Introduction
Osteosarcoma (OS) is a malignant primary bone
neoplasm that is most commonly diagnosed in children and
adolescents and is the foremost cause of cancer-related mortality
in young people (1,2). Standard treatment protocol for OS
is based on resection surgery and polychemotherapy (3); however, the 5-year survival rate of
patients with is typically <20% (4). Clinically, the residual OS cells
following resection surgery may lead to continued bone destruction,
new lesions in adjacent tissue and even potential recurrence of OS
(5). On the other hand,
conventional clinical chemotherapy agents often encounter
challenges in accessing the tumor site to elicit therapeutic
effects and their non-selective nature tends to engender strong
toxicity to normal cells (6).
Hence, there is need for the advancement of a novel, efficacious
and low-toxicity treatment modality capable of precisely targeting
tumor tissue to improve patient prognosis and quality of life.
Targeted therapies for OS have garnered interest,
with small-molecule targeted inhibitors emerging as one of the most
promising avenues (7). mTOR, a
serine/threonine protein kinase, serves a pivotal role in
regulating cell proliferation, differentiation, senescence and
metabolism (8-10). Zhou et al (10) elucidated that the mTOR expression
in OS tissue derived from 65 patients with primary OS exhibited a
positive correlation with progression of OS; inhibition of AKT/mTOR
signaling has been reported to induce apoptosis in human OS cell
line (11). Considering the
association between mTOR and the occurrence and progression of OS,
inhibitors targeting mTOR have become focal points of research and
development by major pharmaceutical companies and scientific
research institutions (12).
GNE-477, a potent and efficacious dual PI3K/mTOR inhibitor capable
of blocking both mTORC1 and mTORC2 signaling pathways (13), has shown encouraging outcomes in
the treatment of numerous types of cancer, including renal cell
carcinoma (14) and glioblastoma
(15). Nevertheless, clinical
utilization of mTOR inhibitors has not yielded substantial benefits
for patients and causes severe adverse reactions and side effects.
This primarily stems from inadequate tissue selectivity of mTOR
inhibitors, with dosage tolerance and drug toxicity limiting
development and use (16). While
GNE-477 possesses promising potential in OS treatment, control over
dosage, delivery system and application protocol are important for
future clinical use. Consequently, there is need to develop a novel
drug delivery system with good biocompatibility.
Stimulus-responsive self-assembled polymer micelles,
capable of selectively delivering bioactive substances to specific
sites within an organism, represent one of the research hotspots of
controlled drug release systems (17). The underlying principle of
stimulus-responsive drug carriers involves encapsulating or bonding
hydrophobic drugs within stimulus-responsive polymers during the
self-assembly process to form drug-loaded nano micelles. These
micelles are modified and spliced with biologically recognizable
moieties, which improve drug solubility in water, extend
circulation time in the bloodstream, and achieve target-controlled
release of drugs, thereby enhancing the therapeutic efficacy while
decreasing systemic toxicity (18). External or physiological
environmental stimuli are triggers for drug release, including
light, temperature, ultrasound, magnetic force, enzymes, pH and
redox substances (19-22). Reactive oxygen species (ROS),
comprising OH−, H2O2 and
O2–, among others, are a class of free
radicals in cells (23) that are
highly released under disease conditions, particularly at sites of
inflammation (24) and tumor
advancement (25,26). Among ROS,
H2O2 boasts an extended biological lifespan
and facile diffusion both within and between cells (27). Moreover, oxidative stress caused
by H2O2 is implicated in the pathogenesis of
diverse types of disease, such as cancer, Parkinson's disease,
cardiovascular disease (28).
Therefore, the development of H2O2
stimulus-responsive biomaterials holds promise in decreasing drug
toxicity for lesions characterized by heightened oxidative
stress.
A variety of polymers are currently undergoing
pre-clinical scrutiny to investigate potential in micelle
formation, carrying chemotherapeutic agents, and anti-tumor effects
in both in vitro cell experiments and in vivo animal
models (29-32). Several polymeric micelles have
progressed to clinical trials and have proved their efficacy in
human subjects (33,34). Notably, GNE-477 has special amino
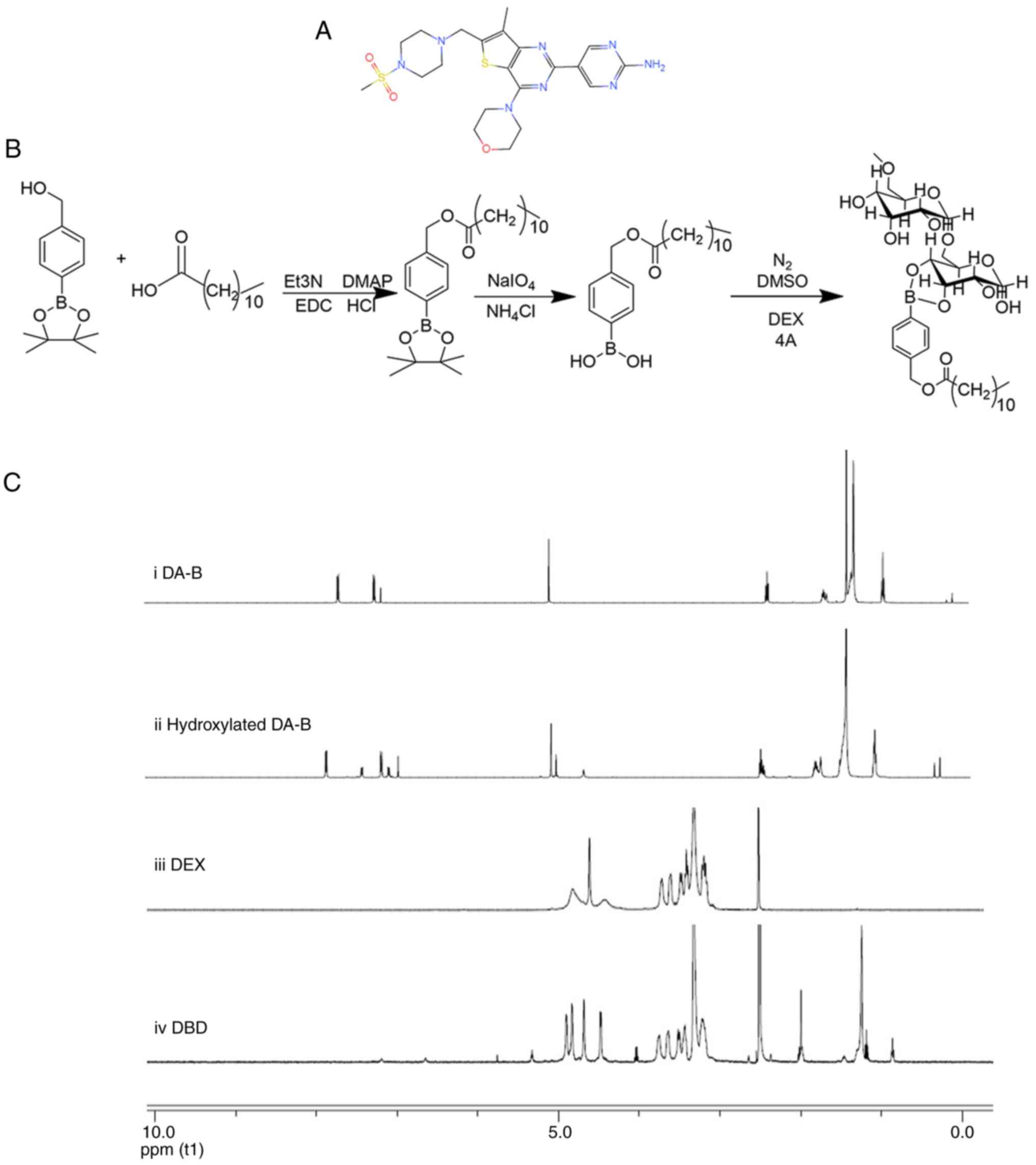
and methanesulfonyl (Fig. 1A)
groups, which facilitate its chemical synthesis and modification
into a micellar structure. Among
H2O2-sensitive moieties, phenylborate ester
(PBAE) with readily modifiable structure and excellent
biocompatibility represents one of the most sensitive structures to
H2O2 (35). Under the induction of
H2O2, the carbon-boron bond undergoes
oxidative cleavage, leading to irreversible decomposition of PBAE
(36). Additionally, dodecanoic
acid (DA) functions as a graft-reactive monomer, while dextran
(DEX) is hydrophobically modified to self-assemble into nano
micelles featuring a shell-core structure. The combination of the
aforementioned components as drug carriers holds promise in
addressing the limitations associated with current antitumor
medications. Thus, the present study synthesized GNE-477-loaded
H2O2 stimulus-responsive dodecanoic
acid-PBAE-dextran (DA-B-DEX) polymeric micelles (GNE-477@DBD) to
release the drug in response to high H2O2
concentration in physiological lesion tissue and the anti-tumor
effect of GNE-477@DBD against OS was investigated in vitro
and in vivo.
Materials and methods
Cell culture
The human osteoblast cell line hFOB1.19 and OS cell
lines MG-63, U2OS, and 143B were procured from iCell Bioscience,
Inc. Cells were cultured in Dulbecco's Modified Eagle Medium/F-12
(DMEM-F12), supplemented with 10% FBS (both Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 μg/ml
streptomycin, under a 5% CO2 atmosphere at 37°C.
Animals
A total of 20 eight-week-old male BALB/c nude mice
(weighted 18-20 g) were obtained from SPF (Beijing) Biotechnology
Co., Ltd. and acclimatized for 1 week. The mice were housed at
constant room temperature (60-65% humidity, 23±2°C) with a 12/12-h
light/dark cycle and free access to standard chow and water. The
research protocol received approval from the Medical Ethics
Committee of the First People's Hospital of Nanning (Guangxi,
China; approval no. 2021-076-01) and complied with the guidelines
of the National Institutes of Health Animal Care and Use Committee
(37).
