Introduction
Adrenic acid (AdA), also known as
cis-7,10,13,16-didodecatetraenoic acid, is an endogenous, 22-carbon
long-chain polyunsaturated fatty acid (PUFA) with the molecular
formula C22H36O2 and a molecular
weight of 332.52 g/mol (Fig. 1).
The primary distinction between AdA and other PUFAs is its distinct
carbon chain length and double bond configuration. AdA structure
encompasses 22 carbon atoms and four double bonds at carbon atoms
at positions 7, 10, 13 and 16. This configuration renders AdA
unique in its physiological functions and metabolic pathways. AdA
is formed via the extension of the 2-carbon chain of arachidonic
acid (AA) (1,2). As a constituent of cell membrane
phospholipids, AdA is predominantly located in the adrenal gland,
liver, brain, kidneys and vascular system and it is classified
under the n-6 family of fatty acids (2).
Although the metabolic pathway of AdA is similar to
that of AA, its unique functional lipid derivatives and metabolites
yield unique biological effects (3). AdA serves a role in several
biological processes beyond its involvement in β-oxidation and the
formation of cell membranes (2,4-9).
It is a biologically active metabolite that participates in cell
signalling and plays an important role in regulating physiological
functions, including the immune response and metabolic homeostasis
(2,4-9).
AdA has been demonstrated to induce inflammatory responses in the
liver and coronary arteries (4-6).
In vitro studies have demonstrated that AdA increases
triglyceride (TG) and cholesteryl ester accumulation in
fibroblasts, thereby serving an important role in regulation of
lipid metabolism (7,8). Furthermore, AdA acts as an
endogenous regulator to maintain vascular function by affecting ion
channels and modulating vasoconstrictors and dilators (2,9).
As a FA, AdA is susceptible to free radical attack, leading to
redox imbalance and generation of lipid peroxidation products that
cause cell membrane damage and cell death (10). Furthermore, AdA can be converted
into other bioactive molecules via enzymatic and non-enzymatic
reactions. These molecules participate in pathological processes,
including oxidative stress, inflammation and cell death. They also
play an important role in the progression of many diseases,
including epilepsy, Alzheimer's disease (AD) (1,6,11). The application of technologies
such as untargeted metabolomics has led to confirmation of a
significant association between AdA levels in peripheral blood and
several disease states, including non-alcoholic fatty liver disease
(NAFLD), non-alcoholic steatohepatitis (NASH), type 2 diabetes
mellitus (T2DM), AD, acute kidney injury (AKI) and pancreatic
ductal adenocarcinoma (PDAC) (12-17). Certain natural products and
synthetic drugs, such as astragaloside IV (AS IV), evodiamine
(EVO), quercetin, kaempferol, Berberine-baicalin (RA) and
prebiotics, have been demonstrated to regulate the metabolism of
AdA, thereby exerting a therapeutic effect in treatment of disease
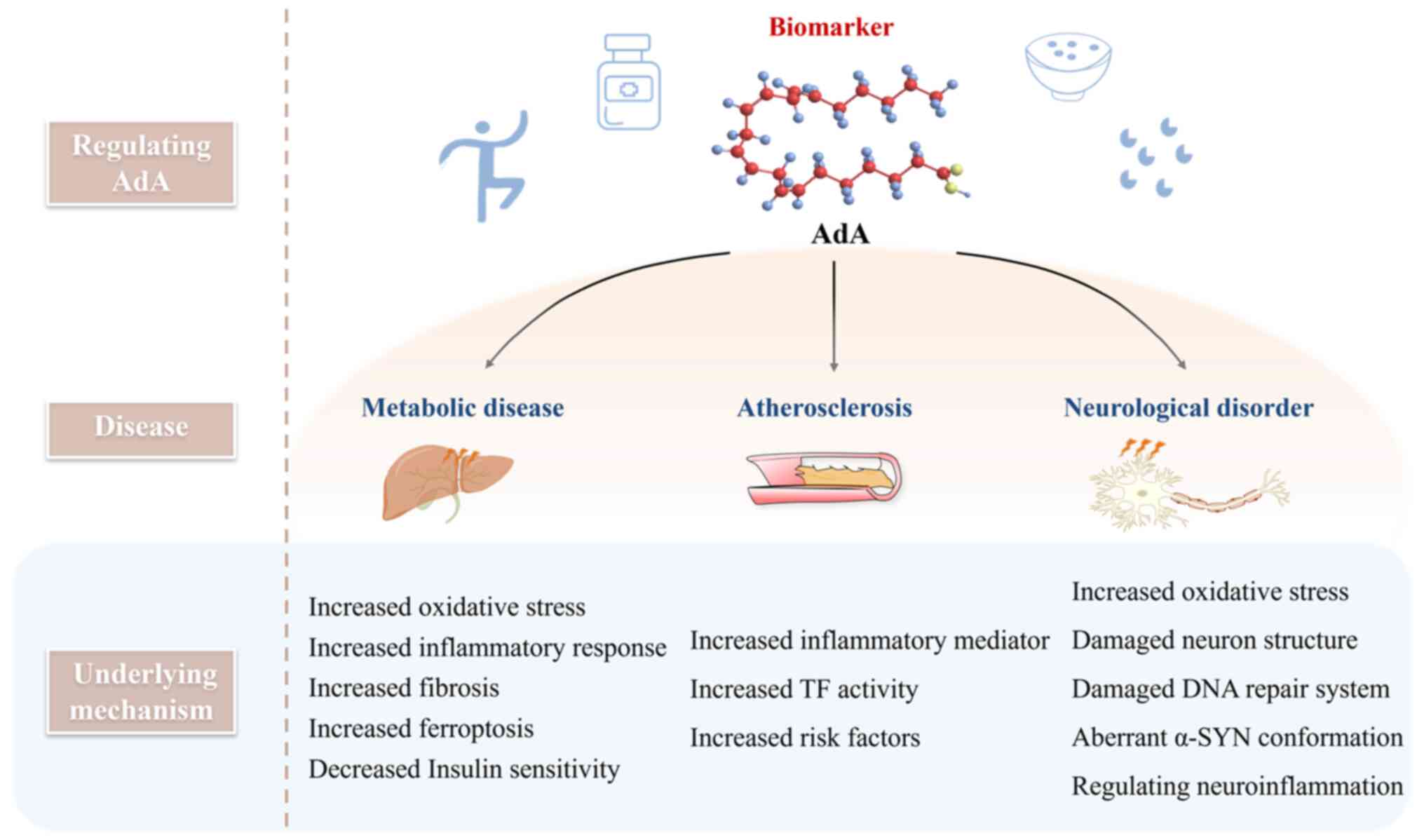
(18-21). These findings indicate that AdA
may serve as a potential biomarker in metabolic, neurodegenerative
and cardiovascular disease and novel target for clinical therapy
and drug development. Thus, the mechanism of action of AdA in
disease and its potential clinical applications must be
investigated. Although AdA plays an important role as a highly
sensitive metabolite in a wide range of diseases, its precise mode
of action remains unclear (22).
Nevertheless, numerous challenges remain to be addressed before
AdA-targeted therapies can be translated into clinical practice. To
the best of our knowledge, there is a lack of clinical studies on
AdA, highlighting the need for further research. Additionally,
translating AdA quantification into clinical practice is
challenging due to measurement complexity, lack of standardization,
and individual metabolic differences.
The present study conducted a comprehensive search
of major databases PubMed (https://pubmed.ncbi.nlm.nih.gov), Google Scholar
(/scholar.google.com), Web of Science
(https://www.webofscience.com) and China
National Knowledge Infrastructure (https://www.cnki.net), up to October 2024 using search
terms 'adrenic acid', '7,10,13,16-docosatetraenoic acid', 'adrenic
acid AND metabolic diseases', 'adrenic acid AND cardiovascular
diseases', and 'adrenic acid AND neurological diseases'. Previous
studies have shown that there is a potential relationship between
AdA and metabolic, cardiovascular and neurological diseases
(12-17). The present study focused on
research on the mechanism of action of AdA in these diseases.
Literature from the past 5-10 years was included. A total of 201
papers were included. The present review summarizes the
biosynthesis and metabolic pathways of AdA, AdA mediation of
metabolic, cardiovascular and neurological disease by regulating
immuno-inflammatory responses, oxidative stress, vascular function
and cell death and its molecular mechanisms. Furthermore, the
regulatory methods of AdA were outlined from the perspectives of
exercise, natural products, dietary nutrition and endocrinology and
the clinical translational role of AdA as a biomarker and
therapeutic target was discussed to provide a new perspective for
further exploring clinical therapeutic targets of common
diseases.
Metabolism of AdA
Synthesis
AdA synthesis process primarily depends on the n-6
FA metabolic pathway. The process commences with ingestion of
linoleic acid (LA) and culminates in the production of AA through a
series of chemical reactions. These include dehydrogenation and
carbon chain extension, which are catalysed by Δ6-desaturase (D6D),
extra-long-chain FA elongase 5 (ELOVL5) and D5D (23). AA serves as the immediate
precursor to AdA, which is catalysed by ELOVL2 and ELOVL5 (2). The carbon chain is extended further
to generate AdA (2). AA, the
direct precursor of AdA, can be obtained directly from food
sources, in addition to being synthesized primarily through the LA
metabolic pathway (24).
Catabolism
AdA is primarily metabolized by the lipoxygenase
(LOX), cyclooxygenase (COX), and cytochrome P450 (CYP450) pathways,
resulting in production of bioactive lipids that play a pivotal
role in the inflammatory response, immunomodulation and vascular
function (4). Furthermore, AdA
can elicit biological effects via non-enzymatic oxidative metabolic
reactions (25). AdA is also
metabolized via the peroxisomal β-oxidation pathway, resulting in
production of ATP for energy (26). In addition to undergoing
metabolic processes, AdA is incorporated into cell membranes for
storage. Upon stimulation of the cell, AdA can be released from the
cell membrane via calcium-independent VIA phospholipase
A2 (iPLA2β), which participates in the
metabolic generation of various active molecules (27).
Enzymatic oxidative metabolic
reaction
AdA is metabolized in platelets via the LOX pathway
to dihomo (DH)-hydroxyeicosatetraenoic acid (28). AdA is metabolized by COX in human
vascular endothelial cells to DH-prostaglandin I2
(DH-PGI2) and DH-thromboxane A2, which
inhibit thrombin-induced platelet aggregation (9). CYP450 mediates oxidative metabolism
of AdA to generate epoxy FAs, particularly
DH-16,17-epoxyeicosatrienoic acids (DH-16,17-EETs). These are
involved in regulation of vascular tone in multiple vascular beds.
DH-16,17-EETs induce concentration-dependent vasodilation via the
activation of coronary vascular smooth muscle K+
channels and hyperpolarisation responses (29). Additionally, they have been
observed to cause the activation of large-conductance
calcium-activated potassium channel (BKCa) in adrenocortical
endothelial and globular zone cells, which mediate adrenocortical
arteriolar diastole and regulate adrenal blood flow (2). Additionally, Singh et al
(30) discovered that in
vitro, the methyl ester regional isomers of DH-EETs markedly
enhances cell viability and diminishes the phosphorylation/total
protein ratios of the unfolded protein response markers, binding
immunoglobulin protein and inositol-requiring enzyme 1α, as well as
the levels of X-box binding protein 1, spliced form in human
embryonic kidney cells that had been subjected to prolonged
clindamycin exposure. This resulted in alleviation of endoplasmic
reticulum stress. In vivo, methyl ester regional isomers of
DH-EETs alleviate carrageenan-induced inflammatory pain in rats
(30).
Non-enzymatic oxidative metabolic
reaction
Non-enzymatic oxidative metabolic reactions are
non-selective and non-specific. F2-DH-isoprostane (IsoPs) and
isofurans are biomarkers of oxidative stress produced via free
radical-driven peroxidation of AdA (25). These markers are used to evaluate
the extent and status of oxidative stress in a range of
pathological conditions, including epilepsy, AD and cerebral white
matter injury (14,15). This approach can potentially
facilitate the early diagnosis and treatment. Furthermore,
F2-dihomo-IsoPs are utilized to evaluate allograft function
throughout the kidney transplantation process (16).
Peroxisome β-oxidation
AdA is β-oxidised, and the carbon chain is shortened
in peroxisomes and translocated to mitochondria, where it
participates in the tricarboxylic acid (TCA) cycle and produces ATP
as an energy source. In peroxisomes, β-oxidation reaction involves
oxidation, hydration, dehydrogenation and thiolysis. This process
results in production of a molecule of acyl-coenzyme A (CoA) that
is shortened by two carbon chains and a molecule of acetyl-CoA. The
shortened acyl-CoA is transferred out of peroxisomes via the
carnitine shuttle system and enters mitochondria to undergo
β-oxidation. Repeated oxidation yields several acetyl CoA
molecules, which participate in the TCA cycle in the mitochondrial
matrix (31). This generates
ATP, which provides energy to the cell (26). A study conducted on isolated
hepatocytes revealed that AdA is β-oxidized by peroxisomes at a
rate 2-3 times faster than that observed for oleic acid, AA and
docosahexaenoic acid (DHA) (32). Furthermore, AdA is
reverse-converted to AA by peroxisomal β-oxidation. This
establishes a dynamic equilibrium between the two, whereby they act
as metabolic precursors for each other. The initial step involves
activation of AdA, which then forms its CoA derivative (AdA-CoA).
In the peroxisome, AdA-CoA undergoes a series of β-oxidation
reactions, in which two carbon atoms are removed, resulting in
formation of a shorter-chain acyl-CoA. Following several
β-oxidation cycles, AdA-CoA is converted to AA-CoA, and AA is
released (33) (Table I; Fig. 2).
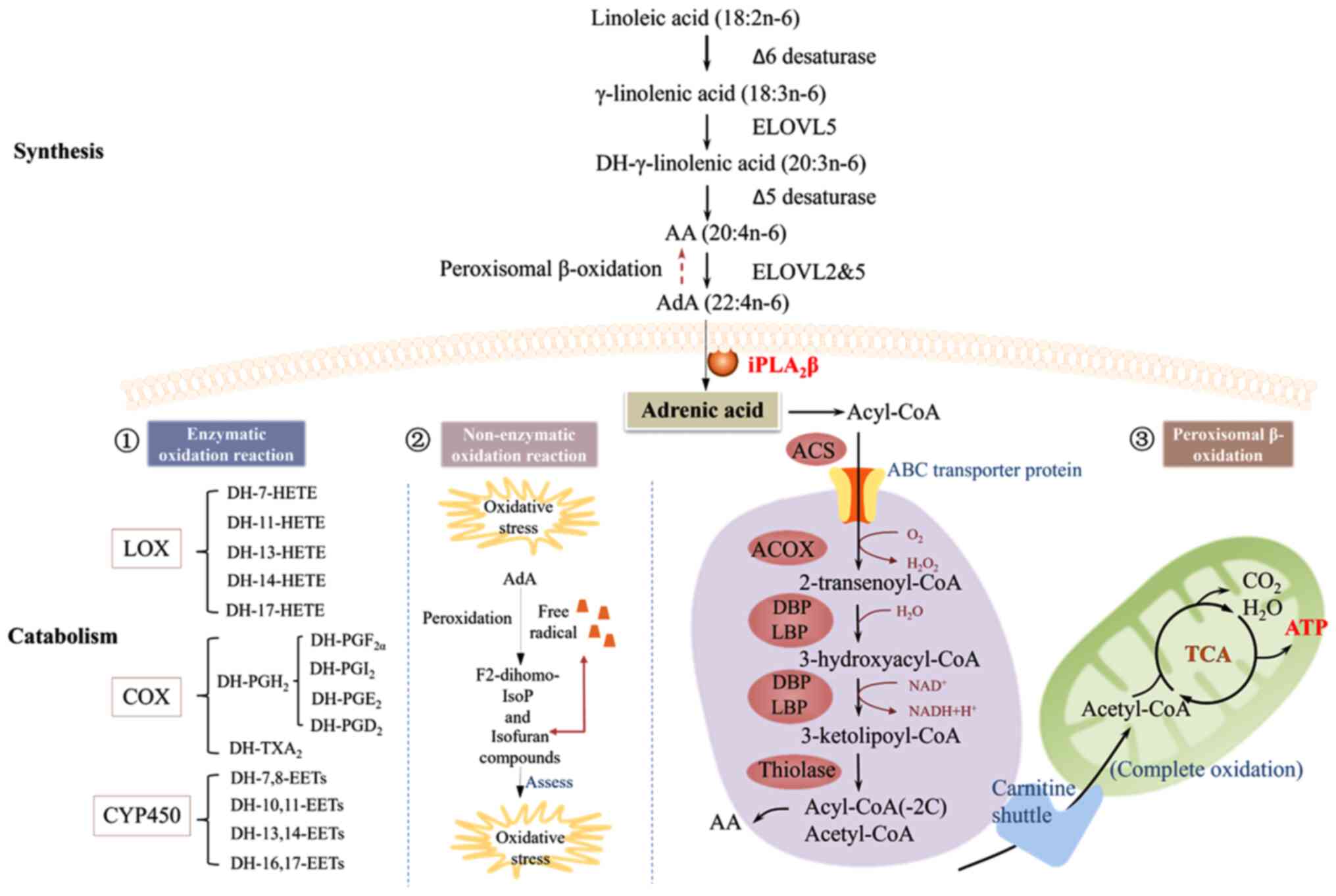 | Figure 2Metabolism of AdA. AdA synthesis
process primarily depends on the n-6 fatty acid metabolic pathway.
