Sepsis is a life-threatening condition characterized
by the dysregulated response of the body to infection, leading to
multiple organ dysfunction syndrome. It is a major factor
contributing to death in patients admitted to the intensive care
unit (ICU) (1-3). Sepsis-induced myopathy (SIM), also
known as ICU-acquired weakness, is a common complication associated
with sepsis. SIM is characterized by symmetrical atrophy, and
weakness of the respiratory system and limb skeletal muscles,
leading to prolonged mechanical ventilation, challenges in weaning
patients off ventilators and limb dysfunction (4-6).
This condition substantially affects the clinical course and
recovery of patients, exacerbating their overall morbidity and
prolonging their stay in the ICU (7,8).
The incidence of SIM is as high as 40% among
critically ill patients in the ICU (5-7).
SIM is associated with an increased risk of mortality and long-term
functional impairment (9-12).
Muscle wasting in sepsis occurs early and rapidly within the first
10 days of ICU admission (13).
A meta-analysis of 10 cohort studies including 2,396 patients with
sepsis found that patients with sepsis with sarcopenia had a
significantly higher risk of early mortality than patients with
sepsis without sarcopenia (8).
Furthermore, critically ill patients who survive often experience
reduced quality of life after discharge, primarily owing to the
decline in physical function caused by skeletal muscle atrophy
(14-16). Despite marked advancements in
medicine and technology, developing effective treatments for SIM
remains challenging.
Mesenchymal stem cells (MSCs) are pluripotent stem
cells derived from the mesoderm, and they are predominantly found
in mesenchymal and connective tissues. MSCs can differentiate into
bone, cartilage, muscle, fat and other tissues, and possess
anti-inflammatory, immunoregulatory and paracrine properties. MSCs
have shown efficacy in the treatment of cardiovascular diseases
(17,18), respiratory diseases (19,20), motor system disorders (21) and sepsis (22). Furthermore, several studies have
indicated that MSCs can enhance the function of organs affected by
sepsis, including the heart, lungs, liver and kidneys (23-26). These findings suggest that MSCs
may possess considerable potential in treating SIM. The present
review aims to summarize the sources and biological characteristics
of MSCs and their therapeutic potential in SIM.
MSCs are a class of pluripotent stem cells
originating from the mesoderm. They were first described by
Friedenstein et al (27)
in 1968 as adherent, fibroblast-like, non-hematopoietic precursor
cells. In 2006, the International Society for Cellular Therapy
established three primary criteria for defining MSCs: i) Adherence
to plastic in standard culture conditions; ii) expression of the
surface markers CD105, CD90 and CD73, along with the absence of
HLA-DR, CD34, CD45, CD19 and CD11b; and iii) the ability to
differentiate into osteoblasts, chondrocytes and adipocytes under
appropriate culture conditions in vitro (28).
MSCs were initially isolated from the bone marrow,
which remains their primary source; therefore, they are often
referred to as bone marrow-derived mesenchymal stromal cells
(BMSCs). In addition, MSCs are found in the adipose tissue, muscle,
tendon, umbilical cord, placenta, spleen, peripheral blood and
dental pulp (29-31). Some studies have identified
perivascular cells as another source of MSCs. These supportive
cells of the vessel wall exhibit chemotactic activity in response
to inflammation and injury, and have an MSC-like phenotype
(32).
Owing to their robust proliferative capacity and
multidirectional differentiation potential, MSCs can differentiate
into myocytes, osteoblasts, chondrocytes, adipocytes, hepatocytes,
stromal cells and other cell types under suitable culture
conditions (29-32). Furthermore, MSCs possess
anti-inflammatory (33) and
immunomodulatory properties (34,35). Upon injury or inflammation, MSCs
migrate to the site of damage and interact with immune cells to
secrete cytokines, scavenge pathogens, suppress inflammatory
responses, and reduce oxidative stress and apoptosis (36). The mechanisms through which MSCs
repair tissue damage include: i) Direct differentiation or cell
fusion; ii) transfer of exosomes, cytokines or organelles to
injured cells through tunnelling nanotubes (TNTs); and iii)
promotion of tissue repair by releasing cytokines in a paracrine
manner (37). MSCs are widely
available, and easy to extract, culture and expand. The lack of
major histocompatibility complex II or co-stimulatory factors
endows MSCs with specific immune-privileged properties (38). These characteristics make MSC
transplantation a viable treatment option for various diseases
(39,40).
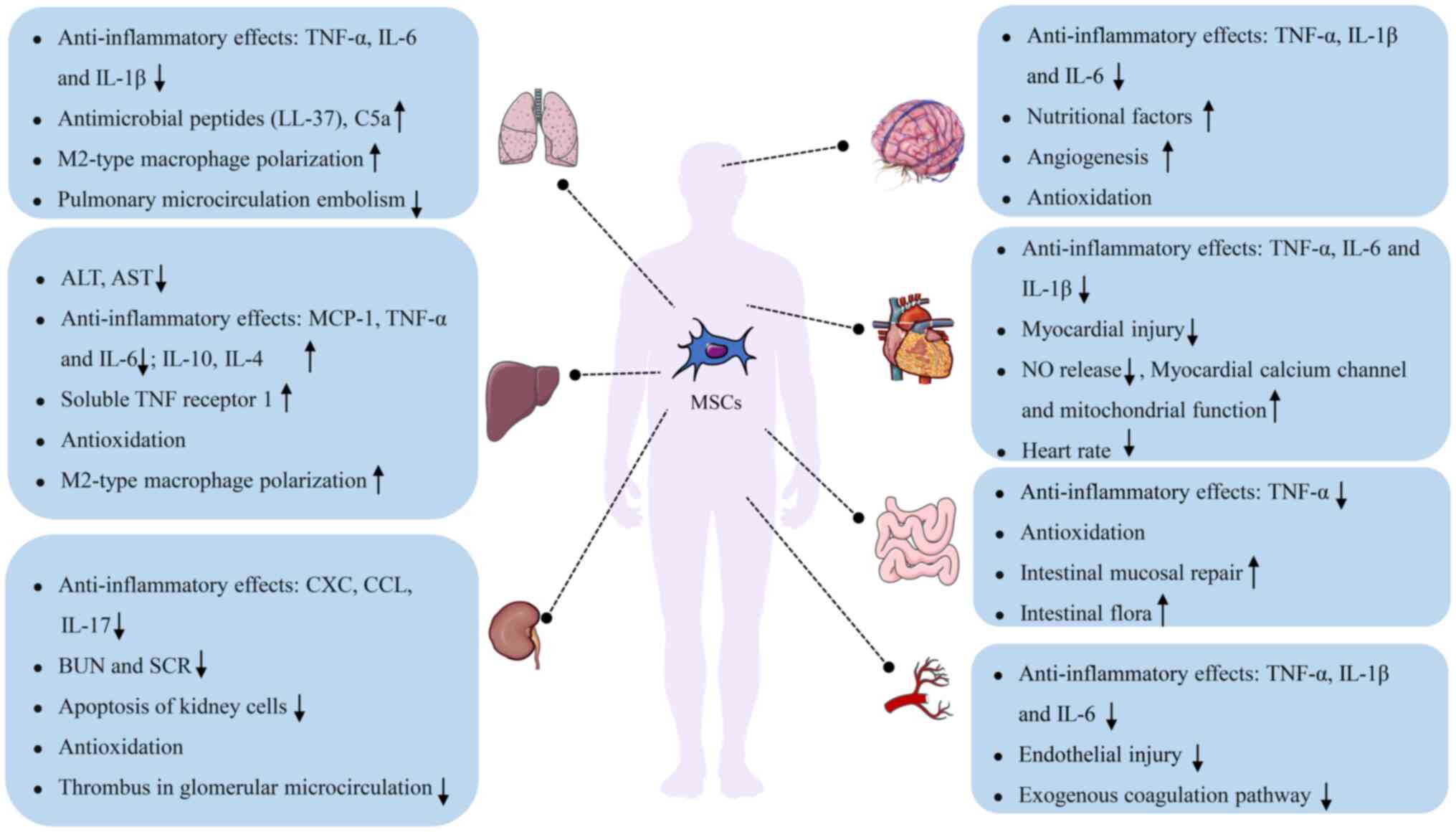
Owing to their immunomodulatory properties, MSCs can
inhibit the release of inflammatory cytokines in the lungs during
sepsis, thereby alleviating lung injury. Studies have demonstrated
that MSCs can reduce the number of inflammatory cells in the
alveolar lavage fluid of septic mice and inhibit the release of
inflammatory factors, such as IL-1β, IL-6 and TNF-α, thereby
correcting the immune imbalance (51-53). Chen et al (54) revealed that MSCs used in
combination with melatonin reduced the number of CD14+
and CD68+ T cells, and the levels of IL-6 in the lungs
during acute lung injury in sepsis, thereby attenuating the
inflammatory response. Moreover, MSCs secrete antimicrobial
peptides (such as LL-37), complement components (such as C5a) and
other antimicrobial substances to inhibit the proliferation of
pathogenic microorganisms and promote macrophage polarization
toward the M2 phenotype, enhancing the phagocytic activity of
macrophages and reducing the intrapulmonary microbial load
(55-59). Activation of the exogenous
coagulation pathway and formation of microthrombi in pulmonary
capillaries are other crucial causes of septic lung injury. Tan
et al (60) validated
that MSCs reduced thrombin levels in pulmonary microcirculation,
alleviated microcirculation embolism and improved pulmonary blood
circulation. Furthermore, MSCs can alleviate sepsis-induced lung
injury by regulating the expression of microRNAs (miRNAs) in
exosomes (61,62).
Sepsis-induced myocardial dysfunction (SIMD) is one
of the most common complications of sepsis. The pathogenic factors
of SIMD include the release of myocardial depressant factor,
upregulation of NO, impairment of myocardial calcium homeostasis
and mitochondrial dysfunction. SIMD manifests primarily as
myocardial ischemia and hypoxia, systolic myocardial dysfunction,
and reduced left ventricular ejection fraction (EF), resulting in
inadequate tissue and organ perfusion (63,64). TLRs on cardiomyocyte membranes
activate downstream mTOR/NF-κB and mTOR/Akt signaling pathways by
recognizing pathogen-associated molecular patterns and
danger-associated molecular patterns, resulting in the release of
inflammatory factors, such as TNF-α and IL-6, which directly
contribute to myocardial injury (65-67). These inflammatory factors
activate myocardial endothelial cells, leading to increased
secretion of inducible NO synthase (iNOS) and excessive production
of NO. Excess NO inhibits type I calcium channels and myocardial
mitochondrial function, resulting in systolic dysfunction and
decreased cardiac output (68-70). Additionally, the ratio of
anti-apoptotic to pro-apoptotic proteins decreases in sepsis,
leading to myocardial damage (71).
Several studies have reported that MSCs attenuate
inflammatory cell infiltration in cardiomyocytes during sepsis,
alleviate myocardial injury, and improve myocardial contractility.
Wu et al (72) reported
that MSCs can markedly improve cardiac EF by reducing the
expression of TLRs; inhibiting the NF-κB signaling pathway; and
decreasing the levels of inflammatory factors, such as IL-1β, IL-6
and TNF-α, in the plasma and myocardium of septic mice. Weil et
al (73) showed that, in
addition to reducing the levels of myocardial inflammatory factors,
MSCs can decrease the frequency of myocardial contraction, thereby
improving myocardial function. Additionally, MSCs have been shown
to secrete exosomes enriched with miRNA-223, which inhibits
myocardial inflammatory responses and alleviates myocardial injury
(74). Taken together, MSCs can
attenuate the inflammatory response, inhibit NO release, improve
myocardial calcium channel activity and mitochondrial function, and
alleviate myocardial injury in sepsis by inhibiting inflammatory
signaling pathways and secreting exosomes.
