Introduction
Breast cancer is the second most common cancer in
the world, affecting 1.9 million individuals and causing 601,000
deaths annually (1). Patients
with breast cancer show rapid tumor progression and metastasis
(2), and treatment strategies
for such patients include surgical resection, radiotherapy,
targeted therapies, and chemotherapy (3). However, these treatment methods
affect not only the cancer cells, but also normal cells, and can
cause adverse outcomes such as resistance, hypertension, venous and
arterial thromboembolism, and metabolic disorders, resulting in
poor prognosis (4). Anticancer
treatments using natural products can prevent or minimize the risk
of side effects, compared to chemotherapy, and such treatment can
exert numerous anticancer activities. Therefore, there is a need
for research on anticancer therapy based on natural products
(5).
Flavonoids are the most common group of plant
polyphenols, and can be largely divided into flavones, flavonols,
flavanones, flavanonols, flavanols or catechins, anthocyanins and
chalcones (6). Among the
flavonols, myricetin is included. Myricetin
(3,3′,4′,5,5′,7-hexahydroxyflavone,
C15H10O8) is abundantly found in
bayberry, nuts, red wine, and green tea (7-9).
Previous studies have demonstrated the anti-oxidant, antiviral,
antibacterial, and anticancer effects of myricetin (10-12). Myricetin has been found to
exhibit anticancer effects against pancreatic (13), liver (14), prostate (15), thyroid (16) and breast cancer (17-19) in vitro. In particular,
myricetin targets the mitochondrial apoptosis pathway to inhibit
cellular proliferation and induce apoptosis (20). However, the anticancer effects of
myricetin in regards to breast cancer SK-BR-3 cells have not been
evaluated. Additionally, there is a lack of studies on the
mechanism of apoptosis caused by myricetin and its relation to
autophagy.
Apoptosis is one of many key anticancer mechanisms
involving programmed cell death, and is characterized by cell
contraction, nuclear condensation, and blebbing of the cell
membrane. Apoptosis is an essential process, and abnormal apoptosis
may cause mutated cells to progress into tumor cells (21). Apoptosis involves two pathways:
extrinsic and intrinsic. The mitochondria play a key role in the
intrinsic pathway, and it is also affected by Bax (a pro-apoptotic
protein) and Bcl-2 (an anti-apoptotic protein). DNA damage and
cellular stress lead to a relative increase in the expression of
the Bax protein, and this increases the permeability of the outer
mitochondrial membrane. During this process, cytochrome c is
released from the intermembrane space of the mitochondria.
Cytochrome c creates the caspase complex, inhibits Bcl-2,
and causes a caspase chain reaction (22) that fragments the PARP protein.
The fragmented PARP protein binds to the ends of DNA and becomes
activated, following which NAD and ATP are depleted in the cells,
causing apoptosis (23,24).
The mitogen-activated protein kinase (MAPK) pathway
is involved in apoptosis via its regulation of pro-apoptotic and
anti-apoptotic proteins through various mechanisms. The main
proteins involved in the MAPK pathway include
extracellular-regulated kinase (ERK), c-Jun N-terminal kinase
(JNK), and p38 mitogen-activated protein kinases (p38). ERKs are
activated via the stimulation of growth factors that cause cell
differentiation and proliferation. ERKs inhibit apoptosis by
promoting anti-apoptotic proteins and suppressing pro-apoptotic
proteins (25,26). In addition, JNK and p38 are
activated by stress, and are involved in maintaining the balance
between cell survival and death. These proteins promote
pro-apoptotic proteins and suppress anti-apoptotic proteins to
stimulate apoptosis (27,28).
Thus, these proteins regulate the MAPK pathway and are fundamental
for the induction of apoptosis.
Autophagy is commonly known to inhibit apoptosis and
inhibit the activity of caspases; however, excessive degradation of
the cellular cytoplasm through autophagy may lead to apoptosis and
cellular death (29). When the
Bcl-2/beclin 1 complex is dissociated, beclin1 recruits autophagic
proteins to initiate autophagy (30,31). These autophagic proteins form a
double membrane to become autophagosomes that combine with
lysosomes and degrade old organelles and proteins. In this process,
phycoerythrin (PE) combines with 1A/1B-light chain 3 (LC 3)-I to
form LC 3-II, which can bind to phagophores. Thus, LC 3 and beclin1
are used as marker proteins that indicate the activation of
autophagy (32).
Therefore, in the present study, inhibition of the
cell proliferation of SK-BR-3 cells, in which the anticancer
efficacy of myricetin is unclear, was confirmed, and whether
inhibition of viability is induced through apoptosis was further
investigated. In addition, the pathway through which apoptosis
occurs was identified, and the association between apoptosis and
autophagy in SK-BR-3 cells treated with myricetin was examined,
which has not been studied previously.
Materials and methods
Reagents and antibodies
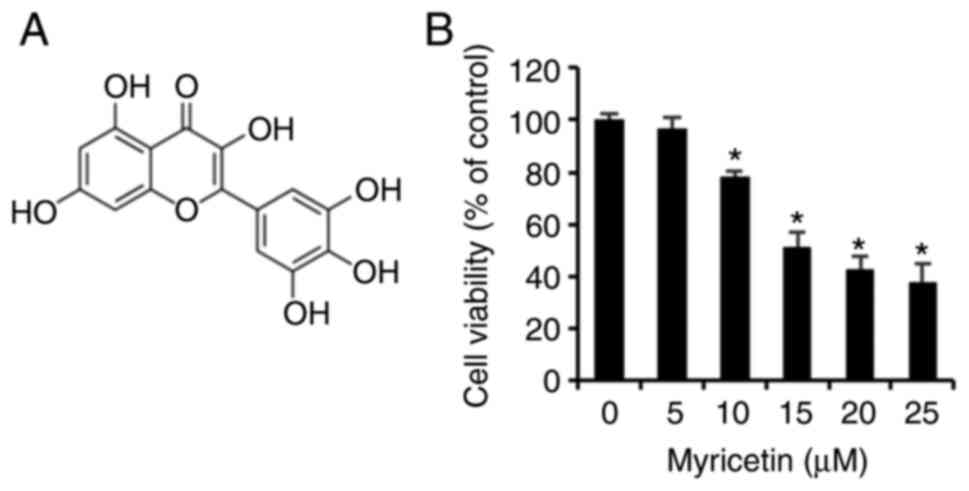
Myricetin (Fig.
