The liver, a central immunological organ, has a
significant role in deoxidation, storing glycogen, secreted protein
synthesis, body metabolism, secreting and excreting bile,
detoxifying, and the maintenance of coagulation and anticoagulation
(1). Chronic and acute liver
diseases cause irreversible liver damage and, eventually, lead to
liver failure (2). Nonalcoholic
fatty liver disease (NAFLD), also termed metabolic
dysfunction-associated fatty liver disease, is one of the most
common chronic liver diseases worldwide (3). The development of NAFLD is fueled
by a sedentary lifestyle, low frequency of physical activity,
unhealthy diet and excessive calorie intake (4). NAFLD is characterized by fat
accumulation in the liver accounting for >5% of hepatic tissue
weight (fatty liver), as well as liver inflammation, which is not
caused by alcohol abuse (3).
NAFLD can range from the simple fatty liver (SFL) to advanced
stages of nonalcoholic steatohepatitis (NASH), which may lead to
liver fibrosis (LF), liver cirrhosis (LC), liver cancer and liver
failure (5). A total of 51% of
obese adults worldwide suffer from NAFLD, with higher incidences in
men than women (6,7). The prevalence of NAFLD among adults
in the general population is 30%, but this is increased to up to
70% in patients with type 2 diabetes mellitus (T2DM) (8,9).
Accumulating evidence suggests that NAFLD is tightly associated
with insulin resistance, obesity, metabolic syndromes (MetS) and
diabetes, and plays a key role in the development of extrahepatic
clinical outcomes, such as heart disease and malignancies, which
are the most common causes of death in patients with NAFLD
(10-13).
NAFLD development involves an aberrant increase in
triacylglycerol (TG) levels and a reduction of TG output. Aberrant
increases in TGs are caused by hyperglycemia, hyperinsulinemia,
obesity and fructose ingestion through the increase of lipogenesis
(14,15). Reduction of TG output represents
decreasing very low-density lipoprotein (LDL) and decreasing
β-oxidation in mitochondria (16). SFL can be induced by aberrant
accumulation of TGs. However, SFL is not accompanied by fibrosis,
inflammation or hepatocyte injury and can be attenuated by
lifestyle modifications. It could develop into NASH after long-term
hepatic steatosis, followed by fibrosis, cirrhosis and hepatic
carcinoma. In addition, insulin resistance, inflammatory cytokines,
oxidative stress, endoplasmic reticulum (ER) stress and lipid
peroxidation can also contribute to the development of NAFLD.
At present, the best treatment to improve NAFLD is
lifestyle modification to achieve weight loss (18). It is well established that the
risk of hepatic steatosis increases after consuming high-calorie,
high-sugar and high-fat foods (19). A notable reduction in NAFLD
activity score, remission of steatohepatitis and regression of
fibrosis were noted in patients with weight loss and diet
alteration (20). In addition,
patients with NAFLD are advised to consume the Mediterranean diet
in the European Association for the Study of the Liver, European
Association for the Study of Diabetes and European Association for
the Study of Obesity Clinical Practice Guidelines for the
management of NAFLD (18). The
Mediterranean diet, a nutritional model, began in the regions
surrounding the Mediterranean sea and consisted of eating nuts,
legumes, fruits, vegetables, fish, white meat and olive oil, which
are rich in monounsaturated fatty acids (MUFAs) and polyunsaturated
fatty acids (PUFAs). The diet also recommends drinking red wine in
moderation, and eating limited amounts of red meat, processed meats
and sweets. Minimizing the intake of processed and high-fructose
food is a feature of the Mediterranean diet, which leads to
decreased ingestion of advanced glycation end products (AGEs)
(21,22). AGEs are associated with diabetes,
are increased in patients with NASH and are positively associated
with insulin resistance (23,24). Fructose promotes alterations in
the gut microbiota, increased intestinal permeability, exacerbated
lipid peroxidation and hepatic steatosis, and increased tumor
necrosis factor-α (TNF-α) production (25). The risk of NAFLD is negatively
associated with omega-3 PUFAs, indicating that patients with NAFLD
and NASH consume fewer omega-3 PUFAs (26). Omega-3 PUFA-rich diets reportedly
reduce apoptosis, the content and activity of fatty acid synthase
and inflammation, and ameliorate glucose homeostasis, thereby
decreasing the development of NAFLD (27). Furthermore, they also correct
glycemia levels, oxidative stress status and metabolic endotoxemia
(28). Individuals who adopted
the Mediterranean diet showed a remarkable reduction in circulating
oxidized LDL and inflammatory markers (29). It has been demonstrated that the
Mediterranean diet reduces the risk of T2DM, obesity, cancer and
cardiovascular diseases, all of which are related to NAFLD
(30-33). In general, the Mediterranean diet
may influence NAFLD via mechanisms including lipid lowering,
antioxidant and anti inflammatory effects. However, the
Mediterranean diet may not be readily accepted or adapted to by
individuals from different countries (34), and may therefore not be a viable
therapy for all patients with NAFLD.
Exercise notably decreases steatosis and the risk of
NAFLD developing into NASH (35,36). Hashida et al (37) suggested that exercise reduced
20-30% of intrahepatic TG by gathering and analyzing 24 studies. A
total of 220 obese patients with NAFLD were randomly divided into
the following groups: i) No exercise (control); ii) moderate
exercise; and iii) vigorous exercise. The individuals were then
evaluated after the 1-year exercise intervention and decreased
waist circumference, intrahepatic TG content, blood pressure and
hepatic fat accumulation were noted in the two exercise groups
compared with the control group (38). A total of 154 patients with NAFLD
were assigned to the intervention group or the control group and
were assessed after a 12-month regular exercise intervention. Wong
et al (39) used this
method to study the efficacy of exercise intervention in non-obese
patients with NAFLD. The results demonstrated that NAFLD could be
relieved in 67% of non-obese patients after exercise intervention
(39). In a maternal
western-style-diet (WSD)-induced obesity mouse model, Kasper et
al (40) discovered that
maternal exercise prevented WSD-induced hepatic steatosis in obese
dams by increased hepatic β-oxidation and suppression of
lipogenesis through activating the adenosine
5'-monophosphate-activated protein kinase (AMPK) and
peroxisome-proliferator-activated-receptor-γ-coactivator-1α
(PGC-1α) signaling pathways. In addition, the offspring also
displayed increased AMPK-PGC1α activity and were protected against
WSD-induced fat accumulation and steatosis of the liver in later
life (40). Furthermore,
Battista et al (41)
described a significant reduction of blood pressure, insulin
resistance and intrahepatic fat after exercise-training
interventions, by analyzing 54 articles. However, the benefits of
exercise are only established after relatively long periods of
time, which causes the majority of individuals to give up in the
process. By contrast, bariatric surgery takes less time to achieve
significant weight loss. In addition, bariatric surgery could not
only achieve long-term weight loss but also attenuate hypertension,
T2DM, insulin resistance, fibrosis and other high-risk factors of
NAFLD (18,42,43).
Laparoscopic sleeve gastrectomy and Roux-en-Y
gastric bypass are the most common means of bariatric surgery that
aim to shrink the stomach. The mechanisms by which they attenuate
NAFLD appear to be reduction of oxidative stress and inflammation.
Liver histological features, oxidative stress and inflammation in
patients with NAFLD were markedly ameliorated 1 year
post-laparoscopic sleeve gastrectomy surgery (44). Laparoscopic sleeve gastrectomy
led to histologic improvement and later reshaped cellular
interactions, thereby reducing liver damage of NAFLD (45).
Roux-en-Y gastric bypass ameliorated hepatic
steatosis in diet-induced obese mice that was mediated by
mechanistic targeting of the mTOR/AKT2/insulin-induced gene 2
signaling pathway (46). NAFLD
was weakened via weight loss and a decrease in steatosis after
Roux-en-Y gastric bypass treatment (47). Roux-en-Y gastric bypass relieved
diabetes, hypertension and NAFLD. However, it frequently caused
diseases related to the lack of nutrients (48).
Duodenal switch and biliopancreatic diversion lead
to notable weight loss, as well as amelioration or resolution in
obesity-related diseases (49).
Several trials proposed that biliopancreatic diversion reversed
whole-body insulin resistance and reduced inflammation, thereby
alleviating NASH (50,51). Liver function and inflammation of
morbidly obese patients were upgraded by biliopancreatic diversion
with duodenal switch (52,53). Furthermore, biliopancreatic
diversion altered insulin sensitivity, and accordingly, reducing
insulin resistance in men with morbid obesity (54).
In addition, duodenal-jejunal bypass, a key
component of bariatric surgery, amended lipid metabolism,
inflammatory responses and insulin sensitivity in diet-induced
obese rats with NASH (55).
Furthermore, bariatric surgery could also regulate other
NAFLD-related factors, such as intraluminal ileal environment, the
gut microbiota and the bile acid (BA) ratio (56,57). However, costs, patient
acceptability and side effects, including vomiting, diarrhea,
infection and even death, limit the implementation of bariatric
surgery (43). Biologically
active substances, which are cheaper and have fewer side effects,
are attractive treatment methods for NAFLD.
Due to the antioxidant, anti-inflammatory, hormonal
and metabolism regulatory functions of peptides, they have
attracted increased attention. In a high-fat diet (HFD)-induced rat
model of NAFLD, lipid accumulation, insulin resistance and
oxidative stress were reduced after treatment with corn peptides
(CPs). Yao et al (58)
found for the first time that CPs could be a potential therapy for
NAFLD by regulating anomalous lipid metabolism in vivo and
in vitro by inhibiting ER stress via activating the
AMPKα/Sirtuin 1 pathway. Lupin protein hydrolysates decreased lipid
accumulation, as well as the hepatic inflammatory and oxidant
situation of WSD-induced NAFLD mice (59). In HFD-induced NAFLD mice,
intraperitoneally injected peptide that was synthesized from potato
protein hydrolysate could reduce hepatic fat deposition and
inflammation by activating the AMPK signaling pathway (60). After dyslipidemia was treated
with Ganoderma lucidum polysaccharide peptide for a month,
hepatic dysfunction, steatosis and insulin resistance were
ameliorated in NAFLD mice. Zhong et al (61) demonstrated that the improvement
was achieved by controlling BA synthesis via the farnesoid X
receptor-small heterodimer partner/fibroblast growth factor
pathway. In vitro, Ganoderma lucidum polysaccharide
peptide also reduced the accumulation of lipid droplets and the TG
content (61). Lipid
peroxidation, oxidative stress and inflammation were reduced in
NAFLD mice with monkfish peptide treatment. The liver function was
repaired by activating the AMPK and nuclear factor
erythroid-2-related factor 2 (Nrf2) pathways (62). Furthermore, R-Tf-DLP4 (a
mitochondrial voltage-dependent anion channel 1-based peptide) and
VHVV (a lipolysis-stimulating peptide) inhibited hepatic apoptosis,
hepatic lipid accumulation, inflammation and fibrosis in a mouse
model, thereby arresting NASH progression (63,64). However, at present, only a small
number of clinical trials is available to support the
aforementioned findings.
Glucagon-like peptide 1 (GLP-1) was able to induce
insulin secretion, and GLP-1 analogues or receptor agonists have
been approved for treating T2DM and obesity. Liraglutide, a GLP-1
analogue, decreased inflammation and fat accumulation, and
normalized glycemic parameters and the composition of the gut
microbiota in HFD-induced NAFLD mice (65). Liraglutide restored autophagic
flux by the GLP-1 receptor-transcription factor EB pathway in
vivo and in vitro, thereby alleviating liver steatosis
(66). On the other hand, it
targeted the renin-angiotensin system through the PI3K/AKT pathway
and ameliorated NAFLD (67).
After subcutaneous injections of liraglutide, NAFLD was attenuated
in 39% of subjects but diarrhea, constipation and loss of appetite
also occurred in the liraglutide treatment group (68). Another GLP-1 receptor agonist,
Dulaglutide, reduced lipid accumulation and normalized the liver
function of patients with NAFLD in a randomized controlled trial
(69). Semaglutide treatment
reduced NASH and had a positive impact on NAFLD therapy through
decreasing lipid accumulation and inflammation, and regulating
blood sugar and liver function (70-72). However, semaglutide led to
vomiting and constipation (70).
Jin et al (73) developed
a GLP-1 receptor agonist candidate, AWRK6 (a synthetic peptide),
and researched its effect in treating NAFLD. They observed that
AWRK6 ameliorated obesity, hepatic steatosis, abnormal lipid and
glucose metabolism in high energy diet-induced NAFLD mice. These
changes were mediated through the PI3K/AKT/AMPK/acetyl-CoA
carboxylase α (ACC) signaling pathway (73). However, another study showed that
the GLP-1 analogue did not reduce the risk of NAFLD development
compared with insulin treatment (74). Hence, further studies are
required to clarify the role of GLP-1 analogues in NAFLD
treatment.
Alkaloids are a class of natural alkaline organic
compounds containing nitrogen that are extracted mainly from
plants, and have been shown to attenuate NAFLD through various
mechanisms, such as lipid lowering, anti-inflammation, autophagy
regulation and anti-oxidative stress. Berberine (BBR;
C20H18NO4), extracted from the
plants Coptidis chinensis Franch, Phellodendron
chinense Schneid or the berberis genus, is used for the
treatment of cardiovascular disease, hypercholesterolemia,
hypertension and diabetes (75,76). The functions of BBR include
lipid-lowering, regulation of metabolism disorder and enhancement
of insulin sensitivity, indicating that it would be a promising
strategy for treating NAFLD (77). A meta-analysis of six random
clinical trials showed that BBR was beneficial for liver function
and blood lipid improvement in patients with NAFLD (78). Mechanically, in preclinical
studies, BBR has been found to exert protective effects through
various pathways. It activates the AMPK signaling pathway (79,80), thereby inhibiting the expression
of stearoyl CoA desaturase-1, a rate-limiting enzyme involved in
de novo fatty acid synthesis. In addition, BBR also exhibits
anti-inflammatory effects in liver and adipose tissue by blocking
NLR family pyrin domain containing 3 (NLRP3) inflammasome assembly
(81) and modulating macrophage
infiltration and polarization (79). Anti-oxidative stress may also
contribute to the hepatic protective effect of BBR. An in
vitro study demonstrated that BBR activated the Nrf2 signaling
pathway and protected against oxidative stress in free fatty
acid-exposed Huh7 cells (82).
