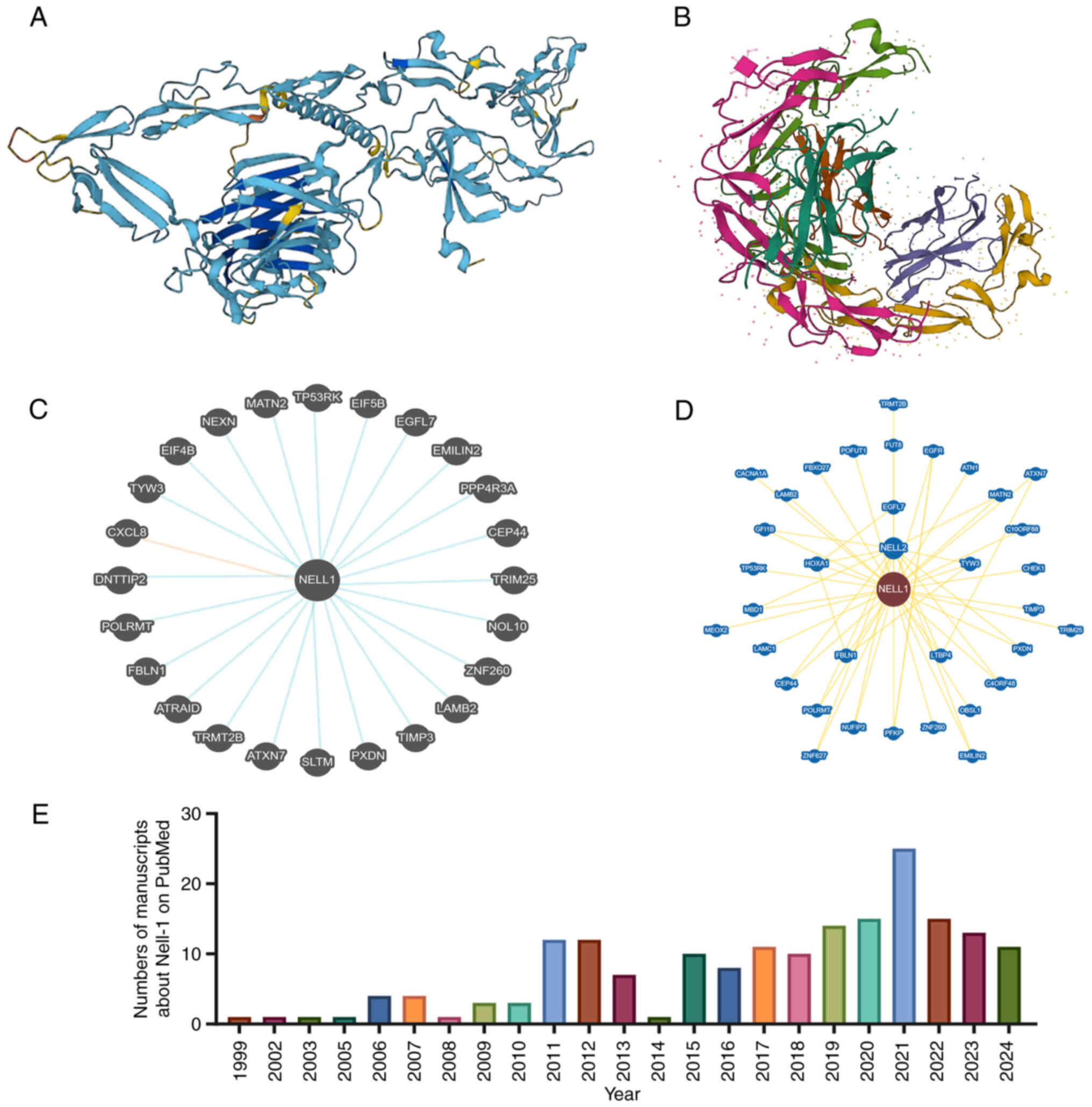
Disruption in the normal formation of bone and/or
cartilage may cause a series of bone diseases, including but not
limited to osteoporosis (1,2),
osteoarthritis (3,4), osteonecrosis (5,6)
and bone defects (7,8). Osteoporosis is the most common bone
disease, with estimates indicating that >200 million individuals
worldwide suffer from it (9).
Osteoarthritis is a common musculoskeletal disease that affects
>10% of the elderly population (10). Osteonecrosis of the jaw has an
incidence of 1.3-10%, with mandibular osteonecrosis being more
prominent than maxillary osteonecrosis (11). Approximately 5-10% of fractures
eventually have delayed union or nonunion, leading to bone defect
(12). Therefore, there is still
a need to discover bone-specific osteogenic anabolic agents for the
treatment of these conditions.
NEL-like molecule-1 (NELL-1), a neuroepidermal
growth factor-like protein, was first discovered by Ting et
al (13) in 1999 and found
to be able treat osteoporotic bone loss (14). Of note, the protein was found in
the cranial tissues of patients with unilateral coronal sclerosis
and was isolated (13). The
protein consists of the following domains: A thrombospondin protein
(TSP)-1-like N-terminal domain, a coiled-coil domain, four von
Willebrand factor (vWF) C-type domains and six epidermal growth
factor (EGF)-like domains (15).
The NELL-1 protein, encoded by the NELL-1 gene,
contains 810 amino acids and has a molecular weight of ~90
kilodaltons (kDa) before N-terminal glycosylation and
oligomerization. The secreted rat NELL-1 is a phosphorylated
homotrimer with a molecular weight of >400 kDa (16). Of note, human recombinant NELL-1
shares 92.6% homology with rat NELL-1. However, the molecular
weight of recombinant NELL-1 expressed in Chinese hamster ovarian
cells was ~140 kDa under reducing conditions and >700 kDa under
nonreducing conditions in sodium dodecyl sulfate gel
electrophoresis, suggesting that NELL-1 may be secreted as a
pentamer. Researchers have speculated that the crimp helical
structure of the 5'-end of NELL-1's first vWF domain may cause
oligomerization similar to that in the cartilage oligomeric matrix
protein (17).
NELL-1 has been considered a TSP-1-like molecule
because of the presence of an N-terminal TSP-1 phospholipid-binding
domain (16). However, NELL-1
lacks some major TSP-1 motifs, including type I and III TSP
repeats, Arg-Gly-Asp binding domains and C-terminal domains.
Phospholipid binding is a typical biochemistry feature of NELL-1.
EGF repeats in NELL-1 are key components that allow its binding to
protein kinase C subunits, and this interaction between EGF repeats
and their corresponding factors is considered a new type of binding
mode (18). The lack of a fifth
EGF repeat NELL-1 shearing isomer may affect this EGF binding by
regulating the binding with calcium. The vWF domain is also thought
to contribute to NELL-1 oligomerization and mediate cell adhesion
(17). Of note, TSP-1 can bind
and activate the potential form of transforming growth factor
(TGF)-β1 (19). However, to
date, no study has confirmed the ability of NELL-1 to bind to
TGF-β1; however, the existence of a consensus repeat junction
domain indicates that NELL-1 can bind with bone morphogenetic
protein (BMP) members of the TGF-β superfamily (20).
In recent years, research on NELL-1 in bone and
cartilage has become increasingly popular (21-30). These articles or reviews have
elaborated on the interaction between NELL-1 and bone/cartilage
from different perspectives. However, most of these studies have
only considered NELL-1 in their research, which is different from
the approach of the current review. A PubMed search using the key
word 'NELL-1' identified several recent manuscripts (Fig. 1E), which confirm NELL-1's close
association with bone formation (31,32). In line with this, the current
study reviews the available literature on NELL-1, focusing on its
molecular mechanism, interactions with other molecules/cells,
molecular-level changes, applications in bone tissue engineering
and its expression in tumors. In other words, this comprehensive
review focused on two aspects: Theoretical study and clinical
application. The theoretical study was conducted on some star
molecules in the signaling pathway that have been extensively
studied, such as BMP, RUNX family transcription factor 2 (RUNX2),
Hedgehog, nuclear factor of activated T-cells (Nfatc) and Wnt.
Clinical applications were explored based on interactions of NELL-1
with other molecules/cells, molecular-level changes and
applications in bone tissue engineering. Finally, the expression of
NELL-1 was assessed in tumors. In conclusion, NELL-1 has a
biological role in treating bone tissue diseases through complex
signaling pathways and its expression can be optimized to amplify
its biological functions. Thus, the present review aimed to
summarize these studies and provide a theoretical basis for the
early widespread clinical application of NELL-1.
This section elaborates on how NELL-1 plays an
osteogenic role at the theoretical level. In other words, it is
described how NELL-1 exerts its osteogenic effect through the
activation and inhibition of a series of signaling pathways. A
literature search revealed that certain key molecules in the
signaling pathway have been extensively studied, such as BMP,
RUNX2, Hedgehog, Nfatc and Wnt. Therefore, the chapters below
elaborate on key molecules in these signaling pathways to make it
easy for the reader to understand the related mechanisms.
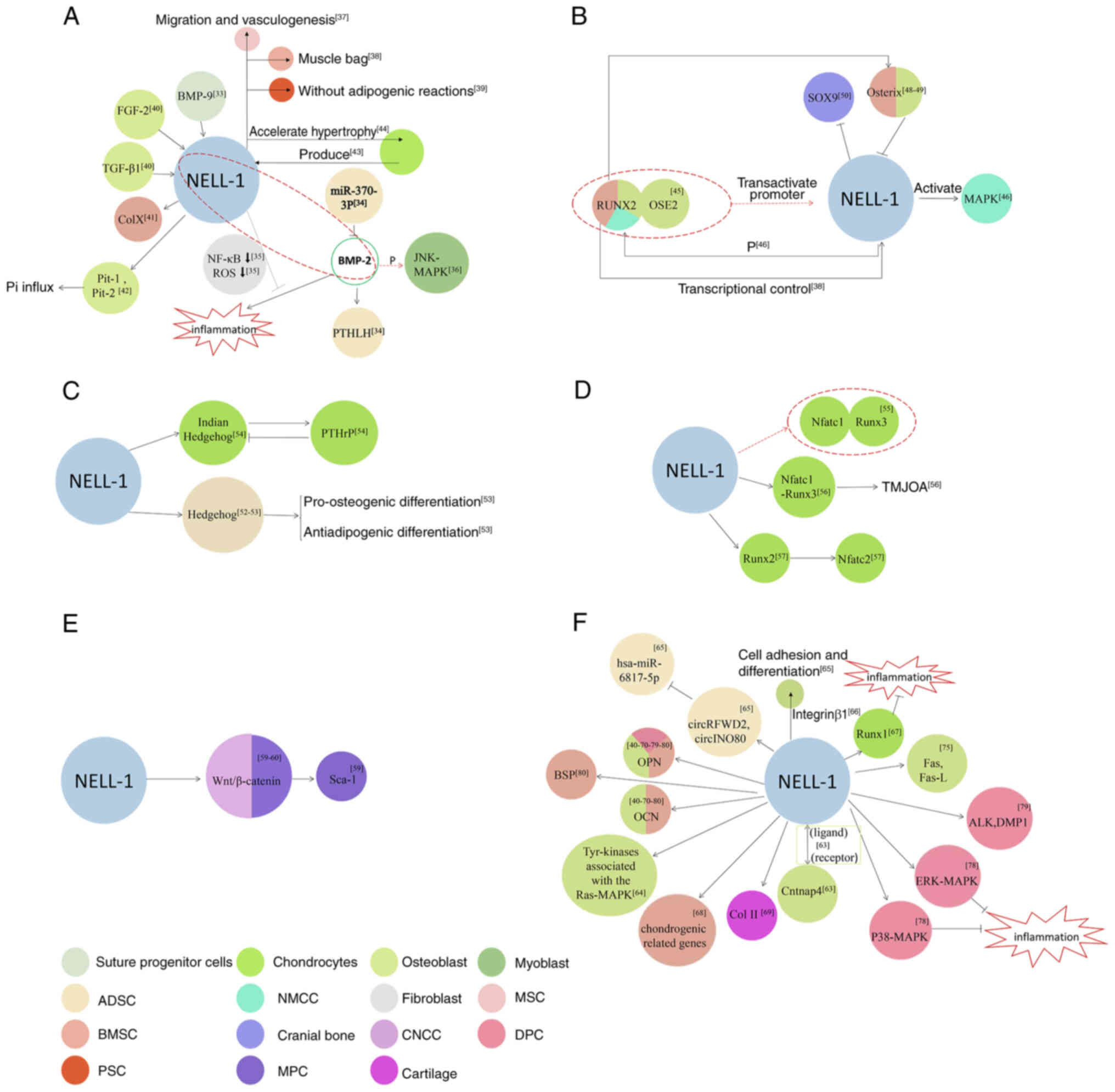
The interaction and differences between NELL-1 and
BMP were first explored in the present review. NELL-1, which is
regulated by BMP-9, participates in the regulation of biological
processes related to osteoblast differentiation and may play an
important role in the healing of cranial sutures (33). During the process of bone
formation stimulated by NELL-1, microRNA (miR)-370-3p can target
BMP-2 and interfere with the expression of parathyroid hormone
(PTH)-like hormone (34). During
bone regeneration and repair, growth factor NELL-1 significantly
attenuates or completely reverses BMP-2-induced inflammation,
perhaps due to NF-κB. This is caused by the reduction in
transcriptional activity or reactive oxygen species production
(35). NELL-1 and BMP-2
synergistically enhance osteogenic differentiation of myoblasts and
phosphorylate the Jun N-terminal kinase (JNK)-mitogen-activated
protein kinase (MAPK) pathway (36).