Synthesis and characterization of
DA-B-DEX polymer
A total of 700.00 4-hydroxymethyl PBAE, 946.25 DA,
76.95 4-dimethylaminopyridine (DMAP), 733.32
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and 79.67 mg
triethylamine were weighed and dissolved in 10 ml of
dichloromethane and nitrogen was injected using a syringe to
protect the system. Following 18 h reaction at room temperature,
DA-B was obtained. A total of 700.00 DA-B, 761.32 NaIO4
190.40 mg and NH4Cl (was dissolved in acetone (10 ml),
with an equivalent volume of water. The reaction mixture was
refluxed at 60°C overnight, resulting in the synthesis of terminal
hydroxylated DA-B. The hydroxylated DA-B (30 mg) was reacted with
DEX (115.18 mg) under 4A molecular sieve (200 mg) and nitrogen
protection at 60°C for 60 h. The molecular sieve was removed via
filtration and the filtrate was slowly dripped into ice-cold
ethanol. A white precipitate emerged, which underwent
centrifugation at 1,006 × g for 5 min at room temperature. The
upper layer was discarded, and the ice-cold ethanol was added,
followed by centrifugation at 1,006 × g for 3 min at room
temperature. After being washed three times in ethanol, the
precipitate yielded the DA-B-DEX polymer. The synthetic route of
the DA-B-DEX polymer is shown in Fig. 1B.
1H nuclear magnetic resonance spectra of
the DA-B-DEX polymers were determined at room temperature using a
Nuclear Magnetic Resonance Spectrometer (Bruker), with deuterium
oxide (D2O) serving as the solvent and trimethylsilane
(TMS) as the internal standard, and the reaction grafting rates
were calculated based on the characteristic proton peaks (38). In the spectrum of hydrogen, the
ratio of the area of hydrogen peaks at specific positions on the
grafting group to the area of hydrogen peaks at certain
characteristic positions on the main chain is termed as the
grafting rates.
Synthesis of DA-B-DEX micelles and
determination of critical micelle concentration (CMC)
A total of 100 mg of the DA-B-DEX polymer was
dissolved in ultra-pure water. Dialysis bags with a molecular
weight cut-off of 3,500 KD were used for dialysis in ultra-pure
water for 48 h, with the water refreshed every 4 h. Then, the
solution in the dialysis bag was freeze-dried in a freeze-drying
machine for 24 h to yield a white solid, designated DA-B-DEX
micelles.
CMC was determined utilizing a pyrene fluorescent
probe, as previously described (39). A total of 0.0051 g pyrene was
dissolved in 25 ml methanol, yielding a 1.01×10−3 mol/l
pyrene solution, which was diluted with methanol to
1.616×10−6 mol/l pyrene solution. The prepared
1.616×10−6 mol/l pyrene solution (150 μl) was
transferred to 10 ml bottles, and the methanol was evaporated at
50°C for 5 min. A total of 10 ml DA-B-DEX solution
(1×10−4, 1×10−3, 1×10−2,
1×10−1, 1, 10, 100 and 1,000 mg/ml) was added to each of
the 10 ml bottles containing a trace amount of pyrene. The solution
underwent ultrasound treatment in a 50°C water bath for 30 min,
followed by standing at room temperature for 2 h. The fluorescence
spectra of each solution were prepared for analysis by fluorescence
photometer. The fluorescence photometer was set with a slit of
excitation and emission at 5 nm, with a scanning range of 300-360
nm and an emission wavelength of 372 nm. The intensity of the
emitted light at 333 (I333) and 339 nm (I339) excitation
wavelengths were measured, regression curves were plotted, and the
ratio of I339/I333 was calculated. The intersection of the two
regression curves was calculated.
Preparation of GNE-477@DBD
A total of 91.5 DA-B-DEX and 8.5 mg GNE-477 (8.5 was
co-dissolved and added to PBS buffer (100 ml). The resulting
solution was then transferred to a dialysis bag with an
interception relative molecular weight of 3,500 kDa and underwent
dialysis in ultra-pure water for 48 h, with the water changed every
4 h. The suspension was filtered using 5.00 and 0.45 μm
syringe filters and freeze-dried (Labconco Freezone 6), yielding a
white powder named GNE-477@DBD.
Evaluation of micelle morphology and
particle size distribution
To evaluate the morphological characteristics of
GNE-477@DBD, samples were dropped onto a copper grid and negatively
stained at room temperature for 2 min using 2% UO2
acetate aqueous solution. Following drying, morphology was observed
under a transmission electron microscope (TEM; FEI Talos F200S;
Thermo Fisher Scientific, Inc.). Additionally, particle size
distribution was determined by a laser particle sizer (Zetasizer
Nano ZS ZEN3600, Malvern Instruments, Ltd.).
Drug release of GNE-477@DBD
The prepared drug-loaded micelles were dissolved in
the following solutions: i) Pure PBS (pH=7.4), PBS solution
containing ii) 10 or iii) 50 μmol/l
H2O2; iv) simulated body fluid (SBF, pH=7.4)
and v) simulated tumor microenvironment (pH=6.5, 100 μM
H2O2). These solutions were sealed in
dialysis bags with a cut-off relative molecular weight of 3,500 kDa
and dialyzed for 50 h in 3,000 ml PBS buffer at 37°C. A total of 1
ml GNE-477@DBD sample was taken and replaced with 1 ml fresh PBS
solution to maintain solution volume. The released GNE-477 was
quantified using high-performance liquid chromatography (HPLC;
Agilent 1260, Agilent Technologies, Inc.). GNE-477 were filtered
using 0.2 μm cellulose acetate filters and analyzed using an
Eclipse XDB-C18 column (150.0×4.6 mm; 5 μm particle size)
operated at 25°C. The mobile phase consisted of 2% (v/v) acetic
acid in water (mobile phase A) and 0.5% acetic acid in water and
acetonitrile (10:90 v/v) (mobile phase B), and at a flow rate of
1.0 ml/min. The injection volume was 10 μl and the
wavelength of the UV detector is set at 280 nm. The quantification
was performed by applying the standard calibration curve.
In vitro uptake of GNE-477@DBD
The uptake of GNE-477@ DBD was assessed in MG-63,
U2OS and 143B cell lines following 2 and 6 h incubation. Cells were
seeded in a 2-multiwell plate at a density of 2×104
cells/well (2 ml/well) and cultured in DMEM for 24 h at 37°C and 5%
CO2. Culture medium was replaced with a dispersion of
GNE-477@ DBD in fresh DMEM (0.25 mg/ml, 1 ml/well), and the cells
were incubated for 2 or 6 h at room temperature. Upon reaching ~90%
confluence, cells were treated with Nile Red (HY-D0718,
MedChemExpress)-loaded GNE-477 for 1 h at room temperature, fixed
and stained with DAPI for 10 min at room temperature using the
method described previously (40). Finally, the slides were analyzed
using a Leica TCS SP8 confocal laser scanning microscope
(Leica-Microsystems GmbH). Nile Red was excited with a 560 nm laser
and emission was collected at 570-620 nm (41).
MTT assay
hFOB1.19, MG-63, U2OS and 143B cells were cultured
in a complete RPMI-1640 medium supplemented with 10% FBS, 100 IU/ml
penicillin and 100 mg/ml streptomycin (all Gibco; Thermo Fisher
Scientific, Inc.). The cells were seeded in 96-well plates
(2×104 cells/well) and incubated overnight at 37°C and
5% CO2. At 24, 48 and 72 h, 10 μl MTT solution
(Wuhan Biofavor Biotechnology Service Co., Ltd.) was added for 4 h
at room temperature and the medium was removed. The formazan
crystals were dissolved in 150 μl DMSO. Blank wells were
used as blank groups. The optical density (OD) at 560 nm was
measured by a microplate reader. Finally, the cell viability was
calculated as follows: Cell viability (%)=OD of treated cells/OD of
control ×100. The experiment was repeated three times.
Flow cytometry
The apoptotic rate (early + late apoptosis) and cell
cycle distribution were assessed using flow cytometry. For
apoptosis assessment, MG-63, U2OS and 143B cells subjected to
different treatments (Control, GNE-477, DBD, and GNE-477@DBD) were
suspended in binding buffer and incubated with 5 Annexin V-FITC and
10 μl propidine iodide (PI; BD Biosciences) for 15 min at
room temperature away from light. The apoptotic cells were
determined on an Attune™ NxT flow cytometer (Thermo Fisher
Scientific, Inc.), and results were analyzed using FlowJo software
(version 9.3.2; FlowJo LLC). For cell cycle distribution, cells
were fixed in 75% ethanol for 2 h at 4°C and treated with RNase A
for 40 min at room temperature. Staining with PI for 40 min at room
temperature, followed by detection on an Attune™ NxT flow cytometer
(Thermo Fisher Scientific, Inc.), and Attune NxT Software (version
3.2.1; Thermo Fisher Scientific, Inc.) to determine the percentage
of cells in G0/G1, S and G2/M phases.
Western blotting
Protein samples were extracted from OS cells or
tissue using RIPA buffer (BioTeke Corporation), and the protein
content was measured using a BCA protein quantification kit
(Beyotime Institute of Biotechnology). 10 μg of protein from
each sample was loaded and separated by 10% SDS-PAGE (Sinopharm
Chemical Reagent Co., Ltd.), followed by transfer onto PVDF
membranes. The membrane was blocked with 5% skimmed milk in
Tris-buffered saline at room temperature for 1 h, followed by
overnight incubation at 4°C with primary antibodies (all Cell
Signaling Technology, Inc.) against caspase 3 (1:1,000; cat. no.
9662), cleaved-caspase 3 (1:1,000; cat. no. 9661), Bax (1:1,000;
cat. no. 2772), Bcl-2 (1:1,000; cat. no. 15071), Cyclin D1
(1:1,000; cat. no. 2922), Cyclin E (1:1,000; cat. no. 20808), AKT
(1:1,000; cat. no. 9272), phosphorylated-AKT (p-AKT; 1:500; cat.
no. 9271), mTOR (1:1,000; cat. no. 2972), p-mTOR (1:1,000; cat. no.