AdA is incorporated into the cell membrane for storage; upon
stimulation of the cell, AdA is released from the cell membrane via
iPLA2β, thereby participating in metabolic generation of
various active molecules. (1)
AdA is primarily metabolized by the LOX, COX and CYP450 pathways to
produce various bioactive lipids. (2) Non-enzymatic oxidation reaction of
F2-DH-IsoPs and isofurans is produced by free radical-driven
non-enzymatic peroxidation of AdA in the presence of oxidative
stress. (3) Peroxisomes
β-oxidise AdA and shorten the carbon chain, which is transferred to
the mitochondria to participate in the TCA cycle and produce ATP as
an energy source. AdA can be reverse-converted by peroxisomal
β-oxidation to AA. AdA, adrenic acid; AA, arachidonic acid;
iPLA2β, calcium-independent VIA phospholipase
A2; LOX, lipoxygenase; COX, cyclooxygenase; CYP,
cytochrome P; F2-DH-IsoP, F2-dihomo-isoprostane; TCA, tricarboxylic
acid; ELOVL, extra-long-chain fatty acid elongase; HETE,
hydroxyeicosatetraenoic acid; PG, prostaglandin; TXA, thromboxane
A; EET, epoxyeicosatrienoic; ACS, acyl-CoA synthase; ABC,
ATP-binding cassette; ACOX, acyl-coenzyme A oxidase; DBP,
D-bifunctional protein. |
 | Table IEnzymes and regulatory mechanisms of
AdA biosynthesis and metabolism. |
Table I
Enzymes and regulatory mechanisms of
AdA biosynthesis and metabolism.
| Metabolic
process | | Substrate | Enzyme | Catalytic
mechanism | Product |
|---|
| Biosynthesis | | LA | Δ6-desaturase |
Dehydrogenation | GLA |
| GLA | ELOVL5 | Carbon chain
extension | DGLA |
| DGLA | Δ5-desaturase |
Dehydrogenation | AA |
| AA | ELOVL2 and 5 | Carbon chain
extension | AdA |
| Cell membrane
storage | | AdA |
iPLA2β | Hydrolysis | AdA |
| Metabolism | Enzymatic oxidative
metabolism | AdA | LOX | Insertion of
molecular oxygen into carbon-carbon double bonds to form
hydroperoxides | DH-HETEs |
| COX | Conversion to
cyclic endoperoxide by two-step oxidation | DH-PGH2
DH-TxA2 |
| CYP450 | Hydroxylation or
epoxi-dation at numerous carbon positions by binding oxygen atoms
in a mono-oxygenase reaction | DH-EETs |
| Non-enzymatic
oxidative metabolic reaction | AdA | - | Radical-driven | F2-dihomo-IsoPs,
isofurans |
| Peroxisome | AdA | ACS | Activation | Acyl-CoA |
| β-oxidation | Acyl-CoA | ACOX | Oxidation |
2-Transenoyl-CoA |
|
2-Transenoyl-CoA | DBP, LBP | Hydration |
3-Hydroxyacyl-CoA |
|
3-Hydroxyacyl-CoA | DBP, LBP |
Dehydro-genation | 3-Ketoacyl-CoA |
| 3-Ketoacyl-CoA | Thiolyase | Thiolation | Acyl-CoA,
Acetyl-CoA |
Mechanisms of AdA in metabolic disease
Metabolic diseases are a group of conditions
resulting from the interaction of genetic, environmental and
lifestyle factors that cause abnormal metabolic function in the
body. This involves dysregulation of glucose homeostasis, lipid
metabolism, immune cells and cytokines (34). Metabolic diseases encompass a
range of conditions, such as diabetes mellitus, NAFLD, obesity and
insulin resistance (IR). The prevalence of metabolic diseases has
been increasing in recent years, leading to an increase in
mortality rates and affecting the quality of life of nearly 2
billion people around the world (35). This has become a notable global
health issue, placing a burden on public health and medical
resources (36). Metabolic
diseases have multifactorial pathological mechanisms that cause a
series of metabolic disorders. These disorders damage tissues and
organs, leading to acute or chronic complications and increasing
risk of cardiovascular and cerebral vascular diseases, cancer and
neurodegenerative disease (37-39). Therefore, the prevention and
treatment of metabolic diseases are key.
AdA and NAFLD
NAFLD is characterized by abnormal fat accumulation
in the liver and hepatic steatosis. The severity of the disease can
range from simple steatosis to steatohepatitis; in certain cases,
it can progress to advanced hepatic fibrosis, cirrhosis and liver
failure (40,41). Patients with advanced liver
disease and cirrhosis often require liver transplantation, which
places a burden on society and the healthcare system. Approximately
30% of the global population is estimated to suffer from NAFLD, and
this percentage is increasing (42). The onset and progression of NAFLD
are typically associated with steatosis (43), IR (44), iron overload (45), oxidative stress (46) and mitochondrial dysfunction
(47). Numerous studies have
demonstrated that AdA is involved in these mechanisms and can
induce oxidative stress, exacerbate inflammatory responses and
cause cellular damage, thereby accelerating the pathological
process of NAFLD (1,5,48,49).
AdA induces hepatocyte injury via
oxidative stress
Oxidative stress, caused by an imbalance between
reactive oxygen species (ROS) and antioxidant defences, contributes
to hepatocyte injury in NAFLD (50). In vitro assay using HepG2
cells has demonstrated that AdA increases intracellular ROS
production, leading to oxidative stress, decreased cell viability
and a dose-dependent increase in cell death (1). Superoxide dismutase (SOD),
glutathione peroxidase (Gpx) and haem oxygenase-1 (HO-1) are
intracellular antioxidant defence enzymes (51). In cells treated with 500
µM AdA, SOD2 and HO-1 mRNA expression increases, while Gpx1
mRNA expression decreases. These findings suggest activation of the
antioxidant defence mechanism in response to ROS generation, which
compensates for the increase in SOD2 and HO-1 expression (1). However, AdA-induced ROS
over-accumulation exceeds the antioxidant defence capacity and
decreases expression of Gpx1, leading to oxidative stress (1). In conclusion, AdA induces cell
oxidative stress injury by increasing the production of ROS and
downregulating expression of Gpx1 (1). Other studies have shown that in a
male Sprague-Dawley rat model of NAFLD induced by intraperitoneal
injection of 0.01, 0.05 and 0.20 µmol/kg
3,3′,4,4′,5-pentachlorobiphenyl for 3 months, the levels of AdA
increase in a dose-dependent manner (52), which results in a decrease in the
hepatic glucose levels and peroxisomal oxidation levels of FAs,
leading to hepatic steatosis and disturbing intrahepatic redox
homeostasis and antioxidant enzyme levels (53-55).
AdA promotes NAFLD progression through an
immune-inflammatory response
The progression of NAFLD to severe stages is driven
by hepatocyte injury and inflammation. Lipotoxic effects occur due
to excessive accumulation of harmful lipids in subcellular
structures, such as cell membranes, endoplasmic reticulum and
mitochondria (56). This leads
to hepatocyte injury and the release of inflammatory cytokines
(57). Injured hepatocytes
release intracellular lipids and harmful molecules from
intracellular organs, contributing to the inflammatory response,
which activates and infiltrates immune cells, exacerbating the
degree of hepatocyte injury and increasing the intensity and
persistence of the immune response. This aggravates pathological
damage to the liver, which may lead to severe consequences such as
cirrhosis and hepatocellular carcinoma (58).
TCRαβ double-negative T (DNT) cells are a
distinctive subset of T cell populations, predominantly present in
the liver. These cells serve as immunomodulatory agents via the
secretion of cytotoxic molecules, including granzyme B (GZMB) and
perforin (59,60). It has been demonstrated that the
apoptosis of TCRαβ DNT cells is markedly enhanced in patients with
NAFLD. The adoptive transfer of DNT cells has been shown to inhibit
accumulation of hepatic fat, reduce inflammation and hepatic
fibrosis and consequently inhibit progression of NAFLD and NASH
(61). AdA has been linked to
the apoptosis of hepatic DNT cells in mice with NAFLD (49). In vitro, AdA induces
apoptosis of TCRαβ DNT cells via downregulation of the PI3K/AKT
signalling pathway and mRNA expression of downstream molecule
c-Myc. Additionally, AdA decreases GZMB secretion, increases levels
of pro-inflammatory CD4 and CD8 cells and decreases levels of
anti-inflammatory CD8+ regulatory T cells (49). This, in turn, has been shown to
reduce the immunosuppressive function of TCRαβ DNT cells and
promote progression of NAFLD (49).
AdA markedly elevates the levels of pro-inflammatory
cytokines and triggers an inflammatory response in hepatocytes.
In vitro, 0.5 mM AdA pretreatment increases the levels of
autocrine tumour necrosis factor-α (TNFα) in HepG2 cells (5). Additionally, AdA pretreatment
enhances TNFα-induced inflammatory response in HepG2 cells, as
evidenced by increased mRNA levels of various pro-inflammatory
cytokines, including IL-8, macrophage inflammatory protein 1β
(MIP1β) and monocyte chemoattractant protein 1 (MCP1) (5). This indicates that AdA not only
stimulates inflammatory pathways directly in hepatocytes but also
significantly enhances TNFα-mediated inflammatory signalling,
thereby potentially contributing to a broader pro-inflammatory
environment in liver pathology.
AdA promotes fibrosis by upregulating
pro-fibrotic chemokine expression
Hepatic fibrosis is the accumulation of collagen
fibres in liver tissue, resulting in the formation of excessive
connective tissue. This can lead to impaired liver function,
cirrhosis and liver failure (62). It is a key predictor of mortality
in patients with NAFLD (63).
The activation, proliferation and transformation of hepatic
stellate cells (HSCs), the principal cell type responsible for
synthesizing the extracellular matrix in the liver, are key steps
in the process of liver fibrosis (64). Chemokines have pro-fibrotic
effects (64). IL-8 activation
stimulates stellate cell activation, whereas MCP1 and MIP1β can
direct stellate cell migration (65,66). HSCs express C-C chemokine
receptor 2 and 5 receptors for MCP1 and MIP1β (67). Horas et al (5) showed that 0.5 mM AdA treatment
increases mRNA expression levels of the chemokines IL-8, MIP1β and
MCP1 and fibroblast cytokine TGFβ1 in HepG2 cells and enhances
activation of hepatic stellate cells, leading to extracellular
matrix synthesis and liver fibrosis. These findings suggested that
AdA promotes fibrotic progression by upregulating hepatocyte
chemokine expression.
AdA may also contribute to progression of NAFLD
through other pathways. Studies have shown that peroxisomal
β-oxidation, the first step catalysed by acyl-coenzyme A oxidase 1
(ACOX1), may be impaired during progression from NAFLD to NASH and
ACOX1 levels are decreased in NASH mice (5,68). Mice lacking the ACOX1 gene
develop severe hepatic steatosis at a young age. By 5 months of
age, hepatocytes begin to undergo apoptosis and form
steatogranulomas that progress to hepatocellular carcinoma
(69). Additionally, expression
of ACOX1 in patients with NASH decreases with disease progression
(70). Thus, impaired catabolism
of AdA may mediate the progression of NAFLD. However, additional
direct evidence is needed.
AdA can serve as a biomarker for liver
injury
An increasing number of studies have shown a
positive association between peripheral blood levels of AdA and
liver injury (1,5). In patients with drug-induced liver
injury (DILI), the ratio of serum ω-6/3 PUFA is significantly
elevated (71). AdA may serve as
an ideal predictive model for chronic risk in the acute phase of
DILI (12). Studies have shown
that esterified and unesterified free AdA is elevated in patients
with NASH (72) and patients
with NASH-associated hepatocellular carcinoma have higher plasma
AdA concentrations than healthy controls (49,73). Furthermore, compared with healthy
children, children with severe steatosis show a trend toward
increased plasma AdA (74). Mice
with NASH induced by high-fat diet (HFD) deficient in choline and
amino acids exhibit significantly greater levels of free AdA and
AdA-containing phospholipid species in the liver and plasma than
mice fed a normal diet (5).
Additional studies have shown the notable increase in free AdA
levels in plasma and liver of NASH mice may be attributed to an
increase in endogenous synthesis and decrease in AdA catabolism in
hepatic peroxisomes (5,20). Furthermore, plasma AdA levels in
patients with NAFLD are positively associated with serum alanine
aminotransferase levels (5,75). Although the aforementioned
studies indicate that AdA levels may be indicative of the extent of
hepatocellular damage, other studies have proposed an alternative
perspective (20,76). In patients with hepatitis B virus
(HBV)-associated cirrhosis, a positive association is observed
between serum AdA levels and expression of peroxisome
proliferator-activated receptor γ (PPARγ) in HSCs, indicating AdA
may play a pivotal role in regulating lipid metabolism. Following
48 weeks of entecavir treatment, a significant restoration of PPARγ
expression is observed in HSCs of patients with HBV-associated
cirrhosis, accompanied by an increase in AdA levels (76). Thus, AdA may affect lipid
metabolism by regulating PPARγ expression, thereby contributing to
the pathological state of cirrhosis.
Taken together, the aforementioned findings
indicated that AdA accumulation mediates disease progression in
NAFLD by inducing oxidative stress, activating the
immune-inflammatory response and promoting the expression of
hepatic fibrosis chemokines. Additionally, AdA serves as a
biomarker of liver injury, reflecting the extent of damage to
hepatocytes. However, in certain diseases such as HBV-associated
cirrhosis, AdA may also promote liver repair (Fig. 3).
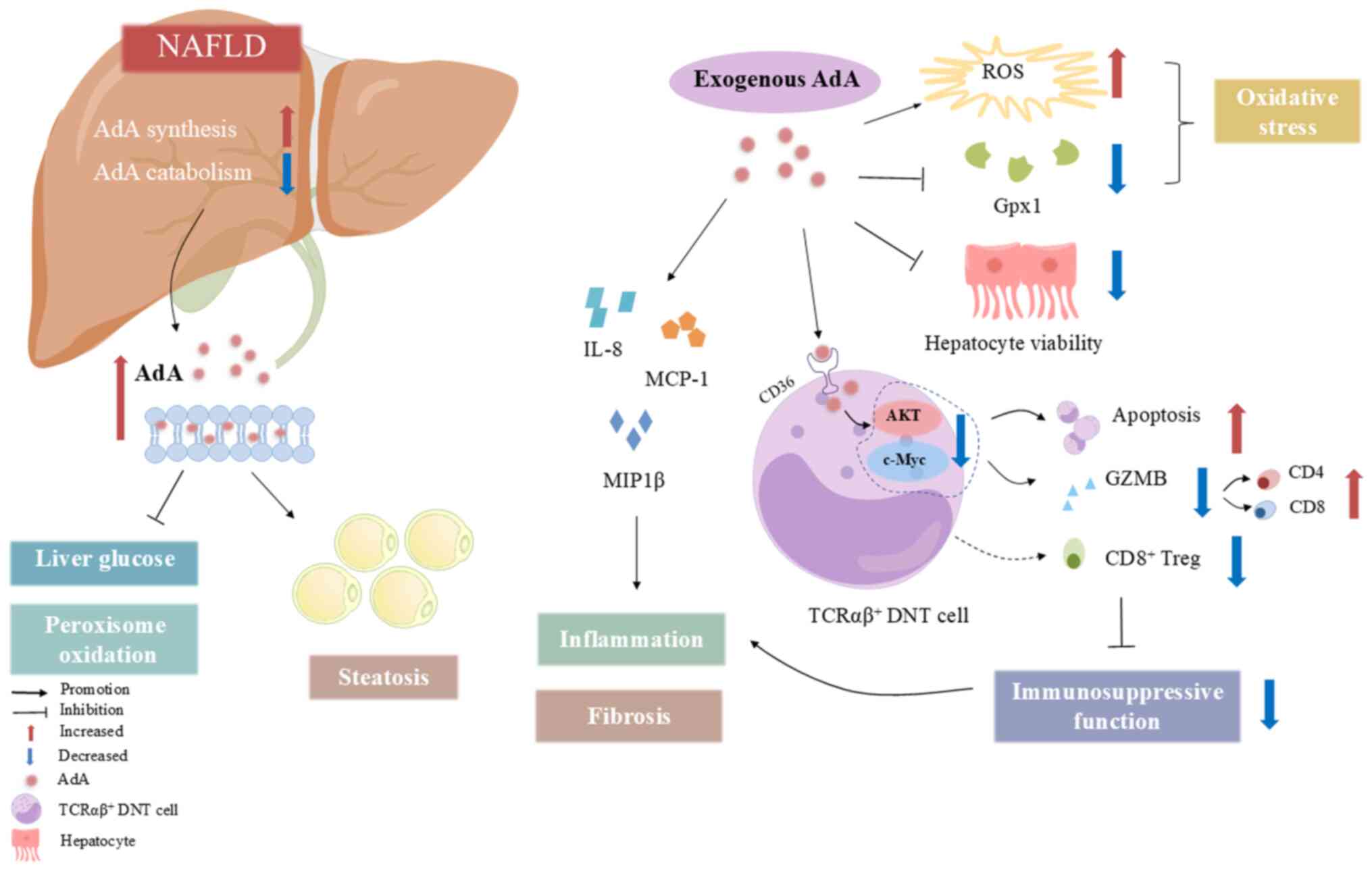 | Figure 3Mechanism of action of AdA in NAFLD.
Enhancing endogenous synthesis and decreasing catabolism of AdA in
hepatic peroxisomes notably increases levels of AdA-free and
AdA-containing phospholipids in the liver and plasma. This can
lower hepatic glucose levels and decrease peroxisomal oxidation,
contributing to liver steatosis. Exogenous AdA treatment elevates
cellular ROS, decreases the expression of the antioxidant enzyme
Gpx1, causes oxidative stress damage and impairs cell viability.
AdA also raises mRNA levels of chemokines IL-8, MIP1β and MCP1,
which promote inflammation and fibrosis. In vitro, exogenous
AdA stimulation of TCR DNT cells induces apoptosis by
downregulating the PI3K/AKT signalling pathway and its downstream
molecule, c-Myc. This reduces GZMB secretion, increases
pro-inflammatory CD4+ and CD8+ T cells and
decreases anti-inflammatory CD8+ Treg levels, thereby
diminishing the immunosuppressive function of TCRαβ DNT cells and
advancing NAFLD progression. AdA, adrenic acid; NAFLD,
non-alcoholic fatty liver disease; ROS, reactive oxygen species;
Gpx, glutathione peroxidase; MIP, macrophage inflammatory protein;
MCP, chemoattractant protein; TCR, T cell receptor; DNT,
double-negative T; GZMB, granzyme B; Treg, regulatory T cell. |
AdA and obesity
There may be a link between obesity and plasma AdA
levels. Studies in humans have shown that the percentage of plasma
AdA is significantly greater in overweight/obese children than in
normal-weight children (74,77). Similarly, an animal study has
shown that rats fed HFD have enlarged adipocytes and significantly
greater plasma AdA levels than rats fed high-fructose diet
(78). These findings suggested
that obesity is associated with elevated plasma AdA levels.
Untargeted metabolomics have shown that AdA is a common metabolite
in IR and obese patients (79)
but to the best of our knowledge, direct evidence linking AdA to
obesity and adipocyte dysfunction is limited (18).
Phospholipases responsible for releasing AdA from
membrane phospholipids may serve a role in obesity-related
processes. Monge et al (27) demonstrated iPLA2β
regulates the mobilization and release of AdA from membrane
phospholipids of innate immune and inflammatory cells. Studies have
shown that iPLA2β is associated with an increased
percentage of body fat and risk of T2DM (80,81). Mice lacking iPLA2β are
at decreased risk of obesity, IR, dyslipidaemia and fatty liver
(82). Additionally, knocking
down iPLA2β in 3T3-L1 inhibits the differentiation of
3T3-L1 cells into adipocytes induced by hormones (83). In conclusion, population studies,
animal models and cellular experiments have shown that
iPLA2β, which mobilizes and releases AdA, promotes
development of obesity.
In conclusion, an association exists between plasma
AdA levels and obesity, indicating that AdA may serve a key role in
the development of obesity and its associated metabolic disorders.
In particular, the role of iPLA2β as a key enzyme for
AdA release in the development of obesity offers novel insight into
the molecular mechanism of obesity. Further research on the
association between AdA and obesity should investigate its impact
on adipocyte differentiation through animal models and 3T3-L1 cell
experiments, examine obesity phenotypes alongside metabolomics and
establish clinical trials to evaluate the effectiveness of
supplements that decrease AdA levels for obese patients.
