Introduction
Recent evidence suggests that primary bone cancer is
a major health problem among children (0-14 years) and adolescents
(15-19 years), comprising the third leading cause of cancer-related
death in children and adolescents in the United States and the
sixth in China (1,2). Among primary bone cancers,
osteosarcoma (OS), an aggressive malignant tumor, which is commonly
accompanied by lung metastasis, is the most frequently occurring
type (3). Since the 1980s,
surgical removal combined with neoadjuvant chemotherapy has been
the mainstay of OS treatment, significantly improving patient
prognosis. However, OS treatment has not been significantly
improved since then and patients with relapsed or primary
metastatic OS still have a dismal prognosis, with a five-year
survival rate of only 20% (4,5).
Moreover, to date, no targeted therapy for OS has been approved by
the US Food and Drug Agency (6).
Thus, novel effective therapies for OS are urgently required.
Cancer stem cells are a small subpopulation of
cancer cells that have stem-like properties and retain pluripotency
and self-renewing abilities, which might be responsible for cancer
metastasis or recurrence (7).
Gibbs et al (8) first
confirmed the existence of OS stem cells (OSCs) in multiple OS cell
lines. Since then, OSCs have been revealed to drive OS initiation,
metastasis and recurrence; therefore, targeting these cells is
considered a novel and attractive strategy to treat OS (9). Several signaling pathways and genes
were found to participate in the regulation of the biological
function of OSCs (10). However,
to date, no treatment has been approved to deplete OSCs. Thus, new
biomarkers and an improved understanding of the molecular biology
of OSCs are needed to develop targeted therapy to treat OS and
improve patient prognosis.
Tumor metastasis results from a complex series
steps, including local migration and invasion, circulation of live
cancer cells, arrest at the secondary site, and tumor initiation
(11). However, due to the lack
of survival signals generated from the extracellular matrix (ECM)
and neighboring cells, most of the cancer cells undergo apoptosis
when they are detached from the ECM during circulation (12). This kind of programmed cell
death, comprising apoptosis upon cell detachment from the ECM, is
called anoikis (13). Anoikis is
not only an important process to prevent normal cells from
colonizing the wrong site in the body, but also provides a strong
barrier against cancer metastasis (14). Thus, in addition to tumor
initiation caused by OSCs, anoikis resistance, which prevents the
key fraction of tumor cells from undergoing apoptosis and cell
death during circulation, is another major cause of tumor
metastasis (15). Recent
studies, including our own, have provided some insights into
anoikis resistance in OS (16,17). Nevertheless, the molecular
mechanisms underlying anoikis resistance in OS remain incompletely
understood.
FOS like antigen 1 (FOSL1), also known as FOS
related antigen 1 (FRA1), is a transcription factor and a member of
the activator protein 1 (AP1) complex, localizing to the cell
nucleus and cytoplasm (18,19). FOSL1 can bind to the
12-O-tetradecanoylphorbol-13-acetate response element (TRE;
TGAC/GTCA), a specific DNA sequence on the promoter or enhancer of
its target genes, thereby mediating multiple tumor cell malignant
phenotypes, such as cell migration, differentiation, apoptosis and
proliferation (19). Previous
evidence suggests that FOSL1 could be a candidate for targeted
therapy for cancer stem cells in some types of cancer, such as head
and neck squamous cell carcinoma, glioma and colorectal cancer
(18,20,21). In addition, FOSL1 has been
reported to be involved in K-Ras-transformed canine renal cell
anoikis resistance (22).
Moreover, FOSL1 mRNA was reported to be highly expressed in OS
tissues, suggesting FOSL1 as a potential therapeutic target for OS
(23). However, the function of
FOSL1 in OS remains poorly understood and its role in maintaining
cancer stemness and regulating anoikis resistance in OS is
unclear.
In the present study, it was demonstrated that FOSL1
is highly expressed in OSCs and facilitates their stem-like
properties, tumorigenic potential and anoikis resistance.
Mechanistically, FOSL1 exerts these functions by directly promoting
SOX2 (encoding SRY-box transcription factor 2) transcription. The
present study highlights the important role of FOSL1 in maintaining
OS cell stemness properties and anoikis resistance, suggesting
FOSL1 as a promising target in OS.
Materials and methods
Cell culture
The OS cell lines 143B and MNNG/HOS were purchased
from the American Type Culture Collection and maintained in
Dulbecco's modified Eagle's medium (DMEM, Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS),
100 μg/ml penicillin and 100 μg/ml streptomycin. All
cells were incubated at 37°C in a humidified atmosphere containing
5% CO2. OS spheres were cultured in 6-well ultra-low
attachment plates (Corning, Inc.) in stem cell medium, which
consisted of serum-free DMEM with BASIC Ham's F-12 Nutrient Mix
(DMEM/F12) medium (Gibco; Thermo Fisher Scientific, Inc.) with 20
ng/ml epidermal growth factor (EGF, PeproTech, Inc.), 20 ng/ml
basic fibroblast growth factor (bFGF, PeproTech, Inc.) and 2 mg/ml
B27 (Gibco; Thermo Fisher Scientific, Inc.). The medium was added
at 1 ml every two days during anchorage-independent
proliferation.
RNA sequencing (RNA-seq) and
bioinformatic analysis
Total RNA was extracted using TRIzol reagent (cat.
no. 15596018CN; Thermo Fisher Scientific, Inc.) following the
manufacturer's proctocol. RNA quantity and purity were analysis of
Bioanalyzer 2100 and RNA 6000 Nano LabChip kit (cat. no. 5067-1511;
Agilent Technologies, Inc.), high-quality RNA samples with RIN
number >7.0 were used to construct sequencing library. Then,
RNA-sequencing library preparation and sequencing were performed on
the Illumina Novaseqä 6000 platform by LC-Bio Technology Co., Ltd.
The average insert size for the final cDNA librarys were 300±50 bp.
At last, the 2×150 bp paired-end sequencing (PE150) was performed
on an Illumina Novaseq™ 6000 (LC-Bio Technology CO., Ltd.)
following the supplier's recommended protocol. Moreover, to get
high quality clean reads, reads were further filtered by Cutadapt
(https://cutadapt.readthedocs.io/en/stable/). Then, the
raw reads were filtered and the clean reads were mapped using
HISAT2. Differential gene expression analysis was performed using
DESeq2 software (https://support.bioconductor.org/tag/DESeq2/) between
two different groups. The genes with parameters of a false
discovery rate <0.05 and absolute fold change ≥2 were considered
as differentially expressed genes (DEGs). The DEGs were then
subjected to enrichment analysis for gene ontology (GO) functions
and Kyoto Encyclopedia of Genes and Genomes pathways. The raw
sequence data have been submitted to the NCBI Short Read Archive
(SRA) datasets with the accession number <PRJNA1080098>. For
the bioinformatic analysis, the UALCAN database (https://ualcan.path.uab.edu/) (24), the R2: Genomics Analysis and
Visualization Platform (https://hgserver1.amc.nl) (25), the TARGET database (using the
TNMplot.com tool (https://tnmplot.com/analysis/)) (26) and the KM-plotter Platform
(http://kmplot.com/) (27) were used according to the
supplier's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR) and western blot analysis
Western blotting and RT-qPCR analyses were performed
as previously described (5,17). The primers used in the present
study were obtained from Sangon Biotech Co., Ltd. and are listed in
Table SI. For western blotting,
proteins (35 μg/lane) were separated using 8-12% SDS
Tris-glycine gels and transferred onto PVDF membranes. Membranes
were blocked with 5% fat-free milk for 2 h at room temperature and
incubated with the appropriate primary antibodies overnight at 4°C.
Antibodies against GAPDH (1:1,000; cat. no. 60004-1-lg) were
purchased from Proteintech Group, Inc. Rabbit anti-human FRA1
(1:1,000; cat. no. 252421), rabbit anti-human SOX2 (1:1,000; cat.
no. 92494), rabbit anti-human NANOG (1:1,000; cat. no. 109250),
rabbit anti-human BAX (1:1,000; cat. no. 32503), rabbit anti-human
Caspase3 (1:1,000; cat. no. 32351) and anti-cleaved Caspase3
antibodies (1:500; cat. no. 32042) were purchased from Abcam.
Rabbit anti-human BCL-2 (1:1,000; cat. no. 15071) antibodies were
purchased from Cell Signaling Technology, Inc. Then, the secondary
antibody (goat anti-rabbit or mouse IgG; 1:5,000; cat. nos.
bs-0295G-HRP and bs-0368G-HRP; BIOSS) was applied. Immunoreactivity
was detected using an ECL Kit (Beyotime Institute of
Biotechnology).
Multiplex immunohistochemistry (mIHC)
staining, immunohistochemistry (IHC), immunofluorescence (IF) and
Transwell assays
The mIHC staining was based on the Tyramine Signal
Amplification (TSA) technology, as previously described (28) and was detected by using a TSA
Fluorescence Triple Staining kit (cat. no. AFIHC024; AiFang
biological; http://afantibody.cn/). Other assays
were performed as previously described (5,29).
IHC was performed using an IHC kit (Zsbio). Sample
sections were deparaffinized through a series of xylene baths,
antigens were retrieved by steam treatment in 10 mM citrate buffer,
blocked with 3% hydrogen peroxide for 15 min at 37°C, pre-incubated
with blocking goat serum solution (cat. no. ZLI-9022; Zsbio) for 30
min at 37°C, and then incubated at 4°C with the primary antibodies
overnight. Subsequently, the ready-to-use undiluted secondary
antibodies conjugated with HRP in the ElivisionTM plus Polyer HRP
(Mouse/Rabbit) IHC kit (cat. no. KIT-9902; Fuzhou Maixin Biotech
Co., Ltd.) were applied for 40 min at 37°C and the nuclei were
counterstained with haematoxylin. The slides were then examined
from non-overlapping cells using a light microscope (Olympus
Corporation). Primary rabbit anti-human FRA1 (1:100, cat. no.
252421; Abcam), mouse anti-human SOX2 (1:100, cat. no. 171380;
Abcam), and ready-to-use mouse anti-human c-Kit (CD117) (cat. no.
kit-0029; Fuzhou Maixin Biotech Co., Ltd.) were used.
For IF, after different interventions, 143B and
MNNG/HOS cells (5×105 cells per well) were fixed with
10% formalin and incubated with 0.2% Triton X-100 in PBS for 10 min
followed by 5% bovine serum albumin (cat. no. GC305010; Wuhan
Servicebio Technology Co., Ltd.) for 60 min at room temperature.
The slides were then incubated with rabbit anti-FRA1 antibody
(1:100) and mouse anti-SOX2 antibody (1:100; cat. no. 171380;
Abcam) at 4°C overnight. After washing with PBS, the slides were
incubated with the corresponding secondary antibody for 60 min at
room temperature. Slides were stained with nuclear dye
4,6-diamidino-2-phenylindole (DAPI; 5 μg/ml; cat. no. C1002;
Beyotime Institute of Biotechnology) and cover-slipped, and FL
images were captured using a fluorescence microscope (CKX53;
Olympus Corporation).
For the Transwell migration assay, cells
(4×105 cells/ml) were resuspended in DMEM without serum
and 200 μl of the cell suspension seeded into the upper
chamber of 8-μm Transwell filters (Merck KGaA). DMEM
containing 15% FBS was added to the lower chambers (24-well plate)
and the cells were incubated 16 h for the migration assay at 37°C
in 5% CO2. The migratory cells were quantified after
0.1% crystal violet staining for 5 min at room temperature in five
randomly selected fields (magnification, ×200) under an inverted
phase-contrast light microscope (Olympus Corporation).
Lentiviral transfection
The pHBLV-CMV-MCS-EF1-puro lentiviral vector for
overexpressing FOSL1 and the control were synthesized and obtained
from Hanbio Biotechnology Co., Ltd. The coding sequence of FOSL1
was amplified using the following primers: Forward, 5'-TAC TAG AGG
ATC TAT TTC CGG TGA ATT CGC CAC CAT GTT CCG AGA CT-3' and reverse,
5'-GAG CGA TCG CAG ATC CTT AGG ATC CTC ACA AAG CGA GGA GGG TTG-3'.
The short hairpin RNA (shRNA) in the pHBLV-U6-MCS-PGK-BSD
lentiviral vector targeting SOX2 and a scrambled sequence were also
purchased from Hanbio Biotechnology Co., Ltd. 293T cells were
transfected with the plasmids using LipofiterTM (cat.
no. HB-TRCF-1000; Hanbio Biotechnology Co., Ltd.), in accordance
with the manufacturer's protocol. After 48 and 72 h the lentiviral
were vectors were collected. When 143B and MNNG/HOS cells'
confluence reached 30-40%, they were infected with the lentiviral
vectors according to the manufacturer's protocol [The multiplicity
of infection (MOI) for h-FOSL1 and h-SOX2 shRNA lentiviral vector
in OS cell lines were 40 and 20] for 24 h at 37°C in 5%
CO2 and then cultured with puromycin (1 μg/ml for
1 week) and blasticidin (2 μg/ml for 4 days) at 37°C in 5%
CO2 as necessary to establish stable cell lines. The
subsequent experimentation began after one-time cell passage. The
shRNA target sequences are listed in Table SII.