2971) and GAPDH (1:1,000; cat. no. 2118). After washing by TBST
(Tris-buffered saline with 0.1% Tween 20), the membrane was
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibodies (Abcam; 1:10,000; cat. no. ab205718) for 2 h
at room temperature. Protein bands were visualized using enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.) and
semi-quantification was performed using Image J software (version
1.54i 03; National Institutes of Health).
Reverse transcription-quantitative (RT-q)
PCR
Total RNA from OS cell lines was isolated using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), followed by RT
into cDNA using PrimeScript™ RT reagent kit (TransGen Biotech Co.,
Ltd.), according to the manufacturer's protocol. Next, qPCR was
performed using AceQ qPCR SYBR Green Master Mix (MedChemExpress
LLC). The analysis of mRNA expression levels was conducted by the
2−ΔΔCq method (42),
with GAPDH as the internal reference. The sequences of the primers
were as follows: Caspase 3, forward, 5′-CGTGCTTCTAAGCCATGGTG-3′ and
reverse, 5′-GTCCCACTGTCCGTCTCAAT-3′; Bax, forward,
5′-TGCCTCAGGATGCATCTACC-3′ and reverse, 5′-AAGTAGAAAAGCGCGACCAC-3′;
Bcl-2, forward, 5′-AGGGCATTCAGTGACCTGAC-3′ and reverse,
5′-CGATCCGACTCACCAATACC-3′; Cyclin D1, forward,
5′-ACCCGACGAGTTACTGCAAAT-3′ and reverse,
5′-TCTGTTTGGTGTCCTCTGCC-3′; Cyclin E, forward,
5′-AGAGGAAGGCAAACGTGACC-3′ and reverse, 5′-TATTGTCCCAAGGCTGGCTC-3′
and GAPDH, forward, 5′-AGGCCGGTGCTGAGTATGTC-3′ and reverse,
5′-TGCCTGCTTCACCACCTTCT-3′. The thermocycling conditions were as
follows: Initial denaturation at 96°C for 5 min, followed by 40
cycles of 95°C for 30 sec and 68°C for 20 sec.
Xenograft model
U2OS tumor-bearing Balb/c mice were generated by
subcutaneously injecting U2OS cells (2×106/mouse). Then,
the mice were randomly divided into four groups (n=5/group) as
follows; Control, GNE-477, DBD and GNE-477@DBD. Every 2 days, the
state of the mice was observed and the body weight was determined.
In addition, the tumor size was measured using vernier calipers and
tumor volume was calculated as follows: Tumor volume=0.5x length ×
width2. Once the inoculated tumor volume reached 100-200
mm3, 25 mg/kg GNE-477, DBD@GNE-477 (containing 25 mg/kg
GNE-477), or an equivalent volume of vehicle control (DBD) and
negative control (PBS) were administered intraperitoneally. Weight
loss ≥20% within a short period or the average tumor diameter
exceeds 20 mm, the experiment was terminated and mice were
euthanized. Mice were anesthetized after 34 days using 3%
isoflurane and then euthanized by cervical dislocation. Cessation
of heartbeat and breathing were considered to confirm death. The
heart, liver, spleen, lung, kidney and tumor were removed, washed
with saline, weighed, and fixed for 24 h at 4 °C in 4% formaldehyde
for subsequent staining.
Hematoxylin and eosin (H&E)
staining
To evaluate the potential side effects of drugs on
organs, H&E staining was performed. The fixed heart, liver,
spleen, lung and kidney tissue was dehydrated, embedded in paraffin
and cut into 4 μm-sections. Thereafter, the samples were
stained with hematoxylin (Beijing Solarbio Science & Technology
Co., Ltd.) for 8 min at room temperature and rinsed with running
water. Samples were differentiated with 5% acetic acid for 1 min,
washed with running water for 10 min, stained with eosin (Beijing
Solarbio Science & Technology Co., Ltd.) for 1 min at room
temperature, and rinsed three times with running water for 5 min.
Finally, the images were captured under a light microscope
(magnification, ×100).
Statistical analysis
All data are presented as the mean ± SD. GraphPad
PRISM 8.0 software (Dotmatics) was used for statistical analysis by
one- or two-way ANOVA followed by Tukey's post hoc test. Each
sample was evaluated in a minimum of three experimental replicates.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of DA-B-DEX polymer
The 1H-nuclear magnetic resonance (NMR)
spectra of DA-B, hydroxylated DA-B, DEX and DA-B-DEX conjugates are
shown in Fig. 1C. In the
spectrum of DA-B, the characteristic single peak of pinacol ester
(1.24 ppm) and the two characteristic peaks of benzene rings were
clearly discernible. The chromatogram of hydroxylated DA-B
exhibited disappearance of the single peak at 1.24 ppm, confirming
the removal of the protective group (pinacol ester). Hydroxylated
DA-B was successfully grafted onto DEX, yielding a calculated
grafting rate of ~3% (Fig. 1C),
meeting the general requirements for formation of amphiphilic
micelles (grafting rate <10%, ensuring the solubility of the
micelles) (43).
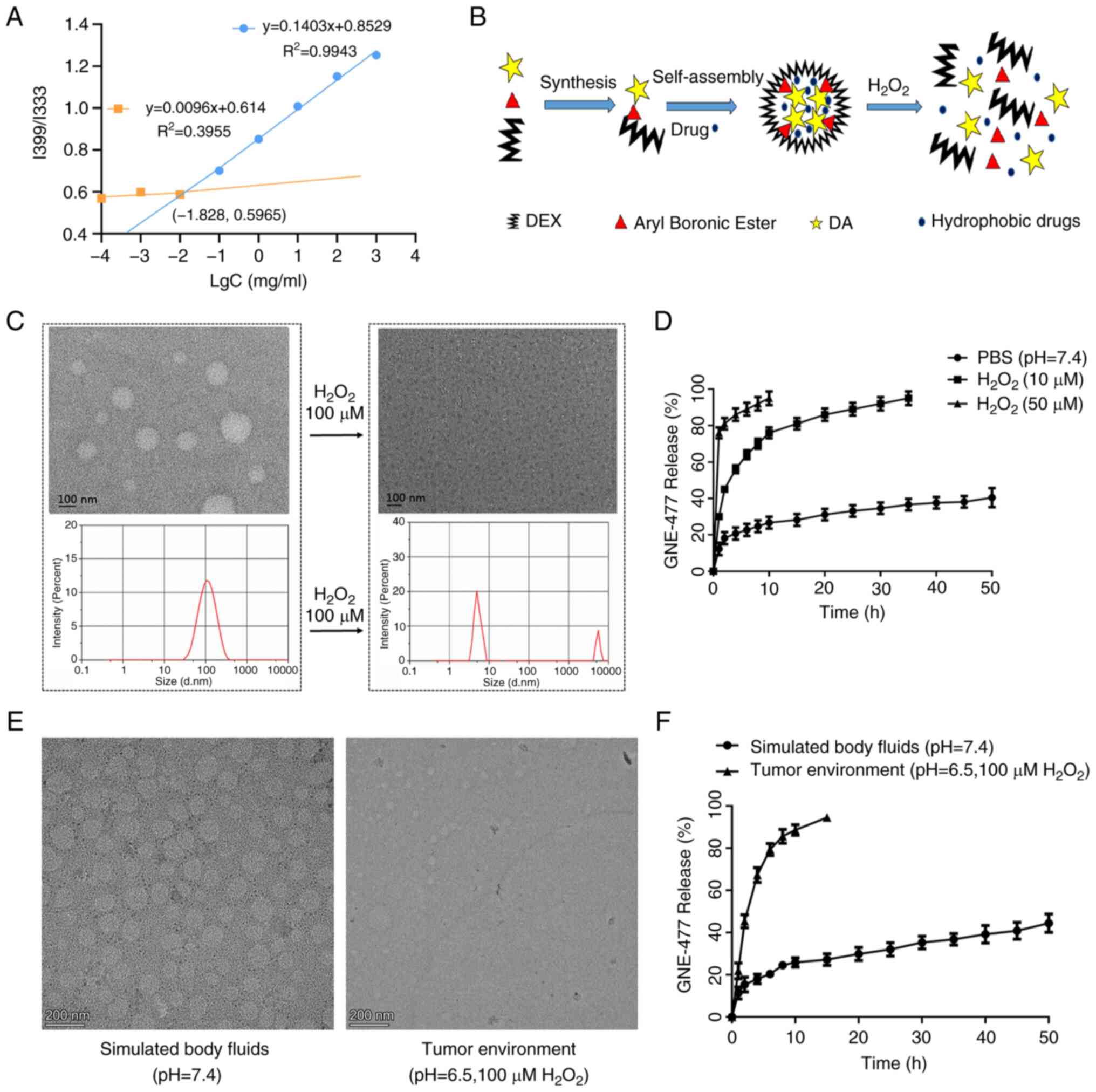
Preparation and release of
GNE-477@DBD
As in Fig. 2A,
the intersection of the two regression curves for the I339/I333
ratio of DA-B-DEX pyrene solution was at (−1.828,0.5965). CMC of
DA-B-DEX micelles was 0.01486 mg/ml; the low CMC signified that
DA-B-DEX readily formed micelles and maintained the core-shell
structure under highly diluted conditions. The degradation process
of DA-B-DEX micelles is shown in Fig. 2B. In the absence of
H2O2, almost all drug-loaded micelles were
spherical and intact, whereas exposure to 100 μmol/l
H2O2 resulted in notable disruption of
micelle structure, accompanied by an alteration in the particle
size distribution of the micelles towards inhomogeneous (Fig. 2C). The in vitro release
profile of GNE-477 revealed a significant disparity in drug release
from DA-B-DEX in PBS, 10 and 50 μmol/l
H2O2 environments. At 2 h, drug release in
the PBS group was 20%, while it reached 41 and 85% at 10 and 50
μmol/l H2O2, respectively (Fig. 2D). TEM was conducted to assess
rupture and release of GNE-477@DBD under conditions of SBF (pH=7.4)
and tumor environment (pH=6.5, 100 μM
H2O2). The micellar structure of GNE-477@DBD
was more susceptible to disruption in the tumor environment
(Fig. 2E), exhibiting a higher
drug release rate compared with SBF (Fig. 2F). These findings indicated that
the DA-B-DEX micelles ruptured and released the drug faster in the
presence of H2O2, thus DA-B-DEX micelles were
H2O2-responsive.