However, notable differences exist between NELL-1
and BMP-2. Of note, NELL-1 may promote bone defect healing through
endogenous cell recruitment and angiogenesis induction, which
differs from BMP-2's mechanism of action (37). Unlike BMP-2, NELL-1 cannot
initiate ectopic bone formation in muscle tissue but can induce
bone marrow stromal cells (BMSCs) to form bone in a mouse muscle
bag model, highlighting the specificity of BMP deficiency (38). While recombinant BMP-2 increases
bone formation in vivo, it also leads to a large number of
adipogenic reactions. By contrast, NELL-1 selectively enhances bone
formation. NELL-1 is a candidate growth factor that can induce
osteogenesis of human perivascular stem cells (PSCs) (39). Fibroblast growth factor-2 and
TGF-β1 can stimulate NELL-1 expression, but BMP-2 has no direct
effect (40). Although BMP-2
induced a greater bone mass, the central cavity of the bone was
filled with adipose bone marrow tissue. Despite the lower bone mass
induced by NELL-1, histologic analysis through immunohistochemistry
of type X collagen confirmed that it was similar to newly formed
mixed cartilaginous bone found in an area of the trabecular bone.
This difference indicates that NELL-1 has potential clinical
advantages in bone tissue engineering and regeneration (41). Recombinant human NELL-1 can
increase matrix mineralization and inorganic phosphate (Pi) influx
in the cell line MC3T3-E1, which is closely associated with the
activation of Pi transporter-1 and -2 channels, with the activation
of the latter being more obvious. Pi transporters induced by
recombinant human BMP-2 are only associated with Pi transporter-1
activation, indicating the fundamental difference between NELL-1
and BMP-2 signals (42).
Chondrocytes in the proliferative zone of the growth plate produce
factors involved in cartilage metabolism and bone formation. One
study found that the expression of BMP-1, -2 and -5-7, as well as
insulin-like growth factor 1, growth differentiation factor 5 and
osteoclast stimulating factor 1 was considerably high, whereas the
mRNA levels of BMP-3, BMP-4 and NELL-1 were exceedingly low
(43). In a study involving
in vitro rat organ culture, although BMP-7 and NELL-1
induced similar bone formation in the stretch suture, the
mechanisms by which they achieved this differed. Accordingly, BMP-7
induced chondrocyte proliferation and differentiation, whereas
NELL-1 accelerated chondrocyte hypertrophy and endochondral bone
formation (Fig. 2A) (44).
The interactions between NELL-1 and RUNX2 in the
nucleus and cytoplasm are described in this chapter.
NELL-1 and RUNX2 are closely related in the nucleus,
with NELL-1 being a key downstream target of RUNX2. RUNX2 directly
combines with osteoblast-specific binding elements 2 and
transactivates the human NELL-1 promoter (45). Under the direct transcriptional
control of RUNX2, NELL-1 is preferentially expressed in osteoblasts
and well-regulated during bone development (38). NELL-1 is a key downstream
functional mediator of RUNX2. RUNX2-regulated NELL-1 promotes
osteoblast differentiation by activating MAPK and enhancing RUNX2
phosphorylation. When NELL-1 is blocked or deleted, RUNX2 activity
is significantly reduced (46).
However, during cartilage formation, the biological potential of
NELL-1 remains unaffected by RUNX2's nuclear introduction and DNA
binding (47).
Osterix is a direct transcriptional regulator that
inhibits the expression of the NELL-1 gene, which helps regulate
the delicate balance between NELL-1 transcription and RUNX2
regulation and may have a key role in the differentiation and
mineralization of osteoblasts (48). Activating NELL-1 expression
enhances implant osseointegration by upregulating the RUNX2/osterix
axis, highlighting the potential of the BMSC lamellar implant
complex in gene therapy (49).
In NELL-1-deficient mice, osteoblast markers, including RUNX2, were
generally reduced, whereas early proliferative Sox9 was enriched.
NELL-1 is an important growth factor that regulates osteochondral
differentiation by controlling the expression of RUNX2 and Sox9 in
the skull (50). In a rat model,
NELL-1 has demonstrated its potential as an effective
osteoinductive molecule. In addition, the regulation of NELL-1 by
RUNX2 indicates that NELL-1's role in osteoblasts is more specific
compared to that of BMP, which influences multiple cell types
(Fig. 2B) (51).
Cells treated with NELL-1 have shown increased
expression of the Hedgehog signaling pathway and the combined
application of the smoothened antagonist cyclopamine reverses the
osteogenic effect of NELL-1 (52). The Hedgehog signal was analyzed
as a potential downstream target of the NELL-1 signal in regulating
excessive osteogenic fat differentiation. NELL-1 is an effective
anti-fat agent. In addition, NELL-1 signaling may inhibit fat
differentiation through a Hedgehog-dependent mechanism (53). NELL-1 is a key regulator of
epiphyseal homeostasis and endochondral ossification.
Chondrocyte-specific NELL-1 inactivation significantly impedes bone
development and leads to dwarfism and premature osteoporosis by
inhibiting Indian Hedgehog (Ihh) signal transduction and changing
the Ihh-PTH-related protein feedback circuit (Fig. 2C) (54).
Nfatc1 is a key transcription factor that mediates
NELL-1-RUNX3 signal transduction. When NELL-1 is used for
processing, Nfatc1 is combined with the promoter 833-810 region of
RUNX3 (55). The Nfatc1-RUNX3
signaling pathway may be involved in osteochondral injury caused by
temporomandibular joint osteoarthritis (TMJOA) (56). Nfatc2 may play an important role
in NELL-1-mediated osteochondral differentiation in vivo and
in vitro. Studies have also found that Nfatc2 is the main
response gene of NELL-1, whereas RUNX2 is the intermediary between
NELL-1 and Nfatc2 (Fig. 2D)
(57).
The newly discovered ability of NELL-1 to stimulate
Wnt signal transduction and inhibit fat production may represent a
new method for treating bone loss in osteoporosis (58). Recombinant human NELL-1 induces
stem cell antigen-1 (Sca-1) transcription in mesenchymal progenitor
cells (MPCs), which requires complete Wnt/β-catenin signal
conduction (59). NELL-1 is a
key regulator of craniofacial nerve crest cells and the mandible.
It also activates Wnt/β-catenin access (Fig. 2E) (60).
This chapter explores the interaction between NELL-1
and other molecules in different parts of bones, craniofacial bones
and teeth.
At the cellular level, the expression of NELL-1 may
regulate osteoblast differentiation and is sufficient and necessary
(61). Apart from NELL-1, nerve
growth factor, Notum, prostaglandin signaling and the activator
protein-1 family can effectively restore the mechanical reactivity
of aging bone (62). In the
process of osteogenesis, NELL-1 exhibits a ligand-receptor-like
association with contactin-associated protein-like 4 (Cntnap4),
which indicates that Cntnap4 may be the cell surface-specific
receptor for NELL-1 (63). A
previous study also indicated that roundabout guidance receptor 2
(Robo2) serves as a receptor for NELL-1 (64). When binding to specific
receptors, NELL-1 transmits osteogenic signals by activating some
Tyr kinases associated with the Ras-MAPK cascade, ultimately
leading to osteogenic differentiation (65). NELL-1 induces the osteogenic
differentiation of MC3T3-E1 by inducing late markers [osteopontin
(OPN) and osteocalcin (OCN)] (40). During the osteogenic
differentiation of human adipose-derived stem cells (ASCs) induced
by NELL-1, circular RNA (circRNA) of COP1 E3 ubiquitin ligase and
circRNA of INO80 complex ATPase subunit upregulate and inhibit the
expression of hsa-miR-6817-5p, respectively, affecting the positive
effects of NELL-1 on osteogenesis (66). The adhesion of NELL-1 to the cell
surface depends on integrin β1, the cell surface target of NELL-1
that plays an important role in promoting cell adhesion and
osteogenic differentiation of NELL-1 (67). NELL-1 can bind to all-trans
retinoic acid-induced differentiation factor (APR3) on the cell
surface, after which APR3 can inhibit osteogenic proliferation but
promote osteogenic differentiation (68). NELL-1 can effectively inhibit the
expression of inflammatory cytokines and their downstream cartilage
catabolic enzymes by upregulating the expression of RUNX1 in
articular cartilage chondrocytes. Therefore, NELL-1 is a promising
candidate for a disease-modifying osteoarthritis drug, as it
promotes chondrogenesis and inhibits inflammation, thereby
preventing and reducing cartilage injury associated with arthritis
(69). In addition, NELL-1
specifically promotes chondrogenesis and differentiation of human
BMSCs in vitro by increasing the expression of
chondrogenesis-related genes and proteins (70). During the process of ameliorating
cartilage loss, NELL-1 enhances alcian blue and saffron-O staining
and increases the deposition of type II collagen (71). In terms of femoral fracture
treatment using NELL-1, data have also shown an increase in the
immunostaining of the bone differentiation markers OPN and OCN.
Therefore, NELL-1 effectively enhances in situ osteogenesis
in the bone marrow (72). The
response of canine PSCs to bone induction signals from NELL-1 is
similar to that of human PSCs (73). NELL-1 gene polymorphisms have
also been associated with osteoporosis (74,75). Only a few studies have been
conducted on the relationship between NELL-1 and
osteoclastogenesis. In one such study, NELL-1 could increase the
osteoprotegerin/RANK ligand expression ratio in BMSCs, thereby
inhibiting osteoclastogenesis (49).