Transfection of short interfering RNA
(siRNA)
A total of two siRNAs targeting FOSL1 were used for
the knockdown experiments (Guangzhou RiboBio Co., Ltd.). Cells were
transfected with 20 nM targeting siRNA (two sequences) or scrambled
siRNA, using the RiboBio-FECT™ CP kit (Guangzhou RiboBio Co.,
Ltd.), according to the manufacturer's protocol at 37°C in 5%
CO2. The knockdown efficiency was assessed using RT-qPCR
and western blot analysis of cells at 48 h after transfection. The
siRNA target sequences were siRNA-1, GTC GAA GGC CTT GTG AACA; and
siRNA2, GGA AGG AAC TGA CCG ACTT.
Spheroid formation assay and plate colony
formation assay
To evaluate the cells' self-renewal capacity, tumor
sphere formation and colony formation assays were used. 143B and
MNNG/HOS cells were cultured in stem cell medium in 96-well
ultra-low attachment plates (Corning Inc.). In total, 20 or 40
cells were seeded into each well, which was supplemented with 20
μl of new stem cell medium every two days. Spheres with a
size >50 μm were obtained and counted. After 10-12 days
of culture, culture wells with spheres were marked and spheres were
counted (30). For the plate
cloning experiment, cells were seeded at a density of 50-200
cells/per well in a regular 6-well plate and cultured in a 5%
CO2 incubator at 37°C. The medium was changed every 3
days. After observing that the proliferation of the cells had
stopped, the cells were washed with phosphate-buffered saline (PBS)
three times, fixed using 4% paraformaldehyde for 15 min at room
temperature and stained with 0.1% crystal violet for 5 min at room
temperature. Colonies with a size >50 μm were obtained
and counted.
Fluorescence activated cell sorting
(FACS) analysis
143B and MNNG/HOS cells were seeded into ultra-low
attachment 6-well plates to culture stem cells and then transfected
with lentivirus or siRNAs as required. Cells were dissociated into
single cells using Accutase (MilliporeSigma) as required, and
incubated with anti-CD117 (also known as KIT proto-oncogene,
receptor tyrosine kinase)-Phycoerythrin (PE)-Cyanine 7 (Cy7)
antibodies (1:25; cat. no. 25-1178-42; Thermo Fisher Scientific,
Inc.) and anti-Stro1-Allophycocyanin (APC) (1:100; cat. no.
MA5-28635; Thermo Fisher Scientific, Inc.) antibodies, or isotype
control antibodies, at 37°C in the dark for 20 min. After staining,
the cells were washed with PBS and then measured using a flow
cytometer (BD FACS CantoTM II; BD Biosciences) to detect
CD117+ and Stro1+ sub-populations.
Anoikis analysis
Cell anoikis was assessed using Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) analysis
as in our previous study (17).
Briefly, an Annexin V-FITC/PI kit (cat. no. 556547; BD Biosciences)
was used according to the manufacturer's protocols. After the cells
were subjected to lentivirus or siRNA transfection, they were
seeded in ultra-low attachment 6-well plates (Corning Inc.) and
cultured for 24 or 48 h in DMEM supplemented with 5% FBS.
Thereafter, the cells were harvested and washed, and an anti-FITC
antibody and anti-PI antibody, in a 3:100 ratio with 1X buffer,
were used to stain the cells in the dark for 15 min. Then, 400
μl of 1X buffer was added, and the cells were assessed using
a flow cytometer (BD biosciences) with FlowJo software (version
10.8.1; BD FACS CantoTM II; FlowJo LLC). Annexin V-FITC positive,
meanwhile PI negative cells and both Annexin V-FITC and PI positive
cells were identified as anoikis cells.
Chromatin immunoprecipitation
(ChIP)-qPCR
Chromatin immunoprecipitation coupled with qPCR was
performed as described previously (20). Briefly, MNNG/HOS cells were
firstly transfected with FOSL1-overexpression lentivirus and the
efficiency of transfection was confirmed. Then, cells were fixed
using 1% formaldehyde for 10 min at room temperature with rotation,
and then quenched using glycine. Total cell lysates were sonicated
to generate 200-1,000-bp DNA fragments. Chromatin complexes were
immunoprecipitated following the instructions of the
SimpleChIP® Enzymatic Chromatin IP Kit (cat. no. 9003;
Cell Signaling Technology, Inc.) with an anti-FRA1 (FOSL1) antibody
(cat. no. sc-28310; Santa Cruz Biotechnology, Inc.). The
precipitated DNA samples were quantified using qPCR. Data are
expressed as the percentage of input DNA. The ChIP-qPCR assays were
performed in triplicate and the data are presented as the mean ±
SD. The SOX2 ChIP-qPCR primers were obtained from MDL Biotech Co.,
Ltd. and are listed in Table
SIII.
Dual-luciferase reporter assays
To create the luciferase reporter gene, the sequence
of NANOG or SOX2 containing the predicted and mutated binding sites
was inserted into the vector psi-CHECK2. These constructs, which
included SOX2-WT (wild-type), SOX2-MT (mutated), NANOG-WT and
NANOG-Mut, were synthesized by Bomaide Gene Technology Co., Ltd.
The detailed sequences are listed Tables SIV and SV. In brief, cells were first seeded
in 6-well plates and cultured to 60-80% confluency. Then,
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was
used to co-transfect the luciferase reporter gene with either
over-FOSL1 or NC plasmids (Biomed) into MNNG/HOS cells. At 48 h
after transfection, Firefly and Renilla luciferase
activities in each well were detected using the Dual Luciferase
Reporter Gene Assay kit (Shanghai Yeasen Biotechnology Co., Ltd.)
according to the manufacturer's protocol.
Animals
A total of 95 female (4 weeks-old) athymic BALB/c
nude mice (weight, 18-20 g) were obtained from Vital River
Laboratory Animal Technology Co., Ltd. Housing conditions included
temperature (22±2°C), humidity (40-60%), 12/12-h light/dark cycle
and feed ad libitum to minimize the distress of animals. No
mouse succumbed before euthanasia; and all the mice were euthanized
by inhaling 40% CO2, with a flow rate of 5 l/min for 5
min, using a carbon dioxide inhalation device and followed by 5 min
monitoring to verify irreversible euthanasia before they were
sacrificed; and the fill rate of carbon dioxide was standardized by
the experimental animal division of Chongqing Medical University.
Animal death is typically verified by checking for absence of
respiration and heartbeat. All the animal care and experimental
procedures were approved (approval no. 2022-224) by the
Institutional Animal Care and Use Committee of the Second
Affiliated Hospital of Chongqing Medical University (Chongqing,
China), and were performed according to the Guide for the Care Use
of Laboratory Animals.
Xenografts and tumor initiation assays in
vivo
Xenograft models were generated as previously
reported (5) and 25 mice were
randomly divided into 5 groups (n=5 mice in each group). Briefly,
MNNG/HOS cell suspensions (2×107 cells/ml) in PBS were
injected subcutaneously into each mouse in a volume of 0.1 ml. The
mice health and weight were monitored every two days and xenografts
were observed and measured every three days. The volume of the
xenografts was calculated as V (mm3)=1/2 × (length ×
width2) (31). All
the mice were sacrificed at 20 or 24 days after injection and
tumors were harvested and measured. The xenografts were fixed,
sectioned, subjected to haematoxylin and eosin (H&E) staining,
and further analyzed using IHC. The tumor initiation assay was
carried out using a limiting-dilution assay in the aforementioned
mice xenograft models with the injection of different low doses
(5×103, 104, 5×104 and
5×105) of MNNG/HOS OSCs and 40 mice were divided into 5
groups (one mouse carried two doses of OSCs). The tumor
re-initiating cell frequency of OSCs was calculated using the ELDA
software (http://bioinf.wehi.edu.au/software/elda/) (32).
In vivo anoikis and lung metastasis
model
The lung metastasis models were established as
previously reported (5,17) and 30 mice were randomly divided
into 5 groups (n=6 mice in each group). Briefly, to emphasize the
influence of anoikis, with appropriate treatments, fewer cells
(2×106) in a volume of 100 μl PBS were injected
into the tail vein of each 4-week-old nude mouse. Mice health and
weight were monitored every two days. Then all the mice were
sacrificed at 20 days after injection. All the lungs were resected
and fixed. Then, the fixed lungs were embedded in paraffin,
sectioned and stained with H&E, followed by counting the
microscopic lung metastases and determining the metastasis
rate.
Statistical analysis
Quantitative data are presented as the mean ± SD and
were analyzed using unpaired Student's t-tests or the Mann-Whitney
test (non-parametric test when the P-value of the F test was
<0.05) for two groups. One-way ANOVA analysis with Tukey's
multiple comparisons was used for comparisons among three or more
groups. All analyses were performed using GraphPad Prism (version
8.00, GraphPad Software, Inc.; Dotmatics). In vitro
functional experiments were performed at least in triplicate.
Results
FOSL1 expression is upregulated in OSCs
and is associated with the stem cell-like phenotype of OS
cells
Previously, FOSL1 was reported to be highly
expressed in human OS tissues compared with that in non-tumor
tissues (23). To further
confirm this, a pan-cancer analysis was first applied by analyzing
The Cancer Genome Atlas samples at the UALCAN database to examine
the FOSL1 expression in multiple human benign tissues and tumors
(Fig. 1A). A higher rate of
abnormal FOSL1 expression was observed in multiple cancers,
including sarcoma, with a relatively high abundance. Then, by
analyzing the TARGET database, it was observed that compared with
that in normal bones, FOSL1 expression was significantly
upregulated in OS tissues (Fig.
1B). Moreover, survival analysis revealed that high expression
of FOSL1 was significantly associated with lower overall survival
and disease-free survival in sarcoma (Fig. 1C). Together, these bioinformatic
findings suggested that FOSL1 had a potential oncogenic role in
OS.