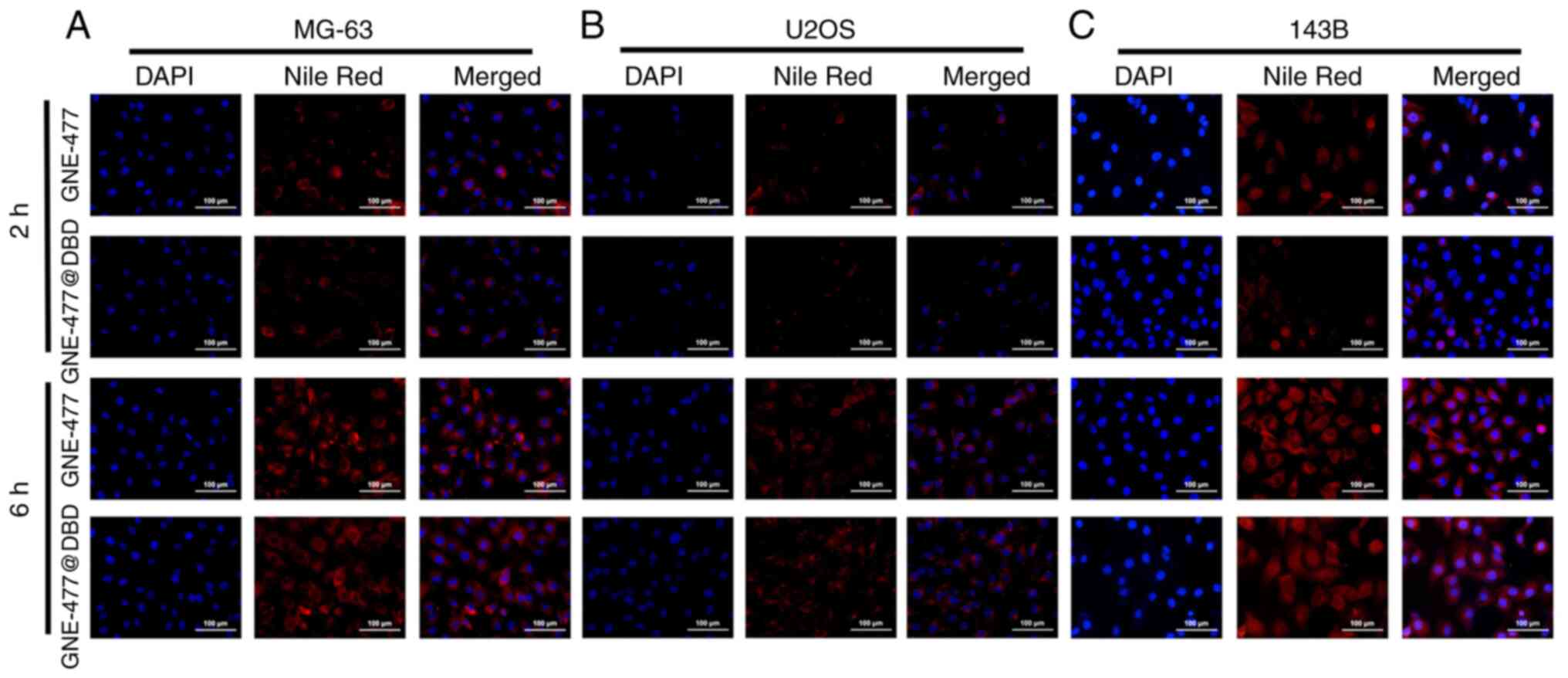
Effect of GNE-477@DBD on drug uptake of
OS cells in vitro
Following 2 h incubation, free GNE-477 was
distributed in both cytoplasm and nucleus, while in the GNE-477@DBD
group, GNE-477 was predominantly located in the cytoplasm,
exhibiting a weaker fluorescence intensity compared with the
GNE-477 group. Following 6 h incubation, the fluorescence intensity
of GNE-477 in the GNE-477@DBD group notably increased, indicating a
progressive internalization of more GNE-477@DBD into the cells,
followed by sustained release of GNE-477 in the cells (Fig. 3A-C).
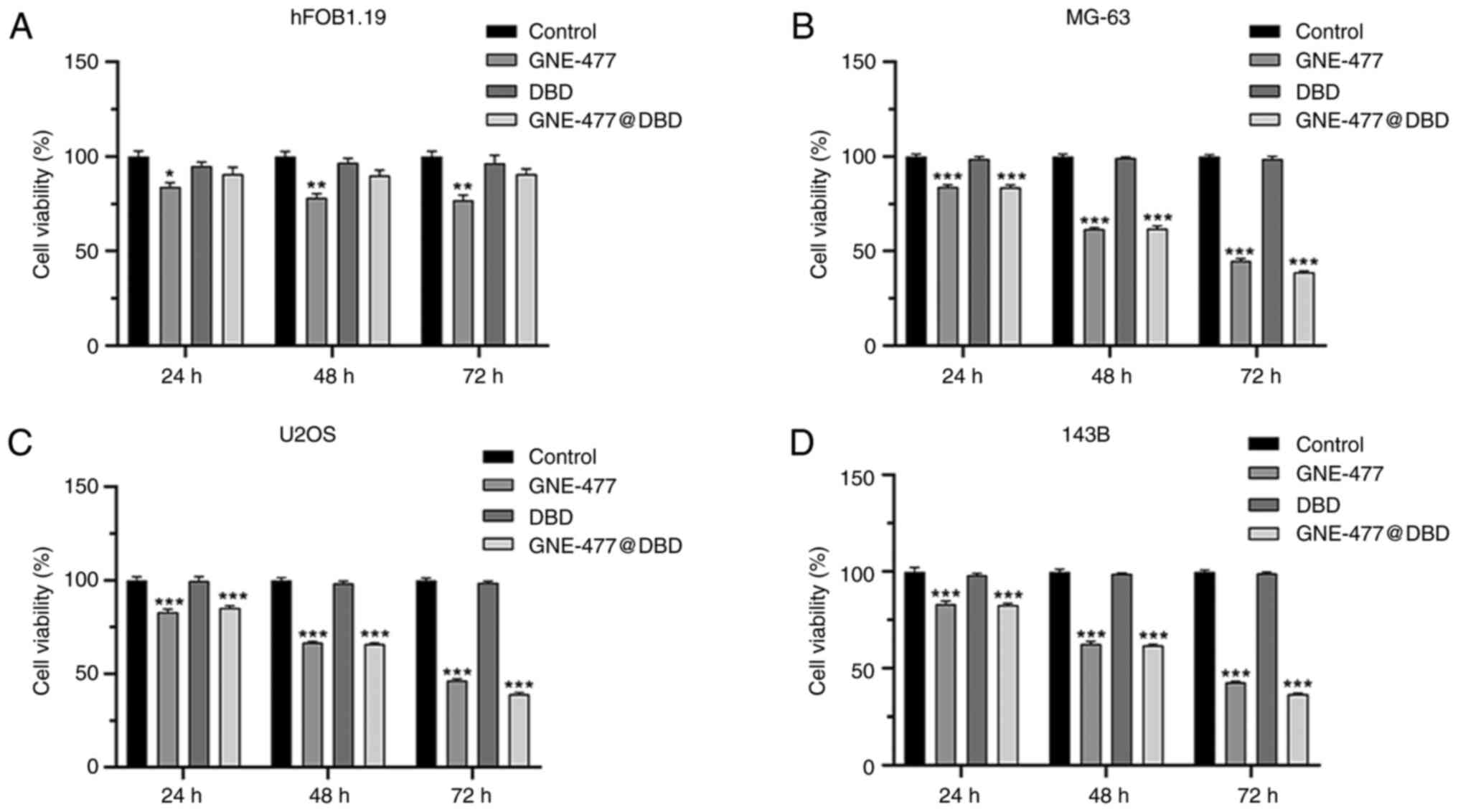
Effect of GNE-477@DBD on viability,
apoptosis and cell cycle of OS cells in vitro
Normal human osteoblast hFOB1.19 and MG-63, U2OS and
143B OS cells were treated with PBS, GNE-477, DBD and GNE-477@DBD