Besides these classic biological pathways, BBR regulated the
metabolism in HFD-treated rats or rats with T2DM via the intestinal
microflora (83-85), indicating that the compound may
modulate lipid metabolism by directly affecting the gut microbiota.
Therefore, BBR is a multi-target compound with hepatic protective
effects; however, its therapeutic potential on NAFLD is still being
elucidated. To date, two clinical trials have been conducted to
further observe the effect of BBR on hepatic steatosis markers, the
cardiometabolic profile and the gut microbiota profile of NAFLD
(clinical trial no. NCT05523024) and evaluate the efficacy and
safety of BBR in NASH (clinical trial no. NCT03198572) are in
progress. Several other alkaloids have also been reported to
exhibit hepatic protective effects. Kukoamine A, a spermine
alkaloid extracted from Lycium chinense Miller, inhibited
the expression of proteins related to the biosynthesis of
cholesterol and fatty acids, attenuated inflammation and hepatic
oxidative stress, thereby attenuating fatty liver and liver injury
in HFD-fed mice (86).
Trigonelline, extracted from fenugreek (Trigonella
foenum-graecum L.) seeds, prevented hepatic steatosis in
high-fat and high-cholesterol diet-fed mice via regulating
autophagy and ER stress (87).
Liensinine, an isoquinoline alkaloid extracted from the seed embryo
of Nelumbo nucifera Gaertn, reduced lipid accumulation in
the liver and improved liver function in HFD-induced NAFLD mice via
suppressing oxidative stress and inflammation (88). However, studies onto these
alkaloids are still in the preclinical stage and their exact effect
on NAFLD and the underlying mechanisms remain to be verified.
Polyphenols, which are mainly derived from plants,
have been shown to decrease blood lipids, lower blood pressure,
have anti-oxidative effects and inhibit tumor growth (89). Curcumin is extracted from
turmeric and has antioxidant and anti-inflammatory activities
(90,91). A total of two randomized
controlled trials revealed that curcumin reduced blood fat and
lipid accumulation, and ameliorated obesity, liver function and
insulin metabolism in patients with NAFLD (92,93). Curcumin moderated NAFLD by
reducing blood glucose, oxidative stress and inflammation (94). Furthermore, Mahmoudi et al
(95) demonstrated that curcumin
may treat NAFLD via 14 target genes, including cytochrome P450 1A2,
NFE2 Like BZIP Transcription Factor 2 and peroxisome
proliferator-activated receptor α (PPARα), through analyzing
curcumin-interacting proteins and NAFLD-associated genes using a
Venn diagram. Resveratrol is widely found in peanuts, mulberries
and grapes and ameliorates atherosclerosis, MetS and NAFLD
(96-99). The blood fat, hepatic steatosis,
hepatic apoptosis, liver function, insulin sensitivity and insulin
metabolism of patients with NAFLD were ameliorated after
supplementation of resveratrol (98,100). Tejada et al (101) proposed that resveratrol reduces
hepatic lipid accumulation and regulates glycaemic and lipid
metabolism via sirtuin 1/AMPK/nuclear factor ĸB (NF-ĸB) signaling
pathways. Resveratrol reduced the HFD-induced methylation of the
Nrf2 promoter and attenuated NAFLD by reducing the expression of
lipogenesis-related genes and reactive oxygen species production
via the Nrf2 signaling pathway (102). Quercetin, a polyphenolic
flavonoid, has anti-apoptosis, antioxidant, anti-inflammation and
anticancer activities, and may be a potential alternative
preventative agent for NAFLD (103). Quercetin activated the
farnesoid X receptor 1/Takeda G-protein-coupled receptor 5
signaling pathways, thus inhibiting oxidative stress, reducing
inflammation and decreasing lipid accumulation (104). Furthermore, quercetin restored
lipid metabolism and the gut microbiota balance through the
toll-like receptor 4/NF-κB signaling pathway (105). Quercetin targeted lipid
metabolism to ameliorate NAFLD via the inositol-requiring enzyme-1α
(IRE1α)/X-box binding protein 1 (XBP1) pathway (106). In addition, regulation of
apoptosis and BA metabolism was shown to be associated with the
attenuation of NAFLD by quercetin (107). Mangiferin originates from mango
leaves and has anti-diabetic, anti-oxidative, anti-inflammatory and
anti-cancer activity (108,109). It activated the AMPK signaling
pathway, accordingly reducing insulin resistance and inflammation
in HFD-induced NAFLD mice (110). By activating the AMPK pathway,
epigallocatechin-3-gallate, a main active ingredient of green tea
polyphenols, reduced cellular lipid accumulation, thus mitigating
NAFLD (111). In addition,
polyphenols activated the AMPK pathway and promoted the expression
of PPARα, thereby increasing lipid catabolic metabolism. However,
in specific cases (high pH and transition metals in the presence of
O2), polyphenols were shown to aggravate oxidative
stress (112).
Silymarin is obtained from the seeds of Silybum
marianum and was shown to be beneficial in the treatment of liver
diseases (113), and it is the
active ingredient of the approved drug Legalon®. In an
infantile model of NASH, silymarin reduced inflammation and
apoptosis, and normalized glycemia, lipid profile, liver function
and liver fibrosis (114). In a
mouse model of NAFLD, hepatic steatosis was attenuated by silymarin
via regulating lipid metabolism and oxidative stress (115). Mechanistically, silymarin
enhanced FXR transcriptional activity and inhibited NF-κB
signaling, and ameliorated insulin resistance, inflammation and the
BA ratio in HFD-fed mice (116). In clinical trials, silymarin
reduced liver fibrosis in adult patients with biopsy-proven NASH
and was safe and well tolerated (117). However, in another clinical
trial, after silymarin treatment, the NAS scores were not reduced
(117). Silymarin plus vitamin
C, vitamin E, coenzyme Q10 and selenomethionine has been proved to
aid the recovery of liver function in patients diagnosed with NAFLD
(118). However, allergic skin
rashes and gastrointestinal disturbances have been reported as side
effects of silymarin (116). As
the effective component of silymarin, silibinin has been shown to
be beneficial in NAFLD treatment in several studies. In
methionine-choline deficiency (MCD)-induced NASH rats, silibinin
showed preventive effects in hepatic steatosis, fibrosis and
inflammation through upregulating Nrf2 target genes and inhibiting
the NF-ĸB signaling pathway (119). The AMPKα signaling pathway was
activated by silibinin, thereby inhibiting lipogenesis and
promoting fatty acid consumption in an NAFLD hamster model
(120). In NASH models,
silibinin regulated the caspase 8 and Fas-associated protein with
death domain-like apoptosis regulator/c-Jun N-terminal kinase (JNK)
pathway and moderated lipid accumulation and oxidative stress
(121). However, currently,
clinical trials of silibinin are lacking.
The regulation of gut microbiota via the
administration of antibiotics may be a promising approach for NAFLD
treatment (122). Rifaximin, a
gut-selective antibiotic, mainly has an effect on the gut
microflora. Rifaximin improves intestinal permeability by repairing
zonula occludens-1 disruption and decreasing portal endotoxin in
NASH mice that were induced by a choline-deficient/l-amino acid
diet (123). Rifaximin
decreased lobular inflammation, hepatic steatosis and fibrogenesis,
as well as accumulation of BAs and deoxycholic acid in mice fed an
MCD diet, and recovered MCD-induced gut microbiome changes.
Therefore, Jian et al (124) proposed that rifaximin treatment
may be a potential NASH therapy. In an observational study,
rifaximin inhibited endotoxin-induced inflammation in patients with
NAFLD (125). Rifaximin reduced
insulin resistance and inflammation in a randomized controlled
trial and rifaximin was reportedly safe and effective in treating
NAFLD (126). Solithromycin, a
highly potent antibiotic, relieved NASH by recovering liver
function and targeting intestinal microbiomes (127,128). In addition, the combination of
polymyxin B and neomycin reduced lipid accumulation and ameliorated
the intestinal barrier in diet-induced NAFLD mice (129). Numerous studies have shown that
antibiotics mainly act to eliminate harmful microbiota and are
effective in treating liver disease. However, due to harmful side
effects, including increased irreversible peripheral neurotoxicity,
long-term clinical use of antibiotics is not recommended (130).
Fatty acids play critical roles in regulating cell
proliferation, inflammation, energy and metabolic homeostasis.
Caffeic acid reduces blood fat and lipid accumulation through
upregulating AMPK/ACC and downregulating sterol-regulatory element
binding protein-1 in damaged HepG2 cells (131). MUFAs, particularly oleic acid,
have shown a dual role in previous studies. Oleic acid could
attenuate apoptosis and activation of stress-related kinases,
reduce lipid levels and inflammation but induce hepatic steatosis
(132-134). Palmitic acid triggered
apoptosis, activation of ER stress and insulin resistance, thereby
exacerbating the development of NAFLD (132). Furthermore, MUFAs including
myristoleic acid, palmitoleic acid and oleic acid were
significantly increased in NAFLD (135). However, the effects of MUFA on
NAFLD require further exploration. The beneficial effects of PUFAs
have been reported, including regulation of inflammation,
steatosis, plasma lipid and obesity (136-140). PUFAs are generally divided into
n-3 and n-6, with n-3 PUFAs functioning to limit hepatic TG
storage. A low intake of n-3 contributed to hepatic steatosis and
insulin resistance, and led to MetS and NAFLD. NAFLD and MetS were
ameliorated after adequate n-3 supplementation (141). Diets with a high n-6 to n-3
ratio induced liver function decline, lipid accumulation, oxidative
stress and inflammation. This was induced by insufficient n-3
intake and excessive n-6 intake (142). In a cross-sectional study, Da
Silva et al (143) found
that >80% of individuals (74 patients with NAFLD) did not ingest
sufficient n-3. Supplementation with n-3 PUFA (>2 g/d) appeared
to be a more effective way to control MetS and NAFDL (144).
Vitamins are necessary for maintaining physical
health with an adjustment function. Vitamin E, an effective
antioxidant agent, was advised for managing NAFLD in the guidance
of the National Institute for Health and Care Excellence and the
American Association for the Study of Liver Diseases (42,145). It ameliorated oxidative stress,
inflammation and fibrosis in HFD-fed mice, accordingly delaying the
progression of NAFLD (146). In
addition, hepatic steatosis, hepatocyte apoptosis and inflammation
of adults with NAFLD were weakened by vitamin E and vitamin E was
well-tolerated (147-149). Vitamin E reduced liver
steatosis and lipid accumulation in an NAFLD model via activating
the Nrf2/carboxylesterase 1 signaling pathway (150). Furthermore, combination
treatment of vitamin E and other drugs, including atorvastatin,
decreased the risk of NAFLD (151-153). However, the incidence of
bladder cancer and prostate cancer increased after vitamin E
administration (154,155). Vitamin D was indicated to have
an important role in regulating phosphorus and calcium homeostasis
and in the pathogenesis of T1DM, T2DM and MetS (156,157). Low levels of vitamin D were
associated with the development of NAFLD and an increased risk for
NAFLD (158-160). Liu et al (161) demonstrated that NAFLD was
exacerbated through Toll-like receptor (TLR) activation under
deficiency of vitamin D. Intestinal function was regulated and
oxidative stress and lipid accumulation were reduced by vitamin D
in HFD-induced NAFLD mice. Liu et al (162) demonstrated that the development
of NAFLD was prevented by vitamin D through the p53 pathway.
Vitamin D enhanced liver function and decreased lipid accumulation,
insulin resistance, blood fat and oxidative stress in patients with
NAFLD (163). Furthermore,
mechanisms by which vitamin D worked on NAFLD included
anti-inflammation, anti fibrosis and regulating metabolism
(164). Xie et al
(165) summarized that
participants with higher serum vitamin C had a lower prevalence of
LF, LC and NAFLD. An inverse association existed between serum
vitamin C and LF, LC and NAFLD (165). Liver function, insulin
sensitivity and intestinal microbiota diversity of patients with
NAFLD were increased after daily intake of vitamin C (166). Gu et al (167) pointed out that vitamin C
attenuated HepG2 cell stresses caused by TNF-α via activating the
fibroblast growth factor (FGF)21/FGF receptor 2/adiponectin
pathway. This may be a mechanism by which vitamin C treats NAFLD
(167). Due to the limited
number of trials in humans studying the role of vitamins in NAFLD
and the lack of consensus on the optimal level of specific vitamins
in the scientific community, the use of vitamins is controversial
in NAFLD treatment (164).
Probiotics are active microorganisms that are
beneficial to the host by changing the composition of the
microbiome in the host, and can improve dysbiosis and prognosis in
patients with NAFLD (168). The
main mechanisms are regulating intestinal barrier function and gut
microbiota (130). In addition,
oxidative stress, inflammation, fibrosis and carcinogenesis were
reduced by probiotic treatment in a mouse model that imitated the
features of human NAFLD (169).