AdA decreases insulin sensitivity by
modulating the insulin signalling pathway
Insulin signalling refers to transmission of
specific biological effects within a cell by insulin through
binding to its receptor and triggering signalling processes.
Insulin activates the phosphatidylinositol 3-kinase (PI3K)/Akt
pathway by binding to the insulin receptor on the cell surface and
phosphorylating insulin receptor substrate 1 (IRS1). This pathway
promotes translocation of glucose transporter 4 to the cell
membrane of adipocytes and muscle cells, allowing glucose uptake
and lowering blood glucose concentrations (84). The catalytic subunit of PI3K,
p110β, is highly sensitive to insulin signalling (84). Additionally, expression levels of
p110 and IRS1 are reduced in the muscle tissue of patients with IR
(85). Studies report increased
levels of AdA in liver of diabetic rats (86,87). Huang et al (78) established a rat model of
HFD-induced IR and found that plasma AdA levels are significantly
elevated. In addition, a human study has reported a negative
association between expression of IRS1 and p110β in the visceral
adipose tissue of obese patients and AdA levels (11). The aforementioned studies
indicate that AdA may impair the IRS1/PI3K signalling pathway,
resulting in decreased insulin sensitivity in various tissues and
organs, including the liver and visceral adipose tissue. This may
contribute to development of IR in the body. However, further
direct evidence is required to validate this hypothesis.
In conclusion, the metabolism of AdA is associated
with insulin sensitivity. Furthermore, elevated levels of AdA
worsen metabolic disturbances in the IR state, highlighting the
potential role of AdA in regulating insulin signalling and
maintaining metabolic homeostasis.
AdA indirectly mediates T2DM and its
complications via ACSL4 in the ferroptosis pathway
T2DM is a syndrome caused by a combination of
etiological factors, including genetic and environmental factors.
Pathophysiological disorders resulting from obesity, high-calorie
diet and sedentary lifestyles impair glucose homeostasis and
predispose individuals to T2DM (88). Forouhi et al (89) found high plasma levels of AdA are
positively associated with the risk of developing T2DM. Therefore,
inhibiting AdA may be a promising treatment for T2DM.
Ferroptosis is a type of programmed cell death
caused by iron-dependent lipid peroxidation and is associated with
development of diabetes and its complications (90). The onset of ferroptosis requires
intracellular free iron, which catalyses the peroxidation of PUFAs
in the lipid bilayer, producing highly reactive lipid peroxides
that disrupt the integrity and stability of the cell membrane.
Multiple metabolic pathways regulate this process, including redox
homeostasis, iron and lipid metabolism and mitochondrial activity
(91).
AdA-containing phosphatidylethanolamines (PE-AdA)
and PE-AA, key phospholipids in initiation of ferroptosis, can be
oxidized by LOX, producing toxic lipid peroxides and inducing
ferroptosis (92,93). Specifically, acyl-CoA synthase
long-chain family member 4 (ACSL4), one of the genes responsible
for initiation of ferroptosis, can selectively catalyse the binding
of AdA and AA to CoA to form AdA-CoA and AA-CoA, which promotes
esterification and activation of AdA and AA; therefore, ACSL4 may
be a potential target or regulator of ferroptosis (94). In addition to its direct
involvement in ferroptosis, AdA indirectly exacerbates lipid
peroxidation and ferroptosis susceptibility in adrenocortical cells
by promoting steroid synthesis (95,96). According to Doll et al
(97), knockdown of ACSL4 in
pituitary fibroblast α (Pfa)1 cells decreases levels of PE-AdA and
PE-AA, thereby inhibiting ferroptosis. Conversely, exogenous
supplementation of AdA/AA renders ACSL4 knockout Pfa1 cells
susceptible to ferroptosis. Furthermore, ferroptosis is associated
with IR and insulin secretion disorder in DM (98). Research has shown that
antihyperglycemic agents, particularly thiazolidinediones, inhibit
ACSL4 expression and decrease ferroptosis, thereby ameliorating
metabolic disorder in diabetes (97). Therefore, ACSL4, AdA and AA may
be potential targets for diabetes treatment.
AdA is associated with diabetic complications. AdA
not only serves as a substrate promoting ferroptosis but also
serves as an important biomarker. As one of the most abundant FAs
in the retina, AdA can undergo lipid peroxidation induced by
pathological conditions such as hyperglycaemia, resulting in
accumulation of ROS and oxidative stress damage. Studies have
demonstrated that serum AdA and its metabolite, DH-IsoP, can be
used to assess the severity of a range of retinopathies, including
diabetic retinopathy (99-101). Systemic chronic inflammation
represents a key pathological feature of T2DM and is a major
contributor to multiorgan complications (102). AdA may function as an
epigenetic regulator of production and secretion of inflammatory
cytokines. A significant positive association has been shown
between the circulating blood levels of AdA and methylation levels
of the pro-inflammatory cytokine TNF (103). This indicates AdA serves as an
intermediate in the alleviation of inflammation by promoting
methylation of TNF-encoding genes (103). This phenomenon is exclusively
observed in the female population, suggesting that sex differences
may influence the epigenetic regulatory effects of AdA (103).
AdA and ACSL4 are potential mediators and
therapeutic targets for T2DM complications. Furthermore, diabetes
can lead to complications including diabetic cardiomyopathy and
neuropathy, vascular injury and pancreatic dysfunction (104). To the best of our knowledge,
there are no population studies or animal experiments on the
association and mechanism of action between these diabetic
complications and AdA, however, several studies have confirmed
associations and potential mechanisms of action of AdA with chronic
kidney disease and vascular endothelial function (105,106). Therefore, AdA is a promising
therapeutic target for research. Future studies should explore the
potential of AdA as a biomarker for monitoring risk and extent of
diabetes and its complications to facilitate development of
individualized preventive and therapeutic programs.
Mechanism of AdA in atherosclerosis
(AS)
AS is a chronic inflammatory disease that affects
the arterial wall. Its occurrence is associated with multiple
factors: One of the early pathological processes of AS is the
accumulation of lipids in the intima-media layer. Low-density
lipoprotein is a plasma lipid that is deposited in the arterial
intima, causing inflammation and oxidative stress, which can cause
endothelial dysfunction. A damaged arterial wall triggers an
inflammatory and fibroproliferative response (107). Leukocytes and monocytes migrate
into the vessel wall, releasing inflammatory mediators such as
cytokines and chemokines that exacerbate the inflammatory response.
The body responds to repair damaged arterial walls via the
proliferation of smooth muscle cells and the synthesis of collagen
fibres, leading to a fibroproliferative response (108). The combination of lipid
accumulation, endothelial dysfunction, inflammatory response and
fibroproliferation leads to vascular smooth muscle cell
proliferation and extracellular matrix changes, forming AS plaques
and thickening of the arterial wall (109).
AdA and its metabolites serve a role in regulation
of vascular tone and function in several vascular beds, triggering
physiological responses. The production of DH-PGI2 and
DH-16,17-EET by AdA via the COX and CYP450 metabolic pathways
activates K+ channels in vascular smooth muscle cells
(29). This results in increased
K+ efflux, leading to hyperpolarisation and vasodilation
in bovine coronary vascular smooth muscle cells (29). This concentration-dependent
relaxation is blocked by COX and CYP450 inhibitors and potassium
channel blockers (110).
Endothelial and glomerular zone cell-derived DH-16,17-EET from the
bovine adrenal cortex activates BKCa channels and exerts a
hyperpolarising effect, which is instrumental in maintaining normal
adrenal function and ensuring appropriate blood flow supply. The
regulatory mechanism is reversed by EET antagonism, hyperkalaemia
and CYP450 inhibitors (2). By
contrast, inhibition of COX has no effect on vasorelaxation in the
adrenal vascular system (2).
Campbell et al (9)
demonstrated that AdA is rapidly metabolized by COX to
1α,1β-dihyperprostaglandin E2 (DH-PGE2) and
DH-PGF2α in human umbilical vein endothelial cells,
thereby inhibiting platelet aggregation. AdA has also been shown to
compete with AA for the conversion of COX, inhibit oxidation of AA
and reduce the synthesis of vasodilator and vasoconstrictor PGs,
thereby modulating the vascular effects of PGs (111). The aforementioned studies
demonstrate the role of AdA and its metabolites in physiological
regulation of vascular function and blood flow supply. Furthermore,
AdA has been found to modulate pathological processes in vascular
disease (9).
AdA may serve as a marker for the risk of
developing cardiovascular disease
A cohort study of patients at high risk of coronary
artery disease found a positive association between AdA and
expression levels of inflammatory markers, including
high-sensitivity C-reactive protein (hs-CRP), IL-6, fibrinogen and
vascular cell adhesion molecule 1 (6). This association directly
contributes to the increased risk of death from coronary artery
disease, with a mortality rate 10% higher than that observed in
healthy controls (6); this was
confirmed by meta-analysis examining the association between n-6
FAs and the likelihood of cardiovascular disease (112). A study discovered that
individuals with elevated serum AdA levels are at increased risk of
coronary heart disease, myocardial infarction and aortic stroke
(113). Additionally, these
patients demonstrate higher levels of fasting glucose,
LDL-cholesterol and high-DL-cholesterol (113). Furthermore, excessive
consumption of dietary oat bran leads to increased levels of AdA
and its oxidized products F2-DH-IsoPs and DH-isofuran compounds in
the cardiac tissue of apolipoprotein E-knockout mice, which is a
model of AS (114);
additionally, pro-inflammatory products HETE and PGF2α
increase, whereas hydroxy DHA, an oxidized product of PUFA with
anti-inflammatory properties, decreases (114). This imbalance in pro- and
anti-inflammatory mediators contributes to disruption in
cardiovascular function and increases susceptibility to
inflammation-driven vascular damage (115), linking high AdA levels with
cardiovascular pathology and systemic inflammation.
AdA contributes to plaque formation by
enhancing tissue factor (TF) activity
TF, which initiates the coagulation process, is
expressed by thrombin-stimulated endothelial cells (116). Tardy et al (106) enriched human endothelial cell
culture medium with FAs (eicosapentaenoic and docosapentaenoic acid
and AdA) and found that only AdA significantly enhanced TF activity
of thrombin-stimulated endothelial cells following 4 h thrombin
stimulation. TF activation initiates a coagulation cascade leading
to thrombus formation. These thrombi obstruct arterial flow,
leading to cardiovascular events. Additionally, they cause release
of pro-inflammatory factors and oxidative stressors from platelets
and inflammatory cells, which worsen endothelial inflammatory
responses and vessel wall damage, thereby contributing to plaque
formation (117,118). TF expression in monocytes,
platelets and platelet-leukocyte aggregates is pro-thrombotic in
patients with acute coronary syndrome (119). In mice, pharmacological
inhibition of TF activity decreases AS plaque formation (120). Consequently, AdA may mediate
formation of thrombotic plaques in AS by activating endothelial
cell TF activity.
AdA mediates AS by modulating AS risk
factors
Hypertension, elevated plasma homocysteine (Hcy)
levels, excessive alcohol consumption and dyslipidaemia are risk
factors for the development of AS (121). Studies have shown that serum
AdA levels are associated with these risk factors and contribute to
development of AS (122-126).
Chronic hypertension causes damage to the
endothelium and triggers inflammatory responses in blood vessels.
This promotes plaque formation and thickening of arterial walls.
Hypertension increases mechanical stress on the vessel wall,
increasing the susceptibility of arteries to structural and
functional abnormality (127).
In spontaneously hypertensive rats (SHRs), a high-DHA diet reduces
AdA levels. This may be attributed to the competitive inhibition of
D5D activity by DHA, which subsequently leads to a decrease in AdA
synthesis and a subsequent decrease in blood pressure (122). Additionally, untargeted serum
metabolomic analysis shows that egg white peptides administered to
SHRs at a dose of 50 mg/kg body weight for 4 weeks is associated
with reduced AdA levels and antihypertensive effects (123). The application of metabolomic
techniques in observational studies has enabled identification of
key metabolic signatures associated with coronary artery disease
(128,129). These signatures have the
potential to facilitate more accurate disease diagnosis and predict
patient prognosis and mortality (130).
Elevated plasma Hcy levels are an independent risk
factor for cardiovascular disease. A study on middle-aged and
elderly hyperlipidaemic patients found a significant positive
association between plasma Hcy and AdA (124). AdA may regulate gene expression
of enzymes that synthesize and metabolize plasma Hcy (124). However, the association between
Hcy and AdA and the molecular mechanisms by which AdA regulates Hcy
require further investigation.
Excessive alcohol consumption is a risk factor for
AS; as alcohol consumption increases in male patients, so do serum
levels of AdA (125). Excessive
alcohol consumption may interfere with absorption, synthesis or
metabolic processes of serum AdA (125).
Additionally, AdA can contribute to accumulation of
TG and cholesteryl esters, leading to lipid deposition and abnormal
distribution (8). By contrast,
decreasing levels of n-6 PUFAs such as AdA in vivo and
lowering the n-6/n-3 PUFA ratio can notably improve lipid
metabolism (126). A recent
study indicated that AdA may facilitate regression of cirrhosis by
regulating lipid metabolism (76). In patients with HBV-associated
cirrhosis, serum AdA is positively associated with PPARγ in HSCs
(76). Furthermore, serum AdA
levels and PPARγ expression in HSCs are significantly elevated
following 48 weeks of entecavir treatment (76). PPARγ may be a key metabolic
regulator of hepatic lipid metabolism and inflammation (131). AdA may confer benefits for
improvement of liver cirrhosis via this signalling pathway.
Notably, a study examining FA composition of breast
milk has demonstrated a positive association between AdA and the
incidence of perinatal and persistent maternal cardiometabolic
disorder (132). Therefore,
regulating AdA activity may be a promising therapeutic approach for
improving AS pathology and clinical outcomes. However, the precise
mechanism of action of AdA in modulating AS risk factors remains
unclear. Further experimental and clinical studies are necessary to
explore this phenomenon.
In conclusion, AdA and its metabolites not only
regulate physiological processes within blood vessels and blood
flow but also regulate pathological processes associated with
vascular disease. AdA contributes to AS plaque aggregation and
thickening of the arterial wall by increasing production of
inflammatory mediators, enhancing the activity of TF, leading to
thrombosis, and modulating the risk factors for AS (high blood
pressure, elevated Hcy levels, excessive alcohol abuse and
dyslipidaemia) to drive the onset and progression AS (Fig. 4).
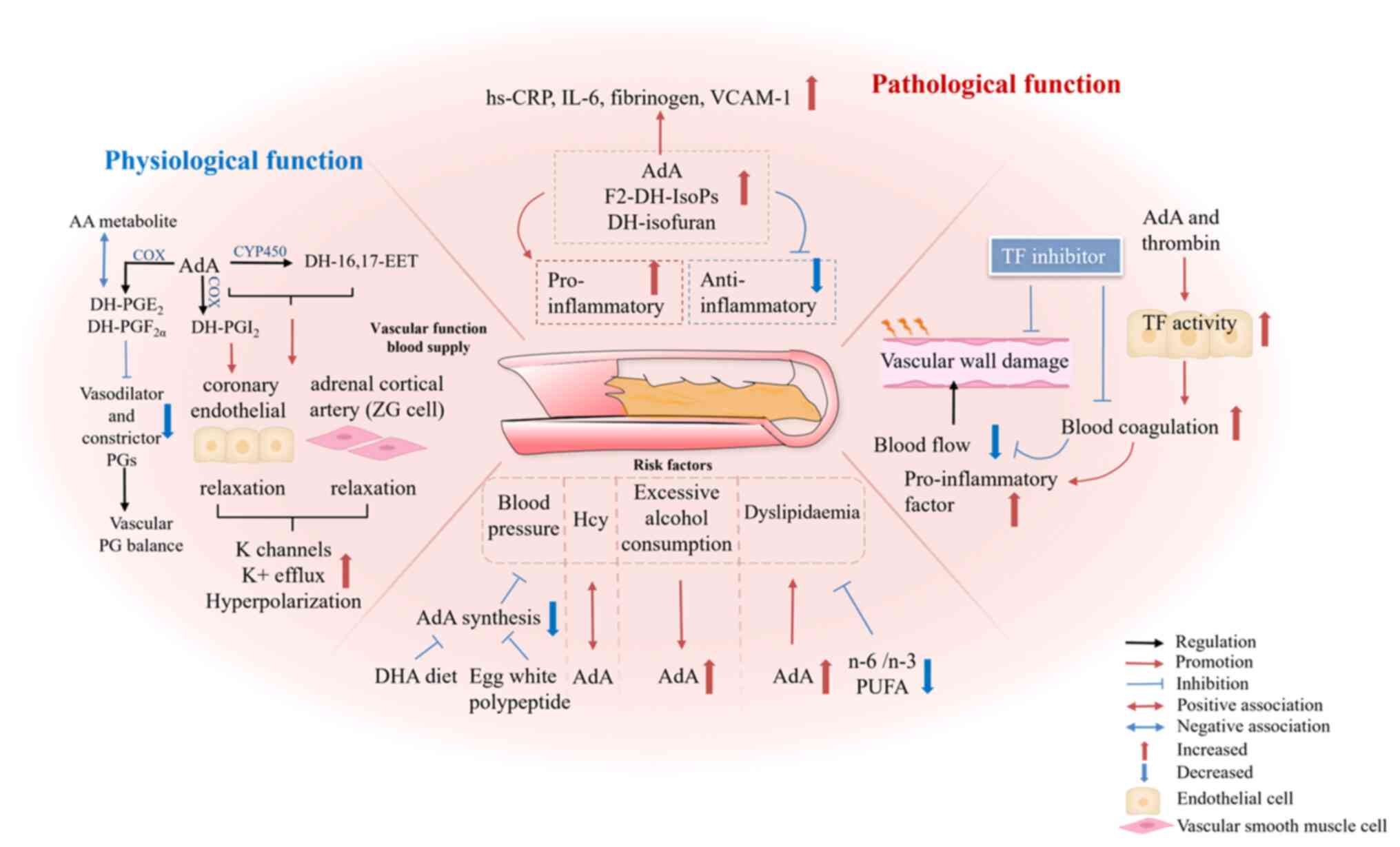 | Figure 4Mechanism of AdA in AS. AdA and its
metabolites regulate physiological vascular function and blood flow
supply. Additionally, AdA modulates pathological processes in
vascular disease. AdA contributes to an elevated risk of coronary
artery disease by increasing levels of inflammatory mediators,
including hs-CRP, IL-6 and VCAM-1. Elevated levels of AdA and its
oxidative products increase pro-inflammatory compounds and decrease
anti-inflammatory hydroxy DHA, which impairs cardiovascular
function. Stimulation of endothelial cells by AdA and thrombin
increases TF activity, which initiates the coagulation cascade and
may lead to thrombosis and obstruction of arterial flow. AdA
affects other risk factors for AS, including high blood pressure,
plasma Hcy levels, excessive alcohol intake and dyslipidaemia. A
diet rich in DHA and egg white peptides inhibits the production of
AdA, thereby lowering blood pressure. There is a positive
association between plasma Hcy levels and AdA. Furthermore, heavy
alcohol consumption increases serum AdA levels. AdA contributes to
TG and cholesterol accumulation, promoting dyslipidaemia. AdA,
adrenic acid; hs-CRP, high-sensitivity C-reactive protein; VCAM,
vascular cell adhesion molecule; DHA, docosahexaenoic acid; TF,
tissue factor; AS, atherosclerosis; Hcy, homocysteine; TG,
triglyceride; IsoP, isoprostane; ZG, zona glomerulosa; CYP,
cytochrome P; DH, dihomo; EET, epoxyeicosatrienoic; PG,
prostaglandin; COX, cyclooxygenase; PUFA, polyunsaturated fatty
acid; AA, arachidonic acid. |
Mechanisms of AdA in neurological
disorder
AdA is the third most prevalent PUFA in grey matter
glycerophospholipids of the brain and is the most abundant PUFA in
white matter ethanolamine phosphoglycerides (133). AdA is key for neuronal growth
and myelin lipid enrichment and is implicated in modulation of
physiological and pathological responses in the nervous system
(134). Wijendran et al
(135) demonstrated that
dietary intake of AA can extend the carbon chain in vivo to
produce AdA, which accumulates in the brain, suggesting that the
extended action of AA can produce AdA in the brain. Nevertheless,
levels of FAs in the brain largely depend on availability of the
peripheral FA pool due to the markedly low activity of enzymes
involved in FA metabolism in the brain (136). Orally ingested AdA can cross
the blood-brain barrier (BBB) as an ethyl ester after metabolic
conversion and accumulates in the brain, indicating AdA exerts
biological activity within the central nervous system (CNS)
(137). AdA has been found to
play a significant role in neurodevelopmental disorder and
neurodegenerative pathologies, mediating key pathophysiological
processes (138,139). The levels of AdA and its
oxidized products in the body may serve as biomarkers in
pathological conditions such as AD, Rett syndrome (RTT),
Parkinson's disease (PD) and epilepsy (15,17,105,140,141). Furthermore, in rats exposed to
heavy metal mixture, serum metabolomic analysis reveals notable
abnormalities in the biosynthetic pathways of unsaturated FAs, as
well as significantly elevated levels of AA and AdA (142). These metabolic changes may be
associated with heavy metal-induced neurological dysfunction
(142).