for 24, 48, and 72 h. The findings revealed that DBD and
GNE-477@DBD exhibited good biocompatibility and low toxicity to
normal cells (Fig. 4A) and both
GNE-477 and GNE-477@DBD demonstrated inhibitory effects on OS cell
viability (Fig. 4B-D). Following
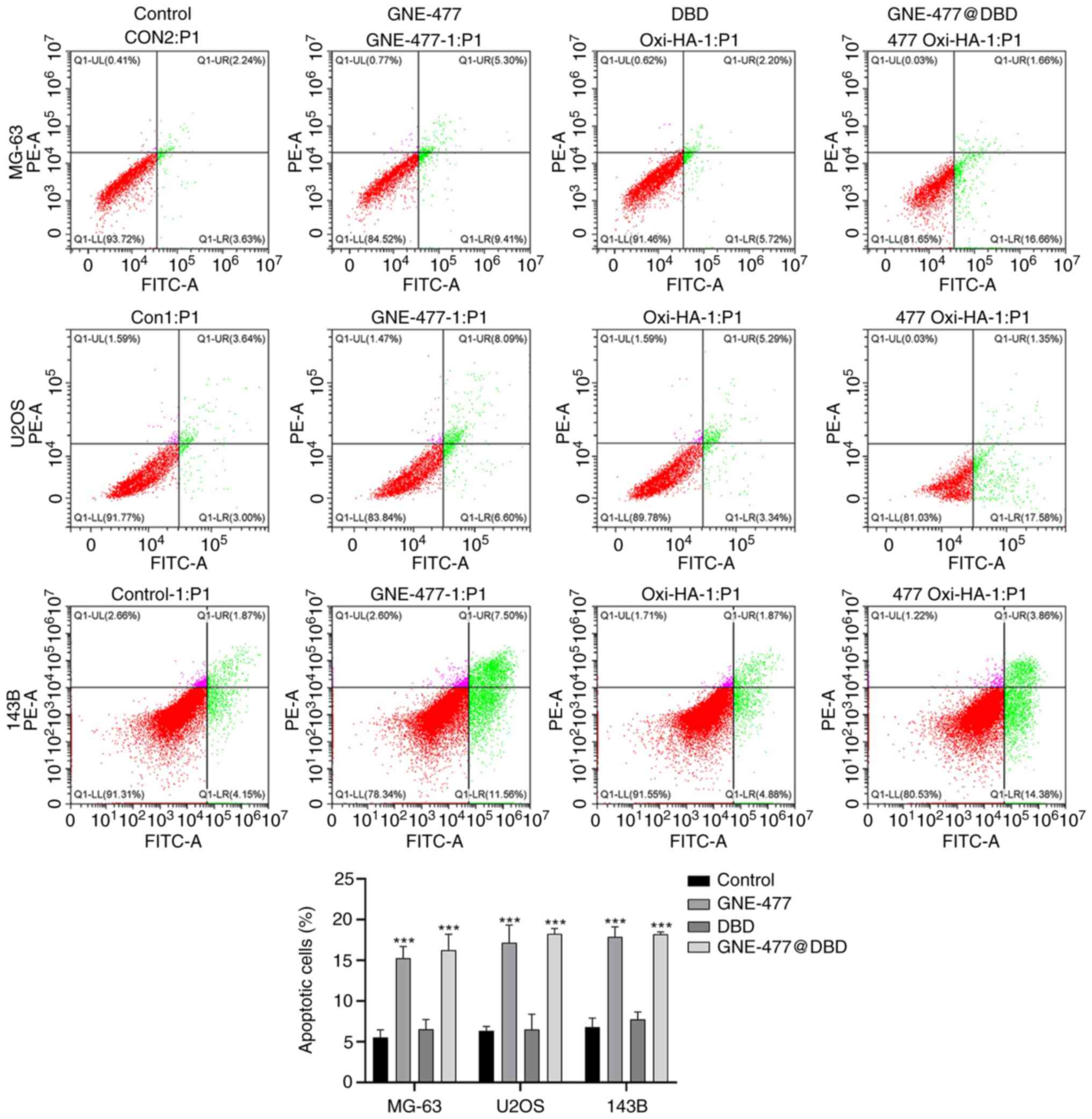
24 h drug treatment, flow cytometry showed that compared with
Control, both GNE-477 and GNE-477@DBD induced apoptosis in OS cell
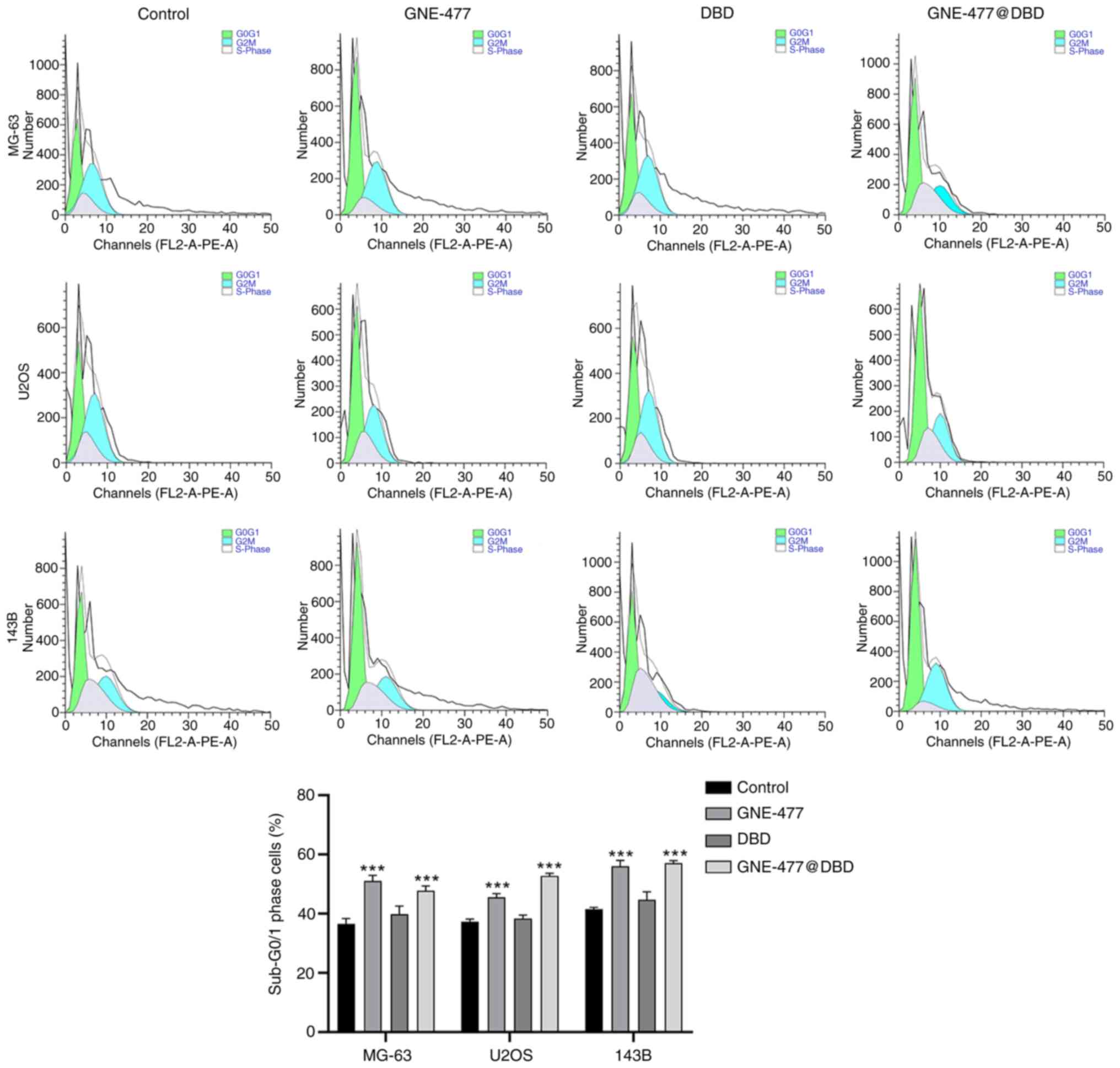
lines (Fig. 5) and elevated the
proportion of cells in G1 phase, suggesting that GNE-477 and
GNE-477@ DBD blocked cell division at G1 phase and prevented cell
cycle progression (Fig. 6).
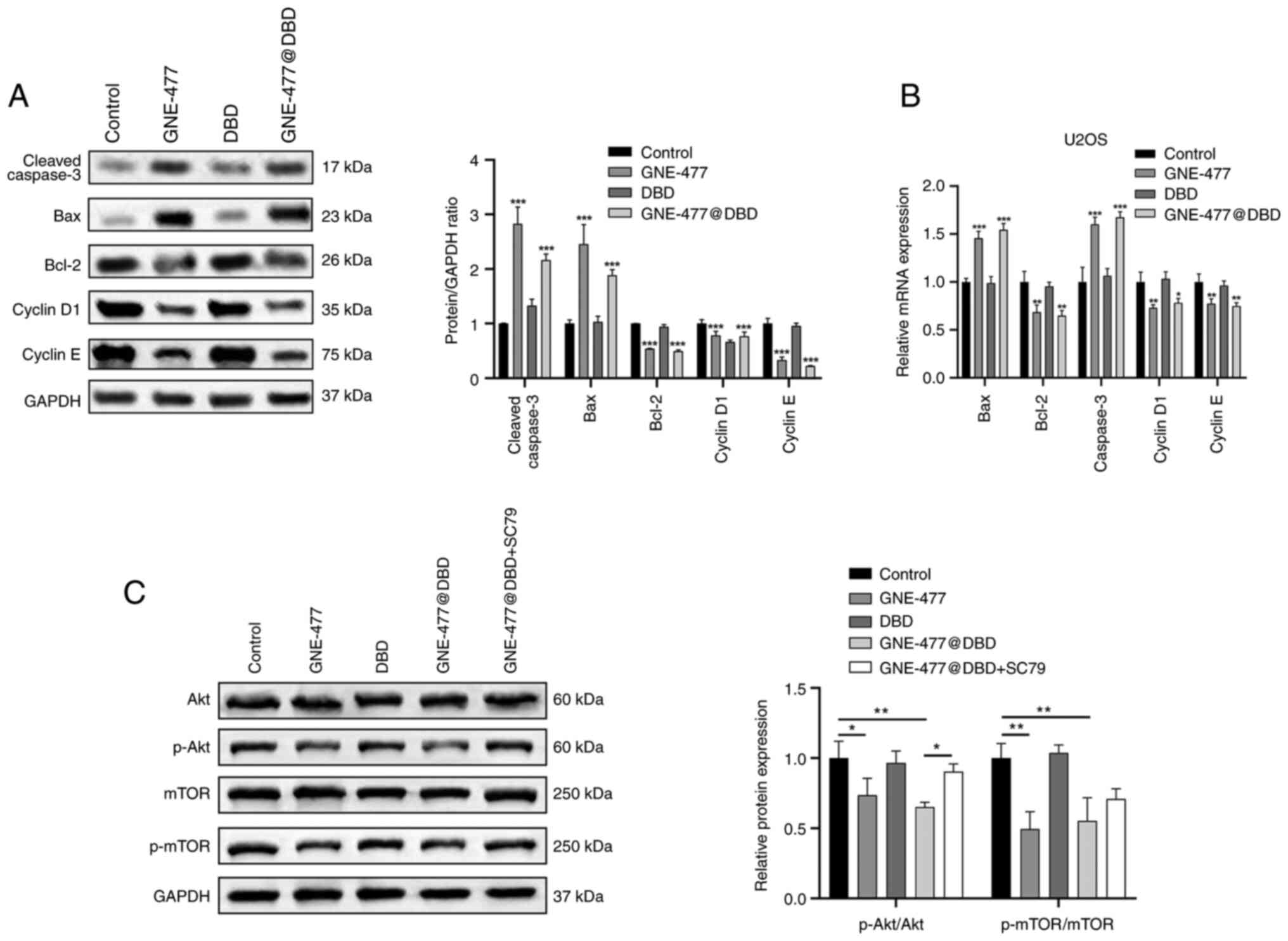
Furthermore, protein and mRNA levels of cell apoptosis- and cell
cycle-associated genes were verified by western blot and RT-qPCR in
U2OS cells (Fig. 7A and B).