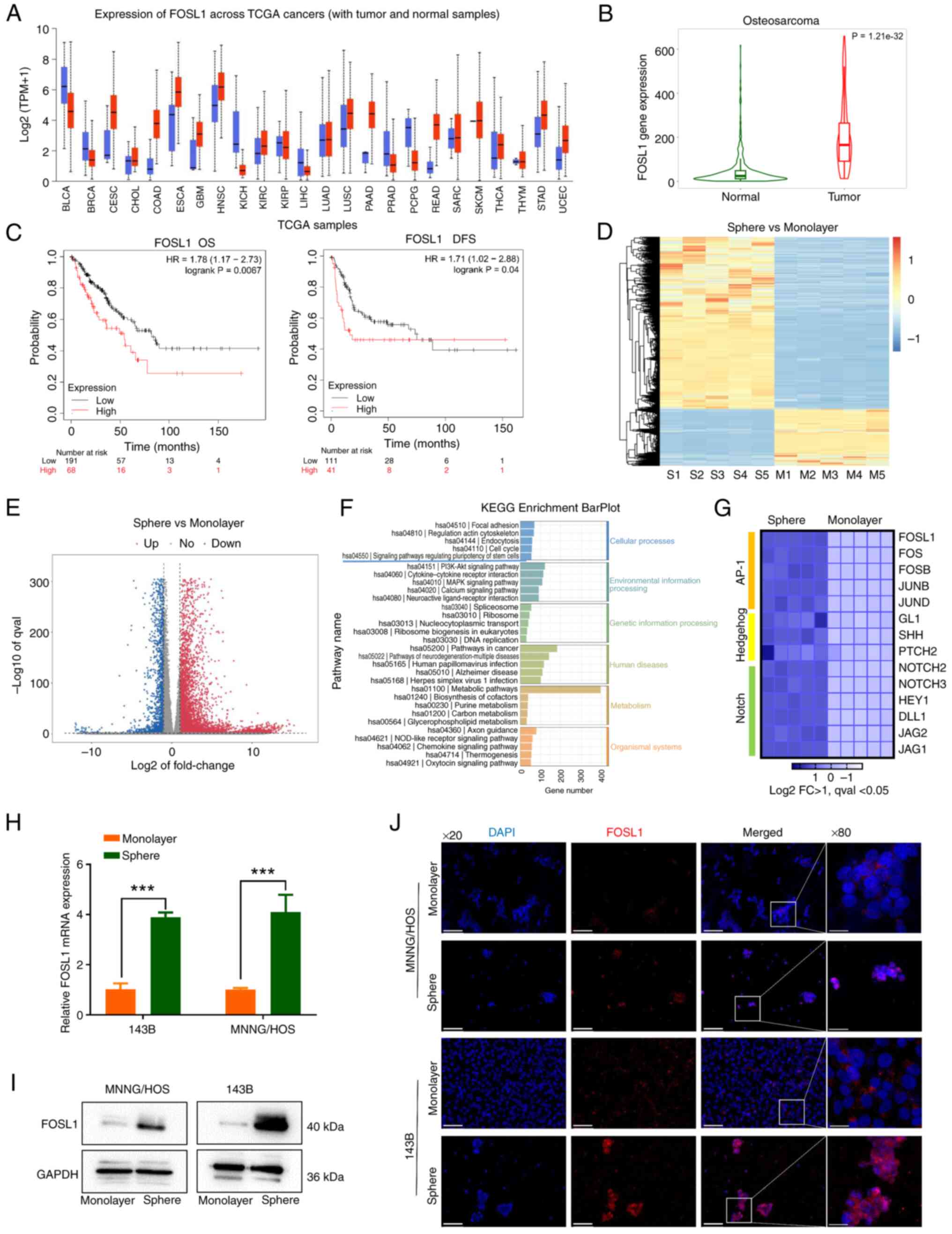 | Figure 1FOSL1 is highly expressed in OS and
upregulated in OSCs. (A) Expression of FOSL1 across a pan-cancer
analysis in TCGA samples, as analyzed at the UALCAN cancer database
(https://ualcan.path.uab.edu/). (B) The
expression of FOSL1 in OS tissues (red) and normal samples (green)
in the TARGET database, assessed using the TNMplot.com tool (https://tnmplot.com/analysis/). (C) Kaplan-Meier
curves of FOSL1 expression related to overall survival and
disease-free survival in sarcoma in the TCGA database, analyzed
using the KM-plotter database (https://kmplot.com/analysis/). (D and E) The heat map
and volcano map of the RNA-seq data, between monolayer groups
(adherent cells) and sphere groups (rich in OSCs). n=5, Log2|FC|
>1, qval <0.05. (F) KEGG Enrichment BarPlot of the RNA-seq
data. (G) Heat map of the differentially expressed genes in the
Notch, AP-1, and Hedgehog pathways, from gene expression profiling
results based on the RNA-seq data. n=5, Log2|FC| >1, qval
<0.05. (H) The mRNA expression of FOSL1 in tumor sphere cells
compared with that in monolayer adherent cells, in the indicated
cell lines. ***P<0.001. (I) FOSL1 expression patterns
in the indicated groups according to western blotting in 143B and
MNNG/HOS cells. (J) The difference in the distribution and
expression of FOSL1 in the monolayer and sphere groups of OS cell
lines (143B and MNNG/HOS) as analyzed using immunofluorescence
staining, Scale bars, 100 μm for the ×20 images and 25
μm for the x80 images. OS, osteosarcoma; OSCs, OS stem
cells; FOSL1, FOS-like antigen 1; TCGA, The Cancer Genome Atlas;
KEGG, Kyoto Encyclopedia of Genes and Genomes. |
Recent evidence suggested that FOSL1 could be a
candidate for targeted therapy for cancer stem cells (21) and OSCs were considered to be the
cause of tumor formation, recurrence and metastasis (9). Therefore, it was decided to
investigate whether FOSL1 plays a critical role in OSCs. Firstly,
OS-spheres were cultured in a serum-free medium supplied with
cytokines to enrich OSCs (30)
(Fig. S1A). In addition, to
confirm the enrichment of OSCs, FACS analysis demonstrated that the
population of CD117+ and Stro-1+ cells, which
have been proven as surface markers of OSCs (33), were significantly increased and
the expression levels of stemness-related transcription factors
SOX2 and NANOG were significantly upregulated, following OS-sphere
culture (Fig. S1B-D).
Thereafter, RNA-seq was employed to analyze the dynamic gene
expression patterns during acquisition of the stem-like properties
by OS cells (five samples for each group). The results are
demonstrated in Fig. 1D-F.
Notch, Hedgehog and AP-1 pathways which have been widely used to
study the maintenance of OS or other cancer cell stemness (10,34,35) are activated. Thus, the
differential expression of the major genes of these signaling
pathways was then explored and it was found that FOSL1 expression
was significantly increased during acquisition of the stem-like
properties in OS cells, which was confirmed using RT-qPCR and
western blotting (Fig. 1G-I).
More importantly, in the IF analysis, besides upregulation of FOSL1
protein levels, FOSL1 was mostly located in the nucleus (Fig. 1J). FOSL1 is a transcription
factor; therefore, these data suggested that FOSL1 might be
involved in the biological function of OSCs.
To validate the functional role of the upregulated
expression of FOSL1 in OSCs, two siRNAs (siRNA-1 and siRNA-2) were
generated to target two different FOSL1 sequences. Both siRNAs were
able to knockdown FOSL1 expression in 143B and MNNG/HOS OS cell
lines (Fig. 2A). As revealed in
Fig. 2A-D, depletion of FOSL1
significantly inhibited the tumor sphere and colony formation
ability of OS cells, and reduced the protein levels of SOX2 and
NANOG in 143B and MNNG/HOS cells. In addition, knockdown of FOSL1
significantly reduced the number of CD117+ and
Stro-1+ cells (Fig.
2E). These results suggested that FOSL1 is involved in
regulating the self-renewal and stemness phenotype of OSCs.
Collectively, the present data suggested that FOSL1 is
significantly upregulated and is a potential regulator of the
stemness phenotype of OSCs.
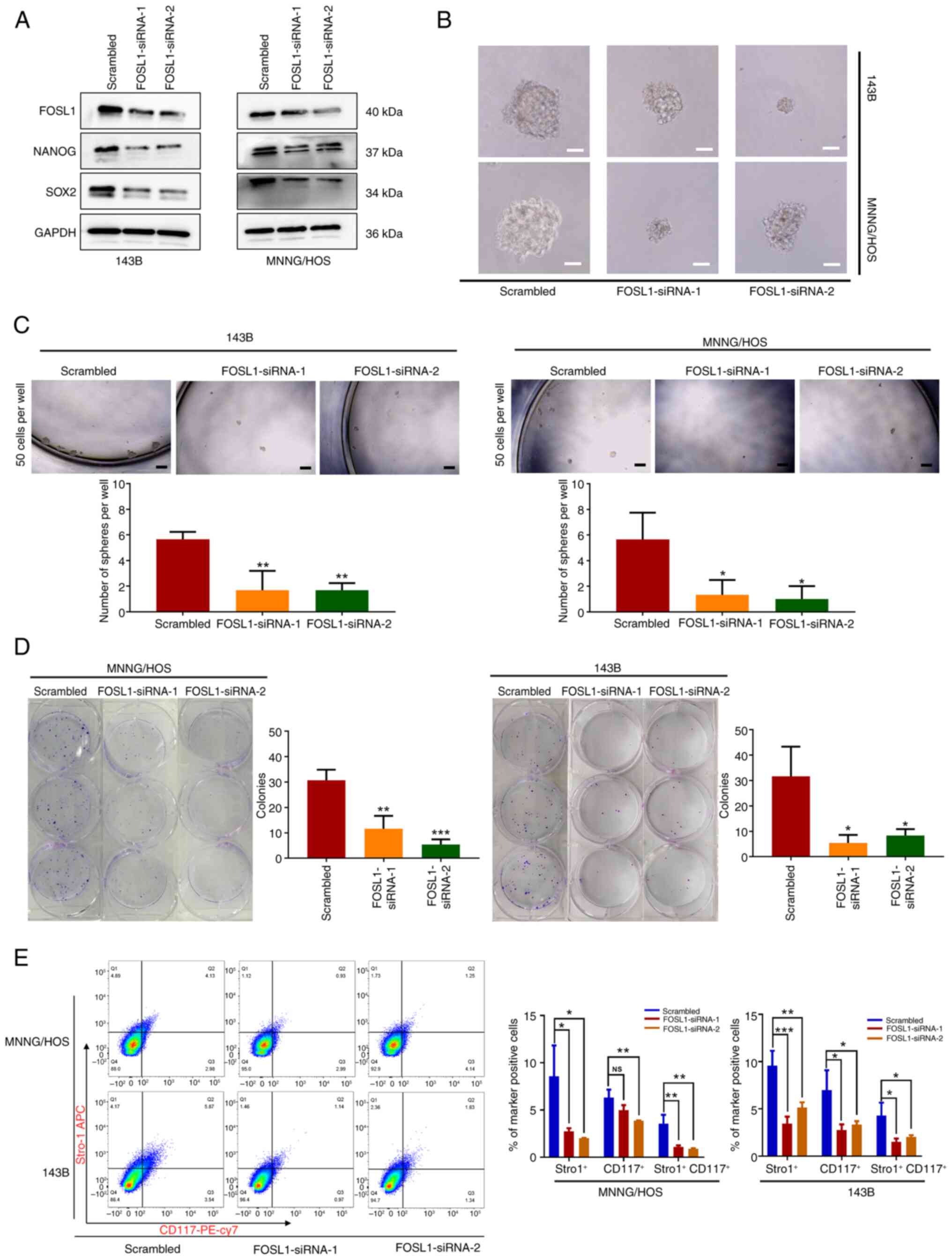 | Figure 2Upregulated expression of FOSL1 in
OSCs is associated with the maintenance of stem-like properties.
(A) Representative images of western blot analysis of FOS1L, NANOG
and SOX2 levels in 143B and MNNG/HOS cells, following transfection
with two siRNAs targeting FOSL1. (B) Results of a sphere formation
test of OSC stemness after transfection with siRNA-FOSL1. Typical
photomicrographs of the newly formed clonal spheres are shown.
Scale bars, 50 μm. (C) Statistical analysis of the numbers
of clonal spheres. scale bars, 100 μm. (D) Transfection with
siRNA-FOSL1 attenuated colony-formation by OS cell lines (143B and
MNNG/HOS). Images of colony formation captured after 12 days of
culture for 143B cells or 13 days of culture for MNNG/HOS cells.
(E) Results of flow cytometric analysis carried out to measure
CD117 and Stro1 expression on 143B and MNNG/HOS OS cells (after
suspension culture) following transfection with siRNA-FOSL1. Each
experiment was conducted three times. *P<0.05,
**P<0.01 and ***P<0.001 compared with
the scrambled group. FOSL1, FOS-like antigen 1; OS, osteosarcoma;
OSCs, OS stem cells; SOX-2, SRY (sex determining region Y)-box 2;
siRNA, small interfering RNA; NS, not significant (P>0.05). |
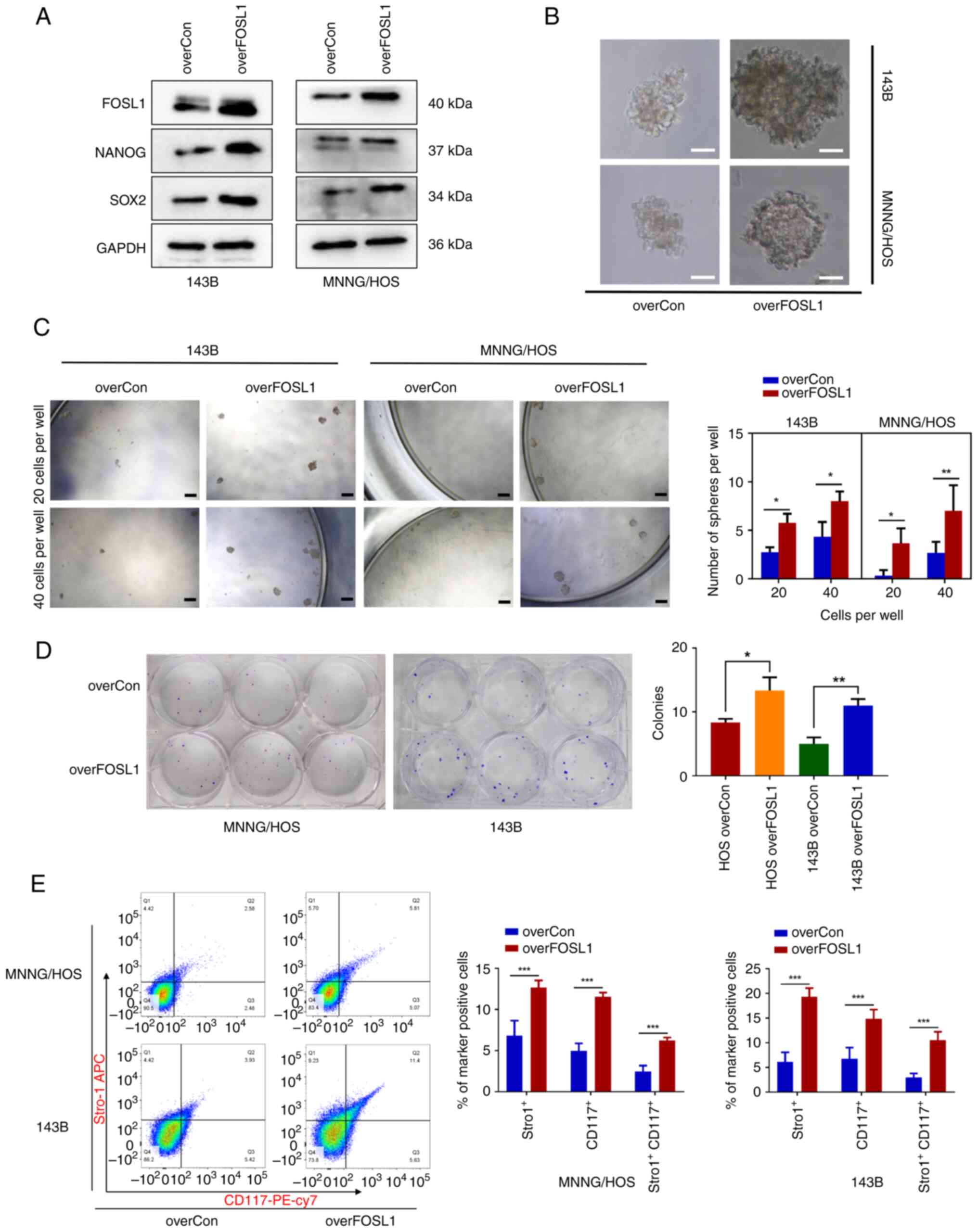
FOSL1 maintains OSC stemness and promotes
anoikis resistance in OS
To further address whether FOSL1 promotes the
stemness phenotype and self-renewal ability in OS in vitro,
FOSL1 was stably overexpressed using the lentivirus-based overFOSL1
plasmid in 143B and MNNG/HOS cells and the effect was examined
using western blotting (Fig.
