergmeister
H, Sunzenauer J, Regele H, Eskandary F, Reindl-Schwaighofer R,
Kainz A and Oberbauer R: miR-182-5p inhibition ameliorates ischemic
acute kidney injury. Am J Pathol. 187:70–79. 2017. View Article : Google Scholar
|
|
13
|
Ren GL, Zhu J, Li J and Meng XM: Noncoding
RNAs in acute kidney injury. J Cell Physiol. 234:2266–2276. 2019.
View Article : Google Scholar
|
|
14
|
Wang C, Cai L, Liu J, Wang G, Li H, Wang
X, Xu W, Ren M, Feng L, Liu P and Zhang C: MicroRNA-30a-5p inhibits
the growth of renal cell carcinoma by modulating GRP78 expression.
Cell Physiol Biochem. 43:2405–2419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Zhang J, Zhang Z, Feng Z, Wei J,
Lu J, Fang Y, Liang Y, Cen J, Pan Y, et al: The putative tumor
suppressor microRNA-30a-5p modulates clear cell renal cell
carcinoma aggressiveness through repression of ZEB2. Cell Death
Dis. 8:e28592017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang X, Bai F, Xu Y, Chen Y and Chen L:
Intensified Beclin-1 mediated by low expression of Mir-30a-5p
promotes chemoresistance in human small cell lung cancer. Cell
Physiol Biochem. 43:1126–1139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Li Y, Chen X, Cheng X, Liao Y and
Yu X: Exosomal transfer of miR-30a between cardiomyocytes regulates
autophagy after hypoxia. J Mol Med (Berl). 94:711–724. 2016.
View Article : Google Scholar
|
|
18
|
Guo D, Ma J, Yan L, Li T, Li Z, Han X and
Shui S: Down-regulation of lncrna MALAT1 attenuates neuronal cell
death through suppressing Beclin1-dependent autophagy by regulating
Mir-30a in cerebral ischemic stroke. Cell Physiol Biochem.
43:182–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the care and use of
laboratory animals. Washington DC: National Academies Press (US):
1996
|
|
20
|
Sun Y, Zhang Y, Zhao D, Ding G, Huang S,
Zhang A and Jia Z: Rotenone remarkably attenuates oxidative stress,
inflammation, and fibrosis in chronic obstructive uropathy.
Mediators Inflamm. 2014:6701062014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Sun J, Chen X, Liu T, Jiang X, Wu Y, Yang
S, Hua W, Li Z, Huang H, Ruan X and Du X: Berberine protects
against palmitate-induced apoptosis in tubular epithelial cells by
promoting fatty acid oxidation. Med Sci Monit. 24:1484–1492. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu F, Ai FY, Zhang DC, Tian L, Yang ZY
and Liu SJ: LncRNA NEAT1 knockdown attenuates autophagy to elevate
5-FU sensitivity in colorectal cancer via targeting miR-34a. Cancer
Med. 9:1079–1091. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hausser J, Berninger P, Rodak C, Jantscher
Y, Wirth S and Zavolan M: MirZ: An integrated microRNA expression
atlas and target prediction resource. Nucleic Acids Res.
37:W266–W272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The http://microRNA.orgurisimplemicroRNA.org resource:
Targets and expression. Nucleic Acids Res. 36:D149–D153. 2008.
