Introduction
Bones are specialised tissues in the human body,
serving not only to protect internal organs, but also to provide
essential mechanical support, store minerals and produce blood
cells through the process of haematopoiesis (1). Fractures are the most frequent type
of traumatic injury and tissue damage, and bones can be restored to
their pre-injury state via a process of regeneration. However, ~10%
of fractures lead to delayed healing or non-union (2). The process of fracture healing
includes several distinct phases, such as inflammation,
angiogenesis, cartilage formation, resorption and bone remodelling,
as well as several cells, including macrophages, stem cells,
osteoblasts and osteoclasts (3,4).
Multiple approaches, such as specific biophysical, local and
systemic therapies, aim to promote skeletal repair, and the most
widely applied strategy is to promote new bone formation (3). For example, the two most
extensively studied therapies for promoting fracture healing use
bone morphogenetic proteins (BMPs), such as BMP2, and parathyroid
hormones (PTHs), such as PTH (1-34), both of which promote new bone
formation; however, these strategies still have some limitations
(3). Even though both fracture
healing and endochondral bone formation have been proven to be
directly mediated by BMPs, such as BMP2 (5), it has been reported that BMPs have
a lack of effect on shortening the time to fracture union and also
on returning bones to their normal function (6).
Therefore, it is important to identify new drugs
that can enhance fracture healing with greater effectiveness,
improved safety and fewer side effects. Recently, there has been
growing interest in naturally occurring compounds that are abundant
in food and plants; in particular, anthocyanins (ACNs) have been
reported to serve a role in bone health, which has led to increased
attention towards the potential application of these compounds
(7). ACNs are water-soluble
natural pigments that are prominent in coloured plants and belong
to the family of flavonoid compounds (8). Several studies have demonstrated
that numerous ACNs can benefit bone homeostasis by promoting the
proliferation and differentiation of pre-osteoblasts, and enhancing
the maturity of osteoblasts (7).
For example, black rice extracts (9) have been shown to promote
osteogenesis via Wnt and TGF-β/BMP pathways, and
delphinidin-3-rutinoside has been reported to regulate the
differentiation and proliferation of osteoblasts through the
PI3K/AKT pathway (7,10).
Enocyanin (ENO) is a type of ACN extracted from
grape skins, and it exerts inhibitory effects on leucine
aminopeptidase, acid phosphatase, γ-glutamyl transpeptidase and
esterase activity (11). In
addition, it has been shown to possess dose-dependent
anti-inflammatory activity in a R3/1-NF-κB luciferase cell model
(12). Polyphenols are a class
of ubiquitous compounds distributed in nature, with inherent
biocompatible, bio-adhesive, antioxidant and antibacterial
properties. However, the role of ENO in bone regeneration and
fracture healing have yet to be determined.
The present study aimed to investigate the potential
role of ENO in promoting osteogenic differentiation in vitro
and bone regeneration in vivo, thereby exploring ENO as a
potential candidate for bone fracture management. Furthermore, the
underlying mechanism of ENO in osteogenesis was investigated based
on mRNA-sequencing analysis.
Materials and methods
Osteogenic induction
The multi-potential bone marrow stromal cell line
KusaO was obtained from Dr Julian W Quinn (The University of
Melbourne, Melbourne, Australia) and was used for the evaluation of
osteogenesis in vitro (13). Osteogenic induction was performed
as previously described (14).
Specifically, cells were cultured at 37°C and 5% CO2 in
osteogenic medium, which consisted of low glucose (LG)-DMEM (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% foetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 nM
dexamethasone (MilliporeSigma), 50 μM ascorbic acid
(MilliporeSigma) and 20 mM β-glycerophosphate (MilliporeSigma).
Cells were also cultured in complete medium, which comprised
LG-DMEM containing 10% FBS, as a control.
ENO treatment
ENO (CAS no. 11029-12-2; MedChemExpress) at
different concentrations (7.5, 15, 30 and 60 μM) was added
to the osteogenic medium to evaluate the effect of ENO on the
osteogenic differentiation of KusaO cells in vitro. The
cells were treated for 24, 48 or 72 h to assess cell proliferation,
3 days for reverse transcription-quantitative PCR (RT-qPCR) and
alkaline phosphatase (ALP) staining, 7 days for western blotting,
and 14 days for alizarin red S (ARS) staining. The medium was
replaced every 3 days during the treatment period. The changes in
osteoblastic genes were detected using RT-qPCR, and ALP staining
was used to assess the osteogenic differentiation of KusaO cells.
Western blotting was used to evaluate the protein expression of
osteoblastic markers. ARS staining was used to assess the
mineralization of KusaO cells.
Cell proliferation
Cell proliferation was assessed using the CellTiter
96® AQueous One Solution Cell Proliferation Assay (MTS)
(Promega Corporation) according to the manufacturer's protocol. The
cells were seeded at a density of 5×103 cells/well in
96-well plates. After a 24-h incubation, the cells were treated
with ENO at various concentrations (7.5, 15, 30 and 60 μM)
for 24, 48 and 72 h at 37°C and 5% CO2. Subsequently, 10
μl MTS was added to each well and incubated at 37°C with 5%
CO2 for 4 h. The absorbance was measured at 490 nm using
a Bio-Rad 680 microplate reader (Bio-Rad Laboratories, Inc.) and
cell proliferation was expressed as a percentage of the control
culture value, which was considered 100% viable.
ALP assay
Cells were cultured in osteogenic medium with or
without various concentrations of ENO (7.5, 15, 30 and 60
μM) and incubated for 3 days at 37°C and 5% CO2.
Subsequently, the Alkaline Phosphatase Detection Kit
(MilliporeSigma) was used, according to the manufacturer's
protocol. Briefly, cells were fixed in 95% ethanol for 10 min at
room temperature and were subsequently incubated with ALP regent at
37°C for another 10 min. After rinsing with PBS; ight microscopy
(Nikon Corporation) was used to capture images of the cells with
purple ALP staining. To semi-quantify positive ALP staining, Fiji
ImageJ2 software was used (15).
ARS staining
Cells were cultured in osteogenic medium containing
different concentrations of ENO (0, 7.5, 15, 30 and 60 μM)
for 14 days at 37°C and 5% CO2. After fixing the cells
with 95% ethanol for 10 min at room temperature, they were stained
with 0.5% ARS (cat. no. A5533; MilliporeSigma) solution (pH 4.2)
for 10 min at room temperature to visualize mineral deposits, which
appeared as red-stained areas. Images were captured using a UMAX
Scanner (PowerLook 2100XL-USB; VueScan) and a light microscope
(Nikon Corporation).
Recombinant MMP9 protein treatment
Cells were cultured in osteogenic medium containing
30 μM ENO, and MMP9 protein (cat. no. 909-MM-010; R&D
Systems, Inc.) was added at different concentrations (100 and 200
ng/ml) to observe whether the effects of ENO on osteogenesis could
be reversed by MMP9. The cells were treated for 3, 7 or 14 days at
37°C and 5% CO2, and the cells were then collected for
further RT-qPCR after 3 days, western blotting after 7 days and ARS
staining after 14 days to examine changes in osteoblastic genes and
proteins, as well as the mineralization of cells.
RT-qPCR
Cells were plated in 24-well plates and were
cultured in osteogenic medium containing different concentrations
of ENO (0, 15, 30 and 60 μM) for 3 days at 37°C and 5%
CO2, with 6 replicates for each concentration (n=6).
RT-qPCR was performed to detect the changes in the expression
levels of osteoblastic genes after ENO treatment, and the
experiment was performed as described in our previous study
(14). Briefly, total RNA was
extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.), and cDNA was synthesized with PrimeScript RT
Master Mix (cat. no. RR036B; Takara Biotechnology, Ltd.), according
to the manufacturers' protocols. The cDNA then underwent qPCR with
Power UP SYBR Green Master Mix (cat. no. A25742, Thermo Fisher
Scientific, Inc.) on an Analytik Jena qTOWER (Analytik Jena GmbH).