3A). Then, in vitro tumor sphere formation and colony
formation assays were performed to detect the self-renewal ability
in OS cells. Unsurprisingly, the sphere-forming and colony
formation capacity of FOSL1-overexpressing cells was significantly
increased compared with that in the overexpression control
(overCon) cells, and the spheres generated from
FOSL1-overexpressing cells were markedly bigger than those
generated from overCon cells (Fig.
3B-D). Subsequently, it was found that overFOSL1 OS cells had
significantly higher protein levels of SOX2 and NANOG compared with
overCon OS cells (Fig. 3A). In
addition, FACS analysis demonstrated that the population of
CD117+ and Stro-1+ OS cells was significantly
increased following FOSL1 overexpression in 143B and MNNG/HOS
sphere cells (Fig. 3E). Thus,
these findings confirmed that FOSL1 is a key regulator that
promotes the stemness phenotype of OS in vitro.
Metastasis is a major factor that predicts poor
prognosis in patients with OS (36). High expression of FOSL1 is
considered to facilitate the metastasis of malignant tumors of
epithelial origin by promoting their migration and invasion ability
(18,37); however, the relationship between
FOSL1 and OS metastasis is unclear. To examine the effect of FOSL1
in regulating OS cell metastasis in vitro, Transwell assays
were first performed to determine whether the change in FOSL1
expression influenced the migration ability of OS cells.
Surprisingly, FOSL1 overexpression did not have a significant
effect on the migration of 143B and MNNG/HOS cells (Fig. 4A). While searching for other
possible mechanisms by which FOSL1 might regulate OS cell
metastasis, our attention was drawn to anoikis resistance, which is
an important process in tumor distant metastasis (17,38). A previous study identified that
FOSL1 is involved in regulating anoikis resistance in the K-Ras
transformed Madin-Darby Canine Kidney (MDCK) cell line (23). Thus, it was investigated whether
FOSL1 could affect anoikis in OS cells using ultra-low attachment
6-well plates to create suspension conditions. After suspension
culture for 24 or 48 h, it was found that overexpression of FOSL1
significantly attenuated the anoikis rate compared with their
respective control group, and an increased percentage of anoikis
cells was observed after transfection with FOSL1 siRNAs in 143B and
MNNG/HOS cells (Fig. 4B and C).
Consequently, the dynamic changes in the levels of apoptotic
proteins were detected. The results demonstrated that
overexpression of FOSL1 not only increased anti-apoptotic protein
B-cell CLL/lymphoma 2 (BCL2) expression, but also reduced the
levels of pro-apoptotic protein BCL2 associated X protein (BAX) and
cleaved Caspase-3 in the 143B and MNNG/HOS cells under suspension
culture, which was reversed by knockdown of FOSL1 (Fig. 4D). These results allowed us to
conclude that FOSL1 could reduce anoikis sensitivity in OS
cells.
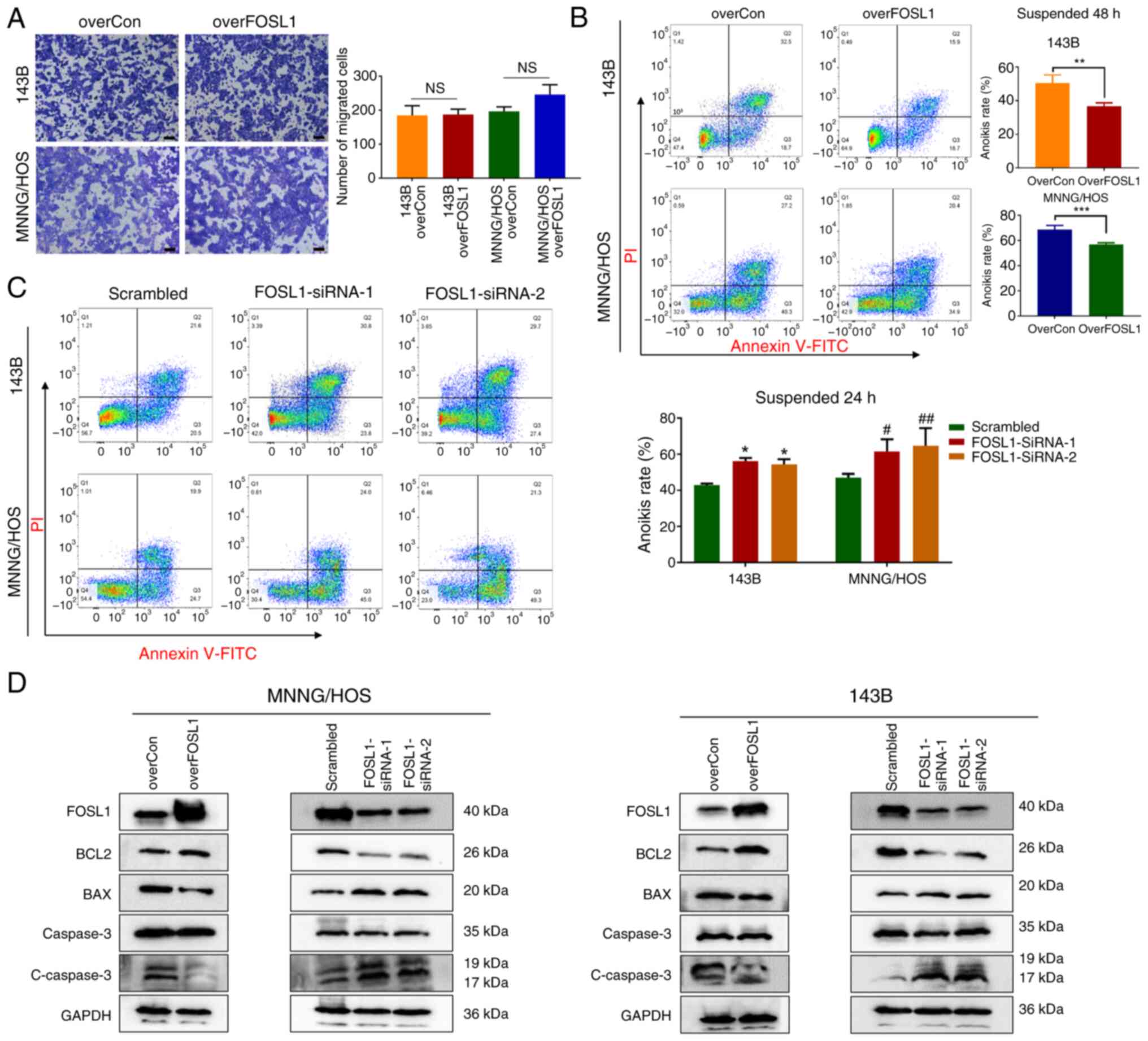 | Figure 4Crucial involvement of FOSL1
expression in OS cells anoikis resistance. (A) Representative
images and statistical analysis of the Transwell assay of cells
overexpressing FOSL1 or not. Scale bars, 50 μm. (B) Results
of an Annexin V-FITC/PI assay for the anoikis rates in the overCon
and overFOSL1 (FOSL1 overexpression) groups of OS cell lines 143B
and MNNG/HOS after incubation for 48 h. **P<0.01 and
***P<0.001. (C) Results of Annexin V-FITC/PI assays
for the anoikis rates in the indicated groups transfected with
siRNA-FOSL1, after suspension culture for 24 h.
*P<0.05 compared with the 143B scrambled group;
#P<0.05 and ##P<0.01, compared with the MNNG/HOS
scrambled group. (D) Representative images of the western blot
analysis for FOSL1, BCL2, BAX, Caspase 3 and cleaved-Caspase 3 in
143B and MNNG/HOS cells. Cells were subjected to FOSL1
overexpression or silencing. FOSL1, FOS-like antigen 1; OS,
osteosarcoma; NS, not significant (P>0.05); overCon,
overexpression control; siRNA, small interfering RNA. |
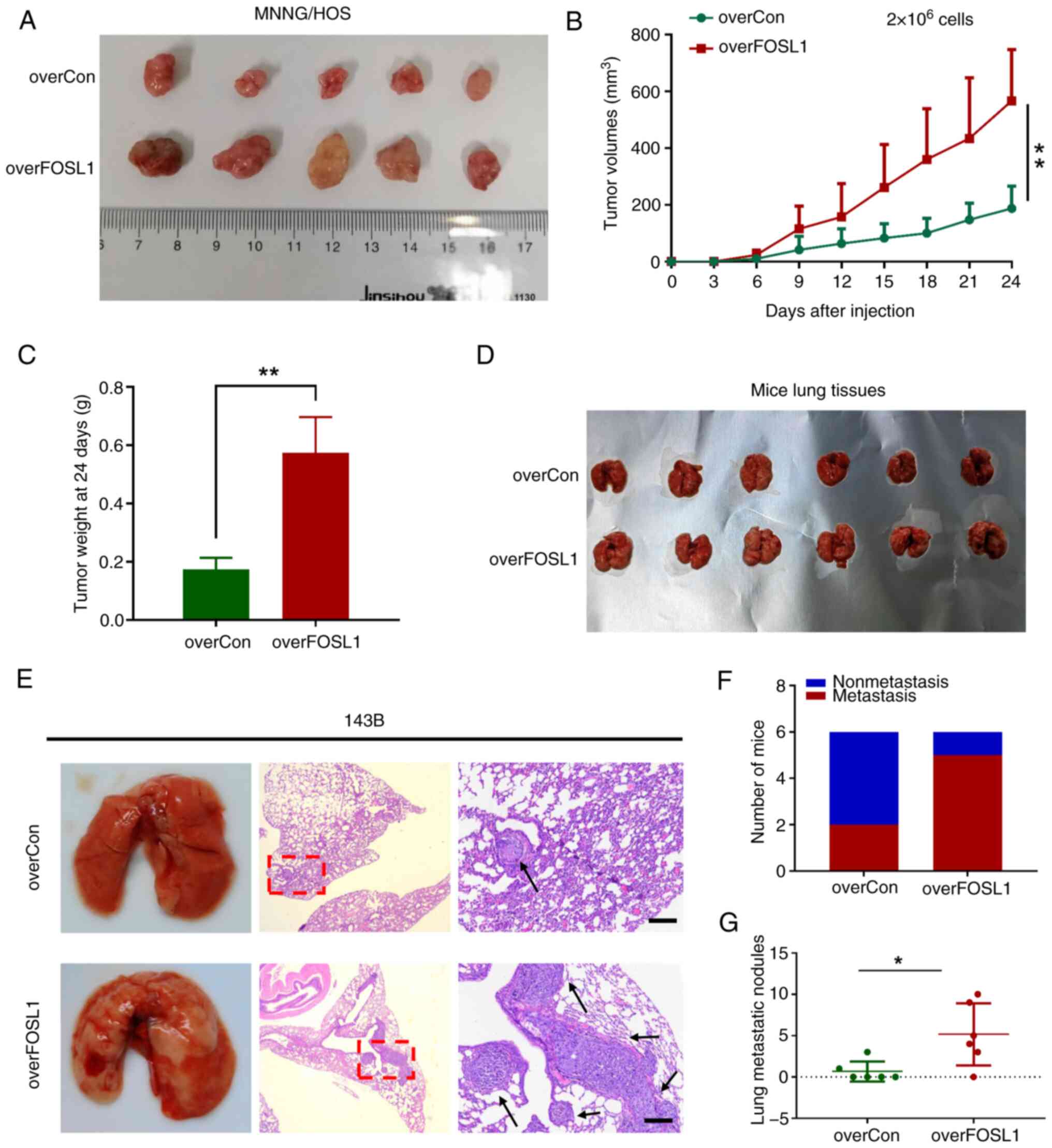
FOSL1 promotes OS cell tumorigenicity and
metastasis in vivo
Having shown that FOSL1 could promote the stemness
phenotype and anoikis resistance in OS cells in vitro, it
was important to determine whether these effects could also be
detected in vivo. Subcutaneous xenograft models were created
with 2×106 tumor cells inoculated subcutaneously into
athymic nude mice after treatment with or without FOSL1
overexpression. In addition, an in vivo limiting-dilution
assay with the subcutaneous xenograft models was performed with two
different low doses (5×104 and 5×105) of
overFOSL1-MNNG/HOS cells and their corresponding control cells. In
agreement with the in vitro results, FOSL1-MNNG/HOS cells
displayed significantly higher tumorigenicity and tumor
re-initiating cell frequency compared with those of the control
cells (Fig. S2). Moreover,
FOSL1 overexpression significantly increased OS growth, as
reflected by the tumor size, volume and tumor weight of the
xenograft tumors (Fig.
