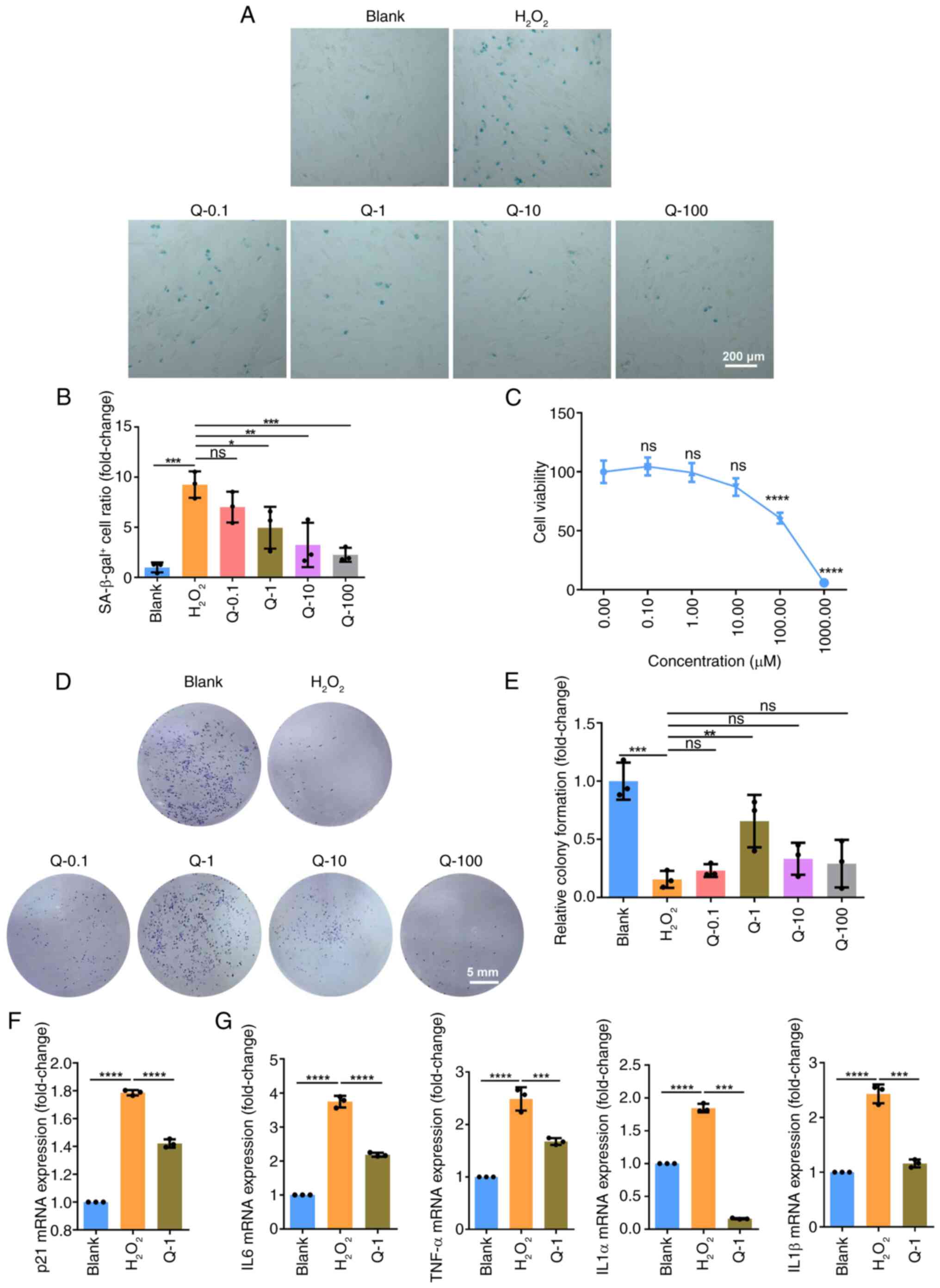
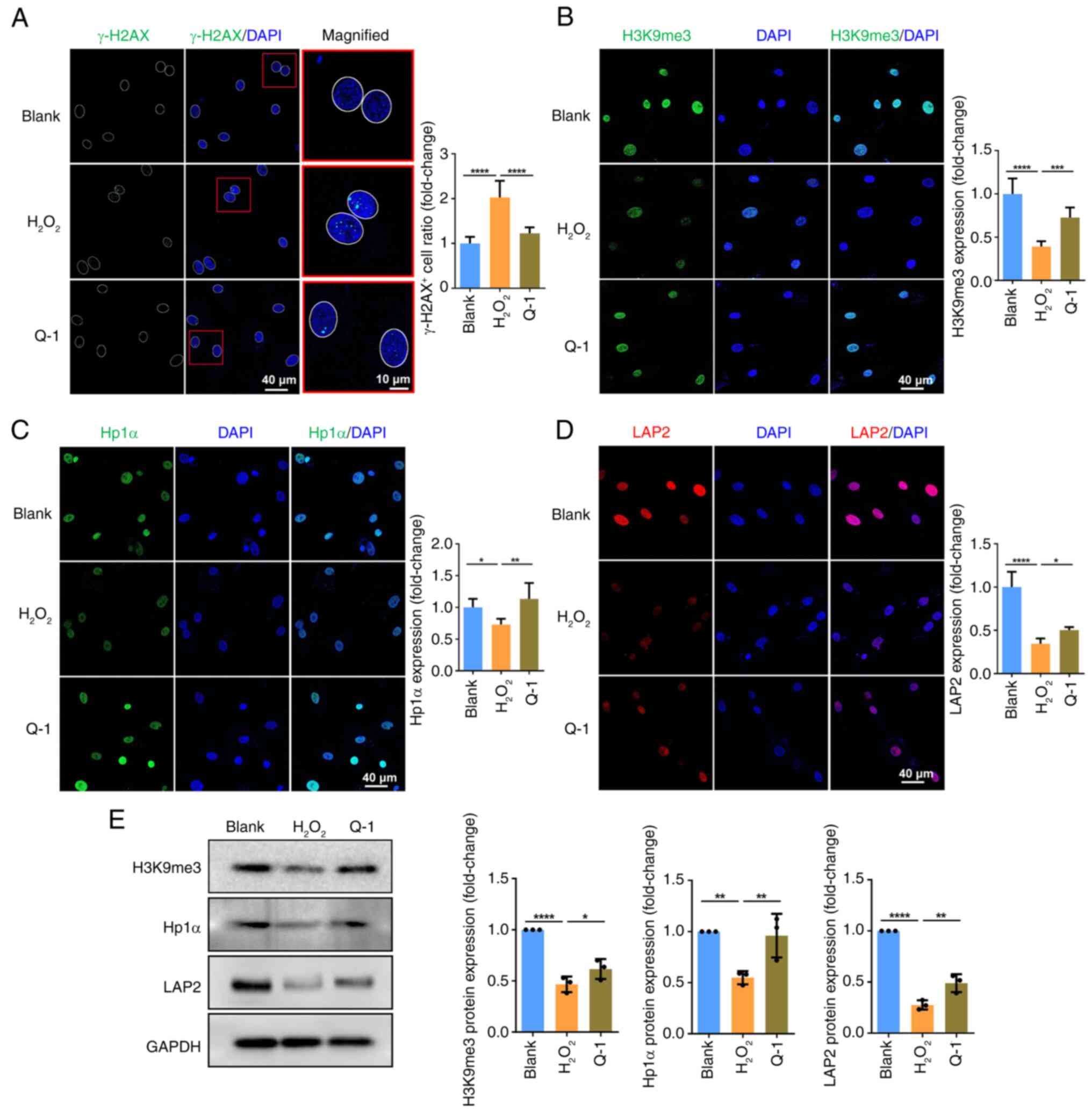
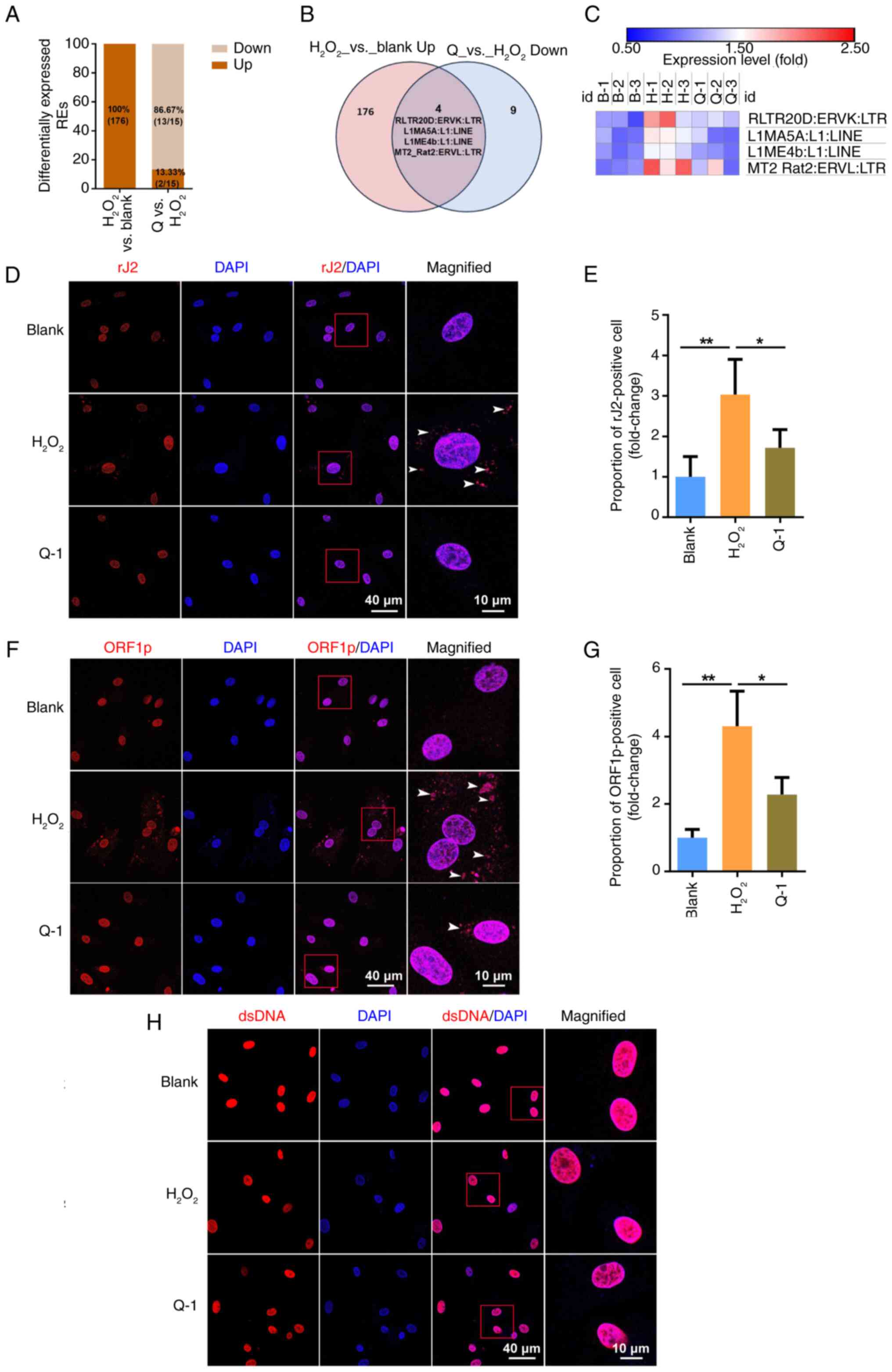
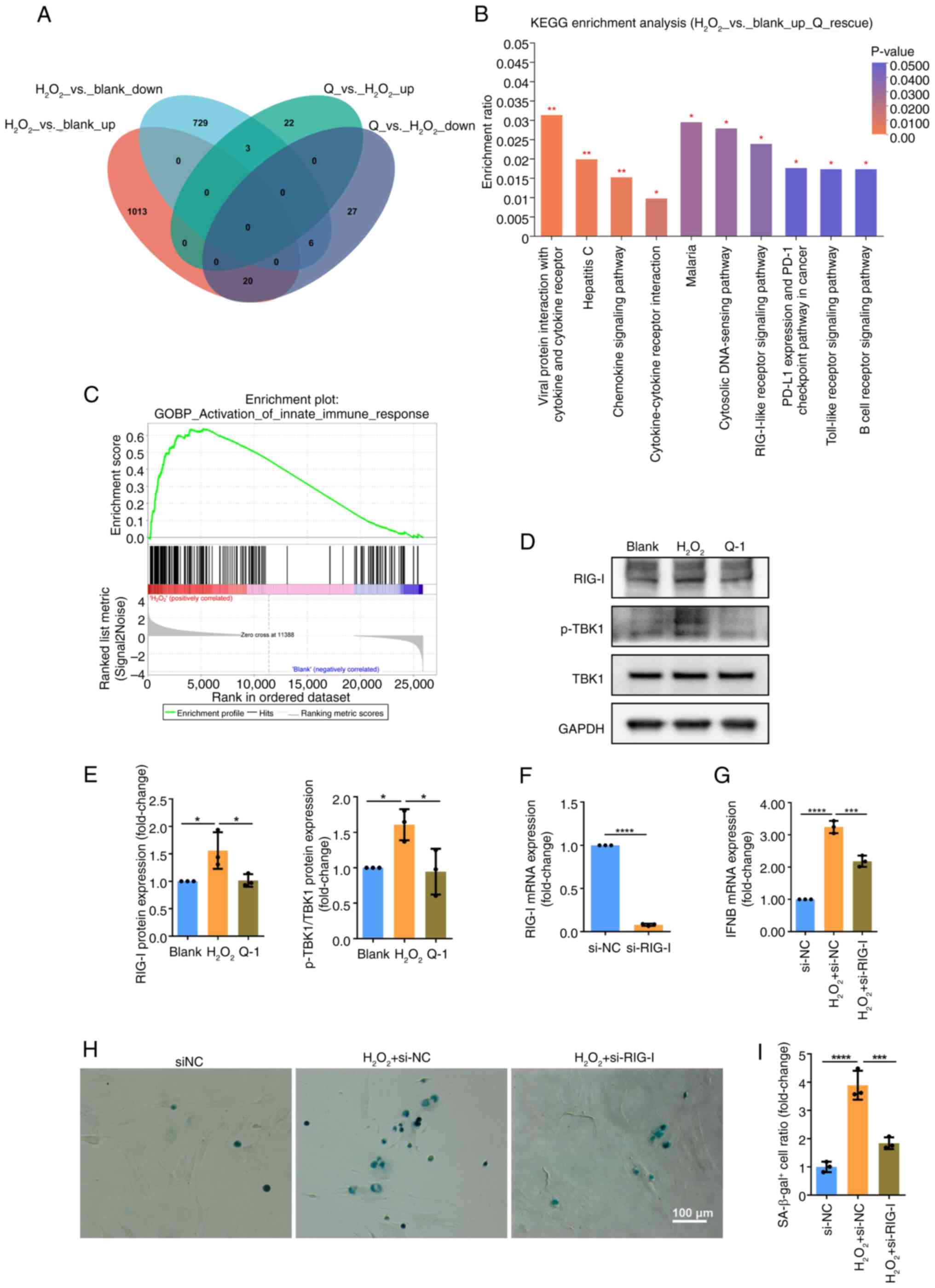