The amplification conditions for qPCR were as follow: 50°C for 2
min and 95°C for 5 min, followed by 40 cycles at 95°C for 15 sec
and 60°C for 1 min, with a melt curve stage of 95°C for 15 sec,
60°C for 1 min and 95°C for 15 sec. The relative mRNA expression
levels of all genes were normalized to the housekeeping gene Gapdh
and were calculated using the 2−ΔΔCq method (16). The primers used were listed in
Table I and Mmp9 primers were
purchased from Sino Biological, Inc. (cat. no. MP200552).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer | Sequence |
|---|
| Gapdh | Forward
5′-AGGTCGGTGTGAACGGATTTG-3′ |
| Reverse
5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
| Bglap | Forward
5′-TGCTTGTGACGAGCTATCAG-3′ |
| Reverse
5′-GAGGACAGGGAGGATCAAGT-3′ |
| Alpl | Forward
5′-CCAACTCTTTTGTGCCAGAGA-3′ |
| Reverse
5′-GGCTACATTGGTGTTGAGCTTTT-3′ |
| Spp1 | Forward
5′-AGCAAGAAACTCTTCCAAGCAA-3′ |
| Reverse
5′-GTGAGATTCGTCAGATTCATCCG-3′ |
| Bsp | Forward
5′-ATGGAGACGGCGATAGTTCC-3′ |
| Reverse
5′-CTAGCTGTTACACCCGAGAGT-3′ |
| Runx2 | Forward
5′-ATGGGACTGTGGTTACCGTCAT-3′ |
| Reverse
5′-AAGGTGAAACTCTTGCCTCGT-3′ |
| Sp7 | Forward
5′-AGCGACCACTTGAGCAAACAT-3′ |
| Reverse
5′-GCGGCTGATTGGCTTCTTCT-3′ |
Western blotting
For western blotting, proteins were extracted from
cells with or without treatment using RIPA lysis and extraction
buffer (Thermo Fisher Scientific, Inc.) with Halt Protease
Inhibitor Cocktail (100X; Thermo Fisher Scientific, Inc.). The
concentration of extracted proteins was determined using the BCA
Protein Assay (Thermo Fisher Scientific, Inc.), and 20 μg
proteins were separated by SDS-PAGE on 10% gels made using TGX
FastCast acrylamide kits (Bio-Rad Laboratories, Inc.). The proteins
were run alongside a molecular weight marker (Pierce; Thermo Fisher
Scientific, Inc.) for 1.5 h at 100 V. The proteins were then
transferred from the gel to PVDF membranes using the Trans-Blot
Turbo Transfer System (Bio-Rad Laboratories, Inc.). The membranes
were incubated in 5% non-fat milk in TBS-1% Tween for 1 h at room
temperature and then incubated overnight at 4°C with primary
antibodies against Runx2 (1:1,000; cat. no. 12556; Cell Signaling
Technology, Inc.), osteopontin (OPN; 1:1,000; cat. no. ab63856;
Abcam) MMP9 (1:500; cat. no. 10375-2-AP; Wuhan Sanying
Biotechnology) and β-actin (1:10,000; cat. no. ab32572; Abcam). The
next day, the membranes were incubated with HRP-conjugated
anti-rabbit and anti-mouse secondary antibodies (1:3,000; cat. nos.
7074 and 7076; Cell Signaling Technology, Inc.) for 1 h at room
temperature. For signal development, BeyoECL Plus reagent (Beyotime
Institute of Biotechnology) was used according to the
manufacturer's recommendations. Images were acquired in the dark
using the ChemiDoc XRS Imaging System (Bio-Rad Laboratories, Inc.)
and protein expression levels were analysed using Image Lab 5.2.1
software (Bio-Rad Laboratories, Inc.).
RNA-sequencing
Total RNA was extracted from KusaO cells with or
without 30 μM ENO treatment under 3-day osteogenic induction
using TRIzol. Purity was assessed using a NanoDrop (OD260/280 and
OD260/230 ratios; NanoDrop; Thermo Fisher Scientific, Inc.) and
integrity was evaluated using an Agilent 4200 TapeStation (Agilent
Technologies, Inc.). Libraries were prepared by Chengqi
Biotechnology, which involved mRNA isolation, fragmentation and
cDNA synthesis. Library quality was assessed using an Agilent 4200
TapeStation. Sequencing was performed on a NovaSeq 6000 platform
(Illumina, Inc.) using NovaSeq6000 S4 Reagent kit v1.5 (cat. no.
20028312; Illumina, Inc.), generating 150 bp strand-specific
paired-end reads. The loading concentration of the final library
was 100 pM.
Bioinformatics analysis
Bioinformatics analysis was carried out by Chengqi
Biotechnology. Briefly, raw sequencing reads underwent quality
control using FastQC (v0.11.9, https://www.bioinformatics.babraham.ac.uk/projects/fastqc/)
and trimming with Trimmomatic (v0.36, http://www.usadellab.org/cms/?page=trimmomatic). Clean
reads were aligned to HISAT2 (2.2.0, https://daehwankimlab.github.io/hisat2/).
FeatureCounts (v2.0.4, https://subread.sourceforge.net/featureCounts.html)
was used to count the read numbers mapped to each gene.
Differential expression analysis was conducted with edgeR (v3.40.2,
https://bioconductor.org/packages/release/bioc/html/edgeR.html),
identifying differentially expressed genes (DEGs) based on a |log2
fold change| >2 and adjusted P<0.05. Functional enrichment
analysis of DEGs was subsequently performed. ClusterProfiler R
(v4.6.2, https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html)
was used to test the statistical enrichment of DEGs in Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathways. Gene Set
Enrichment Analysis (GSEA) can be used to determine whether a
predefined set of genes exhibits statistically significant
differences between control and ENO groups. The local version of
the GSEA tool was used in the present study (http://www.broadinstitute.org/gsea/index.jsp),
and KEGG datasets were used for GSEA.
Femur fracture models
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Ruiye Bio-tech
Guangzhou Co., Ltd. (approval no. RYEth-20210716213), and were
carried out in accordance with The Code of Ethics of the World
Medical Association (17). The
animal experiments were performed at the animal laboratory of Ruiye
Bio-tech Guangzhou Co., Ltd. by the authors. Animals were
maintained in a standard room at 22±2°C with 40-60% humidity, under
a 12-h light/dark cycle, with ad libitum access to food and
water according to the institutional animal guidelines. A total of
12 female Sprague-Dawley rats (weight, 200-250 g) underwent open
femoral fracture surgery as previously reported (18). Rats were anesthetized with an
intraperitoneal injection of 0.6% pentobarbital sodium (40 mg/kg).
Subsequently, the femurs were exposed and cut down the middle to
construct femur fracture models. The fractures were then internally
fixed using intramedullary 1.2-mm Kirschner wires. After surgery,
the rats were housed in individual cages with soft bedding, and
easy access to food and water. To minimize the pain of surgery,
buprenorphine (0.05 mg/kg) was administered subcutaneously every
8-12 h for 48-72 h post-surgery (17). (The rats were randomly divided
into three groups (n=4/group): Saline group, 30 mg/kg ENO group and
60 mg/kg ENO group. After 3 days of recovery from the operation,
ENO was injected locally at the fracture sites every 3 days for 6
weeks, with different concentrations used for each group. The
Saline group of rats received saline injections as a control.
Stereomicroscopy and micro-CT
analysis
A total of 6 weeks after the operation, all rats
were sacrificed by an overdose of pentobarbital sodium (0.6%)
anaesthesia at a dose of 300 mg/kg by intraperitoneal injection.
Death was confirmed by the cessation of heartbeat and respiratory
movements, along with the absence of reflexes. Fractured femurs
were collected for stereomicroscopy (SMZ25; Nikon Corporation) and
were fixed for micro-CT analysis. Femurs were fixed in 10%
neutral-buffered formalin solution for 3 days at room temperature.
To assess the conditions of bone regeneration, the femurs were
scanned using the Skyscan 1176 μCT scanner (Bruker
Corporation). Reconstructive images were generated using NRecon
v1.6 software (Bruker Corporation) and were then analysed by CTAn
v1.9 (Bruker Corporation). Two-and three-dimensional images were
generated by Data-viewer and CTvox software (Bruker Corporation),
respectively. For bone fracture healing analysis, the defect size
of the cortical bone was selected. The bone mass of each group was
analysed using CTAn v1.9. The parameters measured included bone
volume/tissue volume (BV/TV) and bone mass density (BMD).
Histological staining
After micro-CT scanning, all femurs from the rats
were decalcified in 10% EDTA at 37°C for 10 days followed by 1
month in 10% EDTA. Subsequently, the femurs were processed for
paraffin embedding as previously described (19). For further staining, 5-μm
sections were utilized. Haematoxylin and eosin (H&E) and
Safranin O/Fast green were applied to evaluate the healing of femur
fractures as described previously (19), which included bone regeneration
and the expression of proteoglycan at the callus bone areas. Images
were captured using a stereomicroscope (SMZ25; Nikon Corporation)
and a light microscope (Nikon Corporation).
Immunohistochemistry was applied using a Mouse and
Rabbit specific HRP/DAB detection IHC kit (cat. no. SAP-9100;
OriGene Technologies, Inc.) as described previously (19). Sections were treated according to
the manufacturer's protocol, including incubation with primary
antibodies overnight at 4°C. The primary antibodies included OPN
(1:200; cat. no. ab63856; Abcam), Collagen I (1:200; cat. no.
ab6308; Abcam), Collagen II (1:200; cat. no. 32160702;
MilliporeSigma), osteocalcin (OCN; 1:200; cat. no. ab133612; Abcam)
and MMP9 (1:200; cat. no. 10375-2-AP; Wuhan Sanying Biotechnology).
Images were captured under a light microscope (Nikon Corporation)
and were analysed using Fiji ImageJ2 software.
Statistical analysis
Data are presented as the mean ± SD. All data
analyses were performed using GraphPad Prism 10.0 (Dotmatics) by
one-way ANOVA followed by Dunnett's multiple comparisons test. At
least three independent experiments were performed. P≤0.05 was
considered to indicate a statistically significant difference.