Amplification of the inflammatory response is the
primary cause of sepsis-induced liver injury. MSCs can inhibit the
inflammatory response and the migration of inflammatory cells to
the liver, thereby alleviating liver injury. Several studies have
shown that MSCs can inhibit the release of TNF-α, IL-6 and monocyte
chemotactic protein (MCP)-1, and upregulate the anti-inflammatory
factors IL-10 and IL-4 to correct immune imbalance. In addition,
MSCs can suppress the migration and activation of neutrophils in
the liver, thereby alleviating liver damage caused by inflammation
(80-82). Studies have demonstrated that
adipose tissue-derived MSCs secrete TNF receptor 1, and attenuate
apoptosis and inflammatory responses in the liver, thus improving
the survival of septic mice (83,84). Furthermore, MSCs can promote
macrophage polarization toward the M2 phenotype, clear pathogens,
increase intrahepatic glycogen reserves, reduce hepatic oxidative
stress, and decrease the plasma levels of aspartate
aminotransferase and alanine aminotransferase (59).
Sepsis-associated kidney injury (SAKI) involves the
development of structural and functional abnormalities in the
kidney following sepsis in patients without pre-existing kidney
injury (85,86). Crucial factors involved in the
pathogenesis of SAKI include inflammatory responses, abnormal
intrarenal microcirculation, and altered bioenergetic processes of
renal cells (85,86). The global incidence of SAKI in
patients with sepsis ranges from 19 to 23%, whereas that in
patients with septic shock is substantially higher, ranging from 51
to 66.9% (85,86). The excessive release of
inflammatory factors in sepsis causes damage to glomerular
capillary endothelial cells and tubular epithelial cells, resulting
in increased glomerular capillary permeability and decreased
glomerular filtration rate (GFR) (87,88). Furthermore, inflammatory factors
can induce the release of tissue factor and activate the exogenous
coagulation pathway, leading to the formation of microthrombi in
renal microcirculation. Owing to ischemia and hypoxia, the
production of ROS is increased in renal tissues, causing increased
mitochondrial permeability and decreased mitochondrial membrane
potential, leading to mitochondrial dysfunction and worsening of
renal injury (89,90).
Studies have shown that MSCs can reduce the
incidence of SAKI and attenuate renal tissue damage. Luo et
al (91) showed that
treatment with 106 MSCs within 3 h of induction of
sepsis in mice reduced the incidence of SAKI when compared with
control mice. After 24 h of induction, MSCs reduced the release of
inflammatory factors, such as CXC, CCL and IL-17, and inhibited the
migration of neutrophils to the kidney, improving renal tubular
function and prolonging survival in mice with sepsis. Cóndor et
al (92) demonstrated that
umbilical cord Wharton's jelly-derived MSCs suppressed renal cell
apoptosis, NF-κB expression, and the release of IL-1 and IL-6,
thereby improving GFR and renal tubular function in sepsis. The
combined use of adipose-derived MSCs (AMSCs) and exendin-4 (a
glucagon-like peptide-1 analogue) can reduce renal oxidative stress
and fibrosis in sepsis (93),
and the combined use of MSCs and melatonin has been shown to
substantially reduce NF-κB expression and the release of
inflammatory factors (94).
Additionally, MSCs can inhibit thrombosis in glomerular
microcirculation, prevent the destruction of glomerular endothelial
cells and restore renal function (90,92).
Sepsisassociated encephalopathy (SAE) is
characterized by confusion, coma and other changes in consciousness
(95,96). Notably, 10-20% of patients with
SAE experience long-term cognitive dysfunction worldwide (97). The pathogenesis of SAE primarily
involves neuroinflammation, blood-brain barrier (BBB) impairment,
cerebrovascular microcirculation disorder and mitochondrial
dysfunction (95,96). MSCs can inhibit neuroinflammatory
responses and promote the restoration of intracerebral
microcirculation, thereby improving cognitive dysfunction following
sepsis. Studies have demonstrated that MSCs injected via the
internal jugular vein and peripheral vein can cross the BBB and
colonize damaged sites in the brain (98,99). These infiltrating cells have been
shown to inhibit the secretion of IL-6, IL-1β and TNF-α, and the
proliferation and activation of microglia, consequently attenuating
the inflammatory response (100-102). Tan et al (103) and Silva et al (104) demonstrated that MSCs improved
cognitive function in septic mice by inhibiting neurological and
peripheral inflammation. Studies have demonstrated that MSCs can
secrete various trophic factors, including brain-derived
neurotrophic factor, which promotes the differentiation of new
neurons, and vascular endothelial growth factor (VEGF) and
hepatocyte growth factor (HGF), which promote local vascular
regeneration and improve blood circulation in brain tissue
(105-108). MSCs can transfer their
mitochondria to damaged cells via membrane channels (TNTs), thereby
continuing aerobic respiration and reducing ROS production. In
addition, they can alleviate mitochondrial dysfunction and reduce
oxidative stress (109). Liu
et al (110) showed that
olfactory mucosa-derived MSCs may promote the expression of UBIAD1,
improving mitochondrial function. Cao et al (111) and Wang et al (112) demonstrated that MSCs could
inhibit the expression of brain-iNOS and NADPH oxidase, and that
MSC-derived exosomes contain antioxidant components, such as
miRNAs, which may alleviate damage caused by oxidative stress
(113).
Intestinal dysfunction can increase the global
morbidity and mortality rates of patients with sepsis in the ICU to
as high as 41.9% (114). MSCs
can protect against sepsis-induced intestinal dysfunction by
attenuating the intestinal inflammatory response, enhancing
mitochondrial function and improving microbial diversity. Studies
have demonstrated that MSCs may inhibit the production of the
inflammatory factor TNF-α and promote the secretion of the
anti-inflammatory factor IL-10 by modulating dendritic cell
function (115,116). Chen et al (117) and Koliaraki et al
(118) showed that MSCs can
respond to TNF, inhibit the production of TNF-α and IL-12, and
promote the secretion of IL-4 and IL-10. Parikh et al
(119) and Zheng et al
(120,121) showed that MSC-derived
microvesicles could deliver mfn2 and PGC-1α to endothelial cells,
promoting mitochondrial production and delivering the mitochondria
directly to damaged endothelial cells to improve cellular energy
metabolism and reduce oxidative stress-induced damage. Phinney
et al (122)
demonstrated that MSCs can promote mitochondrial autophagy.
Additionally, MSCs have been shown to promote intestinal mucosal
repair and increase the diversity of intestinal flora (123). For example, Valcz et al
(124) showed that MSCs can
colonize damaged intestinal mucosa and differentiate into
intestinal mesenchymal or epithelial cells. Hayashi et al
(125) also found that MSCs can
secrete VEGF and TGF to promote the repair of damaged intestinal
mucosa. Furthermore, MSCs may improve the diversity of intestinal
flora, regulate the secretion of IgA and maintain intestinal
symbiotic homeostasis (126).
SIC is characterized by an enhanced coagulation
response and impairment of anticoagulation mechanisms, leading to
extensive microthrombosis (127). The incidence of SIC in patients
with sepsis is 50-70%, with ~35% of patients progressing to
disseminated intravascular coagulation worldwide (128). The pathogenesis of SIC
primarily includes the release of inflammatory mediators,
endothelial cell injury, activation of the exogenous coagulation
pathway, and inhibition of the anticoagulation and fibrinolytic
systems (129). Studies have
validated that MSCs possess potent anti-inflammatory and
immunomodulatory functions. For example, Wang et al
(130,131) and Miao et al (82) demonstrated that MSCs could
inhibit the activity of the NLRP3 inflammasome, reduce the levels
of IL-1β, IL-6 and TNF-α, and promote the secretion of IL-10.
Furthermore, MSCs can alleviate vascular endothelial cell injury,
thereby improving the anticoagulant function of protein C. Notably,
Baudry et al (132)
showed that MSCs pretreated with interferon-γ (IFNγ) reduced
selectin-E levels and increased intercellular adhesion molecule-1
levels, thereby accelerating leukocyte flow in the blood, reducing
leukocyte adhesion and attenuating vascular endothelial cell injury
in septic mice (132). By
improving the anticoagulant function of protein C, MSCs can
substantially decrease the levels of von Willebrand factor and
tissue factor in plasma, inhibit the exogenous coagulation pathway
and reduce thrombin production, consequently ameliorating SIC
(60).
MSCs possess a notable capacity for directed
differentiation, which enables them to transform into skeletal
muscle cells under appropriate conditions (133,134). In vitro studies have
demonstrated that the transcription factor Pax-3 can induce the
differentiation of MSCs to myogenic cells (135), whereas intracellular structural
domain genes can drive the differentiation of MSCs to skeletal
muscle cells (136).
Additionally, mechanical traction can stimulate BMSCs to
differentiate into skeletal muscle cells (137). An in vivo study
validated that embryonic MSCs (EMSCs) promoted the repair of
injured tibialis anterior muscle in mice, with >60% of EMSCs
differentiating into skeletal myocytes (138).
In addition to their ability to differentiate into
skeletal muscle cells, MSCs can enhance the functional recovery of
injured skeletal muscles. In a previous study, BMSCs isolated from
the tibia and femur of green fluorescent protein-expressing
transgenic Sprague-Dawley rats were cultured and expanded in
vitro. After these BMSCs were transplanted at the site of
skeletal muscle injury, the contractile force of the injured muscle
reached close to pre-injury levels after 1 month. By contrast, the
contractile force in control rats recovered to only ~80% of the
pre-injury levels (139).
Another study showed that MSCs improved skeletal muscle function in
a cell density-dependent manner, with the most significant
treatment effect being observed when the number of transplanted
MSCs was 10×106 (140). Notably, the treatment effect
was not influenced by the timing of MSC transplantation or the sex
of rats (141,142). Furthermore, MSCs have been
shown to promote skeletal muscle angiogenesis and increase blood
flow. In a previous study, transplantation of hypoxic MSCs in the
hindlimb of mice resulted in a 2-fold increase in the number of
skeletal muscle capillaries and a 7-fold increase in the number of
vascular connections and branches when compared with the control
group (139). Additionally,
studies have shown that MSCs hold great promise in the treatment of
various skeletal myopathies, such as Duchenne muscular dystrophy
(143), skeletal muscle
denervation atrophy (144) and
traumatic skeletal muscle injury (145). These studies provide diverse
avenues for further research on MSCs and the treatment of SIM using
MSCs.