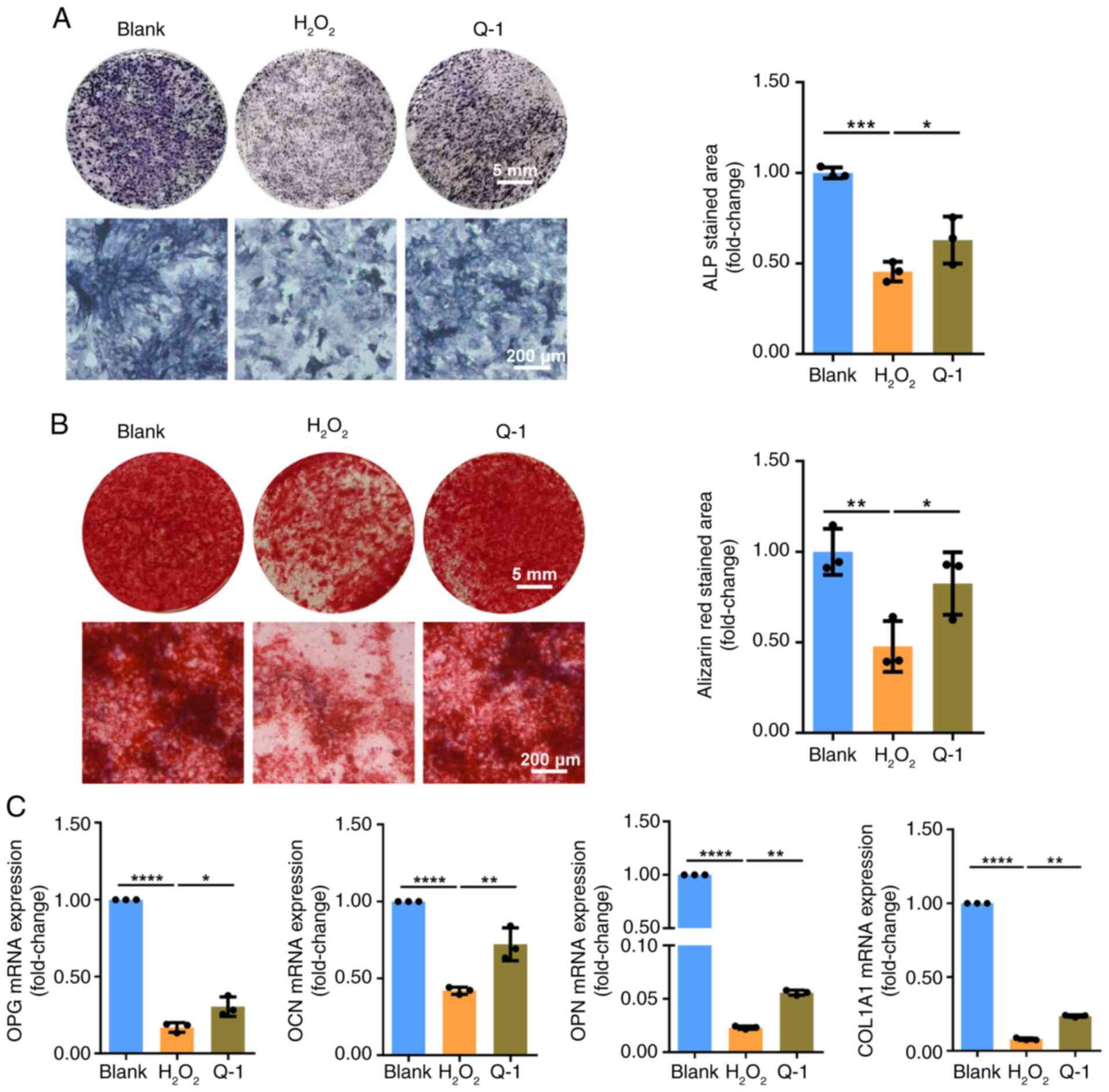
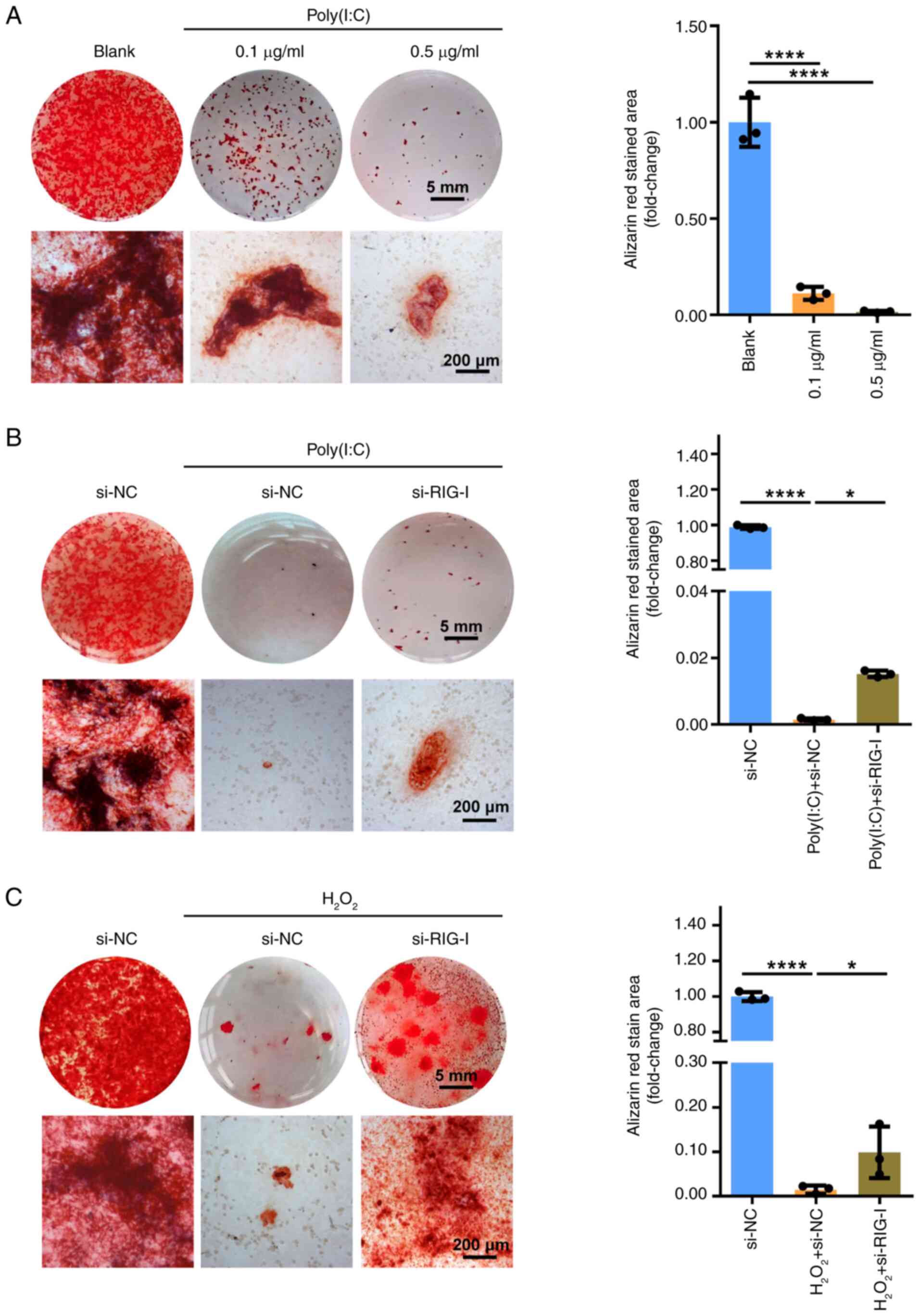
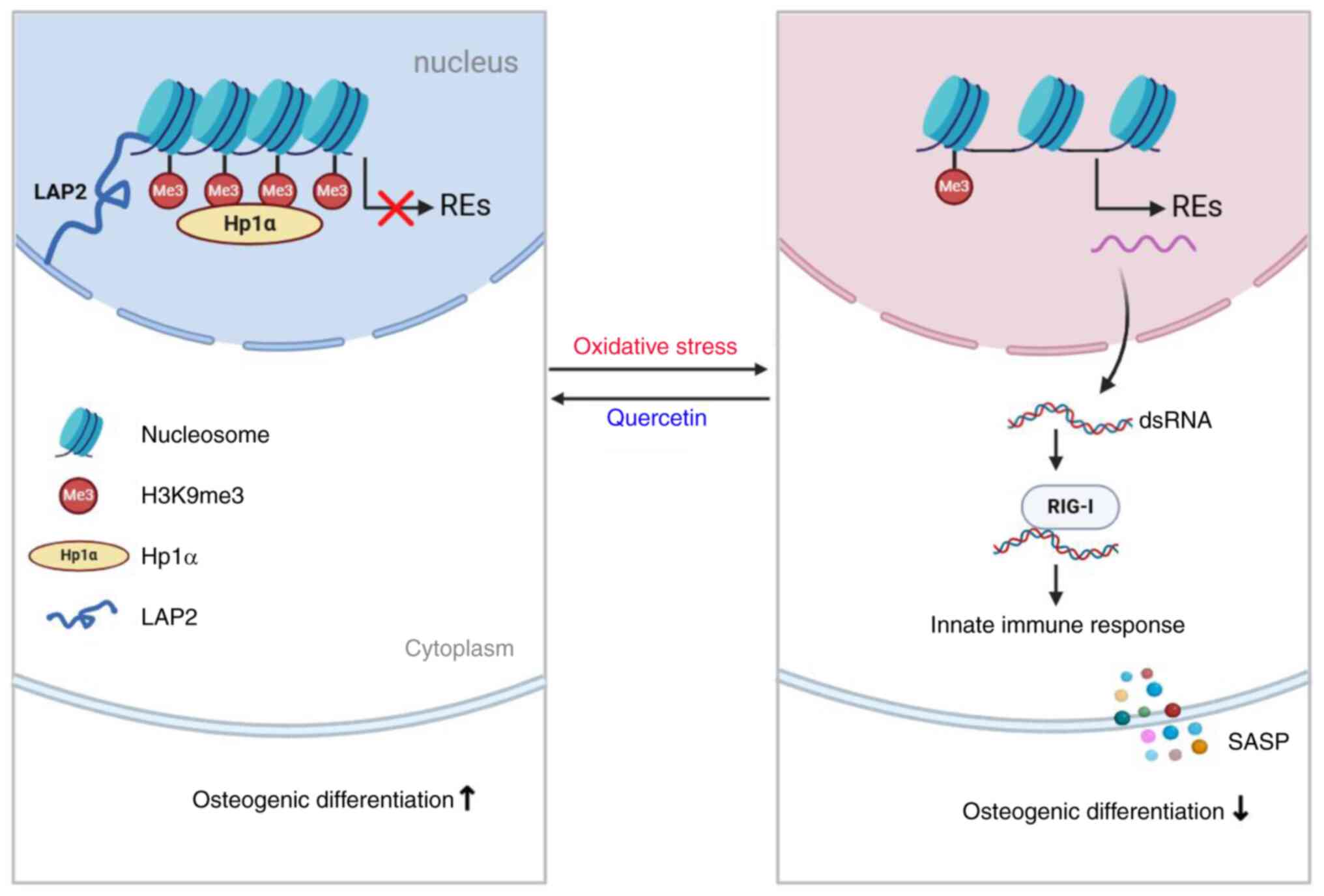
|
1
|
Zheng C, Chen J, Liu S and Jin Y: Stem
cell-based bone and dental regeneration: A view of
microenvironmental modulation. Int J Oral Sci. 11:232019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Azab M, Safi M, Idiiatullina E,
Al-Shaebi F and Zaky MY: Aging of mesenchymal stem cell: Machinery,
markers, and strategies of fighting. Cell Mol Biol Lett. 27:692022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin H, Sohn J, Shen H, Langhans MT and
Tuan RS: Bone marrow mesenchymal stem cells: Aging and tissue
engineering applications to enhance bone healing. Biomaterials.