AdA and neurodevelopmental disorder
Neurodevelopmental disorders are a group of genetic
or environmental conditions that affect brain development and
function. These include attention deficit hyperactivity disorder
(ADHD) and RTT (143). Several
studies have revealed that AdA serves a critical regulatory role in
neurodevelopmental disorder (144-146).
ADHD is a prevalent neurodevelopmental disorder
that impairs capacity to concentrate, consequently influencing
academic, social, and daily living functions. A study has shown a
negative association between AdA and personality stability traits,
including emotional stability, responsibility and agreeableness
(144). Personality traits in
adolescent males with ADHD may be associated with FA composition of
erythrocyte membranes. Therefore, modifying FA content may affect
personality traits and behaviours of adolescent males with ADHD. Wu
et al (145) combined
lipidomics with psychological Bayley-III scale screening and found
that the AdA content in human breast milk lipids is significantly
negatively associated with infant adaptive behaviour development.
The aforementioned study involved supplementing Caenorhabditis
elegans L1-L4 larvae with AdA to simulate AdA uptake of infants
through breast milk: AdA uptake at concentrations >1 µM
during the larval stage of C. elegans negatively impacts
development of locomotive behaviours, foraging ability, chemotaxis
and aggregation behaviours. AdA supplementation during larval
stages impairs neurobehavioral development by upregulating
intracellular ROS levels, blocking 5-hydroxytryptaminergic
synthesis and fragmenting 5-hydroxytryptaminergic neurons (145). Additionally, AdA inhibits
expression of dauer formation abnormal 16 (DAF-16) and
DAF-16-regulated genes metallothionein-like protein 1 (MTL-1) and
-2 and SOD-1 and -3 in the ROS quenching system, leading to the
shortened lifespan of C. elegans. Increased intracellular
ROS damages DNA and the DNA repair system, leading to accumulated
DNA damage and cell death (145). RTT is also a neurodevelopmental
disorder caused by a genetic mutation that predominantly affects
females. Its first stage is most severe and is characterized by
notable loss of neurological function and white matter atrophy
(140). During this stage,
patients exhibit significantly elevated plasma levels of
F2-DH-IsoPs. These levels are positively associated with disease
duration and symptom intensity and negatively associated with brain
white matter score (146).
F2-DH-IsoPs serve as early biomarkers of RTT, reflecting oxidative
damage and dysfunction of brain white matter (146).
In summary, there is a negative association between
AdA and ADHD, which may affect personality traits and behaviour. In
addition, excessively high levels of AdA impair neurobehavioral
development and increase intracellular ROS levels.
AdA and AD
AD is a prevalent neurodegenerative condition that
results in the deterioration of memory and cognitive and
behavioural abilities (147).
Ageing is the most notable risk factor for developing AD. The
hippocampus is one of the first areas to experience atrophy in AD
and is also where neurofibrillary tangles develop initially
(148). Phospholipid changes
serve a critical role in the pathogenesis of AD (149). AdA-containing phospholipids in
the hippocampus and other brain regions decline with age. This
phenomenon has been observed in normal ageing and neurodegenerative
diseases such as AD (150,151). Another study identified a
reduction in AdA in the membrane phospholipid components PE and
phosphatidylserin in the parahippocampal cortex of patients with AD
(152). This remodelling of
membrane lipids may be associated with development of neurological
dysfunction. Amyloid β (Aβ) is a marker of neurodegeneration
associated with AD (153,154). A cross-sectional study has
shown that high serum levels of AdA in older adults with cognitive
impairment are associated with low levels of Aβ aggregation in the
brain (138), indicating that
AdA may exert a protective effect on neurocognitive function.
Furthermore, a study of total lipid fractions from disparate
regions of the brain has demonstrated that AdA levels are markedly
diminished in white matter of the frontal, parietal and
parahippocampal regions of the brain in patients with AD, whereas
they are 3-4 times higher than normal in grey matter (155). This may indicate varying
metabolic and lipid processing patterns in different brain
regions.
Neurodegenerative diseases involve lipid
peroxidation, which is notable due to the high lipid content and
oxygen consumption of brain tissue. The brain is particularly
vulnerable to oxidative damage due to low levels of antioxidant
enzymes, including catalase and glutathione peroxidase (156). Roberts and Milne (157) demonstrated that levels of the
AdA peroxidation products F2-DH-IsoPs are markedly elevated in
patients with AD and brain white matter undergoing oxidative
damage. This suggests that AdA is rapidly peroxidised when white
matter is subjected to oxidative damage, which generates a high
quantity of peroxidation products. This partly explains the
aforementioned reduction in brain tissue and serum AdA levels in
patients with AD.
In conclusion, decreased levels of serum AdA and
AdA-containing phospholipids in brain regions in patients with AD
may indicate that AdA exerts a neuroprotective effect on cognitive
function. However, AdA levels demonstrate contrasting trends in
different brain regions, with elevated levels in grey and decreased
levels in white matter. Thus, metabolic processes and lipid
processing between these regions differ. Further research should
determine the mechanisms through which AdA regulates inflammation,
neuroprotection and lipid metabolism, as well as its potential as a
therapeutic target.
AdA and PD
The primary biochemical characteristic of PD is the
aggregation of aberrant α-synuclein (α-SYN) assemblies in neurons,
which is mediated by misfolded conformational isoforms of the
protein (158). α-SYN is
capable of binding to PUFA, which induces the oligomerization of
α-SYN and subsequently results in neurotoxicity (159). In vitro experiments by
Xylaki et al (160)
utilizing a human neuroblastoma cell line demonstrated that free
cytoplasmic AA and AdA are key factors influencing the type of
intracellular α-SYN oligomerization. Application of cytosolic
phospholipase A2 (cPLA2) inhibitor GK200
results in a 26% decrease in free AA and a 72% decrease in AdA
levels. This regulates the conformational changes of α-SYN, with a
decrease in α-helical multimers and the formation of β-sheet
oligomers (160). This
contributes to the degradation of the α-SYN proteasome and
significantly decreases the intracellular α-SYN levels. Decreased
levels of free AA and AdA results in diminished cell membrane
fluidity and a weakened membrane-binding capacity of α-SYN
(160). This enhances cell
survival and contributes to the alleviation of the pathological
process of PD (160).
In conclusion, AdA and AA serve a key role in PD,
influencing α-SYN aggregation and cytotoxicity. This indicates that
modulating metabolic levels of AdA and AA may represent a promising
therapeutic avenue.
AdA and depression
Depression is a prevalent disorder of the CNS that
notably impacts psychosocial functioning and decreases quality of
life. Its pathogenesis involves alterations in specific
neurotransmitters, dysfunction of the endocrine system and
inflammatory responses (161,162). AdA plays a dual role in
depression risk. A study using Mendelian randomization to
investigate the causal association between FAs and risk of
depression found that high levels of AdA are associated with low
risk of depression (163).
Conversely, a prospective cohort study showed that high serum
levels of AdA may be a risk factor for suicide and major depressive
episodes in early pregnancy (164). The aforementioned study
evaluated the psychiatric state of pregnant patients and their
serum FA composition between the 6 and 13th weeks of gestation. The
results showed that high serum AdA levels are associated with a
high likelihood of suicidality and major depressive episodes in
early pregnancy (164). This
contradicts previous studies and suggests the physiological and
psychological state of pregnant patients may influence the role of
AdA in depression (163,164).
Two studies have investigated the association between serum FA
patterns and depression in adults: Heightened levels of AdA are
associated with increased risk of depression, whereas low levels of
ω-6 FAs, including AdA, may protect against depression (165,166). A cross-sectional study revealed
that levels of AA, AdA and oleic acid are strongly associated with
severity of depressive symptoms as determined by subject work
characteristic curve analysis (167). This suggests they may serve as
biomarkers for the assessment of depression (167).
Furthermore, studies have demonstrated the
potential of AdA to interact with neuroinflammatory processes
(15,168). Alterations in gut microbiota
and its metabolites may influence onset and progression of
depression by regulating the gut-brain axis (169). The active ingredient in the
drug Lily Dihuang Tang is metabolised by intestinal flora into FAs,
including AdA, after intestinal absorption. Network pharmacological
analyses and molecular docking simulations suggest FA metabolism
may be one of the potential therapeutic targets of the
aforementioned drug (168).
Furthermore, the key target gene for depression, FA amide hydrolase
(FAAH), demonstrates the strongest binding affinity with AdA
(168). FAAH is associated with
neuroinflammatory and neurodegenerative disease and FAAH inhibitors
may serve as antidepressant drugs (170,171). Thus, AdA may participate in
FAAH-mediated neuroinflammatory processes by binding with high
affinity to FAAH, influencing development of depression.
Furthermore, AdA levels are markedly altered during bacterial
lipopolysaccharide (LPS)-mediated neuroinflammation and microglia
activation in C57BL/6 mice, serving as a potential biomarker for
differentiating between control and LPS-treated groups (172). This indicates that AdA may be
associated with LPS-induced neuroinflammatory responses and may
regulate inflammatory processes or microglia activation, but the
precise mechanisms have yet to be determined. These findings
underscore the role of AdA in the study of biomarkers associated
with neuroinflammation.
The aforementioned findings demonstrate potential
pathogenesis of depression and its causal association with AdA,
thereby revealing a role for AdA in the risk of depression.
Although certain studies indicate that elevated AdA levels may
provide a preventive effect, they may also increase risk of
depression in pregnant patients and adults (163). Furthermore, AdA may interact
with neuroinflammatory processes. A large-scale longitudinal cohort
study is required to compare the relationship between AdA levels
and incidence of depression in different populations to elucidate
the role of AdA in depression. Furthermore, animal models should be
used to examine the effects of AdA on depressive symptoms and its
underlying mechanisms, particularly for key target genes associated
with depression, such as FAAH. The effect of intestinal flora on
AdA production and its role in depression should be investigated,
as well as the underlying mechanisms of the gut-brain axis.
Hence, AdA is a risk factor for neurodevelopmental
disorder and PD. However, AD may exert a protective effect on
neurocognitive function, with a dual role in risk of depression.
Its peroxidation product, F2-DH-IsoPs, induces oxidative stress in
the CNS and mediates neurological pathology (Fig. 5; Tables II and III).
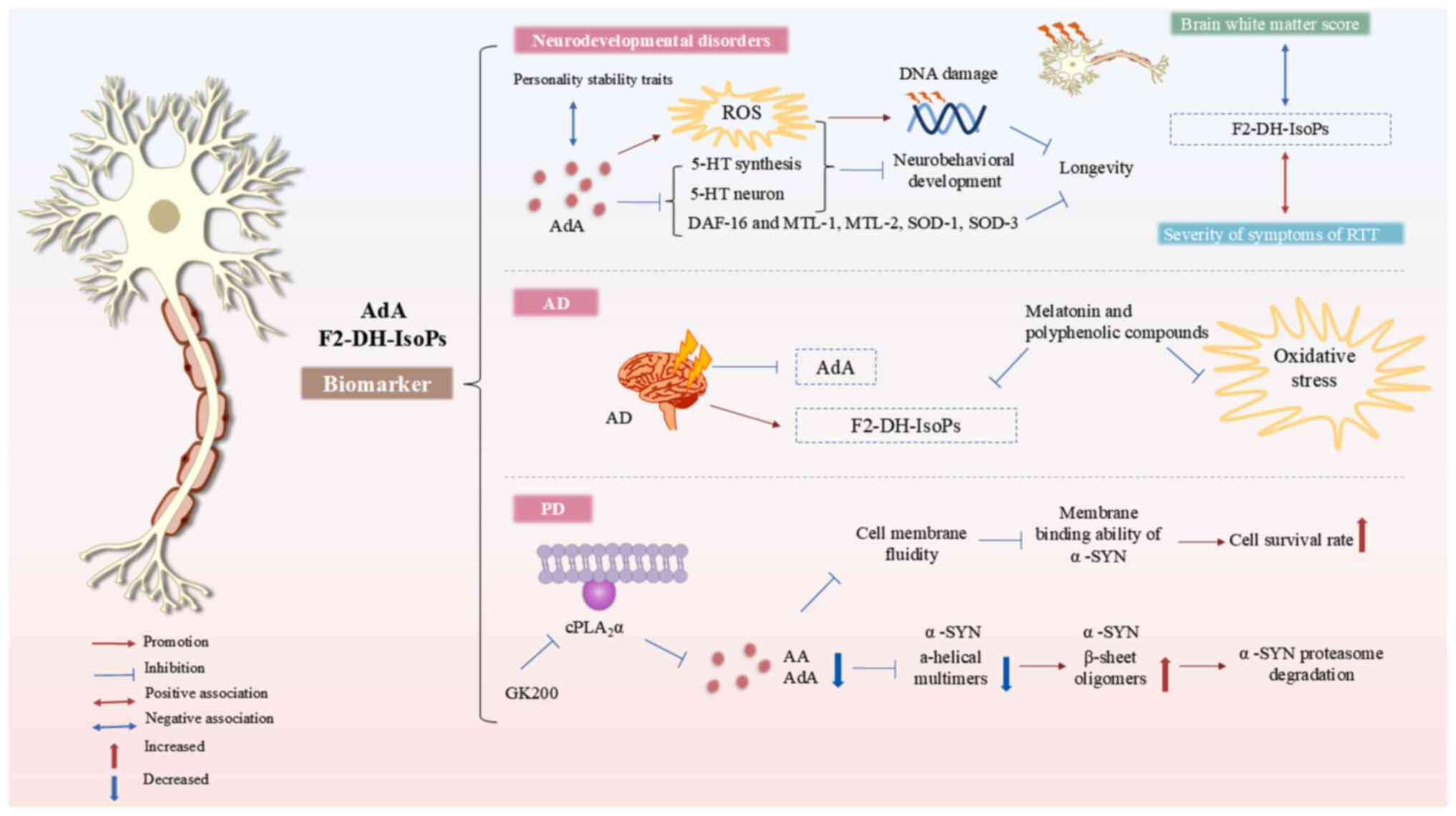 | Figure 5Mechanism of action of AdA in
neurological disease. AdA and its oxidation products are potential
biomarkers for neurodevelopmental and neurodegenerative diseases.