Skeletal muscle, accounting for 35-45% of body
weight in adult humans, is essential for maintaining all
physiological activities. Promoting muscle regeneration and repair
in patients with SIM is crucial for improving prognosis and quality
of life. Factors that lead to skeletal muscle injury and
dysfunction following sepsis include increased protein hydrolysis
and decreased protein synthesis (146,147), oxidative stress (148), release of inflammatory
mediators (149,150), skeletal muscle apoptosis
(151), and skeletal muscle
vascular damage. Skeletal muscle repair involves three stages, as
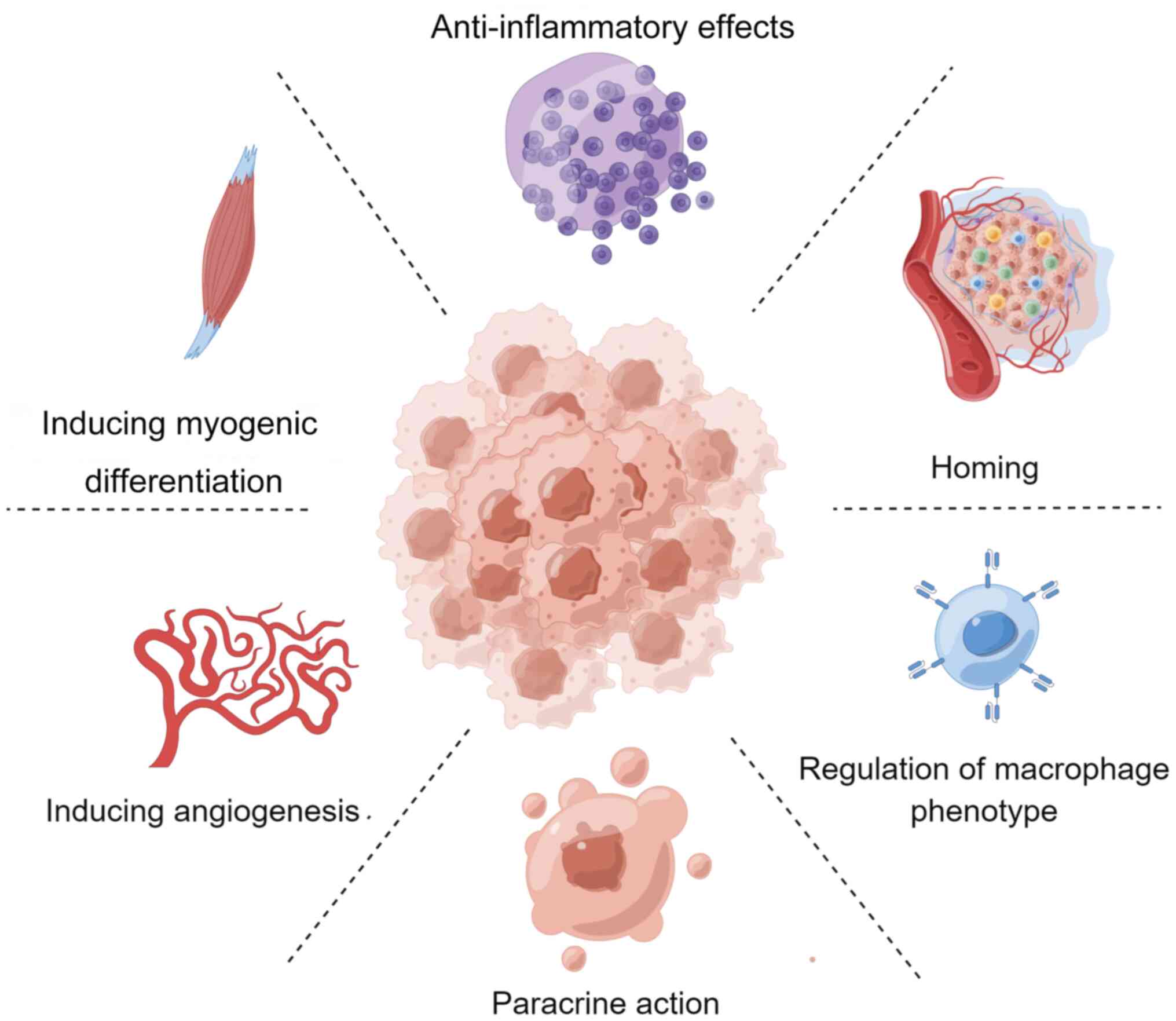
follows: Inflammatory response, repair and remodeling. MSCs may
enhance skeletal muscle function in SIM through numerous mechanisms
(Fig. 2).
Owing to their multidirectional differentiation
potential, MSCs can differentiate into skeletal muscle cells under
appropriate conditions to directly repair muscle injury (152). Orlic et al (153) suggested that different
environments lead to varying MSC differentiation rates, and that
direct contact with injured organs or target cells is crucial for
myogenic differentiation of MSCs. Egusa et al (154) showed that arranging BMSCs
according to skeletal muscle fibers markedly improved the
efficiency of the transformation of BMSCs to skeletal muscle.
Studies have shown that treatment of MSCs with 5-azacytidine
(5-AZA) may result in the formation of multinucleated myotubes
expressing myosin after 7-11 days (155-157). Meligy et al (158) treated BMSCs, AMSCs and skeletal
muscle-derived MSCs with 5-AZA and demonstrated that MSCs highly
expressed myostatin after 1 week of treatment. In addition,
microsatellite cells serve an essential role in the development and
regeneration of skeletal muscles. These cells are dormant under
physiological conditions but are activated upon muscle injury.
Activated microsatellite cells can differentiate into myogenic
cells that fuse to form myotubes (159,160). MSCs have been reported to
secrete fibroblast growth factor (FGF), HGF and insulin-like growth
factor-1 (IGF-1) to induce myogenic differentiation of
microsatellite cells (161,162).
Homing refers to the process by which MSCs are
captured in the vascular system of the target tissue and
subsequently migrate across the vascular endothelium to the target
tissue (163). During
inflammation or injury, various signaling molecules, such as
chemokines, adhesion factors and growth factors, are locally
released from the injured tissue, inducing MSCs to migrate to that
tissue. Homing is crucial for the safe and effective clinical
application of MSCs (164,165). The stromal cell-derived factor
1 (SDF-1)/CXCR-4 axis, comprising SDF-1 and its ligand CXCR-4,
serves a vital role in MSC homing. Upon tissue injury, SDF-1α
secretion increases, and MSCs expressing CXCR-4 migrate to the
injury site along the SDF-1α concentration gradient to participate
in tissue repair (166-169). Additionally, MCP (170,171), VEGF (172), HGF (173) and integrins (174) are essential for MSC homing.
Ferrari et al (175)
used BMSCs to treat injured muscles and found that BMSCs can
migrate to the injury site, differentiating into skeletal muscle
cells and promoting the regeneration of injured muscle fibers.
Winkler et al (176)
used MRI to visualize that labelled MSCs migrated to damaged muscle
fibers and fused with them 24 h post-transplantation.
In sepsis, the immune function becomes dysregulated,
resulting in an uncontrolled inflammatory response, and the release
of large amounts of TNF-α, IL-6 and other inflammatory factors.
TNF-α promotes the secretion of iNOS, leading to muscle injury,
whereas IL-6 has been shown to inhibit the myogenic differentiation
of the C2C12 myotube cell line and the elongation of muscle protein
peptide chains, causing muscle atrophy (149). The excessive release of
inflammatory factors is a key driver of muscle injury (149). MSCs have powerful
anti-inflammatory functions and can restore immune balance, an
essential mechanism by which MSCs repair tissue damage (177). AMSCs have been shown to inhibit
the expression of inflammatory factors, such as TNF-α, IL-6 and
ROS, in injured gastrocnemius muscle while upregulating IL-4 and
IL-10 levels to suppress the inflammatory response and repair
muscle injury (178). BMSCs can
reduce muscle fibrosis by inhibiting TGF-β1 expression. Moreover,
MSCs can inhibit the activity of natural killer cells, preventing
IFN-γ and TNF-α from exerting their effects, and can attenuate the
muscle inflammatory response by inhibiting dendritic cell
maturation.
Macrophages are heterogeneous immune cells that can
be classified into two phenotypes: M1 and M2, based on their
function and markers. M1-type macrophages secrete inflammatory
factors, such as TNF-α, IL-1α, IL-6 and IL-12, which can inhibit
the myogenic differentiation of C2C12 myotubes and lead to skeletal
muscle injury (179-181). Conversely, M2-type macrophages
can secrete anti-inflammatory factors, remove pathogens and
apoptotic cells, promote myogenic differentiation of C2C12 cells,
attenuate the skeletal muscle inflammatory response and facilitate
muscle repair (179-181). When MSCs are co-cultured with
macrophages in vitro, the inflammatory factors secreted by
M1-type macrophages have been shown to activate MSCs. In turn, MSCs
secrete anti-inflammatory factors, such as IL-10, IL-4 and TGF-β,
which can promote the conversion of M1-type macrophages to M2-type
macrophages. The release of these mediators is crucial for
mediating M2-type macrophage polarization (182). MSCs can also induce M2-type
macrophage polarization through the exosome pathway. MSC-derived
exosomes have been reported to promote the conversion of M1-type
macrophages to M2-type macrophages in infarcted myocardium, thereby
reducing the local inflammatory response in a mouse model (182). Exosomal miRNA sequencing has
revealed that miRNA-182 in MSC exosomes may mediate M2-type
macrophage polarization (183-185). Additionally, MSCs can
facilitate M2-type macrophage polarization via the regulatory T
cell pathway (186).
Paracrine secretion underpins the application of
stem cells in tissue regeneration and constitutes a critical
mechanism by which MSCs repair skeletal muscle injury. MSCs
regulate skeletal muscle repair by releasing various growth factors
and exosomes through the paracrine pathway. Studies have confirmed
that MSCs secrete IGF-1, VEGF, sphingosine 1-phosphate (S1P) and
other cellular growth factors involved in skeletal muscle injury
repair (122,137). VEGF has been shown to promote
capillary endothelial cell proliferation and the myogenic
differentiation of C2C12 cells (187), while IGF-1 can enhance
endothelial cell migration to the injury site and repair local
blood circulation (188). S1P
reduces skeletal muscle apoptosis, promotes myogenic proliferation,
and stimulates satellite cell proliferation and differentiation
(189). Moreover, MSC-derived
exosomes serve an essential role in skeletal muscle repair. It has
been found that BMSC-derived exosomes contain FGF-2,
platelet-derived growth factor-BB and recombinant granulocyte
colony-stimulating factor, which are associated with skeletal
muscle regeneration (137,188). Exosomes are also enriched with
miRNAs and proteins crucial for skeletal muscle injury repair. For
example, miRNA-21 in exosomes inhibits apoptosis, and miRNA-494
promotes MSC myogenic differentiation and vascular regeneration
(122,190). Studies have demonstrated that
exosomes are enriched in annexin A1, which is essential for
myofilament repair after skeletal muscle injury (191,192). Similarly, it has been shown
that exosomes contain various proteins related to skeletal muscle
injury repair, such as filament proteins and myosins, which can
promote the proliferation of skeletal muscle satellite cells and
muscle repair (193-195).
Vascular regeneration has a crucial role in
repairing skeletal muscle injury, and studies have shown that MSCs
can promote this process by secreting cytokines. In 2000, Oswald
et al (196) first
applied VEGF to induce BMSCs to differentiate into vascular
endothelial cells, although the differentiation efficiency was low.
Since then, researchers have significantly increased the efficiency
of vascular endothelial cell differentiation by adding VEFG, IGF-1,
FGF and S1P to MSC culture media (197,198). Sassoli et al (187) found that MSCs secrete large
amounts of VEGF, which promotes skeletal muscle vascular
regeneration by activating the Notch-1 signaling pathway. MSCs also
activate the TLR-2/TLR-6 pathway to enhance vascular regeneration
through paracrine secretion. Grote et al (199) significantly increased the
secretion of growth factors and cytokines, such as VEGF and
granulocyte-macrophage colony-stimulating factor, by adding the
TLR-2/TLR-6 agonist MLP-2 to MSC culture media. Additionally,
Leroux et al (200)
found that hypoxia-pretreated MSCs transplanted into skeletal
muscle showed increased Wnt4 expression, and a significant increase
in skeletal muscle vascular regeneration and blood flow, suggesting
that MSCs promote vascular regeneration through the Wnt4 signaling
pathway.
MSCs have significant differentiation potential and
can repair skeletal muscle injury through various mechanisms.
However, several limitations hinder the clinical application of
MSCs in treating skeletal muscle injuries.
Firstly, the optimal tissue source of MSCs remains
undetermined. MSCs are primarily derived from bone marrow, adipose
tissue, cord blood, peripheral blood and dental pulp, with bone
marrow being the most common source. However, acquiring BMSCs
requires invasive procedures, the isolation rate is low
(0.001-0.01%), and the multi-directional differentiation ability of
BMSCs decreases with age. Peripheral blood-derived MSCs are easy to
obtain and possess the most potent immunosuppressive function, but
they take longer to cultivate in vitro (201,202). AMSCs have a similar
immunomodulatory capacity to dental pulp-derived MSCs (DPMSCs);
AMSCs are easy to obtain and have a high cell isolation rate (3%),
whereas DPMSCs have strong proliferative capacity (203). However, cord blood-derived MSCs
exhibit a higher potential for multi-directional differentiation
(203).
Secondly, the administration route for MSCs,
whether local or systemic, remains controversial in terms of
effectiveness. Local application at the injury site is fast-acting
but carries a risk of bleeding (204). Systemic administration methods
include subcutaneous, intramuscular, intravenous and
intraperitoneal injections, each with varying efficacy.
Castelo-Branco et al (205) reported that intraperitoneal but
not intravenous administration of MSCs exhibited better homing and
anti-inflammatory effects, while Gonçalves et al (206) reported conflicting findings.
Roux et al (207)
preferred intraperitoneal injection due to the lower probability of
pulmonary embolism. Intramuscular and subcutaneous injections can
prolong the duration of MSCs in vivo, with intramuscular
injections showing retention for up to 100 days; however, data on
intramuscular injections remain insufficient (204,208).