Results
ENO enhances proliferation and
osteogenesis of KusaO cells in vitro
To investigate the potential impact of ENO on
osteoblast differentiation of mesenchymal stem cells (MSCs), KusaO
cells, which have multi-differentiation abilities in vitro
were employed and cultured in either complete medium or osteogenic
medium supplemented with different concentrations of ENO (0, 7.5,
15, 30 and 60 μM). To determine whether ENO affected the
proliferation of KusaO cells, an MTS assay was used, and the
results showed that ENO had no significant effect after 24, 48 or
72 h of treatment (Fig. 1A). ALP
is recognized as an important early marker of osteoblast
differentiation and was measured to assess the effects of ENO on
early osteogenesis. The results showed the levels of ALP were
significantly increased in a dose-dependent manner after KusaO
cells were treated with different concentrations of ENO (Fig. 1B and C). Additionally, the
mineralization of KusaO cells was observed through ARS staining,
and the results indicated that ENO significantly increased the
mineral deposits of KusaO cells in a dose-dependent manner
(Fig. 1D and E). Consistently,
RT-qPCR results demonstrated that osteoblastic genes, including
Runx2, Alpl, Spp1, Bglap, Bsp and Sp7, were markedly upregulated by
30 μM ENO (Fig. 1F).
Western blotting further confirmed that only 30 μM ENO
promoted the expression levels of the osteoblastic proteins, Runx2
and OPN, which was consistent with the gene expression findings
(Fig. 1G and H). In summary, ENO
promoted the osteogenic differentiation of KusaO cells in
vitro, with the best effects observed in response to 30
μM ENO.
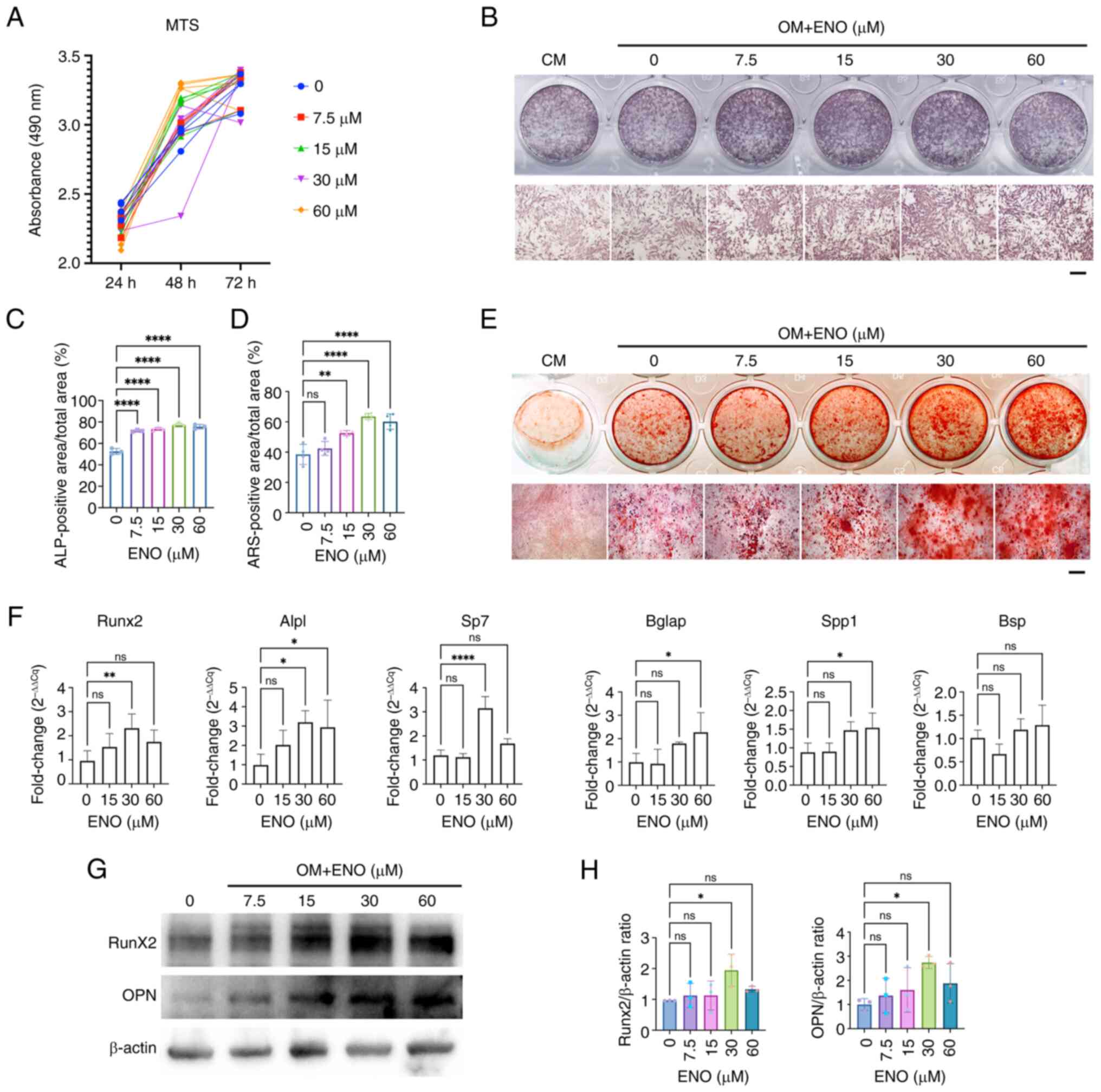 | Figure 1ENO promotes the osteogenesis of
KusaO cells in vitro. (A) MTS assay of the proliferation of
KusaO cells cultured in CM supplemented with different doses of ENO
after 24, 48 or 72 h (n=6). (B) ALP staining of KusaO cells after
treatment with ENO in CM or OM (n=3). Scale bar, 200 μm. (C)
Positive percentage of ALP staining of KusaO cells in each well was
semi-quantified by Fiji ImageJ2 (n=3). (D) Percentage of calcium
nodules in KusaO cells was semi-quantified after ARS staining
(n=4). (E) ARS staining of KusaO cell mineralization after 14 days
of osteogenic induction (n=4). Scale bar, 200 μm. Scale bar,
200 μm. (F) Reverse transcription-quantitative PCR analysis
of the mRNA expression levels of osteoblastic genes, Alpl, Spp1,
Bglap, Runx2, Sp7 and Bsp, in KusaO cells cultured in OM
supplemented with different doses of ENO (n=4). The expression
levels of genes were normalized to Gapdh and were calculated by
2−ΔΔCq. (G) Western blot analysis of the expression of
osteoblastic proteins, Runx2 and OPN (n=3). (H) Runx2 and OPN
expression levels normalized to β-actin were assessed using Image
Lab software (n=3). Data are presented as the mean ± SD.