1A), purity ≥96.0% used in this experiment, was purchased from
Sigma-Aldrich/Merck KGaA. RPMI-1640 medium for cell culturing was
purchased from Welgene, and fetal bovine serum (FBS) and
penicillin/streptomycin were purchased from Gibco BRL/Thermo Fisher
Scientific, Inc.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
4′,6-diamidino-2-phenylindole (DAPI), and acridine orange were
purchased from Sigma-Aldrich/Merck KGaA, and the fluorescein
isothiocyanate (FITC) Annexin V apoptosis detection kit was
purchased from BD Pharmingen. Cell lysis buffer was purchased from
Invitrogen/Thermo Fisher Scientific, Inc. Polyadenosine
diphosphate-ribose polymerase (PARP, rabbit, 1:1,000, #9542), Bcl-2
associated X (Bax, rabbit, 1:1,000, #2772), B cell lymphoma 2
(Bcl-2, rabbit, 1:1,000, #4223), microtubule-associated protein
1A/1B-light chain 3 (LC 3, rabbit, 1:1,000, #4108), beclin 1
(rabbit, 1:1,000, #3738), total c-Jun N-terminal kinase (t-JNK,
rabbit, 1:1,000, #9252), phosphorylated c-Jun N-terminal kinase
(p-JNK, rabbit, 1:1,000, #4668), total extracellular-regulated
kinase (t-ERK, rabbit, 1:1,000, #9102), phosphorylated
extracellular-regulated kinase (p-ERK, rabbit, 1:1,000, #9101),
total mitogen-activated protein kinases (t-p38, rabbit, 1:1,000,
#9212), phosphorylated mitogen-activated protein kinases (p-p38;
rabbit, 1:1,000, #9211), and rabbit IgG (rabbit, 1:1,000, #7074)
antibodies were purchased from Cell Signaling Technology (CST), and
β-actin (mouse, 1:1,000, sc-47778) and mouse IgG (mouse, 1:1,000,
sc-516102) antibodies were purchased from Santa Cruz Biotechnology
Inc. JNK inhibitor (SP600125) was purchased from
Sigma-Aldrich/Merck KGaA and 3-MA was purchased form
MedChemExpress.
Cell culture
Her2-positive SK-BR-3 breast cancer cells were
purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). The
cells were cultured in a 75 cm2 flask using RPMI-1640
medium containing 5% FBS and 1% penicillin/streptomycin at 37.5% in
a CO2 incubator. The cells were passaged when they were
70% confluent in the 75 cm2 flask.
MTT assay
The SK-BR-3 cells were plated in a 96-well plate at
a density of 4×104 cells/ml and placed in an incubator.
After 24 h, the cells were treated for 24 h with the following
concentrations of myricetin: 0, 5, 10, 15, 20, and 25 µM.
Following this, the cells were incubated with 40 µl of MTT
solution at 1 mg/ml for 90 min. The MTT solution was removed, and
the cells were treated with 100 µl of dimethyl sulfoxide
(DMSO). Absorbance (optical density) was measured at 595 nm using
an ELISA reader (Bio-Rad Laboratories) and Microplate
Manager® 6 software (Bio-Rad Laboratories) to calculate
the viability of the cells.
DAPI staining
The SK-BR-3 cells were plated in a 60-mm dish at a
density of 2×105 cells/ml and placed in an incubator for
24 h. The cells were treated with 0, 10, and 20 µM of
myricetin for 24 h. The medium was removed, and the cells were
washed three times with PBS. The cells were fixed in 4%
paraformaldehyde for 15 min and washed three times with PBS. DAPI
was added to the cells and the reaction was allowed to occur for 1
min under dark conditions. The nuclear morphology of the reacted
cells was observed with a fluorescence microscope (Zeiss AG,
magnification, ×200). Apoptotic cells were counted as DAPI-positive
cells/total cells in three random fields.
Acridine orange staining
The SK-BR-3 cells were plated in a 60-mm dish at a
density of 2×105 cells/ml and placed in an incubator for
24 h. The cells were treated with 0, 10, and 20 µM of
myricetin for 24 h. The medium was removed, and the cells were
washed with PBS. Subsequently, the cells were fixed in 4%
paraformaldehyde for 15 min and washed twice with PBS. The cells
were stained with acridine orange and observed under a fluorescence
microscope (Zeiss AG, magnification, ×200).
Annexin V/PI staining
The Annexin V apoptosis detection kit was used for
quantitative analysis of apoptosis. The SK-BR-3 cells were cultured
in a 75 cm2 flask and treated with 0, 10, and 20
µM of myricetin for 24 h. The cells were trypsinized with
trypsin-EDTA and centrifuged (300 × g, 5 min) into a pellet,
following which 1X binding buffer was used to re-suspend the cells
to a concentration of 1×106 cells/ml. The cells were
reacted with FICT-conjugated Annexin V and PE-conjugated propidium
iodide (PI) for 15 min, and analyzed using a FACSCalibur™ flow
cytometer (BD Biosciences).