Exogenous AdA increases ROS levels, damages DNA and inhibits 5-HT
synthesis, impairing neurobehavioral development. AdA decreases
lifespan of Caenorhabditis elegans by downregulating
expression of ROS-quenching genes such as DAF-16, MTL-1 and -2 and
SOD-1 and -3. In RTT, elevated plasma F2-dihomo-IsoPs levels are
associated with disease severity. AdA negatively affects traits
such as emotional stability. In AD, AdA levels fluctuate, but
F2-dihomo-IsoPs remain elevated. Melatonin and polyphenolic
compounds mitigate oxidative stress by decreasing F2-dihomo-IsoP
levels in the central nervous system. The cPLA2
inhibitor GK200 decreases free AA and AdA levels, causing
conformational changes in α-SYN. This leads to fewer α-helical
multimers, more β-sheet oligomers and enhanced α-SYN degradation,
lowering its intracellular levels. Decreased levels of free AA and
AdA also decrease membrane fluidity and weaken α-SYN
membrane-binding, improving cell survival and mitigating
Parkinson's disease pathology. AdA, adrenic acid; ROS, reactive
oxygen species; 5-HT, 5-hydroxytryptaminergic; DAF, dauer formation
abnormal; MTL, metallothionein-like protein; SOD, superoxide
dismutase; DH, dihomo; RTT, Rett syndrome; IsoP, isoprostane; AD,
Alzheimer's disease; cPLA2, cytosolic phospholipase
A2; AA, arachidonic acid; SYN, synuclein. |
 | Table IIAdA levels in different disease
states. |
Table II
AdA levels in different disease
states.
| Disease | Experimental model
| Clinical
research | Results | (Refs.) |
|---|
| In vivo | In
vitro |
|---|
| NAFLD | PCB126-induced SD
male rats | | | Increased AdA
levels | (52) |
| Mice induced by MCD
diet | | | AdA is positively
associated with apoptosis of DNT cells | (49) |
| Patients | AdA is positively
associated with ALT | (5,75) |
| NASH | | | Patients | Increased levels of
esterified and unesterified free AdA in plasma | (72) |
| Mice induced by HFD
lacking choline and restricted amino acids | | | Increased levels of
free AdA and phospholipids containing AdA in liver and plasma and
endogenous synthesis of AdA in liver peroxisome; decreased AdA
catabolism | (5) |
| NASH-associated
hepatocellular carcinoma | | | Patients | Increased plasma
AdA concentration | (49,73) |
| Steatosis | | | Children with
severe steatosis | Increased plasma
AdA concentration | (74) |
| Cirrhosis | | | Patients with
HBV-associated cirrhosis | AdA is positively
associated with PPARγ in HSCs | (76) |
| Obesity | | | Children | Increased plasma
AdA levels | (74,77) |
| HFD-fed rats | | | Increased plasma
AdA levels | (78) |
| IR | Diabetic rats | | | Increased AdA level
in liver | (86,87) |
| Obese patients | Increased AdA
levels | (11) |
| T2DM | | | Patients | Increased AdA
levels in plasma | (89) |
| AS | | | Patients with high
risk of coronary heart disease | Increased AdA
level | (113) |
| Apo E knockout
mice | | | Increased levels of
AdA and its oxidation products in the heart | (114) |
| SHRs fed high
DHAdiet | | | Decreased AdA
synthesis | (122) |
| SHRs fed egg white
peptides | | | Decreased AdA
level | (123) |
| | Elderly patients
with hyperlipidemia | Plasma Hcy is
positively associated with AdA | (124) |
| Alcoholic
patients | Increased serum AdA
levels | (125) |
| Rats exposed to
heavy metals | | | Increased AdA
level | (142) |
| Neurodevelopmental
disorder | | | Adolescent males
with ADHD | AdA is negatively
associated with personality stability traits | (144) |
| AD | | | Healthy elderly
adults | Decreased
AdA-containing phospholipids | (150, 151) |
| | Patients | Decreased PE-AdA
and PS-AdA; increased F2-dihomo-IsoP levels | (152, 157) |
| | Elderly patients
with cognitive impairment | Decreased serum
AdA | (138) |
| PD | | Human neuroblastoma
cells treated with GK200 | | Decreased AdA,
α-helix polymer and α-SYN levels; increased β-sheet levels | (160) |
| Depression | | | Pregnant
patients | Increased serum AdA
levels | (164) |
| | Adults | Elevated AdA levels
are associated with higher risk of depression | (165, 166) |
| LPS-treated C57BL/6
mice | | | | (172) |
 | Table IIIMechanisms underlying effects of
exogenous AdA treatment on different diseases. |
Table III
Mechanisms underlying effects of
exogenous AdA treatment on different diseases.
| Disease | Experimental model
|
Upregulation/activation |
Downregulation/inhibition | (Refs.) |
|---|
| In vivo | In
vitro |
|---|
| NAFLD | | 500 µM
AdA-induced HepG-2 cells | ROS production,
oxidative stress | Cell viability,
expression of antioxidant enzyme Gpx1 | (1) |
| 50 µM
AdA-stimulated TCRαβ and TCRγδ DNT cells | Apoptosis | PI3K/AKT signaling
pathway and its downstream molecule c-Myc mRNA expression, GZMB
secretion, immuno-suppressive function | (49) |
| 0.5 mM AdA +
TNFα-treated HepG2 cells | Autologous TNFα
levels, IL-8, MIP1β and MCP1 mRNA expression, inflammation,
fibrosis | | (5) |
| AS | |
1×10−9-1×10−5 M
AdA/DH-16,17-EET-induced bovine coronary arteries |
Concentration-dependent relaxation of
bovine coronary endothelial cells, K+ channels in
vascular smooth muscle cells, K+ efflux,
hyperpolarization of vascular smooth muscle cells,
vasodilation | | (29) |
|
10−8-10−4 M
AdA-induced bovine adrenal cortical arteries, zona glomerulosa
cells |
Concentration-dependent relaxation of
adrenal cortical artery and K+ channels and efflux,
endothelial cell hyperpolarization, vasodilation | | (2) |
| Human umbilical
vein endothelial cells treated with AdA and AA | Regulation of
homeo-stasis of vascular PGs | Platelet
aggregation, production of PGI2 | (9) |
| AdA-enriched human
EC cells stimulated with thrombin for 4 h | TF activity, blood
clot formation, inflammatory reaction of the blood vessel lining,
damage to the blood vessel wall | Arterial blood
flow | (106, 117, 118) |
| Neuro-developmental
disorder | Supplementationof
AdA inHidradenitiselegans cryptic rodentia larvae | | | Neurobehavioral
development, 5-hydroxytryptamine synthesis,
5-hydroxytrypta-minergic neuron, expression of DAF-16 and its
regulatory genes MTL-1 and -2 and SOD-1-3, longevity | (145) |
Methods of regulating AdA
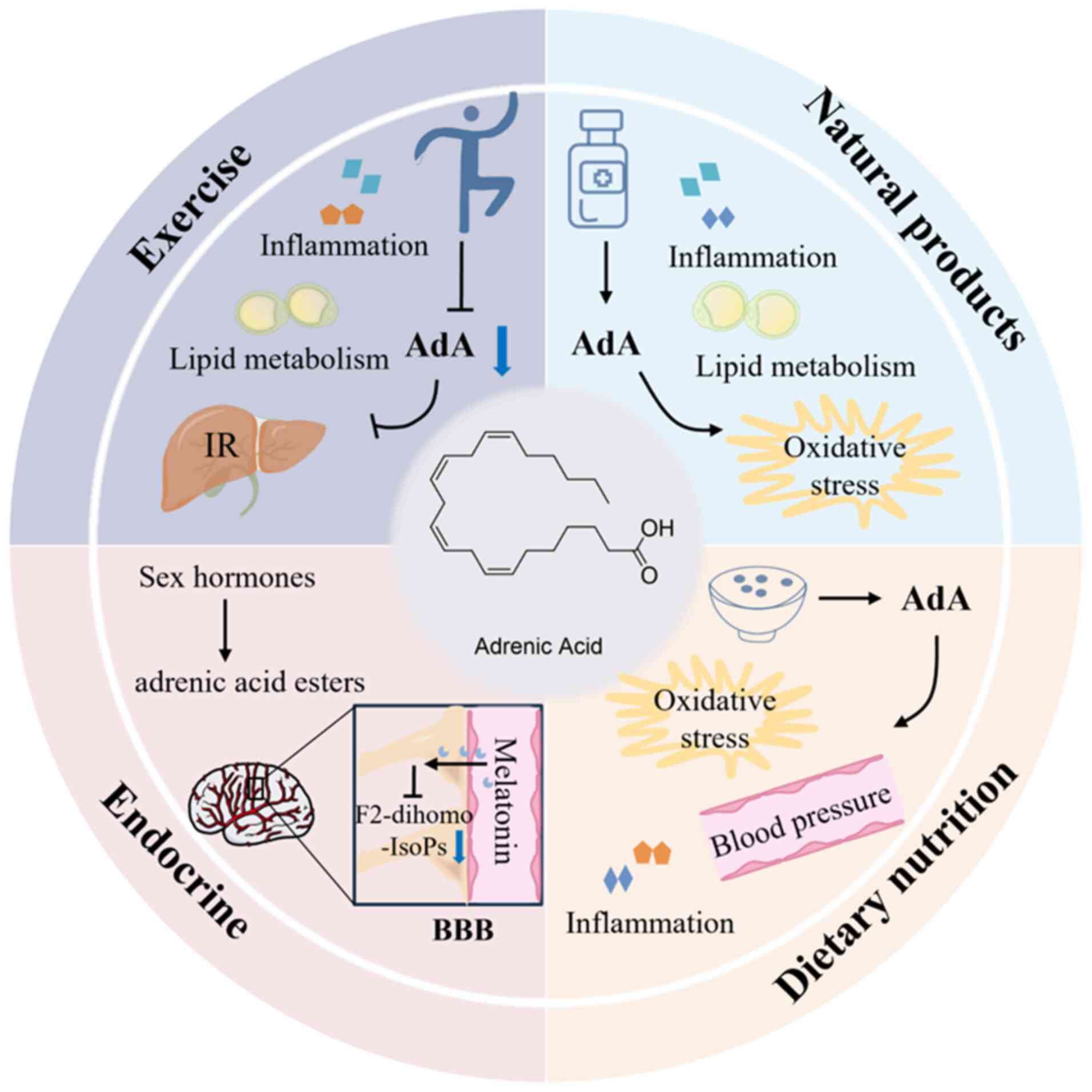
Exercise
Exercise has a wide range of physiological effects
on the human body, and research supports the benefits of exercise
in treating metabolic disorders, improving brain function and
preventing cardiovascular disease and cancer (173-175). Exercise can reduce hepatic
lipid deposition in T2DM mice by decreasing hepatic AdA
concentration, improving IR and lipid metabolism signalling
pathways and decreasing inflammation levels (176). Further ex vivo
experiments have investigated the effects of exogenous AdA
treatment on AML12 hepatocytes: Inflammatory factors such as IL-6,
Il-1β, TNF-α and MIP1β are activated in the cells following AdA
treatment, whereas AdA significantly increases the glucose content
in supernatant and decreases expression levels of IRS1, Akt and
glucose transporter 2 (176).
Furthermore, AdA treatment significantly increases the expression
levels of the lipid metabolism-related genes COX1, medium-chain
acyl-CoA dehydrogenase and FA transport protein 2 but decreases the
expression level of FA transporter molecule microsomal triglyceride
transfer protein. Thus, AdA may be key for improving hepatic IR and
lipid metabolism and decreasing inflammation in T2DM mice via
exercise (176). Furthermore, a
randomized controlled trial demonstrated that long-term
moderate-intensity exercise training significantly decreases plasma
levels of ω-6 FA oxides, such as AdA, and improves overall
metabolic health and cardiovascular function in young, sedentary
adults (177).
In conclusion, the aforementioned findings
underscore the pivotal role of AdA in exercise-induced
physiological adaptations and offer novel avenues for further
investigation into the association between exercise and lipid
metabolism.
Natural products
Several studies have used metabolomic approaches to
investigate the metabolic processes and protective mechanisms of
natural products and synthetic drugs affecting AdA (18-21,178). Oral administration of AS IV has
been demonstrated to decrease serum AdA levels in rats by
influencing the unsaturated FA metabolic pathway (21). Moreover, the effects of AS IV
involve amelioration of inflammatory response and oxidative stress,
alleviating cisplatin-induced AKI. Hence, serum AdA has been
reported as a highly sensitive biomarker for the identification of
AKI (21). EVO alleviates
hyperglycaemia, hyperlipidaemia and IR, whilst also improving
oxidative stress and inflammatory response in rats with T2DM.
Untargeted metabolomic analysis revealed that EVO treatment affects
the levels of 26 metabolites, including AdA (18). Although the precise regulatory
mechanisms remain to be elucidated, the metabolic modulatory
effects of EVO in T2DM rats may include metabolic pathways
associated with AdA (18).
Similarly, quercetin exhibits hepatoprotective effects on NAFLD
rats by regulating levels of FAs such as AdA and metabolites
related to inflammation and oxidative stress (20). According to a previous report,
kaempferol regulates the expression of genes associated with FA
degradation and metabolic pathways, including CYP2b9 and CYP4a12b
(19). This regulation affects
nine serum and three liver metabolites, including AA, and improves
pathophysiological processes such as energy and lipid metabolism,
inflammation and oxidative stress in NASH mice (19). RA improves lipid levels in
hyperlipidaemic mice. The underlying mechanism may pertain to RA
regulation of the metabolic levels of FAs, including AdA, in the
cecum of mice by modulating expression of CYP4a family genes
(CYP4a10, 12b, 31 and 32) associated with FA degradation in the
liver (178). In addition to
its association with metabolic disorder, AdA is associated with
inflammatory disease. The administration of prebiotics
significantly restores faecal metabolite AdA levels in patients
with post-traumatic osteoarthritis (179). Furthermore, there is an
association between AdA and development of post-traumatic
osteoarthritis (179).
The effects of the aforementioned drugs on AdA
metabolism are mediated by common mechanisms and involve
cross-talk. AS IV, EVO, quercetin and prebiotics may affect levels
of AdA by modulating the unsaturated FA metabolic pathway.
Additionally, kaempferol and RA modulate expression of key enzymes
for FA degradation, such as the CYP4a family of genes. Moreover,
these drugs improve metabolism-associated pathophysiological states
by modulating inflammatory responses and oxidative stress. Notably,
metabolomic analyses have identified AdA as a potential biomarker
in diverse experimental models, demonstrating its role in metabolic
regulation (20,123). These shared mechanisms and
cross-talk indicate that distinct pharmacological agents may
interact via analogous metabolic pathways and biomarkers to
regulate AdA levels, improving the pathophysiological process of
associated diseases.
In conclusion, alterations in serum AdA metabolism
are associated with several pharmacological interventions and
influence pathogenesis of numerous pathological conditions,
including T2DM, AKI, NASH and NAFLD, indicating the potential value
of AdA as a biomarker and therapeutic target.
Dietary nutrition
In a study involving healthy volunteers, the daily
consumption of 20 capsules of fish oil concentrate MaxEPA (3.6
eicosapentaenoic acid + 2.4 g DHA) results in a significant
decrease in AdA levels in platelet phospholipids from 7.9 to 3.1
mol% (180). Supplementation
with 30 mg/day of elemental zinc for 24 months increases the
abundance of PUFAs, including AdA, in the erythrocyte membranes of
patients with T2DM and improves membrane flexibility (181). A nutritional supplement
enriched with polyphenolic compounds inhibits oxidative stress in
the CNS by attenuating urinary levels of F2-DH-IsoPs (182). Furthermore, a study analysed
the FA content and oxidized lipids in rainbow trout fillets via gas
chromatography-mass spectrometry: Using high-oleic sunflower oil
for frying reduces AdA levels in fillets, and cooking decreases
levels of oxidized n-6 PUFA-derived lipids in the diet (183).
In addition, animal studies have demonstrated that
diet can modulate AdA levels in vivo (122,123). Consumption of a high-DHA diet
results in reduced levels of AdA in SHRs in vivo,
potentially due to the competitive inhibition of D5D activity by
DHA, which reduces AdA synthesis (122). Similarly, in an SHR model,
administration of 50 mg/kg body weight of egg white peptides for 4
weeks results in a significant decrease in serum AdA levels
(123). Zhou et al
(184) randomly assigned male
Wistar rats to be fed either vitamin A-deficient or an adequate
diet for 7 weeks. The vitamin A-deficient rats had elevated levels
of AdA in the colonic and hepatic tissue, whereas supplementation
with vitamin A decreased the AdA ratio. Feeding Yorkshire sows a
diet containing flaxseed decreases AdA levels in the loin and
abdomen (185). By contrast,
concentrate-fed yak calves experience increased AdA levels in the
pancreas, which result in anti-inflammatory and antibacterial
effects (186). Previous
studies have shown lead poisoning can cause carbon chain elongation
of FAs and induce lipid peroxidation, leading to dysregulation of
the intracellular antioxidant/pro-oxidant balance system (187,188). In mallards fed a diet
containing 200 g/kg lead, plasma cholesterol levels increase,
whereas TG levels decrease (189). A notable decrease in pituitary
cell membrane lipids, particularly membrane phosphoglycerides, AA
and AdA, is observed in young rats that were fed a diet lacking
essential fats for 6 weeks following weaning (190).
In conclusion, metabolic levels of AdA are
regulated by a variety of dietary nutrients. Changes in AdA levels
are associated with physiological and pathological states, both in
healthy individuals and in animal models.
Endocrinology
The metabolism of cholesterol esters in the adrenal
gland is notably influenced by gonadal and sex hormones in
C57BL/10J and DBA/2J mice, and AdA esters are more sensitive to
this hormone-regulated response than other adrenal cholesterol
esters (191). Melatonin serves
as an antioxidant across the BBB, exerting a neuroprotective effect
by decreasing levels of F2-DH-IsoPs to protect AdA from oxidative
attack without significant changes in AdA levels (192).
In conclusion, the regulation of AdA through
various pathways, including exercise, natural products, dietary
nutrition and endocrinology, can effectively improve the
pathological state of disease, mitigate the associated inflammatory
response and promote metabolic health. Therefore, regulation of AdA
not only offers new potential targets for clinical intervention but
also provides an important basis for understanding the interaction
between exercise and nutrition in health management (Fig. 6)
Clinical applications
Recent clinical studies have demonstrated the
potential utility of peripheral AdA levels as a biomarker for
disease diagnosis (138,193).
Firstly, AdA is considered a predictive model for chronic risk in
patients with acute-phase DILI (12) and serves as a non-invasive
biomarker for liver fibrosis in patients with NASH, as well as for
early detection of stage I PDAC (13,17). Furthermore, AdA is used to
evaluate the extent of oxidative stress in neurological disorder,
including epilepsy, AD and cerebral white matter injury, via the
non-enzymatic peroxidation reaction that generates F2-DH-IsoPs and
isofurans (14,15). Additionally, AdA has demonstrated
potential in assessing renal transplantation function (16). Nevertheless, there is a lack of
research examining the clinical applications of targeted AdA
therapy, which represents a key research area for future work.
Dayaker et al (194) found that AdA synthesises a
neuroprotective D1 (NPD1) analogue. Research on NPD1 in animal
models of AD, patients with stroke and human retinal pigment
epithelial cells have confirmed that NPD1 suppresses activation of
the inflammatory factor IL-1β (195,196). NPD1 upregulates the expression
of the anti-apoptotic genes Bcl-2, Bcl-xl and Bfl-1/A1 but
decreases expression of the proapoptotic genes Bax and Bad. These
changes result in anti-inflammatory, anti-apoptotic and
neuroprotective effects (195,196). To the best of our knowledge,
however, the role of NPD1 analogues has not been reported. NPD1
analogues may act similarly to NPD1 by inhibiting inflammatory and
apoptotic signalling, thereby preventing cell degeneration
(197).
In clinical applications, the levels or activity of
AdA are generally measured through analysis of blood or tissue
samples. Commonly employed techniques include gas
chromatography-mass spectrometry and high-performance liquid
chromatography, which are effective for assessment of FA
composition and concentration. Furthermore, ELISA is used for the
identification of bioactive metabolites associated with AdA. These
methods provide quantitative data regarding AdA, facilitating the
assessment of its role in various diseases (76,198). Nevertheless, numerous
challenges are associated with the translation of these techniques
into clinical practice, including complexity of the measurement
process, lack of standardisation of methods and individual
metabolic differences. For example, the variation in AdA
concentrations across different physiological states and metabolic
differences amongst patient populations are crucial factors
influencing the clinical application of AdA (163,164). The interplay between AdA and
physiological and pathological processes complicates its
interpretation as a biomarker, thereby limiting its effectiveness
in disease diagnosis and treatment monitoring. Further research and
standardisation of measurement methods are essential to address
these issues.
Conclusion
AdA is a crucial metabolite implicated in critical
pathophysiological processes across several diseases. AdA is
directly involved in various pathophysiological processes. AdA
promotes oxidative stress by upregulating intracellular ROS levels
and decreasing the expression of antioxidant enzymes, thereby
disrupting redox homeostasis and inducing metabolic and
neurological disorder (1,146).