*P< 0.05, **P <0.01, ****P
<0.0001. ALP, alkaline phosphatase; ARS, Alizarin red S; CM,
complete medium; ENO, enocyanin; ns, not significant; OM,
osteogenic medium; OPN, osteopontin. |
ENO promotes bone regeneration in a rat
model of femur fracture
Based on the in vitro effects of ENO on
promoting the osteoblastic differentiation of KusaO cells, the
present study further explored its effects on bone regeneration in
a rat model of fracture healing in vivo. After 7 days of
post-surgery recovery, 30 and 60 mg/kg ENO were injected into the
fracture sites. Subsequently, stereomicroscopy, micro-CT and
histological staining were applied to detect new bone formation
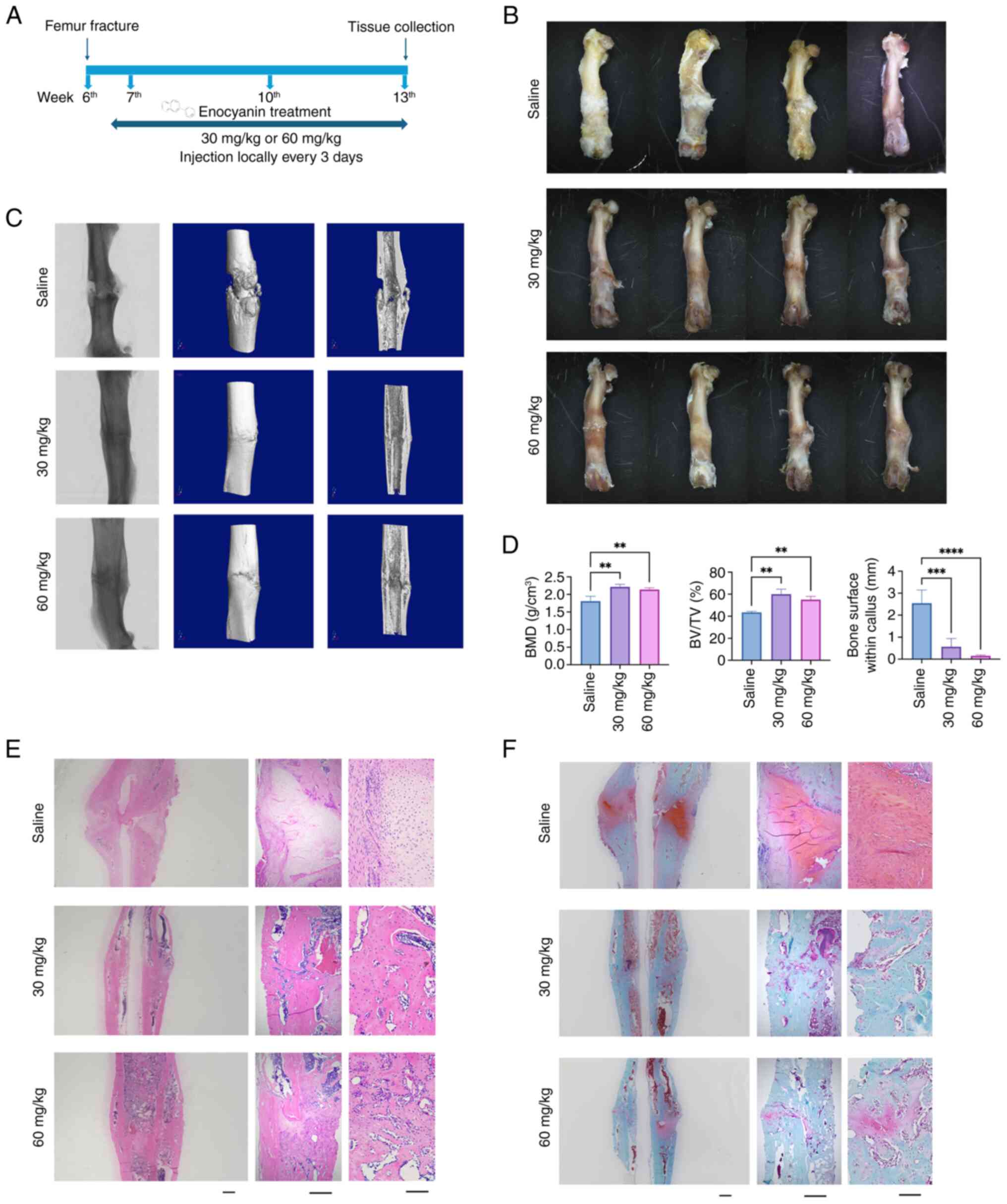
after ENO treatment (Fig. 2A).
The stereomicroscope detected bone callus formation at the fracture
sites, with little callus formation observed in both ENO groups,
whereas more callus formation was found in the Saline group
(Fig. 2B). Similarly, the
micro-CT showed that the size of bone calluses in the ENO treatment
groups was much less than that in the Saline group (Fig. 2D). In addition, non-union was
detected in the Saline group, whereas integration at the fracture
site was markedly better after ENO treatment (Fig. 2C). Furthermore, bone parameters,
including BMD and BV/TV, were significantly increased following ENO
treatment, with the best results observed in the 30 mg/kg ENO group
(Fig. 2D), suggesting that ENO
induced more new bone formation compared with the Saline group.
Consistently, H&E staining showed more bone formation in the
ENO groups compared with that in the Saline group, and woven bone
formation was detected in the ENO groups, indicating faster and
earlier bone remodelling after ENO treatment (Fig. 2E). Fracture healing is considered
the process of endochondral formation, which was measured by
Safranin O/Fast Green staining (Fig.
2F); The present results indicated a notable amount of
proteoglycan (red), and oval or round chondrocyte-like cells at the
bone callus sites in the Saline group, whereas little could be seen
in the ENO groups, suggesting that the process of endochondral
formation was accelerated by ENO. Notably, 30 mg/kg ENO exhibited
the best effects on promoting cartilage resorption and new bone
formation.
ENO promotes the expression of
osteoblastic proteins at the callus sites of the femur in vivo
Immunohistochemical staining was used to evaluate
the expression of osteoblast-related proteins at the callus sites
of the femur (Fig. 3). The
results showed that ENO not only increased the expression of OCN
(Fig. 3A and E), which is a
marker of late osteoblastic differentiation, and OPN (Fig. 3B and F), but also decreased
collagen II expression (Fig. 3D and
H) at the fracture sites. In addition, 60 mg/kg ENO
significantly promoted collagen I expression (Fig. 3C and G). Chondrocytes could be
seen in the Collagen II-positive areas in the Saline group (black
arrows) (Fig. 3D). These
findings are consistent with the results of histological staining,
which showed reduced cartilage and increased new bone formation
after ENO treatment.
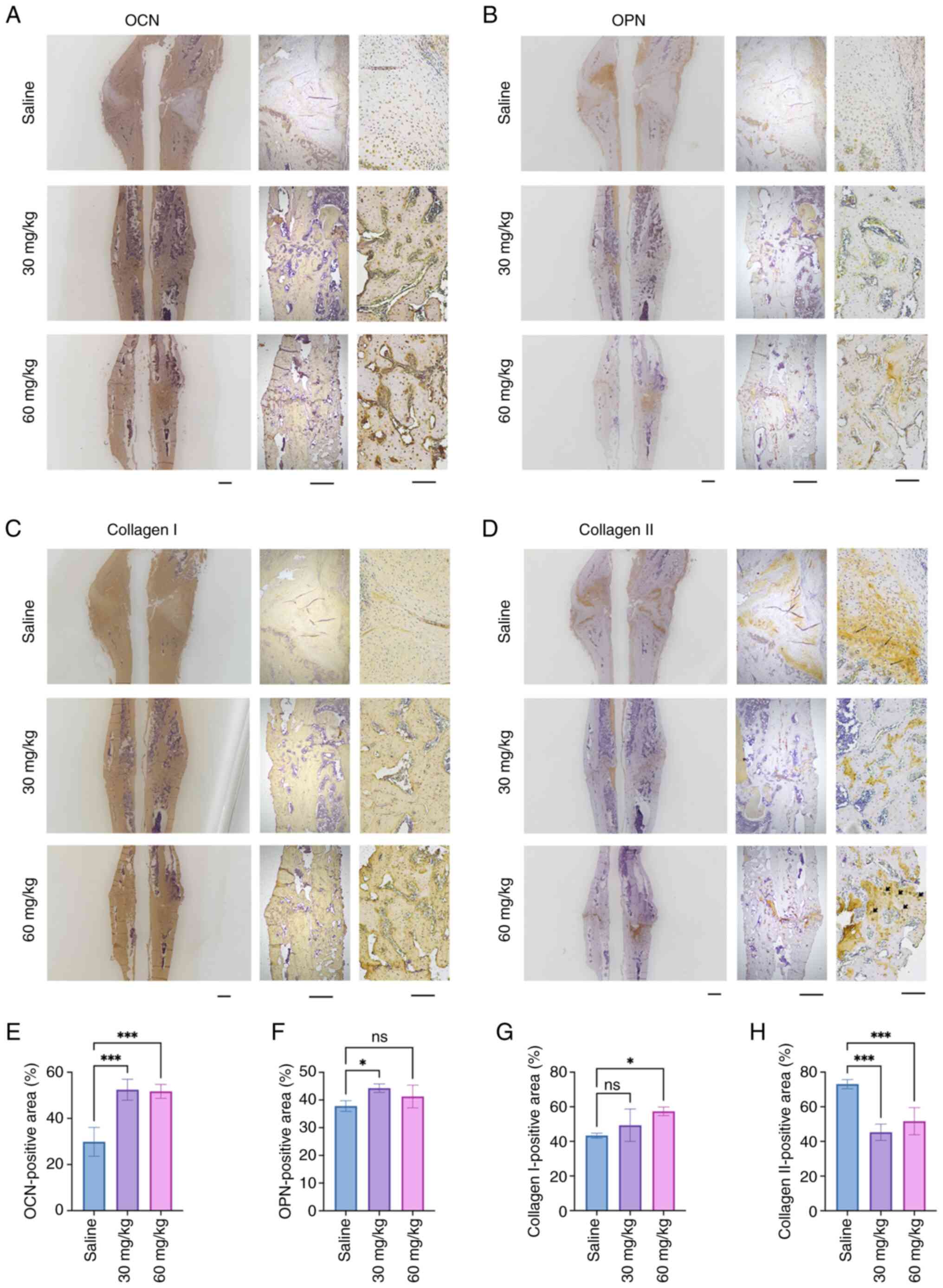 | Figure 3ENO promotes the expression of
osteoblastic proteins at the callus sites of the femur in
vivo. Immunohistochemical analysis was conducted on femur
specimens to evaluate the osteogenic effects of ENO. Positive
expression of (A) OCN, (B) OPN, (C) Collagen I and (D) Collagen II
at the callus sites of the femur with or without ENO treatment
(n=4). Scale bars, 2,000 μm (left), 500 μm (middle)
and 100 μm (right). Semi-quantification of the positive
staining of (E) OCN, (F) OPN, (G) Collagen I and (H) Collagen II
using Fiji ImageJ2 software (n=4). Data are presented as the mean ±
SD. *P< 0.05, *** P< 0.001. ENO,
enocyanin; ns, not significant; OCN, osteocalcin; OPN,
osteopontin. |
ENO alters gene expression profiles in
KusaO cells induced by osteogenic medium
To determine the underlying mechanisms governing the
effects of ENO on MSC osteogenesis, RNA-sequencing was performed.