In the craniofacial bone, the expression of NELL-1
affects bone metabolism. While normal NELL-1 expression regulates
the differentiation and apoptosis of osteoblasts, NELL-1
overexpression deisrupts these pathways, resulting in craniofacial
abnormalities, such as the premature closure of sutures.
NELL-1-induced apoptosis was only observed in osteoblasts but not
in NIH3T3 or primary fibroblasts (76). NELL-1 overexpression was reported
to induce considerable apoptosis of skull osteoblasts through
increased Fas and Fas ligand production (77).
In teeth, NELL-1 can promote bone formation in a
concentration- and time-dependent manner (78,79). NELL-1 can inhibit
lipopolysaccharide-induced inflammation of human dental pulp cells,
which may be mediated by the p38-MAPK and extracellular
signal-regulated kinase-MAPK signaling pathways rather than the
JNK-MAPK signaling pathway (80). HrNELL-1 can increase the activity
of alkaline phosphatase and enhance the expression of important
odontogenic markers in human dental pulp cells, including OPN and
dentin matrix protein 1, thereby promoting odontogenic
differentiation and dentin formation of human dental pulp cells
(81). Under NELL-1 induction,
the expression of bone sialoprotein and OPN increases during the
intermediate stage, whereas OCN expression increases at the later
stage (the entire process ranges from 0-21 days). Alkaline
phosphatase activity and the number of calcium nodules were highest
in the NELL-1 group (Fig. 2F)
(82).
This section focuses on how the therapeutic effect
can be enhanced from the perspective of clinical application by
improving the osteogenic role of NELL-1. Undeniably, NELL-1 on its
own has osteogenic effects, but they can be improved through
modification. In other words, it was explored how clinical
therapeutic outcomes can be optimized through a series of physical
or chemical changes that lead to increased activity of NELL-1.
Through a literature search, it was found that the current
approaches to optimize the efficacy of NELL-1 mainly include the
following: Enhancement of the osteogenic effects of NELL-1 through
interactions with other molecules/cells, through molecular-level
changes and through bone tissue engineering. Therefore, these
optimization strategies were summarized below.
Combined treatment with NELL-1 and BMP exerts
synergistic osteogenic effects, which may be due to the obvious
difference in their signaling pathways, and has a mutually
reinforcing role (38).
Combining NELL-1 with BMP-2 can improve clinical bone regeneration
and exert a mechanism for typical Wnt pathway activity during
NELL-1 and BMP-2 osteogenesis (58). Given that BMP-2 and NELL-1
enhance each other, the simultaneous delivery of both agents can
significantly improve bone healing following tibial distraction
osteogenesis (83). Compared to
BMP-2 alone at a lower dosage, the combination of NELL-1 and BMP-2
showed more mature and complete bone defect healing, as evidenced
by high-resolution micro-computed tomography (CT) and histological
analysis (84). The combined
effects of NELL-1 and BMP-2 in the controlled-release vector may
eventually be applied in clinical practice to avoid the common
adverse reactions of conventional BMP-2 alone. One study reported
that using NELL-1 in controlled-release carriers can improve spinal
fusion rates in clinical settings (85). The synergistic delivery of BMP-2
and NELL-1, an osteochondral-specific signal transduction protein,
has potential therapeutic effects and is of great clinical
significance (36). BMP-2 and
NELL-1 genes exhibit synergistic effects on the osteogenic
differentiation of BMSCs, with one study showing that BMP-2- and
NELL-1-modified BMSCs can promote the formation and maturation of
new bone in a rabbit maxillary sinus model (86). Applying NELL-1 and BMP-2 on
dental pulp can induce dentin tubule repair and dentin formation
and reduce inflammatory cell reaction. These results show that the
combination of NELL-1 and BMP-2 can actively regulate pulp repair
(87). Self-assembled
polyelectrolyte complexes have been prepared to better control the
delivery of BMP-2/NELL-1 through heparin binding and further
enhance the biological activity of growth factors by enhancing
their stability in vivo (88). Liposuction-derived human PSCs
have been identified as a new and rich source of mesenchymal stem
cells (MSCs) for cartilage regeneration, with one study showing
that NELL-1, TGF-β3 and BMP-6 combined with human PSCs can
significantly enhance and accelerate cartilage repair (89).
After combined treatment with the Hedgehog signal
agonist smoothened agonist (SAG) and NELL-1, new bone formation
significantly increased, along with increased defect
vascularization. As a new treatment strategy, NELL-1 plus SAG shows
promise for treating critical-size bone defects. Future research
will focus on optimizing the dosage and delivery strategies for SAG
and NELL-1 combination products (90). The combined application of
Hedgehog and NELL-1 has an additive effect on promoting bone
differentiation and antilipogenic differentiation of ASCs. As such,
the combination of cytokine Sonic Hedgehog-N and NELL-1 may be a
feasible future approach for inducing osteogenic differentiation of
MSCs (52).
Incorporating peroxisome proliferator-activated
receptor (PPAR)γ inhibitors with NELL-1 treatment can enhance bone
formation by promoting anabolic processes, without affecting
NELL-1's ability to inhibit osteoclast activity and adipogenesis.
The combination of PPARγ inhibitors and therapeutic NELL-1 can be
further developed as a new strategy to reverse bone loss in
age-related osteoporosis and reduce bone marrow obesity (91). NELL-1 combined with zoledronic
acid for the treatment of traumatic osteonecrosis in rats can
promote osteoblast activity, inhibit osteoclast activity and
preserve the bone mass and shape of the femoral head, indicating
its significant role in preventing the collapse of femoral head
osteonecrosis. This strategy is expected to reverse the course of
osteonecrosis (92).
The combination of human PSCs and NELL-1 has a
significant additive effect on the angiogenesis and bone formation
of the implant, with studies simultaneously observing the
expression of angiogenic growth factor in vitro. This
combined treatment can improve vascularized bone regeneration
(93). NELL-1 significantly
increases the osteogenic potential of human PSCs in osteoporotic
and nonosteoporotic donors. Accordingly, one study showed that the
combination of human PSCs and NELL-1 can synergistically enhance
spinal fusion in osteoporotic rats (94). Radiographic images and
quantitative analysis from another study in which a mouse
osteonecrosis model was treated with adipose tissue-derived
pericytes and NELL-1 found that this group had the largest bone
formation among the treatment groups, with histomorphology analysis
showing strong bone and vascular formation (95). Pericytes are a potential new
source of cells for future research in bone regeneration medicine.
NELL-1 can significantly induce the proliferation of pericytes and
has been found to promote angiogenesis in vivo and in
vitro. NELL-1 is a candidate growth factor that can induce
osteogenic differentiation of pericytes. A study showed that the
combination of purified human pericytes with NELL-1 has osteogenic
potential (Table II) (96). Overall, the combined treatment of
cells and NELL-1 can be used as a new treatment approach to improve
bone regeneration and treat osteonecrosis and osteoporosis.
The following chapter focuses on how the osteogenic
ability of NELL-1 can be enhanced through changes at the molecular
level. A literature search unveiled that when the osteogenic role
of NELL-1 is examined, changes at the molecular level can make the
osteogenic effect more significant. Currently, these changes mainly
include polyethylene glycol (PEG)ylation of NELL-1 and NELL-1570.
Therefore, these two modifications were summarized to outline the
treatment approaches.
The PEGylation of NELL-1 significantly improves its
thermal stability while maintaining its biological activity in
vitro. Furthermore, PEGylation can significantly increase the
elimination half-life of NELL-1 (5.5-15.5 h) and increase its
content in bone tissues (femur, tibia, spine and skull) by >2-3
times (97). Furthermore,
evidence has shown that PEGylation increases the half-life of
NELL-1 in a mouse model without hampering its osteogenic potential,
thereby improving the pharmacokinetics of its systemic delivery.
Weekly injections of NELL-PEG via the intravenous or
intraperitoneal route successfully improved overall bone quality
(98). Compared to unmodified
NELL-1, all three PEGylation conjugates (three monomer PEG sizes:
5, 20 and 40 kDa) showed enhanced thermal stability and prolonged
circulation time in vivo. In addition, PEGylated NELL-1
maintains its osteogenic activity without any obvious cytotoxicity
(99). NELL-PEG injection
significantly enhances bone regeneration by promoting high bone
turnover, bone formation and mineral adhesion rates.
Immunohistochemical results have also confirmed that the NELL-PEG
treatment group had superior bone remodeling activity (100). Different types of triblock PEG
injectable hydrogels can reach a stable gel state at 37°C and
support the three-dimensional growth of cartilage cells, but the
poly lactide-co-caprolactone (PLCL) block-PEG block-PLCL hydrogel
has a wider gel temperature range and better hydrolytic stability.
Furthermore, its controlled-release curve is closest to zero-order
release kinetics. The PLCL-PEG-PLCL/NELL-1 compound can reverse
osteochondral injury caused by TMJOA (56). Future research should further
focus on developing NELL-1-PEG into a systemic treatment that can
effectively prevent and treat osteoporosis, accelerate fracture
healing and improve overall bone performance. Taken together, this
method has excellent potential for practical application.
NELL-1570 can significantly stimulate the
proliferation of MSCs in multiple MSC-like cell groups, such as
mouse C3H10T1/2 MSCs, mouse primary MSCs and PSCs, which are
considered stem cells of perivascular origin. By contrast,
NELL-1810 (normal molecular weight) only showed limited stimulation
of MSC proliferation. In vivo, NELL-1570 can significantly
induce the regeneration of skull defects given its effect of
increasing cell proliferation (101). The proliferative effect of
NELL-1570 is age-dependent and shows significant induction in adult
mice but not in old mice (Table
III) (102). In other
words, NELL-1570 can potentially be used for bone regeneration
therapy based on cells or hormones.
In this section, it is described how the osteogenic
ability of NELL-1 can be enhanced through bone tissue engineering.
A literature search revealed that when the osteogenic role of
NELL-1 is examined, it is indicated that the osteogenic effect can
be significantly enhanced via bone tissue engineering. Currently,
relevant bone tissue engineering methods mainly include
β-tricalcium phosphate (β-TCP), chitosan (Chi) nanoparticles
(NNPs), polylactic-co-glycolic acid (PLGA) and demineralized bone
matrix (DBM). Therefore, these methods were classified and
summarized to better understand the related solutions.
β-TCP is a bone conductive and biodegradable ceramic
biomaterial that has been successfully used as a bone inducer for
bone regeneration. It can be used as a carrier system for
effectively delivering NELL-1 (103). The protein-carrying capacity of
β-TCP particles can be enhanced and their initial rupture level can
be improved by creating an apatite coating, surface etching with
citric acid solution or immersing them in simulated body fluid. A
study on the release kinetics of protein in modified β-TCP
particles using the novel osteogenic protein NELL-1 as a model
protein showed that the protein-carrying capacity of β-TCP can be
regulated by surface modification, which allows the use of TCP as a
controllable protein carrier (104). Autologous BMSCs modified by the
NELL-1 gene and β-TCP particle scaffold can be used to lift the
maxillary sinus floor in rabbits (86). TCP was modified through
hydroxyapatite (HA) coating, after which a Chi coating was used to
prepare Chi/HA-coated TCP particles. The NELL-1 protein showed a
continuous release mode after being encapsulated in the modified
Chi/HA-TCP particles. The NELL-1-integrated complex of
Chi/HA-coated TCP particles demonstrates the advantages of these
particles as a protein delivery carrier and highlights its
potential as a modified bone matrix for bone regeneration research
(105). The apatite-wrapped
β-TCP vector promoted the sustained release of recombinant human
NELL-1 protein over a prolonged period of time, resulting in the
local inflow of Sca-1-positive MPCs, and induced complete bone
fusion in all samples (100% spinal fusion rate) (59). Using tissue engineering
technology, BMSCs after NELL-1 gene modification were combined with
β-TCP at a concentration of 2×107 cells/ml and implanted
subcutaneously in the backs of nude mice. The percentage of new
bone area in the NELL-1 group (18.1±5.0%) was significantly higher
than that in the non-transfected (11.3±3.2%) and LacZ
(β-galactosidase) groups (12.3±3.1%; P<0.05), suggesting that
NELL-1 is a potential osteogenic gene for bone tissue engineering
(82). One study that
incorporated lyophilized recombinant human NELL-1 protein into a
mixture of β-TCP and HA found that recombinant human NELL-1
vertebral body implantation significantly increased lumbar bone
formation and successfully improved the regeneration of lumbar
cortical and cancellous bones in osteoporotic sheep. This indicates
that bone graft substitutes based on recombinant human NELL-1 have
potential as a new local treatment method (106).