Xue et al (170) showed
that probiotics ameliorated the gut microbiota structure and
hepatic pathology and further delayed the progression of NAFLD via
downregulating lipopolysaccharide/TLR4 signaling in high-sugar and
high-fat diet-fed rats. Clinical trials demonstrated that
probiotics ameliorated lipid accumulation, liver function, blood
fat, insulin resistance and obesity (171,172). Synbiotics consisting of
probiotics and prebiotics could stimulate the growth of probiotics
and were effective in treating obesity, T2DM, insulin resistance
syndrome and NAFLD (173).
After treatment with synbiotics, hepatic fibrosis, inflammation and
lipid accumulation were reduced and liver function was ameliorated
in patients with NAFLD (174,175). Furthermore, synbiotics are
conducive to modifying the gut bacterial flora (176). Further studies are needed to
determine the best dose of probiotics and synbiotics (173).
Lamiaceae mostly include shrubs and herbs and have
potential in ameliorating diabetes, hyperlipidemia, obesity and
NAFLD (177-179). The hepatoprotective and
hypolipidemic functions of chia (Salvia hispanica L.) were
related to its high content of n-3, dietary fiber and phenolics.
Prevention of steatohepatitis and reduced lipids were observed in
rats that were fed a diet with chia for 4 weeks (180). After eight weeks of a
chia-supplemented diet, a study showed that fat accumulation, blood
fat and obesity were decreased in patients with NAFLD. Regression
was observed in a total of 52% of patients with NAFLD (181). Thymbra spicata L. has
antioxidant, anti-inflammatory, anti-hypercholesterolaemic and
anti-steatohepatitic potential that may be attributed to the
richness of phenolic compounds (182). The lipid accumulation,
oxidative stress and inflammation were ameliorated by Thymbra
spicata L. in NAFLD cellular models (183). Scutellaria baicalensis
and its major component, baicalin, exert antioxidant and
anti-inflammatory effects, and regulate lipid metabolism in hepatic
diseases (184). Baicalin
downregulated the sterol-regulatory element binding protein
signaling pathway and upregulated the AMPK signaling pathway,
accordingly decreasing hepatocyte lesions, and blood fat and
hepatic lipid accumulation in NAFLD rats (182). The mechanisms by which
Scutellaria baicalensis extracts treat NAFLD include
suppression of ER stress and thioredoxin-interacting protein/NLRP3
inflammasome activation, and activating the PGC-1α/PPARα signal
pathway (185,186). Due to the small sample size,
the short study time and preliminary data, the effectiveness of
labiatae plants in treating NAFLD remains to be clarified. Clinical
drugs appear to be more effective for urgent treatment. However,
currently, these medicines are undergoing clinical trials have not
been approved for NAFLD treatment.
Statins are mainly used to prevent cardiovascular
disease and decrease LDL-cholesterol levels (187). An increasing number of studies
suggested that statins were useful to reduce the risk of NAFLD. A
systematic review and meta-analysis showed that the liver
conditions of patients with NAFLD were ameliorated by treatment
with statins, and statin treatment was safe (188). Lee et al (189) also summarized that statin use
could reduce the chance of getting NAFLD and hepatic fibrosis.
Atorvastatin reduced the non-invasive score and fibrosis score,
suggesting that atorvastatin weakened NAFLD progression (190). A combination of resveratrol and
atorvastatin attenuated NAFLD by reducing fat accumulation in the
liver of mice with fatty liver (191). Reduced inflammatory markers,
decreased atherosclerosis and inhibition of cholesterol production
in the liver were observed after combined use of ezetimibe and
atorvastatin (192). However,
these combined uses to abate NAFLD lack the support of clinical
trials. Rosuvastatin can improve the histological score and
biochemical biomarkers of NAFLD (193). The combined use of rosuvastatin
and ezetimibe reduced the liver fat of participants with NAFLD
(194). In addition, the
combination of rosuvastatin, ursodeoxycholic acid and ezetimibe
decreased the fibrosis of NAFLD mice (195). Simvastatin protected mice with
a high-fat-high-carbohydrate diet from developing NAFLD by
inhibiting oxidative stress, reducing advanced lipoxidation
end-product receptors of AGEs and decreasing steatosis,
inflammatory parameters and fibrosis (196). However, statin treatment
increased the risk for T2DM, which frequently co-occurs with NAFLD,
by reducing insulin secretion and impairing insulin signal
transduction (197).
PPARs, a class of nuclear receptors, have an
important role in the treatment of NAFLD due to their function that
regulates the transcription of glucose and lipid metabolism. PPAR
agonists are traditionally used for treating MetS by lowering TG
and glucose levels. PPARα regulated the expression of liver fatty
acid binding protein, mitochondrial β-oxidation enzymes and
proteins that are related to fatty acid turnover. On the other
hand, PPARα regulated inflammation by inhibiting NF-ĸB via forming
inhibitory complexes and increasing the expression of the
inhibitory subunit of NF-ĸB α, which is the NF-ĸB inhibitory
protein (198). Wy-14643, a
powerful PPARα agonist, inhibited steatosis and restored insulin
sensitivity, as well as blood lipid and adiponectin levels, thereby
reducing NAFLD that was driven by PPAR-α dysregulation (199,200). Fenofibrate, a fibrate PPARα
agonist, is mainly considered a lipid-lowering drug. Fenofibrate
regulated the IRE1α/XBP1/JNK signaling pathway and lessened lipid
accumulation, apoptosis, inflammation and ER stress, accordingly
attenuating NAFLD in a mouse model (201). In clinical trials, fenofibrate
normalized liver enzymes and blood lipids of patients with NAFLD.
The body mass index of patients was also lowered after fenofibrate
administration (202). PPARδ,
an isoform of the PPAR family, regulated mitochondrial metabolism
and fatty acid β-oxidation, and has a potential role in fibrosis
and inflammation (203).
Clinical trials have shown that after GW501516 (a PPARδ agonist)
treatment, the incidence of cancer was increased. Therefore, NAFLD
treatment with GW501516 was abandoned (204,205). Another PPARδ agonist, GW0742,
alleviated ER stress, improved insulin sensitivity, recovered
hepatic energy metabolism and tackled hepatic inflammation,
accordingly suppressing NAFLD progression in obese mice (206). Elafibranor is an agonist of
PPARα and PPARδ. In a clinical trial, fat accumulation and
inflammation in patients with NAFLD were reduced after treatment
with elafibranor (207). PPARγ
has an essential role in regulating adipogenesis and lipid
metabolism (208).
Pioglitazone, a thiazolidinediones PPARγ agonist, ameliorated liver
function by reducing oxidative stress, inflammation and fibrosis in
HFD-induced NAFLD mice while preventing obesity via insulin
resistance regulation (209).
Several clinical trials indicated that liver function and insulin
sensitivity were recovered and inflammation and lipid accumulation
were reduced in patients with NAFLD after pioglitazone treatment
(149,210). However, pioglitazone caused
weight gain (149).
Saroglitazar, a dual PPARα/γ agonist, ameliorated insulin
sensitivity and lipid parameters. In mice with high-fat and
choline-deficient diet-induced NAFLD, liver function was recovered
and inflammation was decreased after saroglitazar treatment.
Mechanistically, saroglitazar reversed mitochondrial dysfunction
and NF-ĸB phosphorylation, thereby blocking the decrease of
antioxidant biomarkers and the increase of inflammatory markers.
Jain et al (211)
proposed that among saroglitazar, fenofibrate and pioglitazone,
saroglitazar seemed to be the most effective against NAFLD. Lipid
accumulation and insulin resistance of patients with NAFLD/NASH
were ameliorated after receiving saroglitazar (212). Lanifibranor (IVA337) is a PPAR
agonist that activates three isoforms of PPAR (α, δ and γ) and has
strong activity in NAFLD models in vitro and in vivo
(213). The results of several
preclinical models demonstrated that IVA337 normalized insulin
sensitivity and reduced lipid accumulation, inflammation and
oxidative stress. Clinical results have suggested that IVA337
improved histology in patients with NASH (214). Side effects of PPAR agonists,
including bone loss and increased risk of cancer and cardiovascular
complications, are worthy of attention (203).
Inhibition of C-C motif chemokine receptor 2 (CCR2)
reduced the accumulation, migration and infiltration of monocytes
and macrophages to the liver, which decreased hepatic damage
(215). CCR5 participates in
activation of hepatic stellate cells following liver injury,
further aggravating hepatic fibrosis (216). CVC is an oral and dual
antagonist of CCR2 and CCR5. It exerted anti-inflammatory and
antifibrotic effects in a diet-induced NAFLD mouse model and
weakened NAFLD (216). In
clinical trials, CVC ameliorated fibrosis in subjects with NAFLD
(217,218). However, the long-term benefits
of CVC are uncertain (219).
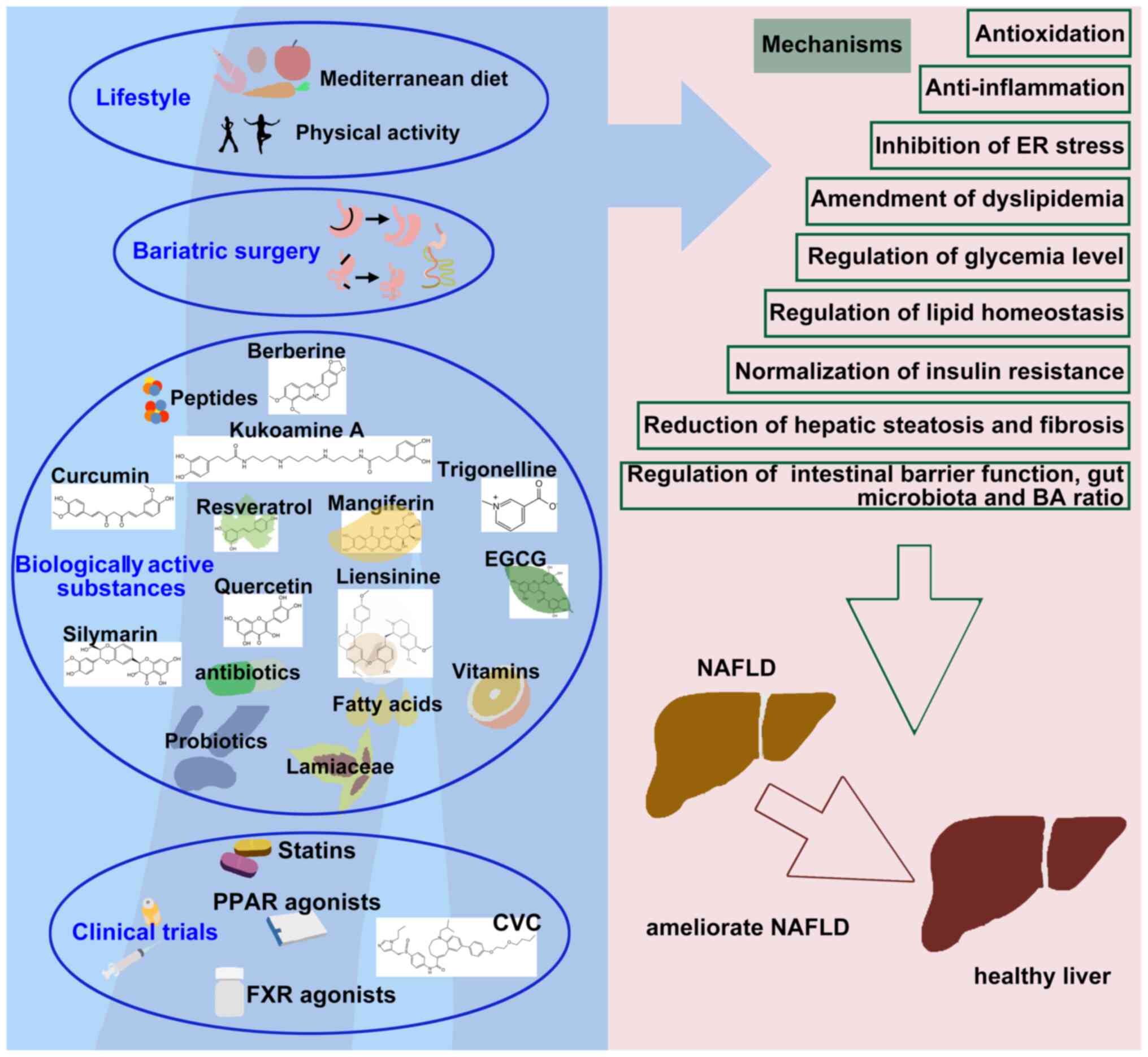
In the present review, the roles of the
Mediterranean diet, exercise, bariatric surgery, drugs and
biologically active substances in the intervention of NAFLD were
summarized, with the aim that this will aid clinical research and
disease treatment. However, in order to better restrain the
processes of NAFLD, there are various suggestions to be
considered.
The development of NAFLD representatively follows
four stages, including liver fat accumulation, early NASH, LF and
LC (227). Due to different
NAFLD stages and individual patients, treatment approaches in
patients with NAFLD should vary.
At present, single interventions for the treatment
of NAFLD appear to result in various side effects and combined use
may be the general trend. For instance, the combined use of
bicyclol and BBR was rather safe; they did not influence each other
and better ameliorated NAFLD (230).
After integrating three fibrosis datasets,
including NAFLD-induced fibrosis, Zhan et al (231) proposed that ATP Binding
Cassette Subfamily B Member 1 may be a novel anti-fibrotic target.
Upregulated CD36 expression drove lipid accumulation in human
hepatocytes and seemed to contribute to hepatic steatosis (232). Exploring new gene targets can
help develop new drugs to treat NAFLD.
Not applicable.