Furthermore, AdA enhances secretion of inflammatory mediators,
promotes local inflammatory responses and decreases the
immunosuppressive function of TCRαβ DNT cells, contributing to the
immune inflammatory response of AS and metabolic disease (5,6,49,114). Furthermore, AdA enhances the
activity of TF in endothelial cells, increasing risk of thrombosis
(106). Additionally, it
facilitates intra-arterial lipid deposition by modulating risk
factors such as high blood pressure, elevated Hcy levels, extreme
alcohol abuse and dyslipidaemia (122-126). In neurological disorder, AdA
damages DNA and DNA repair system, impairs neuronal structure and
modulates aberrant α-SYN conformation, which is associated with
promotion of neurodegenerative pathology (145,160). The dual roles of AdA in
different neurological diseases require further study (163,164). Furthermore, PE-AdA, a key
phospholipid that induces ferroptosis, is susceptible to the lipid
peroxidation-induced ferroptosis pathway (92,93). This suggests that AdA is a key
initiator of iron-dependent cell death in tissue cells. On the
other hand, AdA levels in the periphery are positively associated
with the progression and severity of a range of diseases and organ
damage, including NAFLD, NASH, T2DM, AD, epilepsy, cerebral white
matter injury, DILI, AKI and PDAC (12-17,105,140,141). This emphasises the potential of
AdA as a diagnostic biomarker (Fig.
7).
However, research on AdA is lacking. There is a
lack of direct research and the mechanism of action of AdA requires
further investigation. For example, AA and its metabolites are
typically regarded as proinflammatory and AdA has been shown to
elicit inflammatory responses (5). However, the anti-inflammatory
effects of AdA via inhibition of chemokine leukotriene B4
production have also been documented (199). Further research is required to
clarify the roles of n-6 PUFA. Adipose tissue is essential for
overall metabolic homeostasis as an endocrine organ and energy
reservoir (200,201). However, the association between
AdA and adipocyte differentiation, obesity-associated inflammatory
responses, energy metabolic processes and other obesity-associated
metabolic abnormalities has been rarely investigated (77,79). Consequently, future studies
should investigate the precise mechanisms of action. AdA has been
identified as a highly sensitive marker for several types of
disease, including NAFLD, NASH, T2DM, AD, epilepsy, cerebral white
matter injury, DILI, AKI and PDAC, but the specific molecular
mechanisms underlying its role remain to be elucidated. A
comprehensive investigation of molecular targets and signalling
pathways regulated by AdA, coupled with the identification of
biomarkers, is crucial for enhancing the precision of early
diagnosis and efficacy monitoring. The development of animal models
to mimic the role of AdA in disease is also essential. A second
shortcoming is the lack of clinical studies. At present, most
research on AdA remains at the in vitro and animal
experimentation stage, with a lack of clinical studies. Further
human experimentation and clinical studies are required to
substantiate the efficacy of AdA as a biomarker and therapeutic
target and translate these findings into clinical practice,
facilitating the development of efficacious therapeutic strategies.
Finally, novel therapeutic strategies need to be explored. AdA
metabolism is influenced by lifestyle factors, including exercise
and diet. Therefore, non-pharmacological therapeutic strategies for
AdA should be explored to target its metabolic pathways or modulate
its signalling and to understand the interaction between exercise
and nutrition in health management. The potential of exercise
therapy in addressing health concerns has interest because of its
unique benefits in safety and cost-effectiveness. Nevertheless,
literature examining the association between exercise and AdA is
scarce (176), highlighting the
need for further investigation into the potential of exercise to
improve disease outcomes via AdA. This may provide a theoretical
and practical foundation for prevention and treatment of diseases
through exercise.
In conclusion, AdA is a highly sensitive metabolite
in various types of disease and has broad potential applications.
Although there are currently no clinically applicable drugs
specifically targeting AdA, further studies and technological
developments may allow AdA to serve a promising biomarker and
therapeutic target for many diseases.
Availability of data and materials
Not applicable.
Authors' contributions
ZW conceived the study, performed the literature
review, constructed figures and wrote the manuscript. HG performed
the literature review and wrote the manuscript. XM and DZ edited
the manuscript and constructed figures. LZ conceived the study,
edited the manuscript and constructed figures. WX supervised the
study and constructed figures. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by National Natural Science
Foundation of China (grant no. 32371185), Shanghai Science and
Technology Plan Project (grant no. 23010504200), Shuguang Program
(grant no. 20SG50) funded by the Shanghai Education Development
Foundation and Shanghai Municipal Education Commission, Shanghai
Talent Development Fund (grant no. 2020125), Key Lab of Exercise
and Health Sciences of Ministry of Education (Shanghai University
of Sport; grant no. 2022KF001) and Shanghai Key Lab of Human
Performance (grant no. 11DZ2261100).
References
|
1
|
Zhao J, Nishiumi S, Tagawa R, Yano Y,
Inoue J, Hoshi N, Yoshida M and Kodama Y: Adrenic acid induces
oxidative stress in hepatocytes. Biochem Biophys Res Commun.
532:620–625. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kopf PG, Zhang DX, Gauthier KM,
Nithipatikom K, Yi XY, Falck JR and Campbell WB: Adrenic acid
metabolites as endogenous endothelium-derived and zona
glomerulosa-derived hyperpolarizing factors. Hypertension.
55:547–554. 2010. View Article : Google Scholar
|
|
3
|
Guijas C, Astudillo AM, Gil-de-Gómez L,
Rubio JM, Balboa MA and Balsinde J: Phospholipid sources for
adrenic acid mobilization in RAW 264.7 macrophages. Comparison with
arachidonic acid. Biochim Biophys Acta. 1821:1386–1393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Massey KA and Nicolaou A: Lipidomics of
polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochem
Soc Trans. 39:1240–1246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horas H, Nababan S, Nishiumi S, Kawano Y,
Kobayashi T, Yoshida M and Azuma T: Adrenic acid as an inflammation
enhancer in non-alcoholic fatty liver disease. Arch Biochem
Biophys. 623-624:64–75. 2017. View Article : Google Scholar
|
|
6
|
Delgado GE, März W, Lorkowski S, von
Schacky C and Kleber ME: Omega-6 fatty acids: Opposing associations
with risk-the ludwigshafen risk and cardiovascular health study. J
Clin Lipidol. 11:1082–1090.e14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jarrar MH, Baranova A, Collantes R, Ranard
B, Stepanova M, Bennett C, Fang Y, Elariny H, Goodman Z, Chandhoke
V and Younossi ZM: Adipokines and cytokines in non-alcoholic fatty
liver disease. Aliment Pharmacol Ther. 27:412–421. 2008. View Article : Google Scholar
|
|
8
|
Gavino VC, Miller JS, Dillman JM, Milo GE
and Cornwell DG: Effect of exogenous adrenic acid on the
proliferation and lipid metabolism of cells in tissue culture. Prog
Lipid Res. 20:323–325. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Campbell WB, Falck JR, Okita JR, Johnson
AR and Callahan KS: Synthesis of dihomoprostaglandins from adrenic
acid (7,10,13,16-docosatetraenoic acid) by human endothelial cells.
Biochim Biophys Acta. 837:67–76. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan JY, Lin X, Xu F, Shan SK, Guo B, Li
FX, Wang Y, Zheng MH, Xu QS, Lei LM, et al: Ferroptosis and its
potential role in metabolic diseases: A curse or revitalization?
Front Cell Dev Biol. 9:7017882021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
López-Gómez C, Santiago-Fernández C,
García-Serrano S, García-Escobar E, Gutiérrez-Repiso C,
Rodríguez-Díaz C, Ho-Plágaro A, Martín-Reyes F, Garrido-Sánchez L,
Valdés S, et al: Oleic acid protects against insulin resistance by
regulating the genes related to the PI3K signaling pathway. J Clin
Med. 99:26152020. View Article : Google Scholar
|
|
12
|
Zhao S, Fu H, Zhou T, Cai M, Huang Y, Gan
Q, Zhang C, Qian C, Wang J, Zhang Z, et al: Alteration of bile
acids and omega-6 PUFAs are correlated with the progression and
prognosis of drug-induced liver injury. Front Immunol.
13:7723682022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caussy C, Chuang JC, Billin A, Hu T, Wang
Y, Subramanian GM, Djedjos CS, Myers RP, Dennis EA and Loomba R:
Plasma eicosanoids as noninvasive biomarkers of liver fibrosis in
patients with nonalcoholic steatohepatitis. Therap Adv
Gastroenterol. 13:17562848209239042020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Medina S, Miguel-Elizaga ID, Oger C,
Galano JM, Durand T, Martínez-Villanueva M, Castillo ML,
Villegas-Martínez I, Ferreres F, Martínez-Hernández P and
Gil-Izquierdo Á: Dihomo-isoprostanes-nonenzymatic metabolites of
AdA-are higher in epileptic patients compared to healthy
individuals by a new ultrahigh pressure liquid
chromatography-triple quadrupole-tandem mass spectrometry method.
Free Radic Biol Med. 79:154–163. 2015. View Article : Google Scholar
|
|
15
|
Ferré-González L, Peña-Bautista C, Baquero
M and Cháfer-Pericás C: Assessment of lipid peroxidation in
Alzheimer's disease differential diagnosis and prognosis.
Antioxidants (Basel). 11:5512022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Las Heras-Gómez I, Medina S, Casas-Pina
T, Marín-Soler L, Tomás A, Martínez-Hernández P, Oger C, Galano JM,
Durand T, Jimeno L, et al: Potential applications of lipid
peroxidation products-F4-neuroprostanes,
F3-neuroprostanesn-6 DPA,
F2-dihomo-isoprostanes and F2-isoprostanes-in
the evaluation of the allograft function in renal transplantation.
Free Radic Biol Med. 104:178–184. 2017. View Article : Google Scholar
|
|
17
|
Cao Y, Zhao R, Guo K, Ren S, Zhang Y, Lu
Z, Tian L, Li T, Chen X and Wang Z: Potential metabolite biomarkers
for early detection of stage-I pancreatic ductal adenocarcinoma.
Front Oncol. 11:7446672022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Y, Lu Q and Chen F, Wang S, Niu C, Liao
J, Wang H and Chen F: Serum untargeted metabolomics analysis of the
mechanisms of evodiamine on type 2 diabetes mellitus model rats.
Food Funct. 13:6623–6635. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu Y, Shao M, Xiang H, Zheng P, Wu T and
Ji G: Integrative transcriptomics and metabolomics explore the
mechanism of kaempferol on improving nonalcoholic steatohepatitis.
Food Funct. 11:10058–10069. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Y, Han J, Dong J, Fan X, Cai Y, Li J,
Wang T, Zhou J and Shang J: Metabolomics characterizes the effects
and mechanisms of quercetin in nonalcoholic fatty liver disease
development. Int J Mol Sci. 20:12202019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Y, Hu T, Gao H, Zhai J, Gong J, Zhang
Y, Tao L, Sun J, Li Z and Qu X: Altered metabolic profiles and
biomarkers associated with astragaloside IV-mediated protection
against cisplatin-induced acute kidney injury in rats: An
HPLC-TOF/MS-based untargeted metabolomics study. Biochem Pharmacol.
183:1142992021. View Article : Google Scholar
|
|
22
|
Dai Y, Chen Y, Mo D, Jin R, Huang Y, Zhang
L, Zhang C, Gao H and Yan Q: Inhibition of ACSL4 ameliorates
tubular ferroptotic cell death and protects against fibrotic kidney
disease. Commun Biol. 6:9072023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galano JM, Lee JCY, Gladine C, Comte B, Le
Guennec JY, Oger C and Durand T: Non-enzymatic cyclic oxygenated
metabolites of adrenic, docosahexaenoic, eicosapentaenoic and
α-linolenic acids; bioactivities and potential use as biomarkers.
Biochim Biophys Acta. 1851:446–455. 2015. View Article : Google Scholar
|
|
24
|
Shanab SMM, Hafez RM and Fouad AS: A
review on algae and plants as potential source of arachidonic acid.
J Adv Res. 11:3–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sánchez-Illana Á, Shah V, Piñeiro-Ramos
JD, Di Fiore JM, Quintás G, Raffay TM, MacFarlane PM, Martin RJ and
Kuligowski J: Adrenic acid non-enzymatic peroxidation products in
biofluids of moderate preterm infants. Free Radic Biol Med.
142:107–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Visser WF, van Roermund CW, Ijlst L,
Waterham HR and Wanders RJA: Metabolite transport across the
peroxisomal membrane. Biochem J. 401:365–375. 2007. View Article : Google Scholar :
|
|
27
|
Monge P, Garrido A, Rubio JM, Magrioti V,
Kokotos G, Balboa MA and Balsinde J: The contribution of cytosolic
group iva and calcium-independent group VIA phospholipase
A2s to adrenic acid mobilization in murine macrophages.
Biomolecules. 10:5422020. View Article : Google Scholar
|
|
28
|
VanRollins M, Horrocks L and Sprecher H:
Metabolism of 7,10,13,16-docosatetraenoic acid to
dihomo-thromboxane, 14-hydroxy-7,10,12-nonadecatrienoic acid and
hydroxy fatty acids by human platelets. Biochim Biophys Acta.
833:272–280. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi XY, Gauthier KM, Cui L, Nithipatikom K,
Falck JR and Campbell WB: Metabolism of adrenic acid to
vasodilatory 1alpha,1beta-dihomo-epoxyeicosatrienoic acids by
bovine coronary arteries. Am J Physiol Heart Circ Physiol.
292:H2265–H2274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh N, Barnych B, Wagner KM, Wan D,
Morisseau C and Hammock BD: Adrenic acid-derived epoxy fatty acids
are naturally occurring lipids and their methyl ester prodrug
reduces endoplasmic reticulum stress and inflammatory pain. ACS
Omega. 6:7165–7174. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Osmundsen H, Bremer J and Pedersen JI:
Metabolic aspects of peroxisomal beta-oxidation. Biochim Biophys
Acta. 1085:141–158. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hagve TA and Christophersen BO: Evidence
for peroxisomal retroconversion of adrenic acid [22:4(n-6)] and
docosahexaenoic acids [22:6(n-3)] in isolated liver cells. Biochim
Biophys Acta. 875:165–173. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mann CJ, Kaduce TL, Figard PH and Spector
AA: Docosatetraenoic acid in endothelial cells: Formation,
retroconversion to arachidonic acid, and effect on prostacyclin
production. Arch Biochem Biophys. 244:813–823. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Costello KR and Schones DE: Chromatin
modifications in metabolic disease: Potential mediators of
long-term disease risk. Wiley Interdiscip Rev Syst Biol Med.
10:e14162018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cell Metabolism editorial team: Preventing
metabolic disease: Part I. Cell Metab. 36:2232024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chew NWS, Ng CH, Tan DJH, Kong G, Lin C,
Chin YH, Lim WH, Huang DQ, Quek J, Fu CE, et al: The global burden
of metabolic disease: Data from 2000 to 2019. Cell Metab.
35:414–428.e3. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee YS and Olefsky J: Chronic tissue
inflammation and metabolic disease. Genes Dev. 35:307–328. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
O'Rourke RW: Adipose tissue and the
physiologic underpinnings of metabolic disease. Surg Obes Relat
Dis. 14:1755–1763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhatti JS, Bhatti GK and Reddy PH:
Mitochondrial dysfunction and oxidative stress in metabolic
disorders-A step towards mitochondria based therapeutic strategies.
Biochim Biophys Acta Mol Basis Dis. 1863:1066–1077. 2017.
View Article : Google Scholar
|
|
40
|
Friedman SL, Neuschwander-Tetri BA,
Rinella M and Sanyal AJ: Mechanisms of NAFLD development and
therapeutic strategies. Nat Med. 24:908–922. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pouwels S, Sakran N, Graham Y, Leal A,
Pintar T, Yang W, Kassir R, Singhal R, Mahawar K and Ramnarain D:
Non-alcoholic fatty liver disease (NAFLD): A review of
pathophysiology, clinical management and effects of weight loss.
BMC Endocr Disord. 22:632022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Le MH, Le DM, Baez TC, Wu Y, Ito T, Lee
EY, Lee K, Stave CD, Henry L, Barnett SD, et al: Global incidence
of non-alcoholic fatty liver disease: A systematic review and
meta-analysis of 63 studies and 1,201,807 persons. J Hepatol.
79:287–295. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ye J, Tian X, Wang Q, Zheng J, Yang Y, Xu
B, Zhang S, Yuan F and Yang Z: Monkfish peptides mitigate high fat
diet-induced hepatic steatosis in mice. Mar Drugs. 20:3122022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang CH, Zhou BG, Sheng JQ, Chen Y, Cao
YQ and Chen C: Molecular mechanisms of hepatic insulin resistance
in nonalcoholic fatty liver disease and potential treatment
strategies. Pharmacol Res. 159:1049842020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kowdley KV, Belt P, Wilson LA, Yeh MM,
Neuschwander-Tetri BA, Chalasani N, Sanyal AJ and Nelson JE; NASH
Clinical Research Network: Serum ferritin is an independent
predictor of histologic severity and advanced fibrosis in patients
with nonalcoholic fatty liver disease. Hepatology. 55:77–85. 2012.
View Article : Google Scholar
|
|
46
|
Chen Z, Tian R, She Z, Cai J and Li H:
Corrigendum to 'Role of oxidative stress in the pathogenesis of
nonalcoholic fatty liver disease' [Free Radic. Biol. Med. 152(2020)
116-141]. Free Radic Biol Med. 162:1742021. View Article : Google Scholar
|
|
47
|
Paradies G, Paradies V, Ruggiero FM and
Petrosillo G: Oxidative stress, cardiolipin and mitochondrial
dysfunction in nonalcoholic fatty liver disease. World J
Gastroenterol. 20:14205–14218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vatner DF, Majumdar SK, Kumashiro N,
Petersen MC, Rahimi Y, Gattu AK, Bears M, Camporez JP, Cline GW,
Jurczak MJ, et al: Insulin-independent regulation of hepatic
triglyceride synthesis by fatty acids. Proc Natl Acad Sci USA.
112:1143–1148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li C, Du X, Shen Z, Wei Y, Wang Y, Han X,
Jin H, Zhang C, Li M, Zhang Z, et al: The critical and diverse
roles of CD4−CD8− double negative T cells in
nonalcoholic fatty liver disease. Cell Mol Gastroenterol Hepatol.
13:1805–1827. 2022. View Article : Google Scholar :
|
|
50
|
Masarone M, Rosato V, Dallio M, Gravina
AG, Aglitti A, Loguercio C, Federico A and Persico M: Role of
oxidative stress in pathophysiology of nonalcoholic fatty liver
disease. Oxid Med Cell Longev. 2018:95476132018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mazur-Bialy AI, Kozlowska K, Pochec E,
Bilski J and Brzozowski T: Myokine irisin-induced protection
against oxidative stress in vitro. Involvement of heme oxygenase-1
and antioxidazing enzymes superoxide dismutase-2 and glutathione
peroxidase. J Physiol Pharmacol. 69:117–125. 2018.PubMed/NCBI
|
|
52
|
Kania-Korwel I, Wu X, Wang K and Lehmler
HJ: Identification of lipidomic markers of chronic
3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) exposure in the male rat
liver. Toxicology. 390:124–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Boucher MP, Lefebvre C and Chapados NA:
The effects of PCB126 on intra-hepatic mechanisms associated with
non alcoholic fatty liver disease. J Diabetes Metab Disord.