Total RNA was isolated from cells with or without ENO treatment and
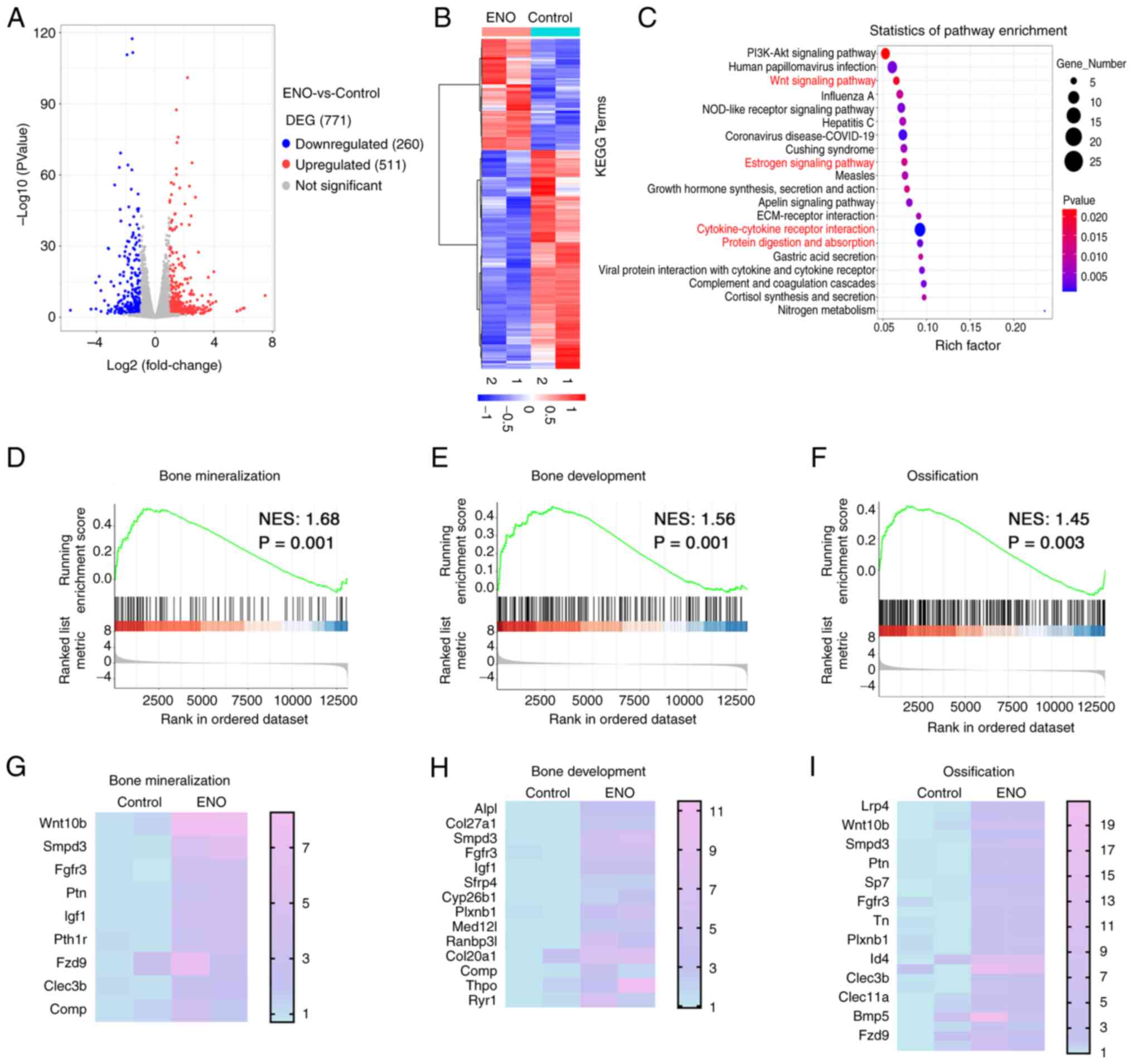
RNA-sequencing analysis was then performed. The results showed that
771 DEGs were identified, with 260 genes showing significant
downregulation and 511 genes showing upregulation after ENO
treatment compared with in the control group (Fig. 4A and B). KEGG analysis revealed
that the cells treated with ENO were enriched in the 'Wnt signaling
pathway' and 'cytokine-cytokine receptor interaction' pathways,
which are classic signalling pathways associated with osteogenesis
(20,21) (Fig. 4C). The 'estrogen signalling
pathway', which is highly related to osteoporosis, was also
involved in the role of ENO. These results highlight the role of
ENO in osteogenesis. In addition, 'protein digestion and
absorption' was involved in the effect of ENO on KusaO cells
induced by osteogenic factors (Fig.
4C), indicating that ENO was involved in regulating matrix
degradation. To further identify the gene sets that are related
with osteogenesis with or without ENO treatment, GSEA was
performed. ENO-induced genes were enriched in 'bone mineralization'
(Fig. 4D and E), 'bone
development' (Fig. 4F and G) and
'ossification' (Fig. 4H and I),
indicating that ENO exerted effects that are highly associated with
osteogenesis and bone formation. Collectively, these RNA-sequencing
data showed that ENO may be highly associated with osteogenic
differentiation and bone formation.
ENO inhibits MMP9 in osteogenesis in
vitro and bone formation in vivo
Since the KEGG pathway analysis showed that 'protein
digestion and absorption' was involved in the role of ENO in
osteogenesis, proteinase-related genes were selected from the DEGs.
Among these genes, Mmp9 was downregulated and Prss35 was
upregulated by ENO treatment (Fig.
5A). Mmp9 is known to be associated with endochondral
ossification (22), while there
is little evidence to support its involvement in osteogenic
differentiation. However, there are few reports on Prss35 in bone
homeostasis. As such, the present study further examined the mRNA
and protein expression levels of MMP9 after ENO treatment in
vitro and in vivo. The results revealed that, in
vitro, both the mRNA and protein expression levels of MMP9 were
decreased after ENO treatment in a dose-dependent manner (Fig. 5B-D). Similarly, in vivo,
the expression of MMP9 at callus sites was significantly decreased
by ENO treatment (Fig. 5E). In
the Saline group, MMP9 was strongly expressed at the callus sites,
whereas much less MMP9-positive staining could be detected in the
ENO groups.