Following 24 h drug treatment, compared with the Control group, the
expression of Bcl-2 and cyclin D1 and E protein and mRNA was
downregulated, while Bax and caspase 3 protein and mRNA expression
levels were upregulated in the GNE-477 and GNE-477@DBD groups,
indicating that GNE-477 exerted anti-tumor effects following
encapsulation in H2O2-responsive nano
micelles. Additionally, western blot analysis revealed that GNE-477
and GNE-477@DBD inhibited expression of proteins involved in the
Akt/mTOR cascade response, confirmed by Akt agonist treatment
(Fig. 7C).
Anti-OS activity and biosafety of
GNE-477@DBD in vivo
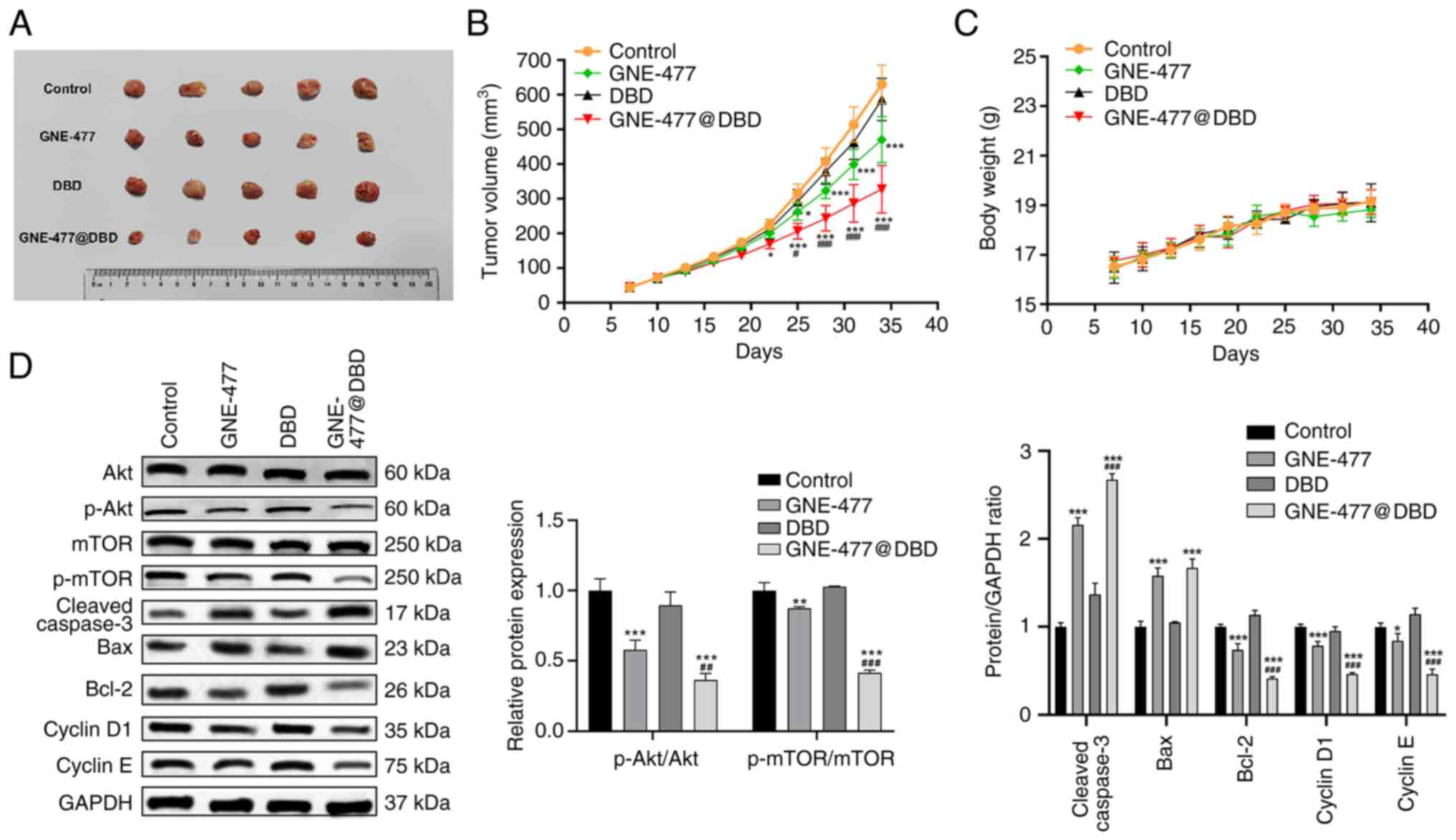
A nude mouse model was established to evaluate in
vivo anti-tumor ability of GNE-477@DBD. GNE-477 and DBD@
GEN-477 groups demonstrated tumor regression and impeded tumor
growth compared with the Control group, notably, the anti-tumor
efficacy of GNE-477@DBD surpassed that of free GNE-477 (Fig. 8A and B). There were no
significant differences in the body weight of mice between groups,
thereby indicating the safety profile of this nanoscale drug
delivery system (Fig. 8C).
Moreover, western blot results corroborated that protein expression
of p-AKT, p-mTOR, Bcl-2 and cyclin D1 and E decreased in the
GNE-477 and GNE-477@DBD groups, while protein expression of Bax and
cleaved-caspase 3 increased. These results suggested that treatment
with GNE-477 and GNE-477@DBD suppressed PI3K/AKT/mTOR pathway
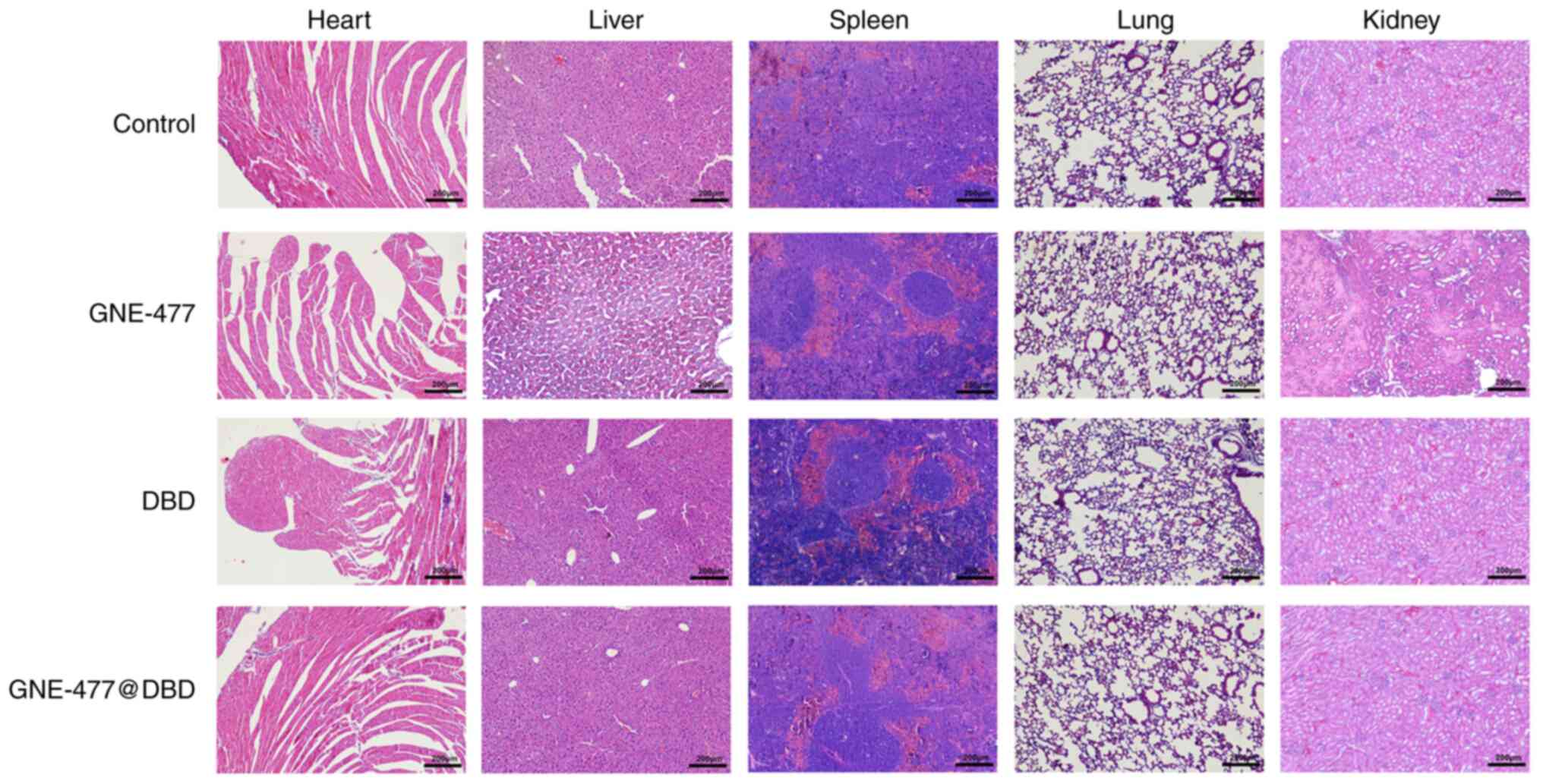
activation and facilitated apoptosis of tumor cells (Fig. 8D). Histopathological examination
of major organs stained with H&E demonstrated varying degrees
of damage in the GNE-477 group, whereas both the DBD and
GNE-477@DBD groups exhibited no discernible organ damage,
indicating that DBD nano micelles had favorable biocompatibility
and mitigated organ damage caused by GNE-477 (Fig. 9).