Finally, the optimal therapeutic dose of MSCs is
not well-defined. Although studies have shown that administering
12×109 MSCs is safe, it does not imply that higher doses
are more effective. MSCs possess anti-apoptotic, self-replicating
and growth-regulating abilities, similar to tumor cells, and
administering large doses may pose safety risks (209,210). Some studies have indicated that
the effective minimum dose ranges from 10×108 to
15×108, with an optimal dose for aging patients at
10×108. Dose response data showing differential efficacy
for improved outcomes were reported in three trials, which
indicated that there was no significant difference between the
therapeutic effects of 10×108 and 20×108
(211-213). Therefore, further studies are
needed to validate the optimal dose for treating skeletal muscle
diseases.
Although advancements in medical technology have
improved the early diagnosis and treatment of sepsis, multiple
organ failure remains a leading cause of death in patients with
sepsis (214). MSCs have
emerged as a promising therapeutic option owing to their
immunomodulatory, anti-inflammatory and regenerative properties
(60,82,104,121). The present review highlights
the therapeutic role of MSCs in sepsis-induced organ failure,
focusing on lung injury, myocardial injury, kidney injury,
encephalopathy and intestinal dysfunction. Existing studies have
suggested that MSCs possess marked potential in the treatment of
sepsis-induced organ failure. An in-depth understanding of the
mechanisms of MSCs and a comparison of their efficacy across
different organs may expand their application, eventually improving
the outcomes and reducing the mortality rate of sepsis.
SIM results from a complex interplay of
inflammation, oxidative stress and metabolic dysregulation
(4-6,215,216). It not only results from sepsis
but also influences the progression and outcomes of the disease
(215). It contributes to
malnutrition and negative nitrogen balance, weakening the overall
condition and immune response of patients, thus making it more
challenging to combat the infection (8,217). Additionally, atrophy of
respiratory muscles, such as the diaphragm, can impair breathing
and increase the likelihood of respiratory failure (218), necessitating prolonged
mechanical ventilation, which in turn increases morbidity and
mortality (218). Therefore,
SIM is closely associated with the progression of sepsis in a cycle
that worsens clinical outcomes. Addressing skeletal muscle atrophy
through early intervention, nutritional support, physical
rehabilitation and novel therapeutic approaches can help disrupt
this cycle, and improve recovery and survival rates in patients
with sepsis.
MSCs possess several characteristics that make them
promising candidates for treating SIM. First, MSCs exert
anti-inflammatory and immunomodulatory effects, which can attenuate
the excessive inflammatory response caused by sepsis (111). Second, MSCs can secrete various
growth factors and cytokines that promote tissue repair and
regeneration (219). They can
enhance the repair and regeneration of skeletal muscles by
secreting exosomes and microvesicles that deliver beneficial
molecules to the damaged site (220). Third, the antioxidant activity
of MSCs can alleviate oxidative stress-induced damage in muscle
cells. In particular, MSCs decrease the production of free radicals
by improving the intracellular redox balance, thereby protecting
muscle cells from oxidative stress-induced injury (221). Although preclinical studies
have strongly supported the potential benefits of MSCs in the
treatment of sepsis, further clinical research is warranted to
translate the findings into effective treatments. A better
understanding of the use of MSCs may help improve clinical outcomes
and reduce the long-term impact of sepsis on skeletal muscle
health.
SIM is a common complication in patients with
sepsis, significantly affecting prognosis and quality of life, with
a lack of effective therapeutic measures. MSCs have emerged as a
novel treatment for certain refractory diseases, and have
demonstrated good clinical efficacy in treating tissue repair,
skeletal muscle diseases and sepsis-related conditions. The present
review specifically focused on the mechanisms and therapeutic
potential of MSCs in SIM. While there have been some studies on the
application of MSCs in sepsis, a focused review on SIM is still
lacking. Secondly, this review integrated the latest experimental
and clinical data, providing comprehensive evidence for the
application of MSCs in SIM. By synthesizing these data, this review
not only highlighted the therapeutic potential of MSCs but also
detailed their mechanisms of action. This may help to fill the
current gaps in the literature, and offers valuable guidance for
future research and clinical practice. Finally, a detailed
comparison of the efficacy of MSCs in treating damage across
different organs was conducted, including the lungs, heart,
kidneys, brain and intestines. This comparative analysis offers a
new perspective, demonstrating the role of MSCs in multi-organ
protection. By elucidating how MSCs can mitigate damage in various
organs, the present study provides a holistic view of their
therapeutic potential, which is crucial for developing effective
treatment strategies for SIM. Therefore, the present study may
improve the understanding and application of MSCs in SIM, and their
underlying mechanisms. However, skeletal muscle repair is a highly
complex biological process. The mechanisms, efficiency and safety
of MSCs in treating SIM have not yet been fully elucidated,
necessitating further research to clarify these aspects. More
clinical studies are required to obtain sufficient data to verify
the safety and therapeutic effects of MSCs in treating SIM. As
research on MSCs progresses, there is reason to believe that MSCs
will become an effective treatment for SIM in the near future.
Not applicable.
All authors contributed to the design of the study
and writing of the manuscript. DW, SQ, LX, YL and CW contributed to
the literature collection, analysis and interpretation for writing
this review. DW and YW wrote the main manuscript text and prepared
the figures. ZL, XB and YL revised the article critically for
important intellectual content. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study was supported by grants from the National Natural
Science Foundation of China (grant nos. 82002101 and 82002096).
|
1
|
Tupchong K, Koyfman A and Foran M: Sepsis,
severe sepsis, and septic shock: A review of the literature. Afr J
Emerg Med. 5:127–135. 2015. View Article : Google Scholar
|
|
2
|
Evans L, Rhodes A, Alhazzani W, Antonelli
M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M,
Prescott HC, et al: Surviving sepsis campaign: International
guidelines for management of sepsis and septic shock 2021. Crit
Care Med. 49:e1063–e1143. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matthaeus-Kraemer CT, Thomas-Rueddel DO,
Schwarzkopf D, Rueddel H, Poidinger B, Reinhart K and Bloos F:
Crossing the handover chasm: Clinicians' perceptions of barriers to
the early detection and timely management of severe sepsis and
septic shock. J Crit Care. 36:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Xu L, Yang Z, Wang D, Li T, Yang F,
Li Z, Bai X and Wang Y: Gut-muscle axis and sepsis-induced
myopathy: The potential role of gut microbiota. Biomed
Pharmacother. 163:1148372023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mankowski RT, Laitano O, Clanton TL and
Brakenridge SC: Pathophysiology and treatment strategies of acute
myopathy and muscle wasting after sepsis. J Clin Med. 10:18742021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Callahan LA and Supinski GS:
Sepsis-induced myopathy. Crit Care Med. 37(10 Suppl): S354–S367.
2009. View Article : Google Scholar
|
|
7
|
Schefold JC, Bierbrauer J and
Weber-Carstens S: Intensive care unit-acquired weakness (ICUAW) and
muscle wasting in critically ill patients with severe sepsis and
septic shock. J Cachexia Sarcopenia Muscle. 1:147–157. 2010.
View Article : Google Scholar
|
|
8
|
Liu W, Hu C and Zhao S: Sarcopenia and
mortality risk of patients with sepsis: A meta-analysis. Int J Clin
Pract. 2022:49744102022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Panahi A, Malekmohammad M, Soleymani F and
Hashemian SM: The prevalence and outcome of intensive care unit
acquired weakness (ICUAW). Tanaffos. 19:250–255. 2020.
|
|
10
|
Dinglas VD, Aronson Friedman L, Colantuoni
E, Mendez-Tellez PA, Shanholtz CB, Ciesla ND, Pronovost PJ and
Needham DM: Muscle weakness and 5-year survival in acute
respiratory distress syndrome survivors. Crit Care Med. 45:446–453.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meyer-Frießem CH, Malewicz NM, Rath S,
Ebel M, Kaisler M, Tegenthoff M, Schildhauer TA, Pogatzki-Zahn EM,
Maier C and Zahn PK: Incidence, time course and influence on
quality of life of intensive care unit-acquired weakness symptoms
in long-term intensive care survivors. J Intensive Care Med.
36:1313–1322. 2021. View Article : Google Scholar
|
|
12
|
Appleton RT, Kinsella J and Quasim T: The
incidence of intensive care unit-acquired weakness syndromes: A
systematic review. J Intensive Care Soc. 16:126–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Andrade-Junior MC, de Salles ICD, de
Brito CMM, Pastore-Junior L, Righetti RF and Yamaguti WP: Skeletal
muscle wasting and function impairment in intensive care patients
with severe COVID-19. Front Physiol. 12:6409732021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herridge MS, Tansey CM, Matté A, Tomlinson
G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart
TE, et al: Functional disability 5 years after acute respiratory
distress syndrome. N Engl J Med. 364:1293–1304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Odden AJ, Rohde JM, Bonham C, Kuhn L,
Malani PN, Chen LM, Flanders SA and Iwashyna TJ: Functional
outcomes of general medical patients with severe sepsis. BMC Infect
Dis. 13:5882013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Huang Y, Chen Y, Shen X, Pan H
and Yu W: Impact of muscle mass on survival in patients with
sepsis: A systematic review and meta-analysis. Ann Nutr Metab.
77:330–336. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan W, Chen Y, Guo Y, Xia Y, Li C, Du Y,
Lin C, Xu X, Qi T, Fan M, et al: Irisin promotes cardiac homing of
intravenously delivered MSCs and protects against ischemic heart
injury. Adv Sci (Weinh). 9:e21036972022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gnecchi M, Danieli P and Cervio E:
Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol.
57:48–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tzouvelekis A, Toonkel R, Karampitsakos T,
Medapalli K, Ninou I, Aidinis V, Bouros D and Glassberg MK:
Mesenchymal stem cells for the treatment of idiopathic pulmonary
fibrosis. Front Med (Lausanne). 5:1422018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sinclair K, Yerkovich ST and Chambers DC:
Mesenchymal stem cells and the lung. Respirology. 18:397–411. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye T, Chen Z, Zhang J, Luo L, Gao R, Gong
L, Du Y, Xie Z, Zhao B, Li Q and Wang Y: Large extracellular
vesicles secreted by human iPSC-derived MSCs ameliorate
tendinopathy via regulating macrophage heterogeneity. Bioact Mater.
21:194–208. 2022.PubMed/NCBI
|
|
22
|
He X, Ai S, Guo W, Yang Y, Wang Z, Jiang D
and Xu X: Umbilical cord-derived mesenchymal stem (stromal) cells
for treatment of severe sepsis: Aphase 1 clinical trial. Transl
Res. 199:52–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cribbs SK and Martin GS: Stem cells in
sepsis and acute lung injury. Am J Med Sci. 341:325–332. 2011.
View Article : Google Scholar
|
|
24
|
Walter J, Ware LB and Matthay MA:
Mesenchymal stem cells: Mechanisms of potential therapeutic benefit
in ARDS and sepsis. Lancet Respir Med. 2:1016–1026. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khosrojerdi A, Soudi S, Hosseini AZ,
Eshghi F, Shafiee A and Hashemi SM: Immunomodulatory and
therapeutic effects of mesenchymal stem cells on organ dysfunction
in sepsis. Shock. 55:423–440. 2021. View Article : Google Scholar
|
|
26
|
Ho MSH, Mei SHJ and Stewart DJ: The
immunomodulatory and therapeutic effects of mesenchymal stromal
cells for acute lung injury and sepsis. J Cell Physiol.
230:2606–2617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friedenstein AJ, Petrakova KV, Kurolesova
AI and Frolova GP: Heterotopic of bone marrow. Analysis of
precursor cells for osteogenic and hematopoietic tissues.
Transplantation. 6:230–247. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini FC, Krause DS, Deans R, Keating A,
Prockop D and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bianco P: 'Mesenchymal' stem cells. Annu
Rev Cell Dev Biol. 30:677–704. 2014. View Article : Google Scholar
|
|
30
|
Rankin S: Mesenchymal stem cells. Thorax.