JS performed the literature search and selection,
analysis and writing-original draft preparation. XJ carried out the
literature search and selection and analysis. YL performed
writing-review and editing. All authors read and approved the final
manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was supported by the General Program of Educational
Department of Liaoning Province (grant no. LJKMZ20221167).
|
1
|
Tao L, Ren X, Zhai W and Chen Z: Progress
and prospects of non-canonical NF-κB signaling pathway in the
regulation of liver diseases. Molecules. 27:42752022. View Article : Google Scholar
|
|
2
|
Hofmann J, Hackl V, Esser H, Meszaros AT,
Fodor M, Öfner D, Troppmair J, Schneeberger S and Hautz T:
Cell-Based regeneration and treatment of liver diseases. Int J Mol
Sci. 22:102762021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eslam M, Newsome PN, Sarin SK, Anstee QM,
Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour
JF, Schattenberg JM, et al: A new definition for metabolic
dysfunction-associated fatty liver disease: An international expert
consensus statement. J Hepatol. 73:202–209. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inoue Y, Qin B, Poti J, Sokol R and
Gordon-Larsen P: Epidemiology of obesity in Adults: Latest trends.
Curr Obes Rep. 7:276–288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Papatheodoridi M and Cholongitas E:
Diagnosis of non-alcoholic fatty liver disease (NAFLD): Current
concepts. Curr Pharm Des. 24:4574–4586. 2018. View Article : Google Scholar
|
|
6
|
Dongiovanni P, Paolini E, Corsini A,
Sirtori CR and Ruscica M: Nonalcoholic fatty liver disease or
metabolic dysfunction-associated fatty liver disease diagnoses and
cardiovascular diseases: From epidemiology to drug approaches. Eur
J Clin Invest. 51:e135192021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lazarus JV, Palayew A, Carrieri P, Ekstedt
M, Marchesini G, Novak K, Ratziu V, Romero-Gómez M, Tacke F,
Zelber-Sagi S, et al: European 'NAFLD Preparedness Index'-Is Europe
ready to meet the challenge of fatty liver disease? JHEP Rep.
3:1002342021. View Article : Google Scholar
|
|
8
|
Younossi ZM, Golabi P, de Avila L, Paik
JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A and Nader F: The
global epidemiology of NAFLD and NASH in patients with type 2
diabetes: A systematic review and meta-analysis. J Hepatol.
71:793–801. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantovani A, Scorletti E, Mosca A, Alisi
A, Byrne CD and Targher G: Complications, morbidity and mortality
of nonalcoholic fatty liver disease. Metabolism. 111s:1541702020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ballestri S, Zona S, Targher G, Romagnoli
D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G and Lonardo A:
Nonalcoholic fatty liver disease is associated with an almost
twofold increased risk of incident type 2 diabetes and metabolic
syndrome. Evidence from a systematic review and meta-analysis. J
Gastroenterol Hepatol. 31:936–944. 2016. View Article : Google Scholar
|
|
11
|
Lonardo A, Nascimbeni F, Mantovani A and
Targher G: Hypertension, diabetes, atherosclerosis and NASH: Cause
or consequence? J Hepatol. 68:335–352. 2018. View Article : Google Scholar
|
|
12
|
Targher G, Lonardo A and Byrne CD:
Nonalcoholic fatty liver disease and chronic vascular complications
of diabetes mellitus. Nat Rev Endocrinol. 14:99–114. 2018.
View Article : Google Scholar
|
|
13
|
Powell EE, Wong VW and Rinella M:
Non-alcoholic fatty liver disease. Lancet. 397:2212–2224. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Woo Baidal JA and Lavine JE: The
intersection of nonalcoholic fatty liver disease and obesity. Sci
Transl Med. 8:323rv12016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Velazquez AM, Bentanachs R, Sala-Vila A,
Lazaro I, Rodríguez-Morató J, Sánchez RM, Alegret M, Roglans N and
Laguna JC: ChREBP-driven DNL and PNPLA3 expression induced by
liquid fructose are essential in the production of fatty liver and
hypertriglyceridemia in a high-fat diet-fed rat model. Mol Nutr
Food Res. 66:e21011152022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mato JM, Alonso C, Noureddin M and Lu SC:
Biomarkers and subtypes of deranged lipid metabolism in
non-alcoholic fatty liver disease. World J Gastroenterol.
25:3009–3020. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petroni ML, Brodosi L, Bugianesi E and
Marchesini G: Management of non-alcoholic fatty liver disease. BMJ.
372:m47472021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
European Association for the Study of the
Liver (EASL); European Association for the Study of Diabetes
(EASD); European Association for the Study of Obesity (EASO):
EASL-EASD-EASO Clinical Practice Guidelines for the management of
non-alcoholic fatty liver disease. J Hepatol. 64:1388–1402. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koopman KE, Caan MW, Nederveen AJ, Pels A,
Ackermans MT, Fliers E, la Fleur SE and Serlie MJ: Hypercaloric
diets with increased meal frequency, but not meal size, increase
intrahepatic triglycerides: A randomized controlled trial.
Hepatology. 60:545–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vilar-Gomez E, Martinez-Perez Y,
Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B,
Gonzalez-Fabian L, Friedman SL, Diago M and Romero-Gomez M: Weight
loss through lifestyle modification significantly reduces features
of nonalcoholic steatohepatitis. Gastroenterology. 149:367–378 e5;
quiz e14-5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uribarri J, del Castillo MD, de la Maza
MP, Filip R, Gugliucci A, Luevano-Contreras C, Macías-Cervantes MH,
Markowicz Bastos DH, Medrano A, Menini T, et al: Dietary advanced
glycation end products and their role in health and disease. Adv
Nutr. 6:461–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lopez-Moreno J, Quintana-Navarro GM,
Delgado-Lista J, Garcia-Rios A, Alcala-Diaz JF, Gomez-Delgado F,
Camargo A, Perez-Martinez P, Tinahones FJ, Striker GE, et al:
Mediterranean diet supplemented with coenzyme Q10 modulates the
postprandial metabolism of advanced glycation end products in
elderly men and women. J Gerontol A Biol Sci Med Sci. 73:340–346.
2018.
|
|
23
|
Asadipooya K, Lankarani KB, Raj R and
Kalantarhormozi M: RAGE is a potential cause of onset and
progression of nonalcoholic fatty liver disease. Int J Endocrinol.
2019:21513022019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Helsley RN, Moreau F, Gupta MK, Radulescu
A, DeBosch B and Softic S: Tissue-Specific fructose metabolism in
obesity and diabetes. Curr Diab Rep. 20:642020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Romero-Gomez M, Zelber-Sagi S and Trenell
M: Treatment of NAFLD with diet, physical activity and exercise. J
Hepatol. 67:829–846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shim P, Choi D and Park Y: Association of
blood fatty acid composition and dietary pattern with the risk of
non-alcoholic fatty liver disease in patients who underwent
cholecystectomy. Ann Nutr Metab. 70:303–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moreira RJ, Castro É, Oliveira TE,
Belchior T, Peixoto AS, Chaves-Filho AB, Moreno MF, Lima JD,
Yoshinaga M, Miyamoto S, et al: Lipoatrophy-Associated insulin
resistance and hepatic steatosis are attenuated by intake of diet
rich in omega 3 fatty acids. Mol Nutr Food Res. 64:e19008332020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Musazadeh V, Dehghan P, Saleh-Ghadimi S
and Abbasalizad Farhangi M: Omega 3-rich Camelina sativa oil in the
context of a weight loss program improves glucose homeostasis,
inflammation and oxidative stress in patients with NAFLD: A
randomised placebo-controlled clinical trial. Int J Clin Pract.
75:e147442021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tosti V, Bertozzi B and Fontana L: Health
benefits of the mediterranean diet: Metabolic and molecular
mechanisms. J Gerontol A Biol Sci Med Sci. 73:318–326. 2018.
View Article : Google Scholar
|
|
30
|
Alonso-Domínguez R, García-Ortiz L,
Patino-Alonso MC, Sánchez-Aguadero N and Gómez-Marcos MA:
Recio-Rodríguez JI: Effectiveness of a multifactorial intervention
in increasing adherence to the mediterranean diet among patients
with diabetes mellitus type 2: A Controlled and Randomized Study
(EMID Study). Nutrients. 11:1622019. View Article : Google Scholar
|
|
31
|
Mohammadi S, Lotfi K, Mirzaei S, Asadi A,
Akhlaghi M and Saneei P: Adherence to mediterranean diet and its
association with metabolic health status in overweight and obese
adolescents. Int J Clin Pract. 2022:99252672022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Torres-Collado L, García-de la Hera M,
Lopes C, Compañ-Gabucio LM, Oncina-Cánovas A, Notario-Barandiaran
L, González-Palacios S and Vioque J: Olive oil consumption and
all-cause, cardiovascular and cancer mortality in an adult
mediterranean population in Spain. Front Nutr. 9:9979752022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martínez-González M, Martín-Calvo N,
Bretos-Azcona T, Carlos S and Delgado-Rodríguez M: Mediterranean
diet and cardiovascular prevention: why analytical observational
designs do support causality and not only associations. Int J
Environ Res Public Health. 19:136532022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zelber-Sagi S, Salomone F and Mlynarsky L:
The Mediterranean dietary pattern as the diet of choice for
non-alcoholic fatty liver disease: Evidence and plausible
mechanisms. Liver Int. 37:936–949. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang XL, Wang TY, Targher G, Byrne CD and
Zheng MH: Lifestyle interventions for non-obese patients both with,
and at risk, of non-alcoholic fatty liver disease. Diabetes Metab
J. 46:391–401. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hinrichs H, Faerber A, Young M, Ballentine
SJ and Thompson MD: Maternal exercise protects male offspring from
maternal diet-programmed nonalcoholic fatty liver disease
progression. Endocrinology. 164:bqad0102023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hashida R, Kawaguchi T, Bekki M, Omoto M,
Matsuse H, Nago T, Takano Y, Ueno T, Koga H, George J, et al:
Aerobic vs. resistance exercise in non-alcoholic fatty liver
disease: A systematic review. J Hepatol. 66:142–152. 2017.
View Article : Google Scholar
|
|
38
|
Zhang HJ, Pan LL, Ma ZM, Chen Z, Huang ZF,
Sun Q, Lu Y, Han CK, Lin MZ, Li XJ, et al: Long-term effect of
exercise on improving fatty liver and cardiovascular risk factors
in obese adults: A 1-year follow-up study. Diabetes Obes Metab.
19:284–289. 2017. View Article : Google Scholar
|
|
39
|
Wong VW, Wong GL, Chan RS, Shu SS, Cheung
BH, Li LS, Chim AM, Chan CK, Leung JK, Chu WC, et al: Beneficial
effects of lifestyle intervention in non-obese patients with
non-alcoholic fatty liver disease. J Hepatol. 69:1349–1356. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kasper P, Breuer S, Hoffmann T, Vohlen C,
Janoschek R, Schmitz L, Appel S, Fink G, Hünseler C, Quaas A, et
al: Maternal exercise mediates hepatic metabolic programming via
activation of AMPK-PGC1α axis in the offspring of obese mothers.
Cells. 10:12472021. View Article : Google Scholar
|
|
41
|
Battista F, Ermolao A, van Baak MA,
Beaulieu K, Blundell JE, Busetto L, Carraça EV, Encantado J, Dicker
D, Farpour-Lambert N, et al: Effect of exercise on cardiometabolic
health of adults with overweight or obesity: Focus on blood
pressure, insulin resistance, and intrahepatic fat-A systematic
review and meta-analysis. Obes Rev. 22(Suppl 4): e132692021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chalasani N, Younossi Z, Lavine JE,
Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM and Sanyal AJ:
The diagnosis and management of nonalcoholic fatty liver disease:
Practice guidance from the American Association for the study of
liver diseases. Hepatology. 67:328–357. 2018. View Article : Google Scholar
|
|
43
|
Nguyen NT and Varela JE: Bariatric surgery
for obesity and metabolic disorders: state of the art. Nat Rev
Gastroenterol Hepatol. 14:160–169. 2017. View Article : Google Scholar
|
|
44
|
Cabré N, Luciano-Mateo F, Fernández-Arroyo
S, Baiges-Gayà G, Hernández-Aguilera A, Fibla M, Fernández-Julià R,
París M, Sabench F, Castillo DD, et al: Laparoscopic sleeve
gastrectomy reverses non-alcoholic fatty liver disease modulating
oxidative stress and inflammation. Metabolism. 99:81–89. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nobili V, Carpino G, De Peppo F, Caccamo
R, Mosca A, Romito I, Overi D, Franchitto A, Onori P, Alisi A and
Gaudio E: Laparoscopic sleeve gastrectomy improves nonalcoholic
fatty liver disease-related liver damage in adolescents by
reshaping cellular interactions and hepatic adipocytokine
production. J Pediatr. 194:100–108.e3. 2018. View Article : Google Scholar
|
|
46
|
Pan Q, Qin T, Gao Y, Li S, Li D, Peng M,
Zhai H and Xu G: Hepatic mTOR-AKT2-Insig2 signaling pathway
contributes to the improvement of hepatic steatosis after Roux-en-Y
Gastric Bypass in mice. Biochim Biophys Acta Mol Basis Dis.
1865:525–534. 2019. View Article : Google Scholar
|
|
47
|
Caiazzo R, Lassailly G, Leteurtre E, Baud
G, Verkindt H, Raverdy V, Buob D, Pigeyre M, Mathurin P and Pattou
F: Roux-en-Y gastric bypass versus adjustable gastric banding to
reduce nonalcoholic fatty liver disease: A 5-year controlled
longitudinal study. Ann Surg. 260:893–898; discussion 898-9. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feng W, Yin T, Chu X, Shan X, Jiang C,
Wang Y, Qian Y, Zhu D, Sun X and Bi Y: Metabolic effects and safety
of Roux-en-Y gastric bypass surgery vs. conventional medication in
obese Chinese patients with type 2 diabetes. Diabetes Metab Res
Rev. 35:e31382019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Malo FC, Marion A, Rioux A, Lebel S, Hould
F, Julien F, Marceau S, Lescelleur O, Lafortune A, Bouvet-Bouchard
L and Biertho L: Long alimentary limb duodenal switch (LADS): An
exploratory randomized trial, results at 2 years. Obes Surg.