Discussion
OS is widely acknowledged as the most prevalent type
of primary bone malignancy, yet conventional chemotherapy drugs
often carry deleterious side effects and lack targeted ability,
compromising the prognosis and quality of life for patients
(44). Biomaterials have shown
promising potential in the treatment of bone-associated disease,
such as the deterioration of human dental enamel, tooth
regeneration and bone tissue repair (45-47). Consequently, development and
application of drug delivery systems hold promise for enhancing the
treatment of OS (48). The
design of nanoplatforms for stable delivery of hydrophobic
anticancer drugs, offering versatility and precision, has garnered
increasing attention (49,50). Here, an
H2O2-responsive DA-B-DEX polymeric micelles
loading GNE-477 system was engineered, demonstrating not only the
therapeutic effect of GNE-477 against OS but also improved drug
uptake and targeted efficacy in both in vitro and in
vivo settings.
The GNE-477-loaded DA-B-DEX delivery system presents
supplementary benefits compared with systemic administration of
GNE-477 for OS treatment. PBAE, serving as the
H2O2 recognition group, triggers degradation
following its conversion to phenol upon exposure to high
concentrations (100 μmol/l) of H2O2.
The phenol undergoes a quinone dimethylformamide rearrangement,
facilitating decomposition of the polyester main chain and
expediting degradation process. In addition, the combination of
PBAE, DA and DEX exhibits excellent synthetic feasibility and
hydrolytic properties as a drug carrier micelle (51). The small molecules generated
following its degradation are readily cleared by the body, allowing
specific targeting and drug release to OS cells (52). Here, characterization indicated
that DA-B-DEX drug-loaded micelles possessed advantages of low CMC,
high loading efficiency, nanoscale diameter, good sensitivity and
controlled release, suggesting their potential as a novel
drug-targeted delivery system. Moreover, the significantly enhanced
drug release rate and drug uptake rate corroborate the
aforementioned results.
Nanodrug carriers not only augment therapeutic
efficacy but also mitigate the toxic side effects during treatment,
rendering them the focus of increased clinical endeavors for the
development of novel drug carriers (53,54). Here, DBD and GNE-477@DBD exerted
lower toxicity against normal cells, indicating the low toxicity
and safety profile. In addition, the anti-tumor efficacy of
GNE-477@DBD was validated both in vitro and in vivo.
GNE-477@DBD inhibited tumor cell viability, arrested cell cycle in
G1 phase and induced apoptosis. To investigate the in vivo
anti-OS activity of GNE-477@DBD, a nude mouse tumor model was
established via subcutaneous injection of U2OS cells. The in
vivo outcomes illustrated that GNE-477 blocked the
PI3K/Akt/mTOR cascade reaction of tumors in vivo and
displayed notable anti-tumor effects, with GNE-477@DBD exhibiting
more positive therapeutic effects compared with the GNE-477 group,
with almost no side effect on organs. This was consistent with
findings of Xu et al (5),
who demonstrated that novel zoledronic acid-loaded hyaluronic
acid/polyethylene glycol/nano-hydroxyapatite nanoparticles
specifically inhibit proliferation of OS cells, while exerting
negligible effects on normal cells. Moreover, the aforementioned
study demonstrated that in vivo local administration of
nanoparticles markedly augmented intratumoral vascular inflammation
and facilitated tumor tissue necrosis and apoptosis. The present
nanomaterial was H2O2-responsive, rendering
it more sensitive and targeted.
The present study developed an amphiphilic
H2O2-stimulated responsive DA-B-DEX nano
micelles system for stable conveyance of hydrophobic anticancer
drugs. GNE-477@DBD nanomaterial demonstrated enhanced selectivity
towards cancer cells in comparison with free drug and potent
anticancer activity and biosafety in vivo. To the best of
our knowledge, there is no prior report of application of DA-B-DEX
polymeric micelles as carriers for anti-cancer drugs such as
GNE-477. Further exploration of synergistic effects arising from
the combination of GNE-477, a dual PI3K/mTOR inhibitor, with
nanomaterial delivery systems holds promise in surmounting the
limitations of GNE-477 in clinical applications.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SP and ZZ conceived and designed the study and wrote
the manuscript. XF and JW analyzed data. HL, YX and ZS performed
the experiments. GL and GF interpreted data. ZH, RG and ML reviewed
the manuscript and interpreted the data. YX analyzed the data and
revised the manuscript. GL and GF confirmed the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Medical
Ethics Committee of the First People's Hospital of Nanning
(approval number: 2021-076-01). The study was reported in
accordance with National Research Council's Guide for the Care and
Use of Laboratory Animals ARRIVE guidelines (arriveguidelines.org) and Basel Declaration. The
euthanasia method in this study was informed by the American
Veterinary Medical Association Guidelines for the Euthanasia of
Animals (2020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by Department of Education of
Guangxi Zhuang Autonomous Region, Project of Enhancing the Basic
Research Ability of Young Teachers in Colleges and Universities
(grant no. 2021KY0105) and Nanning First People's Hospital
Scientific Research and Technology Development Program Project
(grant no. 2021001).
References
|
1
|
Smeland S, Bielack SS, Whelan J, Bernstein
M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC,
Anninga J, et al: Survival and prognosis with osteosarcoma:
Outcomes in more than 2000 patients in the EURAMOS-1 (European and
American Osteosarcoma Study) cohort. Eur J Cancer. 109:36–50. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.
View Article : Google Scholar
|
|
3
|
Heymann MF, Brown HK and Heymann D: Drugs
in early clinical development for the treatment of osteosarcoma.
Expert Opin Investig Drugs. 25:1265–1280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar
|
|
5
|
Xu Y, Qi J, Sun W, Zhong W and Wu H:
Therapeutic effects of zoledronic Acid-loaded hyaluronic
Acid/Polyethylene Glycol/Nano-Hydroxyapatite nanoparticles on
osteosarcoma. Front Bioeng Biotechnol. 10:8976412022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao X, Wu Q, Gong X, Liu J and Ma Y:
Osteosarcoma: A review of current and future therapeutic
approaches. Biomed Eng Online. 20:242021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Yao Q, Peng Y, Dong Z, Tang L, Su
X, Liu L, Chen C, Ramalingam M and Cheng L: Identification of
Small-molecule inhibitors for osteosarcoma targeted therapy:
Synchronizing in silico, in vitro, and in vivo analyses. Front
Bioeng Biotechnol. 10:9211072022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng D, Frank AR and Jewell JL: mTOR
signaling in stem and progenitor cells. Development.
145:dev1525952018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Costa RLB, Han HS and Gradishar WJ:
Targeting the PI3K/AKT/mTOR pathway in triple-negative breast
cancer: A review. Breast Cancer Res Treat. 169:397–406. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Q, Deng Z, Zhu Y, Long H, Zhang S and
Zhao J: mTOR/p70S6K signal transduction pathway contributes to
osteosarcoma progression and patients' prognosis. Med Oncol.
27:1239–1245. 2010. View Article : Google Scholar
|
|
11
|
Miwa S, Sugimoto N, Yamamoto N, Shirai T,
Nishida H, Hayashi K, Kimura H, Takeuchi A, Igarashi K, Yachie A,
et al: Caffeine induces apoptosis of osteosarcoma cells by
inhibiting AKT/mTOR/S6K, NF-κB and MAPK pathways. Anticancer Res.
32:3643–3649. 2012.PubMed/NCBI
|
|
12
|
Zhuo BB, Zhu LQ, Yao C, Wang XH, Li SX,
Wang R, Li Y and Ling ZY: ADCK1 is a potential therapeutic target
of osteosarcoma. Cell Death Dis. 13:9542022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heffron TP, Berry M, Castanedo G, Chang C,
Chuckowree I, Dotson J, Folkes A, Gunzner J, Lesnick JD, Lewis C,
et al: Identification of GNE-477, a potent and efficacious dual
PI3K/mTOR inhibitor. Bioorg Med Chem Lett. 20:2408–2411. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye X, Ruan JW, Huang H, Huang WP, Zhang Y
and Zhang F: PI3K-Akt-mTOR inhibition by GNE-477 inhibits renal
cell carcinoma cell growth in vitro and in vivo. Aging (Albany NY).
12:9489–9499. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Shen H, Sun Q, Zhao L, Liu H, Ye
L, Xu Y, Cai J, Li Y, Gao L, et al: The new PI3K/mTOR inhibitor
GNE-477 inhibits the malignant behavior of human glioblastoma
cells. Front Pharmacol. 12:6595112021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barber NA and Ganti AK: Pulmonary
toxicities from targeted therapies: A review. Target Oncol.
6:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ulbrich K, Hola K, Subr V, Bakandritsos A,
Tucek J and Zboril R: Targeted drug delivery with polymers and
magnetic nanoparticles: Covalent and noncovalent approaches,
release control, and clinical studies. Chem Rev. 116:5338–5431.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
MacEwan SR, Callahan DJ and Chilkoti A:
Stimulus-responsive macromolecules and nanoparticles for cancer
drug delivery. Nanomedicine (Lond). 5:793–806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang H, Feng Q, Shi Y, Zhou J, Wang Q and
Zhong L: Hepatic insulin resistance induced by mitochondrial
oxidative stress can be ameliorated by sphingosine 1-phosphate. Mol
Cell Endocrinol. 501:1106602020. View Article : Google Scholar
|
|
20
|
Do MH, Phan NH, Nguyen TD, Pham TT, Nguyen
VK, Vu TT and Nguyen TK: Activated carbon/Fe(3)O(4) nanoparticle
composite: Fabrication, methyl orange removal and regeneration by
hydrogen peroxide. Chemosphere. 85:1269–1276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng Z, Rong Y, Teng Y, Mu J, Zhuang X,
Tseng M, Samykutty A, Zhang L, Yan J, Miller D, et al:
Broccoli-Derived nanoparticle inhibits mouse colitis by activating
dendritic cell AMP-Activated protein kinase. Mol Ther.
25:1641–1654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dashamiri S, Ghaedi M, Asfaram A, Zare F
and Wang S: Multi-response optimization of ultrasound assisted
competitive adsorption of dyes onto Cu (OH)2-nanoparticle loaded
activated carbon: Central composite design. Ultrason Sonochem.
34:343–353. 2017. View Article : Google Scholar
|
|
23
|
Hou X, Lin H, Zhou X, Cheng Z, Li Y, Liu
X, Zhao F, Zhu Y, Zhang P and Chen D: Novel dual ROS-sensitive and
CD44 receptor targeting nanomicelles based on oligomeric hyaluronic
acid for the efficient therapy of atherosclerosis. Carbohydr Polym.
232:1157872020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S, Luo L, He Y, Li R, Xiang Y, Xing Z,
Li Y, Albashari AA, Liao X, Zhang K, et al: Dental pulp stem
cell-derived exosomes alleviate cerebral ischaemia-reperfusion
injury through suppressing inflammatory response. Cell Prolif.
54:e130932021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He F and Zuo L: Redox roles of reactive
oxygen species in cardiovascular diseases. Int J Mol Sci.
16:27770–27780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon JH, Lee NG, Kang AR, Ahn IH, Choi IY,
Song JY, Hwang SG, Um HD, Choi JR, Kim J, et al: JNC-1043, a novel
podophyllotoxin derivative, exerts anticancer drug and
radiosensitizer effects in colorectal cancer cells. Molecules.
27:2022. View Article : Google Scholar
|
|
27
|
Behera SS, Pramanik K and Nayak MK: Recent
advancement in the treatment of cardiovascular diseases:
Conventional therapy to nanotechnology. Curr Pharm Des.
21:4479–4497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshida K, Ono T, Dairaku T, Kashiwagi Y
and Sato K: Preparation of hydrogen peroxide sensitive nanofilms by
a Layer-by-Layer technique. Nanomaterials (Basel). 8:9412018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumari P, Rompicharla SVK, Muddineti OS,
Ghosh B and Biswas S: Transferrin-anchored poly(lactide) based
micelles to improve anticancer activity of curcumin in hepatic and
cervical cancer cell monolayers and 3D spheroids. Int J Biol
Macromol. 116:1196–1213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiran Rompicharla SV, Trivedi P, Kumari P,
Ghanta P, Ghosh B and Biswas S: Polymeric micelles of
suberoylanilide hydroxamic acid to enhance the anticancer potential
in vitro and in vivo. Nanomedicine (Lond). 12:43–58. 2017.
View Article : Google Scholar
|
|
31
|
Son GM, Kim HY, Ryu JH, Chu CW, Kang DH,
Park SB and Jeong YI: Self-assembled polymeric micelles based on
hyaluronic acid-g-poly(D,L-lactide-co-glycolide) copolymer for
tumor targeting. Int J Mol Sci. 15:16057–16068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo L, He Y, Jin L, Zhang Y, Guastaldi FP,
Albashari AA, Hu F, Wang X, Wang L, Xiao J, et al: Application of
bioactive hydrogels combined with dental pulp stem cells for the
repair of large gap peripheral nerve injuries. Bioact Mater.
6:638–654. 2021.
|
|
33
|
Oerlemans C, Bult W, Bos M, Storm G,
Nijsen JF and Hennink WE: Polymeric micelles in anticancer therapy:
Targeting, imaging and triggered release. Pharm Res. 27:2569–2589.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nam SH, Lee SW, Lee YJ and Kim YM: Safety
and tolerability of weekly Genexol-PM, a Cremophor-Free polymeric
micelle formulation of paclitaxel, with carboplatin in gynecologic
cancer: A phase I study. Cancer Res Treat. 55:1346–1354. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Liu Y, Zang J, Abdullah AAI, Li Y
and Dong H: Design strategies and applications of ROS-Responsive
phenylborate Ester-based nanomedicine. ACS Biomater Sci Eng.
6:6510–6527. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoshida K, Awaji K, Shimizu S, Iwasaki M,
Oide Y, Ito M, Dairaku T, Ono T, Kashiwagi Y and Sato K:
Preparation of microparticles capable of glucose-induced insulin
release under physiological conditions. Polymers (Basel).
10:11642018. View Article : Google Scholar
|
|
37
|
Council NR, Earth Do, Studies L, Research
IfLA, Care CftUotGft and Animals UoL: Guide for the care and use of
laboratory animals. 2010.
|
|
38
|
Ovsianikov A, Deiwick A, Van Vlierberghe
S, Dubruel P, Moller L, Drager G and Chichkov B: Laser fabrication
of three-dimensional CAD scaffolds from photosensitive gelatin for
applications in tissue engineering. Biomacromolecules. 12:851–858.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oommen OP, Duehrkop C, Nilsson B, Hilborn
J and Varghese OP: Multifunctional hyaluronic acid and chondroitin
sulfate nanoparticles: Impact of glycosaminoglycan presentation on
receptor mediated cellular uptake and immune activation. ACS Appl
Mater Interfaces. 8:20614–20624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Neves AR, Queiroz JF, Costa Lima SA,
Figueiredo F, Fernandes R and Reis S: Cellular uptake and
transcytosis of lipid-based nanoparticles across the intestinal
barrier: Relevance for oral drug delivery. J Colloid Interface Sci.
463:258–265. 2016. View Article : Google Scholar
|
|
41
|
Zamani E, Johnson TJ, Chatterjee S,
Immethun C, Sarella A, Saha R and Dishari SK: Cationic π-Conjugated
polyelectrolyte shows antimicrobial activity by causing lipid loss
and lowering elastic modulus of bacteria. ACS Appl Mater
Interfaces. 12:49346–49361. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
43
|
Van Poucke C, Verdegem E, Mangelinckx S
and Stevens CV: Synthesis and unambiguous NMR characterization of
linear and branched N-alkyl chitosan derivatives. Carbohydr Polym.
337:1221312024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Burns J, Wilding CP, L Jones R and H Huang
P: Proteomic research in sarcomas-current status and future
opportunities. Semin Cancer Biol. 61:56–70. 2020. View Article : Google Scholar :
|
|
45
|
Musat V, Anghel EM, Zaharia A, Atkinson I,
Mocioiu OC, Busila M and Alexandru P: A Chitosan-Agarose
Polysaccharide-Based hydrogel for biomimetic remineralization of
dental enamel. Biomolecules. 11:11372021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yuan Z, Nie H, Wang S, Lee CH, Li A, Fu
SY, Zhou H, Chen L and Mao JJ: Biomaterial selection for tooth
regeneration. Tissue Eng Part B Rev. 17:373–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Feng P, Wu P, Gao C, Yang Y, Guo W, Yang W
and Shuai C: A multimaterial scaffold with tunable properties:
Toward bone tissue repair. Adv Sci (Weinh). 5:17008172018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li CJ, Liu XZ, Zhang L, Chen LB, Shi X, Wu
SJ and Zhao JN: Advances in bone-targeted drug delivery systems for
neoadjuvant chemotherapy for osteosarcoma. Orthop Surg. 8:105–110.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mauro N, Utzeri MA, Cillari R, Scialabba
C, Giammona G and Cavallaro G: Cholesterol-Inulin conjugates for
efficient SN38 nuclear delivery: Nanomedicines for precision cancer
therapy. Cancers (Basel). 14:48572022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Song H, Li H, Shen X, Liu K, Feng H, Cui
J, Wei W, Sun X, Fan Q, Bao W, et al: A pH-responsive
cetuximab-conjugated DMAKO-20 nano-delivery system for overcoming
K-ras mutations and drug resistance in colorectal carcinoma. Acta
Biomater. 177:456–471. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang X, Wu J, Lv R, Bai Y, Wang C, Zhang F
and Liu Z: Bioinspired hydrogen peroxide-activated nanochannels and
their applications in cancer cell analysis. Anal Chem.
94:6234–6241. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ma L, Zou X and Chen W: A new X-ray
activated nanoparticle photosensitizer for cancer treatment. J
Biomed Nanotechnol. 10:1501–1508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Marosfoi MG, Korin N, Gounis MJ, Uzun O,
Vedantham S, Langan ET, Papa AL, Brooks OW, Johnson C, Puri AS, et
al: Shear-Activated nanoparticle aggregates combined with temporary
endovascular bypass to treat large vessel occlusion. Stroke.
46:3507–3513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Meng Y, Chen S, Wang C and Ni X: Advances
in composite biofilm biomimetic nanodrug delivery systems for
cancer treatment. Technol Cancer Res Treat.
23:153303382412502442024. View Article : Google Scholar : PubMed/NCBI
|