Western blot analysis
The SK-BR-3 cells were cultured in a 75
cm2 flask and treated with 0, 10, and 20 µM of
myricetin for 24 h. The cells were trypsinized and centrifuged (300
× g, 5 min) into a pellet, and a cell lysis buffer
(Invitrogen/Thermo Fisher Scientific, Inc.) was used to the
separate proteins. The cells were centrifuged at 18,000 × g for 5
min, and the supernatant was collected. The proteins were
quantified using the Bradford protein assay (Bio-Rad Laboratories)
and separated by size using a 12% SDS-PAGE gel. The proteins were
transferred to a nitrocellulose membrane (Bio-Rad Laboratories) and
blocked using 5% skim milk. The primary antibody was reacted
overnight at 4°C. The secondary antibody was reacted at room
temperature for 2 h, and the proteins were reacted with ECL
detection reagents (Pierce/Thermo Fisher Scientific, Inc.).
Multiple proteins were tested on one membrane via stripping.
Protein expression was measured using the Image J Launcher
(provided by NCBI).
Statistical analysis
The results are expressed as mean ± standard
deviation. Statistical analyses were performed using a Student's t
test and using a one-way ANOVA followed by Dunnett's or Tukey's
test, when more than two conditions were compared. Values were
considered statistically significant at P<0.05.
Results
Myricetin inhibits the viability of
SK-BR-3 cells
An MTT assay was conducted to determine the effects
of myricetin on the cell viability of 3 human breast cancer
SK-BR-cells. The SK-BR-3 cells treated with 0, 5, 10, 15, 20, and
25 µM of myricetin for 24 h exhibited viabilities of 96.5%
at 5 µM, 78.1% at 10 µM, 51.4% at 15 µM, 42.5%
at 20 µM, and 37.9% at 25 µM. Thus the SK-BR-3 cells
showed a dose-dependent decrease in viability compared to that of
the control group (Fig. 1B).
Myricetin induces apoptosis in SK-BR-3
cells
To investigate whether the decrease in the viability
of SK-BR-3 cells was mediated by apoptosis, the cells were stained
with DAPI to assess morphological changes. The cells were treated
with 0, 10, and 20 µM of myricetin for 24 h and stained with
DAPI. Cells in the myricetin-treated group showed the presence of
apoptotic bodies such as chromatin condensation, and nuclear
fragmentation. The percentage of apoptotic cells was 1.59% in the
control group, 6.65% in the 10 µM myricetin group, and
12.78% in the 20 µM myricetin group, indicating a
dose-dependent increase in the numbers of apoptotic bodies
(Fig. 2A and C). To analyze the
percentage of apoptosis after DAPI staining, the cells were stained
with Annexin V/PI and analyzed using flow cytometry. The ratio
between cells in early and late apoptosis was 9.8% in the control
group, 12.5% in the 10 µM myricetin group, and 38.5% in the
20 µM myricetin group. This indicated that there was
significant dose-dependent increase in the ratio between cells in
early and late apoptosis (Fig. 2B
and D). To measure the expression levels of PARP, Bax, and
Bcl-2 proteins (apoptosis-regulatory proteins), SK-BR-3 cells were
treated with myricetin at concentrations of 0, 10, and 20 µM
for 24 h, and the proteins were used in a western blot analysis. In
the groups treated with 10 and 20 µM myricetin, the
expression levels of cleaved-PARP and Bax (pro-apoptotic protein)
were increased, whereas that of Bcl-2 (anti-apoptotic protein) was
decreased compared to the expression levels in the control group
(Fig. 3A).
 | Figure 3Myricetin-induced apoptosis occurs
through the MAPK pathway in SK-BR-3 cells. (A) Western blot
analysis of expression levels of cleaved PARP, Bax, and Bcl-2 in
SK-BR-3 cells after myricetin (10, 20 µM) treatment. (B)
Western blot analysis of SK-BR-3 cells treated with myricetin (10
and 20 µM for 24 h) to measure the levels of ERK, p-ERK,
JNK, p-JNK, p38, and p-p38 proteins. The control group (0
µM) was treated with the same amount of DMSO, and β-actin
was used as a loading control. Data are displayed as the mean ± SD
(n=3). *P<0.05 vs. control group. PARP, polyadenosine
diphosphate-ribose polymerase; Bax, Bcl-2 associated X; Bcl-2, B
cell lymphoma 2; MAPK, mitogen-activated protein kinase; ERK,
extracellular-regulated kinase; JNK, c-Jun N-terminal kinase; p38,
p38 mitogen-activated protein kinases; DMSO, dimethyl
sulfoxide. |
Myricetin modulates the MAPK pathway to
induce apoptosis
The results of the previous experiments indicated
that myricetin induces apoptosis in breast cancer SK-BR-3 cells. To
investigate the underlying mechanism, the expression levels of the
ERK, JNK, and p38 proteins in the MAPK pathway were assessed
through western blot analysis. Compared to the control group, the
groups treated with 10 and 20 µM myricetin showed a
dose-dependent increase in p-JNK and p-38 levels. In contrast, the
levels of t-JNK and t-p38 levels were decreased. Compared to the
expression levels in the control group, p-ERK expression was
decreased and t-ERK expression was increased in the groups treated
with 10 and 20 µM myricetin (Fig. 3B). Compared to the control group,
the myricetin treatments showed reduced expression of p-ERK/t-ERK.
In particular, treatment with 20 µM myricetin led to a
significant decrease in p-ERK/t-ERK expression. In contrast,
p-JNK/t-JNK expression was significantly higher in the myricetin
(10 and 20 µM) treatment groups than in the control.
p-p38/t-p38 expression was also significantly higher in the 20
µM myricetin treatment group than in the control.