30:5047–5058. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Russo MF, Lembo E, Mari A, Angelini G,
Verrastro O, Nanni G, Pompili M, Raffaelli M, Vecchio FM, Bornstein
SR and Mingrone G: Insulin resistance is central to long-term
reversal of histologic nonalcoholic steatohepatitis after metabolic
surgery. J Clin Endocrinol Metab. 106:750–761. 2021. View Article : Google Scholar
|
|
51
|
Giannini EG, Coppo C, Romana C, Camerini
GB, De Cian F, Scopinaro N and Papadia FS: Long-term follow-up
study of liver-related outcome after bilio-pancreatic diversion in
patients with initial, significant liver damage. Dig Dis Sci.
63:1946–1951. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hassanian M, Al-Mulhim A, Al-Sabhan A,
Al-Amro S, Bamehriz F, Abdo A, Al Khalidi H and Aldoheyan TA: The
effect of bariatric surgeries on nonalcoholic fatty liver disease.
Saudi J Gastroenterol. 20:270–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aldoheyan T, Hassanain M, Al-Mulhim A,
Al-Sabhan A, Al-Amro S, Bamehriz F and Al-Khalidi H: The effects of
bariatric surgeries on nonalcoholic fatty liver disease. Surg
Endosc. 31:1142–1147. 2017. View Article : Google Scholar
|
|
54
|
Karlsson C, Wallenius K, Walentinsson A,
Greasley PJ, Miliotis T, Hammar M, Iaconelli A, Tapani S, Raffaelli
M, Mingrone G and Carlsson B: Identification of proteins associated
with the early restoration of insulin sensitivity after
biliopancreatic diversion. J Clin Endocrinol Metab.
105:e4157–e4168. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yu HH, Hsieh MC, Wu SY, Sy ED and Shan YS:
Effects of duodenal-jejunal bypass surgery in ameliorating
nonalcoholic steatohepatitis in diet-induced obese rats. Diabetes
Metab Syndr Obes. 12:149–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Talavera-Urquijo E, Beisani M, Balibrea JM
and Alverdy JC: Is bariatric surgery resolving NAFLD via
microbiota-mediated bile acid ratio reversal? A comprehensive
review. Surg Obes Relat Dis. 16:1361–1369. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang X, Zheng J, Zhang S, Wang B, Wu C
and Guo X: Advances in the involvement of gut microbiota in
pathophysiology of NAFLD. Front Med (Lausanne). 7:3612020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yao Z, Song S, Li X, Wang W, Ren P, Wang
H, Xie Y and Li Z: Corn peptides ameliorate nonalcoholic fatty
liver disease by suppressing endoplasmic reticulum stress via the
AMPKα/Sirt1 pathway in vivo and in vitro. Journal of Functional
Foods. 93:1050632022. View Article : Google Scholar
|
|
59
|
Santos-Sanchez G, Cruz-Chamorro I,
Alvarez-Rios AI, Fernández-Santos JM, Vázquez-Román MV,
Rodríguez-Ortiz B, Álvarez-Sánchez N, Álvarez-López AI,
Millán-Linares MDC, Millán F, et al: Lupinus angustifolius protein
hydrolysates reduce abdominal adiposity and ameliorate metabolic
associated fatty liver disease (MAFLD) in Western Diet
Fed-ApoE(-/-) Mice. Antioxidants (Basel). 10:12222021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dumeus S, Shibu MA, Lin WT, Wang MF, Lai
CH, Shen CY, Lin YM, Viswanadha VP, Kuo WW and Huang CY: Bioactive
peptide improves diet-induced hepatic fat deposition and hepatocyte
proinflammatory response in SAMP8 ageing mice. Cell Physiol
Biochem. 48:1942–1952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhong D, Xie Z, Huang B, Zhu S, Wang G,
Zhou H, Lin S, Lin Z and Yang B: Ganoderma lucidum polysaccharide
peptide alleviates hepatoteatosis via modulating bile acid
metabolism dependent on FXR-SHP/FGF. Cell Physiol Biochem.
49:1163–1179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ye J, Tian X, Wang Q, Zheng J, Yang Y, Xu
B, Zhang S, Yuan F and Yang Z: Monkfish peptides mitigate high fat
diet-induced hepatic steatosis in mice. Mar Drugs. 20:3122022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pittala S, Krelin Y, Kuperman Y and
Shoshan-Barmatz V: A Mitochondrial VDAC1-Based peptide greatly
suppresses steatosis and NASH-Associated pathologies in a mouse
model. Mol Ther. 27:1848–1862. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chiang WD, Shibu MA, Lee KI, Wu JP, Tsai
FJ, Pan LF, Huang CY and Lin WT: Lipolysis-stimulating peptide-VHVV
ameliorates high fat diet induced hepatocyte apoptosis and
fibrosis. J Func Foods. 11:482–492. 2014. View Article : Google Scholar
|
|
65
|
Moreira GV, Azevedo FF, Ribeiro LM, Santos
A, Guadagnini D, Gama P, Liberti EA, Saad M and Carvalho C:
Liraglutide modulates gut microbiota and reduces NAFLD in obese
mice. J Nutr Biochem. 62:143–154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fang Y, Ji L, Zhu C, Xiao Y, Zhang J, Lu
J, Yin J and Wei L: Liraglutide alleviates hepatic steatosis by
activating the TFEB-Regulated autophagy-lysosomal pathway. Front
Cell Dev Biol. 8:6025742020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yang M, Ma X, Xuan X, Deng H, Chen Q and
Yuan L: Liraglutide attenuates non-alcoholic fatty liver disease in
mice by regulating the local renin-angiotensin system. Front
Pharmacol. 11:4322020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Armstrong MJ, Gaunt P, Aithal GP, Barton
D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G; LEAN trial
team; et al: Liraglutide safety and efficacy in patients with
non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind,
randomised, placebo-controlled phase 2 study. Lancet. 387:679–690.
2016. View Article : Google Scholar
|
|
69
|
Kuchay MS, Krishan S, Mishra SK, Choudhary
NS, Singh MK, Wasir JS, Kaur P, Gill HK, Bano T, Farooqui KJ and
Mithal A: Effect of dulaglutide on liver fat in patients with type
2 diabetes and NAFLD: Randomised controlled trial (D-LIFT trial).
Diabetologia. 63:2434–2445. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Newsome PN, Buchholtz K, Cusi K, Linder M,
Okanoue T, Ratziu V, Sanyal AJ, Sejling AS and Harrison SA;
NN9931-4296 Investigators: A placebo-controlled trial of
subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J
Med. 384:1113–1124. 2021. View Article : Google Scholar
|
|
71
|
Flint A, Andersen G, Hockings P, Johansson
L, Morsing A, Sundby Palle M, Vogl T, Loomba R and Plum-Mörschel L:
Randomised clinical trial: Semaglutide versus placebo reduced liver
steatosis but not liver stiffness in subjects with non-alcoholic
fatty liver disease assessed by magnetic resonance imaging. Aliment
Pharmacol Ther. 54:1150–1161. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Newsome P, Francque S, Harrison S, Ratziu
V, Van Gaal L, Calanna S, Hansen M, Linder M and Sanyal A: Effect
of semaglutide on liver enzymes and markers of inflammation in
subjects with type 2 diabetes and/or obesity. Aliment Pharmacol
Ther. 50:193–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jin L, Sun Y, Li Y, Zhang H, Yu W, Li Y,
Xin Y, Alsareii SA, Wang Q and Zhang D: A synthetic peptide AWRK6
ameliorates metabolic associated fatty liver disease: Involvement
of lipid and glucose homeostasis. Peptides. 143:1705972021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
van Dalem J, Driessen JHM, Burden AM,
Stehouwer CDA, Klungel OH, de Vries F and Brouwers MCGJ:
Thiazolidinediones and glucagon-like peptide-1 receptor agonists
and the risk of nonalcoholic fatty liver disease: A cohort study.
Hepatology. 74:2467–2477. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhao JV, Yeung WF, Chan YH, Vackova D,
Leung JYY, Ip DKM, Zhao J, Ho WK, Tse HF and Schooling CM: Effect
of berberine on cardiovascular disease risk factors: A mechanistic
randomized controlled trial. Nutrients. 13:25502021. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Majidzadeh H, Araj-Khodaei M, Ghaffari M,
Torbati M, Ezzati Nazhad Dolatabadi J and Hamblin MR: Nano-based
delivery systems for berberine: A modern anti-cancer herbal
medicine. Colloids Surf B Biointerfaces. 194:1111882020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Koperska A, Wesolek A, Moszak M and
Szulinska M: Berberine in non-alcoholic fatty liver disease-A
review. Nutrients. 14:34592022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wei X, Wang C, Hao S, Song H and Yang L:
The therapeutic effect of berberine in the treatment of
nonalcoholic fatty liver disease: A meta-analysis. Evid Based
Complement Alternat Med. 2016:35939512016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Guo T, Woo SL, Guo X, Li H, Zheng J,
Botchlett R, Liu M, Pei Y, Xu H, Cai Y, et al: Berberine
ameliorates hepatic steatosis and suppresses liver and adipose
tissue inflammation in mice with diet-induced obesity. Sci Rep.
6:226122016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhu X, Bian H, Wang L, Sun X, Xu X, Yan H,
Xia M, Chang X, Lu Y, Li Y, et al: Berberine attenuates
nonalcoholic hepatic steatosis through the AMPK-SREBP-1c-SCD1
pathway. Free Radic Biol Med. 141:192–204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vivoli E, Cappon A, Milani S, Piombanti B,
Provenzano A, Novo E, Masi A, Navari N, Narducci R, Mannaioni G, et
al: NLRP3 inflammasome as a target of berberine in experimental
murine liver injury: Interference with P2X7 signalling. Clin Sci
(Lond). 130:1793–1806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sun Y, Yuan X, Zhang F, Han Y, Chang X, Xu
X, Li Y and Gao X: Berberine ameliorates fatty acid-induced
oxidative stress in human hepatoma cells. Sci Rep. 7:113402017.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li D, Zheng J, Hu Y, Hou H, Hao S, Liu N
and Wang Y: Amelioration of intestinal barrier dysfunction by
berberine in the treatment of nonalcoholic fatty liver disease in
rats. Pharmacogn Mag. 13:677–682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang X, Zhao Y, Xu J, Xue Z, Zhang M,
Pang X, Zhang X and Zhao L: Modulation of gut microbiota by
berberine and metformin during the treatment of high-fat
diet-induced obesity in rats. Sci Rep. 5:144052015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cui HX, Hu YN, Li JW and Yuan K:
Hypoglycemic mechanism of the berberine organic acid salt under the
synergistic effect of intestinal flora and oxidative stress. Oxid
Med Cell Longev. 2018:89303742018. View Article : Google Scholar
|
|
86
|
Li G, Zhou F, Chen Y, Zhang W and Wang N:
Kukoamine A attenuates insulin resistance and fatty liver through
downregulation of Srebp-1c. Biomed Pharmacother. 89:536–543. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sharma L, Lone NA, Knott RM, Hassan A and
Abdullah T: Trigonelline prevents high cholesterol and high fat
diet induced hepatic lipid accumulation and lipo-toxicity in
C57BL/6J mice, via restoration of hepatic autophagy. Food Chem
Toxicol. 121:283–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liang L, Ye S, Jiang R, Zhou X, Zhou J and
Meng S: Liensinine alleviates high fat diet (HFD)-induced
non-alcoholic fatty liver disease (NAFLD) through suppressing
oxidative stress and inflammation via regulating TAK1/AMPK
signaling. Int Immunopharmacol. 104:1083062022. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Cai L, Liu S, Sun L, Wang Y, Ji H and Li
J: Application of tea polyphenols in combination with 6-gingerol on
shrimp paste of during storage: Biogenic amines formation and
quality determination. Front Microbiol. 6:9812015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Panahi Y, Hossein i MS, Khalili N, Naimi
E, Simental-Mendía LE, Majeed M and Sahebkar A: Effects of curcumin
on serum cytokine concentrations in subjects with metabolic
syndrome: A post-hoc analysis of a randomized controlled trial.
Biomed Pharmacother. 82:578–582. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Panahi Y, Hosseini MS, Khalili N, Naimi E,
Majeed M and Sahebkar A: Antioxidant and anti-inflammatory effects
of curcuminoid-piperine combination in subjects with metabolic
syndrome: A randomized controlled trial and an updated
meta-analysis. Clin Nutr. 34:1101–1108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Panahi Y, Kianpour P, Mohtashami R, Jafari
R, Simental-Mendía LE and Sahebkar A: Efficacy and safety of
phytosomal curcumin in non-alcoholic fatty liver disease: A
Randomized controlled trial. Drug Res (Stuttg). 67:244–251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Rahmani S, Asgary S, Askari G, Keshvari M,
Hatamipour M, Feizi A and Sahebkar A: Treatment of non-alcoholic
fatty liver disease with curcumin: A Randomized placebo-controlled
trial. Phytother Res. 30:1540–1548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mokgalaboni K, Ntamo Y, Ziqubu K, Nyambuya
TM, Nkambule BB, Mazibuko-Mbeje SE, Gabuza KB, Chellan N, Tiano L
and Dludla PV: Curcumin supplementation improves biomarkers of
oxidative stress and inflammation in conditions of obesity, type 2
diabetes and NAFLD: Updating the status of clinical evidence. Food
Funct. 12:12235–12249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Mahmoudi A, Butler AE, Majeed M, Banach M
and Sahebkar A: Investigation of the effect of curcumin on protein
targets in NAFLD using bioinformatic analysis. Nutrients.