203:96–110. 2019. View Article : Google Scholar :
|
|
4
|
Lopez-Otin C, Blasco MA, Partridge L,
Serrano M and Kroemer G: The hallmarks of aging. Cell.
153:1194–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang W, Li J, Suzuki K, Qu J, Wang P,
Zhou J, Liu X, Ren R, Xu X, Ocampo A, et al: Aging stem cells. A
Werner syndrome stem cell model unveils heterochromatin alterations
as a driver of human aging. Science. 348:1160–1163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soto-Palma C, Niedernhofer LJ, Faulk CD
and Dong X: Epigenetics, DNA damage, and aging. J Clin Invest.
132:e1584462022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pathak RU, Soujanya M and Mishra RK:
Deterioration of nuclear morphology and architecture: A hallmark of
senescence and aging. Ageing Res Rev. 67:1012642021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stamidis N and Żylicz JJ: RNA-mediated
heterochromatin formation at repetitive elements in mammals. EMBO
J. 42:e1117172023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McCarthy RL, Kaeding KE, Keller SH, Zhong
Y, Xu L, Hsieh A, Hou Y, Donahue G, Becker JS, Alberto O, et al:
Diverse heterochromatin-associated proteins repress distinct
classes of genes and repetitive elements. Nat Cell Biol.
23:905–914. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anwar SL, Wulaningsih W and Lehmann U:
Transposable Elements in human cancer: Causes and consequences of
deregulation. Int J Mol Sci. 18:9742017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gorbunova V, Seluanov A, Mita P, McKerrow
W, Fenyö D, Boeke JD, Linker SB, Gage FH, Kreiling JA, Petrashen
AP, et al: The role of retrotransposable elements in ageing and
age-associated diseases. Nature. 596:43–53. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Lu X, Zhang W and Liu GH:
Endogenous retroviruses in development and health. Trends
Microbiol. 32:342–354. 2024. View Article : Google Scholar
|
|
13
|
Ågren JA and Clark AG: Selfish genetic
elements. PLoS Genet. 14:e10077002018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang CR, Burns KH and Boeke JD: Active
transposition in genomes. Annu Rev Genet. 46:651–675. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dumetier B, Sauter C, Hajmirza A, Pernon
B, Aucagne R, Fournier C, Row C, Guidez F, Rossi C, Lepage C, et
al: Repeat element activation-driven inflammation: Role of NFκB and
implications in normal development and cancer? Biomedicines.
10:31012022. View Article : Google Scholar
|
|
16
|
Lanciano S and Cristofari G: Measuring and
interpreting transposable element expression. Nat Rev Genet.
21:721–736. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Copley KE and Shorter J: Repetitive
elements in aging and neurodegeneration. Trends Genet. 39:381–400.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gazquez-Gutierrez A, Witteveldt J, Heras
SR and Macias S: Sensing of transposable elements by the antiviral
innate immune system. RNA. 27:735–752. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Evans TA and Erwin JA:
Retroelement-derived RNA and its role in the brain. Semin Cell Dev
Biol. 114:68–80. 2021. View Article : Google Scholar
|
|
20
|
Miller KN, Victorelli SG, Salmonowicz H,
Dasgupta N, Liu T, Passos JF and Adams PD: Cytoplasmic DNA:
Sources, sensing, and role in aging and disease. Cell.
184:5506–5526. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui Z and Zhao X, Amevor FK, Du X, Wang Y,
Li D, Shu G, Tian Y and Zhao X: Therapeutic application of
quercetin in aging-related diseases: SIRT1 as a potential
mechanism. Front Immunol. 13:9433212022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng D, Chen L, Sun Y, Sun L, Yin Q, Deng
S, Niu L, Lou F, Wang Z, Xu Z, et al: Melanoma suppression by
quercein is correlated with RIG-I and type I interferon signaling.
Biomed Pharmacother. 125:1099842020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nieto M, Konigsberg M and Silva-Palacios
A: Quercetin and dasatinib, two powerful senolytics in age-related
cardiovascular disease. Biogerontology. 25:71–82. 2024. View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Lee SS, Vũ TT, Weiss AS and Yeo GC:
Stress-induced senescence in mesenchymal stem cells: Triggers,
hallmarks, and current rejuvenation approaches. Eur J Cell Biol.
102:1513312023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ritschka B, Storer M, Mas A, Heinzmann F,
Ortells MC, Morton JP, Sansom OJ, Zender L and Keyes WM: The
senescence-associated secretory phenotype induces cellular
plasticity and tissue regeneration. Genes Dev. 31:172–183. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Korotkov A, Seluanov A and Gorbunova V:
Sirtuin 6: Linking longevity with genome and epigenome stability.