5A-C).
Anoikis resistance, as well as extensive
self-renewal potential, are both critical factors that drive tumor
cell metastasis (39). To
further investigate whether FOSL1-induced anoikis resistance and
stemness promotion contribute to metastasis in vivo, a mouse
lung metastasis model was applied by injecting OS cells into the
tail vein. In line with our in vitro findings, it was
demonstrated that overexpression of FOSL1 significantly increased
the ability of 143B cells to produce lung metastases compared with
that of the control cells, as reflected by the increased metastasis
incidence and number of OS metastatic nodules in the lungs
(Fig. 5D-G). Thus, the in
vitro and in vivo results indicated that FOSL1 plays as
a crucial role in facilitating the tumorigenesis and metastasis of
OS by promoting the stemness potential and anoikis resistance of OS
cells.
FOSL1 upregulates SOX2 expression by interacting
with the SOX2 promoter and activating its transcription. Previous
evidence has highlighted FOSL1 as a versatile transcription factor,
with numerous biological roles (40). As demonstrated in Fig. 6A, after transfection with the
lentivirus-based overFOSL1 plasmid, FOSL1 expression was
significantly upregulated. Notably, FOSL1 was localized in the
nucleus of 143B and MNNG/HOS cells, indicating its active role as a
transcription factor. To identify the key downstream factor
promoting the OS stemness phenotype controlled by FOSL1, the R2
database was further analyzed to investigate the correlation
between FOSL1 and some key stemness transcription factors in OS
samples (9,41). The results revealed that there
was a positive correlation between FOSL1 and SOX2 (r=0.322,
P<0.001) (Fig. 6B). It was
shown that overexpression of FOSL1 could upregulate SOX2 protein
levels. To further confirm the regulation at the mRNA level,
RT-qPCR was performed. The results verified that overexpression of
FOSL1 significantly and consistently increased the mRNA expression
levels of SOX2 in the two OS cell lines (Fig. 6C). Based on the aforementioned
results, it would be expected that overexpression of FOSL1 would
enhance SOX2 promoter activity. To confirm this hypothesis, FOSL1
or the negative control were transiently overexpressed in MNNG/HOS
cells cotransfected with the SOX2 promoter-luciferase construct and
then a dual luciferase assay was performed. As expected, SOX2
transcriptional activity was increased after FOSL1 overexpression.
In addition, the potential binding site sequence between FOSL1 and
the SOX2 promoter might be 'ATGACACACC', because the promotion
effect of FOSL1 disappeared after the SOX2 promoter was mutated at
this site (Fig. 6D and Table SII). Similar results were
obtained using ChIP coupled with RT-qPCR, which confirmed that in
overFOSL1 MNNG/HOS cells, FOSL1 could strongly bind to the SOX2
promoter, highlighting that SOX2 is a target of FOSL1 (Fig. 6E). Moreover, mIHC staining and IF
assays showed colocalization of FOSL1 and SOX2 proteins in
overFOSL1 MNNG/HOS xenograft tumor tissues and overFOSL1 OS cells
(Fig. 6F and G). These results
also suggested an interaction between FOSL1 and SOX2 in OS cells.
Additionally, given that NANOG has been reported as a target gene
of FOSL1 (21) and its protein
expression was upregulated in OS cells after FOSL1 overexpression,
RT-qPCR and luciferase reporter assays were applied to investigate
whether NANOG is also a candidate transcriptional target gene of
FOSL1. In contrast to previous results, overexpression of FOSL1
only improved the mRNA expression levels of NANOG in MNNG/HOS cell
line but not obvious in the 143B cell line, and although FOSL1
activated the transcription of NANOG in MNNG/HOS cells, the
increase was not obvious (Fig. 6C
and D). Thus, wit is considered that in OS cells, SOX2 is more
likely to be the key downstream target of FOSL1, rather than NANOG.
Taken together, the results indicated that SOX2 is a major
transcriptional target of FOSL1 in OS cells.
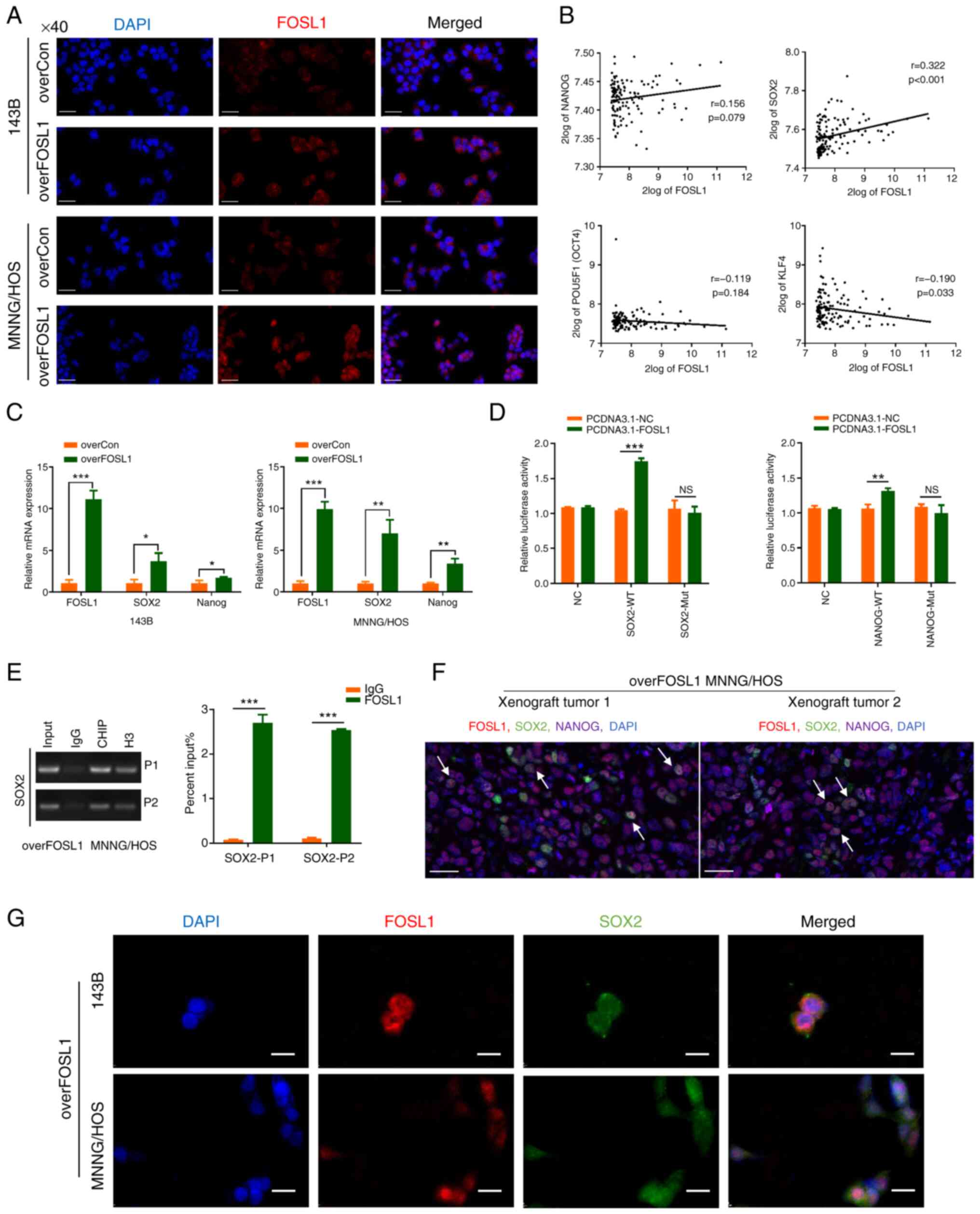 | Figure 6FOSL1 upregulates SOX2 expression by
promoting SOX2 transcriptional activation. (A) Immunofluorescence
staining showing the difference in the distribution and expression
of FOSL1 in monolayer OS cell lines (143B and MNNG/HOS) after
overexpression of FOSL1. Scale bars, 50 μm. (B) Pearson
correlation analysis between FOSL1 and stemness transcription
factor expression levels carried out at the R2 OS gene expression
database (http://hgserver1.amc.nl) using the
dataset named mixed OS-Kuijjer-127-vst-ilmnhwg6v2. (C) The mRNA
expression levels of FOSL1, SOX2 and NANOG after overexpression of
FOSL1 in the indicated cell lines. (D) Luciferase activity of
MNNG/HOS cells transformed with a luciferase reporter plasmid
expressing WT SOX2 or NANOG and Mut SOX2 or NANOG, co-transfected
with PCDNA3.1-FOSL1 or the negative control. (E) The enrichment in
the FOSL1, IgG (negative control) and H3 (positive control) groups
at the SOX2 promoter in overFOSL1 (FOSL1 overexpression) MNNG/HOS
cells, as detected using a ChIP-PCR assay. (F) Multiplex
immunohistochemistry staining using the TSA assay for the xenograft
tumor tissues of overFOSL1 MNNG/HOS cells. Markers were used as
indicated. scale bars: 25 μm. (G) Subcellular localization
of FOSL1 and SOX2 in overFOSL1 143B and MNNG/HOS cells, as analyzed
using confocal laser scanning microscopy. Scale bars, 25 μm.