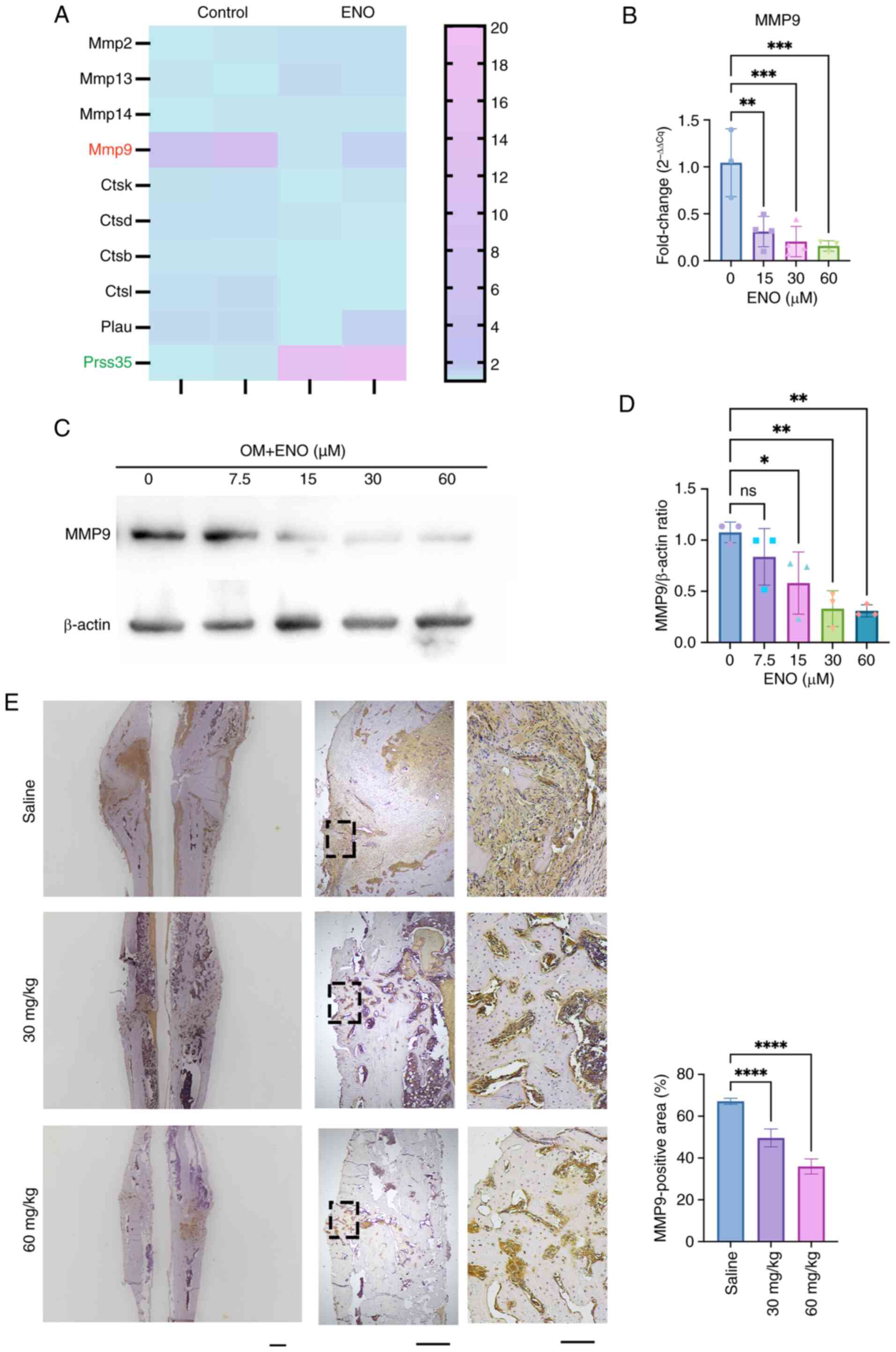 | Figure 5ENO inhibits MMP9 in osteogenesis
in vitro and bone formation in vivo. (A) Heatmap of
the differences in the expression of proteinase-related genes in
cells with or without ENO treatment in osteogenic medium (n=2). (B)
Reverse transcription-quantitative PCR analysis of the mRNA
expression levels of Mmp9 after ENO treatment (n=4). (C) Western
blot analysis showed that the protein levels of MMP9 decreased
after ENO treatment in a dose-dependent manner (n=3). (D) Relative
semi-quantification of the expression of MMP9 normalized to β-actin
as determined by Image Lab (n=3). (E) Immunohistochemical staining
of MMP9 expression at the callus sites in the femur. Scale bars,
2,000 μm (left), 500 μm (middle) and 100 μm
(right). Semi-quantification of the positive staining of MMP9 was
performed using ImageJ software (n=4). *P<0.05,
**P<0.01, ***P <0.001, ****P
<0.0001 (n=3). ENO, enocyanin; MMP9, matrix metalloproteinase 9;
ns, not significant; OM, osteogenic medium. |
Recombinant MMP9 protein reverses the
role of ENO in the osteogenesis of KusaO cells
Based on the findings of RNA-sequencing analysis and
confirmation that MMP9 was decreased after ENO treatment, the
present study further explored whether MMP9 protein treatment could
reverse the role of ENO in the osteogenic differentiation of KusaO
cells. The results showed that ENO increased the mineral deposits
of KusaO cells, which was consistent with the aforementioned
results; however, recombinant MMP9 protein treatment attenuated
their mineralization in the presence of ENO (Fig. 6A and 6B). Moreover, the mRNA expression
levels of several osteoblastic genes, including Alpl, Runx2, Spp1,
Sp7 and Bsp, were downregulated by MMP9 treatment, while there was
no significant difference in the mRNA expression levels of Bglap
between the groups with or without MMP9 treatment (Fig. 6C). Consistent results were
detected regarding the expression levels of osteoblast-related
proteins, including Runx2 and OPN, which were significantly
decreased by 200 ng/ml MMP9 treatment (Fig. 6D and E).
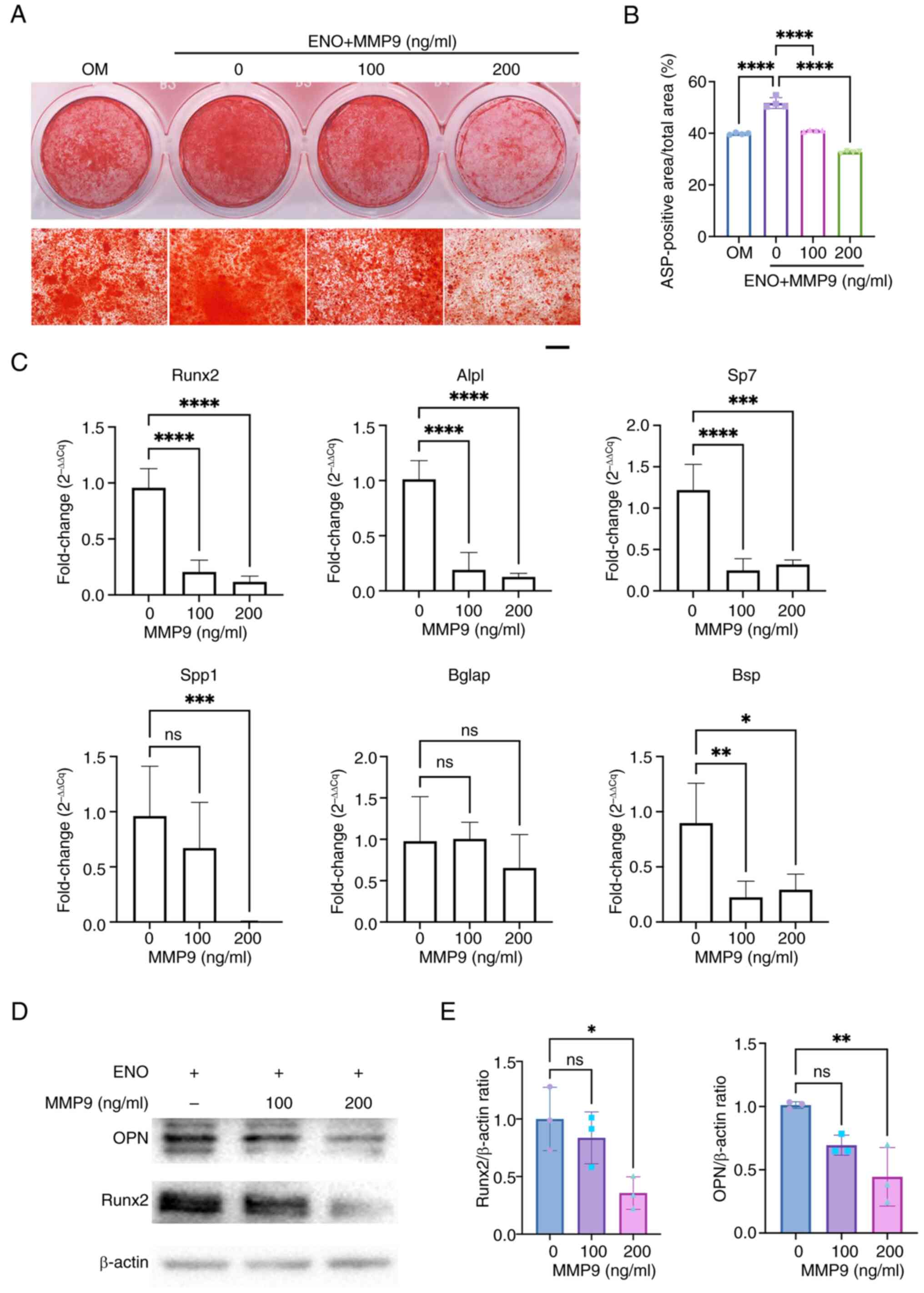 | Figure 6Recombinant MMP9 protein suppresses
the osteogenesis of KusaO cells in the presence of ENO. (A)
Mineralization after treatment with 30 μM ENO and MMP9
protein (n=4). Scale bar, 500 μm. (B) Percentage of calcium
nodules was assessed by Alizarin red S staining and was
semi-quantified (n=4). (C) mRNA expression levels of several
osteoblastic genes, Alpl, Spp1, Sp7, Runx2, Bglap and Bsp, were
downregulated by MMP9 treatment (n=4). (D) Western blot analysis
showed that the expression of osteoblast-related proteins, Runx2
and OPN, was dose-dependently decreased by MMP9 treatment (n=3).
(E) Relative semi-quantification of the normalized expression of
OPN and Runx2 to β-actin was determined by Image Lab (n=3).
*P< 0.05, **P <0.01,
***P<0.001, ****P<0.0001. ENO,
enocyanin; MMP9, matrix metalloproteinase 9; ns, not significant;
OPN, osteopontin. |
Discussion
As the global population ages, osteoporosis has
emerged as a major health concern, which is a serious condition
that can cause bone fractures from even minor trauma, such as
coughing. Notably, drugs for osteoporosis, such as denosumab, have
been developed to improve bone mineral density and reduce
osteoporotic fractures by targeting osteoclasts; however, there are
only two therapeutic agents, teriparatide and abaloparatide, that
could promote osteoblastic lineage cells (23). Even though efforts have been made
in preventing the occurrence of fractures, there were still 178
million new fractures globally in 2019, which represents a 33.4%
increase since 1990 (24).