67:565–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang W and Xu J: Immune modulation by
mesenchymal stem cells. Cell Prolif. 53:e127122020. View Article : Google Scholar :
|
|
32
|
Yianni V and Sharpe PT:
Perivascular-derived mesenchymal stem cells. J Dent Res.
98:1066–1072. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zimmermann JA, Hettiaratchi MH and
McDevitt TC: Enhanced immunosuppression of T cells by sustained
presentation of bioactive interferon-γ within three-dimensional
mesenchymal stem cell constructs. Stem Cells Transl Med. 6:223–237.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yarygin KN, Lupatov AY and Sukhikh GT:
Modulation of immune responses by mesenchymal stromal cells. Bull
Exp Biol Med. 161:561–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Glenn JD and Whartenby KA: Mesenchymal
stem cells: Emerging mechanisms of immunomodulation and therapy.
World J Stem Cells. 6:526–539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saeedi P, Halabian R and Fooladi AAI:
Antimicrobial effects of mesenchymal stem cells primed by modified
LPS on bacterial clearance in sepsis. J Cell Physiol.
234:4970–4986. 2019. View Article : Google Scholar
|
|
37
|
Spees JL, Lee RH and Gregory CA:
Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res
Ther. 7:1252016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Le Blanc K, Tammik C, Rosendahl K,
Zetterberg E and Ringdén O: HLA expression and immunologic
properties of differentiated and undifferentiated mesenchymal stem
cells. Exp Hematol. 31:890–896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View Article : Google Scholar
|
|
40
|
Minguell JJ, Conget P and Erices A:
Biology and clinical utilization of mesenchymal progenitor cells.
Braz J Med Biol Res. 33:881–887. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim WY and Hong SB: Sepsis and acute
respiratory distress syndrome: Recent update. Tuberc Respir Dis
(Seoul). 79:53–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martin GS and Bernard GR: Airway and lung
in sepsis. Intensive Care Med. 27(Suppl 1): S63–S79. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Davis C: Risk factors for the development
of acute lung injury in patients with septic shock: An
observational cohort study. J Emerg Med. 36:P982009. View Article : Google Scholar
|
|
44
|
Schmidt EP, Yang Y, Janssen WJ, Gandjeva
A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX,
et al: The pulmonary endothelial glycocalyx regulates neutrophil
adhesion and lung injury during experimental sepsis. Nat Med.
18:1217–1223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee WL and Slutsky AS: Sepsis and
endothelial permeability. N Engl J Med. 363:689–691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lomas-Neira J, Perl M, Venet F, Chung CS
and Ayala A: The role and source of tumor necrosis factor-α in
hemorrhage-induced priming for septic lung injury. Shock.
37:611–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang L, Taneja R, Razavi HM, Law C, Gillis
C and Mehta S: Specific role of neutrophil inducible nitric oxide
synthase in murine sepsis-induced lung injury in vivo. Shock.
37:539–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grover SP and Mackman N: Tissue factor: An
essential mediator of hemostasis and trigger of thrombosis.
Arterioscler Thromb Vasc Biol. 38:709–725. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Witkowski M, Landmesser U and Rauch U:
Tissue factor as a link between inflammation and coagulation.
Trends Cardiovasc Med. 26:297–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Evans CE and Zhao YY: Impact of thrombosis
on pulmonary endothelial injury and repair following sepsis. Am J
Physiol Lung Cell Mol Physiol. 312:L441–L451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao Y, Yang C, Wang H, Li H, Du J, Gu W
and Jiang J: Therapeutic effects of bone marrow-derived mesenchymal
stem cells on pulmonary impact injury complicated with endotoxemia
in rats. Int Immunopharmacol. 15:246–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee FY, Chen KH, Wallace CG, Sung PH, Sheu
JJ, Chung SY, Chen YL, Lu HI, Ko SF, Sun CK, et al: Xenogeneic
human umbilical cord-derived mesenchymal stem cells reduce
mortality in rats with acute respiratory distress syndrome
complicated by sepsis. Oncotarget. 8:45626–45642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Asami T, Ishii M, Namkoong H, Yagi K,
Tasaka S, Asakura T, Suzuki S, Kamo T, Okamori S, Kamata H, et al:
Anti-inflammatory roles of mesenchymal stromal cells during acute
Streptococcus pneumoniae pulmonary infection in mice. Cytotherapy.
20:302–313. 2017. View Article : Google Scholar
|
|
54
|
Chen HH, Chang CL, Lin KC, Sung PH, Chai
HT, Zhen YY, Chen YC, Wu YC, Leu S, Tsai TH, et al: Melatonin
augments apoptotic adipose-derived mesenchymal stem cell treatment
against sepsis-induced acute lung injury. Am J Transl Res.
6:439–458. 2014.PubMed/NCBI
|
|
55
|
Li W, Chen W, Huang S, Tang X, Yao G and
Sun L: Mesenchymal stem cells enhance pulmonary antimicrobial
immunity and prevent following bacterial infection. Stem Cells Int.
2020:31694692020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Krasnodembskaya A, Song Y, Fang X, Gupta
N, Serikov V, Lee JW and Matthay MA: Antibacterial effect of human
mesenchymal stem cells is mediated in part from secretion of the
antimicrobial peptide LL-37. Stem Cells. 28:2229–2238. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rabani R, Volchuk A, Jerkic M, Ormesher L,
Garces-Ramirez L, Canton J, Masterson C, Gagnon S, Tatham KC,
Marshall J, et al: Mesenchymal stem cells enhance NOX2-dependent
reactive oxygen species production and bacterial killing in
macrophages during sepsis. Eur Respir J. 51:17020212018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yao M, Cui B, Zhang W, Ma W, Zhao G and
Xing L: Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem
cells induces macrophage M2 polarization and ameliorates sepsis.
Life Sci. 264:1186582021. View Article : Google Scholar
|
|
59
|
Krasnodembskaya A, Samarani G, Song Y,
Zhuo H, Su X, Lee JW, Gupta N, Petrini M and Matthay MA: Human
mesenchymal stem cells reduce mortality and bacteremia in
gram-negative sepsis in mice in part by enhancing the phagocytic
activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol.
302:L1003–L1013. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tan L, Huang Y, Pan X, Quan S, Xu S, Li D,
Song L, Zhang X, Chen W and Pan J: Administration of bone marrow
stromal cells in sepsis attenuates sepsis-related coagulopathy. Ann
Med. 48:235–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dos Santos CC, Amatullah H, Vaswani CM,
Maron-Gutierrez T, Kim M, Mei SHJ, Szaszi K, Monteiro APT, Varkouhi
AK, Herreroz R, et al: Mesenchymal stromal (stem) cell therapy
modulates miR-193b-5p expression to attenuate sepsis-induced acute
lung injury. Eur Respir J. 59:20042162022. View Article : Google Scholar
|
|
62
|
Younes N, Zhou L, Amatullah H, Mei SHJ,
Herrero R, Lorente JA, Stewart DJ, Marsden P, Liles WC, Hu P and
Dos Santos CC: Mesenchymal stromal/stem cells modulate response to
experimental sepsis-induced lung injury via regulation of
miR-27a-5p in recipient mice. Thorax. 75:556–567. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bi CF, Liu J, Yang LS and Zhang JF:
Research progress on the mechanism of sepsis induced myocardial
injury. J Inflamm Res. 15:4275–4290. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Aneman A and Vieillard-Baron A: Cardiac
dysfunction in sepsis. Intensive Care Med. 42:2073–2076. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rudiger A and Singer M: Mechanisms of
sepsis-induced cardiac dysfunction. Crit Care Med. 35:1599–1608.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kumar A, Thota V, Dee L, Olson J, Uretz E
and Parrillo JE: Tumor necrosis factor alpha and interleukin 1beta
are responsible for in vitro myocardial cell depression induced by
human septic shock serum. Resuscitation. 32:P1661996. View Article : Google Scholar
|
|
67
|
Zhang X, Lu C, Gao M, Cao X, Ha T,
Kalbfleisch JH, Williams DL, Li C and Kao RL: Toll-like receptor 4
plays a central role in cardiac dysfunction during trauma
hemorrhage shock. Shock. 42:31–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sato R and Nasu M: A review of
sepsis-induced cardiomyopathy. J Intensive Care. 3:482015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Stengl M, Bartak F, Sykora R, Chvojka J,
Benes J, Krouzecky A, Novak I, Sviglerova J, Kuncova J and
Matejovic M: Reduced L-type calcium current in ventricular myocytes
from pigs with hyperdynamic septic shock. Crit Care Med.
38:579–587. 2010. View Article : Google Scholar
|
|
70
|
Kumar A, Brar R, Wang P, Dee L, Skorupa G,
Khadour F, Schulz R and Parrillo JE: Role of nitric oxide and cGMP
in human septic serum-induced depression of cardiac myocyte
contractility. Am J Physiol. 276:R265–R276. 1999.PubMed/NCBI
|
|
71
|
Lv X and Wang H: Pathophysiology of
sepsis-induced myocardial dysfunction. Mil Med Res.
3:302016.PubMed/NCBI
|
|
72
|
Wu Y, Zhou J, Bi L, Huang M, Han Y, Zhang
Q, Zhu D and Zhou S: Effects of bone marrow mesenchymal stem cells
on the cardiac function and immune system of mice with endotoxemia.
Mol Med Rep. 13:5317–5325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Weil BR, Herrmann JL, Abarbanell AM,
Manukyan MC, Poynter JA and Meldrum DR: Intravenous infusion of
mesenchymal stem cells is associated with improved myocardial
function during endotoxemia. Shock. 36:235–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang X, Huang W, Wang Y and Fan GC:
Abstract 12290: Exosomal miR-223 contributes to mesenchymal stem
cell-elicited cardio-protection in polymicrobial sepsis.
Circulation. 1322015.
|
|
75
|
Giovannini I, Chiarla C, Giuliante F,
Vellone M, Ardito F and Nuzzo G: Sepsis-induced cholestasis.
Hepatology. 47:3612008. View Article : Google Scholar
|
|
76
|
Woźnica EA, Inglot M, Woźnica RK and
Łysenko L: Liver dysfunction in sepsis. Adv Clin Exp Med.
27:547–551. 2018. View Article : Google Scholar
|
|
77
|
Gaddam RR, Fraser R, Badiei A, Chambers S,
Cogger VC, Le Couteur DG and Bhatia M: Differential effects of
kupffer cell inactivation on inflammation and the liver sieve
following caecal-ligation and puncture-induced sepsis in mice.
Shock. 47:480–490. 2017. View Article : Google Scholar
|
|
78
|
Wang H and Liu D: Baicalin inhibits
high-mobility group box 1 release and improves survival in
experimental sepsis. Shock. 41:324–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang D, Yin Y and Yao Y: Advances in
sepsis-associated liver dysfunction. Burns Trauma. 2:97–105. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yagi H, Soto-Gutierrez A, Kitagawa Y,
Tilles AW, Tompkins RG and Yarmush ML: Bone marrow mesenchymal
stromal cells attenuate organ injury induced by LPS and burn. Cell
Transplant. 19:823–830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wu KH, Wu HP, Chao WR, Lo WY, Tseng PC,
Lee CJ, Peng CT, Lee MS and Chao YH: Time-series expression of
toll-like receptor 4 signaling in septic mice treated with
mesenchymal stem cells. Shock. 45:634–640. 2016. View Article : Google Scholar
|
|
82
|
Miao CM, Jiang XW, He K, Li PZ, Liu ZJ,
Cao D, Ou ZB, Gong JP, Liu CA and Cheng Y: Bone marrow stromal
cells attenuate LPS-induced mouse acute liver injury via the
prostaglandin E 2-dependent repression of the NLRP3 inflammasome in
Kupffer cells. Immunol Lett. 179:102–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yagi H, Soto-Gutierrez A, Navarro-Alvarez
N, Nahmias Y, Goldwasser Y, Kitagawa Y, Tilles AW, Tompkins RG,
Parekkadan B and Yarmush ML: Reactive bone marrow stromal cells
attenuate systemic inflammation via sTNFR1. Mol Ther. 18:1857–1864.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liang H, Ding X, Yu Y, Zhang H, Wang L,
Kan Q, Ma S, Guan F and Sun T: Adipose-derived mesenchymal stem
cells ameliorate acute liver injury in rat model of CLP
induced-sepsis via sTNFR1. Exp Cell Res. 383:1114652019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Umbro I, Gentile G, Tinti F, Muiesan P and
Mitterhofer AP: Recent advances in pathophysiology and biomarkers
of sepsis-induced acute kidney injury. J Infect. 72:131–142. 2016.