Trends Cell Biol. 31:994–1006. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng JX and Riddle NC: Epigenetics and
genome stability. Mamm Genome. 31:181–195. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Padeken J, Methot SP and Gasser SM:
Establishment of H3K9-methylated heterochromatin and its functions
in tissue differentiation and maintenance. Nat Rev Mol Cell Biol.
23:623–640. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shevelyov YY and Ulianov SV: The nuclear
lamina as an organizer of chromosome architecture. Cells.
8:1362019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cosby RL, Chang NC and Feschotte C:
Host-transposon interactions: Conflict, cooperation, and cooption.
Genes Dev. 33:1098–1116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chuong EB, Elde NC and Feschotte C:
Regulatory evolution of innate immunity through co-option of
endogenous retroviruses. Science. 351:1083–1087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li W, Prazak L, Chatterjee N, Grüninger S,
Krug L, Theodorou D and Dubnau J: Activation of transposable
elements during aging and neuronal decline in Drosophila. Nat
Neurosci. 16:529–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wood JG, Jones BC, Jiang N, Chang C,
Hosier S, Wickremesinghe P, Garcia M, Hartnett DA, Burhenn L,
Neretti N and Helfand SL: Chromatin-modifying genetic interventions
suppress age-associated transposable element activation and extend
life span in Drosophila. Proc Natl Acad Sci USA. 113:11277–11282.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Cecco M, Criscione SW, Peterson AL,
Neretti N, Sedivy JM and Kreiling JA: Transposable elements become
active and mobile in the genomes of aging mammalian somatic
tissues. Aging (Albany NY). 5:867–883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu X, Liu Z, Wu Z, Ren J, Fan Y, Sun L,
Cao G, Niu Y, Zhang B, Ji Q, et al: Resurrection of endogenous
retroviruses during aging reinforces senescence. Cell.
186:287–304.e26. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colombo AR, Elias HK and Ramsingh G:
Senescence induction universally activates transposable element
expression. Cell Cycle. 17:1846–1857. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Di Giorgio E, Ranzino L, Tolotto V, Dalla
E, Burelli M, Gualandi N and Brancolini C: Transcription of
endogenous retroviruses in senescent cells contributes to the
accumulation of double-stranded RNAs that trigger an anti-viral
response that reinforces senescence. Cell Death Dis. 15:1572024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Touma F, Lambert M, Martinez Villarreal A,
Gantchev J, Ramchatesingh B and Litvinov IV: The ultraviolet
irradiation of keratinocytes induces ectopic expression of LINE-1
retrotransposon machinery and leads to cellular senescence.
Biomedicines. 11:30172023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Cecco M, Ito T, Petrashen AP, Elias AE,
Skvir NJ, Criscione SW, Caligiana A, Brocculi G, Adney EM, Boeke
JD, et al: L1 drives IFN in senescent cells and promotes
age-associated inflammation. Nature. 566:73–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wood JG, Hillenmeyer S, Lawrence C, Chang
C, Hosier S, Lightfoot W, Mukherjee E, Jiang N, Schorl C, Brodsky
AS, et al: Chromatin remodeling in the aging genome of Drosophila.
Aging Cell. 9:971–978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pérez-Mancera PA, Young ARJ and Narita M:
Inside and out: The activities of senescence in cancer. Nat Rev
Cancer. 14:547–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu M, Xing L, Zhang L, Liu F, Wang S, Xie
Y, Wang J, Jiang H, Guo J, Li X, et al: NAP1L2 drives mesenchymal
stem cell senescence and suppresses osteogenic differentiation.
Aging Cell. 21:e135512022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xie Y, Han N, Li F, Wang L, Liu G, Hu M,
Wang S, Wei X, Guo J, Jiang H, et al: Melatonin enhances
osteoblastogenesis of senescent bone marrow stromal cells through
NSD2-mediated chromatin remodelling. Clin Transl Med. 12:e7462022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Niepmann ST, Willemsen N, Boucher AS, Stei
M, Goody P, Zietzer A, Bulic M, Billig H, Odainic A, Weisheit CK,
et al: Toll-like receptor-3 contributes to the development of
aortic valve stenosis. Basic Res Cardiol. 118:62023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Khokhani P, Rahmani NR, Kok A, Öner FC,
Alblas J, Weinans H, Kruyt MC and Croes M: Use of therapeutic
pathogen recognition receptor ligands for osteo-immunomodulation.
Materials (Basel). 14:11192021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lou Q, Jiang K, Xu Q, Yuan L, Xie S, Pan
Y, Chen J, Wu J, Zhu J, Jiang L and Zhao M: The RIG-I-NRF2 axis
regulates the mesenchymal stromal niche for bone marrow
transplantation. Blood. 139:3204–3221. 2022. View Article : Google Scholar : PubMed/NCBI
|