Myricetin induces protective
autophagy
Vacuoles, characteristic features typical of
autophagy, were observed in the myricetin-treated SK-BR-3 cells
(Fig. 4A). To determine whether
myricetin induces autophagy in SK-BR-3 cells, acidic vesicular
organelles (AVOs) were identified through acridine orange staining.
A small number of AVOs were observed in the control group; however,
the number of AVOs increased as the concentration of myricetin
increased in the experimental cells (Fig. 4B).
Western blot analysis was conducted to assess the
p-mTOR, beclin 1 and LC 3 proteins, which are related to autophagy.
Compared to the control group, the groups treated with 10 and 20
µM myricetin showed increased expression of beclin1 and LC
3-II/I (Fig. 5A). Conversely,
p-mTOR/t-mTOR expression was significantly decreased. Additionally,
3-MA (an inhibitor of autophagy) was used to evaluate the
association between autophagy and myricetin-induced apoptosis.
First, the MTT assay was conducted to assess cell viability after
the inhibition of myricetin-induced autophagy. The MTT assay
included the following groups: the control group, 3-MA (1 mM, 1 h)
pre-treatment group, myricetin (MYR) (10 µM, 24 h) treatment
group, and 3-MA (1 mM, 1 h) pre-treatment with myricetin (10
µM, 24 h) treatment group. The cell viability in these
groups was as follows: 98.0% in the 3-MA (1 mM, 1 h) pre-treatment
group, 78.5% in the myricetin (10 µM, 24 h) treatment group,
and 70.4% in the 3-MA (1 mM, 1 h) pretreatment and myricetin (10
µM, 24 h) treatment group. Cell viability was significantly
lower in the 3-MA (1 mM, 1 h) pre-treatment with myricetin (10
µM, 24 h) treatment group than in the myricetin (10
µM, 24 h) treatment group (Fig. 5B). Based on these observations,
proteins were collected from each group, and the Bax, Bcl-2, and LC
3 proteins were evaluated through western blot analysis. Compared
to the control group, the myricetin (10 µM, 24 h) treatment
group showed increased levels of Bax and LC 3-II/I and decreased
levels of Bcl-2. The 3-MA (1 mM, 1 h) pre-treatment with myricetin
(10 µM, 24 h) treatment group showed increased levels of Bax
and reduced levels of Bcl-2 and LC 3-II/I compared to these levels
in the myricetin (10 µM, 24 h) treatment group (Fig. 5C and D).
 | Figure 5Myricetin causes cytoprotective
autophagy in SK-BR-3 cells. (A) Western blot analysis of p-mTOR,
beclin 1 and LC 3-II/I expression in SK-BR-3 cells after treatment
with myricetin (10 and 20 µM). (B) Cell viability (as
measured by an MTT assay) of SK-BR-3 cells pretreated with 1 mM
3-MA (autophagy inhibitor) for 1 h, followed by treatment with
myricetin (10 µM for 24 h). (C) Western blot analysis of
Bax, Bcl-2, and LC 3-II/I expression in SK-BR-3 cells. (D)
Densitometric quantification of the bands in C. The control group
(0 µM) was treated with the same amount of DMSO, and β-actin
was used as a loading control. Data are displayed as the mean ± SD
(n=3). *P<0.05 vs. control group;
#P<0.05 vs. myricetin treatment group. MYR,
myricetin; LC 3, microtubule-associated protein 1A/1B-light chain
3; MTT, 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium
bromide; 3-MA, 3-methyladenine; Bax, Bcl-2 associated X; Bcl-2, B
cell lymphoma 2; DMSO, dimethyl sulfoxide. |
Myricetin regulates autophagy through the
activation of JNK
The JNK inhibitor, SP600125, was used to assess the
effects of myricetin-activated JNK on cell viability. This assay
included the following groups: the control group, SP600125 (5
µM, 1 h) pre-treatment group, myricetin (MYR) (10 µM,
24 h) treatment group, and SP600125 (5 µM, 1 h)
pre-treatment and myricetin (10 µM, 24 h) treatment group.
The cell viabilities were as follows: 99.1% in the SP600125 (5
µM, 1 h) pre-treatment group, 79.9% in the myricetin (10
µM, 24 h) treatment group, and 73.7% in the SP600125 (5
µM, 1 h) pre-treatment and myricetin (10 µM, 24 h)
treatment group. The SP600125 (5 µM, 1 h) pre-treatment and
myricetin (10 µM, 24 h) treatment group showed significantly
reduced cell viability compared to that in the myricetin (10
µM, 24 h) treatment group (Fig. 6A). The expression levels of
p-JNK, Bax, Bcl-2, and LC 3, were assessed through western blot
analysis. The myricetin (10 µM, 24 h) treatment group showed
results similar to that in the previous experiment. In the SP600125
pre-treatment and myricetin (10 µM, 24 h) treatment group,
the levels of p-JNK/t-JNK, Bcl-2, and LC 3-II/I were lower, whereas
that of Bax was higher than those in the myricetin (10 µM,
24 h) treatment group (Fig. 6B and
C).
 | Figure 6Myricetin-induced autophagy is
regulated by JNK in SK-BR-3 cell. (A) Cell viability (as measured
by an MTT assay) of SK-BR-3 cells pretreated with SP600125 (a JNK
inhibitor; 5 µM for 1 h) followed by treatment with
myricetin (MYR) (10 µM for 24 h). (B) Western blot analysis
of p-JNK, Bax, Bcl-2, and LC 3-II/I expression in SK-BR-3 cells.