Furthermore, osteoporotic fractures are extremely common in
worldwide. For example, ~1.5 million individuals suffer from
fragility fractures each year in the USA, and one in two women and
one in five men aged >50 years will experience an osteoporotic
fracture in their lifetime (25). Osteoporosis is also known as a
'silent disease'; patients do not always notice the occurrence of
osteoporosis until a fracture happens. Therefore, prevention is
considered more important than cure. As such, naturally occurring
compounds have attracted increasing attention as a means of
preventing and treating osteoporosis, especially fractures.
In the present study, it was revealed that ENO,
which belongs to the ACN family, could accelerate osteoblastic
differentiation both on the cellular and molecular levels in
vitro. Furthermore, when rats were treated with ENO, improved
fracture healing was observed, with the complete union of fracture
sites and increased expression of osteoblast-specific proteins.
Based on RNA-sequencing data, it was demonstrated that the roles of
ENO in osteogenesis were highly related to Wnt signalling and MMP9
expression levels. Moreover, the present study demonstrated that
treatment with the recombinant MMP9 protein could reverse the
effects of ENO on osteogenesis. ENO is abundant in foods, such as
blackberries and black rice, and its potential role in bone
regeneration makes it a promising candidate for fracture healing as
a natural remedy (18,26).
ACNs are a class of naturally occurring compounds
known for their anti-inflammatory and anti-oxidative effects
(27,28). As individuals age, low-grade
inflammation and the production of reactive oxygen species
increases, leading to an imbalance in bone homeostasis (29). In the present study, it was
revealed that ENO promoted the mineralization, as well as the
expression levels of osteoblastic genes and proteins in
vitro, without affecting the proliferation of MSCs. This
finding is highly consistent with the effects of other ACNs, such
as delphinidin, malvidin and petunidin, which have been reported to
positively affect osteoblastic differentiation by upregulating the
expression of osteoblastic genes or promoting mineral deposits
in vitro (30,31). For example, malvidin induced a
significantly higher accumulation of calcium deposits in MSCs than
untreated MSCs, upregulated osteoblast-specific genes BMP-2 and
Runx-2 expression, and induced BMP-2 secretion (30). However, these previous studies
were conducted in vitro, and the in vivo effect of
ACNs on new bone formation remains unexplored.
Based on the effects of ENO on promoting
osteogenesis in vitro, the present study further explored
the roles of ENO in fracture healing in vivo. The results
showed that ENO significantly accelerated femur fracture healing,
as evidenced by improved union of fractures, increased bone mass
and expression of osteoblastic proteins. Furthermore, the process
of endochondral ossification, new bone formation and cartilage
tissue resorption at the callus sites are indicators of bone
remodelling during fracture healing, whereas the failure of
fracture healing, such as bone non-union, is often due to a lack of
initial bone remodelling (32).
In the present study, ENO was shown to significantly accelerate the
process of bone remodelling, as evidenced by the observation of
less proteoglycan and more trabecular bone at the callus sites.
Furthermore, the expression of osteoblastic and chondrogenic
proteins at the callus sites was highly consistent with the
aforementioned findings. Overall, these results suggested that ENO
had a positive impact on fracture healing and could be considered a
promising therapeutic agent for the treatment of bone injuries.
To explore the underlying mechanisms of ENO in
osteogenesis, RNA-sequencing was performed, which revealed a strong
association between ENO, and the 'Wnt signalling pathway' and
'cytokine-cytokine receptor interaction'; both of these are
important pathways involved in osteogenic differentiation (20,21). Activating the Wnt or BMP pathway
leads to increased bone mass and strength, making it a target for
therapeutic intervention in millions of patients at risk of
fractures (20,21). GSEA further confirmed that ENO
was highly associated with 'bone mineralization', 'bone
development' and 'ossification', all of which are known to be
involved in osteogenesis and bone formation. These analyses further
confirmed that ENO could be a promising candidate for bone
regeneration via promoting osteogenesis and new bone formation.
The present study also observed that the KEGG
pathway 'protein digestion and absorption' was involved in the
osteogenic role of ENO. The in vivo data showed that ENO
significantly accelerated the bone remodelling process, leading to
faster fracture healing. As is well-known, the healing of fractures
involves bone formation and bone resorption (32). The proteinases, including MMPs,
are a class of enzymes that serve a vital role in various
biological processes by cleaving and degrading proteins (33). There are several types of
proteinases involved in bone remodelling, including MMPs, serine
proteinases, cysteine proteinases and aspartic proteinases, such as
cathepsin K (34). Among all of
the proteinases known to be involved in bone metabolism and
remodelling, there were only two genes, Mmp9 and Prss35, that had
significant differences after ENO treatment. The present study
further confirmed that ENO inhibited the expression of MMP9 both
in vitro and in vivo. Furthermore, the addition of
exogenous MMP9 protein attenuated the ENO-mediated differentiation
and mineralization of KusaO cells. These results indicated that
MMP9 was involved in the role of ENO in bone remodelling.
MMP9 has a crucial role in regulating the
remodelling of skeletal tissues by coordinating matrix degradation,
and the recruitment and differentiation of osteoprogenitors
(35). However, contrary to
expectations, a previous study on Mmp9-knockout mice showed no
significant effects on adult bone mass (36). Mmp9 is highly associated with the
endochondral ossification process (37,38). Wang et al suggested that
in Mmp9−/− mice stabilized fractures were healed via
endochondral ossification, whereas in wild-type mice they were
healed via intramembranous ossification (37). In addition, in another study,
Mmp2 was shown to be involved in intramembranous ossification,
whereas Mmp9 could specifically impact the endochondral
ossification process (38,39). Therefore, ENO may facilitate the
process of cartilage formation by decreasing MMP9 expression, which
in turn could increase new bone formation.
Notably, Mmp9 is released by osteoclasts, suggesting
that it may participate in the intracellular communication between
osteoblasts and osteoclasts, which is crucial in bone remodelling
(40,41). Nevertheless, a previous study
indicated that Mmp9-null osteoclast fusion and function are largely
unaffected in vitro or in vivo (36), suggesting that the role of Mmp9
in bone may not specifically target osteoclasts. Given this, we
aimed to knockdown Mmp9 in KusaO cells, in order to further confirm
the role of MMP9 in osteogenesis; however, it was found that KusaO
cells without osteogenic induction did not express Mmp9 (data not
shown).
One limitation of the present study is that the role
of ENO was not explored in osteoporotic fractures, even though ENO
was revealed to be closely associated with Wnt signalling, protein
digestion and absorption pathways, and oestrogenic signalling,
which also have fundamental effects on osteoporotic fracture
healing. The reason why we did not explore the role of ENO in
osteoporotic fractures is because hyperactive osteoclasts serve a
crucial role under osteoporotic conditions, and we did not observe
significant effects of ENO on osteoclastogenesis (data not shown).
However, the effects of ENO on bone resorption of mature
osteoclasts have not yet been investigated, and thus further
experiments are needed in the future to explore this
possibility.
In conclusion, in the present study it was observed
that ENO had a positive effect on the osteoblastic differentiation
of MSCs in vitro, and it also promoted fracture healing of
the femur in vivo. The mechanisms involved in the role of
ENO in osteogenesis included the activation of Wnt signalling, and
the regulation of protein digestion and absorption pathways, all of
which have vital roles in bone remodelling. Additionally, the
present results suggested that Mmp9 might be a target of ENO in
promoting bone defect healing.
Availability of data and materials
The data generated in the present study may be found
in the NCBI public database under accession number PRJNA1138174 or
at the following URL: https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1138174.
The other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
SNQ, JKX and AGL contributed to the study design.
WM, YFZ, WCZ, JRY, ZYL, PLH, GDH, GWH, HC and JYL carried out
experiments. HC, GDH, WM, PLH, WCZ, ZYL and GWH contributed to data
collection and analysis. WM, SNQ and AGL contributed to the
drafting and revision of the manuscript. SNQ and AGL confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Ruiye Bio-tech
Guangzhou Co., Ltd. (approval no. RYEth-20210716213), and were
carried out in accordance with The Code of Ethics of the World
Medical Association.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
The authors would like to acknowledge the equipment
support from Professor Kathleen Davern, Dr Kevin Li and Dr Quang
Nguyen (Harry Perkins Institute of Medical Research), and Professor
Kathy Fuller (School of Biomedical Science, The University of
Western Australia).
Funding
This work was supported by the Guangzhou Science and Technology
Project (grant nos. 202002030049 and 2023A03 J0987) and the Oversea
Study Program of Guangzhou Elite Project to SQ.