14:882015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lai I, Chai Y, Simmons D, Luthe G, Coleman
MC, Spitz D, Haschek WM, Ludewig G and Robertson LW: Acute toxicity
of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley
rats: Effects on hepatic oxidative stress, glutathione and metals
status. Environ Int. 36:918–923. 2010. View Article : Google Scholar
|
|
55
|
Gadupudi GS, Klaren WD, Olivier AK,
Klingelhutz AJ and Robertson LW: PCB126-induced disruption in
gluconeogenesis and fatty acid oxidation precedes fatty liver in
male rats. Toxicol Sci. 149:98–110. 2016. View Article : Google Scholar :
|
|
56
|
Weinberg JM: Lipotoxicity. Kidney Int.
70:1560–1566. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Svegliati-Baroni G, Pierantonelli I,
Torquato P, Marinelli R, Ferreri C, Chatgilialoglu C, Bartolini D
and Galli F: Lipidomic biomarkers and mechanisms of lipotoxicity in
non-alcoholic fatty liver disease. Free Radic Biol Med.
144:293–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Peiseler M, Schwabe R, Hampe J, Kubes P,
Heikenwalder M and Tacke F: Immune mechanisms linking metabolic
injury to inflammation and fibrosis in fatty liver disease-novel
insights into cellular communication circuits. J Hepatol.
77:1136–1160. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hillhouse EE and Lesage S: A comprehensive
review of the phenotype and function of antigen-specific
immunoregulatory double negative T cells. J Autoimmun. 40:58–65.
2013. View Article : Google Scholar
|
|
60
|
Brandt D and Hedrich CM:
TCRαβ+CD3+CD4−CD8−
(double negative) T cells in autoimmunity. Autoimmun Rev.
17:422–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sun G, Zhao X, Li M, Zhang C, Jin H, Li C,
Liu L, Wang Y, Shi W, Tian D, et al: CD4 derived double negative T
cells prevent the development and progression of nonalcoholic
steatohepatitis. Nat Commun. 12:6502021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Roehlen N, Crouchet E and Baumert TF:
Liver fibrosis: Mechanistic concepts and therapeutic perspectives.
Cells. 9:8752020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zisser A, Ipsen DH and Tveden-Nyborg P:
Hepatic stellate cell activation and inactivation in
NASH-fibrosis-roles as putative treatment targets? Biomedicines.
9:3652021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mederacke I, Hsu CC, Troeger JS, Huebener
P, Mu X, Dapito DH, Pradere JP and Schwabe RF: Fate tracing reveals
hepatic stellate cells as dominant contributors to liver fibrosis
independent of its aetiology. Nat Commun. 4:28232013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Seki E, de Minicis S, Inokuchi S, Taura K,
Miyai K, van Rooijen N, Schwabe RF and Brenner DA: CCR2 promotes
hepatic fibrosis in mice. Hepatology. 50:185–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Schwabe RF, Bataller R and Brenner DA:
Human hepatic stellate cells express CCR5 and RANTES to induce
proliferation and migration. Am J Physiol Gastrointest Liver
Physiol. 285:G949–G958. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Krenkel O and Tacke F: Macrophages in
nonalcoholic fatty liver disease: A role model of pathogenic
immunometabolism. Semin Liver Dis. 37:189–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yan J, Nie Y, Liu Y, Li J, Wu L, Chen Z
and He B: Yiqi-bushen-tiaozhi recipe attenuated high-fat and
high-fructose diet induced nonalcoholic steatohepatitis in mice via
gut microbiota. Front Cell Infect Microbiol. 12:8245972022.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fan CY, Pan J, Usuda N, Yeldandi AV, Rao
MS and Reddy JK: Steatohepatitis, spontaneous peroxisome
proliferation and liver tumors in mice lacking peroxisomal fatty
acyl-CoA oxidase. Implications for peroxisome
proliferator-activated receptor alpha natural ligand metabolism. J
Biol Chem. 273:15639–15645. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mitsuyoshi H, Yasui K, Harano Y, Endo M,
Tsuji K, Minami M, Itoh Y, Okanoue T and Yoshikawa T: Analysis of
hepatic genes involved in the metabolism of fatty acids and iron in
nonalcoholic fatty liver disease. Hepatol Res. 39:366–373. 2009.
View Article : Google Scholar
|
|
71
|
Warner DR, Warner JB, Hardesty JE, Song
YL, King TN, Kang JX, Chen CY, Xie S, Yuan F, Prodhan MAI, et al:
Decreased ω-6:ω-3 PUFA ratio attenuates ethanol-induced alterations
in intestinal homeostasis, microbiota, and liver injury. J Lipid
Res. 60:2034–2049. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
de Almeida IT, Cortez-Pinto H, Fidalgo G,
Rodrigues D and Camilo ME: Plasma total and free fatty acids
composition in human non-alcoholic steatohepatitis. Clin Nutr.
21:219–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Muir K, Hazim A, He Y, Peyressatre M, Kim
DY, Song X and Beretta L: Proteomic and lipidomic signatures of
lipid metabolism in NASH-associated hepatocellular carcinoma.
Cancer Res. 73:4722–4731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hua MC, Su HM, Yao TC, Kuo ML, Lai MW,
Tsai MH and Huang JL: Alternation of plasma fatty acids composition
and desaturase activities in children with liver steatosis. PLoS
One. 12:e01822772017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ezaizi Y, Kabbany MN, Conjeevaram
Selvakumar PK, Sarmini MT, Singh A, Lopez R, Nobili V and Alkhouri
N: Comparison between non-alcoholic fatty liver disease screening
guidelines in children and adolescents. JHEP Rep. 1:259–264. 2019.
View Article : Google Scholar
|
|
76
|
Fan HN, Zhao ZM, Huang K, Wang XN, Dai YK
and Liu CH: Serum metabolomics characteristics and
fatty-acid-related mechanism of cirrhosis with histological
response in chronic hepatitis B. Front Pharmacol. 14:13292662023.
View Article : Google Scholar
|
|
77
|
Viitasalo A, Ågren J, Venäläinen T,
Pihlajamäki J, Jääskeläinen J, Korkmaz A, Atalay M and Lakka TA:
Association of plasma fatty acid composition with plasma irisin
levels in normal weight and overweight/obese children. Pediatr
Obes. 11:299–305. 2016. View Article : Google Scholar
|
|
78
|
Huang JP, Cheng ML, Hung CY, Wang CH,
Hsieh PS, Shiao MS, Chen JK, Li DE and Hung LM: Docosapentaenoic
acid and docosahexaenoic acid are positively associated with
insulin sensitivity in rats fed high-fat and high-fructose diets. J
Diabetes. 9:936–946. 2017. View Article : Google Scholar
|
|
79
|
Marco-Ramell A, Tulipani S,
Palau-Rodriguez M, Gonzalez-Dominguez R, Miñarro A, Jauregui O,
Sanchez-Pla A, Macias-Gonzalez M, Cardona F, Tinahones FJ and
Andres-Lacueva C: Untargeted profiling of concordant/discordant
phenotypes of high insulin resistance and obesity to predict the
risk of developing diabetes. J Proteome Res. 17:2307–2317. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lu Y, Day FR, Gustafsson S, Buchkovich ML,
Na J, Bataille V, Cousminer DL, Dastani Z, Drong AW, Esko T, et al:
New loci for body fat percentage reveal link between adiposity and
cardiometabolic disease risk. Nat Commun. 7:104952016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ma ZA, Zhao Z and Turk J: Mitochondrial
dysfunction and β-cell failure in type 2 diabetes mellitus. Exp
Diabetes Res. 2012:7035382012. View Article : Google Scholar
|
|
82
|
Deng X, Wang J, Jiao L, Utaipan T,
Tuma-Kellner S, Schmitz G, Liebisch G, Stremmel W and Chamulitrat
W: iPLA2β deficiency attenuates obesity and hepatic steatosis in
ob/ob mice through hepatic fatty-acyl phospholipid remodeling.
Biochim Biophys Acta. 1861:449–461. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Su X, Mancuso DJ, Bickel PE, Jenkins CM
and Gross RW: Small interfering RNA knockdown of
calcium-independent phospholipases A2 beta or gamma inhibits the
hormone-induced differentiation of 3T3-L1 preadipocytes. J Biol
Chem. 279:21740–21748. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Asano T, Fujishiro M, Kushiyama A, Nakatsu
Y, Yoneda M, Kamata H and Sakoda H: Role of phosphatidylinositol
3-kinase activation on insulin action and its alteration in
diabetic conditions. Biol Pharm Bull. 30:1610–1616. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bandyopadhyay GK, Yu JG, Ofrecio J and
Olefsky JM: Increased p85/55/50 expression and decreased
phosphotidylinositol 3-kinase activity in insulin-resistant human
skeletal muscle. Diabetes. 54:2351–2359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Takahashi R, Horrobin DF, Watanabe Y, Kyte
V and Billard V: Short-term diabetes increases triacylglycerol
arachidonic acid content in the rat liver. Biochim Biophys Acta.
921:151–153. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chanussot B, Asdrubal P, Huang YS and
Poisson JP: Adrenic acid delta4 desaturation and fatty acid
composition in the liver of marine-oil fed streptozotocin diabetic
rats. Prostaglandins Leukot Essent Fatty Acids. 57:539–544. 1997.
View Article : Google Scholar
|
|
88
|
No authors listed. Type 2 diabetes
mellitus. Nat Rev Dis Primers. 1:150392015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Forouhi NG, Imamura F, Sharp SJ, Koulman
A, Schulze MB, Zheng J, Ye Z, Sluijs I, Guevara M, Huerta JM, et
al: Association of plasma phospholipid n-3 and n-6 polyunsaturated
fatty acids with type 2 diabetes: The EPIC-interact case-cohort
study. PLoS Med. 13:e10020942016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sha W, Hu F, Xi Y, Chu Y and Bu S:
Mechanism of ferroptosis and its role in type 2 diabetes mellitus.
J Diabetes Res. 2021:99996122021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao
N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell
Death Dis. 11:882020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yang WS, Kim KJ, Gaschler MM, Patel M,
Shchepinov MS and Stockwell BR: Peroxidation of polyunsaturated
fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci
USA. 113:E4966–E4975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Stoyanovsky DA, Tyurina YY, Shrivastava I,
Bahar I, Tyurin VA, Protchenko O, Jadhav S, Bolevich SB, Kozlov AV,
Vladimirov YA, et al: Iron catalysis of lipid peroxidation in
ferroptosis: Regulated enzymatic or random free radical reaction?
Free Radic Biol Med. 133:153–161. 2019. View Article : Google Scholar :
|
|
94
|
Shimbara-Matsubayashi S, Kuwata H, Tanaka
N, Kato M and Hara S: Analysis on the Substrate specificity of
recombinant human Acyl-CoA synthetase ACSL4 variants. Biol Pharm
Bull. 42:850–855. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Igarashi Y and Kimura T: Adrenic acid
content in rat adrenal mitochondrial phosphatidylethanolamine and
its relation to ACTH-mediated stimulation of cholesterol side chain
cleavage reaction. J Biol Chem. 261:14118–14124. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Weigand I, Schreiner J, Röhrig F, Sun N,
Landwehr LS, Urlaub H, Kendl S, Kiseljak-Vassiliades K, Wierman ME,
Angeli JPF, et al: Active steroid hormone synthesis renders
adrenocortical cells highly susceptible to type II ferroptosis
induction. Cell Death Dis. 11:1922020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar :
|
|
98
|
Ansari IUH, Longacre MJ, Stoker SW,
Kendrick MA, O'Neill LM, Zitur LJ, Fernandez LA, Ntambi JM and
MacDonald MJ: Characterization of Acyl-CoA synthetase isoforms in
pancreatic beta cells: Gene silencing shows participation of ACSL3
and ACSL4 in insulin secretion. Arch Biochem Biophys. 618:32–43.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liu A, Chang J, Lin Y, Shen Z and
Bernstein PS: Long-chain and very long-chain polyunsaturated fatty
acids in ocular aging and age-related macular degeneration. J Lipid
Res. 51:3217–3229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Torres-Cuevas I, Millán I, Asensi M, Vento
M, Oger C, Galano JM, Durand T and Ortega ÁL: Analysis of lipid
peroxidation by UPLC-MS/MS and retinoprotective effects of the
natural polyphenol pterostilbene. Antioxidants (Basel). 10:1682021.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yuan Q, Zhu S, Yue S, Han Y, Peng G, Li L,
Sheng Y and Wang B: Alterations in faecal and serum metabolic
profiles in patients with neovascular age-related macular
degeneration. Nutrients. 15:29842023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Rohm TV, Meier DT, Olefsky JM and Donath
MY: Inflammation in obesity, diabetes, and related disorders.
Immunity. 55:31–55. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hussey B, Steel RP, Gyimah B, Reynolds JC,
Taylor IM, Lindley MR and Mastana S: DNA methylation of tumour
necrosis factor (TNF) alpha gene is associated with specific blood
fatty acid levels in a gender-specific manner. Mol Genet Genomic
Med. 9:e16792021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Cole JB and Florez JC: Genetics of
diabetes mellitus and diabetes complications. Nat Rev Nephrol.
16:377–390. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Szczuko M, Kaczkan M, Małgorzewicz S,
Rutkowski P, Dębska-Ślizień A and Stachowska E: The C18:3n6/C22:4n6
ratio is a good lipid marker of chronic kidney disease (CKD)
progression. Lipids Health Dis. 19:772020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tardy B, Bordet JC, Berruyer M, Ffrench P
and Dechavanne M: Priming effect of adrenic acid [22:4(n-6)] on
tissue factor activity expressed by thrombin-stimulated endothelial
cells. Atherosclerosis. 95:51–58. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ
and Han M: Inflammation and atherosclerosis: Signaling pathways and
therapeutic intervention. Signal Transduct Target Ther. 7:1312022.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Grootaert MOJ and Bennett MR: Vascular
smooth muscle cells in atherosclerosis: Time for a re-assessment.
Cardiovasc Res. 117:2326–2339. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Falk E: Pathogenesis of atherosclerosis. J
Am Coll Cardiol. 47:C7–C12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Campbell WB and Fleming I:
Epoxyeicosatrienoic acids and endothelium-dependent responses.
Pflugers Arch. 459:881–895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cagen LM and Baer PG: Adrenic acid
inhibits prostaglandin syntheses. Life Sci. 26:765–770. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Shi F, Chowdhury R, Sofianopoulou E,
Koulman A, Sun L, Steur M, Aleksandrova K, Dahm CC, Schulze MB, van
der Schouw YT, et al: Association of circulating fatty acids with
cardiovascular disease risk: Analysis of individual-level data in
three large prospective cohorts and updated meta-analysis. Eur J
Prev Cardiol. zwae3152024.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mazidi M, Shekoohi N, Katsiki N and Banach
M: Omega-6 fatty acids and the risk of cardiovascular disease:
Insights from a systematic review and meta-analysis of randomized
controlled trials and a Mendelian randomization study. Arch Med
Sci. 18:466–479. 2021.
|
|
114
|
Lee JCY, AlGhawas DS, Poutanen K, Leung
KS, Oger C, Galano JM, Durand T and El-Nezami H: Dietary oat bran
increases some proinflammatory polyunsaturated fatty-acid oxidation
products and reduces anti-inflammatory products in apolipoprotein
E−/− mice. Lipids. 53:785–796. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Henein MY, Vancheri S, Longo G and
Vancheri F: The role of inflammation in cardiovascular disease. Int
J Mol Sci. 23:129062022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Stojkovic S, Kaun C, Basilio J, Rauscher
S, Hell L, Krychtiuk KA, Bonstingl C, de Martin R, Gröger M, Ay C,
et al: Tissue factor is induced by interleukin-33 in human
endothelial cells: A new link between coagulation and inflammation.
Sci Rep. 6:251712016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
ten Cate H: Tissue factor-driven thrombin
generation and inflammation in atherosclerosis. Thromb Res.
129(Suppl 2): S38–S40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Badimon L and Vilahur G: Thrombosis
formation on atherosclerotic lesions and plaque rupture. J Intern
Med. 276:618–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Brambilla M, Camera M, Colnago D, Marenzi
G, De Metrio M, Giesen PL, Balduini A, Veglia F, Gertow K, Biglioli
P and Tremoli E: Tissue factor in patients with acute coronary
syndromes: Expression in platelets, leukocytes, and
platelet-leukocyte aggregates. Arterioscler Thromb Vasc Biol.
28:947–953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Grover SP and Mackman N: Tissue factor in
atherosclerosis and atherothrombosis. Atherosclerosis. 307:80–86.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Libby P: The changing landscape of
atherosclerosis. Nature. 592:524–533. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Engler MM, Bellenger-Germain SH, Engler
MB, Narce MM and Poisson JP: Dietary docosahexaenoic acid affects
stearic acid desaturation in spontaneously hypertensive rats.
Lipids. 35:1011–1015. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yu Z, Wang L, Wu S, Xue W, Zhao W and Li
J: Potential mechanisms of the anti-hypertensive effects of RVPSL
on spontaneously hypertensive rats using non-targeted serum
metabolomics. Food Funct. 12:8561–8569. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Li D, Yu XM, Xie HB, Zhang YH, Wang Q,
Zhou XQ, Yu P and Wang LJ: Platelet phospholipid n-3 PUFA
negatively associated with plasma homocysteine in middle-aged and
geriatric hyperlipaemia patients. Prostaglandins Leukot Essent
Fatty Acids. 76:293–297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Simon JA, Fong J, Bernert JT Jr and
Browner WS: Relation of smoking and alcohol consumption to serum
fatty acids. Am J Epidemiol. 144:325–334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yang LG, Song ZX, Yin H, Wang YY, Shu GF,
Lu HX, Wang SK and Sun GJ: Low n-6/n-3 PUFA ratio improves lipid
metabolism, inflammation, oxidative stress and endothelial function
in rats using plant oils as n-3 fatty acid source. Lipids.
51:49–59. 2016. View Article : Google Scholar
|
|
127
|
Hurtubise J, McLellan K, Durr K, Onasanya
O, Nwabuko D and Ndisang JF: The different facets of dyslipidemia
and hypertension in atherosclerosis. Curr Atheroscler Rep.
18:822016. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Talmor-Barkan Y, Bar N, Shaul AA, Shahaf
N, Godneva A, Bussi Y, Lotan-Pompan M, Weinberger A, Shechter A,
Chezar-Azerrad C, et al: Metabolomic and microbiome profiling
reveals personalized risk factors for coronary artery disease. Nat
Med. 28:295–302. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Fan Y, Li Y, Chen Y, Zhao YJ, Liu LW, Li
J, Wang SL, Alolga RN, Yin Y, Wang XM, et al: Comprehensive
metabolomic characterization of coronary artery diseases. J Am Coll
Cardiol. 68:1281–1293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Smith ML, Bull CJ, Holmes MV, Davey Smith
G, Sanderson E, Anderson EL and Bell JA: Distinct metabolic
features of genetic liability to type 2 diabetes and coronary
artery disease: A reverse Mendelian randomization study.