Preloading NELL-1 into Chi NNP resulted in a
significantly longer release time and greater released biological
activity of NELL-1 compared to directly adding NELL-1 to the
scaffold. As such, NELL-1 and dual-release scaffolds have potential
applications in cartilage tissue engineering (70). A multifunctional polycaprolactone
nano-HA Chi NNP composite fiber with long-term biological activity
and bone induction was successfully prepared by electrospinning.
Subsequent in vivo research found that this composite
material effectively prolongs NELL-1 release and shows good cell
compatibility, indicating its superior ability to induce osteogenic
differentiation. This makes it a promising scaffold for bone tissue
engineering applications (107). Hyaluronic acid hydrogel is
mixed with two types of particles (decalcified bone powder for bone
conduction and biomimetic apatite-coated sodium alginate/Chi NNP
for controlled NELL-1 delivery) to achieve the plasticity of
biomaterials and improve the spinal fusion rate (108). Recombinant human NELL-1 has
shown chondrogenic potential in a three-dimensional sodium alginate
hydrogel microenvironment containing rabbit chondrocytes.
Incorporating NELL-1 into Chi NNP can provide controlled delivery
function and maximize its biological efficiency (109). NELL-1, incorporated into Chi
NNP and embedded in alginate saline gel, can repair bone defects
and promote obvious cartilage regeneration, closely mimicking the
histological properties of natural cartilage. This makes it a
promising candidate for treating various pathologies, such as
cartilage defects and degeneration, using tissue engineering
(71).
Transplanting the NELL-1 protein-coated PLGA
scaffold into a rat skull defect showed that the osteogenic
potential of NELL-1-induced bone regeneration was equivalent to
that of BMP-2, revealing its potential therapeutic effects and
establishing it as a currently recognized alternative to bone
regeneration technology (40).
After precoating a culture dish or PLGA scaffold with NELL-1, the
degrees of cell adhesion and osteogenic differentiation increased
significantly (67). The
NELL-1-modified bone marrow MSC/PLGA group showed strong and rapid
repair effects at 6 weeks, resulting in fibrocartilage regeneration
and a completely repaired natural articular cartilage and
subchondral bone at 24 weeks. Therefore, the NELL-1-modified bone
marrow MSC/PLGA composite can rapidly repair large-area
osteochondral defects of the mandibular condyle and promote the
regeneration of the natural fibrocartilage and subchondral bone
(110).
In sheep, NELL-1, an independent and effective
osteogenic molecule, is easy to use when combined with DBM
(111). Micro-CT revealed that
NELL-1 in DBM exerted a significant effect on spinal fusion,
showing increased bone formation, endochondral ossification and
vascularization (112).
β-TCP/DBM, which is a carrier system for the efficient delivery of
biologically active NELL-1, can improve the biochemical stability
and biological efficiency of NELL-1 (103). In rats, NELL-1 in DBM carriers
can significantly and dose-dependently promote bone regeneration in
critical-size femoral segmental defects. NELL-1 is an effective
bone-specific growth factor, which can be used as a substitute for
bone transplantation in various clinical scenarios, including
repairing severe bone loss when autologous bone is limited or
unavailable (Table IV)
(113).
NELL-1 is also expressed, to a certain extent, in
bone tissue tumors and plays an important role. Sarcoma, as the
most common malignant tumor of bone tissue, should be examined.
Studies have revealed that NELL-1 is stably and reliably expressed
in chondrogenic bone tumors (114) and exhibits extensive and
reliable expression in benign, nonmalignant osteogenic tumors
(115). Recombinant human
NELL-1 mainly increases the activation of the JNK pathway, which is
necessary for mediating the final osteogenic differentiation of
Saos-2 osteosarcoma cells (116). Upregulation of NELL-1 has been
positively associated with the metastasis of rhabdomyosarcoma and
negatively associated with prognosis (117).
Considering the limited research on osteosarcoma, to
enrich the content of the present review, a bioinformatics analysis
was further conducted. The differential expression of NELL-1 in
sarcomatous tissues was analyzed using several databases and
bioinformatics methods.
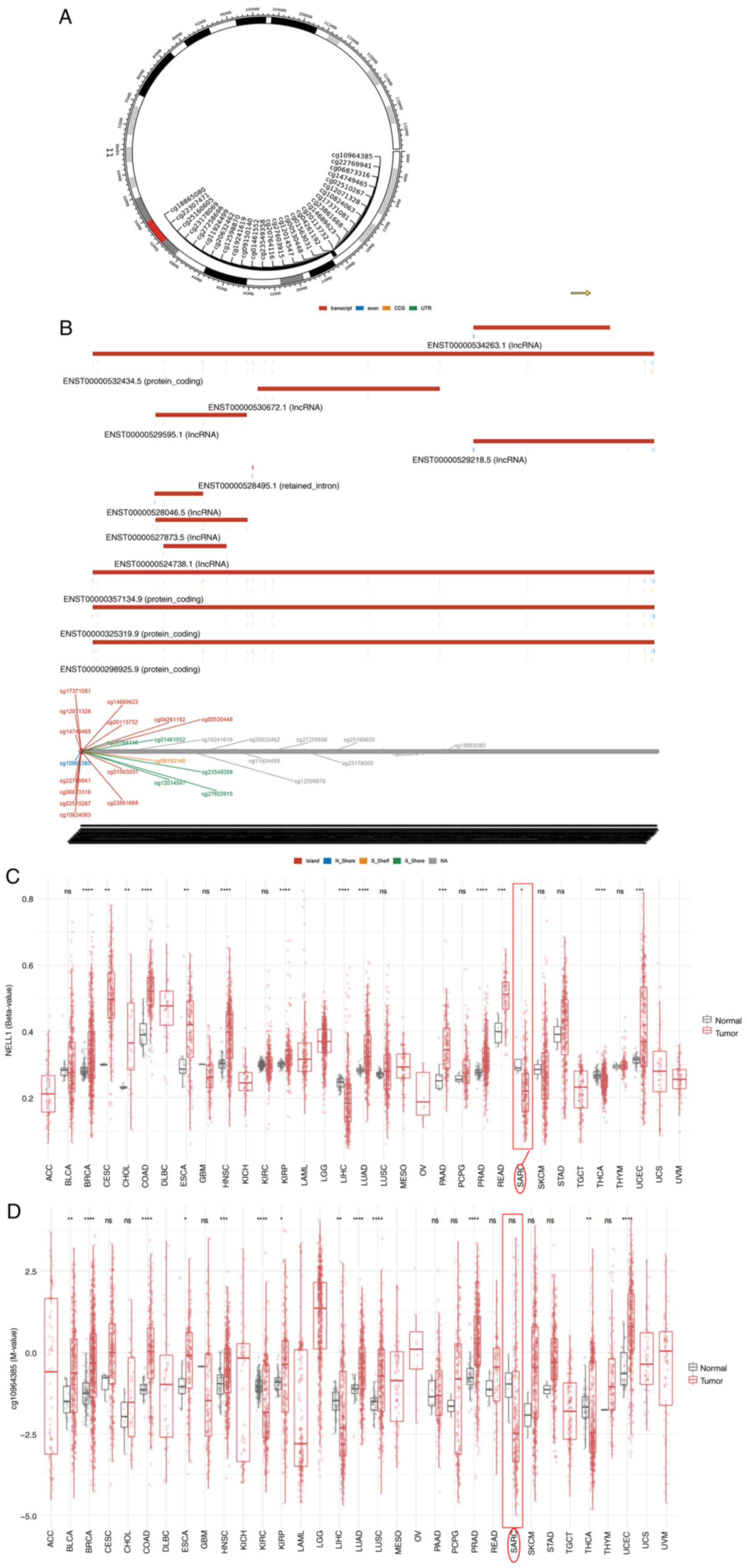
DNA methylation is closely related to tumors and
changes in the methylation level of NELL-1 may be a key factor in
osteosarcoma development (118). NELL-1 methylation levels in
sarcoma tissues vary. Analysis on the SMART platform (http://www.bioinfo-zs.com/smartapp/) revealed the
chromosomal distribution of the methylation probes associated with
NELL-1 (Fig. 3A) and provided
detailed genomic information on NELL-1 (Fig. 3B). The results showed that the
CpG-aggregated methylation value (β-value) of NELL-1 in sarcomatous
tissues was significantly lower than that in normal tissues
(Fig. 3C), indicating that a
decrease in NELL-1 methylation levels can cause osteosarcoma.
Continuing this in-depth research, the probe of cg10964385 was
selected for closer examination; however, no significant difference
between tumor and normal tissue was found for the methylation value
of cg10964385 (Fig. 3D). The
specific significant methylation probe will also become the main
focus of research in the future.