*P<0.05, **P<0.01 and
***P<0.001. FOSL1, FOS-like antigen; SOX-2, SRY (sex
determining region Y)-box 2; OS, osteosarcoma; WT, wild-type; Mut,
mutant; NS, not significant (P>0.05); overCon, overexpression
control. |
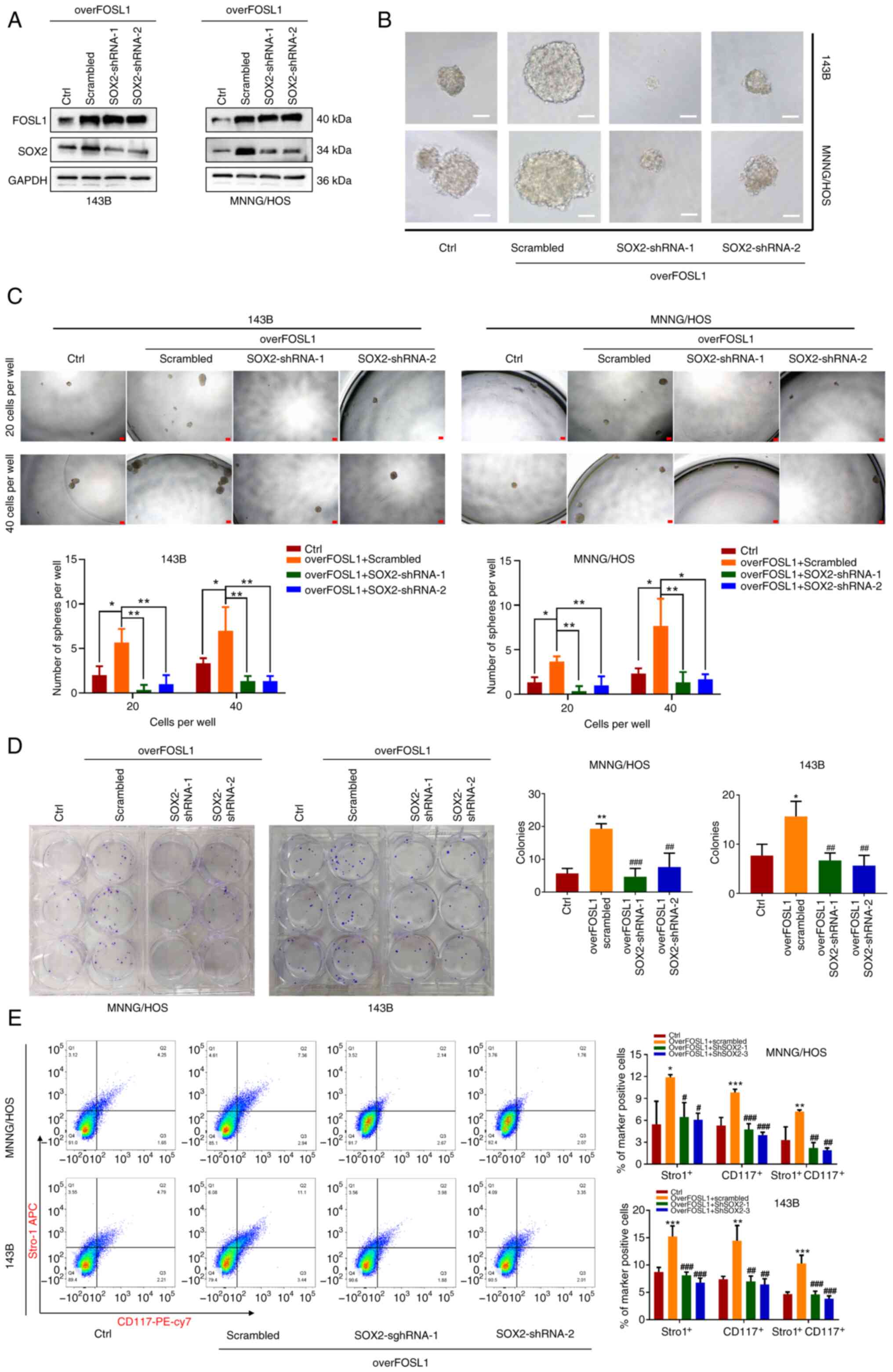
SOX2 contributes to the FOSL1-promoted stemness
phenotype and anoikis resistance in OS cells. SOX2 was reported to
be a key protein related to the maintenance of a stem-like
phenotype in several type of tumors, including OS (42). Thus, to determine whether the
FOSL1-promoted stemness phenotype of OS cells was related to SOX2,
SOX2 expression was knocked down using shRNA lentiviruses (two
sequences), which were transfected into FOSL1-overexpressing cells,
with western blot analysis confirming this result (Fig. 7A). It was found that knockdown of
SOX2 significantly abrogated FOSL1 overexpression-induced promotion
of sphere formation and colony formation in 143B and MNNG/HOS cells
(Fig. 7B-D). Subsequently, it
was found that knockdown of SOX2 also attenuated the FOSL1
overexpression-induced increase in the population of
CD117+ and Stro-1+ OS cells among 143B and
MNNG/HOS cells, as assessed using FACS assays (Fig. 7E). These data indicated that
FOSL1 promotion of the self-renewal ability of OS cell lines is
related to SOX2.
Moreover, compared with the extensive research on
cell stemness maintenance, relatively little attention had been
paid to SOX2 in the field of cell resistance to anoikis. A recent
study reported that SOX2 could also be a driver of the acquisition
of anoikis resistance in ovarian carcinoma cells (43). However, the exact role of SOX2 in
the regulation of anoikis in OS remains unclear. Therefore, to
investigate whether SOX2 contributes to the FOSL1-induced
suppression of anoikis in OS cells, shSOX2-1 and shSOX2-2
lentiviruses were used to eliminate the upregulated expression of
SOX2 after transfection with the FOSL1-overexpression lentiviruses.
As shown in Fig. 8A-C, as
expected, after anchorage-independent suspension culture,
overexpression of FOSL1 significantly reduced the anoikis rate of
143B and MNNG/HOS cells, while the anoikis rates were partly
restored after knockdown of SOX2. This was consistent with the
change in the anoikis rate induced by the FOSL1
overexpression-mediated increase in BCL2 protein levels, and the
reduction of BAX and cleaved Caspase-3 protein levels in OS cells
under the aforementioned suspension culture conditions, which was
also partially reversed by knockdown of SOX2 (Fig. 8D). These data demonstrated that
SOX2 is necessary for FOSL1-induced promotion of the stemness
phenotype and anoikis resistance in OS.
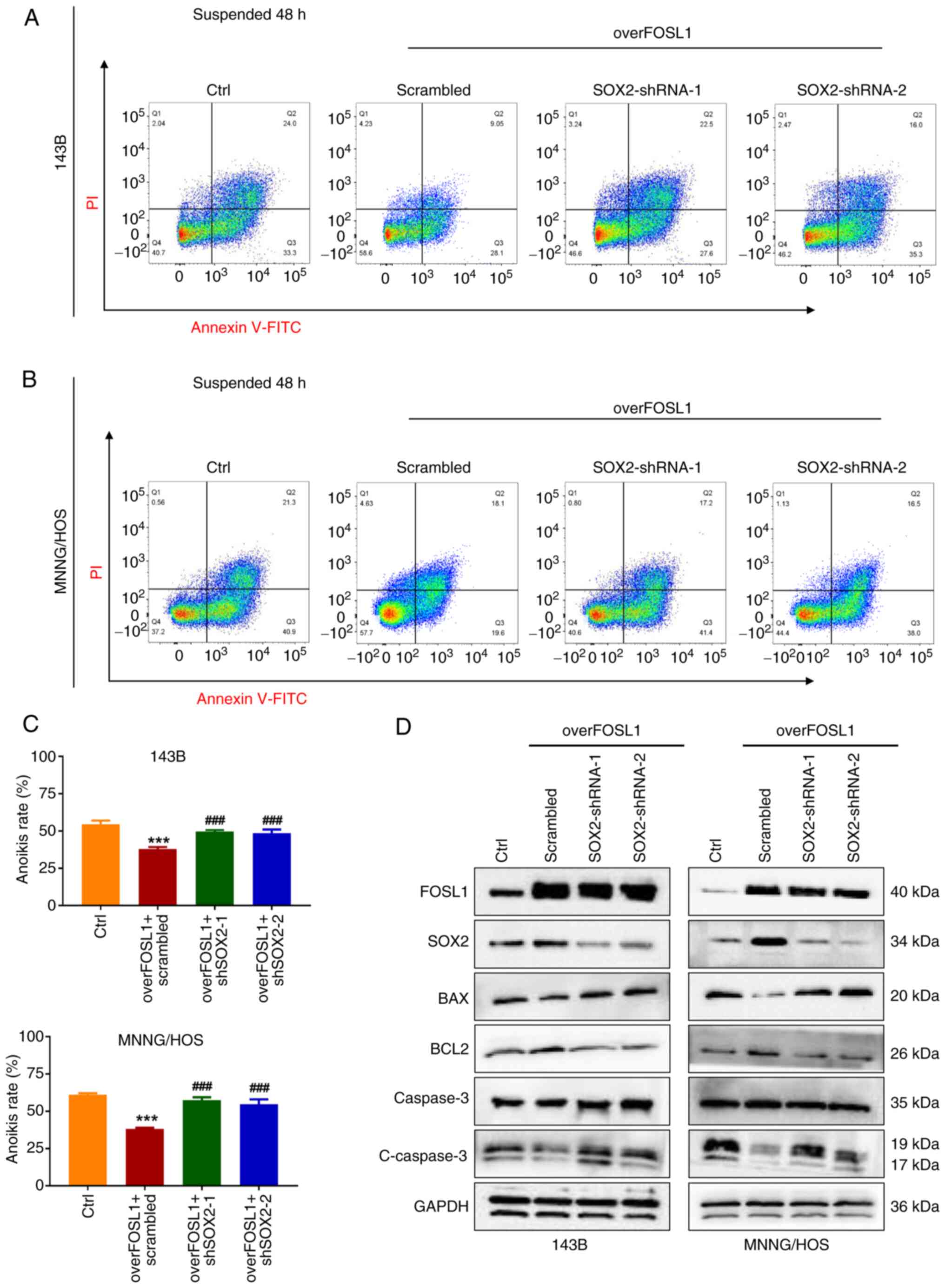 | Figure 8SOX2 is required for FOSL1-mediated
promotion of the anoikis resistant properties of OS cells. (A and
B) The anoikis rates were detected by an Annexin V-FITC/PI assay in
the four groups of OS cell lines 143B and MNNG/HOS, as indicated,
after suspension culture for 48 h. (C) The statistical analysis of
the quantitative flow cytometry data in A and B.
***P<0.001 compared with the Ctrl group;
###P<0.001 compared with overFOSL1 (FOSL1
overexpression) and scrambled group. (D) Representative images of
the western blot analysis of FOSL1, SOX2, BCL2, BAX, Caspase 3 and
cleaved-Caspase 3 in 143B and MNNG/HOS cells. Cells were subjected
to FOSL1 overexpression and SOX2 silencing, as indicated. SOX-2,
SRY (sex determining region Y)-box 2; FOSL1, FOS-like antigen; OS,
osteosarcoma; shRNA, short hairpin RNA. |
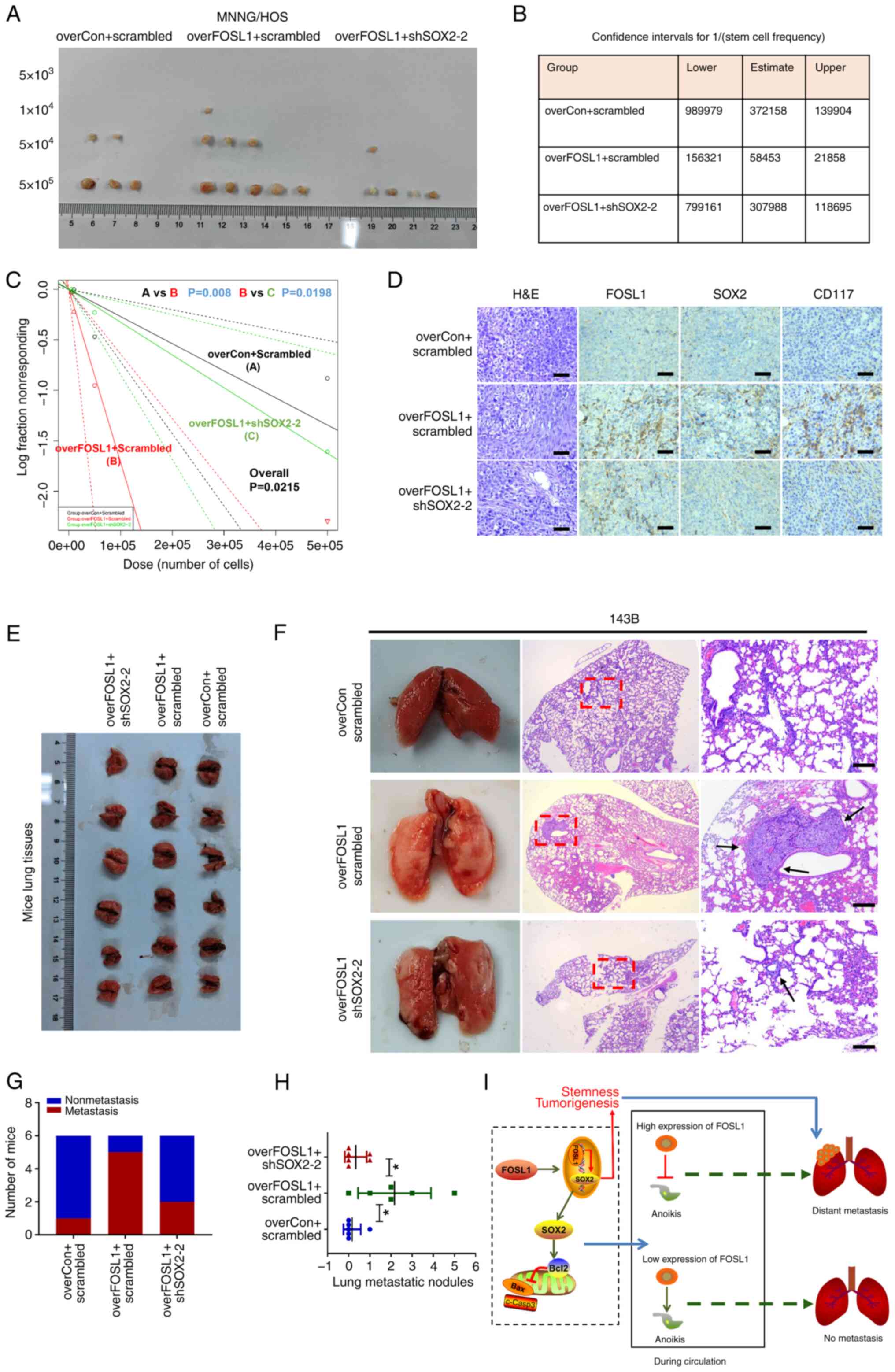
Targeting SOX2 inhibits FOSL1-associated promotion
of tumorigenesis and metastasis in OS. To further investigate
whether SOX2 contributes to FOSL1-induced promotion of
tumorigenesis and metastasis in OS in vivo, subcutaneous
xenograft models were first established by stably transfecting
overFOSL1 MNNG/HOS cells, with or without shSOX2, into nude mice.