(C) Densitometric quantification of the bands in B. The control
group (0 µM) was treated with the same amount of DMSO, and
β-actin was used as a loading control. Data are displayed as the
mean ± SD (n=3). *P<0.05 vs. control group;
#P<0.05 vs. myricetin treatment group. MTT,
3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide; JNK,
c-Jun N-terminal kinase; Bax, Bcl-2 associated X; Bcl-2, B cell
lymphoma 2; LC 3, microtubule-associated protein 1A/1B-light chain
3; DMSO, dimethyl sulfoxide. |
Discussion
Although breast cancer can be treated through
various surgical and therapeutic means, these treatments have known
side effects. However, anticancer treatments using natural products
can reduce the risk of side effects, compared to chemotherapy, and
such treatments can exert many anti-cancer activities. Therefore,
anticancer treatments using natural substances have been
increasingly endorsed (5). In
the present study, we assessed whether myricetin induces apoptosis
and autophagy in breast cancer SK-BR-3 cells and investigated the
correlation between myricetin-induced apoptosis and autophagy.
The experiment was performed after establishing a
non-toxic concentration in normal cells (13,33). In the MTT assay, the viability of
SK-BR-3 cells was significantly decreased after treatment with
myricetin in a dose-dependent manner. According to Phillips et
al (13), when pancreatic
cancer cells were treated with myricetin, cell viability decreased
significantly in a concentration-dependent manner, from 12.5
µM myricetin. Similarly, in the present experiment,
significant results were observed following 10 µM myricetin
treatments, and cell viability was decreased in a
concentration-dependent manner. Therefore, myricetin is thought to
decrease the viability of SK-BR-3 cells.
Apoptotic bodies are formed by the condensation of
the nucleus and chromatin, and are a key feature of apoptosis.
Apoptotic bodies can be observed under the microscope through DAPI
staining (34). Therefore, the
SK-BR-3 cells were treated with myricetin, stained with DAPI, and
observed under a fluorescence microscope. The myricetin treatment
groups showed reduced cell viability compared to that in the
control group, and the myricetin-treated cells showed apoptotic
bodies. Compared to the control group, both the myricetin-treated
groups had significantly higher numbers of apoptotic cells.
According to Jo et al (16), when myricetin was used to treat
thyroid cancer cells, apoptotic bodies increased in a
concentration-dependent manner. Similarly, in the SK-BR-3 cells,
the number of apoptotic bodies was increased in the
myricetin-treated group in a concentration-dependent manner. Cells
undergoing apoptosis exhibit distinct changes to their morphology,
including the surface exposure of phosphatidylserine which is
usually present inside the phospholipid bilayer of the cell
membrane. Apoptosis can be divided into early and late stages
depending on the severity of cell membrane damage (35). To assess these changes, the cells
were stained with Annexin V/PI, and cellular apoptosis was
quantitated through flow cytometry. The myricetin treatment groups
had higher numbers of cells in early and late apoptosis than those
in the control group. These results suggest that the
myricetin-induced decrease in cell viability is mediated by
apoptosis.
Furthermore, our results confirmed the
myricetin-induced expression of apoptosis-related proteins.
Apoptosis is mainly regulated by the Bcl-2 family of proteins,
Bcl-2/Bcl-XL (anti-apoptotic) and Bax and Bak (pro-apoptotic), and
the relative ratio of these proteins may inhibit or promote
apoptosis (36). DNA damage and
cellular stress leads to a relative increase in the expression of
the pro-apoptotic Bax protein, which increases the permeability of
the outer mitochondrial membrane. This causes the release of
cytochrome c, which inhibits the anti-apoptotic Bcl-2
protein and induces a caspase chain reaction. Subsequently, the
PARP protein is fragmented, and NAD and ATP are depleted in cells,
resulting in apoptosis (22-24). In this study, myricetin increased
the levels of the pro-apoptotic Bax protein, cleaved PARP, and
reduced the levels of the anti-apoptotic Bcl-2 protein. These
results suggest that myricetin induces apoptosis in SK-BR-3 cells
by regulating the levels of PARP, Bax, and Bcl-2.
The MAPK pathway regulates the pro-apoptotic Bax and
anti-apoptotic Bcl-2 proteins, and is involved in apoptosis. The
downstream molecules of the MAPK pathway include the ERK1/2, JNK,
and p38 proteins. The activation of ERK promotes Bcl-2 and
suppresses Bax, thus inhibiting apoptosis (25,26), and JNK and p38 play important
roles in balancing cell death and survival. Activated JNK and p38
increase Bax expression and decrease Bcl-2 expression to promote
apoptosis (27,28). These regulatory mechanisms of
proteins in the MAPK pathway are fundamental for the induction of
apoptosis. According to Innajak et al (37), when goniothalamin was used to
treat SK-BR-3 cells, apoptosis was induced by decreasing p-ERK,
while increasing the expression of p-JNK and p-p38 proteins.
Similarly, when SK-BR-3 cells were treated with myricetin, the same
results were obtained. This suggests that myricetin treatments
induce apoptosis in SK-BR-3 breast cancer cells through the MAPK
pathway which inhibits ERK and activates JNK and p38.
SK-BR-3 cells treated with myricetin showed
vacuoles, one of the hallmarks of autophagy. Autophagy is commonly
known to inhibit apoptosis and suppress the activity of caspases;
however, excessive degradation of the cellular cytoplasm through
autophagy may lead to apoptosis and cellular death (29). Acidic vesicular organelles (AVOs)
are characteristic of cells in autophagy, and can be identified
through the accumulation of acridine orange in the acidic
compartments (38). Here, the
SK-BR-3 cells were stained with acridine orange to examine whether
myricetin induces autophagy. Although cells in the control group
had a small number of AVOs, the myricetin (10, 20 µM)
treatment groups showed a high number of AVOs.