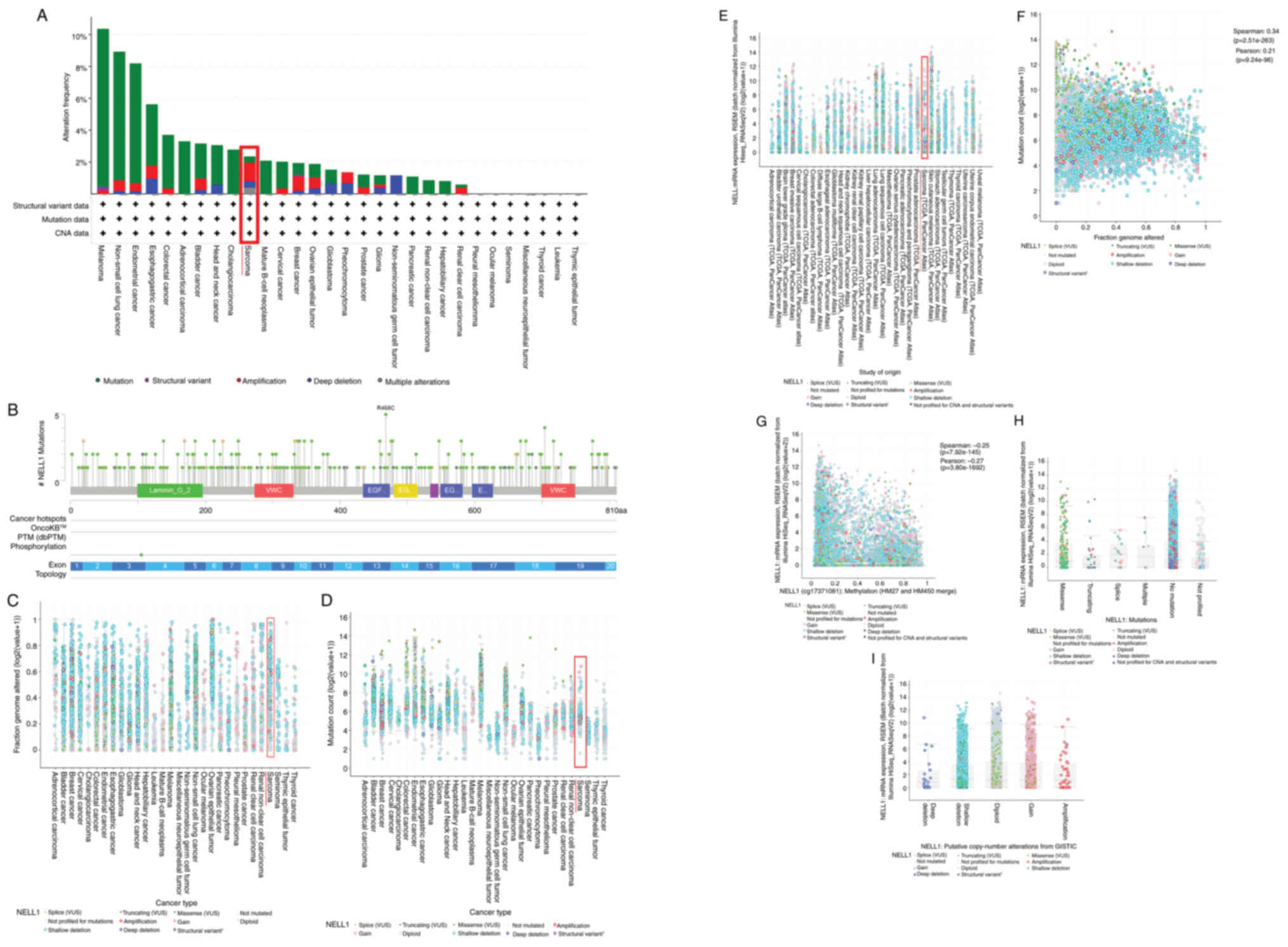
The most common cause of tumor occurrence is genetic
mutations. Mutation in the NELL-1 gene is closely linked to sarcoma
occurrence (119). Using the
cBioPortal platform (https://www.cbioportal.org), the alteration frequency
of NELL-1 in sarcomatous tissues was assessed. A certain level of
mutation, structural variation, amplification, deep deletion and
multiple alterations in sarcomas were found (Fig. 4A); hence, the frequency of
mutation sites was further analyzed (Fig. 4B). Varying degrees of mutation
sites can be observed throughout the NELL-1 sequence. Among them,
the 468th amino acid showed the highest frequency. Subsequently,
data on the fraction genome altered, mutation count and mRNA
expression RNA-sequencing by Expectation-Maximization (RSEM) for
NELL-1 across various tumors were obtained (Fig. 4C-E). For the fraction genome
altered of NELL-1, a shallow deletion of NELL-1 was most commonly
observed in sarcoma (Fig. 4C).
For the mutation count of NELL-1, a shallow deletion of NELL-1 was
most commonly observed in sarcoma (Fig. 4D). For mRNA expression (RSEM) of
NELL-1, a shallow deletion of NELL-1 was most commonly observed in
sarcoma (Fig. 4E). Finally, the
following relationships were analyzed using Spearman and Pearson
correlation: Fraction genome altered and mutation count
(statistically significant; Spearman: 0.34,
P=2.51×10−263; Pearson: 0.21, P=9.24×10−96)
(Fig. 4F); metrology and NELL-1
mRNA expression (statistically significant; Spearman: −0.25,
P=7.92×10−145; Pearson: −0.27, P=3.80×10−162)
(Fig. 4G); NELL1 mutations and
NELL1 mRNA expression (RSEM) [among mutations of NELL-1 (missense,
truncation, splice, multiple, no mutation and not profiled), a
shallow deletion of no mutation was most closely related to NELL1
mRNA expression (RSEM)] (Fig.
4H); and putative copy number alterations from Genomic
Identification of Significant Targets in Cancer (GISTIC) and NELL-1
mRNA expression (RSEM) [among putative copy number alterations from
GISTIC of NELL-1 [deep deletion, shallow deletion, diploid, gain
and amplification], shallow deletion, diploid and gain were similar
and most closely related to NELL1 mRNA expression (RSEM)] (Fig. 4I).
Recently, immune infiltration, a biological process,
has been reported to be closely related to tumor occurrence and
development (120). NELL-1
expression in sarcomatous tissues correlates with immune
infiltration. First, the The Cancer Genome Atlas (TCGA) database
(https://www.cancer.gov/ccg/research/genome-sequencing/tcga)
was used to analyze the correlation between NELL-1 expression and
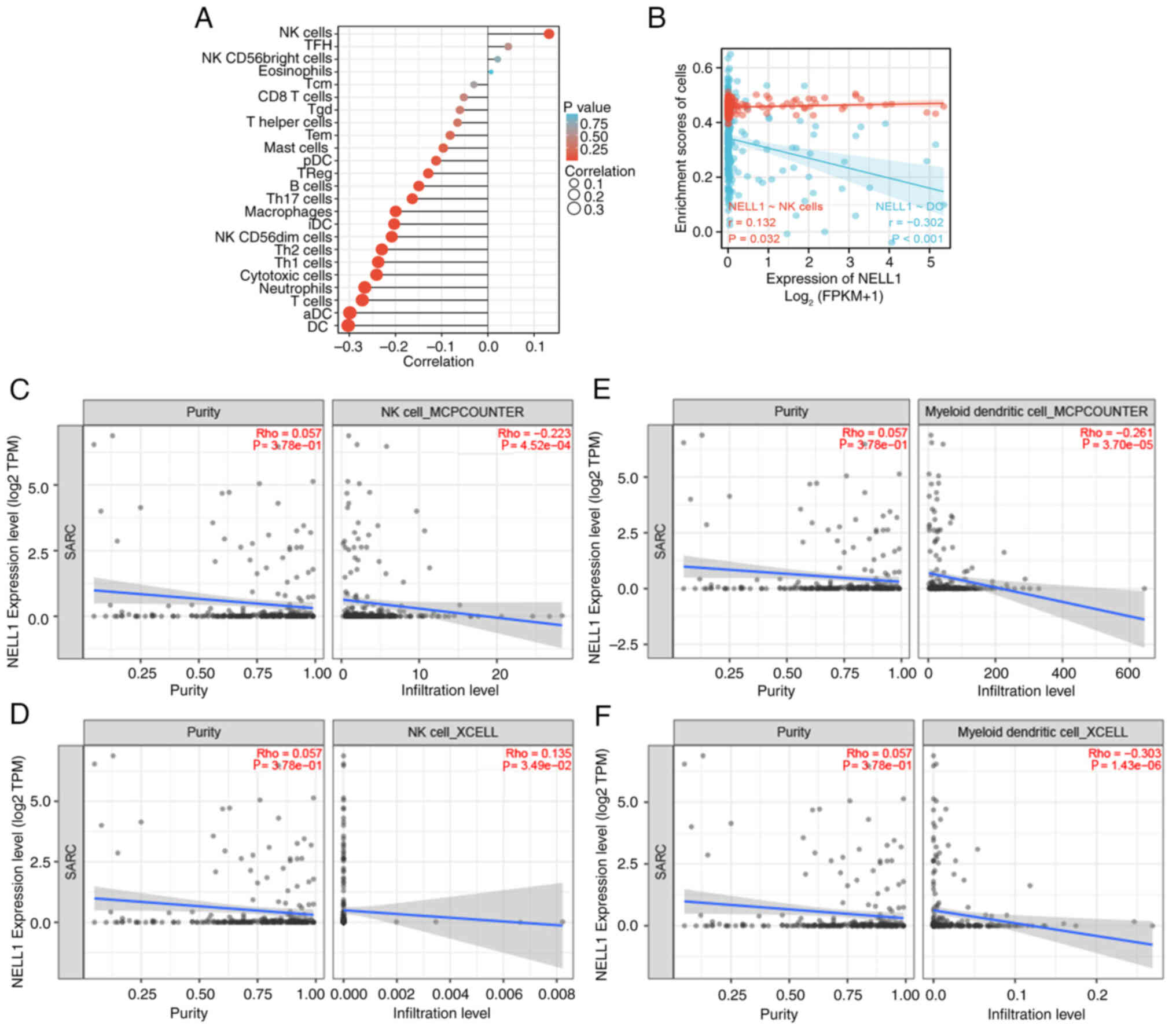
immune cell infiltration into sarcomatous tissues (Fig. 5A). It was found that the levels
of natural killer (NK) cells and NELL-1 had the highest positive
correlation, whereas those of dendritic cells (DCs) and NELL-1 had
the highest negative correlation. Next, the relationship between
the enrichment fraction of NK cells and DCs and the expression
level of NELL-1 was studied, which was subsequently determined to
be statistically significant (P<0.05 in NK cells, P<0.001 in
DC; Fig. 5B). Finally, the
relationship between the purity and infiltration levels of NK cells
and DCs and the expression level of NELL-1 in sarcomatous tissues
were studied using the TIMER2.0 tool (http://timer.cistrome.org/), and the results are
summarized in Fig. 5C-F.
Specifically, the first focus was on NK cells. Using the MCPCOUNTER
algorithm, no significant correlation between the purity of NK
cells and NELL-1 expression levels was found
(P=3.78×10−1); however, a negative correlation was found
between the infiltration level of NK cells and NELL-1 expression
levels (P=4.52×10−4) (Fig. 5C). Using the XCELL algorithm, no
significant correlation was found between the purity of NK cells
and NELL-1 expression levels (P=3.78×10−1); however, a
negative correlation was detected between the infiltration level of
NK cells and NELL-1 expression levels (P=3.49×10−2)
(Fig. 5D). The subsequent focus
was on DCs. Using the MCPCOUNTER algorithm, no significant
correlation was found between the purity of DCs and NELL-1
expression levels (P=3.78×10−1); however, a negative
correlation was noted between the infiltration level of DCs and
NELL-1 expression levels (P=3.70×10−5) (Fig. 5E). Using the XCELL algorithm, no
significant correlation between the purity of DCs and NELL-1
expression levels was found (P=3.78×10−1); however, a
negative correlation was noted between the infiltration level of
DCs and NELL-1 expression levels (P=1.43×10−6) (Fig. 5F).
Future research is needed to further clarify the
basic biological, diagnostic and prognostic significance of NELL-1
in bone tumors.
Research on NELL-1 and bone tissue diseases is
gaining increasing attention. To date, >80 relevant studies,
including 10 reviews on NELL-1 and bone tissue, have been
published; however, the current review was mainly conducted from a
partial perspective and the findings (NELL-1 can induce
osteogenesis) are not comprehensive. For instance, certain studies
reviewed the therapeutic effects of NELL-1 in orthopedic surgeries
such as spinal fusion; however, NELL-1 only makes up a small part
of an array of other growth factors (21,23,24,26,30). Another study reviewed the
efficacy of NELL-1 in osteoporosis; however, osteoporosis is only a
small aspect of metabolic diseases (27). A study reviewed the relationship
between NELL-1 and osteogenesis, but it was only limited to stem
cells (29). Another study
explored the relationship between NELL-1 and RUNX2 in dental
diseases (22). Other reviews
were conducted on the osteogenic effects of NELL-1, similar to the
current review, but from a different perspective, such as different
functions of NELL-1, different sites of NELL-1 and different
applied models of NELL-1 (25,28,38). Therefore, the present review
provides a more systematic and comprehensive overview of all
relevant studies in the literature.