View Article : Google Scholar
|
|
86
|
Gómez H and Kellum JA: Sepsis-induced
acute kidney injury. Curr Opin Crit Care. 22:546–553. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zarjou A and Agarwal A: Sepsis and acute
kidney injury. J Am Soc Nephrol. 22:999–1006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yoshimoto K, Komaru Y, Iwagami M and Doi
K: Acute kidney injury in sepsis: Evidence from Asia. Semin
Nephrol. 40:489–497. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Manrique-Caballero CL, Del Rio-Pertuz G
and Gomez H: Sepsis-associated acute kidney injury. Crit Care Clin.
37:279–301. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bellomo R, Kellum JA, Ronco C, Wald R,
Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y,
Vaara ST and Schneider A: Acute kidney injury in sepsis. Intensive
Care Med. 43:816–828. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Luo CJ, Zhang FJ, Zhang L, Geng YQ, Li QG,
Hong Q, Fu B, Zhu F, Cui SY, Feng Z, et al: Mesenchymal stem cells
ameliorate sepsis-associated acute kidney injury in mice. Shock.
41:123–129. 2014. View Article : Google Scholar
|
|
92
|
Cóndor JM, Rodrigues CE, Sousa Moreira RD,
Canale D, Volpini RA, Shimizu MH, Camara NO, Noronha Ide L and
Andrade L: Treatment with human Wharton's Jelly-derived mesenchymal
stem cells attenuates sepsis-induced kidney injury, liver injury,
and endothelial dysfunction. Stem Cells Transl Med. 5:1048–1057.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen CH, Cheng BC, Chen KH, Shao PL, Sung
PH, Chiang HJ, Yang CC, Lin KC, Sun CK, Sheu JJ, et al: Combination
therapy of exendin-4 and allogenic adipose-derived mesenchymal stem
cell preserved renal function in a chronic kidney disease and
sepsis syndrome setting in rats. Oncotarget. 8:100002–100020. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chen HH, Lin KC, Wallace CG, Chen YT, Yang
CC, Leu S, Chen YC, Sun CK, Tsai TH, Chen YL, et al: Additional
benefit of combined therapy with melatonin and apoptotic
adipose-derived mesenchymal stem cell against sepsis-induced kidney
injury. J Pineal Res. 57:16–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Polito A, Eischwald F, Maho AL, Polito A,
Azabou E, Annane D, Chrétien F, Stevens RD, Carlier R and Sharshar
T: Pattern of brain injury in the acute setting of human septic
shock. Crit Care. 17:R2042013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Catarina AV, Branchini G, Bettoni L, De
Oliveira JR and Nunes FB: Sepsis-associated encephalopathy: From
pathophysiology to progress in experimental studies. Mol Neurobiol.
58:2770–2779. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Prescott HC and Angus DC: Enhancing
recovery from sepsis: A review. JAMA. 319:62–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Oh SH, Choi C, Chang DJ, Shin DA, Lee N,
Jeon I, Sung JH, Lee H, Hong KS, Ko JJ and Song J: Early
neuroprotective effect with lack of long-term cell replacement
effect on experimental stroke after intra-arterial transplantation
of adipose-derived mesenchymal stromal cells. Cytotherapy.
17:1090–1103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Jiang W, Liang G, Li X, Li Z, Gao X, Feng
S, Wang X, Liu M and Liu Y: Intracarotid transplantation of
autologous adipose-derived mesenchymal stem cells significantly
improves neurological deficits in rats after MCAo. J Mater Sci
Mater Med. 25:1357–1366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Huang P, Gebhart N, Richelson E, Brott TG,
Meschia JF and Zubair AC: Mechanism of mesenchymal stem
cell-induced neuron recovery and anti-inflammation. Cytotherapy.
16:1336–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yoo SW, Chang DY, Lee HS, Kim GH, Park JS,
Ryu BY, Joe EH, Lee YD, Kim SS and Suh-Kim H: Immune following
suppression mesenchymal stem cell transplantation in the ischemic
brain is mediated by TGF-β. Neurobiol Dis. 58:249–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Redondo-Castro E, Cunningham C, Miller J,
Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM and
Pinteaux E: Interleukin-1 primes human mesenchymal stem cells
towards an anti-inflammatory and pro-trophic phenotype in vitro.
Stem Cell Res Ther. 8:792017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tan L, Cheng Y, Wang H, Tong J and Qin X:
Peripheral transplantation of mesenchymal stem cells at sepsis
convalescence improves cognitive function of sepsis surviving mice.
Oxid Med Cell Longev. 2022:68977652022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Silva AYO, Amorim ÉA, Barbosa-Silva MC,
Lima MN, Oliveira HA, Granja MG, Oliveira KS, Fagundes PM, Neris
RLS, Campos RMP, et al: Mesenchymal stromal cells protect the
blood-brain barrier, reduce astrogliosis, and prevent cognitive and
behavioral alterations in surviving septic mice. Crit Care Med.
48:e290–e298. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li X, Zheng W, Bai H, Wang J, Wei R, Wen H
and Ning H: Intravenous administration of adipose tissue-derived
stem cells enhances nerve healing and promotes BDNF expression via
the TrkB signaling in a rat stroke model. Neuropsychiatr Dis Treat.
12:1287–1293. 2016.PubMed/NCBI
|
|
106
|
Han C, Song L, Liu Y, Zou W, Jiang C and
Liu J: Rat cortex and hippocampus-derived soluble factors for the
induction of adipose-derived mesenchymal stem cells into
neuron-like cells. Cell Biol Int. 38:768–776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gutiérrez-Fernández M, Rodriguez-Frutos B,
Ramos-Cejudo J, Otero-Ortega L, Fuentes B, Vallejo-Cremades MT,
Sanz-Cuesta BE and Díez-Tejedor E: Comparison between xenogeneic
and allogeneic adipose mesenchymal stem cells in the treatment of
acute cerebral infarct: Proof of concept in rats. J Transl Med.
13:462015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ribeiro CA, Fraga JS, Grãos M, Neves NM,
Reis RL, Gimble JM, Sousa N and Salgado AJ: The secretome of stem
cells isolated from the adipose tissue and Wharton jelly acts
differently on central nervous system derived cell populations.
Stem Cell Res Ther. 3:182012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mahrouf-Yorgov M, Augeul L, Da Silva CC,
Jourdan M, Rigolet M, Manin S, Ferrera R, Ovize M, Henry A, Guguin
A, et al: Mesenchymal stem cells sense mitochondria released from
damaged cells as danger signals to activate their rescue
properties. Cell Death Differ. 24:1224–1238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu J, Huang Y, He J, Zhuo Y, Chen W, Ge
L, Duan D, Lu M and Hu Z: Olfactory mucosa mesenchymal stem cells
ameliorate cerebral ischemic/reperfusion injury through modulation
of UBIAD1 expression. Front Cell Neurosci. 14:5802062020.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cao D, Qiao H, He D, Qin X, Zhang Q and
Zhou Y: Mesenchymal stem cells inhibited the inflammation and
oxidative stress in LPS-activated microglial cells through AMPK
pathway. J Neural Transm (Vienna). 126:1589–1597. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wang SS, Jia J and Wang Z: Mesenchymal
stem cell-derived extracellular vesicles suppresses iNOS expression
and ameliorates neural impairment in Alzheimer's disease mice. J
Alzheimers Dis. 61:1005–1013. 2018. View Article : Google Scholar
|
|
113
|
Cai G, Cai G, Zhou H, Zhuang Z, Liu K, Pei
S, Wang Y, Wang H, Wang X, Xu S, et al: Mesenchymal stem
cell-derived exosome miR-542-3p suppresses inflammation and
prevents cerebral infarction. Stem Cell Res Ther. 12:22021.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Fleischmann-Struzek C, Mellhammar L, Rose
N, Cassini A, Rudd KE, Schlattmann P, Allegranzi B and Reinhart K:
Incidence and mortality of hospital- and ICU-treated sepsis:
Results from an updated and expanded systematic review and
meta-analysis. Intensive Care Med. 46:1552–1562. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar
|
|
116
|
Li YP, Paczesny S, Lauret E, Poirault S,
Bordigoni P, Mekhloufi F, Hequet O, Bertrand Y, Ou-Yang JP, Stoltz
JF, et al: Human mesenchymal stem cells license adult CD34+
hemopoietic progenitor cells to differentiate into regulatory
dendritic cells through activation of the Notch pathway. J Immunol.
180:1598–1608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen X, Zhang Y, Wang W, Liu Z, Meng J and
Han Z: Mesenchymal stem cells modified with heme oxygenase-1 have
enhanced paracrine function and attenuate
lipopolysaccharide-induced inflammatory and oxidative damage in
pulmonary microvascular endothelial cells. Cell Physiol Biochem.
49:101–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Koliaraki V, Pallangyo CK, Greten FR and
Kollias G: Mesenchymal cells in colon cancer. Gastroenterology.
152:964–979. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Parikh SM, Yang Y, He L, Tang C, Zhan M
and Dong Z: Mitochondrial function and disturbances in the septic
kidney. Semin Nephrol. 35:108–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zheng K, Chen S and Hu X: Peroxisome
proliferator-activated receptor gamma coactivator-1 alpha: A
double-edged sword in prostate cancer. Curr Cancer Drug Targets.
22:541–559. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Zheng D, Zhou H, Wang H, Zhu Y, Wu Y, Li
Q, Li T and Liu L: Mesenchymal stem cell-derived microvesicles
improve intestinal barrier function by restoring mitochondrial
dynamic balance in sepsis rats. Stem Cell Res Ther. 12:2992021.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Phinney DG, Di Giuseppe M, Njah J, Sala E,
Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, et
al: Mesenchymal stem cells use extracellular vesicles to outsource
mitophagy and shuttle microRNAs. Nat Commun. 6:84722015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Weber D, Hiergeist A, Weber M, Dettmer K,
Wolff D, Hahn J, Herr W, Gessner A and Holler E: Detrimental effect
of broad-spectrum antibiotics on intestinal microbiome diversity in
patients after allogeneic stem cell transplantation: Lack of
commensal sparing antibiotics. Clin Infect Dis. 68:1303–1310. 2019.
View Article : Google Scholar
|
|
124
|
Valcz G, Krenács T, Sipos F, Leiszter K,
Tóth K, Balogh Z, Csizmadia A, Muzes G, Molnár B and Tulassay Z:
The role of the bone marrow derived mesenchymal stem cells in
colonic epithelial regeneration. Pathol Oncol Res. 17:11–16. 2011.
View Article : Google Scholar
|
|
125
|
Hayashi Y, Tsuji S, Tsujii M, Nishida T,
Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N and
Kawano S: Topical implantation of mesenchymal stem cells has
beneficial effects on healing of experimental colitis in rats. J
Pharmacol Exp Ther. 326:523–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Nagashima K, Sawa S, Nitta T, Tsutsumi M,
Okamura T, Penninger JM, Nakashima T and Takayanagi H:
Identification of subepithelial mesenchymal cells that induce IgA
and diversify gut microbiota. Nat Immunol. 18:675–682. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Levi M: Current understanding of
disseminated intravascular coagulation. Br J Haematol. 124:567–576.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Levi M, de Jonge E and van der Poll T:
Sepsis and disseminated intravascular coagulation. J Thromb
Thrombolysis. 16:43–47. 2003. View Article : Google Scholar
|
|
129
|
Semeraro N, Ammollo CT, Semeraro F and
Colucci M: Coagulopathy of acute sepsis. Semin Thromb Hemost.
41:650–658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang B, Wu S, Wang T, Ma Z and Liu K: Bone
marrow-derived mesenchymal stem cells-mediated protection against
organ dysfunction in disseminated intravascular coagulation is
associated with peripheral immune responses. J Cell Biochem.