Several proteins are activated in the process of
autophagy. At initial stages, the Bcl-2/beclin 1 complex is
dissociated, following which beclin 1 recruits autophagic proteins
to initiate autophagy (30,31). The autophagic proteins form a
double membrane to form autophagosomes that then combine with
lysosomes. The resulting autolysosome degrades old cellular
organelles and proteins (32).
mTOR is an important protein that inhibits autophagy. Therefore, a
decrease in p-mTOR affects the induction of autophagy (39). In this process, LC 3 combines
with phagophores. Through our western blot analysis, we observed
the expression of p-mTOR, beclin 1 and LC 3, which confirmed the
presence of autophagy-related proteins in myricetin-treated SK-BR-3
cells. The myricetin (10, 20 µM) treatment groups showed
significantly higher levels of the beclin 1 protein than the
control group. Similarly, the levels of LC 3-II/I were
significantly increased in the myricetin (20 µM) treatment
group. Taken together, the morphological features of autophagy
(such as increased numbers of vacuoles and AVOs) and the increased
levels of beclin 1 (a marker of autophagy) and LC 3-II/I suggest
that myricetin induces autophagy in SK-BR-3 cells.
To assess the effects of myricetin-induced autophagy
on cell viability, we conducted an MTT assay using 3-MA, an
inhibitor of autophagy. Zhu et al (40) observed a tendency of decreased
cell viability in 3-MA treatments. Similarly, in this experiment,
when myricetin and 3-MA were applied in combination, cell viability
showed a tendency to decrease further. Moreover, as a result of
western blotting, the pro-apoptosis protein Bax increased and the
anti-apoptosis protein Bcl-2 decreased. Accordingly, these findings
indicate that the inhibition of autophagy induces apoptosis and
reduces cell viability, suggesting that myricetin-induced autophagy
exerts protective effects on SK-BR-3 cells.
To examine the effects of the JNK protein on cell
viability in the previous experiment, we conducted an MTT assay
using SP600125, a JNK inhibitor. Studies by Yu et al
(41) and Chen et al
(42) demonstrated that JNK
inhibition leads to a further decrease in cell viability, and our
experiments showed similar results. To confirm that the reduced
cell viability after the inhibition of JNK was related to
autophagy, we evaluated the expression levels of LC 3 and
apoptosis-related proteins through western blot analysis. Yu et
al (41) reported that
SP600125 pre-treatment followed by treatment with a natural
compound resulted in a lower level of LC 3-II/I expression than in
the natural compound treatment group. Similarly, in our study,
SP600125 (20 µM, 1 h) pre-treatment followed by myricetin
(10 µM, 24 h) treatment led to reduced expression levels of
the LC 3-II/I protein compared to that in the myricetin (10
µM, 24 h) treatment group. Additionally, Bcl-2/Bax
expression levels were also reduced. These finding suggest that
autophagy is mediated by JNK, as previously reported by Chen et
al (42). JNK degrades the
Bcl-2/beclin 1 complex, thus allowing beclin 1 to initiate
autophagy. This leads to the increased expression of the LC 3
protein and promotes autophagy (43). These results indicate that
myricetin induces apoptosis via the JNK pathway in SK-BR-3 breast
cancer cells, and that autophagy is regulated by the JNK pathway.
Furthermore, myricetin-induced autophagy also had a protective role
in SK-BR-3 cells. Therefore, JNK inhibition suppressed autophagy,
which protected the cells and led to increased apoptosis.
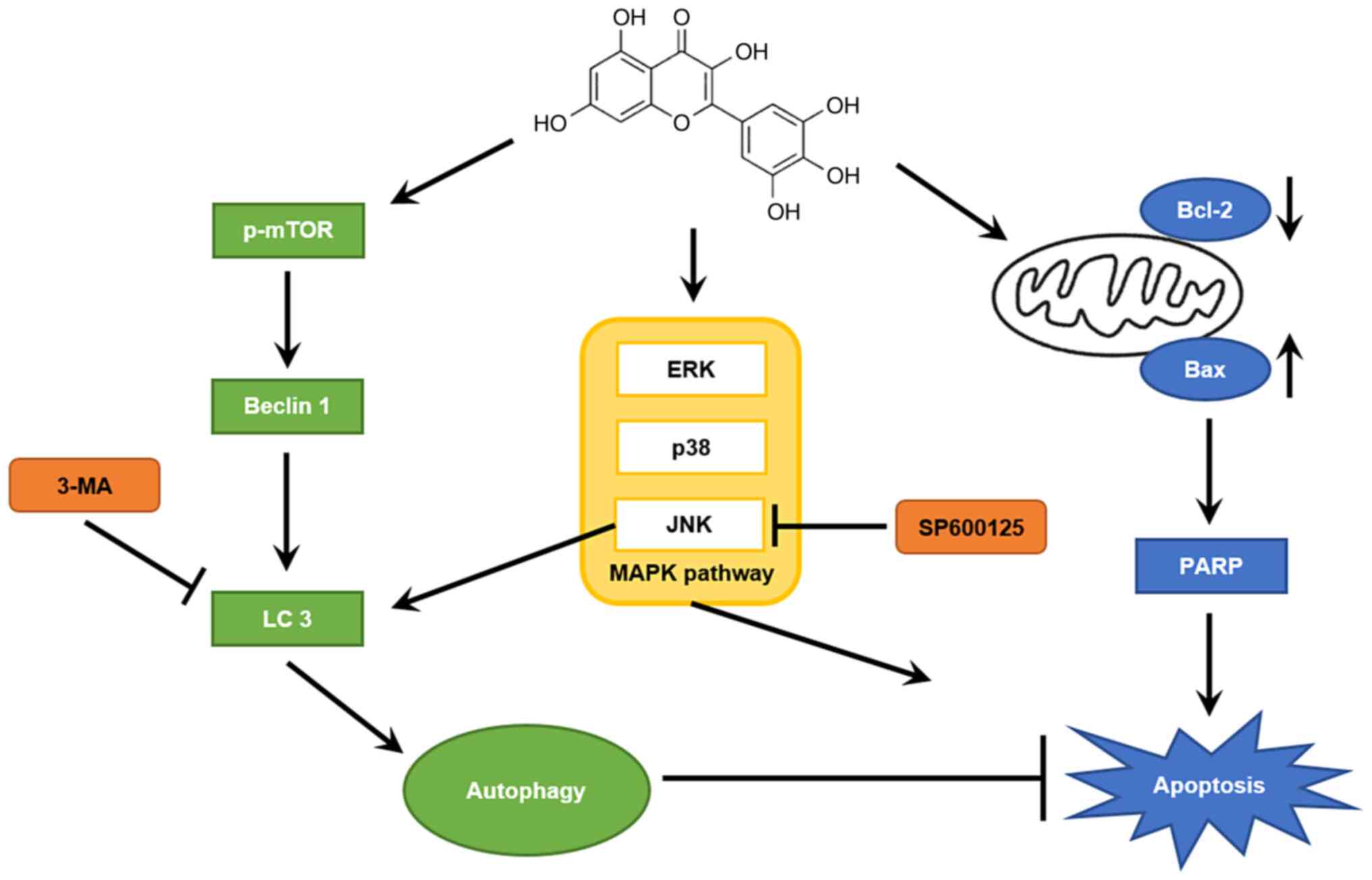
In conclusion, myricetin significantly inhibited the
viability of breast cancer SK-BR-3 cells (in a dose-dependent
manner) by promoting apoptosis. Western blot analysis showed that
myricetin increased the expression of the pro-apoptotic PARP and
Bax proteins, and reduced the expression of the anti-apoptotic
Bcl-2 protein. In addition, the myricetin-activated MAPK pathway
induced apoptosis in SK-BR-cells. The expression levels of
autophagic proteins (beclin 1 and LC 3-II/I) were also increased,