This review had certain limitations. Although it was
attempted to collect all relevant research data, the results may
not be complete because of limitations in search platforms and
language. Furthermore, the present review mainly focused on
categorizing and summarizing the results and the scope for
discussion was slightly insufficient.
Not applicable.
YT contributed to the study's conception and design,
performed the literature selection/review and wrote and edited the
manuscript. ZL performed language editing, tabulation and drawing,
and bioinformatics predictions. All authors have read and approved
the final version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This study received funding from the Liaoning Natural Science
Foundation Project (grant no. 2023-BS-035).
|
1
|
Barrio-Hernandez I, Jafari A, Rigbolt KTG,
Hallenborg P, Sanchez-Quiles V, Skovrind I, Akimov V, Kratchmarova
I, Dengjel J, Kassem M and Blagoev B: Phosphoproteomic profiling
reveals a defined genetic program for osteoblastic lineage
commitment of human bone marrow-derived stromal stem cells. Genome
Res. 30:127–137. 2020. View Article : Google Scholar :
|
|
2
|
Mousa A, Cui C, Song A, Myneni VD, Sun H,
Li JJ, Murshed M, Melino G and Kaartinen MT: Transglutaminases
factor XIII-A and TG2 regulate resorption, adipogenesis and plasma
fibronectin homeostasis in bone and bone marrow. Cell Death Differ.
24:844–854. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zengini E, Hatzikotoulas K, Tachmazidou I,
Steinberg J, Hartwig FP, Southam L, Hackinger S, Boer CG,
Styrkarsdottir U, Gilly A, et al: Genome-wide analyses using UK
Biobank data provide insights into the genetic architecture of
osteoarthritis. Nat Genet. 50:549–558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jones IA, Togashi R, Wilson ML, Heckmann N
and Vangsness CT Jr: Intra-articular treatment options for knee
osteoarthritis. Nat Rev Rheumatol. 15:77–90. 2019. View Article : Google Scholar :
|
|
5
|
Lopes D, Martins-Cruz C, Oliveira MB and
Mano JF: Bone physiology as inspiration for tissue regenerative
therapies. Biomaterials. 185:240–275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu H, Cao F, Zhou W, Wang G, Liu G, Xia T,
Liu M, Mi B and Liu Y: Long Noncoding RNA FAM83H-AS1 Modulates
SpA-Inhibited Osteogenic Differentiation in Human Bone Mesenchymal
Stem Cells. Mol Cell Biol. 40:e00362–19. 2020. View Article : Google Scholar :
|
|
7
|
Zhu H, Kimura T, Swami S and Wu JY:
Pluripotent stem cells as a source of osteoblasts for bone tissue
regeneration. Biomaterials. 196:31–45. 2019. View Article : Google Scholar
|
|
8
|
Petersen A, Princ A, Korus G, Ellinghaus
A, Leemhuis H, Herrera A, Klaumünzer A, Schreivogel S, Woloszyk A,
Schmidt-Bleek K, et al: A biomaterial with a channel-like pore
architecture induces endochondral healing of bone defects. Nat
Commun. 9:44302018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukuda M, Yoshizawa T, Karim MF, Sobuz SU,
Korogi W, Kobayasi D, Okanishi H, Tasaki M, Ono K, Sawa T, et al:
SIRT7 has a critical role in bone formation by regulating lysine
acylation of SP7/Osterix. Nat Commun. 9:28332018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han B, Li Q, Wang C, Patel P, Adams SM,
Doyran B, Nia HT, Oftadeh R, Zhou S, Li CY, et al: Decorin
regulates the aggrecan network integrity and biomechanical
functions of cartilage extracellular matrix. ACS Nano.
13:11320–11333. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scheper MA, Badros A, Chaisuparat R,
Cullen KJ and Meiller TF: Effect of zoledronic acid on oral
fibroblasts and epithelial cells: A potential mechanism of
bisphosphonate-associated osteonecrosis. Br J Haematol.
144:667–676. 2009. View Article : Google Scholar :
|
|
12
|
Ho-Shui-Ling A, Bolander J, Rustom LE,
Johnson AW, Luyten FP and Picart C: Bone regeneration strategies:
Engineered scaffolds, bioactive molecules and stem cells current
stage and future perspectives. Biomaterials. 180:143–162. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ting K, Vastardis H, Mulliken JB, Soo C,
Tieu A, Do H, Kwong E, Bertolami CN, Kawamoto H, Kuroda S and
Longaker MT: Human NELL-1 expressed in unilateral coronal
synostosis. J Bone Miner Res. 14:80–89. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
James AW, Shen J, Zhang X, Asatrian G,
Goyal R, Kwak JH, Jiang L, Bengs B, Culiat CT, Turner AS, et al:
NELL-1 in the treatment of osteoporotic bone loss. Nat Commun.
6:73622015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura Y, Hasebe A, Takahashi K, Iijima
M, Yoshimoto N, Maturana AD, Ting K, Kuroda S and Niimi T:
Oligomerization-induced conformational change in the C-terminal
region of Nel-like molecule 1 (NELL1) protein is necessary for the
efficient mediation of murine MC3T3-E1 cell adhesion and spreading.
J Biol Chem. 289:9781–9794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuroda S, Oyasu M, Kawakami M, Kanayama N,
Tanizawa K, Saito N, Abe T, Matsuhashi S and Ting K: Biochemical
characterization and expression analysis of neural
thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res
Commun. 265:79–86. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kajava AV: Modeling of a five-stranded
coiled coil structure for the assembly domain of the cartilage
oligomeric matrix protein. Proteins. 24:218–226. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuroda S and Tanizawa K: Involvement of
epidermal growth factor-like domain of NELL proteins in the novel
protein-protein interaction with protein kinase C. Biochem Biophys
Res Commun. 265:752–757. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bornstein P: Diversity of function is
inherent in matricellular proteins: An appraisal of thrombospondin
1. J Cell Biol. 130:503–506. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garcia Abreu J, Coffinier C, Larrain J,
Oelgeschlager M and De Robertis EM: Chordin-like CR domains and the
regulation of evolutionarily conserved extracellular signaling
systems. Gene. 287:39–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duarte RM, Varanda P, Reis RL, Duarte ARC
and Correia-Pinto J: Biomaterials and Bioactive Agents in Spinal
Fusion. Tissue Eng Part B Rev. 23:540–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng L, He H, Sun M, Gong X, Zhou M, Hong
Y, Wu Y, Chen X and Chen Q: Runx2 and Nell-1 in dental follicle
progenitor cells regulate bone remodeling and tooth eruption. Stem
Cell Res Ther. 13:4862022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith B, Goldstein T and Ekstein C:
Biologic adjuvants and bone: Current use in orthopedic surgery.
Curr Rev Musculoskelet Med. 8:193–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cottrill E, Ahmed AK, Lessing N,
Pennington Z, Ishida W, Perdomo-Pantoja A, Lo SF, Howell E, Holmes
C, Goodwin CR, et al: Investigational growth factors utilized in
animal models of spinal fusion: Systematic review. World J Orthop.
10:176–191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin XY, Zhao HX, Zhang Q, Chen F and Lin
JX: NELL-1: A novel highly efficient and specific growth factor.
Beijing Da Xue Xue Bao Yi Xue Ban. 48:380–383. 2016.In Chinese.
PubMed/NCBI
|
|
26
|
Zhang Y, Jiang Y, Zou D, Yuan B, Ke HZ and
Li W: Therapeutics for enhancement of spinal fusion: A mini review.
J Orthop Translat. 31:73–79. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng X, Shi J, Jia Z, Ha P, Soo C, Ting
K, James AW, Shi B and Zhang X: NELL-1 in Genome-Wide Association
Studies across Human Diseases. Am J Pathol. 192:395–405. 2022.
View Article : Google Scholar :
|
|
28
|
Li C, Zhang X, Zheng Z, Nguyen A, Ting K
and Soo C: Nell-1 is a key functional modulator in
osteochondrogenesis and beyond. J Dent Res. 98:1458–1468. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pakvasa M, Alverdy A, Mostafa S, Wang E,
Fu L, Li A, Oliveira L, Athiviraham A, Lee MJ, Wolf JM, et al:
Neural EGF-like protein 1 (NELL-1): Signaling crosstalk in
mesenchymal stem cells and applications in regenerative medicine.
Genes Dis. 4:127–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
James AW: Review of signaling pathways
governing MSC osteogenic and adipogenic differentiation.
Scientifica (Cairo). 2013:6847362013.
|
|
31
|
Setzer B, Bächle M, Metzger MC and Kohal
RJ: The gene-expression and phenotypic response of hFOB 1.19
osteoblasts to surface-modified titanium and zirconia.
Biomaterials. 30:979–990. 2009. View Article : Google Scholar
|
|
32
|
Iwan A, Moskalewski S and Hyc A: Growth
factor profile in calcified cartilage from the metaphysis of a calf
costochondral junction, the site of initial bone formation. Biomed
Rep. 14:542021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song D, Huang S, Zhang L, Liu W, Huang B,
Feng Y, Liu B, He TC, Huang D and Reid RR: Differential
Responsiveness to BMP9 between patent and fused suture progenitor
cells from craniosynostosis patients. Plast Reconstr Surg.
145:552e–562e. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu L, Cen X, Xia K, Huang X, Sun W, Zhao Z
and Liu J: microRNA expression profiles and the potential competing
endogenous RNA networks in NELL-1-induced human adipose-derived
stem cell osteogenic differentiation. J Cell Biochem.
121:4623–4641. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen J, James AW, Zara JN, Asatrian G,
Khadarian K, Zhang JB, Ho S, Kim HJ, Ting K and Soo C: BMP2-induced
inflammation can be suppressed by the osteoinductive growth factor
NELL-1. Tissue Eng Part A. 19:2390–2401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cowan CM, Jiang X, Hsu T, Soo C, Zhang B,
Wang JZ, Kuroda S, Wu B, Zhang Z, Zhang X and Ting K: Synergistic
effects of Nell-1 and BMP-2 on the osteogenic differentiation of
myoblasts. J Bone Miner Res. 22:918–930. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fahmy-Garcia S, van Driel M, Witte-Buoma
J, Walles H, van Leeuwen JPTM, van Osch GJVM and Farrell E: NELL-1,
HMGB1, and CCN2 enhance migration and vasculogenesis, but not
osteogenic differentiation compared to BMP2. Tissue Eng Part A.
24:207–218. 2018. View Article : Google Scholar
|
|
38
|
Zhang X, Zara J, Siu RK, Ting K and Soo C:
The role of NELL-1, a growth factor associated with
craniosynostosis, in promoting bone regeneration. J Dent Res.
89:865–878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
James AW, Zara JN, Zhang X, Askarinam A,
Goyal R, Chiang M, Yuan W, Chang L, Corselli M, Shen J, et al:
Perivascular stem cells: A prospectively purified mesenchymal stem
cell population for bone tissue engineering. Stem Cells Transl Med.