118:3184–3192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wang B, Wu SM, Wang T, Liu K, Zhang G,
Zhang XQ, Yu JH, Liu CZ and Fang CC: Pre-treatment with bone
marrow-derived mesenchymal stem cells inhibits systemic
intravascular coagulation and attenuates organ dysfunction in
lipopolysaccharide-induced disseminated intravascular coagulation
rat model. Chin Med J (Engl). 125:1753–1759. 2012.PubMed/NCBI
|
|
132
|
Baudry N, Starck J, Aussel C, Lund K,
Aletti M, Duranteau J, Banzet S, Lataillade JJ, Vicaut E and
Peltzer J: Effect of preconditioned mesenchymal stromal cells on
early microvascular disturbance in a mouse sepsis model. Stem Cells
Dev. 28:1595–1606. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ye Q, Qiu X, Wang J, Xu B, Su Y, Zheng C,
Gui L, Yu L, Kuang H, Liu H, et al: MSCs-derived apoptotic
extracellular vesicles promote muscle regeneration by inducing
Pannexin 1 channel-dependent creatine release by myoblasts. Int J
Oral Sci. 15:72023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Sassoli C, Zecchi-Orlandini S and Formigli
L: Trophic actions of bone marrow-derived mesenchymal stromal cells
for muscle repair/regeneration. Cells. 1:832–850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Gang EJ, Bosnakovski D, Simsek T, To K and
Perlingeiro RCR: Pax3 activation promotes the differentiation of
mesenchymal stem cells toward the myogenic lineage. Exp Cell Res.
314:1721–1733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Dezawa M, Ishikawa H, Itokazu Y, Yoshihara
T, Hoshino M, Takeda SI, Ide C and Nabeshima YI: Bone marrow
stromal cells generate muscle cells and repair muscle degeneration.
Science. 309:314–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Haghighipour N, Heidarian S, Shokrgozar MA
and Amirizadeh N: Differential effects of cyclic uniaxial stretch
on human mesenchymal stem cell into skeletal muscle cell. Cell Biol
Int. 36:669–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Ninagawa NT, Isobe E, Hirayama Y, Murakami
R, Komatsu K, Nagai M, Kobayashi M, Kawabata Y and Torihashi S:
Transplantated mesenchymal stem cells derived from embryonic stem
cells promote muscle regeneration and accelerate functional
recovery of injured skeletal muscle. Biores Open Access. 2:295–306.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Natsu K, Ochi M, Mochizuki Y, Hachisuka H,
Yanada S and Yasunaga Y: Allogeneic bone marrow-derived mesenchymal
stromal cells promote the regeneration of injured skeletal muscle
without differentiation into myofibers. Tissue Eng. 10:1093–1112.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Winkler T, von Roth P, Matziolis G, Mehta
M, Perka C and Duda GN: Dose-response relationship of mesenchymal
stem cell transplantation and functional regeneration after severe
skeletal muscle injury in rats. Tissue Eng Part A. 15:487–492.
2009. View Article : Google Scholar
|
|
141
|
Winkler T, von Roth P, Radojewski P,
Urbanski A, Hahn S, Preininger B, Duda GN and Perka C: Immediate
and delayed transplantation of mesenchymal stem cells improve
muscle force after skeletal muscle injury in rats. J Tissue Eng
Regen Med. 6(Suppl 3): s60–s67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
von Roth P, Duda GN, Radojewski P,
Preininger B, Perka C and Winkler T: Mesenchymal stem cell therapy
following muscle trauma leads to improved muscular regeneration in
both male and female rats. Gend Med. 9:129–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Meregalli M, Farini A, Belicchi M,
Parolini D, Cassinelli L, Razini P, Sitzia C and Torrente Y:
Perspectives of stem cell therapy in Duchenne muscular dystrophy.
FEBS J. 280:4251–4262. 2013. View Article : Google Scholar
|
|
144
|
Jiang J, Yao P, Gu Y, Xu L, Xu J and Tan
H: Adult rat mesenchymal stem cells delay denervated muscle
atrophy. Cell Mol Neurobiol. 32:1287–1298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Merritt EK, Cannon MV, Hammers DW, Le LN,
Gokhale R, Sarathy A, Song TJ, Tierney MT, Suggs LJ, Walters TJ and
Farrar RP: Repair of traumatic skeletal muscle injury with
bone-marrow-derived mesenchymal stem cells seeded on extracellular
matrix. Tissue Eng Part A. 16:2871–2881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Stana F, Vujovic M, Mayaki D, Leduc-Gaudet
JP, Leblanc P, Huck L and Hussain SNA: Differential regulation of
the autophagy and proteasome pathways in skeletal muscles in
sepsis. Crit Care Med. 45:e971–e979. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Khalil R: Ubiquitin-proteasome pathway and
muscle atrophy. Adv Exp Med Biol. 1088:235–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Peruchi BB, Petronilho F, Rojas HA,
Constantino L, Mina F, Vuolo F, Cardoso MR, Gonçalves CL, Rezin GT,
Streck EL and Dal-Pizzol F: Skeletal muscle electron transport
chain dysfunction after sepsis in rats. J Surg Res. 167:e333–e338.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Thoma A and Lightfoot AP: NF-kB and
inflammatory cytokine signalling: Role in skeletal muscle atrophy.
Adv Exp Med Biol. 1088:267–279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Pelosi M, De Rossi M, Barberi L and Musarò
A: IL-6 impairs myogenic differentiation by downmodulation of
p90RSK/eEF2 and mTOR/p70S6K axes, without affecting AKT activity.
Biomed Res Int. 2014:2060262014. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhu S, Nagashima M, Khan MAS, Yasuhara S,
Kaneki M and Martyn JAJ: Lack of caspase-3 attenuates
immobilization-induced muscle atrophy and loss of tension
generation along with mitigation of apoptosis and inflammation.
Muscle Nerve. 47:711–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Okamura LH, Cordero P, Palomino J,
Parraguez VH, Torres CG and Peralta OA: Myogenic differentiation
potential of mesenchymal stem cells derived from fetal bovine bone
marrow. Anim Biotechnol. 29:1–11. 2018. View Article : Google Scholar
|
|
153
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Egusa H, Kobayashi M, Matsumoto T, Sasaki
J, Uraguchi S and Yatani H: Application of cyclic strain for
accelerated skeletal myogenic differentiation of mouse bone
marrow-derived mesenchymal stromal cells with cell alignment.
Tissue Eng Part A. 19:770–782. 2013. View Article : Google Scholar
|
|
155
|
Drost AC, Weng S, Feil G, Schäfer J,
Baumann S, Kanz L, Sievert KD, Stenzl A and Möhle R: In vitro
myogenic differentiation of human bone marrow-derived mesenchymal
stem cells as a potential treatment for urethral sphincter muscle
repair. Ann N Y Acad Sci. 1176:135–143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Maeda Y, Yonemochi Y, Nakajyo Y, Hidaka H,
Ikeda T and Ando Y: CXCL12 and osteopontin from bone marrow-derived
mesenchymal stromal cells improve muscle regeneration. Sci Rep.
7:33052017. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wakitani S, Saito T and Caplan AI:
Myogenic cells derived from rat bone marrow mesenchymal stem cells
exposed to 5-azacytidine. Muscle Nerve. 18:1417–1426. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Meligy FY, Shigemura K, Behnsawy HM,
Fujisawa M, Kawabata M and Shirakawa T: The efficiency of in vitro
isolation and myogenic differentiation of MSCs derived from adipose
connective tissue, bone marrow, and skeletal muscle tissue. In
Vitro Cell Dev Biol Anim. 48:203–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Asakura A: Stem cells in adult skeletal
muscle. rends Cardiovasc Med. 13:123–128. 2003. View Article : Google Scholar
|
|
160
|
Dumont NA, Wang YX and Rudnicki MA:
Intrinsic and extrinsic mechanisms regulating satellite cell
function. Development. 142:1572–1581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Thomas K, Engler AJ and Meyer GA:
Extracellular matrix regulation in the muscle satellite cell niche.
Connect Tissue Res. 56:1–8. 2015. View Article : Google Scholar :
|
|
162
|
Tonkin J, Temmerman L, Sampson RD,
Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musarò A and
Rosenthal N: Monocyte/macrophage-derived IGF-1 orchestrates murine
skeletal muscle regeneration and modulates autocrine polarization.
Mol Ther. 23:1189–1200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Karp JM and Teo GSL: Mesenchymal stem cell
homing: The devil is in the details. Cell Stem Cell. 4:206–216.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Zhou Q, Yang C and Yang P: The promotional
effect of mesenchymal stem cell homing on bone tissue regeneration.
Curr Stem Cell Res Ther. 12:365–376. 2017. View Article : Google Scholar
|
|
165
|
Ringe J, Strassburg S, Neumann K, Endres
M, Notter M, Burmester GR, Kaps C and Sittinger M: Towards in situ
tissue repair: Human mesenchymal stem cells express chemokine
receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with
CXCL8 but not CCL2. J Cell Biochem. 101:135–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Chen L, Li Y, Chen W, Han N, Li K, Guo R,
Liu Z and Xiao Y: Enhanced recruitment and hematopoietic
reconstitution of bone marrow-derived mesenchymal stem cells in
bone marrow failure by the SDF-1/CXCR4. J Tissue Eng Regen Med.
14:1250–1260. 2020.PubMed/NCBI
|
|
167
|
Moll NM and Ransohoff RM: CXCL12 and CXCR4
in bone marrow physiology. Expert Rev Hematol. 3:315–322. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Pozzobon T, Goldoni G, Viola A and Molon
B: CXCR4 signaling in health and disease. Immunol Lett. 177:6–15.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Mousavi A: CXCL12/CXCR4 signal
transduction in diseases and its molecular approaches in
targeted-therapy. Immunol Lett. 217:91–115. 2020. View Article : Google Scholar
|
|
170
|
Guo J, Zhang H, Xiao J, Wu J, Ye Y, Li Z,
Zou Y and Li X: Monocyte chemotactic protein-1 promotes the
myocardial homing of mesenchymal stem cells in dilated
cardiomyopathy. Int J Mol Sci. 14:8164–8178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Schenk S, Mal N, Finan A, Zhang M,
Kiedrowski M, Popovic Z, McCarthy PM and Penn MS: Monocyte
chemotactic protein-3 is a myocardial mesenchymal stem cell homing
factor. Stem Cells. 25:245–251. 2007. View Article : Google Scholar
|
|
172
|
Ahn SY, Park WS, Kim YE, Sung DK, Sung SI,
Ahn JY and Chang YS: Vascular endothelial growth factor mediates
the therapeutic efficacy of mesenchymal stem cell-derived
extracellular vesicles against neonatal hyperoxic lung injury. Exp
Mol Med. 50:1–12. 2018.
|
|
173
|
Shams S, Mohsin S, Nasir GA, Khan M and
Khan SN: Mesenchymal stem cells pretreated with HGF and FGF4 can
reduce liver fibrosis in mice. Stem Cells Int. 2015:7472452015.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Kumar S and Ponnazhagan S: Bone homing of
mesenchymal stem cells by ectopic alpha 4 integrin expression.
FASEB J. 21:3917–3927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Ferrari G, Cusella-De Angelis G, Coletta
M, Paolucci E, Stornaiuolo A, Cossu G and Mavilio F: Muscle
regeneration by bone marrow-derived myogenic progenitors. Science.
279:1528–1530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Winkler T, von Roth P, Schuman MR, Sieland
K, Stoltenburg-Didinger G, Taupitz M, Perka C, Duda GN and
Matziolis G: In vivo visualization of locally transplanted
mesenchymal stem cells in the severely injured muscle in rats.
Tissue Eng Part A. 14:1149–1160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Le Blanc K and Mougiakakos D: Multipotent
mesenchymal stromal cells and the innate immune system. Nat Rev
Immunol. 12:383–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
da Justa Pinheiro CH, de Queiroz JC,
Guimarães-Ferreira L, Vitzel KF, Nachbar RT, de Sousa LGO, de Souza
AL Jr, Nunes MT and Curi R: Local injections of adipose-derived
mesenchymal stem cells modulate inflammation and increase
angiogenesis ameliorating the dystrophic phenotype in
dystrophin-deficient skeletal muscle. Stem Cell Rev Rep. 8:363–374.