View Article : Google Scholar
|
|
26
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang IK, Sun KT, Tsai TH, Chen CW, Chang
SS, Yu TM, Yen TH, Lin FY, Huang CC and Li CY: MiR-20a-5p mediates
hypoxia-induced autophagy by targeting ATG16L1 in ischemic kidney
injury. Life Sci. 136:133–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang L, Liu XQ, Ma Q, Yang Q, Gao L, Li
HD, Wang JN, Wei B, Wen J, Li J, et al: Hsa-miR-500a-3P alleviates
kidney injury by targeting MLKL-mediated necroptosis in renal
epithelial cells. FASEB J. 33:3523–3535. 2019. View Article : Google Scholar
|
|
29
|
Shen WC, Chou YH, Huang HP, Sheen JF, Hung
SC and Chen HF: Induced pluripotent stem cell-derived endothelial
progenitor cells attenuate ischemic acute kidney injury and cardiac
dysfunction. Stem Cell Res Ther. 9:3442018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu N, Jiang N, Guo R, Jiang W, He QM, Xu
YF, Li YQ, Tang LL, Mao YP, Sun Y and Ma J: MiR-451 inhibits cell
growth and invasion by targeting MIF and is associated with
survival in nasopharyngeal carcinoma. Mol Cancer. 12:1232013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lorenzen JM, Haller H and Thum T:
MicroRNAs as mediators and therapeutic targets in chronic kidney
disease. Nat Rev Nephrol. 7:286–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chau BN, Xin C, Hartner J, Ren S, Castano
AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, et al: MicroRNA-21
promotes fibrosis of the kidney by silencing metabolic pathways.
Sci Transl Med. 4:121ra1182012. View Article : Google Scholar
|
|
33
|
Schwienbacher C, Foco L, Picard A, Corradi
E, Serafin A, Panzer J, Zanigni S, Blankenburg H, Facheris MF,
Giannini G, et al: Plasma and white blood cells show different
miRNA expression profiles in parkinson's disease. J Mol Neurosci.
62:244–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Xu X, Wang W, Yu Z, Wen L, He K
and Fan H: MicroRNA-30a-5p promotes replication of porcine
circovirus type 2 through enhancing autophagy by targeting 14-3-3.
Arch Virol. 162:2643–2654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun T, Liu Y, Liu L and Ma F: MicroRNA-544
attenuates diabetic renal injury via suppressing glomerulosclerosis
and inflammation by targeting FASN. Gene. 723:1439862020.
View Article : Google Scholar
|
|
36
|
Wu J, Huang Q, Li P, Wang Y, Zheng C, Lei
X, Li S, Gong W, Yin B, Luo C, et al: MicroRNA-145 promotes the
epithelial-mesenchymal transition in peritoneal dialysis-associated
fibrosis by suppressing fibroblast growth factor 10. J Biol Chem.
294:15052–15067. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fan Y, Chen H, Huang Z, Zheng H and Zhou
J: Emerging role of miRNAs in renal fibrosis. RNA Biol. 17:1–12.
2020. View Article : Google Scholar :
|
|
38
|
Li H, Cai H, Deng J, Tu X, Sun Y, Huang Z,
Ding Z, Dong L, Chen J, Zang Y and Zhang J: TGF-β-mediated
upregulation of Sox9 in fibroblast promotes renal fibrosis. Biochim
Biophys Acta Mol Basis Dis. 1864:520–532. 2018. View Article : Google Scholar
|
|
39
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie Y, Jiang D, Xiao J, Fu C, Zhang Z, Ye
Z and Zhang X: Ischemic preconditioning attenuates
ischemia/reperfusion-induced kidney injury by activating autophagy
via the SGK1 signaling pathway. Cell Death Dis. 9:3382018.
View Article : Google Scholar
|
|
41
|
Liu Z, Zeng W, Wang S, Zhao X, Guo Y, Yu
P, Yin X, Liu C and Huang T: A potential role for the Hippo pathway
protein, YAP, in controlling proliferation, cell cycle progression,
and autophagy in BCPAP and KI thyroid papillary carcinoma cells. Am
J Transl Res. 9:3212–3223. 2017.PubMed/NCBI
|
|
42
|
Bejarano E, Yuste A, Patel B, Stout RF Jr,
Spray DC and Cuervo AM: Connexins modulate autophagosome
biogenesis. Nat Cell Biol. 16:401–414. 2014. View Article : Google Scholar : PubMed/NCBI
|




