1:510–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aghaloo T, Cowan CM, Chou YF, Zhang X, Lee
H, Miao S, Hong N, Kuroda S, Wu B, Ting K and Soo C: Nell-1-induced
bone regeneration in calvarial defects. Am J Pathol. 169:903–915.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aghaloo T, Jiang X, Soo C, Zhang Z and
Zhang X, Hu J, Pan H, Hsu T, Wu B, Ting K and Zhang X: A study of
the role of nell-1 gene modified goat bone marrow stromal cells in
promoting new bone formation. Mol Ther. 15:1872–1880. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cowan CM, Zhang X, James AW, Kim TM, Sun
N, Wu B, Ting K and Soo C: NELL-1 increases pre-osteoblast
mineralization using both phosphate transporter Pit1 and Pit2.
Biochem Biophys Res Commun. 422:351–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hyc A, Moskalewski S and Osiecka-Iwan A:
Growth factors in the initial stage of bone formation, analysis of
their expression in chondrocytes from epiphyseal cartilage of rat
costochondral junction. Folia Histochem Cytobiol. 59:178–186. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cowan CM, Cheng S, Ting K, Soo C, Walder
B, Wu B, Kuroda S and Zhang X: Nell-1 induced bone formation within
the distracted intermaxillary suture. Bone. 38:48–58. 2006.
View Article : Google Scholar
|
|
45
|
Truong T, Zhang X, Pathmanathan D, Soo C
and Ting K: Craniosynostosis-associated gene nell-1 is regulated by
runx2. J Bone Miner Res. 22:7–18. 2007. View Article : Google Scholar
|
|
46
|
Zhang X, Ting K, Bessette CM, Culiat CT,
Sung SJ, Lee H, Chen F, Shen J, Wang JJ, Kuroda S and Soo C:
Nell-1, a key functional mediator of Runx2, partially rescues
calvarial defects in Runx2(+/−) mice. J Bone Miner Res. 26:777–791.
2011. View Article : Google Scholar
|
|
47
|
Li C, Jiang J, Zheng Z, Lee KS, Zhou Y,
Chen E, Culiat CT, Qiao Y, Chen X, Ting K, et al: Neural EGFL-Like
1 is a downstream regulator of runt-related transcription factor 2
in chondrogenic differentiation and maturation. Am J Pathol.
187:963–972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen F, Zhang X, Sun S, Zara JN, Zou X,
Chiu R, Culiat CT, Ting K and Soo C: NELL-1, an osteoinductive
factor, is a direct transcriptional target of Osterix. PLoS One.
6:e246382011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lai K, Xi Y, Du X, Jiang Z, Li Y, Huang T,
Miao X, Wang H, Wang Y and Yang G: Activation of Nell-1 in BMSC
sheet promotes implant osseointegration through regulating
Runx2/Osterix Axis. Front Cell Dev Biol. 8:8682020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang X, Ting K, Pathmanathan D, Ko T,
Chen W, Chen F, Lee H, James AW, Siu RK, Shen J, et al: Calvarial
cleidocraniodysplasia-like defects with ENU-induced Nell-1
deficiency. J Craniofac Surg. 23:61–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu SS, Zhang X, Soo C, Hsu T, Napoli A,
Aghaloo T, Wu BM, Tsou P, Ting K and Wang JC: The osteoinductive
properties of Nell-1 in a rat spinal fusion model. Spine J.
7:50–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
James AW, Pang S, Askarinam A, Corselli M,
Zara JN, Goyal R, Chang L, Pan A, Shen J, Yuan W, et al: Additive
effects of sonic hedgehog and Nell-1 signaling in osteogenic versus
adipogenic differentiation of human adipose-derived stromal cells.
Stem Cells Dev. 21:2170–2178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
James AW, Pan A, Chiang M, Zara JN, Zhang
X, Ting K and Soo C: A new function of Nell-1 protein in repressing
adipogenic differentiation. Biochem Biophys Res Commun.
411:126–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Qi H, Kim JK, Ha P, Chen X, Chen E, Chen
Y, Li J, Pan HC, Yu M, Mohazeb Y, et al: Inactivation of Nell-1 in
chondrocytes significantly impedes appendicular skeletogenesis. J
Bone Miner Res. 34:533–546. 2019. View Article : Google Scholar
|
|
55
|
Li C, Zheng Z, Zhang X, Asatrian G, Chen
E, Song R, Culiat C, Ting K and Soo C: Nfatc1 Is a Functional
Transcriptional Factor Mediating Nell-1-Induced Runx3 Upregulation
in Chondrocytes. Int J Mol Sci. 19:1682018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang C, Wang Y, Wang C, Liu C, Li W, Hu S,
Wu N, Jiang S and Shi J: Therapeutic application of 3B-PEG
injectable hydrogel/Nell-1 composite system to temporomandibular
joint osteoarthritis. Biomed Mater. 17:0150042021. View Article : Google Scholar
|
|
57
|
Chen W, Zhang X, Siu RK, Chen F, Shen J,
Zara JN, Culiat CT, Tetradis S, Ting K and Soo C: Nfatc2 is a
primary response gene of Nell-1 regulating chondrogenesis in ATDC5
cells. J Bone Miner Res. 26:1230–1241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shen J, James AW, Zhang X, Pang S, Zara
JN, Asatrian G, Chiang M, Lee M, Khadarian K, Nguyen A, et al:
Novel Wnt Regulator NEL-Like Molecule-1 antagonizes adipogenesis
and augments osteogenesis induced by bone morphogenetic protein 2.
Am J Pathol. 186:419–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
James AW, Shen J, Tsuei R, Nguyen A,
Khadarian K, Meyers CA, Pan HC, Li W, Kwak JH, Asatrian G, et al:
NELL-1 induces Sca-1+ mesenchymal progenitor cell expansion in
models of bone maintenance and repair. JCI Insight. 2:e925732017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen X, Wang H, Yu M, Kim JK, Qi H, Ha P,
Jiang W, Chen E, Luo X, Needle RB, et al: Cumulative inactivation
of Nell-1 in Wnt1 expressing cell lineages results in craniofacial
skeletal hypoplasia and postnatal hydrocephalus. Cell Death Differ.
27:1415–1430. 2020. View Article : Google Scholar :
|
|
61
|
Zhang X, Kuroda S, Carpenter D, Nishimura
I, Soo C, Moats R, Iida K, Wisner E, Hu FY, Miao S, et al:
Craniosynostosis in transgenic mice overexpressing Nell-1. J Clin
Invest. 110:861–870. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chermside-Scabbo CJ, Harris TL, Brodt MD,
Braenne I, Zhang B, Farber CR and Silva MJ: Old mice have less
transcriptional activation but similar periosteal cell
proliferation compared to young-adult mice in response to in vivo
mechanical loading. J Bone Miner Res. 35:1751–1764. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li C, Zheng Z, Ha P, Chen X, Jiang W, Sun
S, Chen F, Asatrian G, Berthiaume EA, Kim JK, et al: Neurexin
superfamily cell membrane receptor contactin-associated protein
Like-4 (Cntnap4) Is Involved in Neural EGFL-Like 1
(Nell-1)-Responsive Osteogenesis. J Bone Miner Res. 33:1813–1825.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yamamoto N, Kashiwagi M, Ishihara M,
Kojima T, Maturana AD, Kuroda S and Niimi T: Robo2 contains a
cryptic binding site for neural EGFL-like (NELL) protein 1/2. J
Biol Chem. 294:4693–4703. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bokui N, Otani T, Igarashi K, Kaku J, Oda
M, Nagaoka T, Seno M, Tatematsu K, Okajima T, Matsuzaki T, et al:
Involvement of MAPK signaling molecules and Runx2 in the
NELL1-induced osteoblastic differentiation. FEBS Lett. 582:365–371.
2008. View Article : Google Scholar
|
|
66
|
Huang X, Cen X, Zhang B, Liao Y, Zhao Z,
Zhu G, Zhao Z and Liu J: The roles of circRFWD2 and circINO80
during NELL-1-induced osteogenesis. J Cell Mol Med. 23:8432–8441.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shen J, James AW, Chung J, Lee K, Zhang
JB, Ho S, Lee KS, Kim TM, Niimi T, Kuroda S, et al: NELL-1 promotes
cell adhesion and differentiation via Integrinβ1. J Cell Biochem.
113:3620–3628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zou X, Shen J, Chen F, Ting K, Zheng Z,
Pang S, Zara JN, Adams JS, Soo C and Zhang X: NELL-1 binds to APR3
affecting human osteoblast proliferation and differentiation. FEBS
Lett. 585:2410–2418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li C, Zheng Z, Ha P, Jiang W, Berthiaume
EA, Lee S, Mills Z, Pan H, Chen EC, Jiang J, et al: Neural EGFL
like 1 as a potential pro-chondrogenic, anti-inflammatory
dual-functional disease-modifying osteoarthritis drug.
Biomaterials. 226:1195412020. View Article : Google Scholar :
|
|
70
|
Wang C, Hou W, Guo X, Li J, Hu T, Qiu M,
Liu S, Mo X and Liu X: Two-phase electrospinning to incorporate
growth factors loaded chitosan nanoparticles into electrospun
fibrous scaffolds for bioactivity retention and cartilage
regeneration. Mater Sci Eng C Mater Biol Appl. 79:507–515. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Siu RK, Zara JN, Hou Y, James AW, Kwak J,
Zhang X, Ting K, Wu BM, Soo C and Lee M: NELL-1 promotes cartilage
regeneration in an in vivo rabbit model. Tissue Eng Part A.
18:252–261. 2012. View Article : Google Scholar :
|
|
72
|
Kwak J, Zara JN, Chiang M, Ngo R, Shen J,
James AW, Le KM, Moon C, Zhang X, Gou Z, et al: NELL-1 injection
maintains long-bone quantity and quality in an ovariectomy-induced
osteoporotic senile rat model. Tissue Eng Part A. 19:426–436. 2013.
View Article : Google Scholar :
|
|
73
|
James AW, Zhang X, Crisan M, Hardy WR,
Liang P, Meyers CA, Lobo S, Lagishetty V, Childers MK, Asatrian G,
et al: Isolation and characterization of canine perivascular
stem/stromal cells for bone tissue engineering. PLoS One.
12:e01773082017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Karasik D, Hsu YH, Zhou Y, Cupples LA,
Kiel DP and Demissie S: Genome-wide pleiotropy of
osteoporosis-related phenotypes: The Framingham Study. J Bone Miner
Res. 25:1555–1563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Inaba H, Cao X, Han AQ, Panetta JC, Ness
KK, Metzger ML, Rubnitz JE, Ribeiro RC, Sandlund JT, Jeha S, et al:
Bone mineral density in children with acute lymphoblastic leukemia.
Cancer. 124:1025–1035. 2018. View Article : Google Scholar
|
|
76
|
Zhang X, Carpenter D, Bokui N, Soo C, Miao
S, Truong T, Wu B, Chen I, Vastardis H, Tanizawa K, et al:
Overexpression of Nell-1, a craniosynostosis-associated gene,
induces apoptosis in osteoblasts during craniofacial development. J
Bone Miner Res. 18:2126–2134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang X, Cowan CM, Jiang X, Soo C, Miao S,
Carpenter D, Wu B, Kuroda S and Ting K: Nell-1 induces acrania-like
cranio-skeletal deformities during mouse embryonic development. Lab
Invest. 86:633–644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tang R, Wang Q, Du J, Yang P and Wang X:
Expression and localization of Nell-1 during murine molar
development. J Mol Histol. 44:175–181. 2013. View Article : Google Scholar
|
|
79
|
Wang B, Wu Y, Yu H, Jiang L, Fang B and
Guo Q: The effects of NELL on corticotomy-assisted tooth movement
and osteogenesis in a rat model. Biomed Mater Eng. 29:757–771.