14:13312022. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Korsholm AS, Kjær TN, Ornstrup MJ and
Pedersen SB: Comprehensive metabolomic analysis in blood, urine,
fat, and muscle in men with metabolic Syndrome: A Randomized,
placebo-controlled clinical trial on the effects of resveratrol
after four months' treatment. Int J Mol Sci. 18:5542017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Méndez-del Villar M, González-Ortiz M,
Martínez-Abundis E, Pérez-Rubio KG and Lizárraga-Valdez R: Effect
of resveratrol administration on metabolic syndrome, insulin
sensitivity, and insulin secretion. Metab Syndr Relat Disord.
12:497–501. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Faghihzadeh F, Adibi P and Hekmatdoost A:
The effects of resveratrol supplementation on cardiovascular risk
factors in patients with non-alcoholic fatty liver disease: A
randomised, double-blind, placebo-controlled study. Br J Nutr.
114:796–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cheng C, Li Z, Zhao X, Liao C, Quan J,
Bode AM, Cao Y and Luo X: Natural alkaloid and polyphenol compounds
targeting lipid metabolism: Treatment implications in metabolic
diseases. Eur J Pharmacol. 870:1729222020. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chen S, Zhao X, Ran L, Wan J, Wang X, Qin
Y, Shu F, Gao Y, Yuan L, Zhang Q and Mi M: Resveratrol improves
insulin resistance, glucose and lipid metabolism in patients with
non-alcoholic fatty liver disease: A randomized controlled trial.
Dig Liver Dis. 47:226–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tejada S, Capó X, Mascaró CM,
Monserrat-Mesquida M, Quetglas-Llabrés MM, Pons A, Tur JA and
Sureda A: Hepatoprotective effects of resveratrol in non-alcoholic
fatty live disease. Curr Pharm Des. 27:2558–2570. 2021. View Article : Google Scholar
|
|
102
|
Hosseini H, Teimouri M, Shabani M, Koushki
M, Babaei Khorzoughi R, Namvarjah F, Izadi P and Meshkani R:
Resveratrol alleviates non-alcoholic fatty liver disease through
epigenetic modification of the Nrf2 signaling pathway. Int J
Biochem Cell Biol. 119:1056672020. View Article : Google Scholar
|
|
103
|
Ebrahimpour S, Zakeri M and Esmaeili A:
Crosstalk between obesity, diabetes, and alzheimer's disease:
Introducing quercetin as an effective triple herbal medicine.
Ageing Res Rev. 62:1010952020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yang H, Yang T, Heng C, Zhou Y, Jiang Z,
Qian X, Du L, Mao S, Yin X and Lu Q: Quercetin improves
nonalcoholic fatty liver by ameliorating inflammation, oxidative
stress, and lipid metabolism in db/db mice. Phytother Res.
33:3140–3152. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Porras D, Nistal E, Martínez-Flórez S,
Pisonero-Vaquero S, Olcoz JL, Jover R, González-Gallego J,
García-Mediavilla MV and Sánchez-Campos S: Protective effect of
quercetin on high-fat diet-induced non-alcoholic fatty liver
disease in mice is mediated by modulating intestinal microbiota
imbalance and related gut-liver axis activation. Free Radic Biol
Med. 102:188–202. 2017. View Article : Google Scholar
|
|
106
|
Zhu X, Xiong T, Liu P, Guo X, Xiao L, Zhou
F, Tang Y and Yao P: Quercetin ameliorates HFD-induced NAFLD by
promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s
pathway. Food Chem Toxicol. 114:52–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Chen L, Liu J, Mei G, Chen H, Peng S, Zhao
Y, Yao P and Tang Y: Quercetin and non-alcoholic fatty liver
disease: A review based on experimental data and bioinformatic
analysis. Food Chem Toxicol. 154:1123142021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Saha S, Sadhukhan P and Sil PC:
Mangiferin: A xanthonoid with multipotent anti-inflammatory
potential. BioFactors. 42:459–474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Gold-Smith F, Fernandez A and Bishop K:
Mangiferin and cancer: Mechanisms of action. Nutrients. 8:3962016.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yong Z, Ruiqi W, Hongji Y, Ning M, Chenzuo
J, Yu Z, Zhixuan X, Qiang L, Qibing L, Weiying L and Xiaopo Z:
Mangiferin Ameliorates HFD-Induced NAFLD through Regulation of the
AMPK and NLRP3 inflammasome signal pathways. J Immunol Res.
2021:40845662021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chen C, Liu Q, Liu L, Hu YY and Feng Q:
Potential biological effects of (−)-epigallocatechin-3-gallate on
the treatment of nonalcoholic fatty liver disease. Mol Nutr Food
Res. 62:17004832018. View Article : Google Scholar
|
|
112
|
Chen Q and Wang T, Li J, Wang S, Qiu F, Yu
H, Zhang Y and Wang T: Effects of natural products on
fructose-induced nonalcoholic fatty liver disease (NAFLD).
Nutrients. 9:962017. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Abenavoli L, Izzo AA, Milic N, Cicala C,
Santini A and Capasso R: Milk thistle (Silybum marianum): A concise
overview on its chemistry, pharmacological, and nutraceutical uses
in liver diseases. Phytother Res. 32:2202–2213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Marin V, Gazzin S, Gambaro SE, Dal Ben M,
Calligaris S, Anese M, Raseni A, Avellini C, Giraudi PJ, Tiribelli
C and Rosso N: Effects of oral administration of silymarin in a
juvenile murine model of non-alcoholic steatohepatitis. Nutrients.
9:10062017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ni X and Wang H: Silymarin attenuated
hepatic steatosis through regulation of lipid metabolism and
oxidative stress in a mouse model of nonalcoholic fatty liver
disease (NAFLD). Am J Transl Res. 8:1073–1081. 2016.PubMed/NCBI
|
|
116
|
Gu M, Zhao P, Huang J, Zhao Y, Wang Y, Li
Y, Li Y, Fan S, Ma YM, Tong Q, et al: Silymarin ameliorates
metabolic dysfunction associated with diet-induced obesity via
activation of farnesyl X receptor. Front Pharmacol. 7:3452016.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wah Kheong C, Nik Mustapha NR and Mahadeva
S: A Randomized trial of silymarin for the treatment of
nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol.
15:1940–1949.e8. 2017. View Article : Google Scholar
|
|
118
|
Curcio A, Romano A, Cuozzo S, Nicola AD,
Grassi O, Schiaroli D, Nocera GF and Pironti M: Silymarin in
combination with vitamin C, vitamin E, coenzyme Q10 and
selenomethionine to improve liver enzymes and blood lipid profile
in NAFLD patients. Medicina (Kaunas). 56:5442020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ou Q, Weng Y, Wang S, Zhao Y, Zhang F,
Zhou J and Wu X: Silybin alleviates hepatic steatosis and fibrosis
in NASH mice by inhibiting oxidative stress and involvement with
the Nf-κB pathway. Dig Dis Sci. 63:3398–3408. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Cui CX, Deng JN, Yan L, Liu YY, Fan JY, Mu
HN, Sun HY, Wang YH and Han JY: Silibinin Capsules improves high
fat diet-induced nonalcoholic fatty liver disease in hamsters
through modifying hepatic de novo lipogenesis and fatty acid
oxidation. J Ethnopharmacol. 208:24–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Liu Y, Xu W, Zhai T, You J and Chen Y:
Silibinin ameliorates hepatic lipid accumulation and oxidative
stress in mice with non-alcoholic steatohepatitis by regulating
CFLAR-JNK pathway. Acta Pharm Sin B. 9:745–757. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Cassard AM and Ciocan D: Microbiota, a key
player in alcoholic liver disease. Clin Mol Hepatol. 24:100–107.
2018. View Article : Google Scholar :
|
|
123
|
Fujinaga Y, Kawaratani H, Kaya D, Tsuji Y,
Ozutsumi T, Furukawa M, Kitagawa K, Sato S, Nishimura N, Sawada Y,
et al: effective combination therapy of angiotensin-II receptor
blocker and rifaximin for hepatic fibrosis in rat model of
nonalcoholic steatohepatitis. Int J Mol Sci. 21:55892020.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jian J, Nie MT, Xiang B, Qian H, Yin C,
Zhang X, Zhang M, Zhu X and Xie WF: Rifaximin ameliorates
non-alcoholic steatohepatitis in mice through regulating gut
microbiome-related bile acids. Front Pharmacol. 13:8411322022.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Gangarapu V, Ince AT, Baysal B, Kayar Y,
Kılıç U, Gök Ö, Uysal Ö and Şenturk H: Efficacy of rifaximin on
circulating endotoxins and cytokines in patients with nonalcoholic
fatty liver disease. Eur J Gastroenterol Hepatol. 27:840–845. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Abdel-Razik A, Mousa N, Shabana W, Refaey
M, Elzehery R, Elhelaly R, Zalata K, Abdelsalam M, Eldeeb AA, Awad
M, et al: Rifaximin in nonalcoholic fatty liver disease: Hit
multiple targets with a single shot. Eur J Gastroenterol Hepatol.
30:1237–1246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sumida Y and Yoneda M: Current and future
pharmacological therapies for NAFLD/NASH. J Gastroenterol.
53:362–376. 2018. View Article : Google Scholar :
|
|
128
|
Rotman Y and Sanyal AJ: Current and
upcoming pharmacotherapy for non-alcoholic fatty liver disease.
Gut. 66:180–190. 2017. View Article : Google Scholar
|
|
129
|
Brandt A, Jin CJ, Nolte K, Sellmann C,
Engstler AJ and Bergheim I: Short-Term intake of a fructose-, fat-
and cholesterol-rich diet causes hepatic steatosis in mice: Effect
of antibiotic treatment. Nutrients. 9:10132017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Suk KT and Kim DJ: Gut microbiota: novel
therapeutic target for nonalcoholic fatty liver disease. Expert Rev
Gastroenterol Hepatol. 13:193–204. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Liao CC, Ou TT, Huang HP and Wang CJ: The
inhibition of oleic acid induced hepatic lipogenesis and the
promotion of lipolysis by caffeic acid via up-regulation of
AMP-activated kinase. J Sci Food Agric. 94:1154–1162. 2014.
View Article : Google Scholar
|
|
132
|
Pardo V, González-Rodríguez Á, Muntané J,
Kozma SC and Valverde ÁM: Role of hepatocyte S6K1 in palmitic
acid-induced endoplasmic reticulum stress, lipotoxicity, insulin
resistance and in oleic acid-induced protection. Food Chem Toxicol.
80:298–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Reyes-Quiroz ME, Alba G, Saenz J,
Santa-María C, Geniz I, Jiménez J, Ramírez R, Martín-Nieto J,
Pintado E and Sobrino F: Oleic acid modulates mRNA expression of
liver X receptor (LXR) and its target genes ABCA1 and SREBP1c in
human neutrophils. Eur J Nutr. 53:1707–1717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Gu LY, Qiu LW, Chen XF, Lü L and Mei ZC:
Oleic acid-induced hepatic steatosis is coupled with downregulation
of aquaporin 3 and upregulation of aquaporin 9 via activation of
p38 signaling. Horm Metab Res. 47:259–264. 2015.
|
|
135
|
Miyake T, Furukawa S, Matsuura B, Yoshida
O, Miyazaki M, Shiomi A, Kanzaki S, Nakaguchi H, Sunago K, Nakamura
Y, et al: Plasma fatty acid composition is associated with
histological findings of nonalcoholic steatohepatitis.
Biomedicines. 10:25402022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Rodrigues PO, Martins SV, Lopes PA, Ramos
C, Miguéis S, Alfaia CM, Pinto RM, Rolo EA, Bispo P, Batista I, et
al: Influence of feeding graded levels of canned sardines on the
inflammatory markers and tissue fatty acid composition of Wistar
rats. Br J Nutr. 112:309–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Tapia G, Valenzuela R, Espinosa A,
Romanque P, Dossi C, Gonzalez-Mañán D, Videla LA and D'Espessailles
A: N-3 long-chain PUFA supplementation prevents high fat diet
induced mouse liver steatosis and inflammation in relation to
PPAR-α upregulation and NF-κB DNA binding abrogation. Mol Nutr Food
Res. 58:1333–1341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wang M, Ma LJ, Yang Y, Xiao Z and Wan JB:
n-3 Polyunsaturated fatty acids for the management of alcoholic
liver disease: A critical review. Crit Rev Food Sci Nutr. 59(sup1):
S116–S129. 2019. View Article : Google Scholar
|
|
139
|
Smid V, Dvorak K, Sedivy P, Kosek V,
Leníček M, Dezortová M, Hajšlová J, Hájek M, Vítek L, Bechyňská K
and Brůha R: Effect of Omega-3 polyunsaturated fatty acids on lipid
metabolism in patients with metabolic Syndrome and NAFLD. Hepatol
Commun. 6:1336–1349. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Albracht-Schulte K, Kalupahana NS,
Ramalingam L, Wang S, Rahman SM, Robert-McComb J and
Moustaid-Moussa N: Omega-3 fatty acids in obesity and metabolic
syndrome: A mechanistic update. J Nutr Biochem. 58:1–16. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Pacifico L, Giansanti S, Gallozzi A and
Chiesa C: Long chain omega-3 polyunsaturated fatty acids in
pediatric metabolic syndrome. Mini Rev Med Chem. 14:791–804. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Jeyapal S, Kona SR, Mullapudi SV, Putcha
UK, Gurumurthy P and Ibrahim A: Substitution of linoleic acid with
alpha-linolenic acid or long chain n-3 polyunsaturated fatty acid
prevents Western diet induced nonalcoholic steatohepatitis. Sci
Rep. 8:109532018. View Article : Google Scholar
|
|
143
|
Da Silva HE, Arendt BM, Noureldin SA,
Therapondos G, Guindi M and Allard JP: A cross-sectional study
assessing dietary intake and physical activity in Canadian patients
with nonalcoholic fatty liver disease vs healthy controls. J Acad
Nutr Diet. 114:1181–1194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Silva Figueiredo P, Inada AC, Ribeiro
Fernandes M, Granja Arakaki D, Freitas KC, Avellaneda Guimarães RC,
Aragão do Nascimento V and Aiko Hiane P: An overview of novel
dietary supplements and food ingredients in patients with metabolic
Syndrome and non-alcoholic fatty liver disease. Molecules.