An in vivo limiting-dilution assay with the subcutaneous
xenograft models was first performed with four different low doses
(5×103, 104, 5×104 and
5×105) of cells. Interestingly, the tumorigenicity and
tumor re-initiating cell frequency in the overFOSL1 group was
increased compared with those in the control group, but were
significantly reduced after knockdown of SOX2 (Fig. 9A-C). Moreover, the FOSL1-induced
increase in OS growth ability in vivo was also reduced after
knockdown of SOX2 (Fig. S3). In
addition, the fact that overexpression of FOSL1 enhanced SOX2
expression and knockdown of SOX2 decreased its protein expression
were further confirmed in vivo using IHC staining for FOSL1
and SOX2 proteins in mouse xenograft tumor tissues (Fig. 9D). As expected, the rate of
positive CD117 IHC staining was low in the control groups,
significantly increased in the FOSL1-overexpressing group, and
reduced in the FOSL1-overexpressing + SOX2-knockdown group,
suggesting that SOX2 contributes to FOSL1-induced promotion of
stemness and tumorigenesis in vivo (Fig. 9D). Consistent with our in
vitro findings, it was found that FOSL1 could significantly
increase the ability of OS cells to produce lung metastases
compared with that of the control cells. However, knockdown of SOX2
reduced the ability of FOSL1-overexpressing cells to establish lung
metastases, as reflected by the decreased metastasis incidence and
number of OS metastatic nodules in the lungs compared with those in
the overFOSL1 group (Fig. 9E-H).
These results, together with our in vitro findings,
highlighted a functional role for FOSL1 and SOX2 in the regulation
of tumorigenesis and metastasis in OS (Fig. 9I).
Discussion
Considering that OS treatment has not significantly
improved over the past 40 years, there is an urgent clinical need
to develop novel and effective treatments for this common sarcoma
(29). Although the average
5-year survival rate of patients with OS has increased to ~65%
(1), the survival rate reduces
markedly in patients with overt metastases (4). Indeed, the main reason for poor
prognosis of OS can be attributed to metastases. Therefore, there
is a pressing need to find an effective therapy to reduce OS
metastasis. Emerging evidence suggests that cancer stem cells cause
cancer metastasis, local recurrence and therapy resistance in
multiple types of cancer, including OS (7,10,44). Theoretically, according to their
tumor initiation ability, OSCs are considered as important 'seeds',
which enter the blood circulation and initiate colonization at the
secondary site, thus forming a distant metastasis. However, most
circulating cancer cells will be eliminated during blood
circulation; therefore, an anoikis resistance ability is needed to
allow the surviving cells to eventually reach the secondary site of
metastasis (11). Thus,
metastasis formation is considered to be restricted to, and driven
by, a rare subpopulation of tumor cells with a distinctive set of
characteristics, including resistance to anoikis and extensive
self-renewal potential (39).
Interestingly, Guha et al (45) found that anoikis-resistant cancer
cells exhibited similar biological characteristics to cancer stem
cells, reflecting that these cells can express high levels of
cancer stem cell biomarkers such as CD44, CD133, OCT4, NANOG and
SOX2. Thus, it was aimed to identify a collective target for OSCs
and anoikis resistance to provide a new insight into OS therapy.
Accordingly, in the present study, it was demonstrated that FOSL1
could be a new target, not only for OSCs, but also for anoikis
resistance in OS cells.
FOSL1, as a component of the activated protein-1
(AP-1) transcription factor complex, has been reported as a key
regulator that maintains stem cell-like characteristics in various
cancers, such as glioblastoma (46), head and neck squamous cell
carcinoma (18) and colorectal
cancer (21). However, the
functional role of FOSL1 in OS is infrequently discussed and
remains poorly understood. In the present study, the role of FOSL1
was investigated in OS and OSCs. In agreement with the results of a
previous study (23), it was
found that FOSL1 mRNA expression was upregulated in OS tissues
compared with that in normal tissues, and might be a predictor for
poor prognosis in patients with sarcomas. Previous evidence
suggests that overexpression of different AP-1 complex members
could induce genesis of OS in immortalized human mesenchymal stem
cells and exhibited an OS phenotype, indicating a process of tumor
initiation (35). OSCs are tumor
initiation cells and FOSL1 is a member of the AP-1 complex, acting
as a stemness regulator in other kinds of cancers; therefore, it
was hypothesized that FOSL1 could also regulate the OS stemness
phenotype. As expected, based on the RNA-seq results, it was found
that numerous AP-1 related genes were upregulated in OSCs,
including FOSL1. Confirmatory experiments demonstrated that the
FOSL1 protein level is upregulated in OSCs and importantly,
depletion of FOSL1 severely attenuated the self-renewal ability of
OSCs. Consistent with our hypothesis, studies have suggested that
silencing FOSL1 expression using RNA interference is a promising
option to deplete cancer stem cells (18,47). Moreover, it was revealed that
overexpression of FOSL1 not only promoted the self-renewal ability
and stemness biomarker expression levels (CD117, Stro-1, SOX2 and
NANOG) in OS cells, but also decreased their anoikis rate. Indeed,
the effect of FOSL1 in facilitating lung metastasis was confirmed
using the mouse tail vein model to simulate anoikis. Accumulating
evidence has highlighted the importance of triggering the integrin
pathway and suppressing intrinsic pathways to avoid anoikis
(13,17). Zhang et al (22) found that FOSL1 could upregulate
α6-integrin expression in K-RasV12-transformed MDCK
cells. Thus, western blot analysis was used to clarify the
relationship between FOSL1 and BCL-2 family proteins, which are the
common downstream targets for the integrin pathway during anoikis
regulation. Consequently, it was demonstrated that FOSL1 regulated
OS cell anoikis by upregulating the anti-apoptotic protein BCL2,
and decreasing BAX expression. In addition, the FOSL1-induced
reduction in the occurrence of cell death observed when
anchorage-dependent cells detached from the ECM might partly
contribute to its promotion of an increased OSC population. Cancer
stem cells are considered to have an innate ability to evade
anoikis and are capable of anchorage-independent proliferation
(48). Moreover, there is
evidence that cancer stem cells could protect non-stem cells from
anoikis (49). Thus, it is not
unlikely that the increase in the proportion of OSCs could decrease
the anoikis rate of OS cells. Moreover, FOSL1 was reported to
facilitate tumor cell migration and invasion in various cancers
(37,50). However, surprisingly, after FOSL1
overexpression, a significant effect on OS cell migration ability
was not observed in two OS cell lines, which might be explained by
heterogeneity among different kinds of tumors. Overall, the present
findings highlight a novel role for FOSL1 as a target for OSCs and
anoikis resistance in OS.
In OS, SOX2 and NANOG were both reported as key
genes related to the maintenance of a stem-like phenotype (9,41) and the current findings showed
that both SOX2 and NANOG protein levels are upregulated after FOSL1
overexpression. However, in contrast to the study of Wang et
al (21) that FOSL1 plays
transcriptional regulation role in NANOG expression in colorectal
cancer cells, it was found that SOX2 is more likely to be a direct
transcriptional target of FOSL1 in OS cells. Studies have
emphasized the pluripotency-inducing transcription factor function
of SOX2 in OS (9,51); therefore, our observation that
SOX2 contributes to FOSL1-induced promotion of OC cell self-renewal
was not unexpected. However, the function of SOX2 in anoikis
resistance in OS cells remains unclear. In the present study, it
was identified that SOX2 is an essential factor through which FOSL1
regulates BCL2, BAX and cleaved-Caspase 3 protein levels, thereby
regulating OS cell anoikis. Unfortunately, whether BCL2 or BAX are
direct or indirect transcriptional targets of SOX2 is beyond the
scope of the present study. However, consistent with the present
findings, a recent study showed that SOX2 could drive the anoikis
resistance ability of ovarian carcinoma cells by regulating
apoptotic pathway genes (43).
Moreover, another study also reported that inhibition of SOX2 could
induce changes in cell apoptosis and BCL2 protein family expression
through phosphatidylinositol-4,5-bisphosphate 3-kinase
(PI3K)/protein kinase B (AKT) pathways in Ewing's sarcoma cells
(52). Collectively, the results
of the present study confirmed the crucial involvement of SOX2 in
FOSL1-mediated stemness and anoikis in OS; however, future
experiments should focus on gaining a more detailed understanding
of the mechanism of SOX2 in the regulation of cell anoikis.
Taken together, the present data highlight the
importance of FOSL1 in OSCs. Moreover, FOSL1 is an important
regulator that promotes stemness and anoikis resistance to
facilitate tumorigenesis and metastasis in OS. In addition, SOX2 is
the direct transcriptional target of FOSL1. Thus, FOSL1 might
represent an attractive target for therapeutic intervention to
treat OS.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The data generated in the
present study may be found in the Sequence Read Archive database
under accession number PRJNA1080098 or at the following URL:
https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1080098.
Authors' contributions
GSZ, CL and YW designed the experiments. YW, QH and
GSZ participated in the whole experiment, and collected and
analyzed the data. GSZ performed data analysis, prepared the
figures and wrote the manuscript. YC and LY participated in the
cell culture and animal experiments. YFW contributed to the
bioinformatics analysis. SZ and YB provided technical and
theoretical support. QH, LY and HL participated in the western blot
analysis and manuscript preparation. GSZ, CL and YY provided
financial and administrative support. GSZ, CL and YW confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All the animal care and experimental procedures
were approved (approval no. 2022-224) by the Institutional Animal
Care and Use Committee of the Second Affiliated Hospital of
Chongqing Medical University (Chongqing, China), and were performed
according to the Guide for the Care Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
FOSL1
|
FOS-like antigen 1
|
|
FRA1
|
FOS-related antigen 1
|
|
OS
|
osteosarcoma
|
|
OSCs
|
OS stem cells
|
|
IHC
|
immunohistochemistry
|
|
SOX2
|
SRY (sex determining region Y)-box
2
|
|
siRNA
|
small interfering RNA
|
|
shRNA
|
short hairpin RNA
|
|
H&E
|
hematoxylin and eosin
|
|
ChIP
|
chromatin immunoprecipitation
|
|
IF
|
immunofluorescence
|
|
ECM
|
extracellular matrix
|
Acknowledgements
The authors would like to thank Professor Qiao-Nan
Guo and Professor Yang-Fan Lv from the Xinqiao Hospital (Army
Medical University) for their guidance on the present study. The
authors are also grateful to the Department of Pathology of Xinqiao
Hospital for providing parts of the experimental apparatus and for
their assistance with immunohistochemical techniques. The authors
also appreciate the support of LC Bio Technology Co., Ltd. for
assisting with sequencing and/or bioinformatics analysis.
Funding
The present study was supported by the National Natural Science
Foundation of China (NSFC) (grant nos. 82303465 and 82303712), the
Natural Science Foundation of Chongqing, China (grant no.
CSTB2022NSCQ-MSX0103), the Special Financial Aid to the Post doctor
Research Project of Chongqing (grant no. 2022CQBSHTB3079) and the
China Postdoctoral Science Foundation (grant no. 2023MD734132).
References
|
1
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng R, Zhang S, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2016. J Natl Cancer Cent. 2:1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferguson JL and Turner SP: Bone Cancer:
Diagnosis and treatment principles. Am Fam Physician. 98:205–213.