EBioMedicine. 90:1045032023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Chen H, Tan H, Wan J, Zeng Y, Wang J, Wang
H and Lu X: PPAR-γ signaling in nonalcoholic fatty liver disease:
Pathogenesis and therapeutic targets. Pharmacol Ther.
245:1083912023. View Article : Google Scholar
|
|
132
|
Scime NV, Turner S, Miliku K, Simons E,
Moraes TJ, Field CJ, Turvey SE, Subbarao P, Mandhane PJ and Azad
MB: Association of human milk fatty acid composition with maternal
cardiometabolic diseases: An exploratory prospective cohort study.
Breastfeed Med. 19:357–367. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Svennerholm L: Distribution and fatty acid
composition of phosphoglycerides in normal human brain. J Lipid
Res. 9:570–579. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wilson R and Sargent JR: Lipid and fatty
acid composition of brain tissue from adrenoleukodystrophy
patients. J Neurochem. 61:290–297. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wijendran V, Lawrence P, Diau GY, Boehm G,
Nathanielsz PW and Brenna JT: Significant utilization of dietary
arachidonic acid is for brain adrenic acid in baboon neonates. J
Lipid Res. 43:762–767. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Rapoport SI: Translational studies on
regulation of brain docosahexaenoic acid (DHA) metabolism in vivo.
Prostaglandins Leukot Essent Fatty Acids. 88:79–85. 2013.
View Article : Google Scholar
|
|
137
|
Yoshinaga K, Ishikawa H, Beppu F and Gotoh
N: Incorporation of dietary arachidonic and docosatetraenoic acid
into mouse brain. J Agric Food Chem. 69:2457–2461. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Grande de França NA, Díaz G, Lengelé L,
Soriano G, Caspar-Bauguil S, Saint-Aubert L, Payoux P, Rouch L,
Vellas B, de Souto Barreto P and Sourdet S: Associations between
blood nutritional biomarkers and cerebral amyloid-β: Insights from
the COGFRAIL cohort study. J Gerontol A Biol Sci Med Sci.
79:glad2482024. View Article : Google Scholar
|
|
139
|
Hammouda S, Ghzaiel I, Khamlaoui W,
Hammami S, Mhenni SY, Samet S, Hammami M and Zarrouk A: Genetic
variants in FADS1 and ELOVL2 increase level of arachidonic acid and
the risk of Alzheimer's disease in the Tunisian population.
Prostaglandins Leukot Essent Fatty Acids. 160:1021592020.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Durand T, De Felice C, Signorini C, Oger
C, Bultel-Poncé V, Guy A, Galano JM, Leoncini S, Ciccoli L,
Pecorelli A, et al: F(2)-Dihomo-isoprostanes and brain white matter
damage in stage 1 Rett syndrome. Biochimie. 95:86–90. 2013.
View Article : Google Scholar
|
|
141
|
Zhou K, Jia L, Mao Z, Si P, Sun C, Qu Z
and Wang W: Integrated macrogenomics and metabolomics explore
alterations and correlation between gut microbiota and serum
metabolites in adult epileptic patients: A pilot study.
Microorganisms. 11:26282023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Xie J, Zhou F, Ouyang L, Li Q, Rao S, Su
R, Yang S, Li J, Wan X, Yan L, et al: Insight into the effect of a
heavy metal mixture on neurological damage in rats through combined
serum metabolomic and brain proteomic analyses. Sci Total Environ.
895:1650092023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Thapar A, Cooper M and Rutter M:
Neurodevelopmental disorders. Lancet Psychiatry. 4:339–346. 2017.
View Article : Google Scholar
|
|
144
|
Sumich AL, Matsudaira T, Heasman B, Gow
RV, Ibrahimovic A, Ghebremeskel K, Crawford MA and Taylor E: Fatty
acid correlates of temperament in adolescent boys with attention
deficit hyperactivity disorder. Prostaglandins Leukot Essent Fatty
Acids. 88:431–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wu CH, Hsu WL, Tsai CC, Chao HR, Wu CY,
Chen YH, Lai YR, Chen CH and Tsai MH: 7,10,13,16-Docosatetraenoic
acid impairs neurobehavioral development by increasing reactive
oxidative species production in Caenorhabditis elegans. Life Sci.
319:1215002023. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
De Felice C, Signorini C, Durand T, Oger
C, Guy A, Bultel-Poncé V, Galano JM, Ciccoli L, Leoncini S,
D'Esposito M, et al: F2-dihomo-isoprostanes as potential early
biomarkers of lipid oxidative damage in Rett syndrome. J Lipid Res.
52:2287–2297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Khan S, Barve KH and Kumar MS: Recent
advancements in pathogenesis, diagnostics and treatment of
Alzheimer's disease. Curr Neuropharmacol. 18:1106–1125. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Liu RM: Aging, cellular senescence, and
Alzheimer's disease. Int J Mol Sci. 23:19892022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Kosicek M and Hecimovic S: Phospholipids
and Alzheimer's disease: Alterations, mechanisms and potential
biomarkers. Int J Mol Sci. 14:1310–1322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Hancock SE, Friedrich MG, Mitchell TW,
Truscott RJW and Else PL: Decreases in phospholipids containing
adrenic and arachidonic acids occur in the human hippocampus over
the adult lifespan. Lipids. 50:861–872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hancock SE, Friedrich MG, Mitchell TW,
Truscott RJW and Else PL: Changes in phospholipid composition of
the human cerebellum and motor cortex during normal ageing.
Nutrients. 14:24952022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Corrigan FM, Horrobin DF, Skinner ER,
Besson JA and Cooper MB: Abnormal content of n-6 and n-3 long-chain
unsaturated fatty acids in the phosphoglycerides and cholesterol
esters of parahippocampal cortex from Alzheimer's disease patients
and its relationship to acetyl CoA content. Int J Biochem Cell
Biol. 30:197–207. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Cullen NC, Novak P, Tosun D, Kovacech B,
Hanes J, Kontsekova E, Fresser M, Ropele S, Feldman HH, Schmidt R,
et al: Efficacy assessment of an active tau immunotherapy in
Alzheimer's disease patients with amyloid and tau pathology: A post
hoc analysis of the 'ADAMANT' randomised, placebo-controlled,
double-blind, multi-centre, phase 2 clinical trial. EBioMedicine.
99:1049232024. View Article : Google Scholar
|
|
154
|
Pascoal TA, Aguzzoli CS, Lussier FZ,
Crivelli L, Suemoto CK, Fortea J, Rosa-Neto P, Zimmer ER, Ferreira
PCL and Bellaver B: Insights into the use of biomarkers in clinical
trials in Alzheimer's disease. EBioMedicine. 108:1053222024.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Skinner ER, Watt C, Besson JA and Best PV:
Differences in the fatty acid composition of the grey and white
matter of different regions of the brains of patients with
Alzheimer's disease and control subjects. Brain. 116:717–725. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Miller E, Morel A, Saso L and Saluk J:
Isoprostanes and neuroprostanes as biomarkers of oxidative stress
in neurodegenerative diseases. Oxid Med Cell Longev.
2014:5724912014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Roberts LJ II and Milne GL: Isoprostanes.
J Lipid Res. 50(Suppl): S219–S223. 2009. View Article : Google Scholar :
|
|
158
|
Calo L, Wegrzynowicz M, Santivañez-Perez J
and Grazia Spillantini M: Synaptic failure and α-synuclein. Mov
Disord. 31:169–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ugalde CL, Lawson VA, Finkelstein DI and
Hill AF: The role of lipids in α-synuclein misfolding and
neurotoxicity. J Biol Chem. 294:9016–9028. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Xylaki M, Boumpoureka I, Kokotou MG,
Marras T, Papadimitriou G, Kloukina I, Magrioti V, Kokotos G,
Vekrellis K and Emmanouilidou E: Changes in the cellular fatty acid
profile drive the proteasomal degradation of α-synuclein and
enhance neuronal survival. FASEB J. 34:15123–15145. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Malhi GS and Mann JJ: Depression. Lancet.
392:2299–2312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Aguilar-Valles A, Kim J, Jung S, Woodside
B and Luheshi GN: Role of brain transmigrating neutrophils in
depression-like behavior during systemic infection. Mol Psychiatry.
19:599–606. 2014. View Article : Google Scholar
|
|
163
|
Zeng L, Lv H, Wang X, Xue R, Zhou C, Liu X
and Yu H: Causal effects of fatty acids on depression: Mendelian
randomization study. Front Nutr. 9:10104762022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Vaz JS, Kac G, Nardi AE and Hibbeln JR:
Omega-6 fatty acids and greater likelihood of suicide risk and
major depression in early pregnancy. J Affect Disord.
152-154:76–82. 2014. View Article : Google Scholar
|
|
165
|
Chen H, Wang J, Zheng B, Xia W, Tan G, Wu
H, Wang Y, Deng Z, Wang Y, Zhang J and Zhang H: Association of
serum fatty acid pattern with depression in U.S. adults: Analysis
of NHANES 2011-2012. Lipids Health Dis. 23:1772024. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Wang M, Yan X, Li Y, Li Q, Xu Y, Huang J,
Gan J and Yang W: Association between plasma polyunsaturated fatty
acids and depressive among US adults. Front Nutr. 11:13423042024.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Liu T, Wang L, Guo J, Zhao T, Tang H, Dong
F, Wang C, Chen J and Tang M: Erythrocyte membrane fatty acid
composition as a potential biomarker for depression. Int J
Neuropsychopharmacol. 26:385–395. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Pan J, Lu Y, Wang S, Ma T, Xue X, Zhang Z,
Mao Q, Guo D and Ma K: Synergistic neuroprotective effects of two
natural medicinal plants against CORT-induced nerve cell injury by
correcting neurotransmitter deficits and inflammation imbalance.
Phytomedicine. 121:1551022023. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Liu L, Wang H, Chen X, Zhang Y, Zhang H
and Xie P: Gut microbiota and its metabolites in depression: From
pathogenesis to treatment. EBioMedicine. 90:1045272023. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Shang Y, Wang M, Hao Q, Meng T, Li L, Shi
J, Yang G, Zhang Z, Yang K and Wang J: Development of
indole-2-carbonyl piperazine urea derivatives as selective FAAH
inhibitors for efficient treatment of depression and pain. Bioorg
Chem. 128:1060312022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Tripathi RKP: A perspective review on
fatty acid amide hydrolase (FAAH) inhibitors as potential
therapeutic agents. Eur J Med Chem. 188:1119532020. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Oliveira-Lima OC, Carvalho-Tavares J,
Rodrigues MF, Gomez MV, Oliveira ACP, Resende RR, Gomez RS, Vaz BG
and Pinto MCX: Lipid dynamics in LPS-induced neuroinflammation by
DESI-MS imaging. Brain Behav Immun. 79:186–194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Celik O and Yildiz BO: Obesity and
physical exercise. Minerva Endocrinol (Torino). 46:131–144.
2021.
|
|
174
|
Cassilhas RC, Tufik S and de Mello MT:
Physical exercise, neuroplasticity, spatial learning and memory.
Cell Mol Life Sci. 73:975–983. 2016. View Article : Google Scholar
|
|
175
|
Rodríguez-Cañamero S, Cobo-Cuenca AI,
Carmona-Torres JM, Pozuelo-Carrascosa DP, Santacruz-Salas E,
Rabanales-Sotos JA, Cuesta-Mateos T and Laredo-Aguilera JA: Impact
of physical exercise in advanced-stage cancer patients: Systematic
review and meta-analysis. Cancer Med. 11:3714–3727. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Dong G: Swimming exercise ameliorates
liver insulin resistance in type 2 diabetic mice-the role of
adrenic acid. Shanghai University of Sport; 2021, View Article : Google Scholar
|
|
177
|
Jurado-Fasoli L, Di X, Sanchez-Delgado G,
Yang W, Osuna-Prieto FJ, Ortiz-Alvarez L, Krekels E, Harms AC,
Hankemeier T, Schönke M, et al: Acute and long-term exercise
differently modulate plasma levels of oxylipins, endocannabinoids,
and their analogues in young sedentary adults: A sub-study and
secondary analyses from the ACTIBATE randomized controlled-trial.
EBioMedicine. 85:1043132022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Gao Y, Sun YY, Bai D and Wu XX: Mechanism
of the components compatibility of Scutellariae Radix and Coptidis
Rhizoma on mice with hyperlipidemia by regulating the Cyp4a family.
J Ethnopharmacol. 331:1182632024. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Mi Y, Yi N, Xu X, Zeng F, Li N, Tan X,
Gong Z, Yan K, Kuang G and Lu M: Prebiotics alleviate cartilage
degradation and inflammation in post-traumatic osteoarthritic mice
by modulating the gut barrier and fecal metabolomics. Food Funct.
14:4065–4077. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Aukema HM and Holub BJ: Effect of dietary
supplementation with a fish oil concentrate on the alkenylacyl
class of ethanolamine phospholipid in human platelets. J Lipid Res.
30:59–64. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Hernández MC, Rojas P, Carrasco F,
Basfi-Fer K, Valenzuela R, Codoceo J, Inostroza J and Ruz M: Fatty
acid desaturation in red blood cell membranes of patients with type
2 diabetes is improved by zinc supplementation. J Trace Elem Med
Biol. 62:1265712020. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Arcusa R, Carillo JÁ, Cerdá B, Durand T,
Gil-Izquierdo Á, Medina S, Galano JM, Zafrilla MP and Marhuenda J:
Ability of a polyphenol-rich nutraceutical to reduce central
nervous system lipid peroxidation by analysis of oxylipins in
urine: A randomized, double-blind, placebo-controlled clinical
trial. Antioxidants (Basel). 12:7212023. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Flaskerud K, Bukowski M, Golovko M,
Johnson L, Brose S, Ali A, Cleveland B, Picklo M Sr and Raatz S:
Effects of cooking techniques on fatty acid and oxylipin content of
farmed rainbow trout (Oncorhynchus mykiss). Food Sci Nutr.
5:1195–1204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Zhou D, Zaiger G, Ghebremeskel K, Crawford
MA and Reifen R: Vitamin A deficiency reduces liver and colon
docosahexaenoic acid levels in rats fed high linoleic and low
alpha-linolenic acid diet. Prostaglandins Leukot Essent Fatty
Acids. 71:383–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Martínez-Ramírez HR, Kramer JKG and de
Lange CFM: Retention of n-3 polyunsaturated fatty acids in trimmed
loin and belly is independent of timing of feeding ground flaxseed
to growing-finishing female pigs. J Anim Sci. 92:238–249. 2014.
View Article : Google Scholar
|
|
186
|
Jiao Y, Liu S, Zhou Y, Yang D, Li J and
Cui Z: The effect of supplemental concentrate feeding on the
morphological and functional development of the pancreas in early
weaned yak calves. Animals (Basel). 12:25632022. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Dua TK, Dewanjee S, Khanra R, Joardar S,
Barma S, Das S, Zia-Ul-Haq M and De Feo V: Cytoprotective and
antioxidant effects of an edible herb, enhydra fluctuans lour.
(Asteraceae), against experimentally induced lead acetate
intoxication. PLoS One. 11:e01487572016. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Xia J, Lu L, Jin C, Wang S, Zhou J, Ni Y,
Fu Z and Jin Y: Effects of short term lead exposure on gut
microbiota and hepatic metabolism in adult zebrafish. Comp Biochem
Physiol C Toxicol Pharmacol. 209:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Mateo R, Beyer WN, Spann JW and Hoffman
DJ: Relation of fatty acid composition in lead-exposed mallards to
fat mobilization, lipid peroxidation and alkaline phosphatase
activity. Comp Biochem Physiol C Toxicol Pharmacol. 135:451–458.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Aléssio ML, Léger CL, Rasolonjanahary R,
Wandscheer DE, Clauser H, Enjalbert A and Kordon C: Selective
effect of a diet-induced decrease in the arachidonic acid
membranephospholipid content on in vitro phospholipase C and
adenylate cyclase-mediated pituitary response to angiotensin II.
Neuroendocrinology. 60:400–409. 1994. View Article : Google Scholar
|
|
191
|
Stylianopoulou F and Clayton RB:
Strain-dependent gonadal effects upon adrenal cholesterol ester
concentration and composition in C57BL/10J and DBA/2J mice.
Endocrinology. 99:1631–1637. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Marhuenda J, Medina S, Martínez-Hernández
P, Arina S, Zafrilla P, Mulero J, Oger C, Galano JM, Durand T,
Ferreres F and Gil-Izquierdo A: Melatonin and hydroxytyrosol
protect against oxidative stress related to the central nervous
system after the ingestion of three types of wine by healthy
volunteers. Food Funct. 8:64–74. 2017. View Article : Google Scholar
|
|
193
|
Parada H Jr, Wu T, Hoh E, Rock CL and
Martinez ME: Red blood cell polyunsaturated fatty acids and
mortality following breast cancer. Cancer Epidemiol Biomarkers
Prev. 33:944–952. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Dayaker G, Durand T and Balas L: Total
synthesis of neuroprotectin D1 analogues derived from omega-6
docosapentaenoic acid (DPA) and adrenic acid (AdA) from a common
pivotal, late-stage intermediate. J Org Chem. 79:2657–2665. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Bazan NG: Neuroprotectin D1 (NPD1): A
DHA-derived mediator that protects brain and retina against cell
injury-induced oxidative stress. Brain Pathol. 15:159–166. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Stark DT and Bazan NG: Neuroprotectin D1
induces neuronal survival and downregulation of amyloidogenic
processing in Alzheimer's disease cellular models. Mol Neurobiol.
43:131–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Dayaker G, Durand T and Balas L: A
versatile and stereocontrolled total synthesis of dihydroxylated
docosatrienes containing a conjugated E,E,Z-triene. Chemistry.
20:2879–2887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Messamore E, Hoffman WF and Yao JK: Niacin
sensitivity and the arachidonic acid pathway in schizophrenia.
Schizophr Res. 122:248–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Brouwers H, Jónasdóttir HS, Kuipers ME,
Kwekkeboom JC, Auger JL, Gonzalez-Torres M, López-Vicario C, Clària
J, Freysdottir J, Hardardottir I, et al: Anti-inflammatory and
proresolving effects of the omega-6 polyunsaturated fatty acid
adrenic acid. J Immunol. 205:2840–2849. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Song T and Kuang S: Adipocyte
dedifferentiation in health and diseases. Clin Sci (Lond).
133:2107–2119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Villarroya F, Cereijo R, Gavalda-Navarro
A, Villarroya J and Giralt M: Inflammation of brown/beige adipose
tissues in obesity and metabolic disease. J Intern Med.
284:492–504. 2018. View Article : Google Scholar : PubMed/NCBI
|