indicating that myricetin induced autophagy in SK-BR-3 cells. An
MTT assay with the 3-MA inhibitor revealed that myricetin-induced
autophagy had a protective effect in cells. Moreover, the JNK
protein was shown to play an important role in regulating autophagy
(Fig. 7). Taken together, these
results suggest that myricetin has anticancer efficacy against
breast cancer SK-BR-3 cells and that inhibition of autophagy
increases anticancer efficacy through apoptosis. However,
additional studies are required to examine whether myricetin
induces autophagy as well as apoptosis in vivo and to
examine the relationship between the two mechanisms.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHH, CC and JYJ designed the study. SHH, JHL and JSW
performed the experiments and GHJ, SHJ and EJH performed the
reagent preparation and analyzed the data. SKK and BKP developed
the methodology. SHH wrote the manuscript and YSP, BSK and JYJ
revised the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work, in particular the data, are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interest.
Acknowledgments
Not applicable.
Funding
This study was supported by Basic Science Research Program
through the National Research Foundation of Korea (NRF) funded by
the Ministry of Education, Science and Technology
(2021R1A2C1010912).
References
|
1
|
Global Burden of Disease Cancer
Collaboration; Fitzmaurice C, Abate D, Abbasi N, Abbastabar H,
Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I,
et al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2017:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 5:1749–1768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tao Z, Shi A, Lu C, Song T, Zhang Z and
Zhao J: Breast cancer: Epidemiology and etiology. Cell Biochem
Biophys. 72:333–338. 2015. View Article : Google Scholar
|
|
3
|
Brown LC, Mutter RW and Halyard MY:
Benefits, risks, and safety of external beam radiation therapy for
breast cancer. Int J Womens Health. 7:449–458. 2015.PubMed/NCBI
|
|
4
|
Seltzer JH, Gintant G, Amiri-Kordestani L,
Singer J, Koplowitz LP, Moslehi JJ, Barac A and Yu AF: Assessing
cardiac safety in oncology drug development. Am Heart J.
214:125–133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitra S and Dash R: Natural products for
the management and prevention of breast cancer. Evid Based
Complement Alternat Med. 2018:83246962018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5:e472016. View Article : Google Scholar
|
|
7
|
Song X, Tan L, Wang M, Ren C, Guo C, Yang
B, Ren Y, Cao Z, Li Y and Pei J: Myricetin: A review of the most
recent research. Biomed Pharmacother. 134:1110172021. View Article : Google Scholar
|
|
8
|
Ross JA and Kasum CM: Dietary flavonoids:
Bioavailability, metabolic effects, and safety. Annu Rev Nutr.
22:19–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Devi KP, Rajavel T, Habtemariam S, Nabavi
SF and Nabavi SM: Molecular mechanisms underlying anticancer
effects of myricetin. Life Sci. 142:19–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masuda T, Miura Y, Inai M and Masuda A:
Enhancing effect of a cysteinyl thiol on the antioxidant activity
of flavonoids and identification of the antioxidative thiol adducts
of myricetin. Biosci Biotechnol Biochem. 77:1753–1758. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng AW, Chen YQ, Zhao LQ and Feng JG:
Myricetin induces apoptosis and enhances chemosensitivity in
ovarian cancer cells. Oncol Lett. 13:4974–4978. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ong KC and Khoo HE: Biological effects of
myricetin. Gen Pharmacol. 29:121–126. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phillips PA, Sangwan V, Borja-Cacho D,
Dudeja V, Vickers SM and Saluja AK: Myricetin induces pancreatic
cancer cell death via the induction of apoptosis and inhibition of
the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer
Lett. 308:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Chen J, Yu X, Xu S, Li D, Zheng Q
and Yin Y: Myricetin suppresses the propagation of hepatocellular
carcinoma via down-regulating expression of YAP. Cells. 8:3582019.