2018.PubMed/NCBI
|
|
4
|
Mensali N, Köksal H, Joaquina S, Wernhoff
P, Casey NP, Romecin P, Panisello C, Rodriguez R, Vimeux L,
Juzeniene A, et al: ALPL-1 is a target for chimeric antigen
receptor therapy in osteosarcoma. Nat Commun. 14:33752023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao GS, Gao ZR, Zhang Q, Tang XF, Lv YF,
Zhang ZS, Zhang Y, Tan QL, Peng DB, Jiang DM and Guo QN: TSSC3
promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR
pathway to suppress tumorigenesis and metastasis in osteosarcoma,
and predicts a favorable prognosis. J Exp Clin Cancer Res.
37:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim YI, Tseng YC, Ayaz G, Wang S, Yan H,
du Bois W, Yang H, Zhen T, Lee MP, Liu P, et al: SOX9 is a key
component of RUNX2-regulated transcriptional circuitry in
osteosarcoma. Cell Biosci. 13:1362023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang S, Zhu N, Li HF, Gu J, Zhang CJ,
Liao DF and Qin L: The lipid rafts in cancer stem cell: A target to
eradicate cancer. Stem Cell Res Ther. 13:4322022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gibbs CP, Kukekov VG, Reith JD,
Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN and
Steindler DA: Stem-like cells in bone sarcomas: Implications for
tumorigenesis. Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan GN, Lv YF and Guo QN: Advances in
osteosarcoma stem cell research and opportunities for novel
therapeutic targets. Cancer Lett. 370:268–274. 2016. View Article : Google Scholar
|
|
10
|
Martins-Neves SR, Sampaio-Ribeiro G and
Gomes CMF: Self-Renewal and pluripotency in osteosarcoma stem
cells' chemoresistance: Notch, Hedgehog, and Wnt/β-Catenin
Interplay with Embryonic Markers. Int J Mol Sci. 24:84012023.
View Article : Google Scholar
|
|
11
|
Ullmann P, Rodriguez F, Schmitz M, Meurer
SK, Qureshi-Baig K, Felten P, Ginolhac A, Antunes L, Frasquilho S,
Zügel N, et al: The miR-371~373 cluster represses colon cancer
initiation and metastatic colonization by inhibiting the TGFBR2/ID1
signaling axis. Cancer Res. 78:3793–3808. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taddei ML, Giannoni E, Fiaschi T and
Chiarugi P: Anoikis: An emerging hallmark in health and diseases. J
Pathol. 226:380–393. 2012. View Article : Google Scholar
|
|
13
|
Paoli P, Giannoni E and Chiarugi P:
Anoikis molecular pathways and its role in cancer progression.
Biochim Biophys Acta. 1833:3481–3498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han YH, Wang Y, Lee SJ, Jin MH, Sun HN and
Kwon T: Regulation of anoikis by extrinsic death receptor pathways.
Cell Commun Signal. 21:2272023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sattari Fard F, Jalilzadeh N, Mehdizadeh
A, Sajjadian F and Velaei K: Understanding and targeting anoikis in
metastasis for cancer therapies. Cell Biol Int. 47:683–698. 2023.
View Article : Google Scholar
|
|
16
|
Sun T, Zhong X, Song H, Liu J, Li J, Leung
F, Lu WW and Liu ZL: Anoikis resistant mediated by FASN promoted
growth and metastasis of osteosarcoma. Cell Death Dis. 10:2982019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao GS, Zhang Q, Cao Y, Wang Y, Lv YF,
Zhang ZS, Zhang Y, Tan QL, Chang Y, Quan ZX, et al: High expression
of ID1 facilitates metastasis in human osteosarcoma by regulating
the sensitivity of anoikis via PI3K/AKT depended suppression of the
intrinsic apoptotic signaling pathway. Am J Transl Res.
11:2117–2139. 2019.PubMed/NCBI
|
|
18
|
Zhang M, Hoyle RG, Ma Z, Sun B, Cai W, Cai
H, Xie N, Zhang Y, Hou J, Liu X, et al: FOSL1 promotes metastasis
of head and neck squamous cell carcinoma through
super-enhancer-driven transcription program. Mol Ther.
29:2583–2600. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang X, Xie H, Dou Y, Yuan J, Zeng D and
Xiao S: Expression and function of FRA1 protein in tumors. Mol Biol
Rep. 47:737–752. 2020. View Article : Google Scholar
|
|
20
|
Guo S, Ramar V, Guo AA, Saafir T,
Akpobiyeri H, Hudson B, Li J and Liu M: TRPM7 transactivates the
FOSL1 gene through STAT3 and enhances glioma stemness. Cell Mol
Life Sci. 80:2702023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang T, Song P, Zhong T, Wang X, Xiang X,
Liu Q, Chen H, Xia T, Liu H, Niu Y, et al: The inflammatory
cytokine IL-6 induces FRA1 deacetylation promoting colorectal
cancer stem-like properties. Oncogene. 38:4932–4947. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang K, Myllymäki SM, Gao P, Devarajan R,
Kytölä V, Nykter M, Wei GH and Manninen A: Oncogenic K-Ras
upregulates ITGA6 expression via FOSL1 to induce anoikis resistance
and synergizes with αV-Class integrins to promote EMT. Oncogene.
36:5681–5694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen H, Wang W, Ni B, Zou Q, Lu H and Wang
Z: Exploring the molecular mechanisms of osteosarcoma by the
integrated analysis of mRNAs and miRNA microarrays. Int J Mol Med.
42:21–30. 2018.PubMed/NCBI
|
|
24
|
Chandrashekar DS, Karthikeyan SK, Korla
PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne
U, et al: UALCAN: An update to the integrated cancer data analysis
platform. Neoplasia. 25:18–27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao SJ, Jiang YQ, Xu NW, Li Q, Zhang Q,
Wang SY, Li J, Wang YH, Zhang YL, Jiang SH, et al: SPARCL1
suppresses osteosarcoma metastasis and recruits macrophages by
activation of canonical WNT/β-catenin signaling through
stabilization of the WNT-receptor complex. Oncogene. 37:1049–1061.
2018. View Article : Google Scholar
|
|
26
|
Bartha Á and Győrffy B: TNMplot.com: A web
tool for the comparison of gene expression in normal, tumor and
metastatic tissues. Int J Mol Sci. 22:26222021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Győrffy B: Discovery and ranking of the
most robust prognostic biomarkers in serous ovarian cancer.
Geroscience. 45:1889–1898. 2023. View Article : Google Scholar
|
|
28
|
Yang Y, Huang H, Li L and Yang Y:
Multiplex Immunohistochemistry Staining for Paraffin-embedded lung
cancer tissue. J Vis Exp. 2023. View
Article : Google Scholar
|
|
29
|
Wang Y, Zhang L, Zhao G, Zhang Y, Zhan F,
Chen Z, He T, Cao Y, Hao L, Wang Z, et al: Homologous targeting
nanoparticles for enhanced PDT against osteosarcoma HOS cells and
the related molecular mechanisms. J Nanobiotechnology. 20:832022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan GN, Tang XF, Zhang XC, He T, Huang YS,
Zhang X, Meng G, Guo DY, Lv YF and Guo QN: TSSC3 represses
self-renewal of osteosarcoma stem cells and Nanog expression by
inhibiting the Src/Akt pathway. Oncotarget. 8:85628–85641. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
32
|
Hu Y and Smyth GK: ELDA: Extreme limiting
dilution analysis for comparing depleted and enriched populations
in stem cell and other assays. J Immunol Methods. 347:70–78. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Adhikari AS, Agarwal N, Wood BM, Porretta
C, Ruiz B, Pochampally RR and Iwakuma T: CD117 and Stro-1 identify
osteosarcoma tumor-initiating cells associated with metastasis and
drug resistance. Cancer Res. 70:4602–4612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marques C, Unterkircher T, Kroon P,
Oldrini B, Izzo A, Dramaretska Y, Ferrarese R, Kling E, Schnell O,
Nelander S, et al: NF1 regulates mesenchymal glioblastoma
plasticity and aggressiveness through the AP-1 transcription factor
FOSL1. Elife. 10:e648462021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gambera S, Abarrategi A, Rodríguez-Milla
MA, Mulero F, Menéndez ST, Rodriguez R, Navarro S and García-Castro
J: Role of Activator Protein-1 complex on the phenotype of human
osteosarcomas generated from mesenchymal stem cells. Stem Cells.
36:1487–1500. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Whittle SB, Offer K, Roberts RD, LeBlanc
A, London C, Majzner RG, Huang AY, Houghton P, Alejandro Sweet
Cordero E, Grohar PJ, et al: Charting a path for prioritization of
novel agents for clinical trials in osteosarcoma: A report from the
Children's Oncology Group New Agents for Osteosarcoma Task Force.
Pediatr Blood Cancer. 68:e291882021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hyakusoku H, Sawakuma K, Sano D, Takahashi
H, Hatano T, Sato K, Isono Y, Shimada S, Takada K, Kuwahara T, et
al: FosL1 regulates regional metastasis of head and neck squamous
cell carcinoma by promoting cell migration, invasion, and
proliferation. Anticancer Res. 41:3317–3326. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dai Y, Zhang X, Ou Y, Zou L, Zhang D, Yang
Q, Qin Y, Du X, Li W, Yuan Z, et al: Anoikis
resistance-protagonists of breast cancer cells survive and
metastasize after ECM detachment. Cell Commun Signal. 21:1902023.
View Article : Google Scholar
|
|
39
|
Celià-Terrassa T and Kang Y: Distinctive
properties of metastasis-initiating cells. Genes Dev. 30:892–908.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee BK, Uprety N, Jang YJ, Tucker SK, Rhee
C, LeBlanc L, Beck S and Kim J: Fosl1 overexpression directly
activates trophoblast-specific gene expression programs in
embryonic stem cells. Stem Cell Res. 26:95–102. 2018. View Article : Google Scholar :
|
|
41
|
Qi XT, Li YL, Zhang YQ, Xu T, Lu B, Fang
L, Gao JQ, Yu LS, Zhu DF, Yang B, et al: KLF4 functions as an
oncogene in promoting cancer stem cell-like characteristics in
osteosarcoma cells. Acta Pharmacol Sin. 40:546–555. 2019.
View Article : Google Scholar :
|
|
42
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5:82020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shonibare Z, Monavarian M, O'Connell K,
Altomare D, Shelton A, Mehta S, Jaskula-Sztul R, Phaeton R, Starr
MD, Whitaker R, et al: Reciprocal SOX2 regulation by SMAD1-SMAD3 is
critical for anoikis resistance and metastasis in cancer. Cell Rep.
40:1110662022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guha D, Saha T, Bose S, Chakraborty S,
Dhar S, Khan P, Adhikary A, Das T and Sa G: Integrin-EGFR
interaction regulates anoikis resistance in colon cancer cells.
Apoptosis. 24:958–971. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Z, Wang S, Li HL, Luo H, Wu X, Lu J,
Wang HW, Chen Y, Chen D, Wu WT, et al: FOSL1 promotes
proneural-to-mesenchymal transition of glioblastoma stem cells via
UBC9/CYLD/NF-κB axis. Mol Ther. 30:2568–2583. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li R, Che W, Liang N, Deng S, Song Z and
Yang L: Silent FOSL1 enhances the radiosensitivity of glioma stem
cells by down-regulating miR-27a-5p. Neurochem Res. 46:3222–3246.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Talukdar S, Pradhan AK, Bhoopathi P, Shen
XN, August LA, Windle JJ, Sarkar D, Furnari FB, Cavenee WK, Das SK,
et al: MDA-9/Syntenin regulates protective autophagy in
anoikis-resistant glioma stem cells. Proc Natl Acad Sci USA.
115:5768–5773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim SY, Hong SH, Basse PH, Wu C, Bartlett
DL, Kwon YT and Lee YJ: Cancer stem cells protect non-stem cells
from Anoikis: Bystander effects. J Cell Biochem. 117:2289–2301.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiong G, Ouyang S, Xie N, Xie J, Wang W,
Yi C, Zhang M, Xu X, Chen D and Wang C: FOSL1 promotes tumor growth
and invasion in ameloblastoma. Front Oncol. 12:9001082022.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Basu-Roy U, Seo E, Ramanathapuram L, Rapp
TB, Perry JA, Orkin SH, Mansukhani A and Basilico C: Sox2 maintains
self renewal of tumor-initiating cells in osteosarcomas. Oncogene.
31:2270–2282. 2012. View Article : Google Scholar
|
|
52
|
Ren C, Ren T, Yang K, Wang S, Bao X, Zhang
F and Guo W: Inhibition of SOX2 induces cell apoptosis and G1/S
arrest in Ewing's sarcoma through the PI3K/Akt pathway. J Exp Clin
Cancer Res. 35:442016. View Article : Google Scholar : PubMed/NCBI
|