2012. View Article : Google Scholar
|
|
179
|
Németh K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E(2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49. 2009.
View Article : Google Scholar
|
|
180
|
Chen L, Tredget EE, Wu PY and Wu Y:
Paracrine factors of mesenchymal stem cells recruit macrophages and
endothelial lineage cells and enhance wound healing. PLoS One.
3:e18862008. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Bencze M, Negroni E, Vallese D,
Yacoub-Youssef H, Chaouch S, Wolff A, Aamiri A, Di Santo JP,
Chazaud B, Butler-Browne G, et al: Proinflammatory macrophages
enhance the regenerative capacity of human myoblasts by modifying
their kinetics of proliferation and differentiation. Mol Ther.
20:2168–2179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Waterman RS, Tomchuck SL, Henkle SL and
Betancourt AM: A new mesenchymal stem cell (MSC) paradigm:
Polarization into a pro-inflammatory MSC1 or an Immunosuppressive
MSC2 phenotype. PLoS One. 5:e100882010. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
de Couto G, Gallet R, Cambier L,
Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP and Marbán E:
Exosomal MicroRNA transfer into macrophages mediates cellular
postconditioning. Circulation. 136:200–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Xu R, Zhang F, Chai R, Zhou W, Hu M, Liu
B, Chen X, Liu M, Xu Q, Liu N and Liu S: Exosomes derived from
pro-inflammatory bone marrow-derived mesenchymal stem cells reduce
inflammation and myocardial injury via mediating macrophage
polarization. J Cell Mol Med. 23:7617–7631. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X,
Gao L, Xie J and Xu B: Mesenchymal stromal cell-derived exosomes
attenuate myocardial ischaemia-reperfusion injury through
miR-182-regulated macrophage polarization. Cardiovasc Res.
115:1205–1216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Simovic Markovic B, Gazdic M, Arsenijevic
A, Jovicic N, Jeremic J, Djonov V, Arsenijevic N, Lukic ML and
Volarevic V: Mesenchymal stem cells attenuate cisplatin-induced
nephrotoxicity in iNOS-dependent manner. Stem Cells Int.
2017:13153782017. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Sassoli C, Pini A, Chellini F, Mazzanti B,
Nistri S, Nosi D, Saccardi R, Quercioli F, Zecchi-Orlandini S and
Formigli L: Bone marrow mesenchymal stromal cells stimulate
skeletal myoblast proliferation through the paracrine release of
VEGF. PLoS One. 7:e375122012. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Murphy MB, Moncivais K and Caplan AI:
Mesenchymal stem cells: Environmentally responsive therapeutics for
regenerative medicine. Exp Mol Med. 45:e542013. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Sassoli C, Frati A, Tani A, Anderloni G,
Pierucci F, Matteini F, Chellini F, Zecchi Orlandini S, Formigli L
and Meacci E: Mesenchymal stromal cell secreted sphingosine
1-phosphate (S1P) exerts a stimulatory effect on skeletal myoblast
proliferation. PLoS One. 9:e1086622014. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Nakamura Y, Miyaki S, Ishitobi H,
Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y and Ochi M:
Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle
regeneration. FEBS Lett. 589:1257–1265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Leoni G, Neumann PA, Kamaly N, Quiros M,
Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G, et
al: Annexin A1-containing extracellular vesicles and polymeric
nanoparticles promote epithelial wound repair. J Clin Invest.
125:1215–1227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Bizzarro V, Petrella A and Parente L:
Annexin A1: Novel roles in skeletal muscle biology. J Cell Physiol.
227:3007–3015. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Kim S, Lee MJ, Choi JY, Park DH, Kwak HB,
Moon S, Koh JW, Shin HK, Ryu JK, Park CS, et al: Roles of
exosome-like vesicles released from inflammatory C2C12 myotubes:
Regulation of myocyte differentiation and myokine expression. Cell
Physiol Biochem. 48:1829–1842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Choi JS, Yoon HI, Lee KS, Choi YC, Yang
SH, Kim IS and Cho YW: Exosomes from differentiating human skeletal
muscle cells trigger myogenesis of stem cells and provide
biochemical cues for skeletal muscle regeneration. J Control
Release. 222:107–115. 2016. View Article : Google Scholar
|
|
195
|
Forterre A, Jalabert A, Berger E, Baudet
M, Chikh K, Errazuriz E, De Larichaudy J, Chanon S, Weiss-Gayet M,
Hesse AM, et al: Proteomic analysis of C2C12 myoblast and myotube
exosome-like vesicles: A new paradigm for myoblast-myotube cross
talk? PLoS One. 9:e841532014. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Oswald J, Boxberger S, Jørgensen B,
Feldmann S, Ehninger G, Bornhäuser M and Werner C: Mesenchymal stem
cells can be differentiated into endothelial cells in vitro. Stem
Cells. 22:377–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Wang C, Li Y, Yang M, Zou Y, Liu H, Liang
Z, Yin Y, Niu G, Yan Z and Zhang B: Efficient differentiation of
bone marrow mesenchymal stem cells into endothelial cells in vitro.
Eur J Vasc Endovasc Surg. 55:257–265. 2018. View Article : Google Scholar
|
|
198
|
Lu W, Xiu X, Zhao Y and Gui M: Improved
proliferation and differentiation of bone marrow mesenchymal stem
cells into vascular endothelial cells with sphingosine 1-phosphate.
Transplant Proc. 47:2035–2040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Grote K, Petri M, Liu C, Jehn P, Spalthoff
S, Kokemüller H, Luchtefeld M, Tschernig T, Krettek C, Haasper C
and Jagodzinski M: Toll-like receptor 2/6-dependent stimulation of
mesenchymal stem cells promotes angiogenesis by paracrine factors.
Eur Cell Mater. 26:66–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Leroux L, Descamps B, Tojais NF, Séguy B,
Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamazière JM, et
al: Hypoxia preconditioned mesenchymal stem cells improve vascular
and skeletal muscle fiber regeneration after ischemia through a
Wnt4-dependent pathway. Mol Ther. 18:1545–1552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Berebichez-Fridman R and Montero-Olvera
PR: Sources and clinical applications of mesenchymal stem cells:
State-of-the-art review. Sultan Qaboos Univ Med J. 18:e264–e277.
2018. View Article : Google Scholar
|
|
202
|
Mushahary D, Spittler A, Kasper C, Weber V
and Charwat V: Isolation, cultivation, and characterization of
human mesenchymal stem cells. Cytometry A. 93:19–31. 2018.
View Article : Google Scholar
|
|
203
|
Spath L, Rotilio V, Alessandrini M,
Gambara G, De Angelis L, Mancini M, Mitsiadis TA, Vivarelli E, Naro
F, Filippini A and Papaccio G: Explant-derived human dental pulp
stem cells enhance differentiation and proliferation potentials. J
Cell Mol Med. 14:1635–1644. 2010. View Article : Google Scholar
|
|
204
|
Ueda N, Atsuta I, Ayukawa Y, Yamaza T,
Furuhashi A, Narimatsu I, Matsuura Y, Kondo R, Watanabe Y, Zhang X
and Koyano K: Novel application method for mesenchymal stem cell
therapy utilizing its attractant-responsive accumulation property.
Appl Sci. 9:49082019. View Article : Google Scholar
|
|
205
|
Castelo-Branco MTL, Soares IDO, Lopes DV,
Buongusto F, Martinusso CA, do Rosario A Jr, Souza SA, Gutfilen B,
Fonseca LM, Elia C, et al: Intraperitoneal but not intravenous
cryopreserved mesenchymal stromal cells home to the inflamed colon
and ameliorate experimental colitis. PLoS One. 7:e333602012.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Gonçalves Fda C, Schneider N, Pinto FO,
Meyer FS, Visioli F, Pfaffenseller B, Lopez PL, Passos EP,
Cirne-Lima EO, Meurer L and Paz AH: Intravenous vs intraperitoneal
mesenchymal stem cells administration: What is the best route for
treating experimental colitis? World J Gastroenterol.
20:18228–18239. 2014. View Article : Google Scholar
|
|
207
|
Roux C, Saviane G, Pini J, Belaïd N, Dhib
G, Voha C, Ibáñez L, Boutin A, Mazure NM, Wakkach A, et al:
Immunosuppressive mesenchymal stromal cells derived from
human-induced pluripotent stem cells induce human regulatory T
cells in vitro and in vivo. Front Immunol. 8:19912018. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Braid LR, Wood CA, Wiese DM and Ford BN:
Intramuscular administration potentiates extended dwell time of
mesenchymal stromal cells compared to other routes. Cytotherapy.
20:232–244. 2018. View Article : Google Scholar
|
|
209
|
Rangarajan A, Hong SJ, Gifford A and
Weinberg RA: Erratum to species- and cell type-specific
requirements for cellular transformation [Cancer Cell 6, (2004)
171-183]. Cancer Cell. 24:394–398. 2013. View Article : Google Scholar
|
|
210
|
Wang Y, Han ZB, Song YP and Han ZC: Safety
of mesenchymal stem cells for clinical application. Stem Cells Int.
2012:6520342012. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Golpanian S, DiFede DL, Khan A, Schulman
IH, Landin AM, Tompkins BA, Heldman AW, Miki R, Goldstein BJ,
Mushtaq M, et al: Allogeneic human mesenchymal stem cell infusions
for aging frailty. J Gerontol A Biol Sci Med Sci. 72:1505–1512.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Tompkins BA, DiFede DL, Khan A, Landin AM,
Schulman IH, Pujol MV, Heldman AW, Miki R, Goldschmidt-Clermont PJ,
Goldstein BJ, et al: Allogeneic mesenchymal stem cells ameliorate
aging frailty: A phase II randomized, double-blind,
placebo-controlled clinical trial. J Gerontol A Biol Sci Med Sci.
72:1513–1522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Kabat M, Bobkov I, Kumar S and Grumet M:
Trends in mesenchymal stem cell clinical trials 2004-2018: Is
efficacy optimal in a narrow dose range? Stem Cells Transl Med.
9:17–27. 2020. View Article : Google Scholar
|
|
214
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Mankowski RT, Laitano O, Darden D, Kelly
L, Munley J, Loftus TJ, Mohr AM, Efron PA and Thomas RM:
Sepsis-Induced myopathy and gut microbiome dysbiosis: Mechanistic
links and therapeutic targets. Shock. 57:15–23. 2022. View Article : Google Scholar :
|
|
216
|
Liu Y, Wang D, Li T, Yang F, Li Z, Bai X
and Wang Y: The role of NLRP3 inflammasome in inflammation-related
skeletal muscle atrophy. Front Immunol. 13:10357092022. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Liu D, Huang SY, Sun JH, Zhang HC, Cai QL,
Gao C, Li L, Cao J, Xu F, Zhou Y, et al: Sepsis-induced
immunosuppression: Mechanisms, diagnosis and current treatment
options. Mil Med Res. 9:562022.PubMed/NCBI
|
|
218
|
Kramer CL: Intensive care unit-acquired
weakness. Neurol Clin. 35:723–736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Fu X, Liu G, Halim A, Ju Y, Luo Q and Song
AG: Mesenchymal stem cell migration and tissue repair. Cells.
8:7842019. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Song J, Liu J, Cui C, Hu H, Zang N, Yang
M, Yang J, Zou Y, Li J, Wang L, et al: Mesenchymal stromal cells
ameliorate diabetes-induced muscle atrophy through exosomes by
enhancing AMPK/ULK1-mediated autophagy. J Cachexia Sarcopenia
Muscle. 14:915–929. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Abrigo J, Rivera JC, Aravena J, Cabrera D,
Simon F, Ezquer F, Ezquer M and Cabello-Verrugio C: High fat
diet-induced skeletal muscle wasting is decreased by mesenchymal
stem cells administration: Implications on oxidative stress,
ubiquitin proteasome pathway activation, and myonuclear apoptosis.
Oxid Med Cell Longev. 2016:90478212016. View Article : Google Scholar : PubMed/NCBI
|