References
|
1
|
Maksimkin AV, Senatov FS, Anisimova NY,
Kiselevskiy MV, Zalepugin DY, Chernyshova IV, Tilkunova NA and
Kaloshkin SD: Multilayer porous UHMWPE scaffolds for bone defects
replacement. Mater Sci Eng C Mater Biol Appl. 73:366–372. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ho-Shui-Ling A, Bolander J, Rustom LE,
Johnson AW, Luyten FP and Picart C: Bone regeneration strategies:
Engineered scaffolds, bioactive molecules and stem cells current
stage and future perspectives. Biomaterials. 180:143–162. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Einhorn TA and Gerstenfeld LC: Fracture
healing: Mechanisms and interventions. Nat Rev Rheumatol. 11:45–54.
2015. View Article : Google Scholar :
|
|
4
|
Murata K, Ito H, Yoshitomi H, Yamamoto K,
Fukuda A, Yoshikawa J, Furu M, Ishikawa M, Shibuya H and Matsuda S:
Inhibition of miR-92a enhances fracture healing via promoting
angiogenesis in a model of stabilized fracture in young mice. J
Bone Miner Res. 29:316–326. 2014. View Article : Google Scholar
|
|
5
|
Axelrad TW and Einhorn TA: Bone
morphogenetic proteins in orthopaedic surgery. Cytokine Growth
Factor Rev. 20:481–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aro HT, Govender S, Patel AD, Hernigou P,
Perera de Gregorio A, Popescu GI, Golden JD, Christensen J and
Valentin A: Recombinant human bone morphogenetic protein-2: a
randomized trial in open tibial fractures treated with reamed nail
fixation. J Bone Joint Surg Am. 93:801–808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mao W, Huang G, Chen H, Xu L, Qin S and Li
A: Research progress of the role of anthocyanins on bone
regeneration. Front Pharmacol. 12:7736602021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levis S and Lagari VS: The role of diet in
osteoporosis prevention and management. Curr Osteoporos Rep.
10:296–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang WS, Seo CR, Jang HH, Song NJ, Kim JK,
Ahn JY, Han J, Seo WD, Lee YM and Park KW: Black rice (Oryza sativa
L.) extracts induce osteoblast differentiation and protect against
bone loss in ovariectomized rats. Food Funct. 6:265–275. 2015.
View Article : Google Scholar
|
|
10
|
Casati L, Pagani F, Fibiani M, Lo Scalzo R
and Sibilia V: Potential of delphinidin-3-rutinoside extracted from
Solanum melongena L. as promoter of osteoblastic MC3T3-E1 function
and antagonist of oxidative damage. Eur J Nutr. 58:1019–1032. 2019.
View Article : Google Scholar
|
|
11
|
Sako F, Kobayashi N, Taniguchi N and
Takakuwa E: A study on the toxicity of natural food dyes-toxicity
and enzyme inhibition in Paramecium caudatum. J Toxicol Sci.
3:127–136. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Della Vedova L, Ferrario G, Gado F,
Altomare A, Carini M, Morazzoni P, Aldini G and Baron G: Liquid
Chromatography-High-Resolution Mass Spectrometry (LC-HRMS)
profiling of commercial enocianina and evaluation of their
antioxidant and anti-inflammatory activity. Antioxidants (Basel).
11:11872022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura A, Ly C, Cipetić M, Sims NA,
Vieusseux J, Kartsogiannis V, Bouralexis S, Saleh H, Zhou H, Price
JT, et al: Osteoclast inhibitory lectin (OCIL) inhibits osteoblast
differentiation and function in vitro. Bone. 40:305–315. 2007.
View Article : Google Scholar
|
|
14
|
Qin S, Wang W, Liu Z, Hua X, Fu S, Dong F,
Li A, Liu Z, Wang P, Dai L, et al: Fibrochondrogenic
differentiation potential of tendon-derived stem/progenitor cells
from human patellar tendon. J Orthop Translat. 22:101–108. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schindelin J, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Guideline-Rodent Analgesia (Procedure
Specific). T.u.o. queensland: 2022
|
|
18
|
Xu L, Huang S, Hou Y, Liu Y, Ni M, Meng F,
Wang K, Rui Y, Jiang X and Li G: Sox11-modified mesenchymal stem
cells (MSCs) accelerate bone fracture healing: Sox11 regulates
differentiation and migration of MSCs. FASEB J. 29:1143–1152. 2015.
View Article : Google Scholar
|
|
19
|
Wang W, Qin S, He P, Mao W, Chen L, Hua X,
Zhang J, Xiong X, Liu Z, Wang P, et al: Type II collagen sponges
facilitate tendon stem/progenitor cells to adopt more chondrogenic
phenotypes and promote the regeneration of fibrocartilage-like
tissues in a rabbit partial patellectomy model. Front Cell Dev
Biol. 9:6827192021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: From human mutations to treatments.
Nat Med. 19:179–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lowery JW and Rosen V: The BMP pathway and
its inhibitors in the skeleton. Physiol Rev. 98:2431–2452. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reid IR and Billington EO: Drug therapy
for osteoporosis in older adults. Lancet. 399:1080–1092. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
GBD 2019 Fracture Collaborators: Global,
regional, and national burden of bone fractures in 204 countries
and territories, 1990-2019: A systematic analysis from the Global
Burden of Disease Study 2019. Lancet Healthy Longev. 2:e580–e592.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Clynes MA, Harvey NC, Curtis EM, Fuggle
NR, Dennison EM and Cooper C: The epidemiology of osteoporosis. Br
Med Bull. 133:105–117. 2020.PubMed/NCBI
|
|
26
|
He J, Li X, Wang Z, Bennett S, Chen K,
Xiao Z, Zhan J, Chen S, Hou Y, Chen J, et al: Therapeutic anabolic
and anticatabolic benefits of natural Chinese Medicines for the
treatment of osteoporosis. Front Pharmacol. 10:13442019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee YM, Yoon Y, Yoon H, Park HM, Song S
and Yeum KJ: Dietary anthocyanins against obesity and inflammation.
Nutrients. 9:10892017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Samarpita S, Ganesan R and Rasool M:
Cyanidin prevents the hyperproliferative potential of
fibroblast-like synoviocytes and disease progression via targeting
IL-17A cytokine signalling in rheumatoid arthritis. Toxicol Appl
Pharmacol. 391:1149172020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Domazetovic V, Marcucci G, Iantomasi T,
Brandi ML and Vincenzini MT: Oxidative stress in bone remodeling:
Role of antioxidants. Clin Cases Miner Bone Metab. 14:209–216.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saulite L, Jekabsons K, Klavins M,
Muceniece R and Riekstina U: Effects of malvidin, cyanidin and
delphinidin on human adipose mesenchymal stem cell differentiation
into adipocytes, chondrocytes and osteocytes. Phytomedicine.
53:86–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azuma K, Ohyama A, Ippoushi K, Ichiyanagi
T, Takeuchi A, Saito T and Fukuoka H: Structures and antioxidant
activity of anthocyanins in many accessions of eggplant and its
related species. J Agric Food Chem. 56:10154–10159. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marsell R and Einhorn TA: The biology of
fracture healing. Injury. 42:551–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Paiva KBS and Granjeiro JM: Matrix
metalloproteinases in bone resorption, remodeling, and repair. Prog
Mol Biol Transl Sci. 148:203–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Delaissé JM, Engsig MT, Everts V, del
Carmen Ovejero M, Ferreras M, Lund L, Vu TH, Werb Z, Winding B,
Lochter A, et al: Proteinases in bone resorption: obvious and less
obvious roles. Clin Chim Acta. 291:223–234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ortega N, Behonick D, Stickens D and Werb
Z: How proteases regulate bone morphogenesis. Ann N Y Acad Sci.
995:109–116. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu L, Tang Y, Li XY, Keller ET, Yang J,
Cho JS, Feinberg TY and Weiss SJ: Osteoclast-mediated bone
resorption is controlled by a compensatory network of secreted and
membrane-tethered metalloproteinases. Sci Transl Med.
12:eaaw61432020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Yu YY, Lieu S, Yang F, Lang J, Lu
C, Werb Z, Hu D, Miclau T, Marcucio R and Colnot C: MMP9 regulates
the cellular response to inflammation after skeletal injury. Bone.
52:111–119. 2013. View Article : Google Scholar
|
|
38
|
Kalev-Altman R, Janssen JN, Ben-Haim N,
Levy T, Shitrit-Tovli A, Milgram J, Shahar R, Sela-Donenfeld D and
Monsonego-Ornan E: The gelatinases, matrix metalloproteinases 2 and
9, play individual roles in skeleton development. Matrix Biol.
113:100–121. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vu TH, Shipley JM, Bergers G, Berger JE,
Helms JA, Hanahan D, Shapiro SD, Senior RM and Werb Z:
MMP-9/gelatinase B is a key regulator of growth plate angiogenesis
and apoptosis of hypertrophic chondrocytes. Cell. 93:411–422. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cao X: Targeting osteoclast-osteoblast
communication. Nat Med. 17:1344–1346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kular J, Tickner J, Chim SM and Xu J: An
overview of the regulation of bone remodelling at the cellular
level. Clin Biochem. 45:863–873. 2012. View Article : Google Scholar : PubMed/NCBI
|