View Article : Google Scholar :
|
|
15
|
Ye C, Zhang C, Huang H, Yang B, Xiao G,
Kong D, Tian Q, Song Q, Song Y, Tan H, et al: The natural compound
myricetin effectively represses the malignant progression of
prostate cancer by inhibiting PIM1 and disrupting the PIM1/CXCR4
interaction. Cell Physiol Biochem. 48:1230–1244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jo S, Ha TK, Han SH, Kim ME, Jung I, Lee
HW, Bae SK and Lee JS: Myricetin induces apoptosis of human
anaplastic thyroid cancer cells via mitochondria dysfunction.
Anticancer Res. 37:1705–1710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiao D and Zhang XD: Myricetin suppresses
p21-activated kinase 1 in human breast cancer MCF-7 cells through
downstream signaling of the β-catenin pathway. Oncol Rep.
36:342–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soleimani M and Sajedi N: Myricetin
apoptotic effects on T47D breast cancer cells is a P53-independent
approach. Asian Pac J Cancer Prev. 21:3697–3704. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knickle A, Fernando W, Greenshields AL,
Rupasinghe HPV and Hoskin DW: Myricetin-induced apoptosis of
triple-negative breast cancer cells is mediated by the
iron-dependent generation of reactive oxygen species from hydrogen
peroxide. Food Chem Toxicol. 118:154–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morales P and Haza AI: Selective apoptotic
effects of piceatannol and myricetin in human cancer cells. J Appl
Toxicol. 32:986–993. 2012. View Article : Google Scholar
|
|
21
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elumalai P, Gunadharini DN, Senthilkumar
K, Banudevi S, Arunkumar R, Benson CS, Sharmila G and Arunakaran J:
Induction of apoptosis in human breast cancer cells by nimbolide
through extrinsic and intrinsic pathway. Toxicol Lett. 215:131–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly(ADP-ribose)
polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murphy KM, Ranganathan V, Farnsworth ML,
Kavallaris M and Lock RB: Bcl-2 inhibits Bax translocation from
cytosol to mitochondria during drug-induced apoptosis of human
tumor cells. Cell Death Differ. 7:102–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Naturey. 410:37–40. 2001. View Article : Google Scholar
|
|
26
|
Lu Z and Xu S: ERK1/2 MAP kinases in cell
survival and apoptosis. IUBMB Life. 58:621–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He C and Levine B: The beclin 1
interactome. Curr Opin Cell Biol. 22:140–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oberstein A, Jeffrey PD and Shi Y: Crystal
structure of the Bcl-XL-beclin 1 peptide complex: Beclin 1 is a
novel BH3-only protein. J Biol Chem. 282:13123–13132. 2007.
View Article : Google Scholar
|
|
32
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014. View Article : Google Scholar :
|
|
33
|
Huang H, Chen AY, Ye X, Li B, Rojanasakul
Y, Rankin GO and Chen YC: Myricetin inhibits proliferation of
cisplatin-resistant cancer cells through a p53-dependent apoptotic
pathway. Int J Oncol. 47:1494–1502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cummings BS, Wills LP and Schnellmann RG:
Measurement of cell death in Mammalian cells. Curr Protoc
Pharmacol. 56:12.8.1–12.8.24. 2012. View Article : Google Scholar
|
|
35
|
Yoo GS, Lee JM, Lee CH, Jang JB and Lee
KS: Study of apoptosis by scirpi tuber in Hela cell and MCF-7 cell.
J Korean Obstet Gynecol. 24:1–13. 2011.
|
|
36
|
Kale J, Osterlund EJ and Andrews DW: BCL-2
family proteins: Changing partners in the dance towards death. Cell
Death Differ. 25:65–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Innajak S, Mahabusrakum W and
Watanapokasin R: Goniothalamin induces apoptosis associated with
autophagy activation through MAPK signaling in SK-BR-3 cells. Oncol
Rep. 35:2851–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Traganos F and Darzynkiewicz Z: Lysosomal
proton pump activity: Supravital cell staining with acridine orange
differentiates leukocyte subpopulations. Methods Cell Biol.
41:185–194. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu ML, Zhang PM, Jiang M, Yu SW and Wang
L: Myricetin induces apoptosis and autophagy by inhibiting
PI3K/Akt/mTOR signalling in human colon cancer cells. BMC
Complement Med Ther. 20:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu H, Wu CL, Wang X, Ban Q, Quan C, Liu M,
Dong H, Li J, Kim GY, Choi YH, et al: SP600125 enhances C-2-induced
cell death by the switch from autophagy to apoptosis in bladder
cancer cells. J Exp Clin Cancer Res. 38:4482019. View Article : Google Scholar :
|
|
42
|
Chen S, Dobrovolsky VN, Liu F, Wu Y, Zhang
Z, Mei N and Guo L: The role of autophagy in usnic acid-induced
toxicity in hepatic cells. Toxicol Sci. 142:33–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin HO, Hong SE, Park JA, Chang YH, Hong
YJ, Park IC and Lee JK: Inhibition of JNK-mediated autophagy
enhances NSCLC cell sensitivity to mTORC1/2 inhibitors. Sci Rep.
6:289452016. View Article : Google Scholar :
|