23:8772018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Glen J, Floros L, Day C and Pryke R;
Guideline Development Group: Non-alcoholic fatty liver disease
(NAFLD): Summary of NICE guidance. BMJ. 354:i44282016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Presa N, Clugston RD, Lingrell S, Kelly
SE, Merrill AH Jr, Jana S, Kassiri Z, Gómez-Muñoz A, Vance DE,
Jacobs RL and van der Veen JN: Vitamin E alleviates non-alcoholic
fatty liver disease in phosphatidylethanolamine N-methyltransferase
deficient mice. Biochim Biophys Acta Mol Basis Dis. 1865:14–25.
2019. View Article : Google Scholar
|
|
147
|
Amanullah I, Khan YH, Anwar I, Gulzar A,
Mallhi TH and Raja AA: Effect of vitamin E in non-alcoholic fatty
liver disease: A systematic review and meta-analysis of randomised
controlled trials. Postgrad Med J. 95:601–611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Sebastiani G, Saeed S, Lebouche B, de
Pokomandy A, Szabo J, Haraoui LP, Routy JP, Wong P, Deschenes M,
Ghali P, et al: Vitamin E is an effective treatment for
nonalcoholic steatohepatitis in HIV mono-infected patients. AIDS.
34:237–244. 2020. View Article : Google Scholar
|
|
149
|
Sanyal AJ, Chalasani N, Kowdley KV,
McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE,
Tonascia J, Unalp A, et al: Pioglitazone, vitamin E, or placebo for
nonalcoholic steatohepatitis. N Engl J Med. 362:1675–1685. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
He W, Xu Y, Ren X, Xiang D, Lei K, Zhang C
and Liu D: Vitamin E ameliorates lipid metabolism in mice with
nonalcoholic fatty liver disease via Nrf2/CES1 signaling pathway.
Dig Dis Sci. 64:3182–3191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Klaebel JH, Rakipovski G, Andersen B,
Lykkesfeldt J and Tveden-Nyborg P: Dietary intervention accelerates
NASH resolution depending on inflammatory status with minor
additive effects on hepatic injury by vitamin E supplementation.
Antioxidants (Basel). 9:8082020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Farrag SM, Hamzawy MA, El-Yamany MF, Saad
MA and Nassar NN: Atorvastatin in nano-particulate formulation
abates muscle and liver affliction when coalesced with coenzyme Q10
and/or vitamin E in hyperlipidemic rats. Life Sci. 203:129–140.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Klaebel JH, Skjodt M, Skat-Rordam J,
Rakipovski G, Ipsen DH, Schou-Pedersen AMV, Lykkesfeldt J and
Tveden-Nyborg P: Atorvastatin and vitamin E accelerates NASH
resolution by dietary intervention in a preclinical guinea pig
model. Nutrients. 11:28342019. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Xin J, Jiang X, Ben S, Yuan Q, Su L, Zhang
Z, Christiani DC, Du M and Wang M: Association between circulating
vitamin E and ten common cancers: Evidence from large-scale
Mendelian randomization analysis and a longitudinal cohort study.
BMC Med. 20:1682022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Brunner KT, Henneberg CJ, Wilechansky RM
and Long MT: Nonalcoholic fatty liver disease and obesity
treatment. Curr Obes Rep. 8:220–228. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Muscogiuri G, Mitri J, Mathieu C,
Badenhoop K, Tamer G, Orio F, Mezza T, Vieth R, Colao A and Pittas
A: Mechanisms in endocrinology: Vitamin D as a potential
contributor in endocrine health and disease. Eur J Endocrinol.
171:R101–R110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Bea JW, Jurutka PW, Hibler EA, Lance P,
Martínez ME, Roe DJ, Sardo Molmenti CL, Thompson PA and Jacobs ET:
Concentrations of the vitamin D metabolite 1,25(OH)2D and odds of
metabolic syndrome and its components. Metabolism. 64:447–459.
2015. View Article : Google Scholar :
|
|
158
|
Wang Q, Shi X, Wang J, Zhang J and Xu C:
Low serum vitamin D concentrations are associated with obese but
not lean NAFLD: A cross-sectional study. Nutr J. 20:302021.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zeng Y, Luo M, Pan L, Chen Y, Guo S, Luo
D, Zhu L, Liu Y, Pan L, Xu S, et al: Vitamin D signaling maintains
intestinal innate immunity and gut microbiota: potential
intervention for metabolic syndrome and NAFLD. Am J Physiol
Gastrointest Liver Physiol. 318:G542–G553. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Barchetta I, Cimini FA and Cavallo MG:
Vitamin D and metabolic dysfunction-associated fatty liver disease
(MAFLD): An update. Nutrients. 12:33022020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Liu XJ, Wang BW, Zhang C, Xia MZ, Chen YH,
Hu CQ, Wang H, Chen X and Xu DX: Vitamin d deficiency attenuates
high-fat diet-induced hyperinsulinemia and hepatic lipid
accumulation in male mice. Endocrinology. 156:2103–2113. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Liu Y, Wang M, Xu W, Zhang H, Qian W, Li X
and Cheng X: Active vitamin D supplementation alleviates initiation
and progression of nonalcoholic fatty liver disease by repressing
the p53 pathway. Life Sci. 241:1170862020. View Article : Google Scholar
|
|
163
|
Sharifi N, Amani R, Hajiani E and
Cheraghian B: Does vitamin D improve liver enzymes, oxidative
stress, and inflammatory biomarkers in adults with non-alcoholic
fatty liver disease? A randomized clinical trial. Endocrine.
47:70–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Eliades M and Spyrou E: Vitamin D: A new
player in non-alcoholic fatty liver disease? World J Gastroenterol.
21:1718–1727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Xie ZQ, Li HX, Tan WL, Yang L, Ma XW, Li
WX, Wang QB, Shang CZ and Chen YJ: Association of serum vitamin C
With NAFLD and MAFLD among adults in the United States. Front Nutr.
8:7953912022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
He Z, Li X, Yang H, Wu P, Wang S, Cao D,
Guo X, Xu Z, Gao J, Zhang W and Luo X: Effects of oral vitamin C
supplementation on liver health and associated parameters in
patients with non-alcoholic fatty liver disease: A Randomized
clinical trial. Front Nutr. 8:7456092021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Gu X and Luo X, Wang Y, He Z, Li X, Wu K,
Zhang Y, Yang Y, Ji J and Luo X: Ascorbic acid attenuates cell
stress by activating the fibroblast growth factor 21/fibroblast
growth factor receptor 2/adiponectin pathway in HepG2 cells. Mol
Med Rep. 20:2450–2458. 2019.PubMed/NCBI
|
|
168
|
Woodhouse CA, Patel VC, Singanayagam A and
Shawcross DL: Review article: the gut microbiome as a therapeutic
target in the pathogenesis and treatment of chronic liver disease.
Aliment Pharmacol Ther. 47:192–202. 2018. View Article : Google Scholar
|
|
169
|
Arai N, Miura K, Aizawa K, Sekiya M,
Nagayama M, Sakamoto H, Maeda H, Morimoto N, Iwamoto S and Yamamoto
H: Probiotics suppress nonalcoholic steatohepatitis and
carcinogenesis progression in hepatocyte-specific PTEN knockout
mice. Sci Rep. 12:162062022. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Xue L, He J, Gao N, Lu X, Li M, Wu X, Liu
Z, Jin Y, Liu J, Xu J and Geng Y: Probiotics may delay the
progression of nonalcoholic fatty liver disease by restoring the
gut microbiota structure and improving intestinal endotoxemia. Sci
Rep. 7:451762017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Alisi A, Bedogni G, Baviera G, Giorgio V,
Porro E, Paris C, Giammaria P, Reali L, Anania F and Nobili V:
Randomised clinical trial: The beneficial effects of VSL#3 in obese
children with non-alcoholic steatohepatitis. Aliment Pharmacol
Ther. 39:1276–1285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Famouri F, Shariat Z, Hashemipour M,
Keikha M and Kelishadi R: Effects of probiotics on nonalcoholic
fatty liver disease in obese children and adolescents. J Pediatr
Gastroenterol Nutr. 64:413–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Saez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda
FJ, Plaza-Diaz J and Gil A: Effects of probiotics and synbiotics on
obesity, insulin resistance Syndrome, type 2 diabetes and
non-alcoholic fatty liver disease: A review of human clinical
trials. Int J Mol Sci. 17:9282016. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Eslamparast T, Poustchi H, Zamani F,
Sharafkhah M, Malekzadeh R and Hekmatdoost A: Synbiotic
supplementation in nonalcoholic fatty liver disease: A randomized,
double-blind, placebo-controlled pilot study. Am J Clin Nutr.
99:535–542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Mofidi F, Poustchi H, Yari Z, Nourinayyer
B, Merat S, Sharafkhah M, Malekzadeh R and Hekmatdoost A: Synbiotic
supplementation in lean patients with non-alcoholic fatty liver
disease: A pilot, randomised, double-blind, placebo-controlled,
clinical trial. Br J Nutr. 117:662–668. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Khalesi S, Johnson DW, Campbell K,
Williams S, Fenning A, Saluja S and Irwin C: Effect of probiotics
and synbiotics consumption on serum concentrations of liver
function test enzymes: A systematic review and meta-analysis. Eur J
Nutr. 57:2037–2053. 2018. View Article : Google Scholar
|
|
177
|
Gutiérrez-Grijalva EP, Antunes-Ricardo M,
Acosta-Estrada BA, Gutiérrez-Uribe JA and Basilio Heredia J:
Cellular antioxidant activity and in vitro inhibition of
α-glucosidase, α-amylase and pancreatic lipase of oregano
polyphenols under simulated gastrointestinal digestion. Food Res
Int. 116:676–686. 2019. View Article : Google Scholar
|
|
178
|
Pasavei AG, Mohebbati R, Boroumand N,
Ghorbani A, Hosseini A, Jamshidi ST and Soukhtanloo M:
Anti-Hypolipidemic and anti-oxidative effects of hydroalcoholic
extract of origanum majorana on the hepatosteatosis induced with
high-fat diet in rats. Malays J Med Sci. 27:57–69. 2020.PubMed/NCBI
|
|
179
|
Sharifi-Rad J, Quispe C, Turgumbayeva A,
Mertdinç Z, Tütüncü S, Aydar EF, Özçelik B, Anna SW, Mariola S,
Koziróg A, et al: Santalum Genus: phytochemical constituents,
biological activities and health promoting-effects. Z Naturforsch C
J Biosci. 78:9–25. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Fernández-Martínez E, Lira-Islas IG,
Cariño-Cortés R, Soria-Jasso LE, Pérez-Hernández E and
Pérez-Hernández N: Dietary chia seeds (Salvia hispanica) improve
acute dyslipidemia and steatohepatitis in rats. J Food Biochem.
43:e129862019. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Medina-Urrutia A, Lopez-Uribe AR, El
Hafidi M, González-Salazar MDC, Posadas-Sánchez R, Jorge-Galarza E,
Del Valle-Mondragón L and Juárez-Rojas JG: Chia (Salvia
hispanica)-supplemented diet ameliorates non-alcoholic fatty liver
disease and its metabolic abnormalities in humans. Lipids Health
Dis. 19:962020. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Diab F, Zbeeb H, Baldini F, Portincasa P,
Khalil M and Vergani L: The potential of lamiaceae herbs for
mitigation of overweight, obesity, and fatty liver: Studies and
perspectives. Molecules. 27:50432022. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Khalil M, Khalifeh H, Baldini F, Salis A,
Damonte G, Daher A, Voci A and Vergani L: Antisteatotic and
antioxidant activities of Thymbra spicata L. extracts in hepatic
and endothelial cells as in vitro models of non-alcoholic fatty
liver disease. J Ethnopharmacol. 239:1119192019. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Hu Q, Zhang W, Wu Z, Tian X, Xiang J, Li
L, Li Z, Peng X, Wei S, Ma X and Zhao Y: Baicalin and the liver-gut
system: Pharmacological bases explaining its therapeutic effects.
Pharmacol Res. 165:1054442021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhang J, Zhang H, Deng X, Zhang Y and Xu
K: Baicalin protects AML-12 cells from lipotoxicity via the
suppression of ER stress and TXNIP/NLRP3 inflammasome activation.
Chem Biol Interact. 278:189–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Li P, Zhang R, Wang M, Chen Y, Chen Z, Ke
X, Zuo L and Wang J: Baicalein prevents fructose-induced hepatic
steatosis in rats: in the regulation of fatty acid de novo
synthesis, fatty acid elongation and fatty acid oxidation. Front
Pharmacol. 13:9173292022. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Beltran Romero LM, Vallejo-Vaz AJ and
Muniz Grijalvo O: Cerebrovascular disease and statins. Front
Cardiovasc Med. 8:7787402021. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Pastori D, Pani A, Di Rocco A, Menichelli
D, Gazzaniga G, Farcomeni A, D'Erasmo L, Angelico F, Del Ben M and
Baratta F: Statin liver safety in non-alcoholic fatty liver
disease: A systematic review and metanalysis. Br J Clin Pharmacol.