2018.PubMed/NCBI
|
|
80
|
Cao R, Wang Q, Wu J, Liu M, Han Q and Wang
X: Nell-1 attenuates lipopolysaccharide-induced inflammation in
human dental pulp cells. J Mol Histol. 52:671–680. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liu M, Wang Q, Tang R, Cao R and Wang X:
Nel-like molecule 1 contributes to the odontoblastic
differentiation of human dental pulp cells. J Endod. 42:95–100.
2016. View Article : Google Scholar
|
|
82
|
Hu JZ, Zhang ZY, Zhao J, Zhang XL, Liu GT
and Jiang XQ: An ectopic study of tissue-engineered bone with
Nell-1 gene modified rat bone marrow stromal cells in nude mice.
Chin Med J (Engl). 122:972–979. 2009.PubMed/NCBI
|
|
83
|
Zhu S, Song D, Jiang X, Zhou H and Hu J:
Combined effects of recombinant human BMP-2 and Nell-1 on bone
regeneration in rapid distraction osteogenesis of rabbit tibia.
Injury. 42:1467–1473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Aghaloo T, Cowan CM, Zhang X, Freymiller
E, Soo C, Wu B, Ting K and Zhang Z: The effect of NELL1 and bone
morphogenetic protein-2 on calvarial bone regeneration. J Oral
Maxillofac Surg. 68:300–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu L, Lam WMR, Naidu M, Yang Z, Wang M,
Ren X, Hu T, Kumarsing R, Ting K, Goh JC and Wong HK: Synergistic
Effect of NELL-1 and an Ultra-Low Dose of BMP-2 on Spinal Fusion.
Tissue Eng Part A. 25:1677–1689. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xia L, Xu Y, Chang Q, Sun X, Zeng D, Zhang
W, Zhang X, Zhang Z and Jiang X: Maxillary sinus floor elevation
using BMP-2 and Nell-1 gene-modified bone marrow stromal cells and
TCP in rabbits. Calcif Tissue Int. 89:53–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wu J, Wang Q, Han Q, Zhu H, Li M, Fang Y
and Wang X: Effects of Nel-like molecule-1 and bone morphogenetic
protein 2 combination on rat pulp repair. J Mol Histol. 50:253–261.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wing Moon Lam R, Abbah SA, Ming W, Naidu
M, Ng F, Tao H, Goh Cho Hong J, Ting K and Hee Kit W:
Polyelectrolyte complex for heparin binding domain osteogenic
growth factor delivery. J Vis Exp. (114): 542022016.PubMed/NCBI
|
|
89
|
Li CS, Zhang X, Péault B, Jiang J, Ting K,
Soo C and Zhou YH: Accelerated chondrogenic differentiation of
human perivascular stem cells with NELL-1. Tissue Eng Part A.
22:272–285. 2016. View Article : Google Scholar :
|
|
90
|
Lee S, Wang C, Pan HC, Shrestha S, Meyers
C, Ding C, Shen J, Chen E, Lee M, Soo C, et al: Combining
Smoothened Agonist and NEL-Like Protein-1 Enhances Bone Healing.
Plast Reconstr Surg. 139:1385–1396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tanjaya J, Ha P, Zhang Y, Wang C, Shah Y,
Berthiaume E, Pan HC, Shi J, Kwak J, Wu B, et al: Genetic and
pharmacologic suppression of PPARγ enhances NELL-1-stimulated bone
regeneration. Biomaterials. 287:1216092022. View Article : Google Scholar
|
|
92
|
Fan M, Jiang WX, Wang AY, Peng J, Zhang L,
Xu WJ and Lu SB: Combined effects of NEL-like type 1 gene and
zoledronate in preventing collapse of the femoral head. Zhongguo Yi
Xue Ke Xue Yuan Xue Bao. 35:553–560. 2013.In Chinese. PubMed/NCBI
|
|
93
|
Askarinam A, James AW, Zara JN, Goyal R,
Corselli M, Pan A, Liang P, Chang L, Rackohn T, Stoker D, et al:
Human perivascular stem cells show enhanced osteogenesis and
vasculogenesis with Nel-like molecule I protein. Tissue Eng Part A.
19:1386–1397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lee S, Zhang X, Shen J, James AW, Chung
CG, Hardy R, Li C, Girgius C, Zhang Y, Stoker D, et al: Brief
Report: Human perivascular stem cells and nel-like protein-1
synergistically enhance spinal fusion in osteoporotic rats. Stem
Cells. 33:3158–3163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
An HJ, Ko KR, Baek M, Jeong Y, Lee HH, Kim
H, Kim DK, Lee SY and Lee S: Pro-Angiogenic and osteogenic effects
of adipose tissue-derived pericytes synergistically enhanced by
Nel-like Protein-1. Cells. 10:22442021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhang X, Péault B, Chen W, Li W, Corselli
M, James AW, Lee M, Siu RK, Shen P, Zheng Z, et al: The Nell-1
growth factor stimulates bone formation by purified human
perivascular cells. Tissue Eng Part A. 17:2497–2509. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kwak JH, Zhang Y, Park J, Chen E, Shen J,
Chawan C, Tanjaya J, Lee S, Zhang X, Wu BM, et al: Pharmacokinetics
and osteogenic potential of PEGylated NELL-1 in vivo after systemic
administration. Biomaterials. 57:73–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Tanjaya J, Zhang Y, Lee S, Shi J, Chen E,
Ang P, Zhang X, Tetradis S, Ting K, Wu B, et al: Efficacy of
Intraperitoneal Administration of PEGylated NELL-1 for Bone
Formation. Biores Open Access. 5:159–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang Y, Velasco O, Zhang X, Ting K, Soo C
and Wu BM: Bioactivity and circulation time of PEGylated NELL-1 in
mice and the potential for osteoporosis therapy. Biomaterials.
35:6614–6621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tanjaya J, Lord EL, Wang C, Zhang Y, Kim
JK, Nguyen A, Baik L, Pan HC, Chen E, Kwak JH, et al: The Effects
of Systemic Therapy of PEGylated NEL-Like Protein 1 (NELL-1) on
Fracture Healing in Mice. Am J Pathol. 188:715–727. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pang S, Shen J, Liu Y, Chen F, Zheng Z,
James AW, Hsu CY, Zhang H, Lee KS, Wang C, et al: Proliferation and
osteogenic differentiation of mesenchymal stem cells induced by a
short isoform of NELL-1. Stem Cells. 33:904–915. 2015. View Article : Google Scholar
|
|
102
|
Meyers CA, Sun Z, Chang L, Ding C, Lu A,
Ting K, Pang S and James AW: Age dependent effects of NELL-1
isoforms on bone marrow stromal cells. J Orthop. 16:175–178. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Li W, Lee M, Whang J, Siu RK, Zhang X, Liu
C, Wu BM, Wang JC, Ting K and Soo C: Delivery of lyophilized Nell-1
in a rat spinal fusion model. Tissue Eng Part A. 16:2861–2870.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hu J, Hou Y, Park H and Lee M:
Beta-tricalcium phosphate particles as a controlled release carrier
of osteogenic proteins for bone tissue engineering. J Biomed Mater
Res A. 100:1680–1686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang Y, Dong R, Park Y, Bohner M, Zhang
X, Ting K, Soo C and Wu BM: Controlled release of NELL-1 protein
from chitosan/hydroxyapatite-modified TCP particles. Int J Pharm.
511:79–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
James AW, Chiang M, Asatrian G, Shen J,
Goyal R, Chung CG, Chang L, Shrestha S, Turner AS, Seim HB III, et
al: Vertebral Implantation of NELL-1 enhances bone formation in an
osteoporotic sheep model. Tissue Eng Part A. 22:840–849. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Song H, Zhang Y, Zhang Z, Xiong S, Ma X
and Li Y: Hydroxyapatite/NELL-1 Nanoparticles Electrospun Fibers
for Osteoinduction in Bone Tissue Engineering Application. Int J
Nanomedicine. 16:4321–4332. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lee M, Li W, Siu RK, Whang J, Zhang X, Soo
C, Ting K and Wu BM: Biomimetic apatite-coated alginate/chitosan
microparticles as osteogenic protein carriers. Biomaterials.
30:6094–6101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lee M, Siu RK, Ting K and Wu BM: Effect of
Nell-1 delivery on chondrocyte proliferation and cartilaginous
extracellular matrix deposition. Tissue Eng Part A. 16:1791–1800.
2010. View Article : Google Scholar
|
|
110
|
Zhu S, Zhang B, Man C, Ma Y and Hu J:
NEL-like molecule-1-modified bone marrow mesenchymal stem
cells/poly lactic-co-glycolic acid composite improves repair of
large osteochondral defects in mandibular condyle. Osteoarthritis
Cartilage. 19:743–750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Siu RK, Lu SS, Li W, Whang J, McNeill G,
Zhang X, Wu BM, Turner AS, Seim HB III, Hoang P, et al: Nell-1
protein promotes bone formation in a sheep spinal fusion model.
Tissue Eng Part A. 17:1123–1135. 2011. View Article : Google Scholar :
|
|
112
|
Yuan W, James AW, Asatrian G, Shen J, Zara
JN, Tian HJ, Siu RK, Zhang X, Wang JC and Dong J: NELL-1 based
demineralized bone graft promotes rat spine fusion as compared to
commercially available BMP-2 product. J Orthop Sci. 18:646–657.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Li W, Zara JN, Siu RK, Lee M, Aghaloo T,
Zhang X, Wu BM, Gertzman AA, Ting K and Soo C: Nell-1 enhances bone
regeneration in a rat critical-sized femoral segmental defect
model. Plast Reconstr Surg. 127:580–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Shen J, LaChaud G, Shrestha S, Asatrian G,
Zhang X, Dry SM, Soo C, Ting K and James AW: NELL-1 expression in
tumors of cartilage. J Orthop. 12(Suppl 2): S223–S229. 2015.
View Article : Google Scholar
|
|
115
|
Shen J, LaChaud G, Khadarian K, Shrestha
S, Zhang X, Soo C, Ting K, Dry SM and James AW: NELL-1 expression
in benign and malignant bone tumors. Biochem Biophys Res Commun.
460:368–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chen F, Walder B, James AW, Soofer DE, Soo
C, Ting K and Zhang X: NELL-1-dependent mineralisation of Saos-2
human osteosarcoma cells is mediated via c-Jun N-terminal kinase
pathway activation. Int Orthop. 36:2181–2187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Duan C and Townley HE: Isolation of NELL 1
aptamers for rhabdomyosarcoma targeting. Bioengineering (Basel).
9:1742022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Song D, Wang Q, Yan Z, Su M, Zhang H, Shi
L, Fan Y, Zhang Q, Yang H, Zhang D and Liu Q: METTL3 promotes the
progression of osteosarcoma through the N6-methyladenosine
modification of MCAM via IGF2BP1. Biol Direct. 19:442024.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Shen J, LaChaud G, Khadarian K, Shrestha
S, Zhang X, Soo C, Ting K, Dry SM and James AW: NELL-1 expression
in benign and malignant bone tumors. Biochem Biophys Res Commun.
460:368–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ren S, Pan R and Wang Z: Multi-omics and
single cell sequencing analyses reveal associations of
mitophagy-related genes predicting clinical prognosis and immune
infiltration characteristics in osteosarcoma. Mol Biotechnol. Sep
12–2024.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kular J, Tickner J, Chim SM and Xu J: An
overview of the regulation of bone remodelling at the cellular
level. Clin Biochem. 45:863–873. 2012. View Article : Google Scholar : PubMed/NCBI
|