88:441–451. 2022. View Article : Google Scholar
|
|
189
|
Lee JI, Lee HW, Lee KS, Lee HS and Park
JY: Effects of statin use on the development and progression of
nonalcoholic fatty liver disease: A nationwide nested case-control
study. Am J Gastroenterol. 116:116–124. 2021. View Article : Google Scholar
|
|
190
|
Sfikas G, Psallas M, Koumaras C, Imprialos
K, Perdikakis E, Doumas M, Giouleme O, Karagiannis A and Athyros
VG: Prevalence, diagnosis, and treatment with 3 different statins
of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis
in military personnel. do genetics play a role? Curr Vasc
Pharmacol. 19:572–581. 2021. View Article : Google Scholar
|
|
191
|
Yarahmadi S, Farahmandian N, Fadaei R,
Koushki M, Bahreini E, Karima S, Barzin Tond S, Rezaei A,
Nourbakhsh M and Fallah S: Therapeutic potential of resveratrol and
atorvastatin following high-fat diet uptake-induced nonalcoholic
fatty liver disease by targeting genes involved in cholesterol
metabolism and miR33. DNA Cell Biol. 42:82–90. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Husain NE, Hassan AT, Elmadhoun WM and
Ahmed MH: Evaluating the safety of Liptruzet (ezetimibe and
atorvastatin): What are the potential benefits beyond low-density
lipoprotein cholesterol-lowering effect? Expert Opin Drug Saf.
14:1445–1455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Kostapanos MS, Rizos CV and Elisaf MS:
Benefit-risk assessment of rosuvastatin in the treatment of
atherosclerosis and related diseases. Drug Saf. 37:481–500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Cho Y, Rhee H, Kim YE, Lee M, Lee BW, Kang
ES, Cha BS, Choi JY and Lee YH: Ezetimibe combination therapy with
statin for non-alcoholic fatty liver disease: An open-label
randomized controlled trial (ESSENTIAL study). BMC Med. 20:932022.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Seo SH, Lee DH, Lee YS, Cho KJ, Park HJ,
Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, et al: Co-administration
of ursodeoxycholic acid with rosuvastatin/ezetimibe in a
non-alcoholic fatty liver disease model. Gastroenterol Rep (Oxf).
10:goac0372022. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Pereira ENGDS, Araujo BP, Rodrigues KL,
Silvares RR, Martins CSM, Flores EEI, Fernandes-Santos C and Daliry
A: Simvastatin improves microcirculatory function in nonalcoholic
fatty liver disease and downregulates oxidative and ALE-RAGE
stress. Nutrients. 14:7162022. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Brault M, Ray J, Gomez YH, Mantzoros CS
and Daskalopoulou SS: Statin treatment and new-onset diabetes: A
review of proposed mechanisms. Metabolism. 63:735–745. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Ortiz-Lopez N, Fuenzalida C, Dufeu MS,
Pinto-León A, Escobar A, Poniachik J, Roblero JP, Valenzuela-Pérez
L and Beltrán CJ: The immune response as a therapeutic target in
non-alcoholic fatty liver disease. Front Immunol. 13:9548692022.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Du WW, Liu F, Shan SW, Ma XC, Gupta S, Jin
T, Spaner D, Krylov SN, Zhang Y, Ling W and Yang BB: Inhibition of
dexamethasone-induced fatty liver development by reducing miR-17-5p
levels. Mol Ther. 23:1222–1233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Barbosa-da-Silva S, Souza-Mello V,
Magliano DC, Marinho Tde S, Aguila MB and Mandarim-de-Lacerda CA:
Singular effects of PPAR agonists on nonalcoholic fatty liver
disease of diet-induced obese mice. Life Sci. 127:73–81. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Zhang N, Lu Y, Shen X, Bao Y, Cheng J,
Chen L, Li B and Zhang Q: Fenofibrate treatment attenuated chronic
endoplasmic reticulum stress in the liver of nonalcoholic fatty
liver disease mice. Pharmacology. 95:173–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Yaghoubi M, Jafari S, Sajedi B, Gohari S,
Akbarieh S, Heydari AH and Jameshoorani M: Comparison of
fenofibrate and pioglitazone effects on patients with nonalcoholic
fatty liver disease. Eur J Gastroenterol Hepatol. 29:1385–1388.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Liss KH and Finck BN: PPARs and
nonalcoholic fatty liver disease. Biochimie. 136:65–74. 2017.
View Article : Google Scholar :
|
|
204
|
Peters JM, Gonzalez FJ and Müller R:
Establishing the Role of PPARβ/δ in Carcinogenesis. Trends
Endocrinol Metab. 26:595–607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Sahebkar A, Chew GT and Watts GF: New
peroxisome proliferator-activated receptor agonists: Potential
treatments for atherogenic dyslipidemia and non-alcoholic fatty
liver disease. Expert Opin Pharmacother. 15:493–503. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Silva-Veiga FM, Rachid TL, de Oliveira L,
Graus-Nunes F, Mandarim-de-Lacerda CA and Souza-Mello V: GW0742
(PPAR-beta agonist) attenuates hepatic endoplasmic reticulum stress
by improving hepatic energy metabolism in high-fat diet fed mice.
Mol Cell Endocrinol. 474:227–237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Ratziu V, Harrison SA, Francque S, Bedossa
P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M,
Caldwell S, et al: Elafibranor, an agonist of the peroxisome
proliferator-activated Receptor-α and -δ, induces resolution of
nonalcoholic steatohepatitis without fibrosis worsening.
Gastroenterology. 150:1147–1159.e5. 2016. View Article : Google Scholar
|
|
208
|
Choudhary NS, Kumar N and Duseja A:
Peroxisome proliferator-activated receptors and their agonists in
nonalcoholic fatty liver disease. J Clin Exp Hepatol. 9:731–739.
2019. View Article : Google Scholar :
|
|
209
|
van der Veen JN, Lingrell S, Gao X,
Quiroga AD, Takawale A, Armstrong EA, Yager JY, Kassiri Z, Lehner
R, Vance DE and Jacobs RL: Pioglitazone attenuates hepatic
inflammation and fibrosis in phosphatidylethanolamine
N-methyltransferase-deficient mice. Am J Physiol Gastrointest Liver
Physiol. 310:G526–G538. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Cusi K, Orsak B, Bril F, Lomonaco R, Hecht
J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, et al:
Long-Term pioglitazone treatment for patients with nonalcoholic
steatohepatitis and prediabetes or type 2 diabetes mellitus: A
Randomized trial. Ann Intern Med. 165:305–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Jain MR, Giri SR, Bhoi B, Trivedi C, Rath
A, Rathod R, Ranvir R, Kadam S, Patel H, Swain P, et al: Dual
PPARα/ү agonist saroglitazar improves liver histopathology and
biochemistry in experimental NASH models. Liver Int. 38:1084–1094.
2018. View Article : Google Scholar
|
|
212
|
Gawrieh S, Noureddin M, Loo N, Mohseni R,
Awasty V, Cusi K, Kowdley KV, Lai M, Schiff E, Parmar D, et al:
Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A
Randomized controlled double-blind phase 2 trial. Hepatology.
74:1809–1824. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Boubia B, Poupardin O, Barth M, Binet J,
Peralba P, Mounier L, Jacquier E, Gauthier E, Lepais V, Chatar M,
et al: Design, synthesis, and evaluation of a novel series of
indole sulfonamide peroxisome proliferator activated receptor
(PPAR) α/γ/δ triple activators: Discovery of lanifibranor, a new
antifibrotic clinical candidate. J Med Chem. 61:2246–2265. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Wettstein G, Luccarini JM, Poekes L, Faye
P, Kupkowski F, Adarbes V, Defrêne E, Estivalet C, Gawronski X,
Jantzen I, et al: The new-generation pan-peroxisome
proliferator-activated receptor agonist IVA337 protects the liver
from metabolic disorders and fibrosis. Hepatol Commun. 1:524–537.
2017. View Article : Google Scholar
|
|
215
|
Mossanen JC, Krenkel O, Ergen C, Govaere
O, Liepelt A, Puengel T, Heymann F, Kalthoff S, Lefebvre E, Eulberg
D, et al: Chemokine (C-C motif) receptor 2-positive monocytes
aggravate the early phase of acetaminophen-induced acute liver
injury. Hepatology. 64:1667–1682. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Lefebvre E, Moyle G, Reshef R, Richman LP,
Thompson M, Hong F, Chou HL, Hashiguchi T, Plato C, Poulin D, et
al: Antifibrotic effects of the dual CCR2/CCR5 antagonist
cenicriviroc in animal models of liver and kidney fibrosis. PLoS
One. 11:e01581562016. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Friedman SL, Ratziu V, Harrison SA,
Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G,
Kowdley KV, Craxi A, et al: A randomized, placebo-controlled trial
of cenicriviroc for treatment of nonalcoholic steatohepatitis with
fibrosis. Hepatology. 67:1754–1767. 2018. View Article : Google Scholar
|
|
218
|
Ratziu V, Sanyal A, Harrison SA, Wong VW,
Francque S, Goodman Z, Aithal GP, Kowdley KV, Seyedkazemi S,
Fischer L, et al: Cenicriviroc treatment for adults with
nonalcoholic steatohepatitis and fibrosis: Final analysis of the
phase 2b CENTAUR Study. Hepatology. 72:892–905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Tacke F: Cenicriviroc for the treatment of
non-alcoholic steatohepatitis and liver fibrosis. Expert Opin
Investig Drugs. 27:301–311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Abenavoli L, Falalyeyeva T, Boccuto L,
Tsyryuk O and Kobyliak N: Obeticholic Acid: A new Era in the
treatment of nonalcoholic fatty liver disease. Pharmaceuticals
(Basel). 11:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Younossi ZM, Stepanova M, Nader F, Loomba
R, Anstee QM, Ratziu V, Harrison S, Sanyal AJ, Schattenberg JM,
Barritt AS, et al: Obeticholic acid impact on quality of life in
patients with nonalcoholic steatohepatitis: REGENERATE 18-Month
interim analysis. Clin Gastroenterol Hepatol. 20:2050–2058.e12.
2022. View Article : Google Scholar
|
|
222
|
Loomba R, Neuschwander-Tetri BA, Sanyal A,
Chalasani N, Diehl AM, Terrault N, Kowdley K, Dasarathy S, Kleiner
D, Behling C, et al: Multicenter validation of association between
decline in MRI-PDFF and histologic response in NASH. Hepatology.
72:1219–1229. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Neuschwander-Tetri BA, Loomba R, Sanyal
AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy
S, Diehl AM, Hameed B, et al: Farnesoid X nuclear receptor ligand
obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis
(FLINT): A multicentre, randomised, placebo-controlled trial.
Lancet. 385:956–965. 2015. View Article : Google Scholar :
|
|
224
|
Hernandez ED, Zheng L, Kim Y, Fang B, Liu
B, Valdez RA, Dietrich WF, Rucker PV, Chianelli D, Schmeits J, et
al: Tropifexor-Mediated abrogation of steatohepatitis and fibrosis
is associated with the antioxidative gene expression profile in
rodents. Hepatol Commun. 3:1085–1097. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Rau M and Geier A: An update on drug
development for the treatment of nonalcoholic fatty liver disease -
from ongoing clinical trials to future therapy. Expert Rev Clin
Pharmacol. 14:333–340. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Fiorucci S, Biagioli M, Sepe V, Zampella A
and Distrutti E: Bile acid modulators for the treatment of
nonalcoholic steatohepatitis (NASH). Expert Opin Investig Drugs.
29:623–632. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Xu X, Poulsen KL, Wu L, Liu S, Miyata T,
Song Q, Wei Q, Zhao C, Lin C and Yang J: Targeted therapeutics and
novel signaling pathways in non-alcohol-associated fatty
liver/steatohepatitis (NAFL/NASH). Signal Transduct Target Ther.
7:2872022. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Leong PK and Ko KM: Schisandrin B: A
double-edged sword in nonalcoholic fatty liver disease. Oxid Med
Cell Longev. 2016:61716582016. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Parker HM, Johnson NA, Burdon CA, Cohn JS,
O'Connor HT and George J: Omega-3 supplementation and non-alcoholic
fatty liver disease: A systematic review and meta-analysis. J
Hepatol. 56:944–951. 2012. View Article : Google Scholar
|
|
230
|
Li H, Liu NN, Li JR, Dong B, Wang MX, Tan
JL, Wang XK, Jiang J, Lei L, Li HY, et al: Combined use of bicyclol
and berberine alleviates mouse nonalcoholic fatty liver disease.
Front Pharmacol. 13:8438722022. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Zhan Z, Chen Y, Duan Y, Li L, Mew K, Hu P,
Ren H and Peng M: Identification of key genes, pathways and
potential therapeutic agents for liver fibrosis using an integrated
bioinformatics analysis. PeerJ. 7:e66452019. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Rey E, Melendez-Rodriguez F, Maranon P,
Gil-Valle M, Carrasco AG, Torres-Capelli M, Chávez S, Del
Pozo-Maroto E, Rodríguez de Cía J, Aragonés J, et al:
Hypoxia-inducible factor 2α drives hepatosteatosis through the
fatty acid translocase CD36. Liver Int. 40:2553–2567. 2020.
View Article : Google Scholar : PubMed/NCBI
|